Abstract
The prognosis for patients with glioblastoma (GBM), the most common and malignant type of primary brain tumour, is very poor, despite current standard treatments such as surgery, radiotherapy and chemotherapy. Moreover, the immunosuppressive tumour microenvironment hinders the development of effective immunotherapies for GBM. Cytokines such as interleukin-10 (IL-10) play a major role in modulating the activity of infiltrating immune cells and tumour cells in GBM, predominantly conferring an immunosuppressive action; however, in some circumstances, IL-10 can have an immunostimulatory effect. Elucidating the function of IL-10 in GBM is necessary to better strategise and improve the efficacy of immunotherapy. This review discusses the immunostimulatory and immunosuppressive roles of IL-10 in the GBM tumour microenvironment while considering IL-10-targeted treatment strategies. The molecular mechanisms that underlie the expression of IL-10 in various cell types are also outlined, and how this resulting information might provide an avenue for the improvement of immunotherapy in GBM is explored.
Subject terms: Tumour immunology, CNS cancer
Introduction
Gliomas constitute central nervous system (CNS) tumours, thought to arise from mutant glial cells or glial precursor cells, and account for approximately 80% of all malignant brain tumours [1]. Half of all gliomas are glioblastomas (GBMs; also referred to as a grade 4 astrocytoma), the most common and malignant primary brain tumour [2, 3]. Despite standard treatment therapy, which includes maximum safe surgical resection, radiotherapy and chemotherapy, GBM patients are faced with a devastatingly poor prognosis and a median survival of 15 months following diagnosis [4]. As this prognosis has remained stagnant for over a decade [4] and in view of the success of immunotherapy in the context of melanoma and haematological malignancies, efforts to improve the outcome of patients with GBM have turned to strategies that modulate the immune response and the tumour microenvironment (TME) [5]. Unfortunately, clinical trials testing immunotherapies have shown limited efficacy in GBM. The immunotherapy-refractory nature of GBM is thought to be due, in part, to the functional separation of the CNS and the systemic immune system. The CNS has long been considered an immune-privileged organ. This concept was an experimentally defined phenomenon, where cells/tissues grafted into non-CNS tissue are efficiently rejected but show prolonged survival when grafted into the CNS. The basis for this immune privilege was interpreted as CNS isolation from the immune system due to the blood–brain barrier (BBB) and that this is important for protecting neurons during inflammation. It is now increasingly recognised that immune privilege is not absolute but is a highly regulated state, resulting from immunoregulatory mechanisms of CNS-resident cells and their microenvironment. Due to the brain’s immune privilege compared to other organs as well as the nature of the brain TME, both pose a unique challenge for the application of immunotherapy in GBM patients [5].
A major challenge in using immunotherapy for GBM is the presence of an immunosuppressive TME [6]. The GBM TME harbours multiple non-neural cell types including infiltrating immune cells, pericytes, astrocytes, oligodendrocytes and endothelial cells, all contained within a modified extracellular matrix (ECM). These cells communicate with tumour cells and other non-tumour cells through complex signalling networks that involve both direct cell–cell interactions and paracrine signals mediated via soluble proteins, among which are cytokines and chemokines [6] secreted by glioma cells and tumour-associated macrophages (TAMs) [7, 8]. Cytokines and chemokines, in combination with other immune checkpoint molecules, influence the innate and adaptive immune responses by suppressing T cell activity (by inducing T cell apoptosis, for example) and by activating ‘anti-inflammatory’ macrophages [5]. To evade the host immune system and proliferate, tumour cells are highly adaptive and can suppress host anti-tumour immunity through a mechanism termed cancer immunoediting. This is a poorly understood process whereby the immune response not only protects against cancer development but also promotes the outgrowth of tumour cells that can escape immune control. One of the major mechanisms involved includes the expression, activation and secretion of anti-inflammatory cytokines into the TME [9].
Many studies have reported the increased expression of anti-inflammatory cytokines, notably transforming growth factor-β (TGF-β) and interleukin-10 (IL-10) in GBM. Targeting TGF-β has been proposed to promote a less immunosuppressive TME that is more favourable for immunotherapy and to therefore improve survival, but clinical trials using TGF-β inhibitors have not led to improved outcomes [10, 11]. IL-10 is an important pleiotropic immunoregulatory cytokine that, in the context of cancer, confers immunosuppression [12, 13] by inhibiting antigen-presenting cells (APCs), inhibiting T cell proliferation, and inducing the activity of regulatory T (TREG) cells [14]. However, the role of IL-10 in the TME and the therapeutic potential of targeting this cytokine in the context of brain cancer remain unclear. Here we review the role of IL-10 in brain cancer, discussing its functions in immunosuppression and tumour progression. We investigate the molecular mechanisms that might be responsible for regulating the expression of IL-10 in various cell types and consider how targeting this cytokine could potentially improve immunotherapy for patients with GBM.
IL-10 and glioma
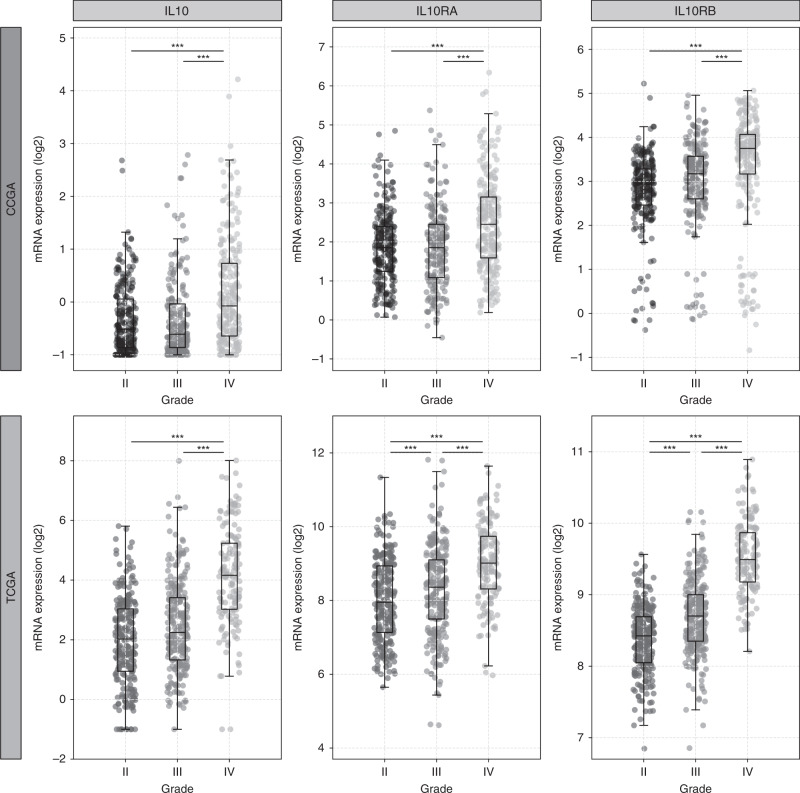
Early evidence for the expression of IL-10 in glioma was reported by Huettner et al. [15], a study which demonstrated elevated levels of IL10 mRNA in 87% of high-grade (grade III and IV) and 4% of lower-grade (grade II) gliomas. Consistent with this finding, analysis of glioma gene expression data from The Cancer Genome Atlas (http://cancergenome.nih.gov/) and the Chinese Glioma Genome Atlas (CGGA) (http://www.cgga.org.cn/) revealed IL10 mRNA expression to be elevated in high-grade glioma (grades III and IV) compared to low-grade (grade II) glioma (Fig. 1). There is limited evidence linking IL-10 expression with glioma patient survival. One study directly investigating this link suggested that a single-nucleotide polymorphism within the promoter region of the IL10 gene (rs1800871 C/T genotype), which leads to decreased IL10 mRNA expression, correlated with a modest increase in overall and progression-free survival in low-grade but not in high-grade glioma patients [16]. It is tempting to speculate that the reported improved patient outcome was due to reduced IL-10 expression in glioma tissue, but the study did not investigate IL-10 expression directly, so a direct link between IL-10 expression and glioma patient prognosis remains to be determined.
Fig. 1. Elevated levels of IL10, and IL-10 receptors, IL10RA and IL10RB mRNA, in glioma patient samples.
Glioma patient samples from the Chinese Glioma Genome Atlas (CCGA) and The Cancer Genome Atlas (TCGA) cohorts were analysed using the GlioVis glioma data portal (http://gliovis.bioinfo.cnio.es/). IL10, IL10RA and IL10RB mRNA expression levels increase with glioma grade (II, III, IV). Statistical significance (Tukey’s Honest Significant Difference) is indicated, ***p < 0.0005. Publicly available mRNA expression data were used to generate the boxplots using the Matplotlib library, Python 3.9.1.
Although the cellular source of IL-10 in glioma has not been determined, evidence suggests that a subpopulation of CD133+ stem-like glioma cells express a higher level of IL10 mRNA and protein compared to CD133– glioma stem-like cells [17]. The expression of IL-10 in CD133+ glioma stem cells requires activation of the Toll-like receptor 4 (TLR4) pathway, which has been shown to support immune evasion [18]. The secretion of IL-10 by tumour cells is thought to activate tumour-infiltrating immune cells such as microglia and macrophages, which are then triggered to produce the majority of IL-10 in the tumour tissue [7, 19, 20]. Despite not being a major source of IL-10, low- and high-grade glioma cells express the IL-10 receptors IL10RA and IL10RB [21, 22], suggesting that glioma cells are responsive to IL-10 (Fig. 1). In glioma cells, the IL-10 receptors activate the Janus kinase–signal transducer and activator of transcription-3 (JAK–STAT3) pathway, which, in turn, regulates tumour cell proliferation, migration and invasion [23, 24].
IL-10 and tumour progression
As well as its immunomodulatory functions with both immunosuppressive and immunostimulatory activities, IL-10 can promote tumour progression by increasing cancer cell proliferation. In the B16 melanoma mouse model, IL-10 enhanced tumour growth by stimulating angiogenesis, immunosuppression and tumour-cell proliferation [25]. Similarly, in vitro studies have demonstrated an IL-10-mediated dose-dependent increase in cell proliferation in U87MG glioma cells [26]. In a mechanism that does not require either IL10RA or IL10RB, IL-10 released from macrophages can bind directly to JAK2 in glioma cells to induce JAK2–STAT3 signalling and thereby promote the proliferation of glioma cells [24]. Alternatively, binding by IL-10 to the IL-10 receptor activates JAK1 and non-receptor tyrosine protein kinase (TYK2), leading to the activation of STAT3 to promote an anti-inflammatory response [27]. In neurodegeneration studies, IL-10 was shown to inhibit apoptosis via IL-10 receptor-mediated phosphatidylinositol-4,5-bisphosphate-3-kinase (PI3K)–Akt-dependent expression of the anti-apoptotic factors B cell lymphoma 2 and B cell lymphoma extra-large and attenuation of the pro-apoptotic caspase-3 [28]. Although IL-10 may play a similar anti-apoptotic role in glioma, this remains to be investigated. In contrast with these findings, however, studies in a 9L glioma mouse model expressing IL-10 and IL-2 suggest that IL-10 promoted a reduction in tumour size and increased T cell infiltration [29]. These conflicting results highlight the spectrum of IL-10 effects and suggest that IL-10 regulates glioma cell apoptosis via direct and indirect mechanisms, depending on the immune cells contexture of the TME.
IL-10 and tumour-cell invasion
The contribution of IL-10 to tumour-cell invasion is unclear. Co-culturing ovarian cancer cells with immune cells such as CD8+ T cells promoted the invasion and migration of the cancer cells through IL-8- and IL-10-mediated upregulation of matrix metalloproteinases (MMPs) and urokinase [30]. Similarly, IL-10-stimulated macrophages are more effective than lipopolysaccharide-stimulated ‘pro-inflammatory’ macrophages at promoting the invasion of gastric and colorectal cancer cells via the induction of MMP-2 and MMP-9 [31]. In patients with glioma, the mRNA and protein levels of IL-10 in the blood correlate with malignancy and glioma grade [32, 33]. Furthermore, cells from higher-grade gliomas exhibit a more invasive capacity than cells from lower-grade gliomas, and notably, IL10 mRNA is selectively expressed in more invasive gliomas compared to less malignant gliomas [34].
Huettner et al. [15] demonstrated that, in addition to contributing to an immunosuppressive TME, IL-10 enhanced cell proliferation and migration in two glioma cell lines. Considering the role of IL-10 in ovarian and colorectal cancer cells outlined above, it is plausible that IL-10 could also promote the expression of invasion-associated molecules such as MMP-2 and MMP-9 in glioma cells. MMPs play a key role in the degradation of the ECM and facilitate the migration of cancer cells throughout the parenchyma [35]. It has also been suggested that IL-10 indirectly increases glioma cell invasion by upregulating the expression of KPNA2 ((karyopherin subunit alpha 2) [26], a nuclear transport protein whose expression correlates with histological glioma grade and invasive activity. Treatment with IL-10 enhanced the invasion of U87MG cells, whereas knockdown of KPNA2 in IL-10-treated U87MG cells inhibited glioma cell invasion [26]. Alternatively, perhaps IL-10- and MMP-expressing TAMs influence glioma cells to adopt pro-invasive properties [36]. Another study demonstrated that IL-10-treated microglia exhibit increased formation of podosomes [37], F-actin-rich structures that are essential for cell migration and ECM degradation, allowing activated microglia to infiltrate the tissue during inflammation [38]. Glioma cells also possess F-actin-rich invadopodia on the surface of their membrane, and these structures facilitate glioma cell invasion into healthy brain tissue [39].
IL-10 in the glioma TME
IL-10 and TAMs
TAMs: Tumour-associated pro- and anti-inflammatory microglia and macrophages
TAMs, which include brain-resident microglia and bone marrow-derived macrophages, constitute up to 50% of the GBM tumour mass, particularly in GBM expressing wild-type isocitrate dehydrogenese [40–44], and are a primary source of IL-10 [7, 45]. Bone marrow-derived macrophages contribute to ~85% of the entire TAM population and predominate in the GBM core, whereas microglia tend to localise in the peritumoural regions [44, 46]. Interestingly, bone marrow-derived macrophages express increased levels of immunosuppressive factors, including IL10, C-C motif chemokine ligand 22 (CCL22) and CXC chemokine receptors, compared to microglia [43]. However, microglia can also express anti-inflammatory markers and secrete anti-inflammatory cytokines, including IL-10 [47]. TAMs exist as a spectrum of various functional forms, depending on their activation state: from pro-inflammatory to immunosuppressive. Immunosuppressive cells are characterised by the production of anti-inflammatory cytokines, including IL-4, IL-10 and TGF-β [48, 49]. However, although IL-10 has often been regarded as an anti-inflammatory marker, it has been discovered that IL-10-expressing TAMs isolated from glioma express both pro- and anti-inflammatory markers, including TGF-β and tumour necrosis factor α (TNF-α) [50].
TAM activation
TAMs undergo activation by secreted factors derived from glioma cells, possibly including IL-10 itself. In vitro studies suggest that IL-10 is secreted by TAMs after incubation with glioma-conditioned medium [20, 45, 51]. Kostianovsky et al. [52] showed that co-culturing human monocytes and GBM tumour cells led to both the production of IL-10 and constitutive activation of STAT3 in both cell types, suggesting that bidirectional signalling might occur via IL-10-induced STAT3 activity between glioma cells and TAMs [52]. IL-10 production by TAMs is also accompanied by an increase in the expression of other anti-inflammatory cytokines and proteins, including CD206, IL-4, CCL2 and TGF-β [53]. The levels of IL-10 also correlate with other tumour characteristics. For example, higher levels of IL-10 and other anti-inflammatory cytokines were secreted by TAMs stimulated by temozolomide-resistant GBM cells, compared to TAMs stimulated with temozolomide-sensitive GBM cells [53].
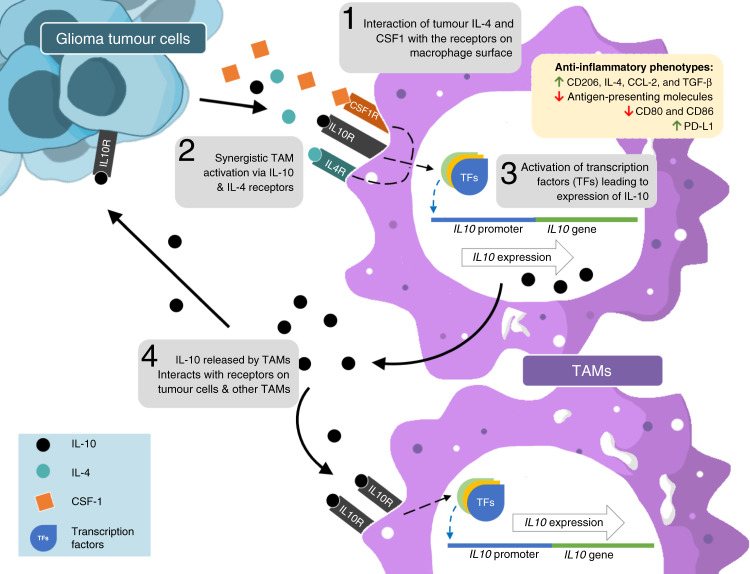
IL-10 alone can drive TAM alternative polarisation to the anti-inflammatory state in vitro [45, 54]. However, some studies suggested that TAM polarisation might occur following stimulation with other cytokines released by glioma cells, such as IL-4 and macrophage colony-stimulating factor (M-CSF or CSF-1), whose cognate receptors are highly expressed in undifferentiated TAMs (Fig. 2) [55–57]. IL-10 and IL-4 can also synergise to activate TAMs. In this scenario, IL-10 is involved in upregulating the expression of the IL-4 receptor in TAMs [55]. The synergy between IL-10 and IL-4 is also supported by another study demonstrating that a combination of IL-10 and IL-4 enhances the expression of anti-inflammatory markers in bone marrow-derived macrophages in vitro compared to those treated with a single cytokine [58]. Collectively, these studies suggest that the ability of TAMs to produce IL-10 is dependent on signals from both tumour cells and the TME.
Fig. 2. IL-10-mediated TAM activation leads to an anti-inflammatory state.
Naive TAMs in the GBM TME receive signals via cytokines released by tumour cells, such as IL-10, IL-4 and M-CSF, which activate a transition to an anti-inflammatory state. IL-10 synergises with IL-4 by increasing the expression of IL-4 receptor (IL-4R) on TAMs. This leads to the expression of IL-10 by TAMs and the induction of anti-inflammatory factors, such as the expression of CD206, IL-4, CCL-2 and TGF-β, downregulation of antigen-presenting and co-stimulatory molecules and upregulation of immune-checkpoint molecules, such as PD-1. IL-10 released by these activated TAMs can interact with target receptors on other TAMs and tumour cells.
The anti-inflammatory phenotype and reduced antigen presentation
Once activated, IL-10-stimulated TAMs adopt anti-inflammatory characteristics and reduce their antigen-presentation capacity (Fig. 2). Antigen-presenting molecules such as major histocompatibility class (MHC) class II and co-stimulatory molecules such as CD86 are downregulated in TAMs as a consequence of the upregulation of the membrane-associated E3 ubiquitin ligase MARCH1 following IL-10 activation. This downregulation of antigen-presenting molecules subsequently impairs CD4+ T cell activation [59] and is consistent with studies showing that TAMs with this anti-inflammatory phenotype express lower levels of MHC class II in comparison with pro-inflammatory TAMs and naive TAMs [57]. The expression of human leucocyte antigen class I (or MHC class I) as well as CD80, CD86 and intercellular adhesion molecule 1 was also attenuated in human monocytes when cultured in the presence of IL-10 [14]. Other than reducing their antigen-presenting capacity, TAMs also upregulate immune checkpoint proteins such as programmed death-ligand 1 and B7-H4 following IL-10 stimulation, which leads to T cell apoptosis and impaired effector function [45, 60].
IL-10 and tumour-associated T cell function
As a result of tumour-mediated angiogenesis and/or MMP secretion, the BBB is disrupted in patients with GBM, enabling peripheral lymphocytes to enter the CNS and infiltrate the tumour [61–63]. Both CD4+ and CD8+ T cells migrate into the brain, where they encounter an immunosuppressive microenvironment. Tumour-infiltrating T cells often accumulate in the perivascular areas; only a small proportion can infiltrate deeper into the tumour core. In the perivascular areas, T cells typically express markers of immune exhaustion [64–66]. Notably, however, T cells can be sequestered in the bone marrow through the tumour-mediated loss of sphingosine-1-phosphate receptor 1; this receptor normally promotes T cell exit from the bone marrow, suggesting that GBM-derived immunosuppression could potentially have a systemic or humoral influence [67]. In general, therefore, the number of infiltrating CD4+ and CD8+ T cells in glioma is relatively low in comparison with other immune cells, especially macrophages [66, 68]. Tumour-infiltrating T cells are likely involved in IL-10 paracrine signalling within the GBM TME, as evidenced by the expression of IL10RA and IL10RB on patient-derived leucocytes, potentially T cells [69], thereby inhibiting cytotoxic T cell function.
TREG cells
TREG cells, which are characterised by the expression of forkhead box protein 3 (FOXP3), represent a low proportion of tumour-infiltrating T cells in glioma patients [66, 70, 71]. TREG cell infiltration occurs to a greater extent in astrocytic gliomas compared with non-astrocytic tumours, suggesting greater TREG-driven immunosuppression in the higher-grade astrocytic cancers [71, 72]. Although most studies suggest that TREG cells are not present in GBM, some studies show that when TREG cells are present, they are a source of IL-10 and TGF-β [73, 74]. The role of TREG cells in modulating tumour immunosuppression was demonstrated by the occurrence of tumour rejection and improved survival, in response to the depletion of TREG cells in a murine glioma model [71]. IL-10, as well as modulating the TME, is crucial for the function of TREG cells. FOXP3-expressing naive T cells require IL-10 and TGF-β to differentiate into TREG cells [75]. Moreover, IL-10 produced by glioma-stimulated dendritic cells facilitates an immunosuppressive response in CD4+ T cells in vitro, as evidenced by high IL-10 and low TNF-α secretion [76]. This TREG cell suppressor function relies on the activation of IL-10R, which further activates the STAT pathway [77].
CD4+ and CD8+ T cells
IL-10 inhibits the function of T cell subtypes, including CD4+ type 1 T helper (Th1), type 2 T helper (Th2) and type 17 T helper (Th17) cells [77–79]. This inhibitory role is mediated by APCs, as demonstrated by the dysfunction of CD4+ Th1 and Th2 effector cells following incubation with IL-10-stimulated APCs [78]. Furthermore, TREG cells are also involved in the IL-10-mediated negative regulation of Th1 and Th17 CD4+ T cells [77, 79], as shown in a B16/F10 mouse model in which depletion of IL-10 downregulates TREG cell differentiation and enhances Th1 and Th17 immunity [79].
The indirect inhibitory roles of IL-10 have also been reported in CD8+ T cells, as shown by their unresponsiveness to antigens and inability to proliferate after activation by monocytes in the presence of IL-10 [14]. Moreover, IL-10-stimulated TREG cells are also involved in inhibiting CD8+ T cell-mediated anti-tumour immunity by increasing the expression of immune checkpoint factors such as programmed cell death protein 1 (PD-1), T cell immunoreceptor with Ig and ITIM domains and lymphocyte activation gene 3 mediated by the transcription factor B lymphocyte-induced maturation protein 1 (Blimp-1) [73]. Finally, the inhibitory roles of tumour-derived IL-10 have been demonstrated in splenic dendritic cells, which lose their ability to stimulate tumour-specific cytotoxic T lymphocytes, and an interferon-γ (IFN-γ) response in CD4+ and CD8+ T cells in MB49 tumour-bearing mice [80]. Together, this evidence indicates that IL-10 negatively regulates CD4+ and CD8+ T cell function and anti-tumour immunity, with the involvement of APCs or TREG cells.
An immunostimulatory role for IL-10?
Although IL-10 is largely involved in immunosuppression, several studies have reported contradictory functions, particularly in regulating the behaviour of CD8+ cytotoxic T cells. Groux et al. [14] demonstrated that IL-10 can stimulate the proliferation of CD8+ T cells in the absence of APCs in vitro. Furthermore, IL-10 has been shown to be necessary for inducing T cell cytotoxicity against tumour cells and for the expression of anti-tumour cytokines, such as IFN-γ, as well as the expression of granzymes and perforin, and the release of molecules necessary for antigen presentation [81]. This immunostimulatory function of IL-10 has been observed in different types of cancer, including cancer of the small intestine [82] and gastric cancer [83]. Emmerich et al. [84] have shown that IL-10 stimulates tumour-resident CD8+ T cells in mice through the activation of the STAT3 and STAT1 pathways, which activate T cells to release IFN-γ [84]. These specific intratumoural CD8+ T cells were reported to express increased levels of IL-10R in comparison to the CD8+ T cells in the nearest draining lymph nodes, suggesting that direct interaction with IL-10 is necessary for the cytolytic activity of the T cells in the tumour [84]. Although this phenomenon has not been reported in glioma or other cancers of the CNS, the stimulatory role of IL-10 in glioma-infiltrating CD8+ T cells requires further investigation.
In addition to CD8+ T cells, in vitro experiments have demonstrated that Th1 CD4+ T cells require IL-10 to adopt anti-tumour functions [85]. After stimulation with dendritic cells loaded with GBM lysate, these Th1 CD4+ T cells release IL-10, as well as IFN-γ, suggesting that IL-10 has a role in T cell antigen uptake and processing. This study also showed that lower levels of IL-10 (~40% of control dendritic cell stimulation) were sufficient for triggering CD4+ T cell-mediated anti-tumour activity. Although the level of IL-10 released by these T cells was relatively low, it was reported that the induction of IL-10 was sustained and correlated with tumour suppression.
It is important to note that the stimulatory role of IL-10 could depend on the balance of pro- and anti-inflammatory cytokines in the TME. This was demonstrated in an in vivo study where a more robust anti-tumour effect was observed in mice transplanted with glioma cells expressing IL-10 and IL-2 compared with those transplanted with tumour cells producing only IL-10 or IL-2 [29]. Enhanced T cell infiltration into the tumour was also seen in the IL-10 IL-2 mice, supporting the notion that IL-10 has a role in T cell anti-tumour activity in the TME, one that must be balanced with the action of pro-inflammatory cytokines. This finding, together with the report from De Vleeschouwer et al. [85], provides evidence that IL-10 might be crucial for CD4+ and CD8+ T cell anti-tumour activity in brain cancer.
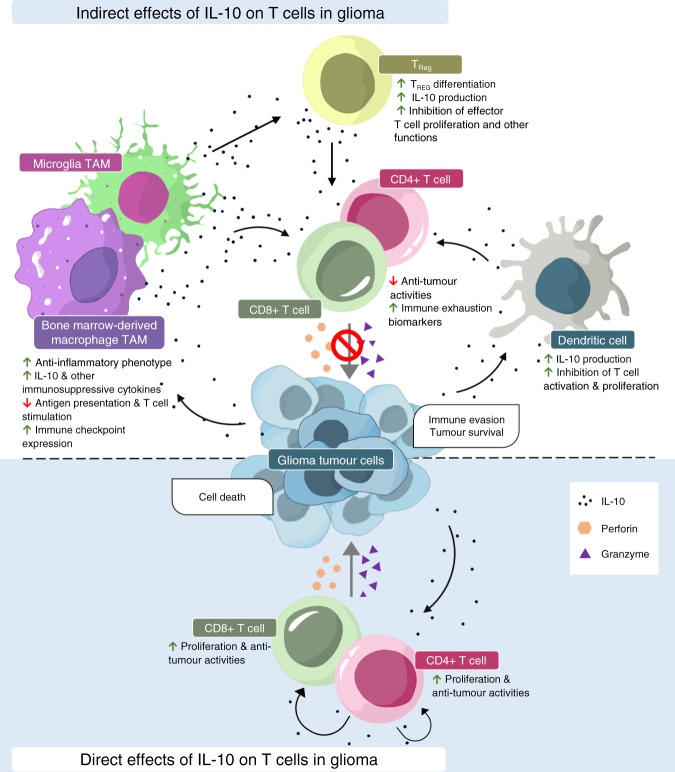
Collectively, most studies investigating the impact of IL-10 on T cell function indicate an indirect inhibitory effect of IL-10 on effector T cells mediated by regulatory immune cells including TAMs, dendritic cells or TREG cells. On the other hand, immunostimulatory roles of IL-10 are achieved when IL-10 directly interacts with effector T cells to activate their anti-tumour functions (Fig. 3).
Fig. 3. Diverse roles of IL-10 in the glioma TME.
The roles of IL-10 in the glioma microenvironment might be onco-stimulatory/immunosuppressive or immunostimulatory, depending on how IL-10 interacts with effector T cells. The immunosuppressive role occurs indirectly and is mediated by alternatively activated anti-inflammatory antigen-presenting cells such as TAMs and dendritic cells, as well as TREG cells. This leads to the inhibition of anti-tumour activity and higher expression of exhaustion markers in CD4+ and CD8+ effector T cells, which thereby promotes tumour immune escape and survival. By contrast, the immunostimulatory roles occur directly via the stimulation of effector T cell proliferation and anti-tumour activity through the activation of STAT proteins, which promote the release of interferons and other anti-tumour factors.
IL-10 and systemic immunosuppression in glioma patients
An increased level of IL-10 is seen in the sera from glioma patients compared with the sera from healthy individuals [86, 87]. Unsurprisingly, the IL-10 serum level is also higher in patients with high-grade glioma compared with low-grade glioma [9, 15, 88]. This evidence indicates that tumour immunomodulation takes place systemically and might be correlated with the characteristics of the immune cells outside the CNS, which exhibit an immunosuppressive phenotype. For example, an elevated number of CD163+ peripheral macrophages were detected in pretreated, newly diagnosed glioma patients compared with healthy individuals. Additionally, the expression of exhaustion markers such as PD-1 on peripheral CD4+ T cells was higher in those patients [89].
Targeting IL-10
Considering the important role of IL-10 in glioma progression and its involvement in the TME, an improvement in the effectiveness of current immunotherapies for GBM could potentially be achieved by targeting the IL-10 axis. However, there is limited research on IL-10-targeted therapies for GBM. To develop effective IL-10-based therapeutic strategies for GBM, it is necessary to not only adopt strategies used in other cancers but to also consider how efficiently systemically delivered therapeutics penetrate the BBB. Broadly, there are two aspects of IL-10-targeted therapy with respect to its biological roles. As IL-10 can impede anti-tumour immunity, its inhibition might be considered for glioma therapy. Alternatively, enhancing IL-10 signalling could be used as an anti-tumour immunity booster.
Inhibiting IL-10 signalling
The rationale for blocking IL-10 in cancer is based on its tumour immunosuppressive role. Neutralising the signalling pathway could therefore reverse immunosuppression, increase anti-tumour T cell-mediated cytotoxicity and improve the efficacy of other immunotherapies, including immune-checkpoint inhibition and anti-tumour vaccination. Blocking IL-10 signalling could be achieved by using IL-10 inhibitors or neutralising antibodies that target the IL-10 receptor. Several agents have demonstrated IL-10 inhibition in human and mouse monocytes and macrophages [90–92]. Compounds such as oleanolic acid were also shown to inhibit IL-10 secretion, attenuate macrophage anti-inflammatory activity and reduce tumour cell proliferation [93]. Inhibiting the IL-10 signalling pathway can also be achieved by using an anti-IL-10 antibody, although such antibodies are most effective when combined with other immunotherapies. For example, concomitant IL-10 and PD-1 inhibition increased the proliferation of patient-derived CD8+ T cells in vitro [94]. Conversely, CD8+ T cells became more sensitive to IL-10 treatment following treatment with PD-1 inhibitors, leading to diminished CD8+ T cell proliferation. These results implicate IL-10 in an immune resistance mechanism in response to PD-1 inhibition. The synergistic effect of PD-1 inhibition and IL-10 blockade was also reported in ovarian cancer-bearing mice [95]. Interestingly, this study reported a systemic increase in IL-10 in the mice following treatment with a PD-1 inhibitor, which might be attributable to a dendritic cell response. The combination of IL-10 blockade and PD-1 inhibition resulted in slower tumour growth, increased immune-cell infiltration, a decrease in myeloid-derived suppressor cell infiltration and prolonged mouse survival, compared to treatment with either agent alone. IL-10 blockade also improved the efficacy of a human papillomavirus (HPV)-derived vaccine (HPV16E7 vaccine/MPLA) in a cervical cancer mouse model. Interestingly, this combined approach increased the number of IL-10-producing T cells, but not FOXP3+ CD4+ TREG cells, in the spleen and draining lymph nodes [96, 97]. These studies suggest that IL-10 might be necessary for splenic CD4+ T cell maturation en route to eliciting an anti-tumour response.
Enhancing IL-10 signalling
As outlined above, some studies suggest that IL-10 can enhance anti-tumour immunity. Pegylated IL-10 has been shown to elicit the proliferation of tumour-reactive CD8+ T cells, as well as an increased production of granzyme B via activation of the STAT3 pathway [98]. The same study also reported that a combination of pegylated IL-10 and a PD-1 inhibitor, pembrolizumab, enhanced CD8+ T cell expansion and tumour rejection in patients with advanced solid tumours, such as melanoma, non-small cell lung cancer or renal cell carcinoma. This treatment regime is currently being investigated in a Phase Ib trial (Table 1, NCT02009449) [99]. The toxicity profile of this combinatorial treatment demonstrates safety and early anti-tumour activity.
Table 1.
Ongoing IL-10-based therapy in clinical trials (2021).
| Trial ID | Condition | Drug | Outcome | Publications |
|---|---|---|---|---|
| NCT02009449 (Phase I) |
Melanoma Prostate cancer Ovarian cancer Breast cancer Renal cell carcinoma Colorectal carcinoma Pancreatic carcinoma Non-small cell lung carcinoma Solid tumours |
Pegylated IL-10 alone or in combination with paclitaxel, docetaxel, carboplatin, cisplatin, FOLFOX, oxaliplatin, leucovorin, 5-fluorouracil, gemcitabine, nab-paclitaxel, capecitabine, pazopanib, pembrolizumab or nivolumab |
Combination with anti-PD-1 antibody demonstrated early anti-tumour activity Toxicity profile of pegylated IL-10 was considered manageable |
[99, 101] |
| NCT02923921 (Phase III) | Pancreatic cancer | Combination of pegylated IL-10 and FOLFOX vs FOLFOX alone as second-line treatment | Pegylated IL-10 did not improve the efficacy of FOLFOX | [100] |
Pegylated IL-10 was also investigated in a Phase III trial alongside chemotherapy (FOLFOX) as a second-line treatment to treat patients with metastatic pancreatic ductal adenocarcinoma, but the addition of pegylated IL-10 did not appear to improve the efficacy of chemotherapy alone (Table 1, NCT02923921) [100]. Although clinical studies have reported tolerable toxicity, systemic adverse effects of IL-10 have been reported in some patients [101]. Consequently, a fusion of IL-10 and an anti-epidermal growth factor receptor (EGFR) antibody, cetuximab, was developed to facilitate the specific delivery of IL-10 to tumours to reduce the adverse effects of IL-10 [102]. This recombinant IL-10–EGFR fusion demonstrated superior efficacy compared with recombinant IL-10 alone, in a syngeneic B16 melanoma mouse tumour model. The study also reported an increased proliferation of CD8+ T cells due to the inhibition of dendritic-cell-induced T cell apoptosis [102]. Despite the promising results in some primary solid cancers, the suitability of IL-10 for brain cancer remains to be investigated.
IL-10 as a potential target in brain cancer
Neutralising the IL-10 signalling pathway or using IL-10 as an immune stimulator results in T cell activation. Although it is difficult to define the context in which IL-10 is immunosuppressive or immunostimulatory, it is tempting to speculate that IL-10 becomes immunosuppressive when T cells are activated by anti-inflammatory TAMs that have previously been stimulated by IL-10, whereas IL-10 may be stimulatory when it directly engages the IL-10 receptor on T cells. Considering that TAMs are the major source of IL-10 in the GBM TME, selectively targeting these cells might significantly reduce the level of IL-10, thus leaving a sufficient level of IL-10 produced by other cells in the TME to elicit an anti-tumour response. For this reason, it is critical to elucidate not only all the cellular sources but also the associated transcriptional regulation of IL-10 in the GBM TME.
Transcriptional regulation of IL-10
The IL10 gene and its promoter have been extensively studied in immune cells [103], with the regulation of expression varying in different cell types. In macrophages and dendritic cells, for example, the levels of IL10 mRNA are coregulated by TLR4 and the mitogen-activated protein kinase (MAPK) pathway [104]. More specifically, the expression of IL-10 relies on specific transcription factors, including the cAMP response element-binding protein (CREB), nuclear factor-κB and CCAAT/enhancer-binding protein β (Table 2). In CD4+ T cells, IL10 transcription is regulated by SMAD family member 4, E4 promoter-binding protein 4, interferon regulatory factor 4 and BLIMP-1. As IL-10 expression is modulated differently in these immune cells, it can be reasoned that it might be possible to modulate IL-10 secretion by targeting transcription factors that regulate IL-10 expression in TAMs.
Table 2.
Transcription factors regulating IL-10 expression in immune cells.
| Immune cell | Transcription factor | Reference |
|---|---|---|
| Macrophages | CREBATF1 | [104, 116, 117] |
| C/EBPβ | [116, 118] | |
| SP1, SP3 | [119, 120] | |
| NF-κB | [121] | |
| C-MAF | [122] | |
| AP-1 | [123] | |
| Microglia | MEF2D | [124] |
| CREB | [125] | |
| Dendritic cells | NF-κB | [126] |
| Th1 cells | SMAD4 | [127, 128] |
| E4BP4 | [129] | |
| IRF4 | [130] | |
| BLIMP-1 | [131] | |
| T-BET, BHLHE40 | [132] | |
| Th2 cells | GATA3 | [133] |
| E4BP4 | [129] | |
| IRF4 | [130] | |
| ETV5 | [134] | |
| Th17 cells | C-MAF | [135] |
| TRIM33, RORγT | [136] | |
| BATF, JUN, IRF4 | [137] | |
| Tr1 cells | C-MAF | [138] |
| Regulatory T cells | E4BP4 | [129] |
| BLIMP 1 | [139, 140] | |
| IRF4 | [140] |
Targeting CREB
Targeting CREB in GBM might attenuate both GBM cell survival and the TAM-mediated release of IL-10. CREB requires phosphorylation on serine 133 to initiate IL10 transcription in TAMs [104], but CREB is also phosphorylated (Ser133) in glioma tumour cells and this phosphorylation correlates with a higher grade of glioma [105]. In GBM tumour cells, phosphorylated CREB sits at the hub of the PI3K and MAPK signalling pathways and is involved in the regulation of various cellular functions, including proliferation, survival and the expression of TGF-β2 [105, 106]. Previous findings showed that CREB deletion attenuates tumour growth in a PI3K-hyperactivated GBM mouse [107], which suggests that targeting CREB could be a potential strategy for GBM therapy.
One potentially useful experimental small-molecule CREB inhibitor is 666–15, which disrupts the interaction between CREB and one of its key transcriptional cofactors, the CREB-binding protein, thus inhibiting transcription of CREB target genes. The potential clinical utility of small-molecule CREB inhibitors is promising. In vivo experiments demonstrate that 666–15 has no demonstrable negative systemic effects but can inhibit tumour growth in mice [108]. Evidence that CREB inhibition decreases immunosuppression comes from data showing reduced IL-10 and increased TNF-α levels in microglia, in vitro [109]. In the same study, 666–15 was injected intraperitoneally in an ischaemic stroke rat model and its pharmacological effects were apparent in the brain 72 h post-injection, indicating that 666–15 crosses the BBB [109]. Moreover, in the context of modulating the immune TME, 666–15 has been shown to improve the tumour immune contexture by boosting the function of infiltrating T cells [110]. In a pancreatic cancer mouse model [111], treatment with 666–15 led to reduced tumour growth, reduced infiltration of TAMs and TREG cells and increased cytotoxic T cell infiltration. And in another study, CREB inhibition downregulated myeloid-derived suppressor cell gene expression and ameliorated CD8+ T cell activity [112].
These findings suggest that targeting CREB could be a novel therapeutic strategy in GBM by affecting tumour cells at two levels: inhibiting tumour cell proliferation and minimising IL-10-mediated immunosuppression, without hindering T cell cytotoxicity. Furthermore, the strategy of targeting tumour cells and IL-10 secretion by TAMs is not limited to inhibiting CREB, as other transcription factors are also involved in coregulating IL-10 expression (Table 3). For example, targeting the transcription factor SP1 with mithramycin A reduces IL-10 expression in alveolar macrophages [113], inhibits glioma migration by blocking the production of MMPs [114] and reduces the astrocytic expression of CCL-2 and CXCL-1, which are crucial for myeloid cell recruitment and tumour progression in GBM [115].
Table 3.
Transcription factor inhibitor activity in experimental cancer models and tumour-associated immune cells.
| Transcription factor | Drug/inhibitor | Biological effect in experimental models | Reference |
|---|---|---|---|
| CREB | 666–15 | Inhibits growth of breast cancer cells in mice | [141] |
|
Reduces growth of pancreatic tumours Reduces tumour-infiltrating myeloid cells and regulatory T cells Increases the number of tumour-infiltrating T cells |
[111] | ||
| Inhibits immunosuppressive myeloid-derived suppressor cell functions from ovarian carcinoma | [112] | ||
| SP1 | Mithramycin A | Reduces the expression of matrix metalloproteinases in glioma | [114] |
| Reduces IL-10 expression in alveolar macrophages | [113] | ||
| Reduces chemokine (CCL2 and CXCL1) expression in astrocytes | [115] | ||
Conclusion
IL-10 is expressed by a wide range of cells in the GBM TME and plays crucial immunosuppressive roles, thereby promoting tumour progression and immune evasion. As IL-10 has also been reported to be required for T cell anti-tumour activities, it will be challenging to design IL-10-targeting treatments that are specific for GBM. Understanding the signalling and transcriptional mechanisms that regulate IL-10 expression in different cell types is crucial for the development of specific targeting strategies aimed at blocking tumour immunosuppression and enhancing anti-tumour immunity. Ultimately, developing novel combination therapies with current immunotherapy drugs that can efficiently cross the BBB will improve treatment efficacy for difficult-to-treat cancers, such as GBM.
Acknowledgements
We thank the members of the Brain Cancer Biology Laboratory for proof-reading the manuscript.
Author contributions
All authors made a substantial contribution to all aspects of the preparation of this paper, including conceiving the work that led to the submission, drafting, and revising the paper and approving the final version. First authorship is shared by S.S.W. and M.D.
Funding
This work was funded by the National Health & Medical Research Council MRFF Accelerated Research (APP1158175), Australian Brain Cancer Mission, Cancer Australia (to S.S.S.), the Department of Surgery Seed Funding, the Department of Microbiology and Immunology, The University of Melbourne, the CASS Foundation and the Brain Foundation Australia.
Data availability
Results generated in Fig. 1 used gene expression data generated by the TCGA Research Network: (https://www.cancer.gov/tcga) and the Chinese Glioma Genome Atlas: (http://www.cgga.org.cn/).
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
There is no data presented that required ethics approval or consent to participate.
Consent to publish
There is no data or material presented that required consent to publish.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Samuel S. Widodo, Marija Dinevska.
References
- 1.Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer Genet. 2012;205:613–21. doi: 10.1016/j.cancergen.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Wesseling P, Kros JM, Jeuken JWM. The pathological diagnosis of diffuse gliomas: towards a smart synthesis of microscopic and molecular information in a multidisciplinary context. Diagn Histopathol. 2011;17:486–94. [Google Scholar]
- 3.Zygogianni A, Protopapa M, Kougioumtzopoulou A, Simopoulou F, Nikoloudi S, Kouloulias V. From imaging to biology of glioblastoma: new clinical oncology perspectives to the problem of local recurrence. Clin Transl Oncol. 2018;20:989–1003. doi: 10.1007/s12094-018-1831-6. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Brown NF, Carter TJ, Ottaviani D, Mulholland P. Harnessing the immune system in glioblastoma. Br J Cancer. 2018;119:1171–81. doi: 10.1038/s41416-018-0258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai K, Hubben A, Ahluwalia M. The role of checkpoint inhibitors in glioblastoma. Target Oncol. 2019;14:375–94. doi: 10.1007/s11523-019-00655-3. [DOI] [PubMed] [Google Scholar]
- 7.Wagner S, Czub S, Greif M, Vince GH, Süss N, Kerkau S, et al. Microglial/macrophage expression of interleukin 10 in human glioblastomas. Int J Cancer. 1999;82:12–6. doi: 10.1002/(sici)1097-0215(19990702)82:1<12::aid-ijc3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Paulus W, Baur I, Huettner C. Effects of transforming growth factor-β1 on collagen synthesis, integrin expression, adhesion and invasion of glioma cells. J Neuropath Exp Neurol. 1995;54:236–44. doi: 10.1097/00005072-199503000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Albulescu R, Codrici E, Popescu ID, Mihai S, Necula LG, Petrescu D, et al. Cytokine patterns in brain tumour progression. Mediators Inflamm. 2013;2013:979748. [DOI] [PMC free article] [PubMed]
- 10.Brandes AA, Carpentier AF, Kesari S, Sepulveda-Sanchez JM, Wheeler HR, Chinot O, et al. A Phase II randomized study of galunisertib monotherapy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro Oncol. 2016;18:1146–56. doi: 10.1093/neuonc/now009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wick A, Desjardins A, Suarez C, Forsyth P, Gueorguieva I, Burkholder T, et al. Phase 1b/2a study of galunisertib, a small molecule inhibitor of transforming growth factor-beta receptor I, in combination with standard temozolomide-based radiochemotherapy in patients with newly diagnosed malignant glioma. Invest N Drugs. 2020;38:1570–9. doi: 10.1007/s10637-020-00910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trifunović J, Miller L, Debeljak Ž, Horvat V. Pathologic patterns of interleukin 10 expression-a review. Biochem Med. 2015;25:36–48. doi: 10.11613/BM.2015.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perng P, Lim M. Immunosuppressive mechanisms of malignant gliomas: parallels at non-CNS sites. Front Oncol. 2015;5:153. doi: 10.3389/fonc.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groux H, Bigler M, de Vries JE, Roncarolo MG. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol. 1998;160:3188–93. [PubMed] [Google Scholar]
- 15.Huettner C, Czub S, Kerkau S, Roggendorf W, Tonn JC. Interleukin 10 is expressed in human gliomas in vivo and increases glioma cell proliferation and motility in vitro. Anticancer Res. 1997;17:3217–24. [PubMed] [Google Scholar]
- 16.Hu M, Du J, Cui L, Huang T, Guo X, Zhao Y, et al. IL-10 and PRKDC polymorphisms are associated with glioma patient survival. Oncotarget. 2016;7:80680–7. doi: 10.18632/oncotarget.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu B, Zhang D, Wang C, Tao J, Tie X, Qiao Y, et al. IL-10 and TGF-β2 are overexpressed in tumor spheres cultured from human gliomas. Mol Biol Rep. 2011;38:3585–91. doi: 10.1007/s11033-010-0469-4. [DOI] [PubMed] [Google Scholar]
- 18.Che F, Yin J, Quan Y, Xie X, Heng X, Du Y, et al. TLR4 interaction with LPS in glioma CD133+ cancer stem cells induces cell proliferation, resistance to chemotherapy and evasion from cytotoxic T lymphocyte-induced cytolysis. Oncotarget. 2017;8:53495–507. doi: 10.18632/oncotarget.18586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei J, Barr J, Kong L-Y, Wang Y, Wu A, Sharma AK, et al. Glioma-associated cancer-initiating cells induce immunosuppression. Clin Cancer Res. 2010;16:461–73. doi: 10.1158/1078-0432.CCR-09-1983. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Wu A, Wei J, Kong L-Y, Wang Y, Priebe W, Qiao W, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12:1113–25. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donson AM, Apps J, Griesinger AM, Amani V, Witt DA, Anderson RCE, et al. Molecular analyses reveal inflammatory mediators in the solid component and cyst fluid of human adamantinomatous craniopharyngioma. J Neuropathol Exp Neurol. 2017;76:779–88. doi: 10.1093/jnen/nlx061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akil H, Abbaci A, Lalloué F, Bessette B, Costes LMM, Domballe L, et al. IL22/IL-22R pathway induces cell survival in human glioblastoma cells. PLoS ONE. 2015;10:e0119872. doi: 10.1371/journal.pone.0119872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riley JK, Takeda K, Akira S, Schreiber RD. Requirement for two distinct receptor-derived signals for anti-inflammatory action. J Biol Chem. 1999;274:16513–21. doi: 10.1074/jbc.274.23.16513. [DOI] [PubMed] [Google Scholar]
- 24.Qi L, Yu H, Zhang Y, Zhao D, Lv P, Zhong Y, et al. IL-10 secreted by M2 macrophage promoted tumorigenesis through interaction with JAK2 in glioma. Oncotarget. 2016;7:71673–85. doi: 10.18632/oncotarget.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García-Hernández ML, Hernández-Pando R, Gariglio P, Berumen J. Interleukin-10 promotes B16-melanoma growth by inhibition of macrophage functions and induction of tumour and vascular cell proliferation. Immunology. 2002;105:231–43. doi: 10.1046/j.1365-2567.2002.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Huang X, Li J, Fan H, Yang F, Zhang R, et al. Interleukin 10 promotes growth and invasion of glioma cells by up-regulating KPNA 2 in vitro. J Cancer Res Ther. 2019;15:927–32. doi: 10.4103/jcrt.JCRT_284_19. [DOI] [PubMed] [Google Scholar]
- 27.Guven-Maiorov E, Acuner-Ozbabacan SE, Keskin O, Gursoy A, Nussinov R. Structural pathways of cytokines may illuminate their roles in regulation of cancer development and immunotherapy. Cancers. 2014;6:663–83. doi: 10.3390/cancers6020663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobo-Silva D, Carriche GM, Castro AG, Roque S, Saraiva M. Balancing the immune response in the brain: IL-10 and its regulation. J Neuroinflammation. 2016;13:297. doi: 10.1186/s12974-016-0763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Book AA, Fielding KE, Kundu N, Wilson MA, Fulton AM, Laterra J. IL-10 gene transfer to intracranial 9L glioma: tumor inhibition and cooperation with IL-2. J Neuroimmunol. 1998;92:50–9. doi: 10.1016/s0165-5728(98)00172-6. [DOI] [PubMed] [Google Scholar]
- 30.Wang J-J, Siu MK-Y, Jiang Y-X, Chan DW, Cheung AN-Y, Ngan HY-S, et al. Infiltration of T cells promotes the metastasis of ovarian cancer cells via the modulation of metastasis-related genes and PD-L1 expression. Cancer Immunol Immunother. 2020;69:2275–89. doi: 10.1007/s00262-020-02621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardoso AP, Pinto ML, Pinto AT, Pinto MT, Monteiro C, Oliveira MI, et al. Matrix metalloproteases as maestros for the dual role of LPS- and IL-10-stimulated macrophages in cancer cell behaviour. BMC Cancer. 2015;15:456. doi: 10.1186/s12885-015-1466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gieryng A, Pszczolkowska D, Walentynowicz KA, Rajan WD, Kaminska B. Immune microenvironment of gliomas. Lab Invest. 2017;97:498–518. doi: 10.1038/labinvest.2017.19. [DOI] [PubMed] [Google Scholar]
- 33.Prosniak M, Harshyne LA, Andrews DW, Kenyon LC, Bedelbaeva K, Apanasovich TV, et al. Glioma grade is associated with the accumulation and activity of cells bearing M2 monocyte markers. Clin Cancer Res. 2013;19:3776–86. doi: 10.1158/1078-0432.CCR-12-1940. [DOI] [PubMed] [Google Scholar]
- 34.Nitta T, Hishii M, Sato K, Okumura K. Selective expression of interleukin-10 gene within glioblastoma multiforme. Brain Res. 1994;649:122–8. doi: 10.1016/0006-8993(94)91055-3. [DOI] [PubMed] [Google Scholar]
- 35.Jabłońska-Trypuć A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzym Inhib Med Chem. 2016;31:177–83. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 36.Gabrusiewicz K, Ellert-Miklaszewska A, Lipko M, Sielska M, Frankowska M, Kaminska B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS ONE. 2011;6:e23902. doi: 10.1371/journal.pone.0023902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siddiqui T, Lively S, Ferreira R, Wong R, Schlichter LC. Expression and contributions of TRPM7 and KCa2.3/SK3 channels to the increased migration and invasion of microglia in anti-inflammatory activation states. PLoS ONE. 2014;9:e106087. doi: 10.1371/journal.pone.0106087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–26. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stylli SS, Kaye AH, Lock P. Invadopodia: at the cutting edge of tumour invasion. J Clin Neurosci. 2008;15:725–37. doi: 10.1016/j.jocn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 2: Studies of the macrophage content of human brain tumors. J Neurosurg. 1979;50:305–11. doi: 10.3171/jns.1979.50.3.0305. [DOI] [PubMed] [Google Scholar]
- 41.Rossi ML, Hughes JT, Esiri MM, Coakham HB, Brownell DB. Immunohistological study of mononuclear cell infiltrate in malignant gliomas. Acta Neuropathol. 1987;74:269–77. doi: 10.1007/BF00688191. [DOI] [PubMed] [Google Scholar]
- 42.Badie B, Schartner JM. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery. 2000;46:957–61. doi: 10.1097/00006123-200004000-00035. [DOI] [PubMed] [Google Scholar]
- 43.Bowman RL, Klemm F, Akkari L, Pyonteck SM, Sevenich L, Quail DF, et al. Macrophage ontogeny underlies differences in tumor-specific education in brain malignancies. Cell Rep. 2016;17:2445–59. doi: 10.1016/j.celrep.2016.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Z, Feng X, Herting CJ, Garcia VA, Nie K, Pong WW, et al. Cellular and molecular identity of tumor-associated macrophages in glioblastoma. Cancer Res. 2017;77:2266–78. doi: 10.1158/0008-5472.CAN-16-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19:3165–75. doi: 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darmanis S, Sloan SA, Croote D, Mignardi M, Chernikova S, Samghababi P, et al. Single-cell RNA-Seq analysis of infiltrating neoplastic cells at the migrating front of human glioblastoma. Cell Rep. 2017;21:1399–410. doi: 10.1016/j.celrep.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, Alizadeh D, Van Handel M, Kortylewski M, Yu H, Badie B. Stat3 inhibition activates tumor macrophages and abrogates glioma growth in mice. Glia. 2009;57:1458–67. doi: 10.1002/glia.20863. [DOI] [PubMed] [Google Scholar]
- 48.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 49.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Müller S, Kohanbash G, Liu SJ, Alvarado B, Carrera D, Bhaduri A, et al. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol. 2017;18:234. doi: 10.1186/s13059-017-1362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergamin LS, Braganhol E, Figueiró F, Casali EA, Zanin RF, Sévigny J, et al. Involvement of purinergic system in the release of cytokines by macrophages exposed to glioma-conditioned medium. J Cell Biochem. 2015;116:721–9. doi: 10.1002/jcb.25018. [DOI] [PubMed] [Google Scholar]
- 52.Kostianovsky AM, Maier LM, Anderson RC, Bruce JN, Anderson DE. Astrocytic regulation of human monocytic/microglial activation. J Immunol. 2008;181:5425–32. doi: 10.4049/jimmunol.181.8.5425. [DOI] [PubMed] [Google Scholar]
- 53.Li Z, Zhang J, Zheng H, Li C, Xiong J, Wang W, et al. Modulating lncRNA SNHG15/CDK6/miR-627 circuit by palbociclib, overcomes temozolomide resistance and reduces M2-polarization of glioma associated microglia in glioblastoma multiforme. J Exp Clin Cancer Res. 2019;38:380. doi: 10.1186/s13046-019-1371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chuang Y, Hung ME, Cangelose BK, Leonard JN. Regulation of the IL-10-driven macrophage phenotype under incoherent stimuli. Innate Immun. 2016;22:647–57. doi: 10.1177/1753425916668243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–63. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 56.Bender AM, Collier LS, Rodriguez FJ, Tieu C, Larson JD, Halder C, et al. Sleeping beauty-mediated somatic mutagenesis implicates CSF1 in the formation of high-grade astrocytomas. Cancer Res. 2010;70:3557–65. doi: 10.1158/0008-5472.CAN-09-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gabrusiewicz K, Rodriguez B, Wei J, Hashimoto Y, Healy LM, Maiti SN, et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI Insight 2016;1:e85841. [DOI] [PMC free article] [PubMed]
- 58.Makita N, Hizukuri Y, Yamashiro K, Murakawa M, Hayashi Y. IL-10 enhances the phenotype of M2 macrophages induced by IL-4 and confers the ability to increase eosinophil migration. Int Immunol. 2015;27:131–41. doi: 10.1093/intimm/dxu090. [DOI] [PubMed] [Google Scholar]
- 59.Mittal SK, Cho K-J, Ishido S, Roche PA. Interleukin 10 (IL-10)-mediated Immunosuppression: MARCH-I induction regulates antigen presentation by macrophages but not dendritic cells. J Biol Chem. 2015;290:27158–67. doi: 10.1074/jbc.M115.682708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao Y, Ye H, Qi Z, Mo L, Yue Q, Baral A, et al. B7-H4(B7x)-mediated cross-talk between glioma-initiating cells and macrophages via the IL6/JAK/STAT3 pathway lead to poor prognosis in glioma patients. Clin Cancer Res. 2016;22:2778–90. doi: 10.1158/1078-0432.CCR-15-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ratnam NM, Gilbert MR, Giles AJ. Immunotherapy in CNS cancers: the role of immune cell trafficking. Neuro Oncol. 2019;21:37–46. doi: 10.1093/neuonc/noy084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alvarez JI, Teale JM. Multiple expression of matrix metalloproteinases in murine neurocysticercosis: implications for leukocyte migration through multiple central nervous system barriers. Brain Res. 2008;1214:145–58. doi: 10.1016/j.brainres.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dubois LG, Campanati L, Righy C, D’Andrea-Meira I, Spohr TCL, de SE, Porto-Carreiro I, et al. Gliomas and the vascular fragility of the blood brain barrier. Front Cell Neurosci. 2014;8:418. doi: 10.3389/fncel.2014.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wöhrer A, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;17:1064–75. doi: 10.1093/neuonc/nou307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woroniecka K, Chongsathidkiet P, Rhodin K, Kemeny H, Dechant C, Farber SH, et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 2018;24:4175–86. doi: 10.1158/1078-0432.CCR-17-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klemm F, Maas RR, Bowman RL, Kornete M, Soukup K, Nassiri S, et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell. 2020;181:1643.e17–60.e17. doi: 10.1016/j.cell.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Farber SH, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24:1459–68. doi: 10.1038/s41591-018-0135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han S, Zhang C, Li Q, Dong J, Liu Y, Huang Y, et al. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014;110:2560–8. doi: 10.1038/bjc.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zadka L, Kram P, Koscinski J, Jankowski R, Kaczmarek M, Piatek K, et al. Association between interleukin-10 receptors and the CD45-immunophenotype of central nervous system tumors: a preliminary study. Anticancer Res. 2017;37:5777–83. doi: 10.21873/anticanres.12019. [DOI] [PubMed] [Google Scholar]
- 70.El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006;8:234–43. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 72.Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Sun W, Qiao W, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14:5166–72. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- 73.Sawant DV, Yano H, Chikina M, Zhang Q, Liao M, Liu C, et al. Adaptive plasticity of IL-10+ and IL-35+ Treg cells cooperatively promotes tumor T cell exhaustion. Nat Immunol. 2019;20:724–35. doi: 10.1038/s41590-019-0346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Z, Liu X, Guo R, Wang PCD4. Foxp3- type 1 regulatory T cells in glioblastoma multiforme suppress T cell responses through multiple pathways and are regulated by tumor-associated macrophages. Int J Biochem Cell Biol. 2016;81:1–9. doi: 10.1016/j.biocel.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 75.Groux H. Type 1 T-regulatory cells: their role in the control of immune responses. Transplantation. 2003;75:8S–12S. doi: 10.1097/01.TP.0000067944.90241.BD. [DOI] [PubMed] [Google Scholar]
- 76.Akasaki Y, Liu G, Chung NHC, Ehtesham M, Black KL, Yu JS. Induction of a CD4+ T regulatory type 1 response by cyclooxygenase-2-overexpressing glioma. J Immunol. 2004;173:4352–9. doi: 10.4049/jimmunol.173.7.4352. [DOI] [PubMed] [Google Scholar]
- 77.Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich J-M, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–78. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–60. [PubMed] [Google Scholar]
- 79.Wang S, Gao X, Shen G, Wang W, Li J, Zhao J, et al. Interleukin-10 deficiency impairs regulatory T cell-derived neuropilin-1 functions and promotes Th1 and Th17 immunity. Sci Rep. 2016;6:24249. doi: 10.1038/srep24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang AS, Lattime EC. Tumor-induced interleukin 10 suppresses the ability of splenic dendritic cells to stimulate CD4 and CD8 T-cell responses. Cancer Res. 2003;63:2150–7. [PubMed] [Google Scholar]
- 81.Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, et al. IL-10 elicits IFNγ-dependent tumor immune surveillance. Cancer Cell. 2011;20:781–96. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 82.Dennis KL, Saadalla A, Blatner NR, Wang S, Venkateswaran V, Gounari F, et al. T-cell expression of IL10 is essential for tumor immune surveillance in the small intestine. Cancer Immunol Res. 2015;3:806–14. doi: 10.1158/2326-6066.CIR-14-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xi J, Xu M, Song Z, Li H, Xu S, Wang C, et al. Stimulatory role of interleukin 10 in CD8+ T cells through STATs in gastric cancer. Tumour Biol. 2017;39:1010428317706209. doi: 10.1177/1010428317706209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Emmerich J, Mumm JB, Chan IH, LaFace D, Truong H, McClanahan T, et al. IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res. 2012;72:3570–81. doi: 10.1158/0008-5472.CAN-12-0721. [DOI] [PubMed] [Google Scholar]
- 85.De Vleeschouwer S, Spencer Lopes I, Ceuppens JL, Van Gool SW. Persistent IL-10 production is required for glioma growth suppressive activity by Th1-directed effector cells after stimulation with tumor lysate-loaded dendritic cells. J Neurooncol. 2007;84:131–40. doi: 10.1007/s11060-007-9362-y. [DOI] [PubMed] [Google Scholar]
- 86.Kmiecik J, Poli A, Brons NHC, Waha A, Eide GE, Enger PØ, et al. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol. 2013;264:71–83. doi: 10.1016/j.jneuroim.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 87.Nijaguna MB, Patil V, Hegde AS, Chandramouli BA, Arivazhagan A, Santosh V, et al. An eighteen serum cytokine signature for discriminating glioma from normal healthy individuals. PLoS ONE. 2015;10:e0137524. doi: 10.1371/journal.pone.0137524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar R, Kamdar D, Madden L, Hills C, Crooks D, O’Brien D, et al. Th1/Th2 cytokine imbalance in meningioma, anaplastic astrocytoma and glioblastoma multiforme patients. Oncol Rep. 2006;15:1513–6. doi: 10.3892/or.15.6.1513. [DOI] [PubMed] [Google Scholar]
- 89.Vidyarthi A, Agnihotri T, Khan N, Singh S, Tewari MK, Radotra BD, et al. Predominance of M2 macrophages in gliomas leads to the suppression of local and systemic immunity. Cancer Immunol. Immunother. 2019;68:1995–2004. doi: 10.1007/s00262-019-02423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matilainen JM, Husso T, Toropainen S, Seuter S, Turunen MP, Gynther P, et al. Primary effect of 1α,25(OH)2D3 on IL-10 expression in monocytes is short-term down-regulation. Biochim Biophys Acta. 2010;1803:1276–86. doi: 10.1016/j.bbamcr.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 91.Berezhnoy A, Stewart CA, Mcnamara JO, 2nd, Thiel W, Giangrande P, Trinchieri G, et al. Isolation and optimization of murine IL-10 receptor blocking oligonucleotide aptamers using high-throughput sequencing. Mol Ther. 2012;20:1242–50. doi: 10.1038/mt.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ni G, Chen S, Yang Y, Cummins SF, Zhan J, Li Z, et al. Investigation the possibility of using peptides with a helical repeating pattern of hydro-phobic and hydrophilic residues to inhibit IL-10. PLoS ONE. 2016;11:e0153939. doi: 10.1371/journal.pone.0153939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fujiwara Y, Komohara Y, Kudo R, Tsurushima K, Ohnishi K, Ikeda T, et al. Oleanolic acid inhibits macrophage differentiation into the M2 phenotype and glioblastoma cell proliferation by suppressing the activation of STAT3. Oncol Rep. 2011;26:1533–7. doi: 10.3892/or.2011.1454. [DOI] [PubMed] [Google Scholar]
- 94.Sun Z, Fourcade J, Pagliano O, Chauvin J-M, Sander C, Kirkwood JM, et al. IL10 and PD-1 cooperate to limit the activity of tumor-specific CD8+ T cells. Cancer Res. 2015;75:1635–44. doi: 10.1158/0008-5472.CAN-14-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lamichhane P, Karyampudi L, Shreeder B, Krempski J, Bahr D, Daum J, et al. IL10 release upon PD-1 blockade sustains immunosuppression in ovarian cancer. Cancer Res. 2017;77:6667–78. doi: 10.1158/0008-5472.CAN-17-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen S, Ni G, Wu X, Zhu B, Liao Z, Wang Y, et al. Blocking IL-10 signalling at the time of immunization renders the tumour more accessible to T cell infiltration in mice. Cell Immunol. 2016;300:9–17. doi: 10.1016/j.cellimm.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 97.Ni G, Liao Z, Chen S, Wang T, Yuan J, Pan X, et al. Blocking IL-10 signalling at the time of immunization does not increase unwanted side effects in mice. BMC Immunol. 2017;18:40. doi: 10.1186/s12865-017-0224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Naing A, Infante JR, Papadopoulos KP, Chan IH, Shen C, Ratti NP, et al. PEGylated IL-10 (Pegilodecakin) induces systemic immune activation, CD8+ T cell invigoration and polyclonal T cell expansion in cancer patients. Cancer Cell. 2018;34:775.e3–91.e3. doi: 10.1016/j.ccell.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Naing A, Wong DJ, Infante JR, Korn WM, Aljumaily R, Papadopoulos KP, et al. Pegilodecakin combined with pembrolizumab or nivolumab for patients with advanced solid tumours (IVY): a multicentre, multicohort, open-label, phase 1b trial. Lancet Oncol. 2019;20:1544–55. doi: 10.1016/S1470-2045(19)30514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hecht JR, Lonardi S, Bendell JC, Sim H-W, Macarulla T, Lopez CD, et al. Randomized phase III study of FOLFOX alone and with Pegilodecakin as second-line therapy in patients with metastatic pancreatic cancer (SEQUOIA) J Clin Oncol. 2020;38:637. doi: 10.1200/JCO.20.02232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Naing A, Papadopoulos KP, Autio KA, Ott PA, Patel MR, Wong DJ, et al. Safety, antitumor activity, and immune activation of pegylated recombinant human interleukin-10 (AM0010) in patients with advanced solid tumors. J Clin Oncol. 2016;34:3562–9. doi: 10.1200/JCO.2016.68.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qiao J, Liu Z, Dong C, Luan Y, Zhang A, Moore C, et al. Targeting tumors with IL-10 prevents dendritic cell-mediated CD8+ T cell apoptosis. Cancer Cell. 2019;35:901–.e4. doi: 10.1016/j.ccell.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 103.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–81. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 104.Ananieva O, Darragh J, Johansen C, Carr JM, McIlrath J, Park JM, et al. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat Immunol. 2008;9:1028–36. doi: 10.1038/ni.1644. [DOI] [PubMed] [Google Scholar]
- 105.Daniel P, Filiz G, Brown DV, Hollande F, Gonzales M, D’Abaco G, et al. Selective CREB-dependent cyclin expression mediated by the PI3K and MAPK pathways supports glioma cell proliferation. Oncogenesis. 2014;3:e108. doi: 10.1038/oncsis.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodón L, Gonzàlez-Juncà A, Inda M, del M, Sala-Hojman A, Martínez-Sáez E, Seoane J. Active CREB1 promotes a malignant TGFβ2 autocrine loop in glioblastoma. Cancer Discov. 2014;4:1230–41. doi: 10.1158/2159-8290.CD-14-0275. [DOI] [PubMed] [Google Scholar]
- 107.Daniel PM, Filiz G, Brown DV, Christie M, Waring PM, Zhang Y, et al. PI3K activation in neural stem cells drives tumorigenesis which can be ameliorated by targeting the cAMP response element binding protein. Neuro Oncol. 2018;20:1344–55. doi: 10.1093/neuonc/noy068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li BX, Gardner R, Xue C, Qian DZ, Xie F, Thomas G, et al. Systemic inhibition of CREB is well-tolerated in vivo. Sci Rep. 2016;6:34513. doi: 10.1038/srep34513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guan X, Wang Y, Kai G, Zhao S, Huang T, Li Y, et al. Cerebrolysin ameliorates focal cerebral ischemia injury through neuroinflammatory inhibition via CREB/PGC-1α pathway. Front Pharmacol. 2019;10:1245. doi: 10.3389/fphar.2019.01245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaiser M, Wiggin GR, Lightfoot K, Arthur JSC, Macdonald A. MSK regulate TCR-induced CREB phosphorylation but not immediate early gene transcription. Eur J Immunol. 2007;37:2583–95. doi: 10.1002/eji.200636606. [DOI] [PubMed] [Google Scholar]
- 111.Srinivasan S, Totiger T, Shi C, Castellanos J, Lamichhane P, Dosch AR, et al. Tobacco carcinogen-induced production of GM-CSF activates CREB to promote pancreatic cancer. Cancer Res. 2018;78:6146–58. doi: 10.1158/0008-5472.CAN-18-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rodríguez-Ubreva J, Català-Moll F, Obermajer N, Álvarez-Errico D, Ramirez RN, Company C, et al. Prostaglandin E2 leads to the acquisition of DNMT3A-dependent tolerogenic functions in human myeloid-derived suppressor cells. Cell Rep. 2017;21:154–67. doi: 10.1016/j.celrep.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 113.Chanteux H, Guisset AC, Pilette C, Sibille Y. LPS induces IL-10 production by human alveolar macrophages via MAPKinases- and Sp1-dependent mechanisms. Respir Res. 2007;8:71. doi: 10.1186/1465-9921-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seznec J, Silkenstedt B, Naumann U. Therapeutic effects of the Sp1 inhibitor mithramycin A in glioblastoma. J Neurooncol. 2011;101:365–77. doi: 10.1007/s11060-010-0266-x. [DOI] [PubMed] [Google Scholar]
- 115.Koyama Y, Kotani M, Sawamura T, Kuribayashi M, Konishi R, Michinaga S. Different actions of endothelin-1 on chemokine production in rat cultured astrocytes: reduction of CX3CL1/fractalkine and an increase in CCL2/MCP-1 and CXCL1/CINC-1. J Neuroinflammation. 2013;10:51. doi: 10.1186/1742-2094-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ruffell D, Mourkioti F, Gambardella A, Kirstetter P, Lopez RG, Rosenthal N, et al. A CREB-C/EBPβ cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci USA. 2009;106:17475–80. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sanin DE, Prendergast CT, Mountford AP. IL-10 production in macrophages is regulated by a TLR-driven CREB-mediated mechanism that is linked to genes involved in cell metabolism. J Immunol. 2015;195:1218–32. doi: 10.4049/jimmunol.1500146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Csóka B, Németh ZH, Virág L, Gergely P, Leibovich SJ, Pacher P, et al. A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood. 2007;110:2685–95. doi: 10.1182/blood-2007-01-065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brightbill HD, Plevy SE, Modlin RL, Smale ST. A prominent role for Sp1 during lipopolysaccharide-mediated induction of the IL-10 promoter in macrophages. J Immunol. 2000;164:1940–51. doi: 10.4049/jimmunol.164.4.1940. [DOI] [PubMed] [Google Scholar]
- 120.Ma W, Lim W, Gee K, Aucoin S, Nandan D, Kozlowski M, et al. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J Biol Chem. 2001;276:13664–74. doi: 10.1074/jbc.M011157200. [DOI] [PubMed] [Google Scholar]
- 121.Cao S, Zhang X, Edwards JP, Mosser DM. NF-κB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J Biol Chem. 2006;281:26041–50. doi: 10.1074/jbc.M602222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cao S, Liu J, Song L, Ma X. The protooncogene c-Maf is an essential transcription factor for IL-10 gene expression in macrophages. J Immunol. 2005;174:3484–92. doi: 10.4049/jimmunol.174.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gollnick SO, Lee BY, Vaughan L, Owczarczak B, Henderson BW. Activation of the IL-10 gene promoter following photodynamic therapy of murine keratinocytes. Photochem Photobiol. 2001;73:170–7. doi: 10.1562/0031-8655(2001)073<0170:aotigp>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang S, Gao L, Lu F, Wang B, Gao F, Zhu G, et al. Transcription factor myocyte enhancer factor 2D regulates interleukin-10 production in microglia to protect neuronal cells from inflammation-induced death. J Neuroinflammation. 2015;12:33. doi: 10.1186/s12974-015-0258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koscsó B, Csóka B, Selmeczy Z, Himer L, Pacher P, Virág L, et al. Adenosine augments IL-10 production by microglial cells through an A2B adenosine receptor-mediated process. J Immunol. 2012;188:445–53. doi: 10.4049/jimmunol.1101224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TBH. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007;26:605–16. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 127.Kitani A, Fuss I, Nakamura K, Kumaki F, Usui T, Strober W. Transforming growth factor (TGF)-beta1-producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF-beta1-mediated fibrosis. J Exp Med. 2003;198:1179–88. doi: 10.1084/jem.20030917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huss DJ, Winger RC, Cox GM, Guerau-de-Arellano M, Yang Y, Racke MK, et al. TGF-β signaling via Smad4 drives IL-10 production in effector Th1 cells and reduces T-cell trafficking in EAE. Eur J Immunol. 2011;41:2987–96. doi: 10.1002/eji.201141666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Motomura Y, Kitamura H, Hijikata A, Matsunaga Y, Matsumoto K, Inoue H, et al. The transcription factor E4BP4 regulates the production of IL-10 and IL-13 in CD4+ T cells. Nat Immunol. 2011;12:450–9. doi: 10.1038/ni.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee C-G, Hwang W, Maeng K-E, Kwon H-K, So J-S, Sahoo A, et al. IRF4 regulates IL-10 gene expression in CD4(+) T cells through differential nuclear translocation. Cell Immunol. 2011;268:97–104. doi: 10.1016/j.cellimm.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 131.Neumann C, Heinrich F, Neumann K, Junghans V, Mashreghi M-F, Ahlers J, et al. Role of Blimp-1 in programing Th effector cells into IL-10 producers. J Exp Med. 2014;211:1807–19. doi: 10.1084/jem.20131548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin C-C, Bradstreet TR, Schwarzkopf EA, Sim J, Carrero JA, Chou C, et al. Bhlhe40 controls cytokine production by T cells and is essential for pathogenicity in autoimmune neuroinflammation. Nat Commun. 2014;5:3551. doi: 10.1038/ncomms4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shoemaker J, Saraiva M, O’Garra A. GATA-3 directly remodels the IL-10 locus independently of IL-4 in CD4+ T cells. J Immunol. 2006;176:3470–9. doi: 10.4049/jimmunol.176.6.3470. [DOI] [PubMed] [Google Scholar]
- 134.Koh B, Hufford MM, Sun X, Kaplan MH. Etv5 regulates IL-10 production in Th cells. J Immunol. 2017;198:2165–71. doi: 10.4049/jimmunol.1600801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu J, Yang Y, Qiu G, Lal G, Wu Z, Levy DE, et al. c-Maf regulates IL-10 expression during Th17 polarization. J Immunol. 2009;182:6226–36. doi: 10.4049/jimmunol.0900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tanaka S, Jiang Y, Martinez GJ, Tanaka K, Yan X, Kurosaki T, et al. Trim33 mediates the proinflammatory function of Th17 cells. J Exp Med. 2018;215:1853–68. doi: 10.1084/jem.20170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li P, Spolski R, Liao W, Wang L, Murphy TL, Murphy KM, et al. BATF-JUN is critical for IRF4-mediated transcription in T cells. Nature. 2012;490:543–6. doi: 10.1038/nature11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–61. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martins GA, Cimmino L, Shapiro-Shelef M, Szabolcs M, Herron A, Magnusdottir E, et al. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nat Immunol. 2006;7:457–65. doi: 10.1038/ni1320. [DOI] [PubMed] [Google Scholar]
- 140.Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M, et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol. 2011;12:304–11. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 141.Xie F, Li BX, Kassenbrock A, Xue C, Wang X, Qian DZ, et al. Identification of a potent inhibitor of CREB-mediated gene transcription with efficacious in vivo anticancer activity. J Med Chem. 2015;58:5075–87. doi: 10.1021/acs.jmedchem.5b00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Results generated in Fig. 1 used gene expression data generated by the TCGA Research Network: (https://www.cancer.gov/tcga) and the Chinese Glioma Genome Atlas: (http://www.cgga.org.cn/).