Abstract
Background
This study aimed to assess the effects of anterior cervical discectomy and fusion (ACDF) on distraction of the posterior ligamentum flavum (LF) by increasing the intervertebral disc height and positioning a graft in patients with degenerative cervical spine disease.
Methods
Sixty-eight patients with degenerative cervical diseases who underwent single-level ACDF were included in the analysis. The intervertebral disc height, Cobb angle, and transverse thickness of the LF were measured, and magnetic resonance imaging was performed both preoperatively and 6 weeks postoperatively on each patient. Correlation analyses were performed to evaluate the relationships between age, sex, change in intervertebral disc height, Cobb angle, and position of the intervertebral implant according to the postoperative change in LF thickness. The position of the intervertebral implant was categorized as anterior, middle, or posterior. We also evaluated radiological effects according to the implant position.
Results
The mean intervertebral disc height increased from 5.88 mm preoperatively to 7.49 mm postoperatively. The Cobb angle was 0.88° preoperatively and 1.43° postoperatively. Age (p = 0.551), sex (p = 0.348), position of cage (p = 0.312), pre- and postoperative intervertebral disc height (p = 0.850, p = 0.900), Cobb angle (p = 0.977, p = 0.460), and LF thickness (p = 0.060, p = 1.00) were not related to changes in postoperative LF thickness. Postoperative increase in disc height was related to Cobb angle (r = 0.351, p = 0.038). No other factors were significantly related. The position of the cage was not related with the change of Cobb angle (p = 0.91), LF thickness (p = 0.31), or disc height (p = 0.54).
Conclusions
Change in the intervertebral disc height and the position of the intervertebral implant after ACDF did not affect the thickness of the LF after surgery in patients with degenerative cervical spine disease.
Keywords: Cervical spine, Anterior cervical discectomy and fusion, Ligamentum flavum, Indirect decompression
Anterior cervical discectomy and fusion (ACDF) has become one of the most frequently performed surgical techniques for the management of symptomatic cervical disc herniation. Since introduced by Smith and Robinson,1) it has been regarded as the standard of care for the surgical treatment of cervical degenerative diseases. One of the advantages of ACDF for the treatment of cervical radiculopathy is that it allows for additional indirect decompression of the uncovertebral joint through distraction of the intervertebral disc space after full discectomy. Restoration of intervertebral disc height using a large intervertebral graft during ACDF can lead to indirect decompression of foraminal stenosis.2,3,4) In lumbar spinal stenosis, indirect decompression of the spinal canal can be achieved with anterior decompression and fusion by stretching the yellow ligament using a large cage;5,6) however, no studies have evaluated the relationship between anterior distraction and posterior indirect decompression in patients with central cervical stenosis. This study therefore aimed to assess the effects of ACDF on distraction of the posterior ligamentum flavum (LF) by increasing the intervertebral disc height and positioning a graft in patients with degenerative cervical spine disease.
METHODS
After obtaining approval from the Institutional Review Board of Haeundae Paik Hospital (IRB No. 2019-08-111-009), we retrospectively reviewed 68 patients diagnosed with degenerative cervical disease who had undergone single-level ACDF using an allograft and additional plate fixation between January 2017 and January 2019. Written informed consents were obtained. Surgical procedures were performed by a single surgeon (HC) at a single institution. An independent surgeon (BWC), who was not involved in the diagnosis and treatment of the patients, analyzed the data. We excluded (1) patients with less than 1-year follow-up, (2) non-degenerative cases such as trauma, infection, or tumorous conditions, and (3) revision cases and adjacent level disease.
Operative Technique
Patients were operated in the supine position under general anesthesia. Using the usual Smith–Robinson approach, the anterior vertebral body and disc space were exposed; after complete decompression by removing the osteophytes and disc materials, the endplate cartilage was detached with a curette and a high speed burr until bleeding was noted. After trials, we press-fitted appropriate intervertebral spacers with intervertebral allografts. Additional plate fixation was performed using fixed plating (Atlantis Anterior Cervical Plate System; Medtronic, North Haven, CT, USA). A Philadelphia cervical orthosis was applied for 6 weeks after operation.
Data Analysis
Demographic data including age, sex, and diagnosis at operation were collected. Magnetic resonance imaging was performed both preoperatively and 6 weeks postoperatively in every case.
Evaluation Criteria
We measured the intervertebral disc height, segmental Cobb angle, and transverse thickness of LF preoperatively and 6 weeks postoperatively using T2-weighted imaging (Fig. 1). Segmental Cobb angles were measured between the end plates from cranial to caudal end plates, which were subject to surgery. If there is an osteophyte of the involved vertebra, we measured the base at the center of end plates. Transverse thickness of LF was measured in the central area perpendicular to the running direction of LF and the thickest length was taken as the measurement value. All measurements were undertaken using a picture archiving communication system (Marosis M-view; Infinitt, Seoul, Korea).
Fig. 1. Radiological parameters. a: Cobb angle, b: intervertebral disc height, c: transverse thickness of the ligamentum flavum.
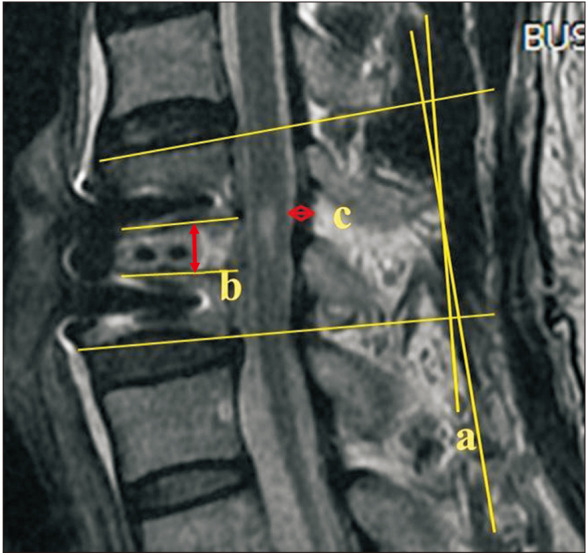
We categorized patients according to the change in LF thickness: group A (no change, less than 0.1 mm), group B (decreased thickness, more than 0.1 mm), and group C (increased thickness, more than 0.1 mm). The position of the intervertebral implant was categorized as anterior, middle, or posterior.7) We also evaluated the radiological results according to the implant position.
Statistical Analysis
Statistical analyses were done using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Correlation analyses were performed to evaluate the relationships between age, sex, change in intervertebral disc height, Cobb angle, and position of intervertebral implant according to the postoperative change in LF thickness. Analysis of variance or the chisquare test was performed as appropriate according to the group. Turkey’s test was used for post hoc analysis. A p < 0.05 was considered to indicate statistical significance.
RESULTS
The mean age of the patients was 51.67 years, and there were 49 men and 19 women. According to the diagnosis at the time of surgery, 35 patients had cervical radiculopathy and 33 had myelopathy. There were 3 cases involving C3–4, 11 cases involving C4–5, 36 cases involving C5–6, and 18 cases involving C6-7.
The mean intervertebral disc height increased from 5.88 ± 0.86 mm preoperatively to 7.49 ± 0.94 mm postoperatively. The Cobb angle was 0.88° (± 2.63°) preoperatively and 1.43° (± 2.67°) postoperatively. The mean thickness of LF decreased from 2.31 mm (± 0.76 mm) preoperatively to 2.2 mm (± 0.73 mm) mm postoperatively.
Patients were categorized according to the change in LF thickness after operation: group A (no change, n = 41), group B (decreased thickness, n = 20), and group C (increased thickness, n = 7). When analyzed according to group, age (p = 0.551), sex (p = 0.348), position of cage (p = 0.312), pre- and postoperative intervertebral disc height (p = 0.850, p = 0.900), Cobb angle (p = 0.977, p = 0.460), and LF thickness (p = 0.060, p = 1.00) were not related to changes in postoperative LF thickness. Postoperative increases in disc height were related to Cobb angle (r = 0.351, p = 0.038); no other factors were significantly related (Table 1). On the post hoc analysis, there was no significant difference between three groups in Turkey’s post hoc test.
Table 1. Correlation Analysis According to Changed Value after Operation.
| Variable | Change of disc height | Change of Cobb angle | Change of LF thickness | |
|---|---|---|---|---|
| Change of disc height | ||||
| Pearson ratio | 1 | 0.351 | 0.150 | |
| p-value | 0.038 | 0.221 | ||
| Change of Cobb angle | ||||
| Pearson ratio | 1 | 0.193 | ||
| p-value | 0.115 | |||
| Change of LF thickness | ||||
| Pearson ratio | 1 | |||
| p-value | ||||
LF: ligamentum flavum.
When we evaluated each case according to the position of the cage, the anterior, middle, and posterior positions represented 39, 27, and 2 cases, respectively. Anterior positioning of the cage was shown to increase disc height (p = 0.54), decrease the Cobb angle (p = 0.91), and decrease LF thickness (p = 0.31); however, none of these findings were statistically significant (Table 2). On the post hoc analysis, there was also no significant difference according to the position of cage in Turkey’s post hoc test.
Table 2. Radiological Result According to the Position of Intervertebral Cage.
| Variable | Change of disc height | Change of Cobb angle | Change of LF thickness |
|---|---|---|---|
| Anterior position | 1.69 ± 0.98 | 0.68 ± 3.67 | 1.8 ± 0.81 |
| Middle position | 1.54 ± 1.07 | 0.41 ± 3.30 | 0.09 ± 0.55 |
| Posterior position | 0.93 ± 0.65 | –0.15 ± 0.21 | 0.05 ± 0.50 |
| p-value | 0.54 | 0.91 | 0.31 |
Values are presented as mean ± standard deviation.
LF: ligamentum flavum.
DISCUSSION
Surgical management of neurological symptoms in spinal disease can be accomplished by decompression of compressed neural elements related with preoperative symptoms. There are two ways of decompression methods; direct and indirect decompression techniques.4) Direct decompression can be achieved by removing the compressed bone, ligaments, and disc material.8) However, severe complications such as spinal cord injury, nerve root injury, and dural tear can occur during the procedure. And a procedure near the spinal cord and nerve root can lead to epidural hematoma and epidural fibrosis.9,10,11,12) Conversely, indirect decompression techniques allow for more safe decompression of neural tissue without resection of compressing tissue and manipulation of neural elements. An indirect decompression procedure performed with the main purpose of distracting the intervertebral space results in widened neural foramen and central epidural space. It also can be done by using posterior spinal instrumentation or by directly widening the disc space using a huge intervertebral graft.4)
In the cervical spine, indirect decompression is performed using ACDF; restoring disc height by a large intervertebral graft expands the diameter of the foramen, leads to decompression of the nerve root, and relieves the radicular pain.13) When the implanted graft height is larger, distraction in the spinal canal and neural foramen can theoretically increase. Albert et al.14) reported an average foraminal area increase of 33% after ACDF. An et al.15) also showed the foraminal cross-sectional area in the cervical spine was increased during ACDF by increasing the interbody graft height using cadaveric spines and computed tomographic analysis. The increase in the foraminal cross-sectional area represents the increased space for the existing nerve root; it may result in improved vascular supply and relieves direct compression on an already compromised nerve. By contrast, subsidence of the interbody graft during the follow-up has an adverse effect on the maintenance of indirect decompression effect. Fujibayashi et al.16) compared clinical and radiological outcomes between the stand-alone interbody cage and autologous iliac bone grafting with an anterior plate. Although the clinical results did not differ, the cage-treated group showed a high incidence of cage subsidence and loss of acquired alignment; thus, plating may prevent subsidence and kyphotic change.
Indirect spinal canal decompression can be achieved by reduction of disc bulging and elongation of the hypertrophied LF through the restoration of disc height, especially in the lumbar spine. Recently developed anterior retroperitoneal fusion using a transpsoas and anteropsoas procedure is being increasingly used in lumbar pathology. Oliveira et al.17) described that substantial dimensional improvement was evidenced by a 13.5%, 24.7%, and 33.1% increase in foraminal height, foraminal area, and central canal diameter, respectively. Kepler et al.18) also reported improvement of the foraminal area by as much as 35% in degenerative foraminal stenosis using extreme lateral interbody fusion (XLIF). The oblique lateral interbody fusion procedure was introduced more recently and has several theoretical advantages over the XLIF procedure, which include less invasion of the psoas muscle and lumbar plexus, a decreased need for neuromonitoring, and access to the L4–5 level with a high-riding pelvis, as well as the L5–S1 level.19) These lateral lumbar interbody fusion technique has proven effective, allowing for indirect decompression of the lumbar spine and relieving the symptoms of lumbar spinal stenosis. Sato et al.20) also showed significant increases in the spinal canal (19%) and intervertebral foramen (21%–39%) areas. Similar concepts are possible in the cervical spine. Bayley et al.21) also reported similar results through a cadaver study. They concluded that anterior discectomy and distraction with strut graft can significantly improve the space available for the cord and foraminotomy, which carries the risk of iatrogenic injury to the cord, but may not always be necessary for improving clinical radiculopathy and myelopathy. However, overdistraction in the cervical spine can lead to adverse effects. Taghvaei et al.22) reported neurological deterioration after an anterior cervical discectomy due to LF buckling. LF buckling should therefore be considered as a potential cause of acute neurologic deterioration after ACDF. Overdistraction by inserting a large graft material was generally considered to lead to postoperative neck pain because of posterior facet joint distraction or posterior neck muscle spasm. However, Chang et al.23) reported that an increase in the intervertebral space or interfacet distance by the insertion of a large graft material while performing ACDF for the treatment of degenerative cervical disease was not related with the change in visual analog scale scores for neck and arm pain and Neck Disability Index scores postoperatively and during the follow-up period.
The position of a cage in the disc space may be an important factor for cage subsidence in cervical ACDF.7) According to the instruction manual for rectangular cage devices, anterior placement is recommended. Mende et al. evaluated the position of the cage and its effect on subsidence and radiological findings.24) Cage placement in the sagittal plane showed characteristic subsidence patterns for certain positions; subsidence occurred most often for posteriorly implanted cages, and cages implanted at the anterior position showed a kyphotic subsidence pattern similar to 31.8% of all anteriorly placed and 31.1% of all centrally placed and subsided cages. Our results showed that the anterior position of the cage led to a more lordotic curve, while LF thickness was decreased; however, we could not find a statistical significance.
This is the only report to evaluate LF changes after ACDF. Although we can imagine the positive effect of indirect decompression on distraction of the LF by distracting the disc space, unfortunately, there was no previous effort to evaluate this.
We assessed the effects of ACDF on distraction of the posterior LF by increasing the intervertebral disc height and positioning a graft in patients with degenerative cervical spine disease. Our results showed that changes in intervertebral disc height or position of the intervertebral implant after ACDF did not affect the thickness of the LF after surgery in patients with degenerative cervical spine disease. Even though ACDF is still effective for foraminal indirect decompression by distracting the intervertebral disc height, there was a limitation for central cervical stenosis.
The limitation of this study is that clinical analysis was not possible due to the small number of cases; additionally, diverse pathological conditions were included in the analysis. According to our results, indirect decompression by distracting the LF was possible, while the clinical effect was minimal. This is the first analysis of the effect of ACDF on the thickness of the LF after surgery. The attempt itself was meaningful, and further, well-designed prospective analyses would provide more accurate information. The change in the intervertebral disc height and position of the intervertebral implant did not affect the thickness of the LF after ACDF in patients with degenerative cervical spine disease.
ACKNOWLEDGEMENTS
This work was supported by grant from Inje University 2019.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40(3):607–624. [PubMed] [Google Scholar]
- 2.Shen FH, Samartzis D, Khanna N, Goldberg EJ, An HS. Comparison of clinical and radiographic outcome in instrumented anterior cervical discectomy and fusion with or without direct uncovertebral joint decompression. Spine J. 2004;4(6):629–635. doi: 10.1016/j.spinee.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Ko S, Choi W, Lee J. The prevalence of cervical foraminal stenosis on computed tomography of a selected community-based Korean population. Clin Orthop Surg. 2018;10(4):433–438. doi: 10.4055/cios.2018.10.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshihara H. Indirect decompression in spinal surgery. J Clin Neurosci. 2017;44:63–68. doi: 10.1016/j.jocn.2017.06.061. [DOI] [PubMed] [Google Scholar]
- 5.Lin GX, Akbary K, Kotheeranurak V, et al. Clinical and radiologic outcomes of direct versus indirect decompression with lumbar interbody fusion: a matched-pair comparison analysis. World Neurosurg. 2018;119:e898–e909. doi: 10.1016/j.wneu.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Hayama S, Nakano A, Nakaya Y, et al. The evaluation of indirect neural decompression after lateral lumbar interbody fusion using intraoperative computed tomography myelogram. World Neurosurg. 2018;120:e710–e718. doi: 10.1016/j.wneu.2018.08.146. [DOI] [PubMed] [Google Scholar]
- 7.Barsa P, Suchomel P. Factors affecting sagittal malalignment due to cage subsidence in standalone cage assisted anterior cervical fusion. Eur Spine J. 2007;16(9):1395–1400. doi: 10.1007/s00586-006-0284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malham GM, Parker RM, Goss B, Blecher CM, Ballok ZE. Indirect foraminal decompression is independent of metabolically active facet arthropathy in extreme lateral interbody fusion. Spine (Phila Pa 1976) 2014;39(22):E1303–E1310. doi: 10.1097/BRS.0000000000000551. [DOI] [PubMed] [Google Scholar]
- 9.Airaksinen O, Herno A, Turunen V, Saari T, Suomlainen O. Surgical outcome of 438 patients treated surgically for lumbar spinal stenosis. Spine (Phila Pa 1976) 1997;22(19):2278–2282. doi: 10.1097/00007632-199710010-00016. [DOI] [PubMed] [Google Scholar]
- 10.Jang IT, Lee SW, Atienza PM, You JS. Decompressive surgery alone for lumbar spinal stenosis in elderly patients. Korean J Spine. 2008;5(2):83–88. [Google Scholar]
- 11.Kou J, Fischgrund J, Biddinger A, Herkowitz H. Risk factors for spinal epidural hematoma after spinal surgery. Spine (Phila Pa 1976) 2002;27(15):1670–1673. doi: 10.1097/00007632-200208010-00016. [DOI] [PubMed] [Google Scholar]
- 12.Turner JA, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis: attempted meta-analysis of the literature. Spine (Phila Pa 1976) 1992;17(1):1–8. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Celik SE, Kara A, Celik S. A comparison of changes over time in cervical foraminal height after tricortical iliac graft or polyetheretherketone cage placement following anterior discectomy. J Neurosurg Spine. 2007;6(1):10–16. doi: 10.3171/spi.2007.6.1.3. [DOI] [PubMed] [Google Scholar]
- 14.Albert TJ, Smith MD, Bressler E, Johnson LJ. An in vivo analysis of the dimensional changes of the neuroforamen after anterior cervical diskectomy and fusion: a radiologic investigation. J Spinal Disord. 1997;10(3):229–233. [PubMed] [Google Scholar]
- 15.An HS, Evanich CJ, Nowicki BH, Haughton VM. Ideal thickness of Smith-Robinson graft for anterior cervical fusion: a cadaveric study with computed tomographic correlation. Spine (Phila Pa 1976) 1993;18(14):2043–2047. doi: 10.1097/00007632-199310001-00020. [DOI] [PubMed] [Google Scholar]
- 16.Fujibayashi S, Neo M, Nakamura T. Stand-alone interbody cage versus anterior cervical plate for treatment of cervical disc herniation: sequential changes in cage subsidence. J Clin Neurosci. 2008;15(9):1017–1022. doi: 10.1016/j.jocn.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976) 2010;35(26 Suppl):S331–S337. doi: 10.1097/BRS.0b013e3182022db0. [DOI] [PubMed] [Google Scholar]
- 18.Kepler CK, Sharma AK, Huang RC, et al. Indirect foraminal decompression after lateral transpsoas interbody fusion. J Neurosurg Spine. 2012;16(4):329–333. doi: 10.3171/2012.1.SPINE11528. [DOI] [PubMed] [Google Scholar]
- 19.Rabau O, Navarro-Ramirez R, Aziz M, et al. Lateral lumbar interbody fusion (LLIF): an update. Global Spine J. 2020;10(2 Suppl):17S–21S. doi: 10.1177/2192568220910707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato J, Ohtori S, Orita S, et al. Radiographic evaluation of indirect decompression of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for degenerated lumbar spondylolisthesis. Eur Spine J. 2017;26(3):671–678. doi: 10.1007/s00586-015-4170-0. [DOI] [PubMed] [Google Scholar]
- 21.Bayley JC, Yoo JU, Kruger DM, Schlegel J. The role of distraction in improving the space available for the cord in cervical spondylosis. Spine (Phila Pa 1976) 1995;20(7):771–775. doi: 10.1097/00007632-199504000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Taghvaei M, Meybodi KT, Zeinalizadeh M. Ligamentum flavum buckling causing immediate post-operative neurological deterioration after an anterior cervical discectomy: case report. Turk Neurosurg. 2018;28(4):678–681. doi: 10.5137/1019-5149.JTN.17403-16.1. [DOI] [PubMed] [Google Scholar]
- 23.Chang H, Baek DH, Choi BW. The relationship between increased intervertebral disc height and evelopment of postoperative axial neck pain after anterior cervical fusion. J Korean Neurosurg Soc. 2014;55(6):343–347. doi: 10.3340/jkns.2014.55.6.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mende KC, Eicker SO, Weber F. Cage deviation in the subaxial cervical spine in relation to implant position in the sagittal plane. Neurosurg Rev. 2018;41(1):267–274. doi: 10.1007/s10143-017-0850-z. [DOI] [PubMed] [Google Scholar]


