Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) binds to PACAP-specific (PAC1) receptors in multiple hypothalamic areas, especially those regulating energy balance. PACAP neurons in the ventromedial nucleus (VMN) exert anorexigenic effects within the homeostatic energy balance circuitry. Since PACAP can also reduce the consumption of palatable food, we tested the hypothesis that VMN PACAP neurons project to the ventral tegmental area (VTA) to inhibit A10 dopamine neurons via PAC1 receptors and KATP channels, and thereby suppress binge-like consumption. We performed electrophysiological recordings in mesencephalic slices from male PACAP-Cre and tyrosine hydroxylase (TH)-Cre mice. Initially, we injected PACAP (30 pmol) into the VTA, where it suppressed binge intake in wildtype male but not female mice. Subsequent tract tracing studies uncovered projections of VMN PACAP neurons to the VTA. Optogenetic stimulation of VMN PACAP neurons in voltage clamp induced an outward current and increase in conductance in VTA neurons, and a hyperpolarization and decrease in firing in current clamp. These effects were markedly attenuated by the KATP channel blocker tolbutamide (100 μM) and PAC1 receptor antagonist PACAP6–38 (200 nM). In recordings from A10 dopamine neurons in TH-Cre mice, we replicated the outward current by perfusing PACAP1–38 (100 nM). This response was again completely blocked by tolbutamide and PACAP6–38, and associated with a hyperpolarization and decrease in firing. These findings demonstrate that PACAP activates PAC1 receptors and KATP channels to inhibit A10 dopamine neurons and sex-dependently suppress binge-like consumption. Accordingly, they advance our understanding of how PACAP regulates energy homeostasis via the hedonic energy balance circuitry.
Keywords: sex difference, estradiol, pituitary adenylate cyclase-activating polypeptide, dopamine, obesity, binge eating
INTRODUCTION
The hypothalamus is known for its regulation of energy balance. The regions primarily responsible for this regulation are the ventromedial nucleus (VMN), paraventricular nucleus (PVN), arcuate nucleus (ARC), dorsomedial nucleus (DMN), and lateral hypothalamic area (LHA) (Berthoud and Morrison, 2008;Wagner, 2016). Within the VMN, expression of the transcription factor steroidogenic factor 1 (SF-1) impacts the development of this region, such that the deletion of SF-1 or leptin receptors (LepR) in SF-1 neurons leads to obesity in mice (Dhillon et al., 2006;Kim et al., 2011;Majdic et al., 2002). Pituitary adenylate cyclase-activating polypeptide (PACAP) is colocalized with SF-1 in the VMN, and the transcript levels of PACAP in the VMN depend on leptin signaling. It was also reported that the anorexigenic PACAP released by the VMN can mediate leptin’s anti-obesity action (Hawke et al., 2009). VMN SF-1/PACAP neurons project to the ARC and produce excitatory inputs onto pro-opiomelanocortin (POMC) neurons (Lindberg et al., 2013). As mentioned, PACAP is highly expressed in the VMN, and it is known that PACAP stimulates c-Fos expression in POMC neurons and increases POMC and melanocortin-4 receptor (MC4R) transcripts in the ARC (Mounien et al., 2009). We have previously reported that PACAP regulates energy homeostasis via stimulation of POMC neurons (Chang et al., 2020), this stimulation may link to the activation of melanocortin system through MC4R in the PVN resulting in the reduction of food intake and increase in energy expenditure (Cheng et al., 2011;Mounien et al., 2009). Beside the VMN, PACAP also colocalizes with a subpopulation of POMC neurons within the ARC (Dürr et al., 2007), a region that projects to both intrahypothalamic (e.g. VMN, DMN, LH, and PVN) and extrahypothalamic areas (e.g. the mesolimbic reward system as well as the nucleus of the tractus solitarius hunger and satiety sites) (Berthoud and Morrison, 2008). The type I PACAP receptor is termed the PACAP-specific (PAC1) receptor, which has high binding affinity and selectivity for PACAP. The PACAP/PAC1 receptor system is highly expressed in the VMN, PVN, ARC, DMN, and LHA, which further reinforces its important role in appetite regulation.
Previous reports have shown that PACAP suppresses appetite and feeding by intracerebroventricular (ICV) injection in mice (Morley et al., 1992), goldfish (Matsuda et al., 2005), and chick (Tachibana et al., 2003), as well as by intraperitoneal (IP) injection in goldfish (Matsuda et al., 2005) and mice (Vu et al., 2015). More specifically, PACAP regulates energy balance when injected into the VMN by reducing food intake, increasing core body temperature while decreasing interscapular brown adipose tissue (iBAT) triglyceride level and retroperitoneal white adipose tissue (WAT) (Resch et al., 2011;Resch et al., 2013;Rudecki and Gray, 2016). In addition, PACAP also plays a role in regulating glycemia as ICV injected PACAP stimulate hepatic glucose production in response to hypoglycemia (Rudecki and Gray, 2016). Finally, in contrast to the role PACAP plays in glucose-induced insulin secretion (Filipsson et al., 1999), chemogenetic stimulation of VMN PACAP neurons inhibits insulin secretion and increases glucose levels (Khodai et al., 2018).
Ion channels play an important role in the central regulation of energy and glucose homeostasis (Sohn, 2013;Sun and Feng, 2013). The K+ channel of particular interest to this study is the ATP-sensitive K+ (KATP) channel, which has proved to be critical in the central regulation of energy expenditure, food intake, and glucose homeostasis (Sohn, 2013;Sun and Feng, 2013). KATP (aka. Kir6) channels belong to a subfamily of the weak inward rectifier K+ channels that are classified into Kir6.1 and Kir6.2 subtypes (Hibino et al., 2010). These channels are negatively gated by ATP. The acute inhibitory effects of leptin and insulin are mediated by the activation of KATP channels in neuropeptide Y/Agouti-related protein (NPY/AgRP) neurons in the ARC (Qiu et al., 2014;van den Top et al., 2004), and for insulin these extend to ARC POMC and VMN SF-1 neurons as well (Hill et al., 2008;Klöckener et al., 2011;Plum et al., 2006;Williams et al., 2010). In the ARC, enhanced phosphatidylinositol3,4,5-triphosphate (PIP3) signaling in POMC neurons due to knockdown of PIP3 phosphatase increased KATP channel activity and led to diet-induced obesity and hyperphagia by blocking the excitatory effects of leptin in these cells (Plum et al., 2006). This indicates that the PIP3 produced by phosphatidylinositol 3-kinase (PI3K) signaling is a positive allosteric modulator of KATP channels. Similarly, stimulation of insulin receptors in VMN SF-1 neurons that play a critical role in controlling energy homeostasis suppresses cell excitability via activation of PI3K and KATP channels; resulting in diet-induced obesity (Klöckener et al., 2011). Moreover, it has also been reported that leptin and insulin act on distinct subpopulations of POMC neurons (Williams et al., 2010).
PACAP has also been shown to regulate binge-eating behavior via site-specific actions such that the injection of PACAP into the nucleus accumbens (NAc) mimicked the actions of γ-aminobutyric acid (GABA) agonists by decreasing hedonic feeding while not affecting homeostatic feeding; whereas, the injection of PACAP into the VMN mimicked the actions of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) by reducing homeostatic feeding without altering hedonic feeding (Hurley et al., 2016;Hurley et al., 2019). This indicates that PACAP can regulate both the homeostatic and the hedonic energy balance circuitries (Liu et al., 2015). Hedonic feeding is defined as pleasure-driven to consume palatable food (i.e. reward-related) (Liu et al., 2015). Hedonic eating is linked to the midbrain dopaminergic (DA) system, which is known for its regulation of reward-related behaviors (Liu et al., 2015;Volkow et al., 2017) and its role in binge eating episodes (Avena and Bocarsly, 2012;Rada et al., 2005). There is a close relationship between hedonic feeding and reward/addiction that involves the mesolimbic dopamine (A10) emanating from the ventral tegmental area (VTA) and projecting to the NAc. It is generally accepted that DA is a key neurotransmitter modulating rewards through this very projection (Volkow et al., 2017). There is also a neural circuit conveying information from the homeostatic energy balance circuitry to the hedonic circuitry, where leptin reduces excitatory synaptic strength onto orexin-expressing neurons originating in the LHA project to the VTA and may affect dopamine signaling and feeding behavior (Leinninger et al., 2009;Liu et al., 2017;Liu et al., 2015). In addition, a subpopulation of ARC POMC neurons project to the VTA, where they inhibit A10 dopamine neurons and thereby participate in regulating stress-induced hypophagia, and anhedonia (Qu et al., 2020). On the other hand, while orexin-expressing LHA neurons project to the VTA to regulate neuronal activity by activating orexin receptors (OX1R) in the VTA and promote appetite for a high-fat diet (HFD) (Zheng et al., 2007), MCH-expressing LHA neurons project to the NAc to elevate both food incentive salience and reward reinforcement (Chung et al., 2009;Georgescu et al., 2005;Mul et al., 2011). Incentive salience refers to the ‘wanting’ module established via neural interactions between the VTA, NAc, and amygdala, which is driven by both physiological state and previously learned associations about a reward cue (Berridge, 1996;Liu et al., 2008). Motivated behaviors then prioritize attention stimuli based off of the anticipated rewards (Liu et al., 2015).
Given the PACAP/PAC1 receptor system and its known suppressive effect on the consumption of palatable food when injected into the NAc (Hurley et al., 2019), which is a region containing the terminal fields of A10 dopamine neurons emanating from the VTA (Volkow et al., 2017) - coupled with the presence of PACAP binding sites in the VTA (Masuo et al., 1991) - this is suggestive of a scenario where PACAP may be capable of inhibiting A10 dopamine neurons and suppressing binge-like consumption. We therefore systematically tested the hypothesis that VMN PACAP neurons project to the VTA to inhibit A10 dopamine neurons via the activation of KATP channels and thereby suppress binge feeding.
MATERIALS AND METHODS
Animal Models
We bought tyrosine hydroxylase (TH)-cre mice (19–28 grams; 7–10 weeks old; Stock # 008601; C57BL/6 background) and PACAP-cre mice (18–25 grams; 7–10 weeks old; Stock #030155; C57BL/6 background) from Jackson Laboratories (Bar Harbor, ME, USA), and bred them in-house. We furnished all animals with food and water ad libitum and kept them under constant temperature (25°C) and kept on a 12hour light – 12hour dark schedule (lights on 06:00–18:00). We weaned the pups when they were 21 days old and genotyped them between 21–42 days of age using established PCR procedures. After weaning, we randomly grouped animals into one of two dietary conditions: a standard chow diet (Teklad Rodent Diet, Teklad Diets, Madison, WI, USA) from which animals received 18% of their calories from fat, 24% from protein, and 58% from carbohydrates, or a HFD (Research Diets, New Brunswick, NJ, USA) – from which animals received 45% of their calories from fat, 20% from protein and 35% from carbohydrates, and kept them there for 5–8 weeks prior to experimentation. Our previous studies have shown that consuming this HFD for this duration produces evident adiposity and glucose dysregulation (Fabelo et al., 2018;Hernandez et al., 2019;Qiu et al., 2018). All of our experiments were conducted in consultation with the NIH Guide for the Care and Use of Laboratory Animals and approved by our institutional animal care and use committee.
Surgical Procedures
We ovariectomized (OVX) female TH-cre, PACAP-cre, and wildtype mice after anesthetizing them with 2% isoflurane anesthesia approximately five days prior to experimentation. We focally injected TH-Cre and PACAP-Cre mice with adeno-associated viral vector (AAV) constructs after anesthetizing them with 2% isoflurane and placing them on a stereotaxic frame (Stoelting, Wood Dale, IL, USA). We made an incision to access the skull, and drilled a hole on one or both sides of the mid-sagittal suture so we could slowly lower the injection needle into the VTA (coordinates from bregma: AP, −2.1 mm; ML, ± 0.5 mm; and DV, −4.0 mm from dura) for TH-Cre mice or into the VMN (coordinates from bregma: AP, −0.6 mm; ML, ± 0.3 mm; and DV, −5.6 mm from dura) for PACAP-Cre mice. AAV constructs containing either cation channel rhodopsin-2 (ChR2) (pAAV-Ef1a-DIO hChR2(E123A)-EYFP.YFP.WPRE.jGH; 7.2 × 1012 genomic copies/mL; 300 nL total volume; a gift from Karl Deisseroth; Addgene plasmid #35507) or its enhanced yellow fluorescent protein (eYFP) blank control (pAAV-Ef1a-DIO EYFP; 1.0 × 1013; 300 nL total volume; Addgene plasmid #27056), as well as the retrograde tracer Fluorogold (Conde K et al., 2016) (Fluorochrome, LLC, Denver, Colo.,USA; 0.2 μL) were injected bilaterally over 2 minutes. We kept the injection needle in place for 10 minutes to enable adequate diffusion from the tip, after which we slowly retracted it from the brain. Animals were ready for use 2–3 weeks after administration of the vectors and one week after delivery of the tracer.
We performed guide cannula implantations into the VTA of wildtype mice similar to the above-described procedure. Briefly, we secured an anesthetized animal into a stereotaxic frame and cut a midline incision through the scalp. We then drilled a hole in the skull and lowered a 26-gauge guide cannula (Plastics One, Roanoke, VA, USA) to a point 1 mm above the VTA (AP, −2.1 mm, ML, ± 0.5 mm, DV, −4.0 mm). We affixed the guide cannulas in place with C&B Metabond cement (Parkell, Edgewood, NY, USA) that we applied to the surgical field. Finally, we inserted a stylet into the guide cannula to ensure patency of the lumen. We allowed the animals to recover 1 week before beginning the experiment. When injecting, we inserted a needle until its tip extended 1 mm below the tip of the guide cannula shaft and into the area of interest. We possess considerable experience in accessing structures deep within the brain (Borgquist et al., 2013;Conde et al., 2016;Hernandez et al., 2019). We used only those animals where the injection sites or guide cannulas were correctly located within the VMN and VTA (Figure 1).
Figure 1.
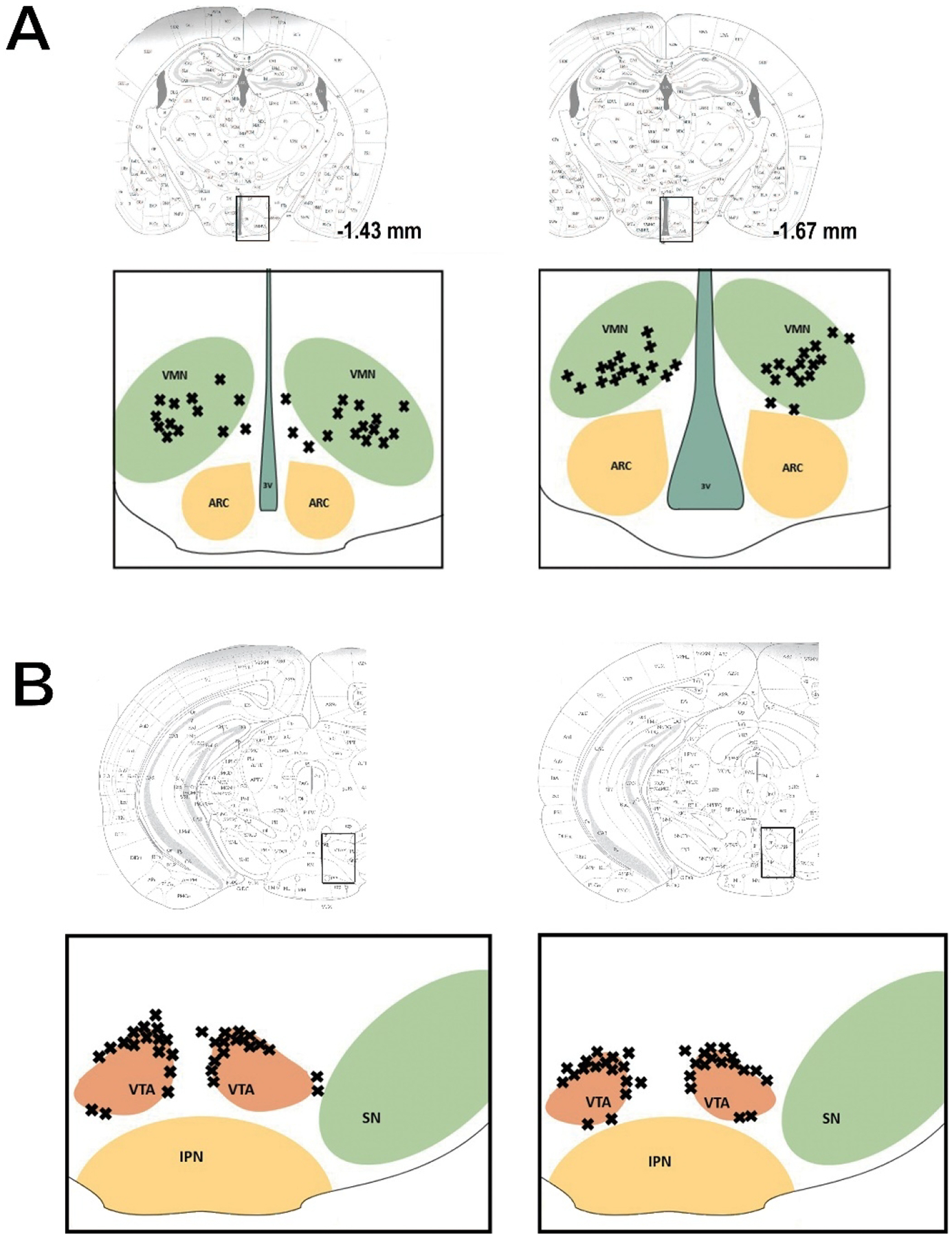
Coronal schematics illustrating the accuracy of our bilateral AAV ChR2 injections in the VMN (A) and the guide cannula implantations and AAV eYFP injections in the VTA (B).
Drugs
We acquired all drugs from Tocris Bioscience/ R&D Systems (Minneapolis, MN, USA) unless otherwise specified. For electrophysiological experiments, the following drugs were used and their stock solutions prepared as detailed below: octahydro-12-(hydroxymethyl)-2-imino-5,9:7,10a-dimethano-10aH-[1,3]dioxocino-[6,5-d]pyrimidine-4,7,10,11,12-pentol (Tetrodotoxin, TTX; 1 mM stock solution in UltraPure H20, diluted with aCSF to 500 nM). PACAP1–38 (100 μM stock solution in UltraPure H2O, diluted with aCSF to 100 nM). PACAP6–38 (200 μM stock solution in UltraPure H2O, diluted with aCSF to 200 nM). Tolbutamide (1-butyl-3-(4-methylphenyl) sulfonylurea; 100 mM stock solution in UltraPure H20, diluted with aCSF to 100 μM).
For the behavioral experiments, we made a 150 μM stock solution of PACAP1–38 by solvating it in filtered saline, and then we administered it directly into the VTA (30 pmol; 0.2 μL). Estradiol benzoate (EB; Steraloids, Newport, RI, USA) was originally formulated as a 1 mg/mL stock solution in punctilious ethanol. We then added a known quantity of this stock solution to a volume of sesame oil that produced a final concentration of 20 μg/kg upon vaporization of the ethanol. We stored all aliquots of our stock solutions at either four or −20 °C until we needed them.
Brain Slice Preparation
On the day of experimentation, the animal was sedated with 32% isoflurane and euthanized by decapitation. We extracted the brain from the skull and further dissected it to obtain hypothalamic or mesencephalic slices in the coronal plane. The resultant block was then attached to a cutting platform that we inserted into a vibratome well containing an ice-cold, oxygenated (95% O2, 5%CO2) sucrose-containing cutting solution (NaHCO3 26; dextrose 10, HEPES 10; sucrose 208; KCl 2; NaH2PO4 1.25; MgSO4 2; CaCl2 1; in mM). In this way, we were able to generate two to three slices (250μm) through the rostrocaudal range of the VTA. We transferred the slices to a storage chamber filled with oxygenated aCSF at room temperature, and kept there until we started recording.
Electrophysiology
We performed whole-cell patch clamp electrophysiological recordings from VTA neurons using biocytin-containing electrodes in mesencephalic slices prepared from intact male TH-Cre and PACAP-Cre mice. We maintained the slices during recordings in a chamber perfused with warmed (35°C), oxygenated aCSF in which we increased the CaCl2 concentration to 2 mM. All drugs (diluted with aCSF) were suffused via peristaltic pump at a rate of 1.5 mL/min. We fabricated patch electrodes from borosilicate glass (World Precision Instruments, Sarasota, FL, USA; 1.5 mm OD) pulled on a P-97 Flaming Brown puller (Sutter Instrument Co., Novato, CA, USA), and filled with an internal solution that included the following (in mM): potassium gluconate 128; NaCl 10; MgCl2 1; EGTA 11; HEPES 10; ATP 1; GTP 0.25; 0.5% biocytin; pH adjusted to 7.3 with KOH; osmolality: 286–320 mOsm. Electrode resistances ranged from 3 to 8 MΩ.
We made visualized recordings on an Olympus BX51 W1 fixed stage microscope equipped with infrared differential interference contrast (DIC) video imaging. Potentials were amplified and current passed current through the electrode via Multiclamp 700A or B preamplifiers (Molecular Devices). Membrane currents and voltages underwent analog-digital transformation with Digidata 1550A or B interfaces (Molecular Devices) linked to pClamp 10.6 or 11.0 software. We monitored the access resistance, resting membrane potential (RMP), and input resistance during the entire recording period. If the access resistance changed more than 10% of its original value, we ended the recording. We conducted low-pass filtering of the currents at a frequency of 2 kHz. We calculated a liquid junction potential of −10 mV, which we corrected for during data analysis using pClamp software. We performed all recordings at a holding potential of −60 mV (in voltage clamp) or at rest (in current clamp).
To ascertain the postsynaptic effects caused by bath-applied PACAP, recordings were performed in slices from TH-Cre mice injected 2–3 weeks beforehand with an eYFP blank-containing AAV into the VTA. We initially established a baseline current-voltage (I/V) relationship from a holding potential of −60 mV using a ramp protocol (75 mV/sec; from −110 to −30 mV). For voltage clamp experiments, we generated baseline I/V relationships with 500 nM TTX on board. We added PACAP1–38 [100 nM] (along with TTX) after the baseline I/V, and monitored the membrane current continuously until a new steady-state value was reached, at which time we generated a second I/V relationship. During the PACAP washout, we again monitored the membrane current until it came back to its original baseline value, and then ran the ramp I/V protocol one final time to confirm reversibility of the PACAP-induced effect. For current clamp experiments, we monitored the membrane potential and firing rate from rest without any TTX on board until new PACAP-induced steady-state levels were achieved, and then evaluated for 10–20 minutes more to allow for the return to baseline. To examine whether these postsynaptic effects are caused by PAC1 receptors and KATP channels, these same recordings were conducted in slices pretreated with PACAP6–38 [200 nM] and tolbutamide [100 μM], respectively.
For the optogenetic experiments, we performed recordings in slices from PACAP-Cre mice that were given a ChR2-containing AAV into the VMN 2–3 weeks before the experiment. Once we encountered PACAP-expressing fibers (visualized with eYFP) impinging on VTA neurons, we ascertained functional synaptic connectivity by delivering high-frequency stimulation (10-ms pulses delivered at 20 Hz for 10 s) from a light-emitting diode (LED) blue light source (470 nm) controlled by a variable 2A driver (ThorLabs, Newton, NJ, USA) that directed the light path through the Olympus 40X water-immersion lens. For voltage clamp experiments, we generated baseline ramp I/V relationships with TTX (500 nM) on board. After doing this, we photo-stimulated to generate the slow outward current (in voltage clamp) or hyperpolarization and decrease in firing (in current clamp) Once a new steady-state current (or voltage) was achieved, we generated another ramp I/V, after which the membrane current (or voltage) was allowed to resettle to its original baseline value, at which time we executed a final ramp I/V protocol to affirm reversibility of the optogenetic stimulus.
Behavioral Studies
We conducted the behavioral studies in a four-station Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, Ohio, USA) as previously characterized and authenticated (Farhang B et al., 2010). We examined energy intake, meal pattern and energy expenditure in intact male and OVX female wildtype mice; and we injected these females with either EB (20 μg/kg; s.c.) or its sesame oil vehicle (1 mL/kg; s.c.) on alternate days during the experiment. First, we allowed the animals to acclimatize to the experimental chambers over three days. We weighed and handled the animals every afternoon before returning them to their respective chambers. After acclimation, we started a five-day monitoring phase. The binge feeding paradigm was performed as previously described (Hernandez et al., 2020;Rospond et al., 2015). Briefly, we exposed animals to the HFD from 4–5 pm for five successive days following the acclimation period, and quantified energy intake, meal pattern and energy expenditure for that 60-minute time frame. We provided standard chow ad libitum for the remaining 23 hours. Just before beginning the one-hour HFD exposure, we injected animals with PACAP1–38 (30 pmol, 0.2 μL) or its 0.9% saline vehicle (0.2 μL) straight into the VTA.
Immunohistochemistry
We fixed slices from wildtype and TH-Cre mice overnight with 4% paraformaldehyde (4% PFM) in Sorenson’s phosphate buffer (pH 7.4). We then immersed them for three days in 20% sucrose dissolved in Sorensen’s buffer, which we replaced daily, followed by snap freezing in 2-methylbutane (EMD Millipore Corporation, Burlington, MA, USA) the next day. We cut coronal sections (20 μm) through the VTA on a cryostat and mounted them on chilled slides. We then washed these sections with 0.1 M sodium phosphate buffer (PBS; pH 7.4), and then incubated them overnight with either a monoclonal antibody directed against TH (Immunostar, Inc., Hudson, WI, USA; 1:4000 dilution) or a polyclonal antibody aimed against PACAP (Chang et al., 2020;Hu et al., 2020;Woodley et al., 2019) (Abcam, Cambridge, MA, USA; 1:200 dilution). We followed this up the next day with two 15-minute PBS washes, and then a two-hour application with either biotinylated goat anti-rabbit (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) or goat anti-mouse (Life Technologies, Carlsbad, CA, USA, 1:300 for both) secondary antibodies. Following another round of three 15-minute PBS washes, we did a last two-hour overlay with streptavidin-Alexa Flour (AF) 546 (Molecular Probes, Inc., Eugene, OR, PA, USA; 1:600), and concluded with a final series of three 30-minute PBS washes and cover slipping the slides. We evaluated the slides via fluorescence immunohistochemistry using a Zeiss Axioskop 2 Plus microscope (Carl Zeiss, Gӧttingen, Germany).
Statistical Analyses
We made comparisons amongst two groups with the Student’s t-test, Mann-Whitney U test or the signed rank test. When there were greater than two groups, we performed either the one-way or repeated measures, multifactorial analysis of variance (ANOVA; with rank transformation when necessary), and then the Least Significant Difference (LSD) test. We considered differences statistically significant when the alpha probability was less than 0.05.
RESULTS
Direct Injection of PACAP into the VTA Significantly Decreases the Consumption of Palatable Food in Wildtype Male but not Wildtype Female Mice
Previous reports have shown that PACAP significantly reduced food intake either by ICV (Matsuda et al., 1992;Mounien et al., 2009;Tachibana et al., 2003), IP (Matsuda et al., 2005;Vu et al., 2015), intra-VMN (Hurley et al., 2016;Resch et al., 2011;Resch et al., 2013), intra-PVN (Resch et al., 2013), or intra-ARC injection (Chang et al., 2020;Gastelum et al., 2021) under both ad libitum-fed and fasting conditions. We now wanted to ascertain whether PACAP could dampen the hedonic, binge-like consumption of palatable food. Here, we exposed wildtype mice to HFD for one hour every day for five days from 4–5 pm; with standard chow provided throughout the remainder of the time. Energy intake, meal pattern and energy expenditure were monitored around the clock. We found that as early as day 2 of the monitoring period, the animals started to exhibit clear signs of anticipatory locomotor activity immediately preceding the presentation of the HFD (Supplemental Figure 1). This is accompanied by an increase in metabolic heat production and O2 consumption during the binge-feeding episode that is maintained for several hours after the HFD exposure (Figure 2A: repeated measures ANOVA/LSD, F = 3.69, df = 8, p < 0.002 and 2B: repeated measures ANOVA/LSD, F = 5.13, df = 8, p < 0.0001) (Supplemental Figures 2 and 3). This limited intermittent access to HFD causes a rapid, dramatic escalation in consumption (Figure 2C). When averaged across the five days (Figure 2D), the energy intake during these binge episodes far exceeds the consumption seen in strictly chow- or HFD-fed animals during the same time period (Hernandez et al., 2020), to such an extent that these otherwise chow-fed males end up eating over 35% of their daily caloric intake during that one-hour exposure (Figure 2E). PACAP administered directly into the VTA (30 pmol; 0.2 μL) suppresses this binge-like escalation (Figures 2C: repeated measures multifactorial ANOVA/LSD, Fday = 10.18, df = 4, p < 0.0001, FPACAP = 16.87, df = 1, p < 0.0003, Finteraction= 0.42, df = 4, p < 0.80; 2D: Student’s t-test, t = 2.928, p < 0.006; 2E: Mann-Whitney U test, W = 121.0, p < 0.04) without affecting chow consumption during the other 23 hours (Figure 2F: Student’s t-test, t = −0.078175, p < 0.94), and this suppression is associated with reductions in meal bout size and duration (Figures 2G: Student’s t-test, t = 2.095, p < 0.05 and 2H: Student’s t-test, t = 3.137, p < 0.004). These results indicate that PACAP reduced binge-like consumption.
Figure 2.
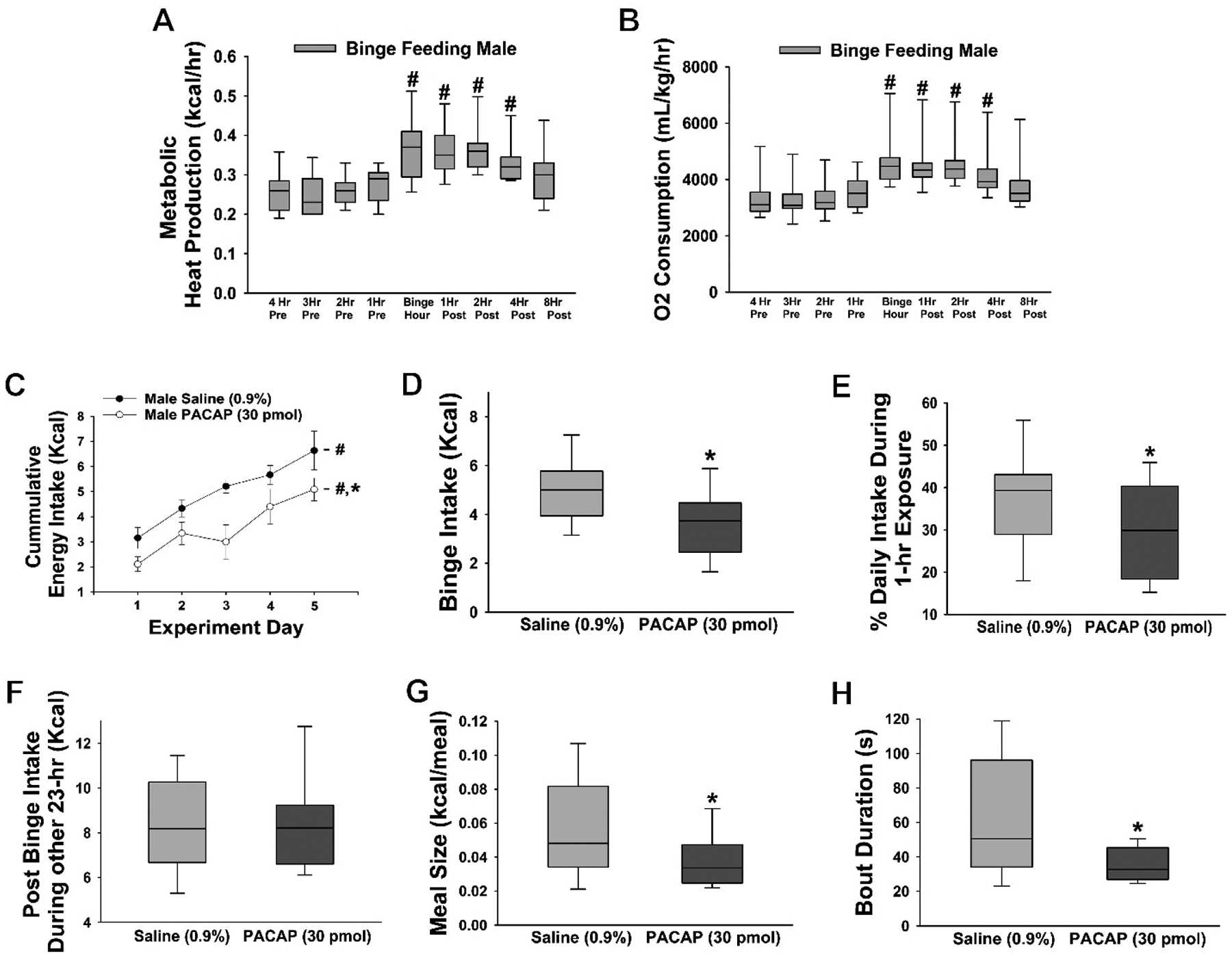
PACAP reduces binge feeding accompanied by decreases in meal size and bout duration. A & B, Pronounced increases in energy expenditure triggered by the binge episode. C-E, The escalation of the daily consumption is significantly dampened by PACAP (30 pmol; VTA; n = 6) as compared to saline-treated controls (n = 6). F, No changes in chow consumption over the remaining 23 hours were observed. G & H, PACAP-induced changes in meal pattern. Symbols represent means and lines 1 SEM. The bars of the box-and-whisker plot represent the median, the 25th and 75th quartiles, whereas the lines depict the 5th and the 95th percentiles. #, p < 0.05 with respect to time (A & B) or day (C), repeated measures ANOVA/LSD; *, p < 0.05; with respect to saline, repeated measures multifactorial ANOVA/LSD (C), Student’s t-test (D, F-H), Mann-Whitney U-test (E).
Similar to male mice, OVX wildtype female mice displayed the anticipatory increase in locomotor activity manifested by increases in both metabolic heat production (Figure 3A) and O2 consumption (Figure 3B) that developed during the binge hour and extended out to at least 4-hour post binge. The increase in metabolic heat production but not O2 consumption was significantly enhanced by EB treatment (20 μg/kg; s.c.) compared to the oil-treated mice (Figures 3A: repeated measures multifactorial ANOVA/LSD, Ftime = 19.22, df = 8, p < 0.0001, FEB = 0.80, df = 1, p < 0.38, Finteraction= 0.31, df = 8, p < 0.97 and 3B: repeated measures multifactorial ANOVA/LSD, Ftime = 17.79, df = 8, p < 0.0001; FEB = 4.01, df = 1, p < 0.05; Finteraction= 0.50, df = 8, p < 0.86). Consistent with what we and others have shown previously (Richard et al., 2017; Hernandez et al., 2020), EB produced its characteristic reduction in binge consumption (Figure 3C) and the percent daily intake ingested during the binge episode (Figure 3D), with no effect on cumulative chow consumption during the remaining 23 hours (Figure 3E). In contrast to male mice, however, PACAP showed no effect on binge intake and increased the percent daily intake during the 1-hour HFD in EB-treated animals (Figures 3C: repeated measures multifactorial ANOVA/LSD, FEB = 52.77, df = 1, p < 0.0001, FPACAP = 0.05, df = 1, p < 0.83, Finteraction= 0.02, df = 1, p < 0.88 and 3D: rank-transformed, repeated measures multi-factorial ANOVA/LSD: FEB = 19.65, df = 1, p < 0.0001, FPACAP = 1.25, df = 1, p < 0.27, Finteraction= 5.24, df = 1, p < 0.03; one-way ANOVA/LSD, F = 9.56, df = 3, p < 0.0001). Interestingly, the post binge intake during other 23-hours in which OVX mice were exposed to ad libitum chow was significantly suppressed in animals treated both EB and PACAP (Figure 3E: repeated measures multi-factorial ANOVA/LSD: FEB = 4.97, df = 1, p < 0.03, FPACAP = 5.46, df = 1, p < 0.03, Finteraction= 17.61, df = 1, p < 0.0001; one-way ANOVA/LSD, F = 8.29, df = 3, p < 0.0001); suggesting a synergistic anorexigenic effect in OVX female mice. Overall, our results indicate a sexual dimorphism in the ability of PACAP to blunt the binge-like consumption of palatable food.
Figure 3.
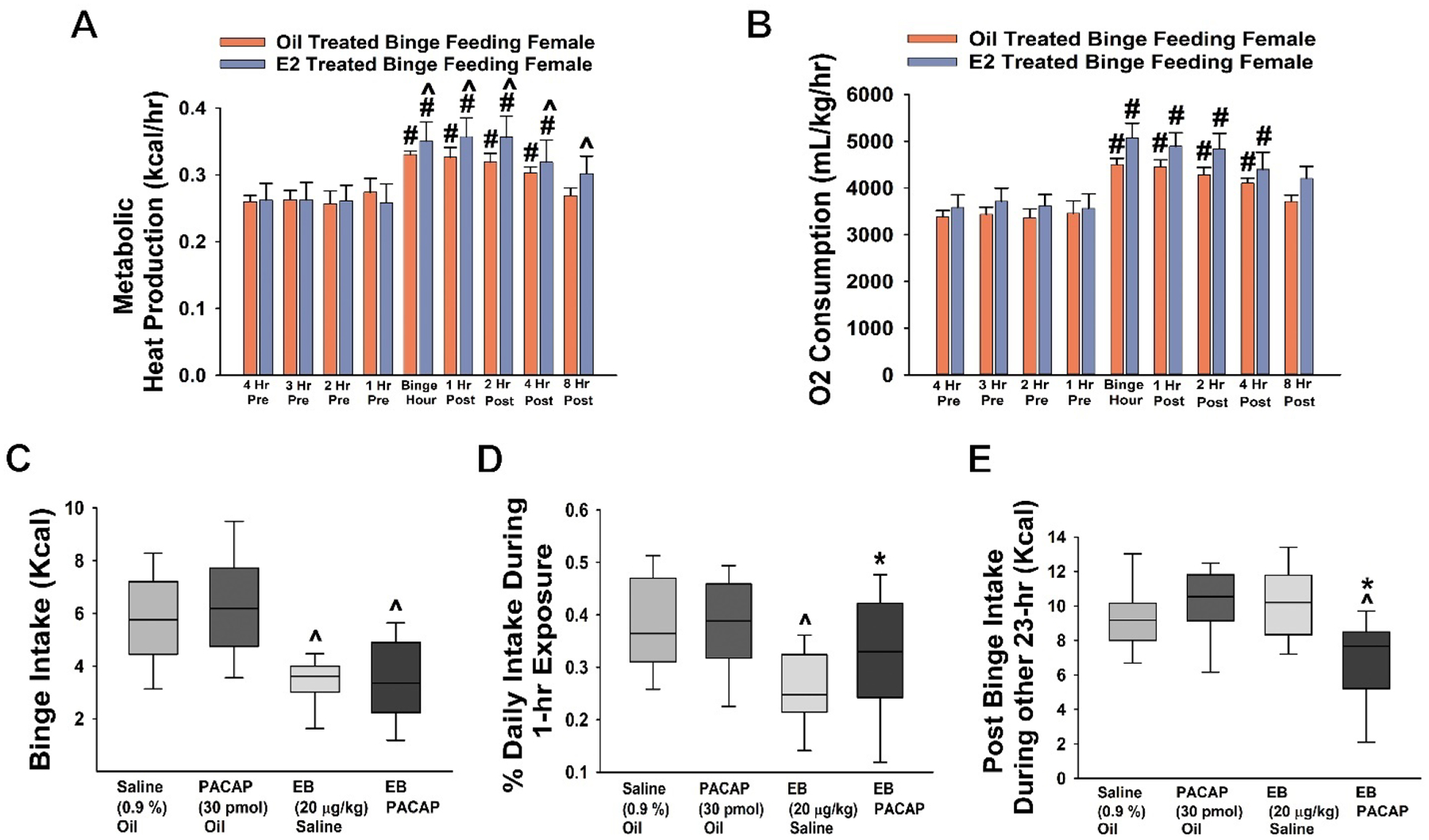
PACAP shows no effect in OVX wildtype female mice. A & B, Pronounced increases in energy expenditure triggered by the binge episode; with heat production being potentiated by EB (A). C & D, EB (20 μg/kg; s.c.; n = 6) but not PACAP (30 pmol; VTA; n = 6) decreases binge intake as well as the percent of the daily intake consumed during the binge hour as compared to their sesame oil (n = 6) and saline controls (n = 6). E, Chow consumption over the remaining 23 hours is significantly reduced in EB- and PACAP-treated animals. Bars represent means and lines 1 SEM. Box-and-whisker plots illustrate the median, 25th and 75th quartiles, as well as the 5th and 95th percentiles. #, p < 0.05 with respect to time (A & B); *, p < 0.05; with respect to saline; ^, p < 0.05 with respect to sesame oil; repeated measures, multi-factorial ANOVA/LSD (A – C, E), rank-transformed, repeated measures, multi-factorial ANOVA/LSD (D).
VMN PACAP Neurons Project to the VTA
The fact that PACAP delivered directly into the VTA suppressed binge feeding behavior suggested that the peptide may also be capable of regulating the excitability of A10 dopamine neurons of the hedonic energy balance circuitry. We then set to determine whether there are endogenous PACAP inputs to the VTA, and from where these inputs originated. To do this, we first performed retrograde tract tracing as described previously (Conde et al., 2016). We injected fluorogold (0.2 μL) into the VTA region of wildtype mice (Figure 4A), which is taken up by the nerve terminals in the VTA and transported back to the cell body. This revealed that the VTA receives strong innervation from the hypothalamus; including large clusters of neurons emanating from the VMN and LHA, with scattered inputs from the ARC (Figures 4B and 4C). Given the prominent population of PACAP neurons in the VMN (Chang et al., 2020;Hawke et al., 2009), we then stained for PACAP immunoreactivity and found a sizable population of immunopositive cells in this region (Figures 4D and 4F). This includes a significant number (~10%) of the fluorogold-filled somata exhibiting colocalization with immunostaining for PACAP (Figures 4E and 4F).
Figure 4.
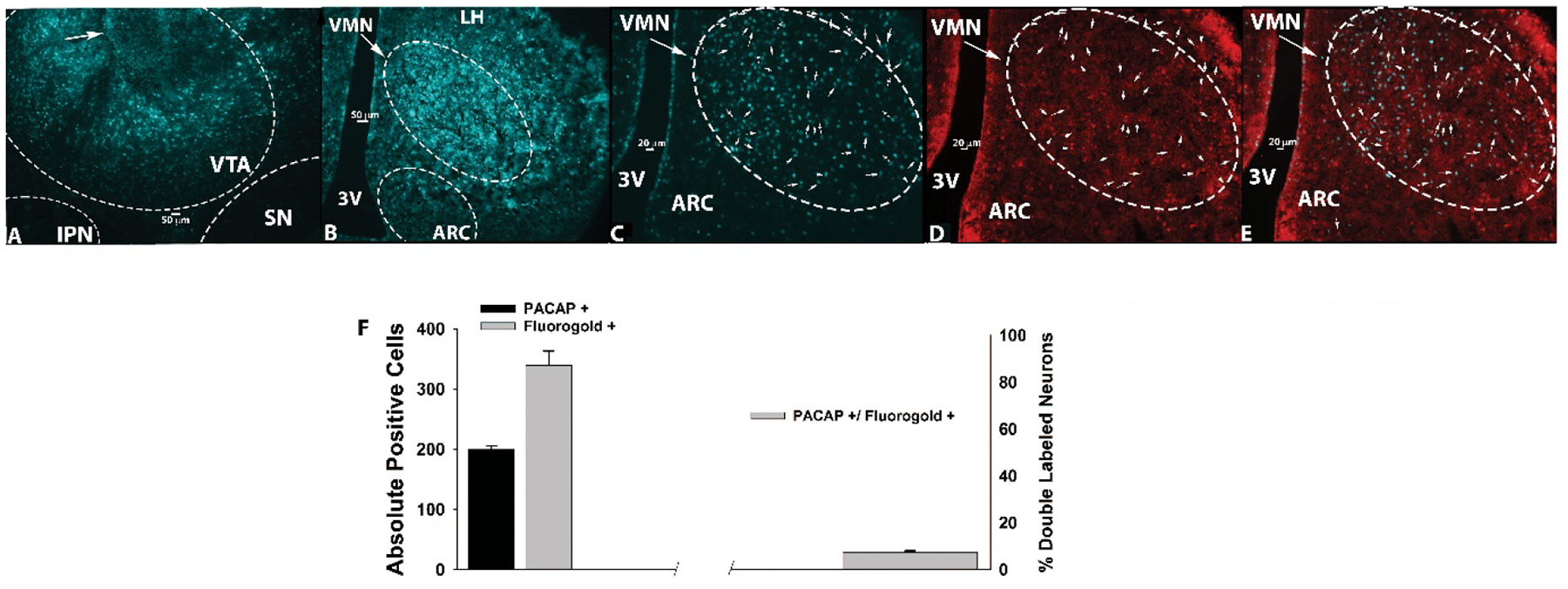
Retrograde tract tracing revealing a subpopulation of PACAP-containing, VTA-projecting VMN neurons. A, The injection site seen within the VTA (5X; denoted by the arrow). B, Fluorogold labelling in the VMN and surrounding areas (5X). C, Fluorogold labelling in the VMN (10X). D, PACAP immunostaining in the VMN as visualized with AF546. E, Composite overlay. Bars represent means and vertical lines 1 SEM. D–E were also photographed at 10X.
In advance of being able to optogenetically stimulate PACAP nerve terminals in the VTA, we then injected a ChR2-containing AAV linked to an eYFP reporter into the VMN of PACAP-cre mice. We have shown previously that the ChR2 expression in the VMN is confined to the vast majority of PACAP-immunoreactive neurons (Chang et al., 2020). Two to three weeks after injection, we observed robust ChR2 expression in PACAP cell bodies in the VMN (Figures 5A and 5B), and equally intense ChR2 expression in PACAP nerve terminals in the VTA (Figure 5C and 5D). This effectively demonstrates connectivity between VMN PACAP neurons and postsynaptic targets in the VTA.
Figure 5.
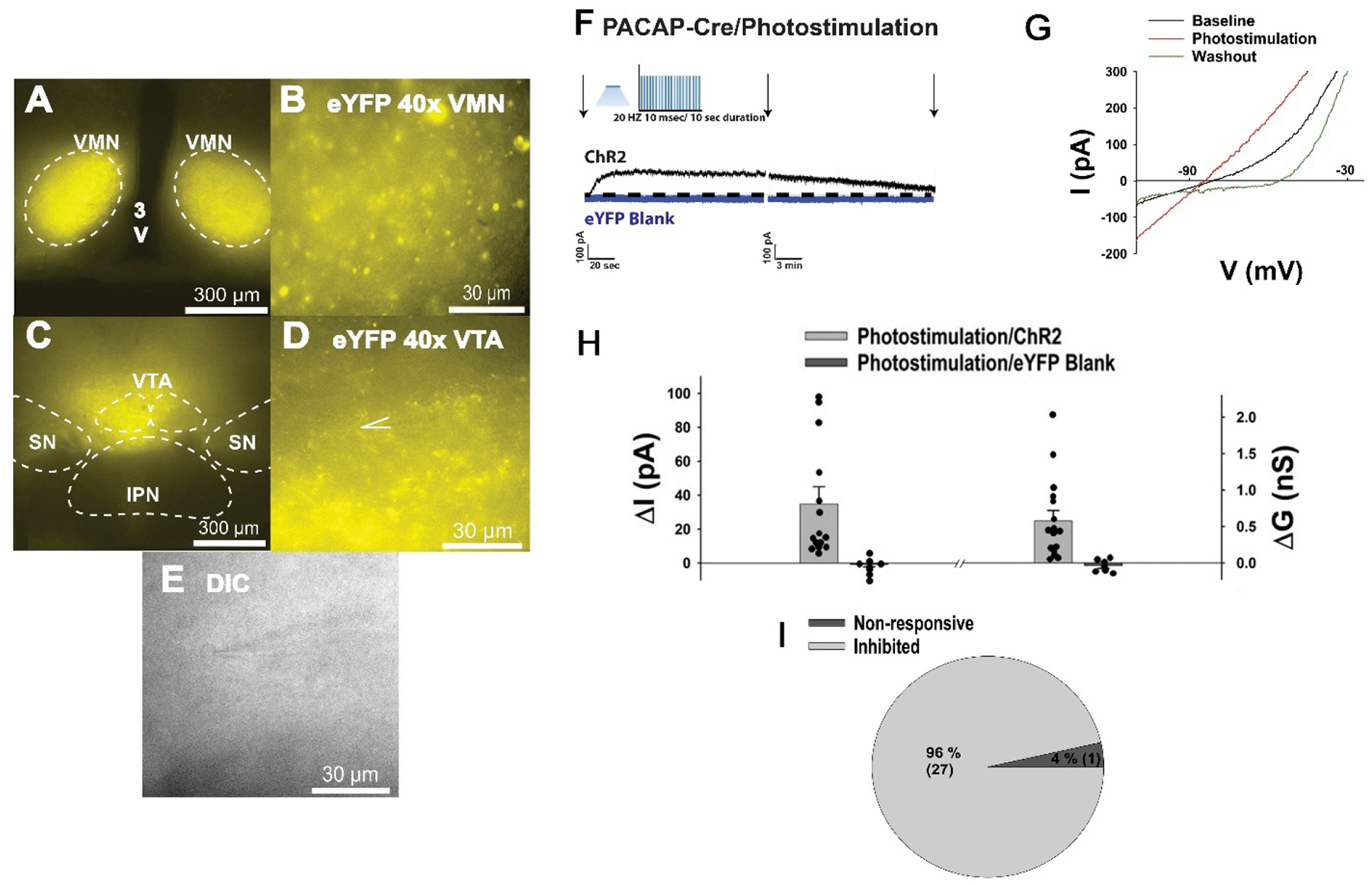
Photostimulation of VMN PACAP neurons inhibits VTA neurons in PACAP-Cre mice. A & B, eYFP ChR2 reporter signal in the VMN (4X & 40X). C & D, eYFP ChR2 reporter signal in the VTA (4X & 40X). E, DIC image (40X) of a recorded VTA neuron. F-I, Optogenetic stimulation of VMN PACAP neurons produces an outward current associated with an increased slope conductance and reversal of polarity at ~ −90 mV in the vast majority of VTA neurons (n = 15). Arrows indicate where I/Vs were conducted.
VMN PACAP Neurons Induce a PAC1 Receptor-Mediated Outward Current Associated with Hyperpolarization in A10 Dopamine Neurons via Activation of KATP Channels
Based off of our in vivo behavioral and tract tracing studies, we then hypothesized that PACAP inhibits A10 dopamine neurons via activation of the KATP channels to suppress binge-like consumption of palatable food. We wanted to test this via optogenetic stimulation of PACAP nerve terminals during visualized whole-cell patch clamp recordings in mesencephalic slices through the VTA from male PACAP-cre mice (Figures 5A–5E). Optogenetic stimulation of PACAP nerve terminals in the VTA rapidly produced a powerful and reversible outward current in voltage clamp in the presence of TTX (500 nM; Figures 5F and 5H) in the majority of VTA neurons (Figure 5I). Identical to what we have explicitly shown previously (Chang et al., 2020), optogenetic stimulation in slices from PACAP-cre mice that were sham-injected or injected with an eYFP blank control AAV failed to elicit any postsynaptic response (Figure 5F and 5H). This outward current is associated with an increase in the slope conductance, and the polarity of the response reverses near the equilibrium potential for K+ (Figures 5G and 5H). The outward current is linked to a reversible hyperpolarization measured under current clamp, and associated with a decrease in firing (Figure 6A & 6D), which are completely reversed in the presence of the KATP channel blocker tolbutamide (100 μM; Figure 6B & 6D) and also significantly attenuated by the PAC1 receptor antagonist PACAP6–38 (200 nM; Figure 6C–6D: one-way ANOVA/LSD, F = 32.22, df = 2, p < 0.0001). It should be noted that these inhibitory effects of endogenous PACAP released via optogenetic stimulation occur in A10 dopamine neurons identified post-hoc via immunohistofluorescence as described previously (Wagner et al., 1998). The change in membrane potential (Vm) was significantly reversed in the presence of tolbutamide and also neutralized when PACAP6–38 was introduced (Figure 6D).
Figure 6.
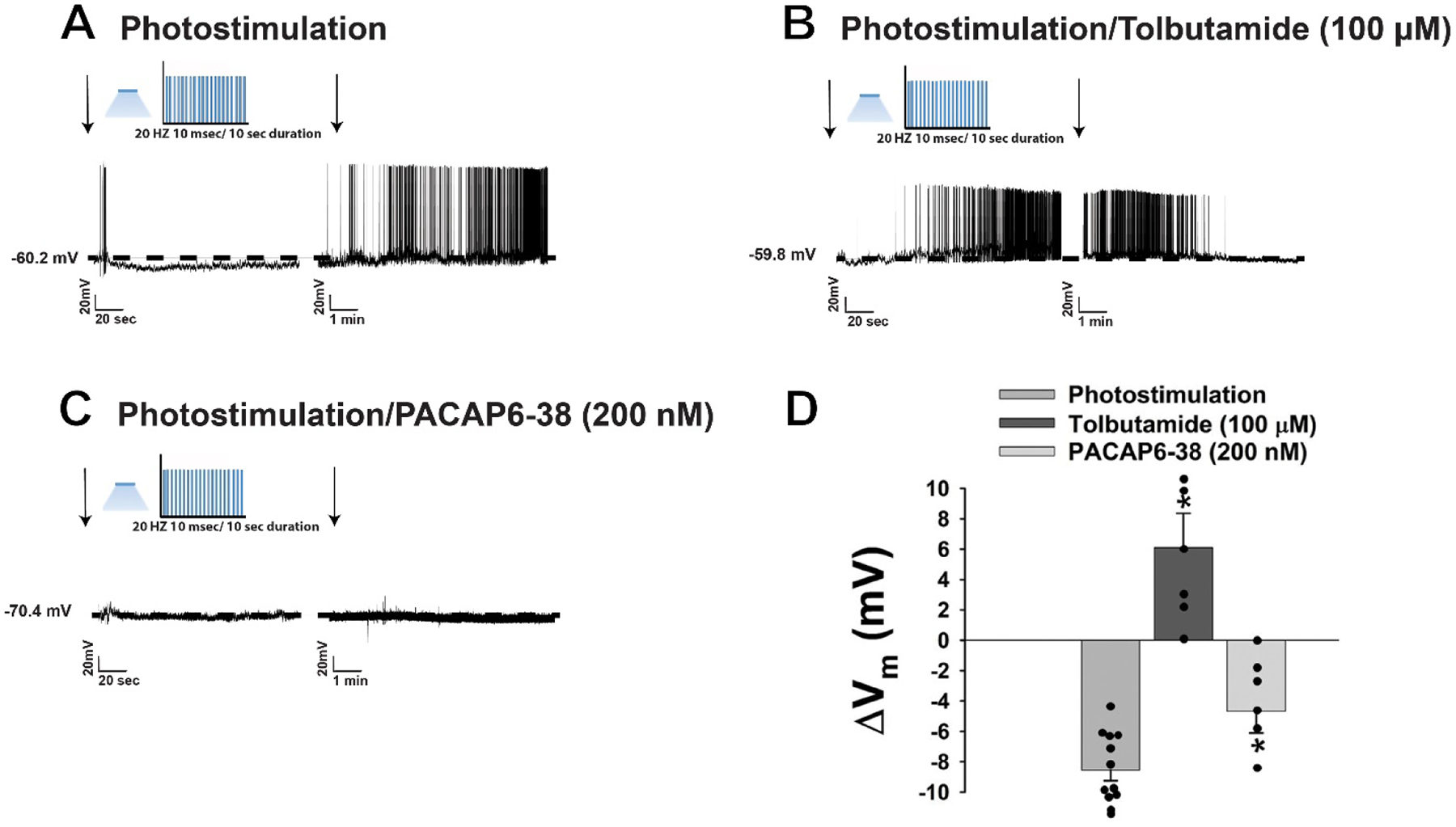
Optogenetic stimulation of VMN PACAP neurons in PACAP-Cre mice also hyperpolarizes VTA neurons and decreases their firing. A & D, Current clamp trace showing that photo-stimulation produces a reversible hyperpolarization (n = 12). B & D, The hyperpolarization is completely reversed in the presence of tolbutamide (100 μM; n = 6). C PACAP6–38 (200 nM; n = 6) significantly attenuated the hyperpolarization. D, Bar represents means and lines 1SEM of the change in membrane potential (ΔVm mV). *, p < 0.05 relative to PACAP alone, one-way ANOVA/LSD.
As a complimentary follow-up study, we also performed visualized patch clamp recordings in mesencephalic slices from male TH-cre mice injected with an eYFP-containing AAV injected into the VTA 2–3 weeks prior to experimentation in order to assess whether exogenously administered PACAP could replicate the inhibitory effect of the peptide on pre-identified A10 dopamine neurons. The robust immunolabelling of TH perikarya was observed in the vast preponderance of eYFP-expressing neurons in the VTA (Figures 7A–7C). Voltage clamp recordings of these eYFP-containing A10 dopamine neurons in the VTA (Figures 7D–7F) revealed that bath application of PACAP (100 nM) elicited a robust and reversible outward current (Figures 7G and 7N) in the considerable majority of A10 dopamine neurons (Figure 7O) that was associated with an increase in slope conductance (Figures 7H & 7M). This inhibitory effect was completely negated in the presence of the tolbutamide (Figures 7I, 7J, 7M: one-way ANOVA/LSD, F = 12.04, df = 2, p < 0.0005, and 7N: one-way ANOVA/LSD, F = 18.48, df = 2, p < 0.0001) and the PAC1 receptor antagonist PACAP6–38 (Figures 7K and 7L–7N).
Figure 7.
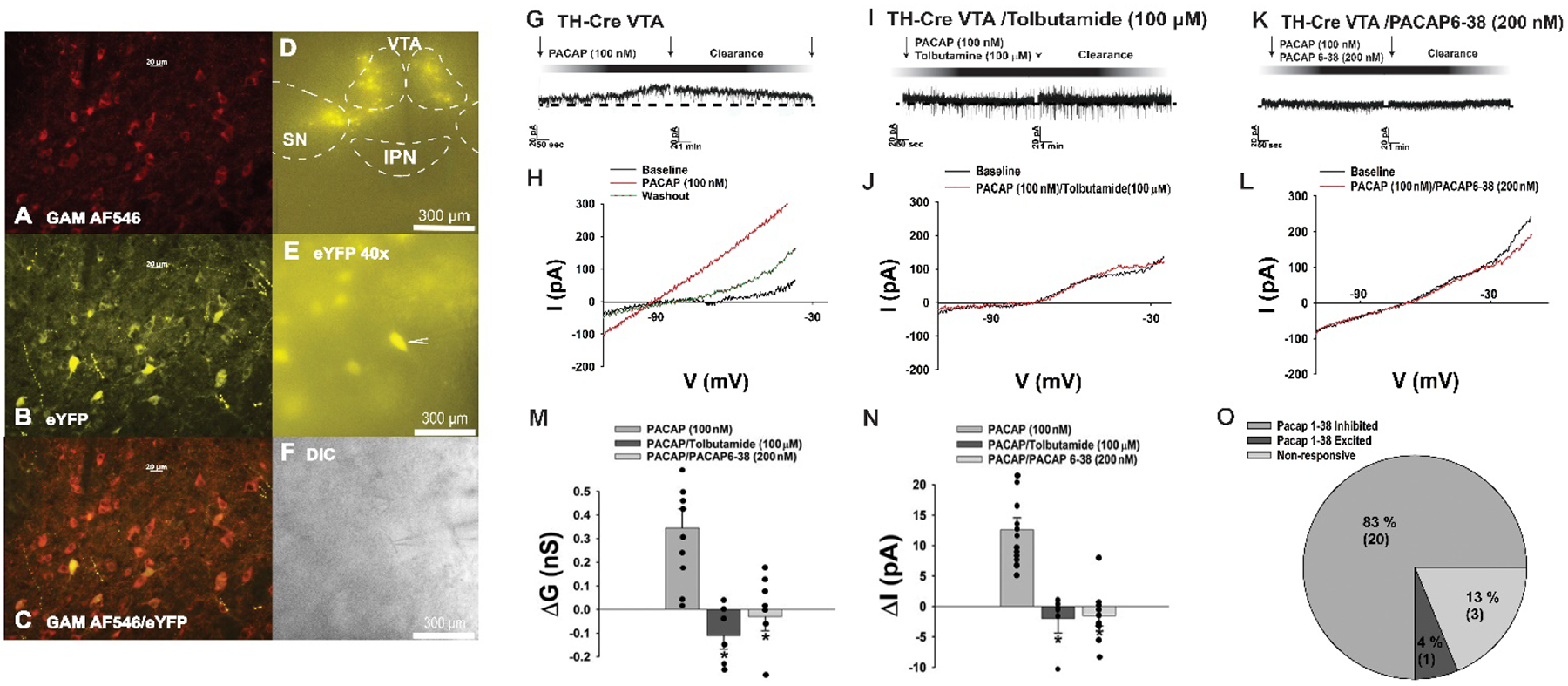
PACAP inhibits A10 dopamine neurons in slices from male TH-cre mice via a PAC1 receptor-mediated activation of KATP channels. A, TH immunostaining in the VTA. B, eYFP signal seen in these A10 dopamine neurons. C composite overlay. D & E, eYFP signal from A10 dopamine neurons in VTA slices seen at 4X and 40X. F, DIC image of the recorded A10 dopamine neuron seen in E. G-O, PACAP (100 nM; n = 12) produces a robust and reversible outward current in A10 dopamine neurons that is associated with an increased K+ conductance and abrogated by PACAP6–38 (n = 9) and tolbutamide (n = 6). Arrows indicate where I/Vs were conducted. Bars represent means and lines 1 SEM of the change in membrane current (M; pA) membrane slope conductance (M; nS) and current (N; pA). *p < 0.05 relative to PACAP alone, one-way ANOVA/LSD.
Similarly, the outward current is also linked to a reversible hyperpolarization measured under current clamp (Figure 8A) that significantly lowered the membrane potential (Vm; Figure 8B: Student’s t-test, t = 6.961, p < 0.001), and was associated with a decrease in firing (Figure 8C: signed rank test, test statistic = 2.10819, p < 0.04). Collectively, these data effectively demonstrate that VMN PACAP neurons inhibit A10 dopamine neurons within the hedonic energy balance circuitry via PAC1 receptor-mediated activation of KATP channels.
Figure 8.
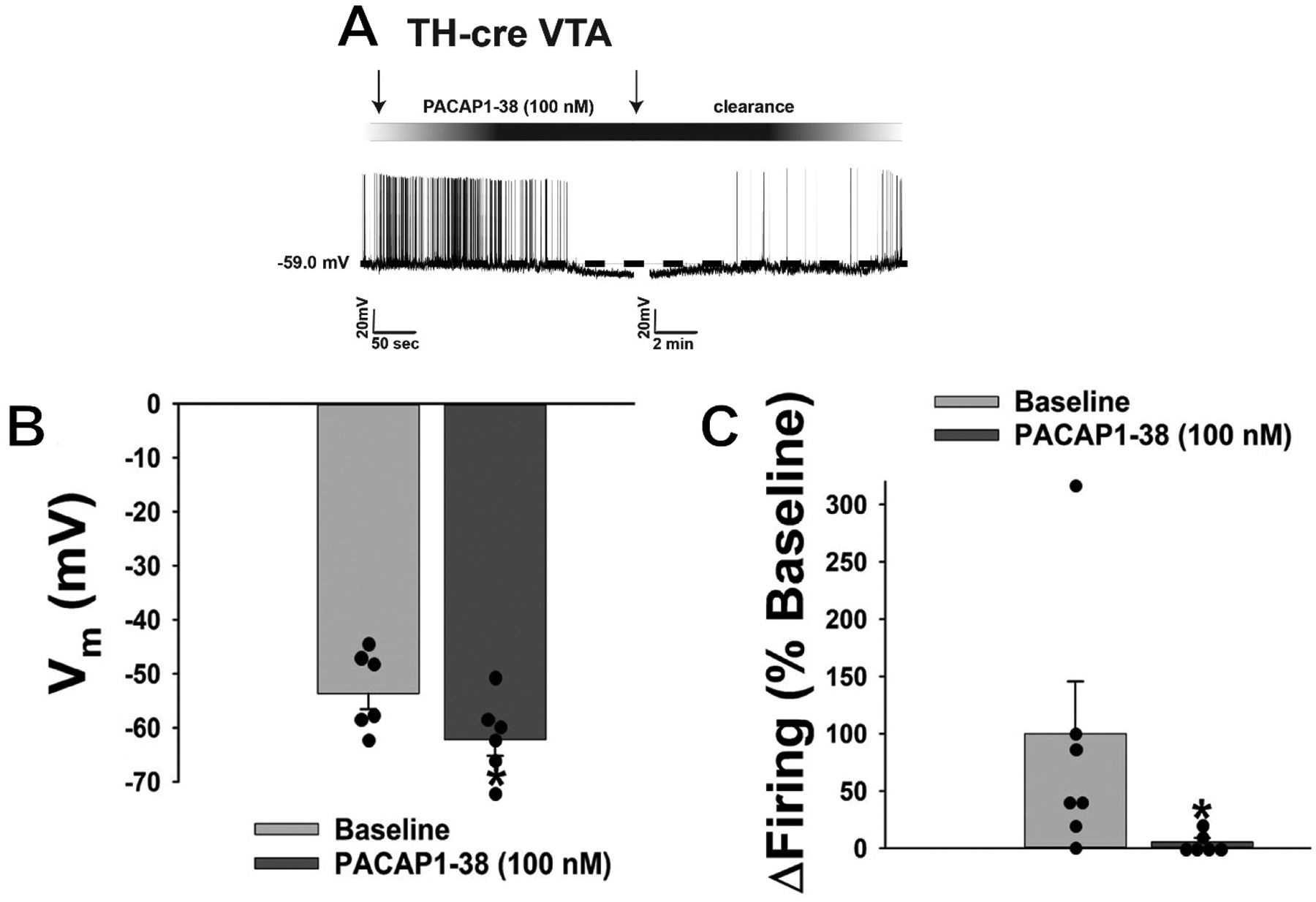
The outward current obtained in A10 dopamine neurons from TH-cre mice is also associated with a hyperpolarization and decrease in firing. A, Current clamp trace illustrating that bath application of PACAP1–38 produces reversible hyperpolarization that significantly lowers the membrane potential (Vm; B) and suppresses firing (C). B & C, Bars represent means and lines 1 SEM (n = 6). *, p < 0.05 relative to PACAP alone, Student’s t-test (B), signed rank test (C).
DISCUSSION
The results generated from this project demonstrate that VMN PACAP neurons can dampen the hedonic, binge-like consumption of palatable food in a sex-dependent manner through inhibition of A10 dopamine neurons. These findings are based on the following observations: 1) PACAP administered directly into the VTA suppresses binge-like consumption in male but not female mice, 2) tract tracing reveals that VMN PACAP neurons innervate the VTA, 3) optogenetic stimulation of VMN PACAP neurons induces a prominent, reversible outward current in VTA A10 dopamine neurons that is associated with an increase in slope conductance as well as hyperpolarization and decrease in firing, 4) the inhibitory effect of PACAP endogenously released from VTA nerve terminals on A10 dopamine neurons is mimicked by exogenous administration of the peptide.
PACAP Administered Directly into the VTA Suppresses Binge-Feeding Behavior in a Sexually Differentiated Manner
Here we uncover the role of PACAP and its predominant receptor PAC1 in the regulation of palatable food consumption. Consistent with what we and others have shown previously (Bello et al., 2003;Hernandez et al., 2020;Kreisler et al., 2017), intermittent exposure to a palatable HFD dramatically accelerates binge-like consumption. Our results further demonstrate that intra-VTA PACAP administration significantly suppresses binge-like consumption that is associated with reductions in meal size and bout duration in male but not female mice. These findings coincide with other reports that not only ICV or IP but also direct injections of PACAP into discrete hypothalamic nuclei causes a reduction in energy intake under various energy states (Chang et al., 2020;Gastelum et al., 2021;Hurley et al., 2016;Matsuda et al., 2005;Morley et al., 1992;Resch et al., 2011;Resch et al., 2013;Tachibana et al., 2003;Vu et al., 2015).
Our findings are also in agreement with the fact that microinjections of PACAP into the NAc mimic the actions of GABA agonists by reducing hedonic but not homeostatic feeding (Hurley et al., 2016), and acute microinjection of PACAP into the NAc attenuates palatable food consumption while microinjection of PACAP into the hypothalamus does not attenuate palatable feeding (Hurley et al., 2019). This further reinforces the idea that PACAP regulates binge-eating behavior in a site-specific manner. As mentioned, PACAP regulates the homeostatic energy balance circuitry in a pleiotropic, energy balance state-dependent manner – it is powerfully anorexigenic in sated animals with unlimited access to food and water (Morley et al., 1992;Mounien et al., 2009;Resch et al., 2011;Resch et al., 2013;Tachibana et al., 2003), but this can be reversed under conditions of negative energy balance (Gastelum et al., 2021). In addition, PACAP increases O2 consumption, metabolic heat production, and core body temperature (Chang et al., 2020;Resch et al., 2011;Resch et al., 2013). Reduction in body weight in these animals is consistently observed after PACAP administration, and pair-feeding studies suggest that the weight loss is not entirely due to food intake but also involves factors like increased activity and metabolic rate (Hawke et al., 2009;Resch et al., 2013).
The lack of effect of PACAP in both oil- and E2-treated OVX females was a great surprise to us, given that E2 potentiates the PACAP-induced excitation of anorexigenic POMC neurons and the corresponding reduction in homeostatic feeding (Chang et al., 2020;Gastelum et al., 2021) and attenuates the N/OFQ-induced inhibition of A10 dopamine neurons and the corresponding reduction in hedonic feeding (Hernandez et al., 2020). E2 per se did decrease binge-like consumption, which is consistent with the fact that it reduces the hedonic drive to consume palatable food (Richard et al., 2017). This also coincides with previous reports that E2 significantly reduces energy intake, body weight gain, and meal size as well as frequency (Geary and Asarian, 1999;Hernandez et al., 2019;Roepke et al., 2010), and this effect was seen at E2 doses ranging from 2.0 and 20.0 μg, respectively (Butera et al., 2010;Geary and Asarian, 1999). The dose of EB used in the present study (20 μg/kg) administered every other day over the behavioral experiment produces circulating levels typically seen during the late follicular phase or proestrus – a stage wherein energy intake is at its nadir in humans and non-human primates (Johnson et al., 1994;Kemnitz et al., 1989)- and consistently produces anorexigenic effects in guinea pigs and other rodent species (Fabelo et al., 2018;Hernandez et al., 2019;Hernandez et al., 2020;Kellert et al., 2009;Washburn et al., 2013). Thus, the ability of E2 to diminish hedonic feeding must occur through a neural substrate other than VMN PACAP neurons.
Binge-Feeding Behavior and the Relation to Dopamine Level and Food Addiction
Although the hedonic and homeostatic energy balance circuitries are regionally distinct, there are neural connections between the two that allow the conveyance of information from one to the other. This neural connection is established through the orexin-expressing LHA neurons in the LHA that innervate the VTA to regulate A10 dopamine neurons (Liu et al., 2017;Liu et al., 2015). These MCH and orexin expressing neurons project to the NAc as well as the VTA, to promote appetite and modulate reward (Chung et al., 2009;Georgescu et al., 2005;Mul et al., 2011;Zheng et al., 2007). These two energy balance circuitries are similar in that they are both modulated by leptin action, such that leptin directly regulates a population of leptin receptor (LepR)-expressing inhibitory neurons in the LHA and decreases feeding and body weight (Leinninger et al., 2009). In addition, leptin is known for its heterogenous effects in which it inhibits ARC NPY/AgRP neurons via activation of KATP channels (van den Top et al., 2004), and excites both POMC and SF-1/PACAP neurons via activation of TRPC5 channels (Dhillon et al., 2006;Hawke et al., 2009;Qiu et al., 2010) to regulate food intake. On the other hand, leptin also modulates behaviors associated with dopamine reward circuit and decreases hedonic feeding via administration of leptin into the VTA (Hommel et al., 2006;Morton et al., 2009).
A10 dopamine neurons play an important role on binge eating behaviors. In this study, we showed that intermittent access to HFD dramatically increases binge-like consumption, and we think that this binge eating may be associated with increased dopamine release from A10 dopamine neurons. This was indeed the case in rats exposed to the intermittent dietary-induced binge eating (DIBE) schedule with a sugar solution (10% sucrose) demonstrate increased accumbens dopamine levels (Rada et al., 2005). However, with repeated exposure to the food reward, the dopamine response habituates, and this habituation of dopamine signaling can be attenuated if animals are food restricted (Bassareo and Di Chiara, 1999;Bello et al., 2003). Therefore, receiving a palatable food during periods of calorie restriction serves to not only increase accumbal dopamine release, but to prevent habituation of dopamine signaling due to repeated stimulus exposure. Recent studies also report that short-term consumption of palatable food (i.e. binge feeding) is mediated by the strengthening of excitatory synaptic transmission onto VTA dopamine neurons, and injection of insulin (an anorexigenic hormone) into the VTA suppresses these excitatory inputs and abolishes food consumption (Liu et al., 2016). This is congruent with what we show in this paper; that administration of PACAP, which is also an anorexigenic hormone, directly into the VTA significantly suppresses binge-like consumption.
As additional proof that dopaminergic stimulation augments hedonic feeding, prolonged dopamine replacement therapy in Parkinson’s disease (PD) enhances dopamine receptor activation, and in parkinsonian animal models daily treatment with levodopa (L-Dopa) significantly escalates chocolate consumption in a time-dependent manner (Mineo et al., 2019). This implies that the restoration of dopaminergic neural activity can promote binge feeding. Likewise, in PD patients, inadequate control of dopamine replacement therapy with either dopamine agonists or high doses of L-Dopa may manifest in the form of behavioral addictions. (Beaulieu-Boire and Lang, 2015).
Our findings reveal that PACAP is without effect on binge intake in OVX female mice but significantly suppresses binge intake in male mice. This provides yet another example of sex differences in the regulation of energy homeostasis. Although the prevalence of obesity is similar between men and women, women seem to have a greater risk of developing eating disorders and extreme obesity (Hoek, 2006;Yang and Colditz, 2015). The investigation of food addiction in young women using the Yale Food Addiction Scale (YFAS) revealed that participants who possessed a greater number of food addiction features exhibited greater activation in the amygdala, dorsolateral prefrontal cortex, and medial orbitofrontal cortex (OFC) during exposure to a palatable food cue such as a picture of a milkshake (Gearhardt et al., 2011). In addition, binge-prone female rats were found to tolerate significantly high levels of foot shock for access to Oreo cookies than their binge-resistant counterparts, confirming the “continued use despite negative consequences” criteria for substance dependence (Oswald et al., 2011). This is consistent with other lines of evidence illustrating that women have a reduced ability to control food desire, higher cortical and limbic activation when presented with visual, gustatory, olfactory cues as well as increased susceptibility to episodes of food-craving compared to men (Uher et al., 2006;Wang et al., 2004;Wang et al., 2009;Weingarten and Elston, 1991).
VMN PACAP Neurons Inhibits A10 Dopamine Neurons Via the PAC1 Receptor-Mediated Activation of KATP Channels
PACAP is highly expressed in the VMN, and colocalizes extensively with SF-1 in VMN neurons (Chang et al., 2020;Hawke et al., 2009). These SF-1/PACAP neurons project to the ARC and synapse with anorexigenic POMC neurons (Chang et al., 2020;Lindberg et al., 2013). Presently, we have demonstrated through both anterograde and retrograde tract tracing that a subpopulation of VMN PACAP neurons projects to the VTA and forms functional synapses with A10 dopamine neurons. We show that the optogenetic stimulation of VMN PACAP neurons results in a robust but reversible outward current that is abrogated by PAC1 receptor antagonism and KATP channel blockade. While PACAP6–38 has long been touted as a PAC1 receptor antagonist, it is also capable of blocking VPAC2 receptors (Robberecht et al., 1992). PACAP binds the PAC1 receptors with high affinity and selectivity, whereas both PACAP and vasoactive intestinal peptide (VIP) bind the VPAC2 receptor with comparable affinity (Vaudry et al., 2009). While both PACAP and VIP inhibit food intake in goldfish and chick, only the appetite suppressant effect of PACAP is antagonized by PACAP6–38 (Matsuda et al., 2005;Tachibana et al., 2003) This indicates that the endogenous PACAP released by photo-stimulation binds and activates PAC1 receptors in A10 dopamine neurons within the hedonic energy balance circuit, which leads to the opening of KATP channels and K+ efflux. The fact that this was done in the presence of TTX confirms that it was a direct effect of PACAP itself, and not through some intermediary neurotransmitter such as GABA that could conceivably activate KATP channels and inhibit the A10 dopamine neurons. This is consistent with the inhibitory effects on these cells previously reported for insulin and leptin that also occur through the activation of KATP channels (Hommel et al., 2006;Morton et al., 2009). It is also congruent with other examples of PACAP-induced activation of KATP channels in vascular smooth muscle, as well as PACAP-induced increases in KATP channel subunit expression in the striatum, that accounts in part for the respective vasodilatory and neuroprotective effects of the peptide (Koide et al., 2014;Wang et al., 2008). The outward current and corresponding hyperpolarization elicited by optogenetic stimulation of VMN PACAP neurons were fully replicated when PACAP is perfused exogenously. In both instances the hyperpolarization caused by K+ efflux through KATP channels greatly suppresses neuronal firing in A10 dopamine neurons. Therefore, the PACAP/PAC1 receptor system can impact the neuronal activity of A10 dopamine neurons and the function of hedonic energy balance circuitry.
The activation of KATP channels play an important role in the regulation of energy homeostasis. It has been demonstrated in vitro that PIP3 activates KATP channels directly (MacGregor et al., 2002). PIP3 is the product generated from PI3K activity, which also is closely associated with downstream signaling molecules like protein kinase B, or Akt. (Denley et al., 2009). The PI3K/Akt pathway can be elicited by the activation of the PAC1 receptor via Gq signaling (Chang et al., 2020;Vaudry et al., 2009), which implies that PACAP may couple to KATP channels directly through this pathway. Furthermore, we have previously shown that the PAC1 receptor-mediated activation of TRPC5 channels in POMC neurons occurs through the Gq-coupled stimulation of PI3K/PLC/PKC signaling (Chang et al., 2020). Within the hedonic energy balance circuitry, activation of another important energy sensing molecule, AMP-dependent protein kinase (AMPK), potentiates KATP channel-mediated currents (Wu et al., 2017) and the PACAP/PAC1R-mediated activation of KATP channels inhibits A10 dopamine neurons, which is associated with hyperpolarization and decrease in firing. This inhibition leads to decreased dopamine neuronal activity and suppresses binge-like consumption. Within the homeostatic energy balance circuitry, however, elevated PIP3 levels in POMC neurons due to the selective knockout of PIP3 phosphatase causes activation of KATP channels to induce hyperphagia and sexually dimorphic diet-sensitive obesity (Plum et al., 2006). Similarly, augmented AMPK activity in POMC neurons promotes a switch in PAC1 receptor coupling from TRPC5 channels under ad libitum-fed conditions to KATP channels under fasting conditions; resulting in a hyperphagic response seen during refeeding (Gastelum et al., 2021). Taken together, this clearly demonstrates that precise control of PI3K and AMPK signaling as well as KATP channel activity is crucial for the maintenance of energy homeostasis.
While we certainly acknowledge that bath application and intra-VTA administration of exogenous PACAP do not entirely replicate the physiological effects of endogenous PACAP released from nerve terminals of VMN PACAP neurons in the VTA, the convergence of the evidence set forth here leads us to conclude that PACAP regulates hedonic feeding via inhibition of A10 dopamine neurons. It does this by postsynaptically activating PAC1 receptors and KATP channels in these cells in a sex-dependent manner.
Supplementary Material
Highlights.
VMN PACAP neurons project to the VTA
These neurons inhibit the excitability of A10 dopamine neurons
This occurs via activation of PAC1 receptors and KATP channels
PACAP suppresses binge-feeding behavior in a sexually dimorphic way
Thus, PACAP inhibits the hedonic feeding circuitry and the intake of palatable food
Acknowledgements
N.L., J.H., and C.G. performed all stereotaxic and survival surgeries. N.L. and J.H. performed all electrophysiological recordings. N.L., J.H., C.G., L.P., I.V. and S.S. performed all metabolic studies. N.L., J.H. and E.J.W. performed data analysis for all electrophysiology and metabolic studies. N.L, J.H., and E.J.W. created all figures and performed all statistical analyses. E.J.W. generated the manuscript, while all authors edited the final manuscript. E.J.W. and N.L. designed the experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by PHS Grant DA024314 and intramural funding from Western University of Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avena NM, Bocarsly ME (2012), Dysregulation of brain reward systems in eating disorders: neurochemical information from animal models of binge eating, bulimia nervosa, and anorexia nervosa. Neuropharmacology 63:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G (1999), Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci 11:4389–4397. [DOI] [PubMed] [Google Scholar]
- Beaulieu-Boire I, Lang AE (2015), Behavioral effects of levodopa. Mov Disord 30:90–102. [DOI] [PubMed] [Google Scholar]
- Bello NT, Sweigart KL, Lakoski JM, Norgren R, Hajnal A (2003), Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. Am J Physiol Regul Integr Comp Physiol 284:R1260–R1268. [DOI] [PubMed] [Google Scholar]
- Berridge KC (1996), Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev 20:1–25. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R, Morrison C (2008), The brain, appetite and obesity. Annu Rev Psychol 59:55–92. [DOI] [PubMed] [Google Scholar]
- Borgquist A, Kachani M, Tavitian N, Sinchak K, Wagner EJ (2013), Estradiol negatively modulates the pleiotropic actions of orphanin FQ/nociceptin at proopiomelanocortin synapses. Neuroendocrinology 98:60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butera PC, Wojcik DM, Clough SJ (2010), Effects of estradiol on food intake and meal patterns for diets that differ in flavor and fat content. Physiol Behav 99:142–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R, Hernandez J, Gastelum C, Guadagno K, Perez L, Wagner EJ (2020), Pituitary adenylate cyclase-activating polypeptide excites proopiomelanocortin neurons: implications for the regulation of energy homeostasis. Neuroendocrinology:doi: 10.1159/000506367. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Chu JY, Chow BK (2011), Central and peripheral administration of secretin inhibits food intake in mice through the activation of the melanocortin system. Neuropsychopharmacology 36:459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Hopf FW, Nagasaki H, Li CY, Belluzzi JD, Bonci A, Civelli O (2009), The melanin-concentrating hormone system modulates cocaine reward. Proc Natl Acad Sci U S A 106:6772–6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde K, Meza C, Kelly MJ, Sinchak K, Wagner EJ (2016), Estradiol rapidly attenuates ORL-1 receptor-mediated inhibition of proopiomelanocortin neurons via Gq-coupled, membrane-initiated signaling. Neuroendocrinology 103:787–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denley A, Gymnopoulos M, Kang S, Mitchell C, Vogt PK (2009), Requirement of phosphatidylinositol(3,4,5)trisphosphate in phosphatidylinositol 3-kinase-induced oncogenic transformation. Mol Cancer Res 7:1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, et al. (2006), Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203. [DOI] [PubMed] [Google Scholar]
- Dürr K, Norsted E, Gömüç B, Suarez E, Hannibal J, Meister B (2007), Presence of pituitary adenylate cyclase-activating polypeptide (PACAP) defines a subpopulation of hypothalamic POMC neurons. Brain Res 1186:203–211. [DOI] [PubMed] [Google Scholar]
- Fabelo C, Hernandez J, Chang R, S. S, Alicea N, Tian S, Conde K, Wagner EJ (2018), Endocannabinoid signaling at hypothalamic steroidogenic factor-1/proopiomelanocortin synapses is sex- and diet-sensitive. Front Mol Neurosci 11: 10.3389/fnmol.2018.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhang B, Pietruszewski L, Lutfy K, Wagner EJ (2010), The role of the NOP receptor in regulating food intake, meal pattern, and the excitability of proopiomelanocortin neurons. Neuropharmacology 59:190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipsson K, Sundler F, Ahren B (1999), PACAP is an islet neuropeptide which contributes to glucose-stimulated insulin secretion. Biochem Biophys Res Commun 256:664–667. [DOI] [PubMed] [Google Scholar]
- Gastelum C, Perez L, Hernandez J, Le N, Vahrson I, Sayers S, Wagner EJ (2021), Adaptive Changes in the Central Control of Energy Homeostasis Occur in Response to Variations in Energy Status. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD (2011), Neural correlates of food addiction. Arch Gen Psychiatry 68:808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N, Asarian L (1999), Cyclic estradiol treatment normalizes body weight and test meal size in ovariectomized rats. Physiol Behav 67:141–147. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolaños CA, Marsh DJ, Bednarek MA, Bibb JA, et al. (2005), The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci 25:2933–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke Z, Ivanov TR, Bechtold DA, Dhillon H, Lowell BB, Luckman SM (2009), PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J Neurosci 29:14828–14835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez J, Fabelo C, Perez L, Moore C, Chang R, Wagner EJ (2019), Nociceptin/orphanin FQ modulates energy homeostasis through inhibition of neurotransmission at VMN SF-1/ARC POMC synapses in a sex- and diet-dependent manner. Biol Sex Diff 10: 10.1186/s13293-13019-10220-13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez J, Perez L, Soto R, Le N, Gastelum C, Wagner EJ (2020), Nociceptin/orphanin FQ neurons in the Arcuate Nucleus and Ventral Tegmental Area Act via Nociceptin Opioid Peptide Receptor Signaling to Inhibit Proopiomelanocortin and A 10 Dopamine Neurons and Thereby Modulate Ingestion of Palatable Food. Physiol Behav 228:doi: 10.1016/j.physbeh.2020.113183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y (2010), Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90:291–366. [DOI] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, et al. (2008), Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest 118:1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek HW (2006), Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Curr Opin Psychiatry 19:389–394. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu Z-W, Gao X-B, Thurmon JJ, Marinelli M, et al. (2006), Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51:801–810. [DOI] [PubMed] [Google Scholar]
- Hu P, Liu J, Maita I, Kwok C, Gu E, Gergues MM, Kelada F, Phan M, et al. (2020), Chronic Stress Induces Maladaptive Behaviors by Activating Corticotropin-Releasing Hormone Signaling in the Mouse Oval Bed Nucleus of the Stria Terminalis. J Neurosci 40:2519–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley MM, Maunze B, Block ME, Frenkel MM, Reily MJ, Kim E, Chen Y, Li Y, et al. (2016), Pituitary adenylate-cyclase activating polypeptide regulates hunger- and palatability-induced binge eating. Front Neurosci 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley MM, Robble MR, Callan G, Choi S, Wheeler RA (2019), Pituitary adenylate cyclase-activiating polypeptide (PACAP) acts in the nucleus accumbens to reduce hedonic drive. Int J Obesity 43:928–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WG, Corrigan SA, Lemmon CR, Bergeron KB, Crusco AH (1994), Energy regulation over the menstrual cycle. Physiol Behav 56:523–527. [DOI] [PubMed] [Google Scholar]
- Kellert BA, Nguyen MC, Nguyen C, Nguyen QH, Wagner EJ (2009), Estrogen rapidly attenuates cannabinoid-induced changes in energy homeostasis. Eur J Pharmacol 622:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemnitz JW, Gibber JR, Lindsay KA, Eisele SG (1989), Effects of ovarian hormones on eating behaviors, body weight and glucoregulation in rhesus monkeys. Horm Behav 23:235–250. [DOI] [PubMed] [Google Scholar]
- Khodai T, Nunn N, Worth AA, Feetham CH, Belle MDC, Piggins HD, Luckman SM (2018), PACAP neurons in the ventromedial hypothalamic nucleus are glucose inhibited and their selective activation induces hyperglycemia. Front Endocrinol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Zhao L, Donato J Jr., Kohno D, Xu Y, Elias CF, Lee C, Parker KL, et al. (2011), Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proc Natl Acad Sci U S A 108:10673–10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöckener T, Hess S, Belgardt BF, Paeger L, Verhagen LAW, Husch A, Sohn J-W, Hampel B, et al. (2011), High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. nature neuroscience 14:911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide M, Syed AU, Braas KM, May V, Wellman GC (2014), Pituitary adenylate cyclase activating polypeptide (PACAP) dilates cerebellar arteries through activation of large-conductance Ca(2+)-activated (BK) and ATP-sensitive (K ATP) K (+) channels. J Mol Neurosci 54:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisler AD, Garcia MG, Spierling SR, Hui BE, Zorilla EP (2017), Extended vs. brief intermittent access to palatable food differently promote binge-like intake, rejection of less preferred food, and weight cycling in female rats. Physiol Behav 177:305–316. [DOI] [PubMed] [Google Scholar]
- Leinninger GM, Jo Y-H, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, et al. (2009), Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Neuron 10:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg D, Chen P, Li C (2013), Conditional viral tracing reveals that steroidogenic factor 1-positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to the autonomic centers of the hypothalamus and hindbrain. J Comp Neurol 521:3167–3190. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Bello NT, Pang ZP (2017), Presynaptic Regulation of Leptin in a Defined Lateral Hypothalamus-Ventral Tegmental Area Neurocircuitry Depends on Energy State. J Neurosci 37:11854–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JJ, Mukherjee D, Haritan D, Ignatowska-Jankowska B, Liu J, Citri A, Pang ZP (2015), High on food: the interaction between the neural circuits for feeding and for reward. Front Biol (Beijing) 10:165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Globa AK, Mills F, Naef L, Qiao M, Bamji SX, Borgland SL (2016), Consumption of palatable food primes food approach behavior by rapidly increasing synaptic density in the VTA. Proc Natl Acad Sci U S A 113:2520–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZH, Shin R, Ikemoto S (2008), Dual role of medial A10 dopamine neurons in affective encoding. Neuropsychopharmacology 33:3010–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor GG, Dong K, Vanoye CG, Tang L, Giebisch G, Hebert SC (2002), Nucleotides and phospholipids compete for binding to the C terminus of KATP channels. Proc Natl Acad Sci U S A 99:2726–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL (2002), Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology 143:607–614. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Ohtaki T, Masuda Y, Nagai Y, Suno M, Tsuda M, Fujino M (1991), Autoradiographic distribution of pituitary adenylate cyclase activating polypeptide (PACAP) binding sites in the rat brain. Neurosci Lett 126:103–106. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Maruyama K, Nakamachi T, Miura T, Uchiyama M, Shioda S (2005), Inhibitory effects of pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) on food intake in the goldfish, Carassius auratus. Peptides 26:1611–1616. [DOI] [PubMed] [Google Scholar]
- Mineo D, Cacace F, Mancini M, Vannelli A, Campanelli F, Natale G, Marino G, Cardinale A, et al. (2019), Dopamine drives binge-like consumption of a palatable food in experimental Parkinsonism. Mov Disord 34:821–831. [DOI] [PubMed] [Google Scholar]
- Morley JE, Horowitz M, Morley PM, Flood JF (1992), Pituitary adenylate cyclase activating polypeptide (PACAP) reduces food intake in mice. Peptides 13:1133–1135. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Blevins JE, Kim F, Matsen M, Figlewicz DP (2009), The action of leptin in the ventral tegmental area to decrease food intake is dependent on Jak-2 signaling. Am J Physiol Endocrinol Metab 297:E202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounien L, Do Rego J-C, Bizet P, Boutelet I, Gourcerol G, Fournier A, Brabet P, Costentin J, et al. (2009), Pituitary adenylate cyclase-activating polypeptide inhibits food intake in mice through activation of the hypothalamic melanocortin system. Neuropsychopharmacol 34:424–435. [DOI] [PubMed] [Google Scholar]
- Mul JD, la Fleur SE, Toonen PW, Afrasiab-Middelman A, Binnekade R, Schetters D, Verheij MM, Sears RM, et al. (2011), Chronic loss of melanin-concentrating hormone affects motivational aspects of feeding in the rat. PLoS One 6:e19600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald KD, Murdaugh DL, King VL, Boggiano MM (2011), Motivation for palatable food despite consequences in an animal model of binge eating. Int J Eat Disord 44:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Münzberg H, Shanabrough M, Burdakov D, et al. (2006), Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest 116:1886–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Meza C, Navarro U-V, Nestor CC, Wagner EJ, Rønnekleiv OK, Kelly MJ (2018), Estradiol protects proopiomelanocortin neurons against insulin resistance. Endocrinology 159:647–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ (2010), Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci 30:1560–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Zhang C, Borgquist A, Nestor CC, Smith AW, Bosch MA, Ku S, Wagner EJ, et al. (2014), Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metabolism 19:682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu N, He Y, Wang C, Xu P, Yang Y, Cai X, Liu H, Yu K, et al. (2020), A POMC-originated circuit regulates stress-induced hypophagia, depression, and anhedonia. Mol Psychiatry 25:1006–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG (2005), Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience 134:737–744. [DOI] [PubMed] [Google Scholar]
- Resch JM, Boisvert JP, Hourigan AE, Muller CR, Yi SS, Choi S (2011), Stimulation of the hypothalamic ventromedial nuclei by pituitary adenylate cyclase-activating polypeptide induces hypophagia and thermogenesis. Am J Physiol Regul Integr Comp Physiol 301:R1625–R1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch JM, Maunze B, Gerhardt AK, Magnuson SK, Phillips KA, Choi S (2013), Intrahypothalamic pituitary adenylate cyclase-activating polypeptide regulates energy balance via site-specific actions on feeding and metabolism. Am J Physiol Endocrinol Metab 305:E1452–E1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JE, Lopez-Ferreras L, Anderberg RH, Olandersson K, Skibicka KP (2017), Estradiol is a critical regulator of food-reward behavior. Psychoneuroendocrinology 78:193–202. [DOI] [PubMed] [Google Scholar]
- Robberecht P, Gourlet P, De Neef P, Woussen-Colle M-C, Vandermeers-Piret M-C, Vandermeers A, Christophe J (1992), Structural requirements for the occupancy of pituitary adenylate-cyclase-activating-peptide (PACAP) receptors and adenylate cyclase activation in human neuroblastoma NB-OK-1 cell membranes: Discovery of PACAP(6–38) as a potent antagonist. Eur J Biochem 207:239–246. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlova-Wuttke D, Wuttke W, Scanlan TS, et al. (2010), Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology 151:4926–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rospond B, Szpigiel J, Sadakierska-Chudy A, Filip M (2015), Binge eating in pre-clinical models. Pharmacol Rep 67:504–512. [DOI] [PubMed] [Google Scholar]
- Rudecki AP, Gray SL (2016), PACAP in the defense of energy homeostasis. Trends Endocrinol Metab 27:620–632. [DOI] [PubMed] [Google Scholar]
- Sohn JW (2013), Ion channels in the central regulation of energy and glucose homeostasis. Front Neurosci 7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HS, Feng ZP (2013), Neuroprotective role of ATP-sensitive potassium channels in cerebral ischemia. Acta Pharmacol Sin 34:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana T, Tomonaga S, Oikawa D, Saito S, Takagi T, Saito ES, Boswell T, Furuse M (2003), Pituitary adenylate cyclase activating polypeptide and vasoactive intestinal peptide inhibit feeding in the chick brain by different mechanisms. Neurosci Lett 348:25–28. [DOI] [PubMed] [Google Scholar]
- Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC (2006), Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res 169:111–119. [DOI] [PubMed] [Google Scholar]
- van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D (2004), Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. nature neuroscience 5:493–494. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BKC, et al. (2009), Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61:283–357. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA, Baler R (2017), The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci 18:741–752. [DOI] [PubMed] [Google Scholar]
- Vu JP, Goyal D, Luong L, Oh S, Sanghu R, Norris J, Parsons W, Pisegna JR, et al. (2015), PACAP intraperitoneal treatment suppresses appetite and food intake via PAC1 receptor in mice by inhibiting ghrelin and increasing GLP-1 and leptin. Am J Physiol Gastrointest Liver Physiol 309:G816–G825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EJ (2016), Sex differences in cannabinoid-regulated biology: a focus on energy homeostasis. Front Neuroendocrinol 40:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EJ, Rønnekleiv OK, Grandy DK, Kelly MJ (1998), The peptide orphanin FQ inhibits β-endorphin neurons and neurosecretory cells in the hypothalamic arcuate nucleus by activating an inwardly-rectifying K+ conductance. Neuroendocrinology 67:73–82. [DOI] [PubMed] [Google Scholar]
- Wang G, Pan J, Tan Y-Y, Sun X-K, Zhang Y-F, Zhou H-Y, Ren R-J, Wang X-J, et al. (2008), Neuroprotective effects of PACAP27 in mice model of Parkinson’s disease involved in the modulation of K(ATP) subunits and D2 receptors in the striatum. Neuropeptides 42:267–276. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M, Zhu W, Wong CT, et al. (2004), Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage 21:1790–1797. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, Zhu W, Wong CT, et al. (2009), Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn N, Borgquist A, Wang K, Jeffery GS, Kelly MJ, Wagner EJ (2013), Receptor subtypes and signal transduction mechanisms contributing to the estrogenic attenuation of cannabinoid-induced changes in energy homeostasis. Neuroendocrinology 97:160–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten HP, Elston D (1991), Food cravings in a college population. Appetite 17:167–175. [DOI] [PubMed] [Google Scholar]
- Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK (2010), Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci 30:2472–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley PK, Min Q, Li Y, Mulvey NF, Parkinson DB, Dun X (2019), Distinct VIP and PACAP functions in the distal nerve stump during peripheral nerve regeneration. Front Neurosci: 10.3389/fnins.2019.01326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YN, Shen KZ, Johnson SW (2017), Differential actions of AMP kinase on ATP-sensitive K(+) currents in ventral tegmental area and substantia nigra zona compacta neurons. Eur J Neurosci 46:2746–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Colditz GA (2015), Prevalence of Overweight and Obesity in the United States, 2007–2012. JAMA Intern Med 175:1412–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR (2007), Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci 27:11075–11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


