Abstract
The liver is an essential organ that produces several serum proteins, stores vital nutrients, and detoxifies many carcinogenic and xenobiotic compounds. Various growth factors positively regulate liver growth, but only a few negative regulators are known. Among the latter are the transforming growth factor β (TGF-β) superfamily members TGF-β1 and activin A. To study the function of novel activin family members, we have cloned and generated mice deficient in the activin βC and βE genes. Expression analyses demonstrated that these novel genes are liver specific in adult mice. Here, we show by RNase protection that activin βC transcripts are present in the liver beginning at embryonic day 11.5 (E11.5) whereas activin βE expression is detected starting from E17.5. Gene targeting in embryonic stem cells was used to generate mice with null mutations in either the individual activin βC and βE genes or both genes. In contrast to the structurally related activin βA and βB subunits, which are necessary for embryonic development and pituitary follicle-stimulating hormone homeostasis, mice deficient in activin βC and βE were viable, survived to adulthood, and demonstrated no reproductive abnormalities. Although activin βC and βE mRNAs are abundantly expressed in the liver of wild-type mice, the single and double mutants did not show any defects in liver development and function. Furthermore, in the homozygous mutant mice, liver regeneration after >70% partial hepatectomy was comparable to that in wild-type mice. Our results suggest that activin βC and βE are not essential for either embryonic development or liver function.
Growth factors and hormones play an extremely important role in regulating biological processes from patterning of the early embryo to regulating the function of tissues and organs. The largest family of growth factors is the transforming growth factor β (TGF-β) superfamily of secreted dimeric proteins (12). Members of this family include activins, TGF-βs, bone morphogenetic proteins (BMPs), and growth differentiation factors and demonstrate diverse functions including roles in left-right asymmetry, skeletal development, reproduction, and oncogenesis (29, 52). Both activins (β-β dimers) and inhibins (α-β dimers) have historically been shown to be regulators of follicle-stimulating hormone (FSH) secretion from the pituitary gland (54). These earlier results were later confirmed by in vivo analysis of activin βB-, activin receptor type IIA (ActRIIA)-, and α-inhibin-deficient mice (32–34). In studies involving Xenopus laevis oocytes (36), activins were tested for their ability to induce mesoderm formation. However, this observation is not true for mice (35), suggesting that the results obtained with X. laevis oocyte injection experiments may be due to nonphysiological effects of the activin ligands.
The adult liver detoxifies the blood through the actions of various enzymes, synthesizes normal serum proteins such as the acute-phase proteins and albumin, and produces bile, which is critical for normal fat absorption (9). Activin A and TGF-β1 have been shown to affect liver growth and function. These two proteins can inhibit mitogen-induced DNA synthesis in hepatocytes (5, 41, 51, 57), induce hepatocellular apoptosis in vitro (7, 21, 42, 48), and stimulate glycogenolysis from cultured hepatocytes (39). In vivo, pharmacological levels (e.g., intravenous infusion of recombinant activin A [21, 48]) or pathophysiologically high levels of activins (8, 30, 32) cause a reduction in liver mass by inducing hepatocellular necrosis around the central vein.
Liver regeneration occurs following liver injury that results in loss of liver mass. The liver regenerates by a process of hypertrophy and a near-synchronous proliferation of the remaining cells through several cycles of replication. Several cytokines are thought to play early roles in the regeneration process: interleukin-1 (IL-1), IL-6, and tumor necrosis factor alpha (reviewed in reference 38). IL-6 has been shown to play a critical role in the progression of liver regeneration but not for initiation (10). While the interleukins and tumor necrosis factor alpha are thought to be important during the early stages of liver regeneration, TGF-β1 to -3 and activins are thought to be negative regulators during this process (27, 38), since mRNAs encoding these ligands are up-regulated during this process (3, 57). However, this latter hypothesis has not yet been tested in vivo.
Recently, our groups (15, 28) and others (19, 43, 47) have cloned three new members of the TGF-β superfamily which demonstrate highest amino acid identity in the mature peptide region to the activin (βA and βB) subfamily. Two of these new members, designated activin βC (actβC) and activin βE (actβE), have been cloned in mammals (15, 19, 28, 47), while the third, activin βD, has been found only in X. laevis (43). Weak induction of a secondary axis has been observed when activin βD mRNA is injected into the ventral blastomeres of Xenopus embryos (43), but activin βC and βE have not yet been functionally tested by any in vitro or in vivo bioassays. Expression of actβC and actβE is primarily liver specific in the adult (14, 28, 47), unlike activin βA and activin βB, which are widely expressed in multiple tissues in rodents (16, 37) and humans (53).
Based on the highly restricted tissue expression pattern, we hypothesized that activins βC and βE may play critical roles in liver physiology. To compare the in vivo functions of these novel liver-restricted mammalian activin βC and βE genes to those of the known activin βA and βB genes, we generated null mutations in actβC, actβE, or both genes in mice. Our studies show that activin βC and βE are not essential for liver development, liver function, or reproduction.
MATERIALS AND METHODS
Construction of targeting vectors and generation of mutant mice.
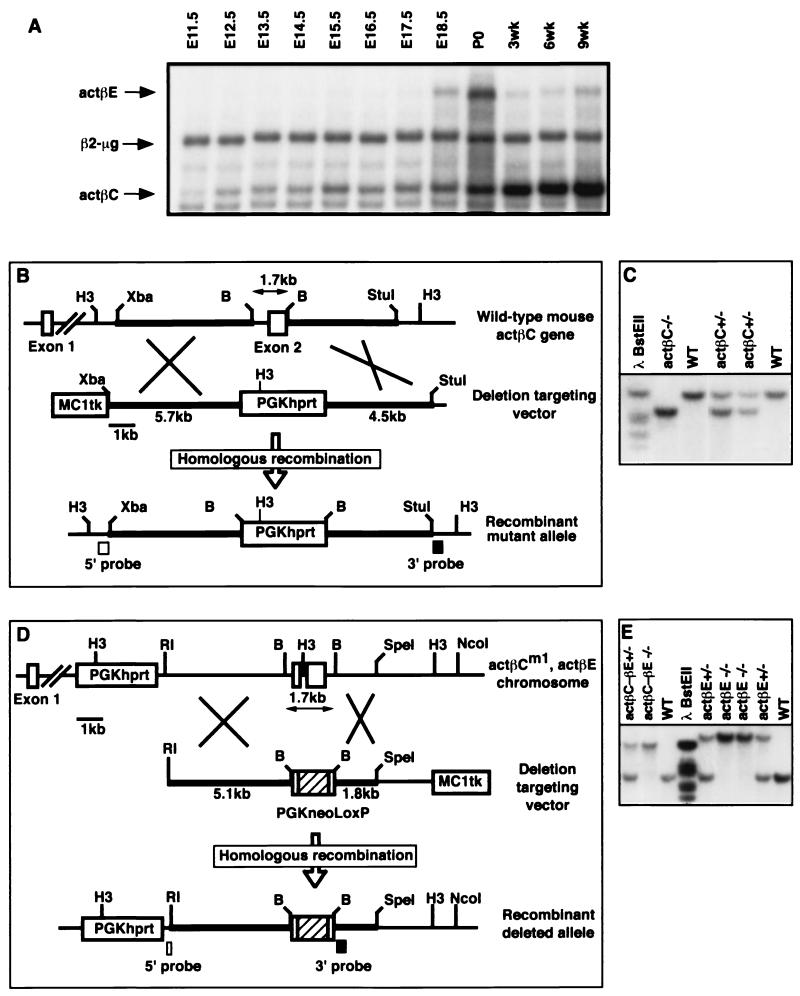
The replacement targeting vector for actβC contained 5.7 kb of sequence for the 5′ homology arm, a PGKhprt selectable marker cassette which replaced a 1.7-kb BamHI-BamHI region of the locus including exon 2 of the activin βC gene, 4.5 kb of sequence for the 3′ homology arm, and an MC1tk expression cassette for negative selection (Fig. 1B). Twenty-five micrograms of the KpnI-linearized targeting vector was electroporated into 107 AB2.1 embryonic stem (ES) cells (a gift of Allan Bradley, Baylor College of Medicine). ES cells were then selected in medium containing hypoxanthine-aminopterin-thymidine and 1-(2′-deoxy-2′-fluoro-β-d-arabinofuranosyl)-5′-iodouracil (FIAU). Culturing of ES cells and collection and injection of blastocysts have been previously described (33). For genomic Southern blot analysis, HindIII-digested DNA was transferred to a GeneScreen Plus nylon membrane (NEN Life Science Products, Boston, Mass.) and probed with an external ∼300-bp NcoI-XbaI fragment (5′ probe). An external ∼380-bp StuI-BamHI fragment (3′ probe) was also used to distinguish the wild-type and activin βC null (actβCm1) alleles (Fig. 1B).
FIG. 1.
Expression of activin βC and activin βE in the livers of embryos and adult mice and gene targeting constructs for activins βC and βE. (A) Expression of actβC and actβE in mouse embryonic and adult livers was detected using RNase protection assays. β2-Microglobulin was used as the internal control. Five micrograms of total RNA from C57BL/6 mice was hybridized to probes specific for activin βC, activin βE, and β2-microglobulin. Shown is a representative autoradiograph repeated in four independent experiments. (B) The targeting strategy used to delete exon 2 of the mouse activin βC gene. The construct contains an MC1tk expression cassette for negative selection and a PGKhprt expression cassette for positive selection. Homologous recombination will delete all of the coding sequence in exon 2. (C) Southern blot analysis of tail DNA from F2 mice at weaning. The 3′ probe identifies the wild-type 13.8-kb band and the 8.3-kb mutant band. (D) The targeting construct to delete all of the coding exons of the actβE gene. Homologous recombination within the homology arms will replace the entire coding region of activin βE with a floxed (vertical-box-flanked) PGKneo-positive selectable marker cassette. The MC1tk cassette is used for negative selection. Due to the close physical proximity of activin βC and βE loci on the same chromosome, ES cells carrying the actβCm1 mutation (cell line βC45-F1, which was used previously for germ line transmission of actβCm1) were electroporated. (E) Southern blot analysis showing weaned F2 mice homozygous for the actβEm1 mutation (19.2-kb band) or the double mutant allele (actβCm1-actβEm1; 13.8-kb band). The wild-type band migrates at 5.3 kb. H3, HindIII; B, BamHI; Xba, XbaI; RI, EcoRI; WT, wild type.
The actβE targeting vector was composed of a 4.85-kb EcoRI-BamHI fragment as the 5′ homology arm, a loxP-PGKneo-loxP selectable marker cassette (a gift from Richard Behringer, University of Texas-M. D. Anderson Cancer Center) that replaced a 1.7-kb BamHI-BamHI region of the locus including the entire coding region of activin βE, a 1.8-kb BamHI-SpeI fragment as the 3′ homology arm, and an MC1tk selectable marker cassette. This vector was linearized with KpnI, and 25 μg of the linearized plasmid was electroporated into 107 βC45-F1 cells, an AB2.1 ES cell line heterozygous for the actβCm1 allele, which was generated as described above. The mutant ES cells were selected in G418 and FIAU. HindIII, which distinguishes the various targeted and wild-type alleles, was used as the diagnostic restriction enzyme. The probes were a 5′ external ∼250-bp BamHI-EcoRI fragment and an internal ∼500-bp BamHI-XhoI fragment (Fig. 1D).
Chimeras were generated by blastocyst injections of the mutant ES cells, per standard methods (33). Chimeric males were mated to C57BL/6 females for the generation of F1 hybrid mice or to 129S5/SvEvBrd female mice to generate F1 inbred mice.
RNA isolation.
Timed matings of wild-type C57BL/6 mice were established, and embryonic livers were collected at various time points for RNA expression analysis. RNA collected at embryonic day 11.5 (E11.5) was liver enriched by using only trunk tissue (i.e., rostral and caudal tissues were excluded). Adult tissues from wild-type 129/SvEv-C57BL/6 hybrid mice were also collected. When necessary, tissues from several mice were pooled. Tissues were immediately homogenized in RNA STAT-60 (Leedo Medical Laboratories, Houston, Tex.) using a Tissue Tearor electronic homogenizer (Biospec Products, Bartlesville, Okla.). RNA was extracted per Leedo Medical Laboratories' instructions and stored under 70% ethanol at −80°C until required. Some samples were stored in RNA Later (Ambion, Austin, Tex.) until required for processing in accordance with the manufacturer's instructions.
RNase protection assay.
Probe plasmids were obtained to analyze the RNA expression levels of actβC, actβE, β-actin (Ambion), and junB (Ambion) and β2-microglobulin levels using RNase protection assays. β2-Microglobulin was used as a quantitative control since its expression is constant during liver regeneration (17). The plasmids containing probe DNA fragments generated in the laboratory were sequenced bidirectionally for accuracy. The mouse activin βC probe was composed of a BamHI-EcoRI fragment from the 3′ untranslated region (+1184 to +1434 of the cDNA; GenBank accession no. U40773) cloned from a 129/SvEv genomic library as previously described (28). The mouse activin βE probe fragment was derived from exon 2 (+750 to +972 of the cDNA; GenBank accession no. U96386) and was generated using PCR primers actβE-F2 (5′-GAGACCACTATGTAGACTTCC) and actβE-R4 (5′-AGAGAGAGGCCTTCGTGCAGT). Two different mouse β2-microglobulin probes were used. For embryonic studies, the β2-microglobulin probe β2-MG3 (+1 to +317 of the cDNA) was derived from pHuActβ2m (a gift from Elizabeth Bikoff, Harvard University [2]). For all other experiments, a second β2-microglobulin probe (β2-MG5, +114 to +306 of the cDNA; GenBank accession no. X01838) was isolated by reverse transcription-PCR using forward primer β2-MGF1/H3 (5′-ACTGCAAGCTTAACACAG-3′), which inserts a HindIII site at the SnaBI site, and the reverse primer β2-MGR1 (5′-TAACTCTGCAGGCGTATG-3′).
Linearized probe plasmids were transcribed in vitro to produce antisense RNA using the Ribomax in vitro transcription kit (Promega, Madison, Wis.). Since some messages are more abundant than others, cold UTP was added to all reactions to limit the protected band intensities to approximately the same range. The amount of cold UTP added was as follows: actβC, actβE, and junB, 0.1 nmol of cold UTP; β-actin and β2-microglobulin, 2.5 nmol of cold UTP. The [α-32P]UTP-radiolabeled RNA probes were purified by gel electrophoresis and eluted at 37°C overnight in elution buffer as described by the manufacturer (RPAII kit; Ambion).
The RPAII RNase protection assay kit (RPAII kit) was used as described by the manufacturer with minor modifications. In brief, 2.5 μl of total RNA (2 μg/μl) and 3 μl of probe mix were added to the hybridization buffer. The mixture was denatured at 95°C for 3 min. The reaction mixtures were then incubated overnight at 45°C followed by digestion with a 1:100 dilution of the RNase cocktail, containing RNase A and RNase T1, at 37°C for 1 h. The protected RNA-RNA hybrids were precipitated and separated on a nondenaturing 5% acrylamide gel except for the junB probe, which was separated on an 8% acrylamide gel. Gels were vacuum dried at 80°C, exposed, and visualized by autoradiography.
Following autoradiography, dried gels were also exposed to a Storage Phosphor Screen (Molecular Dynamics, Sunnyvale, Calif.). Exposed screens were then read with a Storm 860 scanner (Molecular Dynamics), and resulting images were analyzed by ImageQuaNT version 4.2a software (Molecular Dynamics). Data, following integration, were analyzed by Student's t test using Microsoft Excel98 (Microsoft Corp., Redmond, Wash.) for statistical significance. A P value of <0.05 was considered significant for all statistical analyses. For RNase protection assays of RNA collected after partial hepatectomy, a minimum of three mice were used at each time point, and each sample was analyzed in duplicate.
Serum analysis.
Adult 129/SvEv-C57BL/6 hybrid mice at 42 to 45 days were anesthetized using Metofane (Schering-Plough Animal Health, Union, N.J.), and blood was obtained by closed cardiac puncture. Serum was separated in Microtainer tubes (Becton Dickinson, Franklin Lakes, N.J.) and stored at −20°C. Serum samples were analyzed for alanine aminotransferase, aspartate aminotransferase, albumin, random glucose, and total protein by the Comparative Pathology Laboratory (Baylor College of Medicine) on a Roche Cobas MIRA analysis machine. Serum FSH levels were measured by a rat FSH radioimmunoassay with a sensitivity of 10 ng/ml using a National Institute of Diabetes and Digestive and Kidney Diseases kit (National Hormone and Pituitary Distribution Program, National Institute of Diabetes and Digestive and Kidney Disease, National Institutes of Health) as previously described (26). Sera from five to six male mice of each genotype were analyzed for FSH levels.
Morphological and histological analysis.
Mice were anesthetized and sacrificed by cervical dislocation, and tissues were harvested immediately. The wet weights of relevant tissues from mice 42 to 45 days of age were recorded. For testis weights, 11 to 37 mice were analyzed per genotype. All tissues except for testis were fixed overnight at 4°C in 10% buffered formalin (pH 7.2). The testis samples were fixed overnight in Bouin's reagent and washed extensively in 70% ethanol. In some cases, prostate glands were also collected from >1-year-old mice and placed into formalin. The fixed tissue was then embedded in paraffin and sectioned at 5 μm for staining in hematoxylin and eosin for the liver. The ovaries and testes were stained with hematoxylin–periodic acid-Schiff reagent. Embedding and staining were performed per standard procedure by the Baylor College of Medicine Pathology Core Services Laboratory.
Partial hepatectomy.
The partial hepatectomy procedure was performed as described previously with minor modifications (18). Adult 129 × Sv/Ev-C57BL/6 hybrid female mice, 9 to 12 weeks of age and weighing 18 to 22 g, were included in the experiment. IsoFlo (isoflurane; Abbott Laboratories, North Chicago, Ill.) was used as the anesthetic. The partial hepatectomy procedure removes the left medial, right medial, and left lateral lobes. The right half of the medial lobe was removed with one ligature. Both the left half of the medial lobe and the left lateral lobe were removed with a second ligature. During the procedure, care was taken to prevent damage to the gall bladder and the surrounding ducts. Only mice that had >70% of the liver removed were used in our analyses.
RESULTS
Expression of two novel activins in the embryonic liver.
The cloning and tissue expression patterns of the actβC and actβE genes in adult mice have been previously reported by us and others (14, 28, 47). Since both genes appear to be primarily liver specific, we analyzed the temporal expression profiles of actβC and actβE in the embryonic liver using RNase protection (Fig. 1A). Expression of actβC was readily detectable by E11.5 and reached a maximum in the adult at 9 weeks. In contrast, actβE was first detectable at E17.5 and appeared to be maximally expressed at birth (Fig. 1A).
Targeting of the actβC and actβE genes.
To determine the function of activin βC and activin βE, we generated ES cell lines containing single mutations in each of the two genes and a linked (double) mutation in both genes. A serial targeting strategy was employed since the actβC and actβE genes are separated by 5.5 kb on mouse chromosome 10 (14, 47). Initial targeting deleted the entire coding region of actβC exon 2, which encodes the mature (active) peptide domain (actβCm1), to produce a putative null allele (Fig. 1B and C). For the second targeting event, in which a putative null mutation in activin βE (actβEm1) was generated, we used ES cells carrying the actβCm1 allele instead of wild-type ES cells (Fig. 1D and E). Targeting of the actβCm1 heterozygous cell line with the activin βE targeting vector would generate two sets of chromosomes, a cis set and a trans set, with respect to the actβCm1 and actβEm1 alleles. If targeting of the actβE locus occurs on the same chromosome as the previously targeted actβC locus, then a cis set of chromosomes will be generated (actβCm1, actβEm1/+, +), eventually allowing us to generate double-homozygous mutant mice. Conversely, if targeting of the actβE locus occurred on the wild-type chromosome (trans; actβCm1, +/+, actβEm1), then the two mutations segregated and permitted us to generate homozygous mice carrying only the actβEm1 allele. The actβEm1 allele is also predicted to be a null allele since both coding exons have been replaced (Fig. 1D). Utilizing the above strategy, these constructs were electroporated into ES cells, and subsequently, chimeric mice were generated. Germ line transmission of two cell lines for each mutation (actβCm1, actβEm1, and the double mutant actβCm1-actβEm1) was achieved (Fig. 1C and D).
General phenotypes.
Heterozygous mice were intercrossed, and all lines were bred separately. The offspring from the heterozygous crosses were genotyped at 3 weeks of age, and all lines genotyped showed the expected Mendelian ratio of 1:2:1 (Table 1). Therefore, activin βC and/or activin βE is not required during embryogenesis. At 42 days, homozygous mutant mice from all three mutations were grossly indistinguishable from their control littermates, indicating that growth and development were unaffected by the individual or combined null mutations.
TABLE 1.
Genotype distribution from crosses of various mutant mice
| Mutant cell line | Data for mouse group
|
|||||
|---|---|---|---|---|---|---|
| +/− × +/−
|
−/− × −/−; avg litter size (total progeny/ litter no.)b | |||||
| No. with genotype (%)
|
Avg litter size (total progeny/litter no.)b | Male/female ratio (no. male/female) | ||||
| Wild type | Heterozygous | Homozygous | ||||
| βC45-F1a | 92 (26.5) | 159 (46.0) | 95 (27.5) | 9.18 (349/38) | 1.06 (181/171) | 8.03 (233/29) |
| βE82-A3 | 81 (27.1) | 152 (50.8) | 66 (22.1) | 8.82 (300/34) | 1.20 (163/136) | 8.65 (173/20) |
| βE82-C12 | 58 (25.9) | 117 (52.2) | 49 (21.9) | 8.30 (224/27) | 0.95 (109/114) | 7.20 (123/17) |
| βCβE82-A9 | 72 (29.9) | 110 (45.6) | 59 (24.5) | 8.03 (241/30) | 0.85 (111/130) | —c |
| βCβE82-G12 | 64 (25.8) | 128 (51.6) | 56 (22.6) | 8.55 (248/29) | 0.89 (116/130) | 7.40 (140/19) |
Data for βC46-H3 are not shown due to small sample size and few F2 progeny.
Total litter size was used, including nongenotyped mice.
—, not determined.
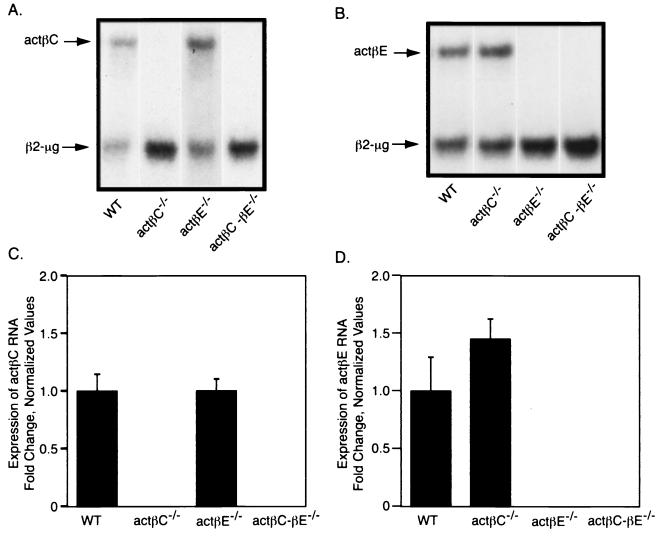
Since actβC and actβE are closely linked on the same chromosome, the integration of a selectable marker cassette into one locus could have effects on the transcription of the other locus. We examined the expression of both genes in the actβCm1/actβCm1, actβEm1/actβEm1, and double-homozygous mutant mice. The expression of actβC was not changed in actβEm1/actβEm1 mice (Fig. 2A and C). However, there appeared to be a slight elevation of actβE message in the actβCm1/actβCm1 mice, although the difference was not statistically significant (P > 0.22) when data were quantitated because of the high variability of expression of actβE in wild-type mice (Fig. 2B and D). Homozygous mutant mice lacked any detectable expression from the corresponding gene, further demonstrating that our gene targeting strategy produced actβC and actβE null alleles.
FIG. 2.
Analysis of activin βC and βE expression in actβCm1/actβCm1, actβEm1/actβEm1, and actβCm1-actβEm1 homozygous mice. RNase protection was used to examine the expression of actβC (A) and actβE (B) RNA expression in actβCm1/actβCm1, actβEm1/actβEm1, and actβCm1-actβEm1 homozygous mutant mice. β2-Microglobulin was used as the internal control. Representative lanes from a single experiment are shown. Phosphorimaging was used to quantitate the expression of actβC (C) and actβE (D). All values were normalized to the average ratio of activin subunit expression to β2-microglobulin expression in wild-type mice. The bars show means ± standard errors of the means. Values were not statistically significant (P > 0.05) compared to the wild type. n = 5 for all genotypes. WT, wild type; actβC, activin βC; actβE, activin βE; actβC-βE, activin βC-βE; β2-μg, β2-microglobulin.
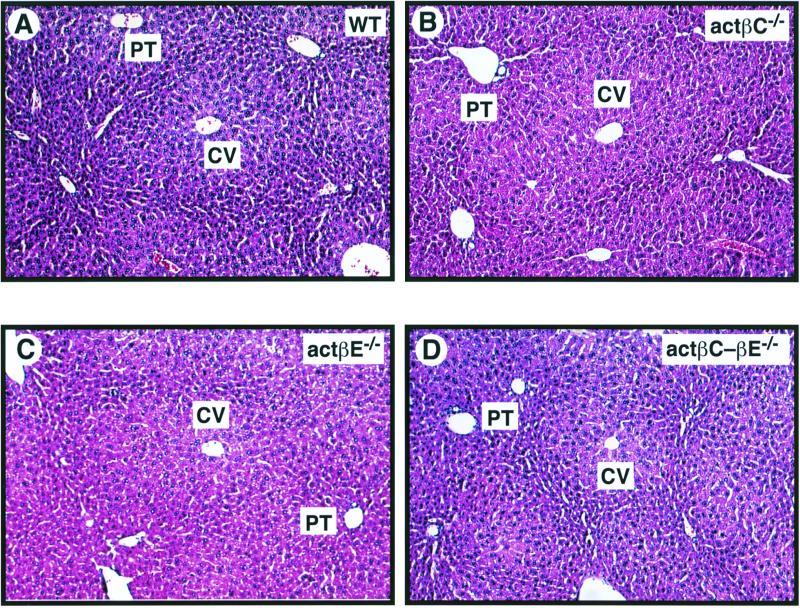
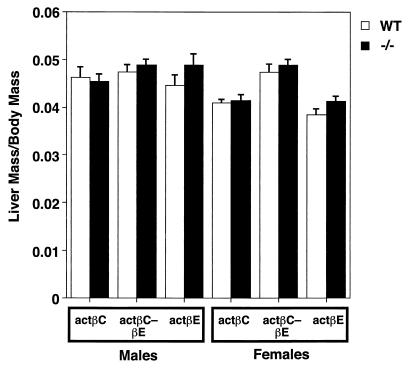
Despite the restricted expression pattern of both actβC and actβE genes, liver lobular morphology and histology appeared normal at the light microscopy level in the homozygous mutant mice (Fig. 3). We analyzed if there were changes in liver growth in the null mice by comparing the ratio of wet liver mass to body mass. Even though related members such as TGF-β1 and activin A have been implicated in growth regulation of hepatocytes (5, 7, 21, 41, 42, 48, 51, 57), mice deficient in either actβC or actβE or both show no statistically significant differences in liver mass/body mass ratio compared to their wild-type littermates (Fig. 4).
FIG. 3.
Histological analysis of livers from 42-day-old wild-type and null mice. Liver tissue from C57BL/6-129/SvEv hybrid mice was formalin fixed and stained with hematoxylin and eosin. The liver histology of the homozygous mutant mice compared to that of wild-type mice showed the expected liver lobular organization of the hepatic parenchyma. All sections were photographed at a magnification of ×100. (A) Wild-type mouse (WT); (B) actβCm1/actβCm1 mouse (actβC−/−); (C) actβEm1/actβEm1 mouse (actβE−/−); (D) actβCm1-actβEm1 (actβC-βE−/−) homozygous mutant mouse. CV, central vein; PT, portal triad.
FIG. 4.
Liver mass/body mass ratios of male and female knockout mice compared to those of wild-type littermates at 42 to 45 days of age. Each point represents the mean ± standard error of the mean. There was no ratio from the homozygous mutant groups that statistically differed significantly from the wild-type ratio (P > 0.05). Mice analyzed in this study are as follows: activin βC, wild-type males, n = 7; actβCm1/actβCm1 males, n = 16; wild-type females, n = 6; actβCm1/actβCm1 females, n = 5; activin βE, wild-type males, n = 9, actβEm1/actβEm1 males, n = 13; wild-type females, n = 7; actβEm1/actβEm1 females, n = 15; actβCm1-actβEm1, wild-type males, n = 10; actβCm1-actβEm1 −/− males, n = 17; wild-type females, n = 6; actβCm1-actβEm1 −/− females, n = 12.
Liver function analysis.
Although there appeared to be no morphological or histological differences between the wild-type and the homozygous mutant mice, a defect in liver function could still exist. Activin A has been shown to affect glycogenolysis in cultured hepatocytes (39). To test whether the liver-restricted activin βC and activin βE genes may function similarly in the liver, various components in the serum obtained from 42-day-old male and female mice were analyzed (Table 2). Markers for hepatocellular damage, such as alanine aminotransferase and aspartate aminotransferase, were not significantly different between wild-type mice and null mice. Female actβCm1/actβCm1 mice demonstrated a significant decrease in serum albumin levels (P < 0.04). In contrast, both actβCm1/actβCm1 males and actβCm1-actβEm1 double homozygous females have normal albumin levels. There were no differences in random glucose levels among the groups (Table 2). These results suggest that adult mice at 42 days lacking activin βC and βE have normal liver function.
TABLE 2.
Serum analysis of wild-type and homozygous mutant mice to study liver functiona
| Genotype | Glucose (mg/dl) | Albumin (g/ml) | AST (IU/liter) | ALT (IU/liter) | Total protein (g/dl) |
|---|---|---|---|---|---|
| Male | |||||
| Wild type | 199 ± 15 | 3.31 ± 0.12 | 103 ± 16 | 21.3 ± 1.6 | 4.87 ± 0.11 |
| actβCm1 | 212 ± 15 | 3.26 ± 0.12 | 128 ± 33 | 19.7 ± 3.3 | 4.96 ± 0.05 |
| actβEm1 | 193 ± 8 | 3.26 ± 0.10 | 136 ± 22 | 26.3 ± 3.4 | 4.87 ± 0.09 |
| actβCm1-actβEm1 | 201 ± 11 | 3.53 ± 0.10 | 104 ± 10 | 24.4 ± 2.2 | 5.10 ± 0.16 |
| Female | |||||
| Wild type | 195 ± 12 | 3.69 ± 0.09 | 135 ± 28 | 16.3 ± 2.3 | 4.95 ± 0.06 |
| actβCm1 | 216 ± 14 | 3.31 ± 0.14b | 84 ± 11 | 21.0 ± 3.4 | 5.1 ± 0.09 |
| actβEm1 | 164 ± 15 | 3.79 ± 0.09 | 114 ± 27 | 20.6 ± 2.2 | 4.98 ± 0.10 |
| actβCm1-actβEm1 | 185 ± 10 | 3.62 ± 0.12 | 141 ± 40 | 22.8 ± 2.3 | 4.81 ± 0.16 |
Mice at 42 to 45 days of age were used. For all data points, n = 8 to 10. AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Student's t test, P < 0.04; P > 0.05 for all other values in comparing mutant groups to wild-type mice.
To check which phenotypes, if any, would eventually develop later at adult stages in the mutant mice, mice were observed for more than 6 months. Weekly weight data were considered as a gross indicator of overall health. There were no significant differences between wild-type mice and any of the homozygous mutant mice for up to 6 months (data not shown). Homozygous mutant mice survived for more than 1 year and were indistinguishable from wild-type cage mates (data not shown). This suggests that activin βC and activin βE do not play an essential role in regulating systemic processes related to liver metabolism.
Regulation of FSH secretion.
Since activin βA and βB are known regulators of the hypothalamic-pituitary-gonadal axis, we analyzed the serum FSH and reproductive tracts from homozygous mutant mice. Serum FSH levels in male actβCm1/actβCm1 (30.1 ± 2.9 ng/ml), actβEm1/actβEm1 (35.5 ± 5.1 ng/ml), and actβCm1/actβEm1 homozygous (31.2 ± 2.1 ng/ml) mice were not statistically different (P > 0.05 by Student's t test) from those of male wild-type mice (27.7 ± 2.7 ng/ml). Morphology and histology of the gonads from the null mice were normal (data not shown). Testis weights at 6 weeks of age were not significantly different (P > 0.05) between wild-type (82.3 ± 1.4 mg) and actβCm1/actβCm1 (86.7 ± 2.5 mg), actβEm1/actβEm1 (79.7 ± 2.0 mg), and actβCm1-actβEm1 homozygous (79.2 ± 2.4 mg) mice. Furthermore, the male and female homozygous mutant mice bred normally, showed no reproductive defects, and produced normal litter sizes (Table 1). Thus, in contrast to activin βA and βB, activin βC and activin βE are not essential regulators of FSH secretion or reproductive function.
Partial hepatectomy in activin βC and βE knockout mice.
Since there was no gross liver phenotype associated with the loss of actβC and/or actβE under normal physiological conditions, we performed 70% partial hepatectomy on these homozygous mutant mice to create a physiological stress paradigm and induce liver regeneration. To estimate the mass of the liver prior to the partial hepatectomy procedure, the average liver mass/body mass ratio in hybrid female mice for each mutation was calculated (wild-type; 0.040 ± 0.004, n = 31; actβCm1/actβCm1, 0.041 ± 0.003, n = 31; actβEm1/actβEm1, 0.043 ± 0.004, n = 22; and actβCm1-actβEm1 −/−; 0.043 ± 0.001, n = 21). We analyzed the expression of actβC, actβE, β-actin, and junB at various time points (0, 3, 6, 12, 24, 48, and 72 h) after partial hepatectomy.
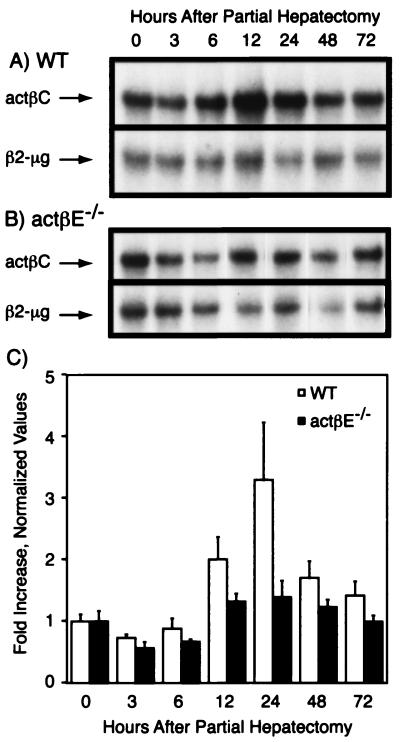
In wild-type (Fig. 5A and C) and activin βE knockout (Fig. 5B and C) mice, actβC expression tended to be reduced 3 to 6 h following partial hepatectomy (Fig. 5C). By 12 h, activin βC expression appeared to be induced and appeared to peak at 24 h after partial hepatectomy. By 72 h, the actβC RNA was near basal levels (Fig. 5C). On the other hand, actβC levels in actβEm1/actβEm1 mice did not show a trend of increased induction (Fig. 5C). Though there was a trend toward lowered induction kinetics of actβC expression in actβEm1/actβEm1 mice, the values were not statistically significant (e.g., wild type at 0 h versus wild type at 24 h, P > 0.18).
FIG. 5.
Expression of actβC before and after partial hepatectomy. (A and B) Activin βC expression levels in the whole or remnant livers were determined before partial hepatectomy (0 h) and at several time points (3, 6, 12, 24, 48, and 72 h) after partial hepatectomy in wild-type (WT) (A) and actβEm1/actβEm1 (actβE−/−)(B) mice. Representative autoradiographs are shown. (C) Autoradiographs were quantitated and visualized as fold increase over the 0-h point ± standard error of the mean for each genotype. There was no significant difference between time zero and all other time points (P > 0.05). The following numbers of mice were analyzed per time point: three to five (wild type) and three to four (actβEm1/actβEm1). β2-μg, β2-microglobulin.
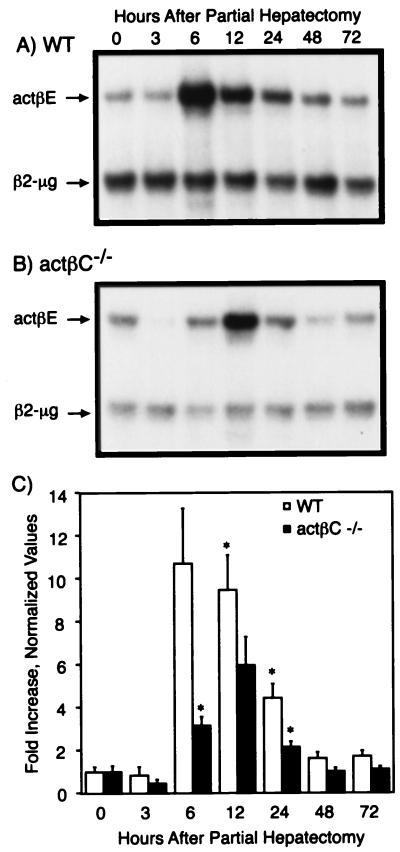
Next, we examined the expression of actβE during liver regeneration following partial hepatectomy (Fig. 6). Like activin βC expression, actβE expression tended to be reduced 3 h after partial hepatectomy in both wild-type (Fig. 6A and C) and actβCm1/actβCm1 mice (Fig. 6B and C). Activin βE was rapidly induced and appeared to peak around 6 h following partial hepatectomy in wild-type mice. Expression of actβE was still high at 12 h and dropped to near-basal levels by 48 h in wild-type mice. However, in actβCm1/actβCm1 mice, actβE expression appeared to peak between 6 and 24 h after the surgery (Fig. 6C).
FIG. 6.
Expression of actβE before and after partial hepatectomy. (A and B) Remnant or whole livers from wild-type (WT) (A) and actβCm1/actβCm1 (actβC−/−) (B) mice were collected at 0, 3, 6, 12, 24, 48, and 72 h after partial hepatectomy. Representative autoradiographs are shown. Expression of actβE in the livers was assayed by RNase protection with β2-microglobin (β2-μg) as the internal control. (C) The protected bands were quantitated and visualized as fold increase over the 0-h point ± standard error of the mean for each genotype. An asterisk denotes time points where expression of actβE in activin βC knockout or wild-type mice was statistically significantly different from the time zero value for the same genotype (P < 0.05) by Student's t test. The numbers of mice analyzed at each time point were three to four for wild type and actβCm1/actβCm1.
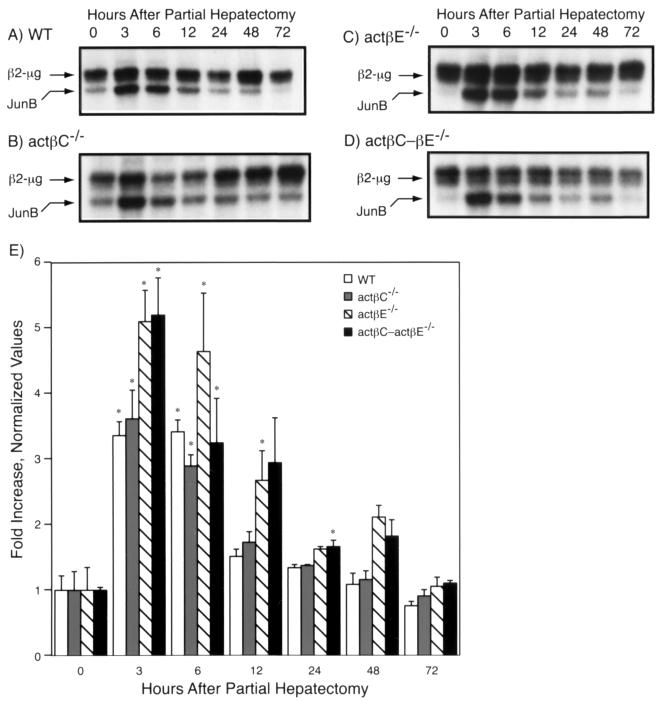
In addition, we examined the expression of junB (Fig. 7), an immediate-early gene that has been previously characterized in the regenerating liver of wild-type C57BL/6 mice (10). Basal expression of junB in the whole liver was barely detectable in actβEm1/actβEm1 and actβCm1-actβEm1 mutant mice compared to wild-type or actβCm1/actβCm1 mice (Fig. 7C and D). However, during liver regeneration, the kinetics of junB expression were similar between wild-type (Fig. 7A and E) and homozygous mutant (Fig. 7B, C, D, and E) mice. Interestingly, a second low-level junB expression peak was seen at the 48-h point in actβEm1/actβEm1 and actβCm1-actβEm1 homozygous mutant mice (Fig. 7C and D).
FIG. 7.
Expression of junB before and after partial hepatectomy. The expression of junB and the internal control β2-microglobulin (β2-μg) in the liver was analyzed by RNase protection. (A to D) Representative autoradiographs from wild-type (WT) (A), actβCm1/actβCm1 (actβC−/−) (B), actβEm1/actβEm1 (actβE−/−) (C), and actβCm1-actβEm1 knockout (actβC-βE−/−) mice (D) are shown. (E) Quantitative results were obtained by normalizing values to the ratio of junB to β2-microglobulin at time zero for each genotype (mean ± standard error of the mean). Asterisks denote values that were statistically significantly different from the value for time zero of the same genotype (P < 0.05). The number of mice was three to four for all genotypes at all time points.
We also characterized the expression of β-actin, a growth-related gene (17), during liver regeneration in wild-type mice. In general, β-actin expression in the null mice was very similar compared to its expression in wild-type mice from 0 to 72 h after partial hepatectomy (data not shown).
DISCUSSION
In this study, we have used gene targeting to determine the essential in vivo functions of the novel activin subunits, activin βC and activin βE. Mice deficient in these two genes, either singly or in combination, appear grossly normal. Despite the dynamic embryonic expression patterns of these two subunit mRNAs, mice with either single or double mutations in these genes were recovered in the expected Mendelian ratios, showing that these genes are dispensable for normal embryonic and postnatal development. Since there was no embryonic or perinatal lethality and no sign of mandibular defects, these data strongly suggest that lack of activin βC and/or activin βE signaling is not the cause of the perinatal defects seen in ActRIIA knockout mice (34). Deleting actβC and/or actβE also does not produce phenotypes similar to those of activin receptor type IIB-deficient mice, the majority of which die perinatally from axial and left-right asymmetry defects (44). The receptors that transduce the activin C and/or activin E signals are not known. Our mutant mice can be a good resource, combined with recombinant activin C, E, or CE, to identify their cognate receptors, assuming that they will be up-regulated in the absence of ligands.
Loveland et al. (31) have shown that actβC is expressed in human and rat gonads, and others have shown that human activin βC is expressed in prostate, kidney, heart, and ovary among other tissues listed in the UniGene database (http://www.nbci.nlm.nih.gov/UniGene/). However, our studies using Northern blot analysis (28) and RNase protection have not detected any actβC message in mouse ovary or testis. Histological analysis of other tissues, including the heart, testis, ovary, and prostate, did not show any gross differences. Additional phenotypes were not seen in homozygous mutant mice over 1 year of age, suggesting that activin βC and βE are not critical regulators of any systemic processes.
We tested whether activin βC and activin βE play essential roles in the regulation of FSH secretion from the pituitary gland since activin βA and βB were originally isolated for this activity. We did not detect any differences in the serum FSH levels among the various mutants. This is in contrast to the results we have observed with other knockout models of activins, inhibins, and the activin signaling pathway (33, 34, 55). As predicted by the current model, mice lacking α-inhibin and activin βB have higher serum FSH levels (33, 55), while ActRIIA knockout mutant mice show a decrease in serum FSH levels (34). Therefore, unlike activins A and B, activin βC and activin βE are not essential (positive) regulators of pituitary FSH.
Normally, liver size is proportional to body habitus. Numerous studies have previously shown in different organisms (including humans), that, upon transplantation, the liver will modulate its size to “fit” the body (27). Another good example of this modulation is the restorative hyperplasia that occurs during liver regeneration (18, 27, 38). Many positive growth factors for hepatocytes such as hepatocyte growth factor/scatter factor, epidermal growth factor, insulin-like growth factor 1, and norepinephrine are known, but very few negative regulators have been discovered (27, 38). TGF-β1 has been shown to negatively regulate hepatocyte proliferation (5, 41, 50, 51), although TGF-β1 is inhibitory only 72 h after partial hepatectomy (46). Activin A has also been implicated as a negative regulator since injection of recombinant activin A intraportally into the regenerating liver delayed liver regeneration. In contrast, follistatin, an activin β-subunit binding protein, injected into the portal vein accelerated liver regeneration (24). Both activin βA and TGF-β1 genes are induced during liver regeneration, but with different kinetics. Expression of activin βA is low in the quiescent liver but is rapidly up-regulated by 24 h after partial hepatectomy (57). TGF-β1 expression is also low in the quiescent liver, but during liver regeneration induced by partial hepatectomy, expression increases from 3 h to peak at 48 to 72 h (3). These initial studies suggested a negative regulatory role for the TGF-β superfamily ligands during liver regeneration. We have also observed an increase in actβC and actβE message levels in α-inhibin knockout mice (data not shown), further suggesting that these genes play roles during liver regeneration. We did not observe an increased rate of liver regeneration in the activin βC and βE mutant mice, but the induction patterns of activin βC and βE appeared to be affected in the corresponding null mice (Fig. 5 and 6). This suggests that the induction of each of these genes is mutually dependent on that of the other. However, this dependency occurs only during liver regeneration, since basal levels of activin βC and βE are normal in the corresponding mutant mice (Fig. 2). Despite the change in activin βC and βE expression profiles during liver regeneration, the mRNA expression profiles of junB and β-actin suggest that liver regeneration initiates and progresses normally in the homozygous mutant mice.
Since the liver is the major site for the production of acute-phase proteins, activin βC and activin βE may play a role during the acute-phase response. TGF-β superfamily ligands have also been shown to affect inflammation and the acute-phase response. One of the phenotypes seen in the TGF-β1 knockout mouse is a hyperstimulated inflammatory response (49). Activin A has also been shown to affect inflammatory response through inhibition of IL-6 action (45, 58) and was isolated biochemically based on this function (4). Activin A may play a role in wound repair since activin βA and βB mRNA is induced at the site of wounding (20) and skin wound healing is enhanced if activin βA is expressed from the human keratin K14 promoter in the skin (40). Further experiments are needed to understand what role(s) actβC and actβE may play during acute-phase response and wound repair.
The expression of many members of the TGF-β superfamily and their signaling components in the liver suggests highly redundant roles. Expression of TGF-β1, TGF-β2, TGF-β3 (22), BMP-6 (23), BMP-9 (6), growth differentiation factor 10 (11), and activin βA (16, 37) among others has all been detected in the liver. BMP-9 has also been shown to be a liver-restricted gene (6). Previous studies have suggested a degree of promiscuity between different receptor and ligand combinations (1, 13, 56). This suggests possible large overlaps between the functions of multiple members of the TGF-β superfamily and their downstream signaling components, especially in such an important organ as the liver.
In conclusion, we have generated and characterized mutant mice lacking actβC and actβE genes. Targeted overexpression of activin βC and βE in the livers of transgenic mice and the production of functional recombinant activin C, E, and CE proteins will be invaluable in the future to study gain-of-function effects of these two genes in vivo and in vitro. Kron et al. have recently reported the production of recombinant human activin βC protein using an insect expression system (25). Purified recombinant active protein can be tested in several functional assays such as hepatocellular apoptosis. Production of anti-activin βC and anti-activin βE antibodies is under way, and the antibodies will be useful for immunohistochemistry to localize the protein. We are also interested in analyzing the circulating levels of activins C and E and identifying the tissues to which these novel activins specifically bind.
ACKNOWLEDGMENTS
We thank P. Wang and Q. Guo for technical assistance; R. R. Behringer, A. Bradley, G. Eichele, M. J. Finegold, and S. Varani for help and advice in these experiments; and C. Brown for help, advice, and critical review of the manuscript.
This work is supported in part by National Institutes of Health grant HD32067 and a sponsored research agreement from the Genetics Institute. A.L.L. is a student in the Program of Developmental Biology, supported in part by National Science Foundation grant BIR-9413237. K.N. was funded in part by the Yoshida Science Foundation.
REFERENCES
- 1.Attisano L, Carcamo J, Ventura F, Weis F M B, Massague J, Wrana J L. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell. 1993;75:671–680. doi: 10.1016/0092-8674(93)90488-c. [DOI] [PubMed] [Google Scholar]
- 2.Bikoff E K, Jaffe L, Ribaudo R K, Otten G R, Germain R N, Robertson E J. MHC class I surface expression in embryo-derived cell lines inducible with peptide or interferon. Science. 1991;354:235–238. doi: 10.1038/354235a0. [DOI] [PubMed] [Google Scholar]
- 3.Braun L, Mead J E, Panzica M, Mikumo R, Bell G I, Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci USA. 1988;85:1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brosh N, Sternberg D, Honigwachs-Sha'anani J, Lee B C, Shav-Tal Y, Tzehoval E, Shulman L M, Toledo J, Hacham Y, Carmi P, et al. The plasmacytoma growth inhibitor restrictin-P is an antagonist of interleukin 6 and interleukin 11. Identification as a stroma-derived activin A. J Biol Chem. 1995;270:29594–29600. doi: 10.1074/jbc.270.49.29594. [DOI] [PubMed] [Google Scholar]
- 5.Carr B I, Hayashi I, Branum E L, Moses H L. Inhibition of DNA synthesis in rat hepatocytes by platelet-derived type beta transforming growth factor. Cancer Res. 1986;46:2330–2334. [PubMed] [Google Scholar]
- 6.Celeste A J, Song J J, Cox K, Rosen V, Wozney J M. Bone morphogenetic protein-9, a new member of the TGF-beta superfamily. J Bone Miner Res. 1994;9:S136. doi: 10.1002/jbmr.5650091113. [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Woodruff T K, Mayo K E. Activin A-induced HepG2 liver cell apoptosis: involvement of activin receptors and smad proteins. Endocrinology. 2000;141:1263–1272. doi: 10.1210/endo.141.3.7361. [DOI] [PubMed] [Google Scholar]
- 8.Coerver K A, Woodruff T K, Finegold M J, Mather J, Bradley A, Matzuk M M. Activin signaling through activin receptor type II causes the cachexia-like symptoms in inhibin-deficient mice. Mol Endocrinol. 1996;10:534–543. doi: 10.1210/mend.10.5.8732684. [DOI] [PubMed] [Google Scholar]
- 9.Crawford J M. The liver and the biliary tract. In: Cotran R S, Kumar V, Robbins S L, editors. Robbins: pathologic basis of disease. 5th ed. Philadelphia, Pa: W. B. Saunders Company; 1994. pp. 831–896. [Google Scholar]
- 10.Cressman D E, Greenbaum L E, DeAngelis R A, Ciliberto G, Furth E E, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham N S, Jenkins N A, Gilbert D J, Copeland N G, Reddi A H, Lee S J. Growth/differentiation factor-10: a new member of the transforming growth factor-beta superfamily related to bone morphogenetic protein-3. Growth Factors. 1995;12:99–109. doi: 10.3109/08977199509028956. [DOI] [PubMed] [Google Scholar]
- 12.Dube J L, Wang P, Elvin J, Lyons K M, Celeste A J, Matzuk M M. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol. 1998;12:1809–1817. doi: 10.1210/mend.12.12.0206. [DOI] [PubMed] [Google Scholar]
- 13.Ebner R, Chen R H, Lawler S, Zioncheck T, Derynck R. Determination of type I receptor specificity by the type II receptors for TGF-beta or activin. Science. 1993;262:900–902. doi: 10.1126/science.8235612. [DOI] [PubMed] [Google Scholar]
- 14.Fang J, Wang S Q, Smiley E, Bonadio J. Genes coding for mouse activin beta-C and beta-E are closely linked and exhibit a liver-specific expression pattern in adult tissues. Biochem Biophys Res Commun. 1997;231:655–661. doi: 10.1006/bbrc.1997.6162. [DOI] [PubMed] [Google Scholar]
- 15.Fang J, Yin W, Smiley E, Wang S Q, Bonadio J. Molecular cloning of the mouse activin beta-E subunit gene. Biochem Biophys Res Commun. 1996;228:669–674. doi: 10.1006/bbrc.1996.1715. [DOI] [PubMed] [Google Scholar]
- 16.Feijen A, Goumans M J, van den Eijnden-van Raaij A J M. Expression of activin subunits, activin receptors and follistatin in postimplantation mouse embryos suggests specific developmental functions for different activins. Development. 1994;120:3621–3637. doi: 10.1242/dev.120.12.3621. [DOI] [PubMed] [Google Scholar]
- 17.Haber B A, Mohn K L, Diamond R H, Taub R. Induction patterns of 70 genes during nine days after hepatectomy define the temporal course of liver regeneration. J Clin Investig. 1993;91:1319–1326. doi: 10.1172/JCI116332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins G M, Anderson R M. Experimental pathology of the liver. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 19.Hotten G, Neidhardt H, Schneider C, Pohl J. Cloning of a new member of the TGF-beta family: a putative new activin betaC chain. Biochem Biophys Res Commun. 1995;206:608–613. doi: 10.1006/bbrc.1995.1086. [DOI] [PubMed] [Google Scholar]
- 20.Hubner G, Hu Q, Smola H, Werner S. Strong induction of activin expression after injury suggests an important role of activin in wound repair. Dev Biol. 1996;173:490–498. doi: 10.1006/dbio.1996.0042. [DOI] [PubMed] [Google Scholar]
- 21.Hully J R, Chang L, Schwall R H, Widmer H R, Terrell T G, Gillett N A. Induction of apoptosis in the murine liver with recombinant human activin A. Hepatology. 1994;20:854–862. doi: 10.1002/hep.1840200413. [DOI] [PubMed] [Google Scholar]
- 22.Jakowlew S B, Mead J E, Danielpour D, Wu J, Roberts A B, Fausto N. Transforming growth factor-beta (TGF-beta) isoforms in rat liver regeneration: messenger RNA expression and activation of latent TGF-beta. Cell Regul. 1991;2:535–548. doi: 10.1091/mbc.2.7.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knittel T, Fellmer P, Muller L, Ramadori G. Bone morphogenetic protein-6 is expressed in nonparenchymal liver cells and upregulated by transforming growth factor-beta 1. Exp Cell Res. 1997;232:263–269. doi: 10.1006/excr.1997.3504. [DOI] [PubMed] [Google Scholar]
- 24.Kogure K, Omata W, Kanzaki M, Zhang Y Q, Yasuda H, Mine T, Kojima L. A single intraportal administration of follistatin accelerates liver regeneration in partially hepatectomized rats. Gastroenterology. 1995;108:1136–1142. doi: 10.1016/0016-5085(95)90212-0. [DOI] [PubMed] [Google Scholar]
- 25.Kron R, Schneider C, Hotten G, Bechtold R, Pohl J. Expression of human activin C protein in insect larvae infected with a recombinant baculovirus. J Virol Methods. 1998;72:9–14. doi: 10.1016/s0166-0934(97)00215-2. [DOI] [PubMed] [Google Scholar]
- 26.Kumar T R, Low M J, Matzuk M M. Genetic rescue of follicle-stimulating hormone beta-deficient mice. Endocrinology. 1998;139:3289–3295. doi: 10.1210/endo.139.7.6111. [DOI] [PubMed] [Google Scholar]
- 27.LaBrecque D. Liver regeneration: a picture emerges from the puzzle. Am J Gastroenterol. 1994;89:S86–96. [PubMed] [Google Scholar]
- 28.Lau A L, Nishimori K, Matzuk M M. Structural analysis of the mouse activin betaC gene. Biochim Biophys Acta. 1996;1307:145–148. doi: 10.1016/0167-4781(96)00061-9. [DOI] [PubMed] [Google Scholar]
- 29.Lau A L, Shou W, Guo Q, Matzuk M M. Transgenic approaches to study the functions of the transforming growth factor-beta superfamily members. In: Aono T, Sugino H, Vale W W, editors. Inhibin, activin and follistatin: regulatory functions in system and cell biology. New York, N.Y: Springer-Verlag; 1997. pp. 220–243. [Google Scholar]
- 30.Li Q, Karam S M, Coerver K A, Matzuk M M, Gordon J I. Stimulation of activin receptor II signaling pathways inhibits differentiation of multiple gastric epithelial lineages. Mol Endocrinol. 1998;12:181–192. doi: 10.1210/mend.12.2.0060. [DOI] [PubMed] [Google Scholar]
- 31.Loveland K L, McFarlane J R, de Kretser D M. Expression of activin beta C subunit mRNA in reproductive tissues. J Mol Endocrinol. 1996;17:61–65. doi: 10.1677/jme.0.0170061. [DOI] [PubMed] [Google Scholar]
- 32.Matzuk M M, Finegold M J, Mather J P, Krummen L, Lu H, Bradley A. Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc Natl Acad Sci USA. 1994;91:8817–8821. doi: 10.1073/pnas.91.19.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matzuk M M, Finegold M J, Su J J, Hsueh A J W, Bradley A. Alpha-inhibin is a tumor suppressor gene with gonadal specificity in mice. Nature. 1992;360:313–319. doi: 10.1038/360313a0. [DOI] [PubMed] [Google Scholar]
- 34.Matzuk M M, Kumar T R, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995;374:356–360. doi: 10.1038/374356a0. [DOI] [PubMed] [Google Scholar]
- 35.Matzuk M M, Kumar T R, Vassalli A, Bickenbach J R, Roop D R, Jaenisch R, Bradley A. Functional analysis of activins during mammalian development. Nature. 1995;374:354–356. doi: 10.1038/374354a0. [DOI] [PubMed] [Google Scholar]
- 36.McDowell N, Gordon J B. Activin as a morphogen in Xenopus mesoderm induction. Semin Cell Dev Biol. 1999;10:311–317. doi: 10.1006/scdb.1999.0307. [DOI] [PubMed] [Google Scholar]
- 37.Meunier H, Rivier C, Evans R M, Vale W W. Gonadal and extragonadal expression of inhibin alpha, beta A, and beta B subunits in various tissues predicts diverse functions. Proc Natl Acad Sci USA. 1988;85:247–251. doi: 10.1073/pnas.85.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michalopoulos G K, DeFrances M C. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 39.Mine T, Kojima I, Ogata E. Stimulation of glucose production by activin-A in isolated rat hepatocytes. Endocrinology. 1989;125:586–591. doi: 10.1210/endo-125-2-586. [DOI] [PubMed] [Google Scholar]
- 40.Munz B, Smola H, Engelhardt F, Bleuel K, Brauchle M, Lein I, Evans L W, Huylebroeck D, Balling R, Werner S. Overexpression of activin A in the skin of transgenic mice reveals new activities of activin in epidermal morphogenesis, dermal fibrosis and wound repair. EMBO J. 1999;18:5205–5215. doi: 10.1093/emboj/18.19.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura T, Tomita Y, Hirai R, Yamaoka K, Kaji K, Ichihara A. Inhibitory effect of transforming growth factor-beta on DNA synthesis of adult rat hepatocytes in primary culture. Biochem Biophys Res Commun. 1985;133:1042–1050. doi: 10.1016/0006-291x(85)91241-0. [DOI] [PubMed] [Google Scholar]
- 42.Oberhammer F A, Pavelka M, Sharma S, Tiefenbacher R, Purchio A F, Bursch W, Schulte-Hermann R. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor beta 1. Proc Natl Acad Sci USA. 1992;89:5408–5412. doi: 10.1073/pnas.89.12.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oda S, Nishimatsu S, Murakami K, Ueno N. Molecular cloning and functional analysis of a new activin beta subunit: a dorsal mesoderm-inducing activity in Xenopus. Biochem Biophys Res Commun. 1995;210:581–588. doi: 10.1006/bbrc.1995.1699. [DOI] [PubMed] [Google Scholar]
- 44.Oh S P, Li E. The signaling pathway mediated by the type IIB activin receptor controls axial patterning and lateral asymmetry in the mouse. Genes Dev. 1997;11:1812–1826. doi: 10.1101/gad.11.14.1812. [DOI] [PubMed] [Google Scholar]
- 45.Russell C E, Hedger M P, Brauman J N, de Kretser D M, Phillips D J. Activin A regulates growth and acute phase proteins in the human liver cell line, HepG2. Mol Cell Endocrinol. 1999;148:129–136. doi: 10.1016/s0303-7207(98)00226-3. [DOI] [PubMed] [Google Scholar]
- 46.Russell W E, Coffey R J, Jr, Ouellette A J, Moses H L. Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci USA. 1988;85:5126–5130. doi: 10.1073/pnas.85.14.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmitt J, Hötten G, Jenkins N A, Gilbert D J, Copeland N G, Pohl J, Schrewe H. Structure, chromosomal localization, and expression analysis of the mouse inhibin/activin betaC (Inhbc) gene. Genomics. 1996;32:358–366. doi: 10.1006/geno.1996.0130. [DOI] [PubMed] [Google Scholar]
- 48.Schwall R H, Robbins K, Jardieu P, Chang L, Lai C, Terrell T G. Activin induces cell death in hepatocytes in vivo and in vitro. Hepatology. 1993;18:347–356. doi: 10.1016/0270-9139(93)90018-i. [DOI] [PubMed] [Google Scholar]
- 49.Shull M M, Ormsby I, Kier A B, Pawlowski S, Diebold R J, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strain A J, Frazer A, Hill D J, Miller R D. Transforming growth factor beta inhibits DNA synthesis in hepatocytes isolated from normal and regenerating rat liver. Biochem Biophys Res Commun. 1987;145:436–442. doi: 10.1016/0006-291x(87)91340-4. [DOI] [PubMed] [Google Scholar]
- 51.Strain A J, Hill D J, Miller R D. Divergent action of transforming growth factor beta on DNA synthesis in human foetal liver cells. Cell Biol Int Rep. 1986;10:855–860. doi: 10.1016/0309-1651(86)90102-5. [DOI] [PubMed] [Google Scholar]
- 52.Tamura K, Yonei-Tamura S, Belmonte J C. Molecular basis of left-right asymmetry. Dev Growth Differ. 1999;41:645–656. doi: 10.1046/j.1440-169x.1999.00465.x. [DOI] [PubMed] [Google Scholar]
- 53.Tuuri T, Eramaa M, Hilden K, Ritvos O. The tissue distribution of activin beta A- and beta B-subunit and follistatin messenger ribonucleic acids suggests multiple sites of action for the activin-follistatin system during human development. J Clin Endocrinol Metab. 1994;78:1521–1524. doi: 10.1210/jcem.78.6.8200957. [DOI] [PubMed] [Google Scholar]
- 54.Vale W W, Bilezikjian L M, Rivier C. Reproductive and other roles of inhibins and activins. In: Knobil E, Neill J D, editors. The physiology of reproduction. 2nd ed. New York, N.Y: Raven Press, Ltd.; 1994. pp. 1861–1878. [Google Scholar]
- 55.Vassalli A, Matzuk M M, Gardner H A R, Lee K-F, Jaenisch R. Activin/inhibin betaB subunit gene disruption leads to defects in eyelid development and female reproduction. Genes Dev. 1994;8:414–427. doi: 10.1101/gad.8.4.414. [DOI] [PubMed] [Google Scholar]
- 56.Yamashita H, ten Dijke P, Huylebroeck D, Sampath T K, Andries M, Smith J C, Heldin C H, Miyazono K. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J Cell Biol. 1995;130:217–226. doi: 10.1083/jcb.130.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yasuda H, Mine T, Shibata H, Eto Y, Hasegawa Y, Takeuchi T, Asano S, Kojima I. Activin A: an autocrine inhibitor of initiation of DNA synthesis in rat hepatocytes. J Clin Investig. 1993;92:1491–1496. doi: 10.1172/JCI116727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu E W, Dolter K E, Shao L E, Yu J. Suppression of IL-6 biological activities by activin A and implications for inflammatory arthropathies. Clin Exp Immunol. 1998;112:126–132. doi: 10.1046/j.1365-2249.1998.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]