1. Introduction
Since the industrial revolution, food staples in wealthy countries have become energy-enriched. Meanwhile the prevalence of obesity has escalated (Cordain et. al., 2005). The average American consumes roughly 58% of their daily energy from processed foodstuffs (Gupta et. al., 2020). By 2030, the majority of Americans are projected to be overweight or obese (Wang et. al., 2020). Processed food consumption has been associated with obesity, metabolic syndrome, hypertension, and depression (Gupta et. al., 2020). Medical complications of obesity include type 2 diabetes, hypertension, cancer, and mortality (Makaronidis & Batterham, 2018). Worldwide, smoking and alcohol are rapidly being displaced by obesity as a leading contributor to morbidity and mortality (Kleinert et. al., 2018).
Despite these effects on public health, reliable weight loss therapies are lacking. Sustained weight loss is unlikely in obese individuals that attempt healthy lifestyle changes. Even after initially successful weight loss, the majority relapse to prior eating habits and regain (van Meer et. al., 2016; Natvik et. al., 2018). While invasive, the most effective treatment for obesity is bariatric surgery (Kittrell & Graber, 2018; Makaronidis & Batterham, 2018). In addition to limiting food intake volume, this surgery also reduces preference for sugar or fat in some patients (Guyot et. al., 2021). These changes in food preference have also been found to be a strong predictor of postoperative weight loss (Neilson et. al., 2021; Kittrell & Graber, 2018). In both animal and man, bariatric surgery appears to diminish energy-dense food reward value (Nielson et. al., 2021). In rodents, this surgery impairs energy selectivity (Bueter et al., 2011; Mathes and Spector, 2012). The biological underpinnings of food preference are an active area of research (Neilson et. al., 2021; Kittrell & Graber, 2018).
Food energy-density influences energy intake, body weight, and adiposity (Teo et. al., 2020). It guides food and flavor preferences in healthy adults and children (Meiselman et. al., 1974; Kern et. al., 1993). Animals also prefer energy-dense foods and characteristics associated with high nutrient densities (Stubbs & Whybrow, 2004; Tordoff et. al., 2017; van Meer et. al., 2016). Obese individuals consume diets higher in energy-density as compared to those with healthy weight (Vernarelli et. al., 2015). They may also pay more attention to cues associated with high energy-density; and show indifference to cues associated with low energy-density (van Meer et. al., 2016; Watson et. al. 2017). Recent rodent studies show changes in food preference after exposure to energy-dense diet (Steele et. al., 2019; Mazzone et. al., 2020; Altherr et. al., 2021).
Mouse models are widely used to study other pathological adaptations to energy-dense diet, such as glucose intolerance, insulin resistance, and hypertension (Avtanski et. al., 2019). Several mouse strains, including the C57BL/6, will selectively feed on energy-dense food (Smith et. al., 2000; Mazzone et. al., 2020). C57BL/6 mice will also develop characteristics resembling human metabolic syndrome when fed energy-dense diet, though males tend to be more susceptible. Approximately 60% of all preclinical research utilizes C57BL/6 mice, in part due to the availability of genetic tools and well-characterized phenotyping protocols (Kleinert et. al., 2018). A standardized technique for studying food preference in this mouse strain would also be a useful tool in the pre-clinical study of obesity (van Meer et. al., 2016).
Here we establish a simple methodology for measuring food preference in C57BL/6 mice. Food preference was scored as the proportion of daily food intake attributable to the energy-superior test food. In this way, food preference assay did not require food restriction or liquid diet. Scores were not affected by satiety. Behavioral testing could be performed using standard housing equipment. Choice tests also captured food intake, permitting the dissociation of treatment effects on appetite and preference. In this way, our results were consistent with recent studies that demonstrate aphagia for less calorie-dense (healthier) foods after exposure to energy-dense diet (Mazzone et. al., 2020; Altherr et. al., 2021).
The present study confirms that food preference can be reliably measured in C57BL/6 mice and is influenced by energy-density. Here, we have characterized behavioral scores generated by a range of test foods. We have investigated the time course of food preference acquisition. Finally, we tested the effects of energy-dense diet on preference. Test foods and maintenance diets were selected from common manufacturers for reproducibility (Envigo, Research Diets Inc., BioServ®). Our work validates food preference assay as a measure of energy selectivity and nutritional state. Data presented here establish that changes in food preference are a measurable effect of energy-dense diet exposure.
2. Materials and Methods
2.1. Animals
All experiments were conducted in accordance with the NIH Guidelines for the Use of Laboratory Animals and were approved by the University of Virginia Institutional Animal Care and Use Committee (protocol number 4191). Mice were housed in standard ventilated caging with corncob bedding, cotton nesting material (Nestlets™, Ancare, Bellmore, NY, USA) and a paper soufflé cup (product F400, Genpak, Charlotte, NC). Housing rooms maintained 12-hour light/ dark cycles. Food and water were provided ad libitum in the home cage prior to behavioral assay. All C57BL/6J mice were bred in-house, with founders purchased from The Jackson Laboratory (Bar Harbor, ME, USA).
2.2. Maintenance Diets
Control mice received standard vivarium chow: Envigo 8664 Teklad F6 Rodent Diet (Teklad 8664) Huntingdon, United Kingdom. Experimental groups received: Envigo TD.88137 Adjusted calories diet (Teklad 88137), Open Source Diets product D12450B (Open Source D12450B; Research Diets, New Brunswick NJ, USA), Open Source Diets product D12492 (Open Source D12492), or BioServ Product S3472 Transgenic Dough Diet™ (BioServ S3472; Flemington NJ, USA). All diets were pelleted, except BioServ S3472 which was formed into a ball, placed on the cage floor, and replaced every other day. Because BioServ S3472 is also a sterile diet, it was supplemented with Envigo Teklad 2019 Global 19% Protein Extruded Rodent Diet (Teklad 2019) to encourage intake, and prevent potential vitamin deficiency (Hirayama et. al., 2007). On average, mice fed BioServ S3472 and Teklad 2019, consumed ~95.7% of their total daily calories from BioServ S3472 (data not shown). See Supplementary Table 1 for additional nutritional information.
2.3. Test Foods
All test foods were in biscuit (pelleted) form. All were pigmented and thereby easily distinguished by experimenters. Soft or crumbly foods were not selected for choice testing. Test foods presented during the first experiment (Fig. 1) were as follows: test 1 Teklad 8664 and Teklad 2019, test 2 Bio-Serv Product F3028 Rodent Diet Grain-Based Control (BioServ F3028) and Bio-Serv Product F3156 Rodent Diet AIN-93G (BioServ F3156), test 3: Open Source D12450B and Open Source Diets product D12451 (Open Source D12451).
Fig. 1. Food preference tests in 10-week old C57BL6 mice.
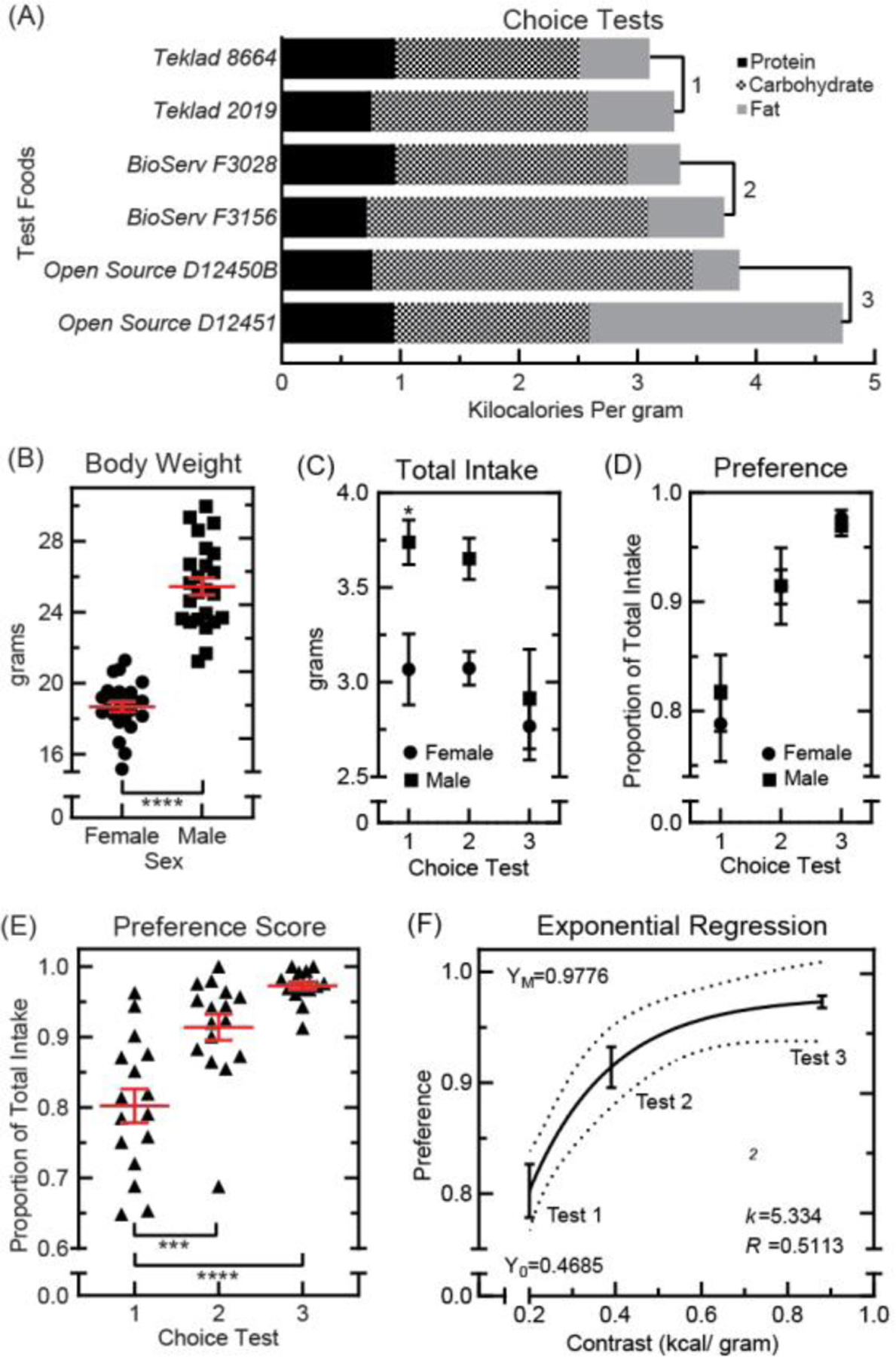
Experimentally naive mice were raised on standard chow (Teklad 8664, 3.1 kilocalories per gram) to 10 weeks of age. During three unique choice tests, concurrent intake of two test foods was measured for 24-hours. (A): Nutritional composition of test foods used in choice tests 1–3. (B): Body weight at test start in female (n=24, ●) and male (n=24, ■) mice, compared using two-tailed t test (t=12.04, p<0.0001, df=46; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Means ±s.e.m. (C): Total food eaten during the 24-hour test period (n=8 per group; male ■, female ●). Results were compared using two-way ANOVA [sex: F(1,42)=11.45, p=0.0016; choice test: F(2,42)=7.029, p=0.0023; interaction: F(2,42)=1.402, p=0.2573] with Bonferroni correction for multiple comparisons (*p<0.05). Means ±s.e.m. (D): Proportion of total intake attributable to the energy-superior test food (n=8 per group; male ■, female ●). Results were compared using two-way ANOVA [sex: F(1,42)=0.1067, p=0.7455; intake test: F(2,42)=22.31, p<0.0001; interaction: F(2,42)=0.2651, p=0.7684] with Bonferroni correction for multiple comparisons. Means ±s.e.m. (E): Food preference scores in all mice during choice tests 1–3 (n=16 per group, ▲). Results were compared using one-way ANOVA [F(2,45)=23.54, p<0.0001] with Bonferroni correction for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Means ±s.e.m. (F): Exponential plateau regression analysis used to model preference score based on the difference in test food energy-density. Function is plotted with 95% confidence intervals [df=45, sum of squares=0.2293, Sy.x=0.07139]. Means ±s.e.m.
Choice experiments 2–4 were performed exclusively with BioServ F3028 and BioServ F3156 (choice test 2, Fig. 1). These test foods were selected for all experiments to follow (Fig. 2–4) because both test foods were novel, and preference scores retained dynamic range. In addition, these foods were marketed as control diets, and were similar to standard feed in energy-density and macronutrient composition. See Supplementary Table 1 for additional nutritional information.
Fig. 2. Consecutive food preference testing in 14-week old C57BL6 mice.
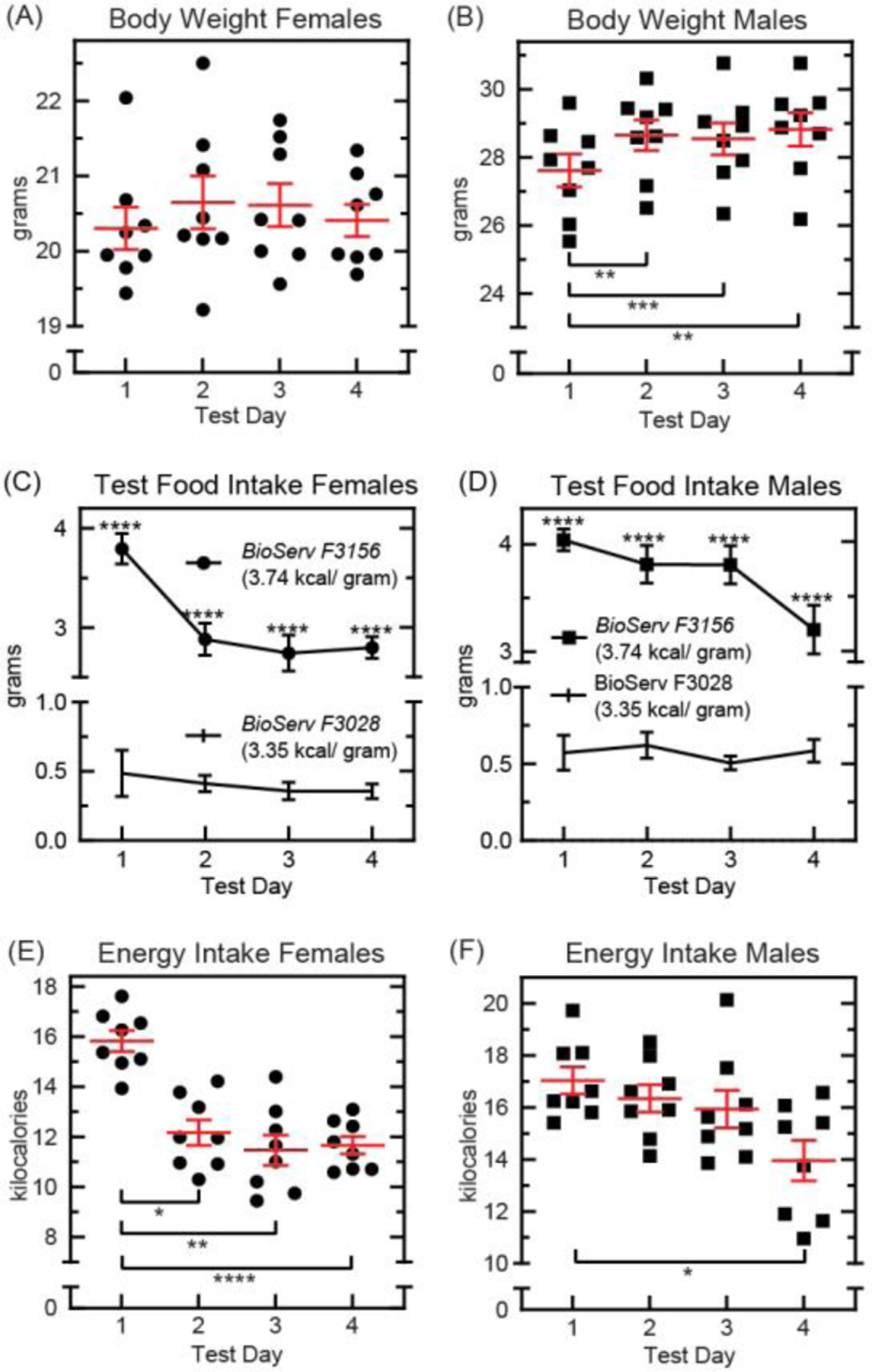
Mice were raised on standard chow (Teklad 8664, 3.1 kilocalories per gram). Concurrent intake of two novel test foods (BioServ F3028, 3.35 kilocalories per gram and Bioserv F3156, 3.74 kilocalories per gram) was measured during 4, consecutive 24-hour test periods. (A): Body weight at the start of each test day in female mice (n=8, ●). Results were compared using repeated measures one-way ANOVA [F(1.962,13.73)=1.809, p=0.2011] with Bonferroni correction for multiple comparisons. Means ±s.e.m. (B): Body weight at the start of each test day in male mice (n=8, ■). Results were compared using repeated measures one-way ANOVA [F(2.267,15.87)=25.00, p<0.0001] with Bonferroni correction for multiple comparisons (*p≤0.05, **p≤0.01, ***p≤0.001). Means ±s.e.m. (C): Daily consumption of test foods in female mice (n=8, ●). Results were compared using repeated measures two-way ANOVA [test food: F(1,14)=921.0, p<0.0001; test day: F(2.422,33.91)=9.045, p=0.0004; test food × test day: F(3,42)=5.661, p=0.0024] with Bonferroni correction for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Means ±s.e.m. (D): Daily consumption of test foods in male mice (n=8, ■). Results were compared using repeated measures two-way ANOVA [test food: F(1,14)=520.5, p<0.0001; test day: F(2.390,33.46)=4.940, p=0.0096; test food × test day: F(3,42)=5.322, p=0.0034] with Bonferroni correction for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Means ±s.e.m. (E): Estimated daily energy intake in female mice (n=8, ●). Results were compared using repeated measures one-way ANOVA [F(2.128, 14.89)=15.87, p=0.0002] with Bonferroni correction for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Means ±s.e.m. (F): Estimated daily energy intake in male mice (n=8, ■). Results were compared using repeated measures one-way ANOVA [F(2.272,15.91)=6.332, p=0.0078] with Bonferroni correction for multiple comparisons (*p<0.05). Means ±s.e.m.
Fig. 4. Food choice after acute exposure to diets enriched with sugar or fat.
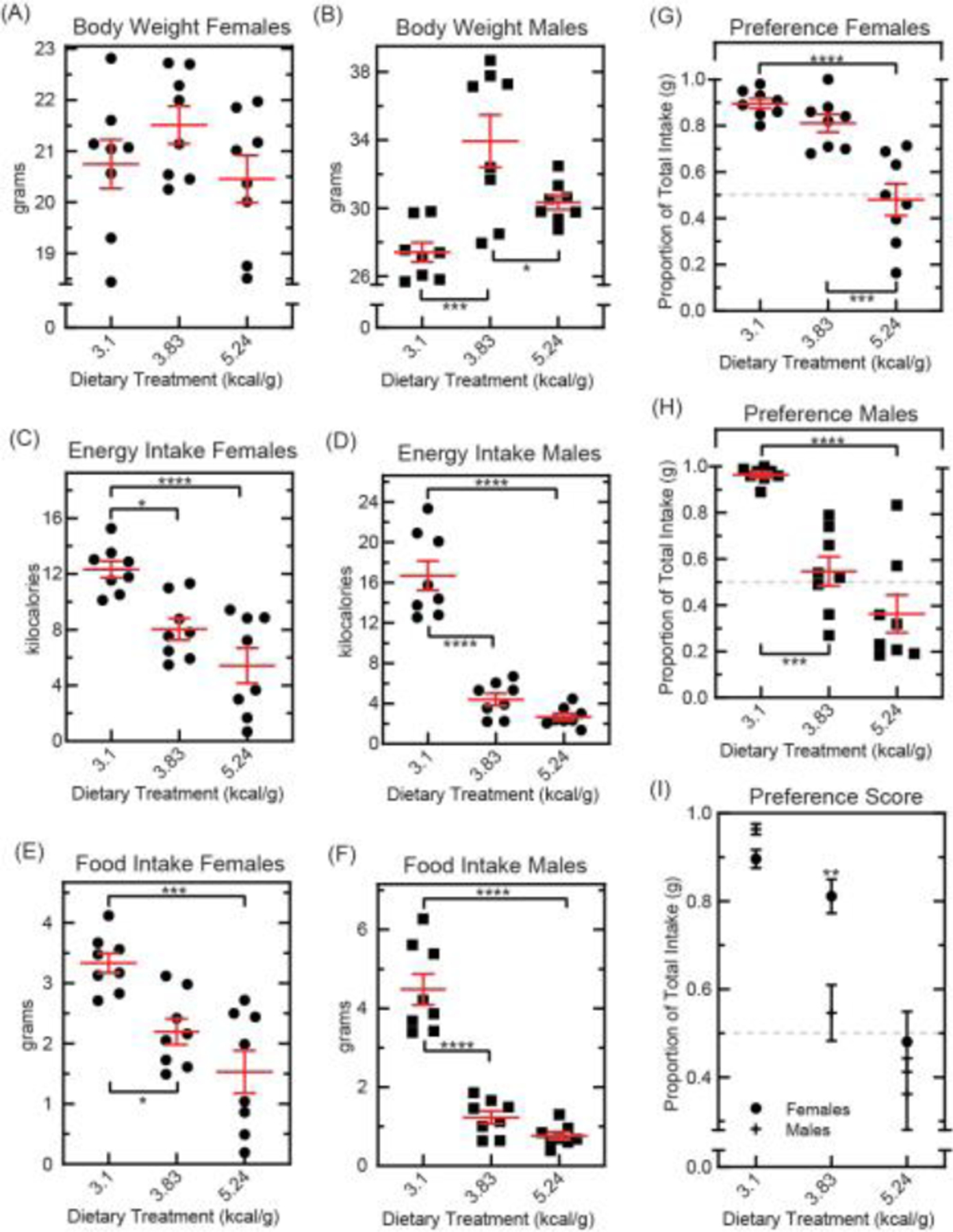
Mice were raised to 10 weeks on standard chow (Teklad 8664, 3.1 kilocalories per gram). Experimental groups were then transitioned to an energy-dense diet for four weeks prior to behavioral assay (BioServ S3472, 3.83 kilocalories per gram, 45% sugar; or Open Source D12492, 5.24 kilocalories per gram, 60% fat). At 14 weeks of age, concurrent intake of two test foods was measured for 24 hours: BioServ F3028 (3.35 kilocalories per gram) and Bioserv F3156 (3.74 kilocalories per gram). (A): Body weight in female mice at the start of each test day (n=8 per group, ●). Results were compared using one-way ANOVA [F(2,21)=1.545, p=0.2367] with Bonferroni correction for multiple comparisons. Means ±s.e.m. (B): Body weight in male mice at the start of each test day (n=8 per group, ■). Results were compared using one-way ANOVA [F(2,21)=11.23, p=0.0005] with Bonferroni correction for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001). Means ±s.e.m. (C): Total energy intake in female mice (n=8 per group, ●) compared using one-way ANOVA [F(2,21)=14.43, p=0.0001] with Bonferroni correction for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Means ±s.e.m. (D): Total energy intake in male mice (n=8 per group, ■) compared using one-way ANOVA [F(2,21)=66.51, p<0.0001] with Bonferroni correction for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Means ±s.e.m. (E): Total food eaten by female mice during the test period (n=8 per group, ●). Results were compared using one-way ANOVA [F(2,21)=12.63, p=0.0002] with Bonferroni correction for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001). Means ±s.e.m. (F): Total food eaten by male mice during the 24-hour test period (n=8 per group, ■). Results were compared using one-way ANOVA [F(2,21)=64.31, p<0.0001] with Bonferroni correction for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Means ±s.e.m. (G): Proportion of total intake from the energy-superior test food in female mice (n=8 per group, ●). Results were compared using one-way ANOVA [F(2,21)=21.78, p<0.0001] with Bonferroni correction for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Means ±s.e.m. (H): Proportion of total intake from the energy-superior test food in male mice (n=8 per group, ■). Results were compared using one-way ANOVA [F(2,21)=26.50, p<0.0001] with Bonferroni correction for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Means ±s.e.m. (I): Proportion of total intake from the energy-superior test food in all mice (n=8 per group). Results were compared using two-way ANOVA [sex: F(1,42)=5.758, p=0.0209; dietary treatment: F(2,42)=44.61, p<0.0001; interaction: F(2,42)=4.792, p=0.0133] with Bonferroni correction for multiple comparisons (*p<0.05, **p<0.01). Means ±s.e.m.
2.4. Food Preference Assay
Prior to intake test, all mice were individually housed for three days. Mice in experiments 1, 3 and 4 were then habituated to test foods for an additional three days (as described in Altherr et. al., 2021). Mice in experiment 2 were not habituated to test foods prior to data collection (Fig. 2; as described in Rainwater et. al., 2017). During test food habituation, maintenance diets were available ad libitum in the wire cage top, and novel test foods were presented mixed together on the cage floor. Fresh test food was supplied each day, in excess of what could be consumed in a 24-hour period (~20 grams per test food).
Test food consumption was tracked via manual weighing of food (Ali & Kravitz, 2018). At the start of the test period, mice were weighed and all food in the home cage was withdrawn. Fresh test food rations were weighed and added to the cage floor. 24-hours later, all remaining food was retrieved and weighed. Food intake was estimated as the post-test change in food weight to the nearest 0.1 gram. Spillage was defined as food crumbs or dust that could not be retrieved from the cage. Spillage was not quantified in the present study, as these quantities could not be easily extracted from soilage and bedding material. In addition, a prior report demonstrated such quantities are very small for pelleted rodent diet (<0.1 grams per mouse, per day; Bachmanov et. al., 2001).
2.5. Statistics
Statistical analysis was performed using Prism GraphPad software. Appropriate bivariate tests were selected according to experimental design. Where means were compared across two independent groups, t test was used to evaluate statistical differences (Fig. 1B). Where >2 independent variables were tested, and means were compared within a single group, one-way analysis of variance (ANOVA) was used (Fig. 1E, 3A–F, 3H, 4A-H). For experiments where within-group comparisons were made across time points, repeated measures one-way ANOVA was used (Fig. 2A–F). Where >2 independent variables were tested, and means were compared between two groups, two-way ANOVA was used (Fig. 1C–D, 3G, 4I). The complete set of statistical results are provided in the figure legends.
Fig. 3. Food choice in mice fed energy-dense diets.
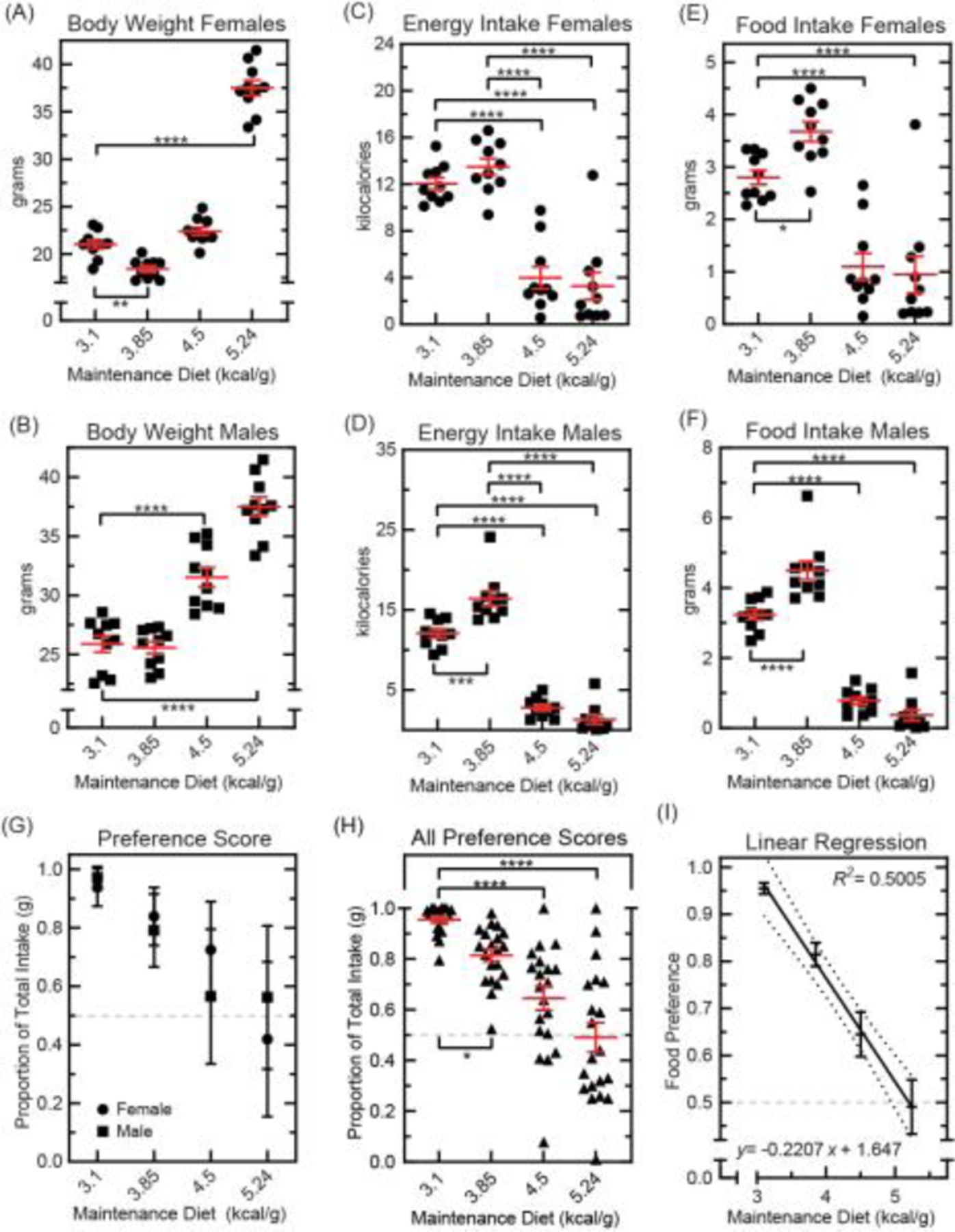
Mice were raised to 14 weeks on standard chow (Teklad 8664, 3.1 kilocalories per gram) or energy-dense diet (Open Source D12450B, 3.85 kilocalories per gram; Teklad 88317, 4.5 kilocalories per gram; Open Source D12492, 5.24 kilocalories per gram). At 14 weeks, consumption of two test foods was measured for 24 hours: BioServ F3028 (3.35 kilocalories per gram) and Bioserv F3156 (3.74 kilocalories per gram). (A): Body weight in female mice at the start of each test day (n=10 per group, ●). Results were compared using one-way ANOVA [F(3,36)=265.4, p<0.0001] with Bonferroni correction for pairwise comparison with controls (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Means ±s.e.m. (B): Body weight in male mice at the start of each test day (n=10 per group, ■). Results were compared using one-way ANOVA [F(3,36)=61.43, p<0.0001] with Bonferroni correction for pairwise comparison with controls (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Means ±s.e.m. (C): Total energy intake in female mice (n=10 per group, ●) compared using one-way ANOVA [F(3,36)=37.58, p<0.0001] with Bonferroni correction for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Means ±s.e.m. (D): Total energy intake in male mice (n=10 per group, ■) compared using one-way ANOVA [F(3,36)=132.3, p<0.0001] with Bonferroni correction for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Means ±s.e.m. (E): Total food eaten by female mice during the test period (n=10 per group, ●). Results were compared using one-way ANOVA [F(3,36)=29.30, p<0.0001] with Bonferroni correction for pairwise comparison with control (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Means ±s.e.m. (F): Total food eaten by male mice during the test period (n=10 per group, ■). Results were compared using one-way ANOVA [F(3,36)=125.8, p<0.0001] with Bonferroni correction for pairwise comparison with controls (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Means ±s.e.m. (G): Proportion of total intake from the energy-superior test food in female (n=10 per group, ●) and male (n=10 per group, ■) mice. Results were compared using two-way ANOVA [sex: F(1,72)=0.03910, p=0.8438; maintenance diet: F(3,72)=26.97, p<0.0001; interaction F(3,72)=2.703, p=0.0518] with Bonferroni correction for multiple comparisons. Means ±s.e.m. (H): Proportion of total intake from the energy-superior test food in all mice (n=20 per group). Results were compared using one-way ANOVA [F(3,76)=25.57, p<0.0001] with Bonferroni correction for pairwise comparison with controls (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Means ±s.e.m. (I): Linear regression analysis of preference score and maintenance diet energy-density plotted with 95% confidence intervals.
3. Results
3.1. Energy-density influences food preference in C57BL6J mice
We first characterized the effects of test food energy-density by measuring intake during three unique choice tests. Test food pairs were selected such that they differed by increasing amount of energy-density in each test (Fig. 1A). In choice test 1, test foods differed by 0.2 kilocalories per gram (3.1 and 3.3 kilocalories per gram). In choice test 2, foods differed by 0.39 kilocalories per gram (3.35 and 3.74 kilocalories per gram). In test 3, foods differed by 0.88 kilocalories per gram (3.85 and 4.73 kilocalories per gram). All mice were raised on standard vivarium chow (Teklad 8664; 3.1 kilocalories per gram). At 10 weeks of age, female and male mice were divided into groups and tested on one of these three food choices.
Body weight was measured in all subjects at the start of the 24-hour test period and pooled for comparison between sexes (Fig. 1B). As expected, male mice weighed significantly more than females. Total food intake was calculated as the amount consumed from both test foods. When compared between sexes (Fig. 1C), males consumed statistically more food than female mice during choice test 1 (Fig. 1C). Variability in energy intake was greater where differences in energy-density were larger (tests 2–3) for both sexes (Supplementary Fig. 1).
Food preference scores were then calculated as the proportion of total food intake represented by the energy-superior test food (Fig. 1D). Mice with no preference were expected to consume roughly equal volumes of test food per unit weight (~50% preference). During all three tests, all mice ate more of the energy-superior test food (Fig. 1D–E). Further, there were no observable differences between females and males in performance on the food preference assay (Fig. 1D). Scores were thus considered appropriate for compilation for comparison between choice tests (Fig. 1E). When choice tests were compared, we found that preference scores were significantly higher where test foods differed more in energy-density (Fig. 1E). Food preference scores could also be modeled based on this difference in energy-density using an exponential regression analysis (Figure 1F). This analysis suggested that approximately 51% of variance in preference score could be explained by differences in test food energy-density (Figure 1F).
Results from regression analysis also predicted that on average, mice would continue to sample both foods, even where preferences were strong, and the differences between foods unambiguous. According to our model, the highest preference score achievable on any two-food test is ~97.8% (calculated as maximum preference value, YM=0.9776; Figure 1F). These results implied that performance scores on choice test 3 (average 97.3%) had approached this maximum value. However, average preference scores on choice test 2 (91.4%) were not as close to this ceiling effect (Figure 1E). There was also less variability in scores as compared to test 3. Choice test 2 was therefore selected for further study, because scores retained dynamic range and both test foods were novel.
3.2. Food preference develops within first 24 hours of encounter
In our second experiment, we tracked the acquisition of food preference and behavioral performance over time. Consumption of two test foods (BioServ F3156 and BioServ F3028) was measured over four consecutive days, with no habituation period. Using this strategy, we hoped our results may reveal the transient effects of food novelty, and thereby confirm the appropriate length of time for test food habituation. Body weight and food intake were measured every 24 hours. All mice were raised on standard vivarium chow (Teklad 8664) to 14 weeks of age.
Body weight was measured at the start of each 24-hour test period, and values were compared across days (Fig. 2A, B). No significant changes in body weight were observed in female mice (Fig. 2A). On average, male mice gained 1.04 ±0.14 (mean ±s.e.m.) grams after the first day, and a total of 1.21 ±0.17 (mean ±s.e.m.) grams by day four (Fig. 2B). This increase in body weight could be explained by increased energy intake. Both males and females consumed significantly more energy during the first 24 hours with novel test foods (Fig. 2E, F). In female mice, this hyperphagia subsided more quickly than in male mice (Fig. 2E). However, by day four both sexes consumed significantly less total energy than they did on day one. Based on these results, we determined that three days was a reasonable length of time for test food habituation.
Over test days, all mice consumed significantly more BioServ F3156 (3.74 kilocalories per gram) as compared to BioServ F3028 (3.35 kilocalories per gram; Fig. 2C, D). In addition, all mice formed this preference quickly- within the first 24 hours of encounter. As predicted, all mice continued to sample the lesser test food (F3028) at a stable, albeit low, rate (Fig. 2E, F). These results confirmed that food preference behavior was equivalent, and remained consistent over time, in both females and males (Supplementary Fig. 2). However, because preferences were fully formed by the first 24-hour time point, we failed to capture a true learning curve. More frequent sampling methods will be needed in future experiments to better expose the time course of food preference learning.
3.4. Energy-dense diet dose-dependently disrupts food preference
For our next experiment, we characterized the effects of energy-dense diet on food preference, using test foods marketed as control diets (BioServ F3028, 3.35 kilocalories per gram versus BioServ F3156, 3.74 kilocalories per gram). We expected these dietary treatments to disrupt test food intake (lower in energy) and preference (discrimination between test foods). For this experiment, we raised mice to 14 weeks on either standard chow or energy-dense diet. Control mice received Teklad 8664 (3.1 kilocalories per gram, 19% fat). Experimental groups received Open Source D12450B (3.85 kilocalories per gram, 10% fat), Teklad 88137 (4.5 kilocalories per gram, 42% fat), or Open Source D12492 (5.24 kilocalories per gram, 60% fat; Fig. 3). Prior to behavioral assay, all mice were habituated to test foods for three days. Consumption of test foods was then measured over 24-hours.
At test start, body weight was measured, and within-sex comparisons were made between maintenance diet groups (Fig. 3A, B). Females raised on Open Source D12492 (5.24 kcal/g) were significantly heavier than controls (Fig. 3A). Female mice raised Open Source D12450B (3.85 kcal/g) were significantly lighter than controls, despite consuming a diet higher in energy (Fig. 3A). This reduction in body weight may have been attributable to D1240B’s low fat content (10%) or textural hardness (possibly making it more difficult for smaller female mice to consume). Males raised on Teklad 88137 (4.5 kcal/g) or Open Source D12492 (5.24 kcal/g) were significantly heavier than controls (Fig. 3B).
Total energy and food intake was calculated for each subject and within-sex comparisons were made between group means. Both females and males raised on high-fat diets (Teklad 88137, Open Source D12492) consumed significantly less test food than their standard-chow counterparts (Fig. 3C–E). Mice raised on a low-fat diet (Open Source D12450B) however, consumed significantly more than controls during choice assay (Fig. 3C–F). We believe this increase in appetite was caused by the comparatively higher fat content present in test foods (13.4% and 17.1% respectively) as compared to that present in D12450B (10%; Supplementary Table 1).
Preference scores were then calculated as the percent of total intake attributable to the energy-superior test food (BioServ F3156). Within-group comparisons between female and male preference scores revealed no significant differences between sexes (Fig. 3G). Scores for both sexes were thus combined, and additional comparisons were made between maintenance diet groups. All energy-dense diet groups scored significantly lower than controls on food preference assay. Furthermore, maintenance diet energy-density appeared to dose-dependently impair choice performance (Fig. 3H).
The average preference score in mice fed the diet highest in energy (OpenSource D12492, 5.24 kilocalories per gram) was near chance, 49.1% (±5.8, mean ±s.e.m.). Such scores demonstrate roughly equivalent sampling of test foods per unit weight, evidence that these mice were indifferent to test food qualities. Furthermore, preference scores were diminished by energy-dense diet in a dose-dependent manner (Fig. 3H). These results support the conclusion that food preferences adapted to energy-dense diet. From these data, a linear equation was generated to model food preference based on maintenance diet energy-density (Fig. 3G). This model suggested that approximately 50% of variance in preference scores were attributable to maintenance diet energy-density. This analysis also predicted that optimal performance on food choice assay (y = 98% preference; YM Fig. 1F) should be observed in mice fed a diet containing 3.02 kilocalories per gram- similar to the energy-density present in most standard rodent feeds.
3.5. Acute exposure to energy-dense diet disrupts food preference
Results from the previous experiment demonstrated that a lifetime of energy-dense diet disrupted preference and suppressed intake of healthy test foods. For our final experiment, we sought to establish whether a shorter exposure to energy-dense diet may also be sufficient to impair choice test performance. To these ends, we raised mice on a standard vivarium chow (Teklad 8664, 3.1 kilocalories per gram) to 10 weeks of age, then transitioned experimental groups to energy-dense diet for four weeks before test. Energy-dense maintenance diets were particularly enriched in sugar (BioServ S3472, 3.83 kilocalories per gram, 45% sugar) or fat (Open Source D12492, 5.24 kilocalories per gram, 60% fat; see Supplementary Table 1). All mice received three days to habituate to test foods prior to 24-hour food preference procedure at 14 weeks.
At the start of the test period, body weight was measured. Within-sex comparisons were made between dietary treatment groups (Fig. 4A, B). No significant differences in weight were observed in female mice (Fig. 4A). However, males fed high-sugar diet (3.83 kilocalories per gram) weighed significantly more than controls; and more than their counterparts fed a high-fat diet (Figure 4B). Total food intake for the 24-hour test was calculated, and within-sex comparisons were made between dietary treatment groups. Results showed that both energy-enriched diet groups were aphagic during preference assay. Average food and energy intake for all groups fed energy-dense diet were significantly lower than controls (Fig. 4C–F).
Preference scores were then calculated and within-sex comparisons made between maintenance diet groups (Fig. 4G, H). Results of these analyses showed that preference scores were near chance in both females and males fed high-fat diet (5.24 kcal/g; Fig. 4G–H). However, a high-sugar diet (3.83 kcal/g) was insufficient to disrupt preference performance in both sexes. Male mice fed high-sugar diet had significantly lower food preference scores as compared to male controls (Fig. 4F); these scores were also significantly lower than females fed the same diet (Fig. 4I). Preference scores in females fed BioServ S3472 did not statistically differ from controls (Fig. 4G). This suggested that female mice were not affected by an acute exposure to high-sugar diet in the same way as males.
These results support findings that female C57BL/6 are comparatively resistant to the deleterious effects of energy-dense diet. This performance profile also shows that adaptations to energy-dense diet are likely macronutrient dependent. Both effects may be due to protective effects of estrogen in preserving insulin activity and glucose tolerance in female mice (Mauvais-Jarvis et. al., 2013). This sex difference also revealed that maintenance diet effects on appetite and food preference were dissociable. While females fed high-sugar diet consumed little, they continued to select the energy-superior test food during choice test (Fig. 4E,G).
4. Discussion
Changes in food preference may be a behavioral hallmark of successful weight loss therapy (Gero et. al., 2017). However, a standardized methodology for studying food choice has not been established in animal or man. Rodent models make particularly good subjects for studies of nutrition and food preference. Diet can be carefully controlled in laboratory rodents, and these species lack many biases (e.g. cultural, egocentric body image) that confound human studies of food intake. Mice also possess the economical advantages of small size, high reproductive output, and a short time to maturity (Kleinert et. al., 2018). A validated methodology for evaluating food preference in C57BL/6 mice could therefore be a useful tool.
Here, we have developed a simple behavioral assay for measuring food preference in laboratory mice. We show that healthy female and male mice robustly discriminate between foods on the basis of energy-density. Our results show that food preference strength varied as a function of energy-density. The present study demonstrates that food preference is a sensitive behavioral measure, that can be used to detect statistically significant differences between dietary treatment groups and sexes. Our data establishes food preference behavior as replicable and stable over time.
However, there are some caveats to this study. For instance, differences in test food energy accounted for only ~51% of variance in choice test score (Fig. 1F). While test food energy content was clearly a primary driver of food selection, these results showed that other factors (e.g. texture or ingredient composition) also influenced preference. Test food ingredient quality (grain-based or purified) may have also contributed, but alone could not account for our observations. More specifically, all mice preferred energy-superior foods during all three unique choice tests in experiment 1 (Fig. 1). Choice test 1 presented two grain-based, chow diets (Teklad 8664 and Teklad 2019). Test 2 offered two specialty control diets, one grain-based (BioServ F3028) and one purified (BioServ F3156). Test 3 juxtaposed two purified diets (Open Source D12492 and Open Source D12450B). In all instances, healthy mice selected the energy-superior food regardless of ingredient quality (Fig. 1).
Preference scores were also influenced by the total amount of food consumed. A correlation analysis of preference scores and total food intake (from Fig. 3) suggested that the quantity eaten during test accounted for ~19% (R2=0.1888, p=0.0051) of the variance in female preference scores, and ~27% (R2=0.2677, p= 0.0006) in male scores (Supplementary Fig. 3A–B). Low food intakes may represent a limitation because aphagic mice may not have had adequate opportunity to demonstrate a preference. This also raises questions regarding the influence of spillage and evaporation on scores calculated from such small intake volumes. Additional experiments are needed to clarify the effect of total intake on the acquisition and expression of food preference behavior.
On the other hand, this potential caveat also revealed a macronutrient-specific effect on appetite. Groups fed energy-dense diets enriched in fat were aphagic, whereas groups fed energy-dense diet enriched in carbohydrate were hyperphagic (Fig. 3C, D). Fat has a potent effect on food preference in humans and rodents (Gaillard et. al., 2008). The ability of the GI tract to detect fat is also influenced by dietary exposure (Little & Feinle-Bisset, 2011). Experiments 3–4 revealed that these effects on food intake were dissociable from food preference. Regardless of whether test food consumption was scant or robust, preference scores were dose-dependently disturbed by energy-dense diet.
Despite some limitations, our data confirm that food preference is guided by energy-density in healthy mice. We show that ~50% (R2=0.5005, p<0.0001) of variance in preference scores were attributable to maintenance diet energy-density (Fig. 3G). Our work also substantiates findings that energy-dense diet exposure causes changes in food preference behavior (Mazzone et. al., 2020; Altherr, 2021). Importantly, our data also shows that changes in food preference were measurable, predictable, and reproducible. Together, these findings help validate food preference assay as a faithful behavioral measure of adaptation to energy-dense diet.
5. Conclusion
Food reward value is a biological function derived from nutrient density. Under free-choice conditions where energy-dense foods are plentiful, preferences favor overnutrition (Huang et. al., 2021). After prolonged exposure to energy-dense foods, high-calorie loads become unexceptional, and low-energy foods imperceivable. Recidivism after successful weight loss therapy may occur, not because energy-dense foods are more stimulating, but because they are sensed as status quo. Such changes in food preference correlate with changes in the brains of obese humans (Harding et. al., 2018) and mice fed energy-dense diet (Mazzone et. al., 2020; Altherr et. al., 2021). Bariatric surgery can reverse these effects, but the underlying physiology is not well understood (Guyot et. al., 2021).
We found that energy-density was a primary driver of food choice. Food selection was influenced by energy-density in two ways: 1) preference scores scaled to differences in test food energy-density, and 2) preference scores were dose-dependently diminished by increasing maintenance diet energy-density. Thus, food preference assay can be used to evaluate food intake, integrated nutrient sensing, and value-based decision making. These functions influence energy intake, and are therefore relevant to the study of overweight and obesity. This assay also provides a minimally invasive screening approach for the effects of energy-dense diet. Food preference assay is a simple and practical method to study food reward value and nutrient sensing in C57BL/6 mice.
Supplementary Material
Highlights.
Appetite and discrimination between ordinary foods is diminished after chronic consumption of energy-dense diet
Food preference is scaled to relative differences in food energy-density
Food preference assay can be used to detect adaptations to energy-dense diet
Dietary adaptations in food preference are sexually dimorphic and macronutrient specific in C57BL6 mice
Acknowledgements
This work was supported by NIH National Institute of General Medicinal Sciences R01GM11937 (ADG), UVA Brain Institute 2018 Seed Funding Award (ADG) and University of Virginia Faculty Start-up Funds (ADG). The authors thank Ryan Grippo for helpful discussion and Elena Tenore for assistance with animal care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT authorship contribution statement
Aundrea Rainwater: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing - original draft. Ali Guler: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- Ali MA, and Kravitz AV (2018). Challenges in quantifying food intake in rodents. Brain research, 1693, 188–191. 10.1016/j.brainres.2018.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altherr E, Rainwater A, Kaviani D, Tang Q, and Güler AD (2021). Long-term High Fat Diet Consumption Reversibly Alters Feeding Behavior via a Dopamine-Associated Mechanism in Mice. Behavioural Brain Research, 113470. 10.1016/j.bbr.2021.113470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avtanski D, Pavlov VA, Tracey KJ, and Poretsky L (2019). Characterization of inflammation and insulin resistance in high-fat diet-induced male C57BL/6J mouse model of obesity. Animal models and experimental medicine, 2(4), 252–258. 10.1002/ame2.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Tordoff MG, Price RA, and Beauchamp GK (2001). Nutrient preference and diet-induced adiposity in C57BL/6ByJ and 129P3/J mice. Physiology and behavior, 72(4), 603–613. 10.1016/S0031-9384(01)00412-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueter M, Miras AD, Chichger H, Fenske W, Ghatei MA, Bloom SR, Unwin RJ, Lutz TA, Spector AC, and le Roux CW (2011). Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiology & behavior, 104(5), 709–721. 10.1016/j.physbeh.2011.07.025 [DOI] [PubMed] [Google Scholar]
- Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, and Brand-Miller J (2005). Origins and evolution of the Western diet: health implications for the 21st century. The American journal of clinical nutrition, 81(2), 341–354. 10.1093/ajcn.81.2.341 [DOI] [PubMed] [Google Scholar]
- Gaillard D, Passilly-Degrace P, and Besnard P (2008). Molecular mechanisms of fat preference and overeating. Annals of the New York Academy of Sciences, 1141(1), 163–175. 10.1196/annals.1441.028 [DOI] [PubMed] [Google Scholar]
- Gero D, Steinert RE, le Roux CW, and Bueter M (2017). Do food preferences change after bariatric surgery? Current Atherosclerosis Reports, 19(9), 38. 10.1007/s11883-017-0674-x [DOI] [PubMed] [Google Scholar]
- Gupta A, Osadchiy V, and Mayer EA (2020). Brain–gut–microbiome interactions in obesity and food addiction. Nature Reviews Gastroenterology and Hepatology, 17(11), 655–672. 10.1038/s41575-020-0341-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot E, Dougkas A, Nazare JA, Bagot S, Disse E, and Iceta S (2021). A systematic review and meta-analyses of food preference modifications after bariatric surgery. Obesity Reviews. 1–18. 10.1111/obr.13315 [DOI] [PubMed] [Google Scholar]
- Harding IH, Andrews ZB, Mata F, Orlandea S, Martínez-Zalacaín I, Soriano-Mas C, Stice E, and Verdejo-Garcia A (2018). Brain substrates of unhealthy versus healthy food choices: influence of homeostatic status and body mass index. International Journal of Obesity, 42(3), 448–454. 10.1038/ijo.2017.237 [DOI] [PubMed] [Google Scholar]
- Hirayama K, Uetsuka K, Kuwabara Y, Tamura M, and Itoh K (2007). Vitamin K deficiency of germfree mice caused by feeding standard purified diet sterilized by γ-irradiation. Experimental animals, 56(4), 273–278. 10.1538/expanim.56.273 [DOI] [PubMed] [Google Scholar]
- Huang FY, Sutcliffe MP, and Grabenhorst F (2021). Preferences for nutrients and sensory food qualities identify biological sources of economic values in monkeys. Proceedings of the National Academy of Sciences, 118(26). 10.1073/pnas.2101954118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery RW, Epstein LH, Wilson GT, Drewnowski A, Stunkard AJ, and Wing RR (2000). Long-term maintenance of weight loss: current status. Health psychology, 19(1S), 5. 10.1037/0278-6133.19.Suppl1.5 [DOI] [PubMed] [Google Scholar]
- Johnson PM, and Kenny PJ (2010). Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature neuroscience, 13(5), 635. 10.1038/nn.2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern DL, McPhee L, Fisher J, Johnson S, and Birch LL (1993). The postingestive consequences of fat condition preferences for flavors associated with high dietary fat. Physiology & Behavior, 54(1), 71–76. 10.1016/0031-9384(93)90045-H [DOI] [PubMed] [Google Scholar]
- Kleinert M, Clemmensen C, Hofmann SM, Moore MC, Renner S, Woods SC, Huypens P, Beckers J, de Angelis MH, Schürmann A, Bakhti M, Klingenspor M, Heiman M, Cherrington AD, Ristaw M, Lickert H, Wolf Eckhard, Havel PJ, Müller TD and Tschöp MH (2018). Animal models of obesity and diabetes mellitus. Nature Reviews Endocrinology, 14(3), 140. 10.1038/nrendo.2017.161 [DOI] [PubMed] [Google Scholar]
- Kittrell H, Graber W, Mariani E, Czaja K, Hajnal A, and Di Lorenzo PM (2018). Taste and odor preferences following Roux-en-Y surgery in humans. PloS one, 13(7). 10.1371/journal.pone.019950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TJ, and Feinle-Bisset C (2011). Effects of dietary fat on appetite and energy intake in health and obesity—oral and gastrointestinal sensory contributions. Physiology & behavior, 104(4), 613–620. 10.1016/j.physbeh.2011.04.038 [DOI] [PubMed] [Google Scholar]
- Makaronidis JM, and Batterham RL (2018). Obesity, body weight regulation and the brain: insights from fMRI. The British journal of radiology, 91(1089). 10.1259/bjr.20170910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathes CM, and Spector AC (2012). Food selection and taste changes in humans after Roux-en-Y gastric bypass surgery: a direct-measures approach. Physiology & behavior, 107(4), 476–483. 10.1016/j.physbeh.2012.02.013 [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Clegg DJ, and Hevener AL (2013). The role of estrogens in control of energy balance and glucose homeostasis. Endocrine reviews, 34(3), 309–338. 10.1210/er.2012-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone CM, Liang-Guallpa J, Li C, Wolcott NS, Boone MH, Southern M, Kobzar NP, de Araujo Salgado I, Reddy DM, Sun F, Zhang Y, Li Y, Cui G, and Krashes MJ (2020). High-fat food biases hypothalamic and mesolimbic expression of consummatory drives. Nature Neuroscience, 23(10), 1253–1266. 10.1038/s41593-020-0684-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiselman HL, Waterman D, and Symington LE (1974). Armed forces food preferences. ARMY NATICK DEVELOPMENT CENTER MA. [Google Scholar]
- Natvik E, Råheim M, Andersen JR, and Moltu C (2018). Living a successful weight loss after severe obesity. International Journal of Qualitative Studies on Health and Well-being, 13(1), 1487762. 10.1080/17482631.2018.1487762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MS, Christensen BJ, Ritz C, Holm L, Lunn S, Tækker L, Schmidt JB, Bredie WLP, Albrechtsen NJW, Holst JJ, Hilbert A, leRoux CW, and Sjödin A (2021). Factors Associated with Favorable Changes in Food Preferences After Bariatric Surgery. Obesity Surgery, 1–11. 10.1007/s11695-021-05374-1 [DOI] [PubMed] [Google Scholar]
- Rainwater A, Sanz E, Palmiter RD, and Quintana A (2017). Striatal GPR88 modulates foraging efficiency. Journal of Neuroscience, 37(33), 7939–7947. 10.1523/JNEUROSCI.2439-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, and Ackroff K (2012). Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 302(10), R1119–R1133. 10.1152/ajpregu.00038.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, and DiFeliceantonio AG (2019). Processed foods and food reward. Science, 363(6425), 346–347. 10.1126/science.aav0556 [DOI] [PubMed] [Google Scholar]
- Smith BK, Andrews PK, and West DB (2000). Macronutrient diet selection in thirteen mouse strains. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 278(4), R797–R805. 10.1152/ajpregu.2000.278.4.R797 [DOI] [PubMed] [Google Scholar]
- Stubbs RJ, and Whybrow S (2004). Energy density, diet composition and palatability: influences on overall food energy intake in humans. Physiology and behavior, 81(5), 755–764. 10.1016/j.physbeh.2004.04.027 [DOI] [PubMed] [Google Scholar]
- Steele CC, Pirkle JR, Davis IR, and Kirkpatrick K (2019). Dietary effects on the determinants of food choice: Impulsive choice, discrimination, incentive motivation, preference, and liking in male rats. Appetite, 136, 160–172. 10.1016/j.appet.2019.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo PS, van Dam RM, Whitton C, Tan LWL, and Forde CG (2020). Consumption of foods with higher energy intake rates is associated with greater energy intake, adiposity, and cardiovascular risk factors in adults. The Journal of Nutrition. November 26, nxaa344. 10.1093/jn/nxaa344. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Pearson JA, Ellis HT, and Poole RL (2017). Does eating good-tasting food influence body weight? Physiology and behavior, 170, 27–31. 10.1016/j.physbeh.2016.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer F, Charbonnier L, and Smeets PA (2016). Food decision-making: effects of weight status and age. Current diabetes reports, 16(9), 84. 10.1038/s41593-020-0684-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernarelli JA, Mitchell DC, Rolls BJ, and Hartman TJ (2015). Dietary energy density is associated with obesity and other biomarkers of chronic disease in US adults. European journal of nutrition, 54(1), 59–65. 10.1007/s00394-014-0685-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P, Wiers RW, Hommel B, Gerdes VE, and de Wit S (2017). Stimulus control over action for food in obese versus healthy-weight individuals. Frontiers in Psychology, 8, 580. 10.3389/fpsyg.2017.00580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Beydoun MA, Min J, Xue H, Kaminsky LA, and Cheskin LJ (2020). Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. International Journal of Epidemiology. 49 (3), 810–823. 10.1093/ije/dyz273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RR, and Phelan S (2005). Long-term weight loss maintenance. The American journal of clinical nutrition, 82(1), 222S–225S. 10.1093/ajcn/82.1.222S [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


