Abstract
Background and Purpose:
Many older patients presenting with acute ischemic stroke were already taking aspirin prior to admission. However, the management strategy for patients with aspirin treatment failure has not been fully established.
Methods:
We used data from the American Heart Association Get With The Guidelines® (GWTG) Stroke Registry to describe discharge antithrombotic treatment patterns among Medicare beneficiaries with ischemic stroke who were taking aspirin prior to their stroke and were discharged alive from 1734 hospitals in the United States between October 2012 and December 2017.
Results:
Of 261,634 ischemic stroke survivors, 100,016 (38.2%) were taking aspirin monotherapy prior to stroke. Among them, 44.4% of patients remained on aspirin monotherapy at discharge (20.9% 81 mg, 18.2% 325 mg, 5.3% other or unknown dose). The next most common therapy choice was DAPT (24.6%), followed by clopidogrel monotherapy (17.8%). The remaining 13.2% of patients were discharged on either aspirin/dipyridamole, warfarin or non-vitamin K antagonist oral anticoagulants with or without antiplatelet, or no antithrombotic therapy at all.
Conclusions:
Nearly half of patients with ischemic stroke while on preventive therapy with aspirin are discharged on aspirin monotherapy without changing antithrombotic class, while the other half are discharged on clopidogrel monotherapy, DAPT, or other less common agents. These findings emphasize the need for future research to identify best management strategies for this very common and complex clinical scenario.
Introduction
More than 40% of adults over the age of 70 in US take aspirin for primary prevention of cardiovascular disease, and more than 70% of patients of any age with a history of cardiovascular disease take aspirin daily.1,2 While aspirin is commonly used for cardiovascular disease and stroke prevention, many patients taking aspirin monotherapy still experience an ischemic stroke (so called “aspirin failure”).3 Although increasing the dose of aspirin, adding a second drug, or switching to an alternative antiplatelet agent are often considered, there is no evidence of superiority of any of these approaches3,4. This study evaluates the prevalence of aspirin failure among older patients presenting with acute ischemic stroke and describes their discharge prescription patterns of antithrombotic therapy for secondary stroke prevention.
Methods
The authors declare that all supporting data are available within the article and its online supplementary files.
Data Source
We utilized the Get With The Guidelines® (GWTG) - Stroke (GWTG-Stroke) program, a nationwide stroke registry sponsored by the American Heart Association5. Standardized registry data collected includes patient demographics, medical history, medications prior to admission, in-hospital treatment, outcomes, and discharge medication. The validity and reliability of data collection have been previously reported6. All participating sites receive approval for human research to enroll consecutive patients without individual consent under the Common Rule or were authorized and waived from subsequent review by their Institutional Review Board. IQVIA, Inc. serves as the data collection and coordination center. The Duke Clinical Research Institute serves as the data analysis center and has an agreement to analyze the aggregate de-identified data. This study was approved by the institutional review board of Duke University.
Study Population and Variables
This is a registry-based observational cohort of Medicare beneficiaries without atrial fibrillation who were discharged alive for ischemic stroke from 1734 GWTG-Stroke hospitals in the United States between Oct 2012 and Dec 2017. Details of inclusion and exclusion criteria can be found in the Supplemental Figure. Aspirin treatment failure was defined as documentation of patients taking aspirin monotherapy (without any other antithrombotic) within 7 days before hospital arrival. The outcome of interest was discharge antithrombotic treatment.
Statistical Analysis
Medians and percentages were used to describe the distribution of continuous and categorical variables, respectively. Ordinal logistic regression was performed to identify factors associated with escalation of discharge antithrombotic medication from no antithrombotics, to single antiplatelet therapy [aspirin or clopidogrel monotherapy], dual antiplatelet therapy of aspirin and clopidogrel [DAPT], and anticoagulant with or without antiplatelet therapy. This study follows the RECORD reporting guidelines. The completed RECORD checklist can be found in the supplement file.
Results
Of 261,634 ischemic stroke survivors, 100,016 (38.2%) were taking aspirin monotherapy prior to stroke (median age 78 years; 53% female; 79.4% initial stroke, 20.6% recurrent stroke). The distribution of discharge antithrombotic therapy is reflected in Figure. Overall, 44.4% of patients remained on aspirin monotherapy at discharge. The next most common therapy choice was DAPT (24.6%), followed by clopidogrel monotherapy (17.8%).
Figure.
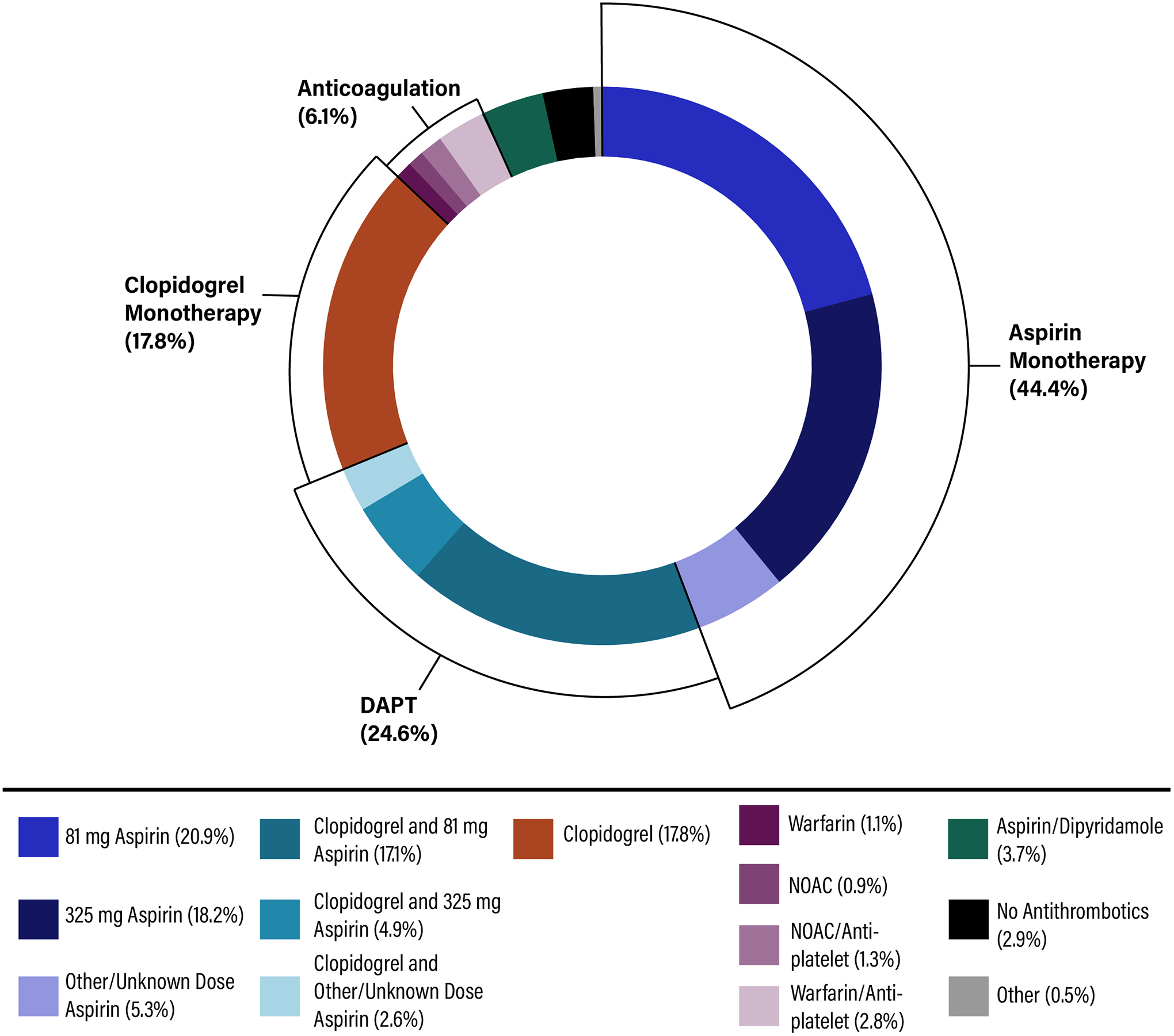
Discharge Antithrombotics for Ischemic Stroke Patients with Aspirin Failure.
Baseline characteristics varied by discharge antithrombotic therapy group (Table 1). Patients discharged on aspirin monotherapy or clopidogrel monotherapy had fewer cardiovascular risk factors than those who received DAPT. Patients discharged on clopidogrel or DAPT tended to have less severe strokes (NIHSS 0–3), whereas patients discharged on aspirin monotherapy, warfarin, NOACs, or no antithrombotic tended to have more severe strokes (NIHSS >10). Ordinal logistic regression suggested that older age, female, patients with renal insufficiency, and patients with less severe strokes were less likely to receive higher intensity antithrombotic therapy (Table 2). By contrast, patients with coronary artery disease or prior myocardial infarction, carotid stenosis, peripheral vascular disease, hypertension, dyslipidemia, heart failure, prior stroke, or prior transient ischemic attack (TIA) were more likely to receive higher-intensity antithrombotic therapy at discharge.
Table 1.
Baseline Characteristics by Discharge Antithrombotic Therapy.
| Variable | Aspirin monotherapy (n=44,000) | Clopidogrel monotherapy (n=17,824) | DAPT (n=24,614) | Other Antiplatelet w/o OAC (n=4,193) | Warfarin w/wo antiplatelet (n=3,803) | NOAC w/wo antiplatelet (n=2,266) | No antiplatelet or oral anticoagulant (n=2,916) |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age, mean (SD) | 79.1 (8.3) | 78.6 (7.9) | 77.6 (7.6) | 78.7 (7.9) | 76.8 (7.3) | 77.9 (7.6) | 81.1 (8.7) |
| Female | 24,729 (55.7%) | 9,350 (52.5%) | 11,789 (47.9%) | 2,119 (50.5%) | 1,727 (45.4%) | 1,099 (48.5%) | 1,683 (57.7%) |
| Race/Ethnicity | |||||||
| Non-Hispanic White | 34,518 (77.7%) | 14,783 (82.9%) | 20,182 (82.0%) | 3,413 (81.4%) | 3,056 (80.4%) | 1,872 (82.6%) | 2,315 (79.4%) |
| Non-Hispanic Black | 5,925 (13.3%) | 1,709 (9.6%) | 2472 (10.0%) | 458 (10.9%) | 483 (12.7%) | 239 (10.5%) | 328 (11.2%) |
| Hispanic | 1,414 (3.2%) | 511 (2.9%) | 681 (2.8%) | 114 (2.7%) | 116 (3.1%) | 57 (2.5%) | 98 (3.4%) |
| Asian | 854 (1.9%) | 326 (1.8%) | 461 (1.9%) | 70 (1.7%) | 59 (1.6%) | 34 (1.5%) | 64 (2.2%) |
| Other | 1,689 (3.8%) | 495 (2.8%) | 818 (3.3%) | 138 (3.3%) | 89 (2.3%) | 64 (2.8%) | 111 (3.8%) |
| Medical History | |||||||
| Prior Stroke | 8,607 (19.4%) | 3,539 (19.9%) | 5,319 (21.6%) | 996 (23.8%) | 969 (25.5%) | 522 (23.0%) | 658 (22.6%) |
| Prior TIA | 4,118 (9.3%) | 2,016 (11.3%) | 2,842 (11.5%) | 503 (12.0%) | 457 (12.0%) | 243 (10.7%) | 240 (8.2%) |
| CAD/Prior MI | 12,868 (29.0%) | 5,249 (29.4%) | 9,024 (36.7%) | 1,429 (34.1%) | 1,658 (43.6%) | 789 (34.8%) | 905 (31.0%) |
| Carotid Stenosis | 1,904 (4.3%) | 785 (4.4%) | 1,617 (6.6%) | 241 (5.7%) | 213 (5.6%) | 103 (4.5%) | 109 (3.7%) |
| Diabetes Mellitus | 15,991 (36.0%) | 6,556 (36.8%) | 9,621 (39.1%) | 1,646 (39.3%) | 1,424 (37.4%) | 773 (34.1%) | 1,000 (34.3%) |
| PVD | 2,126 (4.8%) | 832 (4.7%) | 1,499 (6.1%) | 243 (5.8%) | 322 (8.5%) | 142 (6.3%) | 154 (5.3%) |
| Hypertension | 37,145 (83.7%) | 14,966 (84.0%) | 20,995 (85.3%) | 3,586 (85.5%) | 3,173 (83.4%) | 1,907 (84.2%) | 2,405 (82.5%) |
| Smoking | 4,537 (10.2%) | 1,760 (9.9%) | 2,858 (11.6%) | 434 (10.4%) | 432 (11.4%) | 185 (8.2%) | 223 (7.6%) |
| Dyslipidemia | 24,724 (55.7%) | 10,788 (60.5%) | 15,316 (62.2%) | 2,555 (60.9%) | 2,326 (61.2%) | 1,343 (59.3%) | 1,512 (51.9%) |
| Heart Failure | 3,424 (7.7%) | 1,226 (6.9%) | 1,840 (7.5%) | 283 (6.7%) | 580 (15.3%) | 257 (11.3%) | 298 (10.2%) |
| Chronic Kidney Disease | 4,055 (5.1%) | 1,593 (8.9%) | 2,218 (9.0%) | 370 (8.8%) | 392 (10.3%) | 175 (7.7%) | 286 (9.8%) |
| NIHSS | |||||||
| Median (IQR) | 3 (1,6) | 3 (1,7) | 2 (1,5) | 2 (1,5) | 3 (1,8) | 3 (1,6) | 6 (2,14) |
| 0–3 | 20,842 (46.9%) | 9,735 (54.6%) | 13,540 (55.0%) | 2,142 (51.1%) | 1,722 (45.3%) | 1,165 (51.4%) | 840 (28.8%) |
| 4–9 | 10,375 (23.4%) | 4,128 (23.2%) | 5,704 (23.2%) | 910 (21.7%) | 866 (22.8%) | 509 (22.5%) | 659 (22.6%) |
| ≥10 | 6,377 (14.4%) | 1,759 (9.9%) | 2,374 (9.6%) | 396 (9.4%) | 639 (16.8%) | 342 (15.1%) | 835 (28.6%) |
| Missing NIHSS | 6,806 (15.3%) | 2,202 (12.4%) | 2,996 (12.2%) | 745 (17.8%) | 576 (15.1%) | 250 (11.0%) | 582 (20.0%) |
Abbreviations: DAPT: Dual Antiplatelet Therapy; OAC: Oral Anticoagulant; NOAC: Non-Vitamin K Antagonist Oral Anticoagulant
Table 2.
Factors Associated with Escalation of Discharge Antithrombotic Medication Among Ischemic Stroke Patients with Aspirin Failure*
| Variable | Odds Ratio (95% CI) | P value |
|---|---|---|
| Demographics | ||
| Age, per one year increase | 0.98 (0.98–0.98) | <0.001 |
| Female | 0.85 (0.82–0.87) | <0.001 |
| Race/ethnicity | ||
| Asian vs. non-Hispanic White | 0.92 (0.83–1.02) | 0.59 |
| Black vs. non-Hispanic White | 0.85 (0.81–0.89) | 0.02 |
| Hispanic vs. non-Hispanic White | 0.85 (0.79–0.92) | 0.12 |
| Other vs. non-Hispanic White | 0.88 (0.81–0.96) | 0.54 |
| Medical History | ||
| Prior Stroke | 1.17 (1.13–1.21) | <0.001 |
| Prior TIA | 1.20 (1.15–1.25) | <0.001 |
| CAD/Prior MI | 1.30 (1.27–1.34) | <0.001 |
| Carotid Stenosis | 1.22 (1.15–1.30) | <0.001 |
| Diabetes Mellitus | 1.00 (0.97–1.03) | 0.83 |
| PVD | 1.18 (1.11–1.25) | <0.001 |
| Hypertension | 1.07 (1.03–1.11) | <0.001 |
| Smoking | 0.97 (0.93–1.01) | 0.17 |
| Dyslipidemia | 1.11 (1.08–1.15) | <0.001 |
| Heart Failure | 1.16 (1.10–1.22) | <0.001 |
| Chronic Kidney Disease | 0.93 (0.86–0.98) | 0.004 |
| NIHSS | ||
| 4–9 vs. 0–3 | 0.89 (0.87–0.92) | <0.001 |
| ≥10 vs. 0–3 | 0.68 (0.65–0.71) | <0.001 |
From no antithrombotics, to single antiplatelet [aspirin or clopidogrel monotherapy], dual antiplatelet therapy of aspirin and clopidogrel [DAPT], and anticoagulant with or without antiplatelet therapy
Discussion
In this study, three broad categories of treatment made up the bulk of discharge antithrombotic therapy: aspirin monotherapy, clopidogrel monotherapy, and DAPT. Although current guidelines recommend any of these medications for secondary stroke prevention, there are no data to indicate whether one strategy provides additional protection against future ischemic stroke.
The relatively high frequency of discharge on aspirin/clopidogrel DAPT likely reflects practice based on the CHANCE and POINT trials7,8. However, many DAPT patients had a NIHSS greater than 3, indicating that there is still significant heterogeneity of discharge therapy in this cohort of patients. Future studies are needed to evaluate off-guideline treatment with DAPT for secondary prevention in patients with aspirin failure or larger strokes.
Our study has limitations. First, study findings may not be generalizable beyond Medicare ischemic stroke survivors in the GWTG-Stroke registry. Second, there is a risk of selection bias due to lack of aspirin dose information prior to admission. It is possible that some patients with low dose aspirin prior to stroke switch to high dose aspirin at discharge. Third, the GWTG-Stroke registry only collects medication at discharge but does not document reasons for specific antithrombotic medication prescribed.
In conclusion, there are multiple prevalent antithrombotic treatment strategies for secondary stroke prevention in patients with aspirin treatment failure. Future research is needed to evaluate the efficacy and safety of various treatment strategies for this complex but common clinical scenario.
Supplementary Material
Funding Sources:
The GWTG-Stroke program is provided by the American Heart Association/American Stroke Association. GWTG-Stroke is sponsored, in part, by Novartis, Boehringer Ingelheim Lilly, Novo Nordisk, Sanofi, AstraZeneca, Bayer and Portola Pharmaceuticals. Research reported in this publication was supported by the National Institute On Aging of the National Institutes of Health under Award Number R01AG062770. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures:
Mr. Lusk, Ms Xu, Dr. Matsouaka have no relevant disclosures.
Dr. Peterson discloses research Grant from Janssen, Genetech, BMS, AHA and consultant/advisory Board: Janssen, Boehringer Ingelhiem, Sanofi, Bayer, Merck, Astra Zeneca, Signal Path, and Venable. Personal fees from Novartis.
Dr. Deepak L. Bhatt discloses the following relationships - Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Lexicon, Lilly, Medtronic, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, The Medicines Company, 89Bio; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Philips, Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Takeda.
Dr Fonarow reports grants from American Heart Association and grants from Patient Centered Outcome Research Institute outside the submitted work; and Employee of UC Regents that have a patent on an endovascular device.
Dr. Smith reports consulting fees from Bayer.
Dr. Schwamm reports work as a scientific consultant regarding trial design and conduct to Genentech (late window thrombolysis) and Member of steering committee (TIMELESS NCT03785678); consultant on user interface design and usability to LifeImage; member of a Data Safety Monitoring Boards (DSMB) for Penumbra (MIND NCT03342664) and for Diffusion Pharma (PHAST-TSC NCT03763929); Serving as National PI for Stroke AF, Medtronic (NCT02700945); National Co-PI, late window thrombolysis trial, NINDS (P50NS051343, MR WITNESS NCT01282242; and alteplase provided free of charge to Massachusetts General Hospital as well as supplemental per-patient payments to participating sites by Genentech); Site PI, StrokeNet Network NINDS (New England Regional Coordinating Center U24NS107243)
Dr. Xian discloses research support from National Institute on Aging R01AG062770, the American Heart Association, Genentech, Daiichi Sankyo, Janssen Pharmaceuticals; honoraria from Boehringer Ingelheim and Portola.
Non-Standard Abbreviations and Acronyms
- CHANCE
Clopidogrel with Aspirin in Acute Minor Stroke or Transient Ischemic Attack trial
- DAPT
Dual antiplatelet therapy
- GWTG
Get With The Guidelines®
- NIHSS
National Institutes of Health Stroke Scale
- NOAC
Non-vitamin K antagonist oral anticoagulants
- POINT
Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke trial
- RECORD
Reporting of Studies Conducted Using Observational Routinely-Collected Health Data
Footnotes
References:
- 1.O’Brien CW, Juraschek SP, Wee CC. Prevalence of Aspirin Use for Primary Prevention of Cardiovascular Disease in the United States: Results From the 2017 National Health Interview Survey. Ann. Intern. Med 2019;171:596–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansa BE, Hoffman Z, Lewis N, Savoy C, Hickson A, Stone R, Johnson T. Aspirin Use among Adults with Cardiovascular Disease in the United States: Implications for an Intervention Approach. J. Clin. Med [Internet]. 2019. [cited 2020 Oct 29];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6406947/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee M, Saver JL, Hong K-S, Rao NM, Wu Y-L, Ovbiagele B. Antiplatelet Regimen for Patients With Breakthrough Strokes While on Aspirin: A Systematic Review and Meta-Analysis. Stroke. 2017;48:2610–2613. [DOI] [PubMed] [Google Scholar]
- 4.Powers William J., Rabinstein Alejandro A., Ackerson Teri, Adeoye Opeolu M, Bambakidis Nicholas C, Becker Kyra, Biller José, Brown Michael, Demaerschalk Bart M., Hoh Brian, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. [DOI] [PubMed] [Google Scholar]
- 5.Schwamm LH, Fonarow GC, Reeves MJ, Pan W, Frankel MR, Smith EE, Ellrodt G, Cannon CP, Liang L, Peterson E, et al. Get With the Guidelines-Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation. 2009;119:107–115. [DOI] [PubMed] [Google Scholar]
- 6.Xian Y, Fonarow GC, Reeves MJ, Webb LE, Blevins J, Demyanenko VS, Zhao X, Olson DM, Hernandez AF, Peterson ED, et al. Data quality in the American Heart Association Get With The Guidelines-Stroke (GWTG-Stroke): results from a national data validation audit. Am. Heart J 2012;163:392–398, 398.e1. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, et al. Clopidogrel with Aspirin in Acute Minor Stroke or Transient Ischemic Attack. N. Engl. J. Med 2013;369:11–19. [DOI] [PubMed] [Google Scholar]
- 8.Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, Kim AS, Lindblad AS, Palesch YY. Clopidogrel and Aspirin in Acute Ischemic Stroke and High-Risk TIA. N. Engl. J. Med 2018;379:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


