Abstract
Genetic knockout or knockdown of fat-mass and obesity-associated protein (FTO), a demethylase that participates in RNA N6-methyladenosine modification in injured dorsal root ganglion (DRG), has been demonstrated to alleviate nerve trauma-induced nociceptive hypersensitivities. However, these genetic strategies are still impractical in clinical neuropathic pain management. The present study sought to examine the effect of intrathecal administration of two specific FTO inhibitors, meclofenamic acid (MA) and N-CDPCB, on the development and maintenance of nociceptive hypersensitivities caused by unilateral L5 spinal nerve ligation (SNL) in rats. Intrathecal injection of either MA or N-CDPCB diminished dose-dependently the SNL-induced mechanical allodynia, heat hyperalgesia, cold hyperalgesia, and spontaneous ongoing nociceptive responses in both development and maintenance periods, without altering acute/basal pain and locomotor function. Intrathecal MA also reduced the SNL-induced neuronal and astrocyte hyperactivities in the ipsilateral L5 dorsal horn. Mechanistically, intrathecal injection of these two inhibitors blocked the SNL-induced increase in the histone methyltransferase G9a expression and rescued the G9a-controlled downregulation of mu opioid receptor and Kv1.2 proteins in the ipsilateral L5 DRG. These findings further indicate the role of DRG FTO in neuropathic pain and suggest potential clinical application of the FTO inhibitors for management of this disorder.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-021-01053-2.
Key Words: FTO, Meclofenamic acid, N-CDPCB, Intrathecal injection, G9a, Mu opioid receptor, Kv1.2, Dorsal root ganglion, Neuropathic pain
Introduction
Peripheral neuropathic pain, resulting from nerve trauma, is a debilitating clinical condition with both medical and socioeconomic consequences [1]. Several categories of medication are currently used for management of this disorder, including acetaminophen, antidepressants, topical agents, non-steroidal anti-inflammatory drugs, antiepileptic drugs (e.g., gabapentinoids), and opioids [2]. However, the majority of neuropathic pain patients report unsatisfactory pain control and/or severe side effects [3]. Understanding the molecular and cellular mechanisms that underlie the induction and maintenance of neuropathic pain may provide a new potential approach for treatment of this disorder.
Peripheral nerve trauma leads to alterations in pain-associated gene expression at both transcriptional and translational levels in the dorsal root ganglion (DRG) [4, 5]. These alterations are believed to participate in neuropathic pain development and maintenance [6–8]. Recent evidence has demonstrated that epitranscriptomic modifications including RNA N6-methyladenosine (m6A) modification influence the changes in the expression of pain-associated genes in injured DRG, contributing to neuropathic pain genesis [9–11]. The fat-mass and obesity-associated protein (FTO), a demethylase, erases m6A methylation in mRNA [12]. Peripheral nerve trauma increased the expression of FTO in injured DRG neurons [10]. This increase catalyzed reversal of m6A in euchromatic histone lysine methyltransferase 2 (Ehmt2) mRNA, reduced the binding of YTH m6A RNA binding protein 2 to 3′-untranslated region of Ehmt2 mRNA, stabilized the increased expression of Ehmt2 mRNA and its coding protein G9a, and downregulated opioid receptors and Kv1.2 in injured DRG [10]. Genetic knockdown or knockout of FTO in injured DRG alleviated nerve trauma-induced nociceptive hypersensitivities by reversing a loss of m6A sites in Ehmt2 mRNA, destabilizing the increased Ehmt2 mRNA/G9a and subsequently rescuing nerve trauma-induced downregulation of opioid receptors and Kv1.2 in injured DRG [10]. FTO is likely a potential new target for neuropathic pain management.
Given that the application of a genetic strategy is still impractical in clinical neuropathic pain management, the present study examined the effect of intrathecal administration of two specific FTO inhibitors, N-CDPCB [13] and meclofenamic acid (MA) [14, 15], on unilateral fifth lumbar (L5) spinal nerve ligation (SNL)-induced nociceptive hypersensitivity during the development and maintenance periods. We also examined the effect of intrathecal FTO inhibitors on the SNL-induced neuronal and astrocyte hyperactivities in the ipsilateral L5 dorsal horn. Finally, we observed whether intrathecal FTO inhibitors affected the SNL-induced increase of G9a and downregulation of mu opioid receptor (MOR) and Kv1.2 in injured DRG.
Materials and Methods
Animal Preparation
Male Sprague–Dawley rats weighing 180–250 g from Charles River were used. Rats were housed separately with food and water provided ad libitum on a 12:12-h light–dark cycle. All animal experimental procedures followed ethical guidelines produced by the National Institutes of Health, the International Association for the Study of Pain, and the guidelines for Animal Research: Reporting of In Vivo Experiments (ARRIVE). Animal protocols were approved by the Rutgers New Jersey Medical School Animal Care and Use Committee. Every effort was made to minimize the number of animals used and any subsequent suffering. Animals were randomly assigned to experimental groups (n = 6 rats/group) and exposed to habituation for 2 days prior to use. All experimenters were blind to treatment condition.
Neuropathic Pain Model
L5 SNL-induced neuropathic pain model was carried out as described previously [10, 16, 17]. In brief, after the animals were anesthetized by isoflurane, an incision on the lower back was made and the left L6 transverse process was removed to expose the L5 spinal nerve. After the underlying L5 spinal nerve was isolated, a tight ligature was then made with 4–0 silk and the nerve was transected distal to this ligature. The skin and muscles were finally closed in layers. In sham-operated rats, the left L5 spinal nerve was isolated, but without the ligature and/or transection.
Intrathecal Catheterization and Drug Delivery
Intrathecal catheterization was performed following the procedures described previously [18, 19]. Briefly, after rats were fully anesthetized with 2% isoflurane, a 1-cm longitudinal incision was made over the spinous processes of L4 and L5 vertebrae. The fascia was opened, and superficial muscles around the spinous process were dissected. The dura was carefully punctured using a 22-gauge needle, and a polyethylene-10 (PE-10) catheter was inserted into the subarachnoid space. The residual catheter was anchored to the muscles and tunneled under the skin to the neck area. The catheter location was confirmed by the administration of 10 μl of 2% lidocaine 2 days after the surgery. Only rats that showed complete paralysis of the tail and bilateral hind legs within 30 s followed by total recovery in 30 min after lidocaine injection were considered to have a successful catheter insertion. Seven days following catheter implantation, rats without any locomotor deficits or poor grooming habits were used for the experiments.
The MA (Millipore-Sigma, St. Louis, MO) was dissolved in 0.01 M phosphate-buffered saline (PBS), while N-CDPCB (kindly provided by Dr. Junbiao Chang from Zhengzhou University) was dissolved in 10% DMSO. Vehicle 1 (0.01 M PBS), vehicle 2 (10% DMSO, dissolved in 0.01 M PBS), MA, or N-CDPCB was intrathecally injected 30 min before surgery and once daily for 5 days post-surgery, or once daily for 5 days starting on post-operative day 9. The drugs or vehicles were administrated intrathecally in a 10-μl volume followed by 12 μl of saline for flushing. Intrathecal dosages of the drugs were determined by the previous reports [20, 21] and our pilot study.
Behavioral Tests
Mechanical, heat, and cold tests, as well as locomotor function test and conditioned place preference (CPP) test were carried out as described [10, 16, 17]. There was a 1-h interval between two tests.
To assess mechanical sensitivity, paw withdrawal thresholds were determined as the hind paw withdrawal response to von Frey hair stimulation using the up-and-down method, following previously described procedure [22, 23]. In brief, each rat was placed in an individual Plexiglas chamber on an elevated mesh screen. Von Frey hairs (Stoelting Co., Wood Dale, IL) in log increments of force (0.407, 0.692, 1.202, 2.041, 3.63, 5.495, 8.511, 15.14, 26.0 g) were applied to the plantar surface of the left and right hind paws, beginning with the 3.63-g Von Frey hair. A positive response was defined as a clear paw withdrawal or shaking. Whenever a positive response occurred, the next lower hair was applied. In contrast, whenever a negative response occurred, the next higher hair was applied. The test was terminated under either one of two following conditions: i) a negative response was obtained with the highest force (26.0 g) and ii) three stimuli were applied after the first positive response. The pattern of response was converted to a 50% von Frey threshold, using the formula described previously [22, 23].
Heat hypersensitivity was determined by measuring the paw withdrawal latency with a Model 336 Analgesia Meter (IITC Inc. Life Science Instruments. Woodland Hills, CA) according to the method described [10, 16, 17]. Briefly, the animal was placed in an individual Plexiglas chamber on a glass plate. A beam of light that provided radiant heat was aimed at the middle of the plantar surface of each hind paw through the glass plate. The light beam was automatically turned off when the animal withdrew its foot. The paw withdrawal latency was defined as the time (s) between the start of the light beam and the foot response. The cutoff time is 20 s to avoid tissue damage. Each trial was repeated five times at 5-min interval for each side.
Paw withdrawal latencies to noxious cold (0 °C) were measured with a cold aluminum plate, which was monitored continuously by a thermometer. The paw withdrawal latency was recorded by the length of time between the placement of the animals on the plate and a quick paw withdrawal with or without paw flinching/shaking. Each test was repeated three times at 10-min intervals on the ipsilateral side. To avoid tissue damage, a cut-off time of 60 s was used.
The locomotor function was examined after above-described behavioral tests and determined by the following three reflexes: 1) Placing reflex: The placed positions of the hind limbs were slightly lower than those of the forelimbs and the dorsal surfaces of the hind paws were brought into contact with the edge of a table. Whether the hind paws were placed on the table surface reflexively were recorded. 2) Grasping reflex: After the animal was placed on a wire grid, whether the hind paws grasped the wire on contact were recorded. 3) Righting reflex: When the animal was placed on its back on a flat surface, whether it immediately assumed the normal upright position was recorded. Each trial was repeated five times with 5-min intervals and the scores for placing, grasping, and righting reflexes were recorded based on counts of each normal reflex exhibited in five trials. In addition, the animal’s general behaviors, including spontaneous activity, were observed.
The CPP test was carried out as described previously [24]. In brief, the rats were first preconditioned with full access to two different Plexiglas chambers connected through an internal door (MED Associates Inc., St. Albans, VT) for 30 min. The photobeam detectors installed along the chamber walls monitored and automatically recorded the movement of each animal and time spent in each chamber using MED-PC IV CPP software. At the end of the preconditioning phase, the basal duration time spent in each chamber was recorded within 15 min (900 s). Rats spending greater than 80% or less than 20% of the time in a chamber were excluded from further testing. The conditioning protocol was performed for the following 3 days with the internal door closed. The rat was injected intrathecally with saline (10 μl) paired with one conditioning chamber in the early morning. The lidocaine (0.8% in 10 μl saline) was injected intrathecally paired with another conditioning chamber in the later afternoon. The injection order of saline and lidocaine was switched each consecutive day. On testing day, the rats were placed in one chamber with free access to both chambers. The length of time spent in each chamber was recorded for 15 min. Difference in scores were defined as post-conditioning time subtracted from preconditioning time spent in the lidocaine-paired chamber.
Western Blot Analysis
Western blot analysis was performed according to our previous published procedures [10, 16, 17]. Briefly, the bilateral L5 DRGs and ipsilateral L5 spinal dorsal horn were harvested and placed temporarily in liquid nitrogen. To achieve enough proteins, two ipsilateral L5 DRGs from 2 rats were pooled together. The samples were homogenized with ice-cold lysis buffer (10 mM Tris, 1 mM phenylmethylsulfonyl fluoride, 5 mM MgCl2, 5 mM EGTA, 1 mM EDTA, 1 mM DTT, 40 μM leupeptin, 250 mM sucrose, and 1% phosphatase inhibitor cocktail II and III) and centrifuged at 4 °C for 15 min at 1,000 g. The supernatant was collected for cytosolic proteins and the pellets for nuclear proteins. After the protein concentrations were measured, the samples (30–40 μg/sample) were heated for 5 min at 99 °C and loaded onto a 4–15% stacking/7.5% separating SDS–polyacrylamide gel (Bio-Rad Laboratories). The proteins were then electrophoretically transferred onto a nitrocellulose membrane (Bio-Rad Laboratories). After the membranes were blocked with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 for 1 h, they were incubated at 4 °C overnight with the following primary antibodies: mouse anti-FTO (1:1000, Abcam, Cambridge, MA), rabbit anti-G9a (1:800, Abcam), mouse anti-Kv1.2 (1:800, NeuroMab, Davis, CA), rabbit anti-MOR (1:1,000, Immunostar), rabbit anti-phospho-ERK1/2 (Thr202/Tyr204, 1:1,000, Cell Signaling), rabbit anti-ERK1/2 (1:1,000, Cell Signaling), mouse anti-GFAP (1:1,000, Cell Signaling), rabbit anti-GAPDH (1:2,000, Santa Cruz, Dallas, TX), or rabbit anti-histone H3 (1:2,000, Cell Signaling). The proteins were detected by horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibody (1:3,000, Jackson ImmunoResearch), developed by western peroxide reagent and luminol/enhancer reagent (Clarity Western ECL Substrate, Bio-Rad), and visualized by ChemiDoc XRS and System with Image Lab software (Bio-Rad). The intensity of blots was quantified with densitometry using Image Lab software (Bio-Rad). All cytosol protein bands were normalized to H3 or GAPDH.
Statistical Analysis
All data values were expressed as mean ± S.E.M. Although no statistical power calculation was conducted, the sample size of each experimental group was based on our previous similar studies and was similar to those generally used in the field [10, 18, 19]. The data were statistically analyzed with one-way or two-way analysis of variance (ANOVA) with repeated measures followed by post hoc Tukey testing (SigmaStat, San Jose, CA). The Mann–Whitney U-test was used for non-parametric data (SigmaStat). Significance was set at P < 0.05.
Results
Effect of Intrathecal FTO Inhibitors on the Development of Nerve Trauma-Induced Nociceptive Hypersensitivities
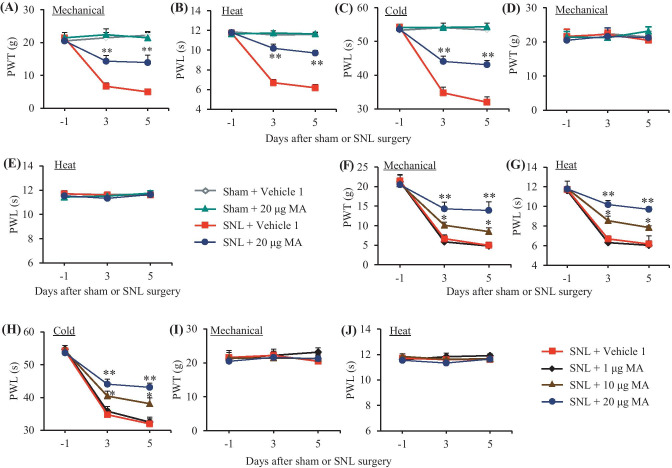
We first examined the effect of intrathecal MA on the development of SNL-induced nociceptive hypersensitivities. Consistent with our previous studies [10, 17, 24], SNL produced long-term mechanical allodynia, heat hyperalgesia, and cold hyperalgesia on the ipsilateral, but not contralateral, side in the vehicle 1-injected SNL rats (Fig. 1A–E). The paw withdrawal thresholds in response to von Frey filament stimulation and the paw withdrawal latencies in response to heat and cold stimuli were markedly decreased on days 3 and 5 post-SNL (Fig. 1A–C). These nociceptive hypersensitivities were attenuated by pre-intrathecal injection of MA, in a dose-dependent fashion (Fig. 1A–C, F–H). Intrathecal MA at both 10 and 20 μg significantly reversed the SNL-induced decreases in paw withdrawal thresholds to mechanical stimuli and paw withdrawal latencies to heat and cold stimuli on the ipsilateral side (Fig. 1A–C, F–H). Intrathecal MA at 1 μg did not affect the SNL-induced decreases in the paw withdrawal thresholds and latencies on the ipsilateral side (Fig. 1F–H). Intrathecal administration of neither MA at three doses used nor vehicle 1 altered basal paw withdrawal responses on the contralateral side in SNL rats and on both ipsilateral and contralateral sides in sham rats (Fig. 1A–J).
Fig. 1.
Effect of intrathecal pre-administration of meclofenamic acid (MA) on the SNL-induced nociceptive hypersensitivities during the development period. MA at three doses (1, 10, and 20 μg) or vehicle 1 (0.01 M PBS) was administered intrathecally (i.th.) starting 30 min before SNL or sham surgery and once daily for 5 days after surgery. Behavioral tests were performed one day before surgery and on days 3 and 5 after surgery. Effect of 20 µg MA or vehicle 1 on paw withdrawal threshold (PWT) to mechanical stimulation (A and D) and paw withdrawal latency (PWL) to heat (B and E) and cold (C) stimuli on the ipsilateral (A–C) and contralateral (D and E) sides. n = 6 rats per group. Two-way ANOVA with repeated measures followed by post hoc Tukey test, Fgroup (3, 60) = 33.26 in (A), 131.5 in (B) and 98.4 in (C), **P < 0.01 versus the vehicle 1-treated SNL rats at the corresponding time point. Effect of MA at 1, 10, and 20 µg or vehicle 1 on paw withdrawal threshold (PWT) to mechanical stimulation (F and I) and paw withdrawal latency (PWL) to heat (G and J) and cold (H) stimuli on the ipsilateral (F–H) and contralateral (I and J) sides. n = 6 rats per group. Two-way ANOVA with repeated measures followed by post hoc Tukey test, Fgroup (3, 60) = 11.47 in (F), 50.96 in (G), and 14.85 in (H), *P < 0.05, **P < 0.01 versus the vehicle 1-treated SNL rats at the corresponding time points
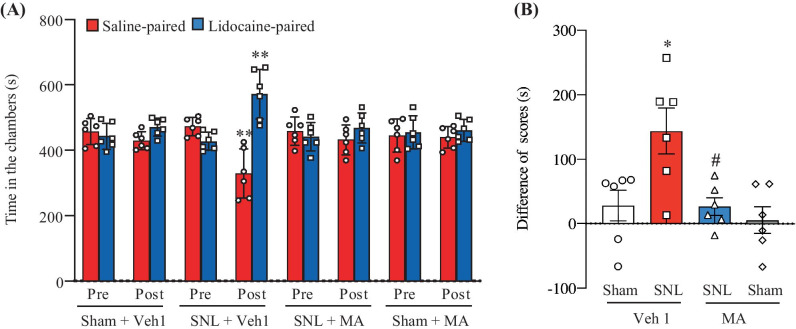
In addition to stimulation-induced evoked nociceptive hypersensitivities, we also examined the effect of intrathecal MA on the SNL-induced spontaneous ongoing nociceptive responses using the CPP paradigm. As expected, the vehicle 1-injected SNL rats exhibited an obvious preference (that is, they spent more time) for the lidocaine-paired chamber on day 5 post-SNL (Fig. 2A, B), indicating stimulation-independent spontaneous nociceptive responses. In contrast, SNL rats pre-injected intrathecally with 20 µg MA, like the vehicle 1- or 20-µg MA-injected sham rats, did not display significant preference toward either the saline- or lidocaine-paired chamber on day 5 post-SNL, indicating no marked spontaneous nociceptive responses (Fig. 2A, B).
Fig. 2.
Effect of intrathecal pre-administration of meclofenamic acid (MA; 20 µg) on the SNL-induced spontaneous ongoing nociceptive responses as assessed by the conditioned place preference paradigm. (A) Time spent in each chamber. (B) Difference in scores for chamber preference were calculated by subtracting preconditioning preference time from post-conditioning time spent in the lidocaine-paired chamber. Pre: preconditioning, Post: post-conditioning, Veh1: vehicle 1. n = 6 rats per group. (A) Two-way ANOVA with repeated measures followed by post hoc Tukey test, Ftraining (1, 40) = 15.14, **P < 0.01 versus the corresponding preconditioning. (B) Two-way ANOVA with repeated measures followed by post hoc Tukey test, Fmodel (1, 20) = 7.67, *P < 0.05 versus the vehicle 1-treated sham group, Ftreatment (1, 20) = 7.80, #P < 0.05 versus the vehicle 1-treated SNL rats
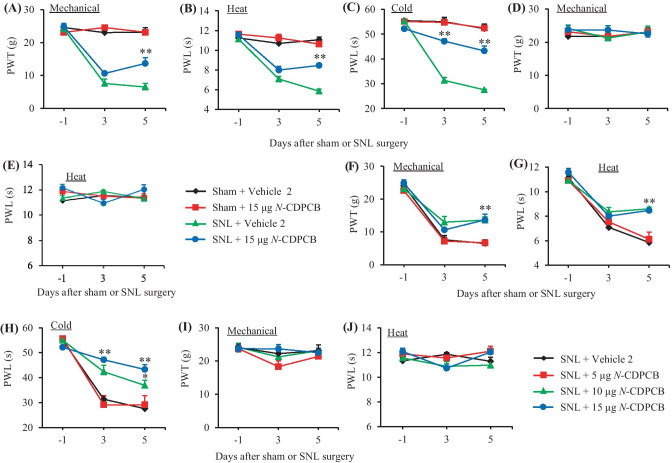
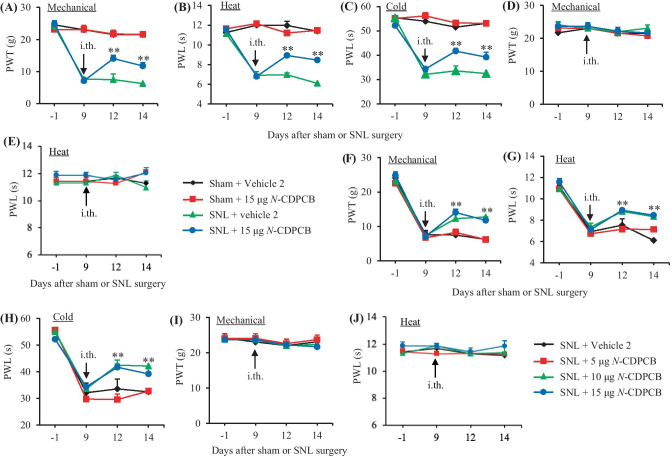
MA also has an anti-inflammatory property [14, 15]. To further verify the pharmacological effect of the FTO inhibitors on neuropathic pain development, we intrathecally pre-injected another FTO inhibitor, N-CDPCB, in SNL rats. Similar behavioral responses were seen after intrathecal injection of N-CDPCB at three doses (5, 10, and 15 µg) or vehicle 2 on days 3 and 5 post-SNL or sham surgery (Fig. 3A–J).
Fig. 3.
Effect of intrathecal pre-administration of N-CDPCB on the SNL-induced nociceptive hypersensitivities during the development period. N-CDPCB at three doses (5, 10, and 15 μg) or vehicle 2 (10% DMSO) was administered intrathecally (i.th.) starting 30 min before SNL or sham surgery and once daily for 5 days after surgery. Behavioral tests were performed one day before surgery and on days 3 and 5 after surgery. Effect of 15 µg N-CDPCB or vehicle 2 on paw withdrawal threshold (PWT) to mechanical stimulation (A and D) and paw withdrawal latency (PWL) to heat (B and E) and cold (C) stimuli on the ipsilateral (A–C) and contralateral (D and E) sides. n = 6 rats per group. Two-way ANOVA with repeated measures followed by post hoc Tukey test, Fgroup (3, 60) = 67.51 in (A), 98.40 in (B), and 137.50 in (C). **P < 0.01 versus the vehicle 2-treated SNL rats at the corresponding time point. Effect of N-CDPCB at 5, 10, and 15 µg or vehicle 2 on paw withdrawal threshold (PWT) to mechanical stimulation (F and I) and paw withdrawal latency (PWL) to heat (G and J) and cold (H) stimuli on the ipsilateral (F–H) and contralateral (I and J) sides. n = 6 rats per group. Two-way ANOVA with repeated measures followed by post hoc Tukey test, Fgroup (3, 60) = 4.50 in (F), 13.09 in (G), and 25.52 in (H). *P < 0.05, **P < 0.01 versus the vehicle 2-treated SNL rats at the corresponding time points
Effect of Intrathecal FTO Inhibitors on the Maintenance of Nerve Trauma-Induced Nociceptive Hypersensitivities
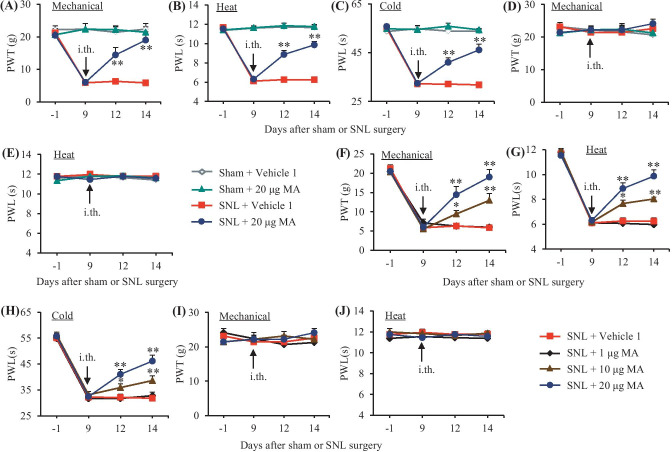
We further examined the effect of intrathecal MA on the maintenance of SNL-induced mechanical, thermal, and cold nociceptive hypersensitivities. MA was intrathecally administered once daily for 5 days starting on day 9 post-surgery, at this time point, SNL-induced nociceptive hypersensitivities were completely developed [10, 17, 24]. On days 12 and 14 post-SNL, mechanical allodynia, heat hyperalgesia, and cold hyperalgesia were dose-dependently reduced by intrathecal MA (Fig. 4A–D, F–H). The paw withdrawal thresholds to mechanical stimuli and paw withdrawal latencies to heat and cold stimuli were markedly higher in the 10 µg or 20 µg MA-injected rats than those in the vehicle 1-injected rats on the ipsilateral side on days 12 and 14 post-SNL (Fig. 4A–D, F–H). In contrast, intrathecal MA at 1 µg did not affect the SNL-induced decreases in paw withdrawal thresholds to mechanical stimuli and paw withdrawal latencies to heat and cold stimuli on days 12 and 14 post-SNL on the ipsilateral side (Fig. 4F–H). Neither MA at the dosages used nor vehicle 1 altered basal paw withdrawal responses on the contralateral side of SNL rats and on the bilateral sides of sham rats during the observation periods (Fig. 4A–H). Similar behavioral responses were seen on days 12 and 14 post-SNL or sham surgery after intrathecal injection of N-CDPCB at three doses or vehicle 2 once daily starting on day 9 post-surgery (Fig. 5A–J).
Fig. 4.
Effect of intrathecal post-administration of meclofenamic acid (MA) on the SNL-induced nociceptive hypersensitivities during the maintenance period. MA at three doses (1, 10, and 20 μg) or vehicle 1 (0.01 M PBS) was administered intrathecally (i.th.) once daily for 5 days starting on day 9 post-surgery. Behavioral tests were performed one day before surgery, prior to MA or vehicle 1 injection on day 9 post-surgery and on days 12 and 14 post-surgery. Effect of 20 µg MA or vehicle 1 on paw withdrawal threshold (PWT) to mechanical stimulation (A and D) and paw withdrawal latencies (PWL) to heat (B and E) and cold (C) stimuli on the ipsilateral (A–C) and contralateral (D and E) sides. n = 6 rats per group. Two-way ANOVA with repeated measures followed by post hoc Tukey test, Fgroup (3, 80) = 63.95 in (A), 294.9 in (B), and 164.3 in (C), **P < 0.01 versus the vehicle 1-treated SNL rats at the corresponding time point. Effect of MA at 1, 10, and 20 µg or vehicle 1 on paw withdrawal threshold (PWT) to mechanical stimulation (F and I) and paw withdrawal latencies (PWL) to heat (G and J) and cold (H) stimuli on the ipsilateral (F–H) and contralateral (I and J) sides. n = 6 rats per group. Two-way ANOVA with repeated measures followed by post hoc Tukey test, Fgroup (3, 80) = 18.5 in (F), 31.67 in (G), and 16.98 in (H), *P < 0.05 or **P < 0.01 versus the vehicle 1-treated SNL rats at the corresponding time points
Fig. 5.
Effect of intrathecal post-administration of N-CDPCB on the SNL-induced nociceptive hypersensitivities during the maintenance period. N-CDPCB at three doses (5, 10, and 15 μg) or vehicle 2 (10% DMSO) was administered intrathecally (i.th.) once daily for 5 days starting on day 9 post-surgery. Behavioral tests were performed one day before surgery, prior to N-CDPCB or vehicle 2 injection on day 9 post-surgery and on days 12 and 14 post-surgery. Effect of 15 µg N-CDPCB or vehicle 2 on paw withdrawal threshold (PWT) to mechanical stimulation (A and D), paw withdrawal latencies (PWL) to heat (B and E) and cold (C) stimuli on the ipsilateral (A–C) and contralateral (D and E) sides. n = 6 rats per group. Two-way ANOVA with repeated measures followed by post hoc Tukey test, Fgroup (3, 80) = 118.50 in (A), 172.10 in (B), and 329.10 in (C). **P < 0.01 versus the vehicle 2-treated SNL rats at the corresponding time point. Effect of N-CDPCB at 5, 10, and 15 µg or vehicle 2 on paw withdrawal threshold (PWT) to mechanical stimulation (F and I) and paw withdrawal latencies (PWL) to heat (G and J) and cold (H) stimuli on the ipsilateral (F–H) and contralateral (I and J) sides. n = 6 rats per group. Two-way ANOVA with repeated measures followed by post hoc Tukey test, Fgroup (3, 80) = 4.171 in (F), 17.56 in (G), and 33.80 in (H). **P < 0.01 versus the vehicle 2-treated SNL rats at the corresponding time points
Effect of Intrathecal MA on the SNL-Induced Central Sensitization in Spinal Cord Dorsal Horn
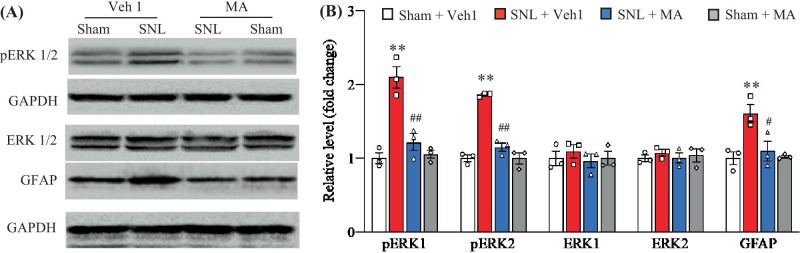
We also examined whether intrathecal injection of MA affected the SNL-induced dorsal horn central sensitization as evidenced by increases in the phosphorylation of extracellular signal regulated kinase 1/2 (p-ERK1/2; a marker for neuronal hyperactivation) and glial fibrillary acidic protein (GFAP; a marker for astrocyte hyperactivation) in the dorsal horn. Consistent with previous studies [25, 26], the amounts of the phosphorylation of ERK1/2 (but not total ERK1/2) and GFAP were significantly increased in the ipsilateral L5 dorsal horn on day 5 after SNL in the vehicle 1-injected SNL rats, but not in the vehicle 1-injected sham rats (Fig. 6A–N). These increases were absent in the SNL rats with pre-intrathecal injection of MA at 20 µg (Fig. 6A, B). Neither MA nor Vehicle 1 changed basal expression of phosphorylation of ERK1/2, total ERK1/2, or GFAP in the ipsilateral L5 dorsal horn of sham rats (Fig. 6A, B).
Fig. 6.
Effect of intrathecal pre-administration of meclofenamic acid (MA; 20 µg) on the SNL-induced dorsal horn central sensitization. The levels of the phosphorylation of ERK1/2 (p-ERK1/2), total ERK1/2 and GFAP in the ipsilateral lumbar 5 dorsal horn on day 5 post-SNL or sham surgery. Representative Western blots (A) and a summary of densitometric analysis (B) are shown. n = 3 biological repeats (6 rats) per group. Two-way ANOVA followed by post hoc Tukey test, Fmodel (1, 8) = 36.63 for p-ERK1, 95.19 for p-ERK2, 0.51 for ERK1, 0.10 for ERK2, and 11.71 for GFAP, **P < 0.01 versus the corresponding vehicle 1 (Veh1)-treated sham group. Ftreatment (1, 8) = 15.60 for p-ERK1, 46.54 for p-ERK2, 0.51 for ERK1, 0.06 for ERK2, and 5.99 for GFAP, #P < 0.05, ##P < 0.01 versus the corresponding vehicle 1-treated SNL group
Effect of Intrathecal FTO Inhibitors on Locomotor Activities
To rule out the possibility that the observed behavioral effects described above were affected by impairing locomotor activities, we examined locomotor functions including grasping, placing, and righting reflexes in the injected rats. MA-, N-CDPCB-, vehicle 1-, and vehicle 2-injected rats displayed normal placing, grasping, and righting reflexes (Table 1). In addition, convulsions and hypermobility were not observed in any of the intrathecal-injected rats. No significant differences in general behaviors, including gait and spontaneous activity, were seen among the intrathecal-injected rats.
Table 1.
Locomotor function
| Treatment groups | Locomotor function test | ||
|---|---|---|---|
| Placing | Grasping | Righting | |
| Sham + vehicle 1 | 5 (0) | 5 (0) | 5 (0) |
| Sham + 20 μg MA | 5 (0) | 5 (0) | 5 (0) |
| SNL + vehicle 1 | 5 (0) | 5 (0) | 5 (0) |
| SNL + 20 μg MA | 5 (0) | 5 (0) | 5 (0) |
| Sham + vehicle 2 | 5 (0) | 5 (0) | 5 (0) |
| Sham + 15 μg N-CDPCB | 5 (0) | 5 (0) | 5 (0) |
| SNL + vehicle 2 | 5 (0) | 5 (0) | 5 (0) |
| SNL + 15 μg N-CDPCB | 5 (0) | 5 (0) | 5 (0) |
MA = meclofenamic acid
n = 6 rats per group. 5 trials. Mean (SEM)
Effect of Intrathecal FTO Inhibitors on the SNL-Induced Increase of G9a and Decreases of Mu Opioid Receptor and Kv1.2 in Injured DRG
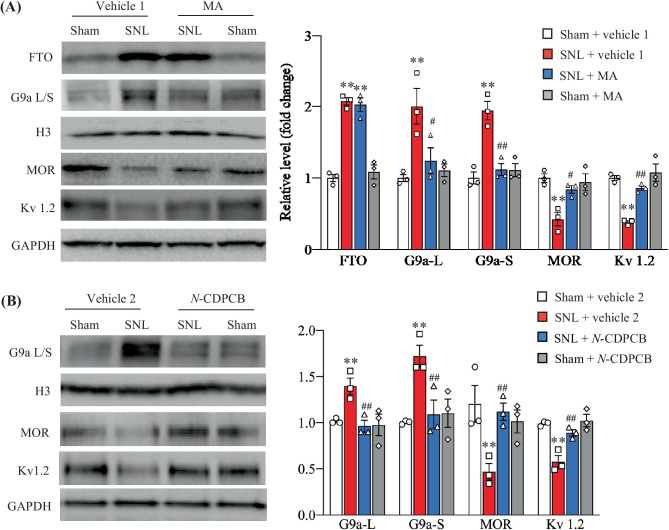
The evidence from our previous study demonstrated that FTO contributed to neuropathic pain through stabilizing nerve trauma-induced upregulation of G9a and subsequently causing the downregulation of mu opioid receptor (MOR) and Kv1.2 in injured DRG [9–11]. Thus, we finally examined whether the antinociceptive effects caused by intrathecal MA or N-CDPCB were mediated by the inhibition of the FTO-triggered increase in G9a and the rescue of G9a-controlled downregulation of MOR and Kv 1.2 in injured DRG. In line with previous studies [27, 28], SNL led to marked increases in the levels of G9a’s two protein isoforms (short and long) and decreases in the levels of MOR and Kv1.2 in the ipsilateral L5 DRG on day 5 post-SNL in the vehicle 1-injected SNL rats (Fig. 7A, B). These changes were significantly blocked by intrathecal pre-injection with MA at 20 µg or N-CDPCB at 15 µg, although the SNL-induced increase of FTO in the ipsilateral L5 DRG was not affected (Fig. 7A, B). Neither MA nor N-CDPCB had the effect on basal levels of G9a’s two protein isoforms, MOR and Kv1.2 in the ipsilateral L5 DRG on day 5 post-sham surgery (Fig. 7A, B).
Fig. 7.
Effect of intrathecal pre-administration of MA or N-CDPCB on the SNL-induced upregulation of G9a and downregulation of MOR and Kv1.2 in injured DRG. L: long isoform. S: short isoform. (A) The levels of FTO, G9a’s two protein isoforms, MOR and Kv 1.2 proteins in the ipsilateral lumbar 5 DRG on day 5 post-surgery in rats with intrathecal injection with 20 μg MA or vehicle 1 (0.01 M PBS) starting 30 min before surgery and once daily for 5 days after surgery. n = 6 rats per group. Two-way ANOVA followed by post hoc Tukey test, Fmodel (1, 8) = 166.9 for FTO, 13.40 for G9a-L, 22.86 for G9a-S, 20.75 for MOR, and 40.65 for Kv 1.2, **P < 0.01 versus the corresponding vehicle 1-treated sham rats, Ftreatment (1, 8) = 0.08 for FTO, 7.04 for G9a-L, 12.86 for G9a-S, 6.26 for MOR, and 17.72 for Kv 1.2, #P < 0.05, ##P < 0.01 versus the corresponding vehicle 1-treated SNL rats. (B) The levels of G9a’s two protein isoforms, MOR and Kv 1.2 proteins in the ipsilateral lumbar 5 DRG on day 5 post-surgery in rats with intrathecal injection with 15 μg N-CDPCB or vehicle 2 (10% DMSO) starting 30 min before surgery and once daily for 5 days after surgery. n = 6 rats per group. Two-way ANOVA followed by post hoc Tukey test. Fmodel (1, 8) = 18.58 for Ga9-L, 24.47 for G9a-S, 16.91 for MOR, and 90.98 for Kv 1.2. **P < 0.01 versus the vehicle 2-treated sham rats. Ftreatment (1, 8) = 24.73 for G9a-L, 13.23 for G9a-S, 41.24 for MOR, and 32.95 for Kv 1.2, ##P < 0.01 versus the vehicle 2-treated SNL rats
Discussion
The present study demonstrated that intrathecal FTO inhibitors, MA and N-CDPCB, dose-dependently mitigated the SNL-induced evoked nociceptive hypersensitivities and spontaneous ongoing nociceptive responses during the development and maintenance periods. Intrathecal MA and N-CDPCB also blocked the SNL-induced increase in G9a expression and rescued the SNL-induced downregulation of MOR and Kv1.2 in injured DRG. Additionally, intrathecal MA attenuated the SNL-induced dorsal horn neuronal and astrocyte hyperactivities. These findings further confirmed a key role of DRG FTO in the peripheral mechanisms of neuropathic pain.
FTO is a demethylase particularly for m6A sites in RNAs. Peripheral nerve trauma increased the expression of FTO in injured DRG neurons [10]. This increase erased m6A in Ehmt2 mRNA, produced a loss in the binding of YTHDF2 to Ehmt2 mRNA, stabilized the increased expression of Ehmt2 mRNA/G9a, and consequently downregulated mu opioid receptors (including MOR) and Kv1.2 in injured DRG [10]. Genetic knockdown or knockout of DRG FTO alleviated nerve trauma-induced nociceptive hypersensitivities [10]. These effects likely resulted from destabilizing nerve trauma-caused G9a upregulation and then rescuing G9a-controlled downregulation of opioid receptors and Kv1.2 in injured DRG. However, these genetic approaches are impractical in current clinical neuropathic pain managements.
The present study revealed that intrathecal administration of two FTO inhibitors, MA and N-CDPCB, not only blocked the SNL-induced increase in G9a and rescued the SNL-induced decreases in MOR and Kv1.2 in injured DRG but also attenuated the SNL-induced mechanical allodynia, heat hyperalgesia, cold hyperalgesia, and spontaneous ongoing nociceptive responses, even if these two inhibitors have different structures [13, 15]. MA appears to have a stronger effect on SNL-induced pain hypersensitivities as compared to N-CDPCB. Pre-intrathecal administration of MA at 10 µg once daily for 5 days significantly blocked mechanical allodynia and heat hyperalgesia at both days 3 and 5 post-SNL, whereas pre-intrathecal injection of N-CDPCB at 10 µg once daily for 5 days markedly attenuated mechanical allodynia and heat hyperalgesia only at day 5 post-SNL. In addition, post-intrathecal administration of MA, but not N-CDPCB, once daily for 5 days produced time-dependent anti-nociceptive effect on the SNL-induced mechanical allodynia, heat hyperalgesia, and cold hyperalgesia. Lower efficacy of N-CDPCB may be associated with its solubility only in DMSO. In contrast, MA not only is water-soluble but also may have other inhibitory effects besides FTO inhibition. For example, it was reported that MA was a gap-junction blocker [20]. Taken together, the present study carried out two distinct FTO inhibitors to further demonstrate DRG FTO as a new potential target for neuropathic pain treatment.
Peripheral nerve trauma-induced downregulation of MOR and Kv1.2 in injured DRG contributes to neuropathic pain genesis. The mice with MOR deficiency displayed increased responses to mechanical stimulation on the contralateral side in addition to enhanced SNL-induced mechanical allodynia on the ipsilateral side [29]. Rescuing downregulation of MOR in injured DRG attenuated the MOR-mediated neurotransmitter release from primary afferents as well as nerve trauma-induced nociceptive hypersensitivity [28, 30, 31]. Similarly, knockdown of DRG Kv1.2 increased the excitability of DRG neurons and led to neuropathic pain-like symptoms in naïve animals [16, 17]. Restoring DRG Kv1.2 downregulation alleviated the nerve trauma-induced nociceptive hypersensitivities [16, 17]. Our previous studies demonstrated that downregulation of MOR and Kv1.2 in injured DRG was determined at least in part by the nerve trauma-induced increase in DRG FTO, since genetic knockout or knockdown of FTO in injured DRG reduced G9a expression and rescued the G9a-controlled downregulation of MOR and Kv1.2 in injured DRG [10]. The present study further verified that intrathecal MA or N-CDPCB had similar effects. Thus, the anti-nociceptive effect of MA or N-CDPCB is likely attributed to the rescue of the G9a-controlled MOR and Kv1.2 donwregulation, the decreases in DRG neuronal hyperexcitability and MOR-mediated neurotransmitter release from primary afferents, and consequent reduction in dorsal horn central sensitization. Consistent with this conclusion, our present study revealed that intrathecal MA attenuated the SNL-induced increases in phosphorylation of ERK1/2 and GFAP, two markers for neuronal and astrocyte hyperactivities, respectively, in the ipsilateral L5 dorsal horn on day 5 post-SNL. It should be noted that intrathecal MA or N-CDPCB partially, but not fully, blocked SNL-induced nociceptive hypersensitivities. These partial effects may be related to multiple mechanisms that participate in nerve trauma-caused downregulation of MOR and Kv1.2 in injured DRG. In additional to the role of FTO/G9a, the increases in DNA methylation of Oprm1 and Kcna2 genes and expression of eukaryotic initiation factor 4 gamma 2 also contributed to nerve trauma-induced downregulation of MOR and Kv1.2 in injured DRG [24, 26, 31–33]. Nerve trauma-induced DRG Kv1.2 downregulation may also be related to nerve trauma-induced increase of endogenous long noncoding Kcan2 antisense RNA in injured DRG [17, 34]. Taken together, the combined different approaches that target multiple mechanisms underlying downregulation of DRG MOR and Kv1.2 likely produce profound effects in neuropathic pain management.
Conclusions
The present study demonstrated the anti-nociceptive effect of intrathecal FTO inhibitors MA and N-CDPCB on both development and maintenance of neuropathic pain. The study dosage used did not alter acute/basal pain and locomotor function. Given that MA is an FDA-approved nonsteroidal anti-inflammatory drug [14, 15], our findings suggest that MA may have potential application for neuropathic pain treatment in a clinical setting.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thanked Dr. Junbiao Chang from Zhengzhou University for generously providing N-CDPCB.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author Contribution
Y.X.T. conceived the project and supervised all experiments. B.X.Z., X.G., and Y.X.T. designed the project. B.X.Z. and X.G. performed the animal model and intrathecal injection, conducted behavioral experiments, and carried out Western blot experiments. B.X.Z., X.G., S.A., J.E., and Y.X.T. analyzed the data. B.X.Z., X.G., S.A., and Y.X.T. wrote and edited the manuscript. All of the authors read and discussed the manuscript.
Funding
This work was supported by the grants (R01NS094224, R01NS111553, R01NS117484 and RFNS113881) from the National Institutes of Health (Bethesda, Maryland, USA).
Declarations
Ethical Approval
All procedures used were approved by the Animal Care and Use Committee at the Rutgers New Jersey Medical School (Newark, NJ).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bi-Xin Zheng and Xinying Guo are contributed equally to this study.
References
- 1.van HO, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 2004;155:654–662. S0304–3959(13)00610–6 [pii];10.1016/j.pain.2013.11.013. [DOI] [PubMed]
- 2.Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. 2014;348:f7656. doi: 10.1136/bmj.f7656. [DOI] [PubMed] [Google Scholar]
- 3.O'Connor AB (2009) Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics 27: 95–112. 2 [pii]. [DOI] [PubMed]
- 4.Wu S, Marie LB, Miao X, et al. Dorsal root ganglion transcriptome analysis following peripheral nerve injury in mice. Mol Pain 2016;12:1–14. 12/0/1744806916629048 [pii];10.1177/1744806916629048. [DOI] [PMC free article] [PubMed]
- 5.Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, Cheng XP, Yan Q, Zhu ZD, Zhang X, Chen Z, Han ZG, Zhang X. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci U S A. 2002;99:8360–8365. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang L, Lutz BM, Bekker A, Tao YX. Epigenetic regulation of chronic pain. Epigenomics. 2015;7:235–245. doi: 10.2217/epi.14.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lutz BM, Bekker A, Tao YX. Noncoding RNAs: new players in chronic pain. Anesthesiology. 2014;121:409–417. doi: 10.1097/ALN.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu S, Bono J, Tao YX. Long noncoding RNA (lncRNA): A target in neuropathic pain. Expert Opin Ther Targets. 2018 doi: 10.1080/14728222.2019.1550075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albik S, Tao YX. Emerging role of RNA m6A modification in chronic pain. Pain. 2021 doi: 10.1097/j.pain.0000000000002219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Guo X, Sun L, Xiao J, Su S, Du S, Li Z, Wu S, Liu W, Mo K, Xia S, Chang YJ, Denis D, Tao YX. N(6)-Methyladenosine Demethylase FTO Contributes to Neuropathic Pain by Stabilizing G9a Expression in Primary Sensory Neurons. Adv Sci (Weinh ) 2020;7:1902402. doi: 10.1002/advs.201902402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng BX, Malik A, Xiong M, Bekker A, Tao YX. Nerve trauma-caused downregulation of opioid receptors in primary afferent neurons: Molecular mechanisms and potential managements. Exp Neurol 2020;337:113572. S0014–4886(20)30403–9 [pii];10.1016/j.expneurol.2020.113572. [DOI] [PMC free article] [PubMed]
- 12.Jia G, Fu Y, Zhao X. et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 2011;7:885–887. nchembio.687 [pii];10.1038/nchembio.687. [DOI] [PMC free article] [PubMed]
- 13.He W, Zhou B, Liu W, Zhang M, Shen Z, Han Z, Jiang Q, Yang Q, Song C, Wang R, Niu T, Han S, Zhang L, Wu J, Guo F, Zhao R, Yu W, Chai J, Chang J. Identification of A Novel Small-Molecule Binding Site of the Fat Mass and Obesity Associated Protein (FTO) J Med Chem. 2015;58:7341–7348. doi: 10.1021/acs.jmedchem.5b00702. [DOI] [PubMed] [Google Scholar]
- 14.Chen B, Li Y, Song R, Xue C, Xu F. Functions of RNA N6-methyladenosine modification in cancer progression. Mol Biol Rep. 2019;46:1383–1391. doi: 10.1007/s11033-018-4471-6. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Yan J, Li Q, et al. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res 2015;43:373–384. gku1276 [pii];10.1093/nar/gku1276. [DOI] [PMC free article] [PubMed]
- 16.Fan L, Guan X, Wang W, et al. Impaired neuropathic pain and preserved acute pain in rats overexpressing voltage-gated potassium channel subunit Kv1.2 in primary afferent neurons. Mol Pain 2014;10:8. 1744–8069–10–8 [pii];10.1186/1744-8069-10-8. [DOI] [PMC free article] [PubMed]
- 17.Zhao X, Tang Z, Zhang H, et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci 2013;16:1024–1031. nn.3438 [pii];10.1038/nn.3438. [DOI] [PMC free article] [PubMed]
- 18.Liang L, Zhao JY, Kathryn T, Bekker A, Tao YX. BIX01294, a G9a inhibitor, alleviates nerve injury-induced pain hypersensitivities during both development and maintenance periods. Transl Perioper Pain Med 2019;6:106–114. 10.31480/2330-4871/097. [DOI] [PMC free article] [PubMed]
- 19.Xu JT, Sun L, Lutz BM, Bekker A, Tao YX. Intrathecal rapamycin attenuates morphine-induced analgesic tolerance and hyperalgesia in rats with neuropathic pain. Transl Perioper Pain Med. 2015;2:27–34. [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashida K, Parker RA, Eisenach JC. Activation of glutamate transporters in the locus coeruleus paradoxically activates descending inhibition in rats. Brain Res 2010;1317:80–86. S0006–8993(09)02784-X [pii];10.1016/j.brainres.2009.12.086. [DOI] [PMC free article] [PubMed]
- 21.Zhou P, Wu M, Ye C, Xu Q, Wang L. Meclofenamic acid promotes cisplatin-induced acute kidney injury by inhibiting fat mass and obesity-associated protein-mediated m(6)A abrogation in RNA. J Biol Chem 2019;294:16908–16917. S0021–9258(20)30532–9 [pii];10.1074/jbc.RA119.011009. [DOI] [PMC free article] [PubMed]
- 22.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. 0165–0270(94)90144–9 [pii]. [DOI] [PubMed]
- 23.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 24.Zhao JY, Liang L, Gu X, et al. DNA methyltransferase DNMT3a contributes to neuropathic pain by repressing Kcna2 in primary afferent neurons. Nat Commun 2017;8:14712. ncomms14712 [pii];10.1038/ncomms14712. [DOI] [PMC free article] [PubMed]
- 25.Wu Q, Wei G, Ji F, Jia S, Wu S, Guo X, He L, Pan Z, Miao X, Mao Q, Yang Y, Cao M, Tao YX. TET1 Overexpression Mitigates Neuropathic Pain Through Rescuing the Expression of mu-Opioid Receptor and Kv1.2 in the Primary Sensory Neurons. Neurotherapeutics. 2019;16:491–504. doi: 10.1007/s13311-018-00689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Zheng B, Du S, et al. Eukaryotic initiation factor 4 gamma 2 contributes to neuropathic pain through downregulation of Kv1.2 and the mu opioid receptor in mouse primary sensory neurones. Br J Anaesth 2020. S0007–0912(20)30911–9 [pii];10.1016/j.bja.2020.10.032. [DOI] [PMC free article] [PubMed]
- 27.Liang L, Gu X, Zhao JY, Wu S, Miao X, Xiao J, Mo K, Zhang J, Lutz BM, Bekker A, Tao YX. G9a participates in nerve injury-induced Kcna2 downregulation in primary sensory neurons. Sci Rep. 2016;6:37704. doi: 10.1038/srep37704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang L, Zhao JY, Gu X, Wu S, Mo K, Xiong M, Bekker A, Tao YX. G9a inhibits CREB-triggered expression of mu opioid receptor in primary sensory neurons following peripheral nerve injury. Mol Pain. 2016;12:1–16. doi: 10.1177/1744806916682242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansikka H, Zhao C, Sheth RN, Sora I, Uhl G, Raja SN. Nerve injury induces a tonic bilateral mu-opioid receptor-mediated inhibitory effect on mechanical allodynia in mice. Anesthesiology 2004;100:912–921. 00000542–200404000–00022 [pii]. [DOI] [PubMed]
- 30.Li Z, Mao Y, Liang L, et al. The transcription factor C/EBPbeta in the dorsal root ganglion contributes to peripheral nerve trauma-induced nociceptive hypersensitivity. Sci Signal 2017;10:eaam5345. 10/487/eaam5345 [pii];10.1126/scisignal.aam5345. [DOI] [PMC free article] [PubMed]
- 31.Sun L, Zhao JY, Gu X, Liang L, Wu S, Mo K, Feng J, Guo W, Zhang J, Bekker A, Zhao X, Nestler EJ, Tao YX. Nerve injury-induced epigenetic silencing of opioid receptors controlled by DNMT3a in primary afferent neurons. Pain. 2017;158:1153–1165. doi: 10.1097/j.pain.0000000000000894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mo K, Wu S, Gu X, et al. MBD1 Contributes to the Genesis of Acute Pain and Neuropathic Pain by Epigenetic Silencing of Oprm1 and Kcna2 Genes in Primary Sensory Neurons. J Neurosci 2018;38:9883–9899. JNEUROSCI.0880–18.2018 [pii];10.1523/JNEUROSCI.0880-18.2018. [DOI] [PMC free article] [PubMed]
- 33.Sun L, Gu X, Pan Z, et al. Contribution of DNMT1 to neuropathic pain genesis partially through epigenetically repressing Kcna2 in primary afferent neurons. J Neurosci 2019. JNEUROSCI.0695–19.2019 [pii];10.1523/JNEUROSCI.0695-19.2019. [DOI] [PMC free article] [PubMed]
- 34.Li Z, Gu X, Sun L, Wu S, Liang L, Cao J, Lutz BM, Bekker A, Zhang W, Tao YX. Dorsal root ganglion myeloid zinc finger protein 1 contributes to neuropathic pain after peripheral nerve trauma. Pain. 2015;156:711–721. doi: 10.1097/j.pain.0000000000000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.