Abstract
Objectives
Fast-track clinics (FTC) have been introduced in different fields and have been reporting significant outcomes in terms of reducing mortality, morbidity, and financial costs. To date, scarce evidence is available for FTC specific for patients suspected of polymyalgia rheumatica (PMR). The primary aim of our paper is to provide an overview of the clinical impact of PMR on patients and the healthcare system by analysing multiple aspects: the median time from onset of symptoms to diagnosis and the burden of the disease both on the healthcare system costs and on patients’ quality of life (QoL). Secondarily, based on these data, we aim to discuss the potential advantages and feasibility of a PMR FTC in everyday clinical practice.
Material and methods
We performed a narrative non-systematic review (PRISMA protocol not followed) of PubMed and Medline (OVID interface) with the following MeSH terms: [polymyalgia rheumatica AND diagnosis OR diagnosis, delayed OR patient care OR early diagnosis OR length of stay OR costs OR healthcare system OR quality of life] or [polymyalgia rheumatica AND glucocorticoids AND side effects]. We decided to exclude every paper that did not report raw data in terms of diagnostic time or delay, hospitalization rate, socio-economic costs on the healthcare system, patients’ QoL, and glucocorticoids-related events in PMR patients. Papers focused primarily on giant cell arteritis patients with overlapping PMR were also excluded. Abstract archives of the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR) congresses of the last 10 years were screened and included in the search if raw data were available. Each paper’s reference list was scanned for additional publications meeting this study’s aims. When papers reported data partially presented in previous articles, we opted to use the most recently published data.
Results
According to our literature review, a PMR FTC might lighten the burden of the disease. Nevertheless, its feasibility depends mostly on the resources of the national health system and of the territorial health district, which are heterogeneously limited. The usefulness of PMR FTCs depends on closer collaboration with the general practitioner because he/she is the first clinician to visit patients with PMR.
Conclusions
Polymyalgia rheumatica fast-track clinics might lighten the burden of the disease. However, it has some limits that should carefully assessed in planning health policies.
Keywords: polymyalgia rheumatica, fast-track clinic, narrative review, public health
Introduction
Polymyalgia rheumatica (PMR) is the one of the most common inflammatory rheumatic conditions in older patients, with a higher prevalence among women and in those of north European ethnicity [1]. Along with the phenomenon of global aging, its incidence is predicted to increase [2]. Aching and stiffness of the shoulders, hip girdle, and neck are its typical manifestations. Its onset is so sudden that the patient usually remembers the exact day and hour [3]. Polymyalgia rheumatica is frequently accompanied by an increase of the inflammatory markers (erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP] concentrations, above all) and in some patients by constitutional symptoms such as fever, fatigue, malaise, and weight loss [4].
On the other hand, normal ESR and CRP should not be a reason to exclude PMR, because PMR with normal ESR and CRP concentrations at the time of diagnosis exists [5, 6], and this applies to 14% of PMR patients, according to a recently published retrospective study [7]. Finally, PMR might present either as an isolated condition or in association (in up to 16–21% of cases) with giant cell arteritis (GCA), a large vessel vasculitis targeting the aorta and its branches [8, 9]. Giant cell arteritis can cause irreversible sight loss if treatment with glucocorticoids (GCs) is procrastinated [10].
Polymyalgia rheumatica can be easily diagnosed when it manifests with typical features. However, atypical presentations are not infrequent in everyday clinical practice, and several diseases, including inflammatory, autoimmune, infective, and malignant processes, can mimic PMR, making its diagnosis more challenging [11].
In Table I, we list the most common PMR-mimicking diseases and the main signs and symptoms that can be useful in their differential diagnosis. If misdiagnosed or inadequately treated, PMR can exert a profound burden on the patient’s quality of life (QoL) [17]. This derives from the impairment due to the inflammatory pain and stiffness, from secondary depressive symptoms and sleep disorders, and chronically from the potential adverse events (AEs) of medium to high dosages of GCs [18–22].
Table I.
Polymyalgia rheumatica–mimicking diseases. Signs and symptoms useful for a correct diagnosis [12–16]
| Disease | Signs and symptoms useful for a correct diagnosis |
|---|---|
| Rheumatoid arthritis | Involvement of some joints of the hands (metacarpophalangeal and proximal interphalangeal), positive results of rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibodies (ACPA), radiographic and ultrasound findings (erosive arthritis, periarticular osteoporosis) |
| Remitting seronegative symmetrical synovitis with pitting oedema (RS3PE) | Symmetric multiple synovitis, seronegative for RF and ACPA, causing boxing-glove swelling with pitting oedema of hands and feet. Ultrasound findings: tenosynovitis of extensor tendon sheath |
| Late-onset inflammatory spondyloarthropathies, including ankylosing spondylitis and psoriatic arthritis | Inflammatory pain in the lumbar region; radiographic findings of sacroiliitis; psoriasis |
| Late-onset systemic lupus erythematosus, scleroderma, Sjogren’s syndrome, vasculitis | Presence of antinuclear antibodies, presence of anti-neutrophil cytoplasmic antibodies |
| Idiopathic inflammatory myopathies (dermatomyositis, polymyositis) | Skin rashes, increased serum levels of creatine kinase |
| Scapulohumeral periarthritis and adhesive capsulitis (“frozen shoulder”) | Restriction of shoulder movements, even passive; ultrasound and magnetic resonance imaging allow one to diagnose the specific inflammation. Inflammatory markers not raised |
| Calcium pyrophosphate deposition disease | Monoarthritis; radiographic and ultrasound findings, examination of synovial fluid |
| Paraneoplastic syndromes | Failure to respond to glucocorticoid therapy or frequent relapses must be considered as elements of suspicion. Furthermore, the presence of atypical clinical manifestations and laboratory findings (among these, macrocytic anaemia or bicytopaenia), and familiarity for neoplasms should be considered as warning signs |
| Fibromyalgia | Inflammatory indices in their normal range, presence of tender points, widespread chronic pain |
Usually the general practitioner (GP) is the first clinician who visits a patient with PMR, but the level of the GP’s diagnostic accuracy is often low [23, 24].
Fast-track approaches have been introduced in different fields and have been reporting significant outcomes in terms of reducing mortality, morbidity, and financial costs. Fast-track clinics (FTC) are already available for the early diagnosis of GCA in different countries worldwide, particularly in the United Kingdom [25, 26], Italy [27], Norway [28], and the United States [29]. Their implementation was proven to significantly reduce the risk of permanent visual impairment and to be more cost-effective by reducing the need for inpatient care [25–29]. Conversely, less evidence is available for FTC specific for patients suspected of PMR. Recently, Danish investigators reported that the introduction of a PMR fast-track pathway in their clinic reduced the time to diagnosis and lowered both prednisone initiation before the rheumatologic assessment and the number of hospital admissions [30].
The primary aim of our paper is to provide an overview of the clinical impact of PMR on patients and the healthcare system by analysing these aspects: the median time from onset of symptoms to diagnosis, and the burden of the disease both on the healthcare system costs and on patients’ QoL. Secondarily, based on these data, we aim to discuss the potential advantages and feasibility of a PMR FTC in everyday clinical practice.
Material and methods
We performed a narrative non-systematic review (PRISMA protocol not followed) on PubMed and Medline (OVID interface) with the following MeSH terms: [polymyalgia rheumatica AND diagnosis OR diagnosis, delayed OR patient care OR early diagnosis OR length of stay OR costs OR healthcare system OR quality of life] or [polymyalgia rheumatica AND glucocorticoids AND side effects].
The articles that did not report raw data in terms of diagnostic time or delay, hospitalization rate, socio-economic costs on the healthcare systems, patients’ QoL, and GCs-related events in PMR patients were excluded from analyses as well as papers focused primarily on the patients with GCA and overlapping PMR. Abstract archives of the European Ligue Against Rheumatism (EULAR) and the American College of Rheumatology (ACR) congresses of the last 10 years were screened and included in the search if raw data were available. The reference list of each paper was scanned for additional publications meeting the aims of the presented study. In the case of presenting the data taken partially from previous articles, we based the analysis on the most recent data.
Results of the search and data interpretation
Time frame from the onset symptoms to the diagnosis of polymyalgia rheumatica and its hospitalization rate
We identified 10 papers reporting the period from symptom onset until diagnosis [23, 30–38]. A wide interval time to PMR diagnosis emerged among the studies, ranging from 22 days to 26 months depending on the considered cohort of patients. Of note, 2 studies also underlined the percentage of misdiagnoses varying from 64.8% to 86.4% [23, 33].
The diversity in the diagnostic performances might be due both to the geographic and time differences among the analysed group of patients and to the pattern of clinical presentations. Besides the typical inflammatory forms with the involvement of the girdles, clinicians may encounter atypical disease spectra; namely: not raised acute phase reactants, malignancy-associated, poor response to GCs, and predominant clinical array of constitutional symptoms without a specific clinical/ultrasound-based girdle involvement.
Additionally, a key role explaining such variability might also be awareness of the disease among GPs. Partnership work and shared training between rheumatologists and GPs proved very effective in raising this awareness and familiarity. For instance, in our experience, the median time from symptom onset until rheumatologic referral was 22 days in the group of trained GPs and 42 days in the group of untrained GPs [23]. Two papers also calculated the rate of hospitalization with contrasting data. In particular, in the retrospective paper of Dalkılıç et al. [31], 29.2% of PMR patients required at least one hospital admission in order to receive the diagnosis. However, no statistically significant differences in the hospitalization rates were shown between PMR and non-PMR patients in the data of Raheel et al. [32].
These results might be explained by differences of the studies in terms of sample size (respectively, 106 vs. 463 patients), study design (retrospective paper vs. population-based study), length of the observation period/follow-up (3 years vs. 19 years), and geographic variability in terms of PMR incidence, clinical patterns, and physicians’ awareness (Turkey vs. US). Table II shows a summary of the raw data.
Table II.
Health-related variables in polymyalgia rheumatica analysed in different counties worldwide
| First author | Dalkılıç [31] | Raheel [32] | Manzo [23] | Do [33] | Kim [34] | Cimmino [35] | Lee [36] | Marsman [7] | Sobrero [37] | Frølund [30] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Turkey | United States | Italy | South Korea | South Korea | Italy | South Korea | Netherlands | Italy | Denmark | |
| Year of publication | 2014 | 2018 | 2018 | 2018 | 2012 | 2011 | 2013 | 2021 | 2021 | 2021 | |
| Sample size | N = 106 | N = 463 | N = 303 | N = 54 | N = 51 | N = 60 | N = 78 | N = 454 | N = 383 | N = 132 | |
| Health-related variable | |||||||||||
| Time frame from symptoms to diagnosis | 13.5 ±13.3 months | n.a. | 24.3 days ±12.5 in trained GPs vs. 42.9 ±15 days in non-trained GPs | 8.1 ±8.6 months | 4 ±11.7 months | 3 months | 10.6 ±13.6 weeks | 10 weeks for patients with increased inflammatory markers vs. 13 weeks for patients with normal values of acute phase reactants | 2 months | 52 days for FTC patients vs. 80 days for the historical cohort | |
| Hospitalization – percentage of admitted patients or number of hospitalizations – LOS | 31/106 patients (29.2%)LOS: 7 ±3 days | 1398 hospitalizations In 8.4 years LOS: 4.4 days | n.a. | n.a. | n.a. | n.a | n.a | n.a | n.a | n.a | |
| Percentage of misdiagnosis | n.a | n.a | 86.4 % | 64.8 % | n.a. | n.a | n.a | n.a | n.a | n.a | |
GPs – general practitioners, LOS – length of stay, n.a. – not assessed.
GC-related events in polymyalgia rheumatica and quality of life in polymyalgia patients
The burden of GC-related complications might be relevant in a significant proportion of PMR patients who need low-dose GC therapy for many years to control the symptoms of the disease.
In a retrospective cohort of 222 PMR patients, low-dose GC treatment was associated with serious adverse events (AEs) such as osteoporosis (24.7%), frailty fractures (13.9%), arterial hypertension (12.1%), and diabetes mellitus (4.9%), with events occurring generally after 2 years of treatment [38]. A lower incidence of cardiovascular events was also reported: acute myocardial infarction (4%), stroke (1.3%), and peripheral arterial disease (0.9%) [39].
However, other studies concluded that treatment with GC in PMR patients is not associated with an increased risk of cardiovascular diseases [40], and a recent meta-analysis investigating the risk ratio of PMR patients for coronary artery disease and cerebrovascular events did not show univocal findings [41].
More recently, the rates of clinically significant GC-related AEs including hypertension, osteoporotic fractures, diabetes mellitus, hyperlipidaemia, and cataracts has been also reported for a larger cohort of 359 patients by Shbeeb et al. [42] (respectively, 16%, 24.9%, 13.5%, and 16%). However, except for the cumulative incidence of cataract in PMR patients (41%), the differences between the other comorbidities were not statistically significant between PMR patients and age- and gender- matched steroid-free comparators. This similarity may result from the generally low GC dosages used. Despite this latter evidence, the use of GCs should always be based on disease activity and tapered in line with international guidelines [43].
Lastly, the diagnosis of a chronic illness often generates psychological sequelae in PMR patients. Indeed, a recent cross-sectional study [19] reported higher prevalence of current depressive symptoms among primary care PMR patients in comparison with matched controls for gender and age. The impaired psychical domain inevitably alters patients’ QoL in association with other potential co-existing symptoms such as sleep disturbances and most notably stiffness, which is closely related to function, often preventing patients from carrying out their daily activities [17–19]. Nevertheless, according to a recent systematic review, grey areas are still present in the assessment of depression and depressive symptoms in patients with PMR [44].
Medical costs of polymyalgia rheumatica
Few literature data are available about the medical costs of PMR and the impact that the disease exerts on the national healthcare system.
An American study was the first to report the financial burden of PMR; in particular, it was observed that individuals with PMR utilized a significantly greater number of outpatient and laboratory services compared with age- and sex- matched controls. More specifically, the authors evaluated that the additional total cost over 5 years ranged from $2.233 to $27.712, respectively, being the 10th percentile and 90th percentile [45].
Additionally, a French paper recently estimated that PMR-related costs increase GCA’s financial burden of 76% in first three years [46]. In detail, the cumulative additional cost incurred by GCA patients due to overlapping PMR totaled €8801 in the first 3 years, and €10,532 during the first 5 years. This was mainly attributed to a greater inpatient stay, drug prescriptions, and paramedical care.
The data of the 2 previously mentioned studies are difficult to confront because of the profound differences between the American and French healthcare systems and the heterogeneity of their populations: the first one considered PMR patients whereas the second focused on GCA individuals with overlapping PMR.
Nevertheless, these findings should stimulate the gathering of further data on the socio-economic impact of PMR from other countries to obtain a better estimate of the financial difference in integrating a PMR FTC in clinical practice.
Can polymyalgia rheumatica fast-track clinics reduce the global disease burden?
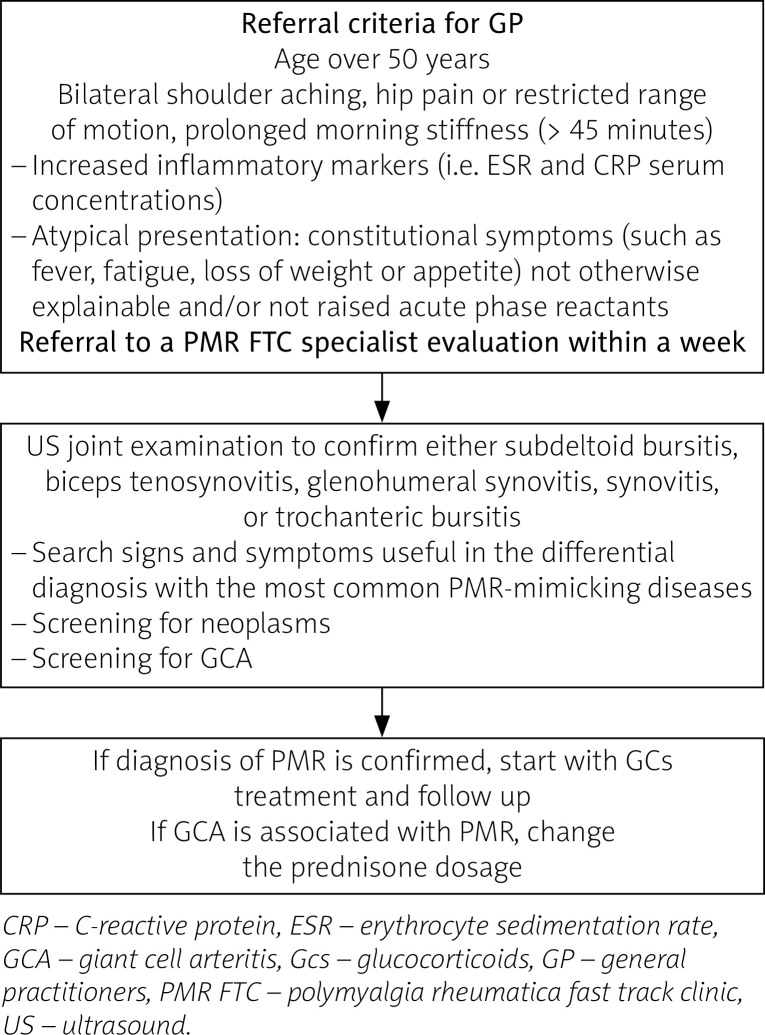
It is easy to understand that the earlier PMR diagnosis is made, the better the health outcomes of the patients. Is an FTC for patients suspected of PMR a viable solution? We suggest a potential algorithm of a PMR fast pathway (Fig. 1).
Fig. 1.
Potential algorithm for a fast-track pathway for polymyalgia rheumatica patients.
On the other hand, an FTC for patients suspected of PMR has some limits that must be highlighted and discussed.
Firstly, a PMR FTC implies the availability of rheumatological clinics to dedicate staff resources with clinical expertise, who are well trained in US joint examination to cover each item of PMR classification criteria [47]. In this way, a finer differential diagnostic can be made with other inflammatory and non-inflammatory musculoskeletal disorders. In addition, clinical and US expertise for diagnosis of GCA is essential.
Secondly, the question of how many national and/or regional health systems could financially support this thorough approach is significant, because if PMR is not an emergency (unlike GCA, for example), how ethically justifiable would be to “favour” these patients, perhaps over others?
Finally, collaboration with the GP should be implemented through partnership work and shared training. Fixed algorithms cannot replace this collaboration.
Conclusions
Polymyalgia rheumatica exerts a profound impact on patients’ lives in multiple aspects: GC-related adverse events, costs on the healthcare system, co-morbidities, and quality of life.
A PMR FTC might lighten the burden of the disease. Nevertheless, its feasibility depends mostly on the resources of the national health systems and of the territorial health districts, which are heterogeneously limited. Also, its usefulness depends on a closer collaboration with the GP because he/she is the first clinician to visit patients with PMR.
Footnotes
The authors declare no conflict of interest.
References
- 1.Gonzalez-Gay MA, Matteson EL, Castaneda S. Polymyalgia rheumatica. Lancet. 2017;390:1700–1712. doi: 10.1016/S0140-6736(17)31825-1. [DOI] [PubMed] [Google Scholar]
- 2.Manzo C. Incidence and prevalence of polymyalgia rheumatica (PMR): the importance of the epidemiological context. The Italian Case. Med Sci (Basel. 2019;7:92. doi: 10.3390/medsci7090092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milchert M, Brzosko M. Diagnosis of polymyalgia rheumatica usually means a favourable outcome for your patient. Indian J Med Res. 2017;145:593–600. doi: 10.4103/ijmr.IJMR_298_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camellino D, Giusti A, Girasole G, et al. Pathogenesis, diagnosis and management of polymyalgia rheumatica. Drugs Aging. 2019;36:1015–1026. doi: 10.1007/s40266-019-00705-5. [DOI] [PubMed] [Google Scholar]
- 5.Manzo C, Milchert M. Polymyalgia rheumatica with normal values of both erythrocyte sedimentation rate and C-reactive protein concentration at the time of diagnosis: a four-point guidance. Reumatologia. 2018;56:1–2. doi: 10.5114/reum.2018.74740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manzo C, Milchert M, Natale M, Brzosko M. Polymyalgia rheumatica with normal values of both erythrocyte sedimentation rate and C-reactive protein concentration at the time of diagnosis. Rheumatology (Oxford. 2019;5:921–923. doi: 10.1093/rheumatology/key431. [DOI] [PubMed] [Google Scholar]
- 7.Marsman DE, den Broeder N, Boers N, et al. Polymyalgia rheumatica patients with and without elevated baseline acute phase reactants: distinct subgroups of polymyalgia rheumatica? Clin Exp Rheumatol. 2021;39:32–37. doi: 10.55563/clinexprheumatol/gdps1r. [DOI] [PubMed] [Google Scholar]
- 8.Clini P, Stimamiglio A, Camellino D, et al. Management of giant cell arteritis among general practitioners from Genoa, Italy: a web-based survey. Reumatismo. 2021;72:207–212. doi: 10.4081/reumatismo.2020.1291. [DOI] [PubMed] [Google Scholar]
- 9.Hysa E, Sobrero A, Camellino D, et al. A seasonal pattern in the onset of polymyalgia rheumatica and giant cell arteritis? A systematic review and meta-analysis. Semin Arthritis Rheum. 2020;50:1131–1139. doi: 10.1016/j.semarthrit.2020.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Tombetti E, Hysa E, Mason JC, et al. Blood biomarkers for monitoring and prognosis of large vessel vasculitides. Curr Rheumatol Rep. 2021;23:17. doi: 10.1007/s11926-021-00980-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manzo C, Camellino D. Polymyalgia rheumatica: diagnostic and therapeutic issues of an apparently straightforward disease. Recenti Prog Med. 2017;108:221–231. doi: 10.1701/2695.27559. [DOI] [PubMed] [Google Scholar]
- 12.Ceccato F, Uńa C, Regidor M, et al. Conditions mimicking polymyalgia rheumatica. Reum Clin. 2011;7:156–160. doi: 10.1016/j.reuma.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Emamifar A, Hess S, Ellingsen T, et al. Prevalence of newly diagnosed malignancies in patients with polymyalgia rheumatica and giant cell arteritis, comparison of 18F-FDG PET/CT scan with chest X-ray and abdominal ultrasound: data from a 40 week prospective, exploratory, single centre study. J Clin Med. 2020;9:3940. doi: 10.3390/jcm9123940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manzo C, Natale M. Polymyalgia rheumatica and cancer risk: the importance of the diagnostic set. Open Access Rheumatol. 2016;8:93–95. doi: 10.2147/OARRR.S116036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manzo C, Emamifar A. Polymyalgia rheumatica and seronegative elderly-onset rheumatoid arthritis: two different diseases with many similarities. EMJ. 2019;4:111–119. [Google Scholar]
- 16.Manzo C. Paraneoplastic syndromes and inflammatory rheumatic diseases: not everything that glitters is gold. The case of polymyalgia rheumatica. J Med Oncl Ther. 2018;3:21–22. doi: 10.35841/medical-oncology.3.2.21-22. [DOI] [Google Scholar]
- 17.Mackie SL, Hughes R, Walsh M, et al. “An Impediment to Living Life”: why and how should we measure stiffness in polymyalgia rheumatica? PLoS One. 2015;10:e0126758. doi: 10.1371/journal.pone.0126758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manzo C, Castagna A. Subjective sleep disturbances at the time of diagnosis in patients with polymyalgia rheumatica and in patients with seronegative elderly-onset rheumatoid arthritis. A pilot study. Reumatologia. 2020;58:196–201. doi: 10.5114/reum.2020.98430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vivekanantham A, Blagojevic-Bucknall M, Clarkson K, et al. How common is depression in patients with polymyalgia rheumatica? Clin Rheumatol. 2018;37:1633–1638. doi: 10.1007/s10067-017-3691-9. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Keeley A, Mallen C, et al. Incidence of infections associated with oral glucocorticoid dose in people diagnosed with polymyalgia rheumatica or giant cell arteritis: a cohort study in England. CMAJ. 2019;191:e680–e688. doi: 10.1503/cmaj.190178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emamifar A, Ellingsen T, Hermann AP, et al. Prognostic impacts of glucocorticoid treatment in patients with polymyalgia rheumatica and giant cell arteritis. Sci Rep. 2021;11:6220. doi: 10.1038/s41598-021-85857-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manzo C, Serra-Mestres J, Castagna A, Isetta M. Behavioral, psychiatric, and cognitive adverse events in older persons treated with glucocorticoids. Medicines (Basel. 2018;5:82. doi: 10.3390/medicines5030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manzo C, Natale M, Traini E. Diagnosis of polymyalgia rheumatica in primary health care: favoring and confounding factors – a cohort study. Reumatologia. 2018;56:131–139. doi: 10.5114/reum.2018.76900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobrero A, Manzo C, Stimamiglio A. The role of the general practitioner and the out-of-hospital public rheumatologist in the diagnosis and follow-up of patients with polymyalgia rheumatica. Reumatismo. 2018;70:44–50. doi: 10.4081/reumatismo.2018.1036. [DOI] [PubMed] [Google Scholar]
- 25.Tedeschi SK, Sobiesczyzk PS, Ford JA, et al. Clinical experience with a multidisciplinary model of vascular ultrasound for the evaluation for giant cell arteritis. ACR Open Rheumatol. 2021;3:147–153. doi: 10.1002/acr2.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patil P, Williams M, Maw WW, et al. Fast track pathway reduces sight loss in giant cell arteritis: results of a longitudinal observational cohort study. Clin Exp Rheumatol. 2015;33(2 Suppl 89):S103–S106. [PubMed] [Google Scholar]
- 27.Monti S, Bartoletti A, Bellis E, et al. Fast-track ultrasound clinic for the diagnosis of giant cell arteritis changes the prognosis of the disease but not the risk of future relapse. Front Med (Lausanne. 2020;7:589794. doi: 10.3389/fmed.2020.589794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diamantopoulos AP, Haugeberg G, Lindland A, Myklebust G. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology (Oxford. 2016;55:66–70. doi: 10.1093/rheumatology/kev289. [DOI] [PubMed] [Google Scholar]
- 29.Sacksen I, Jernberg E, Pollock S, et al. Fast Track Clinic (FTC) for Giant Cell Arteritis (GCA) – the United States Experience [abstract] Arthritis Rheumatol. 2019;71(suppl 10) [Google Scholar]
- 30.Frølund LL, Våben C, Dam M, et al. Fast track clinic for early diagnosis of polymyalgia rheumatica: Impact on symptom duration and prednisolone initiation. Joint Bone Spine. 2021;88:105185. doi: 10.1016/j.jbspin.2021.105185. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Dalkılıç E, Tufan AN, Hafızoğlu E, et al. The process from symptom onset to rheumatology clinic in polymyalgia rheumatica. Rheumatol Int. 2014;34:1589–1592. doi: 10.1007/s00296-014-3034-y. [DOI] [PubMed] [Google Scholar]
- 32.Raheel S, Crowson CS, Achenbach SJ, Matteson EL. Hospitalization rates among patients with polymyalgia rheumatica: a population-based study from 1995–2017 [abstract] Arthritis Rheumatol. 2018;70(suppl 10) [Google Scholar]
- 33.Do JG, Park J, Sung DH. Characteristics of Korean patients with polymyalgia rheumatica: a single locomotive pain clinic cohort study. J Korean Med Sci. 2018;33:e241. doi: 10.3346/jkms.2018.33.e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HA, Lee J, Ha YJ, et al. Induction of remission is difficult due to frequent relapse during tapering steroids in Korean patients with polymyalgia rheumatica. J Korean Med Sci. 2012;27:22–26. doi: 10.3346/jkms.2012.27.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cimmino MA, Parodi M, Montecucco C, Caporali R. The correct prednisone starting dose in polymyalgia rheumatica is related to body weight but not to disease severity. BMC Musculoskelet Disord. 2011;12:94. doi: 10.1186/1471-2474-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JH, Choi ST, Kim JS, et al. Clinical characteristics and prognostic factors for relapse in patients with polymyalgia rheumatica (PMR) Rheumatol Int. 2013;33:1475–1480. doi: 10.1007/s00296-012-2580-4. [DOI] [PubMed] [Google Scholar]
- 37.Sobrero A, Paolino S, Hysa E, et al. Seasonal onset of polymyalgia rheumatica: correlations with the pattern of clinical presentation, disease severity and outcome in 383 patients from a single centre. Clin Exp Rheumatol. 2021;39:564–569. [PubMed] [Google Scholar]
- 38.Mazzantini M, Torre C, Miccoli M, et al. Adverse events during longterm low-dose glucocorticoid treatment of polymyalgia rheumatica: a retrospective study. J Rheumatol. 2012;39:552–557. doi: 10.3899/jrheum.110851. [DOI] [PubMed] [Google Scholar]
- 39.Maradit Kremers H, Reinalda MS, Crowson CS, et al. Glucocorticoids and cardiovascular and cerebrovascular events in polymyalgia rheumatica. Arthritis Rheum. 2007;57:279–286. doi: 10.1002/art.22548. [DOI] [PubMed] [Google Scholar]
- 40.Kang JH, Sheu JJ, Lin HC. Polymyalgia rheumatica and the risk of stroke: a three-year follow-up study. Cerebrovasc Dis. 2011;32:497–503. doi: 10.1159/000332031. [DOI] [PubMed] [Google Scholar]
- 41.Ungprasert P, Koster MJ, Warrington KJ, Matteson EL. Polymyalgia rheumatica and risk of coronary artery disease: a systematic review and meta-analysis of observational studies. Rheumatol Int. 2017;37:143–149. doi: 10.1007/s00296-016-3557-5. [DOI] [PubMed] [Google Scholar]
- 42.Shbeeb I, Challah D, Raheel S, et al. Comparable rates of glucocorticoid-associated adverse events in patients with polymyalgia rheumatica and comorbidities in the general population. Arthritis Care Res (Hoboken. 2018;70:643–647. doi: 10.1002/acr.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dejaco C, Singh YP, Perel P, et al. 2015 Recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis. 2015;74:1799–1807. doi: 10.1136/annrheumdis-2015-207492. [DOI] [PubMed] [Google Scholar]
- 44.Manzo C, Nizama-Via A, Milchert M, et al. Depression and depressive symptoms in patients with polymyalgia rheumatica: discussion points, grey areas and unmet needs emerging from a systematic review of published literature. Reumatologia. 2020;58:381–389. doi: 10.5114/reum.2020.102003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kremers HM, Reinalda MS, Crowson CS, et al. Direct medical costs of polymyalgia rheumatica. Arthritis Rheum. 2005;53:578–584. doi: 10.1002/art.21311. [DOI] [PubMed] [Google Scholar]
- 46.Mounié M, Pugnet G, Savy N, Lapeyre-Mestre M, Molinier L, Costa N. Additional costs of polymyalgia rheumatica with giant cell arteritis. Arthritis Care Res (Hoboken. 2019;71:1127–1131. doi: 10.1002/acr.23736. [DOI] [PubMed] [Google Scholar]
- 47.Dasgupta B, Cimmino MA, Maradit-Kremers H, et al. 2012 provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis. 2012;71:484–492. doi: 10.1136/annrheumdis-2011-200329. [DOI] [PMC free article] [PubMed] [Google Scholar]