Abstract
Objective
To develop a minimum data set, known as a core outcome set, for future abortion randomized controlled trials.
Study design
We extracted outcomes from quantitative and qualitative systematic reviews of abortion studies to assess using a modified Delphi method. Via email, we invited researchers, clinicians, patients, and healthcare organization representatives with expertise in abortion to rate the importance of the outcomes on a 9-point Likert scale. After 2 rounds, we used descriptive analyses to determine which outcomes met the predefined consensus criteria. We finalized the core outcome set during a series of consensus development meetings.
Results
We entered 42 outcomes, organized in 15 domains, into the Delphi survey. Two-hundred eighteen of 251 invitees (87%) provided responses (203 complete responses) for round 1 and 118 of 218 (42%) completed round2. Sixteen experts participated in the development meetings. The final outcome set includes 15 outcomes: 10 outcomes apply to all abortion trials (successful abortion, ongoing pregnancy, death, hemorrhage, uterine infection, hospitalization, surgical intervention, pain, gastrointestinal symptoms, and patients’ experience of abortion); 2 outcomes apply to only surgical abortion trials (uterine perforation and cervical injury), one applies only to medical abortion trials (uterine rupture); and 2 apply to trials evaluating abortions with anesthesia (over-sedation/respiratory depression and local anesthetic systemic toxicity).
Conclusion
Using robust consensus science methods we have developed a core outcome set for future abortion research.
Implications
Standardized outcomes in abortion research could decrease heterogeneity among trials and improve the quality of systematic reviews and clinical guidelines. Researchers should select, collect, and report these core outcomes in future abortion trials. Journal editors should advocate for core outcome set reporting.
Keywords: Surgical abortion, Medical abortion, Clinical trials, Core outcomes
1. Introduction
Clinicians rely on research evidence to inform decisions regarding treatment. Ideally, evidence should come from well-designed and methodologically sound clinical trials. However, inconsistencies in research outcomes, even for a single medical topic, can prevent accurate comparisons between trials or systematic evaluation of outcomes across trials [1]. Studies with poorly measured or inconsistently reported outcomes can compromise the quality of systematic reviews and clinical guidelines [2].
In 2010, researchers, health service users, journal editors, and other key stakeholders came together to establish the Core Outcome Measures in Effectiveness Trials (COMET) initiative. Their goal was to facilitate “the development and application of agreed standardized sets of outcomes, known as core outcome sets [3,4].” The aim of a core outcome set is to define a minimum list of outcomes that researchers should report and measure in any clinical trial on a certain subject. COMET also promotes patient/public involvement in outcome set development [3], [4], [5], [6]. If key stakeholders are not involved in the selection of research outcomes, they may lack relevance [2].
Editors of more than 80 reproductive health journals, including Contraception, endorsed the Core Outcomes in Women's Health (CROWN) initiative, which aims to “harmonize outcome reporting in women's health research” [7]. To date, researchers have developed core outcome sets for a variety of reproductive health topics, including the prevention of preterm birth, endometriosis, and preeclampsia [8], [9], [10], [11].
The Standardizing Abortion Research (STAR) outcomes project aims to define a core outcome set for abortion-related research. Abortion is a common experience worldwide, with an estimated 73 million abortions annually [12]. Robust, well-developed clinical trials and guidelines on abortion provide information that can enhance safety, effectiveness, and acceptability of these ubiquitous services. Thus far, researchers have reported on a variety of outcomes in abortion clinical trials, with some efforts to standardize medical abortion effectiveness and surgical abortion outcomes reporting [13,14]. The STAR project is a 3-stage international and interdisciplinary effort to identify the most relevant outcomes for abortion-related research [15]. Here, we describe the results of an international consensus development study to agree on the core outcomes that future abortion trials and systematic reviews should select, collect, and report.
2. Materials and methods
We developed a protocol for the STAR project, with reference to the COMET handbook and protocols describing the development of other core outcomes in reproductive health [16,17]. We registered the protocol prospectively in the COMET database and the CROWN initiative endorsed the project [15]. At the start, we formed an international advisory group with experience and expertise in abortion care, research, and core outcome set development. The group included service providers, researchers, advocates, nongovernmental organizations (NGO) representatives, and methodologists from countries across the range of World Bank income classifications [18]. World Health Organization (WHO) researchers provided overall study management.
Guided by the COMET initiative guidelines [[8], [9], [10],[19], [20], [21]], the STAR project included three stages with all methods described in our published protocol [15]. In brief, during the first stage, we identified a preliminary list of potential core outcomes by performing systematic reviews of randomized clinical abortion trials and qualitative studies on patient experiences with abortion.
We present the second stage, in which we performed a modified Delphi process and held consensus meetings. We described the methods of the second stage in our published protocol with further details here [15]. We aimed to recruit a sample of approximately 200 participants diverse in their sociodemographic characteristics, location, and experience with abortion via the methods outlined in our protocol. To reach abortion patients/representatives, we contacted abortion activists and group networks to share our recruitment invitation and further disseminate it via snowballing. In the survey, we presented the outcomes in lay terms so all participants could understand them. We duplicated most of the outcomes across the domains of surgical and medical abortion. For example, one outcome was “ongoing viable pregnancy after medical abortion” and another was “ongoing viable pregnancy after surgical abortion.” We wanted to assess whether participants viewed outcome importance differently if it was related to medical versus surgical abortion. We used the COMET Delphi Management platform to administer the surveys (Delphi Manager, University of Liverpool, Liverpool, UK), which only allowed survey access online and in English. We sent e-mail reminders to those who registered but did not complete the survey.
Participants scored outcomes on a Likert scale of 1to 9 with 1to 3 as “not important,” 4to 6 as “important but not critical,” and 7to 9 as “critical,” per Grading of Recommendations Assessment, Development and Evaluation guidelines [22]. Participants could skip outcomes, as desired. At the end of round one, participants could contribute additional outcomes. We carried all outcomes from round 1, including the additional ones, into round 2. We invited all registered participants from round 1 to participate in round 2. We reminded them of their round one scores, shared a graph of how each stakeholder group scored the outcomes, and asked them to rescore all the outcomes. At the completion of round 2, we calculated median and interquartile ranges for each outcome and determined if the participants had reached consensus per the COMET definitions of “consensus in” (at least 70% of participants scored an outcome as “critical” and less than 15% scored it as “not important”), “consensus out” (at least 70% of participants scored an outcome as “not important” and less than 15% scored it as “critical”), or “no consensus” (outcomes not meeting the definition of “consensus in” or “no consensus”) [15,17]. After the second round, the study management team determined that participants had achieved sufficient consensus and there was no need for further rounds.
After the Delphi process, we held a series of virtual consultation meetings from Aprilto June 2020 with the study advisory group and the WHO secretariat. We ratified the outcomes that met criteria for “consensus in” and determined which “no consensus” outcomes to retain. If an outcome was important, however not required, the group could designate it as an outcome for “consideration.” We also discussed the semantics of the included outcomes, which were translated back from lay language into technical terms and aimed to align the terminology with existing outcomes literature [13,14]. We assigned gender-neutral terminology to be inclusive of non-binary abortion patients.
We used R (version 3.0.3, 2014) and SAS Studio (20121, Cary, NC) software, with Kruskal-Wallis testing, for comparison of outcome scores across respondent types at a significance level at 5%. The WHO Ethics Review Committee approved the study.
3. Results
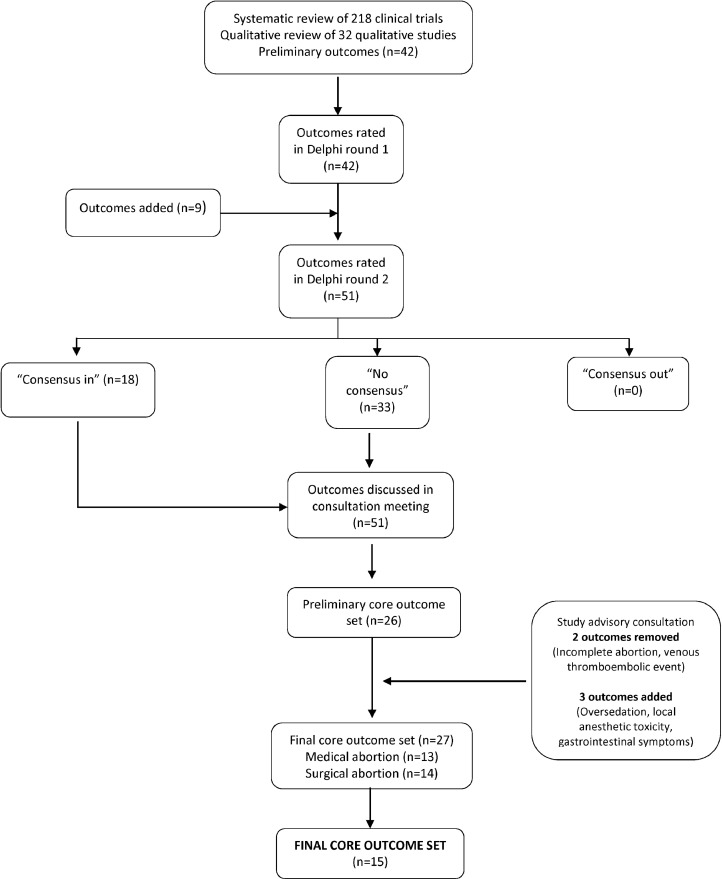
In our literature reviews, we identified 218 abortion clinical trials and 32 qualitative studies, which reported on 177 and 13 outcomes, respectively (Fig. 1). From this initial inventory, we synthesized a preliminary list of 42 outcomes across 15 domains (Appendix A). We entered these domains and outcomes into the first round of the Delphi survey. After round one, we added 9 additional outcomes as suggested by survey respondents. In the second round, respondents evaluated 51 outcomes.
Fig. 1.
Flowchart of identification and selection of core outcomes for abortion trials during the Delphi process and consensus meetings.
Of the 251 invitees who registered to take the surveys, 218 (87%) provided responses of which 203 (81%) completed all questions in round 1. In round 2, 118 of the 218 (42%) participants responded. Table 1 shows the demographic characteristics of the survey and consultation meeting participants. Clinicians were the most represented group in the survey, while the consensus meeting included more researchers. At all stages, most participants identified as white and represented high-income countries in the EURO (Europe) or AMRO (the Americas) regions. The percentage of participants who lived in low and lower-middle income countries ranged from 14 to 18%.
Table 1.
Characteristics of participants in Delphi surveys and consultation meetings conducted to identify core outcomes for abortion trials
| Delphi Surveys |
Consultation meeting (n=16) | ||||
|---|---|---|---|---|---|
| All registered (N=251) | Started round 1 (n=218) | Completed round 1 (n=203) | Completed round 2 (n=118) | ||
| Stakeholder group | |||||
| Clinician | 116 (46) | 101 (46) | 95 (47) | 52 (44) | 5 (31) |
| Researcher | 45 (18) | 40 (18) | 37 (18) | 23 (19) | 8 (50) |
| Patient/representative | 17 (7) | 15 (7) | 14 (7) | 7 (6) | 1 (6) |
| NGO representative | 56 (22) | 49 (22) | 44 (22) | 27 (23) | 0 |
| Other | 17 (7) | 13 (6) | 13 (6) | 9 (8) | 2 (13) |
| Age in years | 43.3 ± 13 | 43.5 ± 13 | 44.1 ± 13 | 43.9 ± 13 | Not available |
| Race | |||||
| Asian | 38 (15) | 35 (16) | 31 (15) | 14 (12) | 3 (19) |
| Black | 27 (11) | 19 (9) | 16 (8) | 13 (11) | 1 (6) |
| Latin American | 13 (5) | 11 (5) | 11 (5) | 8 (7) | 1 (6) |
| Mixed or other | 13 (5) | 6 (3) | 6 (3) | 3 (3) | 5 (31) |
| White | 146 (58) | 136 (62) | 130 (64) | 76 (64) | 5 (31) |
| Prefer not to disclose | 6 (2) | 9 (4) | 7 (3) | 3 (3) | 1 (6) |
| Missing | 8 (3) | 2 (1) | 2 (1) | 1 (1) | 0 |
| Regiona | |||||
| AFRO | 24 (10) | 17 (8) | 12 (6) | 12 (10) | 1 (6) |
| EMRO | 6 (2) | 5 (2) | 5 (2) | 2 (2) | 0 (0) |
| EURO | 47 (19) | 40 (18) | 39 (19) | 18 (15) | 6 (38) |
| AMRO | 134 (53) | 116 (54) | 113 (56) | 67 (57) | 8 (50) |
| SEARO | 13 (5) | 14 (6) | 10 (5) | 6 (5) | 1 (6) |
| WPRO | 27 (11) | 26 (12) | 24 (12) | 13 (11) | 0 |
| Country typeb | |||||
| Low income | 14 (6) | 12 (6) | 10 (5) | 7 (6) | 2 (13) |
| Lower-middle income | 30 (12) | 22 (10) | 18 (9) | 13 (11) | 0 |
| Upper-middle income | 13 (5) | 11 (5) | 9 (4) | 6 (5) | 1 (6) |
| High | 194 (77) | 173 (79) | 166 (82) | 92 (78) | 13 (81) |
All data presented as n (%) or mean ± standard deviation (SD)
WHO regions: AFRO,Africa; AMRO,The Americas EMRO Eastern Mediterranean; EURO, Europe; SEARO (South-East Asia), WPRO (Western Pacific)
According to World Bank classification [18]
Table 2 shows the outcomes that met “consensus in” criteria across all participants. Appendix B lists the outcomes for which there was “no consensus” after round two. No outcomes met criteria for “consensus out.” We show outcome ratings across stakeholder groups in Table 3. Of note, patients/representatives more highly rated “anxiety” (p=0.01) and “sadness” (p=0.002), though even in that group, the average score for these outcomes was below 7. “Ongoing pregnancy” had average scores above 7 in all groups but had higher scores among clinicians and researchers than other groups (p=0.03). “Pain” had mean scores above 7 among patients and NGO representatives but not among other stakeholders (p=0.02).
Table 2.
“Consensus in”a outcomes from all respondent groupsc who participated in the second round (n=118) of a Delphi survey conducted to identify core outcomes for abortion trials
| Core outcome | Participant responses (n=118) |
|---|---|
| Surgical abortion | |
| Damage to internal organs | 116 (98) |
| Death | 115 (97) |
| Potentially life-threatening treatment side effectsb | 115 (97) |
| Ongoing viable pregnancy | 113 (96) |
| All of the pregnancy was passed or removed | 111 (94) |
| Treatments for life threatening side effectsb | 110 (94) |
| Venous thromboembolic eventd | 94 (80) |
| Only a portion of the pregnancy was passed or removed | 93 (79) |
| Cost to participantd | 83 (71) |
| Medical abortion | |
| Potentially life-threatening treatment side effectsb | 117 (99) |
| Death | 115 (97) |
| Treatment for life-threatening side effectsb | 112 (95) |
| All of the pregnancy was passed or removed | 112 (95) |
| Ongoing viable pregnancy | 112 (95) |
| Damage to internal organs | 109 (92) |
| Only a portion of the pregnancy was passed or removed | 97 (83) |
| Venous thromboembolic eventd | 97 (83) |
| Acceptability of experience reported by participant | 86 (73) |
Data are n (%) of participants across all groups who rated the outcome as critical (score 7–9)
“Consensus in” defined as when at least 70% of participants scored an item as 7 to 9 (critical) and less than 15% score it as 1 to 3 (not important)
The term, “side effect,” was used as lay terminology in the survey
Participant groups were: researchers, abortion providers, abortion patients or patient representatives, NGO representatives, and others
Not included in final core outcome set based on consensus discussions
Table 3.
Outcome scoresa that differed across respondent groups in the second round of a Delphi survey conducted to identify core outcomes for abortion trials
| Clinicians | Researchers | Patients/ representatives | NGO representatives | Other | p-valueb | |
|---|---|---|---|---|---|---|
| Anxiety | 5.0 (2.0) | 5.0 (1) | 6.0 (2.0) | 6.0 (2.0) | 6.0 (1.0) | 0.01 |
| Sadness | 5.0 (2.0) | 4.5 (2) | 6.0 (1.0) | 6.0 (1.0) | 6.0 (4.0) | 0.002 |
| Acceptability of surgical abortion experience reported by participant | 7.0 (2.0) | 6.0 (1.0) | 7.0 (1.0) | 8.0 (2.0) | 7.0 (2.0) | 0.05 |
| Return of menstrual cycle | 4.0 (1.0) | 4.0 (1.0) | 4.0 (1.0) | 6.0 (2.0) | 5.0 (2.0) | 0.001 |
| Attendance to postabortion follow-up visits | 4.0 (3.0) | 4.0 (3.0) | 6.0 (2.0) | 5.0 (3.0) | 6.0 (3.0) | 0.008 |
| Ongoing viable pregnancy | 9.0 (0) | 9.0 (0) | 8.0 (2.0) | 9.0 (1.0) | 8.5 (1.5) | 0.03 |
| Pain | 7.0 (2.0) | 6.0 (1.0) | 7.0 (1.0) | 8.0 (2.0) | 6.5 (3.0) | 0.02 |
| Acceptability of medical abortion procedure reported by healthcare provider | 6.0 (3.0) | 5.0 (2.0) | 7.0 (3.0) | 6.5 (4.0) | 6.5 (2.0) | 0.02 |
| Relief/happiness | 6.0 (2.0) | 6.0 (2.0) | 7.0 (2.0) | 6.0 (3.0) | 5.5 (1.5) | 0.04 |
All data presented as median (interquartile range presented as the difference between the 3rd and 1st quartile).
Participants scored outcomes on a scale of 1 to 9 with 1 to 3 as “not important,” 4 to 6 as “important but not critical,” and 7 to 9 as “critical”
Based on Kruskal-Wallis test. This table only shows outcomes for which the Kruskal Wallis test reached statistical significance at a level of 5%.
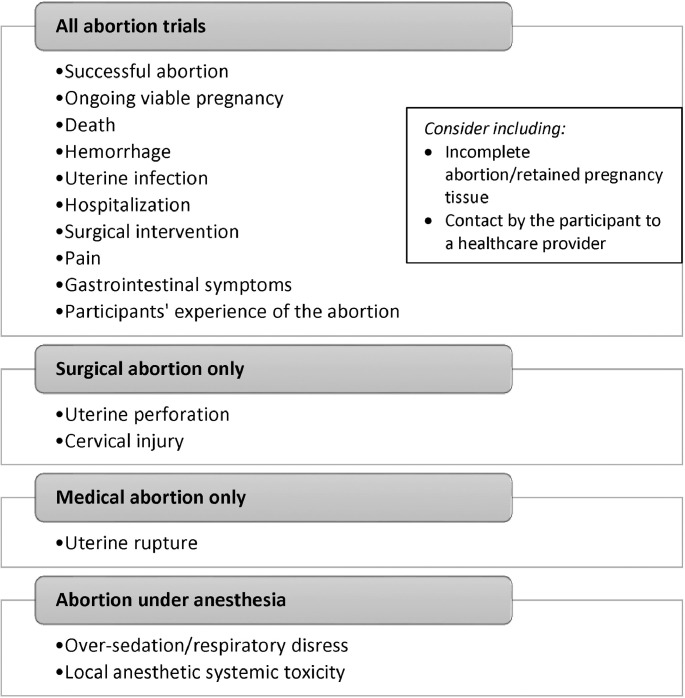
At the consensus meeting, we identified a final list of 13 outcomes for medical abortion and 14 outcomes for surgical abortion. After combining the duplicate outcomes for medical and surgical abortion, we obtained a final list of 15 unique outcomes. While venous thromboembolic event (VTE) met criteria for “consensus in,” the group decided to exclude this outcome in the final set, concluding that VTEs were not related specifically to abortion, but to pregnancy in general. Incomplete abortion met criteria for “consensus in,” however the group concluded that this diagnosis is too vague and not always clinically meaningful. Incomplete abortion was retained as an outcome “for consideration” rather than a requirement. Figure 2 shows the final core outcome set, with 15 required outcomes and 2 outcomes for consideration. Some outcomes apply to all abortions, while others apply only to only medical, surgical, or abortion under anesthesia.
Fig. 2.
Final core outcome set for abortion trials based on the Delphi process and consensus meetings.
4. Discussion
In our project, we used the modified Delphi process and consensus meetings to finalize a core outcome set that future abortion trials, systematic reviews, and clinical guidelines should include. These 15 core outcomes span the domains of abortion success, adverse events, side effects, and personal experience of the abortion. Most of the outcomes are the same for medical and surgical abortion, with the exception of uterine rupture (medical abortion), and uterine perforation and cervical injury (surgical abortion, including for an unsuccessful medical abortion). Two outcomes (over-sedation and toxicity from local anesthesia) only apply to abortions with anesthesia.
To improve consistency and comparability of research studies, researchers should report on relevant core outcomes in future clinical trials on abortion. If core outcomes are not relevant, authors should clarify the reason for not reporting them in the publication. Of course, this minimum list does not constrain researchers from reporting other relevant and interesting outcomes. The expert group included 2 outcomes for consideration, “incomplete abortion/retained pregnancy tissue” and “unanticipated participant contact with a healthcare provider.” The group thought that “incomplete abortion/retained pregnancy tissue” was not always clinically meaningful because this diagnosis is often vague and difficult to distinguish from retained blood clots or the normal spectrum of postabortion recovery. The group felt that “unanticipated participant contact with a healthcare provider” (such as phone calls or outpatient visits) was an important marker of resource utilization for both the healthcare system and the participant but also felt that it might not be feasible for all trials to collect these data. Accordingly, researchers are not instructed to report either of these outcomes or justify their omission.
The systematic review identified significant variations in the outcomes and outcome measures reported by previous abortion trials, a variation that spans across reproductive health [23,24]. When synthesizing the final list of outcomes, we aligned our terminology where possible with other relevant terminologies including the PAIRS framework [14] in the adverse events domain and the MARE guidelines for medical abortion outcomes [13]. We also referred to the Food and Drug Administration, European Medicines Agency, National Health Service, and other international governing bodies’ definitions of serious adverse events when structuring this domain [25], [26], [27].
This study has several strengths. First, we followed the guidelines for developing a core outcome set as dictated by the COMET initiative, including the involvement of patients/representatives [3]. We engaged an international group of participants in the Delphi process and in the study advisory group. The modified Delphi process offers a robust method for reaching consensus without allowing individuals to exert influence on others or dominate a discussion [10]. Conversely, the Delphi process does not allow participants to interact nor present opportunities to clarify misconceptions. In our project, participants included stakeholders across the realm of abortion care from clinicians and researchers to relevant NGO representatives and those with lived experience of abortion. Another strength of our study is that we did not limit the identification of core outcomes to published clinical trials. We also identified outcomes based on a review of qualitative studies focused on participants’ experiences of abortion and drew from patient-centered frameworks in our consensus discussions [28], [29], [30], [31].
One limitation of our study is the high attrition rate (42%) between rounds of the Delphi surveys, possibly due to the slightly more than one-year period between rounds one and two, which may have led to disinterest in participating. This rate, however, fell within the reported range of attrition rates (21%–48%) for other core outcome set development studies in reproductive health [11]. We struggled to reach our goal to recruit equal proportions from each stakeholder group. In particular, we had less representation than desired from abortion patients/representatives (<10% of our population). However because we only allowed survey participants to self-select one stakeholder group, we did not capture those who may have identified with more than 1 group. Reports from other researchers corroborate that it can be challenging to recruit lay people to participate in abortion-related research [32]. We tried to compensate for this underrepresentation by placing a high importance on including a patient-centered outcome in the final outcome set. In addition, we performed a qualitative review on abortion outcomes, which contributed greatly to the entire process and will be reported separately. Despite our efforts to invite participants through a broad network of international societies and organizations, we had low representation by people of color (36%). Per our protocol, we invited all corresponding authors of the abortion trials included in our systematic reviews. Most of these authors lived in Europe or North America, which may have skewed our population. Our consultation group was more racially and ethnically diverse, with 63% identifying as people of color. In the final stage of this project, we will further explore the role of technology, such as mobile applications or social media, and try to forge partnerships with relevant organizations to ensure diversity amongst stakeholders.
While our list advises on the outcomes that researchers should include in future abortion trials, it does not define how to measure them. The Consensus-based Standards for the selection of health Measurement Instruments (COSMIN) initiative has published guidelines on how to select outcome measurement instruments for core outcome set developers [33]. COSMIN describes 4 steps to determining outcome measurement instruments, which include conceptual considerations, review of existing instruments, evaluation of instrument quality, and selection of 1 instrument for each outcome [33]. In the next stage of the STAR project, we will define the measurements for our core outcome set. We intend to build on efforts that are already underway to define the measurement of outcomes in medical abortion [34].
We plan to implement our core outcome set by working with important partners in the field, such as the COMET and CROWN initiatives. We also aim to collaborate with editors of relevant journals to further promote the use of our outcome set. We hope that in time, inclusion, or justification for exclusion, of core outcomes will be required when submitting an abortion clinical trial to a peer-reviewed journal. Researchers should also consider selecting their primary outcome from the core outcome set [35]. We plan to create useful tools, such as generic reporting tables and CONSORT and PRISMA extensions to make it easier for researchers to standardize outcome reporting [20]. These efforts aim to improve the quality of data from abortion trials, systematic reviews and guidelines that use the data from these studies.
Declaration of Competing Interest
Dr. Creinin is a consultant for Danco Laboratories. Dr. Gemzell-Danielsson has received honorariums for giving lectures, participating on international medical advisory boards, or for participation in clinical trials supported by the following organizations: Concept foundation/SunPharma, Exelgyn, HRA Pharma, Mithra, Exeltis, Actavis, Bayer, MSD, Gideon Richter, Natural Cycles, MedinCell, and Cirqle.
Funding
This work was supported by the WHO's UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Sexual and Reproductive Health and Research.
Acknowledgments
The author would like to acknowledge all the participants of the Delphi surveys for the time and input they contributed to this project.
Appendix A. Preliminary list of STAR project outcomes as they appeared to participants of the modified Delphi process
Domain 1: Effectiveness of surgical abortion
Outcome 1: All of the pregnancy was passed or removed
Outcome 2: Only a portion of the pregnancy was passed or removed
Outcome 3: Ongoing viable pregnancy
Outcome 4: Pregnancy is passed before a scheduled abortion
Outcome 5: Time needed to complete abortion (often measured in minutes)
Domain 2: Side effects related to abortion surgery
Outcome 6: Treatment side effects (for example, nausea and vomiting)
Outcome 7: Life threatening treatment side effects (for example, heavy bleeding, allergic reaction, blood infection)
Outcome 8: Blood clots (for example, in the legs and/or lungs)
Outcome 9: Damage to internal organs (for example, poking a hole in the womb)
Domain 3: Treatments for side effects related to abortionsurgery
Outcome 10: Treatments for life threatening side effects related to the abortion surgery (for example, blood transfusion or surgery)
Domain 4: Death from abortion surgery
Outcome 11: Death
Domain 5: Experience of abortion surgery
Outcome 12: Pain
Outcome 13: Anxiety
Outcome 14: Sadness
Outcome 15: Satisfaction with surgical abortion experience reported by woman
Outcome 16: Acceptability of surgical abortion experience reported by woman
Outcome 17: Acceptability of surgical abortion procedure reported by healthcare provider
Domain6: Care after surgical abortion
Outcome 18: Resumption of daily activities (for example, doing daily activities)
Outcome 19: Return of menstrual cycle
Domain 7: Outcomes related to preparing cervix (opening ofwomb) for surgical abortion
Outcome 20: Cervical dilation (The softening and/or opening of the cervix (entrance to the womb)
Domain 8: Utilization of resources associated with surgicalabortion
Outcome 21: Cost to woman
Outcome 22: Cost to healthcare system
Domain 9: Effectiveness of medical abortion (takingmedication to cause abortion)
Outcome 23: All of the pregnancy was passed or removed
Outcome 24: Only a portion of the pregnancy was passed or removed
Outcome 25: Ongoing viable pregnancy
Outcome 26: Time needed to complete abortion (often measured in hours)
Domain 10: Side effects related to medical abortion
Outcome 27: Treatment side effects (for example, nausea and vomiting)
Outcome 28: Life threatening treatment side effects (for example, heavy bleeding, allergic reaction, blood infection)
Outcome 29: Blood clots (for example, in the legs and/or lungs)
Outcome 30: Damage internal organs (for example, rupture of womb)
Domain 11: Treatments for side effects related to medicalabortion
Outcome 31: Treatments for life threatening side effects related to medical abortion (for example, blood transfusion or surgery)
Domain 12: Death from medical abortion
Outcome 32: Death
Domain 13: Experience of medical abortion
Outcome 33: Pain
Outcome 34: Anxiety
Outcome 35: Sadness
Outcome 36: Satisfaction with medical abortion experience reported by woman
Outcome 37: Acceptability of medical abortion experience reported by woman
Outcome 38: Acceptability of medical abortion procedure reported by healthcare provider
Domain14: Care after medical abortion
Outcome 39: Resumption of daily activities (for example, doing daily activities)
Outcome 40: Return of menstrual cycle
Domain 15: Utilization of resources associated with medicalabortion
Outcome 41: Cost to woman
Outcome 42: Cost to healthcare system
Appendix B. Outcomes that were “no consensus” after the second round of a Delphi survey conducted to identify core outcomes for abortion trials
| Surgical abortion (n=17) | Medical abortion (n=16) |
|---|---|
| ■ Time needed to complete abortion | ■ Time needed to complete abortion |
| ■ Treatment side effects | ■ Treatment side effects |
| ■ Pain | ■ Pain |
| ■ Anxiety | ■ Anxiety |
| ■ Sadness | ■ Sadness |
| ■ Relief/happiness | ■ Relief/happiness |
| ■ Satisfaction with surgical abortion experience reported by participant | ■ Satisfaction with medical abortion experience reported by participant |
| ■ Acceptability of surgical abortion procedure reported by healthcare provider | ■ Acceptability of medical abortion procedure reported by healthcare provider |
| ■ Acceptability of surgical abortion procedure as reported by participant's support person/escort | ■ Acceptability of medical abortion procedure as reported by participant's support person/escort |
| ■ Abortion-related violence | ■ Abortion-related violence |
| ■ Resumption of daily activities | ■ Resumption of daily activities |
| ■ Return of menstrual cycle | ■ Return of menstrual cycle |
| ■ Attendance to postabortion follow-up visit | ■ Attendance to postabortion follow-up visit |
| ■ Cost to healthcare system | ■ Cost to healthcare system |
| ■ Cervical dilation | ■ Compliance with medications |
| ■ Pregnancy is passed before a scheduled abortion | ■ Cost to participant |
| ■ Acceptability of surgical abortion experience reported by participant |
References
- 1.Duffy JMN, Bhattacharya S, Herman M, Mol B, Vail A, Wilkinson J, et al. Reducing research waste in benign gynaecology and fertility research. BJOG An Int J Obstet Gynaecol. 2017;124:366–369. doi: 10.1111/1471-0528.14438. [DOI] [PubMed] [Google Scholar]
- 2.Duffy JMN, Ziebland S, von Dadelszen P, McManus RJ. Tackling poorly selected, collected, and reported outcomes in obstetrics and gynecology research. Am J Obstet Gynecol. 2019;220(1) doi: 10.1016/j.ajog.2018.09.023. 71.e1-71.e4. [DOI] [PubMed] [Google Scholar]
- 3.Williamson PR, Altman DG, Blazeby JM, Clarke M, Gargon E. The COMET (Core Outcome Measures in Effectiveness Trials) Initiative. Trials. 2011;12:A70. [Google Scholar]
- 4.Williamson P, Clarke M. The COMET (Core Outcome Measures in Effectiveness Trials) initiative: Its role in improving cochrane reviews. Cochrane Database Syst Rev. 2012;13(5) doi: 10.1002/14651858.ED000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keeley T, Williamson P, Callery P, Jones LL, Mathers J, Jones J, et al. The use of qualitative methods to inform Delphi surveys in core outcome set development. Trials. 2016;7(1):230. doi: 10.1186/s13063-016-1356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffy JMN, Thompson T, Hinton L, Salinas M, McManus RJ, Ziebland S, et al. What outcomes should researchers select, collect and report in pre-eclampsia research? A qualitative study exploring the views of women with lived experience of pre-eclampsia. BJOG An Int J Obstet Gynaecol. 2019;126(5):637–646. doi: 10.1111/1471-0528.15616. [DOI] [PubMed] [Google Scholar]
- 7.CROWN Initiative. Core outcomes in women's and newborn health, http://www.crown-initiative.org/ [accessed April 8, 2021].
- 8.van ’t Hooft J, Duffy JMN, Daly M, Williamson PR, Meher S, Thom E, et al. A core outcome set for evaluation of interventions to prevent preterm birth. Obstet Gynecol. 2016;127(1):49–58. doi: 10.1097/AOG.0000000000001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffy J, Cairns AE, Richards-Doran D, van ’t Hooft J, Gale C, Brown M, et al. A core outcome set for pre-eclampsia research: An international consensus development study. BJOG. 2020;127(12):1516–1526. doi: 10.1111/1471-0528.16319. [DOI] [PubMed] [Google Scholar]
- 10.Duffy JMN, Hirsch M, Vercoe M, Abbott J, Barker C, Collura B, et al. A core outcome set for future endometriosis research: An international consensus development study. BJOG. 2020;127(8):967–974. doi: 10.1111/1471-0528.16157. [DOI] [PubMed] [Google Scholar]
- 11.Duffy JMN, Rolph R, Gale C, Hirsch M, Khan KS, Ziebland S, et al. Core outcome sets in women's and newborn health: A systematic review. BJOG. 2017;124(10):1481–1489. doi: 10.1111/1471-0528.14694. [DOI] [PubMed] [Google Scholar]
- 12.Bearak J, Popinchalk A, Ganatra B, Moller A-B, Tunçalp Ö, Beavin C, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8(9):e1152–e1161. doi: 10.1016/S2214-109X(20)30315-6. [DOI] [PubMed] [Google Scholar]
- 13.Creinin MD, Chen MJ. Medical abortion reporting of efficacy: The MARE guidelines. Contraception. 2016;94:97–103. doi: 10.1016/j.contraception.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Taylor D, Upadhyay UD, Fjerstad M, Battistelli MF, Weitz TA, Paul ME. Standardizing the classification of abortion incidents: The Procedural Abortion Incident Reporting and Surveillance (PAIRS) Framework. Contraception. 2017;96(1):1–13. doi: 10.1016/j.contraception.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Whitehouse KC, Kim CR, Ganatra B, Duffy JMN, Blum J, Brahmi D, et al. Standardizing abortion research outcomes (STAR): A protocol for developing, disseminating and implementing a core outcome set for medical and surgical abortion. Contraception. 2017;95:437–441. doi: 10.1016/j.contraception.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duffy JMN, van ’t Hooft J, Gale C, Brown M, Grobman W, Fitzpatrick R, et al. A protocol for developing, disseminating, and implementing a core outcome set for pre-eclampsia. Pregnancy Hypertens. 2016;6:274–278. doi: 10.1016/j.preghy.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Williamson PR, Altman DG, Bagley H, Barnes KL, Blazeby JM, Brookes ST, et al. The COMET Handbook: Version 1.0. Trials. 2017;18(Suppl 3):280. doi: 10.1186/s13063-017-1978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Bank. World Bank List of Economies 2019. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. [accessed April 8, 2021].
- 19.Duffy JMN, AlAhwany H, Bhattacharya S, Collura B, Curtis C, Evers JLH, et al. Developing a core outcome set for future infertility research: an international consensus development study. Hum Reprod. 2020;35(12):2725–2734. doi: 10.1093/humrep/deaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duffy JMN, Bhattacharya S, Bhattacharya S, Bofill M, Collura B, Curtis C, et al. Standardizing definitions and reporting guidelines for the infertility core outcome set: an international consensus development study. Hum Reprod. 2020;115(1):201–212. doi: 10.1016/j.fertnstert.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Duffy JMN, Cairns AE, Magee LA, von Dadelszen P, van ’t Hooft J, Gale C, et al. Standardising definitions for the pre-eclampsia core outcome set: A consensus development study. Pregnancy Hypertens. 2020;21:208–217. doi: 10.1016/j.preghy.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64(4):395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch M, Duffy JMN, Kusznir JO, Davis CJ, Plana MN, Khan KS. Variation in outcome reporting in endometriosis trials: A systematic review. Am J Obstet Gynecol. 2016;214:452–464. doi: 10.1016/j.ajog.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 24.Duffy JMN, Hirsch M, Kawsar A, Gale C, Pealing L, Plana MN, et al. Outcome reporting across randomized controlled trials evaluating therapeutic interventions for pre-eclampsia. BJOG An Int J Obstet Gynaecol. 2017;124:1829–1839. doi: 10.1111/1471-0528.14702. [DOI] [PubMed] [Google Scholar]
- 25.US Food & Drug Administration. What is a serious adverse event?. https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event [accessed April 8, 2021].
- 26.European Medicines Agency. Serious adverse reaction. https://www.ema.europa.eu/en/glossary/serious-adverse-reaction#:~:text=An adverse reaction that results, or is a birthdefect. [accessed April 8, 2021].
- 27.National Health Service. Classification of adverse events. https://www.nbt.nhs.uk/research-innovation/running-your-study/safety-reporting/classification-adverse-events [accessed April 8, 2021].
- 28.Kingsley C, Patel S. Patient-reported outcome measures and patient-reported experience measures. BJA Educ. 2017;17:137–144. [Google Scholar]
- 29.Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas. 2018;9:353–367. doi: 10.2147/PROM.S156279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darney BG, Powell B, Andersen K, Baum SE, Blanchard K, Gerdts C, et al. Quality of care and abortion: Beyond safety. BMJ Sex Reprod Heal. 2018;44:159–160. doi: 10.1136/bmjsrh-2018-200060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. What is Quality of Care and why is it important?. https://www.who.int/teams/maternal-newborn-child-adolescent-health-and-ageing/quality-of-care [accessed April 8, 2021].
- 32.Dobkin LM, Gould H, Barar RE, Ferrari M, Weiss EI, Foster DG. Implementing a prospective study of women seeking abortion in the United States: Understanding and overcoming barriers to recruitment. Women's. Heal Issues. 2014;24(1):e115–e123. doi: 10.1016/j.whi.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Mokkink LB, Prinsen CAC, Bouter LM, de Vet HCW, Terwee CB. The COnsensus-based standards for the selection of health measurement INstruments (COSMIN) and how to select an outcome measurement instrument. Braz J Phys Ther. 2016;20(2):105–113. doi: 10.1590/bjpt-rbf.2014.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiala C, Cameron S, Bombas T, Parachini M, Agostini A, Lertxundi R, et al. Outcome of first trimester medical termination of pregnancy: Definitions and management. Eur J Contracept Reprod Heal Care. 2018;23(6):451–457. doi: 10.1080/13625187.2018.1535058. [DOI] [PubMed] [Google Scholar]
- 35.Duffy JMN, Hirsch M, Gale C, Pealing L, Kawsar A, Showell M, et al. A systematic review of primary outcomes and outcome measure reporting in randomized trials evaluating treatments for pre-eclampsia. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2017;139:262–267. doi: 10.1002/ijgo.12298. [DOI] [PubMed] [Google Scholar]