Abstract
Purpose
To evaluate whether young women with idiopathic early ovarian aging, as defined by producing fewer oocytes than expected for a given age over multiple in vitro fertilization (IVF) cycles, have changes in telomere length and epigenetic age indicating accelerated biological aging (i.e., increased risk of morbidity and mortality).
Methods
A prospective cohort study was conducted at two Danish public fertility clinics. A total of 55 young women (≤ 37 years) with at least two IVF cycles with ≤ 5 harvested oocytes despite sufficient stimulation with follicle-stimulating hormone (FSH) were included in the early ovarian aging group. As controls, 52 young women (≤ 37 years) with normal ovarian function, defined by at least eight harvested oocytes, were included. Relative telomere length (rTL) and epigenetic age acceleration (AgeAccel) were measured in white blood cells as markers of premenopausal accelerated biological aging.
Results
rTL was comparable with a mean of 0.46 (± SD 0.12) in the early ovarian aging group and 0.47 (0.14) in the normal ovarian aging group. The AgeAccel of the early ovarian aging group was, insignificantly, 0.5 years older, but this difference disappeared when adjusting for chronological age. Sub-analysis using Anti-Müllerian hormone (AMH) as selection criterion for the two groups did not change the results.
Conclusion
We did not find any indications of accelerated aging in whole blood from young women with idiopathic early ovarian aging. Further investigations in a similar cohort of premenopausal women or other tissues are needed to fully elucidate the potential relationship between premenopausal accelerated biological aging and early ovarian aging.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-021-02326-7.
Keywords: Early ovarian aging, Telomere, Accelerated aging, Epigenetics
Introduction
Ovarian aging describes the natural age-related process of loss of the primordial follicles with a concomitant decline in oocyte quality [1]. The age-related reduction in ovarian reserve determines the definite end of reproductive potential at menopause. Up to 10% of females experience early ovarian aging (EOA) with an accelerated loss of primordial follicles leading to either premature (< 40 years) or early menopause (< 45 years) [2], whereas normal ovarian aging results in menopause at an average age of 51 years [3, 4]. Both early and premature menopause are associated with increased risk of several adverse health effects that normally are considered to be indicative of biological aging such as cardiovascular disease (CVD), osteoporosis, and mortality [5–9] indicating that ovarian aging is a marker of biological aging [5, 10]. The association between aging and time of menopause is further supported by studies reporting long life expectancy (longevity) and lower risk of CVD, ischemic heart disease, and all-cause mortality, in women with late reproduction or late menopausal age [7, 11–13].
The early aging process in women with accelerated loss of ovarian reserve may begin before menopause, indicating that the postmenopausal estrogen deficiency may not be the major contributing factor [14]. Studies on young premenopausal women with unexplained low ovarian reserve have shown a more unfavorable CVD risk profile (e.g., increased blood pressure, cholesterol, high-density lipoprotein, fasting glucose), and decreased bone mass density, when compared to young women with normal ovarian reserve [15–20].
Telomere length (TL) has been used as marker of biological aging for decades [21, 22] and consists of repetitive DNA sequences that cap chromosome ends and prevent chromosome degradation and fusion, safeguarding genome integrity [23]. Thus, telomere attrition is associated with aging [24–26]. An accelerated loss of TL has been associated with an increased incidence of age-related events such as CVD [27] and mortality [28], highlighting the potential of TL as marker of biological aging. TL has also been used in relation to ovarian aging [29–35] but with conflicting results on the association of white blood cell (WBC) TL and low ovarian reserve in young women [33–35].
Recently, an epigenetic “clock” that utilizes DNA methylation (DNAm) profiles to produce accurate predictions of chronological age in humans (“Horvath’s Clock”) has been developed [36].The clock has potential to map derivations from normal aging when the predicted DNAm age differs from chronological age. Thus, a DNAm age greater than the chronological age (i.e., age acceleration) has been associated with an increased risk of a variety of age-related diseases (e.g., CVD and cancer) and all-cause mortality [37–39]. Hence, several observations support the use of DNAm age as a new and promising indicator of biological aging [37–39]. In 2018, Horvath’s clock was updated allowing for a more accurate estimation of the chronological age by using a variety of different tissues, including whole blood (“Skin and Blood Clock”) [40, 41].
The use of epigenetic age as a marker of biological age in relation to ovarian aging has only been evaluated in few studies [33, 42–45]. The findings of a negative association between accelerated epigenetic age and age at menopause (i.e., women with late menopause are epigenetically younger) seem to support the concept of ovarian reserve and epigenetic age being interrelated [42]. While the latter study was restricted to postmenopausal women only, a recent smaller study found an accelerated epigenetic age associated with low ovarian reserve evaluated by Anti-Müllerian hormone (AMH) and oocyte yield in premenopausal women [44]. Although these findings further indicate a possible association of premenopausal accelerated epigenetic age and low ovarian reserve, the study by Monseur et al. was not restricted solely to young women with unexplained EOA [44]. Only two studies have evaluated epigenetic age as a marker of biological age in a population of young women with EOA but with opposite results [33, 43].
The limited literature on DNAm age in young premenopausal women and its promising potential as a new marker for biological aging, taken together with the conflicting literature on TL and early ovarian aging, support the necessity to evaluate the possible presence of premenopausal accelerated aging in young women with unexplained low ovarian reserve. IVF treatment offers a unique possibility for identifying young women with idiopathic early ovarian aging.
The association of oocyte output following ovarian stimulation and assessment of ovarian reserve [46–48], as well as its ability to predict the time to menopause [46, 47, 49], makes it a reliable identifier of women with early ovarian aging. Based on previous findings of increased risk of age-related morbidity and mortality in young women with idiopathic ovarian aging, we therefore aimed to evaluate if TL and epigenetic methylation age could support a concept of accelerated premenopausal biological aging in this group of women.
Materials and methods
Young cyclic women undergoing ART with either IVF or ICSI in two public Danish fertility clinics during the period January 2016 to December 2018 were invited to participate in the study prior to oocyte retrieval. To identify women with an exceptional and pathological low ovarian reserve for age, a woman was eligible if she was ≤ 37 years by the time of recruitment and fulfilled the inclusion criteria for either the EOA group or the normal ovarian aging group (NOA). Women were excluded if they had a diagnosis of either cancer, endometriosis, polycystic ovarian syndrome (PCOS), anovulation, Turner syndrome, Fragile-X syndrome, presence of FMR1 mutation, a history of ovarian surgery, chemotherapy/irradiation, or were having ART for the purposes of preimplantation genetic testing (PGT). PGT screening for aneuploidies (PGT-A) in infertile couples is not used in the participating clinics.
EOA was defined by a minimum of two cycles with ≤ 5 oocytes harvested, despite sufficient stimulation (> 250 IU) with follicle-stimulating hormone (FSH). Thus, inclusion to the study was only possible when at least one IVF/ICSI cycle had resulted in harvest of ≤ 5 oocytes. A total of 55 women were included in this group.
A total of 52 women were included in the control group and had normal ovarian aging defined as a minimum of one cycle with ≥ 8 oocytes harvested (i.e., women in the NOA group could be recruited in their first IVF/ICSI cycle). Opposite to the EOA group, there were no requirements of a minimum dose of FSH used for ovarian stimulation. The number of oocytes used for defining the two ovarian reserve groups was based on empirical knowledge combined with suggested thresholds based on the existing literature [14, 33, 43, 50].
Ovarian stimulation/procedure
Controlled ovarian stimulation was carried out with either a short antagonist protocol or a long agonist protocol using urinary or recombinant FSH. Choice of protocol was based on individual factors such as age, ovarian reserve testing (AMH, antral follicle count), or outcomes of previous cycles, and was made regardless of participation in the study. FSH doses were adjusted individually according to the patients’ ovarian response. Follicular development was monitored with ultrasound and ovulation was induced by either hCG administration, when follicles ≥ 17 mm (optimally at least three) were observed, or, in case of increased risk of hyperstimulation syndrome, by GnRH agonist administration. Oocyte retrieval was conducted by transvaginal ultrasound-guided puncture of ovarian follicles 34–36 h after hCG/GnRH agonist administration.
Collection of blood samples, DNA purification, and storage of samples
Peripheral blood samples (EDTA blood) were collected on the day of oocyte retrieval and shipped to the analysis laboratory for DNA purification. Due to practical circumstances, all samples were shipped and stored at room temperature until DNA purification could be carried out. Thus, WBC DNA was isolated within 5 days from retrieval. MagNa Pure Compact (ROCHE®) was used to isolate WBC DNA according to the manufacturer’s instruction. All DNA samples were stored at − 80° C until analysis.
Telomere length
TL was assessed by measuring the relative TL (rTL) of WBC DNA by following the mmqPCR protocol developed by Richard M. Cawthon [51]. Briefly, genomic DNA was diluted with nuclease-free water in a 1.5-ml Eppendorf tube at a concentration of 1 ng/µl DNA as template. The standard curve was prepared with fivefold diluted placenta gDNA in nuclease-free water from 10 to 0.016 ng/µl. All primers used were purchased from Integrated DNA Technologies® and reconstructed in TE buffer at stock concentration of 100 µM. Each reaction contained 5 µl of DNA template, 0.9 µl of each primer at working concentration of 10 µM, 10 µl of SYBR green mix (Bio-Rad®), and 1.4 µl of water. Each sample was set as duplicates. After centrifuging, a 96-well plate was placed in the CFX96 Real-Time System (Bio-Rad®) and the program described in the mmqPCR protocol was carried out [51]. rTL of each sample was calculated by using the standard curve method.
DNA methylation
Genomic DNA was bisulfite-converted using EZ-96 DNA Methylation Kit (Zymo Research®, Irvine, CA, USA) according to the manufacturer’s instructions. DNA methylation was measured using the Infinium MethylationEPIC Kit (Illumina, San Diego, CA, USA) according to the manufacturer’s instruction with the exception that 7-µl bisulfite-converted DNA and 1-µl 0.4 N NaOH was used.
Sensitivity study
Two sensitivity studies were conducted to explore a possible bias of different storage times before WBC DNA purification; blood samples (4 ml EDTA-blood) were collected from each of five volunteers working at the analysis laboratory. To assemble samples with the shortest storage time, DNA was purified 6 h after collection. All samples were stored at room temperature until the second DNA purification 5 days after collection. The purified DNA was stored at − 80° C until analysis. All samples were analyzed together with the EOA and NOA samples.
Sub-analysis
To evaluate if use of AMH to define the two groups could have changed outcomes, a sub-analysis using the AMH value closest to the recruiting cycle was conducted. An AMH value ≤ 7.9 pmol/l was considered EOA and > 7.9 pmol/l as NOA [52]. One woman switched from the EOA to the NOA group when using the AMH as inclusion criterion. Thus, 54 women were included in the EOA group and 53 in the NOA group.
Ethics
The study was approved by the Danish Data protection Agency (nr 1–16-02–320-14) and the Regional committee on Health Research Ethics of Central Region Denmark (jr.no 1–10-72–142-14). All women provided written informed consent upon acceptance of participation.
Statistics
Normally distributed data is given as means (± SD) and variables not following normal distribution as medians (10/90 percentile). Graphical assessments (histogram and probability plots) were used to determine normal distribution. Pearson’s correlation and scatter plots were used to evaluate correlation of biological markers and chronological age by time of oocyte retrieval. A p value < 0.05 was considered statistically significant. All analyses were carried out using Stata IC 16.1 (Stata Corp, College Station, TX, USA) unless otherwise stated.
Statistical analysis of rTL
Graphical visualization of the distribution of rTL in each group was made using a box-plot and comparison of means was conducted using Student’s t test. A linear regression model with rTL as the dependent variable and ovarian reserve status as the independent variable, and chronological age and storage time included as covariates, was conducted to account for any differences in mean age and storage time in the EOA and NOA group, respectively.
Statistical analysis of DNA methylation profiling
DNA methylation data was processed with minfi tools [53]. We used single sample Noob normalization [54] to reduce variation caused by technicalities. Methylation level for each probe was quantified as β = M/(M + U), where M and U denote methylated and unmethylated signals, respectively. Using the individual methylation levels (i.e., β values), we estimated DNA methylation age with the updated Horvath biological clock estimator (“Skin and Blood Clock”) [40] (https://clockbio.com). The estimates of methylation were correlated with chronological age within the EOA and NOA group. Furthermore, we computed the age acceleration (AgeAccel), which we defined as AgeAccel = -age, where is the predicted methylation age, and age is the chronological age. Thus, the AgeAccel describes a predicted biological age older than the chronological age when positive, and the opposite when negative. Test of statistical difference between predicted age and chronological age was obtained using Student’s t test. A linear regression model was conducted to account for any differences in mean age and storage time in the EOA and NOA group respectively, as described for the rTL. Furthermore, DNA methylation age was also estimated using the PhenoAge clock [55] to explore if clock type was important.
Power calculation
To the best of our knowledge, no formal template exists for estimating the power of number of persons needed when estimating accelerated aging using the epigenetic clock. To be able to identify the number of participants needed for an age acceleration of 1, 2, 3, 4 and 5 years, respectively, we therefore used already published data on the epigenetic clock [42, 56, 57]. The approximate size of standard error (SE) regarding estimation of the biological age and the relation of SE and N was determined and the standard deviation (SD) was then calculated on basis of this information. Using one-sample Z test (two sided with a p < 0.05) and the calculated SD, the expected age acceleration (years) for 10, 20, 30, 40, 50, and 100 participants in both groups respectively was calculated. With a power of 80%, fifty participants had to be included in each group to be able to predict an age acceleration of 2 years (further details available upon request from the authors). The evaluation of rTL in ovarian aging was considered exploratory in character, as we did not find data making a meaningful power calculation possible.
Sensitivity study
Wilcoxon signed-rank test was used to compare rTL or predicted DNAm age on day 0 and day 5, respectively.
Results
A total of 107 women with either EOA (n = 55) or NOA (n = 52) were enrolled in the study (Fig. 1). Baseline patient and IVF characteristics are given in Table 1. Mean age was significantly higher in the EOA group compared to the NOA group (32.8 years and 29.3 years, respectively, p < 0.01). As expected, the EOA group had lower AMH levels (median 2.7 (10/90 percentile (1.9/5.7)) pmol/l) and higher maximal FSH dose used for ovarian stimulation (mean (± SD) 368.6 (6.7) IU) compared to the NOA group (median AMH 27.6 (16.9/49.0) pmol/l and maximal FSH dose 185.5 (6.5) IU). Mean storage time from collection of blood samples until DNA purification was comparable in both groups (2.1 (1.7) days and 2.4 (1.3) days, p = 0.32, respectively).
Fig. 1.
Flow-chart of inclusion and exclusion of women in the study. Women who underwent ART with either IVF or ICSI during the period 2016 to 2018 were recruited at two public Danish fertility clinics and divided into two groups depending on their ovarian reserve status
Table 1.
Baseline characteristics of participants by time of inclusion
| Early ovarian aging(≤ 5 oocytes) | Normal ovarian aging(≥ 8 oocytes) | p value | |
|---|---|---|---|
| No | 55 | 52 | |
| Age (years) | 32.8 (3.4) | 29.3 (3.9) | < 0.01a |
| ART characteristics | |||
| AMH (pmol/L)c, median (10/90 percentile) | 2.7 (1.9/5.7) | 27.6 (16.9/49.0) | < 0.01b |
| Max FSH (IU) dose used | 368.6 (6.7) | 181.5 (6.5) | < 0.01b |
| No of oocytes aspirated, median (10/90 percentile) | 3 (0/5) | 13 (9/21) | < 0.01b |
| Blood samples | |||
| Time from collection to DNA purification (days) | 2.1 (1.7) | 2.4 (1.3) | 0.32a |
FSH follicle-stimulating hormone, AMH anti-Müllerian hormone
Numbers are mean (± SD) unless otherwise stated
aStudent’s t test, two sided
bWilcoxon rank sum (Mann–Whitney)
cThe AMH values closest to the recruiting cycle
Relative telomere length
Mean rTL was comparable in the EOA (0.46 (0.14)) and NOA group (0.47 (0.12), p = 0.64)) (Table 2, Fig. 2). The rTL analysis was found to be affected by storage time (Supplementary Table S1), but adjusting for this covariate along with chronological age did not change the overall conclusion of no difference in rTL when comparing EOA to NOA (crude β value: − 0.012, 95% CI (− 0.063 to 0.039), adjusted 0.014 (− 0.039 to 0.067)) (Supplementary Table S2). A relatively poor correlation of rTL and chronological age was observed (Pearson = − 0.15, p = 0.14 with R2 = 0.02) (Supplementary Figure S1).
Table 2.
Description of relative telomere length and epigenetic age according to ovarian reserve status
| Chronological age | Relative telomere length | DNAm age | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean (± SD) | Min/max | Mean (± SD) | Min/max | Predicted age | AgeAccela | |||
| Mean (± SD) | Min/max | Mean (± SD) | Min/max | |||||
| EOA | 32.8 (3.4) | 23/37 | 0.46 (0.14) b | 0.20/0.75 | 31.8 (4.4) | 17.8/42.5 | − 1.02 (2.62)c | − 6.38/5.97 |
| NOA | 29.3 (3.9) | 22/37 | 0.47 (0.12) | 0.23/0.87 | 27.8 (5.4) | 15.2/37.9 | − 1.57 (2.56) | − 6.83/4.81 |
EOA early ovarian aging, NOA normal ovarian aging
aPredicted age—chronological age
bp = 0.64 compared to NOA, Student’s t test
cp = 0.27, Student’s t test
Fig. 2.

Box-plot of relative telomere length according to ovarian reserve status. NOA, normal ovarian aging; EOA, early ovarian aging
Epigenetic age
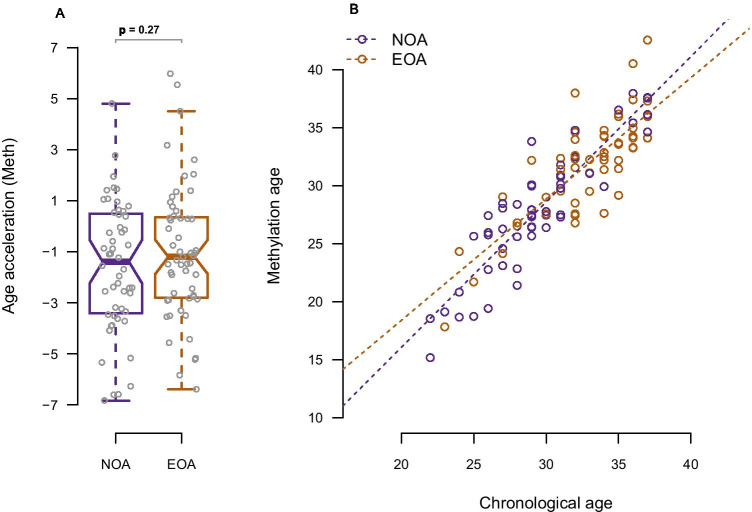
Chronological age and predicted epigenetic age (DNAm age) were strongly positively correlated (=0.88, p ≤ 0.001 with R2 = 0.774) although DNAm age in both groups was found to be slightly younger than the chronological age (Table 2, Fig. 3a and 3b). Comparing the mean AgeAccel, the EOA group had a biological age 0.55 years insignificantly older than the NOA group (mean AgeAccel − 1.02 (2.62) and − 1.57 (2.56), p = 0.27) (Table 2, Fig. 3a). However, this difference disappeared when adjusting for age in the linear regression analysis (crude β value 0.557 (95% CI − 0.437 to 1.551), adjusted β value − 0.004 (95% CI − 1.086 to 1.078) (Supplementary Table S2). The PhenoAge clock showed results similar to the Skin-Blood clock although some variation was observed when comparing the two clocks (=0.398, p ≤ 0.001, R2 = 0.16) (Supplementary Figure S2).
Fig. 3.
a Box-plot of age acceleration according to ovarian reserve group b Methylation age (predicted DNAm age) according to chronological age in the two ovarian reserve groups NOA, normal ovarian aging; EOA, early ovarian aging
Sensitivity study
Due to varying storage time, we analyzed its possible influence on results. A trend towards prolonged rTL was observed with increasing storage time with a median rTL of 0.39 (0.26/0.58) same day as blood sample collection and a median rTL of 0.47 (0.39/0.81) with DNA purified 5 days after sample collection (p = 0.06). Median predicted DNAm age was not influenced in the same manner as rTL when comparing day 0 (26.37 years (25.56/60.73)) to day 5 (28.9 years (25.24/63.55, p = 0.31)) (Supplementary Table S1).
Sub-analysis based on level of AMH as selection criterion
One woman switched from the EOA to the NOA group giving 54 and 53 in the two groups, respectively. Mean age was unchanged, i.e., statistically significantly higher in the EOA group (32.8 ± 3.4 years) than in the NOA group (29.4 ± 3.9 years, p < 0.01). Similarly, mean rTL was comparable when comparing the EOA group (0.46 ± 0.15) to the NOA group (0.47 ± 0.12, p = 0.76) (Supplementary Table S3). Adjusting for chronological age and storage time did not alter the overall conclusion of no difference (crude β value − 0.008, 95% CI (− 0.059 to 0.043), adjusted β value 0.017 CI (− 0.035 to 0.070)) (Supplementary Table S4). As observed in the main analysis, mean AgeAccel was insignificantly slightly higher in the EOA group (− 1.06 ± 2.63, p = 0.36) compared with the NOA group (− 1.52 ± 2.56) but this difference disappeared in the age-adjusted analysis (crude β value 0.465 CI (− 0.530; 1.460), adjusted β value − 0.087 CI (− 1.158; 0.984)) (Supplementary Tables S2 and S3).
Discussion
A hypothesis of accelerated biological aging in young women with low ovarian reserve starting before menopause has been presented previously [14]. In the present study, we did not find changes in WBC rTL or epigenetic age corresponding to an accelerated biological aging process in a group of premenopausal young women with EOA.
In accordance with these findings, Morin et al. (2018) found that neither rTL nor DNAm age indicated an accelerated premenopausal aging in women with low ovarian reserve [33]. However, in a subsequent study from the same group, a statistically significant age acceleration of approximately 2 years was found [43]. Slightly different inclusion criteria and altered age criteria from the first to the second study may explain the different outcomes and thus unfortunately hampers any firm conclusions based on these findings.
Two recently published studies further evaluated the WBC rTL in relation to EOA [34, 35]. Both studies reported shorter WBC rTL in young women with EOA defined as elevated basal FSH levels (≥ 10 IU/l and ≥ 40 IU/l, respectively) compared to young women with FSH levels within the normal range (< 10 IU/l) [34, 35]. The conflicting findings to the present study and the studies by Xu et al. (2017) and Miranda-Furtado et al. (2018) [34, 35] may be explained using FSH levels as inclusion criteria. Thus, some of these women could be peri- or even postmenopausal, which may influence telomere length [58].
Accordingly, the failure to demonstrate WBC rTL changes in young cycling women with EOA but not in young women with EOA closer to the menopausal transition may indicate, opposite to our hypothesis, that postmenopausal estrogen deprivation may play a major role in the accelerated aging process observed in women with a natural premature or early menopause.
Reports on long female reproductive lifespan being associated with changes in aging biomarkers may further support the estrogen deprivation theory. Thus, longer telomeres have been found in women reporting late menopausal age [32] and late age at birth of their last child [31]. This indicates either a delayed timing of aging mechanism(s) per se or an effect of prolonged exposure to normal endogenous estrogen levels. In accordance with the TL findings, it has been found that in postmenopausal women, late age at menopause was associated with a younger epigenetic age, when compared to women with early onset of menopause [42]. In addition, longer time since menopause has been associated with an accelerated epigenetic age, possibly indicating mechanism(s) driven by estrogen deprivation [42].
Nevertheless, whereas both data on TL and epigenetic age indicate delayed timing of aging with a long reproductive life, we still lack consistent knowledge on the status of the two biomarkers when reproductive life is shortened. Thus, the reports of increased premenopausal CVD risk factors in normal cycling women with unexplained very low ovarian reserve [15–19, 59] still point towards the mechanism(s) behind EOA and accelerated aging may be even more complex than could be explained by diminished estrogen levels alone.
Several factors could have influenced the lack to demonstrate premenopausal changes in WBC rTL and methylation age in premenopausal women with accelerated ovarian aging, despite this population has been found to be at increased risk of age-related health events [14]. Small changes in the biomarkers may have been overseen due to the relatively small size of the present cohort. Thus, we only had power to detect age accelerations ≥ 2 years. Regarding the power to detect relevant shortening of WBC rTL, we considered our work to be exploratory, as few studies have investigated the relationship of premenopausal WBC rTL in women with early ovarian aging, and those that did yielded inconsistent results. It is therefore unclear at which level a difference in WBC rTL is to be considered biologically relevant, and we therefore may have been unable to detect discrete, but biologically relevant, changes in the mean WBC rTL.
A recent meta-analysis of observational studies revealed that shorter TL has a rather weak association with increased risks of a range of health outcomes [60]. Furthermore, TL has been found to vary between different tissue within the same individual and whether it is a strong biomarker of aging for a whole organism has been questioned [61, 62]. Accordingly, normal WBC rTL in the EOA group may still be compatible with an increased risk of age-related adverse health events in women with accelerated ovarian aging [14].
A “Telomere Theory of Reproductive Aging” was developed by Keefe et al. [63–66] which connects age-related shortening of telomeres with the normal age-dependent decline of oocyte quality. Thus, telomere dysfunction could contribute to the well-known increasing risk of embryo aneuploidy by age [67, 68], leading to decreasing fertility and increasing risk of miscarriage with age [30, 69]. The hypothesis is supported by several findings, for example, that oocytes with short TL or telomere deficiency was prone to embryonic aneuploidy [70] and young women delivering babies with Down’s syndrome have shorter WBC TL than age-matched peers delivering healthy children [71]. Risk of miscarriage is generally considered a surrogate parameter for aneuploidy and oocyte quality. Accordingly, one may speculate if the normal WBC rTL found in the present cohort of women with accelerated ovarian aging is consistent with a normal-for-age risk of miscarriage in young poor responders [72, 73].
Strength and limitations
The strength of our study is the relatively well-defined population of young women with idiopathic EOA and NOA, which we achieved by excluding patients with factors known to influence the ovarian reserve. Thus, the same exclusion parameters were used for the EOA and NOA group to minimize the possibility of influence from other factors. Moreover, the possibility of women being incorrectly defined as having EOA due to insufficient stimulated cycles was minimized by requiring minimum two cycles with ≤ 5 oocytes with at least 250 IU FSH. The literature seems to support the gain of using higher FSH dosages to increase the oocyte output is very limited and may only result in 1–2 extra oocytes [74–76]. Accordingly, the gap between the two groups regarding oocyte number required (5 versus 8) further minimized potential misclassification of EOA into NOA group due to varying FSH dose. Furthermore, a sub-analysis based on the AMH levels showed that only one woman switched from the EOA group to the NOA group when using AMH as criterion. The findings of no difference in biological age remained in the sub-analysis, supporting the finding that the two groups are representative of idiopathic EOA and NOA, respectively.
There is an ongoing debate on how to define early ovarian aging and which ovarian reserve marker to use. The choice of oocyte number as a marker of ovarian reserve was based on previous findings in a historical follow-up study, where EOA was associated with an increased risk of age-related events when using the same inclusion and exclusion criteria [14]. The Bologna criteria (BC) is a useful tool to standardize selection of women with poor ovarian response (i.e., few oocytes harvested) [52]. However, the BC was primarily developed to investigate and optimize ovarian stimulation protocols in randomized clinical trials [52, 77]. We chose the present selection criteria because repeated harvest of ≤ 3 oocytes is very seldom in our patient population. The analysis of AMH supported that the threshold of repeated cycles with ≤ 5 oocytes may be valid in order to identify EOA in young women. However, it cannot be ruled out that using an even lower number of oocytes harvested as the inclusion criterion could have changed the results, since a possible dose–response relationship of increased risk of age-related events and decreasing oocyte number has been reported [14]. Unfortunately, the size of the present study does not allow a meaningful stratification by oocyte number harvested.
Including two biomarkers of aging further strengthens the conclusions, since WBC TL and the epigenetic clock probably describe different pathways of the aging process. While the above studies relied only on the original pan-tissue clock [33, 36, 43], we further evaluated the PhenoAge clock as it outperforms the epigenetic biomarkers based on chronological age by being a better predictor of mortality, health span, and CVD [55, 78]. The PhenoAge estimator was developed by incorporating age-related biochemical biomarkers in combination with chronological age [55]. Therefore, PhenoAge might be better in capturing epigenetic differences. The PhenoAge clock, however, showed similar results as the Skin and Blood clock with no signs of an accelerated aging in the EOA group (Supplementary Figure S2).
Our study did have some limitations to be taken into consideration when interpreting the results. A difference of 3.5 years in mean chronological age by the time of oocyte retrieval was observed when comparing the EOA to the NOA group. To account for this, linear regression analysis was conducted including age as covariate. No noteworthy changes in neither rTL nor AgeAccel were observed when adjusting for chronological age (Supplementary Table S2). BMI, smoking, and ethnicity have also been reported as potential confounders when studying rTL and DNAm age. However, BMI and smoking status was not systematically measured during the study period and therefore not included in the analyses. In a similar population, we previously found a slightly higher BMI in the EOA group, and comparable smoking status between the two groups [14]. As we did not find any difference in neither rTL nor AgeAccel, it is unlikely that the inability to control for these factors alone may explain the findings. In addition, a large meta-analysis did not find smoking to be associated with telomere attrition [79] and various findings on the effect of BMI and TL have also been reported [62].
Pre-analytical factors such as storage time prior to DNA purification have been reported to impact on DNA quality when conducting molecular testing [80, 81]. Thus, storage of blood samples for up to 5 days prior to DNA purification may constitute a potential bias. However, including storage time as covariate in the linear regression analysis did not change the overall conclusion of no difference in the EOA and EOA group for neither rTL nor the AgeAccel analysis (Supplementary Table S2). In addition, mean storage time was similar in both groups. Thus, the varying storage time prior to DNA purification seems to be of minor importance in the present study.
Another aspect to consider when interpreting the results is the analytic methods used, especially analysis of TL, as it is found to be method dependent [82]. qPCR-based methods have become commonly used due to its high-throughput format, minimal requirement of DNA yield, and less labor-intensive method compared with, e.g., the Southern blot-based methods. However, it is well known that qPCR-based method produces higher measurements errors than Southern blot-based TL analysis. Thus, larger samples sizes are needed to overcome these errors and to maintain statistical power [82–84]. Nevertheless, with the development of the mmqPCR protocol, these measurement errors have been reduced considerably with a strong correlation with the Southern blot-based approach (R2 = 0.844) compared to the correlation of the original singleplex qPCR method and Southern blot-based approach (R2 = 0.677) [51]. Giving mmqPCR has been extensively used and validated since its development, and in its high-throughput format, the mmqPCR approach was chosen over the Southern blot-based analysis.
It has been shown only the shortest TL and not the average TL within a cell determine cellular senescence [85–87]; thus, the percentage of shortest telomeres may be a more precise predictor of lifespan in mammals [86]. Accordingly, methods only measuring average TL may be less sensible to discover these changes. Recently, Lai et al. (2017) developed a promising method to measure the shortest telomeres, but this needs to be further validated in other settings [88]. Nevertheless, future studies may consider this approach when using telomere attrition as measure of biological aging.
Conclusion
In conclusion, our analysis of rTL and epigenetic methylation age did not yield any indication of accelerated general aging in young women with idiopathic early ovarian aging, despite their premature increased risk of age-related health events. Moving forward, these findings should be replicated in larger cohorts.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
All authors contributed to interpretation of data, revised the manuscript critically for important intellectual content, and approved the final version to be published. MWC, HJI, and USK contributed substantially to conception and design of the study and MWC, HJI, USK, and UBK were responsible for data acquisition. Data analyses were performed by MWC, PDR, WF, IC, CS, JBG, ALN, and CSH. MWC wrote the first draft.
Funding
The study was funded by the Health Research Fund of Central Denmark Region, Aase and Ejnar Danielsen Fond, Ferring pharmaceuticals, Denmark, Fonden til Lægevidenskabens fremme (A.P. Moeller foundation) and Grosser L.F. Foghts Fond.
Code availability
Not applicable
Declarations
Ethics approval
The study was approved by the Danish Data protection Agency (nr 1–16-02–320-14) and the Regional committee on Health Research Ethics of Central Region Denmark (jr.no 1–10-72–142-14).
Consent to participate
All women provided written informed consent upon acceptance of participation.
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8(2):141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 2.Nikolaou D, Templeton A. Early ovarian ageing: a hypothesis. Detection and clinical relevance. Human Reprod. 2003;18(6):1137–9. doi: 10.1093/humrep/deg245. [DOI] [PubMed] [Google Scholar]
- 3.Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67(4):604–606. [PubMed] [Google Scholar]
- 4.De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376(9744):911–921. doi: 10.1016/s0140-6736(10)60355-8. [DOI] [PubMed] [Google Scholar]
- 5.Snowdon DA, Kane RL, Beeson WL, Burke GL, Sprafka JM, Potter J, et al. Is early natural menopause a biologic marker of health and aging? Am J Public Health. 1989;79(6):709–714. doi: 10.2105/AJPH.79.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svejme O, Ahlborg HG, Nilsson JA, Karlsson MK. Early menopause and risk of osteoporosis, fracture and mortality: a 34-year prospective observational study in 390 women. BJOG : an international journal of obstetrics and gynaecology. 2012;119(7):810–816. doi: 10.1111/j.1471-0528.2012.03324.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhu D, Chung HF, Dobson AJ, Pandeya N, Giles GG, Bruinsma F, et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. The Lancet Public health. 2019;4(11):e553–e564. doi: 10.1016/s2468-2667(19)30155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mondul AM, Rodriguez C, Jacobs EJ, Calle EE. Age at natural menopause and cause-specific mortality. Am J Epidemiol. 2005;162(11):1089–1097. doi: 10.1093/aje/kwi324. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, Jones M, Mishra GD. Age at natural menopause and development of chronic conditions and multimorbidity: results from an Australian prospective cohort. Human reproduction (Oxford, England) 2020;35(1):203–211. doi: 10.1093/humrep/dez259. [DOI] [PubMed] [Google Scholar]
- 10.Dorland M, van Kooij RJ, te Velde ER. General ageing and ovarian ageing. Maturitas. 1998;30(2):113–118. doi: 10.1016/s0378-5122(98)00066-8. [DOI] [PubMed] [Google Scholar]
- 11.Ossewaarde ME, Bots ML, Verbeek AL, Peeters PH, van der Graaf Y, Grobbee DE, et al. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology. 2005;16(4):556–562. doi: 10.1097/01.ede.0000165392.35273.d4. [DOI] [PubMed] [Google Scholar]
- 12.Otsuki S, Saito E, Sawada N, Abe SK, Hidaka A, Yamaji T, et al. Female reproductive factors and risk of all-cause and cause-specific mortality among women: The Japan Public Health Center-based Prospective Study (JPHC study) Ann Epidemiol. 2018;28(9):597–604.e6. doi: 10.1016/j.annepidem.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Perls TT, Alpert L, Fretts RC. Middle-aged mothers live longer. Nature. 1997;389(6647):133. doi: 10.1038/38148. [DOI] [PubMed] [Google Scholar]
- 14.Christensen MW, Kesmodel US, Christensen K, Kirkegaard K, Ingerslev HJ. Early ovarian ageing: is a low number of oocytes harvested in young women associated with an earlier and increased risk of age-related diseases? Human reproduction (Oxford, England) 2020;35(10):2375–2390. doi: 10.1093/humrep/deaa188. [DOI] [PubMed] [Google Scholar]
- 15.Bleil ME, Gregorich SE, McConnell D, Rosen MP, Cedars MI. Does accelerated reproductive aging underlie premenopausal risk for cardiovascular disease? Menopause. 2013;20(11):1139–1146. doi: 10.1097/GME.0b013e31828950fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cedars MI. Biomarkers of ovarian reserve–do they predict somatic aging? Seminars in reproductive medicine. 2013;31(6):443–451. doi: 10.1055/s-0033-1356480. [DOI] [PubMed] [Google Scholar]
- 17.Verit FF, Keskin S, Omer B, Yalcinkaya S, Sakar N. Is there any relationship between cardiovascular risk markers and young women with diminished ovarian reserve? Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2014;30(10):697–700. doi: 10.3109/09513590.2014.922948. [DOI] [PubMed] [Google Scholar]
- 18.Pal L, Bevilacqua K, Zeitlian G, Shu J, Santoro N. Implications of diminished ovarian reserve (DOR) extend well beyond reproductive concerns. Menopause. 2008;15(6):1086–1094. doi: 10.1097/gme.0b013e3181728467. [DOI] [PubMed] [Google Scholar]
- 19.de Kat AC, Verschuren WM, Eijkemans MJ, van der Schouw YT, Broekmans FJ. The association of low ovarian reserve with cardiovascular disease risk: a cross-sectional population-based study. Human reproduction (Oxford, England) 2016;31(8):1866–1874. doi: 10.1093/humrep/dew159. [DOI] [PubMed] [Google Scholar]
- 20.Zhu D, Chung HF, Pandeya N, Dobson AJ, Hardy R, Kuh D, et al. Premenopausal cardiovascular disease and age at natural menopause: a pooled analysis of over 170,000 women. Eur J Epidemiol. 2019;34(3):235–246. doi: 10.1007/s10654-019-00490-w. [DOI] [PubMed] [Google Scholar]
- 21.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 22.Polonio AM, Chico-Sordo L, Córdova-Oriz I, Medrano M, García-Velasco JA, Varela E. Impact of Ovarian Aging in Reproduction: From Telomeres and Mice Models to Ovarian Rejuvenation. Yale J Biol Med. 2020;93(4):561–569. [PMC free article] [PubMed] [Google Scholar]
- 23.Varela E, Blasco MA. 2009 nobel prize in physiology or medicine: telomeres and telomerase. Oncogene. 2010;29(11):1561–1565. doi: 10.1038/onc.2010.15. [DOI] [PubMed] [Google Scholar]
- 24.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baird DM, Kipling D. The extent and significance of telomere loss with age. Ann N Y Acad Sci. 2004;1019:265–268. doi: 10.1196/annals.1297.044. [DOI] [PubMed] [Google Scholar]
- 26.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. doi: 10.1016/s0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 27.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Njajou OT, Hsueh WC, Blackburn EH, Newman AB, Wu SH, Li R, et al. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2009;64(8):860–864. doi: 10.1093/gerona/glp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson J, Keefe DL. Ovarian aging: breaking up is hard to fix. Sci Transl Med. 2013;5(172):172fs5. doi: 10.1126/scitranslmed.3005579. [DOI] [PubMed] [Google Scholar]
- 30.Kalmbach KH, Antunes DM, Kohlrausch F, Keefe DL. Telomeres and Female Reproductive Aging. Semin Reprod Med. 2015;33(6):389–395. doi: 10.1055/s-0035-1567823. [DOI] [PubMed] [Google Scholar]
- 31.Fagan E, Sun F, Bae H, Elo I, Andersen SL, Lee J, et al. Telomere length is longer in women with late maternal age. Menopause. 2017;24(5):497–501. doi: 10.1097/gme.0000000000000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray KE, Schiff MA, Fitzpatrick AL, Kimura M, Aviv A, Starr JR. Leukocyte telomere length and age at menopause. Epidemiology. 2014;25(1):139–146. doi: 10.1097/ede.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morin SJ, Tao X, Marin D, Zhan Y, Landis J, Bedard J, et al. DNA methylation-based age prediction and telomere length in white blood cells and cumulus cells of infertile women with normal or poor response to ovarian stimulation. Aging (Albany NY) 2018;10(12):3761–3773. doi: 10.18632/aging.101670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu X, Chen X, Zhang X, Liu Y, Wang Z, Wang P, et al. Impaired telomere length and telomerase activity in peripheral blood leukocytes and granulosa cells in patients with biochemical primary ovarian insufficiency. Human reproduction (Oxford, England) 2017;32(1):201–207. doi: 10.1093/humrep/dew283. [DOI] [PubMed] [Google Scholar]
- 35.Miranda-Furtado CL, Luchiari HR, Chielli Pedroso DC, Kogure GS, Caetano LC, Santana BA, et al. Skewed X-chromosome inactivation and shorter telomeres associate with idiopathic premature ovarian insufficiency. Fertil Steril. 2018;110(3):476–85.e1. doi: 10.1016/j.fertnstert.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 36.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16(1):25. doi: 10.1186/s13059-015-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christiansen L, Lenart A, Tan Q, Vaupel JW, Aviv A, McGue M, et al. DNA methylation age is associated with mortality in a longitudinal Danish twin study. Aging Cell. 2016;15(1):149–154. doi: 10.1111/acel.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perna L, Zhang Y, Mons U, Holleczek B, Saum KU, Brenner H. Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a German case cohort. Clin Epigenetics. 2016;8:64. doi: 10.1186/s13148-016-0228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horvath S, Oshima J, Martin GM, Lu AT, Quach A, Cohen H, et al. Epigenetic clock for skin and blood cells applied to Hutchinson Gilford Progeria Syndrome and ex vivo studies. Aging (Albany NY) 2018;10(7):1758–1775. doi: 10.18632/aging.101508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teschendorff AE. Epigenetic clocks galore: a new improved clock predicts age-acceleration in Hutchinson Gilford Progeria Syndrome patients. Aging (Albany NY) 2018;10(8):1799–1800. doi: 10.18632/aging.101533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine ME, Lu AT, Chen BH, Hernandez DG, Singleton AB, Ferrucci L, et al. Menopause accelerates biological aging. Proc Natl Acad Sci USA. 2016;113(33):9327–9332. doi: 10.1073/pnas.1604558113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanson BM, Tao X, Zhan Y, Jenkins TG, Morin SJ, Scott RT, et al. Young women with poor ovarian response exhibit epigenetic age acceleration based on evaluation of white blood cells using a DNA methylation-derived age prediction model. Human reproduction (Oxford, England) 2020;35(11):2579–2588. doi: 10.1093/humrep/deaa206. [DOI] [PubMed] [Google Scholar]
- 44.Monseur B, Murugappan G, Bentley J, Teng N, Westphal L. Epigenetic clock measuring age acceleration via DNA methylation levels in blood is associated with decreased oocyte yield. J Assist Reprod Genet. 2020;37(5):1097–1103. doi: 10.1007/s10815-020-01763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olsen KW, Castillo-Fernandez J, Chan AC, la Cour FN, Zedeler A, Bungum M, et al. Identification of a unique epigenetic profile in women with diminished ovarian reserve. Fertil Steril. 2021;115(3):732–741. doi: 10.1016/j.fertnstert.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Lawson R, El-Toukhy T, Kassab A, Taylor A, Braude P, Parsons J, et al. Poor response to ovulation induction is a stronger predictor of early menopause than elevated basal FSH: a life table analysis. Hum Reprod. 2003;18(3):527–533. doi: 10.1093/humrep/deg101. [DOI] [PubMed] [Google Scholar]
- 47.de Boer EJ, den IT, te Velde ER, Burger CW, van Leeuwen FE. Increased risk of early menopausal transition and natural menopause after poor response at first IVF treatment. Hum Reprod. 2003;18(7):1544–52. doi: 10.1093/humrep/deg278. [DOI] [PubMed] [Google Scholar]
- 48.La Marca A, Dondi G, Sighinolfi G, Giulini S, Papaleo E, Cagnacci A, et al. The ovarian response to controlled stimulation in IVF cycles may be predictive of the age at menopause. Human reproduction (Oxford, England) 2014;29(11):2530–2535. doi: 10.1093/humrep/deu234. [DOI] [PubMed] [Google Scholar]
- 49.de Boer EJ, den TI, te Velde ER, Burger CW, Klip H, van Leeuwen FE. A low number of retrieved oocytes at in vitro fertilization treatment is predictive of early menopause. Fertil Steril. 2002;77(5):978 85 S0015028202029722. doi: 10.1016/S0015-0282(02)02972-2. [DOI] [PubMed] [Google Scholar]
- 50.Morin SJ, Patounakis G, Juneau CR, Neal SA, Scott RT, Seli E. Diminished ovarian reserve and poor response to stimulation in patients <38 years old: a quantitative but not qualitative reduction in performance. Human reproduction (Oxford, England) 2018;33(8):1489–1498. doi: 10.1093/humrep/dey238. [DOI] [PubMed] [Google Scholar]
- 51.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37(3):e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferraretti AP, La MA, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 53.Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30(10):1363–1369. doi: 10.1093/bioinformatics/btu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fortin JP, Triche TJ, Jr, Hansen KD. Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics. 2017;33(4):558–560. doi: 10.1093/bioinformatics/btw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 2018;10(4):573–591. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horvath S, Garagnani P, Bacalini MG, Pirazzini C, Salvioli S, Gentilini D, et al. Accelerated epigenetic aging in Down syndrome. Aging Cell. 2015;14(3):491–495. doi: 10.1111/acel.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horvath S, Langfelder P, Kwak S, Aaronson J, Rosinski J, Vogt TF, et al. Huntington's disease accelerates epigenetic aging of human brain and disrupts DNA methylation levels. Aging (Albany NY) 2016;8(7):1485–1512. doi: 10.18632/aging.101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bayne S, Jones ME, Li H, Pinto AR, Simpson ER, Liu JP. Estrogen deficiency leads to telomerase inhibition, telomere shortening and reduced cell proliferation in the adrenal gland of mice. Cell Res. 2008;18(11):1141–1150. doi: 10.1038/cr.2008.291. [DOI] [PubMed] [Google Scholar]
- 59.de Kat AC, van der Schouw YT, Eijkemans MJC, Broer SL, Verschuren WMM, Broekmans FJM. Can Menopause Prediction Be Improved With Multiple AMH Measurements? Results From the Prospective Doetinchem Cohort Study. J Clin Endocrinol Metab. 2019;104(11):5024–5031. doi: 10.1210/jc.2018-02607. [DOI] [PubMed] [Google Scholar]
- 60.Smith L, Luchini C, Demurtas J, Soysal P, Stubbs B, Hamer M, et al. Telomere length and health outcomes: An umbrella review of systematic reviews and meta-analyses of observational studies. Ageing Res Rev. 2019;51:1–10. doi: 10.1016/j.arr.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Jylhävä J, Pedersen NL, Hägg S. Biological Age Predictors EBioMedicine. 2017;21:29–36. doi: 10.1016/j.ebiom.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev. 2013;35(1):112–131. doi: 10.1093/epirev/mxs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalmbach K, Robinson LG, Jr, Wang F, Liu L, Keefe D. Telomere length reprogramming in embryos and stem cells. Biomed Res Int. 2014;2014:925121. doi: 10.1155/2014/925121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keefe DL, Liu L, Marquard K. Telomeres and aging-related meiotic dysfunction in women. Cellular and molecular life sciences : CMLS. 2007;64(2):139–143. doi: 10.1007/s00018-006-6466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keefe DL, Liu L. Telomeres and reproductive aging. Reprod Fertil Dev. 2009;21(1):10–14. doi: 10.1071/RD08229. [DOI] [PubMed] [Google Scholar]
- 66.Keefe DL, Marquard K, Liu L. The telomere theory of reproductive senescence in women. Curr Opin Obstet Gynecol. 2006;18(3):280–285. doi: 10.1097/01.gco.0000193019.05686.49. [DOI] [PubMed] [Google Scholar]
- 67.Fragouli E, Alfarawati S, Spath K, Wells D. Morphological and cytogenetic assessment of cleavage and blastocyst stage embryos. Mol Hum Reprod. 2014;20(2):117–126. doi: 10.1093/molehr/gat073. [DOI] [PubMed] [Google Scholar]
- 68.Gruhn JR, Zielinska AP, Shukla V, Blanshard R, Capalbo A, Cimadomo D, et al. Chromosome errors in human eggs shape natural fertility over reproductive life span. Science. 2019;365(6460):1466–1469. doi: 10.1126/science.aav7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ. 2000;320(7251):1708–1712. doi: 10.1136/bmj.320.7251.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Treff NR, Su J, Taylor D, Scott RT., Jr Telomere DNA deficiency is associated with development of human embryonic aneuploidy. PLoS Genet. 2011;7(6):e1002161. doi: 10.1371/journal.pgen.1002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Albizua I, Rambo-Martin BL, Allen EG, He W, Amin AS, Sherman SL. Association between telomere length and chromosome 21 nondisjunction in the oocyte. Hum Genet. 2015;134(11–12):1263–1270. doi: 10.1007/s00439-015-1603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haadsma ML, Groen H, Mooij TM, Burger CW, Broekmans FJ, Lambalk CB, et al. Miscarriage risk for IVF pregnancies in poor responders to ovarian hyperstimulation. Reprod Biomed Online. 2010;20(2):191–200. doi: 10.1016/j.rbmo.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 73.Bishop LA, Richter KS, Patounakis G, Andriani L, Moon K, Devine K. Diminished ovarian reserve as measured by means of baseline follicle-stimulating hormone and antral follicle count is not associated with pregnancy loss in younger in vitro fertilization patients. Fertil Steril. 2017;108(6):980–987. doi: 10.1016/j.fertnstert.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 74.Sterrenburg MD, Veltman-Verhulst SM, Eijkemans MJ, Hughes EG, Macklon NS, Broekmans FJ, et al. Clinical outcomes in relation to the daily dose of recombinant follicle-stimulating hormone for ovarian stimulation in in vitro fertilization in presumed normal responders younger than 39 years: a meta-analysis. Hum Reprod Update. 2011;17(2):184–196. doi: 10.1093/humupd/dmq041. [DOI] [PubMed] [Google Scholar]
- 75.Group EREG. 2019 Ovarian stimulation for IVF/ICSI: Guideline of the European Society of Human Reproduction and Embryology.
- 76.Leijdekkers JA, Eijkemans MJC, van Tilborg TC, Oudshoorn SC, McLernon DJ, Bhattacharya S, et al. Predicting the cumulative chance of live birth over multiple complete cycles of in vitro fertilization: an external validation study. Human reproduction (Oxford, England) 2018;33(9):1684–1695. doi: 10.1093/humrep/dey263. [DOI] [PubMed] [Google Scholar]
- 77.Ferraretti AP, Gianaroli L. The Bologna criteria for the definition of poor ovarian responders: is there a need for revision? Human reproduction (Oxford, England) 2014;29(9):1842–1845. doi: 10.1093/humrep/deu139. [DOI] [PubMed] [Google Scholar]
- 78.Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19(6):371–384. doi: 10.1038/s41576-018-0004-3. [DOI] [PubMed] [Google Scholar]
- 79.Bateson M, Aviv A, Bendix L, Benetos A, Ben-Shlomo Y, Bojesen SE, et al. Smoking does not accelerate leucocyte telomere attrition: a meta-analysis of 18 longitudinal cohorts. R Soc Open Sci. 2019;6(6):190420. doi: 10.1098/rsos.190420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang LH, Lin PH, Tsai KW, Wang LJ, Huang YH, Kuo HC, et al. The effects of storage temperature and duration of blood samples on DNA and RNA qualities. PLoS ONE. 2017;12(9):e0184692. doi: 10.1371/journal.pone.0184692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Permenter J, Ishwar A, Rounsavall A, Smith M, Faske J, Sailey CJ, et al. Quantitative analysis of genomic DNA degradation in whole blood under various storage conditions for molecular diagnostic testing. Mol Cell Probes. 2015;29(6):449–453. doi: 10.1016/j.mcp.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 82.Montpetit AJ, Alhareeri AA, Montpetit M, Starkweather AR, Elmore LW, Filler K, et al. Telomere length: a review of methods for measurement. Nurs Res. 2014;63(4):289–299. doi: 10.1097/nnr.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011;39(20):e134. doi: 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eisenberg DT, Kuzawa CW, Hayes MG. Improving qPCR telomere length assays: Controlling for well position effects increases statistical power. Am J Hum Biol. 2015;27(4):570–575. doi: 10.1002/ajhb.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107(1):67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 86.Vera E, Bernardes de Jesus B, Foronda M, Flores JM, Blasco MA. The rate of increase of short telomeres predicts longevity in mammals. Cell Rep. 2012;2(4):732–7. doi: 10.1016/j.celrep.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 87.Zou Y, Sfeir A, Gryaznov SM, Shay JW, Wright WE. Does a sentinel or a subset of short telomeres determine replicative senescence? Mol Biol Cell. 2004;15(8):3709–3718. doi: 10.1091/mbc.e04-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lai TP, Zhang N, Noh J, Mender I, Tedone E, Huang E, et al. A method for measuring the distribution of the shortest telomeres in cells and tissues. Nat Commun. 2017;8(1):1356. doi: 10.1038/s41467-017-01291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable