Abstract
Introduction
Numerous prospective studies have shown that the incorporation of genomic assays into clinical practice significantly impacts the choice of adjuvant treatments for patients with early-stage breast cancer. However, the same evidence does not exist for the treatment of locoregional recurrences.
Hypothesis and objectives
The main objective of this work was to identify the clinicopathological, molecular, and genetic parameters that allow patients to be more precisely categorised into risk groups, in order to create a locoregional recurrence riskclassification tool, the PersonalRT27.
Material and methods
To create PersonalRT27, we retrospective assessed the variables of patients with early breast cancer (stages I or II) who had undergone the OncotypeDx ® and MammaPrint ® genetic tests. These variables and factors included in the tests were categorised and weighted to obtain scores between 1 and 5 pointsto represent a lower or higher risk of relapse, respectively, based on these factors and as determined by the researchers.
Results
The mean follow-up time was 60.5 months (range 25–96 months); locoregional progression-free survival at the time of the analysis was 98.4%, and overall survival was 97.5%, of which 0.6% of the deaths had been cancer specific. The area under the curve for the PersonalRT27 was 0.76 (95% CI [0.70, 0.81]), sensitivity was 78%, and the specificity was 58.9%. We used these factors to create an inhospital web-based nomogram.
Conclusions
The PersonalRT27 is a novel tool that integrates clinical-pathological, molecular, and genetic parameters. External and independent validation will be required to implement its clinical use.
Keywords: Cáncer de mama, Breast cancer, Plataformas genómicas, Genetic assays, Recaída local, Local relapse, Radioterapia, Radiotherapy
Highlights
-
•
Genomic assays impact the choice of adjuvant systemic treatment for patients with early-stage breast cancer.
-
•
However, the same evidence does not exist for decision making regarding adjuvant locoregional therapy.
-
•
In other words, can the clinically approved genomic assays predict the risk of locoregional recurrende as a primary event.
-
•
The main objective of this work was to identify the clinicopathological, molecular, and genetic parameters that allow patients to be more precisely categorised into risk groups, in order to create a locoregional recurrence risk-classification tool, the PersonalRT27.
1. Introduction
The irradiation of breast tissue after conservative surgery as well as histological study of axillary tissues is considered standard in patients with early-stage breast cancer (BC). We know that adjuvant radiotherapy reduces the risk of locoregional recurrence (LRR) and consequently, increases disease-free survival (DFS), cancer-specific survival (CSS), progression-free survival (PFS) and overall survival (OS) [[1], [2], [3]] [[1], [2], [3]] [[1], [2], [3]]. Current trends in oncology are oriented towards personalising treatments, with the aim of de-escalating interventions and hence, reducing the incidence and severity of side effects and, leading to better patient quality of life [4,5].
According to the Spanish Society of Medical Oncology (SEOM: 2020), the incidence of BC has increased in recent decades in Spain. This is attributable to earlier detection, longer overall life expectancies, and a decrease in mortality thanks to screening programs, lifestyle changes, and therapeutic improvements [6,7]. Traditionally, risk estimation has been determined by prognostic factors inherent to the patient such as age, associated comorbidities, general condition, and other tumour-dependent factors such as their clinicopathological stage, expression of hormone receptors, Ki67, and HER2-neu, and the results of genomic assays [[8], [9], [10], [11], [12]].
There is strong evidence for the predictive role genetic profiles may play in foreseeing distant relapses, thereby establishing their use in practice as a tool to help physicians make therapeutic decisions [[13], [14], [15], [16], [17], [18], [19]]. However, their role in exclusively predicting LRR is yet to be fully defined and so classic risk factors are still used alone to establish patient prognoses and therapeutic indications in these cases [[20], [21], [22], [23]]. Therefore, we integrated these genomic profiles along with clinicopathological, epidemiological and molecular factors into, a single novel tool, the PerosnalRT27, which may help improve their collective utility in predicting LRR.
2. Patients and methods
2.1. Elaboration of the study
Between February 2013 and December 2017, we carried out a retrospective, multicentre assessment in patients with early BC (stages I and II) treated at public health departments in the Valencian Community (Spain). The patients included in this work had undergone the OncotypeDx® and MammaPrint® genomic tests to help their physicians discern the best personalised therapeutic approach in each individual case. This study was approved by the Ethics Committee at the Centre for Public Health Research in 2015 and the data from a total of 449 participants were analysed. We created an online data collection notebook to record multiple local and distant relapse variables in these patients in order to predict their OS, locoregional PFS (L-PFS), and metastasis-free survival (MFS).
2.2. Inclusion and exclusion criteria
We applied the following inclusion criteria (1) age at diagnosis less than 75 years, (2) cancer stage T1–T2, N0–N1mic, (3) hormone receptor expression as follows: weak or moderate (10–60% positive) oestrogen receptor (ER) expression, negative, weak, or moderate (10–60% positive) progesterone receptor (PR) expression, and negative Her2 expression; this was important in order to request the genomic tests such as OncotypeDx® and Mammaprint® incorporated into the nomogram in this study; and (4) patients who had undergone conservative or radical surgery and had received systemic adjuvant treatment with hormone therapy (HT) and/or chemotherapy (CT), and/or locoregional radiotherapy (RT).
The intrinsic luminal A and B subtypes were identified according to the St. Gallen guidelines with a Ki67 threshold of 14–20% [24,25]. Patients who underwent genetic testing with the OncotyeDx® platform were stratified into three groups based on their recurrence risk score (RS) obtained using the previously published TAILORx and RxPonder studies [26,27]. In contrast, MammaPrint® was performed in both low and high-risk patients.
Patients for which a genetic test had been performed in a relapse and not the primary tumour, those with HER2 positive and triple negative cancer subtypes, or without adequate follow-up monitoring according to international recommendations were excluded from this study.
The patients were analysed according to the locoregional treatment they had received after surgery, either with conventional fractionation (CF) schemes at 2 Gy or with hypofractionation (HF) at 2.67 Gy on the breast and chest wall plus overprinting on the surgical bed if the margins had been affected or the patient was aged under 60 years. Nodal irradiation was indicated based on the clinical performance protocols available in the academic literature [[28], [29], [30]]. According to the results of the patient genomic tests, systemic adjuvant treatment was administered with CT regimens based on anthracyclines and taxanes, with or without subsequent HT with tamoxifen or aromatase inhibitors [31].
2.3. Statistical analysis
We carried out a descriptive data analysis by calculating the means, dispersion, and 95% confidence intervals (95% CIs) for continuous variables and the distribution of frequencies and percentages for categorical variables. P-values less than 0.05 were considered significant. L-PFS, MFS, and OS were analysed using the Kaplan–Meier method. To calculate the outcome for the PersonalRT27 tool, we used a numerical scoring system for the variables, as represented in frequency tables for each patient. Logistical regression analysis was used to predict the sum of the variables and to compare the PersonalRT27 result with both the MammaPrint® and OncotypeDx® genomic tests results.
The sensitivity of the PersonalRT27 tool, as indicated by receiver operating characteristic (ROC) curves, was analysed by calculating the area under the curve (AUC). Binary logistic regression models were employed to validate the nomogram in our study population and decide if it was a useful tool for detecting disease progression. Student t-tests were used in the univariate analysis of the relationship between the clinical-pathological risk factors and the PersonalRT27 results to compare the quantitative variable and ANOVA test results with the qualitative polytomous variables. All the calculations were performed using SPSS software (version 26.0; IBM Corp., Armonk, NY).
2.4. Definition of the patient evolution outcome variables
PFS-L and MFS were defined as the intervals from surgery to locoregional or distant progression, respectively; OS was defined as the period from the surgical procedure to the date the data were analysed.
3. Results
As shown in Table 1, Table 2, the mean patient age was 54 years (range 31–78 years) and the mean follow-up was 60.5 months (range 25–96 months); 64% of the patients were postmenopausal; 27.4% had type I–II obesity, 28.73% were hypertensive, and 17.1% were active smokers. The mean tumour size was 2.1 cm (range 1.9–2.3 cm), and the predominant stage was cT2 (55.5%). The presence of micrometastases was detected in 16% of the patients and the histological grade was usually G2. Lympho-vascular invasion was present in 7.6% of the patients and Ki67 expression was ≥20% in 42%; the luminal B tumour subtype predominated (66% of the cases).
Table 1.
Distribution of Clinical, Pathological and Molecular Characteristics among patients with early breast cancer.
| Characteristics | n (%) |
|---|---|
| -Age (years) | |
| ≤50 | 162 (36%) |
| 51-60 | 126 (28%) |
| >60 | 160 (35,6%) |
| ND | 1 (0,4%) |
| -Tumor size (cm) | |
| T1 | 200 (44,5%) |
| T2 | 249 (55,5%) |
| -Grade | |
| G1 | 78 (17,4%) |
| G2 | 319 (71%) |
| G3 | 31 (7%) |
| ND | 11 (4,6%) |
| - Lymp nodes | |
| pN0 | 378 (84%) |
| pN1mic | 67 (15%) |
| pN1 | 4 (1%) |
| - Axillary lymph node dissection | 90 (20%) |
| - Sentinel node biopsy | 359 (80%) |
| -Locoregional treatment | |
| - Lumpectomy + RT | 360 (80%) |
| - Mastectomy | 83 (18%) |
| - Mastectomy + RT | 6 (2%) |
| -Margins | |
| R0 | 379 (84%) |
| R1 | 50 (11%) |
| R (1–5 mm) | 20 (5%) |
| - Lymphovascular invasion | |
| Positive | 34 (7,6%) |
| Negative | 298 (66,4%) |
| ND | 117 (26%) |
| -Ki67 | |
| <20 | 258 (57,4%) |
| ≥20 | 191 (42,6%) |
| -Subtipe | |
| Luminal A | 197 (44%) |
| Luminal B | 252 (56%) |
| -Systemic treatment | |
| Chemotherapy + HT | 136 (30%) |
| Hormone therapy (HT) | 313 (70%) |
Table 2.
Risk classification by genetic platforms.
| Genetic test n (%) | Risk classification |
|---|---|
| OncotypeDx® (49%) | RS low (62,7%) RS intermédiate (27,7%) RS high (9,5%) |
| Mammaprint® (51%) | High (38,4%) Low (61,6%) |
3.1. Adjuvant treatments
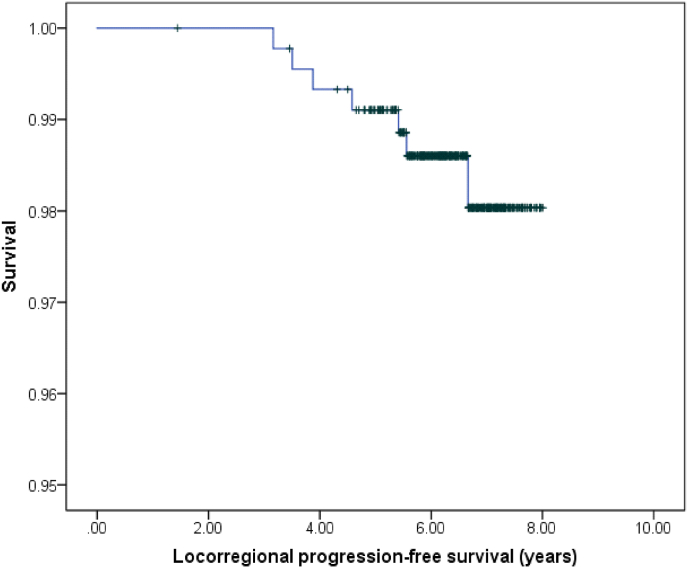
RT was administered after surgical excision in 82% of the cases; CF was used in 49% of patients compared to HF with overprinting in 67.7% of cases. In addition, 67.7% received exclusive HT, and the remaining 36.3% received HT combined with CT. The L-PFS of the patients at the time of study was 98.4% (Fig. 1); 7 locoregional relapses had been detected, with 42% of these being found in the same quadrant. When analysing the L-PFS results, no notable differences were observed according to the genomic test used; the hazard ratio (HR) was 1.02 (95% CI [0.20, 5.06]; p = 0.850). Similarly, there were no notable differences regarding the irradiation scheme used when comparing conservative surgery plus RT versus exclusive radical surgery, with a HR of 1.23 (95% CI [0.14, 10.53]; p = 0.980).
Fig. 1.
Locorregional progression-free survival.
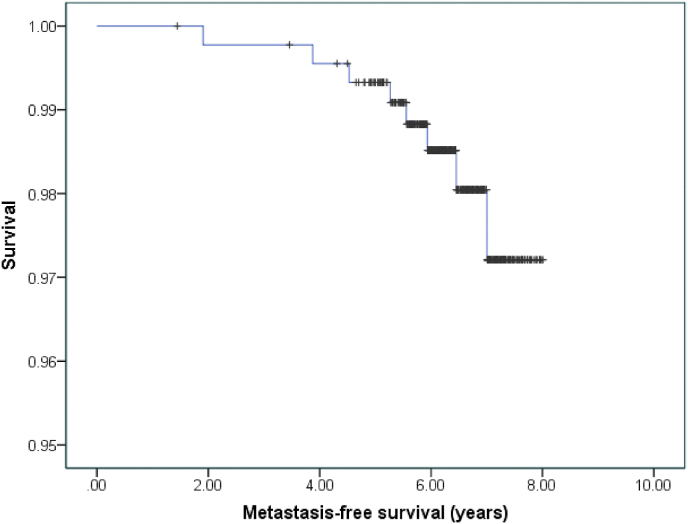
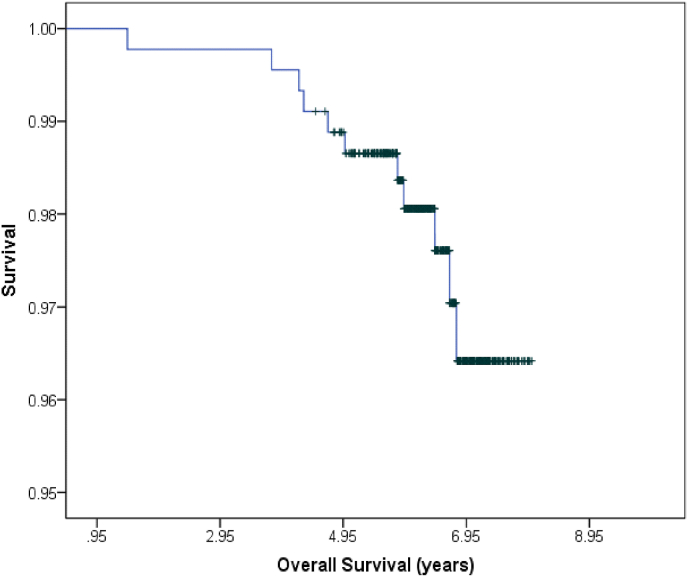
The MFS rate was 98.2%, and when detected in our cohort, the metastases were predominantly distant, in the liver or bone (Fig. 2). The OS was 97.5% (Fig. 3); and cancer-specific death (CSD) was 0.6% at the time of the analysis. The other deaths had been caused by cardiovascular comorbidity factors, especially those related to diabetes mellitus and obesity. No significant differences were observed according to the genomic platform that had been used, OncotypeDx® obtained a HR of 5.06 (95% CI [0.59, 43.38]; p = 0.130). There were also no differences in the OS according to the locoregional treatment used (p = 0.450).
Fig. 2.
Metastasis-free survival.
Fig. 3.
Overall survival.
3.2. Creation of the PersonalRT27 tool
Based on previous evidence [32,33], a relapse-risk score of 1–5 points was assigned for each of the variables analysed, with 5 points representing the highest risk. For example, the patients with the lowest risk of progression were aged over 60 years, had a small (T1), and well-differentiated (G1), luminal subtype A, tumour with negative margins and without lymphovascular invasion and Ki67 expression less than 20%. The results of the genetic platform scores were also categorised by assigning a higher score to patients at a higher risk of recurrence. Individuals who were considered low or high risk for both platforms scored 2 or 5 points respectively; those with an intermediate risk for OncotypeDx® scored 4 points. By summing all the scores for analysed variables, we obtained a score that allowed us to appropriately stratify patients and thereby, individualise their therapeutic strategies.
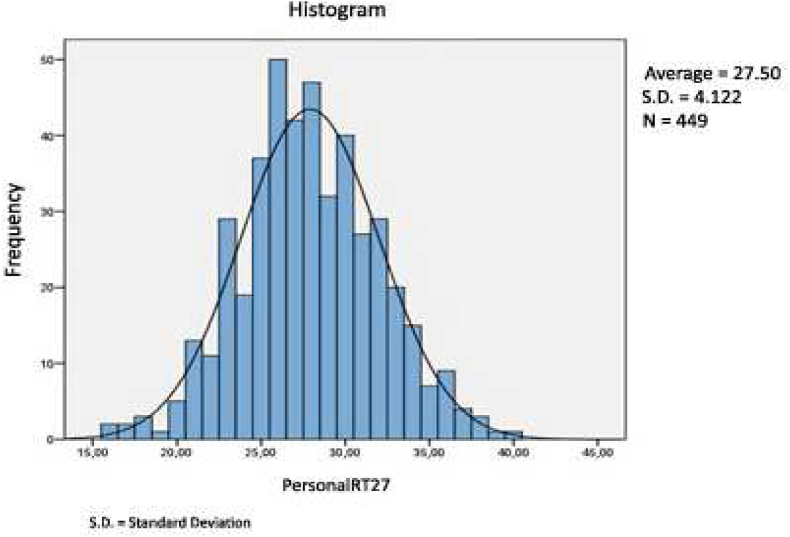
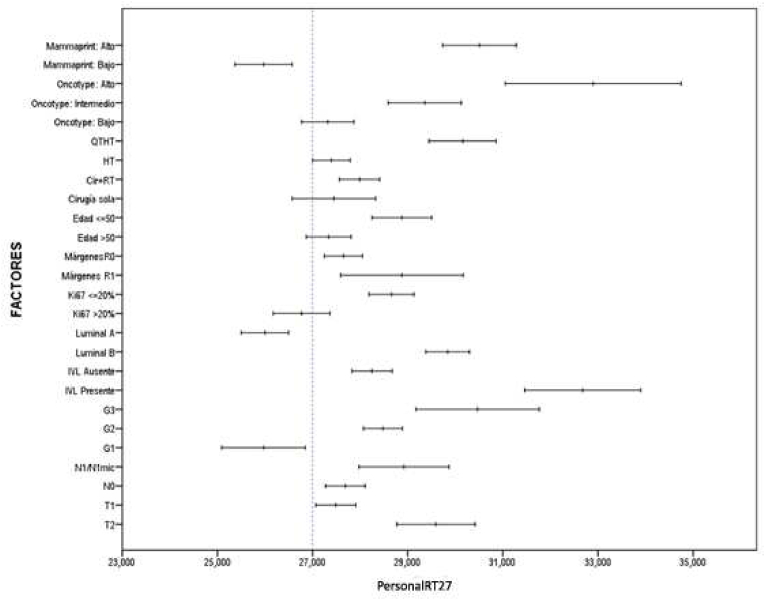
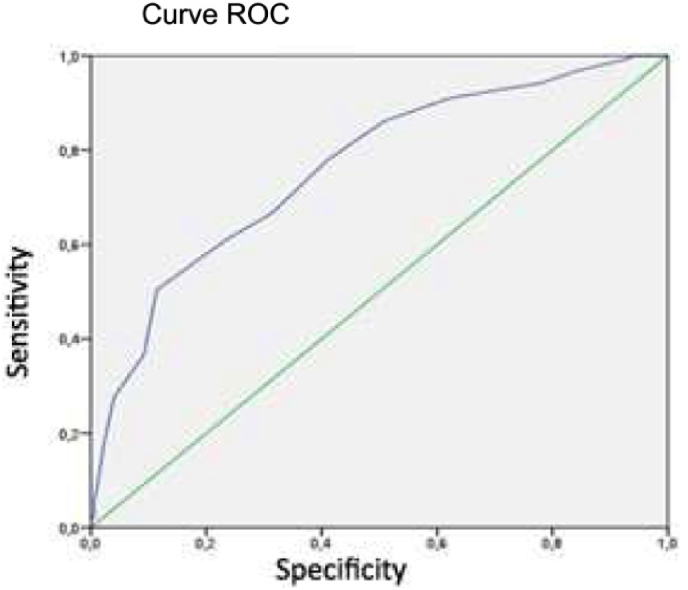
As shown in Table 3, Table 4, 42 patients have obtained a score of 27 for the analysed variables (representing a cumulative percentage of 47,7%). We therefore considered this threshold turning point between a low (<27) and high (≥27) risk of recurrence for this study population and named the tool the PersonalRT27 (Fig. 4). An adjusted univariate analysis to assess the association between the PersonalRT27 score and all the study variables identified a correlation between high scores and the presence of unfavourable prognostic factors. A good correlation was also observed when we stratified risk according to several different classical factors included in other platforms (Fig. 5). When we compared this new tool with the OncotypeDx® and Mammaprint® genetic analysis platforms, we had to join the intermediate and high RS categories for OncotypeDx®, although this did not notably affect the correlation outcome. Thus, an AUC of 0.76 (95% CI [0.70, 0.81]) was obtained for PersonalRT27, with a sensitivity of 78% and a specificity of 58.9% (Fig. 6).
Table 3.
Numerical score according to risk.
| Quantitative variables: Score lowest to highest risk | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Edad | |||||
| ≤ 50años | • | ||||
| 51-59 años | • | ||||
| ≥ 60 años | • | ||||
| Comorbilidad | |||||
| Obesidad(IMC > 30 kg/m2) | • | ||||
| Hipertensión arterial | • | ||||
| Tabaquismo activo | • | ||||
| Diabetes Mellitus | • | ||||
| Anatomía Patológica | |||||
| CDI | • | ||||
| CLI | • | ||||
| Ca in situ | • | ||||
| Tamaño tumoral | |||||
| T1 | • | ||||
| T2 | • | ||||
| Estado ganglionar | |||||
| pN0 | • | ||||
| pN1mi | • | ||||
| pN1a | • | ||||
| Márgenes | |||||
| R0 | • | ||||
| R1 | • | ||||
| R próximos (1–5 mm) | • | ||||
| Invasión linfovascular | |||||
| ILV + | • | ||||
| ILV - | • | ||||
| Grado histológico | |||||
| G1 | • | ||||
| G2 | • | ||||
| G3 | • | ||||
| Subtipo luminal | |||||
| Luminal A | • | ||||
| Luminal B (Her2-) | • | ||||
| Ki67 | |||||
| Ki 67 ≥ 20 | • | ||||
| Ki67 < 20 | • | ||||
| Mammaprint® | |||||
| Alto riesgo | • | ||||
| Bajo riesgo | • | ||||
| OncotypeDx® | |||||
| RS bajo | • | ||||
| RS intermedio | • | ||||
| RS alto | • | ||||
1 Low point; 5 High point; 2-3-4 Intermediate.
Table 4.
PersonalRT27 characteristics.
| Characteristics | Distribution | 95% CI |
|---|---|---|
| Sensitivity | 78.0% | 69.9%–84.5% |
| Specificity | 58.9% | 51.5%–65.9% |
| PPV | 57.1% | 49.6%–64.4% |
| NPV | 79.2% | 71.5%–85.3% |
| Proporción FP | 41.1% | 34.1%–48.5% |
| Proporción FN | 22.0% | 15.5%–30.1% |
| Accuracy | 66.8% | 61.2%–71.9% |
| Oddsratio | 5.09 | 3.02–8.58 |
Abbreviations: PPV (positive predictive value), NPV (negative predictive value), FP (false positive), FN (false negative).
Fig. 4.
PersonalRT27 histograma.
Fig. 5.
Univariante analysis with PersonalRT27.
Fig. 6.
Curve ROC
We also carried out a univariate analysis of the clinicopathological variables and the genetic assays to evaluate their association with progression events; these analyses highlighted a positive correlation with affected margins and the luminal B subtype (Table 5). Furthermore, the association with affected margins was also maintained in the multivariate analysis (Table 6). Given the limited number of events, we created a variable that comprised both locoregional and distant relapses. We wanted to determine if the tool would detect progressions given that relapse events would present scores of 27 or more in the PersonalRT27. Of the 13 progressions we detected among our cohort, 7 were locoregional and 6 were distant; of note, 5 of these patients had a PersonalRt27 score lower than 27, although all of these had also been classified as low-risk by the other two genetic platforms. The remaining patients were classified as high-risk by the PersonalRT27 (scores over 27 points) and so these results were consistent with the evolution of these individuals. Finally, we created an in-hospital nomogram (available at https://form.jotformeu.com/cgarcia84/clingen) that includes all the clinicopathological, molecular, and genetic variables we considered in this work. It was our hope that this nomogram would help physicians easily identify patients with a higher risk which could benefit from targeted treatments.
Table 5.
Univariate analysis of variables with progression.
| Variables | Reference | HR | LCI (95%CIHR) | UCI (95%CIHR) | p-value | |
|---|---|---|---|---|---|---|
| OncotypeDx® | RS low | 0 | ||||
| RS intermediate | 1 | 0.973 | 0.088 | 10.735 | 0.982 | |
| RS high | 2 | 4.871 | 0.387 | 61.359 | 0.221 | |
| Mammaprint® | Low | 0 | ||||
| High | 1 | 0.497 | 0.095 | 2.612 | 0.409 | |
| Age(years) | ≤50 | 1 | 1.608 | 0.424 | 6.101 | 0.485 |
| >50 | 0 | |||||
| Grade | G1 | 0 | ||||
| G2 | 1 | 0.53 | 0.151 | 0.41 | 0.321 | |
| G3 | 2 | 0.986 | ||||
| Lymphovascular invasion | Positive | 0 | ||||
| Negative | 1 | 1.291 | 0.155 | 10.742 | 0.813 | |
| Lymph nodes | N0 | 1 | 0.838 | 0.178 | 3.953 | 0.823 |
| N1/N1mi | 1 | 1.227 | 0.26 | 5.787 | 0.796 | |
| Subtype luminal | A | 0 | ||||
| B | 1 | 0.191 | 0.041 | 0.902 | 0.037 | |
| Margins | R1 | 1 | 4.364 | 1.311 | 14.528 | 0.016 |
| R0 | 0 | 0 | 0 | 0 | ||
| Ki 67 | ≤20% | 0 | ||||
| >20% | 1 | 0.412 | 0.087 | 1.944 | 0.262 | |
| Type of treatment | Surgery + RT | 1 | 0.949 | 0.201 | 4.473 | 0.947 |
| Surgery | 0 | |||||
| Systemic treatment | HT | 0 | ||||
| QT + HT | 1 | 1.251 | 0.366 | 4.273 | 0.721 |
Abbreviations: Surgery (Lumpectomy; Mastectomy), QT (Chemotherapy), HT (hormone therapy).
Table 6.
Multivariate Analysis with progression.
| Variable | Reference | HR | LCI (95%ICHR) | UCI (95%ICHR) | p-value | |
|---|---|---|---|---|---|---|
| Luminal | A | 0 | 0.048 | 1.098 | 0.065 | |
| B | 1 | 0.230 | ||||
| Margins | R0 | 0 | 1.245 | 13.985 | 0.021 | |
| R1 | 1 | 4.172 |
4. Discussion
The natural progression of BC differs because of the inherent heterogeneity of this disease and according to the initial diagnostic stage and baseline status of the patient. In this work, we assessed different therapeutic strategies and their responses, depending on several clinical data variables and/or analytical prognostic and predictive values. We were able to correlate these factors with expected survival based on the so-called prognostic stage [34]. This allowed us to create nomograms that integrated these variables and factors as a tool to assess the risk of distant progression and which can help us to select the most appropriate therapy for each patient based on their individual risk.
Of note, the Adjuvant 8.0 (www.adjuvantonline.com) tool reports the expected OS benefit for individual patients based on the use of CT or HT in these cases [35,36]. In addition, ipsilateral relapse prediction nomograms such as the one designed by Sanghani et al. (IBTR!) that analyses clinicopathological risk variables can correctly calculate the percentage of recurrence after conservative surgery and RT in low and moderate-risk patients [37,38]. Furthermore, genomic tests used in clinical practice can also help us to discriminate patients who could benefit from systemic treatments. The objective of all these tools was to individualise cancer treatments by selecting appropriate profiles for more targeted and effective therapies.
However, precisely because of the heterogeneity of BC, no single platform integrates all these variables and is valid for the prediction of both locoregional and distant BC progression. Therefore, we created the PersonalRT27 to try to improve the selection of patients with early-stage BC for different targeted therapies. This tool, simultaneously considers both the classic factors present in these aforementioned nomograms as well as those included in the OncotypeDx® and Mammaprint® genetic platforms, to predict both local and distant relapses in patients with BC.
Previous work established an association between the classic factors and the OncotypeDx® RS and concluded that the RS adds complementary biological information to the traditional clinicopathological factors when evaluating the probability of relapse [39,40]. Furthermore, these publications also showed that, compared to patients with a low or intermediate risk, more patients with a high RS had ki67 expression exceeding 20%, a high histological grade, lymphovascular invasion, and had undergone CT. Therefore, together with MammaPrint® and other traditional risk factors, the RS incorporated into our tool was a useful predictive variable for patient prognosis. Thus we obtained similar data with the PersonalRT27 tool given that we observed a correlation between affected surgical margins and the luminal B subtype, although only the former was confirmed in our multivariate analysis.
However, other studies have shown a correlation between RS and different variables including age, histological grade, Ki67 expression, and lymph node involvement [41]. In this work, the RS was high in a higher proportion of patients with a G3 morphology, lymphovascular invasion, and who had received CT, compared to patients with intermediate and low RS scores [42].
Most of the patients in our series had received RT after conservative surgery because we presently only consider clinicopathological parameters when deciding the most appropriate locoregional treatment. Thus, when developing PersonalRT27, we considered both these traditional factors and genetic and molecular factors. We also found ongoing studies that had analysed the role of the luminal subtype and RS by other platforms to help decide whether to omit RT in low-risk groups.
Our nomogram was based on a logistic regression analysis of population data; after introducing multiple variables the nomogram obtains a probability value of 0 to 1, as represented by the AUC, which defines the probability of relapse. To determine the sensitivity and specificity of our tool, we determined the AUC and compared it with other nomograms currently in clinical use, including those used to predict the risk of axillary relapse, and obtained values remarkably similar to those published elsewhere [[43], [44], [45], [46], [47]]. However, the work that was most consistent with the variables, population, and development process used for PersonalRT27, was based on the creation and validation of a time-dependent logistic regression model for predicting locoregional recurrence. This tool had integrated multiple clinical-pathological and molecular variables to generate a nomogram to support therapeutic decision-making and clinical follow-ups. Moreover, its AUC for the estimated risk at 5 years was 0.71, which indicated a good discrimination capacity which was lower than that of PersonalRT27, perhaps because we considered genetic profiles in the categorisation of risk. Nonetheless, our tool was novel in the sense that it not only included variables from previously validated nomograms, but it also incorporated independent risk prediction parameters such as intrinsic subtypes and genomic expression profiles.
Regarding the limitations of this work, it is important to note that this was a non-randomised, retrospective study with potential selection bias. We also included relatively few events because the population had a generally very low risk of relapse. However, it is precisely this group that should be targeted with personalised systemic and local treatments that can be adapted to individual patient risk, thus making the treatments less likely to interfere with patient quality of life. In the future, prospective studies must be carried out to allow us to use this tool to evaluate, a priori, which patients subgroups would or would not benefit from the addition of RT to their treatment regimens [[48], [49], [50], [51], [52]].
The PersonalRT27 can help us select the most appropriate locoregional treatments for patients because it can indicate, in advance, which patients have a higher or lower risk of locoregional relapse. For example the PersonalRT27 could predict which patients might benefit from whole-breast RT (those with scores of 27 or more), or on the contrary, less aggressive partial breast RT could be applied in patients with scores under 27 points. Thus, the categorisation tools incorporated into the PersonalRT27 offer a path towards personalised RT in low and intermediate-risk patients. Notwithstanding, the validation of PersonalRT27 to predict the risk of relapse both prospectively and after more than 10 years is still pending, especially in independent populations.
5. Conclusions
PersonalRT27 considers both genetic and clinical factors and in this current work, showed adequate sensitivity and adjusted well to the results of the other genetic testing platforms. Therefore, this tool has a high prognostic value because it correctly categorised patients according to their risk. PersonalRT27 is available online and is easy to use; it allows decisions to be inferred quickly and with some agility. However, independent external validation and a longer follow-up time, including inclusion of a greater number of events will be required before it can be implemented in healthcare practice to help personalise RT treatments.
Contributor Information
Inmaculada Beato Tortajada, Email: inmabeato@gmail.com.
Carlos Ferrer Albiach, Email: ferreralbiach@gmail.com.
Virginia Morillo Macias, Email: vmorill@gmail.com.
References
- 1.Darby S., McGale P., Correa C., Taylor C. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence andthe15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. PMID: 22019144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGale P., Taylor C., Correa C., Cutter D. Effect of radiotherapy after mastectomy and axillary surgeryon 10 year-recurrence and 20 year breast cancer mortality:meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. 10.1016/S0140-6736(14)60488-8. PMID: 24656685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Recht A., Edge S.B., Solin L.J. Postmastectomy radiotherapy: clinical practice guidelines of the American society of clinical oncology. J Clin Oncol. 2001;19:1539–1569. doi: 10.1200/JCO.2001.19.5.1539. PMID: PMID: 11230499. [DOI] [PubMed] [Google Scholar]
- 4.Duffy M.J., Harbeck N., Nap M., Molina R., Nocilini A., Senkus E., et al. Clinical use of biomarkers in breast cancer: update guidelines from the European Group on Tumor Markers (EGTM) Eur J Cancer. 2017;75:284–298. doi: 10.1016/j.ejca.2017.01.017. PMID: 28259011. [DOI] [PubMed] [Google Scholar]
- 5.Hudis C.A., Dickler M. Increasing Precision in adjuvant therapy for breast cancer. N Engl J Med. 2016;375:790–799. doi: 10.1056/NEJMe1607947. 10.1056/NEJMe1607947. PMID: 27557306. [DOI] [PubMed] [Google Scholar]
- 6.SEOM . Disponible en; 2020. Las cifras del cancer en España.https://seom.org/seomcms/images/stories/recursos/Cifras_del_cancer_2020.pdf [Google Scholar]
- 7.Guevara M., Molinuevo A., Salmerón D., Marcos-Gragera R., Chirlaque M.D., Quirós J.R., et al. Red Española de Registros de Cancer (REDECAN); 2019. Supervivencia de Cáncer en España, 2002-2013. [Google Scholar]
- 8.Gospodarowicz M., O′Sullivan B. Prognostic factors in cancer. Semin Surg Oncol. 2003;21(1):13–18. doi: 10.1002/ssu.10016. 10.1002/ssu.10016. PMID: 12923911. [DOI] [PubMed] [Google Scholar]
- 9.Duffy M.J., Harbeck N., Nap M., Molina R., Nocilini A., Senkus E., et al. Clinical use of biomarkers in breast cancer: update guidelines from the European Group on Tumor Markers (EGTM) Eur J Cancer. 2017;75:284–298. doi: 10.1016/j.ejca.2017.01.017. PMID: 28259011. [DOI] [PubMed] [Google Scholar]
- 10.Sparano J.A., Gray R.J., Makower D.F., Kathleen I., Albain K.S., Hayes D.F., et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373:2005–2014. doi: 10.1056/NEJMoa1510764. 10.1056/NEJMoa1510764. PMID: 26412349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts M.C., Miller D.P., Shak S., Petkov V.I. Breast cancer-specific survival in patients with lymph node-positive hormone receptor-positive invasive breast cancer and OncotypeDx® Recurrence Score results in the SEER data base. Breast Cancer Res Treat. 2017;163:303–310. doi: 10.1007/s10549-017-4162-3. PMID: 28243896. [DOI] [PubMed] [Google Scholar]
- 12.Van Poznak C., Somerfield M.R., Bast R.C., Cristofanilli M., Goetz M.P., González-Angulo A.M., et al. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American society of clinical oncology practice guidelines. J Clin Oncol. 2015;33:2695–2704. doi: 10.1200/JCO.2015.61.1459. 10.1200/JCO.2015.61.1459. PMID: 26195705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petkov V., Miller D.P., Howlader N., Gliner N., Howe S., Schussler N., et al. Breast-cancer-specific mortality in patients treated based on the 21-gene assay: a SEER population-based study. NPJ Breast Canc. 2016;2:16017. doi: 10.1038/npjbcancer.2016.17. PMID: 28721379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paik S., Tang G., Shak S., Chungyeul K., Baker J., Cronin W.K., et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–3734. doi: 10.1200/JCO.2005.04.7985. PMID: 16720680. [DOI] [PubMed] [Google Scholar]
- 15.Gluz O., Nitz U.A., Christgen M., Kates R.E., Clemens M., Kraemer S., et al. West German study group phase III PlanB trial: fist prospective outcome data for the 21-gene recurrence score assay and concordance of pronostic markers by central and local pathology assessment. J Clin Oncol. 2016;34:2341–2349. doi: 10.1200/JCO.2015.63.5383. PMID: 26926676. [DOI] [PubMed] [Google Scholar]
- 16.Albain K.S., Barlow W.E., Shak S., Hortobagy G.N., Livingston R.B., Tien Yeh I., et al. Breast Cancer Intergroup of North America. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptorpositive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. PMID: 20005174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardoso F., van’t Veer L., Bogaert J., Slaets L., Viale G., Delaloge S., et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375:717–729. doi: 10.1056/NEJMoa1602253. 10.1056/NEJMoa1602253. PMID: 27557300. [DOI] [PubMed] [Google Scholar]
- 18.Roberts M.C., Miller D.P., Shak S., Petkov V.I. Breast cancer-specific survival in patients with lymph node-positive hormone receptor-positive invasive breast cancer and OncotypeDx® Recurrence Score results in the SEER database. Breast Cancer Res Treat. 2017;163:303–310. doi: 10.1007/s10549-017-4162-3. PMID: 28243896. [DOI] [PubMed] [Google Scholar]
- 19.Van′t Veer L., Dai H., Van de Vijver M.J., He Y.H., Hart A., Mao M., et al. Gene expression profiling predicts outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. 10.1038/415530a. PMID: 11823860. [DOI] [PubMed] [Google Scholar]
- 20.Brauntein L.Z., Taghian A.G., Niemierrko A., Salama L., Capuco A., Bellon J.R., et al. Breast-cancer subtype, age and lymph node status as predictors of local recurrence following breast-conserving therapy. Breast Cancer Res Treat. 2017;161:173–179. doi: 10.1007/s10549-016-4031-5. PMID: 27807809. [DOI] [PubMed] [Google Scholar]
- 21.Solin L.J., Gray R., Goldstein L.J., Recht A., Baehner F.L., Shak S., et al. Prognostic value of biologic subtype and the 21-gene recurrence score relative to local recurrence after breast conservation treatment with radiation for early stage breast carcinoma: results from the Eastern Cooperative Oncology Group E2197 study. Breast Cancer Res Treat. 2012;134:683–692. doi: 10.1007/s10549-012-2072-y. PMID: 22547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voduc K.D., Cheang M.C., Tyldesley S., Gelmon K., Nielsen T.O., Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. PMID: 20194857. [DOI] [PubMed] [Google Scholar]
- 23.Mamounas E.P., Tang G., Fisher B., Paik S., Shak S., Costantino J.P., et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABPB-20. J Clin Oncol. 2010;28(10):1677–1683. doi: 10.1200/JCO.2009.23.7610. 10.1200/JCO.2009.23.7610. PMID: 20065188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldhirsch A., Winer E.P., Coates A.S. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. PMID: 23917950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coates A.S., Winer E.P., Goldhirsch A., et al. Tailoring therapies-improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26:1533–1546. doi: 10.1093/annonc/mdv221. PMID: 25939896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lange S., Scheibler F., Fleer D., et al. Interpretation of the results of the MINDACT Study and consequent recommendations in the updated ASCO clinical practice guideline. J Clin Oncol. 2018;36:429–430. doi: 10.1200/JCO.2017.75.9506. PMID: 29227725. [DOI] [PubMed] [Google Scholar]
- 27.Kalinsky K.M. Tamoxifen citrate, letrozole, anastrozole, or exemestano with or without chemotherapy in treating patients with invasive RxPONDER breast cancer. http://www.clinicaltrials.gov/ct2/show/NCT01272037 Available on:
- 28.Senkus E., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rutgers E. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v8–v30. doi: 10.1093/annonc/mdt284. 10.1093/annonc/mdv298. PMID: 26314782. [DOI] [PubMed] [Google Scholar]
- 29.Giuliano A.E., Ballman K.V., McCall L., Beitsch P.D., Brennan M.B., Keleman P.R., et al. Effect of axillary dissection vs not axillary dissection on 10 year overall survival among women with invasive breast cancer and sentinel node metastasis. J Am Med Assoc. 2017;318(10):918–926. doi: 10.1001/jama.2017.11470. PMID: 28898379 PMCID: PMC5672806 DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NCCN clinical practice guidelines in oncology (NCCN guidelines) version 4. 2020 invasive breast cancer. https://www.nccn.org/professionals/radiation/default.aspx Disponible en:
- 31.Amin M.B., Greene F.L., Edge S.B., Byrd D.R., Brookland R.K., Washington M.K., et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. PMID: 28094848. [DOI] [PubMed] [Google Scholar]
- 32.Almagro E., González C.S., Espinosa E. Prognostic factors of early breast cancer. Med Clin. 2016;146:167–171. doi: 10.1016/j.medcli.2014.12.019. 10.1016/j.medcli.2014.12.019. PMID: 25726309. [DOI] [PubMed] [Google Scholar]
- 33.Arvold N.D., Taghian A.G., Niemierko A., Abi Raad R.F., Sreedhara M., Nguyen P.L., et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011;29(29):3885–3891. doi: 10.1200/JCO.2011.36.1105. PMID: 21900114 PMCID: PMC3189090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galea M.H., Blamey R.W., Elston C.E., Ellis I.O. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat. 1992;22:207–219. doi: 10.1007/BF01840834. PMID: 1391987. [DOI] [PubMed] [Google Scholar]
- 35.Ravdin P.M., Siminoffl, Davis G. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. doi: 10.1200/JCO.2001.19.4.980. PMID: 11181660. [DOI] [PubMed] [Google Scholar]
- 36.Hajage D., de Rycke Y., Bollet M., Savignoni A., Pierga C.M., Horlings H.M., et al. External validation of Adjuvant! Online breast cancer prognosis tool. Prioritising recommendations for improvement. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027446. PMID: 22087319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanghani M., Balk E., Cady B., Wazer D., Wazer D. Predicting the risk of local recurrence in patients with breast cancer: an approach to a new computer-based predictive tool. Am J Clin Oncol. 2007;30:47380. doi: 10.1097/COC.0b013e31805c13d9. PMID: 17921706. [DOI] [PubMed] [Google Scholar]
- 38.Sanghani M., Truong P.T., Raad R.A., Niemierko A., Lesperance M., Olivotto I.A., et al. Validation of a web-based predictive nomogram foripsilateral breast tumor recurrence after breast conserving therapy. J Clin Oncol. 2010;28:718–722. doi: 10.1200/JCO.2009.22.6662. 10.1200/JCO.2009.22.6662. PMID: 20048188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thaker N.G., Hoffman K.E., Stauder M.C., Shaitelman S.F., Strom E.A., TereffeW, et al. The 21-gene recurrence score complements IBTR! Estimates in early-stage, hormone receptor-positive, HER2-normal, lymph node-negative breast cancer. SprinterPlus. 2015;4:36–44. doi: 10.1186/s40064-015-0840-y. e Collection 2015. PMID: 25674496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kilickap S., Kaya Y., Yucel B., Tuncer E., Babacan N.A., Elagoz S., et al. Higher Ki67expression is associates with unfavorable prognostic factors and shorter survival in breast cancer. Asian Pac J Cancer Prev APJCP. 2014;15:1381–1385. doi: 10.7314/apjcp.2014.15.3.1381. PMID: 24606469. [DOI] [PubMed] [Google Scholar]
- 41.Jin Lim Y., Lee S.-W., Choi N., Kwon J., Eom K.Y., Kang E., et al. A novel prognostic Nomogram for predicting risks of distant failure in patients with invasive breast cancer following postoperative adjuvant radiotherapy. Cancer Res Treat. 2018;50:1140–1148. doi: 10.4143/crt.2017.508. PMID: 29216710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delpech Y., Wu Y., Hess K.R., Hsu L., Ayers M., Natowicz R., et al. Ki67 expression in the primary tumor predicts for clinical benefit and time to progression on first-line endocrine therapy in estrogen receptor-positive metastatic breast cancer. Breast Cancer Res Treat. 2012;135:619–627. doi: 10.1007/s10549-012-2194-2. PMID: 22890751. [DOI] [PubMed] [Google Scholar]
- 43.Franco P., Carlo Lorio G., Bartoncini S., Airlodi M., De Sanctis C., Castellano I., et al. De-escalation of breast radiotherapy after conserving surgery in low-risk early breast cancer patients. Med Oncol. 2018;35(5):62. doi: 10.1007/s12032-018-1121-8. 10.1007/s12032-018-1121-8. PMID: 29616366. [DOI] [PubMed] [Google Scholar]
- 44.Houpu Y., Fei X., Yang Y., Fuzhong T., Peng L., Lin C., et al. Use of Memorial Sloan Ketting Cancer nomogram to guide intraoperative sentinel lymph node frozen sections in patients with early breast cancer. J Surg Oncol. 2019;120:587–592. doi: 10.1002/jso.25638. 10.1002/jso.25638. PMID: 31309573. [DOI] [PubMed] [Google Scholar]
- 45.Shimazu K., Sato N., Ogiya A., Sota Y., Yotsumoto D., Ishikawa T., et al. Intraoperative Nomograms Based on One-Step Nucleic Acid Amplification for Prediction of Non-sentinel node Metastasis and four or more axillary node metastases in Breast cancer patients with sentinel node metastasis. Ann Surg Oncol. 2018;25:2603–2611. doi: 10.1245/s10434-018-6633-0. PMID: 29978372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubio I.T., Espinosa-Bravo M., Rodrigo M., Amparo Viguri Diaz M., Hadisson D., Sagasta A., et al. Nomogram including the total tumoral load in the sentinel node assessed by one-step nucleic acid amplification as a new factor for predicting non sentinel lymph node metastasis in breast cancer patientes. Breast Cancer Res Treat. 2014;147:371–380. doi: 10.1007/s10549-014-3108-2. PMID: 25164972. [DOI] [PubMed] [Google Scholar]
- 47.Sweldens C., Peeters S., van Limbergen E., Janssen H., Laenen A., Patil S., et al. Local relapse after breast-conserving therapy for ductal carcinoma in situ. A European single-center experience and extenal validation of the memorial Sloan Kettering cancer center DCIS nomogram. Cancer J. 2014;20(1):1–7. doi: 10.1097/PPO.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 48.Bentzen S.M., Agrawal R.K., Aird E.G., Barrett J.M., Barrett-Lee P.J., Bliss J.M., et al. START Trialists Group. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomized trial. Lancet Oncol. 2008;9:331–341. doi: 10.1016/S1470-2045(08)70077-9. PMID: 18356109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haviland J.S., Owen J.R., Dewar J.A., Agrawal R.K., BarrettJ, Barrett-Lee P.J., et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. PMID: 24055415. [DOI] [PubMed] [Google Scholar]
- 50.Smith B.D., Arthur D.W., Buchholz T.A., Haffy B.G., Hahn C.A., Hardenbergh P.H., et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO) Int J Radiat Oncol Biol Phys. 2009;74:987–1001. doi: 10.1016/j.ijrobp.2009.02.031. PMID: 19545784. [DOI] [PubMed] [Google Scholar]
- 51.Polgár C., Van Limbergen E., Pötter R., Kovacs G., Polo A., Lyczel J., et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the groupe européen de curiethérapi-european society for therapeutic radiology and oncology (CEG-ESTRO) Breast Cancer Working Group based on clinical evidence. Radiother Oncol. 2010;94:264–273. doi: 10.1016/j.radonc.2010.01.014. PMID: 20181402. [DOI] [PubMed] [Google Scholar]
- 52.Polgár C., Fodor J., Major T., Sulyok Z., Kásler M. Breast-conserving therapy with partial or whole breast irradiation: ten-year result of the Budapest randomized trial. Radiother Oncol. 2013;108:197–202. doi: 10.1016/j.radonc.2013.05.008. 10.1016/j.radonc.2013.05.008. PIMD:23742961. [DOI] [PubMed] [Google Scholar]