Abstract
YTHDF1 is the most versatile and powerful reader protein of N6-methyladenosine (m6A)-modified RNA, and it can recognize both G(m6A)C and A(m6A)C RNAs as ligands without sequence selectivity. YTHDF1 regulates target gene expression by different mechanisms, such as promoting translation or regulating the stability of mRNA. Numerous studies have shown that YTHDF1 plays an important role in tumor biology and nontumor lesions by mediating the protein translation of important genes or by affecting the expression of key factors involved in many important cell signaling pathways. Therefore, in this review we focus on some of the roles of YTHDF1 in tumor biology and diseases.
Keywords: YTHDF1, N6-methyladenosine, m6A, protein translation, mRNA stability, tumor biology
Graphical abstract
YTHDF1 is the most versatile and powerful reader protein of N6-methyladenosine (m6A). YTHDF1 regulates target gene expression by promoting translation or regulating the stability of mRNA. YTHDF1 plays an important role in tumor biology and nontumor lesions by mediating different target genes.
Introduction
Post-transcriptional modification of RNA, which includes capping, splicing, and polyadenylation, is regarded as a key factor controlling mammalian protein production. The N6-methyladenosine (m6A) modification, which is the most abundant conserved post-translational modification, is found in a wide range of cellular RNAs. In recent years, m6A modifications have been shown to have an important function in the progression of various metabolic,1 infectious,2 immune system,3 and cardiovascular diseases4 and cancers.5 The RNA base sequence DRACH (D = A/G/U, R = A/G, H = A/C/U) is the consensus site of m6A. m6A modification on RNA polymerase II (pol II) transcribed RNAs such as mRNAs, long noncoding RNAs (lncRNAs), precursors of microRNAs, or circular RNAs can mediate their gene expression.6 m6A is regulated by an evolutionarily conserved methylase complex known as the “writers” complex including ZC3H13, RBM15, KIAA1429, METTL3, METTL14, and WTAP.7,8 It can also be reverted to an unmodified form by a demethylase family of “erasers,” including FTO and ALKBH5.9
m6A produces its effects when it interacts with the YTH protein family of m6A readers, by either directly accommodating the modified residue in a hydrophobic pocket of the YTH domain or having their affinity positively influenced by the presence of m6A. There are five YTH domain-containing proteins in humans: YTHDC1, YTHDC2, YTHDF1, YTHDF2, and YTHDF3.The YTH domains share a conserved α/β fold, including four or five α helices and six β strands.10 Six β strands form a β barrel, with the helices packed against the β strands, which stabilize the hydrophobic core.10 Each kind of reader protein has a different function involved in regulating RNA transcription and post-transcription. For example, YTHDF2 promotes RNA decay when m6A-modified mRNA is recruited to processing bodies (P-bodies). YTHDF3 facilitates mRNA translation with the assistance of YTHDF1 and promotes the decay of m6A-modified transcripts mediated by YTHDF2.11 YTHDC1 is an RNA splicing and nuclear export protein, resulting from its unique localization and enrichment in the nucleus.12 YTHDC2 plays an important role in RNA translation and decay due to its multiple RNA-binding domains.11 YTHDF1 is the most versatile and powerful reader protein of m6A; it can mediate target gene expression and participate in disease processes. In this review, we discuss the mechanisms by which YTHDF1 regulates gene expression and its possible roles in tumor biology and diseases.
The structure and characteristics of YTHDF1
Five α helices, six β strands, and 310 helices following the β5 strand form the YTH domain of YTHDF1. The six β strands, in the order β6-β1-β3-β4-β5-β2, are arranged in an atypical β-barrel fold. β1 and β3 are parallel β strands, whereas the others are antiparallel. Three helices (α1, α2, α3) pack against the β barrel and form a hydrophobic core with a combination of the six β strands. The α1 helix is packed against α0 and α4. A long loop linker follows the α0 helix, whereas α4 is a kink helix with its axis perpendicular to that of α1.13
The pocket of the YTH domain governs m6A-specific recognition. The m6A binding pocket of the YTHDF1 YTH domain is composed of the N terminus of α2, the C termini of β1, α1, and β2, and the loop between β4 and β5. Specifically, m6A is accommodated in a pocket, which is made up of Trp411, Trp465, and Trp470, with the ring planes of Trp411 and Trp470 parallel to each other and perpendicular to the ring plane of Trp465. The N6-methyl moiety and the aromatic cage form CH-π interactions; similarly, the adenine base and the aromatic residues form π-π interactions. As a result, both interactions constitute the basis of m6A-specific recognition.13
YTHDF1 utilizes the D401 residue to form a hydrogen bond with N1 of m6A. N1 of m6A cannot provide hydrogen to make up one hydrogen bond with an aspartic acid residue if intracellular pH conditions are neutral or basic; instead, it serves as the hydrogen acceptor for hydrogen bonding to an asparagine.14 Only under acidic pH conditions was it possible for N1 of m6A to form a hydrogen bond with D401 of YTHDF1.14 The pH-dependent interactions between YTHDF1 and m6A-modified RNA might explain the binding of YTHDF1 to short m6A-modified RNAs. YTHDF1, which is similar to other YTH domain proteins except YTHDC1 with m6A sequence selectivity, specifically recognizes m6A RNA without sequence selectivity. YTHDF1 can recognize both G(m6A)C and A(m6A)C RNAs as ligands,13 whereas YTHDC1 can preferably recognize G(m6A)C RNAs.10 The differences in the domains of YTHDF1 may also be closely related to their functions. Amino acids 100–200 of YTHDF1 are responsible for binding to the translation machinery.15
Mechanisms by which YTHDF1 regulates gene expression
Mechanisms by which YTHDF1 promotes translation
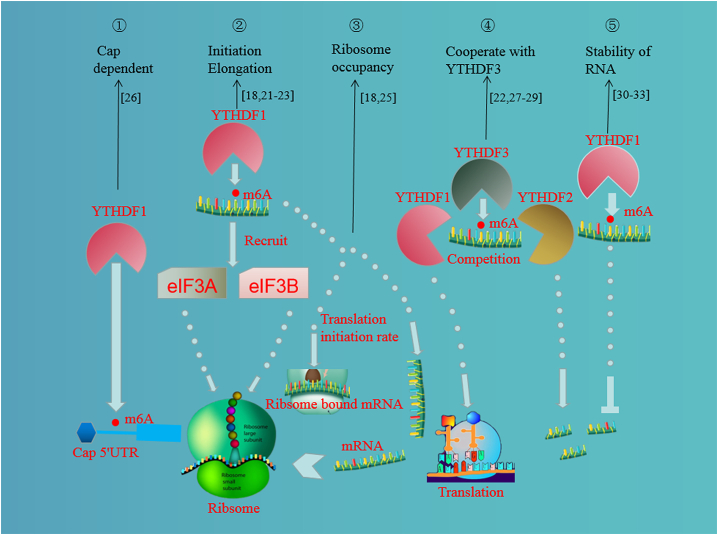
YTHDF1 recognizes m6A-edited mRNA and facilitates its translation, thus promoting its protein expression. However, the corresponding total mRNA levels of target proteins show no changes.16,17 When predicted m6A sites were mutated, YTHDF1 no longer exerted this effect on the regulation of translation.17 The present research shows that the mechanisms by which YTHDF1 promotes protein translation include triggering translation initiation and elongation, in a cap-dependent or cap-independent manner, by promoting ribosome occupancy or through a cooperative relationship with YTHDF3 (Figure 1).
Figure 1.
The mechanisms and functions of YTHDF1
YTHDF1 promotes translation by triggering translational initiation and elongation
A study18 found that YTHDF1 promoted target protein translation by triggering translation initiation through translation initiation factors, such as eIF3A and eIF3B. The expression of eIF3A and eIF3B was significantly inhibited after silencing YTHDF1. Interestingly, when proteins were copurified with YTHDF1, it was found among 13 subunits, 6 subunits belonged to translation initiation factor complex 3 (eIF3). The mutual effect exerted by YTHDF1 and eIF3 was further confirmed by western blotting and immunoprecipitation. YTHDF1 cooperates with the translation initiation machinery to enhance the translation efficiency of target RNAs in mammals.19,20 YTHDF1 enhances YAP translation by recruiting eIF3b to the translation initiation complex.21 Knockdown of eIF3b or YTHDF1 significantly restrains METTL3-mediated translation. Furthermore, eIF3b can bind YAP mRNA with the assistance of YTHDF1; on the other hand, this phenomenon is inhibited in the absence of YTHDF1.21 In addition, a similar study indicated that YTHDF1-promoted YAP mRNA translation was regulated by m6A modification and interaction with eIF3a.22 Furthermore, YTHDF1 depletion significantly downregulated eIF3A and eIF3B.23 YTHDF1 also promotes the translation of EIF3C by binding to m6A-modified EIF3C mRNA and concomitantly argues for the overall translational output; as a result, EIF3C, as a subunit of the protein translation initiation factor, further triggers the translation of other target genes.24 Apart from translation initiation, YTHDF1 is likely responsible for the m6A-induced translation elongation of Snail mRNA through interaction with the elongation factor eEF-2 in cancer cells.25 These studies indicate that YTHDF1 can promote translation by triggering translation initiation and elongation.
YTHDF1 promotes translation in a cap-dependent or cap-independent manner
The 5′ UTR region cap structure of eukaryotes is helpful for improving translation efficiency. mRNA translation of JAK2 was mediated by YTHDF1.26 Rapamycin, an inhibitor of cap-dependent protein translation, can markedly inhibit the increase of JAK2 protein expression promoted by YTHDF1, which indicates that a cap-dependent mechanism may be involved in YTHDF1-mediated translation.26 Maintenance of target protein expression in the presence of rapamycin was not due to an increase in protein stability, as cotreatment of wild-type cells with the protein synthesis inhibitor cycloheximide resulted in the rapid disappearance of the target protein, suggesting that Snail expression was regulated by YTHDF1 through cap-independent translation.25 One study also showed that overexpression of YTHDF1 promoted the translation of target proteins in a cap-independent manner, but the corresponding mRNAs for these target proteins showed no alterations, indicating that protein upregulation resulted from increased translation.16 These studies suggest that YTHDF1 can promote translation mainly in a cap-independent manner and only slightly in a cap-dependent manner.
YTHDF1 promotes translation by promoting ribosome occupancy
Ribosomes are protein-processing plants of cells. Findings regarding YTHDF1 and ribosomal subunits revealed that ribosomal subunits, translation initiation, and enrichment of YTHDF1 coexist in the 40S portion. YTHDF1 is also present in the 80S portion under mild formaldehyde fixation. A total of 119 special proteins were copurified from YTHDF1, 62 of which correlated to translation, including 23 of 47 60S subunits and 27 of 33 40S subunits.18 YTHDF1 altered the association of its target mRNA with ribosomes but did not change the overall methylated mRNA level. The extent of ribosome occupancy was reduced under YTHDF1 knockdown, which correlated with the number of YTHDF1-binding sites on the transcripts and the efficiency of YTHDF1 knockdown. YTHDF1 not only promoted ribosome occupancy of its target mRNA but also promoted ribosome occupancy at translation initiation sites. Thus, YTHDF1 might depend on a core mechanism rather than recruiting mRNA to the translation machinery, which affects the translation initiation rate of ribosome-bound mRNA.18 Lin et al.25 found that YTHDF1 prefers to bind with the coding DNA sequence (CDS) of Snail mRNA and then triggers its association with polysomes (>80S) of ribosomes. Moreover, YTHDF1 interacts with EIF3 to promote the rate-limiting step of translation for m6A-modified mRNAs by increasing their association with polysomes.18 The above studies showed that YTHDF1 could promote translation by promoting ribosome occupancy.
YTHDF1 promotes translation through a cooperative relationship with YTHDF3
YTHDF1 affects protein translation, which may require a very important cooperative relationship with YTHDF3. One report showed that YTHDF1 shares 58% of YTHDF3 targets.11 YTHDF1 shows a decreased binding specificity when YTHDF3 is repressed, indicating that YTHDF1 binding specificity toward its target mRNAs can be altered by the presence of YTHDF3.11 When m6A in pre-mRNA is recognized by YTHDFs, either decay or translation of the mRNA is promoted, depending on the different YTHDFs involved.27 Before undergoing decay through the YTHDF2-mediated pathway in the cytoplasm, YTHDF3 and YTHDF1 may first take part in promoting the translation of m6A-modified transcripts.11 YTHDF1/YTHDF3 preferentially recognizes m6A residues in the ITGA6 3′,UTR and promotes ITGA6 translation. Furthermore, silencing YTHDF1 or YTHDF3, but not YTHDF2, can significantly decrease translation promotion of the m6A-modified ITGA6.28 In summary, the m6A modification of target pre-mRNA (precursor messenger RNA) is first recognized by YTHDF3, followed by competitive binding of YTHDF1 and YTHDF2 to YTHDF3 to determine the fate of decay or translation of target pre-mRNA. Once YTHDF1 binds to YTHDF3, YTHDF1 carries target mRNA to the eIF3a-containing translation initiation complex and then increases target mRNA translation.22
Mechanisms by which YTHDF1 enhances the stability of RNA
Studies have reported that YTHDF1 regulates mRNA stability. An RNA stability assay showed that YTHDF1 silencing could shorten the t1/2 mRNA of c-Myc, which was extended under YTHDF1 overexpression, indicating that YTHDF1 could directly interact with mRNA and mediate RNA stability in an m6A-dependent manner.29 An RNA decay rate assay found that HK2 mRNA half-lives were significantly decreased under YTHDF1 silencing, suggesting that YTHDF1 enhanced HK2 mRNA stability.30 One study also showed that YTHDF1 and YTHDF2 jointly play balancing roles in regulating the gene stabilization and degradation of the lncRNA THOR.31 YTHDF1 promoted the translation of methylated transcripts by increasing both the mRNA and protein levels of MDM2 and YY1, resulting in YTHDF1 not only promoting translation but also affecting the stabilization of mRNA.32 Additionally, YTHDF1 could combine eEF-2 and IGF2BP3 to promote PDK4 translation elongation and mRNA stability by binding with the m6A-modified 5′ UTR of its mRNA.33 One study proved that amino acids 1–100 seemed to be the region of YTHDF1 that caused some RNA stabilization, which led to less protein production.15 All of the studies demonstrated that YTHDF1 could regulate the stability of the target RNAs.
The roles of YTHDF1 in tumors
YTHDF1 affects growth and metastasis of digestive system tumors
YTHDF1 significantly promotes colorectal cancer (CRC) cell tumorigenicity in vitro and murine xenograft tumor growth in vivo and significantly increases the capability of forming colonospheres.34 YTHDF1 recognizes m6A modification of ANKLE1 and affects its expression by altering the ANKLE1 transcriptional efficiency, but not mRNA stability.35 ANKLE1, as a potential tumor suppressor, is critical in inhibiting cell proliferation and facilitating the genomic stability of CRC carcinogenesis.35 Lin et al.23 found that YTHDF1 has a critical effect in regulating the liver cancer cell cycle and cell metabolism by tagging Snail, a key transcription factor of epithelial-mesenchymal transition (EMT), which is an important step in the metastasis of liver cancer cells, whose m6A methylated sites lie in CDS regions. A similar investigation showed that YTHDF1 might promote aggressive phenotypes by facilitating EMT and activating AKT/glycogen synthase kinase-3β/β-catenin signaling.36 Functional studies indicated that YTHDF1 can selectively recognize the m6A site in the frizzled5 (FZD5) mRNA CDS and trigger its translation, subsequently promoting hepatocellular carcinoma cell proliferation and metastasis both in vitro and in vivo. Inhibition of YTHDF1 attenuates hepatocellular carcinoma cell proliferation and tumorigenesis in vitro and in vivo.37 YTHDF1 promotes the translation of a key Wnt receptor, FZD7, via an m6A-dependent mechanism, resulting in hyperactivation of the Wnt/β-catenin pathway and progression of gastric carcinogenesis.38 Another investigation also proved that YTHDF1 facilitated tumorigenesis and metastasis of gastric cancer (GC) by promoting USP14 protein translation in an m6A-dependent manner.39
YTHDF1 affects growth and metastasis of respiratory system tumors
In a study on non-small cell lung cancer (NSCLC), YTHDF1 was proven to increase YAP mRNA translation, which enhances cellular growth, invasion, and EMT of NSCLC cells in vitro and in vivo.22 More specifically, YTHDF1-mediated YAP mRNA translation is controlled by m6A modification and interaction with eIF3a.22 Another study showed that YTHDF1 promotes NSCLC cell proliferation and xenograft tumor formation by mediating the translational efficiency of cyclin D1, cyclin-dependent kinase 2 (CDK2), and CDK4, and that YTHDF1 elimination inhibits de novo lung adenocarcinoma (ADC) progression.40 METTL3 promotes YAP mRNA translation, which significantly induces metastasis of NSCLC by recruiting YTHDF1/3 and eIF3b to the translation initiation complex.21 In addition to mRNA, YTHDF1 can also read m6A motifs, including GA(m6A)CA, GG(m6A)CU, and UG(m6A)CU, which are enriched on lncRNA THOR transcripts and regulate the stability of the lncRNA THOR, thereby regulating the proliferation of lung cancer cells.31 YTHDF1 expresses at a high level in NSCLC cells, and suppresses NSCLC cell apoptosis, facilitates proliferation, and encourages cell aggressiveness.41
YTHDF1 affects growth and metastasis of urogenital system tumors
Jin et al. demonstrated that m6A was highly enriched in ITGA6 transcripts, YTHDF1/YTHDF3 preferentially recognized m6A sites of the ITGA6 3′ UTR and increased ITGA6 translation, and the overexpression of ITGA6 resulted in increased growth and progression of bladder cancer cells in vitro and in vivo.28 Similarly, YTHDF1 binds to the m6A-modified CDCP1 3′ UTR and enhances CDCP1 mRNA modification and translation. CDCP1 upregulation in bladder cancers can play an important role in the progression of bladder cancer.42 In addition to ovarian cancer, YTHDF1 is overexpressed in cancer tissues and is correlated with poor prognosis.24 Mechanistically, YTHDF1 promotes the translation of EIF3C by binding to m6A-modified EIF3C mRNA and concomitantly determines the overall translational output, thereby facilitating tumorigenesis and metastasis of ovarian cancer. EIF3C, as a subunit of the protein translation initiation factor EIF3, also promotes the translation of other proteins in cells.24
YTHDF1 affects growth and metastasis of tumors in other systems
METTL3 accelerates c-Myc stability via YTHDF1-mediated m6A modification, thereby giving rise to tumorigenesis of oral squamous cell carcinoma.29 p53 signaling-related genes, including CDK2, CDK1, RRM2, CCNB1, and CHEK1, are significantly correlated with YTHDF1, which may read m6A modifications of p53 signaling-related genes, further upregulate their expression, and facilitate their roles in inhibiting p53 to suppress tumorigenesis of melanoma.43 Likewise, p53 activation regulators affected by m6A are involved in arsenite-induced transformation of human keratinocytes. YTHDF1 suppresses p53 activation by stimulating translation of YY1 and MDM2 mRNA, which is consistent with p53 inactivation.32 YTHDF1 promotes the proliferation of cell lines and tumor growth of glioma in vivo.44 YTHDF1 regulates such pro-oncogenic features of glioblastoma as increased proliferation and mobility.45 A study on osteosarcoma demonstrated that methylated YAP transcripts were recognized by YTHDF1 to promote its translation; subsequently, upregulation of YAP produced a significant attenuation of antitumor activities.46 In Merkel cell carcinoma, YTHDF1 has high copy gains and high expression. Importantly, there is a relationship between YTHDF1 amplification and MCPyV gene expression. Interestingly, knockdown of YTHDF1 in Merkel cell carcinoma cell lines significantly restrained the translation initiation factor eIF3 and further reduced proliferation and clonogenic capacity in vitro.23 HINT2 acts as a tumor-suppressor gene in ocular melanoma cells, and its translation is affected upon recognition by YTHDF1.47
YTHDF1 affects the formation of tumor stem cells
YTHDF1 silencing decreased CRC stem cell markers, such as CD133, CD44, ALDH1, OCT4, and Lgr5. Additionally, YTHDF1 silencing significantly downregulated the capabilities of CRC cancer stem cells. In terms of mechanics, the authors found that silencing YTHDF1 significantly inhibited Wnt/β-catenin pathway activity in CRC cells.34 Similar research indicated that YTHDF1, as an amplifier of the translation of Wnt signaling effectors including TCF7L2/TCF4 at the translational level, is critical for the maintenance of intestinal stem cells during tumorigenesis and regeneration.48 The levels of cancer stem cell markers, including CD133, NANOG, OCT4, and REX1, and sphere-forming capacity were markedly decreased upon YTHDF1 knockdown, indicating that YTHDF1 is required to maintain cancer stem cell properties in glioblastoma.45
YTHDF1 affects the metabolism of tumor cells
YTHDF1 remarkably interacts with the 5′ UTR of pyruvate dehydrogenase kinase 4 (PDK4) mRNA, and one study revealed that PDK4 promotes glycolysis and ATP generation in an m6A-dependent manner in cervical and liver cancer.33 YTHDF1 positively regulates the translation of m6A-modified TFRC mRNA, which is a critical target gene for increasing iron metabolism. Therefore, YTHDF1 enhances the tumorigenesis of hypopharyngeal squamous cell carcinoma by affecting iron metabolism in vivo and in vitro.49 A further study demonstrated that YTHDF1 enhanced the stability of m6A-modified HK2, thereby enhancing the Warburg effect of cervical cancer, which might provide insight into the treatment of cervical cancer.30
YTHDF1 affects sensitivity to chemotherapy
YTHDF1 significantly increases the resistance to the effects of both 5-fluorouracil and oxaliplatin in HT29 cells. 50 Moreover, YTHDF1 contributes to cancer proliferation and sensitization following exposure to fluorouracil and oxaliplatin.50 Depletion of YTHDF1 facilitates the resistance of cancerous cells to cisplatin treatment of NSCLC, and the Keap1-Nrf2-AKR1C1 axis is regarded as the downstream mediator of YTHDF1.40 The small nuclear ribonucleic protein PRPF6 is positively related to YTHDF1 in lung adenocarcinoma (LUAD) tissues, and knockdown of YTHDF1 can partially inhibit both basal and PRPF6 expression induced by ammonium tetrathiomolybdate (ATTM), which may theoretically be extended to LUAD as a strong copper chelator. Consequently, H2S blocks ATTM-induced anticancer function through YTHDF1-dependent PRPF6 m6A methylation in LUAD cells.51 Temozolomide (TMZ) is the major drug for glioblastoma chemotherapy, and knockdown of YTHDF1 significantly augmented the cytotoxic effect of TMZ on glioblastoma cells.45 METTL3 promotes YAP mRNA translation, which significantly induces drug resistance and metastasis of NSCLC by recruiting YTHDF1/3 and eIF3b to the translation initiation complex.21
YTHDF1 affects tumor immunity
The level of immune cell infiltration and the expression of various immune gene markers in ovarian cancer are closely associated with the expression of YTHDF1, indicating that YTHDF1 is correlated with tumor immune cell infiltration.52 YTHDF1 promotes antigen degradation in the phagosome and restrains cross-presentation of neoantigens in dendritic cells (DCs) by increasing translation of m6A-modified cathepsin mRNA.53 Loss of YTHDF1 in classical DCs increases the cross-presentation of tumor antigen and the cross-priming of CD8+ T cells in vivo. In terms of mechanism, YTHDF1 reads the m6A marked messages of transcripts encoding lysosomal proteases. After binding to these transcripts, YTHDF1 promotes translation of lysosomal cathepsins in DCs, with the inhibition of cathepsins significantly elevating cross-presentation of wild-type DCs.54
YTHDF1 is a promising cancer biomarker for detection, progression, and prognosis
Colorectal cancer patients with high YTHDF1 expression had significantly poorer overall survival, which indicated that YTHDF1 expression is an independent prognostic factor of patients with colorectal cancer.50,55 The expression of YTHDF1 is affected by the T, N, and M (tumor, nodes, and metastasis) of colon adenocarcinoma.55 Additionally, the expression of YTHDF1 is associated with various malignant tumor behaviors, including depth, lymph node metastasis, and poorer colorectal cancer stages.50 Many studies have shown that YTHDF1 is also upregulated in hepatocellular carcinoma and is independent of poor prognosis.36,56,57 The YTHDF1 gene rs6090311 A>G polymorphism is associated with a decreased risk of hepatoblastoma; however, the rs6011668 C>T polymorphism has no similar correlation. Further expression quantitative trait loci evidence suggests that the rs6090311 G allele is significantly associated with decreased BIRC7, RP5-963E22.4, and NKAIN4 levels, which is why the YTHDF1 gene rs6090311 A>G mediates hepatoblastoma risk.58 The expression profile variation of YTHDF1 is significantly associated with the high-risk subtype of GC patients, revealing the potential oncogene roles of YTHDF1 in GC tumors.59 There is an approximately 7% mutation rate in YTHDF1 in GC patients, and high expression of YTHDF1 is correlated with more aggressive tumor progression and poor overall survival.38
YTHDF1 is upregulated in LUAD.60,61 YTHDF1, as one of six genes, together with HNRNPC, METTL3, YTHDC2, KIAA1429, and ALKBH5, was screened to build a risk-scoring signature, which is strongly related to the pathological stages based on clinical features.61 Furthermore, YTHDF1 is relevant to better overall survival and recurrence-free survival and can serve as a biomarker for the prognosis of LUAD61 as well as being a good diagnostic biomarker.62 YTHDF1 was proved to be significantly upregulated in esophageal cancer tissues63 and head and neck squamous cell carcinoma (HNSCC),64 while YTHDF1 was significantly downregulated in thyroid cancer specimens.65 Moreover, YTHDF1 is correlated with the expression of YTHDF1, and patients with HNSCC with high YTHDF1 expression have poor overall survival.66
In ovarian cancer, YTHDF1 is overexpressed in cancer tissues and is correlated with poor prognosis.22 Similarly, YTHDF1 was identified as an important index of the six-m6A regulator signature prognostic model (KIAA1429, HNRNPC, METTL3, YTHDF1, IGF2BP2, and IGF2BP3) in pancreatic cancer by lasso regression.67 YTHDF1 can act as an independent prognostic indicator in cervical squamous cell carcinoma,68 and YTHDF1 and other m6A RNA methylation factors (YTHDC2, YTHDC1, ALKBH5, ZC3H13, and RBMX) were chosen to construct a risk signature of cervical squamous cell carcinoma.69 YTHDF1 is dramatically overexpressed in prostate cancer. Interestingly, YTHDF1 is remarkably higher in lymph node metastasis than in castration-resistant prostate cancer with bone metastasis.70
YTHDF1 is upregulated in breast cancer tissues, and the expression level is significantly correlated with intrinsic subclasses, nodal metastasis, and prognosis.71 Overexpression of YTHDF1 can predict poor prognosis in light of the overall survival of breast cancer patients.72 Similarly, overexpression of YTHDF1 is positively correlated with advanced stages of glioma.44 MSI1 and YTHDF1 were positively related to each other in clinical glioma samples, and their concomitant upregulation was correlated with decreased survival of glioma patients.45
The roles of YTHDF1 in other nontumor diseases
YTHDF1 in nervous system diseases
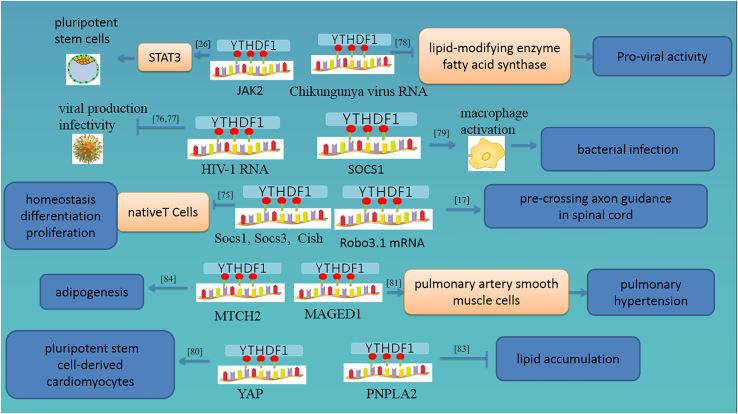
YTHDF1 positively regulates translation of m6A-modified axon guidance receptor Robo3.1 mRNA, which controls precrossing axon guidance in the spinal cord.17 Another study showed that YTHDF1 plays an important role in sciatic nerve lesion-induced global protein synthesis and robust axon regeneration of dorsal root ganglion neurons. Consequently, the extension of regenerating SCG10+ axons was substantially decreased in YTHDF1 knockout mice.73 Genetic deletion of YTHDF1 triggers learning and memory defects, and impairs hippocampal synaptic transmission and long-term potentiation. YTHDF1 re-expression in the hippocampus of adult YTHDF1-KO mice rescues behavioral and synaptic defects, while acute YTHDF1 knockdown in the hippocampus recapitulates hippocampal deficiency.74 These studies suggest that YTHDF1 may play an important role in nervous system diseases, especially in axonal regulation and learning and memory.
YTHDF1 in infectious diseases
Socs1, Socs3, and Cish m6A mRNAs are recognized by YTHDF1–3 and degraded rapidly in wild-type native T cells, which regulate the homeostasis, differentiation, and proliferation of native T cells.75 AIDS is one of the most common infectious diseases and is induced by HIV. A report showed that m6A-modified HIV-1 RNA is recognized by YTHDF1, which inhibits HIV-1 infection in cell lines and primary CD4+ T cells. In addition, the overexpression of YTHDF1 protein in cells inhibits HIV-1 infection mainly by decreasing HIV-1 reverse transcription, while knockdown of YTHDF1 produces the opposite effects.76 Another study showed that YTHDF1 has a substantially higher affinity for m6A-modified HIV-1 RNA in vitro. Moreover, compared with YTHDF2 and YTHDF3, the levels of incoming HIV-1 genomic RNA (gRNA) bound to YTHDF1 appeared higher. Overexpression of YTHDF1 in HIV-1 target cells decreased viral gRNA levels and repressed both early and late reverse transcription. YTHDF1 promoted HIV-1 Gag expression and altered viral production and infectivity in virus-producing cells. In addition, YTHDF1 together with YTHDF2 and HIV-1 Gag form a complex with RNAs in HIV-1-producing cells.77 Chikungunya virus (CHIKV) genomes are also modified by N-methyladenosine, and YTHDF1 binds and suppresses CHIKV replication.78 Research shows that knockout of METTL14 depletion blunts Socs1 m6A methylation and reduces YTHDF1 binding to m6A sites, and m6A-mediated SOCS1 induction plays a critical role in maintaining the negative feedback control of macrophage activation in response to bacterial infection.79 All studies indicated that YTHDF1 could recognize m6A-modified viral RNA and mediate its expression.
YTHDF1 in cardiovascular diseases
In heart regeneration during postnatal and adult injury, YTHDF1 promotes the translation of YAP, further enhancing the proliferative capacity of cardiomyocytes in both humans and mice.80 In human and rodent pulmonary hypertension samples, similar to hypoxic pulmonary artery smooth muscle cells (PASMCs), the expression of YTHDF1 was increased and the levels of m6A were elevated, while deletion of YTHDF1 ameliorated PASMC proliferation, phenotypic switching, and pulmonary hypertension development through promoting the translation of MAGED1 both in vivo and in vitro.81 A study revealed that loss of YTHDF1 triggered significant impairment of cardiomyocyte differentiation.82
The roles of YTHDF1 in pathophysiological processes related to other human diseases
YTHDF1 could affect the translation of m6A-modified PNPLA2, which plays a key role in lipid accumulation.83 Another study found that YTHDF1 plays an important role in regulating adipogenesis through an MTCH2-dependent m6A mechanism.84 In this regard, one study showed that m6A-modified mRNAs were enriched in stress granules (SGs) and that YTHDF1 also played an important role in SG formation and recruitment of mRNAs to SGs. Both the C-terminal m6A-binding YTH domain and N-terminal intrinsically disordered region are critical for SG formation. Super-resolution imaging further demonstrated that YTHDF proteins appear to be in a supersaturated state, forming clusters that often reside in the periphery of or at the junctions between SG core clusters, potentially accelerating SG formation by decreasing the activation energy barrier and critical size for SG condensate formation.85 A further study showed that YTHDF1-mediated JAK2 translation could inhibit the STAT3 pathway and thus the pluripotency of porcine induced pluripotent stem cells.26 Studies have confirmed that the expression of YTHDF1 is significantly higher in female germline stem cells, and upregulated YTHDF1 can regulate female germline stem cell self-renewal, possibly through an m6A-dependent mechanism.86
Conclusion
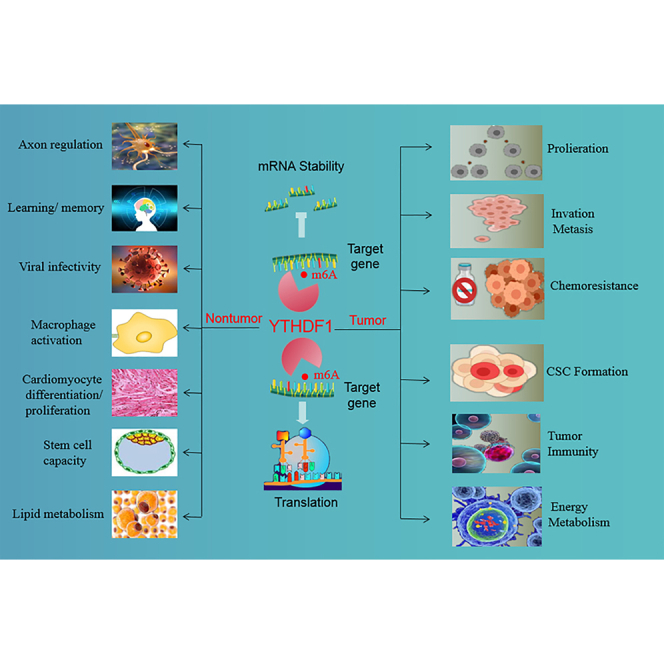
As an important m6A reader, YTHDF1 recognizes m6A-modified target genes and mediates their expression by promoting translation or enhancing the stability of RNA. Alterations in target genes are involved in the progression of many diseases, especially tumors. Therefore, in the present review, we investigated the mechanisms and roles of YTHDF1 in human diseases.
Based on the current research, YTHDF1 promotes translation mainly by triggering translational initiation and elongation, in a cap-dependent or cap-independent manner, by promoting ribosome occupancy, and by cooperating with YTHDF3. In addition, YTHDF1 can regulate the stability of mRNAs to affect the expression of target genes. Furthermore, YTHDF1 participates in the progression of many diseases by mediating target gene expression. In tumors, YTHDF1 affects tumorigenicity, proliferation, invasion and migration, the formation of tumor stem cells, metabolism, and immunity, and inhibits sensitivity to chemotherapy by affecting target gene expression (Table 1). In addition, YTHDF1 participates in the regulation of nontumor lesions such as axonal regulation, learning and memory, cardiovascular diseases, and virus infection through recognizing its target genes (Figure 2). Thus, YTHDF1 may be a promising cancer biomarker for detection, progression, and prognosis and may also become a therapeutic target for human diseases.
Table 1.
Target genes of YTHDF1 and their roles in tumors
| Target RNAs | cis-Elements on RNA | Cancers | Roles of target RNAs | References |
|---|---|---|---|---|
| ANKLE1 | – | colorectal cancer | inhibits cell proliferation and facilitates genomic stability | Tian et al.35 |
| Snail | CDS | liver cancer | promotes epithelial-mesenchymal transition | Orouji et al.23 |
| FZD5 | CDS | gastric carcinoma | promotes hepatocellular carcinoma cells proliferation and metastasis | Liu et al.37 |
| FZD7 | 3′ UTR | gastric carcinoma | regulates Wnt/β-catenin signaling and promotes cell proliferation and tumorigenesis | Pi et al.38 |
| USP14 | 3′ UTR | gastric carcinoma | facilitates tumorigenesis and metastasis | Chen et al.39 |
| YAP | – | non-small cell lung cancer, osteosarcoma | enhances cellular viability, growth, and invasion | Jin et al.,22 Yuan et al.46 |
| CyclinD1, CDK2, CDK4 | – | non-small cell lung cancer | promotes cell proliferation and xenograft tumor formation | Zhou et al.40 |
| lncRNA THOR | CDS | lung cancer | regulates proliferation | Liu et al.31 |
| ITGA6 | 3′ UTR | bladder cancer | promotes cell growth, adhesion, and prognosis | Zhao et al.28 |
| CDCP1 | 3′ UTR | bladder cancer | promotes proliferation, migration, and invasion | Yang et al.42 |
| EIF3C | – | ovarian cancer | facilitates tumorigenesis and metastasis | Liu et al.24 |
| c-Myc | 3′ UTR | oral squamous cell carcinoma | accelerates proliferation, invasion, and migration | Zhao et al.29 |
| YY1, MDM2 | – | arsenic carcinogenesis | mediates arsenite-induced human keratinocyte transformation by suppressing p53 activation | Zhao et al.32 |
| eIF3 | – | Merkel cell carcinoma | promotes proliferation and clonogenic capacity | Orouji et al.23 |
| HINT2 | 3′ UTR | ocular melanoma | inhibits progression of uveal melanoma and conjunctival melanoma | Jia et al.47 |
| TCF7L2/TCF4, | – | colorectal cancer | maintains intestinal stem cells during tumorigenesis and regeneration | Han et al.48 |
| PDK4 | 5′ UTR | cervical cancer, liver cancer | promotes glycolysis and ATP generation | Li et al.33 |
| TRFC | 3′ UTR, 5′ UTR | hypopharyngeal squamous cell carcinoma | increases iron metabolism | Ye et al.49 |
| HK2 | 3′ UTR | cervical cancer | enhances the Warburg effect | Wang et al.30 |
| PRPF6 | – | lung adenocarcinoma | mediates ATTM-induced anticancer effects impeded by H2S | Li et al.51 |
Figure 2.
Target genes of YTHDF1 and their roles in nontumor diseases
Acknowledgments
This review is supported by research grants from the National Natural Science Foundation of China (81773294, 81901147), the Natural Science Foundation of Hunan Province (2019JJ20014, 2019JJ50541), and the Health and Family Planning Commission of Hunan Province (20201929).
Author contributions
All authors contributed to the conceptualization, literature analysis, manuscript writing, editing, validation, and final revision of this review article.
Declaration of interests
The authors declare no competing interests.
References
- 1.Wang J., Wang K., Liu W., Cai Y., Jin H. m6A mRNA methylation regulates the development of gestational diabetes mellitus in Han Chinese women. Genomics. 2021;113:1048–1056. doi: 10.1016/j.ygeno.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Wu F., Cheng W., Zhao F., Tang M., Diao Y., Xu R. Association of N6-methyladenosine with viruses and related diseases. Virol. J. 2019;16:133. doi: 10.1186/s12985-019-1236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang L., Wei X., Li T., Chen Y., Dai Z., Lu C., Zheng G. Emerging perspectives of RNA-methyladenosine (mA) modification on immunity and autoimmune diseases. Front. Immunol. 2021;12:630358. doi: 10.3389/fimmu.2021.630358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin Y., Qiao Y., Li L., Luo E., Wang D., Yao Y., Tang C., Yan G. The mA methyltransferase METTL3 promotes hypoxic pulmonary arterial hypertension. Life Sci. 2021;274:119366. doi: 10.1016/j.lfs.2021.119366. [DOI] [PubMed] [Google Scholar]
- 5.Chen C., Yuan W., Zhou Q., Shao B., Guo Y., Wang W., Yang S., Guo Y., Zhao L., Dang Q., et al. N6-methyladenosine-induced circ1662 promotes metastasis of colorectal cancer by accelerating YAP1 nuclear localization. Theranostics. 2021;11:4298–4315. doi: 10.7150/thno.51342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roignant J., Soller M. mA in mRNA: an ancient mechanism for fine-tuning gene expression. Trends Genetics. 2017;33:380–390. doi: 10.1016/j.tig.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Wang X., Feng J., Xue Y., Guan Z., Zhang D., Liu Z., Gong Z., Wang Q., Huang J., Tang C., et al. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016;534:575–578. doi: 10.1038/nature18298. [DOI] [PubMed] [Google Scholar]
- 8.Knuckles P., Lence T., Haussmann I.U., Jacob D., Kreim N., Carl S.H., Masiello I., Hares T., Villaseñor R., Hess D., et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m(6)A machinery component Wtap/Fl(2)d. Genes Dev. 2018;32:415–429. doi: 10.1101/gad.309146.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer K.D., Jaffrey S.R. Rethinking m6A readers, writers, and erasers. Annu. Rev. Cell Dev. Biol. 2017;33:319–342. doi: 10.1146/annurev-cellbio-100616-060758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu C., Wang X., Liu K., Roundtree I., Tempel W., Li Y., Lu Z., He C., Min J. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat. Chem. Biol. 2014;10:927–929. doi: 10.1038/nchembio.1654. [DOI] [PubMed] [Google Scholar]
- 11.Shi H., Wang X., Lu Z., Zhao B., Ma H., Hsu P., Liu C., He C. YTHDF3 facilitates translation and decay of N-methyladenosine-modified RNA. Cell Res. 2017;27:315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roundtree I., He C. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Trends Genetics. 2016;32:320–321. doi: 10.1016/j.tig.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Xu C., Liu K., Ahmed H., Loppnau P., Schapira M., Min J. Structural basis for the discriminative recognition of N6-methyladenosine RNA by the human YT521-B homology domain family of proteins. J. Biol. Chem. 2015;290:24902–24913. doi: 10.1074/jbc.M115.680389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao S., Sun H., Xu C. YTH domain: a family of N-methyladenosine (mA) readers. Genom. Proteom. Bioinform. 2018;16:99–107. doi: 10.1016/j.gpb.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rauch S., He C., Dickinson B.C. Targeted m6A reader proteins to study epitranscriptomic regulation of single RNAs. J. Am. Chem. Soc. 2018;140:11974–11981. doi: 10.1021/jacs.8b05012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozkurede U., Kala R., Johnson C., Shen Z., Miller R.A., Garcia G.G. Cap-independent mRNA translation is upregulated in long-lived endocrine mutant mice. J. Mol. Endocrinol. 2019;63:123–138. doi: 10.1530/JME-19-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhuang M., Li X., Zhu J., Zhang J., Niu F., Liang F., Chen M., Li D., Han P., Ji S.J. The m6A reader YTHDF1 regulates axon guidance through translational control of Robo3.1 expression. Nucleic Acids Res. 2019;47:4765–4777. doi: 10.1093/nar/gkz157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X., Zhao B.S., Roundtree I.A., Lu Z., Han D., Ma H., Weng X., Chen K., Shi H., He C. N6-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ries R., Zaccara S., Klein P., Olarerin-George A., Namkoong S., Pickering B., Patil D., Kwak H., Lee J., Jaffrey S. mA enhances the phase separation potential of mRNA. Nature. 2019;571:424–428. doi: 10.1038/s41586-019-1374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nettersheim D., Berger D., Jostes S., Kristiansen G., Lochnit G., Schorle H. N6-Methyladenosine detected in RNA of testicular germ cell tumors is controlled by METTL3, ALKBH5, YTHDC1/F1/F2, and HNRNPC as writers, erasers, and readers. Andrology. 2019;7:498–506. doi: 10.1111/andr.12612. [DOI] [PubMed] [Google Scholar]
- 21.Jin D., Guo J., Wu Y., Du J., Yang L., Wang X., Di W., Hu B., An J., Kong L., et al. m6A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1-miR-1914-3p-YAP axis to induce NSCLC drug resistance and metastasis. J. Hematol. Oncol. 2019;12:135. doi: 10.1186/s13045-019-0830-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Jin D., Guo J., Wu Y., Yang L., Wang X., Du J., Dai J., Chen W., Gong K., Miao S., et al. m6A demethylase ALKBH5 inhibits tumor growth and metastasis by reducing YTHDFs-mediated YAP expression and inhibiting miR-107/LATS2–mediated YAP activity in NSCLC. Mol. Cancer. 2020;19:40. doi: 10.1186/s12943-020-01161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orouji P., Orouji H., Utika J. Oncogenic role of an epigenetic reader of m6A RNA modification: YTHDF1 in Merkel cell carcinoma. Cancers. 2020;12:202. doi: 10.3390/cancers12010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu T., Wei Q., Jin J., Luo Q., Liu Y., Yang Y., Cheng C., Li L., Pi J., Si Y., et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 2020;48:3816–3831. doi: 10.1093/nar/gkaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin X., Chai G., Wu Y., Li J., Chen F., Liu J., Luo G., Tauler J., Du J., Lin S., et al. RNA m6A methylation regulates the epithelial mesenchymal transition of cancer cells and translation of Snail. Nat. Commun. 2019;10:2065. doi: 10.1038/s41467-019-09865-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Wu R., Liu Y., Zhao Y., Bi Z., Yao Y., Liu Q., Wang F., Wang Y., Wang X. m6A methylation controls pluripotency of porcine induced pluripotent stem cells by targeting SOCS3/JAK2/STAT3 pathway in a YTHDF1/YTHDF2-orchestrated manner. Cell Death Dis. 2019;10:171. doi: 10.1038/s41419-019-1417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Y., Pei G., Li D., Li R., Shao Y., Zhang Q., Li P. Multivalent mA motifs promote phase separation of YTHDF proteins. Cell Res. 2019;29:767–769. doi: 10.1038/s41422-019-0210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin H., Ying X., Que B., Wang X., Chao Y., Zhang H., Yuan Z., Qi D., Lin S., Min W., et al. N6-methyladenosine modification of ITGA6 mRNA promotes the development and progression of bladder cancer. EBioMedicine. 2019;47:195–207. doi: 10.1016/j.ebiom.2019.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao W., Cui Y., Liu L., Ma X., Qi X., Wang Y., Liu Z., Ma S., Liu J., Wu J. METTL3 facilitates oral squamous cell carcinoma tumorigenesis by enhancing c-myc stability via YTHDF1-mediated m6A modification. Mol. Ther. Nucleic Acids. 2020;20:1–12. doi: 10.1016/j.omtn.2020.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q., Guo X., Li L., Gao Z., Su X., Ji M., Liu J. N-methyladenosine METTL3 promotes cervical cancer tumorigenesis and Warburg effect through YTHDF1/HK2 modification. Cell Death Dis. 2020;11:911. doi: 10.1038/s41419-020-03071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H., Xu Y., Yao B., Sui T., Lai L., Li Z. A novel N6-methyladenosine (m6A)-dependent fate decision for the lncRNA THOR. Cell Death Dis. 2020;11:613. doi: 10.1038/s41419-020-02833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao T., Sun D., Zhao M., Lai Y., Liu Y., Zhang Z. N6-methyladenosine mediates arsenite-induced human keratinocyte transformation by suppressing p53 activation. Environ. Pollut. 2020;259:113908. doi: 10.1016/j.envpol.2019.113908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z., Peng Y., Li J., Chen Z., Chen F., Tu J., Lin S., Wang H. N6-methyladenosine regulates glycolysis of cancer cells through PDK4. Nat. Commun. 2020;11:2578. doi: 10.1038/s41467-020-16306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai Y., Yang C., Wu R., Huang L., Song S., Li W., Yan P., Lin C., Li D., Zhang Y. YTHDF1 regulates tumorigenicity and cancer stem cell-like activity in human colorectal carcinoma. Front. Oncol. 2019;9:332. doi: 10.3389/fonc.2019.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian J., Ying P., Ke J., Zhu Y., Yang Y., Gong Y., Zou D., Peng X., Yang N., Wang X., et al. ANKLE1 N-methyladenosine-related variant is associated with colorectal cancer risk by maintaining the genomic stability. Int. J. Cancer. 2020;146:3281–3293. doi: 10.1002/ijc.32677. [DOI] [PubMed] [Google Scholar]
- 36.Bian S., Ni W., Zhu M., Song Q., Zhang J., Ni R., Zheng W. Identification and validation of the N6-methyladenosine RNA methylation regulator YTHDF1 as a novel prognostic marker and potential target for hepatocellular carcinoma. Front. Mol. Biosci. 2020;7:604766. doi: 10.3389/fmolb.2020.604766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X., Qin J., Gao T., Li C., He B., Pan B., Xu X., Chen X., Zeng K., Xu M., et al. YTHDF1 facilitates the progression of hepatocellular carcinoma by promoting FZD5 mRNA translation in an m6A-dependent manner. Mol. Ther. Nucleic Acids. 2020;22:750–765. doi: 10.1016/j.omtn.2020.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Pi J., Wang W., Ji M., Wang X., Wei X., Jin J., Liu T., Qiang J., Qi Z., Li F., et al. YTHDF1 promotes gastric carcinogenesis by controlling translation of FZD7. Cancer Res. 2020;81:2651–2665. doi: 10.1158/0008-5472.CAN-20-0066. [DOI] [PubMed] [Google Scholar]
- 39.Chen X., Liang R., Yi Y., Fan H., Chen M., Zhang J., Zhu J. The mA reader YTHDF1 facilitates the tumorigenesis and metastasis of gastric cancer via USP14 translation in an mA-dependent manner. Front. Cell Dev. Biol. 2021;9:647702. doi: 10.3389/fcell.2021.647702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi Y., Fan S., Wu M., Zuo Z., Li X., Jiang L., Shen Q., Xu P., Zeng L., Zhou Y., et al. YTHDF1 links hypoxia adaptation and non-small cell lung cancer progression. Nat. Commun. 2019;10:4892. doi: 10.1038/s41467-019-12801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou J., Xiao D., Qiu T., Li J., Liu Z. Loading MicroRNA-376c in extracellular vesicles inhibits properties of non-small cell lung cancer cells by targeting YTHDF1. Technol. Cancer Res. Treat. 2020;19 doi: 10.1177/1533033820977525. 1533033820977525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang F., Jin H., Que B., Chao Y., Zhang H., Ying X., Zhou Z., Yuan Z., Su J., Wu B., et al. Dynamic m6A mRNA methylation reveals the role of METTL3-m6A-CDCP1 signaling axis in chemical carcinogenesis. Oncogene. 2019;38:4755–4772. doi: 10.1038/s41388-019-0755-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li T., Gu M., Deng A., Qian C. Increased expression of YTHDF1 and HNRNPA2B1 as potent biomarkers for melanoma: a systematic analysis. Cancer Cell Int. 2020;20:239. doi: 10.1186/s12935-020-01309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu C., Yuan B., He T., Ding B., Li S. Prognostic values of YTHDF1 regulated negatively by mir-3436 in glioma. J. Cell. Mol. Med. 2020;24:7538–7549. doi: 10.1111/jcmm.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yarmishyn A.A., Yang Y.P., Lu K.H., Chen Y.C., Chien Y., Chou S.J., Tsai P.H., Ma H.I., Chien C.S., Chen M.T., et al. Musashi-1 promotes cancer stem cell properties of glioblastoma cells via upregulation of YTHDF1. Cancer Cell Int. 2020;20:597. doi: 10.1186/s12935-020-01696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan Y., Yan G., He M., Lei H., Li L., Wang Y., He X., Li G., Wang Q., Gao Y., et al. ALKBH5 suppresses tumor progression via an m6A-dependent epigenetic silencing of pre-miR-181b-1/YAP signaling axis in osteosarcoma. Cell Death Dis. 2021;12:60. doi: 10.1038/s41419-020-03315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jia R., Chai P., Wang S., Sun B., Xu Y., Yang Y., Ge S., Jia R., Yang Y.-G., Fan X. m6A modification suppresses ocular melanoma through modulating HINT2 mRNA translation. Mol. Cancer. 2019;18:161. doi: 10.1186/s12943-019-1088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han B., Yan S., Wei S., Xiang J., Liu K., Chen Z., Bai R., Sheng J., Xu Z., Gao X. YTHDF1-mediated translation amplifies Wnt-driven intestinal stemness. EMBO Rep. 2020;21:e49229. doi: 10.15252/embr.201949229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye J., Wang Z., Chen X., Jiang X., Dong Z., Hu S., Li W., Liu Y., Liao B., Han W., et al. YTHDF1-enhanced iron metabolism depends on TFRC m6A methylation. Theranostics. 2020;10:12072–12089. doi: 10.7150/thno.51231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishizawa Y., Konno M., Asai A., Koseki J., Kawamoto K., Miyoshi N., Takahashi H., Nishida N., Haraguchi N., Sakai D., et al. Oncogene c-Myc promotes epitranscriptome mA reader YTHDF1 expression in colorectal cancer. Oncotarget. 2018;9:7476–7486. doi: 10.18632/oncotarget.23554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X., Li N., Huang L., Xu S., Zheng X., Hamsath A., Zhang M., Dai L., Zhang H., Wong J.J.L., et al. Is hydrogen sulfide a concern during treatment of lung adenocarcinoma with ammonium tetrathiomolybdate? Front. Oncol. 2020;10:234. doi: 10.3389/fonc.2020.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Q., Zhang Q., Li Q., Zhang J., Zhang J. Clinicopathological and immunological characterization of RNA m6A methylation regulators in ovarian cancer. Mol. Genet. Genomic Med. 2020;9:e1547. doi: 10.1002/mgg3.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim D.J., Iwasaki A. YTHDF1 control of dendritic cell cross-priming as a possible target of cancer immunotherapy. Biochemistry. 2019;58:1945–1946. doi: 10.1021/acs.biochem.9b00200. [DOI] [PubMed] [Google Scholar]
- 54.Han D., Liu J., Chen C., Dong L., Liu Y., Chang R., Huang X., Liu Y., Wang J., Dougherty U., et al. Anti-tumour immunity controlled through mRNA m6A methylation and YTHDF1 in dendritic cells. Nature. 2019;566:270–274. doi: 10.1038/s41586-019-0916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu T., Li C., Jin L., Li C., Wang L. The prognostic value of m6A RNA methylation regulators in colon adenocarcinoma. Med. Sci. Monitor. 2019;25:9435–9445. doi: 10.12659/MSM.920381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao X., Chen Y., Mao Q., Jiang X., Jiang W., Chen J., Xu W., Zhong L., Sun X. Overexpression of YTHDF1 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Biomark. 2018;21:859–868. doi: 10.3233/CBM-170791. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Z., Yang L., Fang S., Zheng L., Wu F., Chen W., Song J., Chen M., Ji J. The effect of m6A methylation regulatory factors on the malignant progression and clinical prognosis of hepatocellular carcinoma. Front. Oncol. 2020;10:1435. doi: 10.3389/fonc.2020.01435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo Z., Li G., Wang M., Zhu J., Yang Z., Li Y., Zhang J., Xin Y., Li S., Li L., et al. YTHDF1 rs6090311 A>G polymorphism reduces hepatoblastoma risk: evidence from a seven-center case-control study. J. Cancer. 2020;11:5129–5134. doi: 10.7150/jca.46120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu T., Yang S., Cheng Y.P., Kong X.l., Du D.D., Wang X., Bai Y.F., Yin L.H., Pu Y.P., Liang G.Y. The N6-methyladenosine (m6A) methylation gene YTHDF1 reveals a potential diagnostic role for gastric cancer. Cancer Manag. Res. 2020;12:11953–11964. doi: 10.2147/CMAR.S279370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li F., Wang H., Huang H., Zhang L., Wang D., Wan Y. m6A RNA methylation regulators participate in the malignant progression and have clinical prognostic value in lung adenocarcinoma. Front. Genet. 2020;11:994. doi: 10.3389/fgene.2020.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu J., Wang M., Hu D. Deciphering N6-methyladenosine-related genes signature to predict survival in lung adenocarcinoma. Biomed. Res. Int. 2020;2020:2514230. doi: 10.1155/2020/2514230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhuang Z., Chen L., Mao Y., Zheng Q., Li H., Huang Y., Hu Z., Jin Y. Diagnostic, progressive and prognostic performance of m6A methylation RNA regulators in lung adenocarcinoma. Int. J. Biol. Sci. 2020;16:1785–1797. doi: 10.7150/ijbs.39046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu L.C., Pan J.X., Pan H.D. Construction and validation of an m6A RNA methylation regulators-based prognostic signature for esophageal cancer. Cancer Manag. Res. 2020;12:5385–5394. doi: 10.2147/CMAR.S254870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao X., Cui L. Development and validation of a mA RNA methylation regulators-based signature for predicting the prognosis of head and neck squamous cell carcinoma. Am. J. Cancer Res. 2019;9:2156–2169. [PMC free article] [PubMed] [Google Scholar]
- 65.Hou J., Shan H., Zhang Y., Fan Y., Wu B. m6A RNA methylation regulators have prognostic value in papillary thyroid carcinoma. Am. J. Otolaryngol. 2020;41:102547. doi: 10.1016/j.amjoto.2020.102547. [DOI] [PubMed] [Google Scholar]
- 66.Zhou X., Han J., Zhen X., Liu Y., Cui Z., Yue Z., Ding L., Xu S. Analysis of genetic alteration signatures and prognostic values of m6A regulatory genes in head and neck squamous cell carcinoma. Front. Oncol. 2020;10:718. doi: 10.3389/fonc.2020.00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hou J., Wang Z., Li H., Zhang H., Luo L. Gene signature and identification of clinical trait-related m A regulators in pancreatic cancer. Front. Genet. 2020;11:522. doi: 10.3389/fgene.2020.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pan J., Xu L., Pan H. Development and validation of an m6A RNA methylation regulator-based signature for prognostic prediction in cervical squamous cell carcinoma. Front. Oncol. 2020;10:1444. doi: 10.3389/fonc.2020.01444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu H., Dong H., Fu Y., Tang Y., Dai M., Chen Y., Wang G., Wu Y. Expressions of m6A RNA methylation regulators and their clinical predictive value in cervical squamous cell carcinoma and endometrial adenocarcinoma. Clin. Exp. Pharmacol. Physiol. 2021;48:270–278. doi: 10.1111/1440-1681.13412. [DOI] [PubMed] [Google Scholar]
- 70.Wu Q., Xie X., Huang Y., Meng S., Li Y., Wang H., Hu Y. N6-methyladenosine RNA methylation regulators contribute to the progression of prostate cancer. J. Cancer. 2021;12:682–692. doi: 10.7150/jca.46379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anita R., Paramasivam A., Priyadharsini J., Chitra S. YTHDF1The m6A readers and aberrations associated with metastasis and predict poor prognosis in breast cancer patients. Am. J. Cancer Res. 2020;10:2546–2554. [PMC free article] [PubMed] [Google Scholar]
- 72.Liu L., Liu X., Dong Z., Li J., Yu Y., Chen X., Ren F., Cui G., Sun R. N6-methyladenosine-related genomic targets are altered in breast cancer tissue and associated with poor survival. J. Cancer. 2019;10:5447–5459. doi: 10.7150/jca.35053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weng Y., Wang X., An R., Cassin J., Vissers C., Liu Y., Liu Y., Xu T., Wang X., Wong S., et al. Epitranscriptomic mA regulation of axon regeneration in the adult mammalian nervous system. Neuron. 2018;97:313–325.e6. doi: 10.1016/j.neuron.2017.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi H., Zhang X., Weng Y.-L., Lu Z., Liu Y., Lu Z., Li J., Hao P., Zhang Y., Zhang F., et al. m6A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature. 2018;563:249–253. doi: 10.1038/s41586-018-0666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang J., Wang H., Zhang W. Regulation of virus replication and T cell homeostasis by N6-methyladenosine. Virol. Sin. 2019;34:22–29. doi: 10.1007/s12250-018-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tirumuru N., Zhao B., Lu W., Lu Z., He C., Wu L. N(6)-methyladenosine of HIV-1 RNA regulates viral infection and HIV-1 Gag protein expression. eLife. 2016;5:e15528. doi: 10.7554/eLife.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu W., Tirumuru N., St. Gelais C., Koneru P.C., Liu C., Kvaratskhelia M., He C., Wu L. N6-Methyladenosine-binding proteins suppress HIV-1 infectivity and viral production. J. Biol. Chem. 2018;293:12992–13005. doi: 10.1074/jbc.RA118.004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim B., Arcos S., Rothamel K., Jian J., Rose K., McDonald W., Bian Y., Reasoner S., Barrows N., Bradrick S., et al. Discovery of widespread host protein interactions with the pre-replicated genome of CHIKV using VIR-CLASP. Mol. Cell. 2020;78:624–640.e7. doi: 10.1016/j.molcel.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Du J., Liao W., Liu W., Deb D., He L., Hsu P., Nguyen T., Zhang L., Bissonnette M., He C., et al. N-adenosine methylation of Socs1 mRNA is required to sustain the negative feedback control of macrophage activation. Dev. Cell. 2020;55:737–753.e7. doi: 10.1016/j.devcel.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.ZHan Z., Wang X., Xu Z., Cao Y., Gong R., Yu Y., Yu Y., Guo X., Liu S., Yu M., et al. ALKBH5 regulates cardiomyocyte proliferation and heart regeneration by demethylating the mRNA of YTHDF1. Theranostics. 2021;11:3000–3016. doi: 10.7150/thno.47354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu L., Wang J., Huang H., Yu Y., Ding J., Yu Y., Li K., Wei D., Ye Q., Wang F., et al. YTHDF1 regulates pulmonary hypertension through translational control of MAGED1. Am. J. Respir. Crit. Care Med. 2021;203:1158–1172. doi: 10.1164/rccm.202009-3419OC. [DOI] [PubMed] [Google Scholar]
- 82.Wang S., Zhang J., Wu X., Lin X., Liu X., Zhou J. Differential roles of YTHDF1 and YTHDF3 in embryonic stem cell-derived cardiomyocyte differentiation. RNA Biol. 2020;18:1354–1363. doi: 10.1080/15476286.2020.1850628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X., Sun B., Jiang Q., Wu R., Cai M., Yao Y., Liu Q., Shi H., Feng J., Wang Y. mRNA mA plays opposite role in regulating UCP2 and PNPLA2 protein expression in adipocytes. Int. J. Obes. 2018;42:1912–1924. doi: 10.1038/s41366-018-0027-z. [DOI] [PubMed] [Google Scholar]
- 84.Jiang Q., Sun B., Liu Q., Cai M., Wu R., Wang F., Yao Y., Wang Y., Wang X. MTCH2 promotes adipogenesis in intramuscular preadipocytes via an mA-YTHDF1-dependent mechanism. FASEB J. 2019;33:2971–2981. doi: 10.1096/fj.201801393RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fu Y., Zhuang X. mA-binding YTHDF proteins promote stress granule formation. Nat. Chem. Biol. 2020;16:955–963. doi: 10.1038/s41589-020-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao X., Tian G.G., Fang Q., Pei X., Wang Z., Wu J. Comparison of RNA m6A and DNA methylation profiles between mouse female germline stem cells and STO cells. Mol. Ther. Nucleic Acids. 2021;23:431–439. doi: 10.1016/j.omtn.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]