Abstract
Background
Standard equations for estimating glomerular filtration rate (eGFR) employ race multipliers, systematically inflating eGFR for Black patients. Such inflation is clinically significant because eGFR thresholds of 60, 30, and 20 ml/min/1.73m2 guide kidney disease management. Racialized adjustment of eGFR in Black Americans may thereby affect their clinical care. In this study, we analyze and extrapolate national data to assess potential impacts of the eGFR race adjustment on qualification for kidney disease diagnosis, nephrologist referral, and transplantation listing.
Methods
Using population-representative cross-sectional data from the United States National Health and Nutrition Examination Survey (NHANES) from 2015-2018, eGFR values for Black Americans were calculated using the Modification of Diet in Renal Disease (MDRD) equation with and without the 1.21 race-specific coefficient using cohort data on age, sex, race, and serum creatinine.
Findings
Without the MDRD eGFR race adjustment, 3.3 million (10.4%) more Black Americans would reach a diagnostic threshold for Stage 3 Chronic Kidney Disease, 300,000 (0.7%) more would qualify for beneficial nephrologist referral, and 31,000 (0.1%) more would become eligible for transplant evaluation and waitlist inclusion.
Interpretation
These findings suggest eGFR race coefficients may contribute to racial differences in the management of kidney. We provide recommendations for addressing this issue at institutional and individual levels.
Funding
No external funding was received for this study.
Keywords: Race Coefficient, Race Adjustment, eGFR, MDRD, CKD-EPI, Cystatin C, Race-Based Medicine, Racial inequities, Kidney disease, Kidney failure, Nephrology Referral, Kidney transplant, Health Disparities
Research in Context.
Evidence before this study
Analysis of the race adjustment in estimated glomerular filtration rate (eGFR) by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation—which multiplies eGFR by 1.159 if a patient is identified as “Black”—suggests that removal of the adjustment would increase the percentage of Black adults diagnosed with CKD, referred to specialty care, and referred for transplant by 3.5%, 0.2%, and 0.05%, respectively. However, given that most institutions use the Modification of Diet in Renal Disease (MDRD) equation (which includes a racial coefficient of 1.21), these values may be underestimates.
Added value of this study
Using population-level data from the United States, this study finds that removal of the race correction from the MDRD equation would increase the percentage of Black adults diagnosed with CKD, referred to specialty care, and referred for transplant by 10.4%, 0.7%, and 0.1%.
Implications of all available evidence
eGFR race adjustments systematically disadvantage Black patients. Abandoning racialized eGFR calculations dismantles discriminatory and unscientific practices and provides opportunity for more accurate and equitable medicine. Recently, the American Society of Nephrology and the American Kidney Foundation released a new, race-free, creatinine-based eGFR equation and encouraged increased use of cystatin C as a confirmatory measure of kidney function. The data from this study support this recommendation and its implementation throughout the world.
Alt-text: Unlabelled box
1. Introduction
Chronic kidney disease (CKD) involves alteration of the kidney resulting in damage or decreased function for at least three months, regardless of the cause [1]. Estimated glomerular filtration rate (eGFR) emerged as a convenient alternative to 24-hour urine collection and remains the predominant method of assessing kidney function and progression of CKD worldwide. Given its clinical utility, quantitative eGFR thresholds inform referral to specialty care, medication dosing, and evaluation for the kidney transplant waitlist [2].
Two equations for eGFR predominate in North American healthcare systems: the 1999 Modification of Diet in Renal Disease (MDRD) equation, and the 2009 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [3]. These equations include race adjustments that multiply eGFR by 1.21 or 1.16 respectively, if the patient is identified as “Black.” [4]. These race-specific adjustments have spurred growing controversy in recent years [5,6].
Though the MDRD study did demonstrate that as a group, self-reported Black patients had a higher measured GFR when compared to White patients with the same serum creatinine concentrations, it is important to recognize that the process by which Black race was included in the MDRD is specious [7]. Without specifying a functional definition or hypothesis [8], authors of the 1999 study included “Black ethnicity” among the initial predictor variables in stepwise regression modeling effects on GFR [4]. “Black ethnicity” was among six variables that achieved a p-value of less than 0.001. A subsequent abstract narrowed these variables and proposed a “race” coefficient of 1.21 [9].
Black Americans could differ from the referent population in countless social, material and lifestyle dimensions, but the MDRD authors provided only one post hoc justification for inclusion of race in the equation, suggesting that race proxies average differences in muscle mass between Black and White subjects [7]. The three small studies referenced, however, relied on convenience samples, did not support the use of race as an informative substitute for muscle mass, and provided no control for social factors [4,[5], [6], [7], [8], [9], [10], [11], [12], [13]]. Furthermore, the authors did not show that Black and White participants were equivalent for relevant factors besides race. Data from a subset of MDRD participants showed Black participants had excess diabetes, hypertension, and poverty—all independent predictors of CKD—compared to their White counterparts, but this did not factor into the development of the MDRD equation [10].
Despite these flaws, the MDRD's inclusion of race served as proof of concept for race adjustment in the 2009 CKD-EPI equation. Of further concern, though the CKD-EPI cohort included patients of multiple races, authors exclusively categorized individuals as either “Black” or “Non-Black” for purposes of race adjustment, suggesting illogically that Black people are uniquely distinct from all other humans [5,11].
Since 1999, these and further analyses have theorized on racialized physiologic differences and reiterated the importance of racial adjustment [12], [13], [14], [15]. While we acknowledge that such studies demonstrate population-based differences in kidney function measurements, we reiterate that similar errors in methodology, study design, and theorization exist to undermine the conclusion that race-based coefficients are necessary for clinical care. For example, authors in 2020 argued that a model with linear terms for height and weight was less accurate than one including the binary race indicator, although the authors do not justify this particular model specification from among all possible specifications. The estimated GFR based on MDRD and CKD-EPI equations is already standardized to a body surface area of 1.73 m2, with the intention of making it independent of height and weight [12]. This body surface area value is derived from a a sample of 25 year old White Americans in 1927 [16]. Clearly American body habitus has changed over the subsequent century, and there is variation within and between populations that is ignored by using this single standard.
Despite these critiques, eGFR race adjustments remain widely-used and cause far-reaching clinical consequences. Race-specific inflation can restrict access to treatment at critical junctures by requiring Black patients to reach greater kidney dysfunction before meeting benchmarks of disease (See Panel 1.) Recent analysis demonstrated that a significant percentage Black patients would be diagnosed with more severe kidney disease if eGFR race adjustments were eliminated [17,18] The eGFR multiplier can also impede organ receipt, since patients must have a GFR of equal to or less than 20 ml/min/1.73m2 to qualify for the kidney transplant list [2,18,19] (See Panel 2.)
Panel 1.
Panel 1.
Panel 2.
Time on the waitlist is one of the most important factors in determining who receives kidney transplant. Overestimation of eGFR in Black patients means the transplant clock does not start until they are sicker. This delay reduces allocation scores, leading to decreased quality of life, more serious co-morbidities, poorer surgical outcomes, and increased mortality for Black kidney transplant candidates amidst existing racial inequities in kidney disease and kidney transplant referral, listing, and receipt.
Previous analysis of the CKD-EPI race coefficient—which multiplies eGFR by 1.159 if a patient is identified as “Black”—suggests that removal of the adjustment would increase the percentage of Black adults diagnosed with CKD, referred to specialty care, and referred for transplant by 3.5%, 0.2%, and 0.05%, respectively [18]. However, only 4 percent of US laboratories utilize the CKD-EPI equation [20]. Given that most institutions use the MDRD equation (which includes a racial coefficient of 1.21), these values may be underestimates. In this study, we use population-level data from the United States to quantify the potential effect of the race adjustment on potential for CKD diagnosis, nephrology referral, and qualification for kidney transplantation for Black Americans, based on quantitative eGFR thresholds currently used in clinical nephrology.
2. Methods
2.1. Data Source
The National Health and Nutrition Examination Survey (NHANES) is a program of studies conducted by the National Center for Health Statistics (NCHS), a branch of the U.S. Public Health Service in the US Department of Health and Human Services, to examine disease prevalence and trends over time in different cross-sectional representative samples of noninstitutionalized US civilian residents. The survey includes interviews, physical examinations, and laboratory tests, and examines a representative sample of about 5,000 persons annually. NHANES sample characteristics have been previously described at https://www.cdc.gov/nchs/products/databriefs.htm?program=nhanes. This study uses data from the 2015-2016 and 2017-2018 cohorts. The full sample included 10,739 participants. We restricted the analytical sample to Black/African American (non-Hispanic/Latino) persons, arriving at a sample of 2,401 participants. These data are de-identified and publicly available at https://www.cdc.gov/nchs/nhanes/index.htm. Ethical approval was not required, as secondary analysis of NHANES data does not meet the definition of human subjects research.
2.2. Participants
Participants are selected to represent the civilian, noninstitutionalized population of the US. Signed consent is obtained from each eligible individual that grants permission to conduct a household interview, physical examination, to store a small sample of blood and urine for future specimen testing, and to collect a sample for genetic testing. For this study, we used population weighted sampling of self-identified Black adults aged 18 and older. Laboratory methods for determination of serum creatinine have been described elsewhere [21].
2.3. Measures and Statistical Analysis
All analyses were conducted in R. Because the MDRD is more commonly-used in United States laboratories compared to CKD-EPI, we calculated eGFR using the MDRD equation with and without the 1.21 race coefficient using cohort data on age, sex, race, and serum creatinine [4,22,20]. We used three eGFR thresholds to estimate the impact of the race coefficient on care delivery: ≤60 mL/min/1.73 m2 body surface area, at which a patient is considered to have moderate CKD, ≤30, at which general nephrology referral is recommended and the use of nephrotoxic medications are contraindicated, and ≤20, at which specialized transplant nephrology referral is recommended, and patients becomes eligible for registration on the kidney allocation system (KAS) waitlist [23]. Using weighted samples to account for the survey design, we estimated the proportion of Black American adults whose eGFR values were at these thresholds with and without the race coefficient. This study adheres to RECORD reporting guidelines.
2.4. Role of the Funding Source
No external funding was received for this study.
3. Results
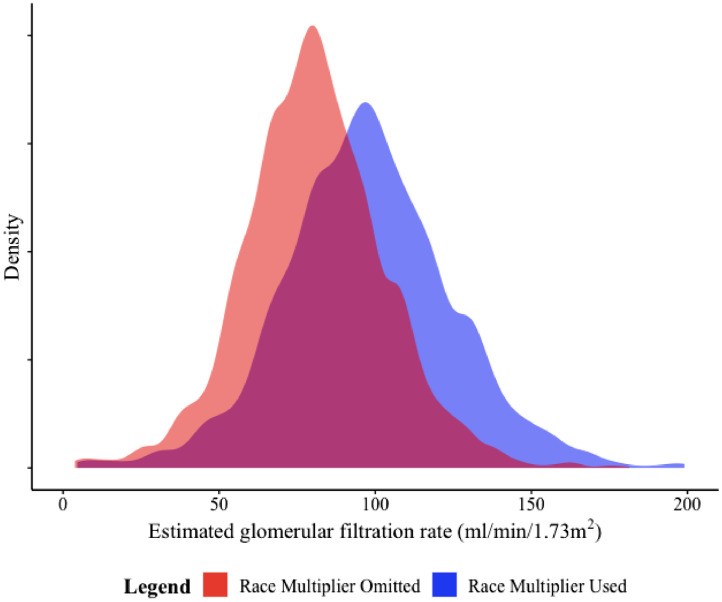
Among Black participants in the 2015-2016 and 2017-2018 cohorts (unweighted sample with n=2,401 Black/African American participants, corresponding to a weighted sample of 26,979,870 Black/African American adults), the distribution of eGFR changes with the elimination of the race multiplier (See Figure 1.) For patients whose eGFR values would otherwise be ≤60 mL/min/1.73 m2, the race coefficient adds a median of 11 mL/min/1.73 m2 and results in a reduction in the proportion of individuals with eGFR values >60 mL/min/1.73 m2 from 93.2% (95% CI: 92.2–94.4%) to 83.7% (95% CI: 82.0–85.0%). When extrapolated to population data, this represents the possibility of missed diagnosis of moderate CKD for 3.3 million Black Americans. (See Table 1.)
Figure 1.
Distribution of estimated glomerular filtration rates among Black/African American (non-Latinx) adults calculated using the Modification of Diet in Renal Disease (MDRD) equation, with and without the use of the race multiplier. The y-axis of the density plot shows the kernel density estimation of the probability density function for eGFR.
Note: Distributions are truncated at the 99th percentile. eGFR values range from 4.6 to 413.7 when the race multiplier is used and from 3.8 to 341.3 when the race multiplier is not used.
Table 1.
Unweighted and weighted sample size by estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease (MDRD) equation with and without a race coefficient, Black/African American adults, National Health and Nutrition Examination Survey, 2015–2018.
| Sample Size |
||
|---|---|---|
| Unweighted | Weighted | |
| Full Sample | 2,401 | 26,979,870 |
| eGFR (MDRD, with coefficient) | ||
| ≥60 mL/min/1.73m2 | 1,975 | 22,603,138 |
| 20 to 60 mL/min/1.73m2 | 174 | 1,491,088 |
| <20 mL/min/1.73m2 | 16 | 153,187 |
| eGFR (MDRD, without coefficient) | ||
| ≥60 mL/min/1.73m2 | 1,730 | 20,284,834 |
| 20 to 60 mL/min/1.73m2 | 416 | 3,792,434 |
| <20 mL/min/1.73m2 | 19 | 170,144 |
This change is largely driven by shifts in persons being classified with eGFR values between 20-60 mL/min/1.73 m2. Eliminating the race-specific factor increases the proportion of Black individuals with an eGFR between 20-60 mL/min/1.73 m2 from 6.2% (95% CI: 5.2–7.0%) to 16.6% (95% CI: 14.1–17.0%), which accounts for a difference of approximately 3.2 million Black Americans.
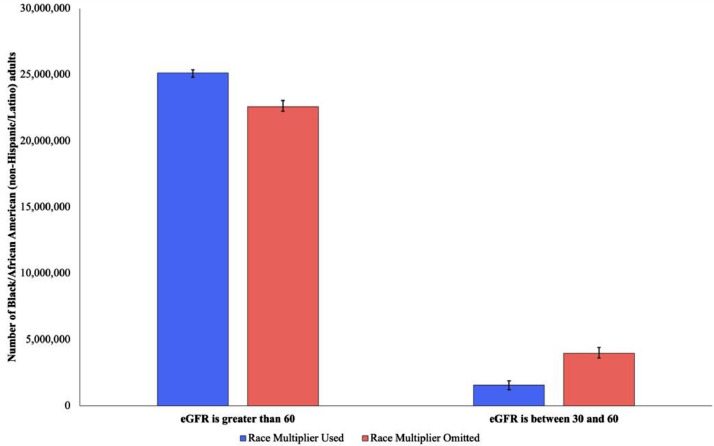
Without the MDRD race adjustment, 1.7% (95% CI: 1.0–3.0%) of Black individuals have eGFR values ≤30 mL/min/1.73 m2, corresponding to 730,000 people. The race coefficient adds a median of 4.6 mL/min/1.73 m2 for patients whose eGFR values would otherwise be ≤30 mL/min/1.73 m2, resulting in a reduction in the proportion of individuals with eGFR values <30 mL/min/1.73 m2 to 1.0% (0.6–2.0%), representing a decrease of 300,000 Black adults (See Figure 2.)
Figure 2.
Number of Black/African American (non-Hispanic/Latino) adults with eGFR values of 60 mL/min/1.73 m2 or greater and between 30 and 60 mL/min/1.73 m2 with and without the use of a race-specific coefficient. Error bars indicate 95% confidence intervals around each point estimate.
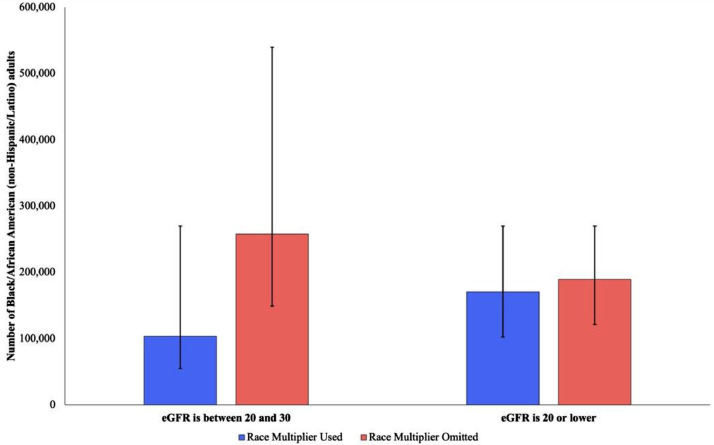
Without the race-specific factor, 0.7% (95% CI: 0.4–1.0%) of Black individuals have eGFR values ≤20 mL/min/1.73 m2, corresponding to 218,500 people. The race adjustment adds a median of 1.9 mL/min/1.73 m2 and results in a decrease in the portion of individuals with eGFR values ≤20 mL/min/1.73 m2 to 0.6% (95% CI: 0.4–1.0%), amounting to 31,000 fewer Black individuals (See Figure 3.)
Figure 3.
Number of Black/African American (non-Hispanic/Latino) adults with eGFR values between 20 and 30 mL/min/1.73 m2 and 20 mL/min/1.73 m2 or less with and without the use of a race-specific coefficient. Error bars indicate 95% confidence intervals around each point estimate.
Sources: N/A Notes: N/a
4. Discussion
Our findings demonstrate that if the MDRD race adjustment were eliminated from US clinical practice, approximately 3.3 million more Black Americans might be classified as having an eGFR of ≤60 mL/min/1.73 m2, increasing the percentage of Black Americans with eGFR values ≤60 from 6.2% to 16.6%. This is one diagnostic threshold for Stage 3 CKD, at which point complications of kidney disease such as hypertension, anemia, and bone disease begin to develop. This suggests that Black Americans may be less likely to receive care for these complications, particularly since moderate kidney disease rarely presents with overt symptoms. In similar analysis examining the CKD-EPI equation, Diao et. al. also found that abandoning the race adjustment would allow thousands more Black patients to qualify for Medicare coverage of medical nutritional therapy and kidney disease education [18]. Given the larger multiplier of the MDRD equation and its prevalence in clinical settings, we find that elimination of race would affect even larger numbers of Black Americans.
In addition, without the MDRD race adjustment, approximately 300,000 additional Black Americans might be classified as having an eGFR ≤30 mL/min/1.73 m2, which is a threshold for ability to safely dose nephrotoxic agents like intravenous contrast and certain oral diabetes medications (e.g., Metformin, SGLT-2 inhibitors.) An eGFR of 30 mL/min/1.73 m2 is also the point at which guidelines recommend nephrology referral, which is associated with decreased hospitalization and mortality rates [23], [24], [25], [26], [27]. Thus, inappropriately inflated eGFR may affect access to specialty care, and could contribute to poorer health outcomes.
We also find that removing the MDRD race multiplier might result in 31,000 more Black Americans meeting the eGFR threshold of ≤20 mL/min/1.73 m2, which is required for referral to specialized transplant nephrologists and inclusion on the kidney transplant waitlist. Kidney transplant significantly increases survival: Whereas individuals with kidney failure experience 134 deaths/1000 patient-years, patients who have received kidney transplant have a mortality rate of 29 deaths/1000 patient years [28]. Women aged 45 to 49 on dialysis live just 8.7 years on average, whereas matched cohorts who receive transplants live 25 more years—almost three times as long [28]. In addition, 58% of dialysis patients die from arrhythmia, cardiac arrest, or withdrawal while only 21% of transplant recipients succumb to such complications [28]. Thus, race-specific eGFR adjustments may affect candidacy for kidney transplant receipt, as well as donation.
Our data alone do not suggest that eliminating race adjustment would automatically alter kidney disease management for Black Americans or increase the number of Black Americans on the KAS waitlist, as clinical care is multifactorial and beset by existing racial inequities. For instance, even Black patients who do qualify for kidney transplant are less likely to be identified as transplant candidates, referred to transplant evaluation, placed on transplant waitlists, or diagnosed while kidney transplant is still a viable option [27]. Still, race-specific eGFR adjustments introduce further systemic barriers that can delay critical management of kidney disease.
Our analysis shows the impact of a racialized algorithm that lacks prima facie validity and has never been substantively justified. Although race remains statistically predictive in the MDRD equation [29], using race as a vague proxy for a slew of considerations including physical activity, diet, social class, inequality, and other behavioral, cultural, and occupational patterns is ad hoc, nonspecific, and unstable over time and place. There is no reason to assume that socio-cultural correlations currently prevailing in the US are consistent internationally, homogenous nationally, or static. MDRD race adjustments do not improve the accuracy of eGFR for Black populations outside the US [30], and indeed, even in the US, some recent investigations have reported no significant racial difference in measured GFR and conclude that race coefficients are unnecessary [31]. Nonetheless, MDRD race adjustments have been applied to global populations on which they have never been developed or validated [3,30,32], and scholars continue to dedicate resources towards developing inconsistent ethnic multipliers fraught with similar issues [11,33].
Finally, regardless of the size, the presence of racialized coefficients falsely reinforces race as an essential determinant of physiology and erroneously presumes uniform differences in Black patients. There is no valid or “gold standard” measure of “Blackness” to estimate consequences to a false precision of 1.21 or 1.16, and it is questionable to inflate kidney function based on a pliable concept such as race, especially without an explicit functional definition [8]. Although prior studies identified significant differences between kidney function of Black patients and patients of other races, this variation likely occurs due to confounding variables such as socioeconomic status and diet. For instance, prior studies have found that racial—or ancestral—associations with health conditions including hypertension and Alzheimer's disease no longer persist after adjustment for education [34,35]. The problematic notion that racial adjustments capture intrinsic distinctions is not supported by careful analysis, embeds damaging notions of racial biology into U.S. culture and scholarship, and has been demonstrated to increase racial prejudice and animosity [36,37].
Our study carries limitations. First, calculation of eGFR using the MDRD equation may oversimplify the model by either including or excluding the 1.21 multiplier for Black patients. Given that the original investigation included “ethnicity” among other predictors, removal of this variable may affect the model in other ways for which we do not account. Second, multiple considerations in addition to eGFR determine if and when patients receive referral to nephrology care, transplant nephrology evaluation, and inclusion on the kidney transplant waitlist. Our study focused exclusively on the impact of eGFR. In addition, our modeling utilized analyses regarding a single measurement of creatinine recorded in NHANES data, though we recognize that CKD classification and diagnosis is based on demonstration of decreased kidney function for three months. Third, these data are based on United States samples and clinical thresholds and are not generalizable to other contexts. Fourth, while the use of data from NHANES allows us to assess the distribution of eGFR in a population-based sample, the underlying sample size in some categories can result in imprecise estimates with wide confidence intervals that should be interpreted with caution. Future research could develop and assess new creatinine-based eGFR estimations that do not include race, consider effects of socioeconomic status, insurance, and experiences of racism on kidney disease and treatment, evaluate the impact of institutional policy changes regarding the use of race in estimations of kidney function, and analyze outcomes in other global populations.
Our findings lend themselves to multiple recommendations. First, we argue that eGFR race adjustment should be abandoned. This is in line with consensus established by the American Society of Nephrology (ASN) and National Kidney Foundation (NKF) Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Diseases, which formally recommends the removal of the eGFR race variable in all laboratories [38]. Though some scholars note that eliminating race adjustment would decrease the number of Black kidney donor candidates [18] or increase individual and institutional costs by prompting treatment of Black patients who previously would not be diagnosed with CKD [18], it is important to note that Black kidney disease patients already receive unequal care and eliminating race coefficients would improve reduce racial inequities [[25], [26], [27],39]. In this case, the benefit of increased sensitivity outweighs the penalty of decreased specificity. More importantly, this type of discussion—where the pros and cons of race adjustment are weighed in competition—creates a false dichotomy and misleading dilemma of restricted options since precise, race-neutral alternatives like Cystatin C already exist [40].
Second, in conjunction with our first recommendation, we call for the widespread implementation of race-free estimations of kidney function [40]. New research suggests that novel eGFR equations that incorporate creatine and cystatin C assays while omitting race are more accurate that existing race-based formulas [41]. These improved approaches should be widely applied and considered alongside other patient factors such as co-morbidities, diet, lifestyle, and acute illness in treatment plans [38].
Fifteen people die while waiting on the kidney transplant list every day: on average, nine of those dead will be Black [42]. Thousands of candidates are removed from the waitlist each year because they have become too sick. Consequently, preemptive listing—which allows patients with an eGFR ≤20 to accumulate waitlist time at earlier disease stages—increases chance of transplant receipt while reducing morbidity and mortality. Although some scholars have argued that transition to Cystatin C estimates may not improve accuracy for all patients and may increase costs [29], we argue that the potential for reduction in nephrological inequities and survival advantage for Black patients again outweigh these potential limitations.
Third, as healthcare institutions and clinicians transition to race-free estimations as standard practice, measures should be taken to eliminate use of the race adjustment when calculating eGFR. Since 1999, race-specific eGFR calculations have shaped clinical landscapes. State legislation in the U.S., for example, require eGFR reporting with retaining measurement, which effectively mandates race discrepant measures of kidney function [43]. These realities impact public spending, health policy, insurance claims, and mortality. Institutions can remove the race coefficient from laboratory reporting. Led by student and faculty activism, several institutions, including Beth Israel Deaconess Medical Center, Zuckerberg San Francisco General Hospital, University of Washington Medicine, Massachusetts General Brigham and Women's Hospital, the Mount Sinai Health System, and Vanderbilt University Medical Center, have already abandoned the race-specific factor. In the absence of institutional commitment against race multipliers, individual providers can advocate for patients by ensuring racialized coefficients do not act as barriers to treatment. Clinicians should utilize a race-conscious approach36—reject race-based clinical adjustments while being mindful of existing health inequities that deprive Black patients of equitable care—and always use the eGFR estimate that offers the most benefit to each patient.
Our study, as well as mounting scholarship and advocacy, suggests that eGFR race adjustments may constitute barriers to CKD diagnosis, nephrology referral, and transplant referral, which systematically disadvantages Black patients [2,5,6,36]. Race coefficients should not be framed as apolitical, empiric calculations necessary for patient care. We argue for reforms at institutional and individual levels to mitigate racialized healthcare inequities. Abandoning racialized eGFR calculations dismantles discriminatory and unscientific practices and provides opportunity for more accurate, thoughtful, and equitable medicine.
Contributors
JWT directed the study execution. JWT and JPC contributed to the study conception and design, interpretation of study data, and manuscript preparation. WCD and JSK contributed to the acquisition, analysis, and interpretation of raw study data. WSA, VG, and MLM contributed to manuscript preparation and provided critical revisions for intellectual content. All authors made the decision to submit the study, approved of the final manuscript, and hold themselves accountable for the accuracy, integrity, and appropriateness of the study.
Data Sharing Statement
Data are publicly available at https://www.cdc.gov/nchs/nhanes/index.htm. Programming code is available upon request.
Funding
No external funding was received for this study.
Declaration of Competing Interest
Author JPC acknowledges support from the National Institutes of Health Medical Scientist Training Program Grant T32GM136651 and the Robert Wood Johnson Health Policy Research Scholars Program. Author WSA has previously acted as a site principal investigator, with fees paid to the institution, for Novartis, Medeor Therapeutics, Amplyx Therapeutics, and InRegen. WSA also serves on the education committee for the American Society of Transplantation. Author MLM previously consulted for BayerAG in 2020. Author VG has previously received payment for educational work for Akebia Pharmaceuticals, the University of New Mexico, Alameda Health System, and the University of Illinois Chicago.
Footnotes
Declaration of Interests: All authors have no conflicts of interest to report.
References
- 1.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. The lancet. 2017;389(10075):1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 2.Kuppachi S, Norman SP, Lentine KL, Axelrod DA. Using race to estimate glomerular filtration and its impact in kidney transplantation. Clinical transplantation. 2020:e14136. doi: 10.1111/ctr.14136. [DOI] [PubMed] [Google Scholar]
- 3.Bhuvanakrishna T, Blake GM, Hilton R, Burnapp L, Sibley-Allen C, Goldsmith D. Comparison of estimated GFR and measured GFR in prospective living kidney donors. International urology and nephrology. 2015;47(1):201–208. doi: 10.1007/s11255-014-0859-y. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Annals of internal medicine. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 5.Grubbs V. Precision in GFR Reporting: Let's Stop Playing the Race Card. Am Soc Nephrol. 2020 doi: 10.2215/CJN.00690120. In: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts DE. Abolish race correction. Lancet (London, England) 2021;397(10268):17–18. doi: 10.1016/S0140-6736(20)32716-1. [DOI] [PubMed] [Google Scholar]
- 7.Braun L, Wentz A, Baker R, Richardson E, Tsai J. Racialized Algorithms for Kidney Function: Erasing Social Experience. Social Science & Medicine. 2020 doi: 10.1016/j.socscimed.2020.113548. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman JS, Cooper RS. In search of the hypothesis. Public Health Reports. 1995;110(6):662. [PMC free article] [PubMed] [Google Scholar]
- 9.Levey A. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:A0828. [Google Scholar]
- 10.Hebert LA, Kusek JW, Greene T, et al. Effects of blood pressure control on progressive renal disease in blacks and whites. Hypertension. 1997;30(3):428–435. doi: 10.1161/01.hyp.30.3.428. [DOI] [PubMed] [Google Scholar]
- 11.Franks CE, Scott MG. On the Basis of Race: The Utility of a Race Factor in Estimating Glomerular Filtration. The Journal of Applied Laboratory Medicine. 2020 doi: 10.1093/jalm/jfaa128. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Tighiouart H, Titan SM, Inker LA. Estimation of glomerular filtration rate with vs without including patient race. JAMA internal medicine. 2020;180(5):793–795. doi: 10.1001/jamainternmed.2020.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis J, Agodoa L, Cheek D, et al. Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. American journal of kidney diseases. 2001;38(4):744–753. doi: 10.1053/ajkd.2001.27691. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Astor BC, Stevens LA, Coresh J. Chronic kidney disease, diabetes, and hypertension: what's in a name? Kidney International. 2010;78(1):19–22. doi: 10.1038/ki.2010.115. [DOI] [PubMed] [Google Scholar]
- 16.Heaf JG. The origin of the 1• 73-m2 body surface area normalization: problems and implications. Clinical physiology and functional imaging. 2007;27(3):135–137. doi: 10.1111/j.1475-097X.2006.00718.x. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed S, Nutt CT, Eneanya ND, et al. Examining the Potential Impact of Race Multiplier Utilization in Estimated Glomerular Filtration Rate Calculation on African-American Care Outcomes. Journal of General Internal Medicine. 2020 doi: 10.1007/s11606-020-06280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diao JA, Wu GJ, Taylor HA, et al. Clinical Implications of Removing Race From Estimates of Kidney Function. JAMA. 2020 doi: 10.1001/jama.2020.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zelnick LR, Leca N, Young B, Bansal N. Association of the estimated glomerular filtration rate with vs without a coefficient for race with time to eligibility for kidney transplant. JAMA network open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.34004. e2034004-e2034004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pathologists TCoA. Current Status Of Reporting Estimated Glomerular Filtration Rate (eGFR) for adults. 2011.
- 21.Control CfD, Prevention. NHANES laboratory procedures manual. Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA.2011.
- 22.Levey AS, Coresh J, Bolton K, et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American Journal of Kidney Diseases. 2002;39(2 SUPPL. 1) [PubMed] [Google Scholar]
- 23.Levin A, Stevens PE, Bilous RW, et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney international supplements. 2013;3(1):1–150. [Google Scholar]
- 24.Lhotta K, Zoebl M, Mayer G, Kronenberg F. Late referral defined by renal function: association with morbidity and mortality. Journal of nephrology. 2003;16(6):855–861. [PubMed] [Google Scholar]
- 25.Jungers P, Massy ZA, Nguyen-Khoa T, et al. Longer duration of predialysis nephrological care is associated with improved long-term survival of dialysis patients. Nephrology Dialysis Transplantation. 2001;16(12):2357–2364. doi: 10.1093/ndt/16.12.2357. [DOI] [PubMed] [Google Scholar]
- 26.Chan MR, Dall AT, Fletcher KE, Lu N, Trivedi H. Outcomes in patients with chronic kidney disease referred late to nephrologists: a meta-analysis. The American journal of medicine. 2007;120(12):1063–1070. doi: 10.1016/j.amjmed.2007.04.024. e1062. [DOI] [PubMed] [Google Scholar]
- 27.Norton JM, Moxey-Mims MM, Eggers PW, et al. Social determinants of racial disparities in CKD. Journal of the American Society of Nephrology. 2016;27(9):2576–2595. doi: 10.1681/ASN.2016010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.System URD. National Institute of Diabetes and Digestive and Kidney Diseases Bethesda. 2018. USRDS 2018 annual report: atlas of chronic kidney disease and end-stage renal disease in the United States. Md. [Google Scholar]
- 29.Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR estimation. Clinical Journal of the American Society of Nephrology. 2020 doi: 10.2215/CJN.12791019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bukabau JB, Sumaili EK, Cavalier E, et al. Performance of glomerular filtration rate estimation equations in Congolese healthy adults: the inopportunity of the ethnic correction. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0193384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanocco JA, Nishida SK, Passos MT, et al. Race adjustment for estimating glomerular filtration rate is not always necessary. Nephron Extra. 2012;2(1):293–302. doi: 10.1159/000343899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bukabau JB, Yayo E, Gnionsahé A, et al. Performance of creatinine-or cystatin C–based equations to estimate glomerular filtration rate in sub-Saharan African populations. Kidney International. 2019;95(5):1181–1189. doi: 10.1016/j.kint.2018.11.045. [DOI] [PubMed] [Google Scholar]
- 33.Rule AD, Teo BW. GFR estimation in Japan and China: what accounts for the difference? American journal of kidney diseases: the official journal of the National Kidney Foundation. 2009;53(6):932. doi: 10.1053/j.ajkd.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Non AL, Gravlee CC, Mulligan CJ. Education, genetic ancestry, and blood pressure in African Americans and Whites. American journal of public health. 2012;102(8):1559–1565. doi: 10.2105/AJPH.2011.300448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Llibre-Guerra JJ, Li Y, Allen IE, et al. Race, genetic admixture and cognitive performance in the Cuban population. The Journals of Gerontology: Series A. 2021 doi: 10.1093/gerona/glab063. [DOI] [PubMed] [Google Scholar]
- 36.Cerdena JP, Plaisime MV, Tsai J. From race-based to race-conscious medicine: how anti-racist uprisings call us to act. Lancet. 2020;396(10257):1125–1128. doi: 10.1016/S0140-6736(20)32076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phelan JC, Link BG, Feldman NM. The genomic revolution and beliefs about essential racial differences: A backdoor to eugenics? American sociological review. 2013;78(2):167–191. doi: 10.1177/0003122413476034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. American Journal of Kidney Diseases. 2021 doi: 10.1053/j.ajkd.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Sehgal AR. American College of Physicians. 2020. Race and the False Precision of Glomerular Filtration Rate Estimates. [DOI] [PubMed] [Google Scholar]
- 40.Diao JA, Inker LA, Levey AS, Tighiouart H, Powe NR, Manrai AK. In Search of a Better Equation—Performance and Equity in Estimates of Kidney Function. New England Journal of Medicine. 2021 doi: 10.1056/NEJMp2028243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inker LA, Eneanya ND, Coresh J, et al. New creatinine-and cystatin C–based equations to estimate GFR without race. New England Journal of Medicine. 2021 doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schold J, Srinivas TR, Sehgal AR, Meier-Kriesche H-U. Half of kidney transplant candidates who are older than 60 years now placed on the waiting list will die before receiving a deceased-donor transplant. Clinical Journal of the American Society of Nephrology. 2009;4(7):1239–1245. doi: 10.2215/CJN.01280209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDonough DP. New Jersey's experience: mandatory estimated glomerular filtration rate reporting. Clin J Am Soc Nephrol. 2007;2(6):1355–1359. doi: 10.2215/CJN.01570407. [DOI] [PubMed] [Google Scholar]