Abstract
Malformations of cortical development (MCDs) represent a range of neurodevelopmental disorders that are collectively common causes of developmental delay and epilepsy, especially refractory childhood epilepsy. Initial treatment with antiseizure medications is empiric, and consideration of surgery is the standard of care for eligible patients with medically refractory epilepsy. In the past decade, advances in next generation sequencing technologies have accelerated progress in understanding the genetic etiologies of MCDs, and precision therapies for focal MCDs are emerging. Notably, mutations that lead to abnormal activation of the mammalian target of rapamycin (mTOR) pathway, which provides critical control of cell growth and proliferation, have emerged as a common cause of malformations. These include tuberous sclerosis complex (TSC), hemimegalencephaly (HME), and some types of focal cortical dysplasia (FCD). TSC currently represents the best example for the pathway from gene discovery to relatively safe and efficacious targeted therapy for epilepsy related to MCDs. Based on extensive pre-clinical and clinical data, the mTOR inhibitor everolimus is currently approved for the treatment of focal refractory seizures in patients with TSC. Although clinical studies are just emerging for FCD and HME, we believe the next decade will bring significant advancements in precision therapies for epilepsy related to these and other MCDs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-021-01122-6.
Keywords: Epilepsy, Malformations of cortical development (MCDs), Precision therapy, Mammalian target of rapamycin (mTOR), Somatic mosaicism
Introduction
Malformations of cortical development (MCDs) refer to a range of neurodevelopmental disorders that are collectively common causes of developmental delay and epilepsy. In an initial classification system, MCDs were divided into three major groups based on the earliest step in brain development that was presumed to be disrupted—proliferation, migration, or cortical organization [1]. In 2012, the classification system was updated to take into account advances in imaging, pathology, genetics, and molecular biology that increased our understanding of development and malformation of the human cerebral cortex [2]. In the current classification system, group 1 refers to malformations secondary to abnormal neuronal and glial proliferation or apoptosis, including congenital microcephaly, megalencephaly, and focal and diffuse dysgenesis and dysplasia; group 2 refers to malformations due to abnormal neuronal migration, including periventricular heterotopia, lissencephaly, subcortical heterotopia, and cobblestone malformation; and group 3 refers to malformations secondary to abnormal post-migrational development, including polymicrogyria, focal dysplasia due to late developmental disturbances, and post-migrational microcephaly [2].
MCDs are an important cause of epilepsy, especially early onset medically refractory epilepsy. Although the true incidence is difficult to determine, MCDs are identified in approximately up to 25% of children and 10% of adults with epilepsy, with relatively wide ranges reported depending on the specific population studied and imaging technologies used, and higher percentages generally reported when focused on non-acquired and focal epilepsy [3–6]. At least 75% of patients with MCDs develop recurrent seizures [7], and MCDs account for up to 40% of children with refractory epilepsy [8]. Initial treatment with antiseizure medications (ASMs) remains empiric, and surgery is the only potential cure for patients with refractory epilepsy and in some cases the only means to achieve palliation of recurrent seizures [9]. Surgery is the current standard of care for eligible patients with medically refractory epilepsy related to MCDs (i.e., patients with focal epilepsy for whom the epileptogenic zone can be localized, typically using stereoelectroencephalography, and is outside eloquent cortex) and can lead to a significant decrease in seizure frequency and even seizure freedom for some patients [10, 11]. In a recent neuropathology study of brain tissue obtained during epilepsy surgery for patients with refractory epilepsy, MCDs were the third most common histopathological diagnosis overall (19.8%) and in adults (12.4%), and the most common histopathological diagnosis in children (39.3%) [12]. Patients with epilepsy related to MCDs had earlier onset of epilepsy compared to patients with epilepsy due to other causes, and over half (58%) of patients with epilepsy related to MCDs who underwent epilepsy surgery had seizure freedom 1 year after surgery [12].
The mechanisms of epileptogenesis in MCDs continue to be elucidated, and studies suggest that multiple factors, including the vulnerable time periods of corticogenesis and synaptogenesis, the initial genetic or non-genetic insult, and the impact of the insult on developing cortical networks, contribute to the development of seizures [13]. Although non-genetic causes, including toxins, infections, and ischemic insults, contribute to some MCDs, in this review, we will focus on genetic causes as a pathway to targeted therapies [14]. As our knowledge of these disorders evolves, MCDs can be classified based on the affected molecular pathways, simplifying diagnoses and providing a path to precision therapies [3]. In the past decade, advances in next generation sequencing (NGS) technologies have accelerated progress in understanding the genetic etiologies of MCDs. Notably, mutations that lead to abnormal activation of the mammalian target of rapamycin (mTOR) pathway, which provides critical control of cell growth and proliferation, have emerged as a common cause of malformations secondary to abnormal proliferation, including tuberous sclerosis complex (TSC), hemimegalencephaly (HME), and some types of focal cortical dysplasia (FCD) [15–20]. In this review, we will focus on focal MCDs for which precision therapies are in the pre-clinical or clinical stages. We will first review TSC as the current best example of a MCD for which targeted therapies for associated epilepsy have been developed based on the underlying genetics and molecular pathways and extensively studied in both pre-clinical models and clinical trials. We will then review the current landscape of such therapies for HME, FCD, and related MCDs. Finally, we will discuss challenges and aspirations for developing precision therapies for epilepsy related to MCDs more broadly.
Tuberous Sclerosis
TSC and Epilepsy
Tuberous sclerosis complex (TSC) is an autosomal dominant multisystem neurocutaneous disorder that affects the central nervous system (CNS), skin, eyes, heart, lungs, and kidneys. TSC occurs in approximately 1 in 6000 live births [21]. The disorder was named by Bourneville in 1880, who described the post-mortem neuropathological findings as “tuberous sclerosis of the cerebral convolutions” in a young patient with epilepsy, hemiparesis, intellectual disability (ID), and renal tumors [22]. Neurological manifestations of TSC include structural abnormalities in the brain (cortical and subcortical tubers, subependymal nodules, and subependymal giant cell astrocytomas [SEGAs]), epilepsy, and a range of neuropsychiatric disorders [23], notably ID in approximately 45% [24] and autism spectrum disorder in approximately 40% [25]. In the 2012 MCD classification, TSC falls in group 1, malformations secondary to abnormal neuronal and glial proliferation or apoptosis, and is further specified as a cortical dysgenesis with abnormal cell proliferation but without neoplasia [2].
Seizures are the most common presentation of TSC, with epilepsy occurring in 80–90% of patients over their lifetimes, including the severe epileptic encephalopathy infantile spasms in 30–40% [26–28]. The majority of patients—almost 75%—develop epilepsy within the first year of life [29], and early onset epilepsy typically presents with infantile spasms or focal seizures [30]. Virtually all seizure types have been reported in TSC, and patients often develop multiple seizure types [26]. The likelihood of developing epilepsy after presenting with a first seizure is essentially 100% for patients with TSC, and thus early treatment with ASMs is warranted, even after a first seizure. Epilepsy related to TSC is medically refractory in up to two-thirds of patients [26]. Alternative treatment options include surgery, the ketogenic diet, and vagus nerve stimulation, and as we will discuss in detail below, there is growing evidence supporting the use of targeted therapy with mTOR inhibitors. Surgical evaluation often localizes the region of seizure origin to a tuber that is then resected, suggesting that tubers may be epileptogenic [31]. Approximately 60% of patients with epilepsy related to TSC who undergo surgery achieve seizure freedom [32].
TSC Genetics and Molecular Pathways
TSC is caused by mutation of one of two tumor suppressor genes: TSC1, which encodes hamartin (TSC1) [20], or TSC2, which encodes tuberin (TSC2) [33]. A pathogenic germline variant in TSC1 or TSC2 is identified in approximately 95% of patients with TSC [34, 35], and a pathogenic somatic mosaic variant may be detected in patients with no identified germline mutation [36]. In the “two-hit” model of Knudson [37], an individual inherits a variant in one allele, which is present in all of the cells, and a post-zygotically acquired variant in the second allele leads to loss of protein function and subsequent disease manifestations, such as overgrowth of specific tissues and/or, in some cases and in some tissues, cancer. In TSC, patients generally have an inherited or de novo germline mutation in one allele of TSC1 or TSC2; somatic mosaic variants of the second allele have been identified in non-nervous system tumors and rarely in cortical tubers or dysplasias [38–41].
TSC1 and TSC2 are negative regulators of the serine/threonine protein kinase mTOR, specifically of the protein complex mTORC1, which consists of mTOR, Raptor, mLST8, DEPTOR, and PRAS40 [42, 43]. The mTOR pathway regulates major cellular processes, including metabolism and proliferation, in response to environmental cues, receiving inputs from upstream energy-sensing and amino acid-sensing signaling pathways [44]. In the CNS, mTOR is a critical regulator of neurodevelopment and influences neuronal excitability [45]. TSC1 and TSC2, along with TBC1D7, form a heterotrimeric complex (TSC) that is a central component of the energy-sensing pathway and mediates responses to growth factors, nucleotides, oxygen, and energy [42, 46]. As one example, growth factor signaling activates PI3K, which leads to activation of AKT, which then inhibits TSC. The tuberous sclerosis complex functions as a GTPase-activating protein for Rheb [47], a GTPase that activates mTOR by directly binding to mTORC1 [48]. Thus, TSC1 and TSC2 inhibit mTOR, and the loss-of-function variants identified in TSC1 and TSC2 in patients with TSC lead to abnormal hyperactivation of the mTOR pathway [49]. Studies have demonstrated increased phosphorylation of downstream targets of mTOR, such as S6 kinase and ribosomal protein S6, in cortical tubers from patients with TSC [50]. The discovery of abnormal hyperactivation of the mTOR pathway in TSC suggested that inhibition of the mTOR pathway could be a promising therapeutic target in patients with TSC. Most pertinent to this review, given the role of the mTOR pathway in cellular processes that influence neuronal excitability, activation of the mTOR pathway was hypothesized to contribute to the development of epilepsy and thus inhibition of this pathway could specifically have an anti-epileptogenic and/or anti-seizure effect [45].
Pre-clinical Studies of mTOR Inhibitors
Rapamycin (also known as sirolimus) and its structural analogs (rapalogs, e.g., everolimus) directly inhibit mTORC1 by forming a complex with FKB12 and binding to mTOR [51]. Numerous studies have provided pre-clinical evidence of beneficial effects of targeted mTOR inhibitors in animal models of TSC, and here we will focus on the initial mouse studies that established anti-epileptogenic effects. Zeng et al. [52] first demonstrated the anti-epileptogenic effect of the mTOR inhibitor rapamycin in Tsc1GFAP conditional knockout mice with conditional inactivation of Tsc1 mainly in glia. Early treatment with rapamycin (before onset of neurological abnormalities) prevented epilepsy and premature death in this mouse model, and late treatment (after the onset of neurological manifestations notably epilepsy) decreased seizures and prolonged survival. Meikle et al. [53], using a mouse neuronal model of TSC in which Tsc1 is ablated in most neurons during cortical development, similarly demonstrated that early treatment with rapamycin or its derivative everolimus prevented spontaneous seizures during the treatment period and prolonged survival. Zeng et al. [54] showed that Tsc2GFAP conditional knockout mice had a more severe epilepsy phenotype compared to Tsc1GFAP conditional knockout mice, and that early treatment with rapamycin decreased seizure frequency and prolonged survival. Taken together, these studies provided support for clinical studies of mTOR inhibitors as targeted therapies for patients with epilepsy related to TSC.
Clinical Studies of mTOR Inhibitors
In the context of TSC, mTOR inhibitors were initially studied for the treatment of TSC-associated tumors, namely, SEGAs and renal angiomyolipomas. In 2006, a case series of four TSC patients with SEGAs reported that treatment with oral rapamycin induced lesion regression [55]. An open label, phase I/II clinical trial of everolimus for SEGAs (NCT00411619) demonstrated that treatment of 28 patients with everolimus for 6 months reduced SEGA volume (primary endpoint), with at least 30% reduction in volume in 75% of patients [56]. Treatment also reduced seizure frequency (secondary endpoint): 9/16 evaluable patients had reduced seizure frequency, 6 had no significant change in seizure frequency, and 1 had increased seizure frequency. An open label extension phase of 25 patients reported that everolimus treatment continued to be effective at 34 months and noted that all patients reported at least 1 adverse event (AE), most commonly upper respiratory infections (URIs), stomatitis, or sinusitis [57]. Final analysis of 22 patients at 5 years revealed a sustained effect on SEGA volume and no new safety concerns [58]. A double-blind, placebo-controlled, phase III clinical trial (EXamining everolimus In a Study of TSC, EXIST-1, NCT00789828), subsequently reported that 35% of patients in the everolimus group had at least 50% SEGA volume reduction compared to none in the placebo group at 6 months (primary endpoint) [59]. Median change from baseline seizure frequency at 6 months (secondary endpoint) was 0 in both the everolimus and placebo groups, but the analysis was inconclusive because the median number of seizures at baseline was also 0. A 2-year open label extension and the final analysis revealed that almost 60% of evaluable patients achieved SEGA response [60, 61]. All but one patient experienced AEs, the most common being stomatitis and mouth ulcerations; 9% of patients experienced an AE that led to discontinuation. Subsequently, the double-blind, placebo-controlled, phase III EXIST-2 clinical trial (NCT00790400) and its open-label extension and 4-year follow-up studies demonstrated that everolimus treatment reduced renal angiomyolipoma volume, with stomatitis and hypercholesterolemia as the most common AEs reported [62–64]. Based on these studies, everolimus was the first drug approved in the USA and Europe for treatment of SEGAs and renal angiomyolipomas in patients with TSC.
Over the past decade, mTOR inhibitors have been studied for the treatment of epilepsy related to TSC. In 2009, Muncy et al. described reduced seizure frequency after treatment with rapamycin in a 10-year-old patient with epilepsy related to TSC [65]. An open label, phase I/II clinical trial (NCT01070316) of everolimus for refractory epilepsy related to TSC demonstrated seizure reduction (median 73%) in 17/20 patients after 12 weeks of treatment, with 4 patients achieving seizure freedom [66]. An open-label extension found that 13/14 patients had at least 50% reduction in seizure frequency at 4 years. Everolimus was well tolerated; although all patients reported at least one AE, most commonly infectious or gastrointestinal/oral, 94% were mild or moderate [67].
The double-blind, placebo-controlled, phase III EXIST-3 clinical trial (NCT01713946) assessed the efficacy and safety of low and high trough exposure concentrations of adjunctive everolimus compared with placebo for 366 patients aged 2–65 years with refractory focal seizures related to TSC [68]. Everolimus treatment significantly reduced seizure frequency compared to placebo after the core phase, with 40% of patients in the high exposure group, 28.2% in the low exposure group, and 15.1% in the placebo group achieving at least 50% reduction in seizure frequency; median reduction in seizure frequency was 14.9% in the placebo group, 29.3% in the low exposure group, and 39.6% in the high exposure group (primary endpoint). An open-label extension for at least 48 weeks found sustained reduced seizure frequency, with a response rate of 46.6% after 1 year and 57.7% after 2 years of treatment [69]. Thus, there was a relationship between everolimus exposure and efficacy, with an increase in response rate over time. The most common AEs were stomatitis, pyrexia, diarrhea, nasopharyngitis, and URIs; 13% of patients discontinued treatment due to AEs, and two deaths were suspected to be related to treatment. Currently, an open label study, Roll-over Study to Collect and Assess Long-term Safety of Everolimus in Patients with TSC and Refractory Seizures Who Have Completed the EXIST-3 Study and Who Are Benefitting From Continued Treatment (NCT02962414), is active and is anticipated to remain open for approximately 10 years from first patient’s first visit to evaluate long-term safety. A post hoc analysis of the 299 pediatric patients in the trial found a higher response rate in both age groups studied (< 6 years and > 6 years) compared to placebo [70]. Seizure reduction was sustained at 1 year, with response rates of 48.9% in the younger subgroup and 47.2% in the older subgroup, and treatment was well tolerated. Everolimus has been approved in the USA and Europe for the adjunctive treatment of focal refractory seizures in patients with TSC who are at least 2 years old. A retrospective study of sirolimus and everolimus in children under 2 years old found increasing use clinically, mainly for refractory epilepsy related to TSC, and largely mild to moderate AEs, most commonly related to infections [71]. Currently, the phase I/II clinical trial Stopping TSC Onset and Progression 2: Epilepsy Prevention in TSC Infants (NCT04595513) is recruiting participants (infants with TSC up to 6 months). The study will include an open-label phase to establish sirolimus dosing followed by a randomized, double-blind, placebo-controlled phase to evaluate the safety and efficacy of early sirolimus to prevent or delay seizure onset in infants with TSC, with primary outcomes as follows: (1) percentage of subjects reporting severe/serious AEs and (2) time to seizure onset.
There have also been several recent smaller, open label studies that evaluated mTOR inhibitors for epilepsy related to TSC. A compassionate use trial of everolimus in 7 patients with TSC and refractory epilepsy showed 25–100% reduction in seizure frequency for 4/6 patients; one patient discontinued early due to an AE of skin flushing [72]. An open-label study including 7 patients with TSC and refractory seizures treated with sirolimus and everolimus found at least 50% reduction in seizure frequency in 5/7 patients, with three patients reporting subjective improvements in learning [73]. A placebo-controlled, open-label, cross-over trial (NTR3178) of 23 children with refractory epilepsy related to TSC found a non-significant 41% reduction in seizure frequency between the adjunctive sirolimus phase compared to the standard care phase; however, the study was underpowered and the mean sirolimus trough level was below target [74]. Of the 14 children who reached the trough target level, there was a significant 61% decrease in seizure frequency. A single-center, open-label study of everolimus for refractory epilepsy related to TSC found a reduction in seizure frequency of at least 50% for 12/15 (80%) children, with 7/12 (58%) seizure free after at least 6 months of treatment [75]. A cohort study of 91 children with epilepsy related to TSC treated with sirolimus for 1 year found that 78% of children responded and 47.2% achieved seizure freedom [76]. There was an indirect relationship between age and response rate, with significantly higher proportion of responders in younger children compared to older children. There was no significant difference in seizure frequency reduction between patients that changed AEDs compared to patients that maintained AEDs while receiving sirolimus, and there were no grade 3 or 4 AEs. Overall, clinical studies of mTOR inhibitors for epilepsy related to TSC have demonstrated efficacy, especially when treatment is started at a young age with higher trough levels and is sustained, as well as a tolerable safety profile.
TSC and Vigabatrin
As mentioned above, infantile spasms occur in 30–40% of patients with TSC, and the ASM vigabatrin is currently approved in the USA for the treatment of infantile spasms in children at least 1 month old as well as the treatment of refractory focal epilepsy in patients at least 2 years old with TSC. Vigabatrin is recommended in the USA and Europe as first-line treatment for infantile spasms related to TSC, with a response rate up to 95% across studies, and in Europe is also recommended as first-line treatment for focal seizures in the first year of life related to TSC, balancing the risk of visual field loss due to irreversible retinal toxicity [77, 78]. Vigabatrin inhibits GABA transaminase and thus GABA catabolism, leading to increased GABA levels in the brain. Interestingly, Tsc1GFAP conditional knockout mice treated with vigabatrin demonstrated not only increased brain GABA concentration but also decreased activation of the mTOR pathway [79], suggesting that some level of inhibition of the mTOR pathway may contribute to its unique efficacy in patients with TSC.
Given studies suggesting that early treatment is associated with improved epilepsy and neurodevelopmental outcomes and the efficacy of vigabatrin, two large clinical trials are investigating the safety and efficacy of its potential preventive use before the appearance of clinical seizures in patients with TSC. The multi-center European Long-Term, Prospective Study Evaluating Clinical and Molecular Biomarkers of Epileptogenesis in a Genetic Model of Epilepsy-Tuberous Sclerosis Complex (EPISTOP, NCT02098759), a randomized controlled trial at 6 sites and an open-label trial at 4 sites, enrolled infants with TSC and no history of seizures [80]. Infants were monitored with monthly video electroencephalography (EEG) and received vigabatrin either preventively (when epileptiform EEG activity was identified before seizures were detected) or conventionally (after the first electrographic or clinical seizure). The time to first clinical seizure (primary endpoint) was significantly longer with preventive compared to conventional treatment with vigabatrin in both the randomized controlled trial and open-label trial (364 days compared to 124 days, and 426 days compared to 106 days, respectively). At 2 years, preventive treatment significantly reduced the risk of clinical seizures, refractory epilepsy, and infantile spasms. Preventive treatment was well tolerated with no related AEs. The US randomized, placebo-controlled, double-blind Preventing Epilepsy Using Vigabatrin In Infants With Tuberous Sclerosis Complex (PREVeNT, NCT02849457) phase II clinical trial is currently ongoing. This trial similarly enrolled infants with TSC and no history of seizures, followed the infants with monthly EEGs, and randomized infants with epileptiform activity detected on EEG to vigabatrin or placebo. The primary outcome is cognitive level as assessed by the Bayley Scales of Infant and Toddler Development at 24 months, and the secondary outcomes include the development of seizures, time to first clinical seizure, and the prevalence of drug-resistant epilepsy. While results of the PREVeNT trial are pending, results from the EPISTOP trial suggest that preventive treatment with vigabatrin may positively change the natural history of TSC. Table 1 summarizes the clinical trials to date on targeted therapies for epilepsy related to TSC.
Table 1.
Previously published registered clinical trials investigating targeted therapy in epilepsy related to MCDs
| Clinical trial number | Phase | Study title | Efficacy results |
|---|---|---|---|
| NCT01070316 | I/II | Everolimus (RAD001) Therapy for Epilepsy in Patients With Tuberous Sclerosis Complex | At least 50% reduction in seizure frequency in 60% (12/20) of patients (at least 2 years old at enrollment) with TSC and refractory epilepsy after 12 weeks of treatment with everolimus [66] |
| [Open-label extension] | At least 50% reduction in seizure frequency in 93% (13/14) of patients with TSC and refractory epilepsy after 4 years of treatment with everolimus [67] | ||
| NCT01713946 | III | A Placebo-controlled Study of Efficacy & Safety of 2 Trough-ranges of Everolimus as Adjunctive Therapy in Patients With Tuberous Sclerosis Complex & Refractory Partial-onset Seizures (EXIST-3) | Significant difference in response rate (at least 50% reduction in seizure frequency) in patients (aged 2–65 years at enrollment) with TSC and refractory focal seizures in the high everolimus trough exposure group (40%, 52/130) and low everolimus trough exposure group (28.2%, 33/117) compared to the placebo group (15.1%, 18/119) after the core phase. Significant difference in median percentage reduction in seizure frequency in the high exposure group (39.6%) and low exposure group (29.3%) compared to the placebo group (14.9%) [68] |
| [Open-label extension] | Response rate 46.6% at 1 year (N = 298 patients) and 57.7% at 2 years (N = 163 patients). Median percentage reduction in seizure frequency 46.7% at 1 year and 56.9% at 2 years [69] | ||
| NCT02098759 | N/A | Long-term, Prospective Study Evaluating Clinical and Molecular Biomarkers of Epileptogenesis in a Genetic Model of Epilepsy—Tuberous Sclerosis Complex (EPISTOP)* | Significantly longer time to first clinical seizure in infants (up to 4 months old at enrollment) with TSC who received preventive treatment (vigabatrin started when epileptiform EEG activity identified before seizures detected) compared to conventional treatment (vigabatrin started after the first electrographic or clinical seizure); 364 vs 124 days in the randomized control trial and 426 vs 106 days in the open-label trial. Preventive treatment significantly reduced the risk of clinical seizures, refractory epilepsy, and infantile spasms at 2 years [80] |
*Further studies needed to determine if vigabatrin is a true targeted therapy
Although further studies on timing, efficacy, and safety of early treatment with mTOR inhibitors and vigabatrin are needed, one can imagine a future in which early diagnosis of TSC prenatally or rapidly postnatally leads to frequent monitoring with EEG for epileptiform activity. This is particularly notable since the standard of care for seizure and epilepsy diagnosis in the general population, without a specific genetic etiology, is to treat only after clinical seizures begin. The very high rate of seizures, as demonstrated by natural history data for patients with TSC, justifies this proactive approach. Detection of abnormal EEG activity prompts early “preventive” treatment with vigabatrin or an mTOR inhibitor. If and when epilepsy develops and necessitates additional treatment, additional ASDs or targeted treatments are started. Treatment options such as the ketogenic diet, vagus nerve stimulation, and epilepsy surgery remain important options, but it is interesting to speculate that the combination of targeted vigabatrin and mTOR inhibitors may show unique efficacy and potential synergism for the treatment of epilepsy related to TSC and may lead to improved epilepsy and neurodevelopmental outcomes.
“mTORopathies”: Focal Cortical Dysplasia Type II and Hemimegalencephaly
FCD II, HME, and Epilepsy
Focal cortical dysplasia (FCD), first described by Taylor et al. in 1971 [81], is a form of MCD characterized by a focal, typically small, region of abnormal cerebral cortex and often its underlying white matter [82]. Based on the classification system developed by the International League Against Epilepsy (ILAE) in 2011, FCD I is defined as an isolated lesion with abnormal cortical lamination, FCD II as an isolated region with abnormal cortical lamination and abnormal cell types (dysmorphic neurons with or without balloon cells in types IIa and IIb, respectively), and FCD III as a lesion with abnormal cortical lamination associated with another principal lesion [82]. The ILAE classification system was integrated into the updated MCD classification system in 2012, with FCD I and III placed in group 3 (malformations due to abnormal post-migrational development), specifically FCDs because of late developmental disturbances, and FCD II placed in group 1 (malformations secondary to abnormal neuronal and glial proliferation or apoptosis), specifically focal cortical dysgenesis with abnormal cell proliferation but without neoplasia, the same subcategory as TSC [2]. Hemimegalencephaly (HME), first reported by Sims in 1835, is a rare MCD characterized by abnormal enlargement of a cerebral hemisphere and is placed in group 1 in the same subcategory as FCD II and TSC [2, 83].
FCD and HME are important causes of epilepsy and especially refractory childhood epilepsy. FCD accounts for approximately 5–10% of patients with focal epilepsy; 40–50% of patients with epilepsy related to FCD and the vast majority of patients with epilepsy related to HME develop refractory epilepsy [84–86]. Patients with FCD or HME generally present with early-onset epilepsy in infancy or childhood; focal seizures are the most common initial presentation but the seizure semiology is heterogeneous [87, 88]. Epilepsy occurs in at least 70% of patients with FCD (though admittedly it is the patients with epilepsy who are most likely to be imaged) and in virtually all patients with HME [86]. In the neuropathology study discussed above, FCD was the most common neuropathological diagnosis and FCD II was the most common specific neuropathological diagnosis (17%) in children with intractable epilepsy who had epilepsy surgery [12]. Patients with FCD or HME may also have developmental delay, ID, and additional neurological deficits. For patients with FCD, the extent of focal neurological deficits relates to the location and extends of the FCD, and for patients with HME, contralateral hemiparesis and hemianopia are commonly reported [84].
FCD II and HME Genetics and Molecular Mechanisms
Compared to MCDs like lissencephaly or microcephaly, FCD and HME are focal MCDs, and it has long been hypothesized that such focal MCDs may be due to somatic mutations that occur post-zygotically during in utero development. FCD II and HME share pathological features with TSC, notably the balloon cells identified in abnormal FCD and HME brain tissue (similar to the giant cells identified in cortical tubers in TSC brain tissue) [50]. As discussed above, TSC is caused by loss of function mutations in TSC1 or TSC2 that leads to abnormal hyperactivation of the mTOR pathway, and evidence of hyperactivation is seen in cortical tubers of patients with TSC. In 2004, increased phosphorylation of downstream targets of the mTOR pathway was detected in surgically resected FCD brain tissue [89, 90]. Subsequent studies specifically showed increased phosphorylation in dysmorphic neurons and balloon cells in FCD II brain tissue, as well as in HME brain tissue [50, 91]. These studies suggested that abnormal hyperactivation of the mTOR pathway may be a common mechanism underlying TSC, FCD II, and HME.
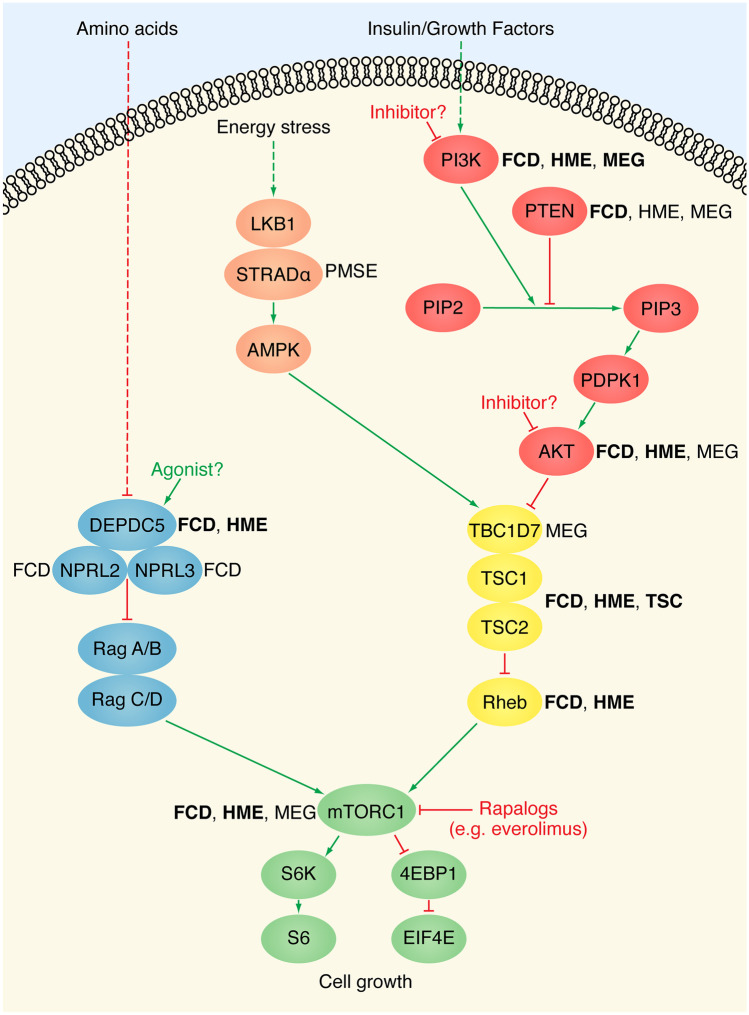
Over the past decade, advances in NGS and single cell technologies have demonstrated that FCD II, HME, and TSC are all “mTORopathies” caused by mutation that leads to abnormal hyperactivation of the mTOR pathway. Evidence for a genetic etiology for HME emerged in 2012, when our group and two others reported somatic mutations in positive regulators of the mTOR pathway (AKT3, PIK3CA, and MTOR) in HME patients [16, 18, 19]. We studied surgically resected brain tissue from eight patients with HME and identified two patients with somatic chromosome 1q copy number increases, which includes the AKT3 locus (encoding a positive regulator of mTOR), and one patient with a somatic activating point mutation in AKT3 [16]. Evidence for a genetic etiology for FCD emerged in 2014, when Scheffer et al. [17] reported germline variants in DEPDC5 (encoding a negative regulator of mTOR) in familial focal epilepsy, including some affected individuals with likely FCD II on neuroimaging. DEPDC5 is part of the GATOR1 complex, which also includes NPRL2 and NPRL3. GATOR1 is a guanine exchange factor for Rag, a GTPase that activates mTORC1 and mediates response to amino acids [42]. Somatic mosaic variants were identified in FCDs in 2015, when Lim et al. studied surgically resected brain tissue from 77 FCDII patients and identified 8 different somatic activating point mutations in MTOR [92]. Multiple studies have since confirmed “single-hit” activating variants in positive regulators of the mTOR pathway in FCD and HME, including variants in AKT1, AKT3, MTOR, PIK3CA, and RHEB [16, 18, 19, 41, 92–95]. Studies have also confirmed loss-of-function variants in negative regulators of the mTOR pathway in FCD and HME, including in DEPDC5, NPRL2, NPRL3, PTEN, TSC1, and TSC2, and in a few cases, a “two-hit” model of germline and somatic mosaic variants has been demonstrated [17, 41, 93, 96–102]. The mTOR pathway and associated “mTORopathies” are summarized in Fig. 1.
Fig. 1.
The mTOR pathway and associated MCDs. Schematic of the mammalian target of rapamycin (mTOR) pathway, with protein components annotated with the malformations of cortical development (MCDs) for which pathogenic mutations have been identified in the respective genes. Bold indicates pathogenic somatic mutations have been identified. FCD, focal cortical dysplasia; HME, hemimegalencephaly; MEG, megalencephaly; PMSE, polyhydramnios, megalencephaly, and symptomatic epilepsy syndrome; TSC, tuberous sclerosis complex
The alternate allele frequency (AAF) of detected somatic mutations in FCD and HME ranges from approximately 1 to 30% [41]. The average AAF for variants associated with FCD is lower than the average AAF for variants associated with HME; while there is some overlap, there appears to be a relationship between the allele frequency and the severity of the phenotype [41]. In general, when blood samples are available, the somatic mosaic variants identified in brain tissue are not detected in blood, suggesting that the mutational events that result in these variants arise relatively late in embryonic development, after gastrulation or in some cases after neurulation. Single cell studies of human brain tissue resected in the course of clinical treatment and mouse studies have suggested that abnormal hyperactivation of the mTOR pathway in neurons is necessary for disease pathogenesis, and further that such hyperactivation in the excitatory neuron lineage is necessary and in some cases sufficient [41]. Overall, FCD II and HME appear to represent a disease continuum, with the size and extent of the lesion dependent on the time (during development) and place (type of progenitor cell) in which the disease-causing somatic mutation occurs. Given the emerging evidence of efficacy and safety of mTOR inhibitors for TSC, use of mTOR inhibitors in the “mTORopathies” FCD II and HME presents a promising option for precision therapy.
Pre-clinical Studies
There is a growing body of literature on pre-clinical mouse and rat models of focal MCDs. In this section, we will highlight several studies that have investigated the anti-epileptogenic effects of mTOR pathway inhibitors in such models. Several models have expressed identified activating variants in positive regulators of the mTOR pathway. In utero electroporation of the variant that results in the Akt3 E17K substitution leads to abnormal cortical architecture, cytomegalic neurons, abnormal neuronal migration, and electrographic seizures that are rescued when rapamycin is administered prenatally but not postnatally [103]. Prenatal conditional expression of Pik3ca mutations leads to megalencephaly, abnormal cortical architecture, cytomegalic neurons, and seizures, and acute postnatal treatment with the PI3K inhibitor BKM120 suppressed seizures [104]. In utero electroporation of the variant that results in Mtor L2427P leads to abnormal neuronal migration, cytomegalic neurons, and spontaneous seizures, and postnatal rapamycin suppressed cytomegalic neurons and seizures [92]. In utero electroporation of the variant that results in Rheb Y35L leads to abnormal neuronal migration, cytomegalic neurons, and seizures, and postnatal rapamycin significantly reduced seizure frequency [95].
In addition, multiple models of loss of function in negative regulators of the mTOR pathway have been studied. Conditional knockout of Pten in neurons leads to megalencephaly, cytomegalic neurons, and seizures, and rapamycin suppressed seizures, including in older mice with established epilepsy [105–107]. Depdc5+/− rats have cytomegalic neurons and balloon-like cells (Depdc5−/− models are embryonic lethal), and prenatal rapamycin suppressed the abnormal cells [108]. Focal mosaic knockout of Depdc5 in mouse brain leads to abnormal cortical lamination, balloon-like cells, and spontaneous epilepsy, and prenatal rapamycin rescued neuronal migration defects [109]. Conditional knockout of Depdc5 in neurons leads to megalencephaly, cytomegalic neurons, and seizures, and postnatal chronic rapamycin prolonged survival and decreased brain size and neuronal soma size [110, 111]. Taken together, studies of mTOR pathway inhibitors in models of FCD and HME suggest promise for use in patients with epilepsy related to FCD or HME, but questions remain regarding safety and efficacy, including the optimal timing of such therapy.
Clinical Studies
Clinical studies of mTOR inhibitors for patients with FCD and HME are just emerging and will be an exciting area in the coming years. Xu et al. [112] described an infant with HME, refractory seizures (after 9 ASMs had been tried), and a somatic mosaic variant in MTOR, for whom rapamycin treatment was started at 3 months of age while awaiting hemispherectomy. The authors observed a greater than 50% reduction in seizures after 1 week of treatment and improved development after 2 weeks of treatment, allowing postponement of surgery to allow for weight gain required to more safely consider surgery because of the risk of blood loss in small infants. Currently, there are two active clinical trials investigating mTOR inhibitors in FCD II. A US study, A Pilot Study To Evaluate The Effects of Everolimus on Brain mTOR Activity and Cortical Hyperexcitability in TSC and FCD, is an open label phase II trial to evaluate the effects of everolimus on brain mTOR signaling in TSC and FCD patients aged 1–40 years who have refractory epilepsy and are having brain surgery, with a primary outcome of number of patients with AEs and a secondary outcome of number of patients with reduced mTOR signaling (NCT02451696). A Korean study, A Study Investigating the Anti-epileptic Efficacy of Afinitor (Everolimus) in Patients With Refractory Seizures Who Have Focal Cortical Dysplasia Type II (FCD II), is a randomized, double-blind, placebo-controlled cross-over phase II trial to evaluate the efficacy and safety of adjunctive everolimus in FCD II patients aged 4–40 years who failed more than 2 AEDs and surgery, with a primary outcome of at least 50% seizure reduction (NCT03198949). Table 2 summarizes currently active clinical trials on targeted therapies for epilepsy related to MCDs.
Table 2.
Currently active registered clinical trials investigating targeted therapy in epilepsy related to MCDs
| Clinical trial number | Phase | Study title | Aims | Primary outcome measures |
|---|---|---|---|---|
| NCT02962414 | III | Roll-over Study to Collect and Assess Long-term Safety of Everolimus in Patients With TSC and Refractory Seizures Who Have Completed the EXIST-3 Study [CRAD001M2304] and Who Are Benefitting From Continued Treatment | Evaluate long-term safety in patients with TSC and refractory seizures in the EXIST-3 trial who are continuing to be treated with and benefitting from treatment with everolimus (enrollment 206 participants) | Adverse events and serious adverse events (10 years) |
| NCT04595513 | I/II | Stopping TSC Onset and Progression 2: Epilepsy Prevention in TSC Infants (STOP2) | Open label phase to verify sirolimus dosing ➔ randomized, double-blind, placebo-controlled phase to evaluate safety and efficacy of early sirolimus to prevent or delay seizure onset in infants (up to 6 months old at enrollment) with TSC (estimated enrollment 65 participants) |
1) Percentage of subjects reporting severe adverse event or serious adverse event 2) Time to seizure onset |
| NCT02451696 | II | A Pilot Study To Evaluate The Effects of Everolimus on Brain mTOR Activity and Cortical Hyperexcitability in TSC and FCD | Evaluate the effects of everolimus on brain mTOR signaling in patients (aged 1–40 years at enrollment) with FCD or TSC and refractory epilepsy who are having brain surgery (enrolled 15 participants) | Number of patients with adverse events |
| NCT03198949 | II | A Study Investigating the Anti-epileptic Efficacy of Afinitor (Everolimus) in Patients With Refractory Seizures Who Have Focal Cortical Dysplasia Type II | Randomized, double-blind, placebo-controlled cross over study to evaluate the efficacy and safety of everolimus in patients (aged 4–40 years at enrollment) with FCD II who have failed more than 2 AEDs and surgery (enrolled 23 participants) | Number of patients with at least 50% seizure reduction |
| NCT02849457 | II | Preventing Epilepsy Using Vigabatrin In Infants With Tuberous Sclerosis Complex* | Randomized, double-blind, placebo-controlled study to evaluate the impact of early identification of EEG biomarkers and early treatment with vigabatrin in infants (up to 6 months at enrollment) with TSC (enrolled 84 participants) | Cognitive assessment scores on the Bayley Scales of Infant and Toddler Development at 24 months |
*Further studies needed to determine if vigabatrin is a true targeted therapy
While rapalogs are a promising targeted therapy, these drugs are unlikely to be a “one size fits all” treatment for patients with epilepsy related to FCD II and HME. It remains to be elucidated how efficacious mTOR inhibitors are for epilepsy related to FCD II and HME, including how early such drugs need to be started, what dosage should be used, and how long the drugs need to be continued for optimal efficacy. Studies thus far have suggested that early and sustained treatment may be needed, notably that chronic treatment may be needed to prevent seizure recurrence or tumor regrowth [56, 113]. Although the AEs of mTOR inhibitors have been studied in the TSC clinical trials described above [114], the long-term effects of early and potentially lifelong treatment with such broad inhibitors on immunosuppression, growth, and development, particularly neurodevelopment and sexual maturation, remain unclear and should prompt caution. Moreover, activation of the energy-sensing pathway (PI3K-PTEN-AKT-TSC-RHEB) versus the amino-acid sensing pathway (GATOR-RAG) that converge on mTOR shows some differences in in vitro studies and animal models, suggesting that the effects of a given activating mTOR pathway mutation will depend on both general hyperactivation of the mTOR pathway and potentially specific effects of the mutated protein [115]. ATP competitive inhibitors of mTOR and PI3K have been used in oncology, and dual PI3K/mTOR inhibitors and AKT inhibitors are also in development, and could be an option in epilepsy related to focal MCDs, with appropriate caution given their similarly relatively broad inhibition [116]. Preclinical and clinical studies in oncology have suggested that careful patient selection based on specific identified mutations may improve efficacy, and that balancing efficacy with systemic toxicity may be an important obstacle [116]. An unexplored but potentially promising target is DEPDC5 agonists, which may be more selective than rapalogs as DEPDC5 levels are highest in the brain and mTOR inhibition would depend on amino acid status [51]. Furthermore, monotherapy with any given therapy, even targeted therapy, is unlikely to be completely efficacious for all patients, and thus stepwise or combination approaches with ideally synergistic effects will be needed. One can imagine a stepwise approach in which early targeted therapy with an mTOR pathway modulator suppresses seizures to a manageable level for some time period, potentially in combination with ASMs, which may be especially helpful in infants with severe refractory epilepsy to allow for weight gain prior to surgery and reduce surgical risk. Studies of the interaction between mTOR pathway inhibitors and ASMs will be needed to ensure safety. The ketogenic diet, which is a treatment option for patients with epilepsy related to focal MCDs, has been shown to reduce levels of phosphorylated S6 and phosphorylated Akt in ketogenic diet-fed rats, suggesting inhibition of the mTOR pathway, potentially due to an amino acid deprivation-like environment. Thus, one can imagine that DEPDC5 agonists and dietary modification may have a synergistic effect on mTOR inhibition [51].
Focal Cortical Dysplasia Type I
As described above, FCD I is defined as an isolated lesion with abnormal cortical lamination and is placed in MCD group 3 (malformations due to abnormal post-migrational development) [2, 82]. Compared to FCD II, less is known about the underlying genetics of FCD I, though genetic etiologies are emerging. Germline and/or somatic variants in diverse genes have been reported in patients with FCD I, including in AKT3, DEPDC5, KCNT1, NPRL2, PCDH19, SCN1A, SLC35A2, and STXBP1, and an underlying pathogenic mechanism remains unclear [117]. Winawer et al. [118] identified somatic mosaic variants in SLC35A2 in surgically resected brain tissue of five patients with refractory focal epilepsy, two of whom had FCDIa confirmed on pathology. Interestingly, phospho-S6 was not increased in the abnormal brain tissue, suggesting a different mechanism than mTOR pathway activation. Somatic mutations in SLC35A2 have also been reported in additional patients with FCD I/mild MCDs, and it has been proposed that some of these cases should be reclassified as mild malformation of cortical development with oligodendroglial hyperplasia in epilepsy (MOGHE) [119–122]. SLC35A2 is a UDP-galactose transporter with a previously established association with congenital disorders of glycosylation. Dietary treatment with galactose supplementation has been reported to improve glycosylation [123] and alertness and interactivity [124] in patients with de novo SLC35A2 mutations, suggesting a potential targeted therapy for MCD patients with similar mutations.
Polyhydramnios, Megalencephaly, and Symptomatic Epilepsy
Polyhydramnios, megalencephaly, and symptomatic epilepsy syndrome (PMSE) is a rare recessive neurodevelopmental disorder discovered in the old order Mennonite population characterized by epilepsy, ID, and focal dysplasias [125, 126]. It is placed in MCD group 1 (malformations secondary to abnormal neuronal and glial proliferation or apoptosis), specifically focal cortical dysgenesis with abnormal cell proliferation but without neoplasia, the same subcategory as TSC, FCD II, and HME [2]. PMSE is caused by loss of function mutations in STRADA, and patients have a homozygous truncating deletion of exons 9 to 13 of STRADA. The deletion prevents STRADA from forming a complex with LKB1, which normally inhibits mTORC1 via AMPK and TSC [127]. Thus, the variant causing PMSE leads to abnormal hyperactivation of the mTOR pathway. Parker et al. [128] demonstrated that treatment with rapamycin prevented abnormal cortical lamination and heterotopic neurons in a PMSE mouse model, and that treatment with sirolimus reduced seizure frequency and improved receptive language in five PMSE patients and was well tolerated. Only one of the five children had a single seizure in the 12 months prior to the publication. Although rigorous clinical trials are needed, this small open-label study provides a promising example of precision treatment in MCD-associated epilepsy.
Conclusion and Future Directions
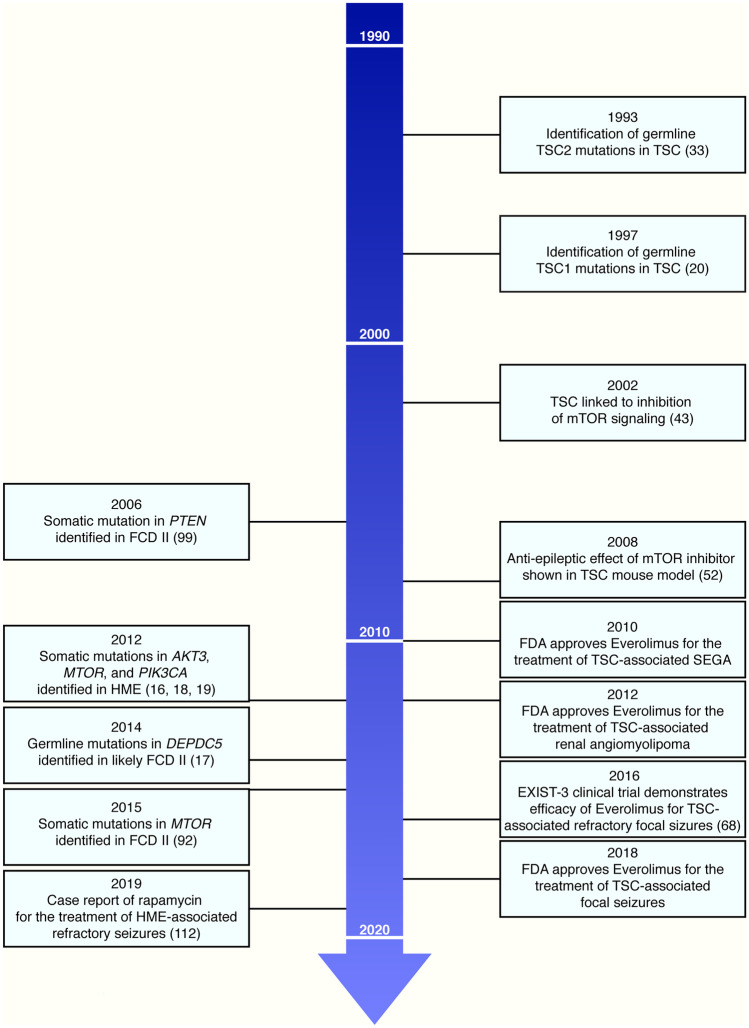
Malformations of cortical development are an important cause of epilepsy, especially refractory childhood epilepsy. Over the past decade, precision therapies for focal MCDs have started to come into focus, bringing hope to a large group of patients with disorders that have only recently been genetically characterized and who have medically refractory epilepsy with all of its comorbidities. TSC represents the current best example of a prototype for the pathway from gene discovery to relatively safe and efficacious targeted therapy for epilepsy related to MCDs. Based on extensive pre-clinical and clinical data, the mTOR inhibitor everolimus is currently approved for the treatment of focal refractory seizures in patients with TSC. It took 25 years from the initial discovery of TSC1 mutations in TSC to the FDA approval of everolimus for TSC-associated focal seizures (Fig. 2). Thus, although clinical studies are just emerging for FCD II, HME, FCD I, and PMSE, we believe the next decade will bring significant advancements in precision therapies for epilepsy related to these and other MCDs. To move forward from aspiration to reality, several challenges remain to be addressed. First, the ability to use a targeted therapy depends on the ability to make a molecular genetic diagnosis—precision diagnosis is required before one can entertain precision therapy, and we are still lagging behind in our translation of the last decade’s molecular discoveries to our patients with focal epilepsy. For germline variants, NGS approaches using clinically accessible tissues like blood or saliva are relatively straightforward. However, for somatic mosaic variants, which appear to occur in a significant proportion of focal MCDs, the disease-causing variants are generally detectable in surgically resected brain tissue but not blood or other tissues (it is possible that some might be detectable in skin because of the shared neuroectodermal lineage of brain and skin, but this has not been demonstrated in patients without skin lesions). Brain tissue is not clinically accessible unless surgery is undertaken, which is arguably too late to be able to initiate treatment and to potentially avoid surgery if possible. Ideally, genetic testing could be performed before epilepsy surgery to have an early impact on guiding management. Proof of concept of using CSF to detect somatic variants in patients with FCD has recently been reported and represents a potentially attractive and less invasive approach [129, 130]. However, even for FCD II and HME patients who undergo resection, many cases remain unsolved. Second, once a molecular genetic diagnosis is made, we need to understand the effects of the specific variant and potentially pair a precision therapy with a specific variant based on the effects of that variant versus broadly trying to pair a precision therapy with all variants in a given gene. An expert panel on “Genetics of Malformations of the Central Nervous System,” including our group and others, is currently curating the genes and variants associated with these malformations. Third, further studies of current targeted therapies (e.g., mTOR inhibitors) are needed to establish dosing and timing for optimal efficacy. For epilepsy related to MCDs, it is possible that treatment with targeted therapies like mTOR inhibitors may need to start early during epileptogenesis lest they be started “too late” to reverse established epilepsy [131]. We need to understand whether therapeutic effects on epilepsy (representing a dynamic process of circuit dysfunction) might be distinct from effects on underlying structural brain abnormalities (representing a completed developmental process). Fourth, further studies are needed to understand the adverse effects of early and likely chronic treatment with these therapies. Perhaps a balance will be required with low or intermittent dosing of mTOR inhibitors to maintain anti-epileptogenic efficacy and minimize AEs [132]. Fifth, we anticipate the need for new types of targeted therapies as we advance our understanding of MCD genetics and of the on- and off-target effects of current therapies. As discussed elsewhere in this issue, delivery of ASO or viral-based therapies intrathecally may increase CNS penetration and limit non-CNS side effects [116], and gene therapy approaches are likely to be developed in the near future. Sixth, clinicians, including oncologists and neurologists, scientists, and regulatory agencies will need to work together to establish an equitable workflow for patient access to future clinical trials and eventually prescription of new and repurposed targeted therapies in both the USA and abroad. Finally, continued studies of molecular and cellular pathways are needed to elucidate how the identified genetic mutations lead to epileptogenesis.
Fig. 2.
mTORopathies timeline. Timeline of key events in the history of tuberous sclerosis complex (TSC) (left) and focal cortical dysplasia (FCD) II and hemimegalencephaly (HME) (right) from gene discovery to the development of precision therapies
Supplementary Information
Below is the link to the electronic supplementary material.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
AMD was supported by T32 HD 098061.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barkovich AJ, Kuzniecky RI, Dobyns WB, Jackson GD, Becker LE, Evrard P. A classification scheme for malformations of cortical development. Neuropediatrics. 1996;27:59–63. doi: 10.1055/s-2007-973750. [DOI] [PubMed] [Google Scholar]
- 2.Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 2012;135:1348–1369. doi: 10.1093/brain/aws019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barkovich AJ, Dobyns WB, Guerrini R. Malformations of cortical development and epilepsy. Cold Spring Harb Perspect Med. 2015;5:a022392. [DOI] [PMC free article] [PubMed]
- 4.Camfield P, Camfield C. Incidence, prevalence and aetiology of seizures and epilepsy in children. Epileptic Disord. 2015;17:117–123. doi: 10.1684/epd.2015.0736. [DOI] [PubMed] [Google Scholar]
- 5.Hunter MB, Yoong M, Sumpter RE, et al. Incidence of early-onset epilepsy: A prospective population-based study. Seizure. 2020;75:49–54. doi: 10.1016/j.seizure.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Papayannis CE, Consalvo D, Kauffman MA, et al. Malformations of cortical development and epilepsy in adult patients. Seizure. 2012;21:377–384. doi: 10.1016/j.seizure.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Leventer RJ, Phelan EM, Coleman LT, Kean MJ, Jackson GD, Harvey AS. Clinical and imaging features of cortical malformations in childhood. Neurology. 1999;53:715–722. doi: 10.1212/wnl.53.4.715. [DOI] [PubMed] [Google Scholar]
- 8.Kuzniecky RI. Magnetic resonance imaging in developmental disorders of the cerebral cortex. Epilepsia. 1994;35(Suppl 6):S44–56. doi: 10.1111/j.1528-1157.1994.tb05988.x. [DOI] [PubMed] [Google Scholar]
- 9.Stevelink R, Sanders MW, Tuinman MP, et al. Epilepsy surgery for patients with genetic refractory epilepsy: a systematic review. Epileptic Disord. 2018;20:99–115. doi: 10.1684/epd.2018.0959. [DOI] [PubMed] [Google Scholar]
- 10.Harvey AS, Cross JH, Shinnar S, Mathern GW, Taskforce IPESS. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia. 2008;49:146–55. [DOI] [PubMed]
- 11.West S, Nevitt SJ, Cotton J, et al. Surgery for epilepsy. Cochrane Database Syst Rev. 2019;6:CD010541. [DOI] [PMC free article] [PubMed]
- 12.Blumcke I, Spreafico R, Haaker G, et al. Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery. N Engl J Med. 2017;377:1648–1656. doi: 10.1056/NEJMoa1703784. [DOI] [PubMed] [Google Scholar]
- 13.Represa A. Why Malformations of Cortical Development Cause Epilepsy. Front Neurosci. 2019;13:250. doi: 10.3389/fnins.2019.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juric-Sekhar G, Hevner RF. Malformations of Cerebral Cortex Development: Molecules and Mechanisms. Annu Rev Pathol. 2019;14:293–318. doi: 10.1146/annurev-pathmechdis-012418-012927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muhlebner A, Bongaarts A, Sarnat HB, Scholl T, Aronica E. New insights into a spectrum of developmental malformations related to mTOR dysregulations: challenges and perspectives. J Anat. 2019;235:521–542. doi: 10.1111/joa.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poduri A, Evrony GD, Cai X, et al. Somatic activation of AKT3 causes hemispheric developmental brain malformations. Neuron. 2012;74:41–48. doi: 10.1016/j.neuron.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheffer IE, Heron SE, Regan BM, et al. Mutations in mammalian target of rapamycin regulator DEPDC5 cause focal epilepsy with brain malformations. Ann Neurol. 2014;75:782–787. doi: 10.1002/ana.24126. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Huynh M, Silhavy JL, et al. De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat Genet. 2012;44:941–945. doi: 10.1038/ng.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riviere JB, Mirzaa GM, O’Roak BJ, et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat Genet. 2012;44:934–940. doi: 10.1038/ng.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Slegtenhorst M, de Hoogt R, Hermans C, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 21.Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Ann N Y Acad Sci. 1991;615:125–127. doi: 10.1111/j.1749-6632.1991.tb37754.x. [DOI] [PubMed] [Google Scholar]
- 22.Gomez MR. History of the tuberous sclerosis complex. Brain Dev. 1995;17(Suppl):55–57. doi: 10.1016/0387-7604(94)00130-8. [DOI] [PubMed] [Google Scholar]
- 23.de Vries PJ, Whittemore VH, Leclezio L, et al. Tuberous sclerosis associated neuropsychiatric disorders (TAND) and the TAND Checklist. Pediatr Neurol. 2015;52:25–35. doi: 10.1016/j.pediatrneurol.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joinson C, O’Callaghan FJ, Osborne JP, Martyn C, Harris T, Bolton PF. Learning disability and epilepsy in an epidemiological sample of individuals with tuberous sclerosis complex. Psychol Med. 2003;33:335–344. doi: 10.1017/s0033291702007092. [DOI] [PubMed] [Google Scholar]
- 25.Richards C, Jones C, Groves L, Moss J, Oliver C. Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2015;2:909–916. doi: 10.1016/S2215-0366(15)00376-4. [DOI] [PubMed] [Google Scholar]
- 26.Chu-Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51:1236–1241. doi: 10.1111/j.1528-1167.2009.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta A, de Bruyn G, Tousseyn S, et al. Epilepsy and Neurodevelopmental Comorbidities in Tuberous Sclerosis Complex: A Natural History Study. Pediatr Neurol. 2020;106:10–16. doi: 10.1016/j.pediatrneurol.2019.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Thiele EA. Managing epilepsy in tuberous sclerosis complex. J Child Neurol. 2004;19:680–686. doi: 10.1177/08830738040190090801. [DOI] [PubMed] [Google Scholar]
- 29.Davis PE, Filip-Dhima R, Sideridis G, et al. Presentation and Diagnosis of Tuberous Sclerosis Complex in Infants. Pediatrics. 2017;140. [DOI] [PMC free article] [PubMed]
- 30.Curatolo P, Moavero R, de Vries PJ. Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol. 2015;14:733–745. doi: 10.1016/S1474-4422(15)00069-1. [DOI] [PubMed] [Google Scholar]
- 31.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 32.Zhang K, Hu WH, Zhang C, Meng FG, Chen N, Zhang JG. Predictors of seizure freedom after surgical management of tuberous sclerosis complex: a systematic review and meta-analysis. Epilepsy Res. 2013;105:377–383. doi: 10.1016/j.eplepsyres.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 33.European Chromosome 16 Tuberous Sclerosis C. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–15. [DOI] [PubMed]
- 34.Northrup H, Koenig MK, Pearson DA, Au KS. Tuberous Sclerosis Complex. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, et al., editors. GeneReviews((R)). Seattle (WA)1993.
- 35.Ogorek B, Hamieh L, Hulshof HM, et al. TSC2 pathogenic variants are predictive of severe clinical manifestations in TSC infants: results of the EPISTOP study. Genet Med. 2020;22:1489–1497. doi: 10.1038/s41436-020-0823-4. [DOI] [PubMed] [Google Scholar]
- 36.Tyburczy ME, Dies KA, Glass J, et al. Mosaic and Intronic Mutations in TSC1/TSC2 Explain the Majority of TSC Patients with No Mutation Identified by Conventional Testing. PLoS Genet. 2015;11:e1005637. [DOI] [PMC free article] [PubMed]
- 37.Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crino PB, Aronica E, Baltuch G, Nathanson KL. Biallelic TSC gene inactivation in tuberous sclerosis complex. Neurology. 2010;74:1716–1723. doi: 10.1212/WNL.0b013e3181e04325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin W, Chan JA, Vinters HV, et al. Analysis of TSC cortical tubers by deep sequencing of TSC1, TSC2 and KRAS demonstrates that small second-hit mutations in these genes are rare events. Brain Pathol. 2010;20:1096–1105. doi: 10.1111/j.1750-3639.2010.00416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tyburczy ME, Jozwiak S, Malinowska IA, et al. A shower of second hit events as the cause of multifocal renal cell carcinoma in tuberous sclerosis complex. Hum Mol Genet. 2015;24:1836–1842. doi: 10.1093/hmg/ddu597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Gama AM, Woodworth MB, Hossain AA, et al. Somatic Mutations Activating the mTOR Pathway in Dorsal Telencephalic Progenitors Cause a Continuum of Cortical Dysplasias. Cell Rep. 2017;21:3754–3766. doi: 10.1016/j.celrep.2017.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Condon KJ, Sabatini DM. Nutrient regulation of mTORC1 at a glance. J Cell Sci. 2019;132. [DOI] [PMC free article] [PubMed]
- 43.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 44.Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;169:361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 45.Citraro R, Leo A, Constanti A, Russo E, De Sarro G. mTOR pathway inhibition as a new therapeutic strategy in epilepsy and epileptogenesis. Pharmacol Res. 2016;107:333–343. doi: 10.1016/j.phrs.2016.03.039. [DOI] [PubMed] [Google Scholar]
- 46.Dibble CC, Elis W, Menon S, et al. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cell. 2012;47:535–546. doi: 10.1016/j.molcel.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 49.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 50.Aronica E, Crino PB. Epilepsy related to developmental tumors and malformations of cortical development. Neurotherapeutics. 2014;11:251–268. doi: 10.1007/s13311-013-0251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myers KA, Scheffer IE. DEPDC5 as a potential therapeutic target for epilepsy. Expert Opin Ther Targets. 2017;21:591–600. doi: 10.1080/14728222.2017.1316715. [DOI] [PubMed] [Google Scholar]
- 52.Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meikle L, Pollizzi K, Egnor A, et al. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeng LH, Rensing NR, Zhang B, Gutmann DH, Gambello MJ, Wong M. Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of tuberous sclerosis complex. Hum Mol Genet. 2011;20:445–454. doi: 10.1093/hmg/ddq491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franz DN, Leonard J, Tudor C, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59:490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 56.Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 57.Krueger DA, Care MM, Agricola K, Tudor C, Mays M, Franz DN. Everolimus long-term safety and efficacy in subependymal giant cell astrocytoma. Neurology. 2013;80:574–580. doi: 10.1212/WNL.0b013e3182815428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franz DN, Agricola K, Mays M, et al. Everolimus for subependymal giant cell astrocytoma: 5-year final analysis. Ann Neurol. 2015;78:929–938. doi: 10.1002/ana.24523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franz DN, Belousova E, Sparagana S, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381:125–132. doi: 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- 60.Franz DN, Belousova E, Sparagana S, et al. Everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2-year open-label extension of the randomised EXIST-1 study. Lancet Oncol. 2014;15:1513–1520. doi: 10.1016/S1470-2045(14)70489-9. [DOI] [PubMed] [Google Scholar]
- 61.Franz DN, Belousova E, Sparagana S, et al. Long-Term Use of Everolimus in Patients with Tuberous Sclerosis Complex: Final Results from the EXIST-1 Study. PLoS One. 2016;11:e0158476. [DOI] [PMC free article] [PubMed]
- 62.Bissler JJ, Kingswood JC, Radzikowska E, et al. Everolimus long-term use in patients with tuberous sclerosis complex: Four-year update of the EXIST-2 study. PLoS One. 2017;12:e0180939. [DOI] [PMC free article] [PubMed]
- 63.Bissler JJ, Kingswood JC, Radzikowska E, et al. Everolimus for renal angiomyolipoma in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: extension of a randomized controlled trial. Nephrol Dial Transplant. 2016;31:111–119. doi: 10.1093/ndt/gfv249. [DOI] [PubMed] [Google Scholar]
- 64.Bissler JJ, Kingswood JC, Radzikowska E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381:817–824. doi: 10.1016/S0140-6736(12)61767-X. [DOI] [PubMed] [Google Scholar]
- 65.Muncy J, Butler IJ, Koenig MK. Rapamycin reduces seizure frequency in tuberous sclerosis complex. J Child Neurol. 2009;24:477. doi: 10.1177/0883073808324535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krueger DA, Wilfong AA, Holland-Bouley K, et al. Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann Neurol. 2013;74:679–687. doi: 10.1002/ana.23960. [DOI] [PubMed] [Google Scholar]
- 67.Krueger DA, Wilfong AA, Mays M, et al. Long-term treatment of epilepsy with everolimus in tuberous sclerosis. Neurology. 2016;87:2408–2415. doi: 10.1212/WNL.0000000000003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.French JA, Lawson JA, Yapici Z, et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388:2153–2163. doi: 10.1016/S0140-6736(16)31419-2. [DOI] [PubMed] [Google Scholar]
- 69.Franz DN, Lawson JA, Yapici Z, et al. Everolimus for treatment-refractory seizures in TSC: Extension of a randomized controlled trial. Neurol Clin Pract. 2018;8:412–420. doi: 10.1212/CPJ.0000000000000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Curatolo P, Franz DN, Lawson JA, et al. Adjunctive everolimus for children and adolescents with treatment-refractory seizures associated with tuberous sclerosis complex: post-hoc analysis of the phase 3 EXIST-3 trial. Lancet Child Adolesc Health. 2018;2:495–504. doi: 10.1016/S2352-4642(18)30099-3. [DOI] [PubMed] [Google Scholar]
- 71.Krueger DA, Capal JK, Curatolo P, et al. Short-term safety of mTOR inhibitors in infants and very young children with tuberous sclerosis complex (TSC): Multicentre clinical experience. Eur J Paediatr Neurol. 2018;22:1066–1073. doi: 10.1016/j.ejpn.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 72.Wiegand G, May TW, Ostertag P, Boor R, Stephani U, Franz DN. Everolimus in tuberous sclerosis patients with intractable epilepsy: a treatment option? Eur J Paediatr Neurol. 2013;17:631–638. doi: 10.1016/j.ejpn.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 73.Cardamone M, Flanagan D, Mowat D, Kennedy SE, Chopra M, Lawson JA. Mammalian target of rapamycin inhibitors for intractable epilepsy and subependymal giant cell astrocytomas in tuberous sclerosis complex. J Pediatr. 2014;164:1195–1200. doi: 10.1016/j.jpeds.2013.12.053. [DOI] [PubMed] [Google Scholar]
- 74.Overwater IE, Rietman AB, Bindels-de Heus K, et al. Sirolimus for epilepsy in children with tuberous sclerosis complex: A randomized controlled trial. Neurology. 2016;87:1011–1018. doi: 10.1212/WNL.0000000000003077. [DOI] [PubMed] [Google Scholar]
- 75.Samueli S, Abraham K, Dressler A, et al. Efficacy and safety of Everolimus in children with TSC - associated epilepsy - Pilot data from an open single-center prospective study. Orphanet J Rare Dis. 2016;11:145. doi: 10.1186/s13023-016-0530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He W, Chen J, Wang YY, et al. Sirolimus improves seizure control in pediatric patients with tuberous sclerosis: A prospective cohort study. Seizure. 2020;79:20–26. doi: 10.1016/j.seizure.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 77.Curatolo P, Nabbout R, Lagae L, et al. Management of epilepsy associated with tuberous sclerosis complex: Updated clinical recommendations. Eur J Paediatr Neurol. 2018;22:738–748. doi: 10.1016/j.ejpn.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 78.van der Poest Clement E, Jansen FE, Braun KPJ, Peters JM. Update on Drug Management of Refractory Epilepsy in Tuberous Sclerosis Complex. Paediatr Drugs. 2020;22:73–84. doi: 10.1007/s40272-019-00376-0. [DOI] [PubMed] [Google Scholar]
- 79.Zhang B, McDaniel SS, Rensing NR, Wong M. Vigabatrin inhibits seizures and mTOR pathway activation in a mouse model of tuberous sclerosis complex. PLoS One. 2013;8:e57445. [DOI] [PMC free article] [PubMed]
- 80.Kotulska K, Kwiatkowski DJ, Curatolo P, et al. Prevention of Epilepsy in Infants with Tuberous Sclerosis Complex in the EPISTOP Trial. Ann Neurol. 2021;89:304–314. doi: 10.1002/ana.25956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taylor DC, Falconer MA, Bruton CJ, Corsellis JA. Focal dysplasia of the cerebral cortex in epilepsy. J Neurol Neurosurg Psychiatry. 1971;34:369–387. doi: 10.1136/jnnp.34.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blumcke I, Thom M, Aronica E, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–174. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sims J. On Hypertrophy and Atrophy of the Brain. Med Chir Trans. 1835;19:315–380. doi: 10.1177/095952873501900120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bast T, Ramantani G, Seitz A, Rating D. Focal cortical dysplasia: prevalence, clinical presentation and epilepsy in children and adults. Acta Neurol Scand. 2006;113:72–81. doi: 10.1111/j.1600-0404.2005.00555.x. [DOI] [PubMed] [Google Scholar]
- 85.Di Rocco C, Battaglia D, Pietrini D, Piastra M, Massimi L. Hemimegalencephaly: clinical implications and surgical treatment. Childs Nerv Syst. 2006;22:852–866. doi: 10.1007/s00381-006-0149-9. [DOI] [PubMed] [Google Scholar]
- 86.Maynard LM, Leach JL, Horn PS, et al. Epilepsy prevalence and severity predictors in MRI-identified focal cortical dysplasia. Epilepsy Res. 2017;132:41–49. doi: 10.1016/j.eplepsyres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 87.Gaitanis JN, Donahue J. Focal cortical dysplasia. Pediatr Neurol. 2013;49:79–87. doi: 10.1016/j.pediatrneurol.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 88.Sasaki M, Hashimoto T, Furushima W, et al. Clinical aspects of hemimegalencephaly by means of a nationwide survey. J Child Neurol. 2005;20:337–341. doi: 10.1177/08830738050200041201. [DOI] [PubMed] [Google Scholar]
- 89.Baybis M, Yu J, Lee A, et al. mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann Neurol. 2004;56:478–487. doi: 10.1002/ana.20211. [DOI] [PubMed] [Google Scholar]
- 90.Miyata H, Chiang AC, Vinters HV. Insulin signaling pathways in cortical dysplasia and TSC-tubers: tissue microarray analysis. Ann Neurol. 2004;56:510–519. doi: 10.1002/ana.20234. [DOI] [PubMed] [Google Scholar]
- 91.Ljungberg MC, Bhattacharjee MB, Lu Y, et al. Activation of mammalian target of rapamycin in cytomegalic neurons of human cortical dysplasia. Ann Neurol. 2006;60:420–429. doi: 10.1002/ana.20949. [DOI] [PubMed] [Google Scholar]
- 92.Lim JS, Kim WI, Kang HC, et al. Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy. Nat Med. 2015;21:395–400. doi: 10.1038/nm.3824. [DOI] [PubMed] [Google Scholar]
- 93.Jansen LA, Mirzaa GM, Ishak GE, et al. PI3K/AKT pathway mutations cause a spectrum of brain malformations from megalencephaly to focal cortical dysplasia. Brain. 2015;138:1613–1628. doi: 10.1093/brain/awv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salinas V, Vega P, Piccirilli MV, et al. Identification of a somatic mutation in the RHEB gene through high depth and ultra-high depth next generation sequencing in a patient with Hemimegalencephaly and drug resistant Epilepsy. Eur J Med Genet. 2019;62:103571. [DOI] [PubMed]
- 95.Zhao S, Li Z, Zhang M, et al. A brain somatic RHEB doublet mutation causes focal cortical dysplasia type II. Exp Mol Med. 2019;51:1–11. doi: 10.1038/s12276-019-0277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baulac S, Ishida S, Marsan E, et al. Familial focal epilepsy with focal cortical dysplasia due to DEPDC5 mutations. Ann Neurol. 2015;77:675–683. doi: 10.1002/ana.24368. [DOI] [PubMed] [Google Scholar]
- 97.D’Gama AM, Geng Y, Couto JA, et al. Mammalian target of rapamycin pathway mutations cause hemimegalencephaly and focal cortical dysplasia. Ann Neurol. 2015;77:720–725. doi: 10.1002/ana.24357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lim JS, Gopalappa R, Kim SH, et al. Somatic Mutations in TSC1 and TSC2 Cause Focal Cortical Dysplasia. Am J Hum Genet. 2017;100:454–472. doi: 10.1016/j.ajhg.2017.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schick V, Majores M, Engels G, et al. Activation of Akt independent of PTEN and CTMP tumor-suppressor gene mutations in epilepsy-associated Taylor-type focal cortical dysplasias. Acta Neuropathol. 2006;112:715–725. doi: 10.1007/s00401-006-0128-y. [DOI] [PubMed] [Google Scholar]
- 100.Sim JC, Scerri T, Fanjul-Fernandez M, et al. Familial cortical dysplasia caused by mutation in the mammalian target of rapamycin regulator NPRL3. Ann Neurol. 2016;79:132–137. doi: 10.1002/ana.24502. [DOI] [PubMed] [Google Scholar]
- 101.Weckhuysen S, Marsan E, Lambrecq V, et al. Involvement of GATOR complex genes in familial focal epilepsies and focal cortical dysplasia. Epilepsia. 2016;57:994–1003. doi: 10.1111/epi.13391. [DOI] [PubMed] [Google Scholar]
- 102.Lee WS, Stephenson SEM, Howell KB, et al. Second-hit DEPDC5 mutation is limited to dysmorphic neurons in cortical dysplasia type IIA. Ann Clin Transl Neurol. 2019;6:1338–1344. doi: 10.1002/acn3.50815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baek ST, Copeland B, Yun EJ, et al. An AKT3-FOXG1-reelin network underlies defective migration in human focal malformations of cortical development. Nat Med. 2015;21:1445–1454. doi: 10.1038/nm.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roy A, Skibo J, Kalume F, et al. Mouse models of human PIK3CA-related brain overgrowth have acutely treatable epilepsy. Elife. 2015;4. [DOI] [PMC free article] [PubMed]
- 105.Ljungberg MC, Sunnen CN, Lugo JN, Anderson AE, D’Arcangelo G. Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Dis Model Mech. 2009;2:389–398. doi: 10.1242/dmm.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nguyen LH, Brewster AL, Clark ME, et al. mTOR inhibition suppresses established epilepsy in a mouse model of cortical dysplasia. Epilepsia. 2015;56:636–646. doi: 10.1111/epi.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou J, Blundell J, Ogawa S, et al. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marsan E, Ishida S, Schramm A, et al. Depdc5 knockout rat: A novel model of mTORopathy. Neurobiol Dis. 2016;89:180–189. doi: 10.1016/j.nbd.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 109.Ribierre T, Deleuze C, Bacq A, et al. Second-hit mosaic mutation in mTORC1 repressor DEPDC5 causes focal cortical dysplasia-associated epilepsy. J Clin Invest. 2018;128:2452–2458. doi: 10.1172/JCI99384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yuskaitis CJ, Jones BM, Wolfson RL, et al. A mouse model of DEPDC5-related epilepsy: Neuronal loss of Depdc5 causes dysplastic and ectopic neurons, increased mTOR signaling, and seizure susceptibility. Neurobiol Dis. 2018;111:91–101. doi: 10.1016/j.nbd.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yuskaitis CJ, Rossitto LA, Gurnani S, Bainbridge E, Poduri A, Sahin M. Chronic mTORC1 inhibition rescues behavioral and biochemical deficits resulting from neuronal Depdc5 loss in mice. Hum Mol Genet. 2019;28:2952–2964. doi: 10.1093/hmg/ddz123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu Q, Uliel-Sibony S, Dunham C, et al. mTOR Inhibitors as a New Therapeutic Strategy in Treatment Resistant Epilepsy in Hemimegalencephaly: A Case Report. J Child Neurol. 2019;34:132–138. doi: 10.1177/0883073818813238. [DOI] [PubMed] [Google Scholar]
- 113.Mingarelli A, Vignoli A, La Briola F, et al. Dramatic relapse of seizures after everolimus withdrawal. Eur J Paediatr Neurol. 2018;22:203–206. doi: 10.1016/j.ejpn.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 114.Davies M, Saxena A, Kingswood JC. Management of everolimus-associated adverse events in patients with tuberous sclerosis complex: a practical guide. Orphanet J Rare Dis. 2017;12:35. doi: 10.1186/s13023-017-0581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Iffland PH, 2nd, Carson V, Bordey A, Crino PB. GATORopathies: The role of amino acid regulatory gene mutations in epilepsy and cortical malformations. Epilepsia. 2019;60:2163–2173. doi: 10.1111/epi.16370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hillmann P, Fabbro D. PI3K/mTOR Pathway Inhibition: Opportunities in Oncology and Rare Genetic Diseases. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed]
- 117.Jesus-Ribeiro J, Pires LM, Melo JD, et al. Genomic and Epigenetic Advances in Focal Cortical Dysplasia Types I and II: A Scoping Review. Front Neurosci. 2020;14:580357. [DOI] [PMC free article] [PubMed]
- 118.Winawer MR, Griffin NG, Samanamud J, et al. Somatic SLC35A2 variants in the brain are associated with intractable neocortical epilepsy. Ann Neurol. 2018;83:1133–1146. doi: 10.1002/ana.25243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baldassari S, Ribierre T, Marsan E, et al. Dissecting the genetic basis of focal cortical dysplasia: a large cohort study. Acta Neuropathol. 2019;138:885–900. doi: 10.1007/s00401-019-02061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bonduelle T, Hartlieb T, Baldassari S, et al. Frequent SLC35A2 brain mosaicism in mild malformation of cortical development with oligodendroglial hyperplasia in epilepsy (MOGHE) Acta Neuropathol Commun. 2021;9:3. doi: 10.1186/s40478-020-01085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schurr J, Coras R, Rossler K, et al. Mild Malformation of Cortical Development with Oligodendroglial Hyperplasia in Frontal Lobe Epilepsy: A New Clinico-Pathological Entity. Brain Pathol. 2017;27:26–35. doi: 10.1111/bpa.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sim NS, Seo Y, Lim JS, et al. Brain somatic mutations in SLC35A2 cause intractable epilepsy with aberrant N-glycosylation. Neurol Genet. 2018;4:e294. [DOI] [PMC free article] [PubMed]
- 123.Dorre K, Olczak M, Wada Y, et al. A new case of UDP-galactose transporter deficiency (SLC35A2-CDG): molecular basis, clinical phenotype, and therapeutic approach. J Inherit Metab Dis. 2015;38:931–940. doi: 10.1007/s10545-015-9828-6. [DOI] [PubMed] [Google Scholar]
- 124.Demos M, Guella I, DeGuzman C, et al. Diagnostic Yield and Treatment Impact of Targeted Exome Sequencing in Early-Onset Epilepsy. Front Neurol. 2019;10:434. doi: 10.3389/fneur.2019.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Orlova KA, Parker WE, Heuer GG, et al. STRADalpha deficiency results in aberrant mTORC1 signaling during corticogenesis in humans and mice. J Clin Invest. 2010;120:1591–1602. doi: 10.1172/JCI41592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Puffenberger EG, Strauss KA, Ramsey KE, et al. Polyhydramnios, megalencephaly and symptomatic epilepsy caused by a homozygous 7-kilobase deletion in LYK5. Brain. 2007;130:1929–1941. doi: 10.1093/brain/awm100. [DOI] [PubMed] [Google Scholar]
- 127.Zeqiraj E, Filippi BM, Deak M, Alessi DR, van Aalten DM. Structure of the LKB1-STRAD-MO25 complex reveals an allosteric mechanism of kinase activation. Science. 2009;326:1707–1711. doi: 10.1126/science.1178377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Parker WE, Orlova KA, Parker WH, et al. Rapamycin prevents seizures after depletion of STRADA in a rare neurodevelopmental disorder. Sci Transl Med. 2013;5:182ra53. [DOI] [PMC free article] [PubMed]
- 129.Ye Z, Chatterton Z, Pflueger J, et al. Cerebrospinal fluid liquid biopsy for detecting somatic mosaicism in brain. Brain Commun. 2021;3:fcaa235. [DOI] [PMC free article] [PubMed]
- 130.Kim S, Baldassari S, Sim NS, et al. Detection of Brain Somatic Mutations in Cerebrospinal Fluid from Refractory Epilepsy Patients. Ann Neurol. 2021. [DOI] [PubMed]
- 131.Jeong A, Wong M. Targeting the Mammalian Target of Rapamycin for Epileptic Encephalopathies and Malformations of Cortical Development. J Child Neurol. 2018;33:55–63. doi: 10.1177/0883073817696814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Curatolo P, Moavero R. mTOR inhibitors as a new therapeutic option for epilepsy. Expert Rev Neurother. 2013;13:627–638. doi: 10.1586/ern.13.49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.