Highlights
-
•
Long non coding RNAs (lncRNAs) are non-protein or low-protein coding transcripts.
-
•
LncRNAs contain more than 200 nucleotides.
-
•
LncRNAs are associated with disease pathogenicity including the neurological disorders.
-
•
LncRNAs mediate pathogenesis by decoy, scaffold, mi-RNA sequestrator, histone modifiers and in transcriptional interference.
-
•
LncRNAs can serve as novel biomarkers for therapeutic aspects.
Keywords: Non coding rnas, Alzheimer's disease, Huntington's disease, Parkinson's disease, Schizophrenia, Regulatory
Abstract
Long non coding RNAs (lncRNAs) are non-protein or low-protein coding transcripts that contain more than 200 nucleotides. They representing a large share of the cell's transcriptional output, demonstrate functional attributes viz. tissue-specific expression, determination of cell fate, controlled expression, RNA processing and editing, dosage compensation, genomic imprinting, conserved evolutionary traits etc. These long non coding variants are well associated with pathogenicity of various diseases including the neurological disorders like Alzheimer's disease, schizophrenia, Huntington's disease, Parkinson's disease etc. Neurological disorders are widespread and there knowing the underlying mechanisms become crucial. The lncRNAs take part in the pathogenesis by a plethora of mechanisms like decoy, scaffold, mi-RNA sequestrator, histone modifiers and in transcriptional interference. Detailed knowledge of the role of lncRNAs can help to use them further as novel biomarkers for therapeutic aspects. Here, in this review we discuss regulation and functional roles of lncRNAs in eight neurological diseases and psychiatric disorders, and the mechanisms by which they act. With these, we try to establish their roles as potential markers and viable diagnostic tools in these disorders.
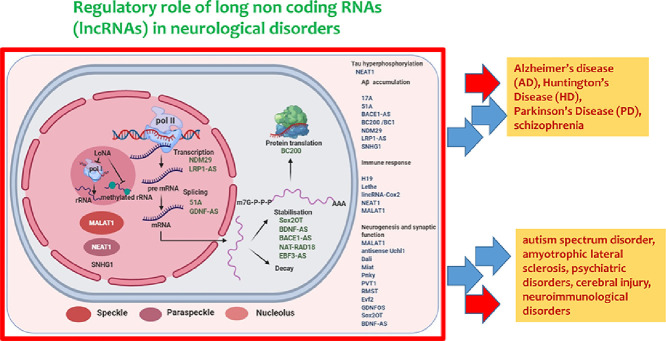
Graphical abstract
1. Introduction
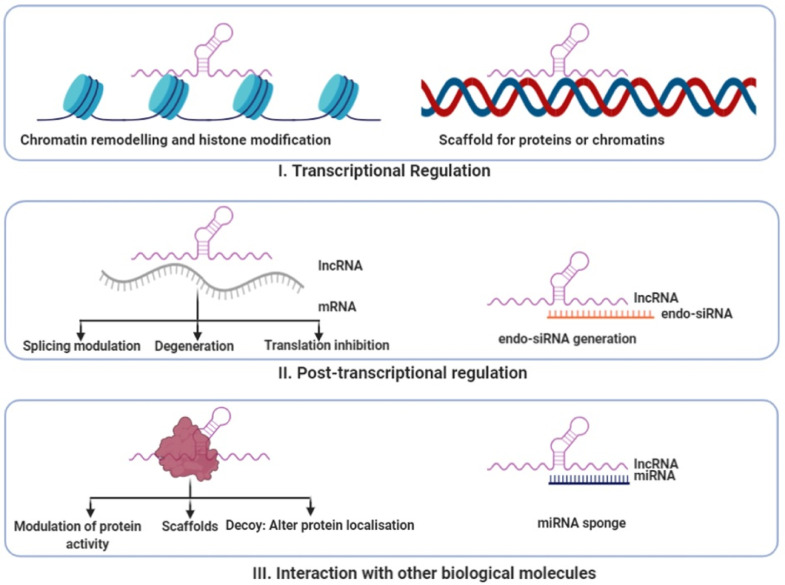
It has now been shown that almost 90% of human genome is transcribed into RNA molecules [1], and only 1.2% of these transcripts are translated into protein molecules [2]. Earlier, these non-coding transcripts were thought to be the degraded products of RNA processing machinery [3]. However, the ENCODE, consortia have re-established that (mostly non-coding) transcripts cover 62%–75% of human genome [4,5]. Once the human genome project was completed, exploration of the biology of these huge amounts of non-coding RNAs (ncRNAs) began and it led to the fact that they serve as important regulators of multiple physiological and cellular functions. Based on their length, ncRNAs are classified into small non coding transcripts like miRNA, snRNA, piwi RNA and long non-coding RNA (lncRNAs) (transcripts longer than 200 nucleotides) [6]. Numerous studies have demonstrated the involvement of small ncRNAs like microRNAs (miRNAs) in various complex diseases [1]. Simultaneously, the importance of lncRNAs as crucial regulators in development, progression and manifestation of metabolic diseases has started to unravel. LncRNAs are classified into different categories based on transcript length, association with annotated protein-coding genes, association with other DNA elements of known function, protein-coding RNA resemblance, association with repeats, association with a biochemical pathway or stability, sequence and structure conservation, expression in different biological states, association with subcellular structures, genome location and context, functioning and targeting mechanism [7,8]. Some of the salient features of lncRNAs include poor sequence conservation across hierarchy and sequences with fewer exons. LncRNAs may or may not be poly-adenylated and these molecules are mostly dependent on their secondary structure for their function and the expression patterns of lncRNAs are tissue specific [9]. Similar to mRNAs, lncRNAs are transcribed by RNA polymerase II, capped at 5′ end, spliced and have promoter regions. Most of them are also polyadenylated at the 3′ end [10]. The functional roles of these lncRNAs can be broadly categorized as- decoy, scaffold, mi-RNA sequestrator, histone modifiers and in transcriptional interference [11,12]. They can be either cis- or trans-acting based on their silencing or gene expression activation on same or different chromosome [9]. LncRNAs are very heterogeneous and exhibit multifaceted biological functions and interact with a variety of other proteins [11]. Depending on their subcellular localization in the nucleus or cytoplasm, lncRNAs can interfere with many transcriptional and post-transcriptional gene regulations by recruiting or inhibiting transcription factors [13,14], alternative splicing 15], as well as mRNA translation [5,11,16]. Nuclear transcripts, for example, can mediate epigenetic gene modifications [17,18] or transcriptional activation and silencing, whereas cytoplasmic lncRNAs often interact with miRNAs to post-transcriptionally regulate gene expression or act as molecular scaffolds for RNA-protein complexes [15,19,20]. Various ways of functioning of lncRNAs are shown in Fig. 1. In past decade, a large number of functional studies have been established and now these transcripts are shown to have regulatory role in fine-tuning of various biological processes.
Fig. 1.
Various ways of functioning of lncRNAs. I. LncRNAs can regulate transcriptional processes by either acting as chromatin remodeler or by modifying histone proteins. It can also act as scaffold for proteins or chromatins. II. LncRNAs can also have post transcriptional regulatory functions. It can module splicing, help in degeneration of mRNA or can inhibit translation. Some lncRNAs can also generate endo siRNA. III. At the level of translation, it can act as modulator of protein activity, scaffold, decoy of as a miRNA sponge.
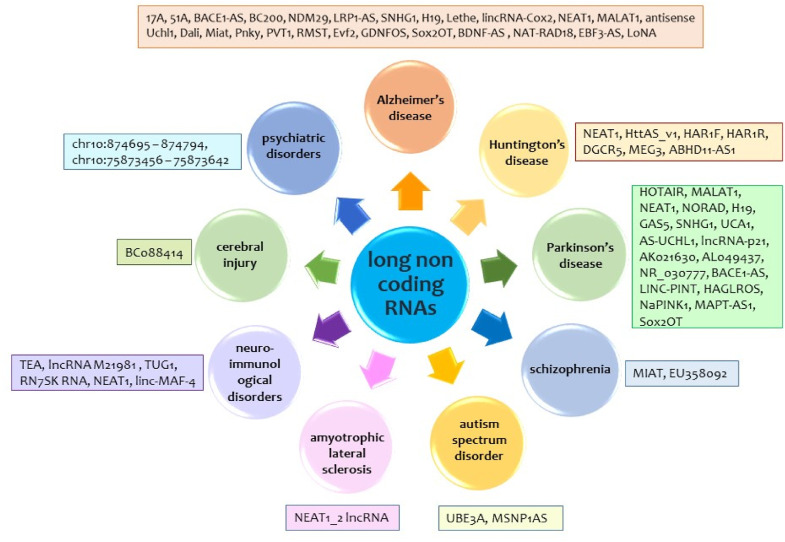
The worldwide prevalence of neurodegenerative disorders makes to of utmost importance. Alzheimer's disease (AD) contribute to over 60% of total 50 million dementia patients worldwide [21], while over ten million people are living with Parkinson's disease (PD) [22]. The worldwide occurrence of Huntington's disease (HD) was estimated to be 2.71 per 100 000 (95% CI: 1.55–4.72) based on a meta-analysis of 13 studies [23]. Motor neuronal disease like amyotrophic lateral sclerosis (ALS) has an incidence rate of 2.2 per 100 000 person-years (py) in European population as estimated by European registry consortium named EURALS, 0.89 per 100 000 py in East Asia and 0.79 per 100 000 py in South Asia [24]. According to WHO, worldwide one in 160 children suffers autism spectrum disorder (ASD) [25] while over 264 million people across all ages suffer from depression globally [26]. LncRNAs are involved in neurological disorders as well. Here we summarize involvement of lncRNAs in eight neurological disorders and psychiatric disorders, namely AD, schizophrenia, HD, PD, ASD, ALS, major depressive disorder, cerebral injury and neuroimmunological disorder.
2. Role of lncRNAs in neurological disorders
2.1. Role of lncRNAs in AD
AD is primarily characterized by accumulation of amyloid beta (Aβ) plaques in the brain tissue and confers subtle contribution to the pathogenesis of the diseases that results in dementia [27]. A membrane bound aspartic protease b-site APP cleaving enzyme 1 (BACE1) is responsible for catalysing the cleavage of amyloid precursor protein (APP) and production of Aβ plaques. A conserved antisense transcript of BACE1, b-site APP cleaving enzyme 1 antisense strand (BACE1-AS) has been found to be up-regulated in brains of Alzheimer's patients [28,29]. BACE1-AS binds with the BACE1 transcripts and stabilizes it, thereby increasing the synthesis of BACE1 enzyme, and consecutively Aβ plaques [28]. A microRNA miR-485–5p has been reported to inhibit BACE1 expression by competitively binding with BACE1-AS [30].
The lncRNA antisense to brain-derived neurotrophic factor (BDNF-AS) is antisense transcript to BDNF, and negatively regulates BDNF level both in vivo and in vitro [31], that further down regulates an immediate-early gene, involved in synaptogenesis and synaptic plasticity called activity-regulated cytoskeleton-associated protein (ARC) [32]. Treatment with Aβ in PC12 cells decreases BDNF concentration but increases BDNF-AS level. Repressing BDNF-AS increases BDNF levels, that promotes cell viability [33]. Early B cell factor 3 (EBF3) (also known as olf), a DNA binding transcription factor, is expressed in the olfactory receptor neurons and their precursors [34] and is involved in neurogenesis, cell cycle arrest and apoptosis [35,36]. The level of EBF3 is found to be elevated in the hippocampus of AD mice. The lncRNA EBF3-AS is transcribed from the opposite strand of EBF3 and is up-regulated in the hippocampus of APP/PS1 mice. In human SH-SY5Y cell, EBF3-AS deficiency decreases EBF3 levels inhibits okadaic acid (OA) or Aβ induced apoptosis, showing its relevance as a biomarker and therapeutic targets of AD [37].
The lncRNA-long nucleolar non-coding RNA (LoNA) binds to nucleolin and decreases its activity, thereby regulates rRNA transcription. It also interacts with fibrillarin and regulates rRNA methylation. Protein translation taking place at neuronal soma has crucial role in synaptic development and plasticity. At the level of translation, LoNA has regulatory activity by modulation of ribosomal components and its assembly [38], [39], [40]. LoNA concentration is significantly up-regulated in the hippocampus of AD mice along with reduced rDNA levels. rDNA silencing has shown to be responsible for AD-related ribosomal deficiency and suppressed rRNA 28 S/18 S ratio [41]. Knocking down LoNA has shown restoration of rRNA levels and improvement of cognitive deficits in AD mice [42]. The mouse lncRNA lincRNA-Cox2 has diverse function of both inducing and repressing immune genes, as it interacts with heterogeneous nuclear ribonucleoprotein A/B and A2/B1 which is required for target gene inhibition [43]. The other mouse lncRNA antisense UchL1 remains partially overlapped with UchL1 mRNA and activates polysomes for its translation [44]. Two other mouse lncRNA MIAT and Pnky are involved in neurogenic commitment and neurogenesis regulation of embryonic and postnatal neural stem cell populations. Dysregulated MIAT causes defective splicing of Wnt7b and has pleiotropic effects on brain development [45], while Pnky mediated neurogenesis regulation of embryonic and postnatal neural stem cell populations takes place by its interaction with splicing factor PTBP1 [46]. The lncRNA PVT1 mediates autophagy protects hippocampal neurons from impaired synaptic plasticity [47], while the lncRNA Evf2 controls expression of Dlx5, Dlx6 and Gad1 by recruiting transcription factors DLX and MECP2 in Dlx5/6 intergenic region [48].
Another lncRNA brain cytoplasmic (BC)−200 RNA (BCYRN1) has been found to be involved in pathogenesis of AD by binding with poly(A)-binding protein 1 (PABP1), a regulator of translation initiation, after being transported as ribonucleoprotein particles to the dendritic processes. Thus, by regulation of the translation process, it modulates the gene expression [49]. It has also been found to be associated with abnormal protein localization by interacting with RNA-binding proteins [50]. Synaptic or dendritic degeneration can take place by over expression of BC-200, as it assumes clustered perikaryal localization in a stress compensation mechanism mediated by dendritic sprouting and remodeling [50]. BC-200 level has also been found higher in AD-affected brain region Brodmann area 9 in Alzheimer's patients compared to the healthy individuals [50]. Further detailed study may provide insights into the role of BC-200 in pathogenesis of AD [51]. The homolog of BC-200 in mouse, called BC1 binds with fragile X syndrome protein (FMRP) and induces translation of APP [52]. Aggregation of Aβ plaque is inhibited in Alzheimer's mice on depleting BC1 or BC1-FMRP complex. It also improves learning and memory in mice [52].
The lncRNA-17A causes excess of Aβ production when overexpressed. It also alternatively splices GABA receptor B (GABAB) and produces an isoform variant of it by directing G-protein coupled receptor 51 (GPR51). The GABA receptor isoform A cannot bind with this isoform variant and cannot produce functional heterodimeric receptor [53]. Another lncRNA, SNHG1 (small nucleolar RNA host gene 1) mediates miR-137 sponge that causes attenuation of the Aβ mediated effect by selectively targeting the untranslated region of an intrinsic pro-apoptotic transmembrane receptor kringle containing transmembrane protein 1 (KREMEN1). Aβ treatment induces expression of SNHG1 while repression of it in Aβ treated cells reduces effectst of Aβ on mitochondrial membrane potential and cell viability [54], [55], [56]. In SH-SY5Y and human primary neuron cells, this takes place by SNHG1 mediated miR-137 sponge that selectively targets the untranslated region of a transmembrane receptor with intrinsic pro-apoptotic activity, called targeting KREMEN1 [56]. SNHG1 also interacts with its protein partners MATR3, Ezh2 [56].
The lncRNA NAT-Rad18 is up-regulated in Alzheimer's and post transcriptionally regulates Rad-18 protein, involved in proliferating cell nuclear antigen (PCNA) ubiquitination, DNA repair and nerve injury and increases the susceptibility to neuronal apoptosis and cell death [57]. Similarly, lncRNA 51A, produced from intron 1 of sorting protein-related receptor 1 (SORL1) gene, helps in accumulation of Aβ42 by altering the spliced form of SORL1 mRNA [58].
An lncRNA–GDNFOS (antisense of glial-cell-line-derived neurotrophic factor) overlaps with 5′–UTR of GDNF (glial-cell-line-derived neurotrophic factor) and negatively regulates the expression of GDNF and promote pathogenesis of AD. In mature temporal gyrus of AD patients, the GDNF peptide is down-regulated showing a halting of GDNF mediated neuroprotective effect [59,60]. The lncRNA LRP1-AS decreases LRP1 expression at both protein and RNA levels; LRP1-AS decreases transcription of LRP1 transcription by reducing LRP1 promoter activity induced by a transcriptional complex consisting of transcription factor Srebp1, which regulates LRP1 transcription and its interacting partner Hmgb2 [61]. In the cerebral cortex of the developing mouse brain, Sox2OT is binds with proteins FUS and YY1 and promotes neurogenesis and neuronal differentiation by repressing Sox2 [62]. Sox2OT is also differentially expressed in early and late disease stages the AD model mouse that suggests its potential role as a biomarker in AD [63].
Neuroblastoma differentiation marker 29 (NDM29), transcribed by RNA polymerase III, leads to inducing Ab secretion, APP synthesis in AD [64]. The lncRNA H19 promotes HDAC1-dependent M1 microglial polarization and causes neuroinflammation [65]. Lethe, a lncRNA in mouse has shown to regulate inflammatory signaling. Lethe- RelA (NF-B subunit RelA) interaction inhibits binding of RelA with DNA and consequently hinders target gene expressions [66].
The lncRNA Dali is involved in neural differentiation regulation by regulation DNA methylation of CpG island associated promoters by the interaction of DNMT1 DNA methyltransferase in trans [67]. Another lncRNA RMST is needed for the binding of promoter regions of neurogenic transcription factors to Sox2 and is involved in neural stem cell fate regulation [68]. The lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1), binds with NONO, SFPQ, PSF, Ezh2 and relocates SFPQ from IL8 promoter to the paraspeckles, that results in transcriptional activation of antiviral cytokines like IL8 [69], [70], [71], [72], [73]. The lncRNA MALAT1 is involved both in Immune response and synaptic density regulation. It promotes Regulation of glucose mediated up-regulation of inflammatory cytokines IL-6 and TNF-alpha by activation of SAA3 expression [74] and regulates synaptic density by modulating the recruitment of serine/arginine-rich (SR) family pre-mRNA-splicing factors (SRSF1,SFPQ) at the transcription site [75], [76], [77]. Polymorphism in lncRNA TCONS_00021856/linc-SLITRK5–11 gene at rs7990916 (T > C) is differentially present in Alzheimer's patients compared to heathy individual [78].
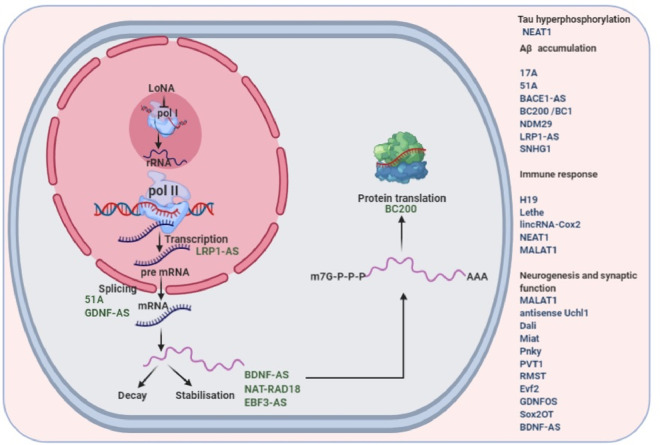
Zhou et al. have found majorly intergenic 84 down-regulated and 24 up-regulated lncRNAs in AD patients, one of these down-regulated lncRNAs, n341006 shows association with protein ubiquitination pathway while another up-regulated lncRNA, n336934, is associated with cholesterol homeostasis after gene set enrichment analysis (GSEA) [79]. Zhang et al. have discovered 114 significantly down-regulated and 97 significantly up-regulated lncRNA transcripts from the SAMP8 (senescence-accelerated mouse prone 8) and SAMR1 (senescence-accelerated mouse resistant 1) model. These transcripts are involved in mitogen activated protein kinase signaling pathway, nerve growth factor term and the AD pathway [80]. Table 1 and Fig. 2 summarises various regulatory mechanisms off lncRNAs in AD.
Table 1.
Role of lncRNAs in Alzheimer's disease.
| Disease | LncRNA | Species | Function | Mechanism | Ref. |
|---|---|---|---|---|---|
| Aβ accumulation | 17A | H | Increases secretion of Aβ and Aβx-42/Aβx-40 peptide ratio | Promotes synthesis of an alternative splicing isoform of GABAB R2 which impairs GABA B2 intracellular signaling | [81] |
| 51A | H | Disease causing; produced as an antisense transcript of intron 1 of the SORL1 gene and leads to increased Aβ formation due to defected processing of APP | Reduces synthesis of SORL1 variant by inducing a splicing shift of SORL | [82,83] | |
| BACE1-AS | H | Involved in production of Aβ1–42 in AD and its downregulation reduces the ability of BACE1 to cleave APP and delays the senile formation of plaques | BACE1-AS produces RNA duplexes by complementing with its protein partner HuD and mRNA BACE1 and therefore increases the stability and expression of BACE1 mRNA which helps to generates additional A1-42 | [84,85,28] | |
| BC200 (in human)/BC1 (in mouse) | H/M | Promotes aggregation and provides protection in memory deficits and spatial learning | In human, gene expression is altered as BC200 binds with PABP1, a regulator of translation initiation, after being transported as ribonucleoprotein particles to the dendritic processes; in mouse, in association with FMRP, APP mRNA translation and aggregation of Ab in brain are induced by BC1 | [86], [87], [88],49] | |
| NDM29 | H | NDM29 is upregulated in patients with neurodegenerative diseases | Increases Aβ secretion and the Aβx-42/Aβx-40 ratio, due to increase in the synthesis of APP, induced by NDM29-dependent cell maturation | [89], [90], [91] | |
| LRP1-AS | H/M | Transcribed antisense to LRP1; negatively regulates the APP trafficking and Aβ processing and Aβ accumulation function of LRP1 | Decreases LRP1 expression at protein and RNA levels by reducing LRP1 promoter activity induced by a transcriptional complex consisting of transcription factor Srebp1, and its interacting partner Hmgb2. | [61] | |
| SNHG1 | H/M | Attenuation of the Aβ mediated effect by selectively targeting KREMEN1 | Aβ treatment induces expression of SNHG1 while repression of it in Aβ treated cells reduces effects of Aβ on MMP and cell viability; SNHG1 mediated miR-137 sponge causes attenuation of the Aβ mediated effect by selectively targeting KREMEN1 | [54], [55], [56] | |
| Genetic polymorphism | TCONS_00021856/ linc-SLITRK5-11 |
H | Polymorphism in lncRNA TCONS_00021856/linc-SLITRK5-11 gene | At rs7990916 (T > C) is differentially present in Alzheimer's patients | [78] |
| Immune response | H19 | H | Neuro-inflammation induction | HDAC1-dependent M1 microglial polarization is induced | [65] |
| Lethe | M | Inflammatory signaling regulation | NF-kB subunit RelA binding with DNA is inhibited by Lethe- RelA interaction and corresponding target gene activation is halted | [66] | |
| lincRNA-Cox2 | M | Distinct classes of immune gene expressions are either induced or inhibited | Interactions of lincRNA-Cox2 with heterogeneous nuclear; ribonucleoprotein A/B and A2/B1 is required for target gene inhibition | [42] | |
| NEAT1 | H/M | Induces innate immune response; helps in activation of antiviral cytokine genes like IL-8 | NEAT1 binds with NONO, SFPQ, PSF, Ezh2 and relocates SFPQ from IL8 promoter to the paraspeckles, that results in transcriptional activation of IL8 | [66,[70], [71], [72], [73] | |
| MALAT1 | H | Regulation of glucose mediated up-regulation of IL-6 and TNF- alpha | By activation of SAA3 expression | [74] | |
| Neurogenesis and synaptic function | MALAT1 | H/M | Synaptic density regulation | By modulating the recruitment of SRSF1,SFPQ at the transcription site | [75], [76], [77] |
| antisense UchL1 | M | By the regulation of UchL1, AS-UchL1 is involved in neurodegenerative diseases and brain function | Antisense Uchl1 RNA helps in overlapping Uchl1 mRNA for activation polysomes in translation purposes | [44] | |
| Dali | H/M | Neural differentiation regulation | DNA methylation of CpG island-associated promoters are regulated in trans by the interaction of DNMT1 DNA methyltransferase in human and mouse | [67] | |
| Miat | M | Neurogenic commitment | Dysregulation of Miat results in defective splicing of Wnt7b and has pleiotropic effects on brain development | [45] | |
| Pnky | M | Neurogenesis regulation of embryonic and postnatal neural stem cell populations | By interacting with PTBP1, the splicing regulator | [46] | |
| PVT1 | M | Autophagic inhibition in diabetes (and diabetes associated AD) decreases PVT1 concentration | Hippocampal neurons are protected from impairment of synaptic plasticity and apoptosis by PVT1-induced autophagy, and helps in betterment of cognitive impairment in diabetes | [47] | |
| RMST | H | Neural stem cell fate regulation | Needed for the binding of promoter regions of neurogenic TFs to Sox2 | [68] | |
| Evf2 | M | In early dentate gyrus and postnatal hippocampus mouse mutants of Evf2 showed less GABAergic interneurons | In the Dlx5/6 intergenic region DLX and MECP2 transcription factors are recruited that controls expression of Dlx5, Dlx6 and Gad1 | [48] | |
| GDNFOS | H | Disease causing; transcribed from the opposite strand of GDNF gene | GDNFOS negatively regulates the expression of GDNF; therefore in mature temporal gyrus of AD patients, the GDNF peptide is downregulated | [59,60] | |
| Sox2OT | H/M/R | Induction and/or maintenance of a TF Sox2 expression | In the cerebral cortex of the developing mouse brain, Sox2OT promotes neurogenesis and neuronal differentiation by repressing Sox2; it is also differentially expressed in early and late disease stages the AD model mouse. | [92,62,63] | |
| BDNF-AS | H/M | Antisense transcript to BDNF, interacts with its protein binding partner PABPC1 and negatively regulates BDNF level | Decrease in BDNF level further down regulates an immediate-early gene, involved in synaptogenesis and synaptic plasticity called ARC | [93,31] | |
| Neurogenesis and synaptic function and neuronal apoptosis | NAT-RAD18 | R | Disease causing and is upregulated in AD; transcribed from the antisense of protein coding gene Rad18 | NAT-Rad18 downregulates the expression of DNA repair protein Rad18 and enhance susceptibility to neuronal apoptosis; involved in PCNA ubiquitination, DNA repair and nerve injury | [57] |
| EBF3-AS | H/M | Transcribed from the opposite strand of EBF3 and is up-regulated in the hippocampus of APP/PS1 mice | EBF3-AS deficiency decreases EBF3 levels inhibits OA or Aβ induced apoptosis in human SH-SY5Y cell | [37] | |
| rRNA transcription and methylation | LoNA | M | Regulates rRNA transcription and methylation | rRNA transcription is regulated by binding to nucleolin and decreases its activity, while methylation is regulated by interaction with fibrillarin | [42,94] |
Fig. 2.
Various roles of lncRNAs in Alzheimer's disease.
2.2. Role of lncRNAs in HD
HD is a hereditary neurodegenerative disorder characterized by psychiatric disturbances, progressive dyskinesias, chorea and dementia, and is caused by an abnormal expansion of CAG trinucleotide in the first exon of the huntingtin gene.The antisense transcript of the Htt gene called as lncRNA HttAS_v1 has lower expression level in the frontal cortex of HD patients, that results in higher expression of Htt mRNA, HD pathogenesis [95]. Htt functions as a modulator of nuclear translocation of the transcriptional repressor RE1 silencing transcription factor/neuron-restrictive silencer factor (REST/NRSF). A mutation in Htt results in abnormal nuclear-cytoplasmic transport of REST/NRSF, leading to abnormal expression of REST target genes [96,97].
Another lncRNA antisense to brain-derived neurotrophic factor (BDNF-OS), upregulates BDNF concentration and has a protective role on neurons and thus it improves the Huntington disease phenotype [98]. The concentration of NEAT1 has been found higher in R6/2 mice and HD patients [99]. It is also essential for the production and maintenance of subnuclear bodies found in mammalian cells called paraspeckles [100].
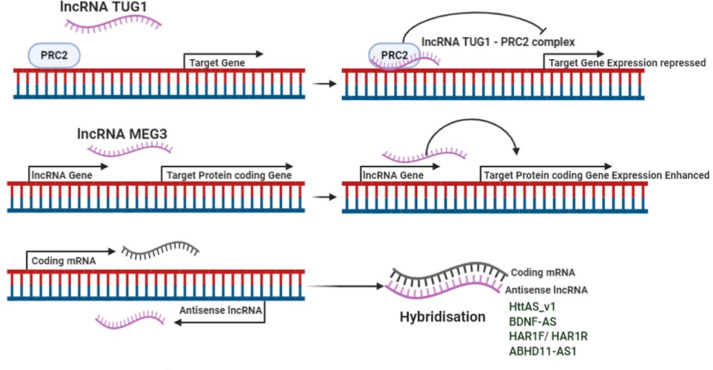
The lncRNAs HAR1F and HAR1R, antisense to HAR1 (human accelerated region 1) gene have been found to be involved in synaptic plasticity, memory structure, neurotransmission in the mature brain, and are down-regulated in striatum of human HD brain as reported [101]. In striatum of HD, excessive REST nuclear- cytoplasmic exchange has been found to effectively repress HAR1 transcriptionally [102]. Another lncRNA DGCR5 (DiGeorge critical region 5) contains a genome binding site for REST and are down-regulated in HD, thus playing a crucial role in pathophysiology of HD [103]. REST has also been found to inhibit downregulation of the lncRNA MEG3 (maternally expressed gene 3), that is otherwise down-regulated in HD brain tissue [104]. In recent studies it has been found that knocking out the lncRNA Abhd11os (ABHD11-AS1 in humans) gene in the HD mouse model produces neurotoxicity, but overexpression of Abhd11os has a neuroprotective effect and neutralises the toxicity of Htt mRNA in murine models of HD [105]. Another lncRNA TUG1, that is up-regulated in HD interacts with PRC2 after being activated by p53 and regulates the downstream genes [104,106]. The lncRNA TUNA is highly expressed in the thalamus and striatum. Deregulation of hTUNA in the caudate nucleus may have involvement in the pathophysiology of HD [107]. Table 2 and Fig. 3 displays roles of lncRNAs in Huntington disease.
Table 2.
Role of lncRNAs in Huntington's disease.
| lncRNAs | Description | Biological Function | Reference |
|---|---|---|---|
| NEAT1 | A nuclear-enriched ncRNA essential for the formation and maintenance of paraspeckles | Protective role in HD; essential for the integrity of the nuclear paraspeckle substructure; increase viability under oxidative stress | [100] |
| HttAS_v1 | Antisense transcript of the Htt gene | Protective role in HD; reduce endogenous HTT transcript levels | [95], [96], [97] |
| HAR1F and HAR1R | Antisense transcripts of the first HAR1 gene | Protective role in HD; involved in neurotransmission, memory structure, and synaptic plasticity in the mature brain | [101,102] |
| DGCR5 | A transcript of DiGeorge critical region 5 | Protective role in HD; include a genome binding site for REST and play an important transcriptional regulatory role in HD | [103] |
| MEG3 | The human homolog of the mouse maternally expressed gene Gtl2, the first imprinted gene identified on the mouse distal chromosome 12 | Disease causing role in HD; alter gene expression in response to neuronal activity | [104] |
| ABHD11-AS1 | Homologous with rodent Abhd11os (ABHD11-AS1 in humans) lncRNA | Protective role in HD; attenuate the toxicity of Htt mRNA | [105] |
Fig. 3.
Regulatory mechanisms of lncRNAs in HD.
2.3. Role of lncRNAs in PD
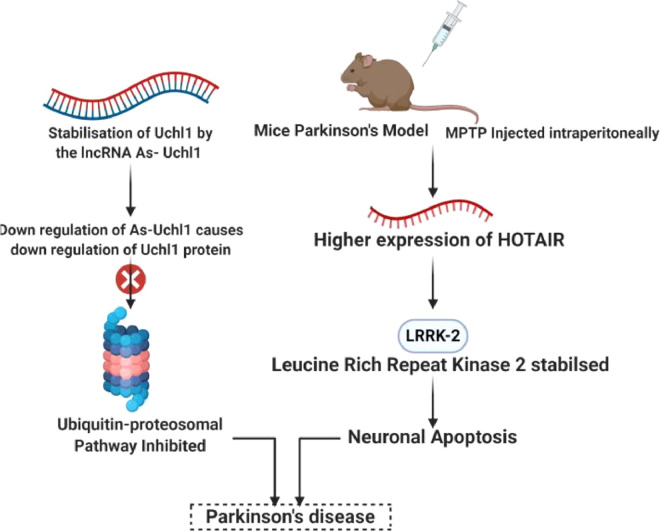
PD is a neurodegenerative disorder caused by depletion of dopamine-secreting neurons, that results in impairments of motor abilities. LncRNAs have crucial role and altered expression profile in PD pathogenesis [108]. The lncRNA antisense ubiquitin carboxy-terminal hydrolase L1 (AS-UchL1) has been found to increase the expression of UchL1 protein, that is closely related to brain function and neurodegenerative diseases, at post-transcriptional level depending on a 5r overlapping sequence and an embedded inverted SINEB2 sequence [67]. As a component of Nurr-1 dependent gene network, down-regulated AS-Uch1 causes decreased translation of UchL1 protein in neurochemical models of PD. This leads to inhibition of ubiquitin-proteasome system [109] (Fig. 5). Impaired motor function or abnormal dopamine release is associated with abnormality in the expression of PTEN-induced kinase 1 (PINK1) [110]. A human specific non-coding RNA NaPINK1 has been found to stabilize PINK1, thereby increasing its expression [111]. The lncRNA metastasis associated lung adenocarcinoma transcript 1 (MALAT1) (also called as NEAT2) is highly expressed in neurons and upregulates α-synuclein production when overexpressed [75,98]. Targeting MALAT1 with β-asarone reduces its level and therefore can serve as a potential therapeutic target for PD [112]. Another commonly known 2.2-kb-long lncRNA HOTAIR (Hox transcript antisense intergenic RNA), is up-regulated in mice Parkinson's model upon intraperitoneal injection of MPTP and stabilises Leucine-rich repeat kinase 2 (LRRK-2) involved in initiation and development of PD [113]. It further induces neuronal apoptosis [114]. Few lncRNAs H19 upstream conserved 1 and 2 (Huc1 and Huc2), lincRNA-p21, MALAT1, SNHG1, and TncRNA have been found to be differentially expressed in PD suggesting their involvement in disease pathogenesis, that is yet to be discovered [115].
Fig. 5.
Regulatory role of HOTAIR and As-UchL1 in PD.
Recent studies have shown that in neuronal SH-SY5Y cells lncRNAs AL049437 and SNGH1 contribute to MPP cytotoxicity [116], [117], [118]. The lncRNA MAPT-AS1 (microtubule-associated protein tau antisense 1) is down-regulated in brains of PD patients, and acts as an epigenetic regulator of MAPT expression that has pathogenic role in PD [119]. In SH-SY5Y cells treated with MPP, and in the substantia nigra of PD patients NEAT1 is significantly up-regulated. It and promotes autophagy and has protective role against oxidative stress and neuronal injury [120], [121], [122]. In MPP-induced SH-SY5Y cells LncRNA-p21 has been found to regulate the neuronal injury via the miR-626-TRMP2 axis [123]. The lncRNA BACE1-AS reduces nitric oxide synthase and prevents oxidative stress by upregulating microRNA-34b-5p in PD rat model [124]. LncRNA HAGLROS is up-regulated in SH-SY5Y cells and PD mouse model and is associated with inhibition of apoptosis and autophagy by the activation of PI3K/Akt/mTOR pathway and regulation of miR-100/ATG10 axis [125]. In mice PD models, the lncRNA H19 that was earlier reported in multiple cancers and heart diseases has been found to show protective role against apoptosis and dopaminergic neuronal loss by regulating miR-301b-3p and miR-585–3p [126,127]. Again, in mice model of PD, the lncRNA GAS5 has been found to promote microglial inflammatory via regulating NLRP3 pathway by sponging miR-223–3p [128]. In MPP treated PD disease model SH-SY5Y cells, NORAD has been found down-regulated. It has protective roles against MPP induced cytotoxicity [129]. The lncRNA UCA1 upregulates SNCA and promotes PD development [130]. The lncRNA LINC-PINT has been found have increased expression in substantia nigra of PD patients. RNAi mediated depletion of this lncRNA shows increased death of cultured N2A and SH‐SY5Y cells under oxidative stress, thereby suggesting a neuro protective function of LINC-PINT in PD pathophysiology [131]. knockdown of AK021630 resulted in decreased mitochondrial mass, mitochondrial transmembrane potential (Δψm), cell viability, and tyrosine hydroxylase (TyrH) secretion in human neuroblastoma SH-SY5Y cell line suggesting protective role of AK021630 in PD[109, 133], and the lncRNA NR_030777 has shown a protective role in Paraquat-induced neurotoxicity via regulating Zfp326 and Cpne5 [133]. In substantia nigra of paraquat and MPTP induced mouse model Nrf2-related lncRNAs have been found to be involved in oxidative stress [134]. In anti-NGF AD11 transgenic mouse the lncRNA Sox2OT is involved in regulating co-transcribed Sox2 gene expression to down neurogenesis [135]. The lncRNAs UchL1-AS, PINK1-AS, HAR1A, Sox2OT, BCYRN1, ANRIL, are reported in PD patients in the Hungarian population. They are involved in interfering with the binding affinity of transcription factors like HNF4A, potentially resulting in abnormal expression of target genes, such as BCYRN1 [136]. The regulatory mechanisms of lncRNA involved in PD are listed in Table 3 and Fig. 4.
Table 3.
Role of lncRNAs in Parkinson's disease.
| lncRNAs in PD | Regulatory mechanisms | Ref. |
|---|---|---|
| HOTAIR | Disease inducing role in PD; HOTAIR induces neuronal apoptosis and autophagy; it also stabilizes LRR for the development of PD | [113,114] |
| MALAT1 | Disease inducing role in PD; upregulates α-synuclein production, regulatory role in neuronal apoptosis | [75,98] |
| NEAT1 | Significantly upregulated in PD patients; promotes autophagy; protective role against oxidative stress and neuronal injury | [120], [121], [122] |
| NORAD | Protective roles against MPP induced cytotoxicity in MPP treated PD disease model SH-SY5Y cells | [129] |
| H19 | Protective role against apoptosis and dopaminergic neuronal loss by regulating miR-301b-3p and miR-585-3p | [126,127] |
| GAS5 | Promote microglial inflammatory via regulating NLRP3 pathway by sponging miR-223-3p | [128] |
| SNHG1 | Contribute to MPP cytotoxicity, involved in autophagy | [116,117] |
| UCA1 | Upregulates SNCA and promoting PD development | [130] |
| AS-UchL1 | Protective role in PD; increases the expression of UchL1 protein, involved in brain function and neurodegenerative diseases; downregulation causes inhibition of ubiquitin-proteasome system | [67,109] |
| lncRNA-p21 | Regulate the neuronal injury via the miR-626-TRMP2 axis | [123] |
| AK021630 | Protective role in PD | [108,132] |
| AL049437 | Contribute to MPP cytotoxicity, regulatory role in neuroinflammation | [116], [117], [118] |
| NR_030777 | Protective role in Paraquat-induced neurotoxicity via regulating Zfp326 and Cpne5 | [133] |
| BACE1-AS | Reduces nitric oxide synthase; prevents oxidative stress by upregulating microRNA-34b-5p in PD rat model | [124] |
| LINC-PINT | Neuroprotective role against oxidative stress in cultured N2A and SH‐SY5Y cells | [131] |
| HAGLROS | Inhibition of apoptosis and autophagy by the activation of PI3K/Akt/mTOR pathway and regulation of miR-100/ATG10 axis in PD mouse model | [125] |
| NaPINK1 | Protective role in PD; Stabilizes PINK1, that is required for regular motor function dopamine release | [111] |
| MAPT-AS1 | Downregulated in brains of PD patients; acts as an epigenetic regulator of MAPT expression | [119] |
| Nrf2-related lncRNAs | Oxidative stress | [134] |
| UchL1-AS, PINK1-AS, HAR1A, Sox2OT, BCYRN1, ANRIL | Interfering with the binding affinity of transcription factor HNF4A, potentially resulting in abnormal expression of target genes, such as BCYRN1 | [126] |
| Sox2OT | Regulating co-transcribed Sox2 gene expression to down neurogenesis | [135] |
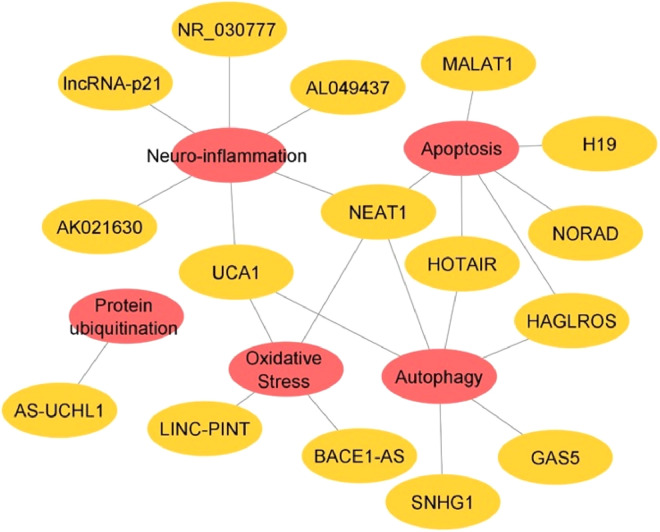
Fig. 4.
Network view of lncRNAs in PD and their involvement in various biological functions like autophagy, apoptosis, oxidative stress, neuroinflammation and protein ubiquitination.
2.4. Role of lncRNAs in schizophrenia
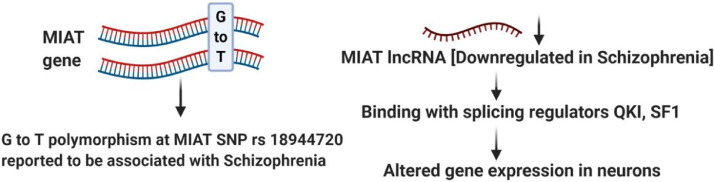
Schizophrenia is a mental disease characterized with neurocognitive impairments. Pathophysiology of schizophrenia is caused by both genetic and environmental factors including lncRNAs [137], [138], [139]. A number of lncRNAs have altered expression in both the periphery and the CNS of patients with schizophrenia [138,[140], [141], [142]. Studies have shown the lncRNA MIAT (residing on chromosome 22q12.1, near to the schizophrenia candidate region, chromosome 22q11.2), is down-regulated in schizophrenia patients [143]. The G to T polymorphism at the MIAT SNP rs18944720 has also been connected to susceptibility to paranoid schizophrenia [144]. MIAT regulates alternative splicing in schizophrenia by binding to the splicing factors, SF1, QKI, SRSF1 and CELF [143,145,146] and is expressed in neuronal populations in CNS, where mature transcripts are nucleus localised [147,148]. Upon neuronal activation the lncRNA MIAT, (also called as Gomafu [143] or RNCR2), is down-regulated in schizophrenia [149] and acts as a competitive endogenous RNA (ceRNA) for miR-150–5p, miR-24, miR-22–3p or miR-150, thereby induces cell proliferation, apoptosis, MIAT can also bind with splicing regulator quaking homolog (QKI) and SF1, and can alter gene expressions in the neuron (Fig. 6). DISC1 (disrupted in schizophrenia 1), ERBB4 (v-erb-a erythroblastic leukemia viral oncogene homolog 4) and alternatively spliced variants of them are all down-regulated because of the upregulation of MIAT in schizophrenia patient postmortem hippocampus region of brains [150], [151], [152] as it acts as a scaffold to affect alternative splicing of these schizophrenia related genes as described earlier [153,154,149]. A novel lncRNA, EU358092 on chromosome 1p21.3, expressed in CNS has been found to be associated with schizophrenia by bioinformatics analysis and GWAS [155]. EU358092 also showed altered expression in SH-SY5Y human neuronal cells in response to the psychoactive drugs [155], thereby showing potential relationships with schizophrenia pathology.
Fig. 6.
Regulatory role of MIAT in schizophrenia.
2.5. Role of lncRNAs in ASD
A group of heterogeneous neuro developmental disorders, characterized by impaired reciprocal social interactions, communication and repetitive stereotyped behaviors, is defined as ASD [156]. A total of 222 differentially expressed lncRNAs have been identified in ASD. It has been shown that number of differentially expressed lncRNAs are higher with control individuals as compared to Autistic samples [157]. Many of the differentially expressed lncRNAs are associated with neurodevelopmental and psychiatric diseases. As for example UBE3A (ubiquitin protein ligase E3A) is involved in Angelman syndrome, that shares common characteristics with ASD. A 3.9 kb lncRNA MSNP1AS, encoded by the antisense strand of the moesin pseudogene 1 (MSNP1), has been identified in genome wide association studies (GWAS) of ASD. It regulates the level of moesin protein is involved in neuronal architecture and immune responses. In postmortem ASD temporal cortex, MSNP1AS is significantly up-regulated [158,159].
2.6. Role of lncRNAs in ALS
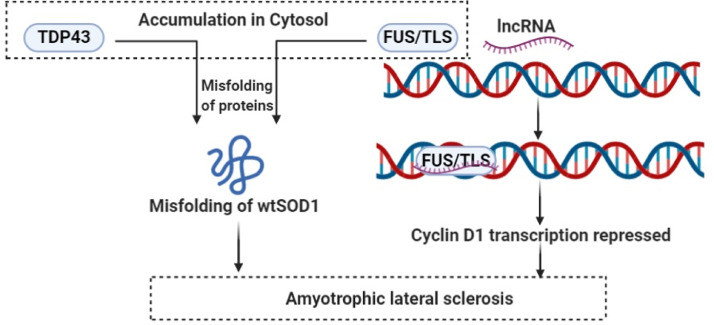
The neurodegenerative disease ALS is characterised by the progressive paralysis of the limbs and muscles and degeneration of spontaneous motor neurons that causes difficulty in speech swallowing, and respiration. The first identified causative mutation in ALS and frontal temporal dementia was the repeated amplification of a six-nucleotide motif (GGGGCC) in the protein-coding gene C9ORF72 (chromosome 9 ORF 72) [160,161]. The bidirectional transcription at C9ORF72 locus that produces both sense and antisense RNAs, [162] are localised in nucleus [163] and both are elevated in ALS patients and the antisense lncRNA can inhibit C9ORF72 mRNA expression. Although it has been found that the corrected disease related gene in fibroblast cannot cure the disease [163]. Two nucleus localised RNA-binding proteins, namely TDP43 (TAR DNA-binding domain protein 43) and FUS/TLS (fused in sarcoma/translated in liposarcoma) are accumulated abnormally in cytosol and lead to misfolding of wtSOD1 (wild-type Cu/Zn superoxide dismutase) in SALS (sporadic ALS) and non-SOD1 FALS (familial ALS), thereby contributing to pathophysiology of ALS [164]. LncRNAs have been found to recruit FUS/TLS to cyclin D1 genomic locus to repress transcription of cyclin D1 [165,166]. (Fig. 7)
Fig. 7.
Regulatory role of lncRNAs in ALS.
2.7. Role of lncRNA in psychiatric disorders
A common psychiatric disorder, major depressive disorder (MDD) is associated with significantly higher level of morbidity, disability and mortality [167]. Three lncRNAs at positions chr10:874,695–874,794, chr10:75,873,456–75,873,642 and chr3:47,048,304–47,048,512 are identified to interact with coding transcripts and are involved in Major depressive disorder [168]. Cui et al., has shown six lncRNAs (TCONS_00019174, ENST00000566208, NONHSAG045500, ENST00000517573, NONHSAT034045 and NONHSAT142707) as down-regulated in MDD patients [193]. These lncRNAs also showed lowered expression in generalized anxiety disorder (GAD) [194]. In another research, Li et al. showed 9 lncRNAs (TCONS_L2_00001212, NONHSAT102891, TCONS_00019174, ENST00000566208, NONHSAG045500, ENST00000591189, ENST00000517573, NONHSAT034045, NONHSAT142707 (P < 0.05) are significantly down-regulated in PBMC of MDD patients [195]. Using microarray genome-wide expression analysis and lncRNA-mRNA co-expression network analysis, Liu et al. have shown that the lncRNAs located at chr10:874,695–874,794, chr10:75,873,456–75,873,642 and chr3:47,048,304–47,048,512 can be crucial in regulating the expression of mRNAs in MDD [196].
2.8. Role of lncRNAs in cerebral injury
Stroke is the second most common cause of death in the world and is caused by haemorrhagic damages or cerebral ischemic in brain [169,170]. Specific temporal and spatial expression pattern of lncRNAs have been found in cerebral ischemia injury as well as in brain damages caused by hypoxic ischemic [171], [172], [173], [174], [175]. Post-ischemic pathophysiology may be modulated by chromatin-modifying proteins (CMPs) activities of lncRNAs. After focal ischemia lncRNAs were found to be dysregulated in rats by middle cerebral artery occlusion [171]. These lncRNAs were homologous to protein coding genes [171]. It was further shown that after brain ischemia, 177 of the 2497 lncRNAs expressed in rat cerebral cortex displayed strong binding to either paired amphipathic helix protein Sin3A (Sin3A) or corepressors of the RE-1 silencing transcription factor (coREST) [172]. It has been recently found that in an in-vitro model of ischemic-reperfusion injury, miR-377 along with lncRNAS can modulate Ncam1 and Negr1 mRNAs maintain neuronal structure and function during neuronal development [173]. In hypoxic-ischemic brains of rats a total of 322 lncRNAs that includes lncRNA BC088414 (related with genes involved in apoptosis) have been found to be differentially expressed [175]. Other than these, after ischemic stroke endothelial-selective lncRNAs has been found to function as a class of novel master regulators in cerebrovascular endothelial pathologies [174].
2.9. Role of lncRNAs in neuroimmunological disorders
LncRNAs are associated with neuroimmunological disorders as well [176,177]. A lncRNA obtained from mouse T early α (TEA) promoter has been found in regulating downstream promoter usage [178]. A good number of lncRNAs are to be dynamically expressed in the process of differentiation and the activation of T-cell [177]. Out of many lncRNAs that are nested in introns of IL2RA gene, the lncRNA M21981 is significantly up-regulated during T-cell activation that suggests partly its regulatory role in pathogenesis of neuroimmunological disorders. LncRNAs have shown significant regulatory relationship in multiple sclerosis, a complex autoimmune disorder. In the peripheral blood mononuclear cells of patients with multiple sclerosis, a total of 2353 up-regulated lncRNAs, 389 down-regulated lncRNAs have been identified [179]. Three lncRNAs namely 7SK small nuclear (RN7SK RNA), taurine up-regulated 1 (TUG1) and NEAT1 have been found to be up-regulated in relapsing remitting Multiple Sclerosis patients compared to healthy controls [180]. The lncRNA linc-MAF-4 that regulates Th1/Th2 differentiation has been found in the pathogenesis of Multiple Sclerosis via in a process of targeting MAF [181]. Table 4 summarises role of lncRNAs in four neurological diseases namely, schizophrenia, ASD, psychiatric disorders and neuroimmunological disorders.
Table 4.
Role of lncRNAs in Schizophrenia, Autism spectrum disorder, psychiatric disorders and other neuro-immunological disorders.
| Disease | lncRNAs | Biological Function | Ref. |
|---|---|---|---|
| Schizophrenia | MIAT | On neuronal activation the lncRNA MIATis down-regulated and acts as a ceRNA for miR-150-5p, miR-24, miR-22-3p or miR-150, and induces cell proliferation, apoptosis; It binds with splicing regulator QKI and SF1, and alter gene expressions in the neuron. DISC1, ERBB4; alternatively spliced variants of these genes are all down-regulated due to the upregulation of MIAT in Schizophrenia patient post-mortem hippocampus region of brains since it acts as a scaffold to affect alternative splicing of these Schizophrenia related genes | [150], [151], [152] |
| EU358092 | Displayed altered expression in SH-SY5Y human neuronal cells in response to the psychoactive drugs | [155] | |
| Autism spectrum disorder | UBE3A | Involved in Angelman syndrome, that shares common characteristics with Autism spectrum disorder | [157] |
| MSNP1AS | Regulates the level of moesin protein is involved in neuronal architecture and immune responses | [158,159] | |
| Psychiatric disorders | chr10:874695–874794, chr10:75873456–75873642 and chr3:47048304 – 47048512 | Interact with coding transcripts | [168] |
| Neuroimmunological disorders | TEA | Regulating downstream promoter usage | [178] |
| lncRNA M21981 | Upregulated during T-cell activation | ||
| TUG1, RN7SK RNA and NEAT1 | Up-regulated in relapsing remitting Multiple Sclerosis patients | [180] | |
| linc-MAF-4 | Regulates Th1/Th2 differentiation | [181] |
3. Potential clinical and therapeutic aspects
LncRNAs are appearing as novel targets for diagnosis and treatment of a range of human diseases in recent days [197], [198], [199], [200] especially against an array of neurological disorders (Fig. 8). Levels of lncRNA transcripts and their post transcriptional modifications can be determined using PCR, RNA sequencing, microarray and the single cell analyzing techniques, like scRNA sequencing. Intracellular trafficking of lncRNA can be measured by the contents of micro-vesicles in blood and cerebrospinal fluid [201]. Oligonucleotide molecular beacons and quantum dot nanoparticles that serves as novel molecular imaging probes are being used in visualizing lncRNAs with a potential to be used further in real time in vivo imaging. This can be utilized in clinical approach by using lncRNAs as molecular markers. As for example Kam et al. reported FIT-PTA molecular beacons for detection of a lncRNA CCAT1 in both living cells as well as human adenocarcinoma colon tissue samples [202]. As a therapeutic strategy, recombinant zinc-finger nuclease (ZFN) with a property of introducing RNA destabilizing elements has shown promising results in silencing the lncRNA NEAT2 [203]. Initial in vitro strategies like using ZFN based therapies for neurological disorder which includes a T cell-oriented strategy for glioblastoma (NCT01082926), shows way to further promising therapeutic potentials. Targeting epigenetic enzymes since these enzymes have regulatory roles in disease context, has shown clear evidence of altered expression of lncRNAs [204]. In summary there had been evidences of using lncRNAs as potentially therapeutic targets, which are to be further investigated in future.
Fig. 8.
Regulatory role of various lncRNAs against neurological and psychiatric disorders.
4. Conclusion
Metabolic abnormalities are diversely complex and are determined by intricate networks and cross-talks between several cellular and tissue-level entities. LncRNAs play roles in fine-tuning of cellular metabolism. Their discovery has given new paradigm shift in understanding fine-tuning of cellular processes. Ease of availability and advent in methodologies to identify lncRNA with very low copy numbers has given new opportunities to establish them as markers. lncRNAs also have multifaceted intracellular regulatory functions and capabilities to alter intercellular communication and interactions [182]. Half-lives of these RNA molecules are relatively shorter than protein-coding transcripts. But their association with RNA-binding proteins and folding into secondary structure provide them enhanced stability and resistance against degradation by RNases. By virtue of its secondary structure and poly-A tail, lncRNAs are able to survive in body fluids [183]. It has been shown that lncRNAs can be detected in wide range of extracellular body fluids such as whole blood, plasma, serum, urine, saliva, gastric juice and display a dynamic alteration upon diseases [11,[184], [185], [186]. LncRNAs can also enter the blood stream encapsulated in exosomes [187] and extracellular vesicles or can be released from the apoptotic bodies [188]. Therefore, with these properties, lncRNAs are transcripts of particular interest to serve as a novel class of non-invasive prognostic and diagnostic marker/biomarker [184,189,190] and they have been well established in various neurological disorder [191,192]. Here we have tried to look into various aspects of lncRNAs and their roles in regulations of various neurological diseases including neurodegenerative disorders. Here in this review we tried to look into the potential of various lncRNAs for being used as therapeutic targets and diagnostic markers in broad range of various neurological and neurodegenerative diseases.
Conflicts of interests
None.
Acknowledgement
We acknowledge Wajid Waheed Bhat (Michigan State University) for his kind support in drawing the figures using Biorender.
References
- 1.Pertea M. The human transcriptome: an unfinished story. Genes. 2012;3(3):344–360. doi: 10.3390/genes3030344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarroux J., Morillon A., Pinskaya M. History, discovery, and classification of lncRNAs. AdvExp Med Biol. 2017;1008:1–46. doi: 10.1007/978-981-10-5203-3_1. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X., Hong R., Chen W., Xu M., Wang L. The role of long noncoding RNA in major human disease. BioorgChem. 2019;92 doi: 10.1016/j.bioorg.2019.103214. [DOI] [PubMed] [Google Scholar]
- 4.Barr A.J. The biochemical basis of disease. Essays Biochem. 2018;62(5):619–642. doi: 10.1042/EBC20170054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalil A.M., Guttman M., Huarte M., Garber M., Raj A., Morales D.R., et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl AcadSci USA. 2009;106(28):11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma L., Bajic V.B., Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10(6):925–933. doi: 10.4161/rna.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Djebali S., Davis C.A., Merkel A., Dobin A., Lassmann T., Mortazavi A., et al. Landscape of transcription in human cells. Nature. 2012;489(7414):101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St Laurent G., Wahlestedt C., Kapranov P. The Landscape of long noncoding RNA classification. Trends Genet. 2015;31(5):239–251. doi: 10.1016/j.tig.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kornienko A.E., Guenzl P.M., Barlow D.P., Pauler F.M. Gene regulation by the act of long non-coding RNA transcription. BMC Biol. 2013;11:59. doi: 10.1186/1741-7007-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z., Zhao W., Wang M., Zhou X. In: Gene expression profiling in cancer. Vlachakis D, editor. Intech Open; London, UK: 2019. The role of long noncoding RNAs in gene expression regulation; pp. 1–17. editor. [Google Scholar]
- 11.Quiat D., Olson E.N. MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment. J Clin Invest. 2013;123(1):11–18. doi: 10.1172/JCI62876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchese F.P., Raimondi I., Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18(1):206. doi: 10.1186/s13059-017-1348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burenina O.Y., Oretskaya T.S., Kubareva E.A. Non-coding RNAs as transcriptional regulators in eukaryotes. Acta Nat. 2017;9(4):13–25. [PMC free article] [PubMed] [Google Scholar]
- 14.Long Y.C., Wang X.Y., Youmans D.T., Cech T.R. How do lncRNAs regulate transcription? SciAdv. 2017;3(9):eaao2110. doi: 10.1126/sciadv.aao2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon J.H., Abdelmohsen K., Gorospe M. Posttranscriptional gene regulation by long noncoding RNA. J Mol Biol. 2013;425(19):3723–3730. doi: 10.1016/j.jmb.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertone P., Stolc V., Royce T.E., Rozowsky J.S., Urban A.E., et al. Global identification of human transcribed sequences with genome tiling arrays. Science. 2004;306(5705):2242–2246. doi: 10.1126/science.1103388. [DOI] [PubMed] [Google Scholar]
- 17.Sawyer I.A., Dundr M. Chromatin loops and causality loops: the influence of RNA upon spatial nuclear architecture. Chromosoma. 2017;126(5):541–557. doi: 10.1007/s00412-017-0632-y. [DOI] [PubMed] [Google Scholar]
- 18.Wang C.G., Wang L.Z., Ding Y., Lu X., Zhang G., Yang J., et al. LncRNA structural characteristics in epigenetic regulation. Int J Mol Sci. 2017;18(12):2659. doi: 10.3390/ijms18122659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon J.H., Abdelmohsen K., Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Semin. Cell Dev Biol. 2014;34:9–14. doi: 10.1016/j.semcdb.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rashid F., Shah A., Shan G. Long non-coding RNAs in the cytoplasm. Genom Proteom Bioinform. 2016;14(2):73–80. doi: 10.1016/j.gpb.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Available from https://www.who.int/news-room/fact-sheets/detail/dementia.
- 22.Available from: https://www.parkinson.org/Understanding-Parkinsons/Statistics#:∼:text=More%20than%2010%20million%20people,have%20Parkinson's%20disease%20than%20women. 2021
- 23.Pringsheim T., Wiltshire K., Day L., Dykeman J., Steeves T., Jette N. The incidence and prevalence of Huntington's disease: a systematic review and meta-analysis. MovDisord. 2012;27(9):1083–1091. doi: 10.1002/mds.25075. [DOI] [PubMed] [Google Scholar]
- 24.Logroscino G., Piccininni M. Amyotrophic lateral sclerosis descriptive epidemiology: the origin of geographic difference. Neuroepidemiology. 2019;52(1–2):93–103. doi: 10.1159/000493386. [DOI] [PubMed] [Google Scholar]
- 25.Available from: https://www.who.int/news-room/fact-sheets/detail/autism-spectrum-disorders#:∼:text=Epidemiology,figures%20that%20are%20substantially%20higher. 2021
- 26.Available from: https://www.who.int/news-room/fact-sheets/detail/depression 2021
- 27.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 28.Faghihi M.A., Modarresi F., Khalil A.M., Wood D.E., Sahagan B.G., Morgan T.E., et al. Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of beta-secretase. Nat Med. 2008;14:723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Modarresi F., Faghihi M.A., Patel N.S., Sahagan B.G., Wahlestedt C., Lopez-Toledano M.A. Knockdown of BACE1-AS nonprotein-coding transcript modulates beta-amyloid-related hippocampal neurogenesis. Int J Alzheimer's Dis. 2011 doi: 10.4061/2011/929042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faghihi M.A., Zhang M., Huang J., Modarresi F., Van der Brug M.P., Nalls M.A., et al. Evidence for natural antisense transcript-mediated inhibition of microRNA function. Genome Biol. 2010;11(5):R56. doi: 10.1186/gb-2010-11-5-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modarresi F., Faghihi M.A., Lopez-Toledano M.A., Fatemi R.P., Magistri M., Brothers S.P., et al. Inhibition of natural antisense transcripts in vivo results in gene-specific transcriptional upregulation. Nat Biotechnol. 2012;30(5):453–459. doi: 10.1038/nbt.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bohnsack J.P., Teppen T., Kyzar E.J., Dzitoyeva S., Pandey S.C., et al. The lncRNA BDNF-AS is an epigenetic regulator in the human amygdala in early onset alcohol use disorders. Transl Psychiatry. 2019;9(1):34. doi: 10.1038/s41398-019-0367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo C.C., Jiao C.H., Gao Z.M. Silencing of LncRNA BDNF-AS attenuates Aβ25-35-induced neurotoxicity in PC12 cells by suppressing cell apoptosis and oxidative stress. Neurol Res. 2018;40(9):795–804. doi: 10.1080/01616412.2018.1480921. [DOI] [PubMed] [Google Scholar]
- 34.Wang M.M., Reed R.R. Molecular cloning of the olfactory neuronal transcription factor Olf-1 by genetic selection in yeast. Nature. 1993;364(6433):121–126. doi: 10.1038/364121a0. [DOI] [PubMed] [Google Scholar]
- 35.Chao H.T., Davids M., Burke E., Pappas J.G., Rosenfeld J.A., McCarty A.J., et al. A syndromic neurodevelopmental disorder caused by De Novo variants in EBF3. Am J Hum Genet. 2017;100(1):128–137. doi: 10.1016/j.ajhg.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao L.Y., Niu Y., Santiago A., Liu J., Albert S.H., Robertson K.D., et al. An EBF3-mediated transcriptional program that induces cell cycle arrest and apoptosis. Cancer Res. 2006;66(19):9445–9452. doi: 10.1158/0008-5472.CAN-06-1713. [DOI] [PubMed] [Google Scholar]
- 37.Gu C., Chen C., Wu R., Dong T., Hu X., Yao Y., et al. Long noncoding RNA EBF3-AS promotes neuron apoptosis in Alzheimer's disease. DNA Cell Biol. 2018;37(3):220–226. doi: 10.1089/dna.2017.4012. [DOI] [PubMed] [Google Scholar]
- 38.Richter J.D., Klann E. Making synaptic plasticity and memory last: mechanisms of translational regulation. Gene Dev. 2009;23(1):1–11. doi: 10.1101/gad.1735809. [DOI] [PubMed] [Google Scholar]
- 39.Riba A., Di Nanni N., Mittal N., Arhné E., Schmidt A., Zavolan M. Protein synthesis rates and ribosome occupancies reveal determinants of translation elongation rates. Proc Natl AcadSci USA. 2019;116(30):15023–15032. doi: 10.1073/pnas.1817299116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin K.C., Ephrussi A. mRNA localization: gene expression in the spatial dimension. Cell. 2009;136(4):719–730. doi: 10.1016/j.cell.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pietrzak M., Rempala G., Nelson P.T., Zheng J.J., Hetman M. Epigenetic silencing of nucleolar rRNA genes in Alzheimer's disease. PLoS One. 2011;6(7):e22585. doi: 10.1371/journal.pone.0022585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li D.F., Zhang J., Wang M., Li X., Gong H., Tang H., et al. Activity dependent LoNA regulates translation by coordinating rRNA transcription and methylation. Nat Commun. 2018;9(1):1726. doi: 10.1038/s41467-018-04072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L., Feng P., Zhu X., He S., Duan J., Zhou D. Long non-coding RNA Malat1 promotes neurite outgrowth through activation of ERK/MAPK signalling pathway in N2a cells. J Cell Mol Med. 2016;20(11):2102–2110. doi: 10.1111/jcmm.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gui Y., Liu H., Zhang L., Lv W., Hu X. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget. 2015;6(35):37043–37053. doi: 10.18632/oncotarget.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aprea J., Prenninger S., Dori M., Ghosh T., Monasor L.S., Wessendorf E., et al. Transcriptome sequencing during mouse brain development identifies long non-coding RNAs functionally involved in neurogenic commitment. EMBO J. 2013;32(24):3145–3160. doi: 10.1038/emboj.2013.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hollands C., Bartolotti N., Lazarov O. Alzheimer's disease and Hippocampal adult neurogenesis; Exploring shared mechanisms. Front Neurosci. 2016;10:178. doi: 10.3389/fnins.2016.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abrous D.N., Koehl M., Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85(2):523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 48.Choi S.H., Bylykbashi E., Chatila Z.K., Lee S.W., Pulli B., Clemenson G.D., et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer's mouse model. Science. 2018;361(6406):1–17. doi: 10.1126/science.aan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muddashetty R., Khanam T., Kondrashov A., Bundman M., Iacoangeli A., Kremerskothen J., et al. Poly(A)-binding protein is associated with neuronal BC1 and BC200 ribonucleoprotein particles. J MolBiol. 2002;321(3):433–445. doi: 10.1016/s0022-2836(02)00655-1. [DOI] [PubMed] [Google Scholar]
- 50.Mus E., Hof P.R., Tiedge H. Dendritic BC200 RNA in aging and in Alzheimer's disease. Proc Natl AcadSci USA. 2007;104(25):10679–10684. doi: 10.1073/pnas.0701532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dieci G., Fiorino G., Castelnuovo M., Teichmann M., Pagano A. The expanding RNA polymerase III transcriptome. Trends Genet. 2007;23(12):614–622. doi: 10.1016/j.tig.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Zhang T., Pang P., Fang Z., Guo Y., Li H., Li X., et al. Expression of BC1 impairs spatial learning and memory in Alzheimer's disease via APP translation. Mol Neurobiol. 2018;55(7):6007–6020. doi: 10.1007/s12035-017-0820-z. [DOI] [PubMed] [Google Scholar]
- 53.Massone S., Vassallo I., Fiorino G., Castelnuovo M., Barbieri F., Borghi R., et al. 17A, a novel non-coding RNA, regulates GABA B alternative splicing and signaling in response to inflammatory stimuli and in Alzheimer disease. Neurobiol Dis. 2011;41(2):308–317. doi: 10.1016/j.nbd.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 54.Yang T.W., Sahu D., Chang Y.W., Hsu C.L., Hsieh C.H., Huang H.C., et al. RNA-binding proteomics reveals MATR3 interacting with lncRNA SNHG1 to enhance neuroblastoma progression. J Proteome Res. 2019;18(1):406–416. doi: 10.1021/acs.jproteome.8b00693. [DOI] [PubMed] [Google Scholar]
- 55.Xu M., Chen X.X., Lin K., Zeng K., Liu X., Pan B., et al. The long noncoding RNA SNHG1 regulates colorectal cancer cell growth through interactions with EZH2 and miR-154-5p. Mol Cancer. 2018;17(1):141. doi: 10.1186/s12943-018-0894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H., Lu B., Chen J. Knockdown of lncRNA SNHG1 attenuated Aβ25-35-induced neuronal injury via regulating KREMEN1 by acting as a ceRNA of miR-137 in neuronal cells. Biochem Biophys Res Commun. 2019;518(3):438–444. doi: 10.1016/j.bbrc.2019.08.033. [DOI] [PubMed] [Google Scholar]
- 57.Parenti R., Paratore S., Torrisi A., Cavallaro S. A natural antisense transcript against Rad18, specifically expressed in neurons and upregulated during beta-amyloid-induced apoptosis. Eur J Neurosci. 2007;26:2444–2457. doi: 10.1111/j.1460-9568.2007.05864.x. [DOI] [PubMed] [Google Scholar]
- 58.Guennewig B., Cooper A.A. The central role of noncoding RNA in the brain. Int Rev Neurobiol. 2014;116:153–194. doi: 10.1016/B978-0-12-801105-8.00007-2. [DOI] [PubMed] [Google Scholar]
- 59.Airavaara M., Pletnikova O., Doyle M.E., Zhang Y.E., Troncoso J.C., Liu Q.R. Identification of novel GDNF isoforms and cis-antisense GDNFOS gene and their regulation in human middle temporal gyrus of Alzheimer disease. J Biol Chem. 2011;286:45093–45102. doi: 10.1074/jbc.M111.310250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wan P.X., Su W.R., Zhuo Y.H. The role of long noncoding RNAs in neurodegenerative diseases. MolNeurobiol. 2017;54:2012–2021. doi: 10.1007/s12035-016-9793-6. [DOI] [PubMed] [Google Scholar]
- 61.Yamanaka Y., Faghihi M.A., Magistri M., Alvarez-Garcia O., Lotz M., Wahlestedt C. Antisense RNA controls LRP1 sense transcript expression through interaction with a chromatin-associated protein, HMGB2. Cell Rep. 2015;11(6):967–976. doi: 10.1016/j.celrep.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knauss J.L., Miao N., Kim S.N., Nie Y., Shi Y., Wu T., et al. Long noncoding RNA Sox2ot and transcription factor YY1 co-regulate the differentiation of cortical neural progenitors by repressing Sox2. Cell Death Dis. 2018;9(8):799. doi: 10.1038/s41419-018-0840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arisi I., D'Onofrio M., Brandi R., Felsani A., Capsoni S., Drovandi G., et al. Gene expression biomarkers in the brain of a mouse model for Alzheimer's disease: mining of microarray data by logic classification and feature selection. J Alzheimer's Dis. 2011;24(4):721–738. doi: 10.3233/JAD-2011-101881. [DOI] [PubMed] [Google Scholar]
- 64.Massone S., Ciarlo E., Vella S., Nizzari M., Florio T., Russo C., et al. NDM29, a RNA polymerase III-dependent non coding RNA, promotes amyloidogenic processing of APP and amyloid b secretion. Biochim Biophys Acta. 2012;1823(7):1170–1177. doi: 10.1016/j.bbamcr.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 65.Wang J., Zhao H., Fan Z., Li G., Ma Q., Tao Z., et al. Long noncoding RNA H19 promotes neuroinflammation in ischemic stroke by driving histone deacetylase 1-dependent M1 microglial polarization. Stroke. 2017;48:2211–2221. doi: 10.1161/STROKEAHA.117.017387. [DOI] [PubMed] [Google Scholar]
- 66.Ng S.Y., Lin L., Soh B.S., Stanton L.W. Long noncoding RNAs in development and disease of the central nervous system. Trends Genet. 2013;29:461–468. doi: 10.1016/j.tig.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 67.Carrieri C., Cimatti L., Biagioli M., Beugnet A., Zucchelli S., Fedele S., et al. Long non-coding antisense RNA controls UchL1 translation through an embedded SINEB2 repeat. Nature. 2012;491:454–457. doi: 10.1038/nature11508. [DOI] [PubMed] [Google Scholar]
- 68.Seaberg R.M., van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22(5):1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ng S.Y., Bogu G.K., Soh B.S., Stanton L.W. The long noncoding RNA RMST interacts with SOX2 to regulate neurogenesis. Mol Cell. 2013;51:349–359. doi: 10.1016/j.molcel.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 70.Yamazaki T., Souquere S., Chujo T., Kobelke S., Chong Y.S., Fox A.H., et al. Functional domains of NEAT1 architectural lncRNA induce paraspeckle assembly through phase separation. Mol Cell. 2018;70(6):1038–1053. doi: 10.1016/j.molcel.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 71.Jiang L., Shao C.W., Wu Q.J., Chen G., Zhou J., Yang B., et al. NEAT1 scaffolds RNA-binding proteins and the Microprocessor to globally enhance pri-miRNA processing. Nat StructMolBiol. 2017;24(10):816. doi: 10.1038/nsmb.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang S.S., Zuo H., Jin J.J., Lv W., Xu Z., Fan Y., et al. Long noncoding RNA Neat1 modulates myogenesis by recruiting Ezh2. Cell Death Dis. 2019;10(7):505. doi: 10.1038/s41419-019-1742-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Govek E.E., Newey S.E., Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19(1):1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- 74.Bernard D., Prasanth K.V., Tripathi V., Colasse S., Nakamura T., Xuan Z., et al. A long nuclear-retained noncoding RNA regulates synaptogenesis by modulating gene expression. EMBO J. 2010;29:3082–3093. doi: 10.1038/emboj.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma P., Li Y., Zhang W., Fang F., Sun J., Liu M., et al. Long non-coding RNA MALAT1 inhibits neuron apoptosis and neuroinflammation while stimulates neurite outgrowth and its correlation with MiR-125b mediates PTGS2, CDK5 and FOXQ1 in Alzheimer's disease. Curr Alzheimer Res. 2019;16(7):596–612. doi: 10.2174/1567205016666190725130134. [DOI] [PubMed] [Google Scholar]
- 76.Tripathi V., Ellis J.D., Shen Z., Song D.Y., Pan Q., Watt A.T., et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39(6):925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen G., Qiu C., Zhang Q., Liu B., Cui Q., et al. Genome-wide analysis of human SNPs at long intergenic noncoding RNAs. Hum Mutat. 2013;34(2):338–344. doi: 10.1002/humu.22239. [DOI] [PubMed] [Google Scholar]
- 78.Zhou X., Xu J. Identification of Alzheimer's disease-associated long noncoding RNAs. Neurobiol Aging. 2015;36(11):2925–2931. doi: 10.1016/j.neurobiolaging.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 79.Zhang S., Qin C., Cao G., Xin W., Feng C., Zhang W., et al. Systematic analysis of long noncoding RNAs in the senescence-accelerated mouse prone 8 brain using RNA sequencing. MolTher Nucl Acids. 2016;5:e343. doi: 10.1038/mtna.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Colucci-D'Amato L., Bonavita V., di Porzio U. The end of the central dogma of neurobiology: stem cells and neurogenesis in adult CNS. NeurolSci. 2006;27(4):266–270. doi: 10.1007/s10072-006-0682-z. [DOI] [PubMed] [Google Scholar]
- 81.Jin K., Zhu Y., Sun Y., Mao X.O., Xie L., Greenberg D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl AcadSci USA. 2002;9(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ciarlo E., Massone S., Penna I., Nizzari M., Gigoni A., Dieci G., et al. An intronicncRNA-dependent regulation of SORL1 expression affecting Abeta formation is upregulated in post-mortem Alzheimer's disease brain samples. Dis Model Mech. 2013;6(2):424–433. doi: 10.1242/dmm.009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramos A.D., Diaz A., Nellore A., Delgado R.N., Park K.Y., Gonzales-Roybal G., et al. Integration of genome-wide approaches identifies lncRNAs of adult neural stem cells and their progeny in vivo. Cell Stem Cell. 2013;12(5):616–628. doi: 10.1016/j.stem.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J., Lucas B.A., Maquat L.E. New gene expression pipelines gush lncRNAs. Genome Biol. 2013;14(5):117. doi: 10.1186/gb-2013-14-5-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kang M.J., Abdelmohsen K., Hutchison E.R., Mitchell S.J., Grammatikakis I., Guo R., et al. HuD regulates coding and noncoding RNA to induce APP–>Abeta processing. Cell Rep. 2014;7(5):1401–1409. doi: 10.1016/j.celrep.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kondrashov A.V., Kiefmann M., Ebnet K., Khanam T., Muddashetty R.S., Brosius J. Inhibitory effect of naked neural BC1 RNA or BC200 RNA on eukaryotic in vitro translation systems is reversed by poly(A)-binding protein (PABP) J Mol Biol. 2005;353(1):88–103. doi: 10.1016/j.jmb.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 87.Li H., Zheng L., Jiang A., Mo Y., Gong Q. Identification of the biological affection of long noncoding RNA BC200 in Alzheimer's disease. Neuroreport. 2018;29(13):1061–1067. doi: 10.1097/WNR.0000000000001057. [DOI] [PubMed] [Google Scholar]
- 88.Qureshi I.A., Mehler M.F. Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease. Nat Rev Neurosci. 2012;13(8):528–541. doi: 10.1038/nrn3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gu L., Guo Z. Alzheimer's Aβ42 and Aβ40 peptides form interlaced amyloid fibrils. J Neurochem. 2013;126(3):305–311. doi: 10.1111/jnc.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Massone S., Ciarlo E., Vella S., Nizzari M., Florio T., Russo C., et al. NDM29, a RNA polymerase III-dependent non coding RNA, promotes amyloidogenic processing of APP and amyloid beta secretion. Bba-Mol Cell Res. 2012;1823(7):1170–1177. doi: 10.1016/j.bbamcr.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 91.Askarian-Amiri M.E., Seyfoddin V., Smart C.E., Wang J., Kim J.E., Hansji H., et al. Emerging role of long non-coding RNA SOX2OT in SOX2 regulation in breast cancer. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0102140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su R., Ma J., Zheng J., Liu X., Liu Y., Ruan X., et al. PABPC1-induced stabilization of BDNF-AS inhibits malignant progression of glioblastoma cells through STAU1-mediated decay. Cell Death Dis. 2020;11(2):1–17. doi: 10.1038/s41419-020-2267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li D.F., Zhang J., Li X.H., Chen Y., Yu F., Liu Q. Insights into lncRNAs in Alzheimer's disease mechanisms. RNA Biol. 2020;18(1):47–63. doi: 10.1080/15476286.2020.1788848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chung D.W., Rudnicki D.D., Yu L., Margolis R.L. A natural antisense transcript at the Huntington's disease repeat locus regulates HTT expression. Hum. Mol Genet. 2011;20(17):3467–3477. doi: 10.1093/hmg/ddr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shimojo M. Huntingtin regulates RE1-silencing transcription factor/neuronrestrictive silencer factor (REST/NRSF) nuclear trafficking indirectly through a complex with REST/NRSF-interacting LIM domain protein (RILP) and dynactin p150 Glued. J Biol Chem. 2008;283(50):34880–34886. doi: 10.1074/jbc.M804183200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zuccato C., Tartari M., Crotti A., Goffredo D., Valenza M., Conti L., et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet. 2003;35(1):76–83. doi: 10.1038/ng1219. [DOI] [PubMed] [Google Scholar]
- 97.Lipovich L., Dachet F., Cai J., Bagla S., Balan K., Jia H., et al. Activity-dependent human brain coding/noncoding gene regulatory networks. Genetics. 2012;192(3):1133–1148. doi: 10.1534/genetics.112.145128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sunwoo J.S., Lee S.T., Im W., Lee M., Byun J.I., Jung K.H., et al. Altered expression of the long noncoding RNA NEAT1 in Huntington's disease. MolNeurobiol. 2017;54(2):1577–1586. doi: 10.1007/s12035-016-9928-9. [DOI] [PubMed] [Google Scholar]
- 99.Clemson C.M., Hutchinson J.N., Sara S.A., Ensminger A.W., Fox A.H., Chess A., et al. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell. 2009;33(6):717–726. doi: 10.1016/j.molcel.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li X., Wu Z., Fu X., Han W. lncRNAs: insights into their function and mechanics in underlying disorders. Mutat Res Rev. 2014;762:1–21. doi: 10.1016/j.mrrev.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 101.Johnson R., Richter N., Jauch R., Gaughwin P.M., Zuccato C., Cattaneo E., et al. Human accelerated region 1 noncoding RNA is repressed by REST in Huntington's disease. Physiol Genom. 2010;41(3):269–274. doi: 10.1152/physiolgenomics.00019.2010. [DOI] [PubMed] [Google Scholar]
- 102.Johnson R., Teh C.H., Jia H., Vanisri R.R., Pandey T., Lu Z.H., et al. Regulation of neural macroRNAs by the transcriptional repressor REST. RNA. 2009;15(1):85–96. doi: 10.1261/rna.1127009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnson R. Long non-coding RNAs in huntington's disease neurodegeneration. Neurobiol Dis. 2012;46(2):245–254. doi: 10.1016/j.nbd.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 104.Francelle L., Galvan L., Gaillard M.C., Petit F., Bernay B., Guillermier M., et al. Striatal long noncoding RNA Abhd11os is neuroprotective against an N-terminal fragment of mutant huntingtin in vivo. Neurobiol Aging. 2015;36(3):1601. doi: 10.1016/j.neurobiolaging.2014.11.014. e7–16. [DOI] [PubMed] [Google Scholar]
- 105.Hwang J.Y., Zukin R.S. REST, a master transcriptional regulator in neurodegenerative disease. CurrOpin Neurobiol. 2018;48:193–200. doi: 10.1016/j.conb.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin N., Chang K.Y., Li Z., Gates K., Rana Z.A., Dang J., et al. An evolutionarily conserved long noncoding RNA TUNA controls pluripotency and neural lineage commitment. Mol Cell. 2014;53(6):1005–1019. doi: 10.1016/j.molcel.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ni Y.H., Huang H., Chen Y.Q., Zhou H., Zhang Y. Investigation of long non-coding RNA expression profiles in the substantia nigra of Parkinson's disease. Cell Mol Neurobiol. 2017;37(2):329–338. doi: 10.1007/s10571-016-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carrieri C., Forrest A.R.R., Santoro C., Persichetti F., Carninci P., Zucchelli S., et al. Expression analysis of the long non-coding RNA antisense to UchL1 (AS UchL1) during dopaminergic cells’ differentiation in vitro and in neurochemical models of Parkinson's disease. Front Cell Neurosci. 2015;9:114. doi: 10.3389/fncel.2015.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chiba M., Kiyosawa H., Hiraiwa N., Ohkohchi N., Yasue H. Existence of Pink1 antisense RNAs in mouse and their localization. Cytogenet Genome Res. 2009;126(3):259–270. doi: 10.1159/000251963. [DOI] [PubMed] [Google Scholar]
- 110.Scheele C., Petrovic N., Faghihi M.A., Lassmann T., Fredriksson K., Rooyackers O., et al. The human PINK1 locus is regulated in vivo by a non-coding natural antisense RNA during modulation of mitochondrial function. BMC Genom. 2007;8(1):74. doi: 10.1186/1471-2164-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang Q.S., Wang Z.H., Zhang J.L., Duan Y.L., Li G.F., Zheng D.L. Beta-asarone protects against MPTP-induced Parkinson's disease via regulating long non-coding RNA MALAT1 and inhibiting a-synuclein protein expression. Biomed Pharmacother. 2016;83:153–159. doi: 10.1016/j.biopha.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 112.Haelterman N.A., Yoon W.H., Sandoval H., Jaiswal M., Shulman J.M., Bellen H.J. A mitocentric view of Parkinson's disease. Ann Rev Neurosci. 2014;37:137–159. doi: 10.1146/annurev-neuro-071013-014317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang S., Zhang X., Guo Y., Rong H., Liu T. The long noncoding RNA HOTAIR promotes Parkinson's disease by upregulating LRRK2 expression. Oncotarget. 2017;8(15):24449–24456. doi: 10.18632/oncotarget.15511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kraus T.F.J., Haider M., Spanner J., Steinmaurer M., Dietinger V., Kretzschmar H.A. Altered long noncoding RNA expression precedes the course of parkinson's disease-a preliminary report. Mol Neurobiol. 2017;54(4):2869–2877. doi: 10.1007/s12035-016-9854-x. [DOI] [PubMed] [Google Scholar]
- 115.Zhang L.M., Wang M.H., Yang H.C., Tian T., Sun G.F., Ji Y.F., et al. Dopaminergic neuron injury in Parkinson's disease is mitigated by interfering lncRNA SNHG14 expression to regulate the miR-133b/α-synuclein pathway. Aging. 2019;11(21):9264–9279. doi: 10.18632/aging.102330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao J., Geng L., Chen Y., Wu Chunfang. SNHG1 promotes MPP+-induced cytotoxicity by regulating PTEN/AKT/mTORsignaling pathway in SH-SY5Y cells via sponging miR-153-3p. Biol Res. 2020;53:1. doi: 10.1186/s40659-019-0267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang L., Wang J., Liu Q., Xiao Z., Dai Q. Knockdown of long non-coding RNA AL049437 mitigates MPP+ -induced neuronal injury in SH-SY5Y cells via the microRNA-205-5p/MAPK1 axis. Neuro Toxicol. 2020;78:29–35. doi: 10.1016/j.neuro.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 118.Coupland K., Kim W.S., Halliday G.M., Hallupp M., Dobson-Stone C., Kwok J.B. Role of the long non-coding RNA MAPT-AS1 in regulation of microtubule associated protein Tau (MAPT) expression in Parkinson's disease. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0157924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Simchovitz A., Hanan M., Niederhoffer N., Madrer N., Yayon N., Bennett E.R., et al. NEAT1 is overexpressed in Parkinson's disease substantia nigra and confers drug-inducible neuroprotection from oxidative stress. FASEB J. 2019;33:11223–11234. doi: 10.1096/fj.201900830R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xie S.P., Zhou F., Li J., Duan S.J. NEAT1 regulates MPP+-induced neuronal injury by targeting miR-124 in neuroblastoma cells. Neurosci Lett. 2019;708 doi: 10.1016/j.neulet.2019.134340. [DOI] [PubMed] [Google Scholar]
- 121.Yan W., Chen Z.Y., Chen J.Q., Chen H.M. LncRNA NEAT1 promotes autophagy in MPTP-induced Parkinson's disease through stabilizing PINK1 protein. Biochem Biophys Res Commun. 2018;496(4):1019–1024. doi: 10.1016/j.bbrc.2017.12.149. [DOI] [PubMed] [Google Scholar]
- 122.Ding X.M., Zhao L.J., Qiao H.Y., Wu S.L., Wang X.H. Long non-coding RNA-p21 regulates MPP+-induced neuronal injury by targeting miR-625 and derepressing TRPM2 in SH-SY5Y cells. Chem Interact. 2019;307:73–81. doi: 10.1016/j.cbi.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 123.Li Y., Fang J., Zhou Z., Zhou Q., Sun S., Jin Z., et al. Downregulation of lncRNA BACE1-AS improves dopamine-dependent oxidative stress in rats with Parkinson's disease by upregulating microRNA-34b-5p and downregulating BACE1. Cell Cycle. 2020;19(10):1158–1171. doi: 10.1080/15384101.2020.1749447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peng T., Liu X., Wang J., Liu Y., Fu Z., Ma X., et al. Long noncoding RNA HAGLROS regulates apoptosis and autophagy in Parkinson's disease via regulating miR-100/ATG10 axis and PI3K/Akt/mTOR pathway activation. Artif. Cells Nanomed Biotechnol. 2019;47(1):2764–2774. doi: 10.1080/21691401.2019.1636805. [DOI] [PubMed] [Google Scholar]
- 125.Jiang J., Piao X., Hu S., Gao J., Bao M. LncRNA H19 diminishes dopaminergic neuron loss by mediating microRNA-301b-3p in Parkinson's disease via the HPRT1-mediated Wnt/beta-catenin signaling pathway. Aging. 2020;12(10):8820–8836. doi: 10.18632/aging.102877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang Y., Xia Q., Lin J. LncRNA H19 attenuates apoptosis in MPTP-induced Parkinson's disease through regulating miR-585-3p/PIK3R3. Neurochem Res. 2020;45(7):1700–1710. doi: 10.1007/s11064-020-03035-w. [DOI] [PubMed] [Google Scholar]
- 127.Xu W., Zhang L., Geng Y., Liu Y., Zhang N. Long noncoding RNA GAS5 promotes microglial inflammatory response in Parkinson's disease by regulating NLRP3 pathway through sponging miR-223-3p. Int Immunopharmacol. 2020;85 doi: 10.1016/j.intimp.2020.106614. [DOI] [PubMed] [Google Scholar]
- 128.Song Q., Geng Y., Li Y., Wang L., Qin J. Long noncoding RNA NORAD regulates MPP+-induced Parkinson's disease model cells. J Chem Neuroanat. 2019;101 doi: 10.1016/j.jchemneu.2019.101668. [DOI] [PubMed] [Google Scholar]
- 129.Lu M., Sun W.L., Shen J., Wei M., Chen B., Qi Y.J., et al. LncRNA-UCA1 promotes PD development by upregulating SNCA. Eur Rev Med PharmacolSci. 2018;22(22):7908–7915. doi: 10.26355/eurrev_201811_16417. [DOI] [PubMed] [Google Scholar]
- 130.Simchovitz A., Hanan M., Yayon N., Lee S., Bennett E.R., Greenberg D.S., et al. AlncRNA survey finds increases in neuroprotective LINC-PINT in Parkinson's disease substantia nigra. Aging Cell. 2020;19(3):e13115. doi: 10.1111/acel.13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Acharya S., Salgado-Somoza A., Stefanizzi F.M., Lumley A.I., Zhang L., Glaab E., et al. Non-coding RNAs in the brain-heart axis: the Case of Parkinson's disease. Int J Mol Sci. 2020;21(18):6513. doi: 10.3390/ijms21186513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang H., Lin Q., Chen N., Luo Z., Zheng C., Li J., et al. LncRNA NR_030777 alleviates paraquat-induced neurotoxicity by regulating Zfp326 and Cpne5. Toxicol Sci. 2020;178(1):173–188. doi: 10.1093/toxsci/kfaa121. [DOI] [PubMed] [Google Scholar]
- 133.Wang L., Yang H., Wang Q., Zhang Q., Wang Z., Zhang Q., et al. Paraquat and MPTP induce alteration in the expression profile of long noncoding RNAs in the substantia nigra of mice: role of the transcription factor Nrf2. Toxicol Lett. 2018;291:11–28. doi: 10.1016/j.toxlet.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 134.Lyu Y., Bai L., Qin C. Long noncoding RNAs in neurodevelopment and Parkinson's disease. Animal Model Exp Med. 2019;2(4):239–251. doi: 10.1002/ame2.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Márki S., Göblös A., Szlávicz E., Török N., Balicza P., Bereznai B., et al. The rs13388259 intergenic polymorphism in the genomic context of the BCYRN1 gene is associated with Parkinson's disease in the Hungarian population. Parkinsons Dis. 2018;2018 doi: 10.1155/2018/9351598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seidman L.J., Mirsky A.F. Evolving notions of schizophrenia as a developmental neurocognitive disorder. J Int Neuropsychol Soc. 2017;23(9–10):881–892. doi: 10.1017/S1355617717001114. [DOI] [PubMed] [Google Scholar]
- 137.Chen S.D., Sun X.Y., Niu W., Kong L., He M., Li W., et al. Aberrant expression of long non-coding RNAs in schizophrenia patients. Med Sci Monitor. 2016;22:3340–3351. doi: 10.12659/MSM.896927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Scholz C.J., Jacob C.P., Buttenschon H.N., Kittel-Schneider S., Boreatti-Hümmer A., Zimmer M., et al. Functional variants of TSPAN8 are associated with bipolar disorder and schizophrenia. Am J Med Genet. 2010;153B(4):967–972. doi: 10.1002/ajmg.b.31057. [DOI] [PubMed] [Google Scholar]
- 139.Hu J., Xu J.Y., Pang L., Zhao H., Li F., Deng Y., et al. Systematically characterizing dysfunctional long intergenic non-coding RNAs in multiple brain regions of major psychosis. Oncotarget. 2016;7(44):71087–71098. doi: 10.18632/oncotarget.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ren Y., Cui Y.H., Li X.R., Wang B., Na L., Shi J., et al. A co-expression network analysis reveals lncRNA abnormalities in peripheral blood in early-onset schizophrenia. Prog. Neuro-Psychopharmacol. Biol Psychiatry. 2015;63:1–5. doi: 10.1016/j.pnpbp.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 141.Cui X.L., Niu W., Kong L.M., He M., Jiang K., Chen S., et al. Can lncRNAs be indicators for the diagnosis of early onset or acute schizophrenia and distinguish major depressive disorder and generalized anxiety disorder?—A cross validation analysis. Am J Med Genet B. 2017;174(4):335–341. doi: 10.1002/ajmg.b.32521. [DOI] [PubMed] [Google Scholar]
- 142.Barry G., Briggs J.A., Vanichkina D.P., Poth E.M., Beveridge N.J., Ratnu V.S., et al. The long non-coding RNA Gomafu is acutely regulated in response to neuronal activation and involved in schizophrenia-associated alternative splicing. Mol Psychiatry. 2014;19(4):486–494. doi: 10.1038/mp.2013.45. [DOI] [PubMed] [Google Scholar]
- 143.Rao S.Q., Hui H.L., Ye N., Shen Y., Xu Q., et al. Genetic variants in long non-coding RNA MIAT contribute to risk of paranoid schizophrenia in a Chinese Han Population. Schizophr Res. 2015;166(1–3):125–130. doi: 10.1016/j.schres.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 144.Tsuiji H., Yoshimoto R., Hasegawa Y., Furuno M., Yoshida M., Nakagawa S. Competition between a noncoding exon and introns: gomafu contains tandem uacuaac repeats and associates with splicing factor-1. Genes Cells. 2011;16:479–490. doi: 10.1111/j.1365-2443.2011.01502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ishizuka A., Hasegawa Y., Ishida K., Yanaka K., Nakagawa S. Formation of nuclear bodies by the lncRNAGomafu-associating proteins Celf3 and SF1. Genes Cells. 2014;19(9):704–721. doi: 10.1111/gtc.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sone M., Hayashi T., Tarui H., Agata K., Takeichi M., Nakagawa S. The mRNA-like noncoding RNA Gomafu constitutes a novel nuclear domain in a subset of neurons. J Cell Sci. 2007;120(15):2498–2506. doi: 10.1242/jcs.009357. [DOI] [PubMed] [Google Scholar]
- 147.Mercer T.R., Dinger M.E., Sunkin S.M., Mehler M.F., Mattick J.S. Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci USA. 2008;105(2):716–721. doi: 10.1073/pnas.0706729105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sun C., Huang L., Li Z., Leng K., Xu Y., Jiang X., et al. Long non-coding RNA MIAT in development and disease: a new player in an old game. J Biomed Sci. 2018;25(1):23. doi: 10.1186/s12929-018-0427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Andrew G., Udawela M., Dean B. Non-Coding RNA as Novel Players in the pathophysiology of schizophrenia. Noncoding RNA. 2018;4(2):11. doi: 10.3390/ncrna4020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nakata K., Lipska B.K., Hyde T.M., Ye T., Newburn E.N., Morita Y., et al. DISC1 splice variants are upregulated in schizophrenia and associated with risk polymorphisms. Proc Natl Acad Sci USA. 2009;106:15873–15878. doi: 10.1073/pnas.0903413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Law A.J., Kleinman J.E., Weinberger D.R., Weickert C.S. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16(2):129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- 152.Roberts T.C., Morris K.V., Wood M.J. The role of long non-coding RNAs in neurodevelopment, brain function and neurological disease. Philos Trans R Soc Lond B Biol Sci. 2014;369 doi: 10.1098/rstb.2013.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu Y.S., Rao S.Q., Xu Y., Zhang F., Wang Z., Zhao X. Changes in the level of long non-coding RNA gomafu gene expression in schizophrenia patients before and after antipsychotic medication. Schizophr Res. 2018;195:318–319. doi: 10.1016/j.schres.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 154.Gianfrancesco O., Warburton A., Collier D.A., Bubb V.J., Quinn J.P. Novel brain expressed RNA identified at the MIR137 schizophrenia-associated locus. Schizophr Res. 2017;184:109–115. doi: 10.1016/j.schres.2016.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tang J., Yu Y.Z., Yang W. Long noncoding RNA and its contribution to autism spectrum disorders. CNS Neurosci Ther. 2017;23(8):645–656. doi: 10.1111/cns.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ziats M.N., Rennert O.M. Aberrant expression of long noncoding RNAs in autistic brain. J Mol Neurosci. 2013;49(3):589–593. doi: 10.1007/s12031-012-9880-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wilkinson B., Campbell D.B. Contribution of long noncoding RNAs to autism spectrum disorder risk. Int Rev Neurobiol. 2013;113:35–59. doi: 10.1016/B978-0-12-418700-9.00002-2. [DOI] [PubMed] [Google Scholar]
- 158.Wang Y., Zhao X., Ju W., Flory M., Zhong J., Jiang S., et al. Genome-wide differential expression of synaptic long noncoding RNAs in autism spectrum disorder. Trans Psychiatry. 2015;5(10):e660. doi: 10.1038/tp.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., Boxer A.L., Baker M., Rutherford N.J., et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zu T., Liu Y., Bañez-Coronel M., Reid T., Pletnikova O., Lewis J., et al. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc Natl Acad Sci USA. 2013;110:E4968–E4977. doi: 10.1073/pnas.1315438110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mizielinska S., Lashley T., Norona F.E., Clayton E.L., Ridler C.E., Fratta P., et al. C9orf72 frontotemporal lobar degeneration is characterised by frequent neuronal sense and antisense RNA foci. ActaNeuropathol. 2013;126:845–857. doi: 10.1007/s00401-013-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lagier-Tourenne C., Baughn M., Rigo F., Sun S., Liu P., Li H.R., et al. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc Natl Acad Sci USA. 2013;110(47):E4530–E4539. doi: 10.1073/pnas.1318835110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pokrishevsky E., Grad L.I., Yousefi M., Wang J., Mackenzie I.R., Cashman N.R. Aberrant localization of FUS and TDP43 is associated with misfolding of SOD1 in amyotrophic lateral sclerosis. PLoS One. 2012;7:e35050. doi: 10.1371/journal.pone.0035050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang X., Arai S., Song X., Reichart D., Du K., Pascual G., et al. Induced ncRNAs allosterically modify RNA-binding proteins in CIS to inhibit transcription. Nature. 2008;454(7200):126–130. doi: 10.1038/nature06992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Doi H., Okamura K., Bauer P.O., Furukawa Y., Shimizu H., Kurosawa M., et al. RNA-binding protein TLS is a major nuclear aggregate-interacting protein in huntingtin exon 1 with expanded polyglutamine-expressing cells. J Biol Chem. 2008;283:6489–6500. doi: 10.1074/jbc.M705306200. [DOI] [PubMed] [Google Scholar]
- 166.Tsuang M.T., Taylor L., Faraone S.V. An overview of the genetics of psychotic mood disorders. J Psychiatr Res. 2004;38(1):3–15. doi: 10.1016/s0022-3956(03)00096-7. [DOI] [PubMed] [Google Scholar]
- 167.Ye C., Zhou J. LncRNAs: macromolecules with big roles in neurobiology and neurological diseases. Metab Brain Dis. 2017;32(2):281–291. doi: 10.1007/s11011-017-9965-8. [DOI] [PubMed] [Google Scholar]
- 168.Wang Z., Gerstein M., Snyder M. RNA-seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10(1):57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Donnan G.A., Fisher M., Macleod M., Davis S.M. Stroke Lancet. 2021;371(9624):1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 170.Dharap A., Nakka V.P., Vemuganti R. Effect of focal ischemia on long noncoding RNAs. Stroke. 2012;43(10):2800–2802. doi: 10.1161/STROKEAHA.112.669465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dharap A., Pokrzywa C., Vemuganti R. Increased binding of strokeinduced long non-coding RNAs to the transcriptional corepressors Sin3A and coREST. ASN Neuro. 2013;5(4):283–289. doi: 10.1042/AN20130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kaur P., Karolina D.S., Sepramaniam S., Armugam A., Jeyaseelan K. Expression profiling of RNA transcripts during neuronal maturation and ischemic injury. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0103525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang F., Gao C., Ma X.F., Peng X.L., Zhang R.X., Kong D.X., et al. Expression profile of long noncoding RNAs in peripheral blood mononuclear cells from multiple sclerosis patients. CNS NeurosciTher. 2016;22(4):298–305. doi: 10.1111/cns.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhao F., Qu Y., Liu J., Liu H., Zhang L., Feng Y., et al. Microarray profiling and Co-expression network analysis of LncRNAs and mRNAs in neonatal rats following hypoxic-ischemic brain damage. Sci Rep. 2015;5:13850. doi: 10.1038/srep13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Qureshi I.A., Mattick J.S., Mehler M.F. Long non-coding RNAs in nervous system function and disease. Brain Res. 2010;1338:20–35. doi: 10.1016/j.brainres.2010.03.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pang K.C., Dinger M.E., Mercer T.R., Malquori L., Grimmond S.M., Chen W., et al. Genome-wide identification of long noncoding RNAs in CD8+ T cells. J Immunol. 2009;182(12):7738–7748. doi: 10.4049/jimmunol.0900603. [DOI] [PubMed] [Google Scholar]
- 177.Abarrategui I., Krangel M.S. Noncoding transcription controls downstream promoters to regulate T-cell receptor alpha recombination. EMBO J. 2007;26(20):4380–4390. doi: 10.1038/sj.emboj.7601866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang F., Liu G., Wei C., Gao C., Hao J. Linc-MAF-4 regulates Th1/Th2 differentiation and is associated with the pathogenesis of multiple sclerosis by targeting MAF. FASEB J. 2016;31(2):519–525. doi: 10.1096/fj.201600838R. [DOI] [PubMed] [Google Scholar]
- 179.Santoro M., Nociti V., Lucchini M., De Fino C., Losavio F.A., Mirabella M. Expression profile of long non-coding RNAs in serum of patients with multiple sclerosis. J Mol Neurosci. 2016;59(1):18–23. doi: 10.1007/s12031-016-0741-8. [DOI] [PubMed] [Google Scholar]
- 180.Zhang J., Yuan L., Zhang X., Hamblin M.H., Zhu T., Meng F., et al. Altered long non-coding RNA transcriptomic profiles in brain microvascular endothelium after cerebral ischemia. ExpNeurol. 2016;277:162–170. doi: 10.1016/j.expneurol.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Angrand P.O., Vennin C., Le Bourhis X., Adriaenssens E. The role of long non-coding RNAs in genome formatting and expression. Front Genet. 2015;6:165. doi: 10.3389/fgene.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Clark M.B., Johnston R.L., Inostroza-Ponta M., Fox A.H., Fortini E., Moscato P., et al. Genome-wide analysis of long noncoding RNA stability. Genome Res. 2012;22(5):885–898. doi: 10.1101/gr.131037.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang L., Duan W., Yan S., Xie Y., Wang C. Circulating long non-coding RNA colon cancer-associated transcript 2 protected by exosome as a potential biomarker for colorectal cancer. Biomed Pharmacother. 2019;113 doi: 10.1016/j.biopha.2019.108758. [DOI] [PubMed] [Google Scholar]
- 184.Terracciano D., Ferro M., Terreri S., Battelli N. Urinary long noncoding RNAs in nonmuscle-invasive bladder cancer: new architects in cancer prognostic biomarkers. Transl Res. 2017;184:108–117. doi: 10.1016/j.trsl.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 185.Martignano F., Rossi L., Maugeri A., Gallà V., Conteduca V., De Giorgi U., et al. Urinary RNA-based biomarkers for prostate cancer detection. ClinChimActa. 2017;473:96–105. doi: 10.1016/j.cca.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 186.Li Q., Shao Y., Zhang X., Zheng T., Miao M., Qin L., et al. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumour Biol. 2015;36(3):2007–2012. doi: 10.1007/s13277-014-2807-y. [DOI] [PubMed] [Google Scholar]
- 187.Viereck J., Kumarswamy R., Foinquinos A., Xiao K., Avramopoulos P., Kunz M., et al. Long noncoding RNA Chast promotes cardiac remodeling. SciTransl Med. 2016;8(326):326ra22. doi: 10.1126/scitranslmed.aaf1475. [DOI] [PubMed] [Google Scholar]
- 188.Gao M., Dong Z., Sun J., Liu W., Xu M., Li C., et al. Liver-derived exosome-laden lncRNA MT1DP aggravates cadmium-induced nephrotoxicity. Environ Pollut. 2020;258 doi: 10.1016/j.envpol.2019.113717. [DOI] [PubMed] [Google Scholar]
- 189.Cai C., Zhang H., Zhu Y., Zheng P., Xu Y., Sun J., et al. Serum exosomal long Noncoding RNA pcsk2-2:1 as a potential novel diagnostic biomarker for gastric cancer. Onco Targets Ther. 2019;12:10035–10041. doi: 10.2147/OTT.S229033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li L., Zhuang Y., Zhao X., Li X. Long non-coding RNA in neuronal development and neurological disorders. Front Genet. 2019;9:744. doi: 10.3389/fgene.2018.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang D.Q., Peng F., Chengye Y., Zhu L.S., Hou T.Y., Chen J.G., et al. Long non-coding RNAs, novel culprits, or bodyguards in neurodegenerative diseases. Mol Ther Nucl Acids. 2018;10:269–276. doi: 10.1016/j.omtn.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cui X., Sun X., Niu W., Kong L., He M., Zhong A., et al. Long non-coding RNA: potential diagnostic and therapeutic biomarker for major depressive disorder. Med Sci Monit. 2016;22:5240–5248. doi: 10.12659/MSM.899372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cui X., Niu W., Kong L., He M., Jiang K., Chen S., et al. Long noncoding RNAs: new evidence for overlapped pathogenesis between major depressive disorder and generalized anxiety disorder. Indian J Psychiatry. 2017;59(1):83–87. doi: 10.4103/psychiatry.IndianJPsychiatry_219_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.He M., Zhu X., Niu W., Kong L., Yao G. Bioinformatics analysis of altered IncRNAs in peripheral blood molecular cells from major depressive disorder (MDD) patients. Int J Blood Res Disord. 2018;5:034. [Google Scholar]
- 195.Liu Z., Li X., Sun N., Xu Y., Meng Y., Yang C. Microarray Profiling and co-expression network analysis of circulating lncRNAs and mRNAs associated with major depressive disorder. PLoS One. 2014;9(3):e93388. doi: 10.1371/journal.pone.0093388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sanchez Y., Huarte M. Long non-coding RNAs: challenges for diagnosis and therapies. Nucl Acid Ther. 2013;23(1):15–20. doi: 10.1089/nat.2012.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Weinberg M.S., Morris K.V. Long non-coding RNA targeting and transcriptional de-repression. Nucl Acid Ther. 2013;23(1):9–14. doi: 10.1089/nat.2012.0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bhartiya D., Kapoor S., Jalali S., Sati S., Kaushik K., Sachidanandan C., et al. Conceptual approaches for lncRNA drug discovery and future strategies. Expert Opin Drug Discov. 2012;7(6):503–513. doi: 10.1517/17460441.2012.682055. [DOI] [PubMed] [Google Scholar]
- 199.Qi P., Du X. The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol. 2013;26(2):155–165. doi: 10.1038/modpathol.2012.160. [DOI] [PubMed] [Google Scholar]
- 200.Shao H., Chung J., Balaj L., Charest A., Bigner D.D., Carter B.S., et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med. 2012;18(12):1835–1840. doi: 10.1038/nm.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kam Y., Rubinstein A., Naik S., Djavsarov I., Halle D., Ariel I., et al. Detection of a long noncoding RNA (CCAT1) in living cells and human adenocarcinoma of colon tissues using FIT-PNA molecular beacons. Cancer Lett. 2013;352(1):90–96. doi: 10.1016/j.canlet.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 202.Gutschner T., Baas M., Diederichs S. Noncoding RNA gene silencing through genomic integration of RNA destabilizing elements using zinc finger nucleases. Genome Res. 2011;21(11):1944–1954. doi: 10.1101/gr.122358.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yang F., Huo X.S., Yuan S.X., Zhang L., Zhou W.P., Wang F., et al. Repression of the long noncoding RNA-LET by histone deacetylase 3 contributes to hypoxiamediated metastasis. Mol Cell. 2013;49(6):1083–1096. doi: 10.1016/j.molcel.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 204.Mercer T.R., Qureshi I.A., Gokhan S., Dinger M.E., Li G., Mattick J.S., et al. Long noncoding RNAs in neuronal-glial fate specification and oligodendrocyte lineage maturation. BMC Neurosci. 2010;11(1):14. doi: 10.1186/1471-2202-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]