Abstract
Background
Mutations in p53, identified in 90% of oesophageal squamous cell carcinoma (ESCC), are associated with unfavourable prognosis and chemo-resistance. APR-246 induces apoptosis by restoring transcriptional ability of mutant p53, and may be a promising therapeutic agent to overcome chemo-resistance in ESCC.
Methods
In ESCC cell lines differing in p53 status, we performed in vitro cell viability and apoptosis assays, evaluated reactive oxygen species (ROS) generation, and assessed signal changes by western blot after APR-246 administration with/without chemo-agent. Antitumour effects and signal changes were evaluated in in vivo experiments using xenograft and patient-derived xenograft (PDX) mouse models.
Results
APR-246 administration induced significant apoptosis by upregulating p73 and Noxa via ROS induction in ESCC cell lines harbouring p53 missense mutations. Moreover, APR-246 plus chemotherapy exerted combined antitumour effects in ESCC with p53 missense mutations. This effect was also mediated through enhanced ROS activity, leading to massive apoptosis via upregulation of p73 and Noxa. These findings were confirmed by xenograft and PDX models with p53 mutant ESCC.
Conclusion
APR-246 strongly induced apoptosis by inducing ROS activity and p73-Noxa signalling, specifically in ESCC with p53 missense mutation. This antitumour effect was further enhanced by combination with 5-FU, which we first confirmed in ESCC preclinical model.
Subject terms: Targeted therapies, Oesophageal cancer
Background
Oesophageal squamous cell carcinoma (ESCC) is among the most aggressive cancers and carries a dismal prognosis compared to other gastrointestinal cancers [1]. Over recent years, multidisciplinary treatments have been developed, and preoperative chemotherapy has become standard treatment for advanced cases. However, with a standard regimen of 5-fluorouracil (5-FU) and cisplatin (CDDP), the response rate is reportedly no more than 37%, and the survival benefit is limited to responders [2]. Therefore, there is an urgent need to enhance chemotherapy responses and thus improve survival of ESCC patients.
The mutation rate of the tumour-suppressor gene p53 accounts for approximately 50% of all cancers [3] and over 90% of ESCCs. Mutations in p53 largely constitute missense mutations and are associated with treatment resistance and poor prognosis in ESCC [4–8]. Therefore, a therapeutic strategy targeting mutant p53 or p53 family members may be a promising means of overcoming treatment resistance and ultimately improving ESCC prognosis.
APR-246 is a low-molecular-weight compound that was recently identified by library screening using cells harbouring mutant p53. It restores DNA-binding ability through sequence-specific modification of mutant p53 protein, thereby promoting its transcriptional ability and inducing apoptosis [9, 10]. Recent studies in other cancer types further reveal that APR-246 exerts antitumour effects by increasing reactive oxygen species (ROS) activity specifically for p53 missense mutations [11–13]. APR-246 also exerts antitumour effects via a p53-independent pathway [14–16]. However, the mechanistic details of these antitumour effects depend on cancer type or p53 mutation status and remain unclear.
In a clinical setting, recent Phase I/II trials have confirmed the safety of APR-246 administration in humans and demonstrated promising results in terms of antitumour effects in haematological cancer [17]. Furthermore, limited evidence supports promising antitumour effects of APR-246 in small cell lung cancer [18] and non-haematological cancer, including ovarian cancer, in preclinical studies [17]. In ESCC, we previously reported that PRIMA-1 (p53 re-activation and induction of massive apoptosis) exerted Noxa-mediated antitumour effects on ESCC with p53 missense mutation [19]. However, the detailed mechanism of APR-246 (also called PRIMA-1MET), and its effect when combined with chemotherapy for ESCC, remain unknown. In the present study, we examined the effect of APR-246 combined with anticancer drugs, and its underlying mechanism, by using a panel of ESCC cell lines with differing p53 status, a xenograft mouse model, and a patient-derived xenograft (PDX) model of ESCC.
Materials and methods
Cell lines and culture
The human oesophageal squamous cell lines TE1, TE4, TE5, TE8, TE9, TE10, TE11, and TE14 were obtained from Riken BioResource Center Cell Bank (Tsukuba, Japan). The KYSE410 and KYSE960 cell lines were obtained from the Japanese cancer research Resource Bank (Osaka, Japan).
Apoptosis analysis with annexin V staining
We assessed apoptosis using the Annexin V-FITC Apoptosis Detection Kit (BD Biosciences). First, cells were incubated for 36–48 h with cisplatin, 5-FU, docetaxel (DTX)], and APR-246 alone or with the combinations of APR-246 plus each anticancer drug. A 100-μL aliquot of cell suspension was mixed with 5 μL of annexin V-FITC and 2.5 μL propidium iodide and incubated for 30 min at room temperature in the dark. Then the samples were analysed by flow cytometry using a FACSCanto II instrument (BD Biosciences, San Jose, CA), and the annexin V-stained cells were considered apoptotic.
Small interfering RNA (siRNA) knockdown
Noxa siRNA, p73 siRNA, and control siRNA were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Cells were transfected for 24 h using Lipofectamine RNAiMAX transfection reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Next, the cells were treated with anticancer drugs, APR-246 alone, or the combination for 36–48 h.
Oesophageal cancer cell xenograft and PDX mouse model
These studies were conducted in accordance with recognised ethical guidelines (Helsinki Declaration, Japanese Human Genome/Gene Analysis Research Ethics Guidelines, and Ethics Guidelines for Medical and Health Research with Humans at Osaka University) and institutional ethics guidelines (Osaka, Japan) on animal experiments. In all, 2.0 × 106 TE8/KYSE410 cells in 100 μL of RPMI1640/Matrigel (BD Biosciences) (1:1 suspension) were injected subcutaneously on both backs of 6 week-old female mice (BALB/c-nu/nu CLEA Japan). As a preclinical model, surgically excised samples were cut into small pieces and implanted subcutaneously in the abdomen of 5-week-old NOD-SCID mice (CLEA Japan). For evaluation of the treatment effect, when the tumour reached approximately 50 mm3, mice were randomly assigned to each group of control (phosphate-buffered saline (PBS)), 5-FU single agent, APR-246 single agent, and 5-FU and APR-246 combined therapy and started receiving treatment. Treatment agent of each group contained 5% dimethyl sulfoxide (DMSO; Wako) and PBS was used as solution for administration. 5-FU was administered intraperitoneally at 5 mg/kg once every 3 days while APR-246 at 25 mg/kg/day. Tumour volume [(short2 × long)/2] was measured once every 3 days from the start of treatment until 21 days by investigator blinded to the group allocation. All control mice received an equal volume of DMSO.
Immunohistochemistry
Subcutaneous tumours were embedded in paraffin, endogenous peroxidase activity was blocked, and 3.5-mm-thick sections were cut and stained with antibodies. To measure ROS activity, we performed alkaline phosphatase immunostaining using the VECTASTAIN ABC-AP Universal Kit (VECTOR). Apoptosis was assessed by performing a terminal dUTP nick-end labelling (TUNEL) assay using the ApopTag Fluorescein In Situ Apoptosis Detection Kit (Chemicon International). ROS activity was evaluated using an In Situ Apoptosis Detection Kit (Chemicon International).
Statistical analysis
Data are shown as mean ± SD of the indicated number of experiments. In xenograft mouse models, data are presented as mean ± SEM. We tested for significant between-group differences using an unpaired Student’s t test. Two-sided P values of <0.05 were considered significant. Statistical analyses were performed using JMP version 14.0 (SAS Institute).
The full methods are described in the Supplementary Materials and Methods.
Results
APR-246 induced significant apoptosis with upregulation of p73 and Noxa signalling in addition to ROS activity specifically in ESCC with p53 missense mutation
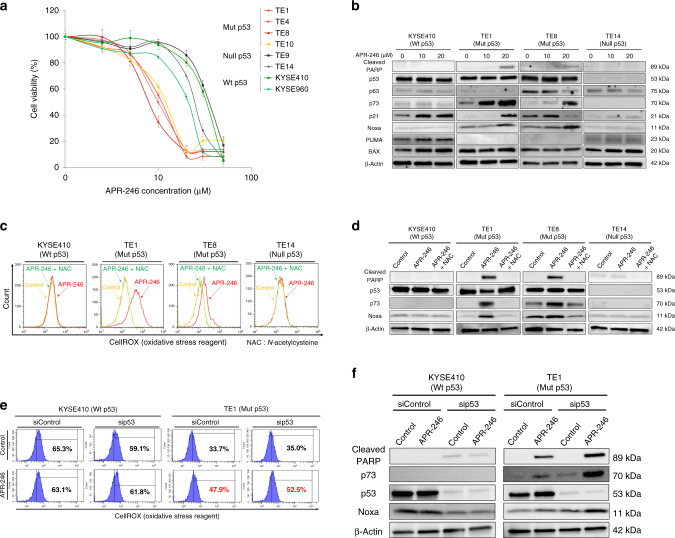
Among a panel of ESCC cell lines that differed in p53 status—including wild-type p53; KYSE410 and KYSE960, which have missense mutations; TE1, TE4, TE5, TE8, and TE10, which have frameshift mutation; TE9, which has a nonsense mutation; and TE14—APR-246 exhibited the most significant antitumour effect in ESCC with p53 missense mutations (Supplementary Table S1). The IC50 values for TE1, TE4, TE5, TE8, and TE10 were 10.5, 9.9, 14.3, 7.9, and 11.7 μmol/L, respectively. On the other hand, APR-246 showed minimal effectiveness in ESCC with other p53 mutation types or wild-type p53, with IC50 values for TE9, TE14, KYSE410, and KYSE960 being 34.6, 24.3, 31.6, and 20.8 μmol/L, respectively (Fig. 1a).
Fig. 1. APR-246 alone induces massive apoptosis via ROS induction leading to upregulation of p73-Noxa in ESCC with p53 missense mutation.
a APR-246 dose–response curve of ESCC cell lines after 48-h treatment with APR-246 as assessed by MTT cell viability assay. Error bars indicate standard deviation. b Western blot to assess relevant protein levels (cleaved PARP, p53, p63, p73, p21, Noxa, PUMA, and BAX) following treatment with APR-246 alone in ESCC cell lines (KYSE410, TE1, TE8, and TE14). c Flow cytometric data comparing ROS activities following administration of control, APR-246 alone, or APR-246 and N-acetylcysteine in ESCC cell lines (KYSE410, TE1, TE8, and TE14). d Comparison of relevant protein levels (cleaved PARP, p53, p73, and Noxa) under the same conditions as in c. e Comparison of ROS activities following administration of control or APR-246 alone in ESCC cell lines (KYSE410 and TE1) transfected with negative control or p53 siRNA. f Comparison of protein expressions under the same conditions as in e. P < 0.05. Wt wild type, Mut missense mutation, Null frameshift or nonsense mutation.
Western blotting confirmed the increased levels of p73 and Noxa, downstream of p53 family signalling along with poly ADP-ribose polymerase (PARP) level (i.e. apoptosis) after APR-246 treatment in ESCC with p53 missense mutation while no significant Noxa induction was detected in ESCC with wild-type and nonsense mutant p53 (Fig. 1b). On the other hand, APR-246 administration significantly increased ROS activity, particularly in ESCC with p53 missense mutation (Fig. 1c). These effects were cancelled by addition of N-acetylcysteine, which specifically suppressed ROS in ESCC with p53 missense mutation (Fig. 1c, d). Importantly, in ESCC cell lines (KYSE410 and TE1) transfected with p53 siRNA, APR-246 mono-treatment still enhanced ROS activity and upregulated the expression levels of cleaved PARP, p73, and Noxa in ESCC with p53 missense mutation (TE1) (Fig. 1e, f). Therefore, APR-246 may have both mut-p53-dependent and mut-p53-independent activities.
APR-246 showed synergistic antitumour effects when combined with chemotherapy agents, particularly 5-FU, in ESCC with p53 missense mutation
We further evaluated the antitumour effects of APR-246 when combined with either CDDP, 5-FU, or DTX, all of which are common preoperative chemotherapy agents for ESCC [20–23]. In ESCC with p53 missense mutations, these treatment combinations generally showed additive or synergic antitumour effects, most prominently with APR-246 plus 5-FU, showing combination index values of 0.58, 0.71, and 0.72 in TE1, TE4, and TE8, respectively. On the other hand, in ESCC with wild-type, nonsense mutation, or frameshift mutation p53, no additive or synergic effects were observed with any anticancer drugs (Table 1).
Table 1.
Combination index calculated by the isobologram method to evaluate antitumour effect of APR-246 in combination with CDDP, 5-FU, or DTX.
| TP53 status | Combination index | |||
|---|---|---|---|---|
| CDDP+APR-246 | 5-FU+APR-246 | DTX+APR-246 | ||
| KYSE410 | Wild type | 1.52 | 1.36 | 1.25 |
| KYSE960 | Wild type | 1.72 | 1.48 | 1.36 |
| TE1 | Missense mutation (p.V272M) | 1.06a | 0.58b | 0.95a |
| TE4 | Missense mutation (p.G245C) | 0.73b | 0.71b | 0.95a |
| TE5 | Missense mutation (p.V272L) | 1.14a | 1.16a | 1.22 |
| TE8 | Missense mutation (p.M237I) | 1.13a | 0.72b | 0.48b |
| TE10 | Missense mutation (p.C242Y) | 1.05a | 1.11a | 1.11a |
| TE9 | Frameshift mutation (p.R273fs) | 1.35 | 1.68 | 1.52 |
| TE14 | Nonsense mutation (p.R213X) | 1.91 | 1.78 | 1.25 |
aAdditive effect.
bSynergistic effect.
Combination of APR-246 plus 5-FU enhances ROS activity, inducing significant apoptosis via p73-Noxa upregulation in ESCC with p53 missense mutation
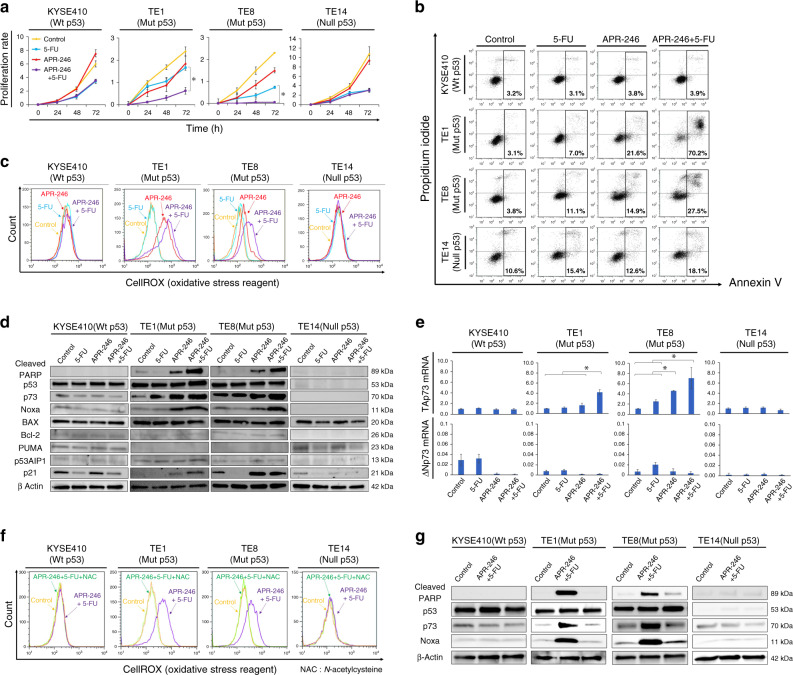
In proliferation assays with wild-type p53 ESCC or nonsense mutation p53, 5-FU, but not APR-246, exerted antitumour effects, while no further inhibition of proliferation was observed in combined use of APR-246 plus 5-FU. In contrast, combined use of APR-246 plus 5-FU induced significant apoptosis in ESCC with p53 missense mutation (Fig. 2a). Apoptosis assays further revealed that APR-246-induced apoptosis was enhanced by combined treatment with APR-246 plus 5-FU in ESCC with p53 missense mutation, but not in ESCC with wild-type or nonsense mutation p53. The percentage of annexin V-positive cells was 3.9% in KYSE410, 70.2% in TE1, 27.5% in TE8, and 18.1% in TE14 (Fig. 2b). In ESCC with p53 missense mutation, APR-246-induced ROS activation was further enhanced by its combination with 5-FU (Fig. 2c). Western blotting revealed a similar trend in terms of p73 level after APR-246 administration in p53 missense mutant ESCC (Fig. 2d).
Fig. 2. APR-246-induced apoptosis was further enhanced by its combination with 5-FU in ESCC with p53 missense mutation.
a Proliferation assay. ESCC cell lines (KYSE410, TE1, TE8, and TE14) were treated with PBS, APR-246 (10 μmol/L), 5-FU (10 μmol/L), or the combination of APR-246 (10 μmol/L) + 5-FU (10 μmol/L) for 72 h. b Apoptosis assay. Percentage of cells with positive annexin V staining after treatment with PBS, APR-246 (20 μmol/L), 5-FU (20 μmol/L), or the combination of APR-246 (20 μmol/L) + 5-FU (20 μmol/L) in ESCC cell lines (KYSE410, TE1, TE8, and TE14). c ROS activities under the same conditions as in b. d Western blotting to assess relevant protein levels (cleaved PARP, p53, p73, Noxa, BAX, Bcl-2, PUMA, p53AIP1, p21, and E2F-1) under the same condition as in b. e PCR results assessing TAp73 and ΔNp73 mRNA levels under the same condition as in e (ΔΔCt method). f Comparison of ROS activities after treatment with control, combination treatment (APR-246 20 μmol/L + 5-FU 20 μmol/L), or combination treatment plus N-acetylcysteine as assessed by flow cytometry in ESCC cell lines (KYSE410, TE1, TE8, and TE14). g Western blotting to assess relevant protein levels under the same conditions as in d. *P < 0.05. Wt wild type, Mut missense mutation, Null frameshift or nonsense mutation.
Among the downstream signals of apoptosis associated with the p53 family, we found that Noxa activation played an important role in APR-246-induced apoptosis, which was further enhanced by the combined use of APR-246 and 5-FU, along with p73 level (Fig. 2d). In terms of p73 induction, APR-246 alone specifically increased TAp73 mRNA expression but not ΔNp73, as assessed by reverse transcriptase polymerase chain reaction (PCR), in p53 missense mutant ESCC, but not in ESCC with wild-type or nonsense mutation p53. Similarly, this induction of TAp73 mRNA was further enhanced by the combination of APR-246 and 5-FU (Fig. 2e). When N-acetylcysteine was co-administered with the combined use of APR-246 and 5-FU, the ROS activation was attenuated and apoptosis induction via p73-Noxa upregulation was cancelled in ESCC with p53 missense mutation (Fig. 2f, g).
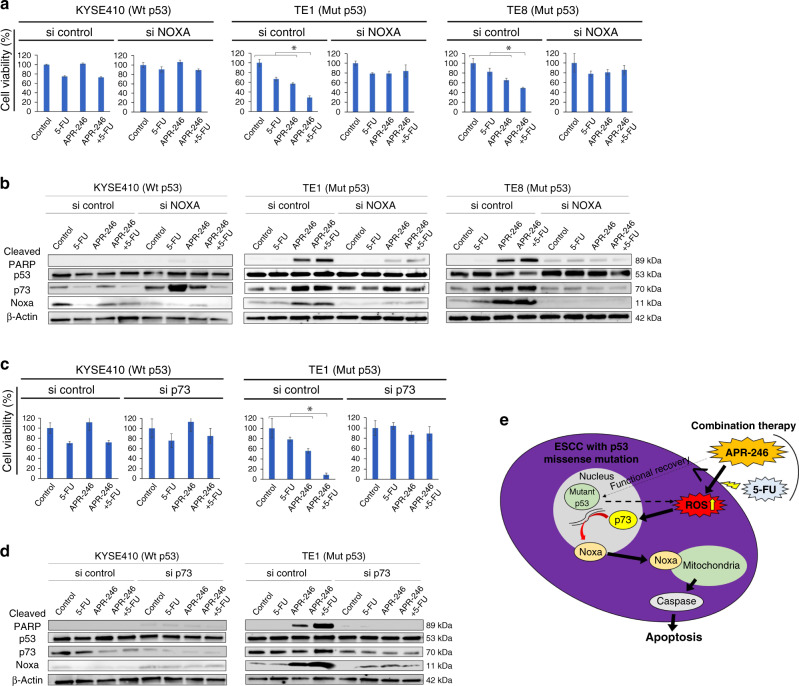
Knockdown of Noxa or p73 by siRNA cancelled induction of apoptotic signals caused by combined use of APR-246 and 5-FU in ESCC with p53 missense mutation
In ESCC with p53 missense mutation (TE1 and TE8), Noxa knockdown using siRNA, as opposed to siControl, cancelled the effects of combined treatment with APR-246 and 5-FU—e.g. decreased cell viability and apoptosis induction (upregulation of cleaved PARP expression on western blot) (Fig. 3a, b). Similarly, p73 knock-down by siRNA cancelled the combined antitumour effect of APR-246 and 5-FU in ESCC with p53 missense mutation (TE1) (Fig. 3c). Western blot analysis revealed that the increased expression levels of Noxa and cleaved PARP caused by the combination of APR-246 and 5-FU were also cancelled by p73 knockdown (si p73) compared to si Control, indicating reversal of Noxa-induced apoptosis by si p73 (Fig. 3d). Overall, these results showed that APR-246 induced significant apoptosis by enhancing ROS activity and the p73-Noxa pathway in ESCC with p53 missense mutation, while limited antitumour effects were exerted through the common p53 apoptotic pathway potentially reactivated by APR-246. This also appeared to be the mechanism underlying the synergistic antitumour effect with combined use of APR-246 and anticancer drugs (Fig. 3e).
Fig. 3. p73-Noxa upregulation as an essential pathway in combination therapy with APR-246 and 5-FU in ESCC with p53 missense mutation.
a Cell viability (%) after 48-h treatment with PBS, 5-FU (20 μmol/L), APR-246 (20 μmol/L), and the combination of APR-246 (20 μmol/L) + 5-FU (20 μmol/L) in ESCC cell lines (KYSE410, TE1, and TE8) transfected with si control or si NOXA. b Western blotting to assess relevant protein levels (cleaved PARP, p53, p73, and Noxa) under the same conditions as in e. c Cell viability (%) after 48-h treatment with PBS, 5-FU (20 μmol/L), APR-246 (20 μmol/L), and the combination of APR-246 (20 μmol/L) + 5-FU (20 μmol/L) in ESCC cell lines (KYSE410 and TE1) transfected with si control or si p73. d Western blotting to assess relevant protein levels (cleaved PARP, p53, p73, and Noxa) under the same condition as in c. e Schematic of the underlying mechanism through which APR-26, alone or in combination with a chemo-agent, induces significant apoptosis in ESCC with p53 missense mutation. *P < 0.05. Wt wild type, Mut missense mutation.
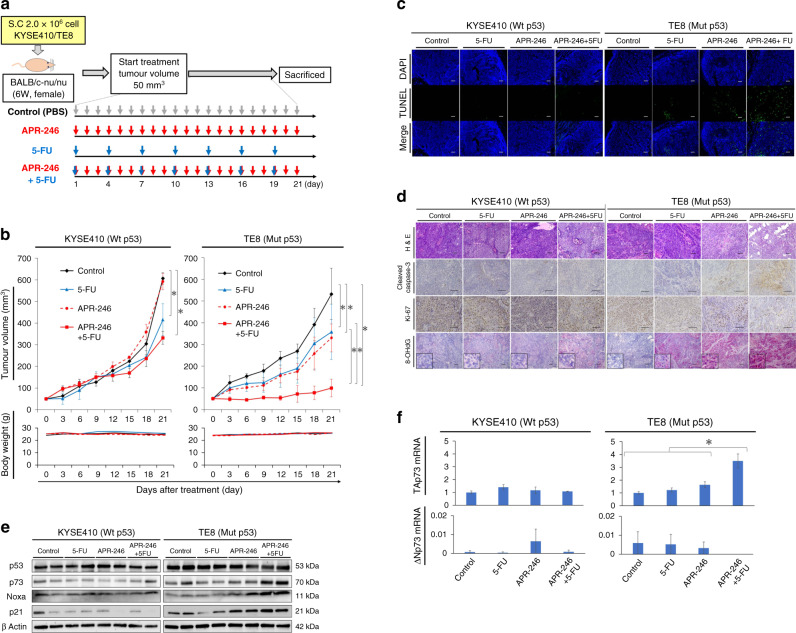
In a xenograft mouse model of ESCC with p53 missense mutation, combined use of APR-246 and 5-FU exerted synergic antitumour effects through activation of ROS and the p73-Noxa pathway without significant adverse events
In a xenograft mouse model generated using p53 missense mutant ESCC (TE8) (Fig. 4a), we observed synergic antitumour effect of 5-FU plus APR-246 compared with each single treatment. On the other hand, in the xenograft tumour model with wild-type p53 ESCC (KYSE410), antitumour effects were observed only with 5-FU treatment but not APR-246, while no synergic antitumour effect of 5-FU plus APR-246 was observed (Fig. 4b). In KYSE410, the tumour volume was 607 ± 26 mm3 with PBS, 416 ± 74 mm3 with 5-FU, 592 ± 38 mm3 with APR-246, and 332 ± 30 mm3 with combination treatment. In TE8, the corresponding tumour volumes were 533 ± 68, 358 ± 37, 330 ± 74, and 99 ± 20 mm3, respectively. Importantly, neither model exhibited significant body weight loss or adverse effect from treatment.
Fig. 4. Combination treatment with APR-246 and 5-FU yields synergic anticancer effect in a xenograft mouse model of ESCC with p53 missense mutation.
a Schematic of the xenograft mouse model using ESCC cell lines with differing p53 status (KYSE410 versus TE8) treated for 21 days with PBS (control), 5-FU monotherapy (5 mg/kg every 3 days), APR-246 monotherapy (25 mg/kg daily), or the combination of 5-FU (5 mg/kg every 3 days) plus APR-246 (25 mg/kg daily). Treatment by intraperitoneal administration was started once tumour volume reached 50 mm3. Each group was sacrificed 21 days after the start of treatment. Each signal was evaluated using samples obtained 7 days after the treatment. b Tumour growth curve and weight change in each treatment group (n = 4). Tumour volume and body weight were measured every 3 days from the start of treatment. c Apoptosis associated with therapeutic effects in the tumour tissue of each treatment group as assessed by the TUNEL assay. d Comparison of immunostaining regarding apoptosis (cleaved caspase-3), cell proliferation ability (ki-67), and ROS activity between each treatment group. 8-OHdG staining: alkaline phosphatase-based immunostaining for the detection of ROS activity. Red staining indicates ROS activity, while purple staining shows haematoxylin and eosin. e Western blotting to assess relevant protein levels (p53, p73, Noxa, and p21) in tumour tissues of each treatment group. f PCR assay to measure the mRNA expression levels of TAp73 and ΔNp73 (PCR ΔΔCt method) in tumour tissues of each treatment group. Scale bars, 100 μm. *P < 0.05. Wt wild type, Mut missense mutation.
In the tumour tissue of group with combination treatment, we observed strongly induced apoptosis as assessed by TUNEL assay and cleaved caspase-3 staining in addition to ROS activity (i.e. 8OH-dG immunostaining; Fig. 4c, d). Furthermore, ki-67 staining revealed strong inhibition of tumour growth after combined treatment in this model (Fig. 4d). Western blot analysis revealed that both p73 and Noxa expression levels were further induced in tumour tissues with combination treatment compared with each single treatment in the xenograft mouse model with TE8 (Fig. 4e). PCR analysis revealed that TAp73 expression was synergistically increased in the combination group (Fig. 4f). On the other hand, in the xenograft mouse model with wild-type p53 ESCC, we detected no further upregulation of TAp73, p73, or Noxa expression levels after combination treatment (Fig. 4e, f).
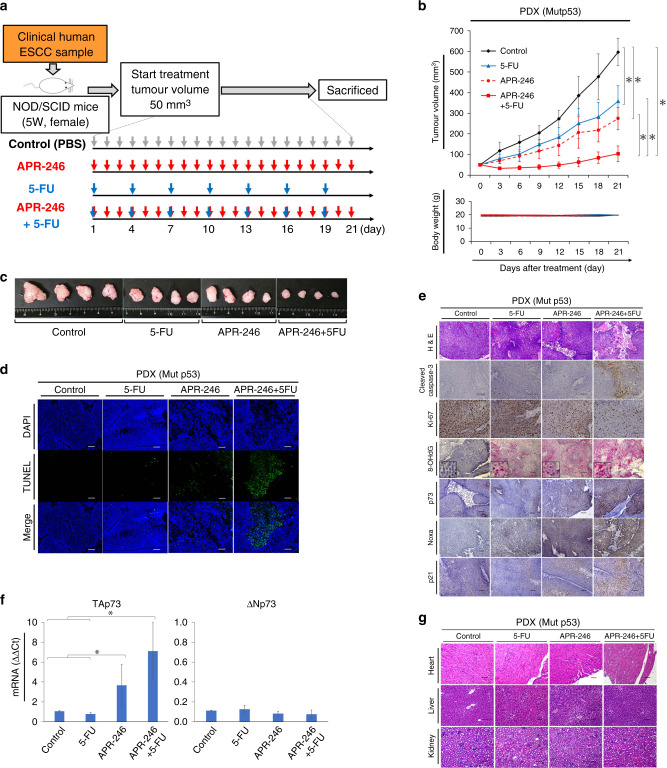
APR-246 plus 5-FU exerted a synergic antitumour effect through the ROS-TAp73-Noxa signalling pathway in a PDX mouse model of ESCC with p53 missense mutation
In a PDX model of ESCC bearing p53 missense mutation (confirmed by Sanger sequencing; Fig. 5a and Supplementary Table S2), we observed synergic antitumour effects following combination treatment compared to control and each mono-treatment group, with a tumour volume of 597 ± 66 mm3 with PBS, 358 ± 76 mm3 with 5-FU, 274 ± 53 mm3 with APR-246, and 104 ± 38 mm3 with combination treatment (Fig. 5b, c). Compared to single treatments, the combined treatment also yielded more significant induction of apoptosis based on TUNEL assay (Fig. 5d) and cleaved caspase-3 immunostaining (Fig. 5e), stronger suppression of cell proliferation as assessed by ki-67 immunostaining (Fig. 5e), and increased ROS activity and expression of p73 (particularly TAp73) and Noxa (Fig. 5e, f). Notably, haematoxylin and eosin staining of essential organs, including the heart, kidneys, and liver, revealed no apparent damage in any treatment group (Fig. 5g).
Fig. 5. PDX mouse model confirmed ROS-TAp73-Noxa-mediated apoptosis by treatment with APR-246 plus 5-FU without any adverse events.
a Schematic of the PDX mouse model using tumour tissue that was confirmed to include p53 missense mutation [p.Val118Phe (exon5) and p.Gly206Val (exon7)] by Sanger sequencing, from a resected specimen from an ESCC patient. Mice were treated with PBS (control), 5-FU monotherapy (5 mg/kg every 3 days), APR-246 monotherapy (25 mg/kg daily), or combined therapy for 21 days. Treatment by intraperitoneal administration was started once tumour volume reached 50 mm3. Each group was sacrificed 21 days after the start of treatment. Samples were obtained on Day 7 for signal evaluation and on Day 21 to evaluate treatment effects on each organ. b Tumour growth curve and weight change in each treatment group (n = 4). Tumour volume and body weight were measured every 3 days from the start of treatment. c Macroscopic findings of resected tumours in each treatment group. d Apoptosis associated with therapeutic effects in tumour tissue from the PDX mouse model of each group, as assessed by TUNEL assay. e Comparison of immunostaining regarding apoptosis (cleaved caspase-3), cell proliferation ability (ki-67), ROS activity (8OH-dG), p73, Noxa, and p21 in tumour tissues of each treatment group. f PCR assay of mRNA expression of TAp73 and ΔNp73 (PCR ΔΔCt method) in tumour tissues of each treatment group. g Haematoxylin and eosin staining of heart, kidney, and liver tissue from each treatment group. Scale bars, 100 μm. *P < 0.05. Mut missense mutation.
Discussion
Our present results showed that APR-246 yielded significant antitumour effects by inducing ROS-p73-Noxa-mediated apoptosis in ESCC cell lines bearing p53 missense mutation. In contrast, the antitumour effect of APR-246 was limited in ESCC with wild-type or nonsense/frameshift mutant p53. Moreover, in p53 missense mutant ESCC, the antitumour effect was further enhanced by the combined use of APR-246 plus anticancer drugs, particularly 5-FU, through increased ROS activity and upregulation of p73-Noxa signalling. These results were confirmed in both xenograft and PDX mouse models of ESCC, in which we observed antitumour effects of combined treatment with APR-246 plus 5-FU via the same underlying mechanism and without any adverse effects. To our knowledge, this is the first demonstration of the detailed mechanism of APR-246, and its antitumour effect in combination with chemotherapy, in a clinical model of ESCC.
We previously revealed that Noxa plays a key role in apoptosis induction by APR-246 treatment for ESCC [19]. Noxa is a downstream signal of the p53 family, and an important pro-apoptotic factor, and a therapeutic strategy involving Noxa activation to induce apoptosis has recently been reported [24–26]. However, Noxa is not necessarily induced only by the p53 pathway [27–30]. In fact, with p53 knockdown, we still observed apoptosis induction mediated by p73 and Noxa after APR-246 administration. In contrast, p73 knockdown suppressed Noxa expression and abrogated apoptotic induction. Therefore, we speculated that the p73-Noxa pathway was significantly more active than the p53-Noxa pathway in terms of inducing significant apoptosis through APR-246 in ESCC with p53 missense mutation, indicating that p73 is a main target of APR-246, as shown for other drugs, including NSC176327, RETRA, etc. [31, 32]. Additionally, ROS induction was identified as being associated with p73-Noxa upregulation in APR-246 treatment for ESCC with p53 missense mutation. This is supported by previous reports of other cancer types [9, 15, 33–35], revealing that APR-246-mediated ROS induction is an important mechanism that specifically occurs in p53 missense mutants [13]. Based on a previous article [13], in the absence of mut-p53 accumulation, NRF2 can transcriptionally regulate cellular redox balance by binding to antioxidant responsive elements on antioxidative stress (AOS) genes. One crucial component of NRF2-mediated redox regulation is the transactivation of SLC7A11 [a key component of the glutamate (Glu)/cysteine (Cys) antiporter system xc−_ (Sxc−_)], resulting in the maintenance of intracellular glutathione (GSH) reserves. Cells without mut-p53 accumulation have sufficient GSH reserves and can thereby mount a normal NRF2-mediated defence against oxidative stress, such as that induced by APR-246. In contrast, in cancer cells with mut-p53 accumulation, mut-p53 entraps NRF2 and impairs its canonical transcriptional activity, resulting in suppressed expression of SLC7A11 and other AOS genes. This reduces GSH reserves and increases resting levels of ROS, rendering mutant-p53 tumours susceptible to oxidative damage [13]. Accordingly, in the present study, APR-246 treatment of p53 wild-type KYSE410 cells was unlikely to lead to significant ROS induction due to the absence of mut-p53 accumulation. On the other hand, in p53-mutated TE1 or TE8 cells, where p73 might play a compensatory role instead of p53 (Fig. 1F), APR-246 administration should lead to ROS-induced apoptosis through the upregulation of p73-Noxa. Although previous reports have shown both p53-dependent [36] and p53-independent [14] p73 induction by APR-246 administration [35], our present results demonstrated that APR-246 induced significant apoptosis via the ROS-p73-Noxa pathway in a p53 status-dependent manner in ESCC.
In contrast to p53, p73 rarely exhibits mutations [37, 38] in tumours and is reportedly associated with chemo-sensitivity [36, 39]. The p73 isoform TAp73 is an activator that induces transcription of various apoptosis-related genes [40, 41], and TAp73β more strongly induces apoptosis compared with TAp73α [16, 42, 43]. Notably, our present study demonstrated that APR-246 administration induced TAp73β at both the transcriptional and protein levels in ESCC with p53 missense mutation. Interaction between p53 and other p53 family members has been reported. A subset of tumour-derived p53 mutants physically interact with the p53 family members p63 and p73 and negatively regulate their pro-apoptotic function [44], although this process remains largely unclear. In head and neck squamous cell carcinoma, p63 reportedly suppresses p73-induced apoptosis, thus facilitating cancer survival [45]. In contrast, our study identified no significant change of p63 expression after APR246 administration in ESCC cell lines harbouring p53 missense mutations as shown in Fig. 1b, implying that p63 does not affect the APR246-induced apoptosis in ESCC. Overall, stabilising p73 or recovering its innate function in cancer tissue might be a pathway to establishing novel cancer treatments. This supports the rationale of APR-246 therapy for ESCC, which induced significant upregulation of the p73-Noxa signal, leading to induction of massive apoptosis in the present study.
With regards to the combined use of APR-246 plus chemo-agents, prior studies have reported enhanced antitumour effects with APR-246 plus CDDP in ovarian cancer, with the estimated mechanism being that APR-246 binds to GSH, suppressing its activity, and enhances CDDP-induced ROS activity. Other chemo-agents used in combination therapy with APR-246 include doxorubicin, taxane, and platinum products [14, 34, 46–49]. Few reports describe combination with 5-FU [35]. Here we found that 5-FU plus APR-246 showed additive or synergistic antitumour effects in ESCC. Previous studies have reported that combination therapy including 5-FU increases ROS activity in other cancer types [50, 51] and that the combination of APR-246 with sulfasalazine or elastin amplifies ROS activity in ESCC [13]. Since 5-FU suppresses signal transcription through RNA dysfunction, 5-FU could induce ROS by downregulating relevant factors that suppress ROS activity, e.g. catalase, glutathione, glutathione peroxidase, etc. Further studies are needed to clarify the detailed mechanism through which the combination of APR-246 and 5-FU increases ROS activity in ESCC.
Limitations of this study
This study has several limitations. First, further investigations are needed to elucidate the mechanism by which combined treatment with APR-246 and other chemo-agents (DTX/CDDP) worked for ESCC. Second, in experiments in the PDX model, we could not evaluate the efficacy of APR-246 plus chemotherapy in ESCC with wild-type p53 because there were too few cases with wild-type p53 ESCC available to generate a PDX model. Third, p73 knockdown using si p73 was found to be lethal in ESCC cell lines and thus could not be used to examine cell lines other than those shown in the present study. Finally, further studies are needed to examine exactly what type of p53 missense mutations influence the combined effect or mechanism of APR-246 (plus chemotherapy) in ESCC.
Conclusion
Our present results demonstrated that APR-246 treatment activated p73-Noxa signalling following ROS induction in a manner dependent on p53 mutation status, leading to significant apoptosis in ESCC with p53 missense mutation. These antitumour effects and signal changes became more pronounced when APR-246 was used in combination with an anticancer drug, particularly 5-FU. These results were observed both in vitro and in vivo in a preclinical model, implying that APR-246 may be a promising means of enhancing chemo-sensitivity in ESCC bearing p53 missense mutation.
Supplementary information
Author contributions
TK and TM contributed to the design and analysis of the results and the writing of the manuscript. KY, TS, KT, TT, YK, MY, and KN contributed to the implementation of the research and analysis of the results. EM, HE, and YD contributed to the design and implementation of the research.
Funding information
This work was supported by a grants-in-aid of the SGH foundation (Kyoto, Japan) and by the Public Trust Surgery Research Fund (Tokyo, Japan) and Japan Society for the Promotion of Science (JSPS).
Data availability
No data sets were generated or analysed during the current study.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Osaka University Animal Experiments Committee had given ethical approval for the animal studies (ethical approval number: 27-061-002).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01561-0.
References
- 1.Colvin H, Mizushima T, Eguchi H, Takiguchi S, Doki Y, Mori M. Gastroenterological surgery in Japan: the past, the present and the future. Ann Gastroenterol Surg. 2017;1:5–10. doi: 10.1002/ags3.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907) Ann Surg Oncol. 2012;19:68–74. doi: 10.1245/s10434-011-2049-9. [DOI] [PubMed] [Google Scholar]
- 3.Beroud C, Soussi T. The UMD-p53 database: new mutations and analysis tools. Hum Mutat. 2003;21:176–81. doi: 10.1002/humu.10187. [DOI] [PubMed] [Google Scholar]
- 4.Sawada G, Niida A, Uchi R, Hirata H, Shimamura T, Suzuki Y, et al. Genomic landscape of esophageal squamous cell carcinoma in a Japanese population. Gastroenterology. 2016;150:1171–82. doi: 10.1053/j.gastro.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 5.Yamasaki M, Miyata H, Fujiwara Y, Takiguchi S, Nakajima K, Nishida T, et al. p53 genotype predicts response to chemotherapy in patients with squamous cell carcinoma of the esophagus. Ann Surg Oncol. 2010;17:634–42. doi: 10.1245/s10434-009-0851-4. [DOI] [PubMed] [Google Scholar]
- 6.Madani K, Zhao R, Lim HJ, Casson AG. Prognostic value of p53 mutations in oesophageal adenocarcinoma: final results of a 15-year prospective study. Eur J Cardiothorac Surg. 2010;37:1427–32. doi: 10.1016/j.ejcts.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Makino T, Yamasaki M, Miyata H, Yoshioka S, Takiguchi S, Fujiwara Y, et al. p53 Mutation status predicts pathological response to chemoradiotherapy in locally advanced esophageal cancer. Ann Surg Oncol. 2010;17:804–11. doi: 10.1245/s10434-009-0786-9. [DOI] [PubMed] [Google Scholar]
- 8.Gao YB, Chen ZL, Li JG, Hu XD, Shi XJ, Sun ZM, et al. Genetic landscape of esophageal squamous cell carcinoma. Nat Genet. 2014;46:1097–102. doi: 10.1038/ng.3076. [DOI] [PubMed] [Google Scholar]
- 9.Lambert JM, Gorzov P, Veprintsev DB, Soderqvist M, Segerback D, Bergman J, et al. PRIMA-1 reactivates mutant p53 by covalent binding to the core domain. Cancer Cell. 2009;15:376–88. doi: 10.1016/j.ccr.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, et al. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med. 2002;8:282–8. doi: 10.1038/nm0302-282. [DOI] [PubMed] [Google Scholar]
- 11.Tessoulin B, Descamps G, Moreau P, Maiga S, Lode L, Godon C, et al. PRIMA-1Met induces myeloma cell death independent of p53 by impairing the GSH/ROS balance. Blood. 2014;124:1626–36. doi: 10.1182/blood-2014-01-548800. [DOI] [PubMed] [Google Scholar]
- 12.Yoshikawa N, Kajiyama H, Nakamura K, Utsumi F, Niimi K, Mitsui H, et al. PRIMA-1MET induces apoptosis through accumulation of intracellular reactive oxygen species irrespective of p53 status and chemo-sensitivity in epithelial ovarian cancer cells. Oncol Rep. 2016;35:2543–52. doi: 10.3892/or.2016.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu DS, Duong CP, Haupt S, Montgomery KG, House CM, Azar WJ, et al. Inhibiting the system xC(-)/glutathione axis selectively targets cancers with mutant-p53 accumulation. Nat Commun. 2017;8:14844. doi: 10.1038/ncomms14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saha MN, Jiang H, Yang Y, Reece D, Chang H. PRIMA-1Met/APR-246 displays high antitumor activity in multiple myeloma by induction of p73 and Noxa. Mol Cancer Ther. 2013;12:2331–41. doi: 10.1158/1535-7163.MCT-12-1166. [DOI] [PubMed] [Google Scholar]
- 15.Peng X, Zhang MQ, Conserva F, Hosny G, Selivanova G, Bykov VJ, et al. APR-246/PRIMA-1MET inhibits thioredoxin reductase 1 and converts the enzyme to a dedicated NADPH oxidase. Cell Death Dis. 2013;4:e881. doi: 10.1038/cddis.2013.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rokaeus N, Shen J, Eckhardt I, Bykov VJ, Wiman KG, Wilhelm MT. PRIMA-1(MET)/APR-246 targets mutant forms of p53 family members p63 and p73. Oncogene. 2010;29:6442–51. doi: 10.1038/onc.2010.382. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann S, Bykov VJ, Ali D, Andren O, Cherif H, Tidefelt U, et al. Targeting p53 in vivo: a first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J Clin Oncol. 2012;30:3633–9. doi: 10.1200/JCO.2011.40.7783. [DOI] [PubMed] [Google Scholar]
- 18.Zandi R, Selivanova G, Christensen CL, Gerds TA, Willumsen BM, Poulsen HS. PRIMA-1Met/APR-246 induces apoptosis and tumor growth delay in small cell lung cancer expressing mutant p53. Clin Cancer Res. 2011;17:2830–41. doi: 10.1158/1078-0432.CCR-10-3168. [DOI] [PubMed] [Google Scholar]
- 19.Furukawa H, Makino T, Yamasaki M, Tanaka K, Miyazaki Y, Takahashi T, et al. PRIMA-1 induces p53-mediated apoptosis by upregulating Noxa in esophageal squamous cell carcinoma with TP53 missense mutation. Cancer Sci. 2018;109:412–21. doi: 10.1111/cas.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makino T, Yamasaki M, Miyazaki Y, Wada N, Takahashi T, Kurokawa Y, et al. Utility of initial induction chemotherapy with 5-fluorouracil, cisplatin, and docetaxel (DCF) for T4 esophageal cancer: a propensity score-matched analysis. Dis Esophagus. 2018. 10.1093/dote/dox130. [DOI] [PubMed]
- 21.Hashimoto T, Makino T, Yamasaki M, Tanaka K, Miyazaki Y, Takahashi T, et al. The pattern of residual tumor after neoadjuvant chemotherapy for locally advanced esophageal cancer and its clinical significance. Ann Surg. 2019;271:875–84. doi: 10.1097/SLA.0000000000003129. [DOI] [PubMed] [Google Scholar]
- 22.Makino T, Yamasaki M, Tanaka K, Masuike Y, Tatsumi M, Motoori M, et al. Metabolic tumor volume change predicts long-term survival and histological response to preoperative chemotherapy in locally advanced esophageal cancer. Ann Surg. 2019;270:1090–5. doi: 10.1097/SLA.0000000000002808. [DOI] [PubMed] [Google Scholar]
- 23.Urakawa S, Makino T, Yamasaki M, Tanaka K, Miyazaki Y, Takahashi T, et al. Lymph node response to neoadjuvant chemotherapy as an independent prognostic factor in metastatic esophageal cancer. Ann Surg. 2021;273:1141–9. doi: 10.1097/SLA.0000000000003445. [DOI] [PubMed] [Google Scholar]
- 24.Hu T, Zhang J, Sha B, Li M, Wang L, Zhang Y, et al. Targeting the overexpressed USP7 inhibits esophageal squamous cell carcinoma cell growth by inducing NOXA-mediated apoptosis. Mol Carcinog. 2019;58:42–54. doi: 10.1002/mc.22905. [DOI] [PubMed] [Google Scholar]
- 25.Grande L, Bretones G, Rosa-Garrido M, Garrido-Martin EM, Hernandez T, Fraile S, et al. Transcription factors Sp1 and p73 control the expression of the proapoptotic protein NOXA in the response of testicular embryonal carcinoma cells to cisplatin. J Biol Chem. 2012;287:26495–505. doi: 10.1074/jbc.M112.376319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flinterman M, Guelen L, Ezzati-Nik S, Killick R, Melino G, Tominaga K, et al. E1A activates transcription of p73 and Noxa to induce apoptosis. J Biol Chem. 2005;280:5945–59. doi: 10.1074/jbc.M406661200. [DOI] [PubMed] [Google Scholar]
- 27.Sharma K, Vu TT, Cook W, Naseri M, Zhan K, Nakajima W, et al. p53-independent Noxa induction by cisplatin is regulated by ATF3/ATF4 in head and neck squamous cell carcinoma cells. Mol Oncol. 2018;12:788–98. doi: 10.1002/1878-0261.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–8. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 29.Shibue T, Takeda K, Oda E, Tanaka H, Murasawa H, Takaoka A, et al. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev. 2003;17:2233–8. doi: 10.1101/gad.1103603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guikema JE, Amiot M, Eldering E. Exploiting the pro-apoptotic function of NOXA as a therapeutic modality in cancer. Expert Opin Ther Targets. 2017;21:767–79. doi: 10.1080/14728222.2017.1349754. [DOI] [PubMed] [Google Scholar]
- 31.Lu C, Wang W, El-Deiry WS. Non-genotoxic anti-neoplastic effects of ellipticine derivative NSC176327 in p53-deficient human colon carcinoma cells involve stimulation of p73. Cancer Biol Ther. 2008;7:2039–46. doi: 10.4161/cbt.7.12.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kravchenko JE, Ilyinskaya GV, Komarov PG, Agapova LS, Kochetkov DV, Strom E, et al. Small-molecule RETRA suppresses mutant p53-bearing cancer cells through a p73-dependent salvage pathway. Proc Natl Acad Sci USA. 2008;105:6302–7. doi: 10.1073/pnas.0802091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambert JM, Moshfegh A, Hainaut P, Wiman KG, Bykov VJ. Mutant p53 reactivation by PRIMA-1MET induces multiple signaling pathways converging on apoptosis. Oncogene. 2010;29:1329–38. doi: 10.1038/onc.2009.425. [DOI] [PubMed] [Google Scholar]
- 34.Mohell N, Alfredsson J, Fransson A, Uustalu M, Bystrom S, Gullbo J, et al. APR-246 overcomes resistance to cisplatin and doxorubicin in ovarian cancer cells. Cell Death Dis. 2015;6:e1794. doi: 10.1038/cddis.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perdrix A, Najem A, Saussez S, Awada A, Journe F, Ghanem G, et al. PRIMA-1 and PRIMA-1(Met) (APR-246): from mutant/wild type p53 reactivation to unexpected mechanisms underlying their potent anti-tumor effect in combinatorial therapies. Cancers. 2017;9:172. [DOI] [PMC free article] [PubMed]
- 36.Kim K-C, Jung C-S, Choi K-H. Overexpression of p73 enhances cisplatin-induced apoptosis in HeLa cells. Arch Pharmacal Res. 2006;29:152–8. doi: 10.1007/BF02974277. [DOI] [PubMed] [Google Scholar]
- 37.Celli J, Duijf P, Hamel BC, Bamshad M, Kramer B, Smits AP, et al. Heterozygous germline mutations in the p53 homolog p63 are the cause of EEC syndrome. Cell. 1999;99:143–53. doi: 10.1016/s0092-8674(00)81646-3. [DOI] [PubMed] [Google Scholar]
- 38.van Bokhoven H, Hamel BC, Bamshad M, Sangiorgi E, Gurrieri F, Duijf PH, et al. p63 gene mutations in EEC syndrome, limb-mammary syndrome, and isolated split hand–split foot malformation suggest a genotype-phenotype correlation. Am J Hum Genet. 2001;69:481–92. doi: 10.1086/323123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irwin MS, Kondo K, Marin MC, Cheng LS, Hahn WC, Kaelin WG., Jr Chemosensitivity linked to p73 function. Cancer Cell. 2003;3:403–10. doi: 10.1016/s1535-6108(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 40.Ozaki T, Naka M, Takada N, Tada M, Sakiyama S, Nakagawara A. Deletion of the COOH-terminal region of p73alpha enhances both its transactivation function and DNA-binding activity but inhibits induction of apoptosis in mammalian cells. Cancer Res. 1999;59:5902–7. [PubMed] [Google Scholar]
- 41.Ueda Y, Hijikata M, Takagi S, Chiba T, Shimotohno K. New p73 variants with altered C-terminal structures have varied transcriptional activities. Oncogene. 1999;18:4993–8. doi: 10.1038/sj.onc.1202817. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez S, Perez-Perez MM, Hernando E, Serrano M, Cordon-Cardo C. p73beta-Mediated apoptosis requires p57kip2 induction and IEX-1 inhibition. Cancer Res. 2005;65:2186–92. doi: 10.1158/0008-5472.CAN-04-3047. [DOI] [PubMed] [Google Scholar]
- 43.Ren J, Datta R, Shioya H, Li Y, Oki E, Biedermann V, et al. p73beta is regulated by protein kinase Cdelta catalytic fragment generated in the apoptotic response to DNA damage. J Biol Chem. 2002;277:33758–65. doi: 10.1074/jbc.M110667200. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Prives C. Are interactions with p63 and p73 involved in mutant p53 gain of oncogenic function? Oncogene. 2007;26:2220–5. doi: 10.1038/sj.onc.1210311. [DOI] [PubMed] [Google Scholar]
- 45.Rocco JW, Leong CO, Kuperwasser N, DeYoung MP, Ellisen LW. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell. 2006;9:45–56. doi: 10.1016/j.ccr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Fransson A, Glaessgen D, Alfredsson J, Wiman KG, Bajalica-Lagercrantz S, Mohell N. Strong synergy with APR-246 and DNA-damaging drugs in primary cancer cells from patients with TP53 mutant high-grade serous ovarian cancer. J Ovarian Res. 2016;9:27. doi: 10.1186/s13048-016-0239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bykov VJ, Zache N, Stridh H, Westman J, Bergman J, Selivanova G, et al. PRIMA-1(MET) synergizes with cisplatin to induce tumor cell apoptosis. Oncogene. 2005;24:3484–91. doi: 10.1038/sj.onc.1208419. [DOI] [PubMed] [Google Scholar]
- 48.Roh JL, Kang SK, Minn I, Califano JA, Sidransky D, Koch WM. p53-Reactivating small molecules induce apoptosis and enhance chemotherapeutic cytotoxicity in head and neck squamous cell carcinoma. Oral Oncol. 2011;47:8–15. doi: 10.1016/j.oraloncology.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu DS, Read M, Cullinane C, Azar WJ, Fennell CM, Montgomery KG, et al. APR-246 potently inhibits tumour growth and overcomes chemoresistance in preclinical models of oesophageal adenocarcinoma. Gut. 2015;64:1506–16. doi: 10.1136/gutjnl-2015-309770. [DOI] [PubMed] [Google Scholar]
- 50.Hu Z, Lv G, Li Y, Li E, Li H, Zhou Q, et al. Enhancement of anti-tumor effects of 5-fluorouracil on hepatocellular carcinoma by low-intensity ultrasound. J Exp Clin Cancer Res. 2016;35:71. doi: 10.1186/s13046-016-0349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zou X, Liang J, Sun J, Hu X, Lei L, Wu D, et al. Allicin sensitizes hepatocellular cancer cells to anti-tumor activity of 5-fluorouracil through ROS-mediated mitochondrial pathway. J Pharmacol Sci. 2016;131:233–40. doi: 10.1016/j.jphs.2016.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data sets were generated or analysed during the current study.