Abstract
Aim
This study aims to examine whether early-life factors are associated with adult ovarian reserve, measured by anti-Müllerian hormone (AMH) levels.
Methods
The work is based on the Jerusalem Perinatal Study (JPS), an extensive birth cohort with detailed information on all pregnancies and deliveries in Jerusalem between 1974 and 1976. A subset of individuals participated in a follow-up study that took place between 2007 and 2009 in which they completed questionnaires and were physically examined at mean age of 32. A blood sample was additionally drawn from each participant, and AMH was measured in a sample of 239 women. The associations between each early-life factors, including birth weight, maternal pre-pregnancy weight, gestational weight gain (GWG), socioeconomic position at birth, and parental smoking during pregnancy, were assessed with AMH levels at the age of 32.Multivariable regression models were used to examine the associations with AMH, adjusting for potential confounders at birth and at the age of 32.
Results
Low birth weight was significantly associated with lower ovarian reserve reflected by lower levels of AMH at age 32 (range 30–36), independent of other early-life factors and after adjusting for confounders (β = 0.180, p = 0.03).
Conclusions
This prospective study demonstrates the association of birth weight and adult ovarian reserve. Underlying mechanisms are yet to be fully understood.
Keywords: Anti-Müllerian hormone, Birth weight, Early-life factors, Ovarian reserve
Introduction
The association between early-life factors, especially during the fetal period, and permanent physiologic and metabolic changes in the developing organism is well-established [1, 2]. The Developmental Origins of Health and Disease (DOHaD) paradigm attributes morphologic development and long-term function of an organism to early-life insults [3, 4]. As early-life environment cannot be measured directly, it is often assessed by factors reflecting this environment. Numerous studies have shown that birth weight represents the intrauterine environment and is hence associated with future health [5–11].
Anti-Müllerian hormone (AMH) is a glycoprotein hormone exclusively produced by the granulosa cells surrounding pre-antral and small antral follicles. The hormone secretion from these cells is independent of the gonadotropic position or the menstrual cycle [12]. As such, AMH is an established marker of ovarian follicle pool [13] and serves as a biochemical marker of ovarian reserve in various clinical conditions [7, 14–16].
Heritability is considered to highly impact ovarian aging [17], and indeed, maternal age at menopause is used for evaluating ovarian reserve in the reproductive age [18]. However, early-life environment may have an additional impact on ovarian reserve later in life. As the number of primordial follicles in the ovary reaches its maximum at 20–26 weeks of gestation [19, 20], adverse intrauterine environment and early-life exposures during this period may thus have an unfavorable effect on follicular development, impairing later life reproductive health. Younger age at menarche and at menopause, polycystic ovarian syndrome, and other reproductive and metabolic conditions have been previously associated with intrauterine nutrition disorders, suggesting a pivotal effect of the intrauterine environment on female reproductive maturation and function [21]. Additionally, some studies have previously demonstrated the association of birth weight and other early-life exposures with AMH levels in infancy and adolescence [22–24].
In this study, using 30-year follow-up data from the Jerusalem Perinatal Study (JPS) birth cohort, which includes detailed information on all births in Jerusalem between 1964 and 1976 [16], we sought to determine if various early-life exposures, including prenatal factors, affect ovarian reserve, estimated by AMH levels, in adult women of child-bearing age.
Materials and methods
The Jerusalem Perinatal Family Follow-up Study cohort
The Jerusalem Perinatal Study (JPS) population-based cohort includes a sub-cohort of all 17,003 births to residents of Western Jerusalem, between years 1974 and 1976, consisting of extensive perinatal data obtained from birth certificates, maternity ward logbooks, and interviews of mothers on the first or second day of postpartum. Detailed information on data collection has been previously described [8, 9].
The JPS Family Follow-up study includes a sample of offspring from the original 1974–1976 birth cohort, all of Jewish ancestry. Eligible for the follow-up study were unrelated singleton offspring born at term (> 36 weeks) without congenital malformations. A stratified sampling approach was carried out based on maternal pre-pregnancy body mass index (BMI) and birth weight. Participants were interviewed and physically examined between 2007 and 2009. Complete interviews, physical examinations, and fasting blood samples were obtained for a final sample of 1,473 offspring, of which 732 (49%) were women [25].
Study population
Selection of women offspring into the current study from the JPS Follow-up cohort was based on birth weight. We used a population-based intrauterine growth chart developed by Nicolaides et al. [26], to define birth weight (BW) percentiles. This growth chart was chosen as it is one of the only charts reflecting intrauterine fetal biometrics, rather than neonatal weight. All women offspring with low BW (i.e., under the 10th percentile) were selected into the study (n = 113). Women with normal BW (i.e., between the 10th and the 90th percentiles) were selected to obtain approximately 1:1 low-to-normal BW ratio using the following approach: normal BW was subdivided into 4 groups (i.e., 10–30th, 30–50th, 50–70th, and 70–90th), and approximately 30 women from each group were randomly selected to ensure the sampling represents the entire distribution of normal BW. The study population selection is described in Fig. 1.
Fig. 1.
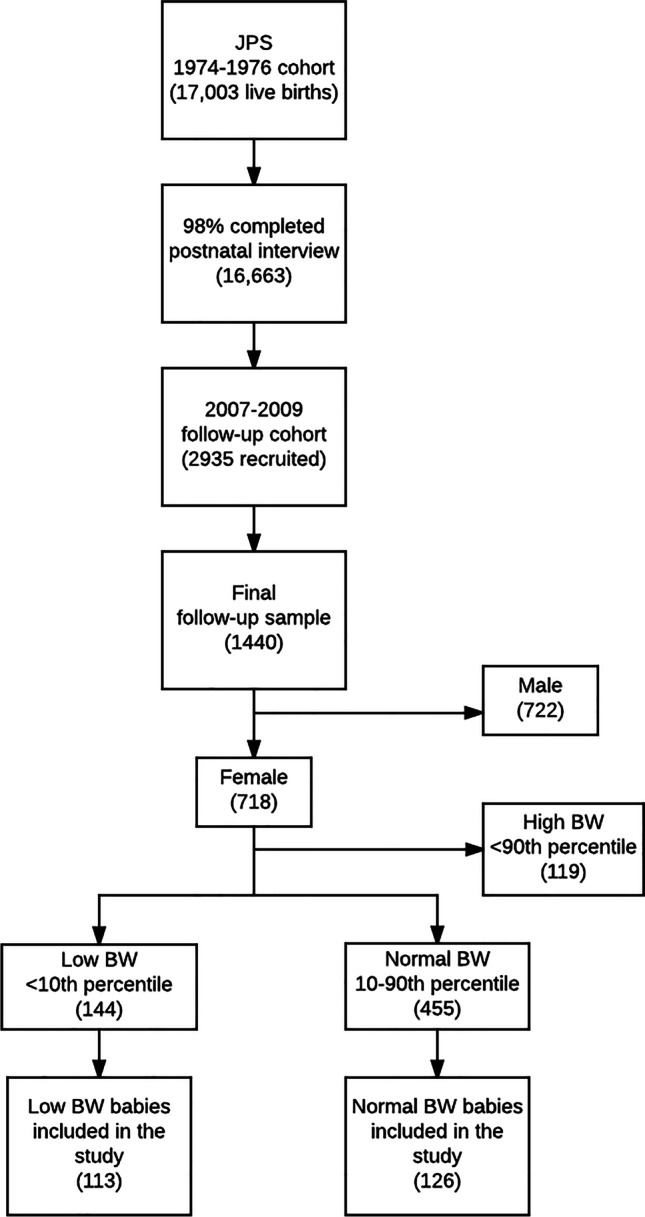
Study population
Anti-Müllerian hormone testing
Frozen plasma samples, stored at − 80 °C, from the selected participants were identified and transferred to a laboratory for AMH measurement. AMH was measured using the automated Ultra-Sensitive AMH/MIS ELISA kit (Ansh labs) with a quantitative three-step sandwich type immunoassay.
Study variables
The primary outcome of the study, ovarian reserve, was estimated by Anti-Müllerian hormone levels and assessed as a continuous variable.
The following factors were investigated: (1) BW (kg, continuous and divided into 6 percentile categories: < 5th, 5–10th, 10–30th, 30–50th, 50–70th, 70–90th); (2) maternal pre-pregnancy body mass index (mppBMI, kg/m2, continuous and divided into quartiles: Q1 < 21.0 kg/m2; Q2 21.0–23.8 kg/m2; Q3 23.9–26.4 kg/m2; Q4 > 26.4 kg/m2); (3) gestational weight gain (GWG, kg, continuous and divided into quartiles: Q1 < 9 kg; Q2 9–11 kg; Q3 12–14 kg; Q4 > 14 kg); and (4) maternal and paternal smoking during pregnancy (as two separate variables, dichotomous).
We addressed the following covariates at time of birth: (1) maternal age at the time of birth (years, continuous); (2) paternal age at the time of birth (years, continuous); (3) maternal medical conditions (dichotomous, based on whether the mother had suffered from any of the following diseases: diabetes, hypertension, heart disease, or toxemia); (4) ethnicity based on mother’s father country of birth (categorized as Israel, other West Asia, North Africa, and Europe/America, and other industrialized countries); (5) average parental years of education at the time of birth (years, continuous); and (6) socioeconomic position (SEP) based on father’s occupation at the time of birth (low, medium, and high). This SEP scale was shown to have predictive validity [27] and has the advantage of allowing classification of occupational groups unique to the Israeli population, such as Rabbis and yeshiva students, in a dimension that differed from education and ethnic group. We also assessed the following covariates measured in adulthood: BMI at age 32 (kg/m2, continuous), smoking status (current vs. else), and use of oral contraceptives. As AMH measurements in 16 of the 239 samples were carried out a few months earlier as part of a pilot study, a dummy variable (pilot study vs. rest) was introduced to all models to adjust for potential batch effects.
Statistical analysis
Values of AMH were log transformed to normalize the distribution, as previously described [4]. Pearson correlation coefficient and one-way analysis of variance (ANOVA) were used to investigate the univariable associations of early-life factors, assessed as continuous and categorical variables, respectively, with AMH at the age of 32. Next, we applied multivariable linear regression models for examining independent associations of early-life factors with AMH. Associations were assessed using the following model: the independent associations of the exposures with AMH were assessed by entering all exposures together into the model, adjusted for ethnicity, parental education and SEP at time of birth, maternal and paternal age at delivery, maternal medical conditions during pregnancy, and the participant’s BMI at age 32. Although only term pregnancies (> 36 weeks) were included in the follow-up study, all models were also adjusted for gestational week to account for residual confounding and for potential batch effect.
We report beta coefficients (β), 95% confidence interval (CI), and two-tailed P values. P value below 0.05 was considered statistically significant.
Analyses were carried out using the IBM SPSS version 24.0 statistical package (SPSS, Inc., Chicago, IL) and Stata 12.0 (StataCorp, College Station, TX).
Power calculations focused on BW demonstrated that this study has sufficient power to detect associations with AMH. Our sample size (n = 239) provides 80% power (α = 0.05) to detect a modest difference of 0.375 standard deviation in AMH levels between women with low BW (approximately 50%) and normal birth weight and a beta coefficient of ± 0.134 for the regression of AMH on BW.
The study was approved by the institutional review board of our medical center. All participants provided informed consent.
Results
Table 1 presents parental and offspring characteristics measured at birth and in adulthood. AMH levels were assessed in a total of 239 adult women. The mean age of women was 32.3 ± 1.2 (range 30–36). The median AMH level of the entire cohort was 3.5 ng/ml (interquartile range 1.9–6.2). There were no significant differences in characteristics measured at birth between the 239 women included in this study and the 479 who were not included (socioeconomic status (P = 0.925), parents’ average years of education (P = 0.620), paternal (P = 0.372), and maternal smoking (P = 0.925)).
Table 1.
Study characteristics at birth and at the age 32 years (n = 239)
| Variable | Mean ± SD or % | Range (min, max) |
Median (Q1, Q3) |
|---|---|---|---|
| Early-life factors | |||
| Birth weight (kg) | 2.96 ± 0.55 | 1.47, 4.2 | |
| Gestational weight gain (kg) | 10.06 ± 4.02 | − 4.0, 23.0 | |
| Maternal pre-pregnancy BMI (kg/m2) | 23.79 ± 3.78 | 16.8, 39.7 | |
| Maternal smoking (%) | 17.57 | ||
| Paternal smoking (%) | 48.32 | ||
| Socioeconomic status (%) | |||
| Low | 22.07 | ||
| Medium | 42.25 | ||
| High | 35.68 | ||
| Characteristics at birth | |||
| Gestational age (weeks) | 39.96 ± 1.56 | 36.1, 43.4 | |
| Maternal age at birth | 27.68 ± 5.37 | 18.7, 42.5 | |
| Paternal age at birth | 31.16 ± 6.26 | 20.9, 51.1 | |
| Average parental years of education | 11.97 ± 2.96 | 3.0, 23.0 | |
| Maternal medical conditiona (%) | 9.62 | ||
| Ethnic origin (%) | |||
| Israel | 14.23 | ||
| Middle east | 28.03 | ||
| North Africa | 19.67 | ||
| Ashkenazi | 38.08 | ||
| Measurements at follow-up | |||
| Age | 32.34 ± 1.23 | 30, 36 | |
| AMH serum concentration (ng/ml) | 4.81 ± 4.52 | 0.13, 28.08 | 3.5 (1.9, 6.22) |
| AMH log transformed | 0.51 ± 0.41 | − 0.89, 1.45 | 0.54 (0.28, 0.8) |
| BMI (kg/m2) | 26.09 ± 5.74 | 17.09, 46.89 | |
| Current smoking | 17.37 | ||
| Use of oral contraceptives | 25.22 | ||
Note: AMH, anti-Müllerian hormone; BMI, body mass index; IQR, interquartile range
aDiabetes, hypertension, heart disease or toxemia
Univariable associations between each early-life factor and AMH levels (log transformed) are presented in Table 2. A statistically significant positive correlation was observed between continuous BW and AMH levels (r = 0.18, P = 0.006). Similarly, while not statistically significant, the mean AMH level at age 32 was lowest in the very low BW group (< 5%) and highest in the highest percentile (70–90%) group. The mean AMH level at age 32 was similar in the different maternal pre-pregnancy BMI and gestational weight gain quartiles, and correlations with continuous maternal characteristics also yielded non-significant results. Additionally, the mean AMH levels did not differ when comparing maternal and paternal smoking status.
Table 2.
Early-life factors and ovarian reserve at age 32
| Variable | Correlation coefficient | Log transformed mean AMHa | P valueb |
|---|---|---|---|
| Birth weight, kg | 0.18 | 0.006 | |
| Birth weight, percentilesc | 0.27 | ||
| < 5% | 0.43 ± 0.44 | ||
| 5–10% | 0.55 ± 0.38 | ||
| 10–30% | 0.49 ± 0.45 | ||
| 30–50% | 0.55 ± 0.35 | ||
| 50–70% | 0.52 ± 0.41 | ||
| 70–90% | 0.64 ± 0.31 | ||
| Maternal pre-pregnancy BMI, kg/m2 | 0.02 | 0.76 | |
| Maternal pre-pregnancy BMI, quartiles | 0.77 | ||
| Q1 | 0.51 ± 0.44 | ||
| Q2 | 0.48 ± 0.42 | ||
| Q3 | 0.50 ± 0.39 | ||
| Q4 | 0.56 ± 0.39 | ||
| Gestational weight gain, kg | − 0.04 | 0.51 | |
| Gestational weight gain, quartiles | 0.90 | ||
| Q1 | 0.53 ± 0.38 | ||
| Q2 | 0.52 ± 0.42 | ||
| Q3 | 0.50 ± 0.45 | ||
| Q4 | 0.46 ± 0.36 | ||
| Maternal smoking during pregnancy | 0.44 | ||
| No smoking | 0.50 ± 0.41 | ||
| Active or past smoking | 0.55 ± 0.38 | ||
| Paternal smoking during pregnancy | 0.62 | ||
| No smoking | 0.50 ± 0.42 | ||
| Active or past smoking | 0.53 ± 0.40 |
aLog-transformed values (base 10) due to asymmetrical distribution
bP values based on either Pearson correlations for exposures assessed as continuous variables or one-way analysis of variance (ANOVA) for exposures assessed as categorical variables
cPercentiles were defined based on a population-based intrauterine growth chart developed by Nicolaides et al. [Poon LCY, Volpe N, Muto B, Syngelaki A, Nicolaides KH. Birthweight with gestation and maternal characteristics in live births and stillbirths. Fetal Diagn Ther 2012;32:156–65]
Note: AMH, anti-Müllerian hormone; BMI, body mass index
Results of multivariable linear regression analyses are presented in Table 3. BW (adjusted for gestational age) was positively associated with AMH serum concentration at the age of 32, independent of all other early-life factors as well as all confounders, including participant’s BMI at the time of AMH measurement (β = 0.180, P = 0.003). Estimates for this associations remained similar in weighted models accounting for the stratified sampling scheme (β = 0.160, P = 0.066).
Table 3.
Associations between early-life factors and anti-Müllerian hormone levels at age 32: multivariable linear regressionsa
| Early-life factors | β | 95% CI | P value |
|---|---|---|---|
| Birth weight (kg) | 0.180 | 0.06, 0.30 | 0.003 |
| Maternal pre-pregnancy BMI (kg/m2) | − 0.003 | − 0.02, 0.01 | 0.73 |
| Gestational weight gain (kg) | − 0.010 | − 0.02, 0.00 | 0.16 |
| Maternal smoking (yes/no) | 0.085 | − 0.07, 0.24 | 0.27 |
| Paternal smoking (yes/no) | 0.002 | − 0.11, 0.11 | 0.97 |
aAll exposures were introduced together into the model, adjusted for gestational week, batch, ethnicity, parental education, socioeconomic position at birth, maternal and paternal age at delivery, maternal medical conditions, and participant’s BMI at age 32
Other early exposures (i.e., mppBMI, GWG and parental smoking) demonstrated a trend of an inverse relationship with ovarian reserve. However, those associations were not found to be statistically significantly.
A set of sensitivity analyses were carried out to assess if several factors potentially related to AMH levels impact the observed associations. In a model excluding six participants who were diagnosed with cancer (all reported having regular menses), results for BW remained largely unchanged (β = 0.171, P = 0.005). Further excluding one woman with surgical premature menopause did not impact the findings. To assess the impact of current smoking and use of oral contraceptives on the reported findings, we introduced both variables to the model, and these too did not impact the BW-AMH association (β = 0.177, P = 0.004). Restricting the analysis to 170 women (75%) who did not use oral contraceptives yielded similar results (β = 0.166, P = 0.022). It is noteworthy that in this study, univariable and multivariable associations of AMH with both current smoking and oral contraceptive use were non-significant. Lastly, although all study participants reported having regular menses (except for the one with surgical menopause), to explore the potential effect of PCOS on the reported results, and given that data on PCOS is unavailable, we reran the analysis excluding 48 women whose AMH levels were at the top 15% of the distribution (AMH > 8.5 ng/ml), as higher AMH levels were shown to be associated with PCOS [28]. Again, the association of BW with AMH remained the same (β = 0.178, P = 0.002).
Discussion
In this study, lower BW was found to be significantly associated with lower ovarian reserve in adulthood, as reflected by decreased AMH serum levels.
The association between BW and AMH serum concentration of the offspring is controversial. The Avon longitudinal study of parents and children (ALSPAC) [24] found no association between small for gestational age BW and AMH levels in adolescence. These findings are in line with other reports as well [23, 29–31]. However, Sir-Petermann et al. demonstrated increased AMH plasma concentration in both high and low birth weight infant girls (measured at 2–3 months of age), suggesting possible ovarian dysfunction at both tails of birth weight distribution [32]. The conflicting results may be related to the wide age range in which AMH was measured in different studies, ranging from infancy [32], through pre-pubertal age 9 and adolescence [23, 24], to young adulthood [30], with the oldest age at assessment being 24 years of age. In our research, AMH level was assessed at 32–34 years of age, resulting in a minimum age difference of almost a decade between the participants in previous and our studies. Follicular dynamics throughout childhood and adolescence [33] compared to an adult population may account for the differences observed. Moreover, the mean age in which women desire to conceive is approximate to the range of 32–34 years of age [34], and therefore, measurement of ovarian reserve in this age group is of high clinical relevance.
Other studies have examined the associations of various early-life exposures and ovarian reserve later in life. Similarly to the aforementioned ALSPAC study [24], in which several factors such as parental age, maternal body mass index, and child’s birth weight were not associated with offspring’s AMH levels, we have not found an association between maternal pre-pregnancy BMI and AMH levels [4]. Similarly, and while maternal smoking was previously associated with end-organ damage in the offspring [35, 36], in our study as well as in others, parental smoking was not linked to offspring ovarian reserve [24, 37]. Socioeconomic position is a complex exposure variable. The PROGRAM/PREMS cohort study has shown lower AMH levels in young adult females (aged 18–24 years) from low socioeconomic position [30] that was determined according to the participant’s educational level at adulthood. Adjustments for cancer diagnosis, smoking status, and use of oral contraceptives showed similar results.
The main strength of this study is its detailed medical, lifestyle, and socio-demographic information on parental and offspring characteristics at pregnancy and birth, as well as the long-term follow-up data at the mean age of 32.
This study has several limitations. Information regarding fragile X mental retardation 1 pre-mutation, chromosome X derangements, ovarian surgery not manifesting in early menopause (unilateral oophorectomy or ovarian cystectomy), and infertility diagnoses such as unexplained infertility, which are possible causes of reduced ovarian reserve and poor ovarian response [38], was not available. Additionally, data regarding familial primary ovarian insufficiency or maternal infertility diagnoses (such as polycystic ovary syndrome) were not collected as well as other ovarian reserve markers, such as follicular-stimulating hormone (FSH) and estradiol levels. Though to date biomarkers indicating diminished ovarian reserve were not shown to be associated with reduced fertility, among the existing biomarkers of ovarian reserve, AMH is the most reliable [39] and only slightly affected by hormonal cycle phase [40] and therefore was chosen as an indicator for this study.
Lastly, we preselected the participants based on their birth weights to assure sufficient power to detect associations with AMH, including non-linear. This may have resulted in decreased power to detect associations with other factors, yet this would be considered a conservative approach where some findings may have been missed, yet more importantly the observed associations are not expected to be biased as a result.
Our study included a selective sub-sample of 239 women out of the total 718 women who participated in the JPS follow-up study. This sub-sample was selected based on birth weight. While approximately half of this sample were of low birth weight, we assured an even dispersion of normal birth weights by using stratified sampling by percentiles within the normal range.
In summary, this study provides further evidence connecting adverse intrauterine environment, reflected by lower birth weight, with subsequent health.
Further research is required to further characterize the relationship of early-life factors, including prenatal exposures, and adult ovarian reserve and its clinical implications.
Author contribution
All authors contributed to the study conception and design. Supervision of the study design and methodology was performed by Uri P Dior, Yechiel Friedlander, and Hagit Hochner. Material preparation, data collection, and analysis were performed by Uri P Dior, Hagit Hochner, Gilad Karavani, and Valerie Soloveichick. Original draft preparation and review and editing were performed by Gilad Karavani, Uri P Dior, Hagit Hochner, and Yechiel Friedlander. All authors read and approved the final manuscript.
Funding
The National Institutes of Health [grant R01HL088884]; the Israeli Science Foundation [grant numbers 1252/07, 552/12]; and the Joint Research fund between the Hebrew University Faculty of Medicine and Hadassah Medical Hospital.
Availability of data and material
Available upon request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dior Uri Pinchas and Karavani Gilad contributed equally to this work.
References
- 1.Barker DJP. Developmental origins of adult health and disease. J Epidemiol Community Heal. 2004;58:114–115. doi: 10.1136/jech.58.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hales CN, Barker DJP. The thrifty phenotype hypothesis: type 2 diabetes. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Hanson M, Gluckman P. Developmental origins of noncommunicable disease: population and public health implications. Am J Clin Nutr. 2011;94:1754S–S1758. doi: 10.3945/ajcn.110.001206. [DOI] [PubMed] [Google Scholar]
- 4.Lucas A. Programming by early nutrition: an experimental approach. J Nutr. 1998;128:401S–S406. doi: 10.1093/jn/128.2.401S. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJP, Osmond C, Golding J, Kuh D, Wadsworth MEJ. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease disease is more closely related to neonatal and maternal. Br J Med. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1:335–47. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 7.White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis. 2009;54:248–261. doi: 10.1053/j.ajkd.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 8.Friedlander Y, Paltiel O, Deutsch L, Knaanie A, Massalha S, Tiram E, et al. Birthweight and relationship with infant, child and adult mortality in the Jerusalem perinatal study. Paediatr Perinat Epidemiol. 2003;17:398–406. doi: 10.1046/j.1365-3016.2003.00522.x. [DOI] [PubMed] [Google Scholar]
- 9.Friedlander Y, Paltiel O, Manor O, Deutsch L, Yanetz R, Calderon-Margalit R, et al. Birthweight of offspring and mortality of parents: the Jerusalem perinatal study cohort. Ann Epidemiol. 2007;17:914–922. doi: 10.1016/j.annepidem.2007.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyengar A, Nesargi S, George A, Sinha N, Selvam S, Luyckx VA. Are low birth weight neonates at risk for suboptimal renal growth and function during infancy? BMC Nephrol. 2016;17:100. doi: 10.1186/s12882-016-0314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tain YL, Hsu CN. Developmental Origins of chronic kidney disease: should we focus on early life? . Int J Mol Sci. 2017;18:E381. doi: 10.3390/ijms18020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weeneen C, Laven JSE, von Bergh ARM, Cranfield M, Groome NP, Visser JA, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Basic Sci Reprod Med. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 13.Kwee J, Schats R, Mcdonnell J, Themmen A. Evaluation of anti-Mullerian hormone as a test for the prediction of ovarian reserve. Am Soc Reprod Med. 2008;90:737–743. doi: 10.1016/j.fertnstert.2007.07.1293. [DOI] [PubMed] [Google Scholar]
- 14.Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20:370–385. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 15.Lutchman Singh K, Muttukrishna S, Stein RC, McGarrigle HH, Patel A, Parikh B, et al. Predictors of ovarian reserve in young women with breast cancer. Br J Cancer. 2007;96:1808–1816. doi: 10.1038/sj.bjc.6603814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harlap S, Davies AM, Deutsch L, Calderon-margalit R, Manor O. The Jerusalem perinatal study cohort, 1964–2005: methods and a review of the main results. Paediatr Perinat Epidemiol. 2007;21:256–273. doi: 10.1111/j.1365-3016.2007.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Younis JS. Ovarian aging: latest thoughts on assessment and management. Curr Opin Obstet Gynecol. 2011;23:427–434. doi: 10.1097/GCO.0b013e32834b92b0. [DOI] [PubMed] [Google Scholar]
- 18.Depmann M, Broer SL, van der Schouw YT, Tehrani FR, Eijkemans MJ, Mol BW, et al. Can we predict age at natural menopause using ovarian reserve tests or mother’s age at menopause? A systematic literature review. Menopause. 2016;23:224–232. doi: 10.1097/GME.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 19.Baker TG. A quantitative and citological study of germ cells in human ovaries. Proc R Soc Lond. 1963;158:417–420. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- 20.Geber S, Megale R, Vale F, Lanna AM, Cabral AC. Variation in ovarian follicle density during human fetal development. J Assist Reprod Genet. 2012;29:969–972. doi: 10.1007/s10815-012-9810-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sloboda DM, Hickey M, Hart R. Reproduction in females: the role of the early life environment. Hum Reprod Update. 2011;17:210–227. doi: 10.1093/humupd/dmq048. [DOI] [PubMed] [Google Scholar]
- 22.Davies MJ, Norman RJ. Programming and reproductive functioning. Trends Endocrinol Metab. 2002;13:386–392. doi: 10.1016/S1043-2760(02)00691-4. [DOI] [PubMed] [Google Scholar]
- 23.Sadrzadeh-broer S, Kuijper EAM, Van WMM, Lambalk CB, Kuijper EAM, Van WMM. Ovarian reserve in young women with low birth weight and normal puberty : a pilot case – control study. Gynecol Endocrinol. 2011;27:641–644. doi: 10.3109/09513590.2010.508544. [DOI] [PubMed] [Google Scholar]
- 24.Fraser A, Mcnally W, Sattar N, Anderson EL, Lashen H, Fleming R, et al. Prenatal exposures and anti-Müllerian hormone in female adolescents: the Avon longitudinal study of parents and children. Am J Epidemiol. 2013;178:1414–1423. doi: 10.1093/aje/kwt137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawrence GM, Siscovick DS, Calderon-Margalit R, Enquobahrie DA, Granot-Hershkovitz E, Harlap S, Manor O, Meiner V, Paltiel O, Kwok PY, Friedlander Y, Hochner H. Cohort profile: the Jerusalem perinatal family follow-up study. Int J Epidemiol. 2016;45:343–352. doi: 10.1093/ije/dyv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poon LCY, Volpe N, Muto B, Syngelaki A, Nicolaides KH. Birthweight with gestation and maternal characteristics in live births and stillbirths. Fetal Diagn Ther. 2012;32:156–165. doi: 10.1159/000338655. [DOI] [PubMed] [Google Scholar]
- 27.Funai EF, Friedlander Y, Paltiel O, Tiram E, Xue X, Deutsch L, et al. Long-term mortality after preeclampsia. Epidemiology. 2005;16:206–215. doi: 10.1097/01.ede.0000152912.02042.cd. [DOI] [PubMed] [Google Scholar]
- 28.Abbara A, Eng PC, Phylactou M, Clarke SA, Hunjan T, Roberts R, Vimalesvaran S, Christopoulos G, Islam R, Purugganan K, Comninos AN, Trew GH, Salim R, Hramyka A, Owens L, Kelsey T, Dhillo WS. Anti-Müllerian hormone (AMH) in the diagnosis of menstrual disturbance due to polycystic ovarian syndrome. Front Endocrinol (Lausanne) 2019;26(10):656. doi: 10.3389/fendo.2019.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lem AJ, Boonstra VH, Renes JS, Breukhoven PE, de Jong FH, Laven JS, et al. Anti-Mullerian hormone in short girls born small for gestational age and the effect of growth hormone treatment. Hum Reprod. 2011;26:898–903. doi: 10.1093/humrep/deq391. [DOI] [PubMed] [Google Scholar]
- 30.Kerkhof GF, Leunissen RWJ, Willemsen RH, de Jong FH, Visser JA, Laven JS, et al. Influence of preterm birth and small birth size on serum anti-Müllerian hormone levels in young adult women. Eur J Endocrinol. 2010;163:937–944. doi: 10.1530/EJE-10-0528. [DOI] [PubMed] [Google Scholar]
- 31.Hart R, Sloboda DM, Doherty DA, Norman RJ, Atkinson HC, Newnham JP, et al. Prenatal determinants of uterine volume and ovarian reserve in adolescence. J Clin Endocrinol Metab. 2009;94:4931–4937. doi: 10.1210/jc.2009-1342. [DOI] [PubMed] [Google Scholar]
- 32.Sir-Petermann T, Marquez L, Carcamo M, Hitschfeld C, Codner E, Maliqueo M, et al. Effects of birth weight on anti-Mullerian hormone serum concentrations in infant girls. J Clin Endocrinol Metab. 2010;95:903–910. doi: 10.1210/jc.2009-1771. [DOI] [PubMed] [Google Scholar]
- 33.Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. Serum anti-mullerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97:4650–4655. doi: 10.1210/jc.2012-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mills M, Rindfuss RR, McDonald P, te Velde E; ESHRE Reproduction and Society Task Force. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17:848–60. [DOI] [PMC free article] [PubMed]
- 35.Oken E, Levitan E, Gillman M. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes. 2008;32:201–210. doi: 10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Högberg L, Cnattingius S, Lundholm C, D’Onofrio BM, Långström N, Iliadou AN. Effects of maternal smoking during pregnancy on offspring blood pressure in late adolescence. J Hypertens. 2012;30:693–699. doi: 10.1097/HJH.0b013e32835168f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bentzen JG, Forman JL, Larsen EC, Pinborg A, Johannsen TH, Schmidt L, et al. Maternal menopause as a predictor of anti-Müllerian hormone level and antral follicle count in daughters during reproductive age. Hum Reprod. 2013;28:247–255. doi: 10.1093/humrep/des356. [DOI] [PubMed] [Google Scholar]
- 38.Younis JS, Ben-Ami M, Ben-Shlomo I. The Bologna criteria for poor ovarian response: a contemporary critical appraisal. J Ovarian Res. 2015;8:76. doi: 10.1186/s13048-015-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20:688–701. doi: 10.1093/humupd/dmu020. [DOI] [PubMed] [Google Scholar]
- 40.Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy Ch, Englert Y. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22:1837–1840. doi: 10.1093/humrep/dem101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available upon request.


