Abstract
Recent advances in molecular and cellular engineering, such as human cell reprogramming, genome editing, and patient-specific organoids, have provided unprecedented opportunities for investigating human disorders in both animals and human-based models at an improved pace and precision. This progress will inevitably lead to the development of innovative drug-screening platforms and new patient-specific therapeutics. In this review, we discuss recent advances that have been made using zebrafish and human-induced pluripotent stem cell (iPSC)–derived neurons and organoids for modeling genetic epilepsies. We also provide our prospective on how these models can potentially be combined to build new screening platforms for antiseizure and antiepileptogenic drug discovery that harness the robustness and tractability of zebrafish models as well as the patient-specific genetics and biology of iPSC-derived neurons and organoids.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-021-01115-5.
Keywords: Epilepsy, Developmental and epileptic encephalopathy (DEE), Drug discovery, Zebrafish, Stem cells, iPSC, Organoids
Introduction
Epilepsy, or ongoing seizures, is the second most common neurological condition, impacting up to 65 million people worldwide [1]. It presents as abnormal electrical activity in the brain detected using electroencephalography (EEG) and various behavioral manifestations [2]. The risk of developing epilepsy has been associated with genetic abnormalities, exposure to toxins, brain trauma, stroke, CNS infection, malformations of cortical development, neurodegenerative disease, and other less well-defined combinations of genetic and environmental factors [2]. Our understanding of pro-epileptic factors has improved in recent years due to better clinical diagnostics, advanced brain imaging techniques, and accumulated knowledge regarding the genetics and neurobiology of seizures [3]. Nevertheless, the cellular and molecular mechanisms disrupted in the brains of patients that cause the development of epilepsy remain poorly understood [4]. As a result, the progress with antiseizure drug discovery has been limited [5]. In fact, approximately 30% of patients with epilepsy suffer from intractable drug-resistant seizures [6, 7], and this number has remained unchanged in decades [8]. This number may need to be updated, as more recently, several new antiseizure medications for rare genetic epilepsies, including stiripentol, cannabadiol, and fenfluramine for Dravet syndrome (DS) [9] and rapamycin for some TSC patients [10], have been approved by FDA. However, there are still no therapies available to cure or prevent epileptogenesis in humans. Such disappointing results urge the identification of barriers that prevent us from developing novel and effective therapies for patients.
A major problem in drug discovery is the lack of preclinical models that recapitulate both patient-specific biology and epilepsy phenotypes and are amenable for drug screening [5, 11] and an exceedingly limited use of new models within the epilepsy community for drug discovery. Thus, there is a need to reinvigorate antiseizure and antiepileptogenic drug discovery by using innovative preclinical models.
One way forward is to focus the initial efforts on patients with refractory drug-resistant epilepsy caused by specific genetic abnormalities early in life—genetic infantile epileptic encephalopathies [12]—and on the discovery of patient-, target-, or variant-specific medication [13]. Interestingly, a recent whole-genome sequencing study of individuals with developmental and epileptic encephalopathy (DEE) identified pathogenic or likely pathogenic variants in all tested individuals [14]. In addition, recent large-scale sequencing efforts identified multiple “high-confidence” genes and genetic abnormalities that are strongly associated with early-onset epilepsy [15–18]. Predictably, a large fraction of the identified variants is in the genes that are well-known regulators of neuronal and network excitability, including voltage-gated ion channels, membrane transporters, and neurotransmitter receptors [19]. Among the most frequently identified mutations are those in the voltage-gated sodium, calcium, and potassium channels, including SCN1A, SCN2A, CACNA1A, CACNA1H, CACNA1E, KCNQ2, KCNQ3, and KCNT1, as well as in the AMPA, NMDA, and GABA receptors, including GRIA2, GRIN2A, GRIN2B, GRIN1, GABRA1, GABRA6, and GABRB1. Other genes identified in these studies are transcription factors, enzymes, and calcium sensors that have unknown or less understood functions in regulating brain development and function. Despite the progress, it remains largely unknown how the identified variants alter the excitability of human neurons and different neural networks during early developmental stages to cause epilepsy phenotypes.
Patient- and genetic variant–specific-induced pluripotent stem cell (iPSC)–derived neurons or organoids have been used successfully to study the effects of pathogenic variants on neuronal excitability [19–21]. On the other hand, zebrafish has emerged as a robust and high-throughput platform for studying epilepsy and drug screening [22]. Theoretically, combining these two models together to generate innovative drug-discovery pipelines may overcome the limitations of existing models and facilitate the development of new medications.
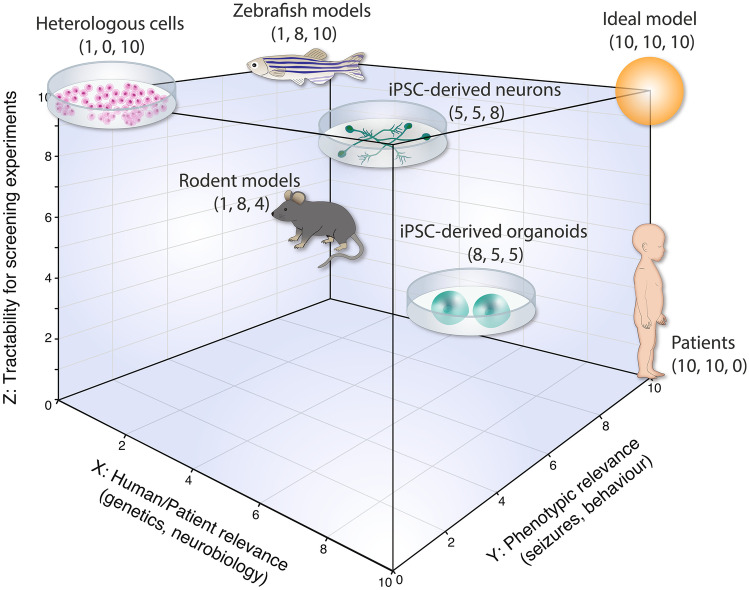
Indeed, the models used in biomedical research and especially in antiseizure drug discovery have limitations (Fig. 1). For example, heterologous cells, such as HEK293 cells or Xenopus oocytes that are frequently used to investigate the impacts of specific variants on protein functions, are amenable for drug-screening experiments [23, 24]. However, a caveat of these models is limited insights into disrupted neurobiology and epilepsy-related neuronal and network excitability deficits. Mouse models, on the other hand, have been incredibly useful for studying the effects of epilepsy genes and mutations on different neural cells, networks, and behaviors [25]. Mutant mice, most often carrying homozygous deletion or mutation, have been developed for many “high-confidence” epilepsy genes, and many invaluable insights into neuronal and network mechanisms disrupted in epilepsy have been obtained using this system [25]. In addition, rodent seizure/epilepsy models have led to the successful discovery and development of more than 30 different FDA-approved antiseizure medications. However, this system is not amenable for high-throughput screening experiments and there are several important differences between human and mouse brain development [26–28], which may need to be considered for studying human DEE. To overcome the limitations of mouse models, human stem cell–based models, such as iPSC-derived neurons and organoids, and organisms such as fruit fly (Drosophila melanogaster) and zebrafish (Danio rerio), have been introduced for studying epilepsy. iPSC-derived neurons and organoids can be generated from patients’ cells to recapitulate the patient-specific genetic background and produce human-specific cell types, such as outer radial glia and their progeny, to model human-specific neurodevelopmental aspects [29–31]. Mutant zebrafish lines can be efficiently generated using contemporary gene editing approaches [32] and used for high-throughput chemical or genetic phenotypic screening [33]. Both iPSC-based models and lower organism models have limitations, including the inability to recapitulate the complex brain environment and epileptic networks in iPSC-based models and the lack of some human disease–related genes, cells, and/or brain regions in zebrafish. Some of these limitations could be mitigated by combining these models together for drug discovery. In the following sections, we describe recent innovations and remaining challenges related to the use of zebrafish and iPSC-based approaches for modeling epilepsy. We also discuss phenotypes detected in different studies using zebrafish and iPSC-derived neurons or organoids in association with epilepsy. Finally, we conclude this review by providing our perspective on how zebrafish could be combined together with patient iPSC-derived neurons or organoids to create new pipelines for antiseizure and antiepileptogenic drug discovery in the future (Fig. 2).
Fig. 1.
Antiseizure drug discovery axis. Different preclinical models are compared to each other based on their relevance to humans (X axis), capability to recapitulate epilepsy phenotypes (Y axis), and tractability for screening (Z axis)
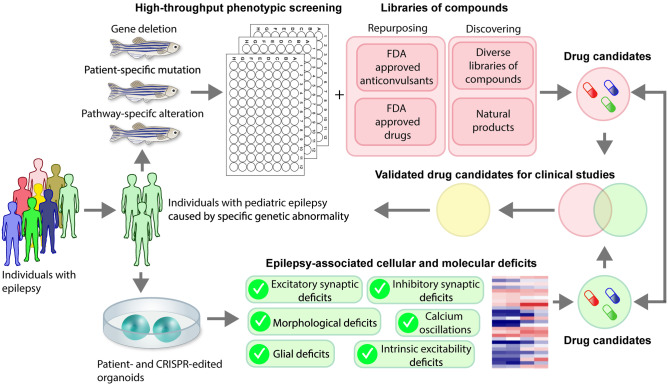
Fig. 2.
Building new drug discovery pipelines. We propose to combine zebrafish and iPSC-derived organoids for antiseizure and antiepileptogenic drug discovery
Zebrafish
With scores of genes and thousands of genetic variants having been associated with epilepsy in humans, there is a need for robust, scalable platforms for modeling and studying the various genetic causes of epilepsy. The zebrafish has become a go-to model for studies that require both an integrated, organismal system and the ability to scale experiments to thousands of conditions or more. As vertebrates, zebrafish share extensive anatomical and physiological conservation with humans, but their small size and fecundity enable large-scale experimentation, including genome-scale forward genetic screens and, importantly, large-scale phenotypic drug screens to identify small molecule modifiers of disease phenotypes. Such screens have already yielded several promising clinical candidates for treating various human diseases [33].
Numerous models of epilepsy in zebrafish have already been described. Among the best established is a model of DS generated by INDEL mutation of the zebrafish Nav1.1 channel (SCN1A) [34]. The scn1Lab allele, identified in a chemical mutagenesis screen, exhibits spontaneous seizure-like convulsions and electrographic traces reminiscent of epilepsy. Known epilepsy treatments including ketogenic diet, valproic acid, and benzodiazepams reduce these phenotypes [34]. This and related DS models have been used extensively and have proven useful for screening chemical libraries for therapeutic candidates [35], assessing metabolic perturbations in DS [36], testing cannabinoids for efficacy in epilepsy [37], elucidating early mechanisms of epileptogenesis [38], and designing optimized drug combinations [39], among others.
In recent years, it has become rather straight-forward to generate INDEL mutations in zebrafish using CRISPR-Cas9 [40]. Generating specific point mutations in zebrafish is significantly less efficient, but several methods have been developed that allow such point mutations to be made [41–44]. More than 80% of human disease–associated genes have a zebrafish ortholog, meaning that the majority of human genetic epilepsies can in theory be modeled in zebrafish [45]. It is important to recognize, however, that a significant minority of zebrafish genes have undergone duplication, so it may be necessary to target two ohnologs of the same gene to achieve complete loss of function. In the case of the scn1Lab allele referenced above, the existence of another SCN1A ohnolog may have proven beneficial by allowing some residual Nav1.1 activity in the presence of the scn1Lab mutation [46].
Another well-established model of seizures in zebrafish is the pentylenetetrazole (PTZ) model in which the convulsant compound PTZ is added to the water surrounding zebrafish larvae, which subsequently exhibit seizure-like convulsions and electrographic discharges that can be reversed by several classes of antiseizure medications [47]. This model has found widespread usage in epilepsy research [48].
Beyond the Dravet and PTZ models, there are numerous other genetic models that have been developed, ranging from potassium channels to synaptic proteins and chromatin remodelers (see ref [48] for an excellent review). Non-genetic models including those based on injury, stress, and environmental factors have also been described, raising the possibility of systematic exploration of gene-environment interactions in epilepsy [49, 50].
One of the great advantages of zebrafish models is that many models can be generated and characterized in parallel. A recent example includes the parallel generation and characterization of 40 genetic epilepsy models [22]. Taking advantage of the scalability of zebrafish experimentation, the models were subjected to systematic studies of their spontaneous seizures, electrophysiology, neuroanatomy, and pharmacological responses. Given that all models were characterized in the same way by the same investigators, powerful comparisons can be made that promise to reveal important distinctions between the models and provide a foundation for future discovery and testing of therapeutic interventions.
While the ability to generate and characterize zebrafish models of epilepsy is impressive, questions remain about the validity of these models for recapitulating human disease. Most published models point to evidence of construct validity (the fact they were generated by targeting a gene or pathway known to cause epilepsy in humans) or face validity (the fact that the zebrafish phenotype resembles human epilepsy in its outward appearance). The level of sophistication surrounding the validation of zebrafish epilepsy models is improving rapidly. With regard to construct validity, it remains much easier to generate gene knockouts than precise models of point mutations or other gene variants, but tools for making precise genome edits have been developed and are increasingly utilized [51]. With regard to face validity, our ability to assess the relevance of epilepsy-related phenotypes has improved dramatically. Whereas early models were determined to be “epilepsy-like” based on rapid seizure-like movements as measured by locomotion in a dish, electrical recordings and functional imaging of zebrafish brains have enabled much more precise determination of the relevance of zebrafish models for human epilepsy [22, 52–54]. Additionally, the conservation of drug responses and drug resistance between zebrafish models and humans has proven a useful approach for validating zebrafish models.
Beyond construct and face validity, predictive validity, or the ability to use a model to predict activity in humans, remains the ultimate test of disease model relevance. Here again, zebrafish models have performed well. A high-profile example involves the scn1Lab model of DS, which was used to screen existing, approved drugs for their ability to suppress hyperlocomotion [55]. In a repurposing screen of approved drugs, clemizole was identified as highly effective in reducing the seizure-like phenotype in scn1Lab zebrafish. Biochemical studies demonstrated that 5-HT receptors were the likely target of clemizole. As clinical-grade clemizole was not available, the investigators turned to another 5-HT receptor agonist lorcaserin and performed a clinical trial in five treatment-resistant children with DS. All five patients treated with lorcaserin exhibited a reduction in total number of seizures [55]. This compelling example demonstrates that carefully constructed and validated zebrafish models can be powerful tools for teasing apart the biology of epilepsy and identifying drug candidates with therapeutic potential.
Although zebrafish models of epilepsy offer clear advantages, they also have some significant limitations. Approximately 82% of human disease-causing mutations have an ortholog in zebrafish [45], meaning that almost a fifth of human disease genes cannot be modeled by simply mutating the zebrafish ortholog. Additionally, there are anatomical, pharmacological, and physiological differences between zebrafish and humans that could theoretically limit the applicability of some zebrafish models and the findings that emerge from their use. These limitations highlight the potential value of pairing zebrafish models with human-derived models such as organoids, and discussed below.
iPSC-Derived Neurons and Organoids
The invention of the cell reprograming technique [56] has allowed researchers to use any accessible cells, including skin fibroblasts or peripheral blood monocytes, for the derivation of cells that are not readily accessible for research, such as brain cells. Using this approach, the accessible cells are first reprogrammed into iPSCs, and then iPSCs are differentiated into the cells or tissue of interest [57]. There have been many protocols developed to generate different types of human brain cells and organized brain tissue, referred to as brain organoids [58]. The access to live patient and control iPSC-derived neurons or organoids permits the performance of research experiments for identifying disease-associated cellular and molecular deficits as well as gain- and loss-of-function experiments for understanding the disrupted mechanisms. The key advantage of this approach to disease modeling is that differentiated cells carry the same mutations and genetic background as the affected cells in the brain. As most epilepsy-associated disorders are polygenic, iPSC-based models provide unique opportunities to investigate the contributions of a potentially pathogenic mutation and genetic background to a specific phenotype.
At present, the majority of epilepsy studies have been performed on 2D cultures of differentiated iPSC-derived neurons generated using neurospheres or the dual-SMAD inhibition protocols for neural induction [58]. Typically, such cultures consist of heterogeneous populations of neural progenitors and immature predominantly deep layer cortical excitatory neurons and inhibitory neurons with forebrain identities. The main advantage of this model is easy access to differentiated cells for mechanistic experiments and drug treatment. Importantly, “virtual patient” iPSC lines can be generated by mutating control lines using CRISPR/Cas9-system and, conversely, the potentially pathogenic variants in patient lines can be corrected to understand their pathogenicity and impacts on development and excitability [20]. This system is also scalable and amenable for screening [59]. The disadvantages of this system are the heterogeneity of differentiated cells and impaired functional and structural maturation of neurons. For example, it remains a major challenge to generate iPSC-derived parvalbumin (PV)–positive fast-spiking inhibitory interneurons that are known to be important for normal network development and disrupted in epilepsy [60]. Some of these disadvantages can be mitigated by using fluorescent reporters to label cells of specific identities and by co-culturing patient and control iPSC-derived cells in the same dish under the same experimental conditions [61]. The co-culture system with fluorescently labeled cells is well suited for studying cell-autonomous deficits and mechanisms; however, it is unsuitable for studying non-cell-autonomous deficits.
2D cultures of differentiated iPSC-derived neurons have been used to investigate functional deficits in Dravet syndrome (DS) [62–68], KCNT1-related DEE [69], DEE type 13 caused by SCN8A mutations [70], and tuberous sclerosis (TSC) [71–73].
DS is a type of catastrophic pediatric epilepsy caused by the loss-of-function mutations in SCN1A [74]. It has already been extensively investigated in various animal models and human iPSC-derived neurons. Collectively, the results of these studies suggest that dysfunctional inhibitory neurons and overexcitable excitatory neurons could be responsible for epilepsy in DS [75]. In addition, it has also been demonstrated that genetic background plays an important role in the development of severe spontaneous seizures in mice [76, 77]. In the first study of iPSC-derived forebrain neurons generated from two DS patient and three unrelated controls, it was demonstrated that the sodium current was significantly increased in patient neurons as compared to control neurons [62]. DS neurons were also hyperexcitable and fired more action potentials in response to somatic depolarizations. These results indicate that the epilepsy phenotype in DS may arise as a result of compensatory overexpression of functional sodium channels and increased neuronal excitability. Similar hyperexcitability phenotypes were detected in another study on iPSC-derived glutamatergic neurons produced from two DS patients [63]. Together, these studies supported the idea of hyperexcitability of excitatory neurons as a potential mechanism for epilepsy in DS. The hyperexcitability phenotypes, however, were not observed in other studies on iPSC-derived neurons from DS patients and genetically engineered lines [64–67, 78]. These studies demonstrated that iPSC-derived inhibitory neurons from DS patients or SCN1A-deficient lines are less excitable as compared to control neurons; while no deficits were detected in excitatory neurons [67]. iPSC-derived inhibitory neurons failed to generate action potentials in response to sustained somatic depolarizations and showed significantly reduced voltage-gated sodium current. It is conceivable that both excitatory and inhibitory neurons are affected in DS, as cannabidiol (CBD), a promising new treatment for DS patients [79], acts by increasing inhibitory and decreasing excitatory neuron excitability [68, 80]. However, a limitation of the studies on iPSC-derived inhibitory neurons is the lack of PV-expressing fast-spiking interneurons that are challenging to produce in vitro [81] and that have been implicated in DS in the mouse studies [82–84].
iPSC-derived forebrain neurons have also been used to investigate excitability deficits in malignant migrating partial seizures of infancy (MMPSI) syndrome caused by heterozygous mutations in the sodium-activated potassium channel KNa1.1 [69]. MMPSI is associated with intractable childhood epilepsy [85]. It was demonstrated that iPSC-derived neurons with homozygous gain-of-function mutation in KCNT1 exhibit increased sodium-activated potassium currents and increased propensity to fire action potentials. This was surprising considering the roles of potassium channels in dampening neuronal excitability. The authors demonstrated that overexcitability deficits likely result from an increased fast afterhyperpolarization that predisposed mutant neurons to fire more action potentials.
Recently, excitability deficits were also investigated in DEE13 using iPSC-derived neurons obtained from three patients with SCN8A mutations and four controls [70]. The study found no significant differences in the amplitude of transient sodium currents, but increased amplitudes of persistent sodium currents in two patients and an increased resurgent sodium current in one patient as compared to the currents in control neurons. The authors noted that the levels of network electrical activity in the cultures of differentiated iPSC-derived neurons were highly variable. Therefore, network epilepsy deficits were investigated in cultures of induced excitatory neurons (discussed below). Although no characterization of persistent or resurgent sodium currents was performed in induced neurons, patient-induced neurons were characterized by increased frequency of spikes in network bursts detected using multielectrode arrays. These deficits were compensated by treatment with phenytoin, a drug often prescribed to patients with SCN8A-related epilepsy, or riluzole, an FDA-approved drug for amyotrophic lateral sclerosis [86, 87]. Remarkably, as a result of this study, riluzole was prescribed off-label to the patients who participated in the study. All treated individuals exhibited a transient reduction in seizure frequency, with only one patient remaining seizure-free during 3 months on riluzole and 11 months after riluzole removal.
Epilepsy-associated deficits were also investigated in several studies using iPSC-derived neurons from patients with TSC or genetically engineered lines with hetero- or homozygous mutations of tuberous sclerosis complex 1 or 2 (TSC1 or TSC2, respectively) [71–73]. In one study, engineered TSC2-/- neurons were characterized by decreased intrinsic excitability due to reduced input resistance and impaired excitatory postsynaptic current [71], which are cellular phenotypes that are inconsistent with epilepsy-related alterations. The network hyperexcitability deficits, however, were observed in two other studies on differentiated iPSC-derived forebrain neurons with genetic abnormalities in TSC1 or TSC2 and induced excitatory neurons with hetero- or homozygous mutations in TSC2 [72, 73]. Interestingly, both hypo- and hyperexcitability deficits reported in these studies were rescued by treatment with a mammalian target of rapamycin (mTOR) inhibitor, rapamycin. This suggests that the types of investigated cells, genetic abnormalities, and/or developmental stages may influence the manifestation of excitability deficits in TSC.
Another approach commonly used for modeling epilepsy in 2D culture is the generation of induced neurons (iNs) [88]. This approach is based on the transient expression of exogenous neurogenic transcription factors without or with microRNAs in human iPSCs or fibroblasts [88–91]. Fibroblast-derived neurons maintain the age-related epigenetic marks and have been used for modeling late-onset neurological and neurodegenerative disorders [92, 93], whereas induced iPSC-derived neurons allow for modeling of intrinsic, synaptic, network deficits associated with neurodevelopmental disorders. Induced excitatory neurons with cortical superficial-like identity can be efficiently produced using temporarily restricted overexpression of Neurog2 or NeuroD2 [94, 95], while inhibitory interneurons with cortical-like identifies can be generated using a combination of two or more transcription factors, such as Ascl1 and Dlx2 [96] or ASCL1, LHX6, DLX2, and miR-9/9*-124 [94]. The biggest advantage of this approach is that iNs demonstrate robust spontaneous electrical activity already after 4–5 weeks post-transduction, which allows for modeling of functional deficits and the performance of drug or genetic screening [97–99]. The disadvantages of this system are predominantly related to the ectopic expression of non-neuronal genes, such as NONOG, NESTIN, and OCT4 in iNs [95, 100], which may influence the properties of neurons; the absent or significantly reduced expression of functional NMDA receptors [100], which are essential for not normal functional maturation of neurons and neural circuits [101]; and the omission of early neurodevelopmental stages that might be essential for the development of disease-related phenotypes [102]. To overcome these limitations, iNs have been used together with directly differentiated iPSC-derived neurons or organoids for disease modeling [103].
The most recent innovation with using iNs for disease modeling is the generation of mixed 2D or 3D cultures that contain defined proportions of induced or differentiated excitatory and inhibitory neurons with primary or induced human astrocytes [104, 105]. This approach could be useful for detecting epilepsy-related excitability deficits and drug discovery.
Excitatory iNs have been used to study epilepsy-related activity deficits in association with SCN8A gain-of-function variants [70], TSC [72], Angelman syndrome (AS) [103], and KCNQ2 encephalopathy [106]. In these studies, iNs were derived using transduction with Neurog2 and subsequent replating on rodent astrocytes for functional assays. Functional deficits were detected using MEA recordings and/or patch-clamp electrophysiology. All these studies have also identified chemicals that rescued functional abnormalities, including riluzole for SCN8A [70], rapamycin for TSC [72], paxilline for AS [103], and apamin and paxilline for KCNQ2 encephalopathy [106]. Interestingly, the studies of AS and KCNQ2 encephalopathy revealed elevated afterhyperpolarization and ectopic upregulation of calcium-activated potassium channels as a potential mechanism for elevated excitability.
To overcome the limitations of 2D cultures for modeling human diseases, particularly those associated with dysfunctional neural networks, 3D cultures of human stem cell-derived self-organized neural tissue (organoids) have been developed [107, 108]. Region-specific organoids have been generated from both iPSCs and iPSC-derived neural progenitors with varying degrees of heterogeneity using intrinsic self-organizing processes and temporally controlled exposure to neuralizing factors and patterning morphogens [109–113].
Thus, in one of the earliest studies, cerebral organoids containing neural cells of forebrain, midbrain, and hindbrain identities were generated from human iPSCs through the generation of embryonic bodies (EBs) and the use of neuronal culture medium with a low concentration of FGF and Matrigel for embedding [110]. Cerebral organoids produced using such “undirected” differentiation protocols are highly heterogeneous [114]. Thus, these organoids may not be particularly suited for disease modeling. However, these organoids can be useful for generating novel cell types using intrinsic differentiation cues, for which there are no differentiation protocols.
Less heterogenous region-specific brain organoids can be generated from stem cell-derived neural progenitors via sequential exposure to specific morphogens, such as the WNT, fibroblast growth factor (FGF), and/or sonic hedgehog (SHH) signaling pathway agonists or antagonists [111, 115]. The organoids produced using defined conditions exhibit a more consistent cellular composition and improved functional maturation [111, 115, 116]. They have also been used to produce later-differentiated glial cell types and for disease modeling [117–120]. In one of the earliest studies, both dorsal and ventral telencephalic organoids were generated from stem cell-derived neural progenitors using different, specifically defined protocols and then fused together into cortical-subpullial assembloids for modeling inhibitory neuron migration deficits associated with Timothy syndrome, a developmental disorder caused by gain-of-function mutations in CACNA1C and associated with cardiac arrhythmia, intellectual disability, and autism [119]. This research demonstrated that inhibitory and excitatory neurons in assembloids become functionally interconnected and that pharmacological treatments that modulate the activity of voltage-gated L-type calcium channels can rescue the migration deficits in inhibitory neurons from patients.
As a result of many recent studies performed on different types of brain organoids, it is becoming increasingly clear that this system provides unique opportunities for generating organized human brain tissue consisting of different cell types for modeling human neurodevelopment and disorders [121]. An important advantage of organoids for studying epilepsy is the ability to produce functional neural networks with physiologically relevant patterns of electrical activity [122]. The disadvantages include the organoid-to-organoid variability due to inconsistent cellular composition and unpredictable number and organization of germinal zones per organoid, the slow functional maturation of neurons and neural networks, and the lack of non-neuronal cells generated outside the brain as well as sensory inputs that are essential to network refinement and maturation. To overcome some of these limitations, organoids with improved organization have recently been generated from isolated single neural rosettes [123], implanted in the mouse brain for vascularization in the brain environment [124], and stimulated with light pulses to modulate visual sensory inputs [114]. Organoids also have been sliced and cultured on porous membranes to improve layering and wiring [125, 126]. Moreover, in the most recent studies, region-specific organoids, such as cortical, thalamic, striatal, and spinal organoids, were generated and fused together for studying interregional anatomical and functional connectivity [127–129].
Although organoids provide several important advantages for studying the development and function of neural circuits, only a few studies so far have organoids for studying epilepsy. In a study of TSC, cortical organoids were generated from genetically engineered stem cell lines with hetero- and homozygous mutations of TSC1 or TSC2 [130]. It was shown that only homozygous loss of TSC1 or TSC2 affected cortical development and led to the generation of dysplastic cells that have been found in TSC patients but not in animal models. It was also demonstrated that biallelic inactivation of TSC2 in neural progenitors is necessary and sufficient to cause the formation of dysplastic cells, providing evidence in support of the second-hit hypothesis of cortical tuber formation in TSC [131]. This study did not investigate epilepsy-associated functional deficits in TSC-deficient organoids.
In another study, cortical organoids were generated from control and isogenic engineered stem cell with homozygous knockout of UBE3A to model epilepsy susceptibility in Angelman syndrome [103]. The results showed that neurons in UBE3A-deficient cortical organoids were more excitable than neurons in isogenic control organoids. Specifically, UBE3A-deficient neurons fired action potentials with a significantly higher frequency in response to increasing somatic depolarizations. In this study, it was also shown that UBE3A-deficient organoids exhibit more synchronized network activity as compared to control organoids and that an inhibitor of BK calcium-activated potassium channels, paxilline, compensates excitability deficits.
Excitability phenotypes were also investigated in association with an early-onset intractable epilepsy caused by mutations in cyclin-dependent kinase-like 5 (CDKL5) [132]. In this study, spontaneous electrical activity in 2–6-month-old control and CDKL5-deficient organoids was measured using surface multielectrode arrays (MEA). CDKL5-deficient organoids showed significantly increased frequency and synchrony of spikes as compared to isogenic control organoids at 3–4 months post-induction (1.5–2.5 months post-plating) but not at later time points (5–6 months post-induction). The early overexcitability deficits in self-organized organoids were also recapitulated in 3D neural spheres composed of defined proportions of human iPSC-derived cortical neurons and astrocytes using calcium imaging. 3D neural spheres were used to reduce variability in high-throughput drug screening. The calcium-imaging screening identified four promising compounds (out of 1112 compounds tested) of different classes, including Ivabradine (hyperpolarization-activated cyclic nucleotide-gated channel blocker), Solifenacin (inhibitor of muscarinic receptors), AZD1080 (GSK3 inhibitor), and Crenigacestat (Notch inhibitor), that could guide the development of new therapeutics for patients with CDKL5 syndrome.
Excitability deficits were also investigated in a recent study on pallial–subpallial assembloids produced from Rett syndrome patients with electroencephalographic abnormalities and seizures [133]. The patient-derived pallial-subpallial assembloids exhibited increased levels of synchronized activity and impaired low frequency and gamma oscillations. Interestingly, by fusing together patient and control pallial and subpallial organoids, the authors demonstrated that the deficits of inhibitory neurons in patient subpallial organoids are likely responsible for the detected functional abnormalities. The study also showed that treatment with an unconventional neuromodulator and p53 inhibitor, Pifithrin-a, compensated network-level abnormalities in patient-derived pallial–subpallial assembloids. Of note, the population of inhibitory neurons in fused organoids was composed of different subtypes, including about 10% of PVALB/SST-expressing interneurons. This suggests that fusing together the pallial and subpallial organoids may promote the specification of PV-expressing interneurons. However, it remains to be determined whether PV-expressing neurons in organoids have functional and morphological characteristics that are similar to native fast-spiking PV-expressing interneurons in the brain.
In summary, tremendous progress has been made with using patient and genetically engineered iPSC-derived neurons and organoids to model the cellular, molecular, and functional deficits associated with pediatric epilepsy. These studies provide insights into the mechanisms and identified novel targets and treatments to compensate overexcitability deficits. It remains unknown whether and how the excitability deficits detected in iPSC-derived neurons and organoids translate into seizures and epilepsy at the organism level and whether the chemicals identified in these studies can be useful for patients. For drug discovery, patient iPSC-based models have unprecedently strong construct validity (patient-specific pathogenic variants and genetic background), face validity defined by the ability to recapitulate the cellular and network excitability deficits, and unknown predictive validity (new drugs identified using iPSC-based models remain to be tested in clinical trials).
New Preclinical Platforms for Antiseizure and Antiepileptogenic Drug Discovery
Despite the availability of many anticonvulsants for epilepsy treatment (~ 86 different drugs), approximately 30% of individuals with epilepsy have drug-resistant seizures [5]. The mechanisms underlying drug resistance remain poorly understood. This is likely attributable to multiple factors, including the use of non-specific animal models with chemically or electrically induced seizures in the preclinical studies and limited information about the targets as well as underlying cellular and molecular mechanisms [5]. We propose that combing together two models—zebrafish for phenotypic screening and iPSC-derived organoids for target-based experiments—into a drug-discovery pipeline may allow the identification of novel therapies for patients with pediatric epilepsy (Fig. 2).
First, new zebrafish lines can be generated by introducing gene deletion or patient-specific mutation using the CRISPR/Cas9 approach, and human telencephalic organoids can be generated from patient and engineered iPSC lines. Next, both zebrafish lines and organoids can be screened for the presence of epilepsy-related phenotypes. For zebrafish, it could be spontaneous electrographic seizures and/or accelerated convulsive-like swimming. For iPSC-derived organoids, the phenotypes could be epileptiform-like activity and/or abnormal network synchrony, elevated intrinsic excitability and/or excitatory synaptic transmission, increased numbers of excitatory synapses and/or spines, reduced inhibitory synaptic transmission and/or number of inhibitory synapses, increased neuronal or glial activity, and/or abnormal ratio of excitatory and inhibitory neurons. If epilepsy-related phenotypes are observed in a zebrafish line, the line can be used for high-throughput phenotypic screening of diverse libraries of compounds, including those that are already FDA-approved (repurposing) and those that are consist of structurally diverse chemicals and/or natural products. The promising compounds that suppress epilepsy-related deficits can be tested next with organoids for their ability to compensate the epilepsy-related cellular and molecular deficits and toxicity. Additional preclinical studies in other animal models will likely be required to validate compounds that aren’t already approved for human use.
If no epilepsy-related phenotypes are detected in mutant zebrafish, alterations can be introduced into a signaling pathway that has been found to be dysregulated in patient or engineered iPSC-derived organoids. For example, the study on CDKL5-deficient organoids found increased mTOR activity as a potential molecular pathway responsible for seizures [132]. Based on this, zebrafish lines with elevated mTOR activity could be tested as a model for epilepsy. Interestingly, zebrafish with knockdown of Depdc5, a negative regulator of mTOR, exhibit epilepsy-related behavioral and electrophysiological phenotypes [134], but no robust epilepsy-related phenotypes were detected in CDKL5-deficient zebrafish [22].
An alternative or parallel approach could be to perform drug screening in organoids. Such effort, however, may be time- and budget-prohibitive as iPSC-derived organoids have been characterized with an increased variability in cellular composition and organization [114, 135], and it requires at least 3–4 months to produce functionally mature organoids [122]. Drug candidates identified in organoids could be tested in zebrafish lines to validate their effects on epilepsy-related phenotypes in vivo.
In summary, we propose that innovative uses of currently available epilepsy models for drug screening may provide new insights into disease and new drug candidates for clinical studies of epilepsy treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aleksandr Shcheglovitov, Email: alexsh@neuro.utah.edu.
Randall T. Peterson, Email: randall.peterson@pharm.utah.edu
References
- 1.Thurman DJ, Beghi E, Begley CE, Berg AT, Buchhalter JR, Ding D, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52:2–26. doi: 10.1111/j.1528-1167.2011.03121.x. [DOI] [PubMed] [Google Scholar]
- 2.Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moshé SL, Perucca E, Ryvlin P. Tomson T. Epilepsy: New advances. Lancet. 2015;385:884–898. doi: 10.1016/S0140-6736(14)60456-6. [DOI] [PubMed] [Google Scholar]
- 4.Scharfman HE. The neurobiology of epilepsy. Curr Neurol Neurosci Rep. 2007;7:348–354. doi: 10.1007/s11910-007-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Löscher W, Klitgaard H, Twyman RE, Schmidt D. New avenues for anti-epileptic drug discovery and development. Nat Rev Drug Discov. 2013;12:757–776. doi: 10.1038/nrd4126. [DOI] [PubMed] [Google Scholar]
- 6.Sillanpää M, Schmidt D. Natural history of treated childhood-onset epilepsy: Prospective, long-term population-based study. Brain. 2006;129:617–624. doi: 10.1093/brain/awh726. [DOI] [PubMed] [Google Scholar]
- 7.Brodie MJ, Barry SJE, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78:1548–1554. doi: 10.1212/WNL.0b013e3182563b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rho JM, White HS. Brief history of anti-seizure drug development. Epilepsia Open. 2018;3:114–119. doi: 10.1002/epi4.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strzelczyk A, Schubert-Bast S. Therapeutic advances in Dravet syndrome: a targeted literature review. Expert Rev Neurother [Internet]. Taylor & Francis; 2020;20:1065–79. [DOI] [PubMed]
- 10.Li M, Zhou Y, Chen C, Yang T, Zhou S, Chen S, et al. Efficacy and safety of mTOR inhibitors (rapamycin and its analogues) for tuberous sclerosis complex: A meta-analysis. Orphanet J Rare Dis. 2019;14:1–9. doi: 10.1186/s13023-019-1012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galanopoulou AS, Buckmaster PS, Staley KJ, Moshé SL, Perucca E, Engel J, et al. Identification of new epilepsy treatments: Issues in preclinical methodology. Epilepsia. 2012;53:571–582. doi: 10.1111/j.1528-1167.2011.03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Deimling M, Helbig I, Marsh ED. Epileptic Encephalopathies—Clinical Syndromes and Pathophysiological Concepts [Internet]. Curr. Neurol. Neurosci. Rep.; 2017. [DOI] [PubMed]
- 13.Dugger SA, Platt A, Goldstein DB. Drug development in the era of precision medicine. Nat Rev Drug Discov. 2018;17:183–196. doi: 10.1038/nrd.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostrander BEP, Butterfield RJ, Pedersen BS, Farrell AJ, Layer RM, Ward A, et al. Whole-genome analysis for effective clinical diagnosis and gene discovery in early infantile epileptic encephalopathy. npj Genomic Med. 2018;3. [DOI] [PMC free article] [PubMed]
- 15.Wang J, Lin ZJ, Liu L, Xu HQ, Shi YW, Yi YH, et al. Epilepsy-associated genes. Seizure. 2017;44:11–20. doi: 10.1016/j.seizure.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 16.EpiPM Consortium A Road Map for Precision Medicine in the Epilepsies EpiPM. Lancet Neurol. 2015;14:1219–1228. doi: 10.1016/S1474-4422(15)00199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng YCA, Howrigan DP, Abbott LE, Tashman K, Cerrato F, Singh T, et al. Ultra-Rare Genetic Variation in the Epilepsies: A Whole-Exome Sequencing Study of 17,606 Individuals. Am J Hum Genet. 2019;105:267–282. doi: 10.1016/j.ajhg.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perucca P, Bahlo M, Berkovic SF. The Genetics of Epilepsy [Internet]. Annu. Rev. Genomics Hum. Genet. Annual Reviews; 2020. p.205–30. [DOI] [PubMed]
- 19.Simkin D, Kiskinis E. Modeling pediatric epilepsy through iPSC-Based technologies. Epilepsy Curr. 2018;18:240–245. doi: 10.5698/1535-7597.18.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niu W, Parent JM. Modeling genetic epilepsies in a dish. Dev Dyn. 2020;249:56–75. doi: 10.1002/dvdy.79. [DOI] [PubMed] [Google Scholar]
- 21.Parent JM, Anderson SA. Reprogramming patient-derived cells to study the epilepsies. Nat Neurosci. 2015;18:360–366. doi: 10.1038/nn.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffin A, Carpenter C, Liu J, Paterno R, Grone B, Hamling K, et al. Phenotypic analysis of catastrophic childhood epilepsy genes. Commun Biol. 2021;4. [DOI] [PMC free article] [PubMed]
- 23.Vanoye CG, Thompson CH, Desai RR, DeKeyser JM, Chen L, Rasmussen-Torvik LJ, et al. Functional evaluation of human ion channel variants using automated electrophysiology. Methods Enzymol; 2021 [DOI] [PubMed]
- 24.Vitko I, Chen Y, Arias JM, Shen Y, Wu XR, Perez-Reyes E. Functional characterization and neuronal modeling of the effects of childhood absence epilepsy variants of CACNA1H, a T-type calcium channel. J Neurosci. 2005;25:4844–4855. doi: 10.1523/JNEUROSCI.0847-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall GF, Gonzalez-sulser A, Abbott CM. Modelling epilepsy in the mouse : challenges and solutions. Dis Model Mech. 2021; [DOI] [PMC free article] [PubMed]
- 26.Silbereis JC, Pochareddy S, Zhu Y, Li M, Sestan N. The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron; 2016. p. 248–68. [DOI] [PMC free article] [PubMed]
- 27.Krienen F, Goldman M, Zhang Q, del Rosario R, Florio M, Machold R, et al. Innovations in Primate Interneuron Repertoire. Nature. 2019;586:262–269. doi: 10.1038/s41586-020-2781-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, Szafer A, et al. Transcriptional landscape of the prenatal human brain. Nature. 2014;508:199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quadrato G, Brown J, Arlotta P. The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nat Med. 2016;22:1220–1228. doi: 10.1038/nm.4214. [DOI] [PubMed] [Google Scholar]
- 30.Hansen D V, Rubenstein JLR, Kriegstein AR. Deriving Excitatory Neurons of the Neocortex from Pluripotent Stem Cells. Neuron; 2011. p. 645–60. [DOI] [PMC free article] [PubMed]
- 31.Di Lullo E, Kriegstein AR. The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci. 2017;18:573–584. doi: 10.1038/nrn.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng Y, Clark KJ, Campbell JM, Panetta MR, Guo Y, Ekker SC. Making designer mutants in model organisms. Dev. 2014;141:4042–4054. doi: 10.1242/dev.102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patton EE, Zon LI, Langenau DM. Zebrafish disease models in drug discovery: from preclinical modelling to clinical trials. Nat Rev Drug Discov; 2021; [DOI] [PMC free article] [PubMed]
- 34.Baraban SC, Dinday MT, Hortopan GA. Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat Commun.; 2013;4. [DOI] [PMC free article] [PubMed]
- 35.Dinday MT, Baraban SC. Large-scale phenotype-based antiepileptic drug screening in a zebrafish model of Dravet syndrome. eNeuro. 2015;2:1–19. [DOI] [PMC free article] [PubMed]
- 36.Kumar MG, Rowley S, Fulton R, Dinday MT, Baraban SC, Patel M. Altered glycolysis and mitochondrial respiration in a zebrafish model of Dravet syndrome. eNeuro. 2016;3:1002–11. [DOI] [PMC free article] [PubMed]
- 37.Griffin A, Anvar M, Hamling K, Baraban SC. Phenotype-Based Screening of Synthetic Cannabinoids in a Dravet Syndrome Zebrafish Model. Front Pharmacol. 2020;11:1–10. doi: 10.3389/fphar.2020.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiraboschi E, Martina S, van der Ent W, Grzyb K, Gawel K, Cordero-Maldonado ML, et al. New insights into the early mechanisms of epileptogenesis in a zebrafish model of Dravet syndrome. Epilepsia. 2020;61:549–560. doi: 10.1111/epi.16456. [DOI] [PubMed] [Google Scholar]
- 39.Ghannad-Rezaie M, Eimon PM, Wu Y, Yanik MF. Engineering brain activity patterns by neuromodulator polytherapy for treatment of disorders. Nat Commun; 2019;10. [DOI] [PMC free article] [PubMed]
- 40.Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. Nature. 2013;31:227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosello M, Vougny J, Czarny F, Mione MC, Concordet JP, Albadri S, et al. Precise base editing for the in vivo study of developmental signaling and human pathologies in zebrafish. Elife. 2021;10:1–27. doi: 10.7554/eLife.65552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simone BW, Martínez-Gálvez G, WareJoncas Z, Ekker SC. Fishing for understanding: Unlocking the zebrafish gene editor’s toolbox. Methods. 2018;150:3–10. doi: 10.1016/j.ymeth.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prykhozhij S V., Caceres L, Berman JN. New Developments in CRISPR/Cas-based Functional Genomics and their Implications for Research Using Zebrafish. Curr Gene Ther. 2017;17. [DOI] [PubMed]
- 44.Petri K, Zhang W, Ma J, Schmidts A, Lee H, Horng JE, et al. CRISPR prime editing with ribonucleoprotein complexes in zebrafish and primary human cells. Nat Biotechnol; 2021; 10.1038/s41587-021-00901-y [DOI] [PMC free article] [PubMed]
- 45.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schoonheim PJ, Arrenberg AB, Del Bene F, Baier H. Optogenetic localization and genetic perturbation of saccade-generating neurons in Zebrafish. J Neurosci. 2010;30:7111–7120. doi: 10.1523/JNEUROSCI.5193-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baraban SC, Taylor MR, Castro PA, Baier H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience. 2005;131:759–768. doi: 10.1016/j.neuroscience.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 48.Gawel K, Langlois M, Martins T, van der Ent W, Tiraboschi E, Jacmin M, et al. Seizing the moment: Zebrafish epilepsy models. Neurosci Biobehav Rev. 2020;116:1–20. doi: 10.1016/j.neubiorev.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 49.Canzian J, Franscescon F, Müller TE, Stefanello F V., Souza TP, Rosa L V., et al. Stress increases susceptibility to pentylenetetrazole-induced seizures in adult zebrafish. Epilepsy Behav.; 2021;114:107557. [DOI] [PubMed]
- 50.Cho SJ, Park E, Telliyan T, Baker A, Reid AY. Zebrafish model of posttraumatic epilepsy. Epilepsia. 2020;61:1774–1785. doi: 10.1111/epi.16589. [DOI] [PubMed] [Google Scholar]
- 51.Raby L, Völkel P, Le Bourhis X, Angrand PO. Genetic engineering of zebrafish in cancer research. Cancers (Basel). 2020;12:1–36. doi: 10.3390/cancers12082168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burrows DRW, Samarut, Liu J, Baraban SC, Richardson MP, Meyer MP, et al. Imaging epilepsy in larval zebrafish. Eur J Paediatr Neurol; 2020;24:70–80. [DOI] [PMC free article] [PubMed]
- 53.Yaksi E, Jamali A, Diaz Verdugo C, Jurisch-Yaksi N. Past, present and future of zebrafish in epilepsy research. FEBS J. 2021;1–13. [DOI] [PubMed]
- 54.Winter MJ, Pinion J, Tochwin A, Takesono A, Ball JS, Grabowski P, et al. Functional brain imaging in larval zebrafish for characterising the effects of seizurogenic compounds acting via a range of pharmacological mechanisms. Br J Pharmacol. 2021;178:2671–2689. doi: 10.1111/bph.15458. [DOI] [PubMed] [Google Scholar]
- 55.Griffin A, Hamling KR, Knupp K, Hong SG, Lee LP, Baraban SC. Clemizole and modulators of serotonin signalling suppress seizures in Dravet syndrome. Brain. 2017;140:669–683. doi: 10.1093/brain/aww342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 57.Inoue H, Nagata N, Kurokawa H, Yamanaka S. iPS cells: a game changer for future medicine. EMBO J. 2014;33:409–417. doi: 10.1002/embj.201387098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki IK, Vanderhaeghen P. Is this a brain which I see before me? Modeling human neural development with pluripotent stem cells. Development. 2015;142:3138–3150. doi: 10.1242/dev.120568. [DOI] [PubMed] [Google Scholar]
- 59.van der Kant R, Langness VF, Herrera CM, Williams DA, Fong LK, Leestemaker Y, et al. Cholesterol Metabolism Is a Druggable Axis that Independently Regulates Tau and Amyloid-β in iPSC-Derived Alzheimer’s Disease Neurons. Cell Stem Cell. 2019;24:363–375.e9. doi: 10.1016/j.stem.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu H, Gan J, Jonas P. Fast-spiking, parvalbumin+ GABAergic interneurons: From cellular design to microcircuit function. Science. 2014;345. [DOI] [PubMed]
- 61.Shcheglovitov A, Shcheglovitova O, Yazawa M, Portmann T, Shu R, Sebastiano V, et al. SHANK3 and IGF1 restore synaptic deficits in neurons from 22q13 deletion syndrome patients. Nature. 2013;503:267–271. doi: 10.1038/nature12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y, Lopez-Santiago LF, Yuan Y, Jones JM, Zhang H, O’Malley HA, et al. Dravet syndrome patient-derived neurons suggest a novel epilepsy mechanism. Ann Neurol. 2013;74:128–139. doi: 10.1002/ana.23897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiao J, Yang Y, Shi Y, Chen J, Gao R, Fan Y, et al. Modeling Dravet syndrome using induced pluripotent stem cells (iPSCs) and directly converted neurons. Hum Mol Genet. 2013;22:4241–4252. doi: 10.1093/hmg/ddt275. [DOI] [PubMed] [Google Scholar]
- 64.Higurashi N, Uchida T, Lossin C, Misumi Y, Okada Y, Akamatsu W, et al. A human Dravet syndrome model from patient induced pluripotent stem cells. Mol Brain. 2013;6:1–12. doi: 10.1186/1756-6606-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim HW, Quan Z, Kim YB, Cheong E, Kim HD, Cho M, et al. Differential effects on sodium current impairments by distinct SCN1A mutations in GABAergic neurons derived from Dravet syndrome patients. Brain Dev. 2018;40:287–298. doi: 10.1016/j.braindev.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 66.Schuster J, Laan L, Klar J, Jin Z, Huss M, Korol S, et al. Transcriptomes of Dravet syndrome iPSC derived GABAergic cells reveal dysregulated pathways for chromatin remodeling and neurodevelopment. Neurobiol Dis;132:104583. [DOI] [PubMed]
- 67.Sun Y, Paşca SP, Portmann T, Goold C, Worringer KA, Guan W, et al. A deleterious Nav1.1 mutation selectively impairs telencephalic inhibitory neurons derived from Dravet Syndrome patients. Elife. 2016;5:1–26. [DOI] [PMC free article] [PubMed]
- 68.Sun Y, Dolmetsch RE. Investigating the therapeutic mechanism of cannabidiol in a human induced pluripotent stem cell (iPSC)-based model of Dravet syndrome. Cold Spring Harb Symp Quant Biol. 2018;83:185–191. doi: 10.1101/sqb.2018.83.038174. [DOI] [PubMed] [Google Scholar]
- 69.Quraishi IH, Stern S, Mangan KP, Zhang Y, Ali SR, Mercier MR, et al. An epilepsy-associated KCNT1 mutation enhances excitability of human iPSC-derived neurons by increasing slack KNa currents. J Neurosci. 2019;39:7438–7449. doi: 10.1523/JNEUROSCI.1628-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tidball AM, Lopez-Santiago LF, Yuan Y, Glenn TW, Margolis JL, Clayton Walker J, et al. Variant-specific changes in persistent or resurgent sodium current in SCN8A-related epilepsy patient-derived neurons. Brain. 2020;143:3025–3040. doi: 10.1093/brain/awaa247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Costa V, Aigner S, Vukcevic M, Sauter E, Behr K, Ebeling M, et al. MTORC1 Inhibition Corrects Neurodevelopmental and Synaptic Alterations in a Human Stem Cell Model of Tuberous Sclerosis. Cell Rep. 2016;15:86–95. doi: 10.1016/j.celrep.2016.02.090. [DOI] [PubMed] [Google Scholar]
- 72.Winden KD, Sundberg M, Yang C, Wafa SMA, Dwyer S, Chen PF, et al. Biallelic mutations in TSC2 lead to abnormalities associated with cortical tubers in human ipsc-derived neurons. J Neurosci. 2019;39:9294–9305. doi: 10.1523/JNEUROSCI.0642-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.G. Nadadhur A, Alsaqati M, Gasparotto L, Cornelissen-Steijger P, van Hugte E, Dooves S, et al. Neuron-Glia Interactions Increase Neuronal Phenotypes in Tuberous Sclerosis Complex Patient iPSC-Derived Models. Stem Cell Reports.; 2019;12:42–56. [DOI] [PMC free article] [PubMed]
- 74.Escayg A, Goldin AL. Sodium channel SCN1A and epilepsy: Mutations and mechanisms. Epilepsia. 2010;51:1650–1658. doi: 10.1111/j.1528-1167.2010.02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lopez-Santiago L, Isom LL. Dravet Syndrome: A Developmental and Epileptic Encephalopathy. Epilepsy Curr. 2019;19:51–53. doi: 10.1177/1535759718822038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rubinstein M, Westenbroek RE, Yu FH, Jones CJ, Scheuer T, Catterall WA. Genetic background modulates impaired excitability of inhibitory neurons in a mouse model of Dravet syndrome. Neurobiol Dis. 2015;73:106–117. doi: 10.1016/j.nbd.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mistry AM, Thompson CH, Miller AR, Vanoye CG, George AL, Kearney JA. Strain- and age-dependent hippocampal neuron sodium currents correlate with epilepsy severity in Dravet syndrome mice. Neurobiol Dis. 2014;65:1–11. doi: 10.1016/j.nbd.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J, Gao C, Chen W, Ma W, Li X, Shi Y, et al. CRISPR/Cas9 facilitates investigation of neural circuit disease using human iPSCs: Mechanism of epilepsy caused by an SCN1A loss-of-function mutation. Transl Psychiatry. 2016;6. [DOI] [PMC free article] [PubMed]
- 79.Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N Engl J Med. 2017;376:2011–2020. doi: 10.1056/NEJMoa1611618. [DOI] [PubMed] [Google Scholar]
- 80.Kaplan JS, Stella N, Catterall WA, Westenbroek RE. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A. 2017;114:11229–11234. doi: 10.1073/pnas.1711351114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fitzgerald M, Sotuyo N, Tischfield DJ, Anderson SA. Generation of cerebral cortical GABAergic interneurons from pluripotent stem cells. Stem Cells. 2020;38:1375–1386. doi: 10.1002/stem.3252. [DOI] [PubMed] [Google Scholar]
- 82.Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, et al. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci.; 2007;27:5903–14. [DOI] [PMC free article] [PubMed]
- 83.Tai C, Abe Y, Westenbroek RE, Scheuer T, Catterall WA. Impaired excitability of somatostatin- and parvalbumin-expressing cortical interneurons in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A. 2014;111:3139–3148. doi: 10.1073/pnas.1411131111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tran CH, Vaiana M, Nakuci J, Somarowthu A, Goff KM, Goldstein N, et al. Interneuron desynchronization precedes seizures in a mouse model of Dravet syndrome. J Neurosci. 2020;40:2764–2775. doi: 10.1523/JNEUROSCI.2370-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barcia G, Fleming MR, Deligniere A, Gazula VR, Brown MR, Langouet M, et al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat Genet. Nature Publishing Group. 2012;44:1255–1259. doi: 10.1038/ng.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Groeneveld GJ, Van Kan HJM, Sastre Toraño J, Veldink JH, Guchelaar HJ, Wokke JHJ, et al. Inter- and intraindividual variability of riluzole serum concentrations in patients with ALS. J Neurol Sci. 2001;191:121–125. doi: 10.1016/s0022-510x(01)00613-x. [DOI] [PubMed] [Google Scholar]
- 87.Rambeck B, Jürgens UH, May TW, Wolfgang Pannek H, Behne F, Ebner A, et al. Comparison of brain extracellular fluid, brain tissue, cerebrospinal fluid, and serum concentrations of antiepileptic drugs measured intraoperatively in patients with intractable epilepsy. Epilepsia. 2006;47:681–694. doi: 10.1111/j.1528-1167.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 88.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang N, Ng YH, Pang ZP, Südhof TC, Wernig M. Induced Neuronal Cells: How to Make and Define a Neuron. Cell Stem Cell [Internet]. Cell Press; 2011;9:517–25. [DOI] [PMC free article] [PubMed]
- 92.Huh CJ, Zhang B, Victor MB, Dahiya S, Batista LFZ, Horvath S, et al. Maintenance of age in human neurons generated by microRNA-based neuronal conversion of fibroblasts. Elife. 2016;5:1–14. doi: 10.7554/eLife.18648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Victor MB, Richner M, Olsen HE, Lee SW, Monteys AM, Ma C, et al. Striatal neurons directly converted from Huntington’s disease patient fibroblasts recapitulate age-associated disease phenotypes. Nat Neurosci. 2018;21:341–352. doi: 10.1038/s41593-018-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun AX, Yuan Q, Tan S, Xiao Y, Wang D, Khoo ATT, et al. Direct Induction and Functional Maturation of Forebrain GABAergic Neurons from Human Pluripotent Stem Cells. Cell Rep. 2016;16:1942–1953. doi: 10.1016/j.celrep.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Pak CH, Han Y, Ahlenius H, Zhang Z, Chanda S, et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang N, Chanda S ham, Marro S, Ng Y-H, Janas J, Haag D, et al. Generation of pure GABAergic neurons by transcription factor programming. Nat Methods. 2017;14:621–8. [DOI] [PMC free article] [PubMed]
- 97.Wang C, Ward ME, Chen R, Liu K, Tracy TE, Chen X, et al. Scalable Production of iPSC-Derived Human Neurons to Identify Tau-Lowering Compounds by High-Content Screening. Stem Cell Reports. 2017;9:1221–1233. doi: 10.1016/j.stemcr.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tian R, Abarientos A, Hong J, Hashemi SH, Yan R, Dräger N, et al. Genome-wide CRISPRi/a screens in human neurons link lysosomal failure to ferroptosis. Nat Neurosci. 2021;24:1020–1034. doi: 10.1038/s41593-021-00862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tian R, Gachechiladze MA, Ludwig CH, Laurie MT, Hong JY, Nathaniel D, et al. CRISPR Interference-Based Platform for Multimodal Genetic Screens in Human iPSC-Derived Neurons. Neuron. 2019;104:239–255.e12. doi: 10.1016/j.neuron.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nehme R, Zuccaro E, Ghosh SD, Li C, Sherwood JL, Pietilainen O, et al. Combining NGN2 Programming with Developmental Patterning Generates Human Excitatory Neurons with NMDAR-Mediated Synaptic Transmission. Cell Rep. 2018;23:2509–2523. doi: 10.1016/j.celrep.2018.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 102.Schafer ST, Paquola ACM, Stern S, Gosselin D, Ku M, Pena M, et al. Pathological priming causes developmental gene network heterochronicity in autistic subject-derived neurons. Nat Neurosci. 2019;22:243–255. doi: 10.1038/s41593-018-0295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun AX, Yuan Q, Fukuda M, Yu W, Yan H, Lim GGY, et al. Potassium channel dysfunction in human neuronal models of Angelman syndrome. Science. 2019;366:1486–1492. doi: 10.1126/science.aav5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saavedra L, Wallace K, Freudenrich TF, Mall M, Mundy WR, Davila J, et al. Comparison of Acute Effects of Neurotoxic Compounds on Network Activity in Human and Rodent Neural Cultures. Toxicol Sci. 2021;180:295–312. doi: 10.1093/toxsci/kfab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sirenko O, Parham F, Dea S, Sodhi N, Biesmans S, Mora-Castilla S, et al. Functional and mechanistic neurotoxicity profiling using human iPSC-Derived neural 3D cultures. Toxicol Sci. 2019;167:249–257. doi: 10.1093/toxsci/kfy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simkin D, Marshall KA, Vanoye CG, Desai RR, Bustos BI, Piyevsky BN, et al. Dyshomeostatic modulation of Ca2+-activated K+ channels in a human neuronal model of KCNQ2 encephalopathy. Elife. 2021;10:1–32. doi: 10.7554/eLife.64434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sasai Y. Next-generation regenerative medicine: Organogenesis from stem cells in 3D culture. Cell Stem Cell. 2013;12:520–530. doi: 10.1016/j.stem.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 108.Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, et al. Self-Organized Formation of Polarized Cortical Tissues from ESCs and Its Active Manipulation by Extrinsic Signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 109.Mariani J, Simonini MV, Palejev D, Tomasini L, Coppola G, Szekely AM, et al. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci. 2012;109:12770–12775. doi: 10.1073/pnas.1202944109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paşca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 2015;12:671–678. doi: 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Watanabe M, Buth JE, Vishlaghi N, de la Torre-Ubieta L, Taxidis J, Khakh BS, et al. Self-Organized Cerebral Organoids with Human-Specific Features Predict Effective Drugs to Combat Zika Virus Infection. Cell Rep. 2017;21:517–532. doi: 10.1016/j.celrep.2017.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Yang SM, Berger DR, et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature. 2017;545:48–53. doi: 10.1038/nature22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G, et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature. 2019;570:523–527. doi: 10.1038/s41586-019-1289-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yoon SJ, Elahi LS, Pașca AM, Marton RM, Gordon A, Revah O, et al. Reliability of human cortical organoid generation. Nat Methods. 2019;16:75–78. doi: 10.1038/s41592-018-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, Caneda C, et al. Human Astrocyte Maturation Captured in 3D Cerebral Cortical Spheroids Derived from Pluripotent Stem Cells. Neuron. 2017;95:779–790.e6. doi: 10.1016/j.neuron.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marton RM, Miura Y, Sloan SA, Li Q, Revah O, Levy RJ, et al. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci. 2019;22:484–491. doi: 10.1038/s41593-018-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, et al. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545:54–59. doi: 10.1038/nature22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, et al. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell. 2015;162:375–390. doi: 10.1016/j.cell.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang G, Shcheglovitov A. Probing disrupted neurodevelopment in autism using human stem cell‐derived neurons and organoids: An outlook into future diagnostics and drug development. Dev Dyn. 2019;1–28. [DOI] [PMC free article] [PubMed]
- 122.Trujillo CA, Gao R, Negraes PD, Gu J, Buchanan J, Preissl S, et al. Complex Oscillatory Waves Emerging from Cortical Organoids Model Early Human Brain Network Development. Cell Stem Cell. 2019;25:558–569.e7. doi: 10.1016/j.stem.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang Y, Chiola S, Yang G, Russell C, Armstrong CJ, Wu Y, et al. Modeling autism-associated SHANK3 deficiency using human cortico-striatal organoids generated from single neural rosettes. bioRxiv.; 2021.: 10.1101/2021.01.25.428022 [DOI] [PMC free article] [PubMed]
- 124.Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, et al. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 2018;36:432–441. doi: 10.1038/nbt.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qian X, Su Y, Adam CD, Deutschmann AU, Pather SR, Goldberg EM, et al. Sliced Human Cortical Organoids for Modeling Distinct Cortical Layer Formation. Cell Stem Cell. 2020;26:766–781.e9. doi: 10.1016/j.stem.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Giandomenico SL, Mierau SB, Gibbons GM, Wenger LMD, Masullo L, Sit T, et al. Cerebral organoids at the air–liquid interface generate diverse nerve tracts with functional output. Nat Neurosci. 2019;22:669–679. doi: 10.1038/s41593-019-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xiang Y, Tanaka Y, Cakir B, Patterson B, Kim KY, Sun P, et al. hESC-Derived Thalamic Organoids Form Reciprocal Projections When Fused with Cortical Organoids. Cell Stem Cell. 2019;24:487–497.e7. doi: 10.1016/j.stem.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miura Y, Li M, Birey F, Ikeda K, Revah O, Thete MV, et al. Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nat Biotechnol. 2020;38:1421–1430. doi: 10.1038/s41587-020-00763-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Andersen J, Revah O, Miura Y, Thom N, Amin ND, Kelley KW, et al. Generation of Functional Human 3D Cortico-Motor Assembloids. Cell. 2020;183:1913–1929.e26. doi: 10.1016/j.cell.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Blair JD, Hockemeyer D, Bateup HS. Genetically engineered human cortical spheroid models of tuberous sclerosis. Nat Med. 2018;24:1568–1578. doi: 10.1038/s41591-018-0139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Crino PB. The mTOR signalling cascade: Paving new roads to cure neurological disease. Nat. Rev. Neurol.; 2016 ; 379–92. [DOI] [PubMed]
- 132.Negraes PD, Trujillo CA, Yu N-K, Wu W, Yao H, Liang N, et al. Altered network and rescue of human neurons derived from individuals with early-onset genetic epilepsy. Mol Psychiatry. 2021 doi: 10.1038/s41380-021-01104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Samarasinghe R, Miranda O, Buth J, Mitchell S, Ferando I, Watanabe M, et al. Identification of neural oscillations and epileptiform changes in human brain organoids. bioRxiv. 2021;1–55. [DOI] [PMC free article] [PubMed]
- 134.de Calbiac H, Dabacan A, Marsan E, Tostivint H, Devienne G, Ishida S, et al. Depdc5 knockdown causes mTOR-dependent motor hyperactivity in zebrafish. Ann Clin Transl Neurol. 2018;5:510–523. doi: 10.1002/acn3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Albanese A, Swaney JM, Yun DH, Evans NB, Antonucci JM, Velasco S, et al. Multiscale 3D phenotyping of human cerebral organoids. Sci Rep. 2020;10:1–17. doi: 10.1038/s41598-020-78130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.