Abstract
Introduction
Fibromyalgia is a complex pain condition that affects mostly women. Given the disease's lack of understanding, patients report poor adherence to medication and mistrust of medical services. This study aims to describe the recruitment characteristics and non-adherence associated factors of fibromyalgia patients to an RCT.
Methods
We performed a retrospective longitudinal analysis with data from our ongoing RCT. We investigated characteristics of subjects recruited, consented, and randomized. Adherence was studied using survival analysis techniques, and its associated factors were identified using Cox proportional hazards regression model.
Results
524 subjects were contacted, 269 were eligible, 61 consented and 40 subjects were randomized. Thirty-eight percent were non-adherent to the protocol with a median of visits of five. The recruitment survey reported that 90% would likely participate in RCTs, 52% had previous participation, and 19% were aware of RCTs by their physicians. Some barriers were investigator-related (staff's friendliness and receiving the results of their trial participation) and center-related (privacy-confidentiality issues and the institution's reputation), without difference between adherent and non-adherent participants. We report significant factors for non-adherence as VAS anxiety score of 5 or more (5.3 HR, p = 0.01), Body Mass Index (BMI) (0.91 HR, p = 0.041) and Quality of Life (QoL) – Personal development subdomain (0.89 HR, p = 0.046).
Conclusion
Recruitment and adherence of fibromyalgia patients is a challenge; however, they seem eager to participate in RCTs. We recommend creating a comfortable, friendly and trusting environment to increase the recruitment rate. Higher anxiety, lower BMI and lower quality of life were associated with a higher attrition rate.
Keywords: Fibromyalgia, Underrepresented population, Clinical trials participation, Recruitment, Therapeutic adherence and compliance
1. Introduction
Fibromyalgia is a complex chronic pain condition predominantly in women [1] with a considerable impact on their physical, mental and social quality of life [2]. The etiology of the disease is not yet well understood, leading to failure in the diagnosis and adherence of chronic pain management and other comorbidities [3]. Ethnic minorities, women, and low socioeconomic status are generally underrepresented in randomized clinical trials making recruitment and adherence a challenge [[4], [5], [6]]. Since 1993, the National Institute of Health (NIH) dictated women and racial minorities' inclusion in clinical trials [7]. This could allow access to the latest treatments and technologies and provide a generalization of the clinical trial results [8].
It is known that recruitment in a randomized clinical trial (RCT) is a sensitive matter, to the point that studies indicate that around 50% had to prolong the recruitment period [[9], [10], [11], [12]]. Recruitment of women has been a problem, around one-fifth of the studies published during the 1990s failed to include women [13]. However, diversity in RCTs has not been significantly increased since then [[14], [15], [16]]. Moreover, women are at greater risk for adverse side effects from chronic pain medications possibly due to a lack of female representation in clinical and pre-clinical trials [17,18] Among the recruitment barriers, some social, economic, and ethnic groups are less likely to accept and finalize the participation in trials due to various factors.
Moreover, the adherence rates in RCTs and their associated factors are an understudied topic. It has been reported that low adherence negatively affects the trials' generalizability and validity, complicates the statistical analysis, and confounds the researcher's ability to confer scientific conclusions [19] by affecting the statistical power and increasing the Type –II error [20,21] Click or tap here to enter text. Despite the considerable rate of non-adherence in RCTs [22], few studies report it or discuss it [23]. Fibromyalgia patients appear to present low adherence to treatment, either pharmacological or non-pharmacological, due to the lack of a single treatment being effective for all the range of symptoms and not being effective for different populations [3,24,25]. They also report higher mistrust of their medical services and providers with higher direct and indirect costs [3,26]. One study [27] reports 60% of non-adherence of fibromyalgia patients to an RCT, including an exercise program. However, to the best of our knowledge, no studies explored the characteristics, barriers, and associated factors to trial adherence in fibromyalgia patients. Having this information would help researchers make an effective strategy to improve clinical trials' design and maximize recruitment and retention.
Therefore, this study aims to describe clinical trial participation identifying the most common barriers and facilitators for recruitment and reporting factors associated with adherence and non-adherence in fibromyalgia patients in an ongoing RCT that combines exercise and a non-invasive brain stimulation technique.
2. Methods
We performed a retrospective longitudinal study, with data obtained from our ongoing RCT [28] covering May 1, 2019 through March 11, 2021. Study data were collected and managed using REDCap electronic data capture tools, a secure, web-based application hosted by Partners HealthCare Research Computing, Enterprise Research Infrastructure [29]. This study obeys the Declaration of Helsinki and was approved by the Institutional Review Board of MassGeneral Brigham's ethics committee under the protocol number 2017P002524. A detailed description of the protocol is published [28].
2.1. Study procedures
The RCT includes a total of 22 visits during 18 weeks, including a preliminary screening call, a screening/consent visit (1st week), a baseline/randomization visit (2 nd week), a period of conditioning (2 nd week), the intervention phase (3rd - sixth week) and a follow-up (18th week).
Four broad types of recruitment strategies were used: (1) Media advertisement (GoogleAds, newspaper (English/Spanish), Facebook, and Hospital (Partners Healthcare Network Rally and ClinicalTrials.gov websites)), (2) Flyers (English/Spanish), (3) mails to potential patients registered to MassGeneral Brigham's Research Patient Data Registry and (4) referral from friends, family members, and health care providers or others. Participants receive US $15 per visit ($330 for the 22 visits), sent once their participation ended. Parking was provided at the research facility.
During an initial preliminary phone call, a trained staff screened potential subjects for preliminary eligibility. Eligible subjects were invited to attend a screening/consent visit and were provided the consent form in advance. As part of the screening/consenting procedures, subjects signed the consent, completed a demographic and a recruitment survey, and perform a pre-training visit to evaluate if they were comfortable walking on the treadmill. Inclusion and exclusion criteria are described in the protocol [28].
During the randomization/baseline visit, subjects were randomized to one of the four following groups: (1) active Transcranial direct current stimulation (tDCS) and aerobic exercise (AE), (2) sham tDCS and AE, (3) active tDCS, and non-aerobic exercise (nAE) or (4) sham tDCS and nAE and underwent baseline assessments.
2.2. Outcomes
The underrepresented population was defined according to NIH standards [30]. This includes female gender, race, ethnicity, low income according to poverty threshold [31] or low education, referring to people without high school diploma or equivalent or higher.
Given the study is an on-site study, adherence of participants in clinical trials was collected by completing the visits the day of the sessions, it is expressed in weeks and/or percentage of the total number of visits (22 visits over 18 weeks), it is described as the extent to which the patients or research participants follow their healthcare provider's or researchers' advice and instructions [32].
Non-adherence was defined as the earliest indicator of adherence failure as the date a participant called or emailed to withdraw from the study, or entirely stopped coming to their scheduled visits.
During the consent/screening visits, we performed a demographic questionnaire and a recruitment survey including 45 items subdivided into three main sections: (1) sociodemographic variables, (2) clinical awareness and experience in RCTs, and (3) perceived factors that might influence clinical trial participation, the latter was an adaptation of a previous survey used to measure participation in RCTs [33]. Sociodemographic variables included age, sex, ethnicity, race, education, household income, number of people per household and others. Clinical awareness was measured by previous knowledge of RCTs by their physicians and whether they were asked previously to participate in a clinical study. The likelihood to participate was measured by a five point-Likert scale. Perceived factors that might influence participation were measured by a four point-Likert scale.
During the randomization/baseline visit (visit 3) was performed a quantitative sensory testing (conditioned pain modulation (CPM) and temporal slow pain summation (TSPS)), Brief Pain Inventory (BPI), Revised Fibromyalgia Impact Questionnaire (FIQR), Visual Analog Scale (VAS) Pain (as continuous and dichotomized as severe if 7.5 points or more and moderate-low pain if less than 7 points [34]), Quality of Life (QoL) questionnaire and the subdomains [35] (Material and Physical Well-being, Relationships with other People, Social, Community, and Civic Activities, Personal Development and Fulfillment, Recreation subdomains), Patient-Reported Outcomes Measurement Information System, Beck Depression Inventory(BDI) (21 items including cognitive and overall subdomains [36]), VAS Anxiety (as continuous and dichotomized as 5 or more and less than 5 points [37,38]), VAS Sleep, VAS Stress and VAS depression and the Pittsburgh Sleep Quality Index (PSQI). All the questionnaires were available in English, Spanish or Portuguese.
The conditioning period (visit 3 to visit 5) consisted of three visits during one week before the intervention period with 15, 20 and 30 min of exercise, respectively.
2.3. Intervention
The intervention period (visit 6 to visit 21) lasted four weeks (16 sessions). Subjects were requested to attend the research facility five times a week for the first two weeks and three times a week for the next 2 weeks. In total, 16 sessions of tDCS and 12 sessions of exercise (three times a week) were provided.
Exercise: Aerobic exercise consisted of moderate-intensity walking on a treadmill over 30 min (between 60% and 70% of age-predicted maximal heart rate (HR)) and Non-Aerobic exercise consisted of walking on the treadmill for 30 min (5% of baseline HR).
Transcranial direct current stimulation: A 1 × 1 low-intensity DC stimulator, the Soterix Medical 1 × 1 tDCS-Clinical Trial is used. During anodal tDCS, 2 mA constant current is delivered for 20 min. The anode is placed over the left primary motor cortex and the cathode over the contralateral supraorbital area. Sham tDCS uses the same montage and parameters, but the active current is applied for 30 s in the beginning and at the end to simulate the same sensations of the current ramping.
2.4. Statistical analysis
We used descriptive statistics (frequencies and percentages for categorical and dichotomous variables, mean and SD for continuous variables) to variables related to recruitment numbers, fibromyalgia pain, demographics, and a recruitment questionnaire (fibromyalgia characteristics, sociodemographic variables, clinical awareness and experience in clinical trials and perceived factors that might influence clinical trial participation). For the recruitment survey, we compared the survey answers between adherent and non-adherent participants by unpaired t-test or Fisher's exact test, for quantitative or categorical data, respectively. Also, we described the differences between the perceived importance across domains from the Likert scale [39], we coded the four possible answers: 4 = very important, 3 = somewhat important, 2 = not very important and 1 = not at all important. Using these values, we estimated a median score for each domain (investigator, trial protocol, center, patient, and physician-patient) by adding all the values obtained for each subdomain.
Then, our research investigated the non-adherence time point. A Kaplan-Meier plot was used to estimate the survival (non-adherence) over time. We used multiple univariate Cox proportional hazard regression models to investigate which factors at baseline/randomization had an impact on the length of time until the event occurred. The event definition was non-adherence to the study, and time was defined as the number of days between the consent visit and the earliest indicator of adherence failure as (1) the date a participant called or emailed to withdraw from the study, or (2) the date a participant entirely stopped coming to their scheduled visits – lost to follow-up. The censored information was defined as subjects who were adherent to the study: (1) completed the study without becoming non-adherent (Right censored) or (2) did not complete the study and did not become non-adherent (Left censored). This latter includes subjects who (1) were screened-out given our exclusion criteria or (2) ended their participation due to the shut-down of our facility because of COVID-19 pandemic. We used the following as independent variables for the univariate Cox proportional hazard regression models: pain assessments, quality of life, mood and sleep characteristics. Pain domains included the numeric scale assessments of BPI, FIQR and VAS Pain. Quality of life assessment included the Quality-of-Life questionnaire and PROMIS. Mood was assessed by BDI, VAS Anxiety, VAS Sleep, VAS Stress and VAS depression and finally, for sleep we used PSQI. Significant differences (p < 0.05) will be controlled by gender as a possible confounder in a multivariate Cox proportional hazards regression model. We reported Hazard Ratio (HR) in our results. As a secondary analysis, we describe associate characteristics of adherence's main factor in women in our clinical trial. Analyses were conducted using R version 4.0.2.
3. Results
3.1. Recruitment
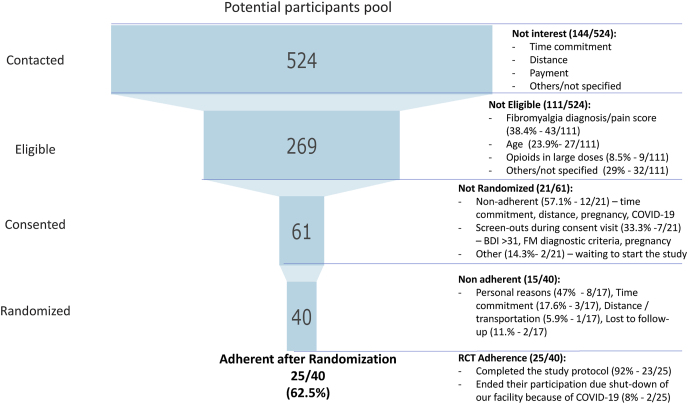
A total of 524 subjects expressed interest in participating in the study, were screened for eligibility, and discussed the study details. Around 51.3% (269/524) of the contacted subjects were eligible and interested in the prescreening. However, only sixty-one subjects were consented to participate, while only 40 subjects were randomized, and 25 were considered adherent to the protocol (See Fig. 1).
Fig. 1.
Participation funnel. RCT: Randomized Clinical Trial.
At the consent visit, around 90.2% were female, with a mean age of 49.4 (SD 10.9) and a range of 20–65 years; the average disease duration was 11.6 years (SD 8.8). Most of them were Caucasian, 39.3% were considered underrepresented given race, ethnicity (19.7% Hispanic) or education. At baseline/randomization visit, 40 subjects were randomized, 87.5% females and the majority Caucasian Non-Hispanic, and underrepresented represented the 42.5%. Finally, 25/40 (62.5%) subjects were adherent to the study visits, 88% were female, and 40% were considered underrepresented (See Supplementary Material 1). There was no significant difference of underrepresented proportion between consented, randomized, and adherent subjects.
Twenty-one subjects consented answered the recruitment questionnaire, 67% (14/21) were randomized and 86% (12/14) completed the study. Underrepresented population was 43% (28.5% Non-Caucasian, 24% Hispanic, 25% low-income and 8.5% low education). Only nineteen percent (4/21) reported being made aware by their physician to participate in an RCT and 90% (19/21) reported they would likely participate by their physician's suggestion. Fifty-two percent (11/21) of responders reported previous participation in an RCT, and 70% of them reported having completed their previous participation. From the subjects who had previously participated in an RCT, the median for study participation was one clinical trial (IQR 1–2.75, max 4). These subjects were recruited to our RCT mainly through Online/internet advertisements (38%) followed by Partners healthcare network (hospital, website or rally) (28.6%) and Social Network (Facebook, Instagram, others) (19%); other resources included Flyers, Doctor/healthcare provider and other. In this sample, 14 subjects were identified as adherent with no significant difference compared to the Non-Adherent group (n = 7). (Table 1).
Table 1.
Responder's characteristics of the recruitment questionnaire.
| Overall, N = 211 | Adherent, N = 141 | Non-Adherent, N = 71 | p-value 2 | |
|---|---|---|---|---|
| Age | 49.57 (10.35) | 49.14 (10.20) | 50.43 (11.40) | 0.64 |
| Female | 19 (90%) | 13 (93%) | 6 (86%) | 0.6 |
| Underrepresented | 9/21 (43%) | 7/14 (50%) | 2/7 (29%) | 0.64 |
| Race | ||||
| Black or African American | 2/21 (9.5%) | 2/14 (14%) | 0/7 (0%) | 0.28 |
| Caucasian | 15/21 (71%) | 10/14 (71%) | 5/7 (71%) | |
| Other | 4/21 (19%) | 2/14 (14%) | 2/7 (29%) | |
| Hispanic | 5/21 (24%) | 3/14 (21%) | 2/7 (29%) | 0.72 |
| Low income | 5/20 (25%) | 5/14 (36%) | 0/7 (0%) | 0.26 |
| Low education | 2/21 (9.5%) | 1/14 (7.1%) | 1/7 (14%) | 0.6 |
| Duration of fibromyalgia (years) | 11.00 (9.14) | 9.64 (8.57) | 13.71 (10.31) | 0.3 |
| Married/Cohabited | 4/21 (19%) | 3/14 (21%) | 1/7 (14%) | 0.69 |
| Religion | ||||
| Yes | 17/21 (70%) | 10/14 (71%) | 7/7 (100%) | 0.54 |
| No | 3/21 (14%) | 3/14 (21%) | 0/7 (0%) | |
| Income | ||||
| Self- Income | 10/21 (48%) | 7/14 (50%) | 3/7 (43%) | 0.48 |
| Other | 11/21 (52%) | 7/14 (50%) | 4/7 (57%) | |
| Government support (Monthly) | ||||
| Yes | 9/20 (45%) | 8/14 (57%) | 1/6 (17%) | 0.16 |
| Employment | ||||
| Employed | 9/21 (42.8%) | 6/14 (43%) | 3/7 (43%) | |
| Retired | 1/21 (4.8%) | 1/14 (7.1%) | 0/7 (0%) | |
| Other | 10/21 (47.6%) | 7/14 (50%) | 4/7 (57%) | |
| Housework daily | ||||
| Less than 3 h | 6/21 (28%) | 5/14 (36%) | 1/7 (14%) | 0.61 |
| 3 or more hours | 15/21 (71%) | 9/14 (64%) | 6/7 (86%) | |
| Beck Depression Inventory | 14.05 (9.07) | 15.21 (8.45) | 11.71 (10.48) | 0.43 |
| How often you have someone you can count on to listen to you when you need to talk about yourself? | ||||
| A little or none of the time | 5/21 (24%) | 2/14 (14%) | 3/7 (43%) | 0.28 |
| Some/most of the time or all of the time | 16/21 (76%) | 12/14 (86%) | 4/7 (57%) | |
| Clinical awareness | ||||
| Has your physician talked to you about clinical trials? | ||||
| Yes | 4/21 (19%) | 2/14 (14%) | 2/7 (29%) | 0.57 |
| Have you ever been asked to participate in clinical trials? | ||||
| Yes | 9/21 (43%) | 6/14 (43%) | 3/7 (60%) | 1 |
| If your doctor found a clinical trial for you and recommended you join; how likely would you be to participate in a clinical trial? | ||||
| Likely or very likely | 19/21 (90%) | 12/14 (86%) | 7/7 (100%) | 0.53 |
| Participation in Clinical trials | ||||
| Yes | 11/21 (52.4%) | 7/14 (50%) | 4/7 (57%) | 0.33 |
| Trial experience | ||||
| I took part and am still in the trial | 1/10 (10%) | 0/6 (0%) | 1/4 (33%) | |
| I took part and completed the trial | 7/10 (70%) | 5/6 (83%) | 2/4 (33%) | |
| I declined to take part in the trial | 2/10 (20%) | 1/6 (17%) | 1/4 (33%) | |
| How did you find out about this clinical trial? | ||||
| Partners healthcare network (hospital, website or rally) | 6/21 (29%) | 5/14 (36%) | 1/7 (14%) | |
| Online/internet advertisements | 8/21 (38%) | 6/14 (43%) | 2/7 (29%) | |
| Social Network (Facebook, Instagram, others) | 4/21 (19%) | 1/14 (7.1%) | 3/7 (43%) | |
| Flyers | 1/21 (4.8%) | 1/14 (7.1%) | – | |
| Doctor/healthcare provider | 1/21 (4.8%) | 1/14 (7.1%) | – | |
| Other | 1/21 (4.8%) | – | 1/7 (14%) | |
1 Mean (SD) or Frequency (%)2 Fisher's exact test; Wilcoxon rank-sum test.
3.2. Perception of participation barriers and facilitators
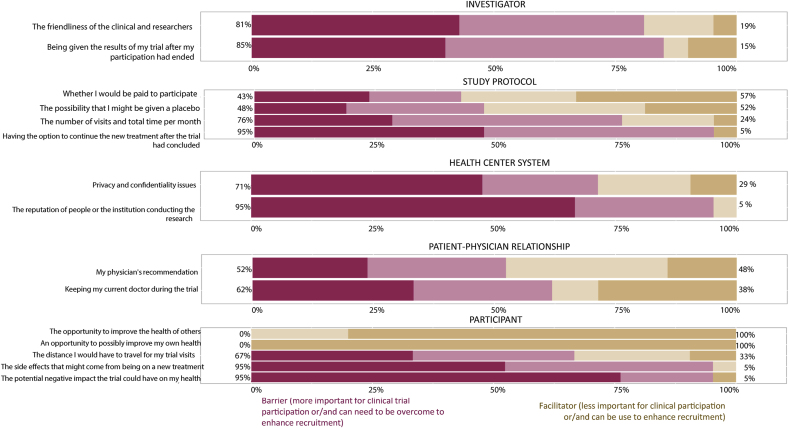
The most important perceived barriers were factors related to the research center, including privacy-confidentiality policies and the institution reputation (8.4 points, from 0 to 10), and to the investigator, including the friendliness of the research staff and the opportunity to receive the results after their clinical trial participation (7.9 points, from 0 to 10). The less critical perceived barriers were factors related to the participant, such as potential side effects or negative impact on health, the distance they need to travel for the visits, and the opportunity to improve their own health or the health of others. There was no significant difference between barriers and facilitators among Adherent (n = 14) and Non-Adherent (n = 7) participants. (Fig. 2 and Table 2).
Fig. 2.
Perception of barriers and facilitators for clinical trial participation of fibromyalgia patients.
Table 2.
Perception on clinical trial participation barriers.
| Not important or not at all important | Important or Very important | p-value | ||
|---|---|---|---|---|
| Investigator | The friendliness of the clinical and researchers | 4 (19%) | 17 (81%) | |
| Adherent | 4 | 10 | 0.72 | |
| Non-Adherent | 0 | 7 | ||
| Being given the results of my trial after my participation had ended | 3 (15%) | 18 (85%) | ||
| Adherent | 2 | 11 | 0.88 | |
| Non-Adherent | 1 | 6 | ||
| Study protocol | The possibility that I might be given a placebo (inactive treatment) | 11 (52%) | 10 (48%) | |
| Adherent | 7 | 7 | 1 | |
| Non-Adherent | 4 | 3 | ||
| The number of visits and total time per month to participate | 5 (24%) | 16 (76%) | ||
| Adherent | 4 | 10 | 0.5 | |
| Non-Adherent | 1 | 6 | ||
| Having the option to continue the new treatment after the trial had concluded | 1 (4.8%) | 20 (95%) | ||
| Adherent | 1 | 13 | 1 | |
| Non-Adherent | 0 | 7 | ||
| Whether I would be paid to participate | 12 (57%) | 9 (43%) | ||
| Adherent | 9 | 5 | 0.25 | |
| Non-Adherent | 3 | 4 | ||
| Health Center System | The reputation of people or the institution conducting the research | 1 (4.8%) | 20 (95%) | |
| Adherent | 0 | 14 | 0.29 | |
| Non-Adherent | 1 | 6 | ||
| Privacy and confidentiality issues | 6 (29%) | 15 (71%) | ||
| Adherent | 4 | 10 | 0.92 | |
| Non-Adherent | 2 | 5 | ||
| Patient-Physician Relationship | Keeping my current doctor during the trial | 8 (38%) | 13 (62%) | |
| Adherent | 7 | 7 | 0.55 | |
| Non-Adherent | 1 | 6 | ||
| My physician's recommendation | 10 (48%) | 11 (52%) | ||
| Adherent | 8 | 6 | 0.26 | |
| Non-Adherent | 2 | 5 | ||
| Participant | The potential negative impact the trial could have on my health | 1 (4.8%) | 20 (95%) | |
| Adherent | – | 14 | 0.5 | |
| Non-Adherent | 1 | 6 | ||
| The distance I would have to travel for my trial visits | 7 (33%) | 14 (67%) | ||
| Adherent | 4 | 10 | 1 | |
| Non-Adherent | 3 | 4 | ||
| The side effects that might come from being on a new treatment | 1 (4.8%) | 20 (95%) | ||
| Adherent | 1 | 13 | 0.76 | |
| Non-Adherent | 0 | 7 | ||
| An opportunity to possibly improve my own health | – | 21 (100%) | – | |
| Adherent | – | 14 | ||
| Non-Adherent | – | 7 | ||
| The opportunity to improve the health of others | – | 20 (100%) | – | |
| Adherent | – | 13 | ||
| Non-Adherent | – | 7 |
3.3. Adherence
After the consent visit, 21/61 (35%) decided to withdraw from the study before randomization/baseline, were screened-out or did not start the study (<13.6% of study visits) (See Fig. 1). After randomization, fifteen subjects (38%) decided to withdraw and twenty-five were considered censored. The majority (92%) of censored cases were right-censored as they were adherent and completed the protocol (23/25). Eight percent (2/25) were left-censored as they were adherent with the visits, but due to the shut-down of our facility because of COVID-19 their participation ended during the second and sixth week of their study participation (Fig. 1).
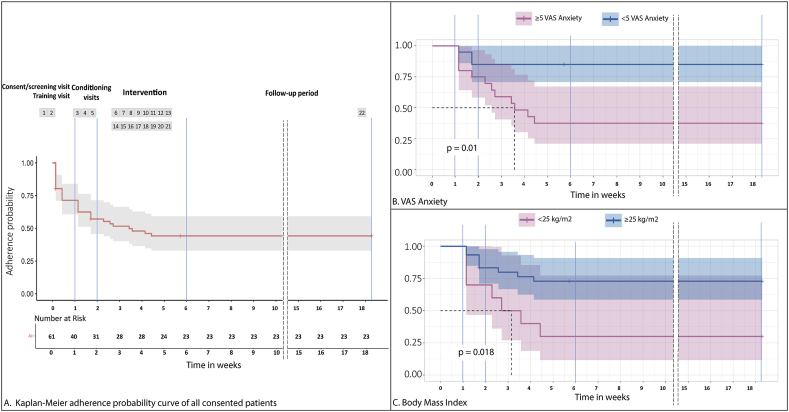
The median of time completed by non-adherent randomized subjects was 5 visits over 2 weeks, during the conditioning phase (∼23% of study visits) of the RCT. There is a major decline during the first two weeks followed by a decline that can be regarded as constant without any specific sensitive weeks to non-adherence (Fig. 3).
Fig. 3.
A. Kaplan-Meier survival curve of all consented patients (N = 61) over 22 visits (18 weeks). B. Kaplan-Meier survival graph displaying the number of days initially compliant patients remained compliant, for subjects with less than 5 on VAS Anxiety scale at baseline (n = 20) and with 5 or more on VAS Anxiety scale (n = 20). C. Kaplan-Meier survival graph displaying the number of days initially compliant patients remained compliant, for subjects not overweight/obesity at baseline (n = 30) and overweight/obesity (n = 10).
Cox's proportional hazards univariate regression models were used to identify variables that explain the length of adherent time. Only five significant associate factors for non-adherence were found, namely VAS anxiety score (HR = 1.3; p = 0.0038), VAS Anxiety dichotomized (HR = 5.3; p = 0.01), Overweight/obesity (HR = 0.29; p = 0.018), BMI (HR = 0.91; p = 0.041) and QoL – Personal development and fulfillment subdomain (HR = 0.89; p = 0.046). VAS Anxiety had the highest hazard ratio, meaning the hazard of non-adherence increases by a factor of 1.3 for every unit increase in VAS Anxiety and having five or more points in the scale increases the hazard in 5.3. Moreover, overweight/obesity reduces the hazard in 71%, and for every unit increase of BMI, there is a reduction of 9% of the hazard. Finally, a unit increase in the QoL scale's personal development and fulfillment subdomain was associated with a decrease of the hazard by 11%. In a multivariate analysis for these variables gender was not found to be a significant confounder (p < 0.05) (see Table 3).
Table 3.
Descriptive and Univariate Cox regression analyses for risk of non-adherence in fibromyalgia subjects in a clinical trial.
| Overall (n = 40)1 | Adherent (n = 25) 1 | Non-Adherent (n = 15) 1 | Univariate Cox Hazard Analysis |
|||
|---|---|---|---|---|---|---|
| β | HR (95% CI for HR) | p-value | ||||
| VAS anxiety | 4.51 (2.74) | 3.40 (2.60) | 6.37 (1.88) | 0.27 | 1.3 (1.1–1.6) | 0.0038 |
| VAS anxiety (≥5) | 20/40 (50%) | 8/25 (32%) | 12/15 (80%) | 1.7 | 5.3 (1.5–19) | 0.01 |
| Overweight/obesity | 30/40 (75%) | 22/25 (88%) | 8/15 (53%) | −1.2 | 0.29 (0.11–0.81) | 0.018 |
| Body Mass Index (kg/m2) | 29.18 (6.55) | 30.84 (6.08) | 26.41 (6.56) | −0.095 | 0.91 (0.83–1) | 0.041 |
| QoL – Personal Development and Fulfillment subdomain | 17.88 (4.97) | 18.92 (5.02) | 16.13 (4.50) | −0.11 | 0.89 (0.8–1) | 0.046 |
| VAS Depression | 3.84 (2.95) | 3.26 (2.99) | 4.79 (2.72) | 0.13 | 1.1 (0.96–1.3) | 0.15 |
| Hispanic | 9/39 (23%) | 4/25 (16%) | 5/14 (36%) | 0.78 | 2.2 (0.73–6.6) | 0.16 |
| PROMIS - Anxiety subdomain | 10.90 (3.85) | 10.24 (4.15) | 12.00 (3.12) | 0.085 | 1.1 (0.97–1.2) | 0.16 |
| QoL total | 70.15 (14.31) | 72.28 (15.23) | 66.60 (12.28) | −0.028 | 0.97 (0.94–1) | 0.16 |
| Obesity | 17/40 (42%) | 13/25 (52%) | 4/15 (27%) | −0.82 | 0.44 (0.14–1.4) | 0.16 |
| BDI total | 18.02 (8.56) | 16.88 (9.82) | 19.93 (5.73) | 0.035 | 1 (0.98–1.1) | 0.22 |
| Caucasian | 27/37 (73%) | 15/23 (65%) | 12/14 (86%) | 0.92 | 2.5 (0.56–11) | 0.23 |
| BDI - cognitive subdomain | 4.97 (3.78) | 4.56 (4.31) | 5.67 (2.66) | 0.075 | 1.1 (0.95–1.2) | 0.23 |
| PSQI total | 12.35 (4.53) | 13.00 (4.74) | 11.27 (4.06) | −0.063 | 0.94 (0.84–1.1) | 0.27 |
| FIQR - Overall subdomain | 11.43 (5.51) | 10.84 (5.21) | 12.40 (6.03) | 0.056 | 1.1 (0.95–1.2) | 0.28 |
| BPI - Worst pain (last 24 h) | 7.18 (1.63) | 6.96 (1.70) | 7.53 (1.51) | 0.18 | 1.2 (0.86–1.7) | 0.29 |
| TSPS | −0.59 (1.94) | −0.82 (2.15) | −0.20 (1.52) | 0.14 | 1.1 (0.87–1.5) | 0.33 |
| QOL - Social, Community, and Civic Activities | 9.35 (2.68) | 9.60 (2.71) | 8.93 (2.69) | −0.098 | 0.91 (0.74–1.1) | 0.35 |
| QOL - Relations with other People | 18.33 (4.81) | 18.96 (5.24) | 17.33 (4.01) | −0.046 | 0.95 (0.86–1.1) | 0.37 |
| QOL - Recreation | 12.97 (3.42) | 13.24 (3.49) | 12.53 (3.38) | −0.068 | 0.93 (0.8–1.1) | 0.4 |
| VAS Stress | 5.33 (3.14 | 4.92 (3.14) | 6.02 (3.11) | 0.07 | 1.1 (0.91–1.3) | 0.41 |
| QOL-Physical and Material Well-being | 7.32 (2.18) | 7.52 (2.55) | 7.00 (1.36) | −0.094 | 0.91 (0.71–1.2) | 0.45 |
| CPM | −0.85 (1.18) | −0.98 (1.10) | −0.64 (1.32) | 0.16 | 1.2 (0.76–1.8) | 0.47 |
| Work - full time | 7/38 (18%) | 5/23 (22%) | 2/15 (13%) | −0.51 | 0.6 (0.13–2.7) | 0.5 |
| PROMIS - Pain total score | 14.55 (3.90) | 14.88 (3.96) | 14.00 (3.85) | −0.037 | 0.96 (0.84–1.1) | 0.58 |
| BPI - Pain interference | 40.50 (14.63) | 39.44 (16.26) | 42.27 (11.71) | 0.0097 | 1 (0.97–1) | 0.59 |
| FIQR - Total Score | 57.67 (18.11) | 56.75 (19.03) | 59.20 (17.00) | 0.0079 | 1 (0.98–1) | 0.61 |
| Duration Fibromyalgia | 11.76 (9.09) | 11.22 (9.37) | 12.67 (8.85) | 0.014 | 1 (0.96–1.1) | 0.61 |
| VAS Pain | 6.10 (1.85) | 5.97 (2.10) | 6.31 (1.36) | 0.069 | 1.1 (0.81–1.4) | 0.63 |
| VAS Pain (≥7.5) | 10/40 (25%) | 7/25 (28%) | 3/15 (20%) | −0.3 | 0.74 (0.21–2.6) | 0.64 |
| FIQR - Symptoms subdomain | 30.73 (8.41) | 30.24 (9.08) | 31.53 (7.38) | 0.013 | 1 (0.95–1.1) | 0.7 |
| Gender - Female | 35/40 (88%) | 22/25 (88%) | 13/15 (87%) | −0.26 | 0.77 (0.17–3.4) | 0.74 |
| BPI - Pain on the AVERAGE | 5.55 (1.69) | 5.48 (1.85) | 5.67 (1.45) | 0.046 | 1 (0.78–1.4) | 0.76 |
| BPI - Pain at its least (last 24 h) | 3.92 (2.37) | 3.80 (2.47) | 4.13 (2.26) | 0.029 | 1 (0.83–1.3) | 0.79 |
| Married | 9/40 (22%) | 6/25 (24%) | 3/15 (20%) | −0.18 | 0.84 (0.24–3) | 0.79 |
| Work - full time/part-time | 12/38 (32%) | 7/23 (30%) | 5/15 (33%) | 0.14 | 1.1 (0.39–3.4) | 0.8 |
| BDI - somatic subdomain | 5.18 (2.34) | 5.20 (2.69) | 5.13 (1.68) | −0.018 | 0.98 (0.8–1.2) | 0.86 |
| PROMIS - fatigue subdomain | 15.55 (3.26) | 15.44 (3.31) | 15.73 (3.28) | 0.013 | 1 (0.86–1.2) | 0.87 |
| Age | 50.20 (11.34) | 49.60 (10.96) | 51.20 (12.27) | 0.0036 | 1 (0.96–1.1) | 0.88 |
| VAS Sleep | 6.06 (2.63) | 5.97 (2.87) | 6.21 (2.26) | 0.013 | 1 (0.83–1.2) | 0.9 |
| FIQR - Function subdomain | 15.52 (7.29) | 15.67 (7.58) | 15.27 (7.04) | 0.00081 | 1 (0.93–1.1) | 0.98 |
1 n/N (%); Mean (SD). CPM: Conditioned pain modulation, TSPS: temporal slow pain summation, VAS: Visual Analog Scale, QoL: Quality of Life, PROMIS: Patient-Reported Outcomes Measurement Information System, BDI: Beck Depression Inventory, PSQI: Pittsburgh Sleep Quality Index, BPI: Brief Pain Inventory, FIQR: Revised Fibromyalgia Impact Questionnaire.
At week 18, 80% (16/20) of randomized subjects with less than 5 points on VAS Anxiety score adhered to the protocol, whereas only 35% (7/20) of the subjects with 5 or more points on VAS Anxiety score at baseline. Therefore, fibromyalgia subjects with less anxiety score measured by VAS had a better adherence for a long study over time than more anxious subjects (p = 0.0038). In the group of fibromyalgia subjects with a BMI of 25 kg/m2 or more, 67% (20/30) continued the study through week 18. In the non-overweight/obesity group, 30% (3/10) completed the protocol. Suggesting fibromyalgia subjects with overweight/obesity have better adherence than patients with less than 25 kg/m2 (p = 0.018). (See Fig. 3).
In a secondary analysis, out of the 35 randomized women, 16 report a higher anxiety (5 points or more on a VAS scale), this showed to be significantly related to higher VAS pain (p = 0.011), BPI- Interference (p < 0.001), FIQR total scale and subscales (overall and symptoms) and VAS- Stress (p = 0.005). (See Supplementary Material 1).
4. Discussion
Fibromyalgia patients were 90% women; there was no significant difference in race or ethnicity proportion among consented, randomized, adherent and non-adherent participants. The recruitment questionnaire reflected that despite the likelihood of fibromyalgia patients participating in clinical trials and that half of them reported previous participation in RCTs, only 19% of them were aware of RCTs by their physician. Two of our primary recruitment resources were Online/internet advertisements and Partners healthcare network (hospital, website or rally). This is indeed a barrier for recruitment as clinicians usually do not have time in their full schedules to explain and provide information on clinical trials [40,41].
The most important recruitment barriers were the health research center, including privacy and confidentiality policies and the institution's reputation; and the investigator, including the friendliness of research staff and the opportunity to receive the results after their participation.
This reflects this population's interest in trying new treatments, regardless of race or ethnicity [42], comparable to the general population [43]. Research demonstrates that minorities in the United States are willing to participate in RCTs, but they are not asked and/or are aware [44]. In fact, the lack of communication regarding trials from health care providers might be related to the difficulty of this population to build a good patient-physician relationship [45]. As seen in other studies, media and online resources have worked as a good tool for recruitment in postmenopausal women [46]. In addition, this method has shown to be cost-effective [47]. Supporting this notion, fibromyalgia patients have higher use of internet resources to look for online health information [48]. Also, other studies have shown the importance of effective communication and presentation of the trial as well as respectfulness, flexibility, and empathy [[49], [50], [51]]. Probably given our sample size, there was no significant difference between adherent and non-adherent participants and their perception of participation in RCTs. Hence, promoting a safe, trusting, and friendly environment by the center and the investigators might improve recruitment and adherence.
Fifty percent of contacted subjects were eligible, but only 23% consented. Some reasons were time commitment and distance to the center. Supporting this finding, another study found that around 36% of withdraws were related to protocol issues as the duration of the study, length of procedures, and other 33% were related to inconvenience as taking time out of work and distance [52]. In fact, as shown in other stimulation sessions, the number of sessions seems to be related to the dropout rate [53]. However, the use of online visits might improve this recruitment and the adherence rate [54]. Thus, the importance of being aware and entertaining potential barriers related to the study population in advance enhanced and designed tailored recruitment strategies [14].
Regarding adherence, our sample has an average pain score of 6, overweight or obesity with low to moderate depression. Out of the 40 subjects randomized, only 62.5% completed the study protocol and from the non-adherent, 60% did not complete the conditioning phase. We found 5 determinants for non-adherence of fibromyalgia patients to our RCT, including VAS anxiety, BMI and the Personal Development and Fulfillment subdomain of the QoL scale.
Participant adherence may become a problem in any RCT. Researchers have been extensively studying adherence since the 1990's identifying factors as study protocol, researcher and participants [19]; minority population report low adherence due to distrust, provider perceptions, and access to care [55,56]. According to a systematic review, the non-adherence rates among chronic pain patients ranged from 8% to 62% with a mean of 40%; related to dosing frequency, polymedication, pain intensity, and concerns about pain medication [57]. Other studies have found that the female gender is a predictor of lower adherence to antiretroviral therapies for HIV [58], another underrepresented population, and less likely to adhere to chronic medications [59]. In contrast, a study with a remote intervention found that female gender, higher resistance to change, higher openness to experience, and higher depressive symptoms were predictors for better maintenance [60]. Regarding exercise studies, rigid timelines were the main difficulty for retention in older adults' resistance training programs, and group-onsite sessions reported a better adherence [61]. These results suggest that reasons for non-adherence vary from different populations and study protocols (on-site vs. remote).
Our findings showed that higher anxiety levels were associated with lower adherence rates. According to a meta-analysis, anxiety had 59% higher odds of poor adherence [62]. However, a study looking for adherence to remote monitoring for Pregnancy-induced hypertension found that higher anxiety, depression, among others, were related to higher adherence [63]. Thus, this variable may be disease-specific or confounded by other factors. In a spinal cord injury pain trial, we found no relationship between dropout and depression levels [64]. However, in fibromyalgia, depression and anxiety are important components of this and thus may impact more the risk of being non-adherent to rehabilitation interventions and medication regimes [65]. Longitudinal multivariate regression modeling analysis had also identified anxiety and depression, among other factors, as significant predictors of treatment adherence [66]. Moreover, in oncology trials, moderate to severe depressive symptoms were associated with a lower adherence [67]. However, a recent review of adherence to fibromyalgia treatment did not include anxiety as a relevant factor for adherence [68]. Mood disorders such as anxiety and depression are likely to be important factors as they have the potential to impair motivation, which may affect the willingness and ability to follow and complete a treatment program. In a secondary analysis of women with 5 or more points on the VAS-Anxiety scale, we showed a significant association with other functional and pain measurements, supporting the importance of understanding fibromyalgia as a multiverse disease. One consideration is how to use this information in future RCTs to improve the design. It may be difficult to prevent, but one consideration is to use this variable as a stratification factor in the randomization to ensure balanced groups.
Also, we showed a direct relationship between BMI and adherence. Supporting this notion, Dobkin PL et al. [27] found a better adherence in fibromyalgia participants with less physical fitness at baseline in a remote-exercise intervention and a higher pain score for aerobic exercise. In contrast, Kaleth et al. [69] show how obesity influences adherence using exercise and a motivational intervention. Based on this, although high BMI has been negatively related to exercise and physical involvement in fibromyalgia patients [70], the low-moderate intensity and simple exercise implemented might motivate low active participants to engage better in the study than those with a higher activity performance.
We did not find a correlation between the probability of withdrawal and the pain outcomes (clinical and experimental measures), but we did find relationships with other covariates. Trials. One important point to consider for dropouts in the future design of fibromyalgia is whether they are related to the outcome or are independent. In the first case, there would be some evidence that missing data was happening not at random and possibly because the subjects dropping out were either improving too much (and not finding the need to continue) or not improving at all (and finding no incentive to continue). In the second case, with dropouts happening unrelated to the outcomes, only to independent variables, there would be some evidence of missingness at random [71]. Given this, our trial would fall in the second case and missing data is possibly happening at random. Therefore, the main issue would be increasing the total sample size to consider the overall dropouts (in fact, during the design of our study we had already increased the sample size by 30% so the trial is not underpowered).
It is recommended that in long-term trials and especially in chronic conditions, screening and enrollment should be meticulous from the beginning to prevent a lack of adherence [22]. Research scientists have also advised that participants must be informed and educated about the protocol, making sure they understand and fully commit to it. It also entails effective communication, including understanding, active listening, verbal and non-verbal communication during all the trial participation [32,72,73]. It has been proposed that pre-randomization screening should be performed on research participants to screen out poor adherents. It is based on the premise that short-term adherence during pre-randomization can predict long-term adherence after enrollment [20]. Secondly, it has been suggested that researchers should keep educating and communicating with participants during the trial and address their concerns to enhance and sustain their adherence. Finally, home-based intervention might help with adherence in this population [74,75].
This study has some limitations. First, we used data from an ongoing clinical trial, therefore the sample size is limited and included a very selected and randomized sample that could not be generalized to all fibromyalgia patients. Another limitation is, despite being an RCT, there is a possibility of other confounders than gender. Regardless of these limitations, this study is a novel contribution to the literature on recruitment and trial adherence of fibromyalgia patients. To the best of our knowledge, this is the first time using a comprehensive demographic and clinical survey and survival analysis to explore recruitment and adherence associated factors in fibromyalgia patients. Future research is indicated to better understand patient perspectives on recruitment and adherence barriers on larger and more representative samples and the development and test of multidimensional strategies to overcome these challenges.
5. Conclusions
Fibromyalgia patients are more likely to participate in clinical trials, however, there is a lack of awareness of RCTs from their primary care physician. Moreover, developing a friendly environment improving trust, communication, and active listening with the participants might improve recruitment and adherence. Therefore, clinical research focusing on minority populations is important to understand the disease's pattern, trend, and behavior, effectiveness, and validity of treatment. Our study shows that VAS anxiety, low BMI, and low QoL were the significant factors for non-adherence. Taken together, these results suggest that reasons for non-adherence vary from population to population. Therefore, it is necessary to assess the targeted patient population's clinical characteristics and design the clinical trial according to these factors to ensure optimal retention.
Data availability
The data that support the findings of this study are available from the corresponding author, FF, upon request.
Funding
This work is supported by the National Institutes of Health (R01 AT009491-01A1).
Author's contribution
FF, AC-R, LC-B and KP-B participated in the Conceptualization and Methodology of the study. AC-R contributed with the Formal analysis. All the authors participated in the Investigation and the drafting of the article. All authors approved the final version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2021.100860.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Marques A.P., Santo A. de S. do E., Berssaneti A.A., Matsutani L.A., Yuan S.L.K. Prevalence of fibromyalgia: literature review update. Revista Brasileira de Reumatologia (English Edition. 2017;57:356–363. doi: 10.1016/j.rbre.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 2.White L.A., Birnbaum H.G., Kaltenboeck A., Tang J., Mallett D., Robinson R.L. Employees with fibromyalgia: medical comorbidity, healthcare costs, and work loss. J. Occup. Environ. Med. 2008;50:13–24. doi: 10.1097/JOM.0b013e31815cff4b. [DOI] [PubMed] [Google Scholar]
- 3.Ghavidel-Parsa B., Bidari A., Amir Maafi A., Ghalebaghi B., Maafi A.A. The iceberg nature of fibromyalgia burden: the clinical and economic aspects. Korean J. Pain. 2015;28:169–176. doi: 10.3344/kjp.2015.28.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coakley M., Fadiran E.O., Parrish L.J., Griffith R.A., Weiss E., Carter C. Dialogues on diversifying clinical trials: successful strategies for engaging women and minorities in clinical trials. J. Wom. Health. 2012;21:713–716. doi: 10.1089/jwh.2012.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman S.H., Cunningham C.O., Lin J., Haramati L.B., Levsky J.M. Having a primary care provider is the strongest predictor of successful follow-up of participants in a clinical trial. J. Am. Board Fam. Med. 2020;33:431–439. doi: 10.3122/jabfm.2020.03.190018. [DOI] [PubMed] [Google Scholar]
- 6.Underwood S.M. Minorities, women, and clinical cancer research: the charge, promise, and challenge. Ann. Epidemiol. 2000;10 doi: 10.1016/s1047-2797(00)00200-3. [DOI] [PubMed] [Google Scholar]
- 7.I. of M. (US) C. on E. and L.I.R. to the I. of W . In: NIH Revitalization Act of 1993 Public Law 103-43. Studies C., Mastroianni A.C., Faden R., Federman D., editors. 1994. https://www.ncbi.nlm.nih.gov/books/NBK236531/ [Google Scholar]
- 8.Cox K., Mcgarry J. Why patients don't take part in cancer clinical trials: an overview of the literature. Eur. J. Cancer Care. 2003;12:114–122. doi: 10.1046/j.1365-2354.2003.00396.x. [DOI] [PubMed] [Google Scholar]
- 9.McDonald A.M., Knight R.C., Campbell M.K., Entwistle V.A., Grant A.M., Cook J.A., Elbourne D.R., Francis D., Garcia J., Roberts I., Snowdon C. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7:9. doi: 10.1186/1745-6215-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakumanu S., Manns B.J., Tran S., Saunders-Smith T., Hemmelgarn B.R., Tonelli M., Tsuyuki R., Ivers N., Southern D., Bakal J., Campbell D.J.T.T. Cost analysis and efficacy of recruitment strategies used in a large pragmatic community-based clinical trial targeting low-income seniors: a comparative descriptive analysis. Trials. 2019;20:577. doi: 10.1186/s13063-019-3652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.J R., A Y., L S., R M., A C., D T., P D. Clinical trial metadata: defining and extracting metadata on the design, conduct, results and costs of 125 randomised clinical trials funded by the National Institute for Health Research Health Technology Assessment programme. Health Technol. Assess. 2015;19:1–166. doi: 10.3310/HTA19110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sj W., I B.D.A.H.-C., O B., L F., D H., RM J., C K., B N., J R., M S., SA J. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open. 2017;7 doi: 10.1136/BMJOPEN-2016-015276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidaver R.M., Lafleur B., Tong C., Bradshaw R., Marts S.A. Women subjects in NIH-funded clinical research literature: lack of progress in both representation and analysis by sex. J. Wom. Health Gend. Base Med. 2000;9:495–504. doi: 10.1089/15246090050073576. [DOI] [PubMed] [Google Scholar]
- 14.Overcoming the barriers to recruitment of underrepresented minorities. https://acrp.digitellinc.com/acrp/sessions/4095/view n.d.)
- 15.Khan M.S., Shahid I., Siddiqi T.J., Khan S.U., Warraich H.J., Greene S.J., Butler J., Michos E.D. Ten‐year trends in enrollment of women and minorities in pivotal trials supporting recent US food and drug administration approval of novel cardiometabolic drugs. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen M.S., Lara P.N., Dang J.H.T., Paterniti D.A., Kelly K. Twenty years post-NIH Revitalization Act: enhancing minority participation in clinical trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual: renewing the case for enhancing minority participation in cancer clinical trials. Cancer. 2014;120(Suppl 7):1091–1096. doi: 10.1002/cncr.28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zucker I., Prendergast B.J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex Differ. 2020;11:32. doi: 10.1186/s13293-020-00308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mogil J.S. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat. Rev. Neurosci. 2020;21:353–365. doi: 10.1038/s41583-020-0310-6. [DOI] [PubMed] [Google Scholar]
- 19.Haynes R.B., Dantes R. Patient compliance and the conduct and interpretation of therapeutic trials. Contr. Clin. Trials. 1987;8:12–19. doi: 10.1016/0197-2456(87)90021-3. [DOI] [PubMed] [Google Scholar]
- 20.Robiner W.N. Enhancing adherence in clinical research. Contemp. Clin. Trials. 2005;26:59–77. doi: 10.1016/j.cct.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Mallayasamy S., Chaturvedula A., Blaschke T., Fossler M.J. A systematic evaluation of effect of adherence patterns on the sample size and power of a clinical study. CPT Pharmacometrics Syst. Pharmacol. 2018;7:818–828. doi: 10.1002/psp4.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiovitz T.M., Bain E.E., McCann D.J., Skolnick P., Laughren T., Hanina A., Burch D. Mitigating the effects of nonadherence in clinical trials. J. Clin. Pharmacol. 2016;56:1151–1164. doi: 10.1002/jcph.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A Andrade, R. De Azevedo, K. Steffens, S. Mendes Sieczkowska, L. Alexandre, P. Tartaruga, G. Torres Vilarino, A systematic review of the effects of strength training in patients with fibromyalgia: clinical outcomes and design considerations, (n.d.). 10.1186/s42358-018-0033-9. [DOI] [PubMed]
- 24.Cui Z., Zhao Y., Novick D., Faries D. Predictors of duloxetine adherence and persistence in patients with fibromyalgia. J. Pain Res. 2012;5:193–201. doi: 10.2147/JPR.S31800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marlow N.M., Simpson K.N., Vaughn I.A., Jo A., Zoller J.S., Short E.B. Healthcare costs and medication adherence among patients with fibromyalgia: combination medication vs. Duloxetine, milnacipran, venlafaxine, and pregabalin initiators. Pain Pract. 2018;18:154–169. doi: 10.1111/papr.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolfe F., Walitt B. Culture, science and the changing nature of fibromyalgia. Nat. Rev. Rheumatol. 2013;9:751–755. doi: 10.1038/nrrheum.2013.96. [DOI] [PubMed] [Google Scholar]
- 27.Dobkin P.L., da Costa D., Abrahamowicz M., Dritsa M., du Berger R., Fitzcharles M.-A., Lowensteyn I. Adherence during an individualized home based 12- week exercise program in women with fibromyalgia. J. Rheumatol. 2006;33(2):333–341. [PubMed] [Google Scholar]
- 28.Castelo-Branco L., Uygur Kucukseymen E., Duarte D., El-Hagrassy M.M., Bonin Pinto C., Gunduz M.E., Cardenas-Rojas A., Pacheco-Barrios K., Yang Y., Gonzalez-Mego P., Estudillo-Guerra A., Candido-Santos L., Mesia-Toledo I., Rafferty H., Caumo W., Fregni F. Optimised transcranial direct current stimulation (tDCS) for fibromyalgia - targeting the endogenous pain control system: a randomised, double-blind, factorial clinical trial protocol. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-032710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Institutes of Health (NIH) https://www.nih.gov/
- 31.U.S.C. Bureau, United States Census Bureau. Poverty Thresholds, (n.d.).
- 32.Probstfield J.L. Adherence and its management in clinical trials: implications for arthritis treatment trials. Arthritis Care Res. 1989;2:A48–A57. doi: 10.1002/anr.1790020314. [DOI] [PubMed] [Google Scholar]
- 33.DasMahapatra P., Raja P., Gilbert J., Wicks P. Clinical trials from the patient perspective: survey in an online patient community. BMC Health Serv. Res. 2017;17:166. doi: 10.1186/s12913-017-2090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boonstra A.M., Preuper H.R.S., Balk G.A., Stewart R.E. Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain. 2014;155:2545–2550. doi: 10.1016/j.pain.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Burckhardt C.S., Anderson K.L. The quality of life scale (QOLS): reliability, validity, and utilization. Health Qual. Life Outcome. 2003;1:60. doi: 10.1186/1477-7525-1-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quilty L.C., Zhang K.A., Bagby R.M. The latent symptom structure of the Beck depression inventory-II in outpatients with major depression. Psychol. Assess. 2010;22:603–608. doi: 10.1037/a0019698. [DOI] [PubMed] [Google Scholar]
- 37.Facco E., Stellini E., Bacci C., Manani G., Pavan C., Cavallin F., Zanette G. Validation of visual analogue scale for anxiety (VAS-A) in preanesthesia evaluation. Minerva Anestesiol. 2013;79:1389–1395. [PubMed] [Google Scholar]
- 38.Appukuttan D., Vinayagavel M., Tadepalli A. Utility and validity of a single-item visual analog scale for measuring dental anxiety in clinical practice. J. Oral Sci. 2014;56:151–156. doi: 10.2334/josnusd.56.151. [DOI] [PubMed] [Google Scholar]
- 39.Zafra-Tanaka J.H., Pacheco-Barrios K., Inga-Berrospi F., Taype-Rondan A. Self-perceived competencies in the diagnosis and treatment of mental health disorders among general practitioners in Lima, Peru. BMC Med. Educ. 2019;19:464. doi: 10.1186/s12909-019-1900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramirez A.G., Chalela P., Suarez L., Muñoz E., Pollock B.H., Weitman S.D. Early phase clinical trials: referral barriers and promoters among physicians. J. Community Med. Health Educ. 2012;2 doi: 10.4172/2161-0711.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahmud A., Zalay O., Springer A., Arts K., Eisenhauer E. Barriers to participation in clinical trials: a physician survey. Curr. Oncol. 2018;25:119–125. doi: 10.3747/co.25.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wendler D., Kington R., Madans J., van Wye G., Christ-Schmidt H., Pratt L.A., Brawley O.W., Gross C.P., Emanuel E. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030019. –0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.U.S. Census Bureau QuickFacts: United States. https://www.census.gov/quickfacts/fact/table/US/PST045219 (n.d.)
- 44.Wendler D., Kington R., Madans J., Van Wye G., Christ-Schmidt H., Pratt L.A., Brawley O.W., Gross C.P., Emanuel E. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030019. –0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freburger J.K., Callahan L.F., Currey S.S., Anderson L.A. Use of the Trust in Physician Scale in patients with rheumatic disease: psychometric properties and correlates of trust in the rheumatologist. Arthritis Rheum. 2003;49:51–58. doi: 10.1002/art.10925. [DOI] [PubMed] [Google Scholar]
- 46.Waltman N.L., Smith K.M., Kupzyk K.A., Lappe J.M., Mack L.R., Bilek L.D. Approaches to recruitment of postmenopausal women for a community-based study. Nurs. Res. 2019;68:307–316. doi: 10.1097/NNR.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferreira I.S., Pinto C.B., Saleh Velez F.G., Leffa D.T., Vulcano de Toledo Piza P., Fregni F. Recruitment challenges in stroke neurorecovery clinical trials. Contemp. Clin. Trials Commun. 2019;15:100404. doi: 10.1016/j.conctc.2019.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basavakumar D., Flegg M., Eccles J., Ghezzi P. Accuracy, completeness and accessibility of online information on fibromyalgia. Rheumatol. Int. 2019;39:735–742. doi: 10.1007/s00296-019-04265-0. [DOI] [PubMed] [Google Scholar]
- 49.Kaur G., Smyth R.L., Powell C.V.E., Williamson P. A survey of facilitators and barriers to recruitment to the MAGNETIC trial. Trials. 2016;17:607. doi: 10.1186/s13063-016-1724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duncan M., Korszun A., White P., Eva G., investigators S. Qualitative analysis of feasibility of recruitment and retention in a planned randomised controlled trial of a psychosocial cancer intervention within the NHS. Trials. 2018;19:327. doi: 10.1186/s13063-018-2728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loutfy M.R., V L.K., Mohammed S., Wu W., Muchenje M., Masinde K., Salam K., Soje L., Gregorovich S., Tharao W. Recruitment of HIV-positive women in research: discussing barriers, facilitators, and research personnel's knowledge. Open AIDS J. 2014;8:58–65. doi: 10.2174/1874613601408010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brintnall-Karabelas J., Sung S., Cadman M.E., Squires C., Whorton K., Pao M. Improving recruitment in clinical trials: why eligible participants decline. J. Empir. Res. Hum. Res. Ethics. 2011;6:69–74. doi: 10.1525/jer.2011.6.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohrtman E.A., Zaninotto A.L., Carvalho S., Shie V.L., Leite J., Ianni C.R., Kazis L.E., Zafonte R., Ryan C.M., Schneider J.C., Fregni F. Longitudinal clinical trial recruitment and retention challenges in the burn population: lessons learned from a trial examining a novel intervention for chronic neuropathic symptoms. J. Burn Care Res. 2019;40:792–795. doi: 10.1093/jbcr/irz084. [DOI] [PubMed] [Google Scholar]
- 54.National Academies of Sciences . In: Virtual Clinical Trials: Challenges and Opportunities: Proceedings of a Workshop. Shore C., Khandekar E., Alper J., editors. National Academies Press (US); Washington (DC): 2019 Jul 23. Engineering, and medicine; health and medicine division; board on health sciences policy; forum on drug discovery, development, and translation.https://www.ncbi.nlm.nih.gov/books/NBK548971/ 2, Opportunities to Improve Clinical Trials. Available from: National Academies Press, 2019. [DOI] [PubMed] [Google Scholar]
- 55.Fisher J.A., Kalbaugh C.A. Challenging assumptions about minority participation in US clinical research. Am. J. Publ. Health. 2011;101:2217–2222. doi: 10.2105/AJPH.2011.300279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamel L.M., Penner L.A., Albrecht T.L., Heath E., Gwede C.K., Eggly S. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control. 2016;23:327–337. doi: 10.1177/107327481602300404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Timmerman L., Stronks D.L., Groeneweg J.G., Huygen F.J. Prevalence and determinants of medication non-adherence in chronic pain patients: a systematic review. Acta Anaesthesiol. Scand. 2016;60:416–431. doi: 10.1111/aas.12697. [DOI] [PubMed] [Google Scholar]
- 58.Tapp C., Milloy M.J., Kerr T., Zhang R., Guillemi S., Hogg R.S., Montaner J., Wood E. Female gender predicts lower access and adherence to antiretroviral therapy in a setting of free healthcare. BMC Infect. Dis. 2011;11:86. doi: 10.1186/1471-2334-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manteuffel M., Williams S., Chen W., Verbrugge R.R., Pittman D.G., Steinkellner A. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J. Wom. Health. 2014;23:112–119. doi: 10.1089/jwh.2012.3972. [DOI] [PubMed] [Google Scholar]
- 60.Mikolasek M., Witt C.M., Barth J. Adherence to a mindfulness and relaxation self-care app for cancer patients: mixed-methods feasibility study. JMIR MHealth and UHealth. 2018;6 doi: 10.2196/11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cyarto E.V., Brown W.J., Marshall A.L. Retention, adherence and compliance: important considerations for home- and group-based resistance training programs for older adults. J. Sci. Med. Sport. 2006;9:402–412. doi: 10.1016/j.jsams.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 62.Wykowski J., Kemp C.G., Velloza J., Rao D., Drain P.K. Associations between anxiety and adherence to antiretroviral medications in low- and middle-income countries: a systematic review and meta-analysis. AIDS Behav. 2019;23:2059–2071. doi: 10.1007/s10461-018-02390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vandenberk T., Lanssens D., Storms V., Thijs I.M., Bamelis L., Grieten L., Gyselaers W., Tang E., Luyten P. Relationship between adherence to remote monitoring and patient characteristics: observational study in women with pregnancy-induced hypertension. JMIR MHealth and UHealth. 2019;7 doi: 10.2196/12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carvalho S., Leite J., Jones F., Morse L.R., Zafonte R., Fregni F. Study adherence in a tDCS longitudinal clinical trial with people with spinal cord injury. Spinal Cord. 2018;56:502–508. doi: 10.1038/s41393-017-0023-5. [DOI] [PubMed] [Google Scholar]
- 65.Bautista L.E., Vera-Cala L.M., Colombo C., Smith P. Symptoms of depression and anxiety and adherence to antihypertensive medication. Am. J. Hypertens. 2012;25:505–511. doi: 10.1038/ajh.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huyser B., Buckelew S.P., Hewett J.E., Johnson J.C. Factor affecting adherence to rehabilitation interventions for individuals with fibromyalgia. Rehabil. Psychol. 1997;42:75–91. doi: 10.1037/0090-5550.42.2.75. [DOI] [Google Scholar]
- 67.Barber B., Dergousoff J., Nesbitt M., Mitchell N., Harris J., O'Connell D., Côté D., Biron V., Seikaly H. Depression as a predictor of postoperative functional performance status (PFPS) and treatment adherence in head and neck cancer patients: a prospective study. J. Otolaryngol. Head Neck Surg. 2015;44 doi: 10.1186/s40463-015-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Oliveira Júnior J.O., Ramos J.V.C. Adherence to fibromyalgia treatment: challenges and impact on the quality of life. Braz. J. Pain. 2019;2:81–87. doi: 10.5935/2595-0118.20190015. [DOI] [Google Scholar]
- 69.Kaleth A.S., Slaven J.E., Ang D.C. Obesity moderates the effects of motivational interviewing treatment outcomes in fibromyalgia. Clin. J. Pain. 2018;34:76–81. doi: 10.1097/AJP.0000000000000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vincent A., Clauw D., Oh T.H., hipple M.O., Toussaint L.L. Decreased physical activity attributable to higher body mass index influences fibromyalgia symptoms. P & M (Philos. Med.) R. 2014;6:802–807. doi: 10.1016/j.pmrj.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 71.Rubin D.B. Inference and missing data. Biometrika. 1976;63:581–592. doi: 10.1093/biomet/63.3.581. [DOI] [Google Scholar]
- 72.Shumaker S.A., Dugan E., Bowen D.J. Controlled Clinical Trials. Elsevier Inc.; 2000. Enhancing adherence in randomized controlled clinical trials; pp. S226–S232. [DOI] [PubMed] [Google Scholar]
- 73.Wilcox S., Shumaker S.A., Bowen D.J., Naughton M.J., Rosal M.C., Ludlam S.E., Dugan E., Hunt J.R., Stevens S. Controlled Clinical Trials. Elsevier; 2001. Promoting adherence and retention to clinical trials in special populations: a Women's Health Initiative workshop; pp. 279–289. [DOI] [PubMed] [Google Scholar]
- 74.Salaffi F., di Carlo M., Farah S., Marotto D., Giorgi V., Sarzi-Puttini P. Exercise therapy in fibromyalgia patients: comparison of a web-based intervention with usual care. Clin. Exp. Rheumatol. 2020;38:S86–S93. https://pubmed.ncbi.nlm.nih.gov/32116212/ [PubMed] [Google Scholar]
- 75.Brietzke A.P., Zortea M., Carvalho F., Sanches P.R.S., Silva D.P.J., Torres I.L. da S., Fregni F., Caumo W. Large treatment effect with extended home-based transcranial direct current stimulation over dorsolateral prefrontal cortex in fibromyalgia: a proof of concept sham-randomized clinical study. J. Pain. 2020;21:212–224. doi: 10.1016/j.jpain.2019.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, FF, upon request.