Abstract
Running, compared to pedaling is a whole-body locomotive movement that may confer more mental health via strongly stimulating brains, although running impacts on mental health but their underlying brain mechanisms have yet to be determined; since almost the mechanistic studies have been done with pedaling. We thus aimed at determining the acute effect of a single bout of running at moderate-intensity, the most popular condition, on mood and executive function as well as their neural substrates in the prefrontal cortex (PFC). Twenty-six healthy participants completed both a 10-min running session on a treadmill at 50% and a resting control session in randomized order. Executive function was assessed using the Stroop interference time from the color-word matching Stroop task (CWST) and mood was assessed using the Two-Dimensional Mood Scale, before and after both sessions. Prefrontal hemodynamic changes while performing the CWST were investigated using functional near-infrared spectroscopy. Running resulted in significant enhanced arousal and pleasure level compared to control. Running also caused significant greater reduction of Stroop interference time and increase in Oxy-Hb signals in bilateral PFCs. Besides, we found a significant association among pleasure level, Stroop interference reaction time, and the left dorsolateral PFCs: important brain loci for inhibitory control and mood regulation. To our knowledge, an acute moderate-intensity running has the beneficial of inducing a positive mood and enhancing executive function coinciding with cortical activation in the prefrontal subregions involved in inhibitory control and mood regulation. These results together with previous findings with pedaling imply the specificity of moderate running benefits promoting both cognition and pleasant mood.
Subject terms: Neuroimmunology, Microglia, Psychiatric disorders
Introduction
Running is not only a principal form of physical exercise, but it also has roots as a major contributing factor to the physiological and anatomical evolution of humans1,2. As a whole-body locomotive movement, running may greatly impact on mental health via stimulating the brains that differ from other forms of exercise such as leg-based pedaling. Given exercise is medicine, the effects of drugs differ depending on the type of drug, different types of exercise such as running and pedaling should be observed to have different effects on mental health and brain activation as well.
Consider the previous studies, including our own, physical exercise has been reported to enhance executive function by predominantly activating the left dorsolateral prefrontal cortex (l-DLPFC) that is the brain loci implicating in inhibitory control, without reporting change of pleasant mood3–5. Almost these studies have used pedaling as a physical exercise, not running. Although running was reported to improve mood as well as neurocognitive function based-on event-related potentials (ERPs), but a neuronal substrate with which running enhances executive function related to prefrontal subregion activation remains unelucidated6–8.
Running is not only an exercise that promotes physical health by enhancing cardiovascular endurance, strengthening muscles and building strong bones as it is a weight-bearing exercise, but also has an implication in lowering mental health burden9–12. To execute the appropriate movement, running requires neuronal top-down feedforward control responses to multi-modal sensory information to control coordinated movements and balance13–15. The prefrontal cortex, a brain region implicated in cognition and mood regulation, is partially involve in running, especially when there is demand for coordinated action14–21. Muscles throughout the body, in particular leg muscles, are recruited continuously to propel the body forward while supporting the body weight22. This evidence may support that running has stronger beneficial effects on mood and executive function related to prefrontal activation compared to other forms of exercise that do not require as much coordination of weight-bearing activity such as pedaling. Furthermore, the mechanical impact of each foot-strike during running has been shown to increase blood circulation peripherally and centrally that may benefit brain activation23,24. However, these findings need more evidence to explain a mechanism. Besides, a mechanical force from vertical head acceleration, the rate of change of head velocity in up and down direction, during running has been shown in animal studies to induce serotonin receptor internalization in the prefrontal cortex, improving emotion and cognitive control25. Based on these properties of running, we hypothesized that running has the potential to enhance mood and executive function with broad prefrontal activation.
Few studies reported that a single bout running influence an inhibitory control, a core executive function which involves being able to control one’s attention, behavior, thoughts and emotions to override strong internal predispositions or external lures16,26–28. The color-word matching Stroop task (CWST) is extensively used in both experimental and clinical settings to measure this aspect of executive function localized in the prefrontal cortex, in particular l-DLPFC29–31. The CWST consisted of three conditions those are neutral, congruent and incongruent; ranging from the lowest to the highest executive function demand. The test requires the participants to name the color in which a word is written rather than reading the word itself as quickly as possible30. Based-on the previous evidence, we hypothesized that an acute moderate-intensity running would be associated with shorter interference times (i.e., difference between response times in neural condition vs. incongruent condition), which could reflect an enhancement of executive function.
With respect to neural mechanisms, it has remained unknow how running induced neural activation in enhancing mood and executive functions. Based-on the properties of running, running may induce arousal levels by activating the reticular-activating system (RAS), a network of neurons located in the brainstem, that regulates ascending projections to the prefrontal cortex that are implicated in cognitive control and mood regulation32–39. To test this assumption, we used functional near-infrared spectroscopy (fNIRS) during the performance of the CWST to measure prefrontal hemodynamic changes, as in our previous studies3–5,40–43.
Considering statistical robustness as regards neuroimaging analysis, we focused on comparison between resting control and running to clarify the effects of an acute bout of running with hypothesizing that acute moderate-intensity running would enhance executive function by greatly impacting on positive mood with broad prefrontal activation.
Results
Verification of moderate intensity running
To verify whether the participants could perform moderate-intensity running, we monitored heart rate (HR) and rate of perceived exertion (RPE) every minute44. During the last minute of running, average HR and RPE were 141.35 ± 11.31 bpm and 10.61 ± 1.72 points, respectively. Based on the guidelines of the American College of Sports Medicine, average HR was in the appropriate range for moderate-intensity exercise whereas RPE was observed to be lower than the reference range (12–13 points)45.
Psychological results
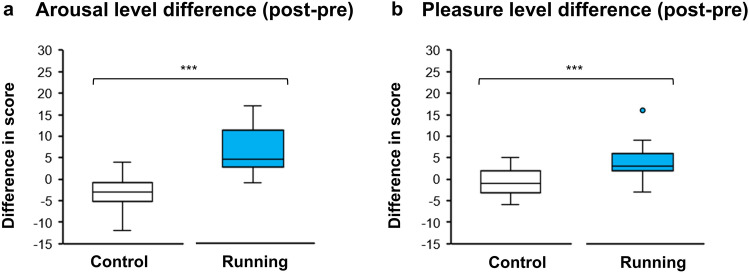
Arousal and pleasure were found to have significant interactions between session (CON, RUN) and time (pre, post) (F(1, 25) = 62.21, p < 0.001 and F(1, 25) = 24.73, p < 0.001, respectively; repeated-measures two-way ANOVA). Changes of arousal and pleasure were subsequently examined revealing that the RUN session had a significantly greater increase of arousal and pleasure than did the CON session (t(25) = 7.89, p < 0.001, Cohen’s d = 1.55 (Fig. 1a) and t(25) = 4.97, p < 0.001 , Cohen’s d = 0.98 (Fig. 1b), respectively; paired t-test). A comparison of baseline arousal and pleasure between CON and RUN were provided in Supplementary A.
Figure 1.
Comparison in mood change [(post-session)—(pre-session)] between control and running: (a) arousal level difference and (b) pleasure level difference. The bottom, middle and top lines of each box indicate the 25th, 50th (median) and 75th percentiles, respectively. Whiskers above and below each box refer to the most extreme point within 1.5 times the interquartile range. Points above the whiskers are outliers. *** = p < 0.001.
Behavioral results
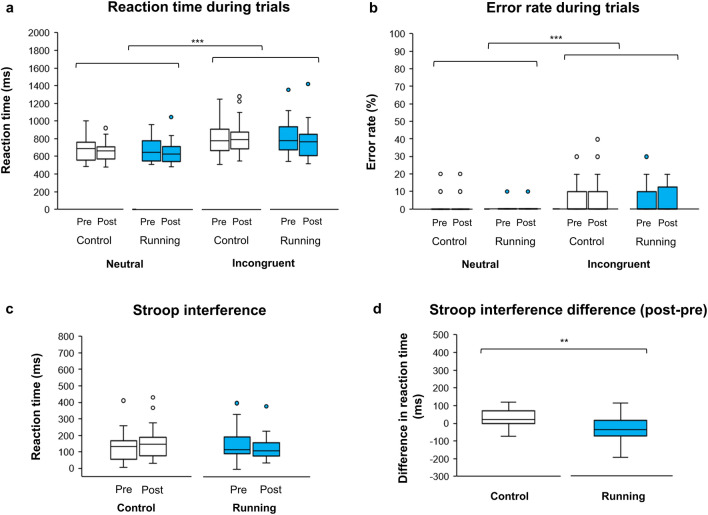
RT and ER had significant main effects of condition (neutral/incongruent; F(1, 25) = 87.47, p < 0.001 (Fig. 2a) and F(1, 25) = 27.50, p < 0.001 (Fig. 2b), respectively; repeated-measures three-way ANOVA) showing that the Stroop interference effect was basically found between the neutral and incongruent conditions in all sessions of this study. Next, the effects of running on Stroop interference RT and ER were examined. Stroop interference RT revealed significant interaction between session (CON, RUN) and time (pre, post) (F(1, 25) = 11.79, p = 0.002; repeated-measures two-way ANOVA; Fig. 2c) whereas Stroop interference ER did not reveal significant interaction (F(1, 25) = 0.013, p = 0.911). Finally, changes in Stroop interference RT were investigated and the results showed that Stroop interference RT in the RUN session was significantly more reduced than in the CON session (t(25) = -3.43, p = 0.002, Cohen’s d = 0.67; paired t-test; Fig. 2d).
Figure 2.
Comparison of Stroop task performance in (a) reaction time and (b) error rate between neutral and incongruent conditions. (c) Difference in Stroop interference [incongruent—neutral] between control and running sessions. (d) Contrast in Stroop interference difference ([incongruent—neutral of post-session]—[incongruent—neutral of pre-session]) between control and running. The bottom, middle and top lines of each box indicate the 25th, 50th (median) and 75th percentiles, respectively. Whiskers above and below each box refer to the most extreme point within 1.5 times the interquartile range. Points above the whiskers are outliers. *** = p < 0.001, ** = p < 0.01.
fNIRS results
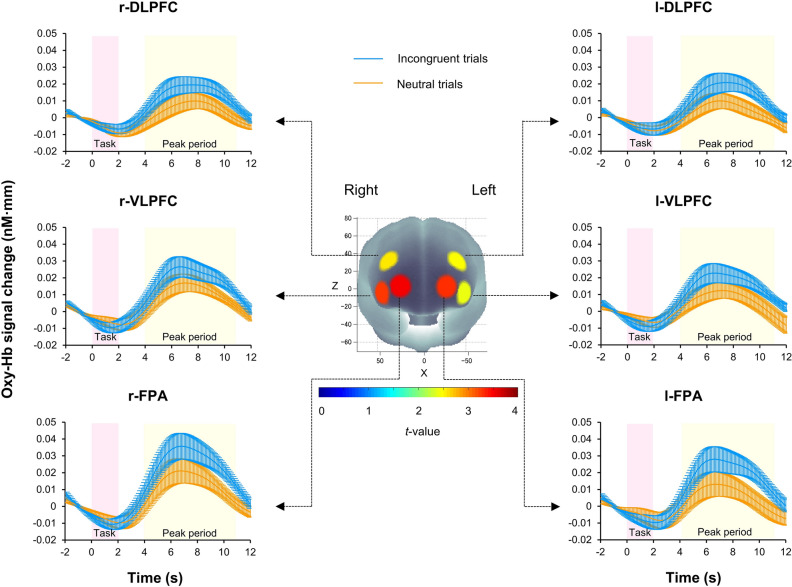
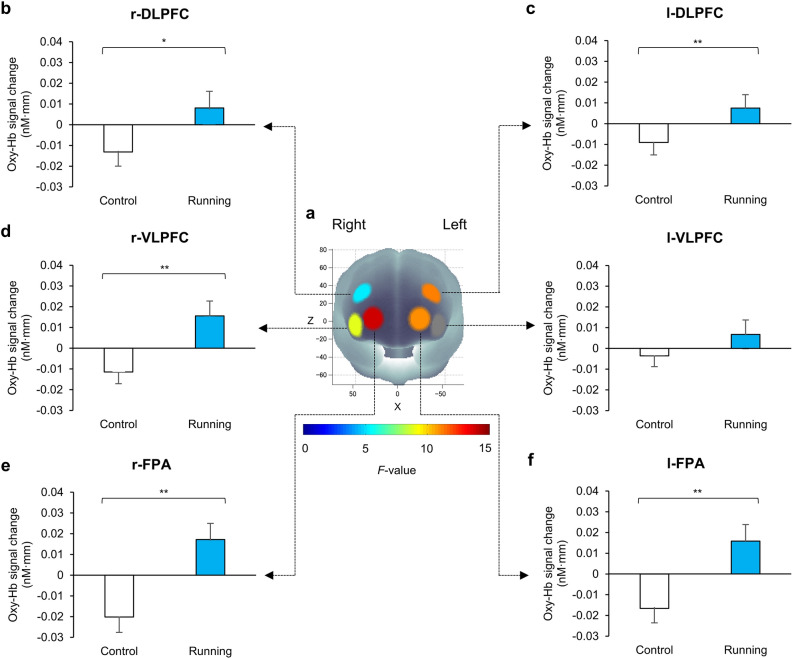
There were no significant differences in pre-sessions of Stroop-interference-related cortical activation between CON and RUN conditions in any ROIs. The pre-session data for the CON and RUN sessions, which were free from any effect of exercise, were averaged and served as substrates for a ROI-wise analysis. Significant Stroop-interference-related cortical activations were found in all ROIs (p < 0.05, one-sample t-test, FDR correction) indicating that the Stroop interference effect could be observed in this study (Fig. 3). The effect of running on prefrontal activation in all ROIs was subsequently determined and the results revealed that Stroop-interference-related cortical activations had significant interaction between session (CON, RUN) and time (pre, post) in 5 ROIs: l-DLPFC (F(1, 25) = 11.26, p = 0.003), l-FPA (F(1, 25) = 11.04, p = 0.003), r-DLPFC (F(1, 25) = 5.36, p = 0.029), r-VLPFC (F(1, 25) = 8.98, p = 0.006) and r- FPA (F(1, 25) = 13.85, p = 0.001) (repeated-measures two-way ANOVA). Finally, changes in oxy-Hb with Stroop interference in these 5 ROIs were examined and the results revealed that the RUN session had a significantly greater increase of oxy-Hb with Stroop interference than did the CON session in all 5 ROIs: l-DLPFC (t(25) = 3.36, p = 0.003, Cohen’s d = 0.66), l-FPA (t(25) = 3.32, p = 0.003, Cohen’s d = 0.65), r-DLPFC (t(25) = 2.31, p = 0.029, Cohen’s d = 0.45), r- VLPFC (t(25) = 3.00, p = 0.006, Cohen’s d = 0.59) and r-FPA (t(25) = 3.72, p = 0.001, Cohen’s d = 0.66) (paired t-test, FDR correction; Fig. 4). Cortical activation patterns during performance of the CWST in the post-session of RUN were provided in Supplementary B.
Figure 3.
Cortical activation patterns during performance of the color-word matching Stroop task (CWST) in the pre-session of control and running. Presented data are average values between the pre-session of control and the pre-session of running. Baseline (2 s before trial onset) is set at zero and peak periods are indicated from 4 to 11 s after trial onset. Significant Stroop-interference-related cortical activations [incongruent—neutral] were found in all regions of interest (ROIs) indicating that the Stroop interference effect can be observed. The t-map demonstrates oxygenated hemoglobin (Oxy-Hb) signal change, t-values are indicated as in the color bar. Data are mean ± SE.
Figure 4.
Running elicits prefrontal activation in response to the Stroop interference difference ([incongruent—neutral of post-session]—[incongruent—neutral of pre-session]). (a) F-map of oxygenated hemoglobin (Oxy-Hb) signal change, F-values are indicated as in the color bar. Among the 6 regions of interest (ROIs), significant differences are found in (b) the right dorsolateral prefrontal cortex, (c) the left dorsolateral prefrontal cortex, (d) the right ventrolateral prefrontal cortex, (e) the right frontopolar area and (f) the left frontopolar area. Data are mean ± SE, **p < 0.01, *p < 0.05.
The association among mood, behavior and fNIRS results
Significant coincidences between running-induce mood change and running-effected behavioral Stroop performance were observed. Increased arousal and pleasure levels significantly coincided with shortened Stroop interference RT (χ2mc (1,26) = 17.05, p < 0.001 and χ2mc (1,26) = 14.06, p < 0.001, respectively; McNemar test). Enhanced arousal levels were consistently found to significantly coincide with all 5 ROIs (l-DLPFC, χ2mc (1,26) = 14.06, p < 0.001; l-FPA, χ2mc (1,26) = 18.05, p < 0.001; r-DLPFC, χ2mc (1,26) = 16.06, p < 0.001; r-VLPFC, χ2mc (1,26) = 15.06, p < 0.001 and r-FPA, χ2mc (1,26) = 17.05, p < 0.001; McNemar test) as were pleasure levels (l-DLPFC, χ2mc (1,26) = 7.56, p = 0.004; l-FPA, χ2mc (1,26) = 14.06, p < 0.001; r-DLPFC, χ2mc (1,26) = 12.07, p < 0.001; r-VLPFC, χ2mc (1,26) = 9.60, p = 0.001 and r-FPA, χ2mc (1,26) = 13.07, p < 0.001; McNemar test) (Table 1). In addition, shortened Stroop interference RT demonstrated significant coincidence with all 5 ROIs (l-DLPFC, χ2mc (1,26) = 7.69, p = 0.003; l-FPA, χ2mc (1,26) = 11.53, p < 0.001; r-DLPFC, χ2mc (1,26) = 9.60, p = 0.001; r-VLPFC, χ2mc (1,26) = 6.72, p = 0.008 and r-FPA, χ2mc (1,26) = 10.56, p = 0.001; McNemar test).
Table 1.
Coincidence frequency between running-induced mood change and sub-regional prefrontal activation for McNemar test.
| Oxy-Hb in l-DLPFC | Total | Oxy-Hb in l-FPA | Total | Oxy-Hb in r-DLPFC | Total | Oxy-Hb in r-VLPFC | Total | Oxy-Hb in r-FPA | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| − | + | − | + | − | + | − | + | − | + | |||||||
| Arousal | + | 9 | 16 | 25 | 5 | 20 | 25 | 7 | 18 | 25 | 8 | 17 | 25 | 6 | 19 | 25 |
| − | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | |
| Total | 9 | 17 | 26 | 5 | 21 | 26 | 7 | 19 | 26 | 8 | 18 | 26 | 6 | 20 | 26 | |
| Pleasure | + | 7 | 14 | 21 | 5 | 16 | 21 | 7 | 14 | 21 | 7 | 14 | 21 | 6 | 15 | 21 |
| − | 2 | 3 | 5 | 0 | 5 | 5 | 0 | 5 | 5 | 1 | 4 | 5 | 0 | 5 | 5 | |
| Total | 9 | 17 | 26 | 5 | 21 | 26 | 7 | 19 | 26 | 8 | 18 | 26 | 6 | 20 | 26 | |
Presented values are number of participants. The contrasts between [(post-session)−(pre-session)] in RUN and [(post-session)−(pre-session)] in CON for each variable was subjected to analysis. Significant coincidences among variables were observed. l-DLPFC = left dorsolateral prefrontal cortex, l-FPA = left frontopolar area, r-DLPFC = right dorsolateral prefrontal cortex, r-VLPFC = right ventrolateral prefrontal cortex, r-FPA = right frontopolar area.
Discussion
Here we used fNIRS to test the hypothesis that acute running stimulates the PFC, which results in increasing positive mood and executive function. We found that a single bout of moderate-intensity running at 50%, the most popular running condition, enhanced mood not only through arousal but also through pleasure and also influenced executive function, as evidenced in the shortening of Stroop interference RT, while activating the cortical areas associated with cognition and mood regulation. These results clearly support the hypothesis that acute moderate running benefits mood and executive function coincident with cortical activation in the prefrontal subregions involved in mood regulation. Referred to our previous evidence that suggested single benefit of moderate pedaling exercise in enhancing cognition without reporting pleasant mood change based-on the TDMS, the current study could demonstrate dual benefits of moderate running exercise in increasing cognition and pleasure level, which may influence the brain in regulating stress, leading to mental health4,5,43,46–48.
Running apparently enhanced arousal levels compared to a resting control, and this is consistent with our previous studies4,5. Moreover, we also revealed that 10 min of moderate-intensity running benefited pleasure levels, which also agrees with previous evidence reporting increased positive affection after a single bout of moderate-intensity running49,50. Based on these findings, running may be considered an exercise mode that benefits mood, which is an important factor for exercise adherence51. Another psychological response observed here is an observed RPE that was lower than the reference range45. Theoretically, RPE is a recognized marker of intensity and homeostatic disturbance during exercise as well as an effective way to monitor physical effort52,53. In regulating RPE, we propose that the prefrontal cortex may be involved in the interpretation of physiological signals, which are integrated between internal and external factors present in exercise environments to determine exercise tolerance52,54. Therefore, running seems to have the potential to influence prefrontal regions to diminish perceived physical effort (lower RPE) that presumably benefits exercise performance. However, this account remains somewhat speculative.
The behavioral response investigated using the CWST revealed longer RT with higher ER in the incongruent condition compared to the neutral condition, demonstrating that the Stroop interference effect could be observed across all sessions. The Stroop interference time was subsequently determined, and the results support our hypothesis that running shortened Stroop interference time compared to the resting control3–5. Moreover, significant coincidence between enhanced mood (increased arousal and pleasure levels) and shortened Stroop interference time was revealed. Together, these results allow us to postulate that a running-induced positive mood could enhance behavioral Stroop performance resulting in increased executive function, although the directionality of this relationship cannot be directly inferred from our data34,37.
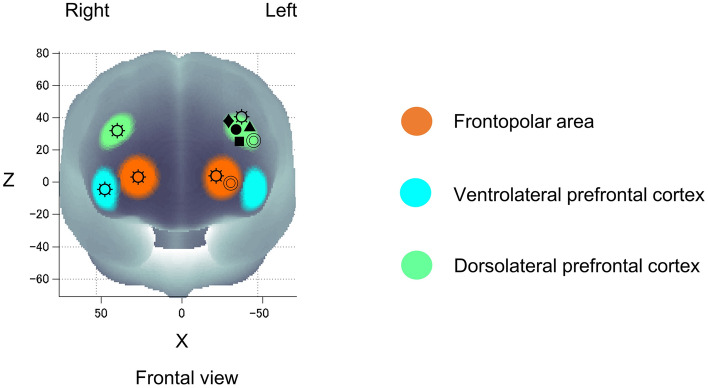
The prefrontal cortex – the neural substrate underpinning the performance of the CWST – revealed significant increases of Oxy-Hb in response to Stroop interference for all ROIs, showing that the Stroop interference effect could be observed as in our previous studies4,5. The effect of running on prefrontal activation was subsequently determined, and the results showed that running led to significant increases in activation in all ROIs except the l-VLPFC. The l-DLPFC, which plays an important role in the implementation of cognitive control, had increase of Oxy-Hb response to Stroop interference, which corresponds to the results of previous studies including our own4,5,30,55,56. Besides, the l-DLPFC was reported to implicate in mood regulation in mood disorders, demonstrating significant coincidence between running-induced positive mood and l-DLPFC activation as we hypothesized19–21. The right DLPFC, which is involved in conflict resolution (faster reaction time after incongruent trials) and Stroop accuracy, was also showed significant activation31,57,58. The right-VLPFC, which is associated with mood and emotion regulation, was observed to have significant activation coinciding with increased arousal and pleasure levels19–21,59. In addition, the FPA, a brain region which generally activates with the DLPFC to response to tasks involving manipulation and monitoring, such as planning for action, was observed to have significant both sides activation60. It is considered that running-induced bilateral prefrontal activation, differing from the previous studies using pedaling exercise that revealed prominent l-DLPFC activation (Fig. 5)3–5,43,61. Taken together, these results support our hypothesis that an acute bout of moderate-intensity running elicits mood improvement and enhances executive function coinciding with prefrontal subregion activations involved in mood regulation.
Figure 5.
Exercise elicits prefrontal activation in response to Stroop interference difference ([incongruent—neutral of post-session]—[incongruent—neutral of pre-session]) of the present moderate-intensity running study (☼) compared to previous studies with pedaling exercise: moderate-intensity exercise (Yanagisawa et al., 2010: ●), moderate-intensity exercise (Endo et al., 2013: ■), mild-intensity exercise (Byun et al., 2014: ◎), high-intensity intermittent exercise (Kujach et al., 2018: ♦) and moderate-intensity exercise with music (Suwabe et al., 2021: ▲). All studies measured cortical activation using fNIRS while participants performed the color-word matching Stroop task (CWST).
The neural mechanisms for running-elicited cortical activation have remained unclear. Running requires top-down feedforward control responses to multi-modal sensory information that partially involve the prefrontal cortex to execute coordinated movement and balance13–15,17,18. Specific features of running, such as foot strike, may benefit the brain activation by enhancing blood flow velocity in the middle cerebral artery24. However, the mechanism of this finding still unclear. Besides, a mechanical force from vertical head acceleration, may induce serotonin receptor internalization in the prefrontal cortex, influencing emotion and cognitive control based-on animal study25. With respect to the theoretical concept of acute exercise-enhanced cognition, it is considered that running has the potential to induce neural activation by activating the RAS to enhance arousal levels, and by projecting neuronal signals to the prefrontal cortex32,33,37–39. This neuronal response is mostly modulated by three neurotransmitter systems: the noradrenergic, dopaminergic and serotoninergic systems, which originate from the brainstem and project profusely throughout the forebrain, playing a role in cognition and mood regulation34–39.
That insufficient physical activity leads to physical and mental illness has been recognized as a global issue. It is important to demonstrate a minimal effective exercise that will benefit both mental and physical health. We have found that even a 10-min single bout of moderate-intensity running can boost both mood and executive function; in particular, broad prefrontal activation as well as enhanced pleasure levels are important for exercise adherence. These fruitful findings are meaningful in terms of health promotion because running is considered a practical exercise and is one of the most popular recreation activities worldwide.
Here we investigated only the effect of running, but not a pedaling because of the following methodological limitations. First, to gain statistical robustness through the process of neuroimaging analysis using fNIRS, we must limit session numbers. Second, we have already found the exact findings on pedaling using the same experimental protocol for ensuring the specificity of running effects4,5,30. Indeed, we successfully revealed the dual effects of running boosting mood and executive function, different from those pedaling (Fig. 5).
Conclusions
The current study reveals that a 10-min single-bout of moderate-intensity running elicits a positive mood and increased executive function by enhancing arousal levels coincidentally with activation in prefrontal subregions involved in mood regulation. We provide evidence for the neural substrates behind mood enhancement and increased executive function induced by acute moderate-intensity running. To this end, these finding are valuable in supporting moderate running effect on mental health since running is an easily accessible form of exercise requiring minimal equipment and sport structure. This should shed light on the specificity of running among a varieties of exercise prescriptions promoting mental health.
Materials and methods
Participants
Twenty-six young adults participated in this study. All participants were Japanese native speakers, right-hand dominant, with normal, or corrected-to-normal vision and normal color vision. No participant reported a history of neurological or psychiatric disorders, or had a disease requiring medical care. The characteristics of participants are presented in Table 2. This study was approved by the Institutional Ethics Committee of the University of Tsukuba, and the protocol was in accordance with the latest version of the Helsinki Declaration. Complete information about the study was given to the participants prior to obtaining written informed consent from all of them.
Table 2.
The characteristics of participants (n = 26).
| Male (n = 18) | Female (n = 8) | |
|---|---|---|
| Age (yr) | 23.22 ± 2.24 | 22.88 ± 1.96 |
| Weight (kg) | 67.78 ± 8.33 | 55.25 ± 10.23 |
| Height (cm) | 172.89 ± 3.87 | 158.66 ± 5.39 |
| BMI (kg/m2) | 22.66 ± 2.59 | 21.80 ± 2.79 |
| (ml/kg/min) | 53.05 ± 7.62 | 39.79 ± 5.63 |
| HRmax (bpm) | 191.06 ± 10.42 | 194.88 ± 5.36 |
Presented values are mean ± SD. BMI = Body Mass Index; = Peak oxygen uptake; HRmax = Maximal Heart Rate.
Experimental procedure
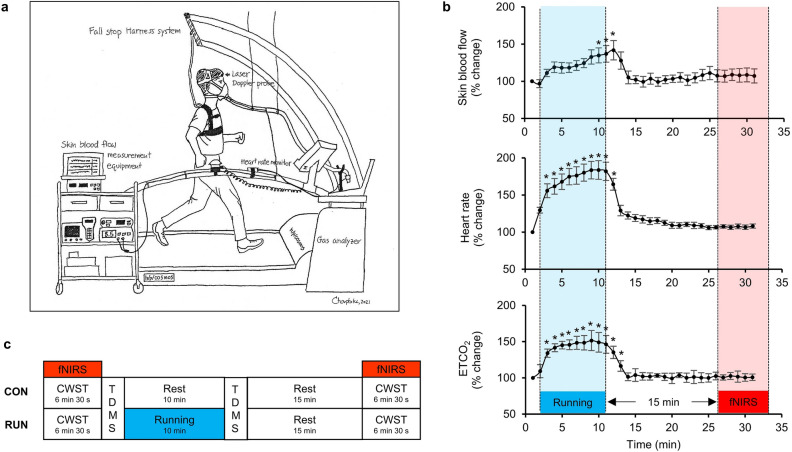
The experimental procedure consisted of three major steps. First, we measured maximal oxygen uptake () to determine the appropriate individual intensity of moderate exercise, which is 50% based on the definition of exercise intensity of the American College of Sports Medicine45. Second, we investigated non-cortical physiological signals induced by running in 6 participants, such as skin blood flow, to specify proper fNIRS measurement times after running in order to eliminate possible contamination of the fNIRS signal62. We found that all physiological variables fully returned to the baseline within 15 min. Therefore, 15 min after running, we started fNIRS measurements to avoid possible signal contamination (Fig. 6a and b). Details of the two preliminary steps are provided in Supplementary C. Finally, we determined the effect of a single bout of moderate-intensity running on mood and executive function. Two sessions, control (CON) and running (RUN), were randomly conducted with a counterbalance measure design on different days. In the RUN session, fNIRS was used to measure prefrontal hemodynamic changes while performing the CWST before and 15 min after running to avoid non-cortical physiological signal contamination. Participants ran for 10 min on a treadmill at a personalized speed. Mood state was assessed before and after running. In the CON session, participants rested for 10 min instead of running (Fig. 6c).
Figure 6.
(a) Illustration of experimental setup for the second preliminary step: non-cortical physiological measurements. A laser Doppler probe was attached to the forehead to assess skin blood flow. (b) Results of non-cortical physiological parameters at baseline (set at 100%), during 10 min of moderate-intensity running and 20 min rest. Time points with a significant change compared to baseline are identified with asterisks (p < 0.05, one-way ANOVA with Dunnett correction). All physiological variables fully returned to baseline within 15 min. Thus, fNIRS measurement was started 15 min after running to eliminate possible signal contamination. ETCO2 = End-tidal carbon dioxide; (c) Experimental design for control (CON) and running (RUN) sessions. CWST = color-word matching Stroop task, TDMS = Two-Dimensional Mood Scale, fNIRS = Functional near-infrared spectroscopy.
Psychological measures
Psychological mood state was measured before and after running using the Two-Dimensional Mood Scale (TDMS)63. The TDMS is a momentary mood scale that consists of eight words describing arousal and pleasure states (energetic, lively, lethargic, listless, relaxed, calm, irritated and nervous). The participants were asked to indicate how they were feeling for each mood-expressing word using a 6-point Likert scale that ranged from 0 = Not at all to 5 = Extremely. Then, arousal and pleasure levels were calculated.
Behavioral measures
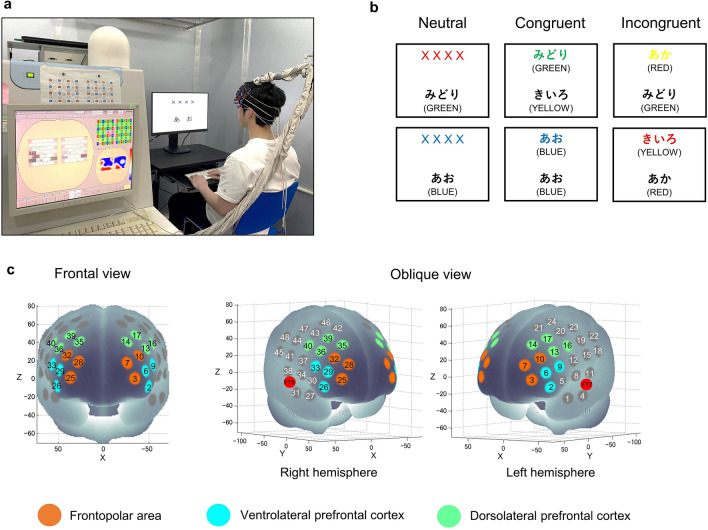
Executive function was investigated using an event-related version of the CWST3,4,43,64. The test appeared on the screen with 2 rows of letters (Fig. 7a). The participants were instructed to decide whether the color of the letters in upper row corresponded to the color name in the lower row. They were also asked to place their index fingers on “yes” and “no” buttons and respond to the test by pressing the appropriate button as fast as they could. Subsequently, reaction time (RT) and error rate (ER) were calculated. The CWST consisted of three conditions: neutral, congruent and incongruent. For the neutral condition, the upper row was presented as a row of X’s (XXXX) printed in red, green, blue or yellow, and the lower row presented the word ‘RED’, ‘GREEN’, ‘BLUE’ or ‘YELLOW’ printed in black. For the congruent condition, the upper row contained the word ‘RED’, ‘GREEN’, ‘BLUE’ or ‘YELLOW’ printed in the congruent color (e.g., ‘RED’ was printed in red), and the lower row presented the same words as in the lower row of the neutral condition. For the incongruent condition, the upper row presented the color word printed in an incongruent color to produce interference between the color word and the color name (e.g., ‘RED’ was printed in green), and the lower row presented the same words as in the lower row of the neutral and congruent conditions (Fig. 7b). Each experimental session consisted of 30 trials, made up of 10 neutral, 10 congruent and 10 incongruent trials, which appeared in random order. The upper row was presented 100 ms before the lower row in order to shift visual attention. The trial remained on the screen until the participant responded or for 2 s, whichever was shorter. Then, a fixation cross appeared on the screen as an inter-stimulus interval for 10–12 s to avoid prediction of the timing of the subsequent trial. Stroop interference, an index of executive function in the prefrontal cortex, was calculated as the difference in reaction time between the incongruent and neutral conditions30. All words were written in Japanese. The participants performed three practice sessions to ensure that they understood and were familiarized with the CWST well before starting the experiment.
Figure 7.
(a) Illustration of experimental setup for fNIRS measurements during the color-word matching Stroop task (CWST). (b) Examples of the CWST neutral, congruent and incongruent conditions. The presented words were written in Japanese. English translations are shown in parentheses. (c) Spatial profiles of fNIRS channels used in the present study. Two sets of probe holders were placed to cover both lateral prefrontal activation foci as in our previous studies. Colors indicate each region of interest (ROI) in the lateral part of the prefrontal cortex.
fNIRS data acquisition
Cortical hemodynamic changes were monitored using the multichannel fNIRS optical topography system ETG-7000 (Hitachi Medical Corporation, Japan) and adopting two wavelengths of near-infrared light (785 and 830 nm). The optical data from fNIRS was analyzed based on the modified Beer-Lambert Law as previously described65,66. With this method, we were able to calculate signals reflecting the oxygenated hemoglobin (oxy-Hb) concentration changes in a unit of millimolar-millimeter (mM∙mm)66. The sampling rate was set at 10 Hz. For fNIRS probe placement, two sets of 4 × 4 multichannel probe holders, which consist of 8 illuminative and 8 detective probes arranged alternately at an inter-probe distance of 3 cm resulting in 24 channels (CH) per set, were placed to cover both lateral prefrontal activation foci as in previous studies3,4. The left probe holder was placed such that probe 5 (between CH4 and CH11) was placed over FT7, with the medial edge of the probe column parallel to the medial line. Likewise, the right probe holder was symmetrically placed on the left hemisphere (Fig. 7c). We adopted a virtual registration method to register fNIRS data to Montreal Neurological Institute (MNI) standard brain space67,68. With this method, we were able to place a virtual probe holder on the scalp by stimulating the holder’s deformation and by registering probes and channels onto a reference brain in the magnetic resonance image (MRI) database69,70. We probabilistically estimated the MNI coordinate values for fNIRS in order to obtain the most likely microanatomical prediction for locations of the given channels as well as the spatial variability of the estimation71. Finally, a MATLAB function was used to label the estimated locations in a macro-anatomical brain atlas72.
Analysis of fNIRS data
Prefrontal Oxy-Hb changes that occurred while performing the CWST were calculated as shown in our previous studies3,4,40–43. Individual timeline data for each channel were preprocessed with a band-pass filter using a high-pass filter (0.04 Hz) to remove baseline drift and a low-pass filter (0.30 Hz) to screen out heartbeat pulsations. Then, channel-wise and subject-wise contrasts were calculated by the inter-trial mean of differences between peak (4–11 s after trial onset) and baseline (0–2 s before trial onset) periods. The contrasts obtained were subsequently subjected to a second level of random effects group analysis. This study adopted LBPA40, a widely used method among anatomical labeling systems, to combine 3—4 neighboring channels to form each region of interest (ROI)72. The regions included the DLPFC (l-DLPFC: channels 13, 14, 16 and 17; r-DLPFC: channels 35, 36, 39 and 40), the VLPFC (l-VLPFC: channels 2, 6 and 9; r-VLPFC: channels 26, 29 and 33) and the FPA (l-FPA: channels 3, 7 and 10; r-FPA: channels 25, 28 and 32). LBPA40 is considered valid because optical properties of neighboring channels are known to be similar73. However, with this method, optical properties in different ROIs can cause systematic bias during statistical analysis. Therefore, we limited analyses to ROI-wise and used a false discovery rate (FDR) to control the low proportion of false positives74.
Statistical analysis
Psychological mood state was subjected to repeated-measures two-way ANOVA with session (CON, RUN) and time (pre, post) as within-subject factors. Bonferroni’s post hoc test was subsequently processed after a significant F value was observed. Regarding behavioral Stroop performance, RT and ER were first subjected to repeated-measures three-way ANOVA with condition (neutral, incongruent), session (CON, RUN) and time (pre, post) as within-subject factors to examine whether the general tendencies for the Stroop task could be reproduced in all conditions. Then, the effect of running on Stroop task performance was analyzed by repeated-measures two-way ANOVA with session (CON, RUN) and time (pre, post) as within-subject factors followed by Bonferroni’s post hoc test. For the fNIRS data, pre-sessions of cortical activation for CON and RUN sessions, which were free from any effect of running, were first examined to determine whether the Stroop interference effect (the difference in oxy-Hb between incongruent and neutral conditions) could be observed. The Stroop interference levels for the CON and RUN sessions were averaged and served as substrates for a ROI-wise analysis with FDR for controlling the low proportion of false positives. Only significant ROIs for Stroop interference were subsequently analyzed for the effect of running on prefrontal activation using repeated-measures two-way ANOVA with session (CON, RUN) and time (pre, post) as within-subject factors followed by FDR correction. This study adopted the McNemar test to assess the association among running-influenced mood change, running-effected behavioral Stroop performance and running-elicited cortical activation in a binomial manner. The contrasts between [(post-session)—(pre-session)] in RUN and [(post-session)—(pre-session)] in CON for each variable was subjected to the McNemar test. Details of the McNemar test are provided in Supplementary D. The statistical significance level was set a priori at p < 0.05. SPSS Statistical Packages version 24 (SPSS, Inc., USA) was used for statistical analysis.
Supplementary Information
Acknowledgements
This work was supported in part by the Japan Society for the Promotion of Science (JSPS) Grant 16H06405 (H. S.), 18H04081 (H. S.), and 21H04858 (H. S.) and the Japan Science and Technology Agency (JST) Grant JPMJMI19D5 (H. S.). The authors thank members of the Laboratory of Exercise Biochemistry and Neuroendocrinology for their assistance with data collection and took a picture (Fig. 7a) that obtained permission to use from them. The illustration (Fig. 6a) was drawn by the author. They express their gratitude to M. Noguchi (ELCS English Language Consultation, Japan) for helping with the manuscript.
Author contributions
C.D., R.K., K.S., G.O., T.F. and H.S. contributed to design the study. C.D. played a role in collecting data with assistance from R.K., K.S., G.O., Y.Y. and K.A. Data analysis was done by C.D., R.K., K.S., G.O., Y.Y. and H.S. The manuscript was written by C.D., and edited by H.S., M.A.Y., K.S., R.K., K.A. and W.C. H.S. contributed to suggest concept of the study and funding acquisition. All authors have read and approved the final version of the manuscript.
Data availability
All data that support the findings of this study are available from the corresponding author by request with no restrictions.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-01654-z.
References
- 1.Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345–352. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- 2.Schulkin J. Evolutionary basis of human running and its impact on neural function. Front. Syst. Neurosci. 2016;10:1–10. doi: 10.3389/fnsys.2016.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanagisawa H, et al. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage. 2010;50:1702–1710. doi: 10.1016/j.neuroimage.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 4.Byun K, et al. Positive effect of acute mild exercise on executive function via arousal-related prefrontal activations: An fNIRS study. Neuroimage. 2014;98:336–345. doi: 10.1016/j.neuroimage.2014.04.067. [DOI] [PubMed] [Google Scholar]
- 5.Kujach S, et al. A transferable high-intensity intermittent exercise improves executive performance in association with dorsolateral prefrontal activation in young adults. Neuroimage. 2018;169:117–125. doi: 10.1016/j.neuroimage.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt A, et al. Modulation of distinct intrinsic resting state brain networks by acute exercise bouts of differing intensity. Brain Plast. 2019;5:39–55. doi: 10.3233/BPL-190081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu CH, et al. Acute exercise and neurocognitive development in preadolescents and young adults: An ERP Study. Neural Plast. 2017;2017:14–16. doi: 10.1155/2017/2631909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kao SC, Westfall DR, Soneson J, Gurd B, Hillman CH. Comparison of the acute effects of high-intensity interval training and continuous aerobic walking on inhibitory control. Psychophysiology. 2017;54:1335–1345. doi: 10.1111/psyp.12889. [DOI] [PubMed] [Google Scholar]
- 9.Jones AM, Carter H. The effect of endurance training on parameters of aerobic fitness. Sport. Med. 2000;29:373–386. doi: 10.2165/00007256-200029060-00001. [DOI] [PubMed] [Google Scholar]
- 10.Lee DC, et al. Running as a key lifestyle medicine for longevity. Prog. Cardiovasc. Dis. 2017;60:45–55. doi: 10.1016/j.pcad.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Garofolini A, Taylor S. The effect of running on foot muscles and bones: A systematic review. Hum. Mov. Sci. 2019;64:75–88. doi: 10.1016/j.humov.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Markoti V, et al. The positive effects of running on mental health. Psychiatr. Danub. 2020;32:233–235. [PubMed] [Google Scholar]
- 13.Skoyles JR. Human balance, the evolution of bipedalism and dysequilibrium syndrome. Med. Hypotheses. 2006;66:1060–1068. doi: 10.1016/j.mehy.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 14.Jordan LM, Sławińska U. The brain and spinal cord networks controlling locomotion. In: Faingold CL, Blumenfeld H, editors. Neuronal Networks in Brain Function, CNS Disorders, and Therapeutics. Academic Press; 2014. pp. 215–233. [Google Scholar]
- 15.Kiely J, Collins DJ. Uniqueness of human running coordination: The integration of modern and ancient evolutionary innovations. Front. Psychol. 2016;7:1–8. doi: 10.3389/fpsyg.2016.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller EK, Cohen JD. An integrate theory of PFC function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 17.Fuster JM. Prefrontal cortex in motor control. In: Brooks V, editor. Handbook of Physiology, Nervous System, vol II: Motor Control. American Physiological Society; 1981. pp. 1149–1178. [Google Scholar]
- 18.Suzuki M, et al. Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: An optical imaging study. Neuroimage. 2004;23:1020–1026. doi: 10.1016/j.neuroimage.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Davidson RJ, et al. Neural and behavioral substrates of mood and mood regulation. Biol. Psychiatry. 2002;52:478–502. doi: 10.1016/s0006-3223(02)01458-0. [DOI] [PubMed] [Google Scholar]
- 20.Drevets WC. Neuroimaging studies of mood disorders. Soc. Biol. Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 21.Fu C, Shi D, Gao Y, Xu J. Functional assessment of prefrontal lobes in patients with major depression disorder using a dual-mode technique of 3D-arterial spin labeling and 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Exp. Ther. Med. 2017;14:1058–1064. doi: 10.3892/etm.2017.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamner SR, Seth A, Delp SL. Muscle contributions to propulsion and support during running. J. Biomech. 2010;43:2709–2716. doi: 10.1016/j.jbiomech.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palatini P, et al. Blood pressure changes during running in humans: The ‘beat’ phenomenon. J. Appl. Physiol. 1989;67:52–59. doi: 10.1152/jappl.1989.67.1.52. [DOI] [PubMed] [Google Scholar]
- 24.Lyngeraa TS, et al. Middle cerebral artery blood velocity during running. Scand. J. Med. Sci. Sport. 2013;23:32–37. doi: 10.1111/sms.12009. [DOI] [PubMed] [Google Scholar]
- 25.Ryu Y, et al. Mechanical regulation underlies effects of exercise on serotonin-induced signaling in the prefrontal cortex neurons. iScience. 2020;23:1–16. doi: 10.1016/j.isci.2020.100874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wohlwend M, Olsen A, Håberg AK, Palmer HS. Exercise intensity-dependent effects on cognitive control function during and after acute treadmill running in young healthy adults. Front. Psychol. 2017;8:1–10. doi: 10.3389/fpsyg.2017.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludyga S, Gerber M, Brand S, Pühse U, Colledge F. Effects of aerobic exercise on cognitive performance among young adults in a higher education setting. Res. Q. Exerc. Sport. 2018;89:164–172. doi: 10.1080/02701367.2018.1438575. [DOI] [PubMed] [Google Scholar]
- 29.Stroop JR. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935;18:643–662. [Google Scholar]
- 30.MacDonald AW, Cohen JD, Andrew Stenger V, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 31.Vanderhasselt MA, de Raedt R, Baeken C. Dorsolateral prefrontal cortex and Stroop performance: Tackling the lateralization. Psychon. Bull. Rev. 2009;16:609–612. doi: 10.3758/PBR.16.3.609. [DOI] [PubMed] [Google Scholar]
- 32.Sanders AF. Towards a model of stress and human performance. Acta Psychol. (Amst) 1983;53:61–97. doi: 10.1016/0001-6918(83)90016-1. [DOI] [PubMed] [Google Scholar]
- 33.Audiffren M, Tomporowski PD, Zagrodnik J. Acute aerobic exercise and information processing: Energizing motor processes during a choice reaction time task. Acta Psychol. (Amst) 2008;129:410–419. doi: 10.1016/j.actpsy.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Ramos BP, Arnsten AFT. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacol. Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robbins TW, Everitt BJ. A role for mesencephalic dopamine in activation: Commentary on Berridge (2006) Psychopharmacology. 2007;191:433–437. doi: 10.1007/s00213-006-0528-7. [DOI] [PubMed] [Google Scholar]
- 36.Meeusen R, Watson P, Hasegawa H, Roelands B, Piacentini MF. Central fatigue: The serotonin hypothesis and beyond. Sports Med. 2006;36:881–909. doi: 10.2165/00007256-200636100-00006. [DOI] [PubMed] [Google Scholar]
- 37.Dietrich A, Audiffren M. The reticular-activating hypofrontality (RAH) model of acute exercise. Neurosci. Biobehav. Rev. 2011;35:1305–1325. doi: 10.1016/j.neubiorev.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 38.McMorris T. Developing the catecholamines hypothesis for the acute exercise-cognition interaction in humans: Lessons from animal studies. Physiol. Behav. 2016;165:291–299. doi: 10.1016/j.physbeh.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Basso JC, Suzuki WA. The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: A review. Brain Plast. 2017;2:127–152. doi: 10.3233/BPL-160040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyodo K, et al. The association between aerobic fitness and cognitive function in older men mediated by frontal lateralization. Neuroimage. 2016;125:291–300. doi: 10.1016/j.neuroimage.2015.09.062. [DOI] [PubMed] [Google Scholar]
- 41.Ochi G, et al. Neural basis for reduced executive performance with hypoxic exercise. Neuroimage. 2018;171:75–83. doi: 10.1016/j.neuroimage.2017.12.091. [DOI] [PubMed] [Google Scholar]
- 42.Kuwamizu R, et al. Spontaneous eye blink rate connects missing link between aerobic fitness and cognition. Med. Sci. Sports Exerc. 2021;53:1425–1433. doi: 10.1249/MSS.0000000000002590. [DOI] [PubMed] [Google Scholar]
- 43.Suwabe K, et al. Positive mood while exercising influences beneficial effects of exercise with music on prefrontal executive function: A functional NIRS study. Neuroscience. 2021;454:61–71. doi: 10.1016/j.neuroscience.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Borg G. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 45.American College of Sports Medicine . ACSM’s Guidelines for Exercise Testing and Prescription. Medicine & Science in Sports & Exercise. Lippincott Williams & Wilkins; 2017. [DOI] [PubMed] [Google Scholar]
- 46.Yamazaki Y, et al. Inter-individual differences in working memory improvement after acute mild and moderate aerobic exercise. PLoS ONE. 2018;13:1–14. doi: 10.1371/journal.pone.0210053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hyodo K, Takashi J, Suwabe K, Soya H, Nagamatsu T. Acute effects of light-intensity, slow-tempo aerobic dance exercise on mood and executive function in older adults. Bull. Phys. Fit. Res. Inst. 2019;117:8–16. [Google Scholar]
- 48.Zschucke E, Renneberg B, Dimeo F, Wüstenberg T, Ströhle A. The stress-buffering effect of acute exercise: Evidence for HPA axis negative feedback. Psychoneuroendocrinology. 2015;51:414–425. doi: 10.1016/j.psyneuen.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 49.Anderson RJ, Brice S. The mood-enhancing benefits of exercise: Memory biases augment the effect. Psychol. Sport Exerc. 2011;12:79–82. [Google Scholar]
- 50.Bernstein EE, McNally RJ. Acute aerobic exercise helps overcome emotion regulation deficits. Cogn. Emot. 2017;31:834–843. doi: 10.1080/02699931.2016.1168284. [DOI] [PubMed] [Google Scholar]
- 51.Ekkekakis P, Hall EE, Petruzzello SJ. The relationship between exercise intensity and affective responses demystified: To crack the 40-year-old nut, replace the 40-year-old nutcracker! Ann. Behav. Med. 2008;35:136–149. doi: 10.1007/s12160-008-9025-z. [DOI] [PubMed] [Google Scholar]
- 52.Eston R. Use of ratings of perceived exertion in sports. Int. J. Sports Physiol. Perform. 2012;7:175–182. doi: 10.1123/ijspp.7.2.175. [DOI] [PubMed] [Google Scholar]
- 53.Travlos AK, Marisi DQ. Perceived exertion during physical exercise among individuals high and low in fitness. Percept. Mot. Skills. 1996;82:419–424. doi: 10.2466/pms.1996.82.2.419. [DOI] [PubMed] [Google Scholar]
- 54.Robertson CV, Marino FE. A role for the prefrontal cortex in exercise tolerance and termination. J. Appl. Physiol. 2016;120:464–466. doi: 10.1152/japplphysiol.00363.2015. [DOI] [PubMed] [Google Scholar]
- 55.Leung HC, Skudlarski P, Gatenby JC, Peterson BS, Gore JC. An event-related functional MRI study of the Stroop color word interference task. Cereb. Cortex. 2000;10:552–560. doi: 10.1093/cercor/10.6.552. [DOI] [PubMed] [Google Scholar]
- 56.Song Y, Hakoda Y. An fMRI study of the functional mechanisms of Stroop/reverse-Stroop effects. Behav. Brain Res. 2015;290:187–196. doi: 10.1016/j.bbr.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 57.Kerns JG, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 58.Egner T, Hirsch J. The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage. 2005;24:539–547. doi: 10.1016/j.neuroimage.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- 61.Endo K, et al. Dynamic exercise improves cognitive function in association with increased prefrontal oxygenation. J. Physiol. Sci. 2013;63:287–298. doi: 10.1007/s12576-013-0267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Byun K, et al. Possible influences of exercise-intensity-dependent increases in non-cortical hemodynamic variables on NIRS-based neuroimaging analysis during cognitive tasks: Technical note. J. Exerc. Nutr. Biochem. 2014;18:327–332. doi: 10.5717/jenb.2014.18.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sakairi Y, Nakatsuka K, Shimizu T. Development of the Two-Dimensional Mood Scale for self-monitoring and self-regulation of momentary mood states. Jpn. Psychol. Res. 2013;55:338–349. [Google Scholar]
- 64.Schroeter ML, Zysset S, Kupka T, Kruggel F, Von Cramon DY. Near-infrared spectroscopy can detect brain activity during a color-word matching stroop task in an event-related design. Hum. Brain Mapp. 2002;17:61–71. doi: 10.1002/hbm.10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cope M, et al. Methods of quantitating cerebral near infrared spectroscopy data. Adv. Exp. Med. Biol. 1988;3:183–189. doi: 10.1007/978-1-4615-9510-6_21. [DOI] [PubMed] [Google Scholar]
- 66.Maki A, et al. Spatial and temporal analysis of human motor activity using noninvasive NIR topography. Med. Phys. 1995;22:1997–2005. doi: 10.1118/1.597496. [DOI] [PubMed] [Google Scholar]
- 67.Tsuzuki D, et al. Virtual spatial registration of stand-alone fNIRS data to MNI space. Neuroimage. 2007;34:1506–1518. doi: 10.1016/j.neuroimage.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 68.Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat. Rev. Neurosci. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- 69.Okamoto M, et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage. 2004;21:99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 70.Okamoto M, Dan I. Automated cortical projection of head-surface locations for transcranial functional brain mapping. Neuroimage. 2005;26:18–28. doi: 10.1016/j.neuroimage.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 71.Singh AK, Okamoto M, Dan H, Jurcak V, Dan I. Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. Neuroimage. 2005;27:842–851. doi: 10.1016/j.neuroimage.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 72.Shattuck DW, et al. Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage. 2008;39:1064–1080. doi: 10.1016/j.neuroimage.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Katagiri A, et al. Mapping of optical pathlength of human adult head at multi-wavelengths in near infrared spectroscopy. Adv Exp Med Biol. 2010;662:205–212. doi: 10.1007/978-1-4419-1241-1_29. [DOI] [PubMed] [Google Scholar]
- 74.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A practical and powerful approach to multiple testing. R. Stat. Soc. 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings of this study are available from the corresponding author by request with no restrictions.