Abstract
Background and Purpose:
Studies of carotid artery disease have suggested that high grade stenosis can affect cognition, even without stroke. The presence and degree of cognitive impairment in such patients have not been reported and compared with a demographically-matched population-based cohort.
Methods:
We studied cognition in 1,000 consecutive CREST-2 patients, a treatment trial for asymptomatic carotid disease. Cognitive assessment was after randomization but before assigned treatment. The cognitive battery was developed in the general population REasons for Geographic And Racial Differences in Stroke (REGARDS) Study, involving Word-List Learning (WLL-Sum), Word-List Recall (WL-Delay), and Word List fluency for animal names and the letter “F”. The carotid stenosis patients were ≥ 45 years old with ≥ 70% asymptomatic carotid stenosis and no history of prevalent stroke. The distribution of cognitive performance for the patients was standardized, accounting for age, race and education using performance from REGARDS, and after further adjustment for hypertension, diabetes, dyslipidemia and smoking. Using the Wald Test, we tabulated the proportion of Z-scores less than the anticipated deviate for the population-based cohort for representative percentiles.
Results:
There were 786 baseline assessments. Mean age was 70 years, 58% men, and 52% right-sided stenosis. The overall Z-score for patients was significantly below expected for higher percentiles (p < 0.0001 for 50th, 75th and 95th percentiles) and marginally below expected for the 25th percentile (p = 0.015). Lower performance was attributed largely to WL-Delay (p<0.0001 for all percentiles) and for WLL-Sum (50th, 75th, and 95th percentiles below expected, p≤0.01). The scores for left vs right carotid disease were similar.
Conclusions:
Baseline cognition of patients with severe carotid stenosis showed below normal cognition compared to the population-based cohort, controlling for demographic and cardiovascular risk factors. This cohort represents the largest group to date to demonstrate that poorer cognition, especially memory, in this disease.
Clinical Trial Registration Information:
https://clinicaltrials.gov/ct2/show/NCT02089217; NCT02089217.
Introduction
Carotid artery stenosis, a major risk factor for ischemic stroke, accounts for 8% of stroke events.1 Although cognitive decline can occur with stroke2, carotid disease with high-grade stenosis can affect cognition3–5, but has never been tested in a large-scale randomized controlled trial. We wanted to determine whether participants have diminished cognition with asymptomatic carotid disease before study treatment. The Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial (CREST-2) addressed this question.
CREST-2 has two trials of primary stroke prevention in patients with high-grade asymptomatic carotid stenosis, comparing: 1) treatment differences between intensive medical management (IMM) alone compared to carotid endarterectomy (CEA) plus IMM, and 2) treatment differences between IMM alone compared to carotid stenting (CAS) (Clinicaltrials.gov no:NCT02089217).6 Randomization began 12/2014 and is ongoing, with a goal of 1,240 patients in each trial.
A secondary outcome in CREST-2 is the impact of treatment on the change in cognitive function, with evaluations at baseline and yearly thereafter. We employ a centrally-administered telephone battery, with tests derived from the Reasons for Geographic And Racial Differences in Stroke (REGARDS) study,7–9 a population sample of 30,239 community-dwelling black and white participants aged 45+ living in the 48 contiguous US states randomly chosen from a commercially available list. They were recruited through a combination of mail and telephone contact between 2003 and 2007. The primary exclusion criteria were having actively-treated cancer, residence in (or on a waiting list for) a nursing home, and non-English preferred language. The estimated participation rate was 34% and annual retention is 97.4%, comparable to other longitudinal cohort studies. The cohort has been accepted as generally representative of the US black and white population (see https://www.uab.edu/soph/regardsstudy/ for details). The REGARDS cognitive test battery, administered by telephone, has been shown to yield normally distributed scores, and is sensitive to cognitive change,8,9 and cerebrovascular risk factors.10 We hypothesized that CREST-2 patients would have significantly lower scores on baseline cognitive testing than participants in the REGARDS cohort with adjustment for: 1) age, education and race, and 2) further adjustment for cardiovascular risk factors (hypertension, diabetes, dyslipidemia and smoking) associated with the development of carotid atherosclerosis11 and cognitive impairment.9 Because of the well-accepted association between prevalent stroke and cognitive performance, we excluded CREST-2 participants with reported stroke at baseline. The analysis of baseline cognitive function was pre-specified, although the N was not determined until CREST-2 had begun. Neverthless, we chose the milestone of 1,000 patients a priori, not by a formal power/sample-size analysis but rather with confidence that this sample size would achieve the aims of this paper.
Methods
Data Availability.
The data for this paper come from CREST-2 and REGARDS. To abide by obligations with NIH/NINDS and their respective IRB’s, these studies facilitate data sharing through data use agreements. Requests for access to baseline CREST-2 data may be sent to Meschia.james@mayo.edu and data related to REGARDS to regardsadmin@uab.edu.
Participants.
We studied the first 1,000 consecutive patients in CREST-2. The list of inclusion-exclusion criteria and definitions of cardiovascular risk factors is published elsewhere.6 In general, eligibility includes men and women ≥ 35 years old who have ≥ 70% asymptomatic stenosis involving the carotid bifurcation with or without involvement of the contralateral internal carotid artery. A patient is considered asymptomatic in the absence of ipsilateral symptoms within 180 days prior to randomization with a modified Rankin Score ≤ 1. Exclusions include history of severe dementia by self-/family-report or the presence of neurologic symptoms that could be confused for stroke or TIA. Patients underwent baseline cognitive assessment either before revascularization or no later than 44 days after randomization if assigned to IMM alone. For inclusion in these analyses, allowing comparison to the REGARDS participants, the CREST-2 patients had to be free of prevalent stroke at baseline, black or white, and age of ≥45 years older.
Cognitive Measures.
The CREST-2 neurocognitive battery is administered via telephone by certified interviewers at the Survey Research Unit at the University of Alabama at Birmingham, blinded to trial (CEA or CAS) and treatment assignment. The battery includes measures (See Table 1) administered in identical fashion (same order) as REGARDS study, and comprised of “Consortium to Establish a Registry for Alzheimer’s Disease” (CERAD) Memory Registration12 (Word List Learning Sum, or WLL-Sum, for 10 words over three trials), Word Fluency for animal names and for the single letter, “F” (Controlled Oral Word Association, or COWA)13, CERAD Recall12 (Word List Recall after a 10-min delay, or WLL-Delay, for the 10 words), and a brief screen for depression.14 This battery follows the harmonization guidelines for the assessment of vascular cognitive impairment.15,16 Additional measures were added to the CREST-2 battery in December 2017 to accommodate the mechanistic goals of the CREST-H ancillary study17, but not included here because few participants had undergone the extended battery at the time of this analysis.
Table 1.
The REGARDS/CREST-2 Measures in Common.
| Test | Cognitive Domain | Outcome |
|---|---|---|
| CERAD Word List Learning (“WLL Sum”) | Learning | Sum of 3 learning trials (0–30) |
| CERAD World List Recall (“WLL-Delay”) | Memory | Number Correct (0–10) |
| “Animal Naming” CERAD; NINDS-CSN | Executive Function | Number correct in 60 sec |
| Letter Fluency (“F”) NINDS-CSN) | Number correct in 60 sec |
CERAD= Consortium to Establish a Registry for Alzheimer’s Disease; NINDS-CSN= National Institute of Neurological Disorders and Stroke - Canadian Stroke Network.
Statistical Methods.
The statistical analysis approach used the population-based REGARDS study as norms for performance on the CREST-2 cognitive tasks after adjustment for: 1) demographic factors (age strata, race and education), and 2) after further adjustment for major cardiovascular risk factors associated with cognitive performance (hypertension, diabetes, dyslipidemia and smoking). Because of the number of factors considered in the development of these norms, stratification would require an unwieldly large number of strata (for the joint consideration of demographic and risk factors: 5 × 2 × 4 × 2 × 2 × 2 × 2 = 640 strata). As such, the expected performance (i.e., the normative standard) for each potential combination of the demographic factors, and demographic plus risk factors, was calculated from regression models. Specifically, for the first analysis adjusting for age, race and education, a cell-means model was used in REGARDS to estimate the stratified mean for the 40 cells (5 age strata, by 2 race strata, by 4 education strata), with the residual mean square error used as the standard deviation, to be used to calculate the deviation score in the CREST population. For the second analysis incorporating further adjustment for risk factors: 1) the mean score for the same 40 cells was estimated in REGARDS using a reference cell approach, with the reference cell being those with no risk factors, and 2) the main effect impact of the 4 risk factors was estimated in the same model. The deviation score for the CREST patients was then calculated by adjusting their age-race-education mean by subtracting off the main effect for each of their prevalent risk factors. Because the distribution of each of the individual cognitive tests is normally distributed, the distribution of the performances of the CREST-2 patients would be a standard normal distribution (“SND,” or a normal curve with a mean of 0.0 and standard deviation of 1.0) if their performances were the same as the general population.
As a result, the extent to which the standardized z-scores from the CREST-2 population matches (or fails to match) the SND provides an assessment of the similarity of their performance compared to the general population, adjusted for demographic factors, or demographic factors plus risk factors. Specifically, the deviates of the SND provide the expected proportion of the population at or below that level of cognitive functioning. For example, under the null hypothesis that the CREST-2 population has the same cognitive performance as the general population, 5% of the CREST-2 population should have a z-score less than −1.64 (the 5th percentile of the SND), 25% less than −0.67 (the 25th percentile of the SND), 50% less than 0.0 (the 50th percentile of the SND), 75% less than 0.67 (75th percentile of the SND), and 95% less than 1.64 (95th percentile of the SND). If the distribution of the CREST-2 participants does not reflect the SND, then there is evidence that the cognitive performance of these patients is different from the general population. For example, if for one of the cognitive assessments the 25th percentile of the z-scores for the CREST-2 patients is −1.00 (rather than the expected −0.67), this represents a lower-than-expected performance. Further, if 40% of CREST-2 patients have a z-score value below −0.67 (rather than the expected 25%), then this represents “too many” CREST-2 patients with lower-than-expected performance. Whether this proportion is significantly below the expected 25% was assessed with a binomial test. We also assessed if there were differences in the distribution if z-scores between those with right versus left target lesions using quantile regression. We also compared Z-score distribution of cognitive scores for each of the four tests between those with left (L) vs right (R) carotid occlusive disease.
Standard Protocol Approvals, Registrations and Participant Consents
The CREST-2 protocol was approved by a Central IRB (CIRB) at the University of Cincinnati, and all participants provided written informed consent prior to randomization. For REGARDS, the protocol was approved by the University of Alabama at Birmingham IRB, and consent was obtained initially by telephone and later in writing during the in-person evaluation. The study methods were approved by the institutional review boards of participating institutions not governed by the CIRB.
Results
Of the first 1000 CREST patients, we removed 113 patients with a previous stroke, and 4 patients with missing data on the presence of a previous stroke. Also removed were 24 patients with missing data for education, 1 missing hypertension, 3 missing diabetes, and 5 missing dyslipidemia. Finally, 62 participants were neither black or white, and were deleted, resulting in a final analytic sample of 786 patients.
Table 2 shows that there was a high prevalence of cardiovascular risk factors (hypertension, elevated lipids, smoking and diabetes). Slightly more than half (52%) of the patients had the target carotid vessel on the right, and all began their assigned treatments after baseline cognitive assessment. About half the patients were enrolled in each of the CEA and CAS trials, respectively. Because only 7% of the total cohort was Black, we were underpowered to perform demographic and cognitive comparisons across racial groups.
Table 2.
Descriptive statistics of the white and black patients included in the first 1,000 CREST randomizations
| All | White | Black | ||
|---|---|---|---|---|
| N | 786 | 722 | 64 | |
| Age (mean (SD)) | 69.6 (7.6) | 69.6 (7.6) | 69.5 (7.0) | |
| Education n (%) | < High School | 99 (13) | 83 (11) | Education n (%) |
| High School Grad | 225 (29) | 211 (29) | 14 (19) | |
| Some College | 303 (39) | 279 (39) | 27 (37) | |
| College Graduate | 159 (20) | 149 (21) | 14 (19) | |
| Male (n (%)) | 458 (58) | 430 (60) | 28 (44) | |
| Hypertension (n (%)) | 673 (86) | 615 (85) | 58 (91) | |
| Diabetes (n (%)) | 279 (35) | 250 (35) | 29 (45) | |
| Dyslipidemia (n (%)) | 727 (92) | 668 (93) | 59 (92) | |
| Right Target Artery (n (%)) | 408 (52) | 373 (52) | 35 (55) | |
Figure 1 and Table 3 summarize our findings for all 786 patients after adjustment for demographic factors (age strata, race and education). The left-most box-and-whisker plot in Figure 1 shows the expected distribution of Z-scores under the null hypothesis (with the dashed lines provided for comparison); corresponding percentile ranks are depicted to the right. The five box-and-whisker plots on the right of Figure 1 correspond to observed distribution for the overall cognitive score and each of the four cognitive tests, with higher Z-scores representing higher levels of cognitive function. The upper percentiles of distribution of the overall cognitive score were significantly lower than expected from the general population (p-values of 0.015 for the 25th percentile, and <0.0001 for other percentiles). Among the four tests, the greatest cognitive differences were detected for WLL (Word List) Delay for which the observed percentiles were significantly lower than the REGARDS cohort for all percentiles considered (p < 0.0001). The WLL Sum (Word List Learning) scores were also below expected at the 50th percentile (p = 0.0012), the 75th percentile (p < 0.0001) and 95th percentile (p < 0.0001). Likewise, the observed CREST-2 distribution for the letter F was below expected at the 50th percentile (p = 0.010) and 75th percentile (p = 0.00001) and the 95th percentile (p < 0.0001) Conversely, the 5th percentile of animal naming was above expected (p = 0.0017), as was the 5th percentile for Letter F (p = 0.015). Hence, the distribution of cognitive performance of the CREST-2 was substantially below that of a general population, with the differences being larger for the higher percentiles and with the differences being primarily attributable to WLL Delay (and to a lesser extent WLL Sum and Letter F).
Figure 1.
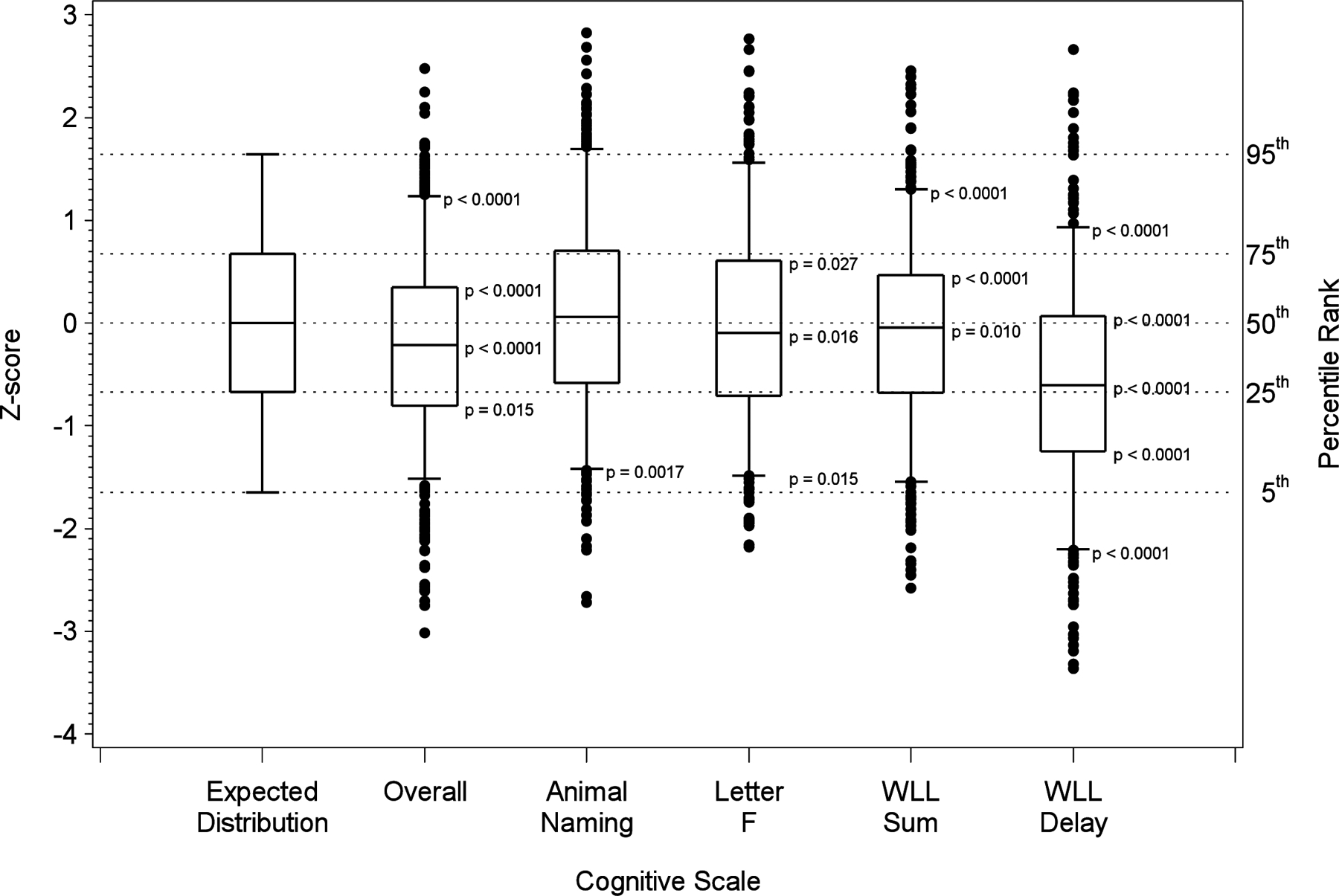
Box-and-whisker plots showing the distribution of cognitive performance in the CREST- 2 value where scores are standardized using the general population values from the REGARDS population. The box-and-whisker plot is drawn with the box showing the 25th and 75th percentiles, the line within the box the 50th percentile, the whiskers the 5th and 95% percentiles, and observations below the 5th percentile or above the 95th percentile.
Table 3:
Percent of observations with a standardized value below the threshold values from a standard normal distribution associated with the specific percentiles (5th = −1.64, 25th = −0.67, 50th = 0.00, 75th = 0.67, 95th = 1.64), and p-value for difference from the expected proportion.
| Percentile | Overall | Animal Naming | Letter F | WLL-Sum | WLL-Delay | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age-Race-Education Adjusted | + Risk Factors | Age-Race-Education Adjusted | + Risk Factors | Age-Race-Education Adjusted | + Risk Factors | Age-Race-Education Adjusted | + Risk Factors | Age-Race-Education Adjusted | + Risk Factors | |
| 5% | 4.1 0.26 |
3.9 0.15 |
2.6 0.0017 |
2.2 0.0003 |
3.1 0.015 |
2.8 0.0058 |
3.8 0.13 |
3.1 0.0012 |
15.3 <0.0001 |
13.4 <0.0001 |
| 25% | 28.8 0.015 |
24.1 0.58 |
22.6 0.12 |
20.7 0.0053 |
25.8 0.60 |
21.9 0.049 |
27.0 0.20 |
22.5 0.11 |
49.0 <0.0001 |
41.5 <0.0001 |
| 50% | 59.7 <0.0001 |
55/2 0.0045 |
48.0 0.26 |
45.7 0.017 |
54.3 0.016 |
50.8 0.64 |
54.6 0.010 |
50.3 0.89 |
74.4 <0.0001 |
72.0 <0.0001 |
| 75% | 85.2 <0.0001 |
83.1 <0.0001 |
72.7 0.13 |
71.4 0.020 |
78.5 0.027 |
74.7 0.85 |
82.4 <0.0001 |
78.4 0.029 |
90.1 <0.0001 |
89.7 <0.0001 |
| 95% | 99.0 <0.0001 |
98.2 <0.0001 |
94.4 0.43 |
94.3 0.34 |
95.5 0.54 |
94.8 0.84 |
98.1 <0.0001 |
97.3 0.0027 |
98.6 <0.0001 |
98.5 <0.0001 |
Table 3 provides a similar assessment of the impact of further adjustment for cardiovascular risk factors associated with cognitive performance (hypertension, diabetes, dyslipidemia and smoking), showing a small downward shift of the distribution for CREST-2 patients, but with the same general pattern of significant differences. A corresponding box-and-whisker plot showing the percentiles for each scale is also provided as Supplemental Figure 1. The striking lower than expected performance for WLL Delay persisted at all percentiles (p < 0.0001), as did the lower-than-expected performance for the upper percentiles of both WLL Sum (p95 percentile = 0.0027, p75 percentile = 0.029) and the overall scores (p95 percentile and p75 percentile < 0.0001, p50 percentile = 0.0045). For animal fluency, the proportion of patients above the expected levels remained significant at the 25th (p = 0.0003), and the non-significantly higher proportions in the age-race-education adjusted models became significantly higher at the 25th (p = 0.0053), 50th (p = 0.017) and 75th (p = 0.020) percentiles. For letter fluency, the proportion of patients above expected also became significant at the 25th percentile (p = 0.049). For Letter F, however, the lower-than-expected scores at the 50th and 75th percentiles were attenuated by the adjustment for risk factors and became non-significant.
Figure 2 shows the similar z-score distribution of cognitive scores for the overall measure and the four tests in our test battery between those with left (L) vs right (R) carotid occlusive disease. Of the 25 percentiles displayed in the figure (5 cognitive assessments times 5 percentiles), none differed significantly between those with a left versus right target lesion (p > 0.05).
Figure 2.
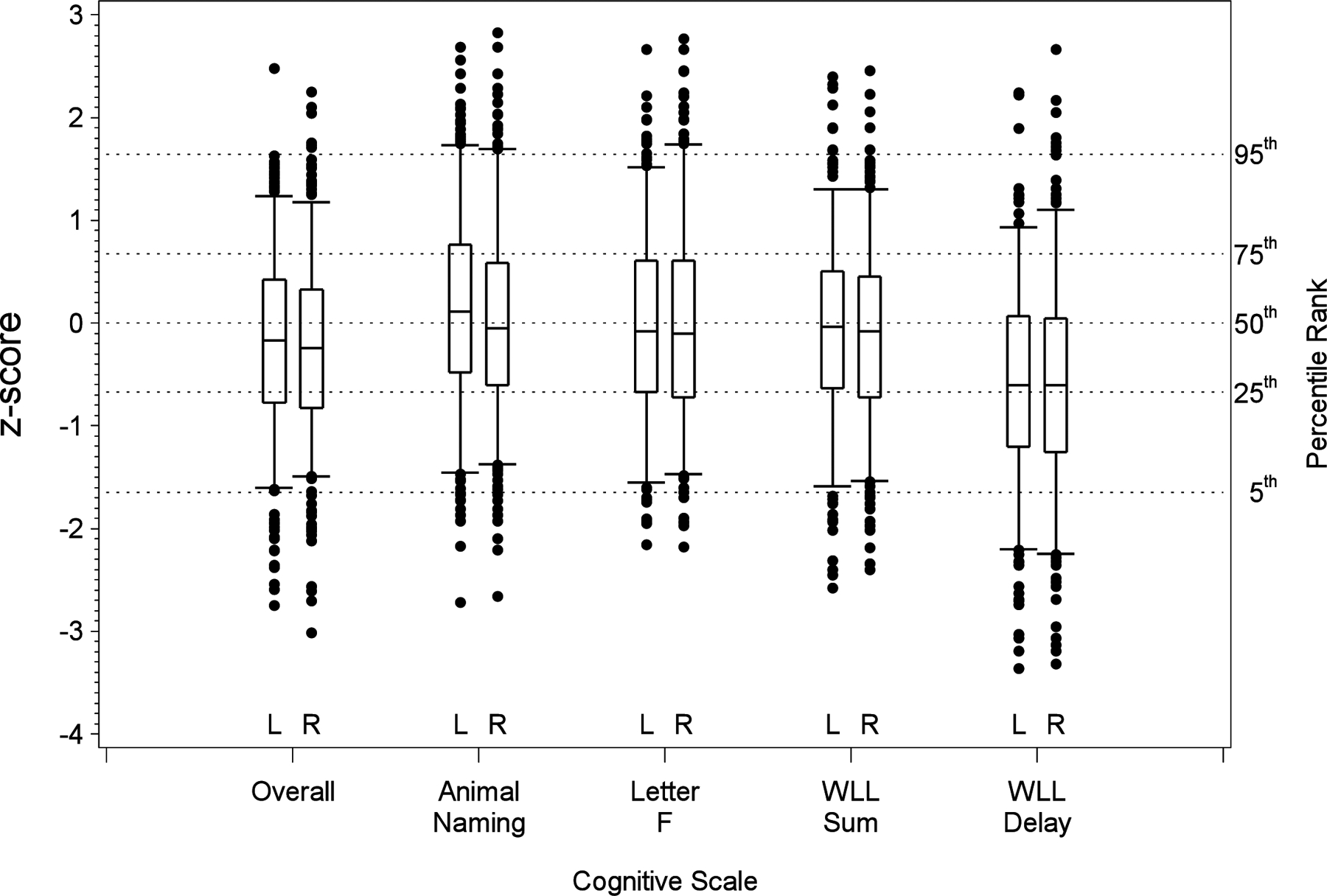
Box-and-whisker plots showing the distribution of cognitive performance in the CREST- 2 value where scores are standardized using the general population values from the REGARDS population shown by the hemisphere of the target artery. The box-and-whisker plot is drawn with the box showing the 25th and 75th percentiles, the line within the box the 50th percentile, the whiskers the 5th and 95% percentiles, and observations below the 5th percentile or above the 95th percentile. Of the 25 percentiles shown (5 cognitive domains times 5 percentiles), only the 5th percentile of WLL Sum differed significantly by hemisphere (p < 0.05).
Discussion
Of the first 1,000 CREST-2 patients, baseline cognitive examination from the 786 stroke-free, evaluable black and white patients with severe, unilateral, asymptomatic carotid stenosis showed a downward shift for those performing at higher levels of cognitive functioning, compared to the REGARDS normative participants, controlling for demographic factors (age, education and race); and with further adjustment for cardiovascular risk factors associated with cognitive outcomes. Performance of CREST-2 patients for WLL Delay was lower than expected across the entire distribution of cognitive performance. Conversely, there appears to be a smaller upward shift in performance for those performing lower on cognitive function for other cognitive domains. The CREST-2 cohort represents the largest group of patients to date to demonstrate that poorer cognition was associated with carotid occlusive disease, an effect only modestly attenuated by further adjustment for cardiovascular risk factors associated with cognitive impairment.
There is increasing evidence that asymptomatic stenosis is associated with alterations in cognitive function.18,19 Among 1,975 stroke-free participants in the Framingham Offspring Study assessed with carotid ultrasound, MRI and neuropsychological tests, carotid stenosis was associated with reduced cognitive performance and indices of cerebral ischemia on imaging.20 Individuals with advanced carotid disease, however, have a higher prevalence of cardiovascular factors conveying risk for both atherosclerosis11 and cognitive impairment,9 including diabetes21, hypertension22, and likely white matter disease and silent brain infarction23. We showed, however, that accounting for the higher risk factor prevalence in those with advanced carotid stenosis was associated with only a modest attenuation of the lower cognitive performance.
We found that CREST-2 patients at with lower cognitive performance (i.e., the above expected performance at lower percentiles) were slightly above expected levels (particularly for fluency measures). This higher-than-expected performance could be attributed to the explicit or implicit exclusion of patients from CREST-2 where potential dementia was concerning, an exclusion that would specifically affect the lower end of the distribution of cognitive performance. The impact of cardiovascular risk factors on cognitive performance was estimated in the REGARDS population using linear regression, an approach that models the “average” (mean) difference for a risk factor being prevalent. Secondly, this average impact of a risk factor was then assumed to have an equal impact across the entire distribution of cognitive performance in CREST-2. In CREST-2, magnitude of the higher-than-expected performance for those with lower cognitive performance increased with adjustment for the cardiovascular risk factors. This larger difference may be a product of risk factors having a smaller impact on cognitive performance (i.e., a floor effect) at the lower end of the distribution cognitive performance. This would result in an underestimate of performance for those with lower cognitive performance in REGARDS, making the performance of lower-performing CREST-2 patients appear better.
There is increasing evidence that chronic cerebral hypoperfusion is associated with cognitive decline in severe carotid stenosis. Unilateral high-grade disease resulting in impaired vasomotor reactivity was found to be associated with cognitive impairment specific to the ipsilateral hemisphere3. Baseline cognitive scores in the Randomized Evaluation of Carotid Occlusion and Neurocognition (RECON) study were compared between patients with carotid occlusion with and without hemodynamic failure by PET oxygen extraction fraction (with failure defined as increased oxygen extraction fraction -OEF), with otherwise no other differences in demographic, clinical or radiological factors between groups. Among individuals with “no stroke” as a qualifying event, those who were PET-positive (increased OEF) had significantly lower average Z-scores than those who were PET-negative, controlling for age, education and side of occlusion.4
Since all of our tests were administered via telephone requiring verbal responses, it might have been expected that scores would have been lower among individuals with left-sided stenosis.24,25 We did not, however, find differences in cognitive scores among those with left vs right disease, with learning and memory carrying the burden of impairment. That memory might have been affected by perfusion failure was not surprising, with known susceptibility of amygdala and hippocampal structures to ischemia.26 Bilateral effects of severe unilateral carotid stenosis was recently addressed by Marshall et al, who investigated the association of regional cortical blood flow and regional cortical thickness in asymptomatic patients.27 Using pseudocontinuous arterial spin labeling (pCASL) MRI, this study found significantly greater thinning of motor cortex in the anterior circulation on the side of stenosis where blood flow was significantly lower than on the non-occluded side. Interestingly, there was also some thinning on the non-occluded side in the anterior circulation, whereas there was no thinning in the visual cortex in the posterior circulation. It was hypothesized that there might have been a synergistic effect of underlying small vessel cerebrovascular disease in the anterior circulation on the non-occluded side, resulting in susceptibility to hemodynamic effects of both small and large vessels to blood flow. Adding to evidence of a bilateral effect is the finding that cognitive function associated with the presumably “unaffected” side in RECON was −0.76 SD’s below the normative mean.4 Conversely, the absence of an effect on word list generation for animal names and the Letter “F” from a critical stenosis in either hemisphere in the current study, more typically seen in executive dysfunction of vascular origin, suggests that not all cognitive domains are comparably affected by carotid disease. Because we controlled for education, the intactness of word list generation indicates that our memory findings were not related to lower than expected verbal intelligence in our study cohort.28
Although an exclusion criterion for CREST-2 participation was a prior diagnosis of dementia, the cognitive performance of the CREST-2 patients was substantially reduced. To put the cognitive findings into perspective, a patient who scored at the 50th percentile among CREST-2 participants on Word List Learning – Delay Recall (WLL-Delay) scored at the 25th percentile of those in the normative REGARDS cohort. Similarly, a patient scoring at 25th percentile among those in CREST-2, ranked at the 8th percentile compared to those in REGARDS. One point regarding clinical relevance is that that a score ≤ 15th percentile (or −1.03 SD below the normative mean) correlates with a change in quality of life in the setting of symptomatic carotid occlusion.29 Moreover, severe carotid stenosis has been associated with increased rates of cognitive deterioration during a 3-year follow-up in 210 patients with asymptomatic disease.30 Importantly, that cohort was functioning no differently than normal controls with comparable risk factors at baseline. That the CREST-2 participants are already functioning worse than the normal population may place them at even greater risk for progression to MCI, or worse, a question that will be answered in CREST-2 since patients will be followed for four years.
Limitations of this study include a limited cognitive battery because of administration over the telephone, made necessary by the large number of participating centers, although there is good validity between our telephone and in-person administration.31 We could not, for example, examine visual-spatial skills, and a broader range of executive skills. Another limitation is that only those who were English speaking were tested, so generalization to non-English speaking groups cannot be made. Third, CREST-2 permits a variety of diagnostic modalities as part of its enrollment criteria. As a result, we are not able to correlate our cognitive findings with specific hemodynamic measurements. Moreover, with ≥70% stenosis being the structural criterion, participants were included with only mild hypoperfusion and as well as those with more severe stenoses having substantial impacts on perfusion. Thus, our patient cohort represented the overall population of individuals under consideration for treatment of their disease. Nevertheless, the finding that memory was the most affected cognitive domain is not typical in vascular cognitive impairment in the absence of frank stroke and hemorrhage, and suggests a possible perfusion-related mechanism.32 Indeed, the absence of impact on word list generation as a marker of processing speed makes small vessel disease a less likely explanation.33 On a related matter, we were unable to adjust for contralateral disease because we could not match cases to REGARDS, which has not collected those data. The hemodynamic threshold for cognitive impairment from carotid disease is not yet known. Fourth, we controlled for the most common confounders, but acknowledge that other unmeasured factors could modify the relationship between this disease and cognition. There are, however, several advantages of centralized assessment. First, we are able to present a standardized administration to patients at more than 100 clinical sites. Second, the REGARDS study provides a large, well-characterized, population-based cohort, enabling us to control for age, education, race, and sex; and additionally, with risk factor assessment allowing for adjustment in the CREST-2 patients. Third, the battery follows the harmonization guidelines for the assessment of vascular cognitive impairment15, and there is established sensitivity to vascular risk factors in predicting incident cognitive impairment, and to change in cognitive status among those with greater risk-factor exposure.9 Nevertheless, a more comprehensive battery may have detected a broader range of cognitive deficits typical of profiles commonly seen in vascular cognitive impairment.33
Conclusions
We have shown that there are below normal cognitive test scores in patients with severe, asymptomatic carotid stenosis about to undergo randomized treatment in a very large clinical trial when compared to a demographically matched population-based cohort. The precise mechanisms for cognitive change are not yet known. Cerebral hypoperfusion is one plausible mechanism since memory was the most impacted function, in contrast to measures of executive function more commonly found in vascular cognitive impairment. Because patients in CREST-2 are followed for several years with annual cognitive assessments, we will uniquely be able to characterize functional trajectories over time.
Supplementary Material
Figure I: Baseline cognitive performance for Crest-2 with risk-factor adjustment.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the CREST-2 trials for their valuable contributions. A full list of participating CREST-2 investigators and institutions can be found at http://www.crest2trial.org. We also thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at https://www.uab.edu/soph/regardsstudy/.
Sources of Funding
The CREST-2 trials are supported by cooperative agreements U01 NS080168, and U01 NS080165 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and by the Centers for Medicare and Medicaid Services (CMS), Department of Health and Human Services. Additional support for CREST-2 comes from StrokeNet U01 NS086872. The CREST-2 Registry is supported by CMS, with additional support from Industry (Abbott Vascular, Boston Scientific, Cardinal Health, Covidien, Gore Medical and Silk Road Medical).
REGARDS is supported by cooperative agreement U01 NS041588 co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA.
Other sources of funding include National Institute of Neurological Disorders and Stroke (NIH) grants U01 NS080165, R01 NS097876 and U24 NS107223, and Oregon Health and Sciences.
Non-Standard Abbreviations
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease
- COWA
Controlled Oral Word Association Test
- CREST-2
Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial
- IMM
Intensive Medical Management
- REGARDS
REasons for Geographic And Racial Differences in Stroke Study
- SND
Standard Normal Distribution
- WL-Sum
Word List Sum
- WL-Delay
Word List Delay
Footnotes
Disclosures
None.
Contributor Information
Ronald M. Lazar, UAB Evelyn F. McKnight Brain Institute, Department of Neurology, The University of Alabama at Birmingham, Birmingham, AL USA.
Virginia G. Wadley, Department of Medicine, The University of Alabama at Birmingham, Birmingham, AL, USA.
Terina Myers, UAB Evelyn F. McKnight Brain Institute, Department of Neurology, The University of Alabama at Birmingham, Birmingham, AL USA.
Michael R. Jones, Cardiology, Baptist Health Lexington, Lexington, KY USA.
Donald V. Heck, Diagnostic Radiology, Novant Health, Winston-Salem, NC USA.
Wayne M. Clark, Department of Neurology, Oregon Health & Science University, Portland, OR USA.
Randolph S. Marshall, Department of Neurology, Columbia University Irving Medical Center, New York NY USA.
Virginia J. Howard, Department of Epidemiology, The University of Alabama at Birmingham, Birmingham, AL USA.
Jenifer H. Voeks, Department of Neurology, Medical University of South Carolina, Charleston, SC. USA.
Jennifer J. Manly, Gertrude H. Sergievsky Center and the Taub Institute for Research in Aging and Alzheimer’s disease, Columbia University Irving Medical Center, New York; NY. USA.
Claudia S. Moy, Department of Health & Human Services, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD USA.
Seemant Chaturvedi, Department of Neurology, University of Maryland School of Medicine, Baltimore, MD USA.
James F. Meschia, Department of Neurology, Mayo Clinic, Jacksonville, FL USA.
Brajesh K. Lal, Department of Surgery, University of Maryland School of Medicine, Baltimore, MD USA.
Thomas G. Brott, Department of Neurology, Mayo Clinic, Jacksonville, FL USA.
George Howard, Department of Biostatistics, University of Alabama School of Public Health, Birmingham, AL. USA.
References
- 1.Flaherty ML, Kissela B, Khoury JC, et al. Carotid artery stenosis as a cause of stroke. Neuroepidemiology. 2013;40(1):36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalaria RN. Cerebrovascular disease and mechanisms of cognitive impairment: evidence from clinicopathological studies in humans. Stroke. 2012;43(9):2526–2534. [DOI] [PubMed] [Google Scholar]
- 3.Silvestrini M, Paolino I, Vernieri F, et al. Cerebral hemodynamics and cognitive performance in patients with asymptomatic carotid stenosis. Neurology. 2009;72(12):1062–1068. [DOI] [PubMed] [Google Scholar]
- 4.Marshall RS, Festa JR, Cheung YK, et al. Cerebral hemodynamics and cognitive impairment. Neurology. 2012;78(4):250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buratti L, Balucani C, Viticchi G, et al. Cognitive deterioration in bilateral asymptomatic severe carotid stenosis. Stroke. 2014;45(7):2072–2077. [DOI] [PubMed] [Google Scholar]
- 6.Howard VJ, Meschia JF, Lal BK, et al. Carotid revascularization and medical management for asymptomatic carotid stenosis: Protocol of the CREST-2 clinical trials. Int J Stroke. 2017;12(7):770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. [DOI] [PubMed] [Google Scholar]
- 8.Wadley VG, Unverzagt FW, McGuire LC, et al. Incident cognitive impairment is elevated in the stroke belt: the REGARDS study. Ann Neurol. 2011;70(2):229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unverzagt FW, McClure LA, Wadley VG, et al. Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology. 2011;77(19):1729–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy RE, Wadley VG, McClure LA, et al. Performance of the NINDS-CSN 5-minute protocol in a national population-based sample. J Int Neuropsychol Soc. 2014;20(8):856–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo SY, Joh JH, Han SA, Park HC. Prevalence and risk factors for atherosclerotic carotid stenosis and plaque: A population-based screening study. Medicine (Baltimore). 2017;96(4):e5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fillenbaum GG, van Belle G, Morris JC, et al. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): the first twenty years. Alzheimers Dement. 2008;4(2):96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14(2):167–177. [PubMed] [Google Scholar]
- 14.Melchior LA, Huba GJ, Brown VB, Reback C. A Short Depression Index for Women. Educational and Psychological Measurement. 1993;53:1117–1125. [Google Scholar]
- 15.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37(9):2220–2241. [DOI] [PubMed] [Google Scholar]
- 16.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall RS, Lazar RM, Liebeskind DS, et al. Carotid revascularization and medical management for asymptomatic carotid stenosis – Hemodynamics (CREST-H): Study design and rationale. Int J Stroke. 2018, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakker FC, Klijn CJM, Jennekens-Schinkel A, Kappelle LJ. Cognitive disorders in patients with occlusive disease of the carotid artery- a systematic review of the literature. Journal of Neurology 2000;247:669–676. [DOI] [PubMed] [Google Scholar]
- 19.Norling AM, Marshall RS, Pavol MA, et al. Is Hemispheric Hypoperfusion a Treatable Cause of Cognitive Impairment? Curr Cardiol Rep. 2019;21(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero JR, Beiser A, Seshadri S, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke. 2009;40(5):1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moran C, Beare R, Wang W, Callisaya M, Srikanth V, Alzheimer’s Disease Neuroimaging I. Type 2 diabetes mellitus, brain atrophy, and cognitive decline. Neurology. 2019;92(8):e823–e830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine DA, Galecki AT, Langa KM, et al. Blood Pressure and Cognitive Decline Over 8 Years in Middle-Aged and Older Black and White Americans. Hypertension. 2019;73(2):310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pendlebury ST, Rothwell PM, Oxford Vascular S. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford Vascular Study. Lancet Neurol. 2019;18(3):248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ott BR, Daiello LA, Dahabreh IJ, et al. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2015;30(3):348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohr JP, Leicester J, Stoddard LT, Sidman M. Right hemianopia with memory and color deficits in circumscribed left posterior cerebral artery territory infarction. Neurology. 1971;21(11):1104–1113. [DOI] [PubMed] [Google Scholar]
- 26.de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol. 2012;2012:367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall RS, Asllani I, Pavol MA, Cheung YK, Lazar RM. Altered cerebral hemodyamics and cortical thinning in asymptomatic carotid artery stenosis. PLoS One. 2017;12(12):e0189727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolla KI, Lindgren KN, Bonaccorsy C, Bleecker ML. Predictors of verbal fluency (FAS) in the healthy elderly. J Clin Psychol. 1990;46(5):623–628. [DOI] [PubMed] [Google Scholar]
- 29.Pavol MA, Sundheim K, Lazar RM, Festa JR, Marshall RS. Cognition and Quality of Life in Symptomatic Carotid Occlusion. J Stroke Cerebrovasc Dis. 2019;28(8):2250–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balestrini S, Perozzi C, Altamura C, et al. Severe carotid stenosis and impaired cerebral hemodynamics can influence cognitive deterioration. 2013;80(23):2145–2150. [DOI] [PubMed] [Google Scholar]
- 31.Marceaux JC, Prosje MA, McClure LA, et al. Verbal fluency in a national sample: Telephone administration methods. Int J Geriatr Psychiatry. 2019;34(4):578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall RS, Lazar RM. Pumps, aqueducts, and drought management: vascular physiology in vascular cognitive impairment. Stroke. 2011;42(1):221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dichgans M, Leys D. Vascular Cognitive Impairment. Circ Res. 2017;120(3):573–591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure I: Baseline cognitive performance for Crest-2 with risk-factor adjustment.
Data Availability Statement
The data for this paper come from CREST-2 and REGARDS. To abide by obligations with NIH/NINDS and their respective IRB’s, these studies facilitate data sharing through data use agreements. Requests for access to baseline CREST-2 data may be sent to Meschia.james@mayo.edu and data related to REGARDS to regardsadmin@uab.edu.


