Abstract
The risk of cancer associated with persons with multiple sclerosis (pwMS) prescribed with disease modifying therapies (DMTs) is not well established. This observational, cross-sectional, pharmacovigilance cohort study examined individual case safety reports from the World Health Organization database: VigiBase®. All consecutive reports of DMTs prescribed to pwMS (alemtuzumab, dimethyl fumarate, fingolimod, glatiramer acetate, interferon-β, natalizumab, ocrelizumab, and teriflunomide), and their serious adverse event cases were eligible, excluding those reporting immunosuppressant DMTs used as anticancer therapies. The primary outcome was the multivariate odds ratio of cancer reporting (r-OR) for DMTs prescribed to pwMS after imputation of missing data. There were 5966 cancer cases from 240,993 reports of DMTs prescribed to pwMS. After adjustments on age, sex, and geographical region, natalizumab (r-OR 1.74, 95% CI 1.63–1.87), interferon-β (r-OR 1.39, 95% CI 1.30–1.49), dimethyl fumarate (r-OR 1.35, 95% CI 1.25–1.46), and fingolimod (r-OR 1.15, 95% CI 1.06–1.24) were significantly associated with a greater cancer reporting, whereas alemtuzumab, glatiramer acetate, ocrelizumab, and teriflunomide were not, in the disproportionality analysis. As exploratory analyses, upper aerodigestive tract, breast, urinary including the male genitourinary tract, and nervous system cancers were associated with natalizumab, interferon-β, and dimethyl fumarate. Fingolimod was only associated with skin cancer types. Cancer cases reporting these four DMTs prescribed to pwMS were younger in age than for non-pwMS drugs in the VigiBase® (p < 0.0001). A close and regular cancer screening in pwMS treated with natalizumab, interferon-β, dimethyl fumarate, and fingolimod may be warranted, even for persons at a younger age.
Trial Registration NCT04237337
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-021-01073-y.
Introduction
In the last 20 years, the therapeutic armamentarium for multiple sclerosis (MS) has drastically improved. Since the first approved disease-modifying therapies (DMTs) in the mid-1990s (including interferon-β and glatiramer acetate as immunomodulators), many new drugs, particularly immunosuppressants, entered the market as first-line (teriflunomide and dimethyl fumarate) or second-line therapies (natalizumab, fingolimod, ocrelizumab, and alemtuzumab [1].
Not all DMTs prescribed for persons with MS (pwMS) are specific of this condition. These non-specific drugs include azathioprine, cladribine, cyclophosphamide, mitoxantrone, mycophenolate mofetil, and rituximab, whereas specific DMTs prescribed to pwMS include alemtuzumab, dimethyl fumarate, fingolimod, glatiramer acetate, interferon-β, natalizumab, ocrelizumab, and teriflunomide. Specific DMTs are those without any anticancer indication. DMTs have effects on the immune system, such as blockade of activation and/or significant depletion of T- and B-cell lines, and improve the control of inflammatory and immune-mediated diseases [2]. Intense immunomodulation combined with an inflammatory background, however, has been suggested to favor tumor growth and increase the risk of cancer [2, 3].
Cancer incidence in pwMS, irrespective of DMTs, was largely investigated through hospital and population registries, case-controls, and prospective studies. Some studies found a reduced or equivalent overall incidence of cancer compared to general population samples [4–7]. Moreover, an increased incidence was observed for specific types of cancer including breast, digestive system, skin, respiratory and genitourinary tract cancers, and lymphoma [4–10].
The role of DMTs in cancer development for pwMS remains unclear. Studies reported that interferon-β and glatiramer acetate were not associated with an increased overall risk of cancer [11–13]. Other studies found glatiramer acetate associated with a non-significant increase of breast cancer in female pwMS, and interferon-β was associated with a non-significant increase of non-breast-related cancers [14]. Furthermore, several DMTs that are not specifically prescribed to pwMS (azathioprine, cyclophosphamide) increased the overall risk of cancer [11]. These studies, however, have not addressed the potential association between cancer and recently marketed DMTs such as alemtuzumab and ocrelizumab. The objective of this study was to evaluate the association between cancer and specific DMTs prescribed to pwMS using a disproportionality analysis with the World Health Organization (WHO) pharmacovigilance database, VigiBase®.
Methods
The STROBE (Strengthening the Reporting of Observational studies in Epidemiology) statement was used to report the cohort study [15]. The study protocol was prospectively registered on https://www.ClinicalTrials.gov, NCT04237337.
Study Design
An international retrospective pharmacovigilance disproportionality analysis was performed in the WHO pharmacovigilance database, VigiBase®, from 1 January 2000 to 1 September 2019.
DMTs Prescribed to the pwMS Cohort
Serious individual case safety reports (ICSRs) were eligible if they reported at least one DMT that was prescribed to pwMS, to limit the indication bias (referred to as the DMTs prescribed to pwMS cohort). DMTs had to be labelled by either the Food and Drug Administration or the European Medicines Agency and could not have an additional indication for treatment of cancer (exclusion of DMTs that are non pwMS prescribed). Alemtuzumab, dimethyl fumarate, fingolimod, glatiramer acetate, interferon-β, natalizumab, ocrelizumab, and teriflunomide were eligible, whereas azathioprine, cladribine, cyclophosphamide, mitoxantrone, mycophenolate mofetil, and rituximab were not eligible (including prior use of these treatments). Drugs with indications for other inflammatory diseases were considered (e.g. natalizumab and Crohn’s disease) as the indication bias only affected malignancies in our setting. We only included reports on serious adverse events (SAEs) and excluded reports concurrently reporting of DMTs that were non-pwMS-prescribed, anticancer drugs or non-SAEs.
Variables
Exposure variables were DMTs prescribed to pwMS identified with their international non-proprietary name in the reports. Potential confounders were age, sex and geographical region. Outcome variables were cancers identified with the Medical Dictionary for Regulatory activities (MedDRA) terms and Standardized Queries (Supplementary Methods for details). The delay from drug initiation to the time to cancer onset and death reporting were also collected.
Data Sources
The VigiBase® database was used to conduct this study and details are available in the Supplementary Methods. VigiBase® characterizes adverse drug reactions (ADRs) related to cancer therapies and to detect potential associations between non-cancer treatment and the development of cancer [16–18]. The extract case level database was used to allow for multiple adjustments on concomitant medications, demographic parameters, and missing data imputation (Supplementary Methods for details).
Quantitative Variables
Age was defined as an ordered categorical variable (“ < 45 years old (yo)”, “45–64 yo”, “65–74 yo”, “ > 75 yo”), and time to cancer onset was defined as a continuous variable, expressed in months.
Objectives
The primary objective was to evaluate the association between DMTs prescribed to pwMS and cancer reportings in the cohort. Secondary objectives included evaluating the changes of the reporting signal of DMTs prescribed to pwMS and cancer reporting over time, age at diagnosis comparison of cancer cases according to DMTs prescribed to pwMS versus all other cancer cases in the VigiBase®, subgroup analyses according to cancer types for DMTs prescribed to pwMS significantly associated with cancer reporting, and a descriptive analysis of cancer cases reported with DMTs prescribed to pwMS significantly associated with cancer reporting.
Statistical Methods
Disproportionality analysis was used to assess the effect of DMTs prescribed to pwMS on cancer reporting. Details to this methodology have been described elsewhere [16, 17, 19, 20]. This method is the reference to assess the association between a drug and an adverse event (AE) in pharmacovigilance databases. Disproportionality analysis compares the proportion of reporting of a specific AE with a suspected drug to an expected proportion assuming the AE with this drug is independently reported. The reporting odds ratio (r-OR) was used to quantify the association. The r-OR was estimated with univariate and multivariate logistic regression models for dichotomous outcomes and with polytomous regression models for categorical outcomes (comparison of age distribution among cancer cases). There was no variable selection, and potential confounders were included in the model based on a priori knowledge (age, sex, and world region). The 95% confidence interval (CI) of the depending variable r-OR was estimated, and p < 0.05 was the threshold for significance. For DMTs prescribed to pwMS associated with an over-reporting of cancer, secondary and exploratory analyses according to cancer types were performed.
Missing data on age and sex were associated with several parameters including administrative variables, DMTs prescribed to pwMS, cancer types (Supplementary Methods for details), and therefore the Missing Completely at Random hypothesis was rejected. Since missing data on age affected more than 5% of the reports, we imputed the missing data with multiple imputations by chained equation algorithms, using 20 imputed datasets (Supplementary Methods for details). Estimates were combined according to Rubin’s rule [21, 22]. Missing data reporting complied with the Guidelines for reporting analysis potentially affected by missing data [23].
Sensitivity analyses for the primary outcome included (i) a restriction of the window period from 2014 to 2019 (to obtain a shorter window period to further improve the comparability between the drugs and limit older treatment bias), (ii) an exclusion of geographical regions in Africa, South-East Asia, and the Eastern Mediterranean region (because MS is mostly affecting patients in the US, European, and Asian regions), (iii) non-imputed datasets analyses, (iv) a consolidated period from 2000 to 2017 (to ascertain cases from individual countries were exhaustively registered in VigiBase®), (v) a comparison against all reports in the database (no restriction to DMT related-reports), and (vi) a post-hoc analysis including non-serious AE reports. Statistical analyses were performed with R, Version 3.5.3 for Windows (R Foundation for Statistical Computing, Vienna, Austria), and R package “mice” [24].
Role of the Funding Source
There was no funding source dedicated to this study. The study was supported by the Caen Normandy University Hospital (CHU Caen Normandie) and the Normandy University (Université de Caen Normandie) in France. The corresponding author had full access to all data in the study and had the responsibility for the decision to submit for publication.
Results
DMTs Prescribed to the pwMS Cohort
Of 20,471,248 reports in the VigiBase®, we identified 240,993 reports related to DMTs prescribed to pwMS and reporting of SAEs, which formed the DMTs prescribed to pwMS cohort (Fig. 1). Interferon-β was reported in 44% of the reports, natalizumab in 15%, fingolimod in 15%, dimethyl fumarate in 10%, glatiramer in 10%, teriflunomide in 5%, alemtuzumab in 3%, and ocrelizumab in 1%. Some reports had more than one DMT prescribed to pwMS (31,845, 13%). In the DMTs prescribed to the pwMS cohort, missing data affected age in 32% (N = 78,179) and sex in 2% (N = 5516). Missing data imputation details are provided in the Supplementary Data. After imputation, patients were mostly female (77%) and aged 45 or older (61%) (Supplementary Table 1 for additional details on baseline characteristics distribution according to missing data).
Fig. 1.
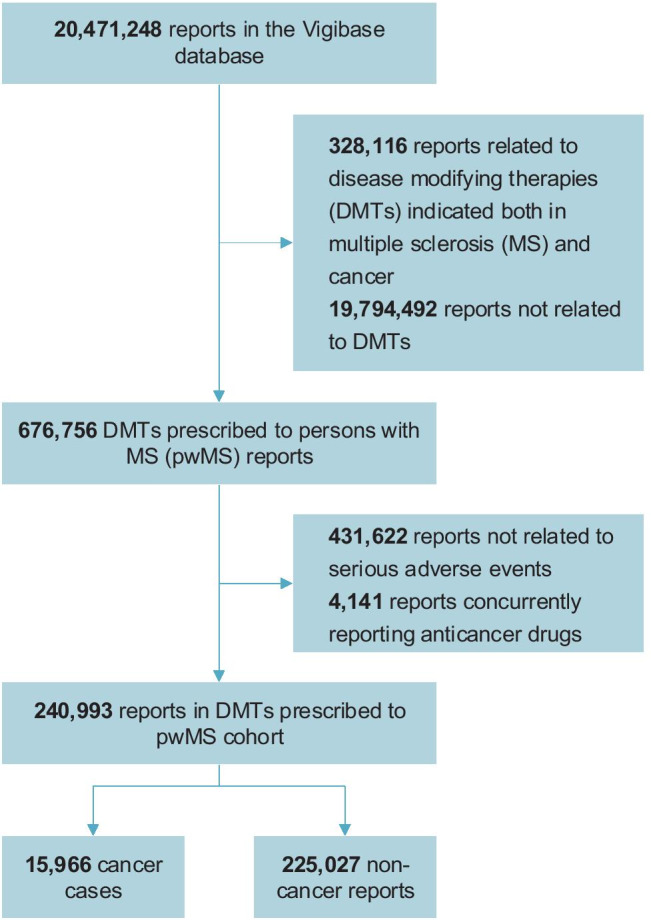
Flowchart of the reports selection (DMT, disease-modifying therapy; MS, multiple sclerosis. DMTs prescribed to persons with MS (pwMS) were alemtuzumab, dimethyl fumarate, fingolimod, glatiramer acetate, interferon-β, natalizumab, ocrelizumab, and teriflunomide)
Association Between Cancer and DMTs Prescribed to pwMS
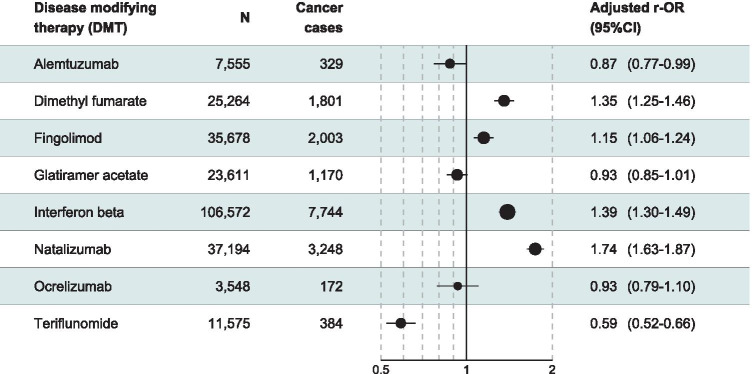
There were 15,966 cancer cases in the cohort of DMTs prescribed to pwMS. In the univariate analysis, dimethyl fumarate, interferon-β, and natalizumab were significantly associated with cancer reporting (Supplementary Table 2). After adjustment on age, sex and geographical region, natalizumab (r-OR 1.74, 95% CI 1.63–1.87; p < 0.0001), interferon-β (r-OR 1.39, 95% CI 1.30–1.49; p < 0.0001), dimethyl fumarate (r-OR 1.35, 95% CI 1.25–1.46; p < 0.0001), and fingolimod (r-OR 1.15, 95% CI 1.06–1.24; p < 0.0001) were significantly associated with cancer reporting, whereas glatiramer acetate, ocrelizumab, alemtuzumab, and teriflunomide were not associated (Fig. 2).
Fig. 2.
Disproportionality analysis for the association of disease-modifying therapies (DMTs) prescribed to persons with multiple sclerosis (pwMS) and cancer reporting (CI, confidence interval; r-OR, reporting odds ratio. Adjustment variables: age, sex, and geographical region)
Changes in the adjusted r-ORs over time showed a constantly increasing signal for natalizumab, a constant signal for interferon-β, and a varying pattern for fingolimod and dimethyl fumarate (Supplementary Fig. 1). Exploratory analyses according to cancer type for DMTs prescribed to pwMS significantly associated with cancer showed that upper aerodigestive tract, breast, urinary including the male genitourinary tract, and nervous system cancers were associated with natalizumab, interferon-β and dimethyl fumarate. Fingolimod was only associated with skin cancer types (Supplementary Table 3). Natalizumab was also associated with hematologic malignancies. Sensitivity analyses are shown in Supplementary Table 2. Inclusion of non-SAE in the analysis resulted in natalizumab associated with cancer, whereas other DMTs were not.
Description of Cancer Cases for DMTs Prescribed to pwMS Significantly Associated with Cancer
Characteristics of cancer cases for the four DMTs prescribed to pwMS associated with cancer are shown in Table 1. Natalizumab, interferon-β, dimethyl fumarate, and fingolimod accounted for 14,211 cancer cases, of whom 79% were female and 74% were aged 45 or older. Cancer cases associated with natalizumab, interferon-β, dimethyl fumarate, and fingolimod were significantly younger than all the other cancer cases in VigiBase® (p < 0.0001 for all four drugs). Time to cancer onset was significantly different among DMTs associated with cancer (p < 0.0001). Time to cancer onset was significantly shorter for natalizumab versus fingolimod and interferon-β cases (p < 0.0001 for both), and not different for dimethyl fumarate cases (p = 0.07). Time to cancer onset was significantly longer for interferon-β versus natalizumab, dimethyl fumarate and fingolimod (p < 0.0001 for all) (Table 1). The highest proportions of death in cancer cases were found for interferon-β and natalizumab (12.7% and 10.4%, respectively), whereas dimethyl fumarate and fingolimod had the lowest fatality reporting rates (5.9 and 3.7%, respectively) (Table 1). The proportion of deaths in cancer cases associated with natalizumab, interferon-β, dimethyl fumarate, and fingolimod was significantly lower than in all other cancer cases in the VigiBase® (25.5% of 350,347 cancer cases, p < 0.0001 for all).
Table 1.
Characteristics of cancer cases for persons with multiple sclerosis (pwMS) prescribed with disease-modifying therapies (DMTs) in the VigiBase® database among serious reports
| Dimethyl fumarate | Fingolimod | Interferon-β | Natalizumab | |
|---|---|---|---|---|
| Number of cancer cases | 1.801 | 2.003 | 7.744 | 3.248 |
| Report source | ||||
| Spontaneous | 813 (45.1%) [1.801] | 1.216 (61%) [1.993] | 4.962 (65.1%) [7.617] | 1.839 (57.5%) [3.201] |
| From study | 933 (51.8%) [1.801] | 754 (37.8%) [1.993] | 2.598 (34.1%) [7.617] | 1.330 (41.5%) [3.201] |
| Other | 55 (3.1%) [1.801] | 23 (1.2%) [1.993] | 57 (0.7%) [7.617] | 32 (1%) [3.201] |
| Age (%)1 | ||||
| < 45 years | 26.8% | 38.8% | 21.9% | 28.9% |
| 45–64 years | 58.4% | 56.2% | 63.7% | 59.0% |
| 65–74 years | 13.1% | 4.6% | 12.7% | 11.2% |
| > 75 years | 1.7% | 0.4% | 1.7% | 0.9% |
| Female (%)1 | 76.9% | 78.1% | 80.1% | 76.5% |
| Drug dosing (IQR) [availability] | 240 mg/day (240–240) [1.291] | 0.5 mg/day (0.5–0.5) [1.150] | 30 µg/week (30–30) [4.024] | 300 mg/months (300–300) [1.705] |
| Time to cancer onset in months, median (IQR) [availability] | 13 (5–22) [136] | 24 (12–45) [452] | 54 (22–100) [1.565] | 19 (8–34) [588] |
| Death (%) | 5.9 | 3.7 | 12.7 | 10.4 |
IQR interquartile range
1Data extracted after multiple imputation, hence it is not possible to provide exact numbers of cases for age and sex in addition to percentages
Discussion
Natalizumab, interferon-β, dimethyl fumarate, and fingolimod were significantly associated with cancer reporting for pwMS, with some cancer types prominently represented than others. In addition, cancer cases associated with patients prescribed with these DMTs were younger at diagnosis than other cancer cases in VigiBase®. We reported the most extensive cohort of cancer cases associated with DMTs prescribed to pwMS, in a worldwide database and evaluated the cancer safety signal for a large panel of such DMTs, including recently approved ones in the treatment for MS patients. The younger age in cancer cases associated with DMTs prescribed to pwMS as compared to other cancer cases in VigiBase® reinforces a potential role of DMTs. This younger age is unlikely to reflect an increased risk of MS itself and immune dysregulation, as studies showed an average or reduced incidence of cancer in pwMS, as compared to the general population [4–7]. Proportion of cancer cases resulting in death among those associated with DMTs was significantly lower compared to all other cancer cases in VigiBase®. This might translate a low aggressivity of these cancers and/or an early diagnosis from surveillance bias.
Cancer incidence in pwMS, irrespective of DMTs, was not different to that of the general population in retrospective populational studies [6, 7, 25], and sometime reduced [4–6]. Reported differences in the incidence of some cancers in pwMS might reflect ascertainment differences rather than true differences [25].
Previous studies which identified cancer cases following natalizumab exposure had a limited number (fewer than 20) of cancer cases. The AFFIRM phase III trial of natalizumab found five cancer cases in the natalizumab arm (627 patients) compared to one in the placebo arm (315 patients) over a 2-year follow-up [26]. A Swedish national population registry and a retrospective study of cancer cases of pwMS found no increased risk of cancer with natalizumab; however, only 17 cancer cases were detected in this group [11, 27]. Our study had more than 3000 cancer cases following natalizumab exposure. We found that the safety signal was constantly increasing over time, thus reducing the likeliness of being affected by the Weber effect (peak of notification early after drug authorization). Also, the time to cancer onset was among the shortest in our descriptive analysis.
The association between interferon-β and cancer is unclear. Our results suggest that interferon-β could be associated with cancer. A trend toward a higher overall risk of cancer was suspected in a longitudinal censored-data analysis, particularly showing a potential risk of breast cancer with interferon-β [14]. This significance was not replicated in retrospective studies [11, 12]. Nonetheless, we found that the time to cancer onset with interferon-β was the longest among DMTs. This may show that studies with mid-term follow-up or spontaneous non-systematic assessment of cancer incidence might be misleading in addressing this association, since it was found that a longer exposure duration to DMTs and use of multiple DMTs could increase the risk of cancer [28].
Our study is the first to assess the link between dimethyl fumarate and cancer to date. This association, although limited, constantly increased over time. Immunosuppressant DMTs are suspected to increase the risk of cancer by attenuating anti-tumour immune responses leading to tumour growth. This could explain an increased risk of cancer with azathioprine, cyclophosphamide, and mitoxantrone in pwMS [28]. Furthermore, this study showed that four DMTs prescribed to pwMS were associated with various cancer types, the most noticeable being the nervous system and gynecologic and genito-urinary tract cancers. Natalizumab may have also been associated with hematologic malignancies. The risk of nervous system cancers in pwMS may be attributable to a surveillance bias [4]. The surveillance bias could also affect several other cancer types because pwMS are more likely to pass medical examinations, for example when developing ADRs other than cancer, which could lead to an unexpected diagnosis of latent malignancies. The likeliness of developing gynecologic and hematologic malignancies as compared to other cancers may reflect the demographic characteristics of pwMS (age), since MS is more likely to occur in young women. Fingolimod was only associated with skin cancer types and is consistent with the low proportion of cases resulting in death in our findings and with the attention engendered in the pivotal trials for an increased risk of skin cancer and suggested dermatological examination. This is further consistent with fingolimod only being associated with an increased but limited risk of cancer in a recent propensity matched cohort [27].
In literature, glatiramer acetate, alemtuzumab, ocrelizumab, and teriflunomide were never associated with a risk of cancer and we did not find evidence to support an association either [11]. This absence of association supports our own setting for detection of association between DMTs and cancer. The relatively recent commercialization and marketing of alemtuzumab and ocrelizumab compared to non-pwMS prescribed DMTs may explain the absence of association since cancer development takes time and can be detected several years after treatment initiation. It might also explain that safety issues relying on case reports of thyroid cancer, melanoma, and lymphoproliferative disorders notified in the FDA label of alemtuzumab were not replicated in our study. Furthermore, additional properties of DMTs could negatively influence the development of cancer. For example, teriflunomide was suspected to downregulate anti-apoptotic proteins and growth factor receptors in cancer cells, interrupt cancer cell survival signalling, induce cancer cell death, abolish cancer stem cells, and disrupt cancer cell mitochondrial function [2]. Although the disproportionality analysis cannot support a protective role of teriflunomide on the development of cancer, it could be an interesting anticancer drug, especially since it shows efficacy at lower doses compared to other drugs that inhibit DNA synthesis (such as methotrexate) [2].
Study Limitations
Studies using pharmacovigilance databases are disadvantaged by underreporting bias, halo bias, lack of clinical information, and sales volumes of drugs. In this study, this was taken into consideration by using the disproportionality analysis which is unaffected by underreporting bias [19]. There were, however, other limitations to the study. We could not access cancer risk factors and could not ensure the exclusion of other non-drug aetiologies, or the prior use of a chemotherapy in some cases (incomplete data), or other treatment history. The likeliness of receiving a DMT reflects the disease activity which may itself interfere with the risk of cancer (indication bias). The case/non-case design of our analyses cannot provide evidence of a causal relationship between DMTs prescribed to pwMS identified and cancer development. In addition, since we chose to restrict the analysis to a DMTs prescribed to the pwMS cohort, we could not assess positive and negative controls. ADRs are more likely to be notified shortly after post marketing and several drugs with distant marketing dates may be difficult to compare on a determined window period [19]. To address this issue, we investigated the changes of the r-ORs over time (secondary outcome) and restricted the setting of our study to reports from January 2000.
The putative causal pathway of developing cancer for pwMS treated with DMTs is highly affected in the case of a prior history of cancer. Therefore, we chose to exclude reports of an anticancer drug as a proxy to a history of cancer. Non-SAEs and SAEs may not have had the same probability of being reported, and data completeness may be different, thus altering multivariate analyses and leading to apparent discordances. Since cancer is almost always reported as a serious condition according to the pharmacovigilance criteria (Supplementary Methods), we excluded non-serious reports to reduce the notification bias. The incidence of SAEs cannot be assessed in pharmacovigilance database, because the denominator (the total number of pwMS treated with DMTs) is not available. However, the incidence of several cancer types had already been assessed in pwMS registries [28].
In conclusion, natalizumab, interferon-β, dimethyl fumarate, and fingolimod were associated with a significantly greater reporting of cancer compared to other DMTs prescribed to pwMS. Cancer was found to occur in younger pwMS and several years after the treatment initiation. Prospective registries for pwMS with systematic assessment of cancer diagnosis are needed to deliver further evidence in the future. In addition to the recommendations already set for the general population, the increase of cancer development in younger pwMS may advocate for regular systematic cancer screening (especially for immunosuppressors) and the need for clinical recommendations based on frequency and monitoring for this screening type. Longitudinal populational studies focusing attention on DMTs, especially the newer, are needed to replicate these findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author Contributions
The results presented in this study do not represent the opinion of the UMC or the World Health Organization. We would like to thank the custom search team at the Uppsala Monitoring Centre (Uppsala, Sweden) research department for providing the VigiBase® extract case level data (VigiBase® and the WHO global database of individual case safety reports), as this study would not have been possible without them. The authors would also like to thank Sarina Yaghobian from AcaciaTools for editing the manuscript.
Funding
There was no funding source dedicated to this study. The study was supported by the Caen Normandy University Hospital (CHU Caen Normandie) and the Normandy University (Université de Caen Normandie) in France.
Data Availability
Data for this study is the property of the World Health Organization and is not available from the corresponding author.
Declarations
Conflict of Interest
Charles Dolladille declares no competing interests.
Basile Chrétien declares no competing interests.
Laure Peyro-Saint-Paul declares no competing interests.
Joachim Alexandre declares no competing interests.
Olivier Dejardin declares no competing interests.
Sophie Fedrizzi declares no competing interests.
Gilles Defer has received personal compensation for the scientific advisory board for Biogen, Novartis, Genzyme, Merck Serono, Roche, and Teva. He has received speaker honoraria and travel grants from Merck Serono, Biogen, Novartis, Roche, Genzyme, and Teva. His institution has received research support in his department from Merck Serono, Biogen, Novartis, and Genzyme.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler J. :25.
- 2.Melamed E, Lee MW. Multiple Sclerosis and Cancer: The Ying-Yang Effect of Disease Modifying Therapies. Front Immunol [Internet]. 2020 Jan 10 [cited 2020 Apr 17];10. Available from: https://www.frontiersin.org/article/10.3389/fimmu.2019.02954/full [DOI] [PMC free article] [PubMed]
- 3.Tan T-T, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19(2):209–216. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Bahmanyar S, Montgomery SM, Hillert J, Ekbom A, Olsson T. Cancer risk among patients with multiple sclerosis and their parents. Neurology. 2009;72(13):1170–1177. doi: 10.1212/01.wnl.0000345366.10455.62. [DOI] [PubMed] [Google Scholar]
- 5.Kingwell E, Bajdik C, Phillips N, Zhu F, Oger J, Hashimoto S, et al. Cancer risk in multiple sclerosis: findings from British Columbia. Canada. Brain J Neurol. 2012;135(Pt 10):2973–2979. doi: 10.1093/brain/aws148. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen NM, Rostgaard K, Rasmussen S, Koch-Henriksen N, Storm HH, Melbye M, et al. Cancer risk among patients with multiple sclerosis: a population-based register study. Int J Cancer. 2006;118(4):979–984. doi: 10.1002/ijc.21437. [DOI] [PubMed] [Google Scholar]
- 7.Midgard R, Glattre E, Grønning M, Riise T, Edland A, Nyland H. Multiple sclerosis and cancer in Norway. A retrospective cohort study. Acta Neurol Scand. 1996 Jun;93(6):411–5. [DOI] [PubMed]
- 8.Hajiebrahimi M, Montgomery S, Burkill S, Bahmanyar S. Risk of Premenopausal and Postmenopausal Breast Cancer among Multiple Sclerosis Patients. PLOS ONE. 2016 Oct 24;11(10):e0165027. [DOI] [PMC free article] [PubMed]
- 9.Sun LM, Lin CL, Chung CJ, Liang JA, Sung FC, Kao C-H. Increased breast cancer risk for patients with multiple sclerosis: a nationwide population-based cohort study. Eur J Neurol. 2014;21(2):238–244. doi: 10.1111/ene.12267. [DOI] [PubMed] [Google Scholar]
- 10.Grytten N, Myhr K-M, Celius EG, Benjaminsen E, Kampman M, Midgard R, et al. Risk of cancer among multiple sclerosis patients, siblings, and population controls: A prospective cohort study. Mult Scler J. 2019;1:135245851987724. doi: 10.1177/1352458519877244. [DOI] [PubMed] [Google Scholar]
- 11.Lebrun C, Vermersch P, Brassat D, Defer G, Rumbach L, Clavelou P, et al. Cancer and multiple sclerosis in the era of disease-modifying treatments. J Neurol. 2011;258(7):1304–1311. doi: 10.1007/s00415-011-5929-9. [DOI] [PubMed] [Google Scholar]
- 12.Kingwell E, Evans C, Zhu F, Oger J, Hashimoto S, Tremlett H. Assessment of cancer risk with β-interferon treatment for multiple sclerosis. J Neurol Neurosurg Psychiatry. 2014;85(10):1096–1102. doi: 10.1136/jnnp-2013-307238. [DOI] [PubMed] [Google Scholar]
- 13.Sandberg-Wollheim M, Kornmann G, Bischof D, Moraga MS, Hennessy B, Alteri E. The risk of malignancy is not increased in patients with multiple sclerosis treated with subcutaneous interferon beta-la: analysis of data from clinical trial and post-marketing surveillance settings. Mult Scler Houndmills Basingstoke Engl. 2011;17(4):431–440. doi: 10.1177/1352458511403642. [DOI] [PubMed] [Google Scholar]
- 14.Achiron A, Barak Y, Gail M, Mandel M, Pee D, Ayyagari R, et al. Cancer incidence in multiple sclerosis and effects of immunomodulatory treatments. Breast Cancer Res Treat. 2005;89(3):265–270. doi: 10.1007/s10549-004-2229-4. [DOI] [PubMed] [Google Scholar]
- 15.STROBE Statement: Home [Internet]. [cited 2018 Feb 4]. Available from: https://www.strobe-statement.org/index.php?id=strobe-home
- 16.Dolladille C, Ederhy S, Sassier M, Cautela J, Thuny F, Cohen AA, et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol [Internet]. 2020 Apr 16 [cited 2020 Apr 18]; Available from: https://jamanetwork.com/journals/jamaoncology/fullarticle/2764543 [DOI] [PMC free article] [PubMed]
- 17.Salem J-E, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chrétien B, Lelong-Boulouard V, Chantepie S, Sassier M, Bertho M, Brazo P, et al. Haematologic malignancies associated with clozapine v. all other antipsychotic agents: a pharmacovigilance study in VigiBase®. Psychol Med. 2020 Feb 10;1–8. [DOI] [PubMed]
- 19.Faillie J-L. Case-non case studies: Principles, methods, bias and interpretation. Therapie. 2018;73(3):247–255. doi: 10.1016/j.therap.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Chrétien B, Dolladille C, Hamel-Sénécal L, Sassier M, Faillie JL, Miremont-Salamé G, et al. Comparative study of hypoglycaemia induced by opioids. Is it a class effect? Expert Opin Drug Saf. 2019 Jul 24;1–6. [DOI] [PubMed]
- 21.Mislevy RJ. Review of Statistical Analysis with Missing Data. J Educ Stat. 1991;16(2):150–155. [Google Scholar]
- 22.Little RJA, Rubin DB. Statistical Analysis with Missing Data, 3rd Edition | Wiley [Internet]. Wiley. 2019 [cited 2020 Apr 18]. (Wiley Series in Probability and Statistics). Available from: https://www.wiley.com/en-us/Statistical+Analysis+with+Missing+Data%2C+3rd+Edition-p-9780470526798
- 23.Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. The BMJ [Internet]. 2009 Jun 29 [cited 2020 Apr 22];338. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2714692/ [DOI] [PMC free article] [PubMed]
- 24.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45(1):1–67. [Google Scholar]
- 25.Marrie RA, Maxwell C, Mahar A, Ekuma O, McClintock C, Seitz D, et al. Cancer Incidence and Mortality Rates in Multiple Sclerosis: A Matched Cohort Study. Neurology. 2021;96(4):e501–e512. doi: 10.1212/WNL.0000000000011219. [DOI] [PubMed] [Google Scholar]
- 26.Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 27.Alping P, Askling J, Burman J, Fink K, Fogdell-Hahn A, Gunnarsson M, et al. Cancer Risk for Fingolimod, Natalizumab, and Rituximab in Multiple Sclerosis Patients. Ann Neurol. 2020;87(5):688–699. doi: 10.1002/ana.25701. [DOI] [PubMed] [Google Scholar]
- 28.Lebrun C, Rocher F. Cancer Risk in Patients with Multiple Sclerosis: Potential Impact of Disease-Modifying Drugs. CNS Drugs. 2018;32(10):939–949. doi: 10.1007/s40263-018-0564-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for this study is the property of the World Health Organization and is not available from the corresponding author.