Abstract
Vitamin D is lipophilic and accumulates substantially in adipose tissue. Even without supplementation, the amount of vitamin D in the adipose of a typical adult is equivalent to several months of the daily reference nutrient intake (RNI). Paradoxically, despite the large amounts of vitamin D located in adipose tissue, individuals with obesity are often vitamin D deficient according to consensus measures of vitamin D status (serum 25‐hydroxyvitamin D concentrations). Thus, it appears that vitamin D can become ‘trapped’ in adipose tissue, potentially due to insufficient lipolytic stimulation and/or due to tissue dysfunction/adaptation resulting from adipose expansion. Emerging evidence suggests that exercise may mobilise vitamin D from adipose (even in the absence of weight loss). If exercise helps to mobilise vitamin D from adipose tissue, then this could have important ramifications for practitioners and policymakers regarding the management of low circulating levels of vitamin D, as well as chronically low levels of physical activity, obesity and associated health conditions. This perspective led us to design a study to examine the impact of exercise on vitamin D status, vitamin D turnover and adipose tissue vitamin D content (the VitaDEx project). The VitaDEx project will determine whether increasing physical activity (via exercise) represents a potentially useful strategy to mobilise vitamin D from adipose tissue.
Keywords: 25(OH)D, 25‐hydroxyvitamin D, adipose, exercise, physical activity, vitamin D
Introduction
Vitamin D has effects far beyond its classical actions on calcium homeostasis and bone metabolism, and vitamin D insufficiency is thought to affect many physiological systems and a wide array of human health outcomes (Dobnig et al. 2008; Ginde et al. 2009; Semba et al. 2010). Vitamin D status is conventionally determined by measuring concentrations of the main circulating form of vitamin D, 25‐hydroxyvitamin D [25(OH)D]. Approximately 30–40% of the UK population have circulating 25(OH)D <25 nmol/l in winter (SACN 2016), and the correction of low systemic concentrations of 25(OH)D is a recognised public health priority (Palacios & Gonzalez 2014; Cashman et al. 2016).
Vitamin D: Overview
The two main forms of vitamin D are vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) (Askew et al. 1930; Windaus et al. 1936). Previtamin D3 is synthesised by the skin when solar ultraviolet (UV) radiation (wavelength 290–320 nm) penetrates the skin and is absorbed by 7‐dehydrocholesterol (Maclaughlin et al. 1982), before spontaneously and rapidly isomerising to vitamin D3. Vitamin D3 is metabolised in the liver to 25(OH)D – the main circulating form of vitamin D (Blunt et al. 1968; Blunt & Deluca 1969). Vitamin D2 and vitamin D3 can be obtained from the diet and from supplements (Holick 2007). Vitamin D2 is synthesised by UV irradiation of ergosterol and intake is primarily through consumption of UV‐irradiated mushrooms or supplements. In contrast, vitamin D3 is more widely distributed in foods and is more commonly used in supplements and fortified foods than vitamin D2. One reason for using serum 25(OH)D as a measure of vitamin D status is that it has a half‐life of ~2–3 weeks and, therefore, quantification of this metabolite is not influenced by transient changes in dietary vitamin D or acute sun exposure to the same extent as other vitamin D metabolites (Jones et al. 2015). The methods of assessing 25(OH)D are reviewed by Le Goff et al. (2015). In this review, reference to ‘vitamin D’ denotes either vitamin D3 (cholecalciferol) or 25(OH)D.
The main metabolically active form of vitamin D in vivo is the seco‐steroid 1,25‐dihydroxyvitamin D [1,25(OH)2D], also referred to as calcitriol. It is 1,25(OH)2D that is generally considered responsible for the physiological functions of vitamin D. The production of 1,25(OH)2D takes place predominantly in the kidney, and its action is mediated through binding to a vitamin D receptor (VDR), which is usually located in the nuclei of target cells, and which regulates target gene expression when bound with 1,25(OH)2D (Haussler et al. 2013). Study of VDR‐ablated mice has demonstrated numerous functions of vitamin D (Bouillon et al. 2013).
Vitamin D binding protein (DBP) in serum binds to different vitamin D metabolites with different affinity (Daiger et al. 1975). Vitamin D is bound to DBP for transport to relevant tissues and to regulate bioavailability (Safadi et al. 1999). Normally ~85% of circulating 1,25(OH)2D is bound to DBP, with ~0.4% being free circulating (Bikle et al. 1984, 1985), and ~88% of circulating 25(OH)D is bound to DBP with ~0.04% free (Bikle et al. 1986). Vitamin D can also be bound to albumin and chylomicrons of lipoproteins at lower affinity (Haddad et al. 1993). Although the majority of circulating vitamin D metabolites are bound to DBP or albumin, there is currently considerable debate about whether bound or unbound forms of vitamin D are biologically active (Bikle et al. 2017), with some tissues requiring uptake of DBP‐bound vitamin D and others appearing to access free or unbound vitamin D (Chun et al. 2014).
Potentially meaningful quantities of vitamin D have been observed in the skin, liver, skeletal muscle and adipose tissue of humans (Mawer et al. 1972), with evidence that extra‐renal tissues (such as the placenta) are capable of metabolising vitamin D (Weisman et al. 1979; Adams & Hewison 2012). The present review will focus on the role of adipose tissue, exploring the accumulation of vitamin D in this sizeable depot, whilst also exploring the potential mechanisms underlying the mobilisation of vitamin D from adipose within the context of well‐established physiological concepts.
Vitamin D accumulation in adipose
Vitamin D is lipophilic and early studies used radioactive isotopes to demonstrate its accumulation in adipose tissue (Rosenstreich et al. 1971; Mawer et al. 1972). Supplementing with 20 000 international units (IU) of vitamin D3 per week for 3–5 years leads to a substantial increase in vitamin D3 content in subcutaneous abdominal adipose tissue, approximately sixfold greater than placebo (Didriksen et al. 2015). The amount of 25(OH)D present in adipose explants remained correlated with serum 25(OH)D concentrations 1 year after supplementation had ceased (Martinaityte et al. 2017). Although it is worth noting that values for serum 25(OH)D and adipose tissue vitamin D3 measured in this study varied considerably between participants, which may be explained by the fourfold variation in body fat mass across the study population. There is a positive linear correlation between vitamin D3 concentrations in subcutaneous abdominal adipose tissue and serum (Blum et al. 2008b). High concentrations of vitamin D3 have been observed in adipose tissue from various sites (perirenal, pericardial, axillary and cervical) taken from humans who were not known to be supplementing with vitamin D (Lawson et al. 1986). Vitamin D storage was highly variable between adipose depots (Lawson et al. 1986), which may be driven by depot‐specific differences in adipose tissue blood flow or metabolism; differences which have been identified when comparing subcutaneous abdominal adipose and gluteofemoral adipose (Manolopoulos et al. 2010). Furthermore, the relative amount of vitamin D (i.e. per gram of subcutaneous abdominal adipose tissue) is highly variable between individuals (Blum et al. 2008b; Pramyothin et al. 2011). It has recently been suggested that vitamin D accumulates in greater concentrations in omental adipose than subcutaneous abdominal adipose (Carrelli et al. 2017). However, given that the mass of subcutaneous adipose tissue is fourfold to sixfold greater than that of visceral adipose tissue (Ross et al. 2000; Merlotti et al. 2017), the absolute capacity and accumulation of vitamin D in subcutaneous adipose depots is likely to be quantitatively more important. Nonetheless, further research is needed to understand both the distribution and inter‐/intra‐individual variability of vitamin D accumulation across different adipose depots.
Whilst adipose can accumulate both vitamin D3 and 25(OH)D, the limited data available suggest that the concentrations of vitamin D3 are much greater (Piccolo et al. 2013; Didriksen et al. 2015). Published values for the amount of vitamin D3 present in subcutaneous adipose tissue vary substantially, ranging from ~4 to ~500 ng/g, suggesting large individual variability and dependency on supplementation status (Didriksen et al. 2015). For an individual weighing 100 kg, with 40% body fat, this may equate to 160–20 000 μg vitamin D3, which is the equivalent to anywhere between 16 and 2000 days of the daily reference nutrient intake (RNI) of total dietary vitamin D (10 μg) for the UK population (Fig. 1). The median value for vitamin D3 in adipose of non‐supplementing humans with overweight or obesity is 32 ng/g (Didriksen et al. 2015), which equates to 128 days of the RNI. Thus, adipose tissue has the potential to accumulate a substantial amount of vitamin D, especially when adipose mass is expanded (i.e. in overweight and obesity).
Figure 1.
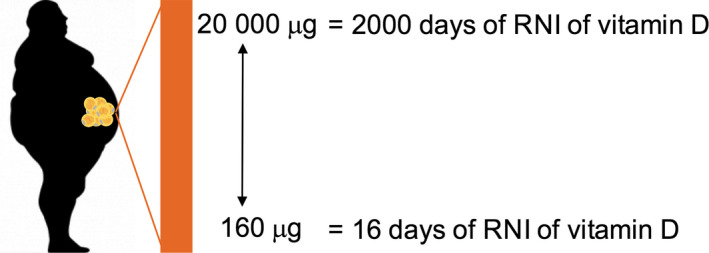
The amount of vitamin D present in adipose tissue is likely to be quantitatively important. When extrapolated, the amounts of vitamin D present in the adipose tissue of an individual weighing 100 kg and with a body fat percentage of 40% may equate to up to 2000 days of the daily recommended nutrient intake (RNI) of vitamin D (10 μg) from dietary sources or supplementation. [Colour figure can be viewed at wileyonlinelibrary.com]
Obesity and vitamin D status
Individuals with obesity have lower circulating 25(OH)D concentrations than individuals without obesity (Compston et al. 1981; Bell et al. 1985; Liel et al. 1988; Vimaleswaran et al. 2013; Pereira‐Santos et al. 2015; Walsh et al. 2016). The mechanisms explaining this have been debated. One suggestion is that reduced sunlight exposure is a behavioural tendency in individuals with obesity (Compston et al. 1981), but there is limited empirical evidence to support this. Wortsman et al. (2000) found that increases in serum 25(OH)D, induced by UV exposure, were attenuated in individuals with obesity, hypothesising that vitamin D is less bioavailable because it is sequestered in the expanded adipose tissue compartment. In support of this suggestion, in individuals supplemented with 700 IU/day of vitamin D for a year, the increase in serum 25(OH)D was inversely related to body mass index (BMI) (Blum et al. 2008a). Another study assessed serum 25(OH)D responses across 90 days with ingestion of a single oral bolus of 300 000 IU of vitamin D3 and showed that the increase in serum 25(OH)D concentration was blunted in individuals with overweight or obesity compared with lean controls (Camozzi et al. 2016). Lower vitamin D status has sometimes been attributed to volumetric dilution, with regression analysis revealing body mass as the strongest univariate predictor of vitamin D status (Drincic et al. 2012). If volumetric dilution was driving this observation, then lower vitamin D status may also be associated with expansion of muscle (and total) mass. However, when comparing winter levels of serum 25(OH)D in a range of non‐supplementing athletes (rugby league players), significantly greater mean serum 25(OH)D was observed compared with non‐supplementing, non‐athlete controls despite markedly greater body mass (reflective of large muscle mass) (Close et al. 2013). Furthermore, in university athletes, fat mass was a significant predictor of serum 25(OH)D concentrations but fat‐free mass was not (Heller et al. 2015). Collectively, these findings indicate that volumetric dilution per se does not explain the negative associations between mass and vitamin D status. This makes sense when considering the lipophilic nature of vitamin D. Indeed, it seems likely that the detrimental effect of obesity on vitamin D status is predominantly due to specific expansion of adipose mass rather than expansion of total mass or volume.
Early studies postulated that increased circulating 1,25(OH)2D with obesity reduces serum 25(OH)D by reducing hepatic synthesis of 25(OH)D via negative feedback (Bell et al. 1984, 1985). However, there is a weak negative relationship between fat mass and circulating 1,25(OH)2D (Parikh et al. 2004), calling into question the existence of a negative feedback mechanism by which production and accumulation of excess 1,25(OH)2D would reduce 25(OH)D synthesis. Alternatively, low circulating vitamin D in individuals with obesity may be attributable to increased metabolic clearance and enhanced uptake of vitamin D by adipose tissue (Liel et al. 1988). An upregulation of enzymes responsible for metabolising 25(OH)D to 1,25(OH)2D is unlikely to contribute to decreased serum 25(OH)D because of the significantly lower circulating levels of 1,25(OH)2D relative to 25(OH)D (~1:400) (Lips 2007). Wamberg et al. (2013) analysed the expression of a number of vitamin D hydroxylases in visceral and subcutaneous abdominal adipose tissue of lean women and women with obesity. There was no difference in CYP24A1 expression (a major enzyme responsible for catabolising vitamin D) between lean individuals and individuals with obesity. Similar results have since been replicated in a cohort of individuals with obesity (Di Nisio et al. 2017). Together, these studies suggest that CYP2J2‐25‐hydroxylation and 1α‐hydroxylation are impaired, rather than upregulated, in the adipose tissue of individuals with obesity, so do not support the idea that the decreased systemic 25(OH)D concentrations observed in obesity are caused by increased metabolism of vitamin D in adipose. Other recent investigations using stable isotopes indicate that there is no difference in the overall 25(OH)D half‐life with obesity (Walsh et al. 2016). Therefore, adipose does not seem to actively metabolise more vitamin D in individuals with obesity and there seems to be no effect of obesity on overall 25(OH)D metabolism and turnover. Instead, the most striking effect of obesity seems to be that adipose becomes a sink or reservoir for vitamin D.
A role for vitamin D within adipose?
Adipose tissue comprises heterogeneous cells including adipocytes, various immune cells and preadipocytes. The cellular composition of adipose impacts on adipose inflammation (Bourlier et al. 2008) and the secretion of inflammatory mediators (Maury & Brichard 2010), which is particularly pertinent to individuals with obesity as it contributes to systemic low‐grade inflammation (Trim et al. 2018).
Treatment of human adipocytes in vitro with 1,25(OH)2D decreases secretion of interleukin 6 (Mutt et al. 2012), suggesting that 1,25(OH)2D inhibits phosphorylation of IкBα, which has an inhibitory effect on NF‐кB (Baeuerle & Baltimore 1988). NF‐кB induces transcription of pro‐inflammatory pathways (Baldwin 1996), so vitamin D is potentially anti‐inflammatory for adipose tissue if available to exert physiological actions. Vitamin D might also be important for the formation of new adipocytes, and this has been reviewed in detail elsewhere (Dix et al. 2018). Peroxisome proliferator‐activated receptor gamma (PPARγ) is thought to be the ‘master regulator’ of adipogenesis, with differentiation maintained by concomitant expression of PPARγ and CCAAT enhancer‐binding protein α (Rosen et al. 2002). Study of human preadipocytes suggests 1,25(OH)2D promotes adipogenesis (Nimitphong et al. 2012). Narvaez et al. (2013) found similar effects in human mesenchymal progenitor cells (hMPCs), where 1,25(OH)2D increased expression of adipogenic marker genes including fatty acid synthase, fatty acid‐binding proteins and PPARγ.
In vitro studies provide intriguing insights into potential functions of vitamin D within adipose tissue itself. Unfortunately, very little is known about the amount/distribution of vitamin D metabolites and the actions of 1,25(OH)2D in adipose tissue in vivo. Furthermore, it is important to note that simple measurement of vitamin D3 or 25(OH)D in adipose biopsy samples does not reveal the extent to which active 1,25(OH)2D is available for biological activity.
The potentially positive effects of vitamin D on adipose tissue physiology and function present a very interesting avenue for further research. However, since vitamin D is likely sequestered in lipid droplets of adipocytes, it might only be expected to exert physiological effects within adipose once mobilised into the cytosol and/or interstitium. It is an intriguing paradox that, in individuals with obesity, vitamin D seems to be ‘trapped’ in the lipid droplets of adipocytes in very close proximity to sites that may potentially benefit if only it could be made bioavailable.
Physical activity/exercise and vitamin D status
Physical activity is a powerful stimulus for lipid mobilisation from adipose tissue (Thompson et al. 2012). It is therefore conceivable that vitamin D ‘trapped’ in adipocytes is mobilised (along with stored lipid) by physical activity. In support of this suggestion, association studies consistently report greater serum 25(OH)D concentrations in individuals who self‐report higher physical activity (Reinehr et al. 2007; Villareal et al. 2008; Chomistek et al. 2011; Mason et al. 2011; Gangloff et al. 2015; Chin et al. 2017). One study using objective measures of physical activity (hip‐based accelerometry) showed a trend for increased serum 25(OH)D at higher physical activity levels (Klenk et al. 2015). Such associations have often been attributed to confounding factors (e.g. active people spending more time outside and receiving additional sunlight exposure), but recent studies indicate that exercise may have a direct and causal effect on vitamin D status. Spoo et al. (2015) found a progressive increase in serum 25(OH)D in sled dogs performing prolonged endurance exercise across 8 days. Dietary vitamin D intake was controlled, there was no weight loss, and confounding from sunlight exposure was mitigated by the dogs’ thick fur coats (How et al. 1994). Furthermore, Sun et al. (2017) showed elevated serum 25(OH)D concentrations in lean humans in response to 30 minutes of cycling exercise at 70% (Fig. 2a,b). The increase in circulating 25(OH)D was observed immediately post‐exercise and persisted for 24 hours. Interestingly, the strongest positive predictor of increased 25(OH)D (incremental area under the curve) across 24 hours was higher body fat percentage (with no such relationship with fat‐free mass). More recently, the same group reported that 5 weeks of progressive endurance cycling exercise (30 minute bouts, three bouts/week, 60–75% ) during winter months prevented any reductions in serum 25(OH)D concentrations in a small group of elderly men (Sun et al. 2018) (Fig. 2c,d), independent from any changes in adipose tissue mass. Collectively, these recent studies indicate that exercise has a direct and causal effect on 25(OH)D concentrations – possibly through the mobilisation of adipose‐derived vitamin D and/or 25(OH)D.
Figure 2.
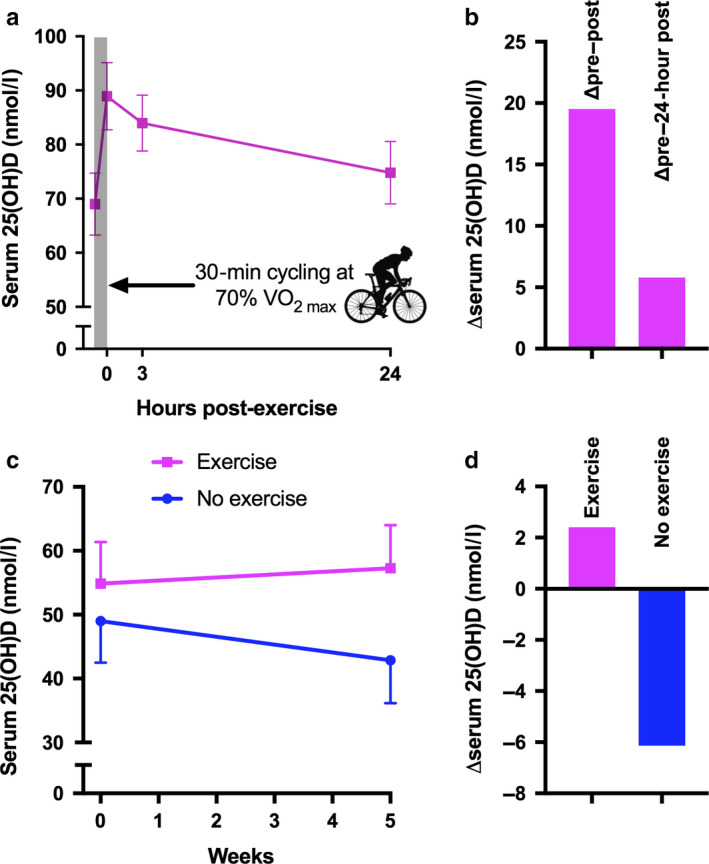
(a) The effect of 30 minutes of cycling exercise at 70% on serum 25(OH)D concentrations in humans [redrawn using data from Sun et al. (2017)]. Data shown are mean ± standard error of the mean (SEM) for men and women combined. (b) Mean change in serum 25(OH)D concentrations following 30 minutes of cycling exercise at 70% . Left‐hand bar represents change from pre‐exercise to immediately post‐exercise, right‐hand bar represents change from pre‐exercise to 24 hours post‐exercise [redrawn using data from Sun et al. (2017)]. (c) The effect of 5 weeks of progressive cycling endurance exercise (30 minute bouts, 3 bouts/week, from 60% to 75% ) during winter months on serum 25(OH)D [redrawn using data from Sun et al. (2018)]. Data shown are mean ±SEM. (d) Mean change in serum 25(OH)D concentrations following 5 weeks of progressive cycling endurance exercise [redrawn using data from Sun et al. (2018)]. [Colour figure can be viewed at wileyonlinelibrary.com]
Lipolysis as a key mechanism of vitamin D mobilisation with exercise?
During exercise, there is a rise in plasma glucagon, adrenaline and atrial natriuretic peptide (ANP) (Galbo et al. 1975; Bloom et al. 1976; Gyntelberg et al. 1977; Jezova et al. 1985; McMurray et al. 1987; Moro et al. 2007) and a decrease in plasma insulin (Hodgetts et al. 1991), concomitant with increased adipose tissue blood flow (Thompson et al. 2012). Glucagon, adrenaline and ANP are stimulatory lipolytic hormones (Arner et al. 1990; Perea et al. 1995; Moro et al. 2007) and suppression of insulin leads to a potent increase in lipolysis (Jensen et al. 1989). This leads to hydrolysis of triacylglycerol from the lipid droplet of adipocytes by the action of adipose triglyceride lipase (ATGL) (Jenkins et al. 2004; Villena et al. 2004; Zimmermann et al. 2004) and hormone‐sensitive lipase (HSL) (Vaughan et al. 1964). Exercise in the fasted or the fed state leads to an approximate twofold to threefold increase in adipose tissue lipolysis (Wolfe et al. 1990; Klein et al. 1994; Enevoldsen et al. 2004) and, when stored triacylglycerol is hydrolysed, vitamin D metabolites may also be released from the lipid droplet (Fig. 3).
Figure 3.
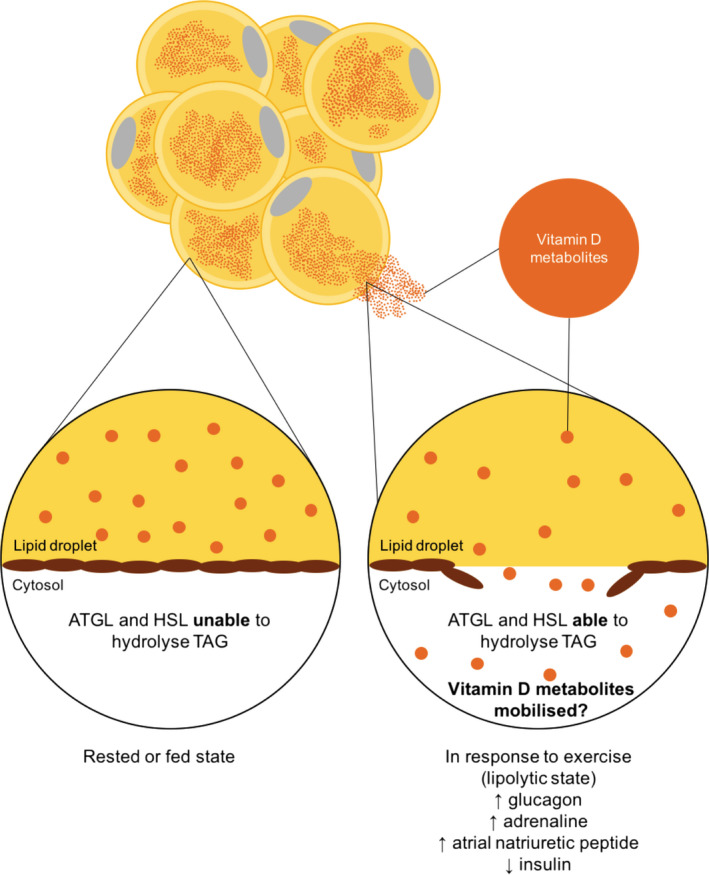
Vitamin D and the adipocyte. We hypothesise that in the rested or fed state triacylglycerol and vitamin D metabolites are trapped in the lipid droplet of adipocytes. When exposed to a lipolytic stimulus, triacylglycerol is liberated and this may coincide with mobilisation of vitamin D metabolites. ATGL, adipose triglyceride lipase; HSL, hormone‐sensitive lipase, TAG, triacylglycerol. [Colour figure can be viewed at wileyonlinelibrary.com]
The lipolytic response of adipose tissue to exercise is impaired in individuals with obesity. Ex vivo adipose tissue explants from individuals with obesity display reduced maximal lipolytic responses to adrenergic stimulation compared with adipose from individuals without obesity (Reynisdottir et al. 1994; Large et al. 1999; Hellstrom & Reynisdottir 2000), coinciding with decreased gene expression of ATGL and HSL (Large et al. 1999; Langin et al. 2005; Jocken et al. 2007; McQuaid et al. 2011). These findings are supported in vivo, as individuals with obesity display lower rates of lipolysis during exercise than lean controls (Stich et al. 2000; Mittendorfer et al. 2004). Whilst this may protect individuals with obesity from elevated circulating fatty acids (McQuaid et al. 2011), it may also contribute to sequestration of vitamin D. In support of this contention, adipose explants taken from individuals with obesity release less vitamin D when stimulated with lipolytic hormones than explants from lean controls (Di Nisio et al. 2017). In this study, mobilisation of vitamin D was proportional to both the lipolytic response (glycerol release) and protein expression of beta‐adrenergic receptors (Di Nisio et al. 2017); further supporting the notion that vitamin D mobilisation is inherently coupled to lipolysis.
Exercise may also target adipose tissue dysfunction in obesity. It is well established that an increase in physical activity chronically improves adipocyte function (Thompson et al. 2012). Exercise training increases sensitivity to various mediators such as insulin and adrenaline in overweight/obese adipose tissue (Thompson et al. 2012). It has also recently been shown that trained individuals exhibit greater protein content of lipolytic enzymes in subcutaneous abdominal adipose tissue (Bertholdt et al. 2018). Thus, in addition to an acute effect on vitamin D release associated with each exercise bout, regular exercise improves adipocyte function and the capacity to respond when stimulated, which may include the capacity to mobilise vitamin D in response to multiple stimuli (e.g. habitual physical activity, fasting and stress).
The effect of exercise on vitamin D mobilisation: The VitaDEx project
The UK Biotechnology and Biological Sciences Research Council (BBSRC) recently supported the VitaDEx project (‘Mobilising Vitamin D sequestered in adipose tissue in humans with Exercise’; running from October 2018 to October 2021). In VitaDEx, we will examine the impact of increased exercise on whole‐body and adipose tissue vitamin D metabolism, along with the pathways involved in vitamin D mobilisation from adipose tissue. We will use a 10‐week randomised controlled trial in overweight men and women to determine the impact of exercise (versus control) on vitamin D status and metabolism. Multiple vitamin D metabolites including vitamin D3, 25(OH)D3, 25(OH)D2, active vitamin D [1,25‐dihydroxyvitamin D, 1,25(OH)2D3] and other metabolites such as 24,25‐dihydroxyvitamin D [24,25(OH)2D3] and 3‐epi‐25(OH)D will be measured in serum using liquid chromatography–mass spectrometry (LC‐MS/MS) (Jenkinson et al. 2016). Turnover of 25(OH)D will be assessed using an established stable isotope technique (with measurement by LC‐MS/MS) (Jones et al. 2016; Walsh et al. 2016). We will determine whether the change in serum 25(OH)D has a demonstrable effect on functional measures of bioavailability by culturing monocytes from a single donor with media supplemented with participant serum to assess the impact of different serum vitamin D concentrations on monocyte function. In addition, measures in subcutaneous abdominal adipose biopsies and fluxes across adipose tissue (arteriovenous differences) will be combined to understand the impact of exercise on the capacity to mobilise vitamin D from adipose tissue. The acquired tissue samples will enable us to characterise the biological pathways and mechanisms that are involved in vitamin D mobilisation. In addition to the intervention study, we will include a lean comparator group to understand the independent effects of adiposity and contextualise the effects of exercise on vitamin D mobilisation, status and metabolism.
Variability in UV‐induced vitamin D synthesis is a potential risk during intervention studies and, therefore, we will structure the experimental work to take place during the winter months when there is little vitamin D synthesis at the latitude where the trial will be conducted (Webb et al. 1988). This restriction in time for experimental work necessitates a pragmatic approach to study design; thus, a 10‐week exercise intervention has been chosen to increase the available window of time for participants to commence the trial. A 10‐week exercise intervention is also long enough to impact on key measures of adipose tissue function (Thompson et al. 2012). Furthermore, we anticipate that serum 25(OH)D will decline during 10 weeks in winter in non‐exercising control participants, such that protective effects of an exercise intervention will be apparent over this timeframe. Notably, all exercise undertaken by the exercise group will occur indoors. Participants in the exercise group will perform cardiovascular exercise training four times per week, predominantly in the form of treadmill walking and cycling. Exercise duration will be progressive, at an intensity that will be personalised to participants, predominantly corresponding to maximal rates of fat oxidation.
Another potential risk during exercise interventions is variability in energy balance and weight loss due to varying degrees of dietary compensation (Turner et al. 2010), so we will compensate for the increase in energy expenditure with food prescribed to fully offset the energy expended during exercise (thus maintaining energy balance). Energy expenditure will be monitored during exercise to enable us to replace the energy expended with foods containing no vitamin D, and we will verify the adequacy of this energy replacement by monitoring changes in body composition via dual X‐ray absorptiometry (DEXA). Dietary intake will be recorded with a 3‐day weighed diet record in the week prior to, and during the final week of, the intervention, along with a retrospective food frequency questionnaire at both time points to more specifically capture dietary sources of vitamin D habitually consumed.
Conclusions
Vitamin D can accumulate in large amounts in adipose tissue where it may become sequestered. Preliminary evidence indicates that exercise may be a potential strategy to mobilise vitamin D from adipose tissue. We will examine this concept in a new research study (the VitaDEx project). This research will help us to understand the impact of exercise on vitamin D status and whether increasing physical activity represents a potentially useful strategy to mobilise vitamin D from adipose tissue. If exercise helps to mobilise vitamin D from adipose, then this could have important ramifications for practitioners and policymakers regarding the management of (i) low vitamin D status, (ii) obesity and associated conditions and (iii) low levels of physical activity. Current public health strategies typically approach vitamin D deficiency by increasing intake and/or synthesis of vitamin D (e.g. dietary supplementation or UV treatment). Notably, overweight/obesity reduces the impact of dietary supplementation with vitamin D on 25(OH)D concentrations (Arunabh et al. 2003; Snijder et al. 2005; Blum et al. 2008a; Beydoun et al. 2010) and the systemic 25(OH)D response to UV irradiation is significantly impaired (Wortsman et al. 2000). Thus, complementing conventional intake/synthesis strategies with techniques to mobilise endogenous vitamin D has the capacity to mutually enhance the overall effectiveness of interventions to improve vitamin D status.
Conflict of interest
The authors have no conflict of interest to disclose.
Author contribution
AH and DT conceptualised the work; AH and DT wrote the first draft; JTG provided initial intellectual insights; KSJ and MH provided further intellectual insights; all authors edited the manuscript and all authors agreed on the final version of the manuscript prior to submission.
Acknowledgements
This work is funded by a grant from BBSRC (BB/R018928/1). KSJ and AK are supported by the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre (IS‐BRC‐1215‐20014). The NIHR Cambridge Biomedical Research Centre is a partnership between Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge, funded by the NIHR. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
The copyright line for this article was changed on 19 March 2020 after original online publication.
References
- Adams JS & Hewison M (2012) Extrarenal expression of the 25‐hydroxyvitamin D‐1‐hydroxylase. Archives of Biochemistry and Biophysics 523: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P, Kriegholm E, Engfeldt P et al. (1990) Adrenergic regulation of lipolysis in situ at rest and during exercise. Journal of Clinical Investigation 85: 893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunabh S, Pollack S, Yeh J et al. (2003) Body fat content and 25‐hydroxyvitamin D levels in healthy women. The Journal of Clinical Endocrinology and Metabolism 88: 157–61. [DOI] [PubMed] [Google Scholar]
- Askew FA, Bourdillon RB, Bruce HM et al. (1930) The distillation of vitamin D. Proceedings of the Royal Society 107: 76–90. [Google Scholar]
- Baeuerle PA & Baltimore D (1988) IкB: a specific inhibitor of the NF‐кB transcription factor. Science 242: 540–6. [DOI] [PubMed] [Google Scholar]
- Baldwin AS (1996) The NF‐кB and IкB proteins: new discoveries and insights. Annual Review of Immunology 14: 649–83. [DOI] [PubMed] [Google Scholar]
- Bell NH, Shaw S & Turner RT (1984) Evidence that 1,25‐dihydroxyvitamin‐D‐3 inhibits the hepatic production of 25‐hydroxyvitamin‐D in man. Journal of Clinical Investigation 74: 1540–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell NH, Epstein S, Greene A et al. (1985) Evidence for alteration of the vitamin D endocrine system in obese subjects. Journal of Clinical Investigation 76: 370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholdt L, Gudiksen A, Stankiewicz T et al. (2018) Impact of training state on fasting‐induced regulation of adipose tissue metabolism in humans. Journal of Applied Physiology 124: 729–40. [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Boueiz A, Shroff MR et al. (2010) Associations among 25‐hydroxyvitamin D, diet quality, and metabolic disturbance differ by adiposity in adults in the United States. The Journal of Clinical Endocrinology and Metabolism 95: 3814–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Gee E, Halloran B et al. (1984) Free 1,25‐dihydroxyvitamin‐D levels in serum from normal subjects, pregnant subjects, and subjects with liver‐disease. Journal of Clinical Investigation 74: 1966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Siiteri PK, Ryzen E et al. (1985) Serum protein binding of 1,25‐dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. The Journal of Clinical Endocrinology & Metabolism 61: 969–75. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Gee E, Halloran B et al. (1986) Assessment of the free fraction of 25‐hydroxyvitamin‐D in serum and its regulation by albumin and the vitamin‐D‐binding protein. The Journal of Clinical Endocrinology & Metabolism 63: 954–9. [DOI] [PubMed] [Google Scholar]
- Bikle D, Bouillon R, Thadhani R et al. (2017) Vitamin D metabolites in captivity? Should we measure free or total 25(OH)D to assess vitamin D status? The Journal of Steroid Biochemistry and Molecular Biology 173: 105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom SR, Johnson RH, Park DM et al. (1976) Differences in the metabolic and hormonal response to exercise between racing cyclists and untrained individuals. The Journal of Physiology 258: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M, Dallal GE & Dawson‐Hughes B (2008a) Body size and serum 25 hydroxy vitamin D response to oral supplements in healthy older adults. Journal of the American College of Nutrition 27: 274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M, Dolnikowski G, Seyoum E et al. (2008b) Vitamin D‐3 in fat tissue. Endocrine 33: 90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunt JW & Deluca HF (1969) The synthesis of 25‐hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry 8: 671–5. [DOI] [PubMed] [Google Scholar]
- Blunt JW, Deluca HF & Schnoes HK (1968) 25‐hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry 7: 3317–22. [DOI] [PubMed] [Google Scholar]
- Bouillon R, Lieben L, Mathieu C et al. (2013) Vitamin D action: lessons from VDR and Cyp27b1 null mice. Pediatric Endocrinology Reviews 10 (Suppl 2): 354–66. [PubMed] [Google Scholar]
- Bourlier V, Zakaroff‐Girard A, Miranville A et al. (2008) Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation 117: 806–15. [DOI] [PubMed] [Google Scholar]
- Camozzi V, Frigo AC, Zaninotto M et al. (2016) 25‐Hydroxycholecalciferol response to single oral cholecalciferol loading in the normal weight, overweight, and obese. Osteoporosis International 27: 2593–602. [DOI] [PubMed] [Google Scholar]
- Carrelli A, Bucovsky M, Horst R et al. (2017) Vitamin D storage in adipose tissue of obese and normal weight women. Journal of Bone and Mineral Research 32: 237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman KD, Dowling KG, Skrabakova Z et al. (2016) Vitamin D deficiency in Europe: pandemic? The American Journal of Clinical Nutrition 103: 1033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K, Zhao D, Tibuakuu M et al. (2017) Physical activity, vitamin D, and incident atherosclerotic cardiovascular disease in whites and blacks: the ARIC study. The Journal of Clinical Endocrinology and Metabolism 102: 1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomistek AK, Chiuve SE, Jensen MK et al. (2011) Vigorous physical activity, mediating biomarkers, and risk of myocardial infarction. Medicine & Science in Sports & Exercise 43: 1884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun RF, Peercy BE, Orwoll ES et al. (2014) Vitamin D and DBP: the free hormone hypothesis revisited. The Journal of Steroid Biochemistry and Molecular Biology 144: 132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close GL, Russell J, Cobley JN et al. (2013) Assessment of vitamin D concentration in non‐supplemented professional athletes and healthy adults during the winter months in the UK: implications for skeletal muscle function. Journal of Sports Sciences 31: 344–53. [DOI] [PubMed] [Google Scholar]
- Compston JE, Vedi S, Ledger JE et al. (1981) Vitamin D status and bone histomorphometry in gross obesity. The American Journal of Clinical Nutrition 34: 2359–63. [DOI] [PubMed] [Google Scholar]
- Daiger SP, Schanfield MS & Cavallisforza LL (1975) Group‐specific component (Gc) proteins bind vitamin D and 25‐hydroxyvitamin D. Proceedings of the National Academy of Sciences of the United States of America 72: 2076–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nisio A, De Toni L, Sabovic I et al. (2017) Impaired release of vitamin D in dysfunctional adipose tissue: new cues on vitamin D supplementation in obesity. The Journal of Clinical Endocrinology and Metabolism 102: 2564–74. [DOI] [PubMed] [Google Scholar]
- Didriksen A, Burild A, Jakobsen J et al. (2015) Vitamin D‐3 increases in abdominal subcutaneous fat tissue after supplementation with vitamin D‐3. European Journal of Endocrinology 172: 235–41. [DOI] [PubMed] [Google Scholar]
- Dix CF, Barcley JL & Wright ORL (2018) The role of vitamin D in adipogenesis. Nutrition Reviews 76: 47–59. [DOI] [PubMed] [Google Scholar]
- Dobnig H, Pilz S, Scharnagl H et al. (2008) Independent association of low serum 25‐hydroxyvitamin d and 1,25‐dihydroxyvitamin d levels with all‐cause and cardiovascular mortality. Archives of Internal Medicine 168: 1340–9. [DOI] [PubMed] [Google Scholar]
- Drincic AT, Armas LAG, Van Diest EE et al. (2012) Volumetric Dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity 20: 1444–8. [DOI] [PubMed] [Google Scholar]
- Enevoldsen LH, Simonsen L, Macdonald IA et al. (2004) The combined effects of exercise and food intake on adipose tissue and splanchnic metabolism. The Journal of Physiology 561: 871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbo H, Holst JJ & Christensen NJ (1975) Glucagon and plasma catecholamine responses to graded exercise and prolonged exercise in man. Journal of Applied Physiology 38: 70–6. [DOI] [PubMed] [Google Scholar]
- Gangloff A, Bergeron J, Pelletier‐Beaumont E et al. (2015) Effect of adipose tissue volume loss on circulating 25‐hydroxyvitamin D levels: results from a 1‐year lifestyle intervention in viscerally obese men. International Journal of Obesity 39: 1638–43. [DOI] [PubMed] [Google Scholar]
- Ginde AA, Scragg R, Schwartz RS et al. (2009) Prospective study of serum 25‐hydroxyvitamin D level, cardiovascular disease mortality, and all‐cause mortality in older U.S. adults. Journal of the American Geriatrics Society 57: 1595–603. [DOI] [PubMed] [Google Scholar]
- Gyntelberg F, Rennie MJ, Hickson RC et al. (1977) Effect of training on the response of plasma glucagon to exercise. Journal of Applied Physiology 43: 302–5. [DOI] [PubMed] [Google Scholar]
- Haddad JG, Matsuoka LY, Hollis BW et al. (1993) Human plasma transport of vitamin D after its endogenous synthesis. Journal of Clinical Investigation 91: 2552–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler MR, Whitfield GK, Kaneko I et al. (2013) Molecular mechanisms of vitamin D action. Calcified Tissue International 92: 77–98. [DOI] [PubMed] [Google Scholar]
- Heller JE, Thomas JJ, Hollis BW et al. (2015) Relation between vitamin D status and body composition in collegiate athletes. International Journal of Sport Nutrition and Exercise Metabolism 25: 128–35. [DOI] [PubMed] [Google Scholar]
- Hellstrom L & Reynisdottir S (2000) Influence of heredity for obesity on adipocyte lipolysis in lean and obese subjects. International Journal of Obesity and Related Metabolic Disorders 24: 340–4. [DOI] [PubMed] [Google Scholar]
- Hodgetts V, Coppack SW, Frayn KN et al. (1991) Factors controlling fat mobilization from human subcutaneous adipose tissue during exercise. Journal of Applied Physiology 71: 445–51. [DOI] [PubMed] [Google Scholar]
- Holick MF (2007) Vitamin D deficiency. The New England Journal of Medicine 357: 266–81. [DOI] [PubMed] [Google Scholar]
- How KL, Hazewinkel HA & Mol JA (1994) Dietary vitamin D dependence of cat and dog due to inadequate cutaneous synthesis of vitamin D. General and Comparative Endocrinology 96: 12–8. [DOI] [PubMed] [Google Scholar]
- Jenkins CM, Mancuso DJ, Yan W et al. (2004) Identification, cloning, expression, and purification of three novel human calcium‐independent phospholipase A(2) family members possessing triacylglycerol lipase and acylglycerol transacylase activities. Journal of Biological Chemistry 279: 48968–75. [DOI] [PubMed] [Google Scholar]
- Jenkinson C, Taylor AE, Hassan‐Smith ZK et al. (2016) High throughput LC‐MS/MS method for the simultaneous analysis of multiple vitamin D analytes in serum. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences 1014: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD, Caruso M, Heiling V et al. (1989) Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes 38: 1595–601. [DOI] [PubMed] [Google Scholar]
- Jezova D, Vigas M, Tatar P et al. (1985) Plasma testosterone and catecholamine responses to physical exercise of different intensities in man. European Journal of Applied Physiology and Occupational Physiology 54: 62–6. [DOI] [PubMed] [Google Scholar]
- Jocken JW, Langin D, Smit E et al. (2007) Adipose triglyceride lipase and hormone‐sensitive lipase protein expression is decreased in the obese insulin‐resistant state. The Journal of Clinical Endocrinology & Metabolism 92: 2292–9. [DOI] [PubMed] [Google Scholar]
- Jones KS, Assar S, Vanderschueren D et al. (2015) Predictors of 25(OH)D half‐life and plasma 25(OH)D concentration in The Gambia and the UK. Osteoporosis International 26: 1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KS, Assar S, Prentice A et al. (2016) Vitamin D expenditure is not altered in pregnancy and lactation despite changes in vitamin D metabolite concentrations. Scientific Reports 6: 26795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Coyle EF & Wolfe RR (1994) Fat metabolism during low‐intensity exercise in endurance‐trained and untrained men. American Journal of Physiology 267: E934–40. [DOI] [PubMed] [Google Scholar]
- Klenk J, Rapp K, Denkinger M et al. (2015) Objectively measured physical activity and vitamin D status in older people from Germany. Journal of Epidemiology and Community Health 69: 388–92. [DOI] [PubMed] [Google Scholar]
- Langin D, Dicker A, Tavernier G et al. (2005) Adipocyte lipases and defect of lipolysis in human obesity. Diabetes 54: 3190–7. [DOI] [PubMed] [Google Scholar]
- Large V, Reynisdottir S, Langin D et al. (1999) Decreased expression and function of adipocyte hormone‐sensitive lipase in subcutaneous fat cells of obese subjects. Journal of Lipid Research 40: 2059–66. [PubMed] [Google Scholar]
- Lawson DEM, Douglas J, Lean M et al. (1986) Estimation of vitamin D3 and 25‐hydroxyvitamin D3 in muscle and adipose tissue of rats and man. Clinica Chimica Acta 157: 175–81. [DOI] [PubMed] [Google Scholar]
- Le Goff C, Cavalier E, Souberbielle JC et al. (2015) Measurement of circulating 25‐hydroxyvitamin D: a historical review. Practical Laboratory Medicine 2: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liel Y, Ulmer E, Shary J et al. (1988) Low circulating vitamin D in obesity. Calcified Tissue International 43: 199–201. [DOI] [PubMed] [Google Scholar]
- Lips P (2007) Relative value of 25(OH)D and 1,25(OH)2D measurements. Journal of Bone and Mineral Research 22: 1668–71. [DOI] [PubMed] [Google Scholar]
- Maclaughlin JA, Anderson RR & Holick MF (1982) Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science 216: 1001–3. [DOI] [PubMed] [Google Scholar]
- Manolopoulos KN, Karpe F & Frayn KN (2010) Gluteofemoral body fat as a determinant of metabolic health. International Journal of Obesity 34: 949–59. [DOI] [PubMed] [Google Scholar]
- Martinaityte I, Kamycheva E, Didriksen A et al. (2017) Vitamin D stored in fat tissue during a 5‐year intervention affects serum 25‐hydroxyvitamin D levels the following year. The Journal of Clinical Endocrinology & Metabolism 102: 3731–8. [DOI] [PubMed] [Google Scholar]
- Mason C, Xiao L, Imayama I et al. (2011) Effects of weight loss on serum vitamin D in postmenopausal women. The American Journal of Clinical Nutrition 94: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury E & Brichard SM (2010) Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Molecular and Cellular Endocrinology 314: 1–16. [DOI] [PubMed] [Google Scholar]
- Mawer EB, Stanbury SW, Backhous J et al. (1972) The distribution and storage of vitamin D and its metabolites in human tissues. Clinical Science 43: 413–31. [DOI] [PubMed] [Google Scholar]
- McMurray RG, Forsythe WA, Mar MH et al. (1987) Exercise intensity‐related responses of β‐endorphin and catecholamines. Medicine and Science in Sports and Exercise 19: 570–4. [PubMed] [Google Scholar]
- McQuaid SE, Hodson L, Neville MJ et al. (2011) Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes 60: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlotti C, Ceriani V, Morabito A et al. (2017) Subcutaneous fat loss is greater than visceral fat loss with diet and exercise, weight‐loss promoting drugs and bariatric surgery: a critical review and meta‐analysis. International Journal of Obesity 41: 672–82. [DOI] [PubMed] [Google Scholar]
- Mittendorfer B, Fields DA & Klein S (2004) Excess body fat in men decreases plasma fatty acid availability and oxidation during endurance exercise. American Journal of Physiology‐Endocrinology and Metabolism 286: E354–62. [DOI] [PubMed] [Google Scholar]
- Moro C, Pillard F, de Glisezinski I et al. (2007) Sex differences in lipolysis‐regulating mechanisms in overweight subjects: effect of exercise intensity. Obesity 15: 2245–55. [DOI] [PubMed] [Google Scholar]
- Mutt SJ, Karhu T, Lehtonen S et al. (2012) Inhibition of cytokine secretion from adipocytes by 1,25‐dihydroxyvitamin D(3) via the NF‐kappaB pathway. The FASEB Journal 26: 4400–7. [DOI] [PubMed] [Google Scholar]
- Narvaez CJ, Simmons KM, Brunton J et al. (2013) Induction of STEAP4 correlates with 1,25‐dihydroxyvitamin D3 stimulation of adipogenesis in mesenchymal progenitor cells derived from human adipose tissue. Journal of Cellular Physiology 228: 2024–36. [DOI] [PubMed] [Google Scholar]
- Nimitphong H, Holick MF, Fried SK et al. (2012) 25‐hydroxyvitamin D‐3 and 1,25‐dihydroxyvitamin D‐3 promote the differentiation of human subcutaneous preadipocytes. PLoS ONE 7: e52171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios C & Gonzalez L (2014) Is vitamin D deficiency a major global public health problem? The Journal of Steroid Biochemistry and Molecular Biology 144: 138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh SJ, Edelman M, Uwaifo GI et al. (2004) The relationship between obesity and serum 1,25‐dihydroxy vitamin D concentrations in healthy adults. The Journal of Clinical Endocrinology & Metabolism 89: 1196–9. [DOI] [PubMed] [Google Scholar]
- Perea A, Clemente F, Martinell J et al. (1995) Physiological effect of glucagon in human isolated adipocytes. Hormone and Metabolic Research 27: 372–5. [DOI] [PubMed] [Google Scholar]
- Pereira‐Santos M, Costa PR, Assis AM et al. (2015) Obesity and vitamin D deficiency: a systematic review and meta‐analysis. Obesity Reviews 16: 341–9. [DOI] [PubMed] [Google Scholar]
- Piccolo BD, Dolnikowski G, Seyoum E et al. (2013) Association between subcutaneous white adipose tissue and serum 25‐hydroxyvitamin D in overweight and obese adults. Nutrients 5: 3352–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramyothin P, Biancuzzo RM, Lu Z et al. (2011) Vitamin D in adipose tissue and serum 25‐hydroxyvitamin D after Roux‐en‐Y Gastric Bypass. Obesity 19: 2228–34. [DOI] [PubMed] [Google Scholar]
- Reinehr T, de Sousa G, Alexy U et al. (2007) Vitamin D status and parathyroid hormone in obese children before and after weight loss. European Journal of Endocrinology 157: 225–32. [DOI] [PubMed] [Google Scholar]
- Reynisdottir S, Ellerfeldt K, Wahrenberg H et al. (1994) Multiple lipolysis defects in the insulin resistance (metabolic) syndrome. Journal of Clinical Investigation 93: 2590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Hsu CH, Wang XZ et al. (2002) C/EBP alpha induces adipogenesis through PPAR gamma: a unified pathway. Genes & Development 16: 22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich SJ, Rich C & Volwiler W (1971) Deposition in and release of vitamin D3 from body fat: evidence for a storage site in the rat. Journal of Clinical Investigation 50: 679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Dagnone D, Jones PJ et al. (2000) Reduction in obesity and related comorbid conditions after diet‐induced weight loss or exercise‐induced weight loss in men. a randomized, controlled trial. Annals of Internal Medicine 133: 92–103. [DOI] [PubMed] [Google Scholar]
- SACN (Scientific Advisory Committee on Nutrition) (2016) Vitamin D and Health. SACN: London, UK. [Google Scholar]
- Safadi FF, Thornton P, Magiera H et al. (1999) Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. Journal of Clinical Investigation 103: 239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Houston DK, Bandinelli S et al. (2010) Relationship of 25‐hydroxyvitamin D with all‐cause and cardiovascular disease mortality in older community‐dwelling adults. European Journal of Clinical Nutrition 64: 203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder MB, van Dam RM, Visser M et al. (2005) Adiposity in relation to vitamin D status and parathyroid hormone levels: a population‐based study in older men and women. The Journal of Clinical Endocrinology & Metabolism 90: 4119–23. [DOI] [PubMed] [Google Scholar]
- Spoo JW, Downey RL, Griffitts C et al. (2015) Plasma vitamin D metabolites and C‐reactive protein in stage‐stop racing endurance sled dogs. Journal of Veterinary Internal Medicine 29: 519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stich V, De Glisezinski I, Crampes F et al. (2000) Activation of alpha(2)‐adrenergic receptors impairs exercise‐induced lipolysis in SCAT of obese subjects. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology 279: R499–504. [DOI] [PubMed] [Google Scholar]
- Sun X, Cao ZB, Taniguchi H et al. (2017) Effect of an acute bout of endurance exercise on serum 25(OH)D concentrations in young adults. The Journal of Clinical Endocrinology & Metabolism 102: 3937–44. [DOI] [PubMed] [Google Scholar]
- Sun X, Cao ZB, Tanisawa K et al. (2018) Effects of chronic endurance exercise training on serum 25(OH)D concentrations in elderly Japanese men. Endocrine 59: 330–7. [DOI] [PubMed] [Google Scholar]
- Thompson D, Karpe F, Lafontan M et al. (2012) Physical activity and exercise in the regulation of human adipose tissue physiology. Physiological Reviews 92: 157–91. [DOI] [PubMed] [Google Scholar]
- Trim W, Turner JE & Thompson D (2018) Parallels in immunometabolic adipose tissue dysfunction with ageing and obesity. Frontiers in Immunology 9: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JE, Markovitch D, Betts JA et al. (2010) Nonprescribed physical activity energy expenditure is maintained with structured exercise and implicates a compensatory increase in energy intake. The American Journal of Clinical Nutrition 92: 1009–16. [DOI] [PubMed] [Google Scholar]
- Vaughan M, Berger JE & Steinberg D (1964) Hormone‐sensitive lipase and monoglyceride lipase activities in adipose tissue. Journal of Biological Chemistry 239: 401–9. [PubMed] [Google Scholar]
- Villareal DT, Shah K, Banks MR et al. (2008) Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: a one‐year randomized controlled trial. The Journal of Clinical Endocrinology & Metabolism 93: 2181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena JA, Roy S, Sarkadi‐Nagy E et al. (2004) Desnutrin, an adipocyte gene encoding a novel patatin domain‐containing protein, is induced by fasting and glucocorticoids. Journal of Biological Chemistry 279: 47066–75. [DOI] [PubMed] [Google Scholar]
- Vimaleswaran KS, Berry DJ, Lu C et al. (2013) Causal relationship between obesity and vitamin D status: bi‐directional mendelian randomization analysis of multiple cohorts. PLOS Medicine 10: e1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JS, Evans AL, Bowles S et al. (2016) Free 25‐hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. The American Journal of Clinical Nutrition 103: 1465–71. [DOI] [PubMed] [Google Scholar]
- Wamberg L, Christiansen T, Paulsen SK et al. (2013) Expression of vitamin D‐metabolizing enzymes in human adipose tissue‐the effect of obesity and diet‐induced weight loss. International Journal of Obesity 37: 651–7. [DOI] [PubMed] [Google Scholar]
- Webb AR, Kline L & Holick MF (1988) Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. The Journal of Clinical Endocrinology & Metabolism 67: 373–8. [DOI] [PubMed] [Google Scholar]
- Weisman Y, Harell A, Edelstein S et al. (1979) 1 alpha, 25‐Dihydroxyvitamin D3 and 24,25‐dihydroxyvitamin D3 in vitro synthesis by human decidua and placenta. Nature 281: 317–9. [DOI] [PubMed] [Google Scholar]
- Windaus A, Schenck FR & Fv Werder (1936) The anti‐rachitically active irradiation product from 7‐dehydro‐cholesterol. Hoppe‐Seyler's Zeitschrift fur physiologische Chemie 241: 100–3. [Google Scholar]
- Wolfe RR, Klein S, Carraro F et al. (1990) Role of triglyceride‐fatty acid cycle in controlling fat metabolism in humans during and after exercise. American Journal of Physiology 258: E382–9. [DOI] [PubMed] [Google Scholar]
- Wortsman J, Matsuoka LY, Chen TC et al. (2000) Decreased bioavailability of vitamin D in obesity. The American Journal of Clinical Nutrition 72: 690–3. [DOI] [PubMed] [Google Scholar]
- Zimmermann R, Strauss JG, Haemmerle G et al. (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306: 1383–6. [DOI] [PubMed] [Google Scholar]


