Abstract
Introduction
There is, so far, no universal definition of severe asthma. This definition usually relies on: number of exacerbations, inhaled therapy, need for oral corticosteroids, and respiratory function. The use of such parameters varies in the different definitions used. Thus, according to the parameters chosen, each patient may result in having severe asthma or not. The aim of this study was to evaluate how the choice of a specific definition of severe asthma can change the allocation of patients.
Methods
Data collected from the Severe Asthma Network Italy (SANI) registry were analyzed. All the patients included were then reclassified according to the definitions of U-BIOPRED, NICE, WHO, ATS/ERS, GINA, ENFUMOSA, and TENOR.
Results
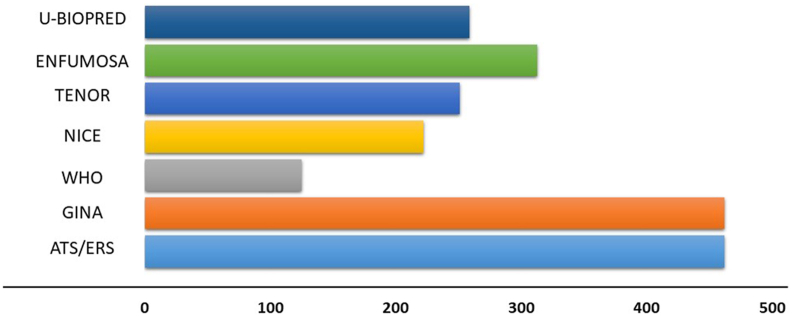
540 patients, were extracted from the SANI database. We observed that 462 (86%) met the ATS/ERS criteria as well as the GINA criteria, 259 (48%) the U-Biopred, 222 (41%) the NICE, 125 (23%) the WHO, 313 (58%) the Enfumosa, and 251 (46%) the TENOR criteria. The mean eosinophil value were similar in the ATS/ERS, U-Biopred, and Enfumosa (528, 532 and 516 cells/mcl), higher in WHO and Tenor (567 and 570 cells/mcl) and much higher in the NICE classification (624 cells/mcl). Lung function tests resulted similarly in all groups, with WHO (67%) and ATS/ERS-GINA (73%), respectively, showing the lower and upper mean FEV1 values.
Conclusions
The present observations clearly evidence the heterogeneity in the distribution of patients when different definitions of severe asthma are used. However, the recent definition of severe asthma, provided by the GINA document, is similar to that indicated in 2014 by ATS/ERS, allowing mirror reclassification of the patients examined. This lack of homogeneity could complicate the access to biological therapies. The definition provided by the GINA document, which reflects what suggested by ATS/ERS, could partially overcome the problem.
Keywords: Severe asthma, Classification, Definition, Biological treatment
Background
Among chronic respiratory diseases, asthma is one of the most common, affecting all age groups. It reaches a prevalence of about 20% in children1 and 5–10% in adulthood.2 Out of these patients, a percentage varying between 5 and 10% suffers from a severe form of the disease, characterized by poor symptom control despite a correctly prescribed maximal inhaled therapy. The severe form of asthma (SA) appears to be a serious burden from the clinical point of view, characterized by a poor quality of life due to frequent exacerbations, extra visits, and hospitalizations. Moreover, even though it affects a small percentage of patients, severe asthma is also an important socio-economic encumbrance, due to the use of most of the economic resources invested in asthmatic subjects.3,4 Traditionally, to achieve control of the disease, these patients must be treated with oral corticosteroids (OCS), given as courses or regularly, with the well-known side effects caused by these drugs (ie, cataract, obesity, osteoporosis, diabetes).5 Furthermore, it is well known that chronic OCS6,7 therapy is burdened by direct and indirect annual costs between 600€ to about 5000€ in severe asthmatic patients.8,9 The recent and more accurate knowledge of the mechanisms underlying asthma10 has allowed the development of new biological drugs able to reduce or interrupt the OCS intake, reduce exacerbations, and improve quality of life, with a satisfactory safety profile.11, 12, 13, 14 So far, these drugs can be prescribed and reimbursed only when a patient is clearly defined as having SA. Therefore, a careful analysis of the clinical-instrumental characteristics is pivotal to define patients as severe or not.
So far, there are several definitions to identify patients as severe, or difficult to control, that must be taken into consideration in daily clinical practice. Analyzing the main definitions of severe/uncontrolled asthma, relevant differences emerge in the inclusion criteria. Thus, using one definition rather than another may or not allow the patient to be considered severe and therefore eligible or not for a biological drug. The objective of this study was to observe how the difference in inclusion criteria, of the main definition of severe/difficult to treat asthma, may be able to exclude patients and if so, in patients who resulted to have severe asthma, were there differences in the main clinical, laboratory or functional parameters.
Methods
The data of patients included in the registry of the Severe Asthma Network Italy (SANI),12 were fully available until January 29, 2019. Those patients were reclassified according to the definition of severe uncontrolled asthma of ATS/ERS (American Thoracic Society/European Respiratory Society) guidelines,13 ENFUMOSA (European Network For Understanding Mechanisms Of Severe Asthma),14 TENOR (The Epidemiology and Natural History, Outcome and Treatment Regimens),15 severe asthma of WHO (World Health Organization)16 and GINA (Global INitiative on Astma),17 difficult asthma of NICE,18 and severe refractory asthma of U-Biopred19 (Table 1).
Table 1.
Principal criteria of severe asthma in guidelines and main clinical trials.
| Exacerbations in previous year | FEV1 | Symptoms control | Therapy | OCS | |
|---|---|---|---|---|---|
| Enfumosa | ≥1 (requiring OCS) | Not required | Not required | ≥1200 μg beclometasone or equivalent | Not required |
| WHO | >2 | FEV1 <60% | Poor | Maximal dose | Daily |
| TENOR | ≥2 (requiring OCS)a | Not required | Not required | ≥3 drugsa | >5 mg prednisone or equivalent |
| ATS-ERS and GINA | ≥2 (requiring OCS) or ≥1 hospitalizationa | FEV1 < 80% FEV1/FVC < LLNa | ACQ >1.5, ACT<20a | ≥1000 μg beclometasone or equivalent | >50% of previous yeara |
| U-Biopred | ≥2 (requiring OCS) | Not required | ACQ >1.5 or equivalent score | ≥1000 μg fluticasone or equivalenta | Daily usea |
| NICE | 1 life threatening or 2 hospitalizationa | FEV1 < 70%a | Not required | ≥1000 μg beclometasone or equivalent | >50% of previous year (>7.5 mg prednisone or equivalent)a |
OCS: oral corticosteroid; FEV1: forced expiratory volume in 1 s.
Refers to the presence of at least one of these
For all patients, the main demographic data, exacerbations and hospitalizations in the previous 12 months, age onset of the disease, main asthma comorbidities (rhinitis, nasal polyposis, reflux disease, bronchiectasis), lung function data (FEV1, FVC), eosinophils blood sampling, asthma control test (ACT)20 score, and daily therapy were available. All these data were used to reclassify them, according to the aforementioned criteria. The main biological and functional characteristics were then analyzed by comparing them between the groups, with the ANOVA test, t-test, and chi-squared when necessary.
All patients had to have a definite diagnosis of bronchial asthma, with the characteristics that make it definable as uncontrolled, according to the criteria adopted by the various researchers of the centres belonging to SANI. To be included in the study, patients had to be recorded in the registry, with informed consent. No exclusion criteria, except missing data, were applied. Each patient met all the criteria to be included into any of the mentioned definitions of severe asthma. About adherence and inhalation technique, we assume that all patients were correctly instructed on the use of their device, and the drug intake was evaluated before entering the patient into the national registry, as required by the registry's own rules.
Results
The data of 702 patients were available in the SANI registry. Out of them, 162, were not complete and were discharged. Thus the analysis was performed on 540 severe asthmatic patients (mean age 55 ± 13.5; range 15–88), with a mean age onset of the disease of 36 ± 16.7 years. The mean exacerbation rate was 3.1 ± 5.7/year and the hospitalization rate was 0.23 ± 0.66. There were 16 (3%) current smokers and 104 (19%) ex-smokers. Concerning the main comorbidities, 255 (47%) had nasal polyposis (treated at least once surgically), 196 (36%) patients had gastro-oesophageal reflux disease, and 96 (18%) had ascertained bronchiectasis. The forced expiratory volume in 1 s (FEV1) mean value before bronchodilation was 75 ± 23% and the median fractional exhaled nitric oxide level (FeNO) was 31 (IQR 17-61) ppb. The control of asthma was evaluated by the asthma control test (ACT) and its mean value was 18 ± 5.
Using the re-classification of patients, according to the various criteria taken into consideration, important numerical differences between the various sets could be observed. Out of the cohort of patients of the SANI registry 462 (85%) fit the ATS/ERS and GINA criteria,13,17 222 (41%) the NICE, 125 (23%) the WHO, 259 (48%) the U-Biopred, 313 (58%) the ENFUMOSA, and 251 (46%) the TENOR criteria (Fig. 1, Table 2).
Fig. 1.
Distribution of the patients of the Severe Asthma Network Italy using the different definitions.
Table 2.
Comparison between groups.
| Group code | Whole sample | ATS/ERS |
NICE |
WHO |
GINA |
U-BIOPRED |
ENFUMOSA |
TENOR |
|
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | |||
| no. patients | 540 | 462 (85%) | 222 (41%) | 125 (23%) | 462 (85%) | 259 (48%) | 313 (58%) | 251 (46%) | |
| Significance for comparison with groups ∗∗ | |||||||||
| Age | 56 (14) | 56 (13) | 56 (12) | 56 (12) | 56 (13) | 56 (14) | 56 (13) | 57 (13) | |
| Significance for comparison with groups ∗∗ | – | – | – | – | – | – | – | ||
| Male | 233 (43%) | 202 (44%) | 103 (46%) | 53 (42%) | 202 (44%) | 103 (40%) | 137 (44%) | 101 (40%) | |
| Significance for comparison with groups ∗∗ | – | – | – | – | – | – | – | ||
| Age onset | 36 (17) | 36 (16) | 37 (16) | 37 (16) | 36 (16) | 37 (17) | 36 (16) | 38 (16) | |
| Significance for comparison with groups ∗∗ | – | – | – | – | – | – | – | ||
| Exacerbations | 3.1 (5.7) | 3.7 (6.1) | 3.6 (3.5) | 4.1 (3.9) | 3.7 (6.1) | 3.5 (3.7) | 4.4 (6.6) | 4.6 (3.4) | |
| Significance for comparison with groups ∗∗ | FG | FG | – | – | AB | AB | |||
| Hospitalizations | 0.23 (0.66) | 0.28 (0.72) | 0.41 (0.94) | 0.31 (0.79) | 0.28 (0.72) | 0.29 (0.83) | 0.32 (0.79) | 0.39 (0.87) | |
| Significance for comparison with groups ∗∗ | – | – | – | – | – | – | – | ||
| Eosinophils (cell/mcl)§ | 370 (140–720) | 350 (140–700) | 380 (120–730) | 400 (140–737) | 350 (140–700) | 370 (140–730) | 390 (160–750) | 400 (127–752) | |
| Significance for comparison with groups ∗∗ | – | – | – | – | – | – | – | ||
| FeNO§ | 31 (17–61) | 31 (17–61) | 32 (20–61) | 32 (20–61) | 31 (17–61) | 29 (16–62) | 31 (16–61) | 34 (20–61) | |
| Significance for comparison with groups ∗∗ | – | – | – | – | – | – | – | ||
| FEV1% | 75 (23) | 73 (21) | 70 (23) | 67 (23) | 73 (21) | 72 (23) | 71 (21) | 71 (22) | |
| Significance for comparison with groups ∗∗ | – | – | – | – | – | – | – | ||
| FEV1 L | 2.06 (0.89) | 2.04 (0.78) | 1.98 (0.82) | 1.87 (0.78) | 2.04 (0.78) | 1.99 (0.78) | 1.99 (0.77) | 1.96 (0.80) | |
| Significance for comparison with groups ∗∗ | – | – | – | – | – | – | – | ||
| ACT | 18 (5) | 17 (5) | 17 (6) | 14 (4) | 17 (5) | 16 (5) | 17 (5) | 16 (5) | |
| Significance for comparison with groups ∗∗ | C | C | ABDEFG | C | C | C | C | ||
| Smokers¥ | 16 (3%) | 15 (3%) | 7 (3%) | 4 (3%) | 15 (3%) | 10 (4%) | 11 (4%) | 9 (4%) | |
| Significance for comparison with groups ∗∗ | – | – | – | – | – | – | – | ||
| Nasal Polyps¥ | 255 (47%) | 227 (49%) | 107 (48%) | 58 (46%) | 227 (49%) | 77 (30%) | 164 (52%) | 135 (54%) | |
| Significance for comparison with groups ∗∗ | E | E | E | E | ABCDFG | E | E |
Value expressed in mean (SD), ¥ expressed in total number (%), ∗∗p < 0.05, § Median (IQR)
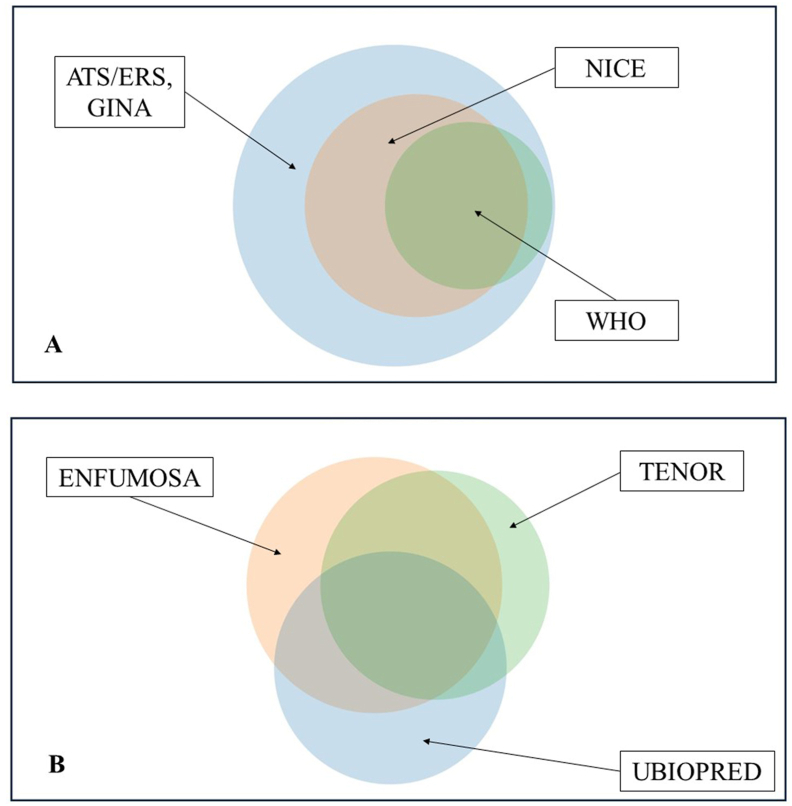
ATS/ERS and GINA criteria enclosed the larger number of patients, followed by the NICE and WHO criteria (Fig. 2a), whose patients remain included in the first definition. Concerning clinical trials, the situation was different, with a percentage of patients around 50% overlapping between all 3 groups, another percentage between 2 distinct groups, and finally a variable part that would be enrolled only in 1 of the trials and not in the other 2 (Fig. 2b).
Fig. 2.
Different distribution in Venn diagram of the Italian SANI patients among guidelines groups (A) and clinical trials definitions (B).
There was no significant difference among the groups of SA patients selected according to the different definitions, apart from the mean number of exacerbations in the last month (higher in patients according to ENFUMOSA or TENOR criteria), the level of asthma control according to ACT (worse in patients selected according to WHO criteria) and the prevalence of nasal polyps (lower in patients selected according to U-BIOPRED criteria) (Table 2).
When patients selected according to ATS/ERS and GINA criteria were compared with the remaining patients (not fulfilling such criteria), this last group included patients with a lower rate of severe exacerbations and hospitalizations, better asthma control according to ACT, lower blood eosinophilia, and greater percentage of current smokers (Table 3), suggesting a different pheno-endotype of SA.
Table 3.
Comparison between patients classifiable or not by ATS/ERS definition.
| Whole sample SANI | ATS/ERS, GINA | NON ATS/ERS | p-value | |
|---|---|---|---|---|
| no. patients | 540 | 462 (85%) | 78 (15%) | n.s. |
| Age | 56 (14) | 56 (13) | 58 (15) | n.s. |
| Age onset | 36 (17) | 36 (16) | 35 (18) | n.s. |
| Exacerbations | 3.1 (5.7) | 3.7 (6.1) | 0.4 (0.7) | <0.05 |
| Hospitalizations | 0.23 (0.66) | 0.28 (0.72) | 0.03 (0.16) | <0.05 |
| Eosinophils (cell/mcl) | 587 (733) | 528 (670) | 390 (400) | <0.05 |
| FeNO | 45 (44) | 48 (47) | 31 (23) | n.s. |
| FEV1% | 75 (23) | 73 (21) | 80 (18) | n.s. |
| FEV1 L | 2.06 (0.89) | 2.04 (0.78) | 2.26 (0.85) | n.s. |
| ACT | 18 (5) | 17 (5) | 23 (2) | <0.05 |
| Smokers¥ | 16 (3%) | 15 (3%) | 17 (22%) | <0.05 |
| Nasal Polyps¥ | 255 (47%) | 227 (49%) | 28 (36%) | n.s. |
| GERD¥ | 196 (36%) | 179 (39%) | 18 (23%) | n.s. |
Value expressed in mean (SD), ¥ expressed in total number (%)
Discussion
The use of a pre-defined specific definition of SA may lead to including or not including each patient into the SA group. We observed in a national Italian database of SA patients that there was a large variability in the allocation of patients, when we re-classified according to different definitions. Currently, we have available several biological drugs limited to SA, which are expensive, and therefore require to be carefully and properly prescribed. Looking at what emerges from the analysis of the observed data, we can see how the GINA definition almost mirrors the ATS/ERS definition; the patients included in the former are in fact numerically and clinically specular. The recent edition of the GINA document takes the ATS/ERS definition of SA as a guidance and makes it its own, thus describing the patient affected by this form of asthma as one who needs a maximum dose of inhalation therapy, but despite this remains symptomatic (referring to what is suggested by ATS/ERS as criteria of poor symptom control). A crucial point in the definition of severe asthma is the adherence to therapy. In fact, the GINA document places great importance on this point, emphasizing the pivotal role of adherence to treatment, without which the patient cannot be considered as having severe asthma.
Despite the large difference in the proportion of patients selected according to the different definitions of severe asthma, the main values of the relevant evaluated parameters are similar. For instance, age and age of onset, FEV1, eosinophils, and FeNO, were similar among the groups. Several differences were detected in exacerbation rate, that was higher in patients defined with ENFUMOSA and TENOR criteria (4.4 ± 6.6 and 4.6 ± 3.4), compared with the Italian sample (3.1 ± 5.7). By dividing the definitions into those of guidelines (AST/ERS, GINA, WHO and NICE) and those derived by criteria used in trials (ENFUMOSA, TENOR, U-BIOPRED) it was observed that guidelines, with their different inclusion criteria, lead to a numerical difference in patients defined as severe asthmatics (Table 2). In particular, the difference in the number of exacerbations, varying from a number ≥ to 1 up to > of 2 (at least 3), but also the inhaled steroid dose necessary to define the patient as severe asthmatic, ranging from “medium-high” of step 4 GINA (as indicated in ATS/ERS), to ≥ 1200 mcg required in the ENFUMOSA study, seems to be a relevant factor. Another discriminating factor concerns the respiratory function, whose limit values vary from an FEV1 ≤ 80% to a cut-off of 60%. A less relevant difference was observed using the definition from clinical trials. Nevertheless, observing the characteristics of the groups it appears that, even in this case, although there is a different distribution, mainly between the definitions of severe asthma guidelines, the clinical/functional characteristics are similar. The major difference is observed in the group with the U-BIOPRED characteristics, where nasal polyposis is much lower than in all the other groups (Table 2). This may be relevant because, although nasal polyposis is an extra-pulmonary disease, it is often present in patients with severe asthma both in clinical trials21 and real life.22 Furthermore, nasal polyposis is not only a frequent comorbidity in severe asthmatic patients, but it seems to be associated with a phenotype in which patients are more severe than those who are not affected by this comorbidity.23
A large proportion of the severe asthma patients enrolled in the SANI registry fulfilled the ATS/ERS and GINA definitions. This could be expected for 2 reasons. First, there where strictly recommended definitions used for enrolling patients in the SANI register. Second, ATS/ERS and GINA definitions are the more comprehensive definition of SA, because they include both the level of current treatment (step 4-5 GINA) and at least 1 among different clinical and functional features (symptom control, exacerbations, hospitalization or reduced pulmonary function).
At this point, there are 2 aspects to be addressed: the of adherence to therapy and the correct disease diagnosis. As discussed above, according to guidelines, the patient cannot be considered as severe if adherence to the prescribed therapy is not confirmed. This point is still controversial, since there are currently few practical tools to be used on a large scale to objectively assess the amount of drugs taken. The most used methodology, the anamnestic report, is simple to do, but heavily burdened by poor objectivity. In recent years, different methods have been proposed, such as electronic devices to be coupled to inhalers to monitor and quantify the drug intake.24,25 The problem of adherence has also been successfully addressed by enhancing the awareness of patients, through functional health education.26 The aspect of the correct diagnosis is propaedeutic to the definition of SA. Indeed, a detailed differential diagnosis in patients who do not respond to maximal inhalation therapy is mandatory. The diagnosis of asthma must be confirmed before labelling a patient as severe asthmatic, as underlined by all international documents. However, there is no universally accepted algorithm for the differential diagnosis, and this remains an open question.
As an additional note, the present data showed that about 15% of patients of the SANI registry did not fit the ERS/ATS criteria. These patients had better symptom control, a lower rate of exacerbations and hospitalizations, and a lower blood eosinophilia in comparison with patients fulfilling the ATS/ERS classification. These seem to belong to a different pheno-endotype of asthma, whose severity is lower responding to less stringent criteria. Thus, a more accurate inclusion/selection of patients is needed, expecially in large registries.
In conclusion, this analysis confirms a certain heterogeneity in the definition of patients with severe asthma, depending on the chosen guideline and classifications. Although there is no relevant difference in the characteristics of asthmatic patients who are defined as severe, there are relevant differences in numbers, which could preclude patients to the access to biological drugs. The latest indications of the GINA document, following what has already been suggested in ATS/ERS, mark a path for a more univocal definition of disease. Finally appropriate protocols on what to do as basic standard investigation for differential diagnosis is desirable.
Abbreviations
ACT: Asthma Control Test, FeNO: fractional exhaled nitric oxide level, FEV1: Forced Expiratory Volume in the first second, FVC: Forced Vital Capacity, OCS: Oral Corticosteroids, SANI: Severe Asthma Network Italy, ERS/ATS: European Respiratory Society/American Thoracic Society, GINA: Global INitiative on Asthma, SA: Severe Asthma.
Funding
No funding has been received for this manuscript.
Authors’ consent for publication
All authors agree to publish this manuscript.
Contributor's statement
DB and GP have designed, written the work and analysed the data. ET, CF help to collect and analyse data. PLP and ML reviewed and correct manuscript. All other authors provided the data and suggested how to proceed with the processing of the manuscript.
Availability of data and materials
Partially available on request.
Ethics approval
All patients signed informed consent.
Declaration of competing interest
None to declare.
Acknowledgements
SANI (Severe Asthma Network Italy) for data.
Footnotes
Full list of author information is available at the end of the article
Contributor Information
Diego Bagnasco, Email: diego.bagnasco@dimi.unige.it.
on behalf of SANI (Severe Asthma Network Italy):
Gabriella Guarnieri, Vincenzo Patella, Foschino Barbaro Maria Pia, Giovanna Elisiana Carpagnano, Anna del Colle, Giulia Scioscia, Pelaia Gerolamo, Francesca Puggioni, Francesca Racca, Elisabetta Favero, Sandra Iannacone, Eleonora Savi, Marcello Montagni, Gianna Camiciottoli, Chiara Allegrini, Carlo Lombardi, Giuseppe Spadaro, Caterina Detoraki, Francesco Menzella, Carla Galeone, Patrizia Ruggiero, Monna Rita Yacoub, Alvise Berti, Nicola Scichilone, Carmen Durante, Maria Teresa Costantino, Chiara Roncallo, Mariachiara Braschi, Alice D’Adda, Erminia Ridolo, Massimo Triggiani, Roberta Parente, D’Amato Maria, Maria Vittoria Verrillo, Giovanni Rolla, Luisa Brussino, Agata Valentina Frazzetto, Zappa Maria Cristina, Marianna Lilli, Nunzio Crimi, Marco Bonavia, Angelo Guido Corsico, Amelia Grosso, Stefano Del Giacco, Margherita Deidda, Luisa Ricciardi, Stefania Isola, Francesca Cicero, Giuliana Amato, Federica Vita, Antonio Spanevello, Patrizia Pignatti, Francesca Cherubino, Dina Visca, Fabio Luigi Massimo Ricciardolo, Vitina Maria Anna Carriero, Francesca Bertolini, Pierachille Santus, Roberta Barlassina, Andrea Airoldi, Giuseppe Guida, Nucera Eleonora, Arianna Aruanno, Angela Rizzi, Cristiano Caruso, Stefania Colantuono, Gianenrico Senna, Marco Caminati, Alessandra Arcolaci, Andrea Vianello, Fulvia Chieco Bianchi, Maria Rita Marchi, Stefano Centanni, Simone Luraschi, Silvia Ruggeri, Rocco Rinaldo, Elena Parazzini, Cecilia Calabrese, Martina Flora, Lorenzo Cosmi, Linda Di Pietro, Enrico Maggi, Laura Pini, Luigi Macchia, Danilo Di Bona, Luca Richeldi, Carola Condoluci, Leonello Fuso, Matteo Bonini, Alessandro Farsi, Giulia Carli, Paolo Montuschi, Giuseppe Santini, Maria Elisabetta Conte, Elisa Turchet, Carlo Barbetta, Francesco Mazza, Simona D’Alo, Stefano Pucci, Maria Filomena Caiaffa, Elena Minenna, Luciana D'Elia, Carlo Pasculli, Vittorio Viviano, Paolo Tarsia, Joyce Rolo, Mariacarmela Di Proietto, and Salvatore Lo Cicero
References
- 1.Lai C.K., Beasley R., Crane J., et al. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC) Thorax. 2009;64(6):476–483. doi: 10.1136/thx.2008.106609. [DOI] [PubMed] [Google Scholar]
- 2.Sears M.R. Trends in the prevalence of asthma. Chest. 2014;145(2):219–225. doi: 10.1378/chest.13-2059. [DOI] [PubMed] [Google Scholar]
- 3.Lang D.M. Severe asthma: epidemiology, burden of illness, and heterogeneity. Allergy Asthma Proc. 2015;36(6):418–424. doi: 10.2500/aap.2015.36.3908. [DOI] [PubMed] [Google Scholar]
- 4.Bagnasco D., Povero M., Pradelli L., et al. Economic impact of mepolizumab in uncontrolled severe eosinophilic asthma, in real life. World Allergy Organ J. 2021 Jan 27;14(2):100509. doi: 10.1016/j.waojou.2021.100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heffler E., Bagnasco D., Canonica G.W. Strategies to reduce corticosteroid-related adverse events in asthma. Curr Opin Allergy Clin Immunol. 2019 Feb;19(1):61–67. doi: 10.1097/ACI.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 6.Sweeney J., Patterson C.C., Menzies-Gow A., et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the optimum patient care research database and the British thoracic difficult asthma registry. Thorax. 2016;71(4):339–346. doi: 10.1136/thoraxjnl-2015-207630. [DOI] [PubMed] [Google Scholar]
- 7.Lefebvre P., Duh M.S., Lafeuille M.H., et al. Burden of systemic glucocorticoid-related complications in severe asthma. Curr Med Res Opin. 2017;33(1):57–65. doi: 10.1080/03007995.2016.1233101. [DOI] [PubMed] [Google Scholar]
- 8.Barry L.E., Sweeney J., O'Neill C., et al. The cost of systemic corticosteroid-induced morbidity in severe asthma: a health economic analysis. Respir Res. 2017;18:129. doi: 10.1186/s12931-017-0614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice J.B., White A.G., Scarpati L.M., Wan G., Nelson W.W. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Therapeut. 2017;39(11):2216–222910. doi: 10.1016/j.clinthera.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Caminati M., Pham D.L., Bagnasco D., Canonica G.W. Type 2 immunity in asthma. World Allergy Organ J. 2018 Jun 26;11(1):13. doi: 10.1186/s40413-018-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busse W.W. Biological treatments for severe asthma. Curr Opin Allergy Clin Immunol. 2018;18(6):509–518. doi: 10.1097/ACI.0000000000000487. [DOI] [PubMed] [Google Scholar]
- 12.Heffler E., Blasi F., Latorre M., et al. T he severe asthma network in Italy: findings and perspectives. J Allergy Clin Immunol Pract. 2019 May-Jun;7(5):1462–1468. doi: 10.1016/j.jaip.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Chung K.F., Wenzel S.E., Brozek J.L., et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 14.Abraham B., Antó J.M., Barreiro E., et al. The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. Eur Respir J. 2003 Sep;22(3):470–477. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- 15.Chipps B.E., Zeiger R.S., Borish L., et al. Key findings and clinical implications from the epidemiology and natural history of asthma: outcomes and treatment Regimens (TENOR) study. J Allergy Clin Immunol. 2012;130(2):332–342. doi: 10.1016/j.jaci.2012.04.014. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bush A., Zar H.J. WHO universal definition of severe asthma. Curr Opin Allergy Clin Immunol. 2011;11(2):115–121. doi: 10.1097/ACI.0b013e32834487ae. [DOI] [PubMed] [Google Scholar]
- 17.GINA Severe asthma definition. https://ginasthma.org/gina-reports/. Last access 05.08.2021.
- 18.Quality standard of asthma, NICE. https://www.nice.org.uk/guidance/qs25/resources/asthma-pdf-2098547456965. Last access 15.11.2021.
- 19.Shaw D.E., Sousa A.R., Fowler S.J., et al. 2015. Clinical and Inflammatory Characteristics of the European U-BIOPRED Adult Severe Asthma Cohort; pp. 1308–1321. [DOI] [PubMed] [Google Scholar]
- 20.Schatz M., Sorkness C.A., Li J.T., et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117(3):549–556. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Langdon C., Mullol J. Nasal polyps in patients with asthma: prevalence, impact, and management challenges. J Asthma Allergy. 2016;9:45–53. doi: 10.2147/JAA.S86251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagnasco D., Milanese M., Rolla G., et al. The North-Western Italian experience with anti IL-5 therapy amd comparison with regulatory trials. World Allergy Organ J. 2018;11(1):34. doi: 10.1186/s40413-018-0210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castillo Vizuete J.A., Sastre J., Del Cuvillo Bernal A., et al. Asthma, rhinitis, and nasal polyp multimorbidities. Arch Bronconeumol (Engl Ed) 2019 Mar;55(3):146–155. doi: 10.1016/j.arbres.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Moore A., Preece A., Sharma R., et al. A randomised controlled trial of the effect of a connected inhaler system on medication adherence in uncontrolled asthmatic patients. Eur Respir J. 2021 Jun 4;57(6):2003103. doi: 10.1183/13993003.03103-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braido F., Paa F., Ponti L., Canonica G.W. A new tool for inhalers’ use and adherence monitoring: the Amiko® validation trial. Int J Eng Res Sci. 2016;2:159–166. [Google Scholar]
- 26.Haahtela T., Tuomisto L.E., Pietinalho A., et al. A 10 year asthma programme in Finland: major change for the better. Thorax. 2006 Aug 1;61(8):663–670. doi: 10.1136/thx.2005.055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Partially available on request.