Summary
Background
Many individuals who survive tuberculosis disease face ongoing disability and elevated mortality risks. However, the impact of post-tuberculosis sequelae is generally omitted from policy analyses and disease burden estimates. We therefore estimated the global burden of tuberculosis, inclusive of post-tuberculosis morbidity and mortality.
Methods
We constructed a hypothetical cohort of individuals developing tuberculosis in 2019, including pulmonary and extrapulmonary disease. We simulated lifetime health outcomes for this cohort, stratified by country, age, sex, HIV status, and treatment status. We used disability-adjusted life-years (DALYs) to summarise fatal and non-fatal health losses attributable to tuberculosis, during the disease episode and afterwards. We estimated post-tuberculosis mortality and morbidity based on the decreased lung function caused by pulmonary tuberculosis disease.
Findings
Globally, we estimated 122 (95% uncertainty interval [UI] 98–151) million DALYs due to incident tuberculosis disease in 2019, with 58 (38–83) million DALYs attributed to post-tuberculosis sequelae, representing 47% (95% UI 37–57) of the total burden estimate. The increase in burden from post-tuberculosis varied substantially across countries and regions, driven largely by differences in estimated case fatality for the disease episode. We estimated 12·1 DALYs (95% UI 10·0–14·9) per incident tuberculosis case, of which 6·3 DALYs (5·6–7·0) were from the disease episode and 5·8 DALYs (3·8–8·3) were from post-tuberculosis. Per-case post-tuberculosis burden estimates were greater for younger individuals, and in countries with high incidence rates. The burden of post-tuberculosis was spread over the remaining lifetime of tuberculosis survivors, with almost a third of total DALYs (28%, 95% UI 23–34) accruing 15 or more years after incident tuberculosis.
Interpretation
Post-tuberculosis sequelae add substantially to the overall disease burden caused by tuberculosis. This hitherto unquantified burden has been omitted from most previous policy analyses. Future policy analyses and burden estimates should take better account of post-tuberculosis, to avoid the potential misallocation of funding, political attention, and research effort resulting from continued neglect of this issue.
Funding
National Institutes of Health.
Introduction
Tuberculosis was estimated to cause 1·4 million deaths in 2019.1 Individuals who develop tuberculosis experience a range of symptoms, including fever, wasting, and cough. Although successful treatment prevents death, many tuberculosis survivors experience ongoing health problems following the disease episode, and there is increasing evidence of long-term disability and elevated mortality risks in this population.2
The term post-tuberculosis describes the range of pathological conditions experienced by tuberculosis survivors. Pulmonary tuberculosis, the commonest disease presentation, causes progressive destruction of lung tissue, and this damage might not fully resolve after treatment.3, 4, 5 Although prompt treatment can minimise lung damage, many tuberculosis survivors experience residual lung pathology, with cross-sectional studies consistently demonstrating substantial pulmonary impairment—including chronic obstructive pulmonary disease (COPD), spirometric restriction, bronchiectasis, and pulmonary hypertension, as well as secondary non-tuberculosis lung infections—among tuberculosis survivors.3, 4, 5 These results are consistent with longitudinal evidence comparing lung function before and after the tuberculosis episode.6, 7 Individuals with chronic respiratory disease experience lower quality of life,8 higher health-care use,9 and reduced economic productivity compared with healthy individuals.10 Individuals with chronic respiratory disease additionally face higher all-cause mortality, even with only mild lung impairment.2, 11 In addition to pulmonary damage, extrapulmonary tuberculosis disease and drug toxicity can cause permanent damage to other organ systems, as well as social and psychological sequelae.5
Research in context.
Evidence before this study
A growing number of cross-sectional and longitudinal studies have described chronic lung disease, long-term disability, and elevated mortality among individuals surviving tuberculosis disease. However, these post-tuberculosis sequelae are omitted from disease burden estimations and policy analyses that quantify the health losses caused by tuberculosis.
Added value of this study
We quantified the global burden of disease caused by incident tuberculosis in 2019, inclusive of post-tuberculosis morbidity and mortality over the lifetime of tuberculosis survivors. The results of this analysis demonstrate that post-tuberculosis burden adds considerably to the health losses caused by incident tuberculosis—globally, we estimated 122 (95% uncertainty interval 98–151) million disability-adjusted life-years (DALYs) due to tuberculosis occurring in 2019, with 58 (38–83) million DALYs attributed to post-tuberculosis sequelae, representing 47% (37–57) of the total burden estimate.
Implications of all the available evidence
When post-tuberculosis sequelae are considered, the disease burden caused by tuberculosis is substantially greater than conventional tuberculosis burden estimates. Accounting for post-tuberculosis in burden estimates and policy analyses will probably lead to revisions in the estimated impact of tuberculosis control efforts.
Historically, post-tuberculosis has not been a major focus of the tuberculosis control agenda. However, the health-care needs of tuberculosis survivors are drawing increasing attention,5 and recent modelling analyses have highlighted the large population of tuberculosis survivors, estimated at 155 million in 2020.12 The mortality and morbidity caused by post-tuberculosis have not traditionally been included in estimates of tuberculosis disease burden,13 nor policy analyses of tuberculosis control interventions.14 For burden estimates, with health losses quantified as disability-adjusted life-years (DALYs), health outcomes are attributed to specific diseases using the International Classification of Diseases (ICD) framework. Although ICD-10 includes a code for tuberculosis sequelae (B90.9), this code is rarely used. In practice, deaths among individuals with post-tuberculosis are commonly attributed to their proximal cause. For this reason, contemporary estimates of tuberculosis burden only include deaths and disability occurring during the tuberculosis disease episode.
Disease burden estimates shape the health research agenda and funding landscape, and they also determine how interventions are prioritised within disease control budgets. By excluding post-tuberculosis, current burden estimates might substantially underestimate the health losses caused by tuberculosis, which could lead to misallocation of funding, political attention, and research effort. Burden estimates for individual settings have provided initial evidence of the biases from omitting post-tuberculosis burden: an analysis estimating tuberculosis burden for India with and without post-tuberculosis found that total DALYs increased by 62% when post-tuberculosis was included.15 A study of tuberculosis burden in Tarrant County, TX, USA, found a similarly large fraction of total burden due to chronic tuberculosis sequelae.16 In this study, we estimated the total DALYs caused globally by incident tuberculosis disease in 2019, including attributable deaths and long-term disability among tuberculosis survivors in subsequent years. We report estimates for each of 186 countries reporting ten or more tuberculosis cases in 2019, to provide a more comprehensive description of the health losses caused by tuberculosis.
Methods
Study design
We constructed a hypothetical cohort of individuals developing tuberculosis in 2019, including pulmonary and extrapulmonary disease, who were stratified by factors related to the burden of tuberculosis or consequences after tuberculosis cure, and for whom sufficient data were available (country, age, sex, HIV status, and whether the individuals received tuberculosis treatment). We synthesised evidence describing quality of life and mortality among individuals with tuberculosis, during the disease episode and over their remaining life. Using this evidence, we simulated lifetime health outcomes for the cohort developing tuberculosis disease in 2019. We used DALYs to summarise the total health losses attributable to tuberculosis.
The WHO Global TB Programme reports annual estimates of the population developing tuberculosis disease, stratified by country, sex, and age group.1 For each country and sex, we interpolated these values to estimate incidence by single year of age in 2019. The fraction receiving treatment (by country, sex, and age) was based on reported case notifications1 divided by estimated incidence. For countries with missing notification data, we used WHO-estimated treatment coverage.1 We removed countries with insufficient data or with less than ten estimated tuberculosis cases for 2019. Applying these criteria, we retained 186 countries, representing 10·0 million tuberculosis cases, 99·99% of global incidence. To stratify cases by HIV status, we took estimates of age-specific HIV prevalence in the general population17 and inflated these by a common odds ratio (OR), to match the reported number of tuberculosis-HIV cases in each country.1 For countries for which this value was missing, we assumed tuberculosis-HIV prevalence was 0%. For each age and sex, the fraction receiving tuberculosis treatment was assumed to be independent of HIV status. We did not stratify the cohort by the presence or absence of multidrug-resistant tuberculosis (MDR-TB), extrapulmonary disease, or other factors that influence the severity of post-tuberculosis sequelae. The appendix (p 2) shows the distribution of the cohort across modelled strata.
Mortality and disability weight estimates during and after tuberculosis
We adopted WHO estimates of tuberculosis case fatality in 2019.1 To apply these average mortality risks to individual strata, we specified ORs describing differences in survival by age (using age-specific data on tuberculosis cases and deaths in the USA18, 19), and by HIV and tuberculosis treatment status, based on mortality risks used in WHO tuberculosis estimations.20 We applied these ORs to each country and calibrated average mortality risks to reproduce WHO country-level case fatality estimates. The appendix (p 2) shows the fraction of the cohort estimated to survive the disease episode, by age.
Tuberculosis disability weights were based on current Global Burden of Disease Study (GBD) valuations21 (table 1). For HIV-uninfected individuals without tuberculosis we assumed no disability. For HIV-infected individuals without tuberculosis we averaged the disability weights for “HIV: symptomatic, pre-AIDS” and “HIV/AIDS: receiving antiretroviral treatment”, weighted by the global fraction of HIV-infected individuals receiving antiretroviral treatment in 2019 (67%).17 We calculated the incremental disability weight associated with tuberculosis disease as the difference between individuals with and without tuberculosis disease, stratified by HIV status. We assumed disability values applied for the duration of the disease episode, and adopted WHO disease duration estimates,20 stratified by treatment and HIV status (table 1).
Table 1.
Values and sources for model parameters included in the uncertainty analysis
| Mean value (95% UI)* | Prior distribution | Source | |
|---|---|---|---|
| Disability weight for tuberculosis | 0·333 (0·224 to 0·454) | Beta | Global Burden of Disease Study (2019)21 |
| Disability weight for tuberculosis and HIV | 0·408 (0·274 to 0·549) | Beta | Global Burden of Disease Study (2019)21 |
| Disability weight for HIV on ART† | 0·078 (0·052 to 0·111) | Beta | Global Burden of Disease Study (2019)21 |
| Disability weight for symptomatic HIV, no ART† | 0·274 (0·184 to 0·377) | Beta | Global Burden of Disease Study (2019)21 |
| Disability weight for mild COPD | 0·019 (0·011 to 0·033) | Beta | Global Burden of Disease Study (2019)21 |
| Disability weight for moderate COPD | 0·225 (0·153 to 0·31) | Beta | Global Burden of Disease Study (2019)21 |
| Disability weight for severe COPD | 0·408 (0·273 to 0·556) | Beta | Global Burden of Disease Study (2019)21 |
| Mortality rate ratio for post-tuberculosis individuals | 2·91 (2·21 to 3·84) | Gamma | Romanowski et al (2019)2 |
| Linear term for log-linear model for mortality by FEV1% | −2·783 (−3·908 to 1·662) | Normal | Duong et al (2019)11 (function fitted to study data) |
| Quadratic term for log-linear model for mortality by FEV1% | 0·8947 (0·2759 to 1·522) | Normal | Duong et al (2019)11 (function fitted to study data) |
| Mortality risk ratio for post-tuberculosis (alternative specification) | 1·78 (1·61 to 1·98) | Gamma | Lee-Rodriguez et al (2020)22 |
| Intercept term for log-linear model for OR of chronic respiratory disease with post-tuberculosis | −1·494 (−3·223 to 0·321) | Normal | Byrne et al (2015)23 (function fitted to study data) |
| Slope term for log-linear model for OR of chronic respiratory disease in post-tuberculosis | 0·45 (0·057 to 0·831) | Normal | Byrne et al (2015)23 (function fitted to study data) |
| Duration of treated tuberculosis | 1·1 (0·2 to 2·0) | Gamma | WHO Global TB Database1 |
| Duration of untreated tuberculosis | 2·5 (1·0 to 4·0) | Gamma | WHO Global TB Database1 |
| Duration of treated tuberculosis, with HIV | 0·51 (0·01 to 1·0) | Gamma | WHO Global TB Database1 |
| Duration of untreated tuberculosis, with HIV | 0·11 (0·01 to 0·2) | Gamma | WHO Global TB Database1 |
| Total global tuberculosis cases in 2019 | 9·96 (8·94 to 11·00) | Gamma | WHO Global TB Database1 |
| Global average tuberculosis case fatality rate in 2019 | 0·14 (0·13 to 0·16) | Beta | WHO Global TB Database1 |
| Fraction of tuberculosis cases untreated | 0·29 (0·20 to 0·35) | Beta | WHO Global TB Database1 |
ART=antiretroviral therapy. COPD=chronic obstructive pulmonary disease. FEV1%=forced expiratory volume in 1 s. OR=odds ratio. UI=uncertainty interval.
Lower and upper bounds represent the 2·5th and 97·5th percentiles of the parameter distribution. Together these represent an equal-tailed 95% UI for the parameter.
Disability weights for HIV without tuberculosis are applied to HIV-infected tuberculosis survivors.
Individuals surviving tuberculosis disease face elevated mortality risks. A recent meta-analysis reported a standardised mortality ratio of 2·91 (95% CI 2·21–3·84) for tuberculosis survivors, compared with individuals without previous tuberculosis.2 These elevated risks reflect a combination of the causal impact of tuberculosis on future mortality risks and the effect of individual characteristics that are correlated with both mortality rates and tuberculosis disease, but not caused by tuberculosis. To decompose these two effects, we assumed that the causal effect of tuberculosis on future mortality can be estimated from the effects of tuberculosis on lung function, and the resulting effects of impaired lung function on mortality rates.
Two systematic reviews have assessed the elevated rates of chronic respiratory disease among tuberculosis survivors.23, 24 The more recent of these reviews described an association between the odds of COPD among individuals with post-tuberculosis and country-level tuberculosis incidence. In this context, incidence is probably a proxy for delayed case detection, recurrent disease episodes, and other risk factors for severe disease.5 We estimated a meta-regression model summarising this association (appendix p 3) and used these results to estimate an OR of chronic respiratory disease among individuals with post-tuberculosis for each country, based on their 2019 tuberculosis incidence (appendix p 4). We used the results of a multisite observational study11 to describe the population distribution of lung function, quantified as country-standardised forced expiratory volume in 1 s (FEV1%), and assumed that tuberculosis induced a downward shift of this distribution. For each country, the reduction in FEV1% among tuberculosis survivors was estimated to match the ORs for chronic respiratory disease in tuberculosis survivors, which was defined as an FEV1% lower than 80%. To model the association between FEV1% and mortality we fitted a quadratic function to mortality rate ratios (RRs) reported for different FEV1% impairment levels,11 and estimated the mortality risk ratio for post-tuberculosis as the average mortality rate for the post-tuberculosis FEV1% distribution compared with the distribution without post-tuberculosis. Country-level mortality RRs estimated for post-tuberculosis varied between 1·02 and 1·34, with a median of 1·14 (appendix p 4), and they were applied to all modelled strata.
We estimated all-cause mortality rates for each country, sex, age, and calendar year, by interpolating UN Population Division abridged life tables.25 To account for excess mortality among HIV-positive individuals we assumed a mortality RR producing an 8-year shorter life expectancy for HIV-positive individuals than for HIV-negative individuals.26 To calculate future mortality rates for tuberculosis survivors we multiplied all-cause general population mortality rates for each stratum by the standardised mortality ratio reported for tuberculosis survivors.2 To calculate future mortality rates for the cohort under a counterfactual where they had not developed tuberculosis, we divided the mortality rates estimated for the tuberculosis scenario by the causal mortality RR estimated for each country.
We adapted COPD disability weights to account for the reduced quality of life of tuberculosis survivors.21 We adopted a mapping between COPD severity levels and FEV1% impairment level from an earlier study of COPD burden,8 and applied these disability weights to the distribution of tuberculosis survivors across FEV1% impairment levels to calculate a post-tuberculosis disability weight for each country. Country-level estimates of the incremental disability caused by post-tuberculosis varied between 0·006 and 0·088, with a median of 0·036 (appendix p 4). These were applied to all model strata over the remaining life expectancy.
Statistical analysis
The primary outcome was DALYs attributable to tuberculosis, stratified by tuberculosis disease and post-tuberculosis periods, using a lifetime horizon. DALYs are calculated as the sum of years of life lost (YLLs) and years lived with disability (YLDs).27 We computed YLLs by multiplying deaths at each age by age-specific life expectancy, based on the GBD reference life table.28 We computed YLDs by multiplying total years lived in each health state by the disability weight for that state. As DALYs arising during the tuberculosis disease episode are also reported by the GBD study,29 we validated our results for this outcome against these existing estimates. As a secondary outcome, we calculated the reductions in life expectancy due to tuberculosis disease and post-tuberculosis compared with a no-tuberculosis counterfactual.
We specified probability distributions to represent uncertainty in model parameters (table 1), and we used second-order Monte Carlo simulation to generate 95% uncertainty intervals (UIs) for study outcomes.30 To do so, we re-estimated results for 1000 Latin hypercube samples from the parameter probability distributions, and calculated intervals as the 2·5th and 97·5th percentiles of the resulting distributions of each outcome. Analyses were conducted in R (v4.0.2).31
The mortality RR for post-tuberculosis used in the main analysis is conservative, as it only captures one of several mechanisms through which tuberculosis affects future mortality risks. As a sensitivity analysis, we recalculated results using a mortality risk ratio for post-tuberculosis of 1·78 (95% CI 1·61–1·98), based on a retrospective cohort study of individuals with and without post-tuberculosis, controlling for multiple demographic and clinical risk factors for mortality.22 We also calculated partial rank correlation coefficients (PRCCs) to report the relative influence of individual parameters on study outcomes.32
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Table 2 shows estimates of the total DALYs attributable to incident tuberculosis cases from 2019. Globally, we estimated 122 (95% UI 98–151) million DALYs, with 64 (54–75) million attributed to the tuberculosis disease episode, and 58 (38–83) million attributed to post-tuberculosis, representing 47% (37–57) of the total burden estimate. Globally, DALY estimates for the tuberculosis disease episode were 8·3% higher than GBD estimates (appendix p 5), largely due to greater tuberculosis mortality estimated by WHO1 than that estimated in the GBD study.29 Results for individual countries were highly consistent (rank correlation 0·97, appendix p 5). Mortality (reflected in the YLL results) represented the large majority (93% [88–96]) of DALYs from the tuberculosis disease episode.
Table 2.
Burden of disease caused by incident tuberculosis in 2019, globally and by WHO region
|
Outcomes by disease stage (millions) |
Percent increase with post-tuberculosis (%)* | |||
|---|---|---|---|---|
| Tuberculosis disease | Post-tuberculosis | Total | ||
| Years of life lost | ||||
| Eastern Mediterranean | 2·52 (2·14–2·99) | 3·55 (2·38–5·01) | 6·07 (4·78–7·68) | 141% (95–206) |
| Europe | 0·88 (0·75–1·02) | 0·64 (0·41–0·93) | 1·51 (1·23–1·83) | 73% (48–109) |
| Africa | 25·35 (21·51–30·03) | 12·46 (8·09–18·11) | 37·81 (31·49–45·26) | 49% (32–73) |
| Western Pacific | 2·87 (2·45–3·36) | 6·91 (4·70–9·75) | 9·78 (7·49–12·68) | 242% (164–351) |
| Americas | 0·75 (0·65–0·88) | 0·75 (0·50–1·08) | 1·51 (1·21–1·86) | 100% (66–148) |
| Southeast Asia | 26·90 (23·06–31·50) | 17·81 (11·92–25·38) | 44·72 (37·02–53·84) | 66% (44–97) |
| WHO high-burden | 50·96 (43·47–59·89) | 37·52 (24·91–53·46) | 88·48 (72·75–107·52) | 74% (49–109) |
| Global | 59·27 (50·56–69·63) | 42·12 (28·32–59·77) | 101·39 (83·64–122·78) | 71% (47–104) |
| Years lived with disability | ||||
| Eastern Mediterranean | 0·44 (0·22–0·77) | 1·36 (0·75–2·13) | 1·80 (1·13–2·63) | 340% (142–686) |
| Europe | 0·10 (0·04–0·19) | 0·21 (0·12–0·35) | 0·31 (0·19–0·49) | 262% (96–596) |
| Africa | 1·13 (0·57–1·94) | 3·43 (1·80–5·44) | 4·56 (2·83–6·65) | 331% (136–659) |
| Western Pacific | 0·78 (0·36–1·44) | 2·75 (1·54–4·28) | 3·53 (2·20–5·18) | 394% (153–809) |
| Americas | 0·11 (0·05–0·21) | 0·33 (0·19–0·52) | 0·44 (0·28–0·67) | 327% (126–691) |
| Southeast Asia | 1·96 (0·92–3·52) | 7·57 (4·13–11·99) | 9·53 (5·73–14·04) | 432% (167–886) |
| WHO high-burden | 3·91 (1·90–6·93) | 14·02 (7·65–22·14) | 17·94 (10·90–26·25) | 397% (156–789) |
| Global | 4·53 (2·21–8·00) | 15·64 (8·61–24·43) | 20·17 (12·48–29·52) | 383% (154–755) |
| Disability-adjusted life-years | ||||
| Eastern Mediterranean | 2·96 (2·47–3·54) | 4·90 (3·27–7·02) | 7·87 (6·05–10·21) | 167% (106–244) |
| Europe | 0·97 (0·82–1·14) | 0·85 (0·55–1·26) | 1·82 (1·45–2·27) | 88% (56–134) |
| Africa | 26·48 (22·46–31·29) | 15·89 (10·19–23·31) | 42·37 (35·01–51·52) | 60% (38–90) |
| Western Pacific | 3·65 (2·98–4·50) | 9·66 (6·50–13·84) | 13·31 (9·89–17·63) | 267% (169–388) |
| Americas | 0·87 (0·73–1·03) | 1·08 (0·71–1·58) | 1·94 (1·51–2·49) | 125% (82–186) |
| Southeast Asia | 28·87 (24·47–33·66) | 25·38 (16·67–36·84) | 54·25 (43·87–67·66) | 88% (56–130) |
| WHO high-burden | 54·88 (46·34–64·69) | 51·54 (33·78–74·85) | 106·42 (85·74–132·97) | 94% (61–140) |
| Global | 63·79 (53·87–75·16) | 57·76 (38·37–82·97) | 1121·55 (98·29–151·19) | 91% (59–133) |
Values in parentheses represent 95% uncertainty intervals.
Compares outcomes with vs without the inclusion of post-tuberculosis.
DALYs attributable to post-tuberculosis have not been included in past tuberculosis burden estimates, and their inclusion substantially increases burden estimates, by 91% (95% UI 59–132) globally. The increase in total DALYs produced by considering post-tuberculosis varied substantially by region, from 60% in Africa to 267% in the Western Pacific. The proportional increase in YLDs (reflecting the burden of non-fatal morbidity) was 383% (154–755), reflecting the extended duration of post-tuberculosis relative to the tuberculosis disease episode. The proportional increase in YLLs was 71% (47–104). Reductions in survival represent the majority (73% [68–79]) of DALYs from the post-tuberculosis period. We also estimated the DALYs from tuberculosis disease and post-tuberculosis for the 30 high-tuberculosis-burden countries identified by WHO (appendix p 8). In these results, the percent increase in DALYs resulting from the inclusion of post-tuberculosis varied widely between countries, driven largely by differences in WHO-estimated tuberculosis case fatality (appendix p 6), rank correlation= –0·74). When we looked at the global distribution of post-tuberculosis DALYs (appendix p 7), there were 7·5 (5·0–10·8) post-tuberculosis DALYs per 1000 individuals globally, and 21 countries with more than 20 post-tuberculosis DALYs per 1000 individuals.
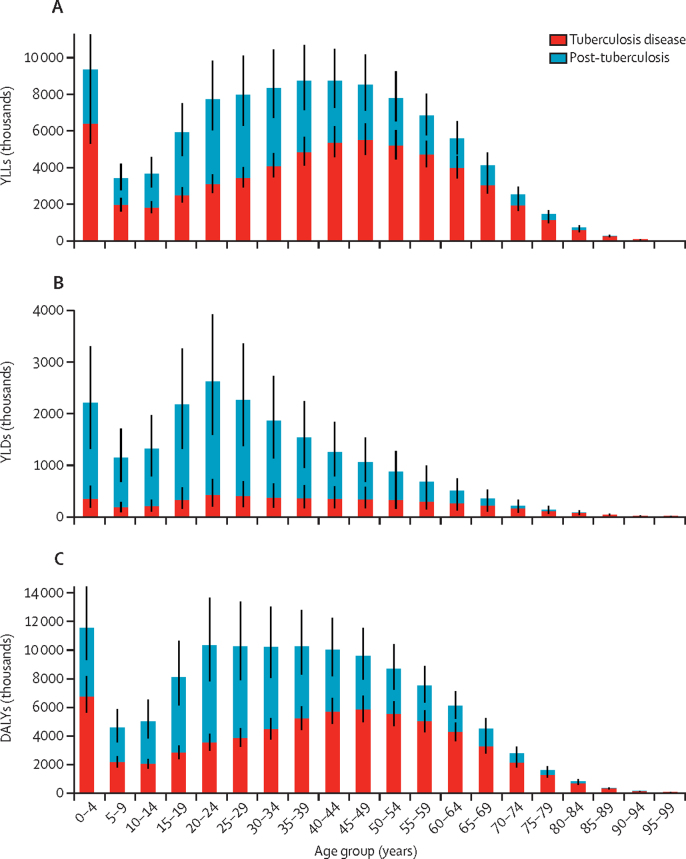
Figure 1 shows global estimates for YLLs, YLDs, and DALYs stratified by age, showing the increase in burden associated with post-tuberculosis in each age group. The proportional increase in DALYs from post-tuberculosis was smaller in older age groups, reflecting higher fatality during tuberculosis disease among older individuals.
Figure 1.
Estimates of YLLs (A), YLDs (B), and DALYs (C) attributable to tuberculosis disease in 2019, stratified by age group of disease incidence, and disease period*
YLL=years of life lost. YLD=years lived with disability. DALYs=disability-adjusted life-years. *Black bars represent 95% uncertainty intervals.
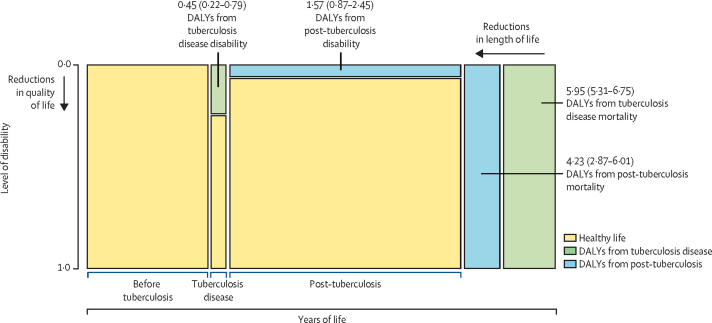
Figure 2 shows a schematic of the contribution of mortality and disability to the average global DALYs per individual developing tuberculosis in 2019, from both the tuberculosis disease episode and post-tuberculosis period. Globally, we estimated 12·1 DALYs (95% UI 10·0–14·9) per incident tuberculosis case, of which 6·3 DALYS (5·6–7·0) were attributable to the disease episode and 5·8 DALYS (3·8–8·3) attributable to post-tuberculosis.
Figure 2.
Average DALYs per incident tuberculosis case from increased disability and mortality rates attributable to tuberculosis, stratified by tuberculosis disease and post-tuberculosis period*
Area of each green and blue rectangle is proportional to the number of DALYs indicated, other dimensions not to scale. Values in parentheses represent 95% uncertainty intervals. DALYs=disability-adjusted life-years. *Total DALYs per incident tuberculosis case are equal to the sum of these values.
Total and per-person DALY estimates varied by individual-level and country-level factors (table 3). The average DALYs per incident case were estimated to be higher for younger individuals, HIV-positive individuals, individuals who do not receive tuberculosis treatment, and individuals living in high-incidence countries.
Table 3.
Total and per-person estimates of the burden of disease caused by incident tuberculosis in 2019
| Total tuberculosis cases (thousands) |
Absolute DALYs (millions) |
DALYs per incident tuberculosis case |
||||||
|---|---|---|---|---|---|---|---|---|
| Tuberculosis disease | Post-tuberculosis | Total | Tuberculosis disease | Post-tuberculosis | Total | |||
| All | 9954 (8952–11 003) | 63·79 (53·87–75·16) | 57·76 (38·37–82·97) | 121·55 (98·29–151·19) | 6·41 (5·69–7·20) | 5·80 (3·83–8·34) | 12·21 (10·02–14·85) | |
| Age group | ||||||||
| 1–4 years | 1185 (1065–1309) | 10·75 (8·97–13·02) | 10·26 (6·51–15·03) | 21·00 (16·61–26·56) | 9·07 (7·92–10·80) | 8·66 (5·52–12·92) | 17·73 (14·16–21·99) | |
| 15–34 years | 3307 (2974–3655) | 14·43 (12·16–17·06) | 24·32 (16·14–35·07) | 38·75 (29·76–50·70) | 4·36 (3·85–4·97) | 7·36 (4·82–10·71) | 11·72 (9·12–15·03) | |
| 35–54 years | 3039 (2733–3359) | 22·06 (18·72–25·77) | 16·32 (10·85–23·19) | 38·37 (31·55–46·87) | 7·26 (6·47–8·18) | 5·37 (3·59–7·65) | 12·63 (10·61–15·02) | |
| 55–74 years | 1998 (1797–2208) | 14·41 (12·26–16·76) | 6·29 (4·27–9·00) | 20·69 (17·61–24·29) | 7·21 (6·41–8·01) | 3·15 (2·17–4·45) | 10·36 (9·09–11·79) | |
| ≥75 years | 426 (383–471) | 2·15 (1·83–2·51) | 0·58 (0·40–0·83) | 2·73 (2·33–3·19) | 5·05 (4·47–5·65) | 1·36 (0·95–1·92) | 6·41 (5·77–7·17) | |
| Sex | ||||||||
| Male | 6158 (5538–6807) | 39·75 (33·58–46·81) | 35·61 (23·69–51·05) | 75·36 (61·12–93·50) | 6·45 (5·73–7·24) | 5·78 (3·85–8·26) | 12·24 (10·10–14·82) | |
| Female | 3796 (3414–4196) | 24·04 (20·29–28·35) | 22·15 (14·63–32·05) | 46·19 (37·13–57·77) | 6·33 (5·62–7·13) | 5·84 (3·81–8·50) | 12·17 (9·93–14·84) | |
| Tuberculosis incidence level (cases per 100 000 population) | ||||||||
| <10 | 51 (46–56) | 0·13 (0·11–0·15) | 0·08 (0·03–0·18) | 0·21 (0·15–0·31) | 2·48 (2·15–2·87) | 1·60 (0·50–3·68) | 4·08 (2·93–6·30) | |
| 10–49 | 331 (298–366) | 1·05 (0·89–1·25) | 1·02 (0·58–1·58) | 2·07 (1·58–2·69) | 3·19 (2·78–3·62) | 3·08 (1·82–4·84) | 6·27 (4·92–8·02) | |
| 50–199 | 4726 (4250–5224) | 29·43 (24·90–34·33) | 23·70 (16·26–34·37) | 53·13 (43·57–64·51) | 6·23 (5·53–6·95) | 5·02 (3·47–7·10) | 11·24 (9·53–13·53) | |
| ≥200 | 4846 (4359–5357) | 33·19 (28·02–39·07) | 32·96 (20·25–49·02) | 66·14 (52·32–83·97) | 6·85 (6·05–7·81) | 6·80 (4·09–10·14) | 13·65 (10·79–17·05) | |
| HIV status | ||||||||
| HIV positive | 999 (899–1104) | 11·84 (10·12–13·84) | 5·81 (3·78–8·44) | 17·65 (14·84–21·04) | 11·85 (10·59–13·39) | 5·82 (3·79–8·51) | 17·67 (15·27–20·45) | |
| HIV negative | 8955 (8053–9898) | 51·95 (43·86–61·16) | 51·95 (34·55–74·36) | 103·90 (83·26–130·27) | 5·80 (5·13–6·54) | 5·80 (3·83–8·36) | 11·60 (9·44–14·24) | |
| Tuberculosis treatment | ||||||||
| Treated | 7224 (6230–8270) | 17·43 (9·75–28·92) | 43·84 (28·17–64·45) | 61·27 (40·87–86·32) | 2·39 (1·48–3·59) | 6·07 (4·09–8·76) | 8·46 (6·10–11·35) | |
| Untreated | 2730 (1999–3530) | 46·36 (33·70–60·53) | 13·92 (8·04–22·31) | 60·28 (43·59–79·15) | 16·99 (15·24–18·81) | 5·10 (3·27–7·55) | 22·09 (19·51–24·84) | |
Values in parentheses represent 95% uncertainty intervals. DALYs=disability-adjusted life-years.
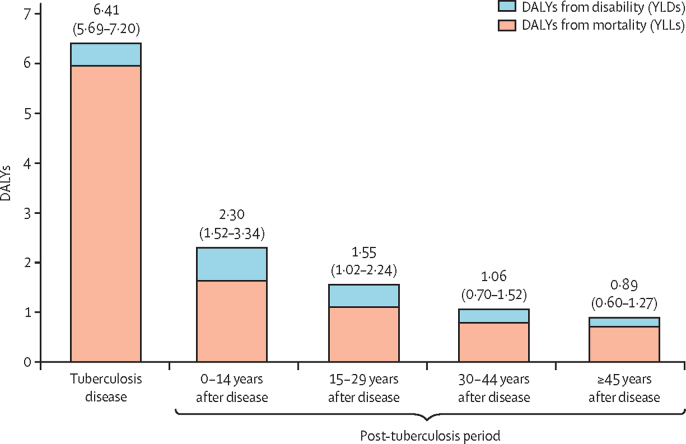
Figure 3 shows the average DALYs per case according to when the burden (ie, the tuberculosis-attributable death, or time spent with disability) occurs. The tuberculosis disease episode represented a short period of high disability and mortality. By contrast, the DALYs from post-tuberculosis were spread over the remaining lifespan, with almost a third (28% [23–34]) of total DALYs accruing 15 or more years after an individual first developed tuberculosis.
Figure 3.
DALYs per incident tuberculosis case, stratified by tuberculosis disease and post-tuberculosis period*
YLL=years of life lost. YLD=years lived with disability. DALYs=disability-adjusted life-years. *Total DALYs per incident tuberculosis case equal to the sum of these values. Values in parentheses represent 95% uncertainty intervals.
The appendix (p 9) shows reductions in life-years lived due to tuberculosis disease and post-tuberculosis, compared with a counterfactual in which individuals do not develop tuberculosis. Per incident tuberculosis case, we estimated an average reduction in life expectancy from mortality during the tuberculosis disease episode of 2·92 years (2·60–3·35) and an additional reduction in life expectancy from post-tuberculosis mortality of 1·87 years (1·21–2·80). The average reduction in life expectancy attributed to incident tuberculosis in 2019 (summing the effects of excess mortality over the life course) was 4·79 years (3·94–5·86).
The appendix (p 10) shows results for the alternative model specification with a higher mortality risk ratio for post-tuberculosis. In these results, total YLLs from post-tuberculosis increased from 42·1 (28·3–59·8) million to 94·1 (78·7–111·6) million. The appendix (p 11) shows PRCCs for each model parameter, for several outcomes. For DALYs due to the tuberculosis disease episode, the most influential parameters were the inputs for total global incidence in 2019 (PRCC=0·89), and case fatality during the disease episode (PRCC=0·89). For DALYs due to post-tuberculosis, the most influential parameters were the OR of respiratory disease with post-tuberculosis (PRCC=0·86), and total global incidence in 2019 (PRCC=0·46). For both total DALYs and the percentage increase in DALYs from post-tuberculosis, the most influential parameter was the OR of respiratory disease with post-tuberculosis (PRCC=0·83 and 0·85, respectively).
Discussion
To our knowledge, this study is the first to estimate the global burden of tuberculosis inclusive of post-tuberculosis morbidity and mortality. Even with conservative assumptions, the inclusion of post-tuberculosis substantially increased the total burden estimate. For the 8·5 million individuals surviving incident tuberculosis in 2019, we estimated that 58 million post-tuberculosis DALYs will accrue over the remaining lifetime, representing almost half of the 122 million DALYs estimated for tuberculosis.
Our burden estimate was approximately twice the GBD estimate for tuberculosis DALYs in the same year.29 Our results do not represent a proposed correction of the GBD estimates, as the two approaches answer different questions. First, we adopted an incidence approach, quantifying DALYs that would accrue over the lifetime due to disease cases arising in a single year. By contrast, GBD uses a prevalence approach to estimate health losses from disability, summing the DALYs realised in a given year irrespective of when the causative disease case occurred. Second, and more fundamentally, the GBD estimates follow ICD disease classification conventions, with the consequence that incremental deaths due to post-tuberculosis are attributed to causes such as respiratory or cardiovascular disease, rather than tuberculosis. Within the GBD framework, the role of post-tuberculosis is more similar to a risk factor, as it increases the risks of other diseases over the lifetime.
The primary disease burden tabulations produced by GBD are calculated to be categorical, such that the sum of DALYs for each individual disease equals the total DALYs from all diseases. However, users of disease burden estimates commonly interpret them to be the burden caused by a given disease, in the sense that they summarise the death and disability avertable through global elimination of that disease. If our results are correct, the causal interpretation of current GBD estimates for tuberculosis disease burden substantially undervalues the potential impact of tuberculosis control efforts. Appropriate valuation of post-tuberculosis disease burden might also lead to reprioritisation within the tuberculosis control portfolio. This could include greater attention to programmatic activities that can prevent tuberculosis from occurring in the first place, to active case-finding that may accelerate diagnosis, and to interventions that can prevent, mitigate, or repair lung damage from tuberculosis. These changes are consistent with a broader definition of treatment success, beyond simply sterilising the causative pathogen. This could include a greater role for end-of-treatment assessments of pathological damage and functional deficits in order to implement interventions that meet the health-care needs of tuberculosis survivors, who in any given year represent a much larger group than the individuals newly developing tuberculosis.12 While initial assessment can be done by tuberculosis programmes, ongoing care will probably require integrated, multi-specialty services.33
The magnitude of post-tuberculosis disease burden also has implications for new research. In particular, further research is needed on the mechanisms of post-tuberculosis impairment, the range of conditions experienced by tuberculosis survivors, and how the extent of impairment changes over time.34 Research is also needed to describe management strategies for post-tuberculosis symptoms and disability, and their effectiveness at improving long-term outcomes for tuberculosis survivors. Policy analyses used to prioritise tuberculosis interventions should also take greater account of post-tuberculosis burden. This new approach may lead to a reweighting of current intervention priorities, and to the identification of a cost-effective portfolio of services for post-tuberculosis individuals. Finally, research is needed to better understand the social and economic implications of post-tuberculosis, including health-care use, social exclusion, and household economic outcomes. This is particularly important given the global target of achieving zero households experiencing catastrophic costs from tuberculosis, described in the End TB Strategy.35
Our analysis has several limitations. First, our results might represent an underestimate of post-tuberculosis burden, as we only considered one pathway through which tuberculosis affects future health outcomes. Tuberculosis disease has deleterious effects on multiple organ systems, and some tuberculosis survivors experience respiratory symptoms even in the absence of clinically impaired lung function.36 Chronic post-tuberculosis disability can also lead to stigma, social exclusion, and poverty, which themselves predispose to additional health risks. A more comprehensive assessment of these effects could produce a larger estimate of post-tuberculosis DALYs. As an indicator of what a more comprehensive assessment might imply, the alternative specification using a higher mortality risk ratio for post-tuberculosis increased the total tuberculosis burden estimate by a further 40%. Second, there is substantial uncertainty associated with our estimates, including parameter uncertainty (quantified as UIs around major outcomes), and structural uncertainty, which represents uncertainty about the functional form of the analytic model, not captured in the reported intervals. A major source of uncertainty is the weakness around the causal attribution of post-tuberculosis health outcomes to tuberculosis (as compared with existing risk factors among individuals developing tuberculosis). These causal assumptions affect the mortality risk ratios and disability weights for tuberculosis survivors. In our analyses, we estimated that the contribution of post-tuberculosis to elevated mortality among tuberculosis survivors2 varied between 2% and 27% across countries (median 12%), yet there is substantial uncertainty around the average value as well as intercountry differences. Age-specific ORs for tuberculosis mortality represent another source of uncertainty. Finally, our analysis did not distinguish conditions for which post-tuberculosis outcomes are likely to be different, including multidrug-resistant and extensively drug-resistant tuberculosis, recurrent tuberculosis, extrapulmonary tuberculosis, or extensive disease at initial presentation. For most of these conditions the burden of post-tuberculosis could be greater than estimated in this analysis.34 Similarly, outcomes might be different for children surviving tuberculosis, and better evidence on paediatric post-tuberculosis is needed.
Many individuals who survive tuberculosis disease face ongoing disability, despite achieving microbiological cure. For these tuberculosis survivors, post-tuberculosis sequelae limit opportunities and enjoyment of life, and increase the risk of early death. Our analysis has quantified these health losses, demonstrating that post-tuberculosis adds substantially to the global disease burden caused by tuberculosis. Future analyses should take better account of post-tuberculosis, to avoid the potential misallocation of funding, political attention, and research effort that would be produced by continued neglect of this issue.
Data sharing
Analytic code and data inputs are available upon request to the corresponding author (nmenzies@hsph.harvard.edu).
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
NAM and TC acknowledge funding from the National Institutes of Health (NIH, R01AI146555). MQ acknowledges funding from the Bill & Melinda Gates Foundation (INV-001754). AKC acknowledges funding from WEHI philanthropy. JM acknowledges funding from the Medical Research Council (MR/S02042X/1). RSW acknowledges funding from Horizon 2020 and EDCTP. RMGJH acknowledges funding from the European Research Council (757699).
Contributors
NAM, MQ, RMGJH, and TC conceptualised the study. BWA, ALB, AKC, ADH, FMM, JM, DP, JAS, SS, SCvK, and RSW contributed to study design. NAM implemented the analysis. MQ, BWA, ALB, AKC, ADH, FMM, JM, DP, JAS, SS, SCvK, RSW, RMGJH, and TC reviewed results. NAM drafted the manuscript. MQ, BWA, ALB, AKC, ADH, FMM, JM, DP, JAS, SS, SCvK, RSW, RMGJH, and TC edited the manuscript. NAM, MQ, RMGJH, and TC have verified the underlying data.
Supplementary Material
References
- 1.WHO Global TB Programme WHO Global TB Database. http://www.who.int/tb/country/data/download/en/
- 2.Romanowski K, Baumann B, Basham CA, Ahmad Khan F, Fox GJ, Johnston JC. Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2019;19:1129–1137. doi: 10.1016/S1473-3099(19)30309-3. [DOI] [PubMed] [Google Scholar]
- 3.Ravimohan S, Kornfeld H, Weissman D, Bisson GP. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev. 2018;27 doi: 10.1183/16000617.0077-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amaral AF, Coton S, Kato B, et al. Tuberculosis associates with both airflow obstruction and low lung function: BOLD results. Eur Respir J. 2015;46:1104–1112. doi: 10.1183/13993003.02325-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allwood BW, van der Zalm MM, Amaral AFS, et al. Post-tuberculosis lung health: perspectives from the First International Symposium. Int J Tuberc Lung Dis. 2020;24:820–828. doi: 10.5588/ijtld.20.0067. [DOI] [PubMed] [Google Scholar]
- 6.Ross J, Ehrlich RI, Hnizdo E, White N, Churchyard GJ. Excess lung function decline in gold miners following pulmonary tuberculosis. Thorax. 2010;65:1010–1015. doi: 10.1136/thx.2009.129999. [DOI] [PubMed] [Google Scholar]
- 7.Plit ML, Anderson R, Van Rensburg CE, et al. Influence of antimicrobial chemotherapy on spirometric parameters and pro-inflammatory indices in severe pulmonary tuberculosis. Eur Respir J. 1998;12:351–356. doi: 10.1183/09031936.98.12020351. [DOI] [PubMed] [Google Scholar]
- 8.Soriano JB, Abajobir AA, Abate KH, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5:691–706. doi: 10.1016/S2213-2600(17)30293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mapel DW, Hurley JS, Frost FJ, Petersen HV, Picchi MA, Coultas DB. Health care utilization in chronic obstructive pulmonary disease. A case-control study in a health maintenance organization. Arch Intern Med. 2000;160:2653–2658. doi: 10.1001/archinte.160.17.2653. [DOI] [PubMed] [Google Scholar]
- 10.Meghji J, Gregorius S, Madan J, et al. The long term effect of pulmonary tuberculosis on income and employment in a low income, urban setting. Thorax. 2021;76:387–395. doi: 10.1136/thoraxjnl-2020-215338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duong M, Islam S, Rangarajan S, et al. Mortality and cardiovascular and respiratory morbidity in individuals with impaired FEV1 (PURE): an international, community-based cohort study. Lancet Glob Health. 2019;7:e613–e623. doi: 10.1016/S2214-109X(19)30070-1. [DOI] [PubMed] [Google Scholar]
- 12.Dodd PJ, Yuen CM, Jayasooriya SM, van der Zalm MM, Seddon JA. Quantifying the global number of tuberculosis survivors: a modelling study. Lancet Infect Dis. 2021;21:984–992. doi: 10.1016/S1473-3099(20)30919-1. [DOI] [PubMed] [Google Scholar]
- 13.Tuberculosis Collaborators GBD The global burden of tuberculosis: results from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2018;18:261–284. doi: 10.1016/S1473-3099(17)30703-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houben RMGJ, Menzies NA, Sumner T, et al. Feasibility of achieving the 2025 WHO global tuberculosis targets in South Africa, China, and India: a combined analysis of 11 mathematical models. Lancet Glob Health. 2016;4:e806–e815. doi: 10.1016/S2214-109X(16)30199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quaife M, Houben RMGJ, Allwood B, et al. Post-tuberculosis mortality and morbidity: valuing the hidden epidemic. Lancet Respir Med. 2020;8:332–333. doi: 10.1016/S2213-2600(20)30039-4. [DOI] [PubMed] [Google Scholar]
- 16.Pasipanodya JG, McNabb SJN, Hilsenrath P, et al. Pulmonary impairment after tuberculosis and its contribution to TB burden. BMC Public Health. 2010;10:259. doi: 10.1186/1471-2458-10-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.UNAIDS . UNAIDS; Geneva, Switzerland: 2021. AIDSinfo Epidemiological Estimates Database.https://aidsinfo.unaids.org/ [Google Scholar]
- 18.US CDC . Centers for Disease Control and Prevention; Atlanta, GA: 2020. Online Tuberculosis Information System (OTIS), National Tuberculosis Surveillance System, United States, 1993–2019.https://wonder.cdc.gov/tb.html [Google Scholar]
- 19.US CDC . Centers for Disease Control and Prevention; Atlanta, GA: 2020. Multiple Cause of Death 1999–2019 on CDC WONDER Online Database.http://wonder.cdc.gov/mcd-icd10.html [Google Scholar]
- 20.WHO Global Tuberculosis Report 2020. Geneva: World Health Organization. https://www.who.int/tb/publications/global_report/en/
- 21.Global Burden of Disease Collaborative Network . Institute for Health Metrics and Evaluation; Seattle, USA: 2020. Global Burden of Disease Study 2019 (GBD 2019) Disability Weights.http://ghdx.healthdata.org/record/ihme-data/gbd-2019-disability-weights [Google Scholar]
- 22.Allwood BW, Myer L, Bateman ED. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration. 2013;86:76–85. doi: 10.1159/000350917. [DOI] [PubMed] [Google Scholar]
- 23.Byrne AL, Marais BJ, Mitnick CD, Lecca L, Marks GB. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis. 2015;32:138–146. doi: 10.1016/j.ijid.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 24.UN Population Division . online edition, rev 1. United Nations, Department of Economic and Social Affairs; Geneva: 2019. World population prospects 2019.https://population.un.org/wpp/Download/Standard/Population/ [Google Scholar]
- 25.Marcus JL, Chao CR, Leyden WA, et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr. 2016;73:39. doi: 10.1097/QAI.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray CJ. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ. 1994;72:429–445. [PMC free article] [PubMed] [Google Scholar]
- 27.Global Burden of Disease Collaborative Network . Institute for Health Metrics and Evaluation; Seattle, USA: 2021. Global Burden of Disease Study 2019 (GBD 2019) Reference Life Table. [DOI] [Google Scholar]
- 28.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making. 2012;32:722–732. doi: 10.1177/0272989X12458348. [DOI] [PubMed] [Google Scholar]
- 30.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: a language and environment for statistical computing. [Google Scholar]
- 31.Lee-Rodriguez C, Wada PY, Hung Y-Y, Skarbinski J. Association of mortality and years of potential life lost with active tuberculosis in the United States. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iman RL, Helton JC. An investigation of uncertainty and sensitivity analysis techniques for computer models. Risk Anal. 1988;8:71–90. [Google Scholar]
- 33.Harries AD, Dlodlo RA, Brigden G, et al. Should we consider a ‘fourth 90’ for tuberculosis? Int J Tuberc Lung Dis. 2019;23:1253–1256. doi: 10.5588/ijtld.19.0471. [DOI] [PubMed] [Google Scholar]
- 34.Allwood BW, Byrne A, Meghji J, Rachow A, van der Zalm MM, Schoch OD. Post-tuberculosis lung disease: clinical review of an under-recognised global challenge. Respiration. 2021;100:751–763. doi: 10.1159/000512531. [DOI] [PubMed] [Google Scholar]
- 35.Uplekar M, Weil D, Lonnroth K, et al. WHO's new end TB strategy. Lancet. 2015;385:1799–1801. doi: 10.1016/S0140-6736(15)60570-0. [DOI] [PubMed] [Google Scholar]
- 36.Allwood BW, Stolbrink M, Baines N, et al. Persistent chronic respiratory symptoms despite TB cure is poorly correlated with lung function. Int J Tuberc Lung Dis. 2021;25:262–270. doi: 10.5588/ijtld.20.0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Analytic code and data inputs are available upon request to the corresponding author (nmenzies@hsph.harvard.edu).