Abstract
Purpose:
To validate a custom algorithm for automated identification and quantification of clinically relevant inflammatory choriocapillaris (CC) lesions from en face swept source optical coherence tomography (SS-OCTA) images.
Design:
observational case series
Methods:
Twenty eyes of 14 patients with posterior uveitis were imaged using the PLEX® Elite 9000. The machine-generated en face OCTA CC slabs were exported to MATLAB where a custom algorithm performed unsupervised lesion boundary delineation and area quantification. Lesions identified by the algorithm (AG) were compared to those identified by two masked human graders (HG1 and HG2), using the Sørensen-Dice coefficient (DSC) and intraclass correlation coefficient (ICC). Intra-grader and intra-visit reliability were determined by coefficient of variation (CV) and DSC.
Results:
The AG demonstrated excellent agreement with both HGs in determination of lesion area (HG1 vs. AG ICC 0.92, 95% CI 0.81–0.97, HG2 vs. AG ICC 0.91, 95% CI 0.78–0.97). The AG demonstrated good spatial overlap (DSC≥0.70) with both HGs in 14/20 (70%) eyes and at least one HG in 16/20 (80%) eyes. Poor spatial overlap (DSC between 0.31 and 0.69) was associated with the presence of a choroidal neovascular membrane and low contrast lesion boundaries. Intra-visit repeatability for the AG was superior to both HGs (CV 2.6% vs >5%).
Conclusion:
This custom algorithm demonstrated a high degree of agreement with human graders in identification of inflammatory CC lesions, and outperformed human graders in reproducibility. Automated CC lesion delineation will support the development of objective and quantitative biomarker of disease activity in patients with posterior uveitis.
Keywords: Choriocapillaris, choroid, swept source optical coherence tomography, optical coherence tomography, optical coherence tomography angiography, OCT, SS-OCT, SS-OCTA, OCTA, uveitis, CC flow-deficit, quantitative image analysis
Table of content
There is currently an unmet need for quantitative biomarkers for measuring disease activity and response to therapy for patients with posterior uveitis. Optical coherence tomography angiography is an excellent modality for use in quantitative disease assessment using either automated or semi-automated image analysis approaches. This article describes and validates a novel automated algorithm using custom software to identify and quantify choriocapillaris lesion boundaries in patients with posterior uveitis.
Introduction
Posterior uveitis frequently manifest with inflammation and ischemia in the choriocapillaris (CC).1–4 Dye-based angiography is the current gold standard for monitoring these diseases, but optical coherence tomography angiography (OCTA) has demonstrated remarkable fidelity in detecting inflammatory disease activity in the CC without the need for intravenous contrast.3,5,6 When viewed as an en face image, the CC OCTA slab from patients with many forms of posterior uveitis demonstrates flow deficits (FDs) that correlate with disease specific findings on fundus examination and other imaging techniques such as angiography, fundus autofluorescence (FAF), and OCT.3,6–11 The majority of studies reporting on the use of OCTA in posterior uveitis have described the presence and extent of CC FDs qualitatively. When the extent of disease is measured quantitatively, the measurements are often performed by thresholding and area summation12,13 or boundary outline by human graders3,14. A comprehensive automated algorithm for CC lesion localization and quantification would allow for a more rapid and reproducible assessment of disease both in clinical practice and in clinical trials.15
OCT/OCTA is an excellent modality for use in quantitative disease assessment using either automated or semi-automated image analysis approaches. These strategies have been applied extensively to diseases of the retinal vasculature, and to a lesser extent to diseases of the choroid.16–18 Using swept source OCTA (SS-OCTA), our group recently reported on the development of an automated image analysis approach that identifies and quantifies the number, size, and density of CC flow deficits (FDs) on an en face CC image.13 Using these metrics, we determined the quantitative characteristics of CC FDs in normal subjects, and identified significant differences in the size and density of FDs in patients with posterior uveitis when compared to normal controls and to patients with anterior and intermediate uveitis. The quantitative metrics in this study were assessed and reported as a single variable for the entire en face image. However, in many forms of posterior uveitis, disease is localized to one or multiple nummular or placoid lesions and clinical progression or response to treatment is judged based on visible changes at the lesion border. Thus, in addition to a quantitative determination of the extent of disease, there is a need to define a lesion border and detect changes in this boundary as well as total lesion area for accurate monitoring of disease activity.
In the current study, we developed and validated an automated algorithm to independently identify and segment the boundary of clinically relevant FD lesions in patients with posterior uveitis and compared the performance of this algorithm (AG) to two human graders (HGs).
Methods
This observational case series was approved by the Institutional Review Board at the University of Washington. Written informed consent was obtained from all patients before study enrollment, and the tenets of the Declaration of Helsinki and the regulations of the Health Insurance Portability and Accountability Act of 1996 were followed.
Patient enrollment
Patients with a diagnosis of posterior uveitis with choroidal involvement underwent swept-source OCTA (SS-OCTA) imaging between 5/2016 to 3/2020. Diagnosis was based on clinical evaluation by an ocular inflammation specialist at the University of Washington Eye Institute. Anatomic location and etiology of inflammation were determined by the treating clinician in accordance with the Standardization of Uveitis Nomenclature (SUN) criteria. All patients underwent complete medical history review, slit lamp biomicroscopy, and fundus examination. Ultra widefield color fundus photography, fluorescein angiography (FA), indocyanine green angiography (ICGA) (Optos camera, Optos PLC, Dunfermline, UK), and laboratory evaluation were performed at the discretion of the treating clinician.
Image acquisition and en face CC slab generation
All patients underwent SS-OCTA imaging with a PLEX® Elite 9000 (Carl Zeiss Meditec, Dublin, CA) device. 6×6mm or 9×9mm scans were acquired for both eyes centered at the fovea, optic disc, or other extrafoveal location as directed by the clinician to best capture the area of pathology.19 The device has a central wavelength of 1060nm and a bandwidth of 100nm, with axial and lateral resolution of ~6 um and ~20 um respectively at the surface of the retina. Images were acquired at a rate of 100,000 A-scans per second.19 FastTrac retinal tracking technology was used for mitigation of motion artifact. The built-in complex optical microangiography (OMAGc) algorithm was used to generate flow data from OCT images. Scans with a signal strength index lower than 7 (manufacturer recommendation), or motion artifact impacting the area of pathology were excluded from further analysis. The CC segmentation was performed using commercially available segmentation software provided in the PLEX® Elite 9000 (version 1.7). The CC slab was defined as 16–31 um below the retinal pigment epithelium (RPE)-fit segmentation line. All RPE segmentation lines were verified for accuracy and manually corrected if necessary. The en face OCTA CC images were generated using maximum projection.
Automated CC lesion boundary identification
En face OCTA CC images were exported to MATLAB (The MathWorks Inc., Natick, Massachusetts) where the remainder of image processing was performed using a custom algorithm. Figure 1 demonstrates the input and output of the subsequent steps of the automated algorithm for lesion boundary identification. Figure 1A shows the raw en face OCTA CC image. Retinal projection artifacts were removed20,21 and a compensation strategy was applied to adjust for signal attenuation as previously described.22 A previously described algorithm identified individual CC FDs23 using a fuzzy clustering approach, whereby pixels that cluster into the lowest intensity group are segmented to define individual CC FDs.23 Figure 1B shows the segmented FDs (in red) and excluded retinal projection artifacts (in yellow). To further segment areas of abnormal CC lesions, a ~50μm sliding window was applied across the entire en face OCTA CC image to determine the local FD density (Figure 1C). Then the local FD density map was binarized (Figure 1D), where regions in white pixels represent abnormally high local FD density. The threshold for binarization was decided by a set of previously published normal subjects.13 To set this threshold, previously published normal eyes were processed to generate local FD density maps as shown in Figure 1C, the mean and standard deviation (SD) values were generated for all eyes and the threshold was set as mean +1.96*SD. Then adjacent areas of high local FD density were combined into individual lesions using morphological operations24 including combining neighboring lesions with a distance (edge to edge) less than 60 μm (morphological closing), filling in inner holes within lesions (flood-fill operation), and excluding (area opening) small islands with an equivalent diameter smaller than 48 μm (~2 times of normal CC intercapillary distance23) (Figure 1E). Finally, the boundaries of the identified CC lesion are extracted by outlining the outer boundaries of resulting binary lesions (Figure 1F) and the total lesion area in the image is calculated in mm2 by summing all pixels within the lesion.
FIGURE 1. Automated choriocapillaris lesion boundary segmentation:
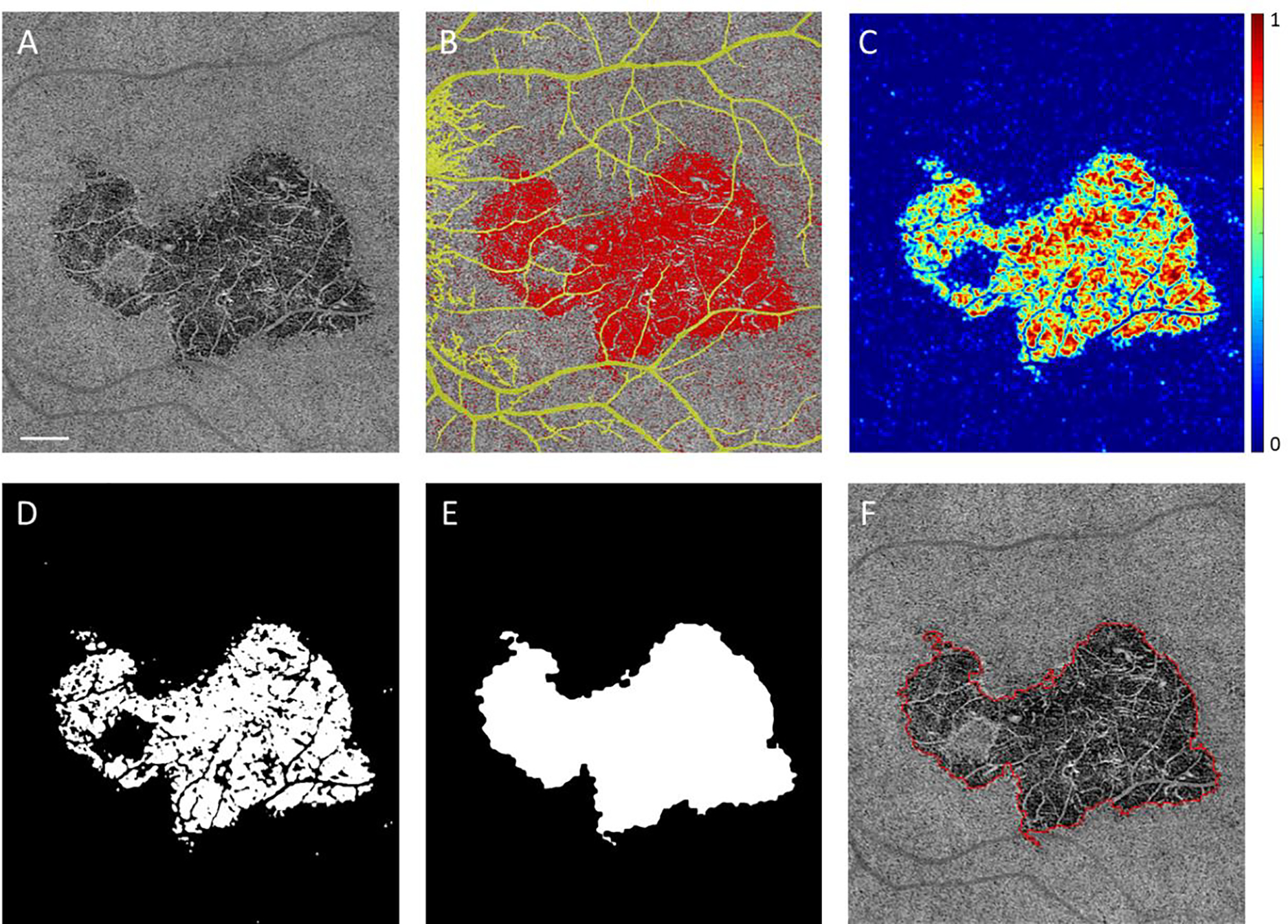
(A) En face image of the choriocapillaris (CC) slab of a patient with macular serpiginous choroiditis (eye 1 on Table 2). (B) Panel A overlaid with automatically identified CC flow deficits (FDs) (in red), and retinal projection artifacts (in yellow). (C) Heat map of local average CC FD density. Generated by applying a ~50 μm moving window onto CC FDs map (in red of panel B). (D) Areas identified as abnormally high local CC FD density (in white), generated by applying a threshold of high local FD density to panel C. (E) Areas segmented as abnormal CC lesion after applying morphological operations on panel D. (F) Final output includes CC lesion boundary (in red) and total lesion area in mm2. Scale bar represents 1 mm.
In order to determine the effect of altering the local average flow deficit density threshold (FDT), the lesion with the lowest agreement between HGs and the AG was outlined by the AG at a range of thresholds and again compared with HGs. Threshold values of high local average CC FD density ranging from 20% to 45% were used instead of the standard definition of 1.96 SD above mean from normal eyes.
Human CC lesion boundary determination
Manual CC lesion boundary identification was performed by two trained HGs (J.K. and A.L.). The graders were trained to the appearance of normal en face OCTA CC images by reviewing a previously published dataset collected from normal subjects.13 CC lesions were defined as areas that were black or dark grey and demonstrated loss of the homogenous appearance seen in the control eye training set. Each human grader (HG), HG1 and HG2, then viewed each en face OCTA CC image and manually applied a lesion boundary using PowerPoint software and the “scribble” tool (Microsoft, Redmond, WA). Hand drawn boundaries were exported as TIFFs and analyzed for spatial overlap using MATLAB software. The graders were masked to diagnosis, additional clinical images, and the CC lesion boundaries generated by the AG and the other HG.
Repeatability and grader agreement
For intra-grader repeatability testing, HGs were asked to draw lesion boundaries on the same images at three separate sessions that were separated in time by at least 24 hours. The AG was also used to perform boundary identification on this same image three times. Intra-grader repeatability was assessed by degree of lesion boundary spatial overlap with calculation of Sørensen-Dice coefficient (DSC) and comparison of lesion area by calculation of coefficient of variation (CV). For intra-visit repeatability analysis, a subset of enrolled patients underwent repeated imaging during one clinic visit. For each subject, 4 consecutive scans were taken at the same location, and en face OCTA CC images were registered rigidly before analysis. Boundaries were outlined by each HG and the AG and compared for spatial overlap with DSC. Lesion areas were calculated and the CV was reported for intra-visit repeatability assessment. For determination of inter-grader agreement, each HG as well as the AG performed lesion boundary delineation a single time on each lesion image. Delineated boundaries for each lesion were then compared in pairwise fashion and spatial overlap was assessed by DSC using binary lesion maps with statistical methods described below.
Statistical analysis:
Statistical analysis was performed in MATLAB and Python (version 3.8.5, Python Software Foundation). Repeatability and inter-grader agreement were assessed using CV and DSC where indicated. A DSC ≥0.70 was considered good spatial overlap,25,26 and a CV less than 10% of lesion area was considered low variability. Intraclass correlation coefficient (ICC) estimates and their 95% confidence intervals (CI) were calculated based on a mean rating (k=3), testing for consistency with a two-way random effects model to assess for intra-grader comparison of lesion area. Values of ICC greater than 0.9 were considered excellent consistency.27 P values less than 0.05 were considered significant and adjusted p values were used for multiple comparisons.
Results
Twenty eyes of 14 patients with choroidal inflammatory diseases including birdshot chorioretinopathy (n=4), serpiginous choroiditis (n=4), multifocal choroiditis (n=3), acute multifocal posterior pigment epitheliopathy (n=1), relentless placoid choroiditis (n=1), and presumed ocular histoplasmosis (n=1), were imaged and analyzed (Table 1). Average patient age was 49 years old (range 23–73 years old). Seventy-one percent of patients were male. At the time of imaging, 10 (71%) patients were taking systemic steroid-sparing immunomodulatory therapy, oral prednisone, or both.
Table 1:
Patient characteristics
| Patients | 14 |
| Age, years | 49 ± 19 |
| Gender | |
| Male | 10 (71%) |
| Female | 4 (29%) |
| Systemic therapy | 10 (71%) |
| Etiology | |
| Birdshot chorioretinopathy | 4 (29%) |
| Serpiginous choroiditis | 4 (29%) |
| Multifocal choroiditis | 3 (21%) |
| Acute multifocal pigment placoid epitheliopathy | 1 (7%) |
| Relentless placoid chorioretinopathy | 1 (7%) |
| Presumed ocular histoplasmosis syndrome | 1 (7%) |
Data presented as mean ± standard deviation or n (%)
To test the ability of the AG to perform segmentation, the lesions from 20 eyes with posterior uveitis were segmented by the AG and two masked HGs (HG1 and HG2). Total lesion area identified in each image and the spatial overlap of the lesions were compared between the three graders (Table 2). In table 2, eyes were ranked according to the degree of spatial overlap (DSC) between the AG and HG2. Agreement in the measurement of lesion area between all graders was evaluated using ICC (Figure 2). The AG demonstrated excellent agreement with each HG in the determination of total lesion area (AG vs. HG1, ICC 0.92, 95% CI 0.81–0.97; AG vs. HG2, ICC 0.91, 95% CI 0.78–0.97). However, the average lesion area from all eyes as measured by the AG (7.30mm2) was significantly smaller than the average lesion area measured by each HG (HG1=10.72mm2 and HG2=10.60mm2, p < 0.01 for both comparisons). There was no significant difference in average lesion area identified by the HGs (p = 0.46), and inter-grader comparison of lesion area identified by both HGs was excellent (ICC 0.99, 95% CI 0.98–1.00).
TABLE 2.
Quantification of Lesion Area and Intergrader Agreement in Lesion Localization
| Lesion Area (mm2) |
Pairwise Intergrader Agreement (DSC) |
Lesion Features |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Eye | Diagnosis | HG1 | HG2 | AG | HG1 vs HG2 | HG1 vs AG | HG2 vs AG | CNVM | Poor-Contrast Boundaries | Disease Status |
| 1 | SC | 15.97 | 15.82 | 16.14 | 0.96 | 0.95 | 0.97 | N | N | Inactive |
| 2 | SC | 3.19 | 3.49 | 3.20 | 0.96 | 0.95 | 0.95 | N | N | Inactive |
| 3 | SC | 4.61 | 4.86 | 4.93 | 0.94 | 0.88 | 0.88 | N | N | Active |
| 4 | BSCR | 0.63 | 0.90 | 0.79 | 0.82 | 0.87 | 0.87 | Ya | N | Inactive |
| 5 | POHS | 5.24 | 4.64 | 3.66 | 0.90 | 0.81 | 0.86 | Ya | N | Inactive |
| 6 | MFC | 2.94 | 3.44 | 2.44 | 0.88 | 0.80 | 0.86 | N | N | Active |
| 7 | RPC | 8.37 | 9.00 | 6.94 | 0.87 | 0.85 | 0.86 | N | Y | Inactive |
| 8 | RPC | 14.75 | 13.35 | 10.34 | 0.90 | 0.80 | 0.85 | N | N | Inactive |
| 9 | SC | 22.02 | 22.56 | 16.11 | 0.99 | 0.84 | 0.83 | N | N | Inactive |
| 10 | SC | 29.77 | 31.48 | 21.90 | 0.96 | 0.84 | 0.82 | N | N | Inactive |
| 11 | MFC | 1.22 | 2.00 | 1.74 | 0.69 | 0.71 | 0.82 | N | N | Inactive |
| 12 | SC | 18.79 | 16.39 | 12.48 | 0.87 | 0.77 | 0.80 | N | Y | Active |
| 13 | BSCR | 9.23 | 9.00 | 6.00 | 0.83 | 0.72 | 0.77 | N | N | Inactive |
| 14 | BSCR | 13.54 | 18.61 | 11.66 | 0.83 | 0.81 | 0.76 | N | Y | Inactive |
| 15 | BSCR | 5.60 | 5.22 | 5.50 | 0.75 | 0.67 | 0.71 | N | Y | Inactive |
| 16 | POHS | 7.24 | 5.40 | 4.62 | 0.81 | 0.70 | 0.66 | N | Y | Inactive |
| 17 | MFC | 11.44 | 11.31 | 5.33 | 0.93 | 0.61 | 0.64 | Y | N | Inactive |
| 18 | MFC | 2.14 | 3.62 | 2.82 | 0.83 | 0.59 | 0.62 | N | Y | Inactive |
| 19 | BSCR | 19.68 | 20.63 | 709 | 0.94 | 0.53 | 0.51 | Y | Y | Inactive |
| 20 | AMPPE | 12.20 | 9.60 | 2.33 | 0.79 | 0.31 | 0.37 | N | Y | Inactive |
| Mean (SD): | 10.72 (8.02) | 10.6 (8.15) | 7.3 (5.70) | 0.87 (0.08) | 0.75 (0.15) | 0.77 (0.15) | ||||
AG = algorithm; AMPPE = acute multifocal pigment placoid epitheliopathy; BSCR = birdshot chorioretinopathy; CNVM = choroidal neovascular membrane; HG1 = human grader 1; HG2 = human grader 2; MFC = multifocal choroiditis; POHS = presumed ocular histoplasmosis syndrome; RPC = relentless placoid chorioretinopathy; SC = serpiginous choroiditis.
Eyes with CNVM reported in the clinical chart, but without neovascularization visible on the choriocapillaris en face image. Twenty eyes were analyzed. The total lesion area (mm2) per image is listed by grader: HG1, HG2, and AG. Agreement in lesion localization between graders was measured by pairwise Sørensen-Dice coefficient (DSC). Eyes are listed in order of highest to lowest DSC between the AG and HG2. Shaded in gray are 4 eyes that demonstrated poor agreement (DSC <0.70) between the AG and both human graders.
FIGURE 2. Agreement between the algorithm and humans is high in the majority of lesions.
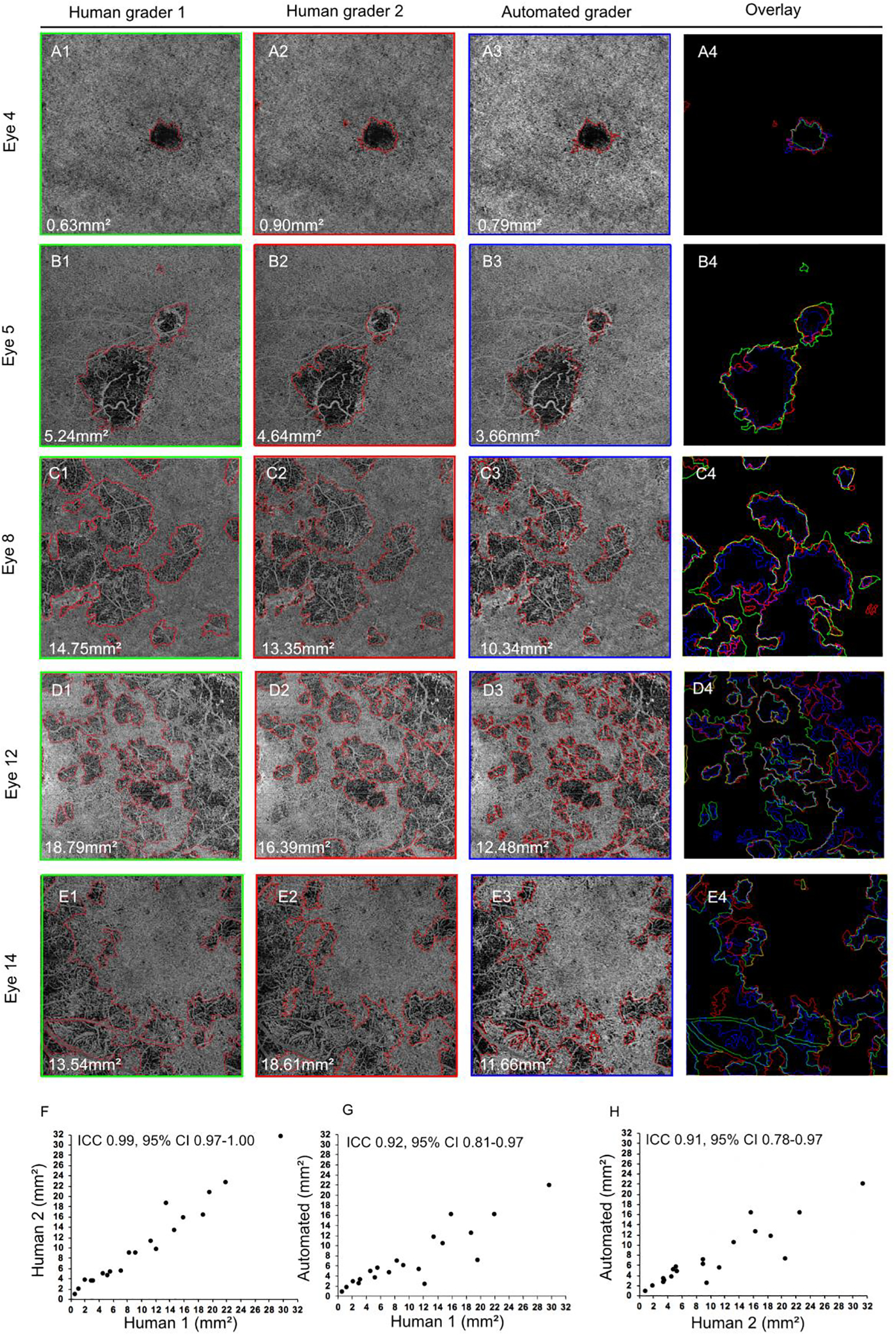
Each row demonstrates a CC lesion boundary identified by each grader. Lesion area is shown in mm2 on each image. The overlays of the human graders (HGs) to the algorithm (AG) are shown in the last panel on the right: HG1 in green, HG2 in red, and the AG in blue. White indicates where the boundaries drawn by all three graders overlap. (A) Eye 4, from a patient with birdshot chorioretinopathy. (B) Eye 5, from a patient with presumed ocular histoplasmosis syndrome. (C) Eye 8, from a patient with relentless placoid chorioretinopathy. D) Eye 12, from a patient with serpiginous-like choroiditis. (E) Eye 14, from a patient with birdshot chorioretinopathy. Panels (F-H) display the intraclass correlation coefficient (ICC) and 95% confidence intervals for the lesion areas identified on all 20 eyes by grader pairs as indicated: (F) HG1 vs. HG2; (G) HG1 vs. AG; (H) HG2 vs. AG.
Next, we assessed agreement in the spatial localization of the lesion by determination of the DSC between each grader pair (Table 2). A DSC ≥ 0.70 was considered to represent good spatial overlap.25,26 The AG demonstrated good spatial overlap with at least one HG in 16/20 (80%) eyes, and with both HGs in 14/20 (70%). Lesions with good spatial overlap between the AG and HGs ranged in appearance from single discrete lesions with high contrast borders (Figure 2A,B) to multifocal lesions with complex border morphology (Figure 2C–E).
In 4/20 (20%) eyes, the AG demonstrated poor spatial overlap (DSC <0.70) with both of the HGs (Table 2, grey shading). These eyes were evaluated qualitatively for a possible common feature that could explain this poor performance (Figure 3). Two eyes with poor AG and HG agreement (Eye #17 and Eye #19) contained a large choroidal neovascular membrane (CNVM). When segmented by the AG, all or most of the CNVM was excluded from within the lesion boundary. In contrast, HGs included the entire CNVM within the lesion boundary. Not all eyes with a clinical history of CNVM demonstrated poor DSC agreement between the AG and HGs.
FIGURE 3. Poor inter-grader agreement is associated with the presence of CNVM and poor contrast lesion boundaries:
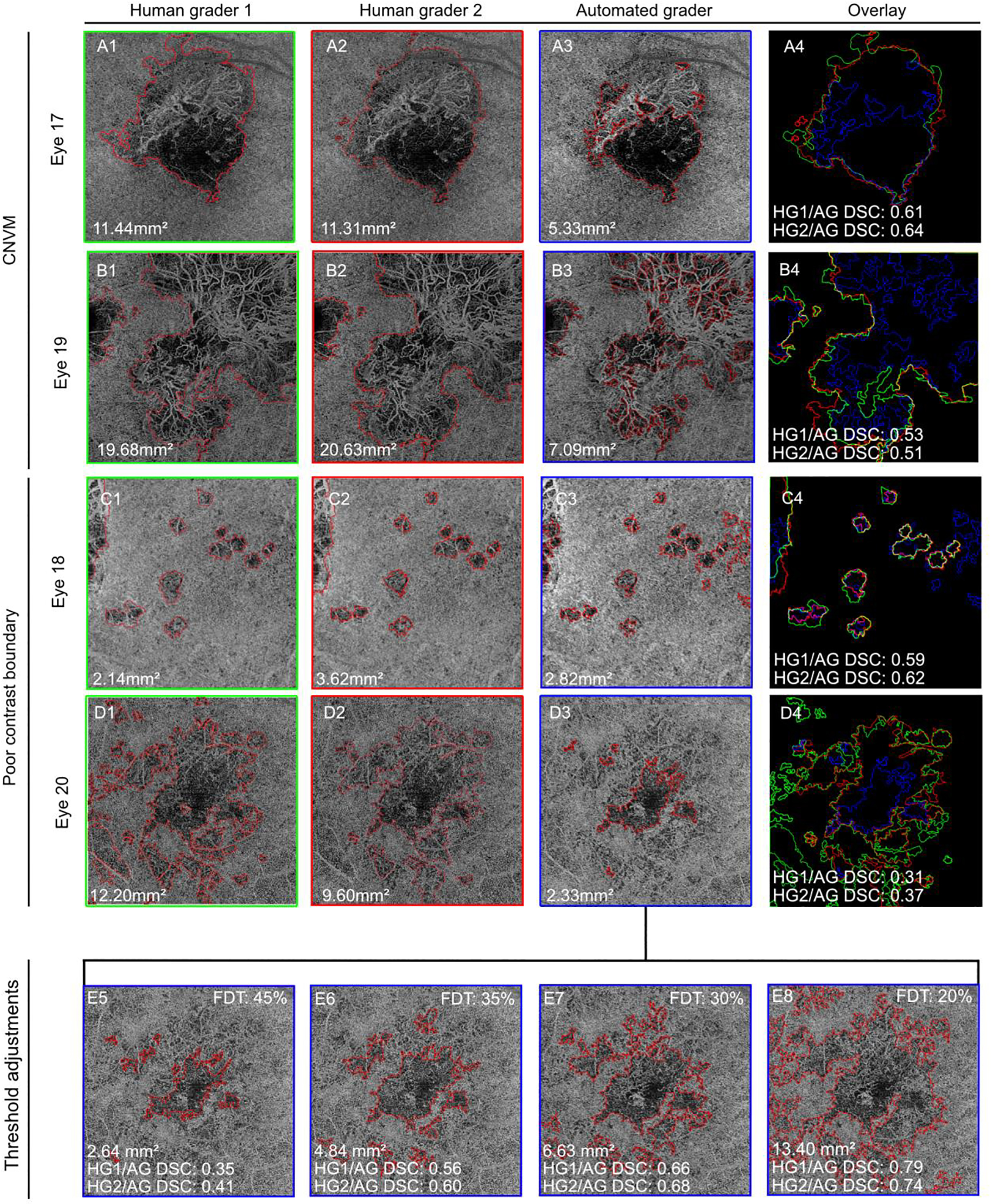
(A) Eye 17, from a patient with multifocal choroiditis. (B) Eye 19, from a patient with birdshot chorioretinopathy. When visible in the choriocapillaris slab, the choroidal neovascular membrane (CNVM) is included in the lesion area by the human graders (HGs) but excluded by the algorithm (AG). (C) Eye 18, from a patient with multifocal choroiditis. Total area is similar, but exact location of the lesion boundaries varied between graders. (D) Eye 20, patient with acute multifocal pigmented placoid epitheliopathy. The AG identified a significantly smaller lesion than both human graders. (E) Alternative AG lesion boundaries generated by decreasing the flow deficit density threshold (FDT). This change allowed the AG to include more subtle FD abnormalities into the lesion area, and improved agreement with human graders.
CNVM = choroidal neovascular membrane, FDT = flow deficit density threshold, HG1 = human grader 1, HG2 = human grader 2, AG = algorithm
For example, chart review indicated the presence of a CNVM in Eyes #4 and #5. Upon unmasked review of the CC slab images from these eyes, a vascular pattern consistent with the presence of a CNVM in the en face OCTA CC image could not be identified.28 These data suggest that a CNVM can impact lesion segmentation by the AG, but only if the neovascular vessels are visible in the en face CC slab. Furthermore, HGs and the AG classify a CNVM differently, as part of or excluded from the lesion respectively, when one is visible in the CC image.
Qualitative review of the two additional eyes with low AG and HG agreement (Eye #18 and #20) identified a feature of both lesions termed “poor contrast boundary”. Poor contrast boundary lesions were defined by the presence of a FD, i.e. a concentrated area of low intensity pixels (black), that was surrounded by pixels of intermediate intensity (dark grey) rather than being surrounded by pixels with a normal CC intensity (bright grey). Eye #20 from a patient with inactive acute multifocal pigmented placoid epitheliopathy (Figure 3D) demonstrated this feature and had the lowest inter-grader agreement between AG and HGs (AG and HG1, DSC 0.31; AG and HG2, DSC 0.37). We hypothesized that in these poor contrast lesions, the AG and HGs were using significantly different pixel intensities to determine where to segment the FD lesion from normal CC. Additionally, we hypothesized that since the humans were not constrained by a set threshold, they were applying a different threshold on these lesions than they used on lesions with higher contrast boundaries. To test this hypothesis, we introduced a range of FDT to the AG, thus altering the threshold CC FD density at which lesion area would be assigned and determined the impact these changes made to the AG output for Eye #20. (Figure 3 E5-E8). We found that by lowering the FDT, the area segmented into the lesion by the AG increased and the spatial overlap with human grader defined lesions improved. At FDT > 20%, the lesion area outlined by the AG (13.40 mm2) was larger than the area outlined by either HG1 (12.20 mm2) or HG2 (9.60 mm2), but achieved good spatial overlap with HG1 (DSC 0.79) and HG2 (DSC 0.74). Taken together, these data suggest that if CNVM area is excluded from the lesion by human graders, and if a sliding scale for the FD density threshold could be applied by a human grader, then agreement between AG lesion and HG lesion segmentation could be further improved on an individual basis.
Repeatability of CC lesion boundary identification
Next, we sought to determine if the AG performed with similar reliability to HGs. First, intra-grader repeatability was determined by comparing the segmentation results obtained on the same image by each grader at three separate sessions (Figure 4 rows A-C). In Figure 4, the boundary segmentation line obtained at each trial is indicated with a different color (red=trial 1, green= trial 2, and blue= trial 3). Areas of perfect overlap between all three trials are shown in white. The AG demonstrated perfect intra-grader repeatability for delineating the same lesion boundary on the same image (average area CV was zero). Neither HG demonstrated perfect repeatability. HG1 demonstrate an average area CV of 4.03% (range 3.03–4.97%), and HG2 an average area CV of 1.50% (range 0.37–3.16%).
FIGURE 4. The algorithm performs better on repeat segmentation of the same image than human graders:
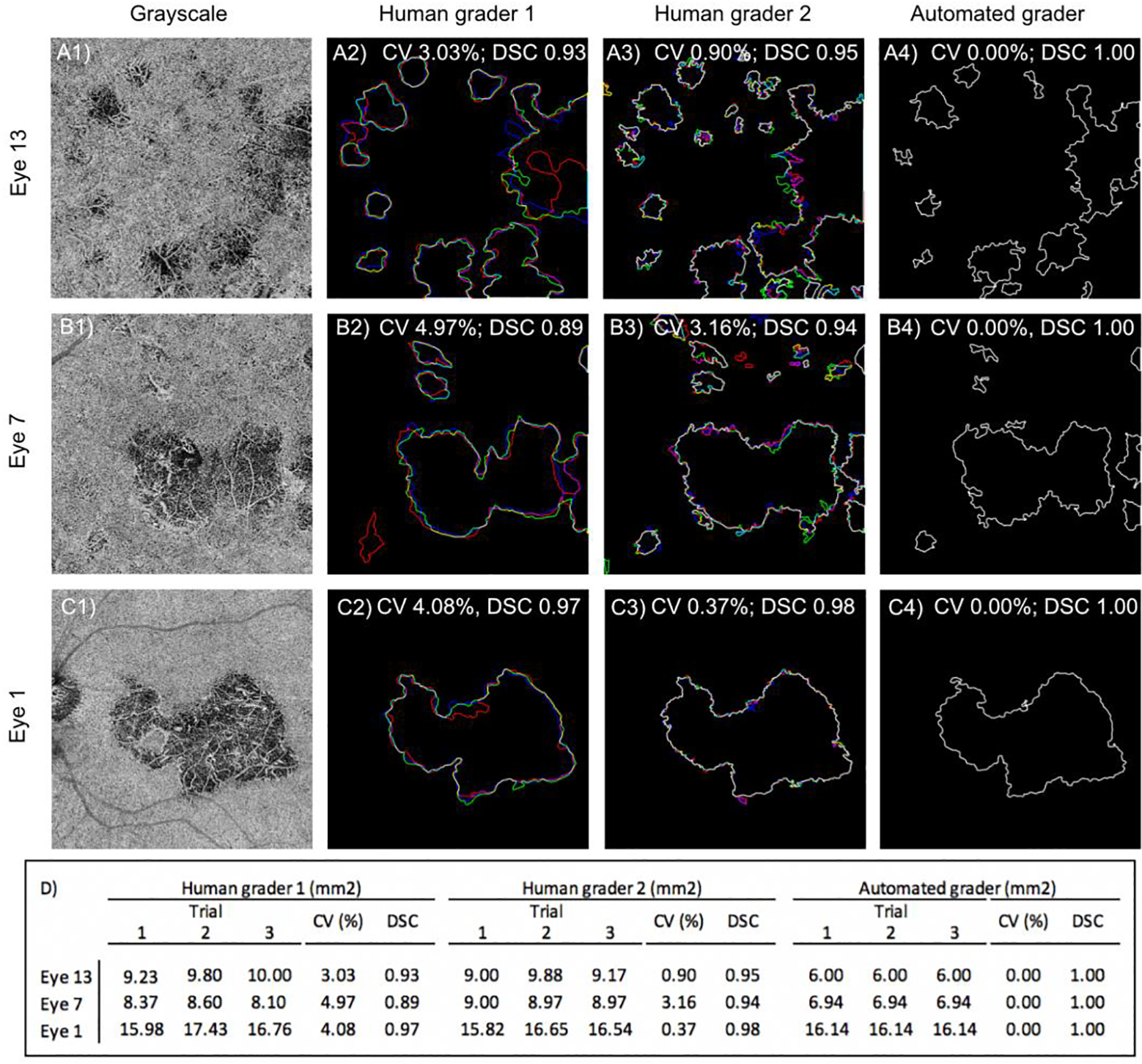
(A) Eye 13, from a patient with birdshot chorioretinopathy. (B) Eye 7, from a patient with relentless placoid chorioretinopathy. (C) Eye 1, from a patient with serpiginous choroiditis. The first column shows the CC en face image that was segmented by each grader. Each column to the right shows the results for HG1, HG2, and the algorithm (AG). For each image, the graders segmented the lesion three times: trial 1 (red), trial 2 (green), trial 3 (blue). Areas of perfect overlap are shown in white. (D) Table showing the total lesion area obtained by each grader at each independent scoring session, coefficient of variance (CV), and average DSC.
The impact of scan-to-scan variability on grader repeatability was also determined. For this analysis, three eyes were imaged four times on the same day, each image was segmented independently by the graders, and the lesion area CV and average DSC agreement between the images scored by the same grader was determined. For the three eyes, the AG demonstrated an average area CV of 2.6% (range 0.54%−5.44%) and average DSC of 0.89 (range 0.80–0.96). HG1 demonstrated an average CV of 5.1% (range 3.49%−7.79%) and average DSC of 0.9 (range 0.82–0.99). HG2 demonstrated an average CV of 5.4% (range 0.33%−10.54%) and average DSC of 0.89 (range 0.82–0.93) (Figure 5). Interscan repeatability was highest for all graders in Eye #2 from a patient with serpiginous choroiditis and was lowest in Eye #20 from a patient with acute multifocal posterior pigment epitheliopathy.
FIGURE 5. The algorithm demonstrates similar performance as human graders on interscan repeatability:
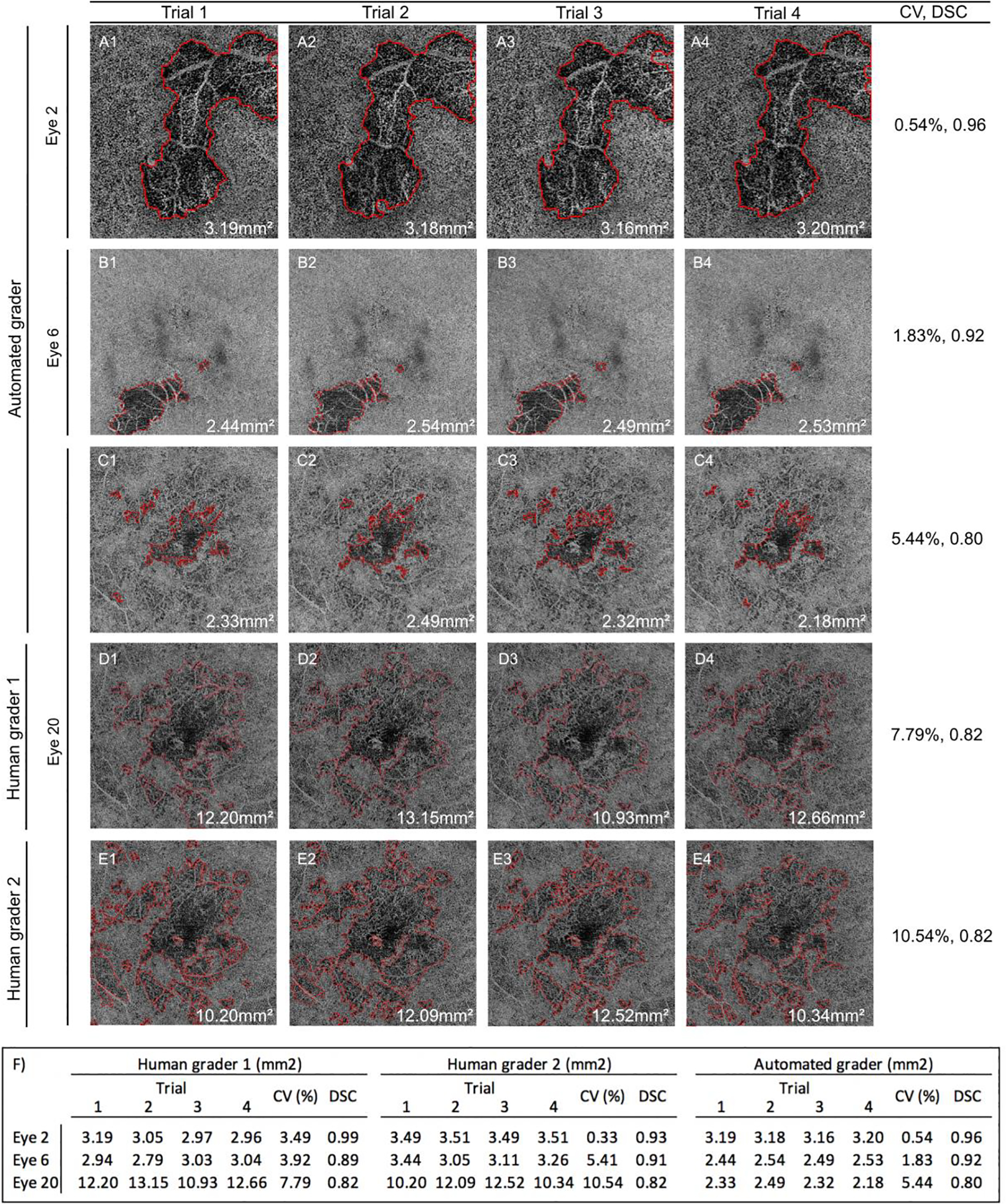
Three eyes were imaged 4 times on the same day and lesion segmentation performed. (A) Eye 2, from a patient with serpiginous choroiditis. Boundaries drawn by the algorithm (AG). (B) Eye 6, from a patient with multifocal choroiditis. Boundaries drawn by the AG. (C) Eye 20, from a patient with acute multifocal pigmented placoid epitheliopathy. Boundaries drawn by the AG. (D) Eye 20. Boundaries drawn by human grader 1 (HG1). (E) Eye 20. Boundaries drawn by HG2. Lesion area, CV, and average DSC were determined for each grader on each image. (F) Table showing the total lesion area obtained by each grader on each image, area coefficient of variance (CV), and average DSC.
Application of automated CC lesion identification to clinically relevant scenarios
To determine if the quantitative results obtained by this automated approach correlated with meaningful changes in CC pathology noted on clinical exam, we compared disease activity to quantitative changes in longitudinal CC images obtained from three patients (Figure 6).
FIGURE 6. Longitudinal CC lesion boundary delineation detects clinically meaningful changes in CC lesions.
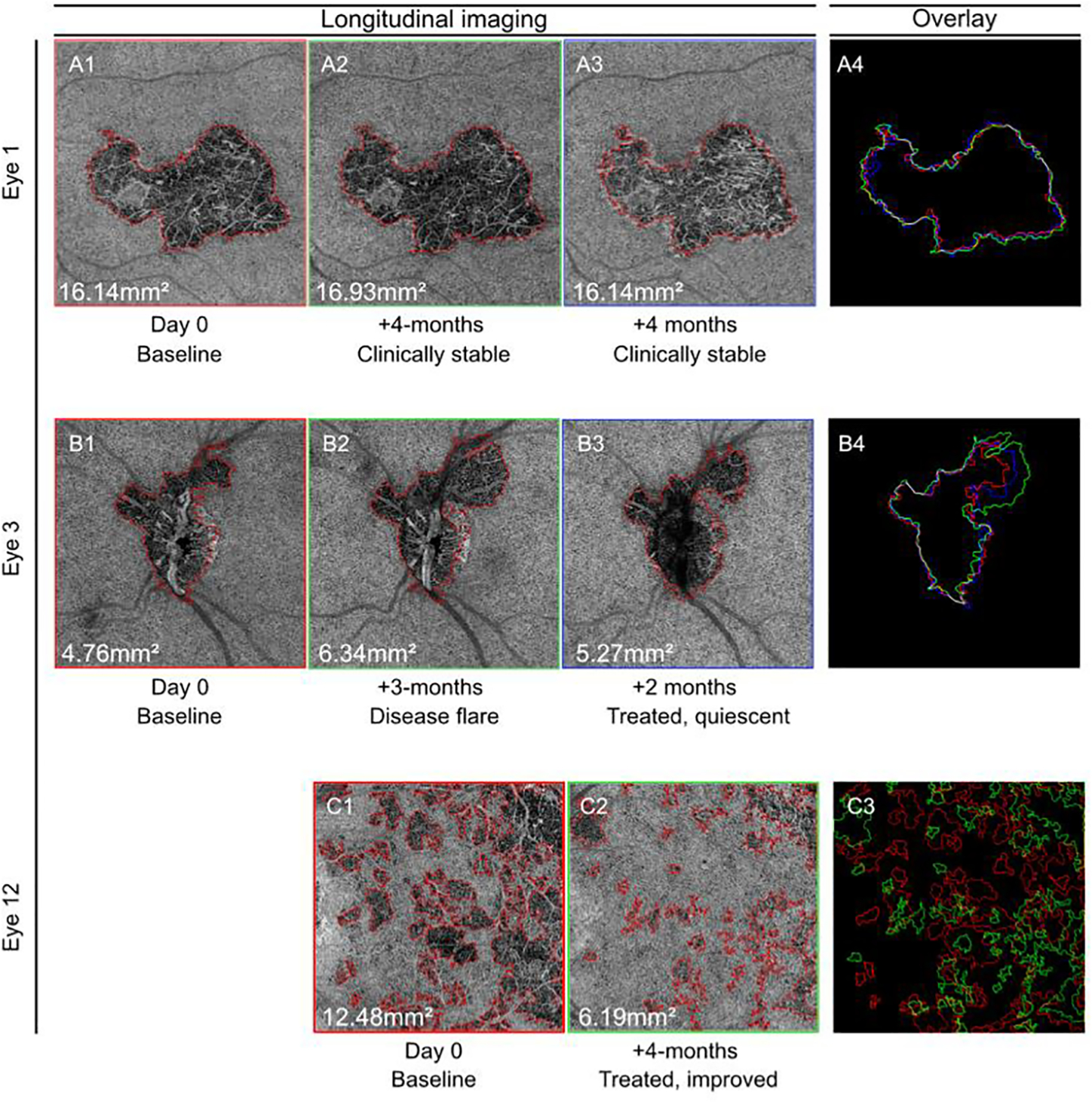
Examples of automated lesion analysis used to measure disease activity on longitudinal images. (A) Eye 1 from a patient with serpiginous choroiditis that did not demonstrate evidence of inflammation on standard of care examination. Lesion area and boundaries determined by the algorithm (AG) were stable over time. (B) Eye 3 from a patient with serpiginous choroiditis that was inactive at the time of baseline scan, but then experienced a clinically evident disease flare. CC lesion area increased from 4.76mm2 to 6.34 mm2 and the superior temporal lesion boundary expanded (green). Following treatment, lesions are decreased to 5.27mm2 and the superior temporal lesion boundary regressed (blue), but did not return to baseline. (C) Eye 12 from a patient with serpiginous-like choroiditis that presented with active choroiditis. Total lesion area at presentation was 12.48mm2. Following treatment with anti-TB therapy and systemic immune suppression, the lesion area decreased to 6.19mm2.
Figure 6 panels A1 to A4 depict Eye #1 from a patient with macular serpiginous choroiditis. The first image was taken 1 week after initiation of high dose (1 mg/kg) oral prednisone and mycophenolate mofetil therapy. At this visit, the disease activity was classified as controlled by the treating physician based on clinical exam and fundus imaging that included FA, ICGA, and FAF. Using the en face OCTA CC image, the total lesion area at this visit was determined to be 16.14mm2, and the lesion boundary drawn by all graders showed good spatial overlap (DSC ≥ 0.95). Four months later, imaging was repeated. The patient was still on mycophenolate mofetil and low dose oral prednisone, and the lesion was classified as inactive and stable by the treating physician after clinical exam and review of repeated clinical imaging. On the en face OCTA CC image, the lesion measured 16.93mm2 (+4.9% from baseline) and had good spatial overlap when compared to baseline (DSC 0.96). At the third visit, 8 months after presentation, the patient had continued on mycophenolate mofetil but had tapered off oral prednisone. The lesion was again classified as inactive and stable based on the results of clinical exam and imaging. At this time point, the lesion area determined from automated segmentation of the en face OCTA CC image was 16.14mm2. Compared to baseline, the area measured at 8 months was the same and the DSC between the two images was 0.95. In this patient with a stable lesion, the area and boundary did vary between visits. However, the inter-visit CV in total lesion area never exceeded the maximum inter-scan CV previously identified for the AG of 5.44%, and the boundary overlay between visits were nearly identical.
Figure 6 panels B1 to B4 depict Eye #3 from a patient with serpiginous choroiditis. At baseline, the patient was taking mycophenolate mofetil, tacrolimus and prednisone and the disease was judged inactive by clinical exam and fundus imaging. At this visit, the en face OCTA CC lesion size was measured as 4.76mm2. Three months after tapering off oral prednisone, the disease flared and the en face OCTA CC lesion size increased to 6.34mm2 (+33% from baseline) with visible expansion of the superior temporal border (in green). After treatment with oral prednisone the lesions area decreased to 5.27mm2 (−17% from prior image), and the lesion border (in blue) regressed toward the baseline border. The change in lesion size at each visit exceeded the maximum inter-scan area CV identified for the AG of 5.44% and was consistent with the clinical impression of flare and recovery.
Figure 6 panels C1 to C3 depict Eye #12 from a patient with TB associated serpiginous-like choroiditis. At the baseline visit, multifocal active choroidal lesions were identified on clinical exam and fundus imaging. The en face OCTA CC lesion area was measured to be 12.48 mm2, and multifocal lesions were outlined within the macular scan. Four months later, after treatment with anti-tuberculosis therapy, oral prednisone, and mycophenolate mofetil, clinical exam and fundus imaging determined that choroidal inflammation was quiescent. Repeat SS-OCTA imaging at this visit revealed marked restoration of CC blood flow and a decrease in the total CC lesion area to 6.19 mm2 (−50.4% from baseline). In the overlay image, the boundaries of the original lesions (red) can be compared with the residual CC lesions (green).
Algorithm testing on images from a separate institution
In order to demonstrate the generalizability of the algorithm and potential utility in a reading center format, we analyzed a new set of unaltered images from an outside institution captured using standard settings on a commercially available PLEX® Elite 9000 machine. These SS-OCTA images were obtained as part of the standard clinical evaluation of patients with posterior uveitis at the University of Bern, Switzerland (MM). The en face CC OCTA slabs of both eyes from 4 patients with posterior uveitis were exported from the OCTA machine and analyzed by the AG. The CC lesions from one patients with serpiginous choroiditis, one patient with chorioretinitis and secondary CNVM associated with undifferentiated connective tissue disease and concomitant small vessel vasculitis, and two patients with multifocal choroiditis (one with secondary CNVM) were automatically segmented and their areas quantified (Figure 7). These images were centered on the macula, optic nerve, or as directed by the clinician to best document the clinically relevant lesion. The AG segmented lesions in all images and provided a single quantitative measure of disease burden for each eye.
Figure 7: Application of automated lesion segmentation and area quantification to images obtained from a second clinical location.
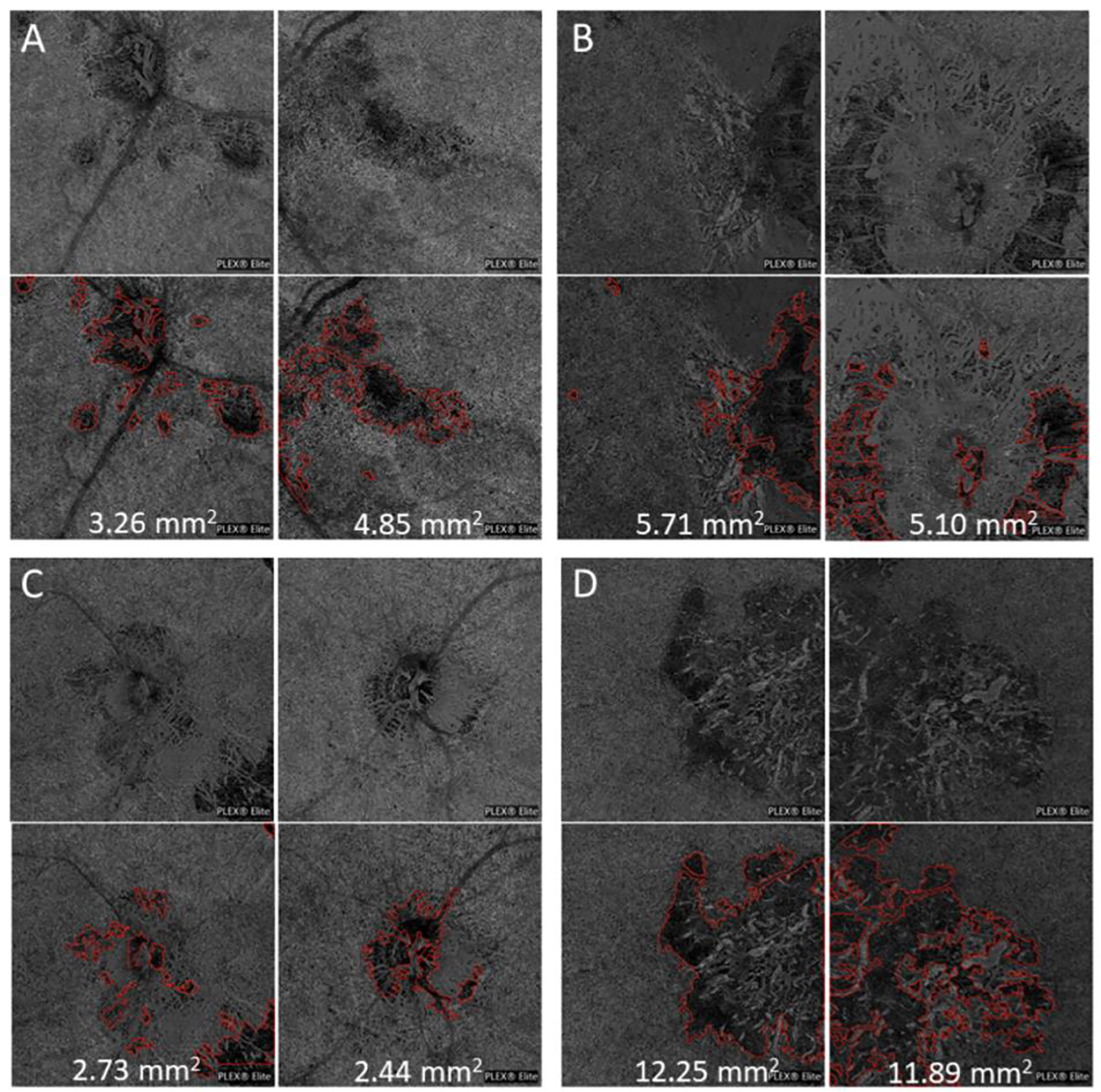
The choriocapillaris (CC) slab from the right and left eyes from four patients with posterior uveitis (top row in each panel) were analyzed and the CC flow deficits were outlined (red) and lesion area quantified in mm2 (bottom row in each panel). (A) Chorioretinitis with secondary peripapillary CNVM associated with undifferentiated connective tissue disease with concomitant small vessel vasculitis (B) Multifocal choroiditis with secondary CNVM (C) Multifocal choroiditis (D) Serpiginous choroiditis.
Discussion:
We developed and validated a novel automated algorithm for segmenting and quantifying CC lesions in patients with posterior uveitis. The AG demonstrated good interscan repeatability as well as similar performance to HGs for identifying lesions from eyes with multiple forms of posterior uveitis. The quantified lesion area and visual output of the lesion boundary provided by en face image segmentation will be useful in future studies utilizing CC FDs as an imaging biomarker of disease activity and response to therapy in patients with posterior uveitis.
A strength of this study was the inclusion of eyes representing a range of diagnoses within the white dot syndrome spectrum. Currently there are no quantitative metrics for disease activity in patients with these forms of uveitis. Due to the primary location of inflammation in the choroid, other well established semiquantitative metrics of inflammation such as anterior chamber cell, anterior chamber flare, and vitreous haze fail to describe disease presence or reflect changes in disease activity in these conditions.
The goal of this study was to test the ability of an algorithm to identify the same CC FD lesions as human graders on otherwise masked en face OCTA CC images, and to provide a quantifiable and automated OCTA-based biomarker of disease activity. Neither the HGs nor the AG had multimodal imaging data to direct their selection of the lesion area. The HGs intuitively identified abnormal areas of CC after reviewing a set of normal en face OCTA CC images. The AG on the other hand, used a predefined threshold based on prior analysis of control patients13 to identify abnormal areas. While we did not validate the overlap of all CC lesions with findings of gold standard multimodal imaging, other studies have established a strong relationship between choroidal lesions detected by conventional imaging and CC FDs on OCTA, and support the use of CC FDs as a useful biomarker of disease activity in the white dot syndromes.6–8,11,14,29–32 We did review clinical assessment and findings of conventional imaging in three eyes, which agreed with the quantitative assessment and boundary changes detected by OCTA. This review supports our underlying assumption that the graders were identifying clinically relevant lesions. Future studies should be designed with concurrent multimodal imaging to confirm the relationships between CC FDs and other signs of clinical disease activity for each specific diagnosis.
In this study, lesions associated with serpiginous choroiditis demonstrated the best DSC agreement between the AG and the HGs. In the majority of these eyes (6/7), the DSC was >0.8 with two eyes demonstrating DSC >0.95 between the AG and the HGs. The sharp contrast border of a typical CC FD lesion in eyes with serpiginous choroiditis has been described previously, and were noted again here.3,14,32 This sharp contrast boundary provides a clear threshold for the AG and contributed to the good agreement scores. Lesions from other forms of choroiditis also demonstrated boundaries with sharp borders, and these lesions tended to produce good DSC agreement between graders. However, agreement was impacted by lesions with poor contrast boundaries, which were observed in the majority of eyes with lower agreement between the AG and HGs. This boundary characteristic was not limited to any specific diagnosis or to the presence or absence of inflammatory disease activity. Determining the factors that contribute to sharply demarcated and poorly demarcated FD lesions and the correspondence of these more subtle lesions with other changes on multimodal imaging will help clarify which FDT produces the most clinically relevant lesion segmentation.
Agreement between the AG and the HGs was markedly impacted by the presence of large CNVM in two eyes. In these cases, HGs judged CNVM vessels as abnormal vasculature in the CC slab and included them within the lesion. The AG instead excluded the high intensity pixels associated with the CNVM leading to exclusion from lesion boundaries. In future studies, this difference in lesion definition could be addressed by incorporating a step for identification of CNVM vasculature28,33 and then adding CNVM area to the automatically determined FD area.
Intra-grader repeatability, whereby the same image was repeatedly analyzed, was predictably perfect for the AG. HGs on the other hand displayed clear differences in lesion boundary segmentation even for repeat grading of an identical image. This type of variability has been noted in other image analysis studies with a similar degree of variation.34 Inter-scan reliability measured by repeat imaging and analysis of the same lesion on the same day was relatively good for all graders (DSC ≥ 0.80). However, the variability in total lesion area as measured by the CV was on average approximately 2 times higher for both HGs than for the AG. This suggests that repeatability and thus reliability of results from the AG is as good as if not better than HGs. Still, inter-scan reliability for the AG was not perfect, suggesting some unmitigated variability likely due to patient factors and variation in device and photographer performance. Identification of CC FDs in uveitis patients has previously shown a high degree of intra-visit repeatability, though these results were assessed for individual CC flow voids rather than comparing bounded lesion areas.12 Imaging in that study was also conducted using spectral domain OCTA rather than swept-source OCTA and assessed with fewer repeatability attempts than in our study. Still, the similarly high degree of repeatability is reassuring for reliable CC FD identification whether for purposes of direct quantification or lesion identification. While not specific to patients with uveitis, the reproducible identification of CC FD using SS OCTA appears to be consistent with a previous report using the same compensation strategy that we employed.22 The significance of repeatability quantification is driven by the need to differentiate pathologic change versus variation inherent to the method of measurement.35 Our results suggest the AG may be as or more sensitive to detect true pathologic changes compared to HGs. Additional studies will be required to clarify the magnitude of change in lesion area and boundary position required to confidently indicate a clinically meaningful change.
There are a number of limitations in this study. OCTA imaging technology is susceptible to motion artifacts, speckle noise and currently has limited lateral resolution. There is a lack of ground truth for CC imaging in our study. We used a curated dataset of high-quality images for which the results may differ from those in clinical practice. We did not obtained images from patients with all posterior uveitis diagnoses or during all phases of disease. Future work will focus on validating this algorithm on a greater number of patients with different ocular inflammatory pathologies and in association with longitudinal changes in disease activity.
Conclusion:
In this study, we present an automated OCTA-based CC lesion identification algorithm, which demonstrated accurate segmentation of a wide range of lesion morphologies in patients with posterior uveitis. Automated lesion localization and area quantification were highly reliable and reproducible in comparison with human graders. Real-time, objective, and quantitative monitoring of choroidal disease activity may be possible using this automated image analysis approach.
Acknowledgements:
a. Funding/Support: Research supported by grants from the National Eye Institute (NEI K08EY023998, R01 EY028753 and R01AG060942), the Cynthia and Joseph Gensheimer Fellowship, Research to Prevent Blindness Career Development Award, Carl Zeiss Meditec, and an unrestricted Departmental Grant from Research to Prevent Blindness. The funding organization had no role in the design or conduct of this research.
b. Financial Disclosures: Dr. Wang received research support from Carl Zeiss Meditec, Inc. Dr. Wang discloses intellectual property owned by the Oregon Health and Science University and the University of Washington. Dr. Wang also receives research support from Moptim Inc, Colgate Palmolive Company and Facebook technologies LLC. He is a consultant to Carl Zeiss Meditec. Dr. Munk is consultant for Carl Zeiss Meditec, Novartis, RetinAI, Oculis, Bayer, Gensight Therapeutics, Allergan and Isarna Therapeutics. The remaining authors have no disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nazari H, Hariri A, Hu Z, Ouyang Y, Sadda SV, Rao NA. Choroidal atrophy and loss of choriocapillaris in convalescent stage of Vogt-Koyanagi-Harada disease: In vivo documentation. J Ophthalmic Inflamm Infect. 2014;4(1):1–9. doi: 10.1186/1869-5760-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouchenaki N, Cimino L, Auer C, Tran VT, Herbort CP. Assessment and classification of choroidal vasculitis in posterior uveitis using indocyanine green angiography. In: Klinische Monatsblatter Fur Augenheilkunde. Vol 219. Klin Monbl Augenheilkd; 2002:243–249. doi: 10.1055/s-2002-30661 [DOI] [PubMed] [Google Scholar]
- 3.Pakzad-Vaezi K, Khaksari K, Chu Z, Van Gelder RN, Wang RK, Pepple KL. Swept-Source OCT Angiography of Serpiginous Choroiditis. Ophthalmol Retin. 2018;2(7):712–719. doi: 10.1016/j.oret.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim W-K, Buggage RR, Nussenblatt RB. Serpiginous choroiditis. Surv Ophthalmol. 50(3):231–244. doi: 10.1016/j.survophthal.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 5.De Carlo TE, Bonini Filho MA, Adhi M, Duker JS. Retinal and choroidal vasculature in birdshot chorioretinopathy analyzed using spectral domain optical coherence tomography angiography. Retina. 2015;35(11):2392–2399. doi: 10.1097/IAE.0000000000000744 [DOI] [PubMed] [Google Scholar]
- 6.Klufas MA, Phasukkijwatana N, Iafe NA, et al. Optical Coherence Tomography Angiography Reveals Choriocapillaris Flow Reduction in Placoid Chorioretinitis. Ophthalmol Retin. 2017;1(1):77–91. doi: 10.1016/j.oret.2016.08.008 [DOI] [PubMed] [Google Scholar]
- 7.Pepple KL, Chu Z, Weinstein J, Munk MR, Van Gelder RN, Wang RK. Use of En Face Swept-Source Optical Coherence Tomography Angiography in Identifying Choroidal Flow Voids in 3 Patients With Birdshot Chorioretinopathy. JAMA Ophthalmol. 2018;136(11):1288–1292. doi: 10.1001/jamaophthalmol.2018.3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heiferman MJ, Rahmani S, Jampol LM, et al. ACUTE POSTERIOR MULTIFOCAL PLACOID PIGMENT EPITHELIOPATHY on OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY. Retina. 2017;37(11):2084–2094. doi: 10.1097/IAE.0000000000001487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke TR, Chu CJ, Salvatore S, et al. Application of OCT-angiography to characterise the evolution of chorioretinal lesions in acute posterior multifocal placoid pigment epitheliopathy. Eye. 2017;31(10):1399–1408. doi: 10.1038/eye.2017.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal K, Agarwal A, Mahajan S, et al. The Role of Optical Coherence Tomography Angiography in the Diagnosis and Management of Acute Vogt–Koyanagi–Harada Disease. Ocul Immunol Inflamm. 2018;26(1):142–153. doi: 10.1080/09273948.2016.1195001 [DOI] [PubMed] [Google Scholar]
- 11.Dingerkus VLS, Munk MR, Brinkmann MP, et al. Optical coherence tomography angiography (OCTA) as a new diagnostic tool in uveitis. J Ophthalmic Inflamm Infect. 2019;9(1):1–28. doi: 10.1186/s12348-019-0176-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caplash S, Kodati S, Cheng SK, et al. Repeatability of Optical Coherence Tomography Angiography in Uveitic Eyes. Transl Vis Sci Technol. 2019;8(6):17. doi: 10.1167/tvst.8.6.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu Z, Weinstein JE, Wang RK, Pepple KL. Quantitative Analysis of the Choriocapillaris in Uveitis Using en face Swept Source Optical Coherence Tomography Angiography. Am J Ophthalmol. May 2020. doi: 10.1016/j.ajo.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macedo S, Pohlmann D, Lenglinger M, Pleyer U, Joussen AM, Winterhalter S. Optical coherence tomography angiography (OCTA) findings in Serpiginous Choroiditis. BMC Ophthalmol. 2020;20(1). doi: 10.1186/s12886-020-01527-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denniston AK, Keane PA, Srivastava SK. Biomarkers and Surrogate Endpoints in Uveitis: The Impact of Quantitative Imaging. Invest Ophthalmol Vis Sci. 2017;58(6):BIO131–BIO140. doi: 10.1167/iovs.17-21788 [DOI] [PubMed] [Google Scholar]
- 16.Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55. doi: 10.1016/j.preteyeres.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taibouni K, Chenoune Y, Miere A, Colantuono D, Souied E, Petit E. Automated quantification of choroidal neovascularization on Optical Coherence Tomography Angiography images. Comput Biol Med. 2019;114. doi: 10.1016/j.compbiomed.2019.103450 [DOI] [PubMed] [Google Scholar]
- 18.Yao X, Alam MN, Le D, Toslak D. Quantitative optical coherence tomography angiography: A review. Exp Biol Med. 2020;245(4):301–312. doi: 10.1177/1535370219899893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunbar MC. Meditec CZ. Carl Zeiss Meditec Plex Elite 9000 OCT 501(k) Premarket Report of FDA, 2016. http://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/default.htm. Accessed November 3, 2019.
- 20.Zhang A, Zhang Q, Wang RK. Minimizing projection artifacts for accurate presentation of choroidal neovascularization in OCT micro-angiography. Biomed Opt Express. 2015;6(10):4130. doi: 10.1364/boe.6.004130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, Zhang A, Lee CS, et al. Projection Artifact Removal Improves Visualization and Quantitation of Macular Neovascularization Imaged by Optical Coherence Tomography Angiography. Ophthalmol Retin. 2017;1(2):124–136. doi: 10.1016/j.oret.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Zheng F, Motulsky EH, et al. A novel strategy for quantifying choriocapillaris flow voids using swept-source OCT angiography. Investig Ophthalmol Vis Sci. 2018;59(1):203–211. doi: 10.1167/iovs.17-22953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu Z, Zhou H, Cheng Y, Zhang Q, Wang RK. Improving visualization and quantitative assessment of choriocapillaris with swept source OCTA through registration and averaging applicable to clinical systems. Sci Rep. 2018;8(1). doi: 10.1038/s41598-018-34826-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q, Chen CL, Chu Z, et al. Automated quantitation of choroidal neovascularization: A comparison study between spectral-domain and swept-source OCT angiograms. Investig Ophthalmol Vis Sci. 2017;58(3):1506–1513. doi: 10.1167/iovs.16-20977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou KH, Warfield SK, Bharatha A, et al. Statistical validation of image segmentation quality based on a spatial overlap index. Acad Radiol. 2004;11(2):178–189. doi: 10.1016/s1076-6332(03)00671-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zijdenbos AP, Dawant BM, Margolin RA, Palmer AC. Morphometric analysis of white matter lesions in MR images: method and validation. IEEE Trans Med Imaging. 1994;13(4):716–724. doi: 10.1109/42.363096 [DOI] [PubMed] [Google Scholar]
- 27.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel R, Wang J, Campbell JP, et al. Classification of choroidal neovascularization using projection-resolved optical coherence tomographic angiography. Investig Ophthalmol Vis Sci. 2018;59(10):4285–4291. doi: 10.1167/iovs.18-24624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zahid S, Chen KC, Jung JJ, et al. OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY of CHORIORETINAL LESIONS DUE to IDIOPATHIC MULTIFOCAL CHOROIDITIS. Retina. 2017;37(8):1451–1463. doi: 10.1097/IAE.0000000000001381 [DOI] [PubMed] [Google Scholar]
- 30.Astroz P, Miere A, Mrejen S, et al. Optical Coherence Tomography Angiography to Distinguish Choroidal Neovascularization From Macular Inflammatory Lesions in Multifocal Choroiditis. Retina. 2018;38(2):299–309. doi: 10.1097/IAE.0000000000001617 [DOI] [PubMed] [Google Scholar]
- 31.Dolz-Marco R, Sarraf D, Giovinazzo V, Freund KB. OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY SHOWS INNER CHOROIDAL ISCHEMIA IN ACUTE POSTERIOR MULTIFOCAL PLACOID PIGMENT EPITHELIOPATHY. Retin Cases Brief Rep. 2017;11(1):S136–S143. doi: 10.1097/ICB.0000000000000473 [DOI] [PubMed] [Google Scholar]
- 32.Desai R, Nesper P, Goldstein DA, Fawzi AA, Jampol LM, Gill M. OCT Angiography Imaging in Serpiginous Choroidopathy. Ophthalmol Retin. 2018;2(4):351–359. doi: 10.1016/j.oret.2017.07.023 [DOI] [PubMed] [Google Scholar]
- 33.Leal I, Tan SZ, Aslam T, Steeples LR, Jones NP, Chhabra R. Intra and inter-rater agreement of inflammatory choroidal neovascular membrane measurements using optical coherence tomography angiography. Graefe’s Arch Clin Exp Ophthalmol. 2020;258(3):647–651. doi: 10.1007/s00417-019-04538-1 [DOI] [PubMed] [Google Scholar]
- 34.Liew SL, Anglin JM, Banks NW, et al. A large, open source dataset of stroke anatomical brain images and manual lesion segmentations. Sci Data. 2018;5. doi: 10.1038/sdata.2018.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visser M, Müller DMJ, van Duijn RJM, et al. Inter-rater agreement in glioma segmentations on longitudinal MRI. NeuroImage Clin. 2019;22. doi: 10.1016/j.nicl.2019.101727 [DOI] [PMC free article] [PubMed] [Google Scholar]


