Abstract
Introduction:
Rates of gestational diabetes mellitus (GDM) are increasing in parallel with rates of overweight and obesity. This analysis examines nationwide trends in the population-attributable fraction for GDM associated with prepregnancy overweight and obesity.
Methods:
A serial, cross-sectional study was performed using U.S. population-based Birth Data Files maintained by the National Center for Health Statistics between 2011 and 2019. Live singleton births to nulliparous women aged 15–44 years were included, and all analyses were stratified by race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, non-Hispanic Asian). Prevalences of prepregnancy overweight (25.0–29.9 kg/m2, 23.0–27.4 kg/m2) and obesity (≥30.0 kg/m2, ≥27.5 kg/m2) based on standard and Asian-specific BMI categories, respectively, were quantified. Logistic regression estimated adjusted associations between prepregnancy overweight and obesity and GDM with normal weight (18.0–24.9 kg/m2, 18.0–22.9 kg/m2) as the ref. Annual population-attributable fractions for GDM associated with prepregnancy overweight and obesity were calculated, which account for both prevalence of the risk factor and the associated risk of GDM.
Results:
Among 11,950,881 included women, mean maternal age was 26.3 years. Population-attributable fractions for GDM associated with overweight were stable (Hispanic: 12.0% to 11.3%, non-Hispanic Asian: 12.1% to 11.6%, p≥0.20) or decreased (non-Hispanic White: 10.8% to 9.4%, non-Hispanic Black: 12.3% to 9.2%, p<0.002); population-attributable fractions for GDM associated with obesity were stable (non-Hispanic Black: 36.3% to 37.9%, p=0.11) or increased (non-Hispanic White: 30.9% to 33.3%, Hispanic: 27.2% to 33.3%, non-Hispanic Asian 12.2% to 15.4%, p<0.001).
Conclusions:
Population-attributable fractions for GDM associated with obesity largely increased in the past decade, underscoring the importance of optimizing weight before pregnancy.
Keywords: gestational diabetes mellitus, Overweight, Obesity, population attributable fraction
INTRODUCTION
Gestational diabetes mellitus (GDM), defined as glucose intolerance that develops in pregnancy and most commonly diagnosed with the oral glucose tolerance test, complicates approximately 6%–8% of all pregnancies in the U.S.1 The American College of Obstetrics and Gynecology recommends universal screening for GDM in pregnant women, usually between 24 and 28 weeks gestation.2 Prevalence of GDM has increased in recent years and is associated with an annual economic burden of nearly $1.6 billion.3,4 Moreover, GDM is a strong risk factor not only for incident type 2 diabetes mellitus (T2DM) after pregnancy but also future subclinical atherosclerosis and symptomatic cardiovascular disease independent of subsequent diabetes status, even among those who maintain normoglycemia.5–7 Additionally, children of mothers with GDM are at higher risk of obesity and impaired glucose tolerance.8,9 Significant and persistent racial/ethnic disparities in GDM exist, and the observed intergenerational transmission of cardiometabolic risk may exacerbate prevalent racial/ethnic disparities in cardiometabolic disease.3 Taken together, these data support the importance of identifying strategies for GDM prevention.
Overweight and obesity prior to pregnancy are important risk factors for GDM. Observational data suggest that prepregnancy weight loss in women with obesity may decrease risk of GDM,10,11 and interventional studies, outside of pregnancy, have demonstrated the benefit of weight loss on related metabolic outcomes such as T2DM .12 By contrast, interventions during pregnancy have been unable to consistently demonstrate a benefit to reduce risk of GDM,13,14 underscoring the importance of prepregnancy intervention, which may have greater benefit. Prevalence of overweight and obesity have also been increasing in recent years, reaching 31.1% and 42.5%, respectively, among adults aged ≥20 years in 2017–2018 in the U.S.; these increases have occurred in parallel with increasing rates of GDM over this same timeframe.3,15 Quantifying the proportion of GDM that could potentially be reduced with optimization of maternal BMI prior to pregnancy may help elucidate the population impact of strategies targeting weight loss in women of reproductive age with overweight or obesity. Population-attributable fractions (PAFs) account for both the prevalence of the risk factor and the excess risk of a disease associated with that risk factor, and are an important metric to inform public health policies. Therefore, this analysis calculates annual estimates of PAFs for GDM associated with prepregnancy overweight and obesity by race and ethnicity between 2011 and 2019, and identifies trends in PAF over time. The objective is to describe the contribution of increasing prevalence of overweight and obesity to rising occurrence of GDM.
METHODS
This study used maternal data from the National Center for Health Statistics Birth Data Files, which captures all live births in the U.S., from 2011 to 2019. Birth records are completed by the medical professional who attended the delivery, using maternal self-report and medical record abstraction when appropriate.16 Pre-gestational DM, GDM, and prepregnancy BMI were included on birth records starting with the 2003 revision of the U.S. Standard Certificate of Live Birth. Individual states variably adopted this revised certificate16 with adoption in a majority of states by 2011 and in all states by 2016.
Study Population
Maternal data were included from all live, singleton births to nulliparous women aged 15–44 years, without pre-gestational DM, who self-identified as non-Hispanic White, non-Hispanic Black, Hispanic, or non-Hispanic Asian (Appendix Figure 1). The analysis was restricted to nulliparous women in order to eliminate differential risk of GDM based on prior pregnancy history of GDM. Women with pre-gestational DM were excluded, as GDM and pre-gestational DM are mutually exclusive on birth certificates. Records that did not use the 2003 revised birth certificate (4.6%) and those missing data on prepregnancy BMI or GDM status (3.1%) were also excluded. This study was exempt from review by the Northwestern University IRB given the de-identified, publicly available nature of the data. This manuscript adheres to the STROBE statement.17
Measures
The exposure was prepregnancy overweight or obesity defined by BMI, which is determined by maternal self-report of prepregnancy height and weight.18 For non-Hispanic White, non-Hispanic Black, and Hispanic women, WHO standard BMI categories (underweight [<18.5 kg/m2], normal weight [18.5–24.9 kg/m2], overweight [25.0–29.9 kg/m2], and obese [≥30.0 kg/m2]) were used. For non-Hispanic Asian women, modified BMI categories according to American Diabetes Association and WHO recommendations (underweight [<18.5 kg/m2], normal weight [18.5–22.9 kg/m2], overweight [23.0–27.4 kg/m2], and obese [≥27.5 kg/m2]) were used.19,20
The outcome was GDM, which was a yes/no binary variable defined as a diagnosis of glucose intolerance during the pregnancy.18 GDM status was determined by the delivery attendant guided by medical record review. Covariates included maternal age, education level, private insurance, receipt of prenatal care, and smoking during pregnancy. Education level was divided into 3 categories based on the highest level of education completed: less than high school, high school graduate, or greater than high school. The remaining variables were coded as yes/no binary variables indicating whether the mother used private insurance, received any prenatal care, or smoked during the pregnancy. All covariate data were based on maternal self-report. As each covariate was missing <3% of data, a complete case analysis was performed.
Statistical Analysis
Owing to known racial/ethnic disparities in GDM,3 all analyses were stratified by race/ethnicity. Descriptive statistics of maternal characteristics were calculated. Age-standardized rates of GDM were then computed using the age distribution of women with live births in 2011 as the reference population. Next, the annual prevalences of overweight and obesity were calculated. Associations between BMI category and GDM were calculated using multivariable logistic regression (normal weight as the ref) adjusted for maternal age at delivery, education, private insurance, receipt of prenatal care, and smoking during pregnancy. Confounding variables were chosen based on known associations with GDM and availability in the data set.
The logistic regression models were then used to determine annual PAFs for GDM associated with overweight and obesity. The statistical module punaf in Stata21 uses the formula described by Rockhill et al.22 to compute PAFs. Specifically, this formula is:
where P(D) is the probability of disease in the population and is the marginal conditional probability of disease averaged over a set of confounders (C) and counterfactual exposures (E). The module punaf uses logistic regression models to compute marginal prevalences of GDM under 2 scenarios: the observed data (representing P(D)) and a counterfactual scenario in which a risk factor, such as obesity, is removed from the population (representing ). The PAF is the difference between the 2 marginal prevalences divided by the marginal prevalence in the observed data and thus varies from 0 to 1, which the authors transformed to percentages (0%–100%). The PAF is interpreted as the percentage of GDM cases that would potentially not occur if the risk factor (overweight or obesity) was eliminated from the population. Differences in PAF between the first and last years of the study period (2011 and 2019) were tested using a method for log-transformed statistics.23 In a secondary analysis, PAFs were calculated within the top 3 Hispanic (Mexican, Puerto Rican, and Central or South American) and non-Hispanic Asian (Asian Indian, Chinese, and Filipina) subgroups by population. All analyses were conducted using Stata, version 15.1. A p-value <0.05 was considered statistically significant.
RESULTS
From 2011 to 2019, of 11,950,881 live births to nulliparous women aged 15–44 years, 57.0% were in non-Hispanic White, 14.1% in non-Hispanic Black, 21.1% in Hispanic (11.9% Mexican, 1.7% Puerto Rican, 3.1% Central or South American), and 7.8% in non-Hispanic Asian (3.1% Asian Indian, 2.2% Chinese, 1.8% Filipina) women. Mean age was 26.3 (SD=5.8) years. Non-Hispanic Black and Hispanic women were, on average, younger than non-Hispanic White and non-Hispanic Asian women, with lower educational attainment and greater use of Medicaid (Table 1). Women who were underweight prior to pregnancy were younger on average than those in other BMI categories, and a greater proportion smoked during pregnancy (Appendix Table 1). For all race/ethnicity groups, the prepregnancy prevalence of both overweight and obesity increased from 2011 to 2019, whereas the prevalence of normal weight decreased (Table 2). In 2019, approximately half of women had prepregnancy overweight or obesity, with prevalence ranging from 48.0% in non-Hispanic Asian women to 58.7% in non-Hispanic Black women.
Table 1.
Maternal Characteristics by Race/Ethnicity in Nulliparous Women in the U.S. (2011‒2019)
| Characteristic | Non-Hispanic White | Non-Hispanic black | Hispanic | Non-Hispanic Asian |
|---|---|---|---|---|
| N | 6,809,923 | 1,683,189 | 2,520,227 | 937,542 |
| Maternal age, mean (SD) | 27.0 (5.6) | 24.3 (5.7) | 24.4 (5.7) | 29.8 (5.0) |
| BMI category, n (%) | ||||
| Underweighta | 274,069 (4.0) | 74,139 (4.4) | 103,128 (4.1) | 88,298 (9.4) |
| Normal weighta | 3,475,075 (51.0) | 680,766 (40.4) | 1,183,361 (47.0) | 450,115 (48.0) |
| Overweighta | 1,620,041 (23.8) | 430,961 (25.6) | 674,395 (26.8) | 267,892 (28.6) |
| Obesea | 1,440,738 (21.2) | 497,323 (29.5) | 559,343 (22.2) | 131,237 (14.0) |
| Education, n (%) | ||||
| Less than high school | 483,548 (7.1) | 254,566 (15.2) | 574,702 (23.0) | 49,838 (5.4) |
| High school graduate | 1,394,432 (20.6) | 547,441 (32.7) | 807,271 (32.3) | 104,792 (11.3) |
| Greater than high school | 4,901,517 (72.3) | 870,609 (52.1) | 1,113,755 (44.6) | 771,467 (83.3) |
| Payment method, n (%) | ||||
| Private insurance | 4470104 (66.2) | 519028 (31.1) | 808620 (32.4) | 637580 (68.4) |
| Medicaid | 1875746 (27.8) | 1034791 (62.0) | 1417045 (56.7) | 209221 (22.5) |
| Other/Self pay | 409,449 (6.0) | 116,438 (6.9) | 273,105 (10.8) | 85,003 (9.1) |
| Received prenatal care, n (%) | 6,627,605 (99.2) | 1,588,633 (97.9) | 2,416,122 (98.1) | 911,669 (99.2) |
| Smoked during pregnancy, n (%) | 611,119 (9.2) | 62,434 (3.8) | 38,696 (1.6) | 6,673 (0.7) |
Underweight (<18.5 kg/m2); Normal weight (18.5–24.9 kg/m2 [18.5–22.9 kg/m2 for Asian women]); Overweight (25.0–29.9 kg/m2 [23.0–27.4 kg/m2 for Asian women]); Obese (≥30.0 kg/m2 [≥27.5 kg/m2 for Asian women]).
Table 2.
Prevalence of Prepregnancy Overweight and Obesity and Associations With Gestational Diabetes in Nulliparous Women
| 2011 | 2015 | 2019 | ||||
|---|---|---|---|---|---|---|
| Race/ethnicity | Prevalence (%) | AOR of GDM (95% CI) | Prevalence (%) | AOR of GDM (95% CI) | Prevalence (%) | AOR of GDM (95% CI) |
| Non-Hispanic White | ||||||
| Normal weight | 53.6 | ref | 51.5 | ref | 47.2 | ref |
| Overweight | 22.9 | 1.85 (1.79, 1.91) | 23.7 | 1.78 (1.73, 1.83) | 25.2 | 1.69 (1.64, 1.73) |
| Obese | 19.0 | 4.06 (3.94, 4.18) | 20.8 | 3.83 (3.74, 3.93) | 24.3 | 3.73 (3.64, 3.82) |
| Non-Hispanic Black | ||||||
| Normal weight | 43.8 | ref | 40.7 | ref | 37.3 | ref |
| Overweight | 25.1 | 1.94 (1.79, 2.11) | 25.4 | 1.70 (1.59, 1.83) | 25.9 | 1.68 (1.57, 1.80) |
| Obese | 26.3 | 3.64 (3.39, 3.92) | 29.5 | 3.12 (2.94, 3.32) | 32.8 | 3.22 (3.04, 3.42) |
| Hispanic | ||||||
| Normal weight | 51.0 | ref | 47.9 | ref | 41.7 | ref |
| Overweight | 25.4 | 1.79 (1.69, 1.89) | 26.5 | 1.89 (1.80, 1.99) | 28.6 | 1.75 (1.67, 1.83) |
| Obese | 19.0 | 3.39 (3.22, 3.57) | 21.4 | 3.79 (3.62, 3.96) | 26.2 | 3.51 (3.37, 3.66) |
| Non-Hispanic Asian | ||||||
| Normal weight | 51.1 | ref | 48.3 | ref | 44.5 | ref |
| Overweight | 26.7 | 1.65 (1.55, 1.76) | 28.3 | 1.71 (1.63, 1.80) | 30.9 | 1.59 (1.52, 1.66) |
| Obese | 11.6 | 2.67 (2.49, 2.88) | 13.8 | 2.70 (2.55, 2.85) | 17.1 | 2.57 (2.45, 2.70) |
GDM, gestational diabetes mellitus.
Across all race/ethnicity groups, age-standardized rates of GDM were successively higher in each higher BMI category and increased from 2011 to 2019 for all BMI categories (Figure 1 and Appendix Table 2). Among women with obesity prior to pregnancy, age-standardized rates of GDM in 2019 ranged from 88.4 per 1,000 live births in non-Hispanic Black women to 169.7 per 1,000 live births in non-Hispanic Asian women.
Figure 1.
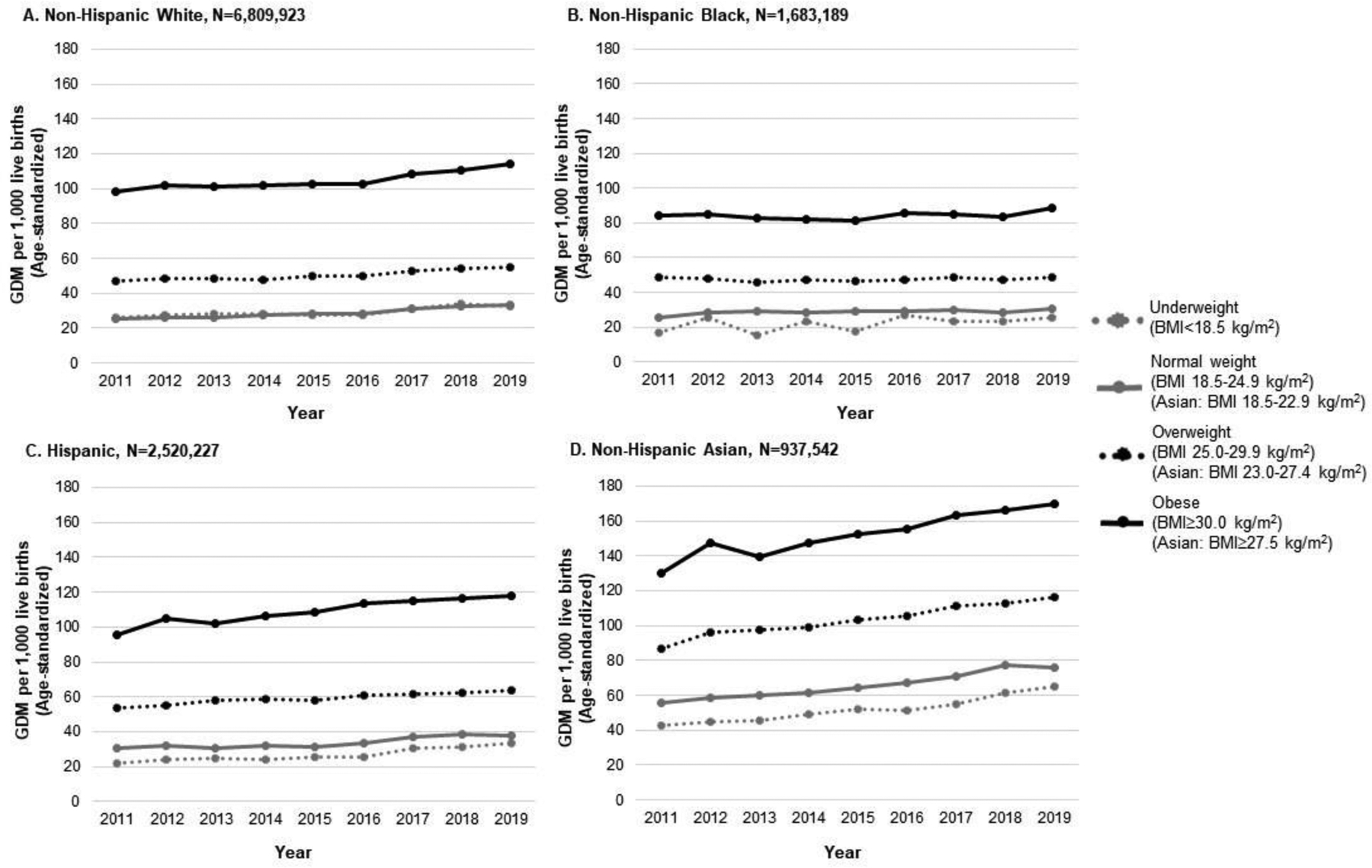
Trends in age-standardized rates of gestational diabetes mellitus stratified by maternal race/ethnicity and BMI category in nulliparous women in the U.S. (2011‒2019).Note: The figure shows annual trends in age-standardized gestational diabetes mellitus rates between 2011 and 2019 stratified by race/ethnicity and BMI category prior to pregnancy. Age-standardized rates of GDM were generally greater with higher prepregnancy BMI category and increased between 2011 and 2019.
GDM, gestational diabetes mellitus.
Prepregnancy overweight and obesity were each associated with GDM from 2011 to 2019 for all race/ethnicity groups (Table 2). Adjusted odds of GDM were higher for prepregnancy overweight and highest for prepregnancy obesity relative to normal weight. For example, in 2019, AORs for Hispanic women with overweight BMI and obesity, respectively, were 1.75 (95% CI=1.67, 1.83) and 3.51 (95% CI=3.37, 3.66). Across race/ethnicity groups in 2019, stratified ORs for prepregnancy overweight ranged from 1.59 (95% CI=1.52, 1.66) in non-Hispanic Asian women to 1.75 (95% CI=1.67, 1.83) in Hispanic women, and for prepregnancy obesity ranged from 2.57 (95% CI=2.45, 2.70) in non-Hispanic Asian women to 3.73 (95% CI=3.64, 3.82) in non-Hispanic White women.
The PAFs for GDM associated with prepregnancy overweight (compared with normal weight) decreased from 2011 to 2019 in non-Hispanic White women from 10.8% (95% CI=10.2, 11.4) to 9.4% (95% CI=8.9, 9.9, p<0.001), and in non-Hispanic Black women from 12.3% (95% CI=10.7, 13.8) to 9.2 (95% CI=8.0, 10.4, p=0.001). Differences in PAFs from 2011 to 2019 were not statistically significant in Hispanic women, changing from 12.0% (95% CI=10.8, 13.2) to 11.3% (95% CI=10.4, 12.3, p=0.20), or in non-Hispanic Asian women, changing from 12.1% (95% CI=10.5, 13.6) to 11.6% (95% CI=10.4, 12.7, p=0.30) (Table 3 and Appendix Table 3).
Table 3.
Population Attributable Fractions for Gestational Diabetes Associated With Prepregnancy Overweight and Obesity in Nulliparous Women
| 2011 | 2015 | 2019 | p-value for differenceb | |
|---|---|---|---|---|
| Race/ethnicity | Population attributable fraction,a % (95% CI) | 2011 vs 2019 | ||
| Non-Hispanic White | ||||
| Normal weight | ref | ref | ref | |
| Overweight | 10.8 (10.2, 11.4) | 10.4 (9.8, 10.9) | 9.4 (8.9, 9.9) | <0.001 |
| Obese | 30.9 (30.2, 31.6) | 31.2 (30.6, 31.8) | 33.3 (32.7, 33.9) | <0.001 |
| Overweight or obese | 41.6 (40.6, 42.6) | 41.5 (40.6, 42.3) | 42.6 (41.7, 43.4) | 0.07 |
| Non-Hispanic Black | ||||
| Normal weight | ref | ref | ref | |
| Overweight | 12.3 (10.7, 13.8) | 9.9 (8.6, 11.2) | 9.2 (8.0, 10.4) | 0.001 |
| Obese | 36.3 (34.4, 38.2) | 34.9 (33.2, 36.6) | 37.9 (36.2, 39.5) | 0.11 |
| Overweight or obese | 48.5 (45.8, 51.1) | 44.7 (42.3, 47.1) | 47.0 (44.7, 49.2) | 0.20 |
| Hispanic | ||||
| Normal weight | ref | ref | ref | |
| Overweight | 12.0 (10.8, 13.2) | 12.8 (11.7, 13.8) | 11.3 (10.4, 12.3) | 0.20 |
| Obese | 27.2 (26.0, 28.5) | 31.4 (30.3, 32.4) | 33.3 (32.3, 34.3) | <0.001 |
| Overweight or obese | 39.2 (37.4, 41.0) | 44.1 (42.5, 45.6) | 44.6 (43.1, 46.1) | <0.001 |
| Non-Hispanic Asian | ||||
| Normal weight | ref | ref | ref | |
| Overweight | 12.1 (10.5, 13.6) | 13.0 (11.8, 14.3) | 11.6 (10.4, 12.7) | 0.30 |
| Obese | 12.2 (11.2, 13.3) | 14.0 (13.1, 14.9) | 15.4 (14.5, 16.3) | <0.001 |
| Overweight or obese | 24.3 (22.3, 26.2) | 27.0 (25.4, 28.6) | 26.9 (25.3, 28.5) | 0.02 |
Selected years shown for simplicity; full results shown in Appendix Table 3.
Boldface indicates statistical significance (p<0.05).
By contrast, PAFs for GDM associated with prepregnancy obesity (compared with normal weight) prior to pregnancy were heterogeneous across race/ethnicity groups and increased from 2011 to 2019, with the exception of non-Hispanic Black women. PAFs were highest in non-Hispanic Black women and increased from 36.3% (95% CI=34.4, 38.2) in 2011 to 37.9% (95% CI=36.2, 39.5) in 2019, although this difference was not statistically significant (p=0.11). PAFs were lowest in non-Hispanic Asian women and increased from 12.2% (95% CI=11.2, 13.3) in 2011 to 15.4% (95% CI=14.5, 16.3) in 2019 (p<0.001) (Table 3 and Appendix Table 3).
The PAFs for the top 3 Hispanic and non-Hispanic Asian subgroups by population largely increased between 2011 and 2019, but were heterogeneous across subgroups (Appendix Table 4).
DISCUSSION
This analysis found that maternal prevalence of prepregnancy overweight and obesity increased from 2011 to 2019, and overweight or obesity was associated with 1.5- to 4-times higher odds of GDM compared with normal weight in all race/ethnicity groups. PAFs for GDM associated with prepregnancy overweight were stable (i.e., did not significantly change) or decreased, and those associated with obesity increased across all race/ethnicity groups except non-Hispanic Black women, in which they were stable, from 2011 to 2019. Overweight and obesity together accounted for up to one half of the population burden of GDM in 2019, with substantial differences by race/ethnicity. Despite the highest rates of GDM in non-Hispanic Asian women, PAFs associated with overweight or obesity were the lowest, even after accounting for Asian-specific BMI definitions.
These results confirm and extend prior findings that the association of maternal excess body weight with GDM was not limited to obesity status, but was also present in overweight in all race/ethnic groups. One prior study, a meta-analysis of cohort data from 3 continents, reported a PAF for GDM associated with prepregnancy overweight of 19.4%,24 which was higher than the estimates in this study (9.2%–11.6% in 2019) and reinforces the important contribution of overweight status to GDM. The PAF estimates for GDM associated with prepregnancy obesity in this study are within the range of those previously reported (8%–42%) in studies of other populations.24–27 These results extend those of prior studies by reporting trends over time and differences between key racial/ethnic subgroups leveraging the National Center for Health Statistics Birth Data Files, which represent the most comprehensive and contemporary source of U.S. population data that include prepregnancy BMI and GDM. These data highlight that a large public health impact of overweight and obesity is being seen in women of reproductive age. This is especially important given long-term adverse cardiometabolic outcomes of GDM in both mothers and their children. Women with a history of GDM have a high lifetime risk of conversion to T2DM5 and a 2-fold higher relative risk of subclinical atherosclerosis and cardiovascular disease independent of diabetes (yes/no) status, even if they attain normoglycemia.6,7 Offspring of women whose pregnancies were complicated by GDM have an elevated risk of impaired glucose tolerance,8 overweight and obesity,9 and early cardiovascular disease,28 suggestive of intergenerational transmission of cardiometabolic risk.
There were substantial racial/ethnic differences in PAFs for GDM associated with prepregnancy body weight. Non-Hispanic Black women had the largest PAF for GDM associated with prepregnancy overweight or obesity (47.0% in 2019). This was due to the greatest prevalence of obesity in this group (32.8% in 2019). By contrast, non-Hispanic Asian women had the smallest PAF for GDM associated with prepregnancy overweight or obesity (26.9% in 2019), although there was heterogeneity between subgroups. This finding was driven by both lower prevalence of overweight and obesity and smaller ORs in non-Hispanic Asian women. However, non-Hispanic Asian women also had the highest age-standardized rates of GDM across the study period in every BMI category, which indicates substantial risk for GDM unrelated to prepregnancy BMI in this subgroup and is similar to patterns previously observed for T2DM risk in Asian Americans at lower BMI categories in the Patient Outcomes Research To Advance Learning (PORTAL) cohort network and in a nationally representative sample.29,30 This is further suggested by higher age-standardized GDM rates in non-Hispanic Asian women with normal prepregnancy BMI compared with those among non-Hispanic White, non-Hispanic Black, and Hispanic women even with overweight BMI category. Interestingly, these findings were based on using the American Diabetes Association cut off for overweight or obesity in Asian Americans (BMI ≥23.0 kg/m2), which is lower than that for other racial/ethnic subgroups (BMI ≥25.0kg/m2).19 Although differences in body fat composition31 and phenotypic heterogeneity of GDM32,33 in Asian women have been observed, the reasons for these differences, which may include variability in dietary patterns34 and other social determinants of health, are unclear.
Increasing obesity prevalence in parallel with increasing PAFs for GDM associated with obesity suggests that unfavorable contemporary trends in GDM may be related to increasing rates of obesity in early adulthood present prior to pregnancy. Obesity affected 40% of young adults in 2017–2018,35 related in part to the high prevalence of suboptimal physical activity levels and poor diet quality among young adults,36 with approximately one half consuming ≥1 sugar-sweetened beverage daily.37 Although calorie reduction and increased physical activity are important components of the prevention and treatment of obesity,38 successful strategies continue to be a challenge for this complex, multifactorial disease. Adjunctive pharmacotherapy or surgical intervention may be appropriate for some patients with obesity to achieve weight loss before conception.39 A loss of 10% of body weight may be an achievable goal that is sufficient to reduce the risk of GDM.11 Potential population-level strategies to promote healthy BMI may include implementation of sugar-sweetened beverage reduction programs,40 including taxation and consumer awareness campaigns, as well as addressing community-level barriers to physical activity, such as availability of public activity spaces41 and neighborhood safety.42
Limitations
This study has several limitations. PAFs are measures of association and should not be interpreted as causal beyond the underlying adjusted logistic regression models. Ascertainment of GDM in birth certificates may be incomplete, and the diagnostic criteria used to code GDM are not available; however, contemporaneous clinical guidelines recommend universal GDM screening between 24 and 28 weeks gestation in the U.S., and GDM status in this data set is identified by the clinician rather than by administrative code. The exclusion of multiparous women may underestimate GDM rates in the total population, as prior pregnancy is associated with increased risk for GDM in subsequent pregnancies. In addition, women who had fetal deaths were not included in the analysis owing to inconsistent reporting across states; however, these account for <0.7% of pregnancies and consequently this exclusion should not alter results materially. BMI is an imperfect indicator of diabetes risk, and the optimal cut points in Asian Americans are debated; however, the definition used for overweight or obesity (BMI ≥23.0 in Asian Americans) is recommended by the American Diabetes Association as a clinical action point. Self-reported weight and height, asked around the time of delivery, may be subject to recall bias and misclassification of prepregnancy BMI category. Gestational weight gain may be an additional important factor related to development of GDM43; however, data on gestational weight gain prior to the diagnosis of GDM are not available in the National Center for Health Statistics Birth Data files. Finally, lifestyle factors such as diet and physical activity, which may partially mediate associations between prepregnancy overweight and obesity and GDM, were not able to be assessed.
CONCLUSIONS
Up to one half of pregnancies complicated by GDM among nulliparous women in the U.S. are associated with overweight or obesity prior to pregnancy. PAFs for GDM associated with obesity increased in all racial/ethnic subgroups except non-Hispanic Black women between 2011 and 2019. Prevalence of overweight and obesity increased throughout the study period with <50% of women entering pregnancy with normal weight by 2019. These data emphasize the importance of promoting healthy weight prior to conception, which may help reverse recent unfavorable trends in GDM and mitigate the subsequent morbidity and mortality related to cardiometabolic disease in young women and their offspring. As population-level efforts to date have been largely unsuccessful, innovative efforts to disrupt the large and increasing burden of overweight- and obesity-related GDM are needed.
Supplementary Material
ACKNOWLEDGMENTS
Supported by grants from the American Heart Association (#19TPA34890060) and NIH (P30DK092939; P30AG059988) to SSK. The funding sponsor did not contribute to design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The authors take responsibility for decision to submit the manuscript for publication. Dr. Khan had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no relevant conflicts of interest to disclose.
REFERENCES
- 1.Martin JA, Hamilton BE, Osterman MJ, Driscoll AK. Births: Final data for 2018. Natl Vital Stat Rep. 2019;68. [PubMed] [Google Scholar]
- 2.ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol. 2018;131(2):e49‒e64. 10.1097/aog.0000000000002501. [DOI] [PubMed] [Google Scholar]
- 3.Deputy NP, Kim SY, Conrey EJ, Bullard KM. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth—United States, 2012–2016. MMWR Morb Mortal Wkly Rep. 2018;67(43):1201‒1207. 10.15585/mmwr.mm6743a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Economic costs of diabetes in the US in 2017. Diabetes Care. 2018;41(5):917‒928. 10.2337/dci18-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862‒1868. 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 6.Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62(6):905‒914. 10.1007/s00125-019-4840-2. [DOI] [PubMed] [Google Scholar]
- 7.Gunderson EP, Sun B, Catov JM, et al. Gestational diabetes history and glucose tolerance after pregnancy associated with coronary artery calcium in women during midlife. Circulation. 2021;143(10):974‒987. 10.1161/circulationaha.120.047320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowe WL, Scholtens DM, Kuang A, et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019;42(3):372‒380. 10.2337/dc18-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nehring I, Chmitorz A, Reulen H, Von Kries R, Ensenauer R. Gestational diabetes predicts the risk of childhood overweight and abdominal circumference independent of maternal obesity. Diabet Med. 2013;30(12):1449‒1456. 10.1111/dme.12286. [DOI] [PubMed] [Google Scholar]
- 10.Glazer NL, Hendrickson AF, Schellenbaum GD, Mueller BA. Weight change and the risk of gestational diabetes in obese women. Epidemiology. 2004;15(6):733‒737. 10.1097/01.ede.0000142151.16880.03. [DOI] [PubMed] [Google Scholar]
- 11.Schummers L, Hutcheon JA, Bodnar LM, Lieberman E, Himes KP. Risk of adverse pregnancy outcomes by prepregnancy body mass index: a population-based study to inform prepregnancy weight loss counseling. Obstet Gynecol. 2015;125(1):133‒ 143. 10.1097/aog.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393‒403. 10.1056/nejmoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poston L, Bell R, Croker H, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3(10):767‒777. 10.1016/s2213-8587(15)00227-2. [DOI] [PubMed] [Google Scholar]
- 14.Dodd JM, Turnbull D, McPhee AJ, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ. 2014;348:g1285. 10.1136/bmj.g1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fryar CD, Carroll MD, Ogden C. Prevalence of overweight, obesity, and severe obesity among adults age 20 and over: United States, 1960‒1962 through 2017‒2018. NCHS Health E-Stats. Published 2020. [PubMed] [Google Scholar]
- 16.National Vital Statistics System. Birth Data. National Center for Health Statistics, Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nvss/births.htm. Published 2020. Accessed February 3, 2021. [PubMed]
- 17.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573‒577. 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. Guide to completing the facility worksheets for the certificate of live birth and report of fetal death (2003 revision). National Center for Health Statistics, Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/data/dvs/GuidetoCompleteFacilityWks.pdf. Published 2019. Accessed February 3, 2021.
- 19.Hsu WC, Araneta MRG, Kanaya AM, Chiang JL, Fujimoto W. BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care. 2015;38(1):150‒158. 10.2337/dc14-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157‒163. 10.1016/s0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 21.Newson RB. Attributable and unattributable risks and fractions and other scenario comparisons. Stata J. 2013;13(4):672‒698. 10.1177/1536867x1301300402. [DOI] [Google Scholar]
- 22.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88(1):15‒19. 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219. 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos S, Voerman E, Amiano P, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American and Australian cohorts. BJOG. 2019;126(8):984‒995. 10.1111/1471-0528.15661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Z, Phung H, Freebairn L, Sexton R, Raulli A, Kelly P. Contribution of maternal overweight and obesity to the occurrence of adverse pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2019;59(3):367‒374. 10.1111/ajo.12866. [DOI] [PubMed] [Google Scholar]
- 26.Lu GC, Rouse DJ, DuBard M, Cliver S, Kimberlin D, Hauth JC. The effect of the increasing prevalence of maternal obesity on perinatal morbidity. Am J Obstet Gynecol. 2001;185(4):845‒849. 10.1067/mob.2001.117351. [DOI] [PubMed] [Google Scholar]
- 27.Oteng-Ntim E, Kopeika J, Seed P, Wandiembe S, Doyle P. Impact of obesity on pregnancy outcome in different ethnic groups: calculating population attributable fractions. PLoS One. 2013;8(1):e53749. 10.1371/journal.pone.0053749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Y, Arah OA, Liew Z, et al. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: population based cohort study with 40 years of follow-up. BMJ. 2019;367:l6398. 10.1136/bmj.l6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Y, Sidell MA, Arterburn D, et al. Racial/ethnic disparities in the prevalence of diabetes and prediabetes by BMI: Patient Outcomes Research To Advance Learning (PORTAL) multisite cohort of adults in the U.S. Diabetes Care. 2019;42(12):2211‒2219. 10.2337/dc19-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng YJ, Kanaya AM, Araneta MRG, et al. Prevalence of diabetes by race and ethnicity in the United States, 2011‒2016. JAMA. 2019;322(24):2389‒2398. 10.1001/jama.2019.19365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araneta MRG, Barrett-Connor E. Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and white women. Obes Res. 2005;13(8):1458‒1465. 10.1038/oby.2005.176. [DOI] [PubMed] [Google Scholar]
- 32.Powe CE, Allard C, Battista M-C, et al. Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care. 2016;39(6):1052‒1055. 10.2337/dc15-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Retnakaran R, Hanley AJG, Connelly PW, Sermer M, Zinman B. Ethnicity modifies the effect of obesity on insulin resistance in pregnancy: a comparison of Asian, South Asian, and Caucasian women. J Clin Endocrinol Metab. 2006;91(1):93‒97. 10.1210/jc.2005-1253. [DOI] [PubMed] [Google Scholar]
- 34.He J-R, Yuan M-Y, Chen N-N, et al. Maternal dietary patterns and gestational diabetes mellitus: a large prospective cohort study in China. Br J Nutr. 2015;113(8):1292‒1300. 10.1017/s0007114515000707. [DOI] [PubMed] [Google Scholar]
- 35.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017‒2018. NCHS Data Brief. 2020;(360):1‒8. [PubMed] [Google Scholar]
- 36.Shay CM, Ning H, Allen NB, et al. Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003‒2008. Circulation. 2012;125(1):45‒56. 10.1161/circulationaha.111.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosinger A, Herrick K, Gahche J, Park S. Sugar-sweetened beverage consumption among U.S. adults, 2011‒2014. NCHS Data Brief. 2017;(270):1‒8. [PubMed] [Google Scholar]
- 38.Chin S-H, Kahathuduwa CN, Binks M. Physical activity and obesity: what we know and what we need to know*. Obes Rev. 2016;17(12):1226‒1244. 10.1111/obr.12460. [DOI] [PubMed] [Google Scholar]
- 39.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25_suppl_2):S102‒S138. 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long MW, Gortmaker SL, Ward ZJ, et al. Cost effectiveness of a sugar-sweetened beverage excise tax in the U.S. Am J Prev Med. 2015;49(1):112‒123. 10.1016/j.amepre.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolch JR, Byrne J, Newell JP. Urban green space, public health, and environmental justice: the challenge of making cities ‘just green enough’. Lands Urban Plan. 2014;125:234‒244. 10.1016/j.landurbplan.2014.01.017. [DOI] [Google Scholar]
- 42.Douglas JA, Briones MD, Bauer EZ, Trujillo M, Lopez M, Subica AM. Social and environmental determinants of physical activity in urban parks: Testing a neighborhood disorder model. Prev Med. 2018;109:119‒124. 10.1016/j.ypmed.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol. 2010;115(3):597‒604. 10.1097/aog.0b013e3181cfce4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


