Abstract
Background
There is increasing interest in persistent interstitial lung disease (ILD) following resolution of acute COVID-19. No studies have yet reported findings in surgical lung biopsies (SLB) from this patient population.
Methods
Our Michigan Medicine pathology database was queried for SLB reviewed between January 2020 and April 2021 from patients with persistent ILD following recovery from acute COVID-19. Slides for our retrospective observational study were independently reviewed by two thoracic pathologists, who were blinded to patient clinical data, radiographic findings, and previous pathologic diagnosis.
Findings
Eighteen cases met inclusion criteria. Of these, nine had usual interstitial pneumonia (UIP). These included two patients with superimposed acute lung injury (ALI). Five cases showed a spectrum of ALI that ranged from persistent diffuse alveolar damage to organizing pneumonia. Four patients had desquamative interstitial pneumonia (1), acute and organizing bronchopneumonia (1), or no diagnostic abnormality (2). Compared to patients without UIP, those with UIP tended to be older and have pre-existing lung disease prior to COVID-19. In patients with UIP, pre-SLB chest computed tomography changes included groundglass with interstitial thickening or peripheral reticulations with bronchiectasis; no UIP patients had groundglass only. The most common radiographic finding in patients without UIP was groundglass opacities only.
Interpretation
UIP was the most common pathologic finding in patients undergoing evaluation for post-COVID-19 ILD. Our preliminary data suggests that CT changes described as interstitial thickening, peripheral reticulations, and/or bronchiectasis may be helpful in identifying patients with underlying fibrotic chronic interstitial pneumonia for which UIP is the chief concern.
Funding
No intramural or extramural funding sources supported this work.
Keywords: coronavirus, interstitial lung disease, lung pathology
Research in context.
Evidence before this study
We searched the published peer reviewed literature for studies describing histopathologic findings in patients with persistent lung disease following acute coronavirus disease 2019 (COVID-19). These works mainly comprise autopsy studies that have focused on the fibrotic phase of diffuse alveolar damage in patients with prolonged hospitalizations for fatal COVID-19 pneumonia and described a pattern of fibrosis resembling nonspecific interstitial pneumonia (NSIP). Lung pathology findings in living patients with a history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are fewer and largely limited to lung transplants specimens that similarly showed NSIP-like fibrosis, sometimes accompanied by histologic evidence of persistent acute lung injury (ALI).
Added value of this study
Our study focuses on surgical lung biopsies (SLB) from patients with persistent evidence of interstitial lung disease (ILD) following recovery from COVID-19. Usual interstitial pneumonia (UIP) was the most frequently encountered histologic finding in our retrospective cohort. A minority of patients without UIP had histologic evidence of persistent ALI.
Implications of all the available evidence
Taken together with previous observations of histopathological findings in selected patients with persistent ILD following COVID-19, SLB in our cohort indicate that only a minority have persistent ALI attributable to SAR-CoV-2 infection. Our preliminary study also suggests that many patients with persistent ILD have diffuse fibrotic lung disease that likely precedes SARS-CoV-2 infection.
Alt-text: Unlabelled box
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic sparked a surge in scientific publications that reflected our scramble to understand, treat, and prevent this devastating, global illness. One and a half years later, the lasting impact to survivors of COVID-19 accounts for much of the second phase of scientific investigation. The earliest descriptions of lung pathologic findings in individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detailed acute and organizing diffuse alveolar damage (DAD) in post-mortem tissue samples from patients who died of severe disease.[1], [2], [3], [4], [5] More recently, autopsy studies have focused on the fibrotic phase of DAD in patients with fatal COVID-19 pneumonia and prolonged hospitalizations prior to death [6,7]. Consistent with earlier studies that described nonspecific interstitial pneumonia (NSIP) as the fibrotic sequela of DAD in a subset of patients [8,9], these COVID-19 studies describe DAD with a pattern of fibrosis resembling NSIP.
Lung pathology findings in living patients with a history of SARS-CoV-2 infection are largely limited to lung transplants in patients suffering from severe COVID-19-related acute respiratory failure. Like the COVID-19 autopsy studies, lung explant specimens have been described as showing NSIP-like fibrosis, sometimes accompanied by histologic evidence of persistent acute lung injury (ALI) [10], [11], [12].
As both hospitalized and non-hospitalized individuals recover from the acute phase of SARS-CoV-2 infection, there is increasing interest in understanding long-term management and outcomes in those with persistent respiratory symptoms or radiographic abnormalities. Current studies have shown that some patients with severe COVID-19 pneumonia develop a persistent restrictive ventilatory defect with reduced diffusion capacity for carbon monoxide; however, this is not true of all patients, particularly those with mild or moderately severe disease [13], [14], [15], [16]. Descriptions of chest computed tomography (CT) changes in these patients include mainly groundglass opacities and/or lower lobe-predominant reticulations [14,16,17]. However, the true incidence of pulmonary fibrosis and corresponding pathologic findings in these patients remains unknown.
To date, there have been no descriptions of lung biopsy findings in living patients with ongoing, post-COVID-19 respiratory symptoms and/or radiographic findings consistent with interstitial lung disease (ILD). Our objective is to report the histologic findings in surgical lung biopsies (SLB) from patients with persistent ILD following resolution of acute SARS-CoV-2 infection and assess the clinical and radiographic data in the context of the pathologic diagnoses.
2. Methods
2.1. Study design
This investigation was a retrospective observational study, using archived specimen materials and records. The study was approved by the University of Michigan's Institutional Review Board (Identifier: HUM00184538). The institutional review board did not require written informed patient consent for this study.
2.2. Case selection
Michigan Medicine Laboratories provides expert extramural consultation services for difficult pathology cases that cover a diverse range of subspecialties, including neoplastic and non-neoplastic problems in thoracic pathology. Our pathology database was queried for lung biopsies received for consultation between January 1, 2020, and April 30, 2021, from patients diagnosed with COVID-19. Patients were included if a SLB was performed for evaluation of ongoing respiratory symptoms and/or persistent radiographic findings consistent with ILD, following recovery from the acute phase of COVID-19. Patients presenting with clinical acute respiratory failure or pneumothorax were excluded. Clinical data was extracted from materials provided with the request for consultation. Chest CT findings were extracted from submitted radiology reports; no imaging studies were available for central review. Exclusion criteria included biopsy procedures other than SLBs, indications for biopsy other than persistent respiratory symptoms and/or diffuse radiological abnormalities after recovery from acute COVID-19 and slides unavailable for review. Slides were requested from referring institutions for cases in which they had already been returned. Five patients who met all other inclusion criteria were missing at random because slides were not returned for re-review.
2.3. Pathologic assessment
Between January 1, 2020, and April 30, 2021, eighteen (18) cases received by our thoracic pathology consultation service met all inclusion criteria. All slides from each of these 18 cases were retrospectively reviewed independently by two fellowship-trained thoracic pathologists who have subspecialty expertise in lung diseases with 7 and 36 years of experience (K.E.K. and J.L.M., respectively). Both reviewing pathologists were blinded to patient clinical data, radiographic findings, and original pathologic diagnoses as well as the diagnosis made at the time of consultation. Histologic diagnoses were rendered in all cases using, when possible, terminology proposed in the 2013 international consensus classification for the idiopathic interstitial pneumonias [18] and the most recent practice guidelines for diagnosis of idiopathic pulmonary fibrosis [19]. Other pathologic findings were also noted. Cases in which there was diagnostic variance between the two reviewers were resolved by consensus.
2.4. Statistical analysis
Continuous variables were reported as the median and interquartile range. Categorical variables were reported as counts and percentages. The distributions of continuous and categorical variables among patients with usual interstitial pneumonia (UIP) were compared to patients without UIP using the Wilcoxon rank-sum test and Fisher exact test, respectively. All statistical analyses were performed using R version 4.1 [20]. P values < 0·05 were considered statistically significant.
2.5. Role of the funding source
No intramural or extramural funding sources supported this work. All authors had full access to the data in the study and share responsibility for the decision to submit for publication.
3. Results
3.1. Patient Characteristics
During the study time period, 38 lung biopsies from patients reported to have a history of SARS-CoV-2 infection were received for consultation. Four transbronchial biopsies and three core needle biopsies were excluded from further study. Seven cases were excluded due to indication for SLB: pneumothorax in four; COVID-19 acute respiratory failure in three; unknown in one. Slides were not available for review in five cases.
The study cohort comprised 18 cases, including ten males and eight females, ages 26 to 76 years (median 56 years). One patient was a current smoker, seven were former smokers, and seven reported as never smokers. Smoking status was not known for three patients. The most common pre-existing underlying medical condition indicated in the available clinical records was hypertension followed by pulmonary disease for which detailed information was frequently lacking. Five patients were reported to have lung disease prior to their COVID-19 diagnosis, including four patients with unexplained chronic respiratory failure or dyspnea and one with chronic obstructive pulmonary disease (COPD). One patient with chronic dyspnea also carried an additional diagnosis of asthma. Two were reported to have underlying rheumatoid arthritis. For two patients, no past medical history was provided. Patient characteristics are detailed in Table 1.
TABLE 1.
Patient Characteristics (n = 18)
| Age, years (median, interquartile range) | 56 (18) |
|---|---|
| Sex | |
| Male | 10 (56%) |
| Female | 8 (44%) |
| Smoker statusa | |
| Current smoker | 1 (6%) |
| Former smoker | 7 (39%) |
| Never smoker | 7 (39%) |
| Pre-existing medical conditionsb | |
| Hypertension | 8 (44%) |
| Pulmonary disease | 5 (28%) |
| Cardiac disease | 3 (17%) |
| Cerebrovascular disease | 3 (17%) |
| Connective tissue disease | 2 (11%) |
| Diabetes | 2 (11%) |
| Obesity | 2 (11%) |
| Chronic kidney disease | 1 (6%) |
Smoking status was unknown for 3 patients.
Medical history was unknown for 2 patients.
The earliest COVID-19 diagnosis was March 2020. The duration of time from COVID-19 diagnosis to biopsy ranged from 2 to 12 months (average 145 days). Eight patients were hospitalized with acute COVID-19 pneumonia, seven patients were not hospitalized, and for three patients, hospitalization for COVID-19 was unknown. Of the eight patients hospitalized for COVID-19 pneumonia, three did not require intubation or mechanical ventilation; details regarding the hospital course for the remaining five patients were unavailable.
Prior to lung biopsy, post-COVID-19 chest CT reports described persistent groundglass opacities as the only finding in seven patients; seven patients had groundglass opacities with interstitial thickening; four had peripheral reticulations with bronchiectasis (Table 2). Along with these radiographic changes, indications for lung biopsy included persistent or worsening dyspnea, cough, and/or hypoxia in all but two patients, who were being evaluated for persistent CT changes. One patient was undergoing evaluation for lung transplant.
TABLE 2.
Post-COVID-19, Pre-Lung Biopsy Chest CT Findings
| Groundglass opacities only | 7 (39%) |
| Groundglass opacities with interstitial thickening | 7 (39%) |
| Peripheral reticulations with bronchiectasis | 4 (22%) |
COVID-19 = coronavirus disease 2019; CT = computed tomography
3.2. Pathologic Findings
Fifteen cases had multiple sites biopsied. The number of submitted sections per case ranged from 2 to 11 (average 7). Three cases were single-site biopsies with two to six sections submitted per case (average four). The pathologic findings are summarized in Table 3.
TABLE 3.
Pathologic Findings
| Usual interstitial pneumonia | n = 9 |
|---|---|
| Definite UIP | 5 |
| Probable UIP | 1 |
| Definite UIP with superimposed ALI | 2 |
| Indeterminate for UIPa | 1 |
| Acute lung injury | 5 |
|---|---|
| Persistent DAD or organizing ALI with fibrosis | 3 |
| Chronic bronchiolitis with organizing pneumonia | 2 |
| Other | 4 |
|---|---|
| Desquamative interstitial pneumonia | 1 |
| Acute and organizing bronchopneumonia | 1 |
| Mild nonspecific abnormalities of uncertain significance | 2 |
ALI = acute lung injury; DAD = diffuse alveolar damage; UIP = usual interstitial pneumonia
Indeterminate for UIP was the consensus diagnosis for a case classified as definite UIP by one reviewer and unclassifiable chronic fibrosing interstitial pneumonia by the other who included UIP and other patterns of fibrotic lung disease in the differential diagnosis.
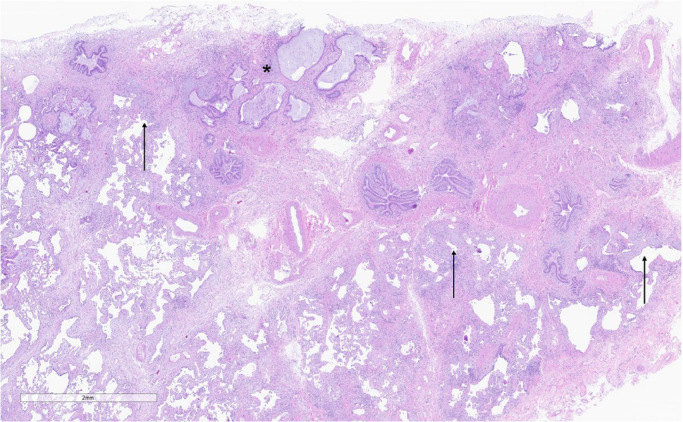
There was complete inter-rater agreement for the final diagnosis in 17 of 18 cases. The single exception was a case showing a fibrotic chronic interstitial pneumonia with prominent peribronchiolar metaplasia, cicatricial organizing pneumonia [21], extensive “NSIP-like” changes, and honeycomb change (Figure 1). One pathologist diagnosed UIP (“UIP with patchy cicatricial OP and prominent PBM”), while the other considered the findings representative of UIP or post-COVID-19-related fibrosis resembling NSIP (“Unclassifiable fibrosing chronic interstitial pneumonia w/ extensive PBM and cicatricial organizing pneumonia”). The diagnostic variance for this case was resolved by consensus resulting in a diagnosis of indeterminate for UIP [19].
Figure 3.
After consensus review, 1 case was classified as indeterminate for usual interstitial pneumonia (UIP). While the presence of tissue-destructive scarring and microscopic honeycomb change were consistent with UIP (3a), this case also showed nonspecific interstitial pneumonia-like changes and extensive peribronchiolar metaplasia (3b). There was also cicatricial organizing pneumonia (3c; arrows). Hematoxylin and eosin-stained slides; magnifications 36x (3a), 66x (3b), and 49x (3c).
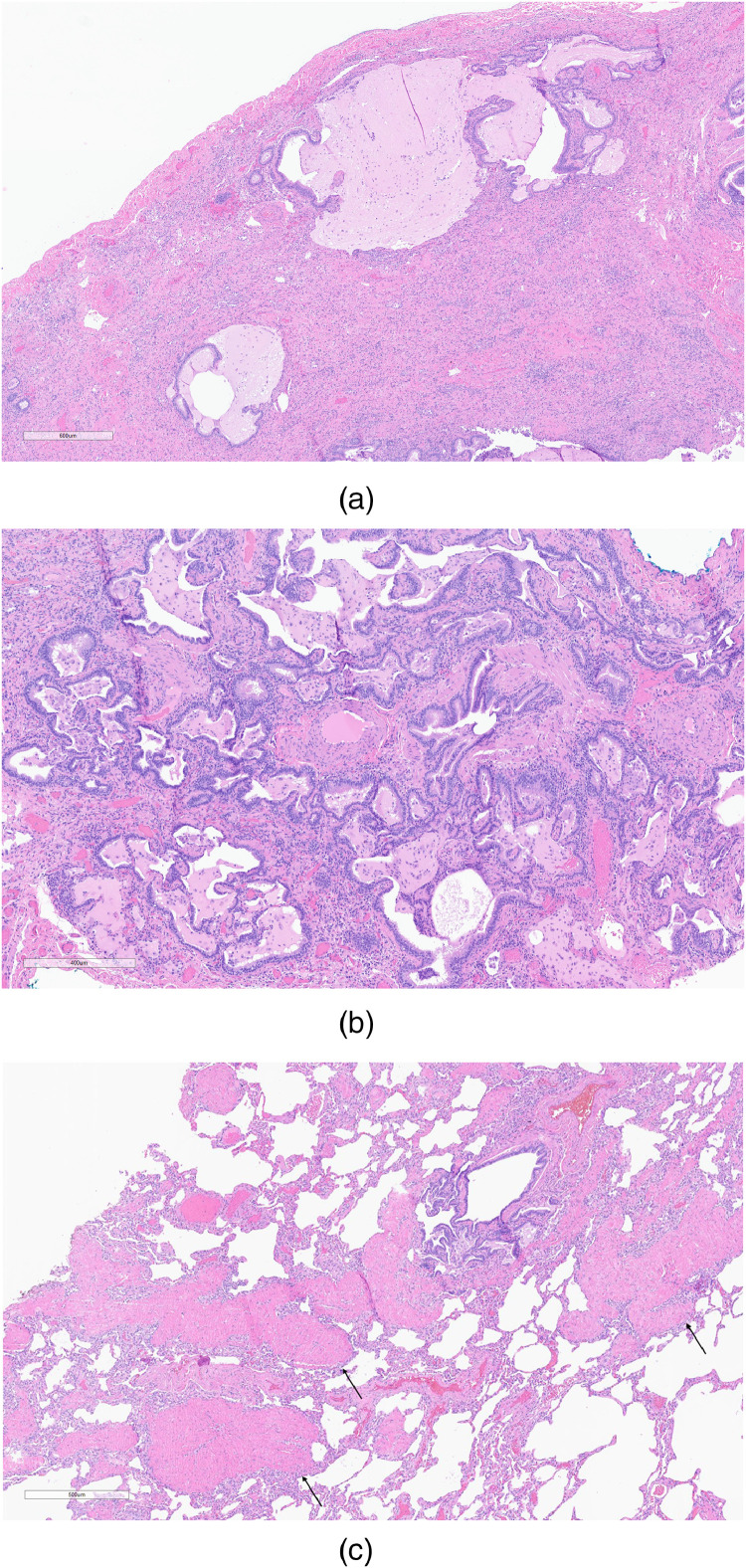
UIP was the most common histopathologic diagnosis, diagnosed in seven patients by both study pathologists (Figure 2). None of these cases showed concomitant follicular lymphoid hyperplasia or associated pleural disease. Two cases with definite UIP were complicated by a superimposed, separate and distinct pattern of organizing ALI (Figure 3). One of these two cases showed hyaline membranes and was classified as organizing DAD. The other case showed a pattern of ALI with overlapping features of organizing pneumonia and organizing DAD; no hyaline membranes were identified. Neither was considered persistent DAD given two temporally distinct patterns of collagen fibrosis and organizing ALI.
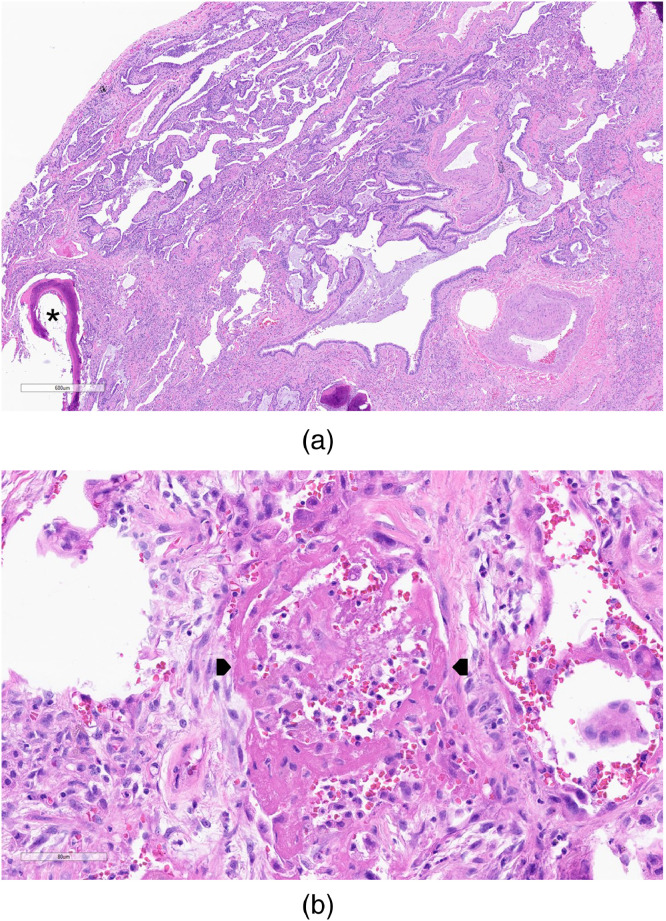
Figure 1.
Cases diagnosed as definite usual interstitial pneumonia showed a characteristic pattern of “patchwork” fibrosis, comprised mainly of dense collagen deposition with scattered fibroblastic foci (arrows). The fibrosis resulted in architectural distortion in the forms of both scarring and microscopic honeycomb change (asterisk). Hematoxylin and eosin-stained slide; magnification 16x.
Figure 2.
Two cases showed usual interstitial pneumonia (2a) with focal osseous metaplasia (asterisk) and a superimposed acute lung injury pattern that in this example, showed hyaline membranes (2b; between arrowheads) diagnostic of diffuse alveolar damage. Hematoxylin and eosin-stained slides; magnifications 33x (2a) and 268x (2b).
One case was diagnosed as probable UIP [19] by both study and referring pathologists. The findings included a distribution of fibrosis typical of UIP with early tissue-destructive scarring in biopsies from the right upper, middle, and lower lobes, but no microscopic honeycomb change.
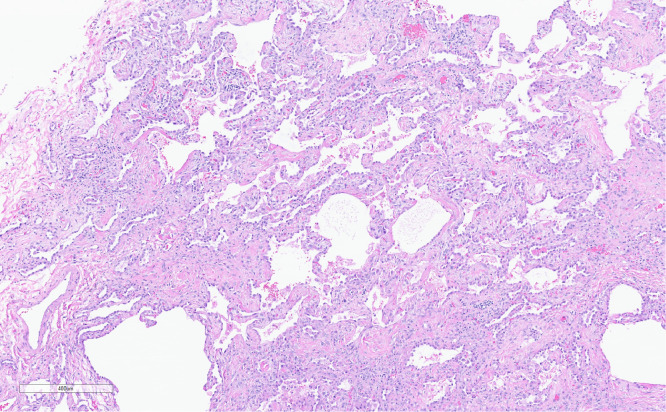
Two cases showed persistent or fibrotic DAD (Figure 4). Both cases showed a spectrum of organizing DAD to fibrotic changes that in 1 case resembled cicatricial organizing pneumonia and the other fibrotic “NSIP-like” changes. One additional case showed organizing ALI with fibrosis resembling NSIP.
Figure 4.
The pattern of fibrosis in persistent diffuse alveolar damage resembled fibrotic nonspecific interstitial pneumonia and resulted in expansion of the interstitium by collagen deposition without significant distortion to the underlying lung architecture. Hematoxylin and eosin-stained slide; magnification 65x.
Two cases showed exquisitely bronchiolocentric changes of chronic bronchiolitis with organizing pneumonia. One case was remarkable for also having extensive squamous metaplasia.
The remaining four cases showed desquamative interstitial pneumonia (DIP) (one), organizing acute bronchopneumonia (one), and mild nonspecific abnormalities of uncertain significance (two). Mild abnormalities of uncertain significance included focal cellular bronchiolitis and pleuritis in a 26-year-old woman who underwent SLB for chronic hypoxic respiratory failure characterized by progressive dyspnea and dry cough. Prior to biopsy she was using supplemental oxygen (2L nasal cannula) at home. She tested positive for COVID-19 42 weeks prior to biopsy and was briefly hospitalized for supportive care without a need for supplemental oxygen or ventilatory support. She developed progressive respiratory symptoms two weeks after discharge for which she was again hospitalized briefly for supportive care one month after initial infection. She was a former smoker, having smoked 0.5 pack per day for four years before quitting three years prior to COVID-19. She was morbidly obese and had a history of polycystic ovary syndrome. A CT scan of her chest done four months prior to SLB reported bilateral groundglass opacities. A COVID-19 test one week prior to biopsy was negative. The second case was a 44-year-old man who underwent SLB 12 weeks after a positive COVID-19 test for which he was not hospitalized. His chief complaints at the time of SLB were intermittent pleuritic chest pain, cough, shortness of breath, and fever. He had a history of systemic hypertension and was morbidly obese. His lungs were clear to auscultation. Multiple serological tests (ANA, anti-DNA, cyclic citrullinated peptide IgG, rheumatoid factor, scl-70, anti-SSA, anti-SSB, anti-RNA, anti-dsDNA, anti-centromere, and anti-Smith) were negative. Chest CT scans, including two CT angiograms, performed 5, 4, and 3 months before, and 6 weeks after, his positive COVID-19 test were negative for pulmonary emboli and showed fleeting, bilateral, groundglass opacities involving his lingula and right lower lobe. SLB showed only very focal partial and complete occlusion of small veins without other histologic features diagnostic of chronic venous hypertension or pulmonary veno-occlusive disease.
3.3. Clinicopathologic Correlation
Definite, probable, and indeterminate for UIP (further referred to as UIP cohort) was the most common histologic diagnosis. Patients in the UIP cohort tended to be older than those without UIP (median 57 versus 53 years, p = 0.042). Patients with UIP were no more likely to have reported underlying lung disease prior to SARS-CoV-2 infection (p = 0.294). UIP patients with pre-existing pulmonary disease included two with unexplained chronic dyspnea, one with chronic dyspnea and asthma, and one with COPD. None of these patients were known to have an underlying connective tissue disease (CTD), occupational, or environmental exposure that might otherwise explain the finding of UIP and were presumed to have idiopathic pulmonary fibrosis (IPF) although to our knowledge none were reviewed at multidisciplinary discussion (MDD). One patient without UIP, who was a former smoker, was reported to have chronic dyspnea prior to COVID-19 and was found to have DIP on lung biopsy.
All patients in the UIP cohort were reported to either have groundglass opacities with interstitial thickening (n = 5) or peripheral reticulations with bronchiectasis (n = 4) on chest CT prior to lung biopsy; none had groundglass opacities only. Patients without UIP were frequently reported to have groundglass opacities only (n = 7); however, two patients, both of whom had persistent or fibrotic DAD, were described as having groundglass opacities with interstitial thickening. Overall, the distribution of patients in each category of the above radiographic changes statistically differed among the UIP cohort and patients without UIP (p-value 0·042). Patients in the UIP cohort (n = 9) were more often reported to have persistent respiratory symptoms as compared to those without UIP (n = 7); however, this finding was not statistically significant and may be due to chance. The characteristics of the UIP cohort compared to patients without UIP are detailed in Table 4.
TABLE 4.
Characteristics of Patients With and Without UIP
| UIP cohort (n = 9) | Patients without UIP (n = 9) | P-value | |
|---|---|---|---|
| Age, years (median, interquartile range) | 57 (12) | 53 (17) | 0·042* |
| Sex | |||
| Male | 5 (56) | 5 (56) | 1·000 |
| Female | 4 (44) | 4 (44) | |
| Smoker statusa | |||
| Current smoker | 0 (0) | 1 (14) | 1·000 |
| Former smoker | 4 (50) | 3 (43) | |
| Never smoker | 4 (50) | 3 (43) | |
| History of pulmonary disease prior to COVID-19 | 4 (44) | 1 (11) | 0·294 |
| Persistent respiratory symptoms post-COVID-19 | 9 (100) | 7 (78) | 0·471 |
| Post-COVID-19 chest CT | |||
| Groundglass opacities only | 0 (0) | 7 (78) | 0·042* |
| Groundglass opacities with interstitial thickening | 5 (56) | 2 (22) | |
| Peripheral reticulations with bronchiectasis | 4 (44) | 0 (0) |
COVID-19 = coronavirus disease 2019; CT = computed tomography; UIP = usual interstitial pneumonia
Smoking status was unknown for 1 UIP cohort patient and 2 patients without UIP.
*Statistically significant
Seven patients had evidence of ALI, including DAD or organizing pneumonia. These included two cases of UIP with superimposed ALI, three cases of persistent DAD or organizing ALI with fibrosis including both patients with RA, and two cases of organizing pneumonia. The duration of time from COVID-19 diagnosis to biopsy was shorter for patients with ALI compared to those without ALI, averaging 85 and 184 days, respectively (p-value 0·016).
Of the seven patients with ALI, five were hospitalized with acute COVID-19 and two were not. Of those that were hospitalized, two were not intubated or mechanically ventilated; it is unknown whether any of the other three required intubation or mechanical ventilation. Of the eight patients hospitalized for acute COVID-19, hospitalization occurred more frequently in patients with ALI (71%) compared to those without ALI (n = 3; 38%). For three patients without ALI, need for hospitalization was unknown. Although patients who were hospitalized more often had ALI on SLB compared to those who were not hospitalized, this finding was not statistically significant (p-value 0·20).
4. Discussion
To our knowledge, this is the first report of SLB findings in patients with clinical and/or radiographic evidence of ILD following recovery from acute COVID-19. This study differs from previous reports of lung pathology in explanted lungs from living patients who underwent lung transplant for severe COVID-19-related acute and nonresolving respiratory failure. The main findings in those studies were persistent ALI with NSIP-like fibrosis [10], [11], [12].
In our patient population, UIP was the most common histologic finding in patients with evidence of ILD following resolution of acute SARS-CoV-2 infection. Patients with UIP were older than patients without UIP, and more likely to have a history of chronic lung disease prior to SARS-CoV-2 infection and persistent respiratory symptoms following recovery. Interstitial thickening and/or peripheral reticulations with bronchiectasis were more common in patients discovered to have UIP on SLB compared to those with other patterns of lung injury.
Our study lacks the robust clinical and radiological review common to MDD, the current diagnostic standard for patients with ILD. Given that IPF is the most common MDD diagnosis in patients with a UIP pattern of fibrosis on SLB, it seems reasonable to speculate that at least a subset of our patients had IPF prior to COVID-19. Two patients had both a definite UIP pattern of fibrosis and superimposed ALI resulting in histologic features identical to those seen in patients with acute exacerbation of IPF, further suggesting that at least some of our patients may have had diffuse fibrotic lung disease that preceded COVID-19. Nonetheless any link between these findings and IPF remains speculative; we cannot categorically assert that these changes are unrelated to SARS-CoV-2 infection given the absence of clinical data and imaging studies to assess for ILD prior to COVID-19 in our cohort. Additional studies, including single institution experiences in which MDD is a common practice, are needed to further understand the significance of a UIP pattern of fibrosis in patients with persistent respiratory symptoms following recovery from the acute phase of COVID-19.
Evidence of ALI that represented a spectrum from DAD to organizing pneumonia was more common in patients who were hospitalized with COVID-19. This is not surprising, since these patients were presumably more ill and required a higher level of clinical care. When fibrosis was present it resembled NSIP, consistent with previous reports [8], [9], [10], [11], [12]. Since the duration of time from SARS-CoV-2 infection to biopsy was shorter for patients with ALI than those without ALI, these findings may represent a more direct effect of SARS-CoV-2 infection. Future studies that include long-term clinical follow-up and testing for SARS-CoV-2 nucleoprotein in biopsy tissues will be helpful to understand the relative contribution of these findings to persistent clinical or radiographic abnormalities.
The main limitations of this study include a selection bias inherent to a single-institution consultation practice in that this service tends to select for more difficult cases, small sample size, and incomplete clinical and radiological data. Additionally, the small dataset also precluded analysis for potential confounding by variables, such as smoking history. Despite these limitations, we were able to make statistically significant preliminary conclusions that will benefit from future studies.
In summary, UIP was found to be the most common pathologic finding in patients undergoing evaluation for post-COVID-19 ILD. We also showed that CT changes described as groundglass with interstitial thickening, peripheral reticulations, and/or bronchiectasis may be helpful in selecting which patients might have a fibrotic chronic interstitial pneumonia should biopsy be pursued for further evaluation.
Author contributions
All authors contributed equally to the conceptualization of the work and gave final approval of the submitted version. In addition, K.E.K. and J.L.M verified all underlying data. K.E.K. contributed to data curation, formal analysis, and writing - original draft. W.P. contributed to data curation, formal analysis, and writing - review & editing. T.H. contributed to writing - review & editing. C.F.F. contributed to formal analysis and writing - review & editing. J.L.M. contributed to data curation, formal analysis, and writing - original draft.
Funding
No intramural or extramural funding sources supported this work.
Data sharing statement
The datasets used for this study are not publicly available. De-identified access can be requested by academic research collaborators upon written request to our corresponding author. All requests will be subject to research team review and approval.
Declaration of Competing Interest
None to declare.
References
- 1.Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ. Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konopka KE, Nguyen T, Jentzen JM, et al. Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 infection is morphologically indistinguishable from other causes of DAD. Histopathology. 2020;77(4):570–578. doi: 10.1111/his.14180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian S, Xiong Y, Liu H, et al. Pathologic study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33(6):1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barisione E, Grillo F, Ball L, et al. Fibrotic progression and radiologic correlation in matched lung samples from COVID-19 post-mortems. Virchows Arch. 2021;478(3):471–485. doi: 10.1007/s00428-020-02934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Wu J, Wang S, et al. Progression to fibrosing diffuse alveolar damage in a series of 30 minimally invasive autopsies with COVID-19 pneumonia in Wuhan, China. Histopathology. 2021;78:542–555. doi: 10.1111/his.14249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katzenstein AL, Fiorelli RF. Non-specific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am J Surg Pathol. 1994;18:136–147. [PubMed] [Google Scholar]
- 9.Cottin V, Donsbeck AV, Revel D, et al. Nonspecific interstitial pneumonia. Individualization of a clinicopathologic entity in a series of 12 patients. Am J Respir Crit Care Med. 1998;158(4):1286–1293. doi: 10.1164/ajrccm.158.4.9802119. [DOI] [PubMed] [Google Scholar]
- 10.Bharat A, Querry M, Markov NS, et al. Lung transplantation for patients with severe COVID-19. Sci Transl Med. 2020;12(574):eabe4282. doi: 10.1126/scitranslmed.abe4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aesif SW, Bribriesco AC, Yadav R, et al. Pulmonary pathology of COVID-19 following 8 weeks to 4 months of severe disease: a report of three cases, including one with bilateral lung transplantation. Am J Clin Pathol. 2021;155(4):506–514. doi: 10.1093/ajcp/aqaa264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bharat A, Machuca TN, Querrey M, et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med. 2021;9(5):487–497. doi: 10.1016/S2213-2600(21)00077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daher A, Balfanz P, Cornelissen C, et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary sequelae. Respir Med. 2020;174 doi: 10.1016/j.rmed.2020.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Tan C, Wu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21(1):163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mo X, Jian W, Su Z, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55(6) doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J. 2021;57(4) doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic pulmonary pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 20.Core Team R. R Foundation for Statistical Computing; Vienna, Austria: 2021. R: A language and environment for statistical computing.https://www.R-project.org/ URL. [Google Scholar]
- 21.Yousem SA. Cicatricial variant of cryptogenic organizing pneumonia. Hum Pathol. 2017;64:76–82. doi: 10.1016/j.humpath.2017.03.018. [DOI] [PubMed] [Google Scholar]