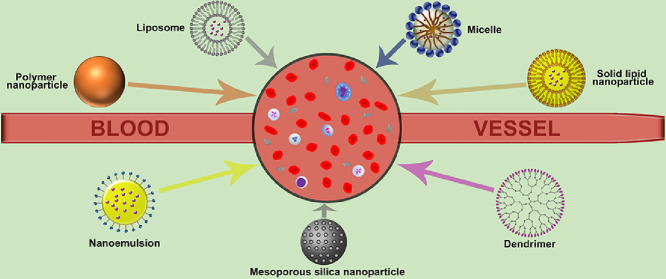
Abstract
This article discusses the various blood interactions that may occur with various types of nano drug-loading systems. Nanoparticles enter the blood circulation as foreign objects. On the one hand, they may cause a series of inflammatory reactions and immune reactions, resulting in the rapid elimination of immune cells and the reticuloendothelial system, affecting their durability in the blood circulation. On the other hand, the premise of the drug-carrying system to play a therapeutic role depends on whether they cause coagulation and platelet activation, the absence of hemolysis and the elimination of immune cells. For different forms of nano drug-carrying systems, we can find the characteristics, elements and coping strategies of adverse blood reactions that we can find in previous researches. These adverse reactions may include destruction of blood cells, abnormal coagulation system, abnormal effects of plasma proteins, abnormal blood cell behavior, adverse immune and inflammatory reactions, and excessive vascular stimulation. In order to provide help for future research and formulation work on the blood compatibility of nano drug carriers.
Keywords: Nano-drug delivery system, Hemocompatibility, Adverse interaction, Improvement strategy
Graphical abstract
1. Introduction
In the past few decades, drug-loaded nanoparticles have developed rapidly in different fields of industry, technology and medicine. Nanostructured systems have been successfully used in the pharmaceutical industry because it can effectively separate several groups of therapeutic agents and change the characteristics and behavior of active substances in biological media. Research involving nanoparticle systems applied to drugs has played an important role in obtaining new or enhanced diagnostic and therapeutic methods for health promotion. Nano formulations can be administered through systemic routes, or be used as a platform, imaging agents, and gene delivery purposes. At the same time, the advent of nano drug-loaded systems has promoted novel research on synthetic methods and bio-conjugation technologies. Various studies on the changes in nanoparticles (NPs) structure, shape, chemical properties, and degradation rate quickly spread to scientific publications.
The development of nanocarriers for drug delivery devices has made considerable progress. However, questions about the biological application of these systems have not been fully answered. One of the main issues related to the safety and effectiveness of such delivery platforms is blood compatibility. Given the size of NPs, the blood compatibility may be lower due to their higher surface area/volume ratio. NPs should simultaneously activate platelets, hemolysis, coagulation factors, or leukocyte signaling molecules, and should not cause endothelial cell damage [1]. These events are related to the occurrence of thrombosis, blood rheology, hemolysis and inflammatory events.
In addition, the respiratory system, skin, and gastrointestinal tract are common ways for NPs to enter the body during daily exposure, while intravenous injection is the most common way for most NPs used in biomedicine. In all cases, the NPs will reach the blood and interact with blood components. If damage occurs between circulating blood cells, materials that can release drug compounds on demand are neither safe nor effective. The harmful effects of blood incompatible systems may include bruising at the injection site, immunogenic reactions and embolism, which may lead to infarction and stroke.
Despite NPs exist great scientific attractiveness and potential, the safety is still an issue of increasing concern. In this review, we will combine previous studies and focus on the issue of blood compatibility. Discuss the possible interactions and adverse reactions of various forms of nano drug delivery systems after entering the blood circulation as foreign. Summarize the relationship between these events and their own structures and properties, and what strategies we can usually adopt to improve its blood compatibility.
2. Possible evaluation and methods
2.1. Hemolysis assess
Hemolysis refers to a phenomenon in which red blood cells (RBCs) rupture and dissolve. The mechanism of hemolytic reaction is complicated. There is no standard preclinical in vivo test method to comprehensively evaluate the hemolytic reaction of pharmaceutical preparations. Therefore, hemolysis of the preparation should be considered in toxicity studies. Hemolysis is the most common blood problem that nano preparations may cause, so hemolysis evaluation is widely used in the research of various types of nano preparations. The hemolysis test is used to study the degree of RBCs destruction induced by the preparation, and is performed by estimating the amount of hemoglobin released after RBCs damage. This hemoglobin is then converted to oxyhemoglobin in the presence of atmospheric oxygen. Oxygenated hemoglobin can be detected and measured spectrophoto metrically. Despite UV spectrophotometry is easily affected by centrifugation temperature, speed, auxiliary materials, and additives, it is still the most commonly used method because of its precise and convenient operation.
Preparation of rat erythrocyte sample: Take the whole fresh rat blood in a centrifuge tube coated with heparin, put it in a triangular flask containing glass beads and shake for 10 min. Remove fibrinogen. 10 times 0.9% sodium chloride solution is added. Shake well and centrifuge at 3000 rpm for 10 min. Discard the upper plasma and then wash the precipitated RBCs with 0.9% sodium chloride solution 2 to 3 times to the supernatant. The resulting RBCs are formulated into a 2% suspension with 0.9% sodium chloride solution [2].
Human RBCs sample preparation: Add 10 ml of fresh blood from a healthy blood donor to an EDTA tube to prevent clotting, and centrifuge at 500 × g for 5 min to separate plasma from human RBCs. The plasma supernatant is removed, and the RBCs are washed 3 times with PBS. Resuspended RBCs in 1 ml PBS to a concentration of 20% (v/v) [3].
Preparation is incubated with RBCs at 37 °C for 1 h. After centrifugation, the supernatant is transferred to a transparent flat-bottom 96-well plate. The absorbance is measured at 540 nm. Generally, sterile filtered PBS (pH 7.4) and 1% Triton X-100 are used as negative and positive controls, respectively. The absorbance value of Triton X-100 is considered to be 100% hemolysis. Percent hemolysis is calculated according to the following formula.
Where ODtreatment, ODPBS and ODTriton X 100 are the optical densities of treatment groups, PBS and Triton X-100, respectively.
Although different types of hemolysis have different thresholds (approximately 10% for humans, 10%–29% for dogs, and 0–37% for rabbits), 10% and 25% of hemolysis are generally considered to be relative limits (<10% is considered non-hemolytic, and >25% are considered hemolytic) [4]. On the other hand, scanning electron microscopy (SEM) is often used to observe RBCs after incubation with ranks to determine whether their morphology has changed.
2.2. Plasma clotting assess
Routine plasma clotting includes prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT), and plasma fibrinogen (Fib). PT mainly reflects the status of exogenous coagulation system. APTT mainly reflects the status of endogenous coagulation system. TT mainly reflects the time when fibrinogen is converted into fibrin. Fib mainly reflects the content of fibrinogen. Among them, the values of PT and APTT are most commonly used to evaluate the effect of the preparation on blood coagulation [5]. The measurement method details can refer to the literature.
The blood is centrifuged at 1344 × g for 15 min and stored at 2–8 °C for 1 d After adding preparations to plasma, APTT and PT are measured and compared with the normal group containing normal saline of equivalent concentration. The sample is stirred at 37 °C for 1 h. To determine APTT, add 100 µl of LIQUICELIN-E to a sample of 100 l of citric acid plasma and keep at 37 °C for 3–5 min. After the end of the incubation period, add 100 µl of preheated calcium chloride solution to the mixture and keep it at 37 °C for 10–15 s. Record the appearance of the gel-like clot. To determine PT, 200 µl of PT reagent is added to 200 µl of citric acid plasma at 37 °C and maintained at 37 °C for 10–15 s. After the end of the incubation period, the samples are checked for coagulation/gel formation. Record the time. The process of recording time will be faster, more accurate and standardized by using a blood coagulometer for quantitative analysis.
2.3. Platelet aggregation test
Platelets in the human blood circulation are scattered, and the adhesion of platelets is called platelet aggregation [1]. Preparations intended for intravenous administration should not cause platelet aggregation. The citrated whole blood is incubated with the NPs. PBS in equal proportion to the preparation is used for spontaneous control. The samples are incubated with whole blood at 37 °C (400 rpm) for 30 min, and a blood smear of each sample is prepared on a slide. After air-drying, stain with Leishman stain, and then rinse. The dried smear is analyzed by an optical microscope in an immersion objective. Before and after the preparation is added, the platelet aggregation in the sample in the whole blood is evaluated by using a hematology counter.
2.4. Complement activation test
Complement is a serum protein that exists in the serum and tissue fluid of humans and vertebrates. It has enzyme activity after activation, which can mediate immune response and inflammatory response. The complement system plays a key role in triggering acute allergic reactions, which may cause inflammatory processes and life-threatening consequences. Although the mechanism has not yet been elucidated, when specific nanodrug carriers are injected into the blood, complement activation may occur. It is necessary to evaluate complement activation for special formulations. The evaluation of complement activation usually uses a kit method to quantitatively analyze the generation of fragments through the ELISA.
2.5. Erythrocyte agglutination test
RBCs agglutination refers to the phenomenon of RBCs agglutination. Some special properties of nano preparations may cause erythrocyte agglutination, which needs to be evaluated [6]. The citric acid blood should centrifuge at 1000 rpm for 10 min at room temperature. 100 µl of the precipitate is mixed with 10 ml of PBS (pH 7.4) and centrifuged again at 1000 rpm for 10 min. Finally, 100 µl of the pellet is mixed with 10 ml of PBS for measurement. After incubating erythrocytes with preparation samples on a 96′U-shaped plate for 2 h, a button-like formation is visually observed. The formation of "button-like" structures confirms their compatibility with hemoglobin at the lowest dose of drug content. Several factors (quality of erythrocytes, laboratory temperature, laboratory equipment, technical expertise of the user) may contribute to slight differences in the interpretation of the test each time it is run.
2.6. Lactate dehydrogenase release from neutrophils
In addition to RBCs, neutrophils are also the main cells of the circulatory system, and the destruction of neutrophils by the preparation sometimes needs to be considered [7]. The total lactate dehydrogenase (LDH) released is determined by lysing neutrophils with 0.1% Triton X-100 at 37 °C for 30 min. NPs are added to the cell culture medium, and then centrifuged. Add substrate assay buffer to the supernatant. After incubating for 30 min, stop solution is added. The sample is measured at 492 nm with a spectrophotometer. Similar to the hemolysis test, the influence of centrifugation temperature, speed, auxiliary materials and additives also need to be considered.
2.7. Platelet secretion assay
In some cases, the effect of nano formulations on platelet secretion should also be investigated [8]. The platelet secretion is determined by measuring the release of ATP using a luciferin/luciferase reagent. 2 min before stimulation, luciferin/luciferase is added to 480 µl of platelet suspension. Pre-incubate the platelets with the preparation and physiological saline for 2 min, and then add ADP with agitation (1000 rpm). Record the platelet secretion in a real-time agglutination instrument at 37 °C. Determine whether the platelet secretion is suppressed.
2.8. Inflammatory cytokine secretion
Inflammatory cytokines refer to various cytokines involved in the inflammatory response. When the nano drug-loading system enters the blood, the secretion of inflammatory cytokines may change. Among the many inflammatory cytokines, they play a great role of TNF-α, IL-1β, IL-6, IL-8, etc. [9]. The evaluation of inflammatory cytokine secretion is usually performed by analyzing its secretion of macrophages by ELISA.
2.9. Serum stability assess
The adsorption of serum proteins on NPs in human serum is one of the main challenges of parenteral drug delivery using nano drug-loading systems. When incubated with serum, nanocarriers may adsorb these proteins and form aggregates. Understanding the interaction of NPs with proteins in plasma is an important factor that helps to promote the application of NPs in the field of biomedicine and reduce/prevent possible adverse effects on biological systems. The internalization and biodistribution of particles may be greatly influenced by specific proteins bound to the particles. At the same time, the structure and function of the protein will be affected by the adsorbed NPs, so the overall bioreactivity of the particles will be changed [10]. Therefore, sometimes it is necessary to ensure that there is no interaction between plasma proteins and materials.
The adsorbed proteins evaluation is difficult because they are always related to the substrate. The choice of analysis method depends on the type of substrate used and the amount of adsorbed protein [11]. Plasma is separated by centrifuging fresh blood (containing anticoagulant, 3.8% sodium citrate) at 750 rpm. The preparation is incubated with 200 µl of plasma for 60 min. The samples are centrifuged at 10 000 rpm for 10 min, and then these separated proteins are subjected to discontinuous natural PAGE analysis. Load 200 µl of supernatant into 8% resolution and 4% stacking gel. Electrophoresis is carried out using an electric swimming pool at 100 V for 90 min. The gel is stained with Coomassie blue, and the image of the gel is recorded using an image analyzer. It is judged that after incubation with human plasma, there is no specific protein binding on the surface of NPs.
2.10. Assessment of blood vessel irritation
Vascular irritation is another safety assessment of nanocarrier injections administered in vivo. Rabbits are always used for the evaluation [12]. Preparation samples are injected into the right ear vein, and an equal volume of saline is injected into the left ear vein as a control. 48 h after the last dose, the rabbits are sacrificed by bleeding, then a piece of vascular tissue is cut at the injection site, fixed in 4% paraformaldehyde, and embedded in paraffin for histopathological examination. Check for discoloration, thrombosis, and tissue edema.
3. Hemocompatibility characteristics or improvement strategies of various nanocarriers
3.1. Polymer nanoparticles
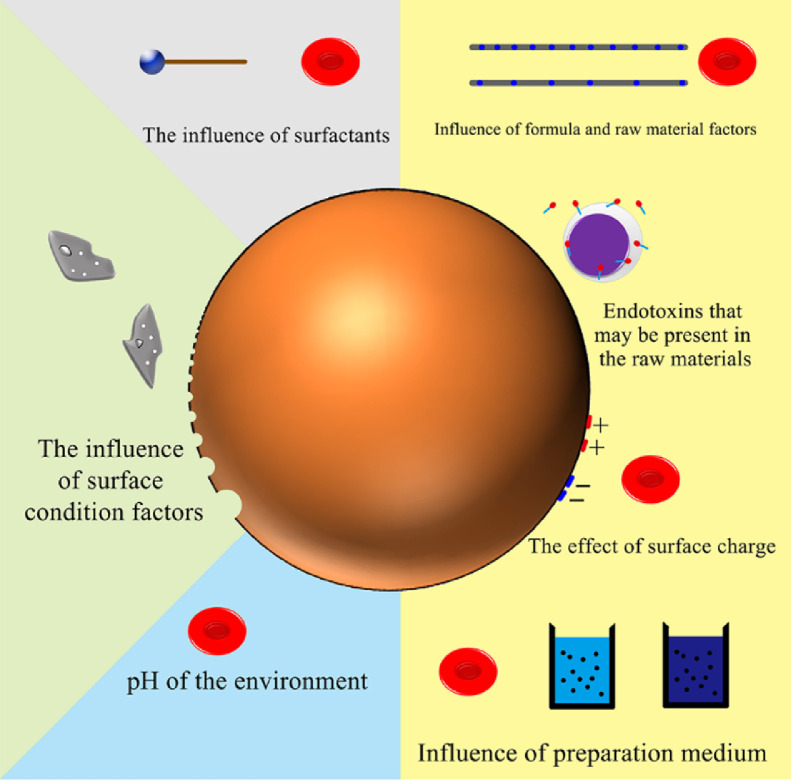
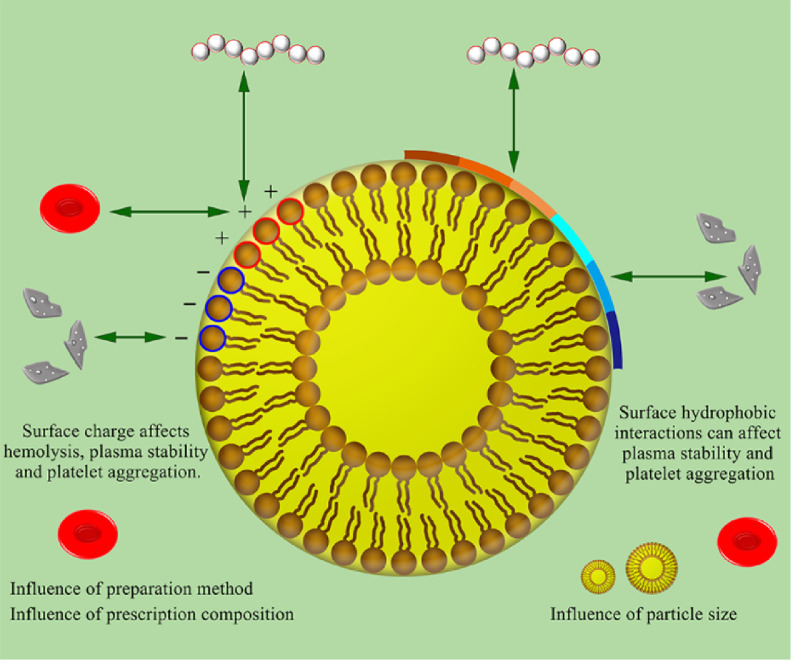
Polymer NPs are an important class of drug delivery systems. They are polymer colloidal particles with a particle size of less than 1 µm, which can be dispersed in water to form a colloidal solution. NPs are used because of their unique properties such as excellent encapsulation ability, extended residence time, and less immune responses. In medicine, NPs make it easier to transport drugs in the body. At the same time, the safety issue after drug administration is a factor that must be paid attention to when designing drug delivery systems (Fig 1). Here, we discuss the blood compatibility-related characteristics and improvement strategies of polymer NPs based on several commonly used materials.
Fig. 1.
Factors affecting haemocompatibility of polymer nanoparticles.
PLA: In synthetic biocompatible materials, saturated poly-α‑hydroxyl esters are more commonly used in the field of biomedicine, especially polylactic acid (PLA). The degradation products of PLA are usually water and carbon dioxide, which will not cause any harm. more importantly, they can be completely removed from body, making PLA the most popular biomedical material on the market. Formulations containing PLA have also been approved by Food and Drug Administration (FDA) for multiple applications. PLA is widely used in the research of polymer NPs in various forms of administration. According to Silva's research, PLA-NP showed no hemolytic activity at concentrations of 250 and 400 µg/ml, respectively. Importantly, these concentrations are very high, far from the actual situation of in vivo administration. Therefore, the results confirmed the blood compatibility of PLA-NP [13]. The residual polyvinyl alcohol in the preparation process may cause RBCs damage, which may be the main hemolytic activity of blank PLA-NP produced by emulsion -solvent evaporation method. In previous studies, we can also find strategies to improve the blood compatibility of PLA nano formulations. The g-HA/PLA composite material prepared by using the characteristics of hydroxyapatite (HA) can improve the hemolytic activity of PLA. Compared with pure PLA, the hemolytic activity decreases as the content of g-HA in the composite increases. PLA functionalized with trifluoromethyl group surfaces can also be used. the fluorinated surface shows relatively few adherent platelets, and those platelets are less activated. In terms of reducing thrombogenicity and reducing platelet reactivity, these fluorinated surfaces will show improved blood compatibility in the body [14]. The use of poly (ethylene glycol) (PEG) to modify PLA-based nanocarriers is a strategy often used in research. This strategy also has excellent effects on improving the blood compatibility of the carriers. It hardly causes platelet adhesion, and no prosthetic feet are observed on the surface either, indicating that platelets are not activated and will not cause blood clotting. This may be due to the hydrophilicity and solubility of PEG causing considerable flexibility and fluidity. PEG chain moves on the polymer surface easily. The movement of the PEG chain prevents protein adsorption or platelet adhesion [15]. On the other hand, the modification of PEG has the ability to improve the erythrocyte morphology and function issues of PLA. This may be because there is no strong interaction between the PEG-PLA copolymer and the RBC surface [16].
PLGA: Polylactic acid-glycolic acid (PLGA) is a copolymer approved by FDA administration for therapeutic excipients. Due to its biodegradability, it is one of the most popular polymers in the medical community. PLGA polymer systems can be engineered with a broad range of properties and degradation kinetics that enable sustained release. Thus, its delivery of drugs in the form of polymer NPs has also been extensively studied. Mittal reported that PLGA NPs are a drug carrier for intravenous administration of paclitaxel. It was found that compared with normal saline, the clotting time of the 10 mg/ml formulation was not statistically different. But at 100 mg/ml, there are significant differences. At the same time, the sample did not cause significant hemolysis at lower concentrations (10 mg/ml) of blood at 8 h of incubation, but at 100 mg/ml, the preparation produced hemolysis [5]. This reflects the ability of PLGA NPs to be used as a drug carrier for intravascular administration within a certain concentration limit. The changes in surface chemistry and physical state associated with polymer NPs can change the blood compatibility of biomaterials significantly. Compared with the original control group, the degradation of PLGA causes slight decrease in platelet adhesion. This difference may be related to changes in polymer surface chemistry or morphology. While contact angle doesn't change significantly, therefore the difference in platelet behavior should be due to the other factors, eg. increased surface roughness or changes in surface energy during the progress of PLGA degradation, other than the hydrophilicity changes that occur during the degradation process [17]. Reasonable modification can also be used as a strategy to improve the blood compatibility of PLGA NPs. Mucinization can control the interaction of PLGA NPs with plasma proteins and blood components through hemolysis, thrombosis, and complement activation. Mucin molecules with a micro-domain structure have alternating hydrophilic oligosaccharides and hydrophobic protein fragments, giving the NPs surface excellent surfactant activity. Modification of PLGA NPs with mucin significantly reduced platelet activation markers PF-4 and P-selectin. On the other hand, all three blood cell fractions (RBC, WBC, platelets) showed good compatibility with the modified NPs [18]. Some other successful blood-compatible PLGA NPs studies are summarized in Table 1.
Table 1.
Studies on the successful preparation of blood-compatible polymer nanoparticles.
| Components or strategy | Preparation method | Route | Drug or purpose | Assessment format | Observations | Ref |
|---|---|---|---|---|---|---|
| PLGA | Nanoprecipitation | i.v. | Paclitaxel | Plasma clotting Haemolysis Platelet aggregation test |
All the evaluated parameters are within the acceptable range and at a concentration of 100 g/ml, the hemolysis was close to the upper limit of acceptance. | [5] |
| FA-modified PEG-PLGA | Double emulsion (W/O/W) method | i.v. | Cisplatin and paclitaxel | Haemolysis Plasma clotting |
All the evaluated parameters are within the acceptable range. | [34] |
| PLGA NP (PNP) surface functionalized with water soluble glycol chitosan (gc) and carboxymethyl chitosan (cmc) | Nano precipitation method | i.v. | Paclitaxel and doxorubicin | Haemolysis Serum protein adsorption |
Concentration of cmcPNP ≥0.8 mg/ml can cause ≥5% hemolysis, while the hemolysis level of gcPNP is similar to the negative control (phosphate buffer). The coated PNP showed a different corona composition of proteins with different molecular weights than the uncoated PNP. cmcPNP is composed of proteins below 30 kDa, and the molecular weight of gcPNP adsorbed is mainly 30–90 kDa. |
[35] |
| Chitosan grafted-PEG methacrylate derivative | Double cross-linking | i.v. | Bevacizumab | Haemolysis | The degree of hemolysis is within an acceptable range. | [36] |
| PEG/lactobionic acid-grafted chitosan | – | i.v. | Anti-liver cancer drug delivery | Plasma rehydration time Haemolysis |
Compared to positive and negative controls, the PRT of PEG/lactobionic acid-grafted chitosan have a significant increase. The degree of hemolysis is within an acceptable range. | [37] |
| Alginic aldehyde and gelatin | Inverse miniemulsion technique | – | Drug- and gene-delivery | Haemolysis Plasma clotting |
All the evaluated parameters are within the acceptable range. | [38] |
| Lipids modified with the polymer gelatin | Double emulsion solvent evaporation method | p.o. | Zidovudine | Blood compatibility studies | All the evaluated parameters are within the acceptable range. | [10] |
| Iron oxide incorporated chitosan-gelatin bioglass composite NPs | Sol-gel process | – | The magnetic hyperthermia treatment and drug delivery | Haemolysis Platelet aggregation test |
All the evaluated parameters are within the acceptable range. | [39] |
| Cross-linked gelatin | Two-step desolvation method | – | Cycloheximide | Haemolysis | The degree of hemolysis is within an acceptable range. | [40] |
Chitosan: Chitosan is a common name for a family of natural polysaccharide polymers obtained from the deacetylation of chitin. Chitosan has been awarded the FDA General Recognized Safety (GRAS) designation and is widely used in dietary supplements and medical devices such as wound dressings and gels. Chitosan is a cationic polymer that is considered nontoxic, biodegradable and biocompatible, so it has been extensively studied in nanobiomedical research. Chitosan can activate blood clotting in the human body, so it has good performance as a hemostatic agent. However, after direct contact with human blood, higher chitosan concentration will lead to a gradual increase in polymorphonuclear neutrophil (PMN) elastase content and an increase in the concentration of complement activation indicator SC5b-9 [19]. Nolte conducted further studies on the pro-inflammatory properties of chitosan reported in the literature. Nolte analyzed the endotoxin content of five different chitosans and investigated their blood and cell compatibility. The data shows that when incubated with human blood or human endothelial cells, the endotoxin content plays an important role in the biological activity of chitosan to exhibit pro-inflammatory or anti-inflammatory properties [20]. The study suggests that before redetermining whether chitosan has pro-inflammatory or anti-inflammatory properties, it is necessary to consider the endotoxin concentration of chitin. In the research, different properties of chitosan are used. The properties of NPs obtained by different formulations and different preparation methods or process parameters will vary greatly [21]. Chitosan 80% NPs are easier to prolong the blood clotting time than chitosan 93% NPs, and 93% NPs are more likely to induce platelet aggregation than 80% NPs. Both NPs have good hemolysis problems and performance and almost no difference.
Compared with the degree of deacetylation of the raw material chitosan, the surface charge of chitosan NPs seems to have a greater impact on its blood compatibility. Many studies have shown that the surface charge of chitosan NPs may be a key factor in blood compatibility question. In the study of Svirshchevskaya et al., regardless of the degree of acetylation, molecular mass and hydrophobicity of chitosan, only positively charged chitosan derivatives can activate platelets, and all positively charged derivatives in this study are activated platelet aggregation, while the negatively charged succinyl derivative does not activate it and is compatible with blood components. The quaternization of chitosan reduces its toxicity at a low degree of substitution and suddenly increases its toxicity at a high degree of substitution. This is most likely because positively charged molecules form an electrostatic interaction with the negatively charged membrane of platelets [22]. Besides, sometimes chitosan NPs play a decisive role in the blood compatibility problem in the preparation of the medium. NPs prepared in acetic acid medium show a higher percentage of hemolysis, while saline dispersed NPs have the best blood compatibility. At the same time, the acidic pH value will prolong the blood clotting time. Only when dispersed in saline, the unmodified and surface-modified chitosan samples neither form thrombus or interact with coagulation factor protein, nor interfere with the plasma coagulation pathway [23]. The type and characteristics of chitosan used for NPs preparation usually have an important effect on the blood compatibility.
Improving blood compatibility through chemical modification is also applicable to chitosan NPs. We can find researches in the field in previous studies. PEG-coated chitosan showed minimal platelet aggregation. The possible cause may be the steric hindrance provided by the PEG chain. There is a delay in the coagulation of EDTA surface-modified NPs, which can be attributed to the inherent anticoagulation properties of EDTA [23]. The hydrophilic sulfo chitosan and hydrophobic lauryl chitosan are encapsulated on the chitosan backbone, and the resulting lauroylated and sulfated chitosan (LSCS) greatly improves hemolysis. At the same time, LSCS NPs did not show the tendency of WBC aggregation and hemostatic effect; the coagulation time increased than CS NPs; complement concentration was within an acceptable area and plasma protein adsorption seemed to be reduced greatly. LSCS has negatively charged sulfate and large lauroyl. The blood compatibility of LSCS is due to electrostatic repulsion on the surface, which reduces the entry of negatively charged blood components. At the same time, there are methyl side groups, flexible bonds and methylene side groups in its structure, which will provide greater flexibility and thus more effectively repel platelets [24]. Liu found that the longer alkyl chain of the modified group of self-assembled chitosan NPs, the better the blood compatibility [25]. Chitosan itself can be fully modified to improve hemolysis, coagulation, or platelet aggregation. Some other successful blood-compatible chitosan NPs studies are summarized in Table 1.
PCL: The biodegradable material poly (ε-caprolactone) (PCL) has FDA and CE approval as a medical device and has also been extensively studied in drug-loaded polymer NPs. Mazzarino prepared xyloglucan block-polycaprolactone (XGO-PCL) copolymer NPs as nanocarriers for drug delivery. The NPs were incubated with blood for 1 h, resulting in a hemolysis rate of less than 1% at all tested concentrations. indicates that XGO-PCL NPs have good blood compatibility [26]. Heparin-loaded polycaprolactone microspheres (PCL-Hep MS) can exhibit a mild anticoagulant effect in the blood. At the same time, the RBC exposed to PCL-Hep MS appeared in a normal double-concave shape. Its hemolysis was better than that of blank PCL microspheres, and its concentration at 200 µg/ml did not show any complement activation [27]. Tan used mPEG-PCL NPs to encapsulate docetaxel and also got blood compatibility results [28]. Sawant used nanoprecipitation to optimize the formulation of PCL NPs loaded with aripiprazole. There is no interaction between the prepared NPs and RBCs, and the shape of RBC has not changed [29]. The PCL microparticle preparations prepared by various methods or processes have shown good blood compatibility.
Gelatin: The exploitation of safe use in pharmaceuticals of gelatin has a long history. Despite it is considered as a GRAS (Generally Regarded As Safe) material by the FDA, it was not fully achieved in this field, gelatin is currently considered to be a very promising biomaterial for establishing advanced delivery systems, and has been used in the preparation of NPs in studies. Nahar prepared NPs (GNP A300, GNP A175, and GNP B75) of different gelatins (type A or B) as a nanocarrier of amphotericin B (AmB) through a two-step desolvation method. In the hemolysis evaluation, the observed percentage of hemolysis of the preparation was GNPA75>GNPA175>GNPA300, and they were all within the acceptable range. Various hematological parameters such as RBC count, hemoglobin content, hematocrit percentage, and platelet count were also evaluated in the mouse model. AmB-carrying GNP A300 showed no significant differences in all these parameters except platelet count decrease (P ≤ 0.05) [30]. Narayanan prepared an injectable ibuprofen sodium-loaded PEG-gelatin NPs using an improved two-step desolvation method. NPs have been shown to be non-hemolytic and do not cause significant platelet activation or induce any platelet aggregation, and nanoparticles do not interact with coagulation factor proteins [31].
Due to the specificity of the molecular structure of gelatin, it has led to some unique features of its NP blood compatibility. The hemolytic activity of gelatin particles is related to the environmental pH. The isoelectric point of human hemoglobin is about 7.1. Gelatin may exhibit a positive charge in all pH ranges that can be reached in the body, or it may be negatively charged. At this time, the ionization state will change. This may result in the existence of a pH range in which hemoglobin and type B gelatin exhibit opposite charges, which facilitates electrostatic interaction between them and increases adsorption. Ye prepared a new type of gel-silica nanoparticles (GSNPs) using the sol-gel method. The cell penetrating peptides Tat, R8 and fusion peptide HA2 were used for modification. At pH 5.0, all synthesized NPs showed a higher positive zeta potential, while at pH 7.0 it was higher. Interestingly, although GS-Tat and GS-R8 showed a higher surface charge than HA2-modified GSNPs at both pH values, GS-HA2 had the strongest ability to destroy the RBC membrane. This shows that the presence of amphiphilic HA2 peptide on the surface improves the blood compatibility of GSNPs [32].
Through appropriate strategies, the blood compatibility of gelatin NPs can be improved. Kumar prepared galactosylated gelatin NPs loaded with primaquine phosphate (PQ) using a one-step desolvation method. The hemolysis test and rat hematology studies have shown that Gel-LA-PQ-NP has lower hemolysis [33]. Other successful studies on blood-compatible gelatin NPs are summarized in Table 1.
3.2. Liposomes and niosomes
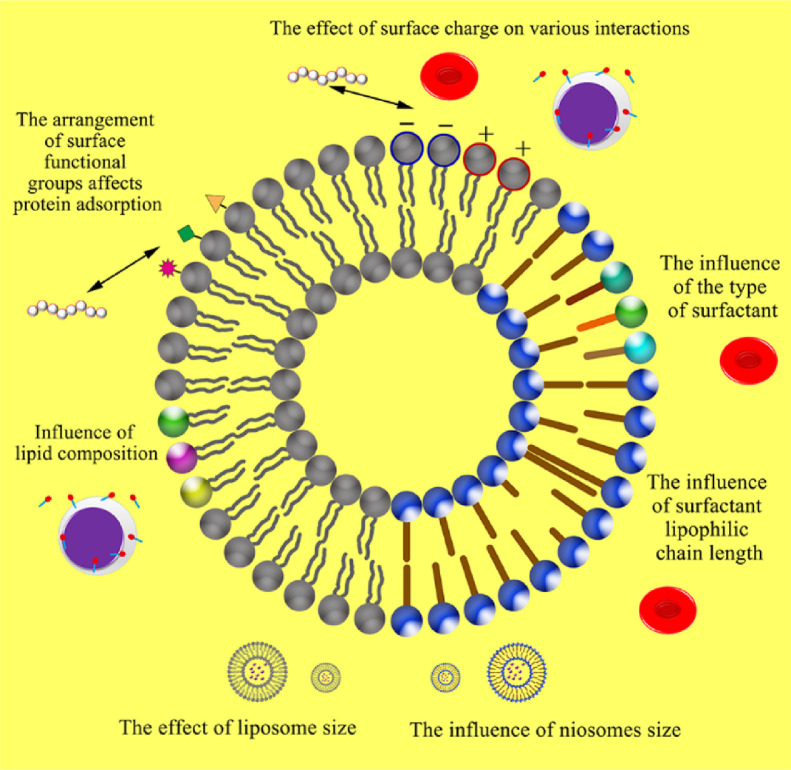
Liposomes: Liposomes are spherical lipid vesicles with a bilayer membrane structure composed of natural, semi-synthetic or synthetic amphiphilic lipid molecules. In the past few decades, liposomes have been widely used in clinic because of their unique characteristics, such as improved pharmacokinetic properties of drugs, reduced system toxicity, and potential application prospects. Liposomes are the first generation of nanocarriers to be approved by FDA in anticancer field [41], and they may have become the most successful nano drug delivery system through these decades. The blood compatibility of liposomes is also one of the factors that are utilized, and it also has characteristics that are different from other nano drug delivery systems (Fig 2). Giselbrecht screened the branched-chain fatty acid lysine conjugate T14diLys (a newly designed cationic lipid for lipid transfection) and prepared a lipid preparation, which achieved good blood compatibility [42]. Kuznetsova used 2% sialic acid Lewis X/A tetrasaccharide based on 8:1 (mol) natural phosphatidylcholine (PC) and phosphatidylinositol (PI) ligand modification, and proved that liposomes can be considered non-hemolytic, and at the same time will reduce the plasma clotting ability [43].
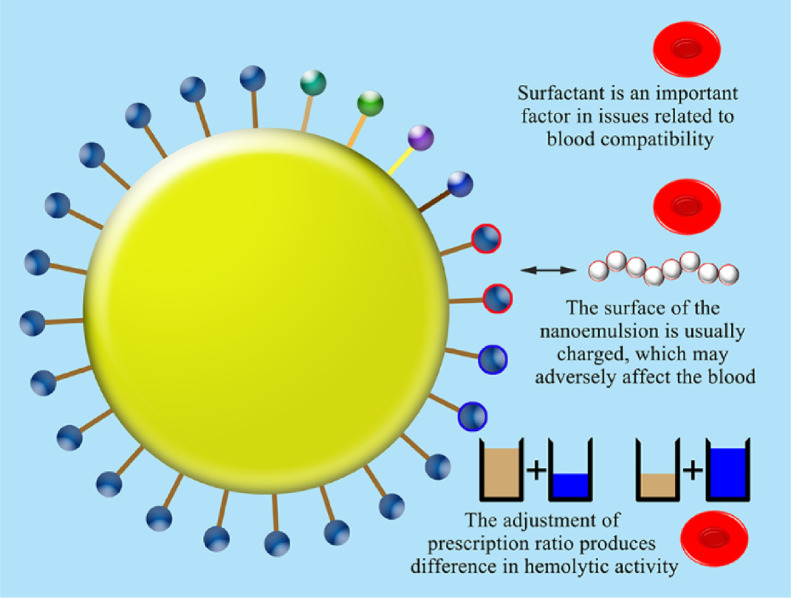
Fig. 2.
Factors affecting haemocompatibility of lipidosomes or niosomes.
When liposomes interact with blood as a drug carrier, surface charge still seems to be one of the most important influencing factors. Kose prepared a multi-layer flow membrane bubble (MLV) containing phosphatidyl choline, cholestrol, and stearylamine by thin-film hydration. The presence of lipid was detected by plasma recalcification time. The positive charge of stearylamine on the body may interact with the blood and interfere with the coagulation cascade, thereby increasing the clotting time [44]. Charge is one of the important factors influencing the effect of liposomes on blood fluid components. When the average zeta negative potential value of liposomes with different compositions increases from −34 to −50 mv, it may promote the binding to rich plasma proteins with positively charged residues, such as coagulation factor XII and high molecular weight actinogen or lipoprotein H. The difference in the charge distribution pattern on the surface of the lipid bilayer may also affect its affinity with blood biomacromolecules.
On the other hand, the secondary structure can be retained or destroyed when interacting with the surface of NPs with a specific curvature. Therefore, the entirety of proteins involved in the hemostasis cascade presented in the plasma, do not dynamically interact with the liposome surface, and adapt the conformation to the surface characteristics, thereby contributing to the role of abnormal cascade functions. Namely, the diameter and size distribution of liposomes, the surface charge and the pattern of the arrangement of functional groups on the surface of the bilayer determine the reactivity of liposomes with plasma coagulation proteins and complexes [43]. The plasma membrane of erythrocytes composed of glycocalyx sialic acid (negatively charged) residues can cause cationic liposomes to exhibit higher interactions. This interaction tends to increase the surface area of the outer lipid monolayer on the RBC than its inner lipid monolayer. This will eventually deform the shape of the RBC and cause decomposition. Therefore, in order for the lipid complex to have a higher degree of blood compatibility, a near-neutral charge is required [2].
The characteristics of the interaction between liposomes and blood can provide guidance for us to design a liposome-based nano drug delivery system, and allow us to consider and solve problems more rationally and comprehensively. Lakkadwala designed a dual-functionalized liposome delivery system with surface modified transferrin (Tf) and a cell penetrating peptide penetrating protein (Pen). Compared to Pen-liposomes, Tf-liposomes show lower hemolysis at low phospholipid concentrations. This may be due to the negative Tf charge on the liposome surface, which reduces the interaction with the RBCs membrane. In contrast, compared to ordinary liposomes, Tf-liposomes show high hemolytic activity at high phospholipid concentrations. This can be explained by the aggregation of high concentrations of Tf-liposomes, which leads to non-specific interactions and instability of the RBCs membrane [45]. In addition, despite the presence of cationic charges on these liposomes can promote a high degree of interaction with the RBCs membrane, Tf-conjugated liposomes improve hemolysis due to the presence of negative charges on the liposomes, so it showed biocompatible for phospholipid concentrations up to 600 nM [46].
Another problem with blood compatibility is the activation of the complement system. In previous studies, some findings on liposome complement activation were also reported, and its complement activation was not detected in the classical pathway. In contrast, the lipid complex induces activation on another pathway, causing 50% of rabbit RBCs to lyse. One possible reason may be that the lipid complex is positively charged in the modifier, because the increased positive charge will increase the possibility of activation of the complement system. Another reason may be the structural change of the coronary protein serum protein, which was confirmed by Giselbrecht's research [42]. Studies on PEGylated liposomes have found that, although the mechanism is not clear, the activation of complement caused by PEGylated liposomes has been attributed to the anionic phosphate portion of the PEG-lipid conjugate. The degree of complement activation of liposomes was found to be related to their lipid composition, and the most important parameters were found to include: lipid saturation, cholesterol content (which causes complement activation in a dose-dependent manner), the presence of charged phospholipids in the lipid bilayer, and the size and curvature of liposomes. Larger liposomes are more effective than smaller liposomes. For the non-targeted PEG liposomes tested, the increase in SC5b-9 levels became higher as the vesicle size increased. Larger PEG nanoliposomes induced a sharp increase in ic3b. There is a clear correlation between vesicle size and the level of activation-related factors [47]. Compared with ordinary PEG liposomes, the surface of specific liposomes may not easily activate the complement system. In any case, controlling the size of liposomes may be a potential solution to minimize any possibility of complement activation. Some other successful blood-compatible liposome studies are summarized in Table 2.
Table 2.
Studies on the successful preparation of blood-compatible lipidosomes or niosomes.
| Components or strategy | Preparation method | Route | Drug or purpose | Assessment format | Observations | Ref. |
|---|---|---|---|---|---|---|
| 3 different lipid compositions: DPPC/Cholesterol (90:10, mol), DPPC/DPPE-mPEG 5000 (95:5, mol) and DPPC/TELM (90:10, mol) | Thin-film hydration method | i.v. | Temoporfin | Haemolysis Plasma clotting |
All the evaluated parameters are within the acceptable range. | [3] |
| RGD (Arg-Gly-Asp) -modified nano-liposomes | Thin-film hydration method | – | Eptifibatide | Haemolysis Evaluation of erythrocyte membrane integrity |
All the evaluated parameters are within the acceptable range. | [55] |
| Conventional or ultra-stable tetraether lipid (TEL) | Thin-film hydration method | i.v. | Photodynamic therapy | Haemolysis Plasma clotting |
The degree of hemolysis is within an acceptable range. The clotting time of normal plasma is 32.8 sec, and the liposome formulation shows an increase in clotting time of 5 to 12 sec. | [56] |
| Injectable liposome and drug-in- cyclodextrin-in-liposome (DCL) formulations | Lipid film hydration technique | i.v. | Estetrol (E4) | Haemolysis Plasma clotting Platelet aggregation test |
All the evaluated parameters are within the acceptable range. | [57] |
| PEGylated liposomes | Ethanol injection method | i.v. | Gambogenic acid (GNA) | Assessment of blood vessel irritation Haemolysis |
There are no degeneration, necrosis and endothelial cell injury with the inner wall of veins. The degree of hemolysis is within an acceptable range. | [58] |
| Soya lecithin-liposomes | Injection method | p.o. | Stavudine | Haemolysis Plasma clotting |
The degree of hemolysis is within an acceptable range. In the SG-LP formulation, it does not form a "button" structure even at the highest concentration. | [6] |
| Pegylated liposomes | Ethanol injection and sonication method | – | Homoharringtonine | Haemolysis Assessment of blood vessel irritation |
The degree of hemolysis is within an acceptable range. In the pathology slide micrograph, after intravenous injection of lclipo-homoharringtonine at a dose of 0.1 mg/kg, no thrombus, swelling, degeneration, or inflammatory cell infiltration were observed. | [59] |
| Distearoylphosphatidylcholine liposomes | Lipid film hydration method | i.v. | Apigenin | Haemolysis | The degree of hemolysis is within an acceptable range. | [60] |
| Stealth liposomal systems functionalized with aspartate-polyoxyethylene stearate conjugate (APS) | Lipid film hydration method | i.v. | Docetaxel | Haemolysis Assessment of blood vessel irritation |
The degree of hemolysis is within an acceptable range. After 5 consecutive days of intravenous injection, only slight irritation was observed in the surrounding tissues in the APS-Lips group. | [61] |
| Sugar-based double-tailed surfactant containing renewable block | Thin film hydration method | p.o. | Levofloxacin | Haemolysis | The degree of hemolysis is within an acceptable range. | [62] |
| Creatinine niosomal | Thin film hydration method | p.o. | Clarithromycin | Haemolysis | The degree of hemolysis is within an acceptable range. | [63] |
Niosomes: Liposomes are the most widely used nano drug delivery system. However, it exists stability, mass production limitations, sterilization and long-term storage issues. Nonionic surfactant-based vesicles (niosomes) are considered a good alternative. Niosomes are vesicles composed of nonionic surfactants, capable of carrying both hydrophilic and hydrophobic drugs in the water or lipid bilayer inside them. According to reports, many amphiphilic molecules in contact with RBCs can induce hemolysis. When the hydrophobic part of the amphiphilic surfactant molecule begins to anchor into the cell membrane. It causes a change in the molar ratio of surfactant to lipid and produces a double layer to micelle transition. However, we can still find many practical niosomes studies based on blood compatible amphiphilic surfactants in predecessors' work. Shehata used three different nonionic surfactants (Brij72, Span 20, and Tween 60) to prepare various naked and PEG niosomes. After incubation for 30 min in PBS (pH 7.4), the hemolysis percentage of all formulations was less than 5% except for the exposed Tween 60 lipid. These formulations are sufficiently biocompatible and stable enough not to release a large amount of free non-ionic surfactants and cause erythrocyte membrane lysis [48]. Ullah studied niosomes based on a new type of biocompatible non-ionic surfactant atocopherol. The hemolytic effect is much lower than that of Tween 80 used as a reference standard, and it behaves good blood compatibility within the allowable limits of biological materials [49].
Because of the unique role of non-ionic surfactants and RBCs, discussions based on the adverse effects of the niosomes drug delivery system on the blood are usually centered around the hemolytic activity of the drug delivery system. Hemolysis may be caused by the destruction of the molecular structure of the RBCs membrane, or it may be the result of the increased permeability of the membrane to external solutes. This process is activated by the absorption of surfactants on the surface of the RBCs and their distribution balance between the aqueous phase and the membrane. At high surfactant concentration, the cell membrane ruptures and the surfactant dissolves the membrane lipid [50]. Tween 80 is a non-ionic surfactant that can be used for intravenous injection. In the research on niosomes, the hemolytic effect of Tween 80 is usually used as an evaluation control standard when studying various new non-ionic surfactants and their niosomes. At the same time, we can also find some available rules on the blood compatibility of various niosomes. Ali developed a membrane-compatible surfactant synthesized from sulfonamide and its self-micellarization, and obtained a hemolysis effect three times lower than Tween 80. they found that the longer alkyl chain length from C14–C18 make synthesized surfactant inter-act with RBCs membrane and contribute in hemolysis [51]. The effect of the alkyl chain (hydrophobic portion) of the non-ionic surfactant on its blood compatibility is often demonstrated in previous studies. Imran developed a novel glycoside nonionic surfactant synthesized by simple etherification of coumarin and bromodecane and obtained a hemolytic activity lower than Tween 80. Imran believes that the number of carbon atoms in the alkyl chain of surfactant not exceeding 11 may be a key factor for the low hemolysis of niosomal nanocarrier [52]. The higher hemolytic activity is affected by the length of the alkyl chain of the surfactant, and the longer chain is more damaging to the RBCs. Surfactants with 14–18 carbon atoms in the alkyl chain of the amphiphile may have a very high damaging effect on the cell membrane [53]. According to the research of Muzzalupo et al., the hemolytic activity of the surfactant is related to the number of carbon atoms in the main chain. The longer the alkyl chain of the sugar-based surfactant is, the stronger the hemolytic effect. N-dodecyl is more hemolytic to RBCs than n-decyl and n-octyl-β-d-glucopyranoside. Generally, short-chain alkyl sugar-based surfactants (C8) have caused hemolysis in the pre-micellar stage, and the toxicity reaches its maximum in the C12–C14 chain. It is speculated that such a length of carbon chain is better inserted into the erythrocyte membrane [50].
The hemolytic ability of the surfactant is also affected by the characteristics of the aggregation gate, which affects the degree to which the surfactant is embedded in the RBCs membrane. These assumptions explain the results obtained in the preparation of niosomes: small niosomes are more toxic than large niosomes, because they easily interact with RBCs, resulting in massive hemolysis. Hemolytic activity is closely related to the chemical structure of the surfactant monomer and its behavior in aqueous solution. Vesicle aggregates of different sizes lead to significantly different hemolytic activity [54]. The same assumption can also explain the fact that the hemolytic capacity in the surfactant solution is always higher than the corresponding niosomes. In fact, it is more difficult for large aggregates to interact with biofilms. Therefore, niosomes represent a strategy to minimize surfactant toxicity. It also ensures the possibility of using vesicles as a drug delivery system in the field of pharmacology [50]. Other successful studies of blood compatible niosomes are summarized in Table 2.
3.3. Micelles
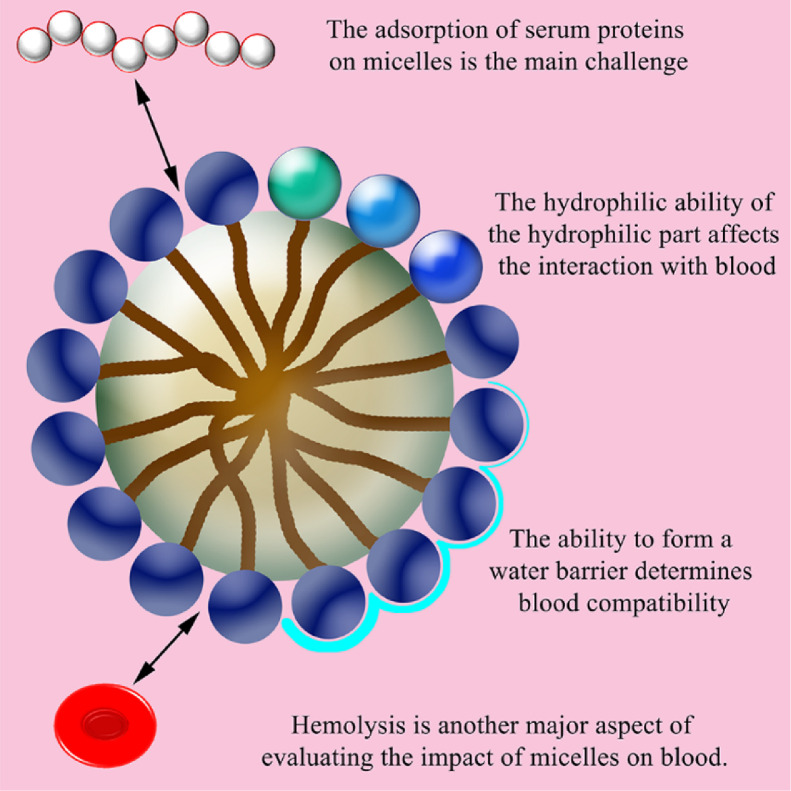
Polymer micelles are a form of drug carrier that has been widely concerned in treatment and research. Micellization provides the unique advantage of improving the solubility of poorly water-soluble drugs, while providing a "core-shell" delivery system for NPs. This unique core-shell structure allows micelles to effectively dissolve poorly water-soluble drugs in the core through hydrophobic action, and still maintain the hydrophilicity of the shell. Some of the materials approved by the FDA for biomedical applications are suitable for the preparation of drug-loaded micelles [64,65]. Compared with other carriers, they have several favorable physicochemical and biological properties, including: nano-sized suitable for drug delivery, hydrophobic core-hydrophilic shell structure that allows loading water-insoluble drugs or protecting sensitive cargo, and tailor customized structure for continuous release or controlled release. With blood, we can also know in previous studies that micelles are a successful carrier form in terms of blood compatibility and the feasibility of entering the circulatory system to play a systemic role (Fig 3). Gao constructed a hydrophobic portion containing poly (ethylene glycol) methyl methacrylate and camptothecin prodrugs and poly [2-(diisopropylamino) ethyl methacrylate] as a hydrophilic chain, which can form stable single molecule micelles. After micelles were injected into healthy mice, blood samples were analyzed. The main parameters of the whole blood test are RBC, WBC, hemoglobin concentration, mean RBCs hemoglobin concentration, mean platelet volume, platelet count hematocrit. All of these parameters in mice blood samples treated with micelles and drug-loaded micelles remained within the normal range, and the difference from the saline control group was negligible [66].
Fig. 3.
Factors affecting haemocompatibility of micelles.
The composition and design of micelles is the determinant of their blood compatibility. In the existing findings, we can still find some general rules about the relationship between micelle composition and blood compatibility. Kumari found that cholesterol modification in the side chain of the copolymer did not produce any obvious effect on the RBC membrane. Dutta observed that the hydrophobic substitution of amino acid side chains in the copolymer did not show any significant cleavage of the RBC membrane. Both Kumari and Dutta found that the activity hemolysis of the copolymer is independent of the hydrophobic composition or the length of the hydrophobic chain [67], [68]. The hydrophilic part of the micelle composition and the hydrophilic ability of the material may have an important influence on the interaction between micelles and blood. Ye found that the zwitterionic modified micelle group showed better anti-hemolytic activity. This is due to the stronger hydration capacity of the zwitterionic portion micelles, which results in a stronger water barrier and prevents the micelles from contacting the blood cell membrane [69]. The ability of some micelles with a specific composition to form a water barrier can be well used to improve blood compatibility. The organized lipid adsorption layer formed by the plasma on the surface of the polymer may also soften the interaction between the copolymer and platelets [70]. Sabra prepared self-assembled polymer micelles composed of Fe3O4 coated with hydrophobic oleic acid and amphiphilic corn lactoferrin (Lf). The micelle possessed a Lf hydrophilic shell to obtain a low hemolysis rate and prevent oxidative damage to the erythrocyte membrane. At the same time, Lf as the hydrophilic shell of these micelles could also enhance the stability of the serum. It could form a brush-like structure, prevented the protein from adsorbing on its surface, and hindered the aggregation of the hydrophobic magnetic core in the water environment [71]. The adsorption of serum proteins on polymeric micelles is the main challenge for parenteral administration using micelles. When incubated with serum, unstable micelles quickly adsorb these proteins, forming aggregates. [72]. Previous work is a good proof that the hydrophilic brush-like structure of the micelle Lf shell can greatly reduce the adsorption of micelles to proteins and protect the hydrophobic core from invasion.
Haemolysis and serum stability are the two main aspects that most related studies evaluate the effect of micelles on blood. Regarding the improvement of the blood compatibility of micelles as drug carriers, the selection of suitable materials and modification of appropriate groups are all feasible strategies. Raveendran studied the amphiphilic Pluronic-PCL block copolymer micelles. The aggregation detected in RBC, WBC, and platelets incubated with micelles was negligible, and micelles did not promote aggregation like the positive control. The non-aggregation of micelles could be attributed to the Pluronic part. The use of Pluronic to improve the blood compatibility of the material had been fully demonstrated, and Pluronic's improved antithrombotic properties could be attributed to the long chain of polyethylene oxide, which could inhibit blood cell adhesion [73].
Strategies commonly used in the field of nanocarriers such as PEG modification can also be used to improve the blood compatibility of micelles. Low hydrophobic content and hydrophilic PEG groups present on the surface of polymer micelles can lead to low hemolysis behavior. Research by Shi showed that RBCs of different MPEG modified micelles at low dose (0.5 mg/ml) exhibited different degrees of RBCs aggregation. Among them, MPEG 2k modified micelles dramatically induced the aggregation of RBCs with micelles, while MPEG 5k modified micelles did not induce any aggregation [74]. Bionic strategies are also worth trying when constructing micelle carriers. As mentioned above, polymer micelles self-assembled by amphiphilic copolymers containing amino groups would decrease their blood compatibility due to the positive charge induced by amino groups. Lin reported a biomimetic phosphorylcholine strategy to improve the blood compatibility of positively charged pH-responsive micelles. Lin speculated that the introduction of biomimetic phosphorylcholine could form a dense hydrated layer through its strong electrostatic force, which shielded the positive charge induced by the amino group, thereby improving blood compatibility. The positive charge on the poly (2-methacryloxy phosphorylcholine) (PMPC)-based micelles prepared by Lin rarely affected platelet activation. Platelet activation played a key role in thromboembolic complications of blood-contacting materials. Some positively charged polymers (such as chitosan) are known for their platelet activation and clot formation. In contrast, it was found that micelles have no effect on platelets in this study, indicating that they were compatible with platelets despite being positively charged [75]. Other successful studies of blood-compatible micelles are summarized in Table 3.
Table 3.
Studies on the successful preparation of blood-compatible micelles.
| Components or strategy | Preparation method | Route | Drug or purpose | Assessment format | Observations | Ref. |
|---|---|---|---|---|---|---|
| Self-assembled nano-colloidal polymeric micelles (LP-PMs) of Soluplus and PF127 | Thin-film hydration method | i.v. | Lapatinib | Haemolysis Platelet aggregation test |
The degree of hemolysis is within an acceptable range. No aggregation was observed in the control or LP-PM treated blood samples. | [76] |
| Amphiphilic maltodextrin-ursodeoxycholic acid (FA and lactobionic acid were coupled to the surface of micelles) | Dialysis method | – | Sulfasalazine and resveratrol | Serum stability Haemolysis |
All the evaluated parameters are within the acceptable range. | [77] |
| Redox-sensitive heparin-β-sitosterol | Probe sonication and aqueous dialysis | i.v. | Doxorubicin | Haemolysis | The preparation has no significant effect on red blood cells. | [78] |
| A series of Chondroitin sulfate/poly (D,L-lactideco-glycolide) block copolymers (CHs-b-PLGA) with different length of hydrophobic block | Sonicate method | i.v. | Doxorubicin | Haemolysis | As the molecular weight of PLGA increases, the hemolysis rate of micelles increases slightly. However, the degree of hemolysis in all formulations is within an acceptable range. | [79] |
| Amphiphilic bifunctional pullulan derivative | Dialysis method | i.v. | Doxorubicin | Haemolysis | The degree of hemolysis is within an acceptable range. | [80] |
| Amphiphilic styrene-maleic acid (SMA) copolymer | Dialysis method | – | 3,4-Difluorobenzylidene curcumin (CDF) | Serum stability | SMA-CDF nanomicelles exposed to fibrinogen or 50% FBS did not find any significant difference in particle size up to 72 h. | [81] |
| Poly(D-lactide), poly(L-lactide) and stereocomplex micelle | Nanoprecipitation method | i.v. | Doxorubicin | Serum stability Haemolysis |
In PBS buffered BSA solution (30.0 mg/ml), all loaded micelles showed excellent stability during the 72-h measurement. The degree of hemolysis is within an acceptable range. | [82] |
| A copolymer composed of carboxybetaine methacrylate (CBMA) as hydrophilic segment and 2-(methacryloyloxy)ethyl lipoate (MAEL) as hydrophobic | Dialysis method | i.v. | Doxorubicin | Serum stability | No increase in the size of drug-loaded micelles was observed in 1 mg / mL PBS solution and 50% FBS at 37 °C. | [83] |
3.4. Solid lipid nanoparticles and nanostructured lipid carriers
Solid lipid nanoparticles: Solid lipid nanoparticles (SLNs) were invented 20 years ago. The lipid NPs are prepared from a lipid matrix that is solid at room temperature and room temperature and stabilized by a suitable surfactant. The first liver-targeting lipid NPs (ONPATTRO) obtained FDA-approval in 2018. they have received more and more attention in pharmaceutical technology research. There are some reports on blood compatibility tests, and the results are encouraging. The low molecular weight heparin-modified isoliquiritigenin-loaded SLNs developed by Zhang, even if the dose is as high as 430 mg/kg, the rabbit will not die and the SLNs concentration of 1.5 mg/ml will not cause hemolysis of rabbit RBCs [84]. Escalona established a reusable double emulsion solvent evaporation method for preparing magnetic SLNs. It is made of an iron oxide core embedded in a solid matrix of trimyristic acid glycerin. No hemolysis on the blood sample was observed (even after 24 h), and platelet activation, complement system activation and plasma clotting time are negligible [85].
Although the effect of SLNs on blood is generally within an acceptable level, as a kind of exogenous NPs with small particle size, large surface area, and surfactants in most cases, it still has some uncertainties. Doktorovova evaluated the constructed SLNs drug-loading system. The SLNs component showed hemolysis < 8%, and SLNs did not change significantly in the presence of serum for up to 120 min (P < 0.05). After 120 min, SLNs changed in size due to the adsorption of serum proteins [86]. The surface charge and surface groups of SLNs are also important factors affecting blood compatibility. In addition, improving the preparation method of SLNs may also be an effective strategy to improve its blood compatibility. Kotmak studied the blood compatibility of SLNs prepared by two different methods, and found that the hemolysis of SLNs prepared by different methods was different. The SLNs preparation prepared using the stepwise method showed lower hemolysis compared to the direct method [87]. Due to the charge interaction with the plasma membrane and proteins, many nanocarriers with cationic charges have been found to be toxic. On the other hand, negatively charged NPs can induce platelet aggregation to a greater extent than cationic or neutral NPs. In addition, the ability of NPs to inhibit platelet aggregation depends on their particle size, surface hydrophobicity and charge. Therefore, the composition of SLNs requires major attention on the issue of blood compatibility in drug-loading systems. Ridha performed folic acid (FA) modification of human serum albumin-coupled cationic SLNs. At a higher concentration (3 mg/ml), the hemolysis rate of the nanocomposite was 2.68%. This result indicates that the nanocarrier has excellent blood safety and suitability for intravenous administration. A suitable value for hemolysis is achieved due to the presence of human serum albumin (HSA) on the surface of the nanocomposite [88]. Other successful studies of blood-compatible SLNs are summarized in Table 4.
Table 4.
Studies on the successful preparation of blood-compatible solid lipid nanoparticles or nanostructured lipid carriers.
| Components or strategy | Preparation method | Route | Drug or purpose | Assessment format | Observations | Ref. |
|---|---|---|---|---|---|---|
| Linseedoil and cetylpalmitate | Solvent emulsification evaporation technique | i.v. | Buprenorphine | Haemolysis LDH release from neutrophils |
All the evaluated parameters are within the acceptable range. | [93] |
| Stearic acid | Microwave-based technique | i.v. | Cisplatin | Haemolysis | The degree of hemolysis is within an acceptable range. | [94] |
| Stearic acid modified with Aloe Vera | Solvent emulsification evaporation technique | p.o. | Zidovudine | Haemolysis Plasma clotting Platelet aggregation test |
All the evaluated parameters are within the acceptable range. | [95] |
| Seventy percent glycerol monostearate as the solid lipid, 30% oleic acid as the liquid lipid | Solvent emulsification evaporation technique | p.o. | Praziquantel | Haemolysis | The degree of hemolysis is within an acceptable range. | [96] |
| Based to sophorolipids and coated with polysorbates | Hot dispersion method | – | Rifampicin | Haemolysis | The degree of hemolysis is within an acceptable range. | [97] |
| The solid lipid and oil with maximum percent partitioning value with propofol | Hot emulsification-probe sonication method | i.v. | Propofol | Haemolysis | The degree of hemolysis is within an acceptable range. | [98] |
| Stearic acid | Hot melt homogenization method | p.o. | Simvastatin | Haemolysis | Treatment of rats with SV preparations will weaken the hemolysis of RBCs, and SV can reverse hyperlipidemia and cause changes in RBCs membrane fluidity. | [99] |
| Cetyl palmitate and caprylic acid | Microemulsion template strategy | Transdermal delivery | Meloxicam | Haemolysis | The degree of hemolysis is within an acceptable range. | [100] |
Nanostructured lipid carriers (NLCs): NLCs is NPs composed of a mixture of solid and liquid lipids, whose lipid matrix is curable at room temperature and body temperature. It is the second generation of solid lipid-based the colloidal carrier and can improve stability and drug encapsulation ability. The research of NLCs on blood interaction is also mainly focused on the research of hemolysis (Fig 4). It is encouraging that NLCs usually also has satisfactory blood compatibility. Nimtrakul established solid lipid formulations for intravenous administration of AmB. The hemolytic effect of NLCs loaded with AmB was significantly reduced. The low hemolysis of AmB when formulated in NLCs can be attributed to the association of drugs and lipid compounds, which may cause structural changes in the drugs without molecular aggregation [89]. The low hemolytic activity of NLCs is entirely reasonable, because hemolytic agents (oil at the core and surfactant at the interface) are restricted at the lipophilic core and the interface, thus hindering Initial contact with RBCs membrane [90]. Therefore, the formation of NLCs usually helps to improve the overall blood compatibility due to its structural characteristics. In the study of the potential of NLCs in the intravenous injection of artemisinin (ARM), Joshi found that glyceryl dilaurate, Tween 80 and Solutol HS 15 in the prescription ingredients showed a certain degree of hemolysis. But what was interesting was that the nano preparations containing Capmul MCM (glyceryl mono/dicaprylate), glycerol dilaurate, Tween 80 and Solutol HS 15 had a higher hemolysis rate than Capmul MCM, but less than the theoretical hemolysis caused by those ingredients. It could be deduced that assembling the above components into an NLCs structure changes the way and degree of their interaction with RBCs [91]. In previous studies, we can understand that factors such as the composition of the prescription, the size of the carrier, and the surface charge generally play an important role in the blood compatibility of NLCs. Nimtrakul prepared three different formulations using different types of solid lipids (stearic acid, palmitic acid and myristic acid). Myristic acid-NLCs showed a higher level of hemolysis. Interestingly, all blank NLCs did not show potential hemolysis at the doses studied, suggesting that NLCs was not responsible for hemolytic potential. Therefore, the hemolysis observed with myristic acid-NLCs was due to the entrapped drug (AmB). This may explain the tendency of the smallest size to be combined with the RBC membrane. Therefore, most of the partially aggregated AmB in the myristic acid-NLCs located on the surface of the particles has more opportunities to destroy RBC [89]. In addition, unlike micelles, NLCs with different alkyl chain lengths (solid phase) do not necessarily exhibit the same trend of hemolytic as alkyl chain lengths [90].
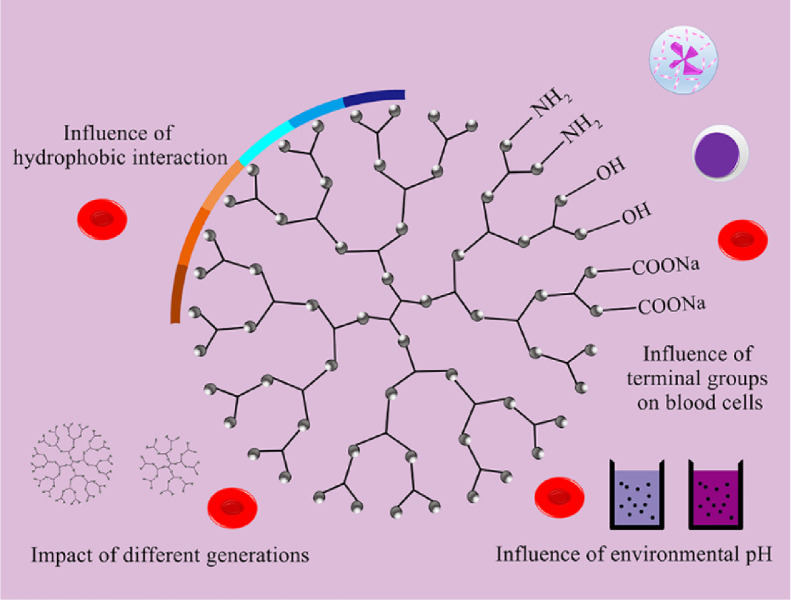
Fig. 4.
Factors affecting haemocompatibility of solid lipid nanoparticles or nanostructured lipid carriers.
Surface charge, as an important property of nanocomposites in terms of blood compatibility, plays an important role in both hemolysis and plasma stability. The absence of interaction between RBC and NLCs may be attributed to the presence of similar charges (negative charges). Since proteins also have a negative charge, this may also be the reason for less interaction with the protein, because the same charge will repel each other, making NLCs stable in serum and without significant interaction with serum proteins. At the same time, the binding of serum proteins not only depends on the surface charge, but other mechanisms, such as hydrophobic interactions. More studies are still needed to confirm [92]. Some other successfully completed studies of blood-compatible nanostructured lipid carriers are summarized in Table 4.
3.5. Nanoemulsion and self-nanoemulsifying drug delivery systems
Nanoemulsion: Nanoemulsion is transparent or translucent oil-in-water (O/W) or water-in-oil (W/O) droplets with an average droplet diameter between 100 and 500 nm. Unlike thermodynamically stable microemulsions, nanoemulsion have kinetic stability in suspension due to the smaller droplets, and have great stability. At the same time, the preparation process of nanoemulsion is relatively simple, which is beneficial to the mass production, which made it widely used and researched. Li prepared an alternative formulation of O/W docetaxel nanoemulsion using high-pressure homogenization. It was composed of medium-chain triglycerides, oleic acid, lecithin, and polycrystalline safe ingredients for intravenous injection. An acceptable level of hemolysis was obtained. After intravenous injection of high-dose docetaxel nanoemulsion, no adverse reactions were found. All experimental mice showed a healthy state and survived for 48 h. No congestion, edema, bleeding and rot were observed at or around the injection site after intravenous injection. In addition, compared to commercial docetaxel injections containing Tween 80, docetaxel nanoemulsion did not cause adverse hypersensitivity reactions [101]. Through appropriate strategies, nanoemulsion can achieve the required blood safety for drug administration (Fig 5). Simultaneously, some FDA-approved gelling agents (such as pluronic) or nanoemulsion formulations can be used as standards for comparative evaluation.
Fig. 5.
Factors affecting haemocompatibility of nanoemulsion or Self-nanoemulsifying drug delivery systems.
Surfactants are used in the acquisition of nanoemulsion, which increases the possibility of hemolysis. Ferreira prepared pomegranate seed oil nanoemulsion containing ketoprofen using pullulan as a polymerization stabilizer. Acceptable hemolytic activity, low hemolytic activity benefits from the use of new surfactants [102]. The surface of the nanoemulsion is usually charged, which becomes another factor that increases its adverse effect on the blood. As mentioned above, the positive or negative charge on the surface of the nanocarrier is likely to increase the interaction between the preparation and the blood component and the positive charge on the surface increases the risk of hemolytic activity, but even for some nanoemulsion that are positively charged on the surface, we can use some preparation methods to achieve acceptable blood compatibility standards. Hussain used Capmul, Labrasol and Acconon with anti-mycobacterial activity to improve the transdermal delivery of rifampicin-loaded cationic nanolatex. The formulations all contained positively charged component oleylamine as a charge inducer. But all nanoemulsion were blood compatible. It showed that the cationic nanoemulsion also had the potential for blood compatibility [103]. Based on above all, the selection of suitable surfactants and the control of the surface charge of the nanoemulsion on its behavior in blood should be considered.
To improve the blood compatibility of nanoemulsion, adjustment of the matrix material and appropriate group modification are common strategies. Fang prepared nanoemulsion using liquid perfluorocarbon and coconut oil as the internal phase core. It was found that the percentage of hemolysis caused by nanoemulsion decreased with increasing oil concentration. The difference in hemolytic activity can be attributed to different emulsifiers and different oil concentrations [104]. Modification of PEG is a commonly used strategy to improve the properties of nano drug delivery systems, and can also be applied to improve the blood compatibility of nanoemulsion. There are a large number of PEG head groups on the surface of PEGylated nanoemulsion droplets, which can hinder its ability to approach RBCs, thereby reducing the direct contact between phospholipids and cell membranes. The PEG chain on the surface of poloxamer nanoemulsion droplets exhibits a brush-like conformation. It is believed that this conformation cannot effectively prevent other hydrophobic surface (such as RBCs) is tightly bound, but the PEG 2000-dspenanomul emulsion droplet is assumed to have a dense brush. It has the ability to shield the droplet surface from contact with nearby surfaces [105]. Other successful hemocompatible nanoemulsion studies are summarized in Table 5.
Table 5.
Studies on the successful preparation of blood-compatible nanoemulsion or SNEDDS.
| Components or strategy | Preparation method | Route | Drug or purpose | Assessment format | Observations | Ref. |
|---|---|---|---|---|---|---|
| Olive oil-based nanoemulsion optimized with seven-factor Box-Behnken | High-speed homogenization method | i.v. | Indinavir | Haemolysis | The degree of hemolysis is within an acceptable range. | [108] |
| Mixing a PEG400 solution of drug and 10% (w/w) blank lipid emulsions (BLEs) | High-speed homogenization method | i.v. | Paclitaxel | Intravenous irritation assessment Haemolysis |
Compared with the saline group during the 3-d administration period, the paclitaxel-loaded lipid nanoemulsion(TPLE) treatment group had no obvious histopathological changes. At a dose of 6.0 mg/kg, TPLE did not stimulate the rabbit ear vein. Even at a paclitaxel concentration of 0.8 mg/ml, TPLE does not cause hemolysis or agglutination at 37 °C. | [12] |
| Peceol nanoemulsion | Slow spontaneous titration method | Transdermal drug delivery | Amphotericin B | Haemolysis | The preparation has no significant effect on red blood cells. | [109] |
| Seed oil nanoemulsion | Spontaneous emulsification | i.v. | Glioma treatment | Haemolysis | The degree of hemolysis is within an acceptable range. | [110] |
| Employing tocol acetate nanoemulsion | Sonication and speed homogenization method | i.v. | Curcumin | Haemolysis | The degree of hemolysis in all formulations is within an acceptable range. | [111] |
| SNEDDS composed of Capryol 90 as an oil phase, Cremophor EL as a surfactant, and Transcutol HP as a cosurfactant | – | p.o. | Cilostazol | Haemolysis | The degree of hemolysis is within an acceptable range. | [112] |
Self-nanoemulsifying drug delivery systems (SNEDDS): SNEDDS are made from a mixture of previously optimized liquid and behave as a dispersible nanoemulsion when diluted with aqueous gastric juice. This liquid mixture is a solid or liquid preparation consisting of an oil phase, a nonionic surfactant and a co-surfactant, which is suitable for use in the gastrointestinal tract or at an ambient temperature (usually refers to a body temperature of 37 °C). Under gentle agitation, it can emulsify spontaneously to form an emulsion with a particle size of about 100–500 nm. Because the ingredients of SNEDDS also contain surfactants, its blood compatibility needs attention. We can use classic or new auxiliary materials that have been proven safe to achieve blood-compatible SNEDDS. Hussain developed a cured SNEDDS loaded with rifampicin. An acceptable hemolysis index was obtained, showing the applicability of the SNEDDS formulation [106]. Khan developed a hydrophobic polyphenol pigment curcumin SNEDDS. According to its hemolysis test, the preparations were found to be quite nontoxic. The nontoxic behavior of these formulations could be attributed to the choice of excipients. The excipients of the preparation are in the GRAS category [107]. Other successfully completed blood-compatible SNEDDS studies are summarized in Table 5.
3.6. Mesoporous silica nanoparticles
Mesoporous silica nanoparticles (MSNs) refer to porous silica NPs with pores having a diameter between 2 and 50 nm. These materials have several attractive properties for drug delivery, such as high surface area and pore volume, easily modified surfaces and pore sizes, excellent biocompatibility and chemical inertness. With recent recognition by the FDA as a safe material for human trials, MSNs are being extensively explored as promising theranostic agents [113]. There are also many unique features in its interaction with blood (Fig 6). MSNs at concentrations below 100 mg/ml showed low RBC hemolysis. The percentage of hemolysis of RBC is positively correlated with the concentration of MSNs in a wide concentration range of 0–500 mg/ml. At a concentration of 500 mg/ml, obvious hemolysis is visible [114]. At the same time, in terms of plasma stability, the bovine serum albumin (BSA) adsorption of pure MSNs is quite high, mainly because the surface silanol groups of MSNs are rich, showing non-specific binding to BSA through hydrogen bonding interactions [115]. The characteristics of the effect of MSNs on blood and strategies to improve blood compatibility should be considered for MSNs research.
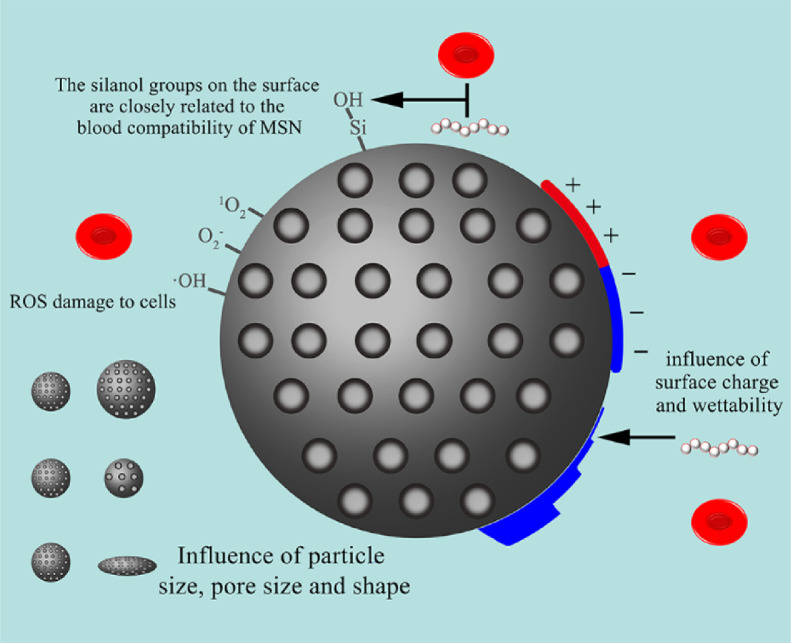
Fig. 6.
Factors affecting haemocompatibility of mesoporous silica nanoparticles.
In general, the hemolysis of silica is considered to be due to the active oxygen on the surface of silica, the electrostatic interaction between silicate and membrane proteins, and the high affinity between silicate tetraalkyl and ammonium groups in RBCs membranes [114]. The ROS on the surface of silica may cause irreversible damage to cells, mainly including singlet oxygen, superoxide anion and hydroxyl radical. In common with other nano-formulations, charge is a factor that must be considered on the blood compatibility of MSNs. The reduction in the surface density of silanol groups contributes to lower hemolysis. Another study found that under certain circumstances, the affinity of silica for RBC decreases with the increase in surface functionality, regardless of the surface charge (−43 to 7 mv) [116]. But increasing the surface charge to a certain threshold (>30 mv) may cause opposite effects, including enhanced interaction of nanoparticles with RBC and increased hemolysis caused by amine-modified mesoporous SiO2. In addition to the surface charge, the hemocompatibility of the material is also affected by the hydrophilicity [117]. The grafted polar carboxyl group enhances the hydrophilicity and shields the ROS on the surface, which can make MSNs circulate in the blood without damaging RBCs.
Li biosynthesized three MSNs using heterocyclic amino acid derivatives as templates, and the MSNs with the most EOH groups on the surface showed the highest wettability and the fastest degradation rate. For silica materials, many factors may lead to hemolysis behavior, and hemolysis performance is closely related to the wettability of the material. It is known that surface properties will ultimately affect blood membrane interactions. The high wettability of MSNs contributes to the high affinity between silica and erythrocyte membrane and causes the erythrocyte membrane to rupture [118]. Silica materials generally have lower protein adsorption capacity, which means that there is less blood reaction and lower clearance rate in the body. The results also indicate that protein adsorption is also related to wettability, and a smaller contact angle will result in a larger contact area and a higher possibility of adsorption.
Silanol groups on the surface of silica particles are closely related to various adverse reactions of blood. When incubated with MSNs at high concentrations, hemolysis of RBCs may be due to silanol groups on the surface that can bind to RBC membranes rich in PC. Wang constructed FA-modified dendritic MSN and found that the hemolytic activity of MSNs after FA modification was reduced to one-eighth of the original, which is considered to be the effect of FA covering silanol groups [119]. After calcination at high temperature, a Si-O three-membered ring can be formed on the surface of amorphous silicon NPs, and further cracked and reacted with water molecules to induce hydroxyl radicals in the solution. The ROS produced by the silicon NPs may lead to the peroxidation of the cellular components of RBC, causing cell lysis [120]. Feng et al. assembled a biocompatible alginic acid/chitosan polyelectrolyte multilayer membrane on the surface of monodisperse silica microspheres, and the hemolysis effect was greatly improved. It might be due to the surface silanol or amino groups of the NPs. It was blocked by the polymer layer, thereby reducing the affinity of the exposed NPs to the RBC membrane, and preserving the integrity of the RBC membrane [121]. Similarly, when the acid silanol on the MSNs is covered by the lipid bilayer, no hemolysis is observed [122]. The strategy for silanol groups on the surface is particularly important in improving the blood compatibility of MSNs.
Morphological factors such as particle size, shape and pore size also affect the blood compatibility of MSN. Zhao studied the interaction of MSNs with different particle sizes and surface properties and human erythrocyte membrane through membrane filtration, flow cytometry and various microscopic techniques. It was found that contrary to the trend of size in some reports. the hemolytic activity of MSNs with larger particle size was higher than that of small particles. Although for blood-compatible MSNs below 225 nm, larger particle sizes may be preferable, increasing the particle size of MSNs beyond this range does not necessarily improve blood compatibility. In addition to particle size, other factors (such as surface area) are also expected to affect the hemolytic potential of MSN [123]. The interaction between MSNs and RBC membrane involves two main processes: (1) the combination of the silanol-rich surface of MSNs and the PC-rich RBC membrane (2) the bending of the RBC membrane to adapt to the rigid surface MSNs. The occurrence of interaction depends on whether the energy released by the combination of MSNs and RBC membrane can overcome the bending membrane and adapt to the free energy required by the surface of MSNs. The energy of the former is related to the external surface area of MSNs, while the latter is proportional to the curvature of the particles or inversely proportional to the square of the radius [123]. Therefore, the interaction and hemolytic activity of MSNs and RBC membrane depends not only on the particle size, but also on its external surface area. The increased pore size will increase the hemolytic activity of MSN. In addition, geometry is another important factor affecting hemolysis. The decrease in surface curvature leads to an increase in the hemolytic activity of MSN. This may be related to the curvature-dependent structural changes of some biomolecules. As we discussed above, the lower MSNs curvature will cause the conformational changes of some proteins to be more pronounced. The interaction between MSNs with lower curvature and RBC may cause significant structural changes of certain proteins on the RBC membrane, which may accelerate RBC hemolysis. Ma speculated that there may be a "threshold" for the surface curvature to affect the hemolytic activity of MSN [124]. Further research indicated that the density of silanol groups of each NP may have a threshold, and above this threshold may cause cell membrane damage immediately after exposure. Therefore, the hemolytic activity depends not only on an external surface area and curvature, but also on the silanol density of each NP exposed to RBC [123].
Both non-porous silica and porous silica can cause damage to the erythrocyte membrane, and the degree of damage is related to concentration and size. Smaller particles have higher hemolytic activity than larger particles. The higher hemolytic activity of the smaller NP may be due to the larger surface area per gram, indicating a higher number of silanol groups present on the cell-contactable surface of the smaller NP. However, further research proved that the size-dependent hemolytic activity is only maintained for NPs with a well-ordered mesoporous structure. Compared with NPs of similar size, the hemolytic activity of NPs is reduced due to the voids on the surface. The pore structure of NPs and the surface area available for contact with cells also affect the hemolytic activity of MSNs [125]. Besides, the pores of mesoporous silica will undergo an aging process. At the same time, during the aging process in phosphate buffer solutions or simulated body fluids, the silica particles will degrade into monomeric oligosilicates. The porous properties will change dynamically, and this structural change will have a significant impact on the use of these porous materials in drug delivery applications. The supernatant of aged NPs is exposed to RBC, and hemolysis is usually not observed. The dissolved silicate material is not a source of increased hemolysis, but the reliable feature of the NPs surface may change. In fact, when the silica dissolves and the pores collapse, although the total surface decreases, by eliminating voids or the longitudinal cracking of the pores to reveal the inside of the pores, the surface area that the cells can still contact May increase [125]. However, the integrity of pores should still be considered in future toxicology studies.
Lakshmi successfully prepared controllable head-tail MSNs using O/W emulsion systems, that is, solid or porous spherical silica head particles with dendritic tails with large pores (1128 nm). It was found that because of the introduction of a tail with large pores and reduced hemolysis, better blood compatibility was obtained. Among all the silica particles tested, compared with the large pore MSN—CC (MSN with a spherical core-cone structure), the small pore dendritic mesoporous silica nanoparticles (DMSN) had low hemolytic activity. However, compared with MSN—CC, the asymmetric particles with a large-pore dendritic tail showed a greatly improved safety of RBCs. The conclusion was that the asymmetric morphology significantly improves blood compatibility, and the concept was applicable to head particles with solid and nanoporous properties. Excellent blood compatibility was due to reduced ROS generation and "flat" contact pattern with RBC membrane [120]. For hollow silica, Zhou prepared hollow silica particles that can control shell thickness and pore size distribution. It was found that a thicker shell resulted in higher hemolytic activity [126]. Therefore, these results revealed the dose-dependent and shell-thickness-dependent hemolytic activity of MSNs with different shell thicknesses. The higher hemolytic activity of MSNs with increased shell thickness may be due to its slightly larger particle size. It results in a higher silanol density on the outer surface of nanoparticles that RBC can access, and resulting in damage to the RBC membrane increase.
In terms of plasma stability, Ma used the three main proteins human serum albumin, human gamma globulin (HGG) and human serum fibrinogen (HSF) in serum as model proteins. It is found that the affinity of serum protein to MSNs is stronger than RBC. The adsorption of serum proteins on MSNs reached equilibrium within 5 min. Because of protein has a higher affinity for MSNs than RBC, after adding MSNs to the mixture of RBC and protein, the protein would first be adsorbed on the surface of MSN. The resulting protein corona could obviously prevent RBC hemolysis [3]. Therefore, serum protein plays an important role and should not be ignored when we examine the biological behavior of intravenous NPs.
MSNs surface modification strategies are often effective in improving blood compatibility. The binding of aminopropyl (AP) and PEG groups on the surface of MSNs retains the elasticity of RBC better than unfunctionalized MSN, especially at higher concentrations (> 20 µg/ml). The adhesion of MSNs on the surface of RBC limits the flexibility of the film and causes the deformability of RBC to be impaired. Conversely, the functional group attached to the surface of the MSNs will reduce the particle affinity of the RBC membrane and allow the cell to stop retaining its deformability [123]. He covalently grafted MSNs using rhodamine B (RhB), which significantly improved the percentage of hemolysis. This may be due to the positive surface potential of the SBA-15 type MSNs used in the study condensing with the quaternary ammonium group may inhibit the damage of the RBC membrane connected to the trimethylammonium head group, because it is believed that the negative surface residues of MSNs will cause irreversible RBC damage. Besides, MSNs-RhB did not activate intrinsic, extrinsic and common coagulation pathways in a wide concentration range of 50–500 µg/ml. The mesophilic mesoporous channels of MSNs-RhB were completely filled in PBS, and there is no room for further absorption of water and plasma when mixing. Therefore, MSNs-RhB did not affect the normal coagulation/anticoagulation function of plasma [127]. Feng used a layer-by-layer method to coat a polyelectrolyte multilayer film composed of polyallylamine and polystyrene sulfonate on the surface of monodisperse silica. The surface silanol groups are shielded by the polyelectrolyte layer, thereby reducing the accessibility of the silanol groups to the RBC and reducing the damage to the RBC membrane. The surface of the coated NPs was negatively charged, which facilitated the interaction between the NPs and positively charged thrombin and reducing the concentration of free thrombin, thereby effectively inhibiting platelet aggregation. Almost all APTT, PT, TT and Fib values were within their normal range [128].
PEG modification is often considered to improve the blood compatibility of MSN, which is an effective strategy. Chen found that hemolytic activity of unmodified MSNs was higher than PEGylated MSN. This result indicated that PEGylation played an important role not only in enhancing the stability of MSNs but also in reducing significant hemolysis [129]. Liu 's study showed that RBC treated by PEG-MSNs had no visible hemolysis, and their hemolysis value was nearly 20 times lower than unmodified MSNs. This may be due to the silanol surface of the nanoparticles being blocked by the polymer layer, thereby reducing the affinity of NPs on the RBC membrane and preserving the integrity of the RBC membrane [130]. During the aging of silica particles, PEG not only masked the surface silanol groups, but also acted as a protective layer, thereby preventing other silanol groups from entering the RBC from the collapsed pores. This simple surface modification strategy is essential to ensure the safety of NP in biomedical applications [125]. The ROS on the outer surface of PEGylated MSNs is thought to be accompanied by a reduction in the surface shielding of long PEG chains, which will also help to inhibit the hemolysis of RBC. At the same concentration, the percentage of hemolysis increased very slowly as the molecular weight of PEG increased. When the PEG chain density can completely and tightly cover the outer surface of MSNs, the ROS shielding effect will become weaker, so the hemolysis percentage is relatively high [114]. After decorating with PEG, the percentage of hemolysis of MSNs dropped sharply. Moreover, as the density of the modified PEG chain increases, the hemolytic effect of RBC decreased. At the same time, the BSA adsorption rate of PEG grafted MSNs decreased sharply [115]. The adsorption degree of HSA has a regularity for all PEGylated MSNs with different molecular weights of PEG xk-silane. A small amount of PEGylation would lead to a sharp decrease in the adsorption degree of HSA, and the adsorption degree of HSA increases slowly when PEGylation reaches a certain level. The "mushroom" and "brush" conformations can be formed at very low and relatively high PEG xk chain densities, respectively. The "brush-like" configuration extends the PEG xk chains away from the MSNs surface and limits their range of motion, which would help to increase the repulsive force against HSA. However, exceeding the high PEG xk chain density may lead to a decrease in flexibility, and reduce the steric hindrance effect on non-specifically bound HAS. The degree of adsorption of HSA exceeds the critical percentage of PEG xk-silane. Such a critical value corresponds to the optimal chain density of PEG xk of different molecular weights. At the same time, the minimum HSA-adsorbed PEG xk chain density and molecular weight of PEG xk–MSNs are related. When the molecular weight of grafted PEG xk increases, the optimal PEG xk chain density decreases for the minimum HSA adsorption of PEG xk-MSN, which is most likely due to the enhanced hydrophilicity of the PEG xk chain [114]. Other successful studies of blood-compatible mesoporous silica NPs are summarized in Table 6.
Table 6.
Studies on the successful preparation of blood-compatible mesoporous silica nanoparticles.
| Components or strategy | Preparation method | Route | Drug or purpose | Assessment format | Observations | Ref |
|---|---|---|---|---|---|---|
| Rod-like MSNs with pH-responsive amphiphilic hyperbranched polyester shells | Sol-gel chemistry method | i.v. | Doxorubicin | Haemolysis | The degree of hemolysis is within an acceptable range. | [131] |
| Gold nanoparticle (GNP)-biotin conjugates were grafted onto the pore outlets of the prepared MSN | Base-catalyzed sol-gel method | i.v. | Doxorubicin | Haemolysis Platelet aggregation test Inflammatory cytokine secretion |
The drug and MSN-GNP-DOX activated GPIIb/IIIa similarly, with no other complications. All the evaluated parameters are within the acceptable range. |
[9] |
| FA functionalized MSNs | Sol-gel chemistry method | p.o. | Etoposide | Haemolysis | The preparation has no significant effect on red blood cells. | [132] |
| Chitosan grafted MSNs | Stober method | p.o. | Raloxifene hydrochloride | Haemolysis | The degree of hemolysis in all formulations is within an acceptable range. | [133] |
| Agarose-loaded mesoporous silica nanoparticle (AMSN) or agarose and heparin-loaded mesoporous silica nanoparticle (AHMSN) | Sol-gel chemistry method | – | Modified on the silicone film surface | Platelet Adhesion Assay Whole Blood Adhesion Tests Hemolysis Plasma clotting |
The AHMSN sample does not affect platelet activation. When silica gel was contacted with fresh rabbit whole blood, a large number of adherent blood cells were observed on Si, MSNs-Si, and AMSN membranes. The preparation has no significant effect on red blood cells. efore loading heparin, the anticoagulation time of MSNs-Si and AMSN was almost the same as that of original Si. On the contrary, the APTT, PT and TT values of AHMSN increased significantly. | [134] |
| Mesoporous core shell NPs surrounded with poly acrylic acid (PAA) layers | Template based synthesis method | p.o. | Bicalutamide | Hemolysis | The degree of hemolysis is within an acceptable range. | [135] |
| MSNs core with hydrophilic and pH responsive PAA functionalization | New template synthesis method | p.o. | Etoposide | Hemolysis | The degree of hemolysis is within an acceptable range. | [136] |
| MSNs coated with a crosslinked polyethyleneimine PEG copolymer | Template based synthesis method | i.v. | SiRNA | Hemolysis Plasma clotting Platelet aggregation test |
All the evaluated parameters are within the acceptable range. | [137] |
3.7. Dendrimers
Dendrimers are monodispersing, three-dimensional, highly branched polymer nanocarriers (1–100 nm) with a specific structure composed of core, branches, and terminal groups. High drug loading, easy synthesis, stability, and tailored functionality are important advantages of dendrimers. The high degree of control over the structure, size, shape, branch length and density of dendrimers and their surface functionality make these compounds suitable for many pharmaceutical applications. Several products using dendrimers as platform have been developed and commercialized upon the approval of the FDA [138]. Regarding blood safety, dendrimers can achieve blood compatibility that meets the requirements (Fig 7). Here we discuss the relevant characteristics and improvement strategies of the blood compatibility of dendrimers.
Fig. 7.
Factors affecting haemocompatibility of dendrimers.
Dendrimers generally start from the core and continue to branch outward. When the algebra is low, it is generally an open molecular configuration. As the algebra increases and branching continues, from the fourth generation, the molecule changes from an open loose state to a for the spherical three-dimensional structure with tight outer and inner loose. Similarly, the blood toxicity of dendrimers is also one of the reasons for limiting the highest algebra that can be used. Different generations of polypropylene imine dendrimers on human erythrocytes toxic behavior increases with the number of generations. The hemolytic activity of 5.0 generation polypropylene imine (5.0 G PPI) dendrimers is more than 3 times that of 4.5 G PPI dendrimers. Kesharwani synthesized PPI dendrimers of different generations (3.0 G, 4.0 G and 5.0 G) and loaded Melphalan. And observed that the common 3.0 G PPI, 4.0 G PPI, 5.0 G PPI, PPI3M, PPI4M and PPI5M formulations showed 36.73%, 42.56%, 73.34%, 33.23%, 39.88% and 69.74% hemolysis rate, respectively. [139]. But the hemolysis results of PP fifth generation at lower concentrations are still within the acceptable range. 5.0 G PPI dendrimers (the highest concentration is 1 mg/ml) show promising performance, which can be developed as a carrier system [140]. The results related to hemolysis and agglutination may be due to the dendrimer partially incorporated into the lipid bilayer, or by removing the outer monolayer.
Generally, polyamindoamine (PAMAM) dendrimers with amino ends are ubiquitous. In the case of PAMAM dendrimers, a generation-dependent hemolysis phenomenon occurs in which the morphology of RBCs changes. In contrast, anions and neutral PAMAM dendrimers with carboxylic sodium and hydroxyl groups, respectively, are usually neither hemolytic nor cytotoxic to cell line trypsin in vitro. Han found a time-dependent hemolysis of cationic PAMAM dendrimers in the study. The high concentration of cationic PAMAM directly leaded to rapid RBC cleavage. Moderate concentrations of cationic PAMAM mainly caused RBC aggregation with or subsequent slow hemolysis, while low concentrations of cationic PAMAM have no significant effect on RBC. At the same time, ethylenediamine with the same concentration of amine cannot induce obvious RBC cleavage [141]. These suggested amino groups and polymer structures play a key role in hemolysis induction, or they cannot work alone. Larger macromolecules and higher surface amine group density can cause more severe hemolysis. Gu found that puerarin-PAMAM dendrimer complex showed less toxicity than dendrimers at equivalent concentrations in dendrimers. It was also speculated that in the case of dendrimer drug complexes, the presence of free amino groups on the surface of pure dendrimers was occupied by drug molecules, which causing a reduction in toxicity [140]. Cui modified the surface charge of G5 PAMAMs. It was found that the negatively charged modified G5 PAMAM had a repulsive force against RBC. At the same time, modified the electrostatically hydrated bottom layer formed by the zwitterionic layer on the surface of the dendrimer could reduce the hemolysis rate. Both of these factors can effectively reduce the interaction between modified G5 PAMAM and RBC [142].
The cytotoxicity of cationic PAMAM dendrimers to erythrocytes can be explained by its surface charge, and the hydrophobicity of dendritic molecules can be used as another possible factor to determine toxicity. Halets found that for cationic dendrimers, the addition of human serum albumin could greatly reduce its blood toxicity. The complex formed based on electrostatic force and hydrophobic force neutralizes the electrostatic and hydrophobic interaction between the dendrimer and the RBCs membrane [143]. In addition, the addition of fetal bovine serum may also provide protection for RBCs [141].
The alkaline pH of cationic PAMAM solution is a factor that cannot be ignored. By adjusting the pH value to physical 7.4, reducing the dosage, and using plasma proteins (or other protective additives, such as negatively charged molecules), the blood compatibility and even biocompatibility of cationic dendrimers can be improved to safety level. The pH of higher concentration cationic PAMAM solutions is usually significantly higher than 7.4, although they are all prepared by dissolving in 7.4 PBS. The study found that after adjusting the pH, the hemolysis caused by different generations of 90 nm cationic PAMAM dendrimers was reduced by 19%–24%. Coincidentally, before adjusting the pH, the original pH of the 90 nm cationic PAMAM dendrimer was about 10.6. Therefore, because of the alkaline solution itself may cause hemolysis and related damage of RBCs, it is necessary to check the blood toxicity caused by alkaline pH [141].
Gu used PAMAM dendrimers linked by Pluronic F127 (PF127) as a new drug carrier. The conjugation of PF127 can significantly reduce the blood toxicity of PAMAM dendrimers, mainly because of the reduction of zeta potential [139].As one of the strategies to improve the blood compatibility of dendrimers, further research had been delivered to surface engineering on dendrimers. The surface engineering of the dendrimer shields the cationic charge, thereby preventing the interaction of RBC and the dendrimer, resulting in the reduction or complete removal of the cationic surface. Compared with the parent dendrimer, it reduces hemolysis and causes better hematological properties.
The improvement of blood compatibility by proper chemical modification or coating is also an effective strategy for dendrimers. Wang modified the main surface amine of the G5 PAMAM dendrimer with different amounts of carboxybetaine acrylamide (CBAA). It also proved that when PAMAM was completely modified by carboxybetaine group, the hemolytic activity of CBAA-PAMAM-20 decreased to undetectable levels [144]. The study by Gupta proved that the FA conjugate on the surface of the dendrimer would interfere with the interaction with RBC and reduce the hemolysis of RBC. The hemolytic toxicity of PPI-FA was about 5 times lower than PPI. The decrease in toxicity might be due to the conjugation with the surface of FA, which caused the doxorubicin (DOX) and main amine groups in the dendritic structure to be shielded, thereby making the surface more biocompatible. At the same time, PPI-FA also showed better white blood cell count and lymphocyte count results in Gupta's research [145]. Zhou introduced different generations of PAMAM dendrites (G = 2,3) into chitosan, which also improved the hemolytic activity [146].
Chen's manipulation of dendrimer sites provides six different chemical surface groups (amines, guanidines, carboxylates, sulfonates, phosphonates and PEG). All molecules showed concentration-dependent and time-dependent hemolysis. The hemolysis in cationic dendrimers was more pronounced than in anionic dendrimers. At the same time, PEG-dendrimers had the least hemolysis [147]. Regarding the PEG modification strategy, Wang found that the hemolysis level of low molecular weight PEG-modified PAMAM is slightly lower than that of PAMAM. For high molecular weight PEG-modified dendrimers, the hemolysis level is much lower than PAMAM [148].
Klajnert reductively aminated the unmodified second-generation to fifth-generation PPI dendrimers under the condition of excessive maltose to synthesize maltose-modified PPI dendrimers. The counterparts of these dendrimers with additional maltose units were found to show almost complete loss of hemolytic activity. Klajnert concluded that the attachment of maltose units to the surface reduced blood toxicity for two generations. The densely organized maltose units form a shell on the surface of the PPI dendrimer, separating the RBCs from the potentially toxic PPI core of the dendrimer [149]. In other studies, coating G5 PPI dendrimers with mannose or galactose can reduce blood toxicity [150,151]. Other successful studies of blood-compatible dendrimers are summarized in Table 7.
Table 7.
Studies on the successful preparation of blood-compatible dendrimers.
| Components or strategy | Route | Drug or purpose | Assessment format | Observations | Ref. |
|---|---|---|---|---|---|
| The triblock dendrimer: polyamidoamine-polyethylene glycol-cyclic RGD | – | – | Hemolysis Plasma clotting Platelet aggregation test Complement activation test |
The preparation has only a minor effect on red blood cells. All the other evaluated parameters are also within the acceptable range. | [152] |
| Cationic PAMAM dendrimer of generation 4.0 (PM4.0) were functionalized by PEG conjugation or by thiolation or the combination of both methods. | – | – | Hemolysis Complement activation test Plasma clotting Platelet aggregation test |
The degree of hemolysis in all formulations is within an acceptable range. Compared with unmodified PM4.0, the thiolation of PM4.0 does not reduce the production of c3a, and compared with unmodified PM4.0, the modification of PM4.0 with PEG alone will moderately reduce the production of c3a. When the PEGylation ratio is increased from 1.8 to 4.0, this effect is more obvious. PM4.0 and PEGylated PM4.0 dendrimers produce higher PT values. Compared with the original PM4.0, PEGylated PM4.0 alone showed a shorter PT, and the effect was more obvious when the PEG ratio was increased. In contrast, bifunctionalized PM4.0 dendrimers and PM3.5 cannot regulate the external coagulation pathway. Similar to PT determination, citrate human plasma treated with various dendrimers showed APTT prolongation or no clotting. Unmodified PM4.0 and PEG-modified PM4.0 showed long-term coagulation exceeding 0.125 mg/ml. The effect of PEGylated and bifunctionalized PM4.0 on collagen-induced platelet aggregation is reduced, suggesting its anticoagulant properties. | [153] |
| Lysine-Cbz (Lys-Cbz) or Arginine-Tos (Arg-Tos) coupled PAMAM G4 dendrimers | – | Antithrombotic drug or a drug delivery vehicle | Hemolysis Platelet aggregation assay Platelet secretion assay Analysis of platelet adhesion |
The proportion of hemolysis of PAMAM dendrimers G4, G4-Lys-Cbz and G5 (concentration of 0.25 mg/ml) were 9.8%, 8.7% and 6.6% respectively. The commercially available PAMAM G4 and G5 dendrimers cause strong aggregation of RBC, while the interaction between PAMAM G4 derivatives and RBC is not large. PAMAMG5 derivatives show moderate RBC agglutination. PAMAM G4 showed only 2% ± 1% inhibition of platelet aggregation. G4-Arg-Tos inhibited platelet secretion in an order of 25% ± 12% (P <0.05). After incubating G4-Arg-Tos in whole blood, platelet coverage was suppressed by 74%. In contrast, PAMAM G5 and its derivatives have no effect on platelet function. Both PAMAM G4-Arg-Tos and G4-Lys-Cbz are effective inhibitors of platelet aggregation induced by ADP. G4-Arg-Tos showed inhibition of platelet secretion. | [8] |
| Cationic G4 PAMAM dendrimers | p.o. | Risperidone | Hemolysis | The preparation has no significant effect on red blood cells. | [154] |
| PEG (molecular weight 5000) was conjugated to G5 and G6 PAMAM dendrimers | i.m. | Gene delivery | Hemolysis | The hemolysis percentage of G5-PEG and G6-PEG was significantly reduced to about 5% (P <0.005). The hemolysis of 2.5 mg/ml PEG-PAMAM dendrimer at high PEG conjugation of 15% was not significantly different from that of 8% PEG conjugation. |
[155] |
4. Prospection
In the past few decades, some nanocarrier systems have been studied, such as polymer NPs, nanocolloids, liposomes, etc. Because of their pharmacokinetic characteristics and functional ability, they have received wide attention. Nano preparations have broad development prospects. However, many nano drug-loading systems cause a series of adverse reactions at high concentrations, which is still difficult to apply in clinical practice, mainly due to they can't perform the intended function in vivo. In which, blood compatibility is a great factor. So it is necessary to discuss the issues in order to benefit future research and application. It is witnessed the evolution of drug delivery systems, from the initial use of controlled-release polymers in macroscopic drug warehouses, implants and suture materials, to injectable micro-controllable drug delivery systems, to nanoscale drug delivery. Therefore, despite many challenges, more practical and industrialized nano drug-loading systems will emerge in the future.
Contributor Information
Yanan Shi, Email: shiyanan001@163.com.
Youxin Li, Email: youxinli@luye.com.
References
- 1.Bender E.A., Adorne M.D., Colome L.M., Abdalla D.S.P., Guterres S.S., Pohlmann A.R. Hemocompatibility of poly(varepsilon-caprolactone) lipid-core nanocapsules stabilized with polysorbate 80-lecithin and uncoated or coated with chitosan. Int J Pharm. 2012;426(1–2):271–279. doi: 10.1016/j.ijpharm.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 2.Kumar Y., Kuche K., Swami R., Katiyar S.S., Chaudhari D., Katare P.B., et al. Exploring the potential of novel pH sensitive lipoplexes for tumor targeted gene delivery with reduced toxicity. Int J Pharm. 2020;573 doi: 10.1016/j.ijpharm.2019.118889. [DOI] [PubMed] [Google Scholar]
- 3.Ali S., Amin M.U., Ali M.Y., Tariq I., Pinnapireddy S.R., Duse L., et al. Wavelength dependent photo-cytotoxicity to ovarian carcinoma cells using temoporfin loaded tetraether liposomes as efficient drug delivery system. Eur J Pharm Biopharm. 2020;150:50–65. doi: 10.1016/j.ejpb.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Amin K., Dannenfelser R.M. In vitro hemolysis: guidance for the pharmaceutical scientist. J Pharm Sci. 2006;95(6):1173–1176. doi: 10.1002/jps.20627. [DOI] [PubMed] [Google Scholar]
- 5.Mittal P., Vardhan H., Ajmal G., Bonde G.V., Kapoor R., Mittal A., et al. Formulation, optimization, hemocompatibility and pharmacokinetic evaluation of PLGA nanoparticles containing paclitaxel. Drug Dev Ind Pharm. 2019;45(3):365–378. doi: 10.1080/03639045.2018.1542706. [DOI] [PubMed] [Google Scholar]
- 6.Nayak D., Boxi A., Ashe S., Thathapudi N.C., Nayak B. Stavudine loaded gelatin liposomes for HIV therapy: preparation, characterization and in vitro cytotoxic evaluation. Mater Sci Eng C Mater Biol Appl. 2017;73:406–416. doi: 10.1016/j.msec.2016.12.073. [DOI] [PubMed] [Google Scholar]
- 7.Liu K.S., Wen C.J., Yen T.C., Sung K.C., Ku M.C., Wang J.J., et al. Combined strategies of apomorphine diester prodrugs and nanostructured lipid carriers for efficient brain targeting. Nanotechnology. 2012;23(9) doi: 10.1088/0957-4484/23/9/095103. [DOI] [PubMed] [Google Scholar]
- 8.Duran-Lara E., Guzman L., John A., Fuentes E., Alarcon M., Palomo I., et al. PAMAM dendrimer derivatives as a potential drug for antithrombotic therapy. Eur J Med Chem. 2013;69:601–608. doi: 10.1016/j.ejmech.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 9.Eskandari P., Bigdeli B., Porgham Daryasari M., Baharifar H., Bazri B., Shourian M., et al. Gold-capped mesoporous silica nanoparticles as an excellent enzyme-responsive nanocarrier for controlled doxorubicin delivery. J Drug Target. 2019;27(10):1084–1093. doi: 10.1080/1061186X.2019.1599379. [DOI] [PubMed] [Google Scholar]
- 10.Joshy K.S., Snigdha S., Kalarikkal N., Pothen L.A., Thomas S. Gelatin modified lipid nanoparticles for anti- viral drug delivery. Chem Phys Lipids. 2017;207(Pt A):24–37. doi: 10.1016/j.chemphyslip.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Martins M.C.L., Sousa S.R., Antunes J.C., Barbosa M.A. Protein adsorption characterization. Methods Mol Biol. 2012;811:141–161. doi: 10.1007/978-1-61779-388-2_10. [DOI] [PubMed] [Google Scholar]
- 12.Chen L., Chen B., Deng L., Gao B., Zhang Y., Wu C., et al. An optimized two-vial formulation lipid nanoemulsion of paclitaxel for targeted delivery to tumor. Int J Pharm. 2017;534(1–2):308–315. doi: 10.1016/j.ijpharm.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Da Silva J., Jesus S., Bernardi N., Colaco M., Borges O. Poly(D,L-Lactic Acid) nanoparticle size reduction increases its immunotoxicity. Front Bioeng Biotechnol. 2019;7:137. doi: 10.3389/fbioe.2019.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khalifehzadeh R., Ciridon W., Ratner B.D. Surface fluorination of polylactide as a path to improve platelet associated hemocompatibility. Acta Biomater. 2018;78:23–35. doi: 10.1016/j.actbio.2018.07.042. [DOI] [PubMed] [Google Scholar]
- 15.Mei T., Zhu Y., Ma T., He T., Li L., Wei C., et al. Synthesis, characterization, and biocompatibility of alternating block polyurethanes based on PLA and PEG. J Biomed Mater Res A. 2014;102(9):3243–3254. doi: 10.1002/jbma.35004. [DOI] [PubMed] [Google Scholar]
- 16.Li C., Ma C., Zhang Y., Liu Z., Xue W. Blood compatibility evaluations of poly(ethylene glycol)-poly(lactic acid) copolymers. J Biomater Appl. 2016;30(10):1485–1493. doi: 10.1177/0885328215627404. [DOI] [PubMed] [Google Scholar]
- 17.Brockman K.S., Lai B.F.L., Kizhakkedathu J.N., Santerre J.P. Hemocompatibility of degrading polymeric biomaterials: degradable polar hydrophobic ionic polyurethane versus poly(lactic-co-glycolic) acid. Biomacromolecules. 2017;18(8):2296–2305. doi: 10.1021/acs.biomac.7b00456. [DOI] [PubMed] [Google Scholar]
- 18.Thasneem Y.M., Rekha M.R., Sajeesh S., Sharma C.P. Biomimetic mucin modified PLGA nanoparticles for enhanced blood compatibility. J Colloid Interface Sci. 2013;409:237–244. doi: 10.1016/j.jcis.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Denzinger M., Hinkel H., Kurz J., Hierlemann T., Schlensak C., Wendel H.P., et al. Hemostyptic property of chitosan: opportunities and pitfalls. Biomed Mater Eng. 2016;27(4):353–364. doi: 10.3233/BME-161591. [DOI] [PubMed] [Google Scholar]
- 20.Nolte A., Hossfeld S., Post M., Niederlaender J., Walker T., Schlensak C., et al. Endotoxins affect diverse biological activity of chitosans in matters of hemocompatibility and cytocompatibility. J Mater Sci Mater Med. 2014;25(9):2121–2130. doi: 10.1007/s10856-014-5244-y. [DOI] [PubMed] [Google Scholar]
- 21.Jesus S., Marques A.P., Duarte A., Soares E., Costa J.P., Colaco M., et al. Chitosan nanoparticles: shedding light on immunotoxicity and hemocompatibility. Front Bioeng Biotechnol. 2020;8:100. doi: 10.3389/fbioe.2020.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svirshchevskaya E.V., Zubareva A.A., Boyko A.A., Shustova O.A., Grechikhina M.V., Shagdarova B.T., et al. Analysis of toxicity and biocompatibility of chitosan derivatives with different physico-chemical properties. Appl Biochem Micro. 2016;52(5):483–490. [PubMed] [Google Scholar]
- 23.Nadesh R., Narayanan D., Sreerekha P.R., Vadakumpully S., Mony U., Koyakkutty M., et al. Hematotoxicological analysis of surface-modified and -unmodified chitosan nanoparticles. J Biomed Mater Res A. 2013;101(10):2957–2966. doi: 10.1002/jbm.a.34591. [DOI] [PubMed] [Google Scholar]
- 24.Shelma R., Sharma C.P. Development of lauroyl sulfated chitosan for enhancing hemocompatibility of chitosan. Colloids Surf B Biointerfaces. 2011;84(2):561–570. doi: 10.1016/j.colsurfb.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Liu C., Wang J., Huang S., Yu L., Wang Y., Chen H., et al. Self-assembled nanoparticles for cellular delivery of peptide nucleic acid using amphiphilic N,N,N-trimethyl-O-alkyl chitosan derivatives. J Mater Sci Mater Med. 2018;29(8):114. doi: 10.1007/s10856-018-6120-y. [DOI] [PubMed] [Google Scholar]
- 26.Mazzarino L., Loch-Neckel G., Dos Santos Bubniak L., Ourique F., Otsuka I., Halila S., et al. Nanoparticles made from xyloglucan-block-polycaprolactone copolymers: safety assessment for drug delivery. Toxicol Sci. 2015;147(1):104–115. doi: 10.1093/toxsci/kfv114. [DOI] [PubMed] [Google Scholar]
- 27.Chen L., Shi T., Xia X., Miao J., Mao C., Huang B., et al. Mild anticoagulant effect of heparin-loaded polycaprolactone microspheres. J Nanosci Nanotechnol. 2015;15(1):144–150. doi: 10.1166/jnn.2015.8772. [DOI] [PubMed] [Google Scholar]
- 28.Tan L., Ma B., QianZhao LanZhang, Chen L., Peng J., et al. Toxicity evaluation and anti-tumor study of docetaxel loaded mPEG-polyester micelles for breast cancer therapy. J Biomed Nanotechnol. 2017;13(4):393–408. [PubMed] [Google Scholar]
- 29.Sawant K., Pandey A., Patel S. Aripiprazole loaded poly(caprolactone) nanoparticles: optimization and in vivo pharmacokinetics. Mater Sci Eng C Mater Biol Appl. 2016;66:230–243. doi: 10.1016/j.msec.2016.04.089. [DOI] [PubMed] [Google Scholar]
- 30.Nahar M., Mishra D., Dubey V., Jain N.K. Development, characterization, and toxicity evaluation of amphotericin B-loaded gelatin nanoparticles. Nanomedicine. 2008;4(3):252–261. doi: 10.1016/j.nano.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Narayanan D., Geena M.G., Lakshmi H., Koyakutty M., Nair S., Menon D. Poly-(ethylene glycol) modified gelatin nanoparticles for sustained delivery of the anti-inflammatory drug ibuprofen-sodium: an in vitro and in vivo analysis. Nanomedicine. 2013;9(6):818–828. doi: 10.1016/j.nano.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Ye S.F., Tian M.M., Wang T.X., Ren L., Wang D., Shen L.H., et al. Synergistic effects of cell-penetrating peptide Tat and fusogenic peptide HA2-enhanced cellular internalization and gene transduction of organosilica nanoparticles. Nanomedicine. 2012;8(6):833–841. doi: 10.1016/j.nano.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Kumar H., Gothwal A., Khan I., Nakhate K.T., Alexander A., Singh V., et al. Galactose-anchored gelatin nanoparticles for primaquine delivery and improved pharmacokinetics: a biodegradable and safe approach for effective antiplasmodial activity against P. falciparum 3D7 and in vivo hepatocyte targeting. Mol Pharm. 2017;14(10):3356–3369. doi: 10.1021/acs.molpharmaceut.7b00376. [DOI] [PubMed] [Google Scholar]
- 34.He Z., Shi Z., Sun W., Ma J., Xia J., Zhang X., et al. Hemocompatibility of folic-acid-conjugated amphiphilic PEG-PLGA copolymer nanoparticles for co-delivery of cisplatin and paclitaxel: treatment effects for non-small-cell lung cancer. Tumour Biol. 2016;37(6):7809–7821. doi: 10.1007/s13277-015-4634-1. [DOI] [PubMed] [Google Scholar]
- 35.Kaur N., Mathur P., Yadav P., Chakraborty S., Shanavas A. Glycol chitosan in situ coating on PLGA nanoparticle curtails extraneous paclitaxel precipitates and imparts protein corona independent hemocompatibility. Carbohydr Polym. 2020;237 doi: 10.1016/j.carbpol.2020.116170. [DOI] [PubMed] [Google Scholar]
- 36.Savin C.L., Popa M., Delaite C., Costuleanu M., Costin D., Peptu C.A. Chitosan grafted-poly(ethylene glycol) methacrylate nanoparticles as carrier for controlled release of bevacizumab. Mater Sci Eng C Mater Biol Appl. 2019;98:843–860. doi: 10.1016/j.msec.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 37.Song X., Wang J., Luo X., Xu C., Zhu A., Guo R., et al. Synthesis, biocompatible, and self-assembly properties of poly (ethylene glycol)/lactobionic acid-grafted chitosan. J Biomater Sci Polym Ed. 2014;25(10):1062–1075. doi: 10.1080/09205063.2014.918465. [DOI] [PubMed] [Google Scholar]
- 38.Sarika P.R., Anil Kumar P.R., Raj D.K., James N.R. Nanogels based on alginic aldehyde and gelatin by inverse miniemulsion technique: synthesis and characterization. Carbohydr Polym. 2015;119:118–125. doi: 10.1016/j.carbpol.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 39.Jayalekshmi A.C., Victor S.P., Sharma C.P. Magnetic and degradable polymer/bioactive glass composite nanoparticles for biomedical applications. Colloids Surf B Biointerfaces. 2013;101:196–204. doi: 10.1016/j.colsurfb.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 40.Verma A.K., Sachin K., Saxena A., Bohidar H.B. Release kinetics from bio-polymeric nanoparticles encapsulating protein synthesis inhibitor- cycloheximide, for possible therapeutic applications. Curr Pharm Biotechnol. 2005;6(2):121–130. doi: 10.2174/1389201053642349. [DOI] [PubMed] [Google Scholar]
- 41.Perez-Herrero E., Fernandez-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. doi: 10.1016/j.ejpb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Giselbrecht J., Wiedemann S., Reddy Pinnapireddy S., Goergen N., Loppnow H., Sedding D., et al. Nucleic acid carrier composed of a branched fatty acid lysine conjugate-interaction studies with blood components. Colloids Surf B Biointerfaces. 2019;184 doi: 10.1016/j.colsurfb.2019.110547. [DOI] [PubMed] [Google Scholar]
- 43.Kuznetsova N.R., Sevrin C., Lespineux D., Bovin N.V., Vodovozova E.L., Meszaros T., et al. Hemocompatibility of liposomes loaded with lipophilic prodrugs of methotrexate and melphalan in the lipid bilayer. J Control Release. 2012;160(2):394–400. doi: 10.1016/j.jconrel.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Kose G.T., Arica M.Y., Hasirci V. Low-molecular-weight heparin-conjugated liposomes with improved stability and hemocompatibility. Drug Deliv. 1998;5(4):257–264. doi: 10.3109/10717549809065756. [DOI] [PubMed] [Google Scholar]
- 45.Lakkadwala S., Dos Santos Rodrigues B., Sun C., Singh J. Dual functionalized liposomes for efficient co-delivery of anti-cancer chemotherapeutics for the treatment of glioblastoma. J Control Release. 2019;307:247–260. doi: 10.1016/j.jconrel.2019.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lakkadwala S., Singh J. Dual functionalized 5-fluorouracil liposomes as highly efficient nanomedicine for glioblastoma treatment as assessed in an in vitro brain tumor model. J Pharm Sci. 2018;107(11):2902–2913. doi: 10.1016/j.xphs.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michanetzis G.P., Markoutsa E., Mourtas S., Missirlis Y.F., Antimisiaris S.G. Hemocompatibility of amyloid and/or brain targeted liposomes. Future Med Chem. 2019;11(7):693–705. doi: 10.4155/fmc-2018-0236. [DOI] [PubMed] [Google Scholar]
- 48.Shehata T., Kimura T., Higaki K., Ogawara K.I. In-vivo disposition characteristics of PEG niosome and its interaction with serum proteins. Int J Pharm. 2016;512(1):322–328. doi: 10.1016/j.ijpharm.2016.08.058. [DOI] [PubMed] [Google Scholar]
- 49.Ullah S., Shah M.R., Shoaib M., Imran M., Shah S.W.A., Ahmed F., et al. Hydrophilically modified self-assembling alpha-tocopherol derivative as niosomal nanocarrier for improving clarithromycin oral bioavailability. Artif Cells Nanomed Biotechnol. 2018;46(3):568–578. doi: 10.1080/21691401.2017.1332633. [DOI] [PubMed] [Google Scholar]
- 50.Muzzalupo R., Tavano L., La Mesa C. Alkyl glucopyranoside-based niosomes containing methotrexate for pharmaceutical applications: evaluation of physico-chemical and biological properties. Int J Pharm. 2013;458(1):224–229. doi: 10.1016/j.ijpharm.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Ali I., Shah M.R., Yousuf S., Ahmed S., Shah K., Javed I. Hemolytic and cellular toxicology of a sulfanilamide-based nonionic surfactant: a niosomal carrier for hydrophobic drugs. Toxicol Res (Camb) 2018;7(5):771–778. doi: 10.1039/c8tx00108a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imran M., Shah M.R., Ullah F., Ullah S., Elhissi A.M., Nawaz W., et al. Glycoside-based niosomal nanocarrier for enhancedin-vivo performance of Cefixime. Int J Pharm. 2016;505(1–2):122–132. doi: 10.1016/j.ijpharm.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 53.Tao L., Uhrich K.E. Novel amphiphilic macromolecules and their in vitro characterization as stabilized micellar drug delivery systems. J Colloid Interface Sci. 2006;298(1):102–110. doi: 10.1016/j.jcis.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 54.Tavano L., Aiello R., Ioele G., Picci N., Muzzalupo R. Niosomes from glucuronic acid-based surfactant as new carriers for cancer therapy: preparation, characterization and biological properties. Colloids Surf B Biointerfaces. 2014;118:7–13. doi: 10.1016/j.colsurfb.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 55.Bardania H., Shojaosadati S.A., Kobarfard F., Morshedi D., Aliakbari F., Tahoori M.T., et al. RGD-modified nano-liposomes encapsulated eptifibatide with proper hemocompatibility and cytotoxicity effect. Iran J Biotechnol. 2019;17(2):e2008. doi: 10.21859/ijb.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plenagl N., Duse L., Seitz B.S., Goergen N., Pinnapireddy S.R., Jedelska J., et al. Photodynamic therapy - hypericin tetraether liposome conjugates and their antitumor and antiangiogenic activity. Drug Deliv. 2019;26(1):23–33. doi: 10.1080/10717544.2018.1531954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palazzo C., Laloy J., Delvigne A.S., Nys G., Fillet M., Dogne J.M., et al. Development of injectable liposomes and drug-in-cyclodextrin-in-liposome formulations encapsulating estetrol to prevent cerebral ischemia of premature babies. Eur J Pharm Sci. 2019;127:52–59. doi: 10.1016/j.ejps.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 58.Tang X., Sun J., Ge T., Zhang K., Gui Q., Zhang S., et al. PEGylated liposomes as delivery systems for Gambogenic acid: characterization and in vitro/in vivo evaluation. Colloid Surface B. 2018;172:26–36. doi: 10.1016/j.colsurfb.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 59.Liu D., Xing J., Xiong F., Yang F., Gu N. Preparation and in vivo safety evaluations of antileukemic homoharringtonine-loaded PEGylated liposomes. Drug Dev Ind Pharm. 2017;43(4):652–660. doi: 10.1080/03639045.2016.1275670. [DOI] [PubMed] [Google Scholar]
- 60.Banerjee K., Banerjee S., Das S., Mandal M. Probing the potential of apigenin liposomes in enhancing bacterial membrane perturbation and integrity loss. J Colloid Interface Sci. 2015;453:48–59. doi: 10.1016/j.jcis.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 61.Luo Q., Yang B., Tao W., Li J., Kou L., Lian H., et al. ATB(0,+) transporter-mediated targeting delivery to human lung cancer cells via aspartate-modified docetaxel-loading stealth liposomes. Biomater Sci. 2017;5(2):295–304. doi: 10.1039/c6bm00788k. [DOI] [PubMed] [Google Scholar]
- 62.Imran M., Shah M.R., Ullah F., Ullah S., Elhissi A.M., Nawaz W., et al. Sugar-based novel niosomal nanocarrier system for enhanced oral bioavailability of levofloxacin. Drug Deliv. 2016;23(9):3653–3664. doi: 10.1080/10717544.2016.1214991. [DOI] [PubMed] [Google Scholar]
- 63.Ullah S., Shah M.R., Shoaib M., Imran M., Elhissi A.M., Ahmad F., et al. Development of a biocompatible creatinine-based niosomal delivery system for enhanced oral bioavailability of clarithromycin. Drug Deliv. 2016;23(9):3480–3491. doi: 10.1080/10717544.2016.1196768. [DOI] [PubMed] [Google Scholar]
- 64.Lamch L., Kulbacka J., Dubinska-Magiera M., Saczko J., Wilk K.A. Folate-directed zinc (II) phthalocyanine loaded polymeric micelles engineered to generate reactive oxygen species for efficacious photodynamic therapy of cancer. Photodiagn Photodyn Ther. 2019;25:480–491. doi: 10.1016/j.pdpdt.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 65.Ozturk-Atar K., Kaplan M., Calis S. Development and evaluation of polymeric micelle containing tablet formulation for poorly water-soluble drug: tamoxifen citrate. Drug Dev Ind Pharm. 2020;46(10):1695–1704. doi: 10.1080/03639045.2020.1820037. [DOI] [PubMed] [Google Scholar]
- 66.Gao Y.E., Bai S., Ma X., Zhang X., Hou M., Shi X., et al. Codelivery of doxorubicin and camptothecin by dual-responsive unimolecular micelle-based beta-cyclodextrin for enhanced chemotherapy. Colloids Surf B Biointerfaces. 2019;183 doi: 10.1016/j.colsurfb.2019.110428. [DOI] [PubMed] [Google Scholar]
- 67.Kumari P., Muddineti O.S., Rompicharla S.V., Ghanta P., Karthik B.B.N.A., Ghosh B., et al. Cholesterol-conjugated poly(D, l-lactide)-based micelles as a nanocarrier system for effective delivery of curcumin in cancer therapy. Drug Deliv. 2017;24(1):209–223. doi: 10.1080/10717544.2016.1245365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dutta P., Dey J., Perumal V., Mandal M. Amino acid based amphiphilic copolymer micelles as carriers of non-steroidal anti-inflammatory drugs: solubilization, in vitro release and biological evaluation. Int J Pharm. 2011;407(1–2):207–216. doi: 10.1016/j.ijpharm.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 69.Ye L., Zhang Y., Yang B., Zhou X., Li J., Qin Z., et al. Zwitterionic-Modified Starch-Based Stealth Micelles for Prolonging Circulation Time and Reducing Macrophage Response. ACS Appl Mater Interfaces. 2016;8(7):4385–4398. doi: 10.1021/acsami.5b10811. [DOI] [PubMed] [Google Scholar]
- 70.Kojima M., Ishihara K., Watanabe A., Nakabayashi N. Interaction between phospholipids and biocompatible polymers containing a phosphorylcholine moiety. Biomaterials. 1991;12(2):121–124. doi: 10.1016/0142-9612(91)90189-h. [DOI] [PubMed] [Google Scholar]
- 71.Sabra S.A., Sheweita S.A., Haroun M., Ragab D., Eldemellawy M.A., Xia Y., et al. Magnetically guided self-assembled protein micelles for enhanced delivery of dasatinib to human triple-negative breast cancer cells. J Pharm Sci. 2019;108(5):1713–1725. doi: 10.1016/j.xphs.2018.11.044. [DOI] [PubMed] [Google Scholar]
- 72.Sabra S.A., Elzoghby A.O., Sheweita S.A., Haroun M., Helmy M.W., Eldemellawy M.A., et al. Self-assembled amphiphilic zein-lactoferrin micelles for tumor targeted co-delivery of rapamycin and wogonin to breast cancer. Eur J Pharm Biopharm. 2018;128:156–169. doi: 10.1016/j.ejpb.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 73.Raveendran R., Bhuvaneshwar G., Sharma C.P. In vitro cytotoxicity and cellular uptake of curcumin-loaded Pluronic/Polycaprolactone micelles in colorectal adenocarcinoma cells. J Biomater Appl. 2013;27(7):811–827. doi: 10.1177/0885328211427473. [DOI] [PubMed] [Google Scholar]
- 74.Shi S., Shi K., Tan L., Qu Y., Shen G., Chu B., et al. The use of cationic MPEG-PCL-g-PEI micelles for co-delivery of Msurvivin T34A gene and doxorubicin. Biomaterials. 2014;35(15):4536–4547. doi: 10.1016/j.biomaterials.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 75.Lin H., Wang Q., Zhong R., Li Z., Zhao W., Chen Y., et al. Biomimetic phosphorylcholine strategy to improve the hemocompatibility of pH-responsive micelles containing tertiary amino groups. Colloids Surf B Biointerfaces. 2019;184 doi: 10.1016/j.colsurfb.2019.110545. [DOI] [PubMed] [Google Scholar]
- 76.Bonde G.V., Ajmal G., Yadav S.K., Mittal P., Singh J., Bakde B.V., et al. Assessing the viability of Soluplus(R) self-assembled nanocolloids for sustained delivery of highly hydrophobic lapatinib (anticancer agent): optimisation and in-vitro characterisation. Colloids Surf B Biointerfaces. 2020;185 doi: 10.1016/j.colsurfb.2019.110611. [DOI] [PubMed] [Google Scholar]
- 77.Anwar D.M., Khattab S.N., Helmy M.W., Kamal M.K., Bekhit A.A., Elkhodairy K.A., et al. Lactobionic/folate dual-targeted amphiphilic maltodextrin-Based micelles for targeted codelivery of sulfasalazine and resveratrol to hepatocellular carcinoma. Bioconjug Chem. 2018;29(9):3026–3041. doi: 10.1021/acs.bioconjchem.8b00428. [DOI] [PubMed] [Google Scholar]
- 78.Debele T.A., Mekuria S.L., Tsai H.C. Synthesis and characterization of redox-sensitive heparin-beta-sitosterol micelles: their application as carriers for the pharmaceutical agent, doxorubicin, and investigation of their antimetastatic activities in vitro. Mater Sci Eng C Mater Biol Appl. 2017;75:1326–1338. doi: 10.1016/j.msec.2017.03.052. [DOI] [PubMed] [Google Scholar]
- 79.Zhang H., Xu J., Xing L., Ji J., Yu A., Zhai G. Self-assembled micelles based on Chondroitin sulfate/poly (d,l-lactideco-glycolide) block copolymers for doxorubicin delivery. J Colloid Interface Sci. 2017;492:101–111. doi: 10.1016/j.jcis.2016.12.046. [DOI] [PubMed] [Google Scholar]
- 80.Chen L., Ji F., Bao Y., Xia J., Guo L., Wang J., et al. Biocompatible cationic pullulan-g-desoxycholic acid-g-PEI micelles used to co-deliver drug and gene for cancer therapy. Mater Sci Eng C Mater Biol Appl. 2017;70(Pt 1):418–429. doi: 10.1016/j.msec.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 81.Kesharwani P., Banerjee S., Padhye S., Sarkar F.H., Iyer A.K. Parenterally administrable nano-micelles of 3,4-difluorobenzylidene curcumin for treating pancreatic cancer. Colloids Surf B Biointerfaces. 2015;132:138–145. doi: 10.1016/j.colsurfb.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 82.Wang J., Xu W., Ding J., Lu S., Wang X., Wang C., et al. Cholesterol-enhanced polylactide-based stereocomplex micelle for effective delivery of doxorubicin. Materials (Basel) 2015;8(1):216–230. doi: 10.3390/ma8010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin W., He Y., Zhang J., Wang L., Wang Z., Ji F., et al. Highly hemocompatible zwitterionic micelles stabilized by reversible cross-linkage for anti-cancer drug delivery. Colloids Surf B Biointerfaces. 2014;115:384–390. doi: 10.1016/j.colsurfb.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 84.Zhang X., Qiao H., Chen Y., Li L., Xia H., Shi Y. Preparation, properties and preclinical pharmacokinetics of low molecular weight heparin-modified isoliquiritigenin-loaded solid lipid nanoparticle. Iran J Pharm Res. 2016;15(3):269–282. [PMC free article] [PubMed] [Google Scholar]
- 85.Munoz de Escalona M., Saez-Fernandez E., Prados J.C., Melguizo C., Arias J.L. Magnetic solid lipid nanoparticles in hyperthermia against colon cancer. Int J Pharm. 2016;504(1–2):11–19. doi: 10.1016/j.ijpharm.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 86.Soni M.P., Shelkar N., Gaikwad R.V., Vanage G.R., Samad A., Devarajan P.V. Buparvaquone loaded solid lipid nanoparticles for targeted delivery in theleriosis. J Pharm Bioallied Sci. 2014;6(1):22–30. doi: 10.4103/0975-7406.124309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kotmakci M., Akbaba H., Erel G., Ertan G., Kantarci G. Improved method for solid lipid nanoparticle preparation based on hot microemulsions: preparation, characterization, cytotoxicity, and hemocompatibility evaluation. AAPS PharmSciTech. 2017;18(4):1355–1365. doi: 10.1208/s12249-016-0606-z. [DOI] [PubMed] [Google Scholar]
- 88.Ridha A.A., Kashanian S., Azandaryani A.H., Rafipour R., Mahdavian E. New folate-modified human serum albumin conjugated to cationic lipid carriers for dual targeting of mitoxantrone against breast cancer. Curr Pharm Biotechnol. 2020;21(4):305–315. doi: 10.2174/1389201020666191114113022. [DOI] [PubMed] [Google Scholar]
- 89.Nimtrakul P., Tiyaboonchai W., Lamlertthon S. Amphotericin B Loaded nanostructured lipid carriers for parenteral delivery: characterization, antifungal and in vitro toxicity assessment. Curr Drug Deliv. 2019;16(7):645–653. doi: 10.2174/1567201816666190729145223. [DOI] [PubMed] [Google Scholar]
- 90.Kanwar R., Gradzielski M., Prevost S., Kaur G., Clemens D., Appavou M.S., et al. Effect of lipid chain length on nanostructured lipid carriers: comprehensive structural evaluation by scattering techniques. J Colloid Interface Sci. 2019;534:95–104. doi: 10.1016/j.jcis.2018.08.066. [DOI] [PubMed] [Google Scholar]
- 91.Joshi M., Pathak S., Sharma S., Patravale V. Design and in vivo pharmacodynamic evaluation of nanostructured lipid carriers for parenteral delivery of artemether: nanoject. Int J Pharm. 2008;364(1):119–126. doi: 10.1016/j.ijpharm.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 92.Kudarha R., Dhas N.L., Pandey A., Belgamwar V.S., Ige P.P. Box-Behnken study design for optimization of bicalutamide-loaded nanostructured lipid carrier: stability assessment. Pharm Dev Technol. 2015;20(5):608–618. doi: 10.3109/10837450.2014.908305. [DOI] [PubMed] [Google Scholar]
- 93.Wang J.J., Liu K.S., Sung K.C., Tsai C.Y., Fang J.Y. Lipid nanoparticles with different oil/fatty ester ratios as carriers of buprenorphine and its prodrugs for injection. Eur J Pharm Sci. 2009;38(2):138–146. doi: 10.1016/j.ejps.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 94.Aldawsari H.M., Singh S. Rapid microwave-assisted cisplatin-loaded solid lipid nanoparticles: synthesis, characterization and anticancer study. Nanomaterials (Basel) 2020;10(3) doi: 10.3390/nano10030510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Joshy K.S., Sharma C.P., Kalarikkal N., Sandeep K., Thomas S., Pothen L.A. Evaluation of in-vitro cytotoxicity and cellular uptake efficiency of zidovudine-loaded solid lipid nanoparticles modified with Aloe Vera in glioma cells. Mater Sci Eng C Mater Biol Appl. 2016;66:40–50. doi: 10.1016/j.msec.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 96.Kolenyak-Santos F., Garnero C., de Oliveira R.N., de Souza A.L., Chorilli M., Allegretti S.M., et al. Nanostructured lipid carriers as a srategy to improve the in vitro schistosomiasis activity of praziquantel. J Nanosci Nanotechnol. 2015;15(1):761–772. doi: 10.1166/jnn.2015.9186. [DOI] [PubMed] [Google Scholar]
- 97.Kanwar R., Gradzielski M., Prevost S., Appavou M.S., Mehta S.K. Experimental validation of biocompatible nanostructured lipid carriers of sophorolipid: optimization, characterization and in-vitro evaluation. Colloids Surf B Biointerfaces. 2019;181:845–855. doi: 10.1016/j.colsurfb.2019.06.036. [DOI] [PubMed] [Google Scholar]
- 98.Shevalkar G., Pai R., Vavia P. Nanostructured lipid carrier of propofol: a promising alternative to marketed soybean oil-based nanoemulsion. AAPS PharmSciTech. 2019;20(5):201. doi: 10.1208/s12249-019-1408-x. [DOI] [PubMed] [Google Scholar]
- 99.Harisa G.I., Alomrani A.H., Badran M.M. Simvastatin-loaded nanostructured lipid carriers attenuate the atherogenic risk of erythrocytes in hyperlipidemic rats. Eur J Pharm Sci. 2017;96:62–71. doi: 10.1016/j.ejps.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 100.Khurana S., Jain N.K., Bedi P.M. Development and characterization of a novel controlled release drug delivery system based on nanostructured lipid carriers gel for meloxicam. Life Sci. 2013;93(21):763–772. doi: 10.1016/j.lfs.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 101.Li X., Du L., Wang C., Liu Y., Mei X., Jin Y. Highly efficient and lowly toxic docetaxel nanoemulsions for intravenous injection to animals. Pharmazie. 2011;66(7):479–483. [PubMed] [Google Scholar]
- 102.Ferreira L.M., Cervi V.F., Gehrcke M., da Silveira E.F., Azambuja J.H., Braganhol E., et al. Ketoprofen-loaded pomegranate seed oil nanoemulsion stabilized by pullulan: selective antiglioma formulation for intravenous administration. Colloids Surf B Biointerfaces. 2015;130:272–277. doi: 10.1016/j.colsurfb.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 103.Hussain A., Altamimi M.A., Alshehri S., Imam S.S., Shakeel F., Singh S.K. Novel approach for transdermal delivery of rifampicin to induce synergistic antimycobacterial effects against cutaneous and systemic tuberculosis using a cationic nanoemulsion gel. Int J Nanomedicine. 2020;15:1073–1094. doi: 10.2147/IJN.S236277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fang J.Y., Hung C.F., Hua S.C., Hwang T.L. Acoustically active perfluorocarbon nanoemulsions as drug delivery carriers for camptothecin: drug release and cytotoxicity against cancer cells. Ultrasonics. 2009;49(1):39–46. doi: 10.1016/j.ultras.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 105.Alayoubi A., Alqahtani S., Kaddoumi A., Nazzal S. Effect of PEG surface conformation on anticancer activity and blood circulation of nanoemulsions loaded with tocotrienol-rich fraction of palm oil. AAPS J. 2013;15(4):1168–1179. doi: 10.1208/s12248-013-9525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hussain A., Shakeel F., Singh S.K., Alsarra I.A., Faruk A., Alanazi F.K., et al. Solidified SNEDDS for the oral delivery of rifampicin: evaluation, proof of concept, in vivo kinetics, and in silico GastroPlus(TM) simulation. Int J Pharm. 2019;566:203–217. doi: 10.1016/j.ijpharm.2019.05.061. [DOI] [PubMed] [Google Scholar]
- 107.Khan M., Nadhman A., Sehgal S.A., Siraj S., Yasinzai M.M. Formulation and characterization of a self-Emulsifying drug delivery system (SEDDS) of curcumin for the topical application in cutaneous and mucocutaneous leishmaniasis. Curr Top Med Chem. 2018;18(18):1603–1609. doi: 10.2174/1568026618666181025104818. [DOI] [PubMed] [Google Scholar]
- 108.Karami Z., Khoshkam M., Hamidi M. Optimization of olive oil-Based nanoemulsion preparation for intravenous drug delivery. Drug Res (Stuttg) 2019;69(5):256–264. doi: 10.1055/a-0654-4867. [DOI] [PubMed] [Google Scholar]
- 109.Hussain A., Singh S., Webster T.J., Ahmad F.J. New perspectives in the topical delivery of optimized amphotericin B loaded nanoemulsions using excipients with innate anti-fungal activities: a mechanistic and histopathological investigation. Nanomedicine. 2017;13(3):1117–1126. doi: 10.1016/j.nano.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 110.Mota Ferreira L., Gehrcke M., Ferrari Cervi V., Eliete Rodrigues Bitencourt P., Ferreira da Silveira E., Hofstatter Azambuja J., et al. Pomegranate seed oil nanoemulsions with selective antiglioma activity: optimization and evaluation of cytotoxicity, genotoxicity and oxidative effects on mononuclear cells. Pharm Biol. 2016;54(12):2968–2977. doi: 10.1080/13880209.2016.1199039. [DOI] [PubMed] [Google Scholar]
- 111.Shukla P., Dwivedi P., Gupta P.K., Mishra P.R. Optimization of novel tocopheryl acetate nanoemulsions for parenteral delivery of curcumin for therapeutic intervention of sepsis. Expert Opin Drug Deliv. 2014;11(11):1697–1712. doi: 10.1517/17425247.2014.932769. [DOI] [PubMed] [Google Scholar]
- 112.Mahmoud D.B., Shukr M.H., Bendas E.R. In vitro and in vivo evaluation of self-nanoemulsifying drug delivery systems of cilostazol for oral and parenteral administration. Int J Pharm. 2014;476(1–2):60–69. doi: 10.1016/j.ijpharm.2014.09.045. [DOI] [PubMed] [Google Scholar]
- 113.Prasad R., Aiyer S., Chauhan D.S., Srivastava R., Selvaraj K. Bioresponsive carbon nano-gated multifunctional mesoporous silica for cancer theranostics. Nanoscale. 2016;8(8):4537–4546. doi: 10.1039/c5nr06756a. [DOI] [PubMed] [Google Scholar]
- 114.He Q., Zhang J., Shi J., Zhu Z., Zhang L., Bu W., et al. The effect of PEGylation of mesoporous silica nanoparticles on nonspecific binding of serum proteins and cellular responses. Biomaterials. 2010;31(6):1085–1092. doi: 10.1016/j.biomaterials.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 115.Wang Y., Han N., Zhao Q., Bai L., Li J., Jiang T., et al. Redox-responsive mesoporous silica as carriers for controlled drug delivery: a comparative study based on silica and PEG gatekeepers. Eur J Pharm Sci. 2015;72:12–20. doi: 10.1016/j.ejps.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 116.Yu T., Malugin A., Ghandehari H. Impact of silica nanoparticle design on cellular toxicity and hemolytic activity. ACS Nano. 2011;5(7):5717–5728. doi: 10.1021/nn2013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu B., Wang J., Li J., Li S., Li H. Superiority of l-tartaric acid modified chiral mesoporous silica nanoparticle as a drug carrier: structure, wettability, degradation, bio-adhesion and biocompatibility. Int J Nanomedicine. 2020;15:601–608. doi: 10.2147/IJN.S233740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li H., Wu X., Yang B., Li J., Xu L., Liu H., et al. Evaluation of biomimetically synthesized mesoporous silica nanoparticles as drug carriers: structure, wettability, degradation, biocompatibility and brain distribution. Mater Sci Eng C Mater Biol Appl. 2019;94:453–464. doi: 10.1016/j.msec.2018.09.053. [DOI] [PubMed] [Google Scholar]
- 119.Wang J., Wang Y., Liu Q., Yang L., Zhu R., Yu C., et al. Rational design of multifunctional dendritic mesoporous silica nanoparticles to load curcumin and enhance efficacy for breast cancer therapy. ACS Appl Mater Interfaces. 2016;8(40):26511–26523. doi: 10.1021/acsami.6b08400. [DOI] [PubMed] [Google Scholar]
- 120.Abbaraju P.L., Meka A.K., Song H., Yang Y., Jambhrunkar M., Zhang J., et al. Asymmetric silica nanoparticles with tunable head-tail structures enhance hemocompatibility and maturation of immune cells. J Am Chem Soc. 2017;139(18):6321–6328. doi: 10.1021/jacs.6b12622. [DOI] [PubMed] [Google Scholar]
- 121.Feng W., Nie W., He C., Zhou X., Chen L., Qiu K., et al. Effect of pH-responsive alginate/chitosan multilayers coating on delivery efficiency, cellular uptake and biodistribution of mesoporous silica nanoparticles based nanocarriers. ACS Appl Mater Interfaces. 2014;6(11):8447–8460. doi: 10.1021/am501337s. [DOI] [PubMed] [Google Scholar]
- 122.Roggers R.A., Joglekar M., Valenstein J.S., Trewyn B.G. Mimicking red blood cell lipid membrane to enhance the hemocompatibility of large-pore mesoporous silica. ACS Appl Mater Interfaces. 2014;6(3):1675–1681. doi: 10.1021/am4045713. [DOI] [PubMed] [Google Scholar]
- 123.Zhao Y., Sun X., Zhang G., Trewyn B.G., Slowing I.I., Lin V.S. Interaction of mesoporous silica nanoparticles with human red blood cell membranes: size and surface effects. ACS Nano. 2011;5(2):1366–1375. doi: 10.1021/nn103077k. [DOI] [PubMed] [Google Scholar]
- 124.Ma Z., Bai J., Wang Y., Jiang X. Impact of shape and pore size of mesoporous silica nanoparticles on serum protein adsorption and RBCs hemolysis. ACS Appl Mater Interfaces. 2014;6(4):2431–2438. doi: 10.1021/am404860q. [DOI] [PubMed] [Google Scholar]
- 125.Lin Y.S., Haynes C.L. Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J Am Chem Soc. 2010;132(13):4834–4842. doi: 10.1021/ja910846q. [DOI] [PubMed] [Google Scholar]
- 126.Zhou X., Cheng X., Feng W., Qiu K., Chen L., Nie W., et al. Synthesis of hollow mesoporous silica nanoparticles with tunable shell thickness and pore size using amphiphilic block copolymers as core templates. Dalton Trans. 2014;43(31):11834–11842. doi: 10.1039/c4dt01138d. [DOI] [PubMed] [Google Scholar]
- 127.He Q., Zhang J., Chen F., Guo L., Zhu Z., Shi J. An anti-ROS/hepatic fibrosis drug delivery system based on salvianolic acid B loaded mesoporous silica nanoparticles. Biomaterials. 2010;31(30):7785–7796. doi: 10.1016/j.biomaterials.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 128.Feng W., Zhou X., He C., Qiu K., Nie W., Chen L., et al. Polyelectrolyte multilayer functionalized mesoporous silica nanoparticles for pH-responsive drug delivery: layer thickness-dependent release profiles and biocompatibility. J Mater Chem B. 2013;1(43):5886–5898. doi: 10.1039/c3tb21193b. [DOI] [PubMed] [Google Scholar]
- 129.Chen Z.A., Wu S.H., Chen P., Chen Y.P., Mou C.Y. Critical features for mesoporous silica nanoparticles encapsulated into erythrocytes. ACS Appl Mater Interfaces. 2019;11(5):4790–4798. doi: 10.1021/acsami.8b18434. [DOI] [PubMed] [Google Scholar]
- 130.Liu X., Ding Y., Zhao B., Liu Y., Luo S., Wu J., et al. In vitro and in vivo evaluation of puerarin-loaded PEGylated mesoporous silica nanoparticles. Drug Dev Ind Pharm. 2016;42(12):2031–2037. doi: 10.1080/03639045.2016.1190742. [DOI] [PubMed] [Google Scholar]
- 131.Bafkary R., Ahmadi S., Fayazi F., Karimi M., Fatahi Y., Ebrahimi S.M., et al. Amphiphilic hyperbranched polyester coated rod mesoporous silica nanoparticles for pH-responsive doxorubicin delivery. J Pharm Sci. 2020;28(1):171–180. doi: 10.1007/s40199-020-00328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Saroj S., Rajput S.J. Etoposide encased folic acid adorned mesoporous silica nanoparticles as potent nanovehicles for enhanced prostate cancer therapy: synthesis, characterization, cellular uptake and biodistribution. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S1115–S1130. doi: 10.1080/21691401.2018.1533843. [DOI] [PubMed] [Google Scholar]
- 133.Shah P.V., Rajput S.J. Facile Synthesis of chitosan capped mesoporous silica nanoparticles: a pH responsive smart delivery platform for raloxifene hydrochloride. AAPS PharmSciTech. 2018;19(3):1344–1357. doi: 10.1208/s12249-017-0949-0. [DOI] [PubMed] [Google Scholar]
- 134.Wu F., Xu T., Zhao G., Meng S., Wan M., Chi B., et al. Mesoporous silica nanoparticles-encapsulated agarose and heparin as anticoagulant and resisting bacterial adhesion coating for biomedical silicone. Langmuir. 2017;33(21):5245–5252. doi: 10.1021/acs.langmuir.7b00567. [DOI] [PubMed] [Google Scholar]
- 135.Saroj S., Rajput S.J. Facile development, characterization, and evaluation of novel bicalutamide loaded pH-sensitive mesoporous silica nanoparticles for enhanced prostate cancer therapy. Drug Dev Ind Pharm. 2019;45(4):532–547. doi: 10.1080/03639045.2018.1562463. [DOI] [PubMed] [Google Scholar]
- 136.Saroj S., Rajput S.J. Tailor-made pH-sensitive polyacrylic acid functionalized mesoporous silica nanoparticles for efficient and controlled delivery of anti-cancer drug Etoposide. Drug Dev Ind Pharm. 2018;44(7):1198–1211. doi: 10.1080/03639045.2018.1438467. [DOI] [PubMed] [Google Scholar]
- 137.Ngamcherdtrakul W., Morry J., Gu S., Castro D.J., Goodyear S.M., Sangvanich T., et al. Cationic polymer modified mesoporous silica nanoparticles for targeted SiRNA delivery to HER2+ breast cancer. Adv Funct Mater. 2015;25(18):2646–2659. doi: 10.1002/adfm.201404629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gautam S.P., Gupta A.K., Gupta R., Gautam T., Singh M.P. Apple Academic Press Inc; 2018. Dendrimers: a glimpse of history, current progress, and applications; pp. 213–236. [Google Scholar]
- 139.Gu Z., Wang M., Fang Q., Zheng H., Wu F., Lin D., et al. Preparation and in vitro characterization of pluronic-attached polyamidoamine dendrimers for drug delivery. Drug Dev Ind Pharm. 2015;41(5):812–818. doi: 10.3109/03639045.2014.908899. [DOI] [PubMed] [Google Scholar]
- 140.Mishra V., Gupta U., Jain N.K. Influence of different generations of poly(propylene imine) dendrimers on human erythrocytes. Pharmazie. 2010;65(12):891–895. [PubMed] [Google Scholar]
- 141.Han M.H., Chen J., Wang J., Chen S.L., Wang X.T. Blood compatibility of polyamidoamine dendrimers and erythrocyte protection. J Biomed Nanotechnol. 2010;6(1):82–92. doi: 10.1166/jbn.2010.1096. [DOI] [PubMed] [Google Scholar]
- 142.Cui Y., Liang B., Wang L., Zhu L., Kang J., Sun H., et al. Enhanced biocompatibility of PAMAM dendrimers benefiting from tuning their surface charges. Mater Sci Eng C Mater Biol Appl. 2018;93:332–340. doi: 10.1016/j.msec.2018.07.070. [DOI] [PubMed] [Google Scholar]
- 143.Halets I., Shcharbin D., Klajnert B., Bryszewska M. Contribution of hydrophobicity, DNA and proteins to the cytotoxicity of cationic PAMAM dendrimers. Int J Pharm. 2013;454(1):1–3. doi: 10.1016/j.ijpharm.2013.06.061. [DOI] [PubMed] [Google Scholar]
- 144.Wang L., Wang Z., Ma G., Lin W., Chen S. Reducing the cytotoxity of poly(amidoamine) dendrimers by modification of a single layer of carboxybetaine. Langmuir. 2013;29(28):8914–8921. doi: 10.1021/la400623s. [DOI] [PubMed] [Google Scholar]
- 145.Gupta U., Dwivedi S.K.D., Bid H.K., Konwar R., Jain N.K. Ligand anchored dendrimers based nanoconstructs for effective targeting to cancer cells. Int J Pharmaceut. 2010;393(1–2):186–197. doi: 10.1016/j.ijpharm.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 146.Zhou Y., Li J., Lu F., Deng J., Zhang J., Fang P., et al. A study on the hemocompatibility of dendronized chitosan derivatives in red blood cells. Drug Des Devel Ther. 2015;9:2635–2645. doi: 10.2147/DDDT.S77105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen H.T., Neerman M.F., Parrish A.R., Simanek E.E. Cytotoxicity, hemolysis, and acute in vivo toxicity of dendrimers based on melamine, candidate vehicles for drug delivery. J Am Chem Soc. 2004;126(32):10044–10048. doi: 10.1021/ja048548j. [DOI] [PubMed] [Google Scholar]
- 148.Wang W., Xiong W., Zhu Y., Xu H., Yang X. Protective effect of PEGylation against poly(amidoamine) dendrimer-induced hemolysis of human red blood cells. J Biomed Mater Res B Appl Biomater. 2010;93(1):59–64. doi: 10.1002/jbm.b.31558. [DOI] [PubMed] [Google Scholar]
- 149.Klajnert B., Appelhans D., Komber H., Morgner N., Schwarz S., Richter S., et al. The influence of densely organized maltose shells on the biological properties of poly(propylene imine) dendrimers: new effects dependent on hydrogen bonding. Chemistry (Easton) 2008;14(23):7030–7041. doi: 10.1002/chem.200800342. [DOI] [PubMed] [Google Scholar]
- 150.Kumar P.V., Asthana A., Dutta T., Jain N.K. Intracellular macrophage uptake of rifampicin loaded mannosylated dendrimers. J Drug Target. 2006;14(8):546–556. doi: 10.1080/10611860600825159. [DOI] [PubMed] [Google Scholar]
- 151.Bhadra D., Yadav A.K., Bhadra S., Jain N.K. Glycodendrimeric nanoparticulate carriers of primaquine phosphate for liver targeting. Int J Pharm. 2005;295(1–2):221–233. doi: 10.1016/j.ijpharm.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 152.Li G., Zhang Y., Tang W., Zheng J. Comprehensive investigation of in vitro hemocompatibility of surface modified polyamidoamine nanocarrier. Clin Hemorheol Microcirc. 2020;74(3):267–279. doi: 10.3233/CH-190641. [DOI] [PubMed] [Google Scholar]
- 153.Liu Y., Pang Y., Toh M.R., Chiu G.N. Dual-functionalized poly(amidoamine) dendrimers with poly(ethylene glycol) conjugation and thiolation improved blood compatibility. J Pharm Pharmacol. 2015;67(11):1492–1502. doi: 10.1111/jphp.12457. [DOI] [PubMed] [Google Scholar]
- 154.Prieto M.J., Temprana C.F., del Rio Zabala N.E., Marotta C.H. Alonso Sdel V. Optimization and in vitro toxicity evaluation of G4 PAMAM dendrimer-risperidone complexes. Eur J Med Chem. 2011;46(3):845–850. doi: 10.1016/j.ejmech.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 155.Qi R., Gao Y., Tang Y., He R.R., Liu T.L., He Y., et al. PEG-conjugated PAMAM dendrimers mediate efficient intramuscular gene expression. AAPS J. 2009;11(3):395–405. doi: 10.1208/s12248-009-9116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]