Significance
Mosquitoes are a global threat to human health. Mosquito female reproduction depends on juvenile hormone (JH), synthesized from inactive precursors through methylation and subsequent epoxidation. The latter occurs in insects but not in earlier-diverging arthropod lineages, such as crustaceans. Our work on genetic knockout mosquitoes lacking either the methylating or the epoxidating enzyme revealed a vital requirement of JH precursor methylation for mosquito development and a key contribution of JH epoxidation to reproductive fitness. Both sexes underperformed in reproduction when deprived of the epoxidated hormone. This finding advances our understanding of mosquito reproductive endocrinology. More generally, our data unveil the importance of JH epoxidation as an evolutionary innovation improving the reproductive performance and increasing the success of insects.
Keywords: Aedes aegypti, corpora allata, juvenile hormone, methyl farnesoate, reproduction
Abstract
Methyl farnesoate (MF) plays hormonal regulatory roles in crustaceans. An epoxidated form of MF, known as juvenile hormone (JH), controls metamorphosis and stimulates reproduction in insects. To address the evolutionary significance of MF epoxidation, we generated mosquitoes completely lacking either of the two enzymes that catalyze the last steps of MF/JH biosynthesis and epoxidation, respectively: the JH acid methyltransferase (JHAMT) and the P450 epoxidase CYP15 (EPOX). jhamt−/− larvae lacking both MF and JH died at the onset of metamorphosis. Strikingly, epox−/− mutants, which synthesized MF but no JH, completed the entire life cycle. While epox−/− adults were fertile, the reproductive performance of both sexes was dramatically reduced. Our results suggest that although MF can substitute for the absence of JH in mosquitoes, it is with a significant fitness cost. We propose that MF can fulfill most roles of JH, but its epoxidation to JH was a key innovation providing insects with a reproductive advantage.
Insects and crustaceans originated from aquatic pancrustacean ancestors that invaded terrestrial ecosystems over 450 million y ago (1–3). Pancrustaceans acquired the ability to biosynthesize sesquiterpenoids, such as farnesoic acid (FA) and methyl farnesoate (MF), which serve signaling functions (4). However, unlike insects, crustaceans lack the capacity to further convert FA and MF into epoxidated juvenile hormones (JHs). These structurally unique hormones stimulate insect reproduction and control their metamorphosis (5, 6). Both MF and JH act through a ligand-activated transcription factor Methoprene-tolerant (Met), a member of the bHLH-PAS protein family established as an intracellular JH receptor (7, 8). The ability of insect (JH) and crustacean (MF) hormones to activate either insect (9, 10) or crustacean (11) Met orthologs suggests that the role of Met in sesquiterpenoid signaling predates the evolutionary separation of the Hexapoda from the crustacean lineages.
The MF epoxidase (EPOX) is an insect-specific P450 enzyme that belongs to the CYP15 family (12). It catalyzes the enantioselective conversion of MF into epoxidated JHs. With the appearance of CYP15 enzymes, JH biosynthetic pathways diverged and generated several structurally different epoxidated JHs, including JH III (5, 6). The ancestral roles of MF diversified extensively in insect lineages, with epoxidated JHs playing vital functions in regulating development, metamorphosis, reproduction, diapause, behavior, and polyphenism (13).
To address the evolutionary significance of MF epoxidation, we generated Aedes aegypti mosquitoes completely lacking either of the two enzymes that catalyze the last steps of MF/JH biosynthesis and epoxidation, the JH acid methyltransferase (JHAMT) (14) and the epoxidase (15) (Fig. 1A). jhamt−/− larvae lacking both MF and JH III died at the onset of metamorphosis. Strikingly, epox−/− mutants, which synthesized MF but no JH III, completed the entire life cycle. These null mutant mosquitoes provided a model to investigate evolutionary relationships between epoxidation of JH and development and reproductive fitness. Our results show that while MF can fulfill most functions of JH III, sesquiterpenoid epoxidation has provided a reproductive advantage, likely contributing to the phenomenal evolutionary success of insects.
Fig. 1.
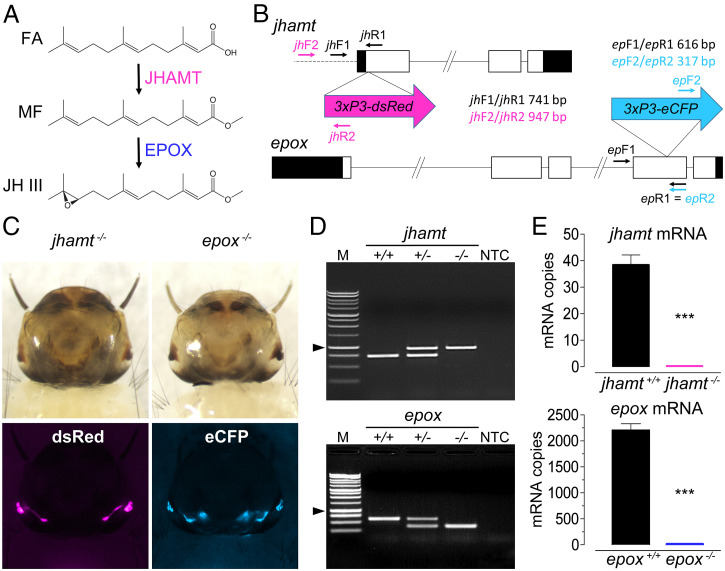
Characterization of jhamt−/− and epox−/− mutants. (A) The last two steps of JH biosynthesis in mosquitoes. (B) Schematics of the A. aegypti jhamt and epox genes. Exons are represented by boxes (open parts depict coding regions) connected by introns (solid lines); the dashed line represents the upstream genomic region of jhamt. The large arrows depict the DNA constructs with marker genes inserted via CRISPR/Cas9-mediated HDR into the jhamt (magenta) and epox (turquoise) loci, respectively. The insertion sites are indicated. Small arrows show the orientation and approximate positions of primers used for genotyping WT (black) and mutant (colored) alleles; the amplicon sizes are given. The scheme is not drawn to scale. (C) Brightfield and fluorescent images of L4-instar larvae expressing the dsRed (jhamt−/−) and eCFP (epox−/−) markers under the 3xP3 enhancer. (Magnification, 20×.) (D) Examples of PCR genotyping using genomic DNA of WT (+/+), heterozygous (+/−), and homozygous (−/−) mutant larvae. M, molecular size marker; NTC, no template control (arrowheads indicate 1 kb). (E) RT-qPCR analysis. For jhamt, RNA was from five independent samples of individual L2-instar larvae; epox RNA was quantified from three independent samples, each with five CA complexes dissected from 5-d-old adult females. Levels of mRNAs are expressed as copy number per 10,000 copies of rpL32 mRNA. Columns represent mean ± SEM (***P ≤ 0.001, unpaired t test).
Results
jhamt and epox Mutants Represent Null Alleles.
To generate A. aegypti mosquitoes deficient in both MF and JH or deficient in just the epoxidated JH, we employed CRISPR/Cas9-mediated homology-dependent repair (HDR) to introduce fluorescent markers into the first exon of the jhamt (dsRed) and the fourth exon of the epox (eCFP) genes, respectively (Fig. 1B). Insertion of the dsRed and eCFP cassettes disrupted both genes in the germline, thus producing stable jhamt and epox mutants, marked with the fluorescent proteins expressed in the eyes (Fig. 1C). The null character of the alleles was established by genotyping (Fig. 1D), and the absence of transcripts for each gene knockout was confirmed using reverse-transcriptase quantitative PCR (RT-qPCR) (Fig. 1E). A stable homozygous epox−/− line was established after seven backcrosses to WT mosquitoes. Due to homozygous lethality, the jhamt null allele had to be kept in the heterozygous jhamt+/− state, and the jhamt−/− genotype was determined using PCR in the genomic DNA of individual larvae. The Mendelian inheritance of the dsRed and eCFP markers was confirmed in G5 outcrosses (phenotypic ratio 1:1) and F1 intercrosses (phenotypic ratio 3:1) (SI Appendix, Fig. S1).
Synthesis of Sesquiterpenoids in jhamt and epox Null Mutants.
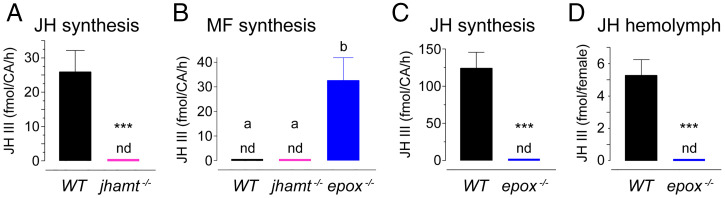
It was critical to examine the anticipated deleterious effects of jhamt and epox loss on sesquiterpenoid production. The quantities of MF and JH III synthesized ex vivo by the corpora allata (CA) glands isolated from WT and mutant mosquitoes were determined using liquid chromatography coupled to electrospray tandem mass spectrometry (LC-MS/MS) (16). Neither MF nor JH III were produced by the CA from jhamt−/− mosquito larvae (Fig. 2 A and B). In contrast, the CA from epox−/− adults synthesized MF but not JH III (Fig. 2 B and C). While normally undetected in the output of WT CA, the MF that could not be metabolized to JH III in the absence of EPOX accumulated in the medium containing the epox−/− CA glands (Fig. 2B). As expected, JH III was detected in the hemolymph of WT but not epox−/− adults (Fig. 2D). JH III was also produced by the CA obtained from females heterozygous for the jhamt null mutation. Although the jhamt+/− CA expressed about 50% of the jhamt mRNA that the WT glands did, the amount of JH III they produced was not lowered (SI Appendix, Fig. S2), indicating that a single dose of the functional jhamt+ gene was sufficient to produce the enzyme for synthesis of normal amounts of JH III. Together, the data demonstrate that we have succeeded in genetically separating the capacity to produce epoxidated JH from the ability to synthesize MF.
Fig. 2.
MF and JH synthesis and hemolymph titers. (A) Average amounts of JH III synthesized by 10 individual CA complexes from L4-instar WT and jhamt−/− larvae. (B and C) Average amounts of MF synthesized by 10 individual CA complexes from L4-instar jhamt−/− larvae (B). Average amounts of MF (B) or JH III (C) synthesized by three CA complexes dissected from four independent groups of sugar-fed WT or epox−/− adult females aged 5 to 6 d. (D) JH III titers in the hemolymph (four independent replicates) pooled from eight sugar-fed WT or epox−/− adult females aged 5 to 6 d. n.d., levels not detectable (below 0.5 fmol). Columns represent mean ± SEM in all panels. Significant differences are marked with asterisks (***P ≤ 0.001, unpaired t test); letters above columns indicate in B, P ≤ 0.05 (one-way analysis of variance with Tukey’s post hoc comparison).
Developmental Phenotypes of jhamt and epox Null Mutants.
The impact of deficiencies of either JH or both MF and JH on mosquito development was followed. Hatching rates did not significantly differ between the WT, epox−/−, and jhamt−/− genotypes, the latter being inferred from the predicted genotype ratio of the jhamt+/− F1 offspring (SI Appendix, Fig. S3). Homozygous jhamt−/− larvae originating from heterozygous parents could be distinguished from jhamt+/− siblings by observing a higher intensity of red fluorescence in the eyes (SI Appendix, Fig. S4). The genotype of selected larvae was subsequently confirmed using PCR (SI Appendix, Fig. S5).
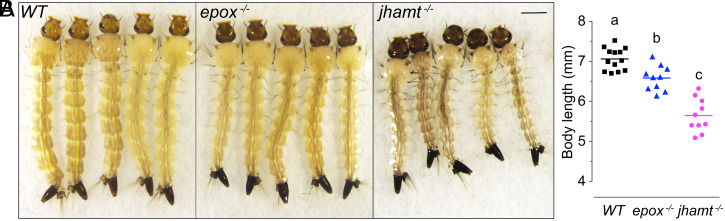
Homozygous jhamt−/− larvae hatched normally but were smaller than WT larvae (SI Appendix, Fig. S6) and developed at a slightly slower pace (SI Appendix, Fig. S7). The size difference became progressively more obvious in the fourth, final-instar larvae (L4) (Fig. 3A). The body length attained by jhamt−/− L4 larvae was 20% shorter than that of WT L4 larvae and was 14% below the size attained by epox−/− L4 larvae (Fig. 3B). The mutants were darker than WT larvae in some parts of their bodies, particularly the respiratory siphons and head capsules (Fig. 3A). Having reached the L4 instar, all homozygous jhamt−/− larvae died just prior to pupation. At the lethal stage, the jhamt−/− animals showed dark respiratory siphons and everted flabella (feeding brushes) (Fig. 3A), two developmental hallmarks normally observed 6 to 7 h before pupation (17). Topical application of the JH receptor agonist Met to jhamt−/− larvae at day 1 of the L4 instar advanced development of a large proportion of the MF- and JH-deficient mutants, so that some could pupate; however, none developed beyond an early pupal stage (SI Appendix, Fig. S8). EPOX-deficient progeny was obtained from the homozygous epox−/− stock. In contrast to jhamt knockout, epox−/− L4 larvae were slightly, albeit significantly, smaller than WT controls (Fig. 3 and SI Appendix, Fig. S9) and developed with a minor delay (SI Appendix, Fig. S10). All of them pupated by day 8 after hatching and reached adulthood.
Fig. 3.
Phenotypes of wild-type (WT) and null mutant larvae. (A) Light microscope images of live, late L4-instar larvae. (Scale bar, 1 mm.) (B) Average larval body size at the L4 S stage (see also SI Appendix, Figs. S6, S8, and S9). ImageJ software was used to measure the body length of individual larvae, represented by the data points. Lines denote mean values, letters above them indicate significant differences (P ≤ 0.05, one-way ANOVA with Tukey’s multiple post hoc comparison).
In summary, neither JHAMT nor EPOX were necessary for mosquitoes to complete embryogenesis and larval development until the final, L4 instar. Without Met treatment, jhamt−/− larvae could not pupate, and regardless of the treatment, these mutants never survived to adulthood. In contrast, epox−/− larvae metamorphosed and successfully reproduced, generating a viable homozygous mutant line. Therefore, while jhamt was required to complete the life cycle, epox was not an essential gene, at least under our laboratory conditions.
Expression of JH-Regulated Genes in jhamt and epox Null Mutants.
To further understand the effects of JHAMT and EPOX deficiency on larval development, we examined the expression of three key transcription factor genes, Krüppel-homolog 1 (Kr-h1), Broad-Complex (Br-C), and E93, which regulate insect development and metamorphosis downstream of JH (18–23). Eggs laid by single jhamt+/− and epox−/− females were hatched, and jhamt−/− and epox−/− homozygous L1 larvae were sorted according to the intensity of fluorescence in their eyes. Larval development was tracked by collecting exuviae of individual instars. Larvae of each instar were sampled, and the expression of the three genes was analyzed using RT-qPCR.
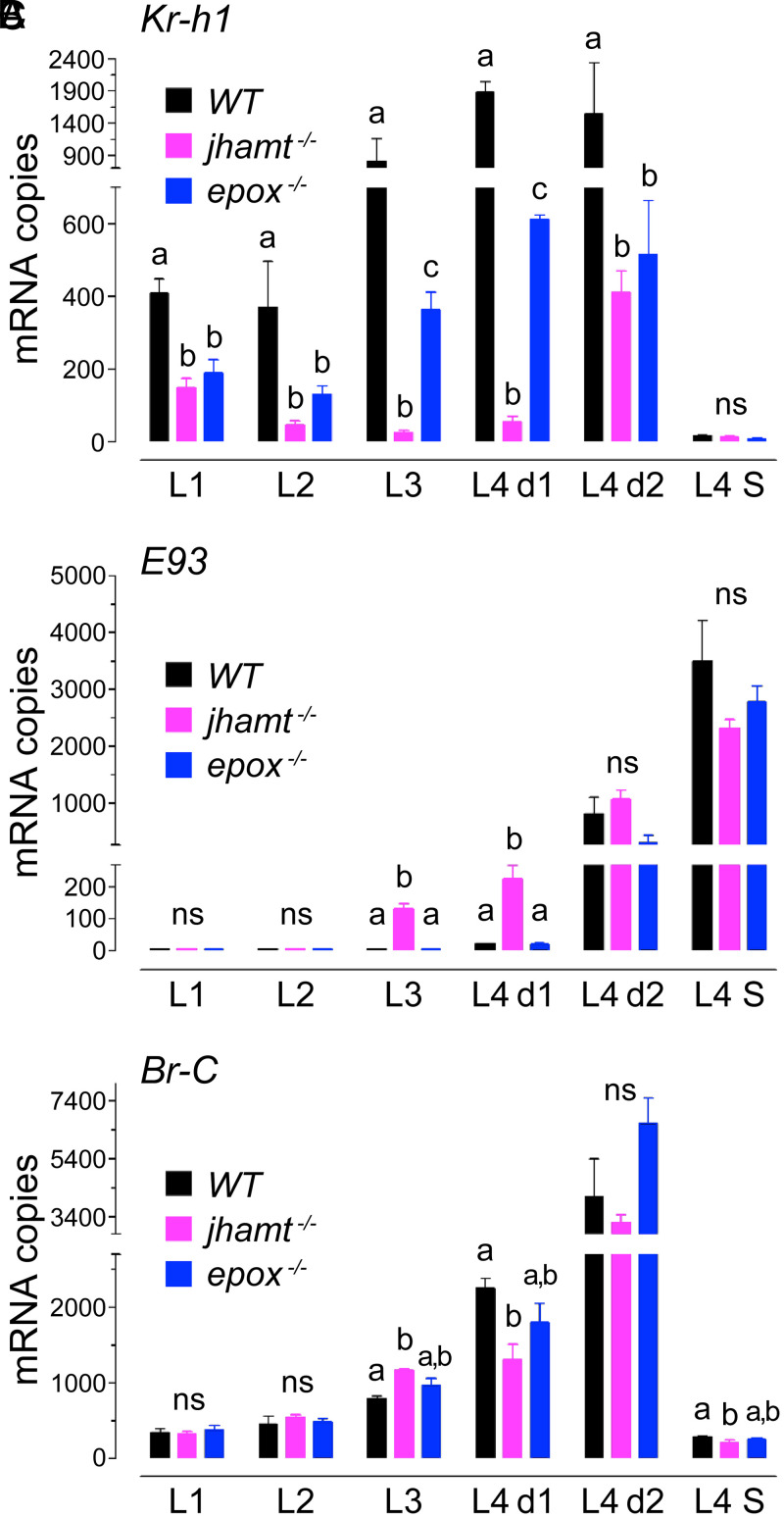
Transcription of Kr-h1 is directly induced by the activated JH-receptor complex (24, 25), and Kr-h1 is required to maintain the larval status in diverse insects (18–23). In WT larvae, Kr-h1 mRNA was highly expressed from L1, reaching maximum levels at the L3 and early L4 instars, then becoming almost undetectable by the last hours before pupation (Fig. 4A). As expected, the levels of Kr-h1 mRNA were drastically decreased in the absence of both MF and JH in jhamt−/− larvae, particularly during the time of highest Kr-h1 expression at the L3 and early L4 instars. While the depletion of epoxidated JH in epox−/− larvae also led to a significant decrease in Kr-h1 transcript levels relative to WT, the levels were much higher when compared with those in jhamt−/− larvae (Fig. 4A). Therefore, MF was sufficient to maintain moderate Kr-h1 expression throughout larval development, consistent with a notion that MF is a weaker JH receptor agonist relative to epoxidated JH (9, 10).
Fig. 4.
Developmental expression of JH-response genes in jhamt and epox null mutants. (A) Kr-h1, (B) E93, and (C) Br-C. Expression of the mRNAs was determined by RT-qPCR in whole body of larvae of the indicated instars; the final-instar stages were day 1 (L4 d1), day 2 (L4 d2), and L4-instar larvae with visible siphons 6 to 7 h before pupation (L4 S). Levels of mRNAs are expressed as copy number per 10,000 copies of rpL32 mRNA. Each data point is average of three to seven independent biological replicates, each from three larvae. Columns represent mean ± SEM in all panels. Significant differences are marked with letters above columns (P ≤ 0.05, one-way ANOVA with Tukey’s multiple post hoc comparison; ns, nonsignificant).
E93 promotes adult development, and it is repressed by JH through Kr-h1 (18–23). Appreciable expression of E93 in WT larvae appeared on day 1 of the L4 instar and increased over 200-fold before pupation (Fig. 4B). In contrast, and consistent with the repressive action of JH on E93, jhamt−/− larvae showed a significant increase in E93 mRNA already by the L3 and early L4 instars, concomitant with the drastically reduced Kr-h1 expression in these mutants. The expression of E93 in epox−/− larvae did not significantly differ from that in WT larvae (Fig. 4B).
Br-C is required for larval–pupal transition in holometabolous insects (18–23). Br-C expression in WT larvae was detectable from the L1 instar but increased for a short pulse during the early-to-mid L4 instar (Fig. 4C). Except for L3 and early L4 instars, Br-C mRNA levels did not significantly differ between jhamt−/− and WT larvae, and no substantial differences appeared in epox−/− larvae.
Epoxidated JH Improves Female Reproductive Fitness.
JH is a central regulator of many aspects of reproductive biology in insects. Therefore, we evaluated how the lack of JH affected reproduction in female and male epox−/− adults. The wing length was employed as a reliable indicator of mosquito size and nutritional status at adult emergence (26, 27).
In the absence of epoxidated JH, female mosquitoes showed a substantial fitness cost. The average wing length in homozygous epox−/− females was 3.056 ± 0.011 mm (n = 37), which was significantly shorter (P ≤ 0.001) than the wings of their epox+/+ (WT; 3.198 ± 0.011, n = 20) female siblings (SI Appendix, Fig. S11A). The wing length was unaffected in heterozygous epox+/− females (3.236 ± 0.009 mm, n = 24), indicating a recessive effect of the mutation and no adverse effect of the eCFP transgene.
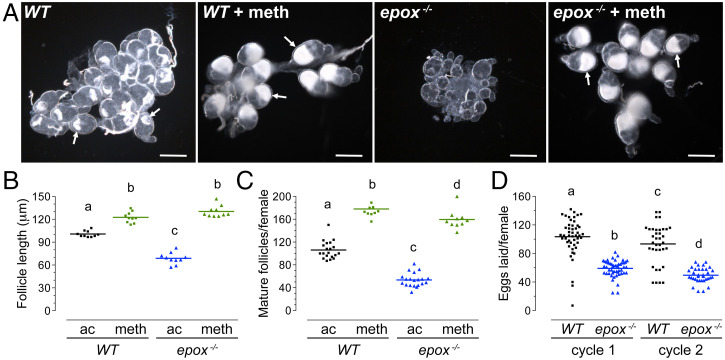
The small epox−/− females presumably emerged with reduced nutritional reserves, which limited their previtellogenic ovarian development. In late female pupae, primary follicles differentiate and separate from the germarium. During the first 3 d after adult emergence, primary follicles grow to the mature previtellogenic size. On observation, mature previtellogenic follicles of epox−/− females were smaller and contained less lipid reserves compared to follicles in WT females (Fig. 5A). They reached on average only 68% the final WT length (P ≤ 0.001) (Fig. 5B). By the end of the previtellogenic stage, ovaries of epox−/− females contained half the number of follicles (53 ± 2, n = 20) present in ovaries of WT females (106 ± 3, n = 20) (P ≤ 0.001) (Fig. 5C). Consequently, after a blood meal, epox−/− females laid significantly fewer eggs (58 ± 2, n = 30) than WT controls (102 ± 4, n = 30) (P ≤ 0.001), and a similar difference persisted in the second gonotrophic cycle (Fig. 5D). Together, these data show that while MF is sufficient to sustain a basal level of female mosquito reproduction, epoxidated JH is required for the level of reproductive fitness that is normally observed in WT females.
Fig. 5.
Reproductive fitness cost in EPOX-deficient females and the effect of Met. (A) Examples of mature previtellogenic follicles from WT and epox−/− females that had been treated with Met (meth) or solvent (ac) at 1 to 4 h after adult eclosion. Note lipid accumulation (white arrows) except in the oocytes of untreated epox−/− females. (Scale bars, 100 μm.) (B and C) Lengths and numbers of mature primary previtellogenic follicles from females of the indicated genotypes with or without the prior Met treatment. Each point represents an average of independent length measurements of 10 follicles from an ovary (n = 10 females) (B), or the number of follicles per ovary (n = 10 to 20 females) (C). (D) Number of eggs laid by WT and epox−/− females during two gonotrophic cycles. Each point represents the number of eggs laid by a female (n = 35 to 50 females). Lines in the scatter plots represent mean values, letters above them mark significant differences (P ≤ 0.05, one-way ANOVA with Tukey’s multiple post hoc comparison).
Topical application of Met to epox−/− females between 1 and 4 h after adult eclosion dramatically increased both the number and size of mature previtellogenic follicles (Fig. 5 A–C), and induced lipid accumulation in the oocytes (Fig. 5A). The potent JH receptor agonist Met not only compensated for the missing epoxidated hormone in epox−/− females, but enhanced oogenesis even in WT females (Fig. 5 A–C).
Epoxidated JH Improves Fitness and Mating Success of Males.
We next examined if the lack of JH might affect the reproductive fitness of males. The average wing length of WT males was 2.427 ± 0.005 mm (n = 20), while epox−/− males had wings 2.349 ± 0.001 mm in length (n = 20) (P ≤ 0.001) (SI Appendix, Fig. S11B). As in females, the male wing size did not significantly differ between WT (epox+/+) and their heterozygous epox+/− siblings carrying the eCFP transgene.
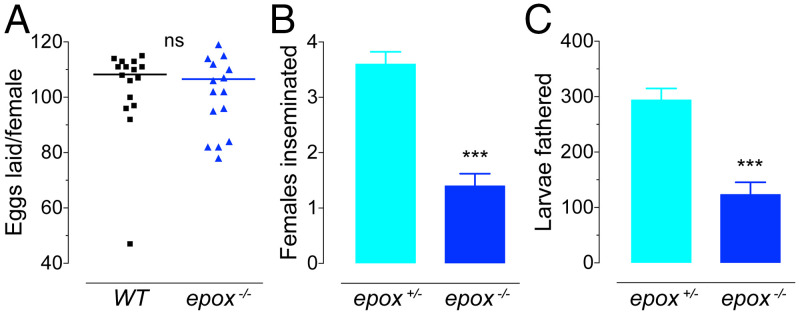
We tested the reproductive fitness of epox−/− males by comparing the fecundity of WT females mated with either WT or epox−/− males. There were no significant differences in the total number of eggs laid by WT females mated to males of either genotype (Fig. 6A). However, epox−/− males proved to perform poorly when exposed to mating competition with males possessing epox+ function. The null mutant males were dramatically outcompeted when pooled in one cage with either epox+/+ (WT) or epox+/− male siblings and allowed to mate with WT females. The homozygous epox−/− mutant males showed significant reductions in both the number of females inseminated (P ≤ 0.001) (Fig. 6B and SI Appendix, Fig. S12), as well as in the total numbers of larvae they had fathered (P ≤ 0.001) (Fig. 6C and SI Appendix, Fig. S12) compared to either WT or epox+/− males. Inclusion of the latter genotype confirmed that the eCFP transgene itself did not affect mating competitiveness of males.
Fig. 6.
Reproductive fitness cost in male epox−/− mutants. (A) Numbers of eggs laid by WT females inseminated by epox+/+ (WT) males or their epox−/− siblings. Each point represents the number of eggs laid by a female (n = 20, line represents the mean). ns, nonsignificant. (B and C) Mating competition between epox+/− and epox−/− males. Five males of each genotype were allowed to mate with five WT females in a mating cage. After genotyping of the larval progeny by the eCFP transgene that marks the epox null mutant allele, the number of females inseminated (B) and the numbers of larvae fathered (C) by males of either genotype were evaluated. Columns represent mean ± SEM of 10 independent assays (***P ≤ 0.001, unpaired t test).
Discussion
Much has been learned since the original discoveries that JH is required to prevent precocious metamorphosis of insect larvae (28, 29). The progress leading from the chemical identification of JH to current knowledge of its gene-regulatory action through the intracellular receptor Met has been reviewed recently (30, 31). Yet, many questions concerning the roles of epoxidated JH receptor agonists and their biosynthetic (and evolutionary) precursors during insect development and reproduction stand out.
To better understand the significance of sesquiterpenoid epoxidation in the evolution of JH signaling, we have chosen the yellow fever mosquito with its well-defined enzymatic conversions, first of FA to MF by JHAMT, followed by MF epoxidation to JH III by the CYP15 family member EPOX (Fig. 1A) (15). This sequence of reactions, although different from that in some other insects (6), enabled us to genetically separate the production of MF from its epoxidation to JH III by generating mutants for the respective jhamt and epox genes. We demonstrate that jhamt−/− larvae synthesize no MF and consequently no JH III, whereas epox−/− mutants only lack the latter, epoxidated hormone. This exclusive set of jhamt and epox knockouts allowed us to examine the requirement of sesquiterpenoid signaling for insect development and, specifically, to assess how MF epoxidation contributes to reproductive fitness.
Sesquiterpenoid Signaling Is Dispensable for Mosquito Embryonic and Larval Development.
The hatching rates of eggs produced by the heterozygous jhamt+/− line relative to hatching rates observed in WT or the homozygous epox−/− line showed no abnormal amount of embryonic lethality inflicted by MF and JH deficiency. Indeed, molecular genotyping of larvae indicated that jhamt−/− progeny of heterozygous jhamt+/− parents accomplished embryonic development in the absence of zygotic jhamt function and, therefore, without autonomous MF and JH synthesis. Insect embryonic CA produce a broad peak of JH during mid-to-late embryogenesis (32, 33), which is mirrored by JH-dependent Kr-h1 mRNA expression (34, 35). This scenario likely applies to A. aegypti embryos, where Kr-h1 mRNA levels were initially low early after egg laying, then increased for the latter half of embryogenesis (SI Appendix, Fig. S13). Importantly, this Kr-h1 expression was significantly reduced in embryos of the null epox−/− line, indicating that in the complete absence of EPOX, JH signaling was diminished without affecting embryogenesis. The remaining Kr-h1 expression in epox−/− embryos might reflect the presence of MF.
Our data concur with those obtained for the silkworm, Bombyx mori, by Daimon et al. (34). Silkworms simultaneously lacking the homologs of JHAMT and EPOX, and therefore any form of JH, completed embryogenesis and, except for a difficulty hatching, produced normal larvae (34). The absence of JH signaling activity in the jhamt−/− knockout Bombyx embryos correlated with loss of Kr-h1 expression, yet it had no deleterious impact on development. Apparently, contradictory data were obtained in A. aegypti where coinjection of three single-guide RNAs (sgRNAs) targeting the jhamt gene reduced hatching rate to 13% (36). However, this effect of CRISPR/Cas9-mediated mutagenesis was observed in embryos of the G0 generation following the injection. As such, these data obtained on mosaic progeny are difficult to reconcile with our analyses of the genetically uniform jhamt and epox knockout lines that had been established upon multiple backcrosses against a WT line to remove potential second-site mutations.
The presence of JH in insect larvae maintains the juvenile status. Disruption of JH signaling therefore often induces precocious metamorphosis (37). Interestingly, in species such as B. mori that normally undergo at least four larval molting cycles (i.e., five larval instars), the antimetamorphic function of JH does not apply to the earliest two larval instars, which appear incompetent to enter metamorphosis (38, 39). This notion has been confirmed in the jhamt−/− and Met1−/− knockout silkworms, which began to metamorphose no earlier than by the L3 instar (34). Similarly, B. mori mod mutants lacking epoxidated JH pupated prematurely at their third or fourth instar (40). Our data contrast with the situation in Bombyx, as the deficiency in MF and JH did not provoke precocious pupation in the mosquito. In the absence of MF and JH, our homozygous jhamt−/− larvae transitioned from L1 to L4 via three complete molting cycles.
While the jhamt−/− and epox−/− knockout mosquito larvae displayed no externally appreciable signs of precocious metamorphosis, they showed alterations at the gene-regulatory level that are consistent with JH signaling deficiency. The antimetamorphic function of JH during penultimate larval instars relies on the activation of the Kr-h1 gene by the JH-receptor complex (18–23, 37, 41). The transcription factor Kr-h1 maintains the larval program by repressing prometamorphic genes, primarily the adult-specifying E93 (18–23). The severely reduced levels of Kr-h1 mRNA in jhamt−/− larvae correspond to the fact that Kr-h1 is a direct JH-inducible target gene in A. aegypti (24, 42). Conversely, the absence of MF and JH resulted in prematurely up-regulated E93 expression during the penultimate and early-L4 instars in jhamt−/− larvae. Why these temporal shifts in Kr-h1 and E93 gene activities did not lead to precocious metamorphosis in Aedes larvae remains to be investigated.
MF Can Substitute for JH in Sustaining Complete Mosquito Development.
Unless aided with Met, homozygous jhamt−/− larvae always failed to initiate the pupation process, and even the Met-treated animals died early upon pupation. Therefore, in mosquitoes jhamt is a vital gene with a function that becomes indispensable during metamorphosis. The lethal phase observed in jhamt−/− mosquitoes resembles that in Drosophila melanogaster lacking either the CA cells (43) or the JH receptor genes Met and gce (9, 44); in both cases the flies die at the onset of pupation. Whether this late phase of requirement for JH signaling is a feature specific to Diptera is currently unknown.
In contrast to the unconditionally lethal jhamt−/− mutants, EPOX-deficient mosquitoes reach adulthood and reproduce, so that a stable, homozygous epox−/− line could be established. This result shows that MF, synthesized by the CA of epox−/− mutants, is sufficient to sustain mosquito development in the absence of JH III. Although the Bombyx mod mutants that lack the corresponding EPOX function experience precocious metamorphosis, some survive to form fertile miniature moths (40), indicating that epoxidated JH is not absolutely vital even in the silkworm. The functionality of MF as a systemic signal in Aedes is evident from the degree of JH-dependent Kr-h1 expression in L3 and early-L4 epox−/− larvae that is reduced relative to WT controls but significantly higher than the barely detectable Kr-h1 mRNA in the MF-deficient jhamt−/− larvae (Fig. 4A). Accordingly, in larvae of the same age, the aberrant precocious up-regulation of E93 transcripts only occurs in the absence of MF and JH III (jhamt−/−) but not in the absence of JH III alone (Fig. 4B). These in vivo data agree with the findings that, like JH III, MF can bind and activate the JH receptor, albeit with a potency lower than that of the epoxidated hormone (9, 10).
Epoxidated JH Confers Reproductive Advantages to Mosquitoes.
The size of adult insects depends on the amount of nutrients and body mass accumulated during the larval feeding stage. Homozygous epox−/− larvae developed and grew slower than their WT siblings, thus reducing the final body size attained by the adults as assessed by the wing length. This smaller size indicates reduced fitness at adult emergence in epox−/− female and male mosquitoes.
EPOX-deficient female mosquitoes suffered a significant reproductive fitness cost. The role of JH in controlling reproductive trade-offs has been extensively studied in female mosquitoes (45–47). JH directs nutrient allocation into the ovaries during the previtellogenic phase, and thus indirectly influences the fate of vitellogenic follicles after a blood meal (48, 49). Depending on nutritional and hormonal homeostasis, the final number of eggs that develop can be adjusted at different times during oogenesis. By the first 72 h after adult emergence, primary follicles grow from 40 μm to over 100 μm (27, 50). This previtellogenic growth depends on nutrients and JH (51). After the follicles reach their maximum previtellogenic size, the final number of eggs produced can again be adjusted through follicular resorption either before or after blood meal (48, 49).
Relative to WT females, the JH-deficient epox−/− mutants developed substantially smaller and fewer mature primary follicles, resulting in reduced numbers of eggs laid after blood meal. Treatment with Met strongly augmented the number and size of mature previtellogenic follicles in epox−/− females (Fig. 5). However, it did not restore normal numbers of eggs laid (SI Appendix, Fig. S14), likely because of the sterilizing effect that long-persisting synthetic JH mimics exert on mosquito oogenesis (52, 53). These results indicate that the reduction in the number of eggs laid by the epox−/− females was determined before blood feeding. The deleterious effects on reproductive output could not be reversed by providing sugar or blood meals to the adults, and continued in the second gonotrophic cycle, therefore imposing a strong and irreversible reproductive fitness cost.
Relative to female reproduction, much less is known about the role of JH in male mosquitoes. We have therefore evaluated the impact of JH deficiency on the reproductive fitness of epox−/− males. Adult epox−/− males were significantly smaller than WT or heterozygous epox+/− siblings, but otherwise showed no obvious phenotypic differences. Secondary sexual characters, such as the erection of the antennal fibrillae and the rotation of the terminal genitalia, developed normally. Therefore, they were able to mate and inseminate females. Males carrying the epox mutation as heterozygotes or homozygotes showed levels of fertility typical of WT as measured by clutch size and larval hatching per mated female. However, homozygous epox−/− males fathered fewer offspring than control males when exposed to competition by either WT or epox+/− males in mating assays with WT females. Our present understanding of mosquito sexual selection and mating success suggests an important role of female mate choice. Females use multiple forms of sexual selection traits and male cues as indicators of both mate and offspring genetic quality (54). The mating competition experiments indicated that females either avoided the smaller epox−/− males or these mutant males were outperformed by WT and epox+/− males. In our experiments, each male had a chance to mate with several females. It is possible that, in addition to being smaller and hence less attractive, epox−/− males could be less fit because of underperforming male accessory glands. JH is known to stimulate the secretory activity of the male accessory glands of A. aegypti (55). Additional studies will be necessary to understand the molecular bases of epox−/− female and male reproductive deficits.
The Importance of Sesquiterpenoid Epoxidation and the Role of the JH Receptor.
Epoxidation of MF by CYP15 enzymes was a major evolutionary novelty in insects (12). Analysis of CYP15 genes suggests that although these genes are highly conserved among insects, they have evolved lineage-specific substrate specificity (12). A monophyletic clade of 38 insect sequences clustered with the A. aegypti epoxidase and the biochemically characterized epoxidases from Diploptera punctata (15), Tribolium castaneum (56), and B. mori (40) (SI Appendix, Fig. S15). All major insect orders are represented in this clade, including Zygentoma but not Archaeognatha. No dipluran, proturan, collembolan, or other arthropod P450s belong to this clade. This suggests that the epoxidase evolved about 420 MYA from related hexapod CYP15 sequences of unknown function. The epoxidase is closely related to CYP303 sequences found only in winged insects and to CYP305. The CYP15 and CYP305 genes are likely products of an ancient tandem duplication, as the two genes are found in synteny in several species (12), including the firebrat, Thermobia domestica (Zygentoma).
Met likely evolved as a receptor for sesquiterpenoid hormones in a pancrustacean common ancestor of crustaceans and insects (11). Met has a hydrophobic ligand-binding pocket flexible enough to accommodate MF and the natural epoxidated JHs, including JH III, the bulkier JH I, and the racemic MF-6,10-diepoxide (10). As the ancestral sesquiterpenoid, MF binds and activates the insect JH receptor, albeit with a reduced potency relative to any epoxidated JH (9, 10, 57). Interestingly, while MF is the natural sesquiterpenoid hormone in crustaceans, the insect JH III is more potent than MF as an agonist of both the insect and the crustacean receptors (58). Together, receptor–hormone interaction studies in insects and crustaceans thus far advocate that evolution of the ligand toward epoxidated JH as a better agonist, rather than evolution of the receptor, is what has driven the selective advantage in insects. In other words, the pancrustacean receptor was preadapted for epoxidated JH, but only in insects did a P450 enzyme evolve to make the more effective, epoxidated hormone.
Conclusions.
It is accepted that the reproductive or gonadotropic action was the ancient role of JH, while the antimetamorphic role evolved later (59). Growth and reproduction are processes that require a tight control of nutritional homeostasis, with JH playing a central role in adjusting resource allocation among growth or reproduction and survival (45). JH has an evolutionarily conserved proreproductive and proaging effect. Down-regulation of JH signaling in response to low nutrient availability switches the physiological state of the insect to a promaintenance, prosurvival mode at the expense of reproduction (60). Our present work shows that mosquitoes can develop and reproduce for generations in the total absence of epoxidated JH. While the epox−/− mutants synthesize no JH, they retain a suboptimal level of signaling reliant on the JH receptor agonist MF. A lethal arrest occurs when MF is also depleted through the mutation of jhamt. While MF is less potent than JH, it allows the mosquitoes to complete the life cycle and reproduce, albeit at a great fitness cost, manifested as compromised oogenesis in females and reduced mating competitiveness in males. In summary, our jhamt and epox null mutants have allowed us to uncover the evolutionary advantage provided to insects by the introduction of epoxidated sesquiterpenoid signaling.
Materials and Methods
Mosquitoes.
A. aegypti (Orlando strain) were reared and maintained at 28 °C, 80% relative humidity under 16:8 h light:dark cycle. Mosquito eggs were hatched in deionized, deoxygenated water containing dissolved tablets of Tetramin tropical fish food (catalog #16152, Tetra). Adult mosquitoes were given ad libitum access to 10% sucrose solution. Four-day-old female mosquitoes were fed pig blood equilibrated to 37 °C, and ATP was added to the blood meal to a final concentration of 1 mM immediately before use, as described previously (49).
CRISPR/Cas9 Reagents.
HDR was employed to introduce gene cassettes encoding fluorescent markers into the first exon of jhamt and the fourth exon of epox, as described previously (61, 62) (Fig. 1A). sgRNAs were designed and checked for potential off-target sites using ZiFit (http://zifit.partners.org/ZiFiT/). To increase the efficiency of HDR, sgRNA pairs, separated by 168 bp (jhamt) and 211 bp (epox), were selected to produce two breaks in the target DNA sequences. The double-stranded DNA templates for generating the sgRNAs were produced using PCR with two overlapping primers (SI Appendix, Table S1), as described previously (61, 62). The sgRNAs were synthesized using the MEGAscript T7 Transcription Kit and purified with the MEGAclear Transcription Clean-Up Kit (both Life Technologies). The Cas9 protein was from PNA Bio. Construction of plasmid vectors for injection to A. aegypti embryos is described in SI Appendix.
Generation of jhamtdsRed and epoxeCFP Mutant Lines.
To generate stable germline mutations, CRISPR/Cas9 reagents were injected into the posterior end of preblastoderm A. aegypti embryos (62, 63). Microinjections were performed at the Insect Transformation Facility at the University of Maryland (https://www.ibbr.umd.edu/). The microinjection mixtures contained 40 ng/μL of each sgRNA of the gene-specific sgRNA pair, 500 ng/μL of the Cas9 protein, and 600 ng/μL of the donor plasmid in nuclease-free water. Details on the numbers of injected embryos, surviving G0 adults, frequencies of occurrence of the dsRed and eCFP eye markers in the G1 progeny, and their inheritance in the following genetic crosses can be found in SI Appendix.
To detect insertion of dsRed and eCFP sequences to the jhamt and epox loci, mosquito genomic DNA was purified using a Genomic DNA extraction mini kit (IBI Scientific) and subjected to PCR with primer pairs jhF2/jhR2 and epF2/epR2, respectively. For genotyping, primer pairs jhF1/jhR1 and epF1/epR1 were used to detect the WT alleles of the jhamt and epox genes, respectively (Fig. 1A and SI Appendix, Table S1). To further verify the site-specific integration of the dsRed donor DNA into exon 1 of jhamt and of the eCFP donor DNA into exon 4 of epox, respectively, the inserted DNAs with additional 150 to 250 bp upstream and downstream of the integration sites were amplified by PCR from genomic DNA of G5 generation mutant mosquitoes. Sequencing of the PCR products confirmed that the donor DNAs were integrated properly to the designated genome loci with no additional insertions or deletions. For each gene, one mutant line with the insertion site verified was selected, back-crossed to the WT strain for another two generations, and used for experiments starting at G7 (SI Appendix).
Genetics.
To determine heritability of the null mutations, heterozygous females from G5 or later generations were backcrossed to WT males, and the progeny was analyzed for the frequency of the dsRed (jhamt) and eCFP (epox) eye markers. The markers segregated in Mendelian fashion with the expected 1:1 ratio, which was confirmed using χ2 test. Mendelian heritability was also verified in F1 generations by analyzing progeny of the crosses of heterozygous males and heterozygous females from generation G5 or later. In this this case, the eye markers segregated with the expected phenotypic ratio 3:1 (SI Appendix, Fig. S1).
JH Biosynthesis Assay.
Mosquitoes were cold-anesthetized and the CA complexes were dissected and incubated at 32 °C for 4 h in 150 μL of the M-199 tissue culture medium (Gibco) containing 2% Ficoll, 25 mM Hepes (pH 6.5), and 100 μM methionine (64). MF and JH III in the tissue culture medium were analyzed by LC-MS/MS, as previously described (16).
Hemolymph Collection.
Hemolymph of adult female mosquitoes was obtained by perfusion as previously described (65). Fine needles were made from 100-μL glass microcapillary tubes using a micropipette puller P-30 (Sutter Instrument), and mounted on a pump (Drummond). Needles were inserted manually through the neck membrane into the thoracic cavity, and insects were perfused with 20 μL of phosphate buffered saline (PBS, pH 7.2). The hemolymph was obtained from a small tear made laterally on the intersegmental membrane of the last abdominal segment. The first drop of perfused hemolymph was collected into a silanized glass tube (Thermo Fisher) placed on ice.
JH III and MF Extraction and Quantification.
Ten microliters of 6.25 ppb of heavy deuterated JH III analog (JH III-D3) in acetonitrile were added to each sample, followed by 600 μL of hexane. Samples were vortexed for 1 min and centrifuged for 5 min at 4 °C and 2,000 × g. The hexane phase was transferred to a new silanized vial and 100 μL of acetonitrile was added. Samples were vortexed for 30 s. Vials were centrifuged 1 min at 4 °C and 2,000 × g. Volume of samples was reduced to 50 μL under nitrogen flow. Samples were transferred to a new silanized vial with a fused 250-μL insert. JH III and MF quantification using LC-MS/MS was done as described previously (16). Briefly, the LC-MS/MS workflow was based on multiple reaction monitoring using the two most abundant fragmentation transitions: JH III: 267 ≥ 235 (primary) and 267 ≥147 (secondary); MF: 251 ≥ 219 (primary) and 267 ≥ 191 (secondary). To accurately quantify the amount of JH III and MF present in the hemolymph or produced by the CA complexes, JH III-D3 served as an internal standard to normalize recoveries during the sample preparation, extraction, and analysis steps. Extraction recovery of 55% or more was routinely observed regardless of the analyte concentration (66).
RT-qPCR.
Total RNA was isolated using Norgen Biotek total RNA purification kit, treated with DNase I, and reverse-transcribed using the Verso cDNA Synthesis Kit (Thermo Fisher). Number of mRNA copies normalized to rpL32 mRNA was quantified in triplicate reactions in a 7300 Real-Time PCR System using the TaqMan Universal PCR Master Mix (Applied Biosystems). Gene accession numbers and primer and probe sequences are listed in SI Appendix, Table S2.
Wing, Larval Body, and Follicle Length Measurements.
Wing length, defined as the distance between the point of articulation and the wing tip excluding the fringe scales (26), was measured under a dissecting microscope using an ocular micrometer. Body lengths of fourth-instar larvae (L4 S stage) were measured from microscope images using the segmented line tool in the ImageJ software (NIH). Ovaries were dissected from abdomens of ice-immobilized adult females and placed in a drop of PBS. The number and lengths of the terminal follicles were measured under a dissecting microscope using an ocular micrometer.
Met Treatment.
Met (500 ng in acetone) (Zoecon) was topically applied (0.5 μL per animal) to larvae or adults. Controls were treated with acetone.
Mating Competition.
Ten groups of five virgin males (aged 4 to 5 d) of epox−/− and either epox+/− or epox+/+ (WT) genotypes were placed in 4.7-l buckets (mating cages) and allowed 5 h for acclimation. Heterozygous epox+/− males were used to ensure that mating differences were not caused by the presence of the eCFP eye marker. Five WT virgin females (sugar-fed, aged 4 to 5 d) were added to each mating cage, and mosquitoes were allowed to mate for 48 h. Females were subsequently, blood fed, and 48 h after blood feeding were placed individually in oviposition vials. Eggs were counted and hatched, and larvae were scored for the presence of eCFP in the eyes. Progeny uniformly positive for eCFP was assigned to an epox−/− father; progeny displaying both eCFP+ and eCFP− eyes was assigned to an epox+/− father.
Statistical Analyses.
All data were processed using the GraphPad Prism Software. Significance of differences were determined either with one-tailed Student's t test, or with one-way ANOVA followed by Tukey’s test.
Acknowledgments
This research was funded by Grants R01AI04554 (to F.G.N.), R21AI153689 (to M.N.), and K22AI112585 (to M.D.) from the NIH–National Institute of Allergy and Infectious Diseases. M.D. was supported by startup funds from Florida International University. M.D. and F.G.N. were also supported by the Centers for Disease Control and Prevention (CDC) Southeastern Center of Excellence in Vector Borne Diseases. V.M. was supported by the Slovak grant agency Grants APVV-16-0395 and APVV-18-0201. M.J. was supported by project 20-05151X from the Czech Science Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the CDC.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2109381118/-/DCSupplemental.
Data Availability
All study data are included in the article and SI Appendix.
References
- 1.Glenner H., Thomsen P. F., Hebsgaard M. B., Sørensen M. V., Willerslev E., Evolution. The origin of insects. Science 314, 1883–1884 (2006). [DOI] [PubMed] [Google Scholar]
- 2.von Reumont B. M., Burmester T., “Remipedia and the evolution of hexapods” inEncyclopedia of Life Sciences (John Wiley & Sons, 2010), pp. 1–6. [Google Scholar]
- 3.Giribet G., Edgecombe G. D., The phylogeny and evolutionary history of arthropods. Curr. Biol. 29, R592–R602 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Qu Z., et al., How did arthropod sesquiterpenoids and ecdysteroids arise? Comparison of hormonal pathway genes in non-insect arthropod genomes. Genome Biol. Evol. 7, 1951–1959 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivera-Pérez C., Clifton M. E., Noriega F. G., Jindra M., “Juvenile hormone regulation and action” in Advances in Invertebrate (Neuro)Endocrinology, Saleuddin S., Lange A. B., Orchard I., Eds. (Apple Academic Press, 2020), vol. 2, pp. 1–76. [Google Scholar]
- 6.Tsang S. S. K., et al., Diversity of insect sesquiterpenoid regulation. Front. Genet. 11, 1027 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charles J.-P., et al., Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc. Natl. Acad. Sci. U.S.A. 108, 21128–21133 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jindra M., Tumova S., Milacek M., Bittova L., A decade with the juvenile hormone receptor. Adv. Insect Physiol. 60, 37–85 (2021). [Google Scholar]
- 9.Jindra M., Uhlirova M., Charles J.-P., Smykal V., Hill R. J., Genetic evidence for function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLoS Genet. 11, e1005394 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bittova L., et al., Exquisite ligand stereoselectivity of a Drosophila juvenile hormone receptor contrasts with its broad agonist repertoire. J. Biol. Chem. 294, 410–423 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyakawa H., et al., A mutation in the receptor Methoprene-tolerant alters juvenile hormone response in insects and crustaceans. Nat. Commun. 4, 1856 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Dermauw W., Van Leeuwen T., Feyereisen R., Diversity and evolution of the P450 family in arthropods. Insect Biochem. Mol. Biol. 127, 103490 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Goodman W. G., Cusson M., “The juvenile hormones” in Insect Endocrinology, Gilbert L. I., Ed. (Academic Press, 2012), pp. 310–365. [Google Scholar]
- 14.Shinoda T., Itoyama K., Juvenile hormone acid methyltransferase: A key regulatory enzyme for insect metamorphosis. Proc. Natl. Acad. Sci. U.S.A. 100, 11986–11991 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helvig C., Koener J. F., Unnithan G. C., Feyereisen R., CYP15A1, the cytochrome P450 that catalyzes epoxidation of methyl farnesoate to juvenile hormone III in cockroach corpora allata. Proc. Natl. Acad. Sci. U.S.A. 101, 4024–4029 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramirez C. E., Nouzova M., Michalkova V., Fernandez-Lima F., Noriega F. G., Common structural features facilitate the simultaneous identification and quantification of the five most common juvenile hormones by liquid chromatography-tandem mass spectrometry. Insect Biochem. Mol. Biol. 116, 103287 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clements A. N., The Biology of Mosquitoes (Chapman and Hall, 1992). [Google Scholar]
- 18.Kayukawa T., Jouraku A., Ito Y., Shinoda T., Molecular mechanism underlying juvenile hormone-mediated repression of precocious larval-adult metamorphosis. Proc. Natl. Acad. Sci. U.S.A. 114, 1057–1062 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ureña E., Chafino S., Manjón C., Franch-Marro X., Martín D., The occurrence of the holometabolous pupal stage requires the interaction between E93, Krüppel-homolog 1 and Broad-Complex. PLoS Genet. 12, e1006020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellés X., Santos C. G., The MEKRE93 (Methoprene tolerant-Krüppel homolog 1-E93) pathway in the regulation of insect metamorphosis, and the homology of the pupal stage. Insect Biochem. Mol. Biol. 52, 60–68 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Martín D., Chafino S., Franch-Marro X., How stage identity is established in insects: The role of the metamorphic gene network. Curr. Opin. Insect Sci. 43, 29–38 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Truman J. W., The evolution of insect metamorphosis. Curr. Biol. 29, R1252–R1268 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Jindra M., Where did the pupa come from? The timing of juvenile hormone signalling supports homology between stages of hemimetabolous and holometabolous insects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20190064 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M., Mead E. A., Zhu J., Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc. Natl. Acad. Sci. U.S.A. 108, 638–643 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kayukawa T., et al., Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proc. Natl. Acad. Sci. U.S.A. 109, 11729–11734 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nasci R. S., Relationship of wing length to adult dry weight in several mosquito species (Diptera: Culicidae). J. Med. Entomol. 27, 716–719 (1990). [DOI] [PubMed] [Google Scholar]
- 27.Caroci A. S., Li Y., Noriega F. G., Reduced juvenile hormone synthesis in mosquitoes with low teneral reserves reduces ovarian previtellogenic development in Aedes aegypti. J. Exp. Biol. 207, 2685–2690 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Wigglesworth V. B., The physiology of ecdysis in Rhodnius prolixus (Hemiptera). II. Factors controlling moulting and ‘metamorphosis’. Q. J. Microsc. Sci. 77, 191–222 (1934). [Google Scholar]
- 29.Wigglesworth V. B., The function of the corpus allatum in the growth and reproduction of Rhodnius prolixus (Hemiptera). Q. J. Microsc. Sci. 79, 91–121 (1936). [Google Scholar]
- 30.Riddiford L. M., Rhodnius, golden oil, and Met: A history of juvenile hormone research. Front. Cell Dev. Biol. 8, 679 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellés X., Insect Metamorphosis: From Natural History to Regulation of Development and Evolution (Elsevier, 2020). [Google Scholar]
- 32.Bergot B. J., Baker F. C., Cerf D. C., Jamieson G., Schooley D. A., “Qualitative and quantitative aspects of juvenile hormone titers in developing embryos of several insect species: Discovery of a new JH-like substance extracted from eggs of Manduca sexta” in Juvenile Hormone Biochemistry, Pratt G. E., Brooks G. T., Eds. (Elsevier, 1981), pp. 33–45. [Google Scholar]
- 33.Maestro J. L., Pascual N., Treiblmayr K., Lozano J., Bellés X., Juvenile hormone and allatostatins in the German cockroach embryo. Insect Biochem. Mol. Biol. 40, 660–665 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Daimon T., Uchibori M., Nakao H., Sezutsu H., Shinoda T., Knockout silkworms reveal a dispensable role for juvenile hormones in holometabolous life cycle. Proc. Natl. Acad. Sci. U.S.A. 112, E4226–E4235 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Nicolas A., Bellés X., Juvenile hormone signaling in short germ-band hemimetabolan embryos. Development 144, 4637–4644 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Zhu G.-H., Jiao Y., Chereddy S. C. R. R., Noh M. Y., Palli S. R., Knockout of juvenile hormone receptor, Methoprene-tolerant, induces black larval phenotype in the yellow fever mosquito, Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 116, 21501–21507 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jindra M., Palli S. R., Riddiford L. M., The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 58, 181–204 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Feyereisen R., Jindra M., The silkworm coming of age—Early. PLoS Genet. 8, e1002591 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smykal V., et al., Importance of juvenile hormone signaling arises with competence of insect larvae to metamorphose. Dev. Biol. 390, 221–230 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Daimon T., et al., Precocious metamorphosis in the juvenile hormone-deficient mutant of the silkworm, Bombyx mori. PLoS Genet. 8, e1002486 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minakuchi C., Namiki T., Shinoda T., Krüppel homolog 1, an early juvenile hormone-response gene downstream of Methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev. Biol. 325, 341–350 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Cui Y., Sui Y., Xu J., Zhu F., Palli S. R., Juvenile hormone regulates Aedes aegypti Krüppel homolog 1 through a conserved E box motif. Insect Biochem. Mol. Biol. 52, 23–32 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riddiford L. M., Truman J. W., Mirth C. K., Shen Y. C., A role for juvenile hormone in the prepupal development of Drosophila melanogaster. Development 137, 1117–1126 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdou M. A., et al., Drosophila Met and Gce are partially redundant in transducing juvenile hormone action. Insect Biochem. Mol. Biol. 41, 938–945 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Zhu J., Noriega F. G., The role of juvenile hormone in mosquito development and reproduction. Adv. Insect Physiol. 51, 93–113 (2016). [Google Scholar]
- 46.Roy S., et al., Regulation of reproductive processes in female mosquitoes. Adv. Insect Physiol. 51, 115–144 (2016). [Google Scholar]
- 47.Roy S., Saha T. T., Zou Z., Raikhel A. S., Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 63, 489–511 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Clifton M. E., Noriega F. G., Nutrient limitation results in juvenile hormone-mediated resorption of previtellogenic ovarian follicles in mosquitoes. J. Insect Physiol. 57, 1274–1281 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clifton M. E., Noriega F. G., The fate of follicles after a blood meal is dependent on previtellogenic nutrition and juvenile hormone in Aedes aegypti. J. Insect Physiol. 58, 1007–1019 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gwadz R. W., Spielman A., Corpus allatum control of ovarian development in Aedes aegypti. J. Insect Physiol. 19, 1441–1448 (1973). [DOI] [PubMed] [Google Scholar]
- 51.Hernández-Martínez S., Mayoral J. G., Li Y., Noriega F. G., Role of juvenile hormone and allatotropin on nutrient allocation, ovarian development and survivorship in mosquitoes. J. Insect Physiol. 53, 230–234 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmed T. H., Saunders T. R., Mullins D., Rahman M. Z., Zhu J., Molecular action of pyriproxyfen: Role of the Methoprene-tolerant protein in the pyriproxyfen-induced sterilization of adult female mosquitoes. PLoS Negl. Trop. Dis. 14, e0008669 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grisales N., et al., Pyriproxyfen-treated bed nets reduce reproductive fitness and longevity of pyrethroid-resistant Anopheles gambiae under laboratory and field conditions. Malar. J. 20, 273 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.League G. P., et al., Sexual selection theory meets disease vector control: Testing harmonic convergence as a “good genes” signal in Aedes aegypti mosquitoes. PLoS Negl. Trop. Dis. 15, e0009540 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramalingam S., Craig G. B., The effects of a JH mimic and cauterization of the corpus allatum complex on the male accessory glands of Aedes aegypti (Diptera: Culicidae). Can. Entomol. 109, 897–906 (1977). [Google Scholar]
- 56.Minakuchi C., et al., Expressional and functional analysis of CYP15A1, a juvenile hormone epoxidase, in the red flour beetle Tribolium castaneum. J. Insect Physiol. 80, 61–70 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Yokoi T., et al., Transcription-inducing activity of natural and synthetic juvenile hormone agonists through the Drosophila Methoprene-tolerant protein. Pest Manag. Sci. 76, 2316–2323 (2020). [DOI] [PubMed] [Google Scholar]
- 58.Miyakawa H., Iguchi T., Comparative luciferase assay for establishing reliable in vitro screening system of juvenile hormone agonists. J. Appl. Toxicol. 37, 1082–1090 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Tobe S. S., Bendena W. G., The regulation of juvenile hormone production in arthropods. Functional and evolutionary perspectives. Ann. N. Y. Acad. Sci. 897, 300–310 (1999). [DOI] [PubMed] [Google Scholar]
- 60.Rodrigues M. A., Flatt T., Endocrine uncoupling of the trade-off between reproduction and somatic maintenance in eusocial insects. Curr. Opin. Insect Sci. 16, 1–8 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Kistler K. E., Vosshall L. B., Matthews B. J., Genome engineering with CRISPR-Cas9 in the mosquito Aedes aegypti. Cell Rep. 11, 51–60 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raji J. I., et al., Aedes aegypti mosquitoes detect acidic volatiles found in human odor using the IR8a pathway. Curr. Biol. 29, 1253–1262.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lobo N. F., Clayton J. R., Fraser M. J., Kafatos F. C., Collins F. H., High efficiency germ-line transformation of mosquitoes. Nat. Protoc. 1, 1312–1317 (2006). [DOI] [PubMed] [Google Scholar]
- 64.Nouzova M., Edwards M. J., Mayoral J. G., Noriega F. G., A coordinated expression of biosynthetic enzymes controls the flux of juvenile hormone precursors in the corpora allata of mosquitoes. Insect Biochem. Mol. Biol. 41, 660–669 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hernández S., et al., Morphological and cytochemical characterization of female Anopheles albimanus (Diptera: Culicidae) hemocytes. J. Med. Entomol. 36, 426–434 (1999). [DOI] [PubMed] [Google Scholar]
- 66.Ramirez C. E., et al., Fast, ultra-trace detection of juvenile hormone III from mosquitoes using mass spectrometry. Talanta 159, 371–378 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are included in the article and SI Appendix.