Significance
The physiological and ecological importance of natural products often remains obscured. Here, we report that Streptomyces-derived marginolactones, a distinct group of soil-borne natural products, specifically trigger the formation of gloeocapsoids, previously undescribed protective aggregate structures produced by the unicellular green alga Chlamydomonas reinhardtii. Gloeocapsoids are distinct palmelloids differing in their protective capability toward azalomycin F. The presence of marginolactone biosynthesis gene clusters in numerous streptomycetes, their ubiquity in soil, and our observation that three different members of this natural product group trigger the formation of gloeocapsoids suggest a cross-kingdom competition with ecological relevance. In the course of evolution, the polysaccharide matrix may have developed from a transient protective feature into the foundation of true multicellularity because of sustained marginolactone stress.
Keywords: microbial interaction, multicellularity, evolution, natural products, morphology
Abstract
Photosynthetic microorganisms including the green alga Chlamydomonas reinhardtii are essential to terrestrial habitats as they start the carbon cycle by conversion of CO2 to energy-rich organic carbohydrates. Terrestrial habitats are densely populated, and hence, microbial interactions mediated by natural products are inevitable. We previously discovered such an interaction between Streptomyces iranensis releasing the marginolactone azalomycin F in the presence of C. reinhardtii. Whether the alga senses and reacts to azalomycin F remained unknown. Here, we report that sublethal concentrations of azalomycin F trigger the formation of a protective multicellular structure by C. reinhardtii, which we named gloeocapsoid. Gloeocapsoids contain several cells which share multiple cell membranes and cell walls and are surrounded by a spacious matrix consisting of acidic polysaccharides. After azalomycin F removal, gloeocapsoid aggregates readily disassemble, and single cells are released. The presence of marginolactone biosynthesis gene clusters in numerous streptomycetes, their ubiquity in soil, and our observation that other marginolactones such as desertomycin A and monazomycin also trigger the formation of gloeocapsoids suggests a cross-kingdom competition with ecological relevance. Furthermore, gloeocapsoids allow for the survival of C. reinhardtii at alkaline pH and otherwise lethal concentrations of azalomycin F. Their structure and polysaccharide matrix may be ancestral to the complex mucilage formed by multicellular members of the Chlamydomonadales such as Eudorina and Volvox. Our finding suggests that multicellularity may have evolved to endure the presence of harmful competing bacteria. Additionally, it underlines the importance of natural products as microbial cues, which initiate interesting ecological scenarios of attack and counter defense.
Soil is an extremely densely populated habitat, and hence, microbial interactions are nearly inevitable (1). Green algae are often overlooked when soil ecosystems are discussed despite their pivotal role as primary producers of organic carbohydrates fixing roughly 39 g carbon per square meter of soil per year (2, 3). As photosynthetic microorganisms, algae convert CO2 to energy-rich organic carbon sources and thereby refuel the carbon cycle. The green alga Chlamydomonas reinhardtii is a common member of wet soils and is hence subject to biotic and abiotic stress (2, 4, 5). Stress leads to the development of adaptive mechanisms that enable survival and increase the evolutionary fitness of microorganisms. Chlamydomonas spp. react to NaCl stress (6), Ca2+ deprivation, ethylenediaminetetraacetic acid (EDTA), organic acids (7, 8), chloroplatinic acid (9), phosphate limitation (10), acidic pH (11), and sublethal levels of pollutants (12) with the production of palmelloids, which are characterized by multiple rounds of failure of daughter cells to escape the parental cell wall after cell division. Chlorella vulgaris forms palmelloids when treated with ecdysteroids (13). The term palmelloid is a catch-all term that originates in reference to the genus of green algae Palmella, which typically grows in a cell cluster (6, 14). Chlamydomonas spp. can also actively produce aggregates which are phenotypically different to palmelloids and serve to defend the algae against harsh stress (14–16).
In addition to abiotic stressors, there are also biotic stressors such as competition and predation. When C. reinhardtii is threatened by the rotifer predator Brachionus calyciflorus, the green alga forms palmelloids (17). Paramecium tetraurelia is also a predator of C. reinhardtii, which likely drove the evolution of C. reinhardtii into multicellular aggregates in response to this constant pressure by predation (18). Palmelloid aggregates are too big in diameter to be engulfed by P. tetraurelia and thus are protected. Another biotic stress is exerted by natural products, low molecular mass compounds with various biological activities often produced by neighboring microorganisms (19). For instance, the bacterium Pseudomonas protegens produces the cyclic lipopeptide orfamide A, which leads to the deflagellation of C. reinhardtii (20). Other microorganisms accompanying algae in wet soils are streptomycetes, known to be prolific producers of natural products (1). These filamentous bacteria are known to readily interact with other microorganisms. A well-known example is given by Streptomyces iranensis that induces otherwise silent natural product biosynthesis gene clusters in the fungi Aspergillus nidulans and Aspergillus fumigatus (21, 22). The products of A. nidulans, orsellinic acid, and lecanoric acid represent metabolites first described in lichens (23). A. fumigatus reacts to the streptomycete by the production of fumigermin, which inhibits the germination of spores of several Streptomyces species (22). However, these interactions are not exclusive to fungi but also include algae. Recently, we demonstrated that S. iranensis specifically released the algicidal marginolactone azalomycin F (SI Appendix, Fig. 1A) in the presence of C. reinhardtii. The alga survived this attack by taking shelter in the mycelium of A. nidulans (24). Whether the alga senses azalomycin F and reacts to the compound remained unknown. Here, we describe the discovery of an alternative strategy for the algae to cope with toxin stress without a fungal partner: the formation of so-called gloeocapsoids, a distinct type of palmelloids differing in function and based on a close resemblance of the gloeocapsoid structure to cyanobacteria of the genera Gloeocapsa and Gloeothece (25). We propose that gloeocapsoid formation is an active protective behavior induced by membrane-targeting marginolactones like azalomycin F, desertomycin A, and monazomycin. Additionally, we propose that the formation of transient cell aggregates like gloeocapsoids could have led to the evolution of multicellular green algae such as Eudorina and Volvox.
Results
Sublethal Azalomycin F Induces the Formation of Multicellular Aggregates.
C. reinhardtii is protected from otherwise lethal concentrations of azalomycin F when it is in contact with the filamentous fungus A. nidulans (24). We aimed to address the question of whether the alga can also survive without a partner microorganism. When grown on Tris-acetate-phosphate (TAP) agar, C. reinhardtii raised the local pH, indicated by the pH indicator dye bromothymol blue (SI Appendix, Fig. 2A) (26). This alkalization is due to an uptake of acetate from the medium. Previously, streptomycetes were shown to raise the surrounding pH by the release of volatile ammonia to inhibit neighboring bacteria (27). Alkaline pH, in turn, decreased killing of C. reinhardtii by S. iranensis as well as the activity of azalomycin F in solid medium (SI Appendix, Fig. 2 B and C). To evaluate reduced toxicity in liquid medium, purified azalomycin F was added to C. reinhardtii cultured in media with a pH range of 7 to 9. As a proxy for viability, the autofluorescence of C. reinhardtii chlorophyll was quantified (28). At pH 7, 5 μg ⋅ mL−1, azalomycin F was sufficient to kill most C. reinhardtii cells (SI Appendix, Fig. 2D), an effect not observed when the alga was cultivated in medium with pH 8 or 9. When C. reinhardtii was grown in TAP medium at pH 8 or 9, the autofluorescence dropped after 24 h but recovered after 3 to 4 d and reached levels of the untreated controls. Recovery from azalomycin F exposure was not observed in C. reinhardtii grown at pH 7. Samples taken from cultures at pH 8 and 9 and treated with azalomycin F formed an algal lawn on TAP agar, demonstrating the survival of algal cells, while agar plates from cultures treated with azalomycin F at pH 7 only showed few algal colonies (SI Appendix, Fig. 3).
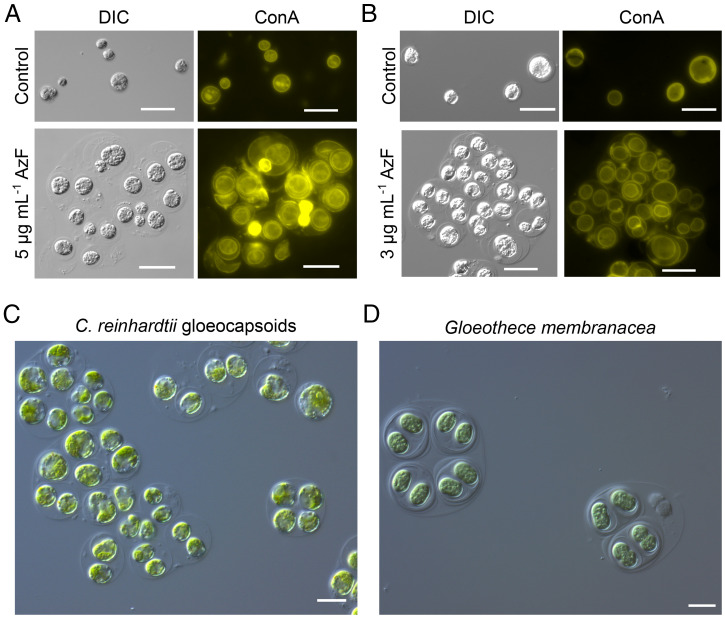
Microscopic inspection of C. reinhardtii treated with 5 μg ⋅ mL−1 azalomycin F at pH 8 after 7 d of incubation revealed that multicellular aggregates surrounded by a spacious matrix had formed (Fig. 1A). The staining of these aggregates with fluorescently labeled concanavalin A binding to α-mannopyranosyl and α-glucopyranosyl residues found in the cell wall and the plasma membrane of C. reinhardtii illustrates the presence of multiple cell envelopes around individual cells (Fig. 1A). To study whether these aggregates are formed only at alkaline pH, C. reinhardtii was treated with 3 μg ⋅ mL−1 azalomycin F at pH 7. In this case, C. reinhardtii formed the same type of aggregate structures (Fig. 1B), suggesting that the activity of azalomycin F decreases at alkaline pH. The staining of the plasma membrane with CellMask indicated the presence of multiple plasma membranes in addition to cell walls surrounding the aggregates (SI Appendix, Fig. 4A). The matrix apparently consisted of acidic polysaccharides as indicated with Alcian blue staining (SI Appendix, Fig. 4B). Alcian blue stains sulfate groups of polysaccharides at pH 0.5 (in 0.5 M HCl), and at pH 2.5 (in 0.5 M acetic acid), it stains other acidic functional groups (29). Blue staining of the matrix was only observed at pH 2.5, which indicates nonsulfate acidic polysaccharides. Due to the similarity of the aggregates to the vegetative growth form of cyanobacteria of the genera Gloeocapsa and Gloeothece (25), we called these structures gloeocapsoids (Fig. 1 C and D). They differ morphologically from both palmelloids and autospores, which lack a spacious matrix and contain fewer cell membranes (14, 30). Although palmelloids can be induced by different stressors, in this study, we applied two stressors, that is, high salt and sodium citrate to induce palmelloids that served as reference. NaCl-induced palmelloids accumulated lipids (6), whereas gloeocapsoids and sodium citrate–induced palmelloids appear to contain few lipid bodies (SI Appendix, Fig. 5). This indicates a certain degree of heterogeneity between palmelloids. In contrast to palmelloids that are surrounded by a single outer membrane (6, 18), gloeocapsoids are surrounded by several membranes. (SI Appendix, Fig. 4A). Gloeocapsoids and palmelloids have in common that in cultures synchronized by light–dark cycles, the mother cells divide during the dark phase, and the daughter cells fail to detach from each other (SI Appendix, Fig. 6) (31). Hence, we suggest gloeocapsoids are a distinct form of palmelloids formed to protect C. reinhardtii from azalomycin F.
Fig. 1.
C. reinhardtii forms gloeocapsoids in contact with sublethal concentrations of azalomycin F. (A) C. reinhardtii in TAP medium at pH 8 treated with or without 5 μg ⋅ mL−1 azalomycin F. (B) C. reinhardtii at pH 7 treated with or without 3 μg ⋅ mL−1 azalomycin F. (Scale bars: 20 μm.) (C) Light microscopy picture of C. reinhardtii gloeocapsoids. (D) Light microscopy picture of vegetative cells of G. membranacea. (Scale bars: 10 μm.)
Gloeocapsoids Form Unusual Pseudomulticellular Structures.
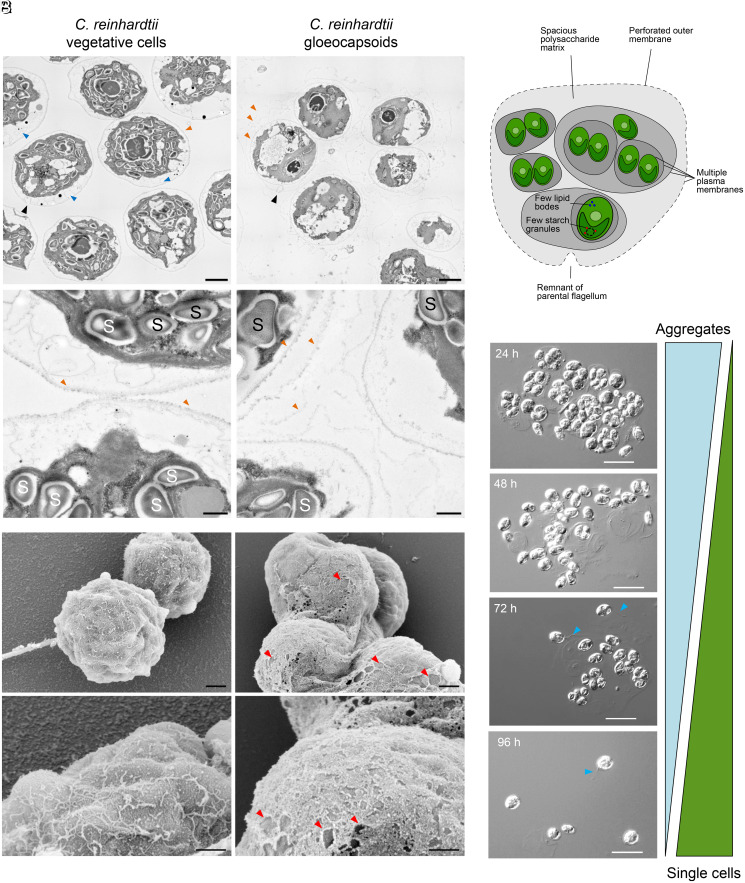
To further substantiate our observation that C. reinhardtii gloeocapsoids are surrounded by multiple cell membranes and to shed further light on their structure, transmission electron microscopy images were taken. As shown in Fig. 2A (orange arrows), gloeocapsoids are characterized by multiple membranes and cell walls. Interestingly, one outer membrane of a gloeocapsoid showed the characteristic indent formed by one of the flagella (Fig. 2A, black arrows) (32), indicating that the outer membrane is a remnant of a formerly flagellated mother cell. It is possible that after division of the mother cell, the daughter cells produced a new membrane but did not separate and instead maintained the original cell membrane. Whether azalomycin F targets the flagella or not remains to be determined. We suggest deflagellation might be due to stress or impaired cell membrane integrity. Additionally, as indicated by scanning electron microscopy, the outermost cell membrane of C. reinhardtii gloeocapsoids appears to be perforated in comparison to vegetative cells (Fig. 2B), forming a net-like structure rather than a coherent membrane. Whether azalomycin F punctures holes into the outer cell membrane or whether the mechanical force exerted by the growing daughter cells is responsible for the torn outer membrane and cell wall remains to be determined.
Fig. 2.
Comparison of the ultrastructure of C. reinhardtii vegetative cells with gloeocapsoids and disassembly of gloeocapsoids after stress relief. (A) Transmission electron microscopy pictures of vegetative C. reinhardtii cells (Left) and gloeocapsoids (Right). Vegetative cells are surrounded by a single membrane (orange arrows) and contain multiple starch granules (“S”; 26.2 ± 7.9 per cell; n = 5). Gloeocapsoids are surrounded by up to three membranes (orange arrows) and a polysaccharide matrix. In the individual cells, there are fewer starch granules (“S”; 6.2 ± 2.9 per cell; n = 5). In their periphery, vegetative cells show secretory vesicles (blue arrows), which are missing in cells embedded in gloeocapsoids. (Scale bars: 2 μm [Top] and 500 nm [Bottom]). (B) Scanning electron microscopy pictures of C. reinhardtii vegetative cells (Left) and gloeocapsoids (Right). The outer membrane of vegetative cells is intact. In gloeocapsoids, the outer membrane exhibits a perforated net-like structure (holes are marked with red arrows). (Scale bars: 1 μm [Top] and 500 nm [Bottom]). (C) Schematic model of the structure of a gloeocapsoid. (D) Disassembly of gloeocapsoids after removal of azalomycin F. Flagellated single cells dominated the culture 96 h after azalomycin F was removed. Visible flagella are indicated with blue arrows. (Scale bars: 20 μm.)
The gloeocapsoids of C. reinhardtii appear to carry fewer intracellular starch granules in proximity to the pyrenoid within the chloroplast. Untreated cells contained 26.2 ± 7.9 (n = 5) starch granules per cell with 6.2 ± 2.9 in the gloeocapsoids (n = 5, Fig. 2A, marked “S”). Additionally, vegetative C. reinhardtii cells showed multiple secretory vesicles in the cell periphery (Fig. 2A, blue arrows), which were completely absent in the gloeocapsoids. The absence of secretory vesicles, which are often associated with cross-species communication (33, 34), supports the idea that C. reinhardtii cells are isolated and thus protected against external toxin stress within gloeocapsoids. The typical gloeocapsoid features described here are summarized in a schematic model in Fig. 2C.
Gloeocapsoids Are Transiently Formed Structures.
Evolution experiments have shown that multicellular aggregates can evolve after repeated subcultivation under selection pressure caused by a predator. These evolved multicellular aggregates remain stable for many generations in the absence of selection pressure (18). By contrast, palmelloids induced by stress conditions were found to be transient (6, 12). Therefore, we addressed the question whether gloeocapsoids would still remain stable when azalomycin F pressure was relieved. As shown in Fig. 2D, gloeocapsoids rapidly disassembled, and flagellated single cells were detected in cultures within 4 d of cultivation after azalomycin F was removed. This indicated that gloeocapsoids are formed transiently as long as cells are exposed to stress.
Desertomycin A and Monazomycin Are Overproduced by Streptomycetes in the Presence of C. reinhardtii.
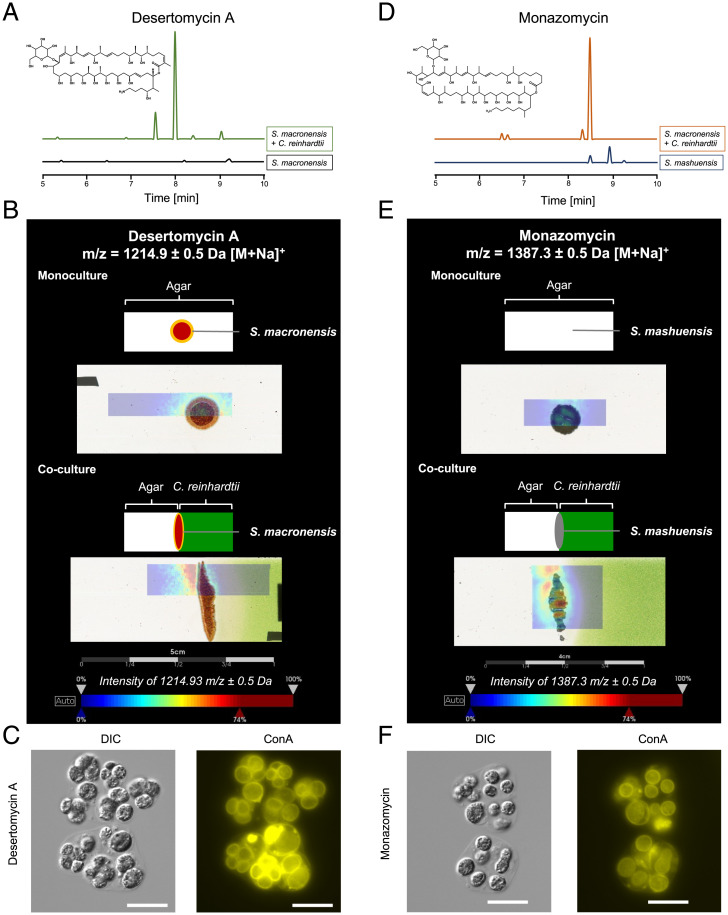
Marginolactones are macrolides whose biosynthesis is initiated by an arginine- or ornithine-derived starter unit (35). The marginolactones azalomycin F, desertomycin A, and monazomycin share similar chemical structures (SI Appendix, Fig. 1 A–C) and are known to disturb cell membranes (36–38). Azalomycin F is specifically released by S. iranensis in the presence of C. reinhardtii (24). Therefore, we tested whether streptomycetes encoding the biosynthetic gene clusters for desertomycin A and monazomycin respond to C. reinhardtii. Liquid chromatography–mass spectrometry analyses demonstrated that in the presence of C. reinhardtii, Streptomyces macronensis overproduced desertomycin A (Fig. 3 A and B). Likewise, Streptomyces mashuensis produced increased amounts of monazomycin in the presence of the alga (Fig. 3 D and E). As shown by matrix-assisted laser desorption/ionization imaging mass spectrometry, both compounds were found in the killing zone of C. reinhardtii produced by the streptomycetes (Fig. 3 B and E). This indicates that marginolactones may serve to specifically target green algae since their production is triggered by C. reinhardtii.
Fig. 3.
Desertomycin A and monazomycin are overproduced by steptomycetes in coculture with C. reinhardtii, colocalize with C. reinhardtii killing zone on solid medium, and induce gloeocapsoid formation. (A) Extracted ion chromatogram of an extract of a coculture of C. reinhardtii with S. macronensis in liquid culture compared with a monoculture of the streptomycete. m/z 1,191.2 to 1,192.2 [M – H]– corresponding to desertomycin A. (B) Matrix-assisted laser desorption/ionization imaging mass spectrometry (MALDI-IMS) for desertomycin A. The microorganisms were grown directly on the object slide. The top picture of the mono- and the coculture represents a schematic overview of the position of the bacterial colony on TAP agar (monoculture) or on a split agar containing C. reinhardtii only in the right agar half (coculture). The bottom pictures show the overlays of the MALDI-IMS image indicating the color-coded abundance of ion m/z 1,214.9 ± 0.5 Da (desertomycin A, [M + Na]+) and the actual culture. (C) C. reinhardtii treated with 1.5 μg ⋅ mL−1 desertomycin A forms gloeocapsoids. (Scale bars: 20 μm.) (D) Extracted ion chromatogram of an extract of a coculture of C. reinhardtii with S. mashuensis compared to a monoculture of the streptomycete. Extracted ion chromatogram of m/z 1,363.5 to 1,364.5 [M – H]– corresponding to monazomycin. (E) MALDI-IMS analysis. (Top) S. mashuensis colony on axenic TAP agar. (Bottom) S. mashuensis on TAP agar containing C. reinhardtii. MALDI-IMS images indicate color-coded abundance of ion m/z 1,387.3 ± 0.5 Da (monazomycin, [M + Na]+). Color code: blue, low abundance and red, high abundance of ion. Mass spectrometry (MS) spectra of liquid chromatography–MS analyses were compared to an authentical standard for identification. (F) C. reinhardtii treated with 2.5 μg ⋅ mL−1 monazomycin with gloeocapsoid formation. (Scale bars: 20 μm.)
Marginolactones Trigger the Formation of Gloeocapsoids.
To investigate whether desertomycin A and monazomycin induce the formation of gloeocapsoids, we added both compounds to C. reinhardtii. Sublethal amounts of both marginolactones triggered gloeocapsoid formation by C. reinhardtii (Fig. 3 C and F and SI Appendix, Fig. 7). This result was not surprising given their considerable structural similarity to azalomycin F. Because these compounds disturb membrane integrity, we reasoned that other membrane-active compounds might also trigger gloeocapsoid formation. This was not the case as concluded from experiments with the membrane-targeting but structurally dissimilar compounds amphotericin B and daptomycin (SI Appendix, Fig. 1 D and E). Amphotericin B is an antimycotic macrolide, which targets ergosterols in algae (39). Sublethal concentrations of amphotericin B did not trigger gloeocapsoid formation in C. reinhardtii (SI Appendix, Fig. 8A). At 2 μg ⋅ mL−1, amphotericin B was lethal to C. reinhardtii (SI Appendix, Fig. 8 B and C). Daptomycin is a well-known cyclic lipopeptide antibiotic, which interferes with bacterial membrane microdomains (40). As shown by the lack of formation of a polysaccharide matrix and missing multiple cell membrane layers, daptomycin only induced the formation of aggregates in C. reinhardtii when applied at sublethal concentrations (SI Appendix, Fig. 9A). An application of 20 μg ⋅ mL−1 daptomycin was lethal for C. reinhardtii (SI Appendix, Fig. 9 B and C). We thus concluded that only marginolactones such as azalomycin F, desertomycin A, and monazomycin induced formation of gloeocapsoids since natural products with a different structural scaffold triggered phenotypically different aggregates or canonical palmelloids.
Gloeocapsoids Protect C. reinhardtii against Alkaline pH and Azalomycin F.
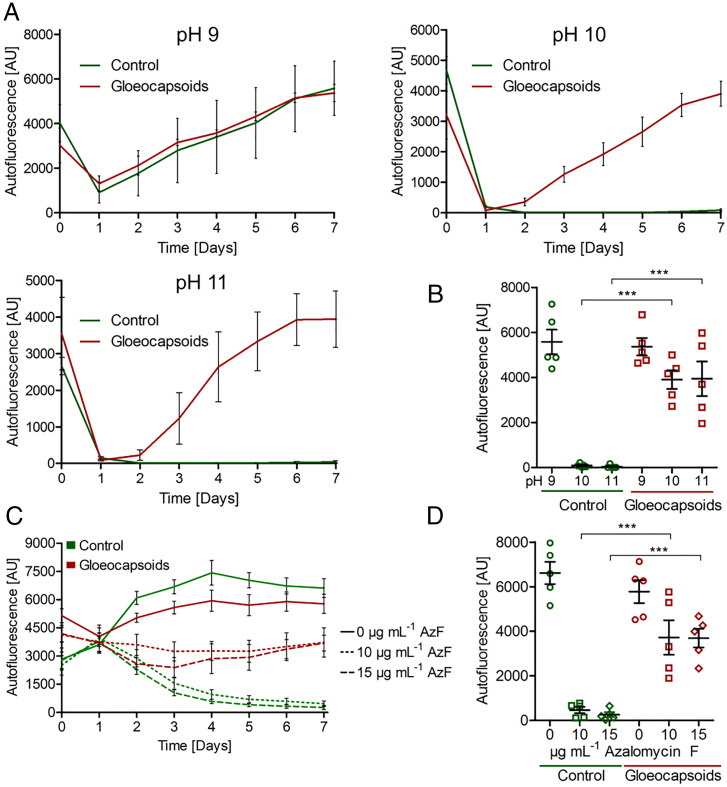
Aggregate structures are often associated with protection (14). As gloeocapsoids are readily formed at neutral and alkaline pH under treatment with azalomycin F, we investigated their capacity to protect encapsulated cells. For this purpose, gloeocapsoid formation was induced by sublethal concentrations of azalomycin F at neutral pH. Then, gloeocapsoids and untreated control cells were inoculated in media with pH values between 9 and 11. After an initial drop, the autofluorescence of gloeocapsoid-derived cells readily recovered at pH 10 and 11, while that of the untreated cells showed no recovery (Fig. 4A). After 7 d of incubation at pH 10 and 11, the autofluorescence of gloeocapsoid-derived cells showed a significantly higher autofluorescence than the control cells (Fig. 4B). This was further demonstrated by plating the cells on TAP agar after 7 d of incubation at pH 9 to 11 (SI Appendix, Fig. 10A), in which the cells derived from gloeocapsoids incubated at pH 10 and 11 formed colonies, an effect not observed for vegetative cells incubated at the same pH.
Fig. 4.
Gloeocapsoid structures confer resistance to internalized cells against external stressors. (A) Control cells and gloeocapsoids of C. reinhardtii were inoculated into TAP media covering the pH range from 9 to 11. At pH 9, both vegetative cells and gloeocapsoid-derived cells grew. At pH 10 and pH 11, gloeocapsoid-derived cells regenerated after an initial drop in autofluorescence, whereas control cells showed no recovery. (B) Autofluorescence at day 7 of C. reinhardtii control cells and gloeocapsoids incubated in TAP medium from pH 7 to 9. (C) C. reinhardtii vegetative cells as well as gloeocapsoids were treated with 10 and 15 μg ⋅ mL−1 azalomycin F, and autofluorescence was monitored for 7 d. (D) Final autofluorescence at day 7 of C. reinhardtii control cells and gloeocapsoids treated with 0, 10, and 15 μg ⋅ mL−1 azalomycin F. For each time point, n ≥ 4; SEMs are shown. ***P ≤ 0.001 calculated using one-way ANOVA.
Next, the resistance of gloeocapsoids against lethal azalomycin F concentrations was tested. While the autofluorescence of untreated cells decreased steadily under the treatment with the toxin, the autofluorescence of gloeocapsoids remained stable (Fig. 4C). After 7 d of incubation, azalomycin F–treated gloeocapsoids showed a significantly higher autofluorescence compared to their azalomycin F–treated vegetative counterparts (Fig. 4D). Additionally, after 7 d of incubation, the cells were plated on TAP agar plates. Gloeocapsoids treated with 10 and 15 μg ⋅ mL−1 azalomycin F formed lawns of C. reinhardtii colonies, while vegetative cells treated with the same amounts of toxin only formed very few colonies (SI Appendix, Fig. 10B). By contrast, palmelloids induced by the addition of 100 mM NaCl and 15 mM sodium citrate to the medium did not protect C. reinhardtii against lethal concentrations of azalomycin F (SI Appendix, Figs. 11–14). Taken together, our data indicate that in contrast to canonical palmelloids, gloeocapsoids protect C. reinhardtii against alkaline pH and against azalomycin F at otherwise lethal concentrations.
Discussion
Multicellular Gloeocapsoids Represent a Defense Strategy against Both Alkaline pH and Azalomycin F.
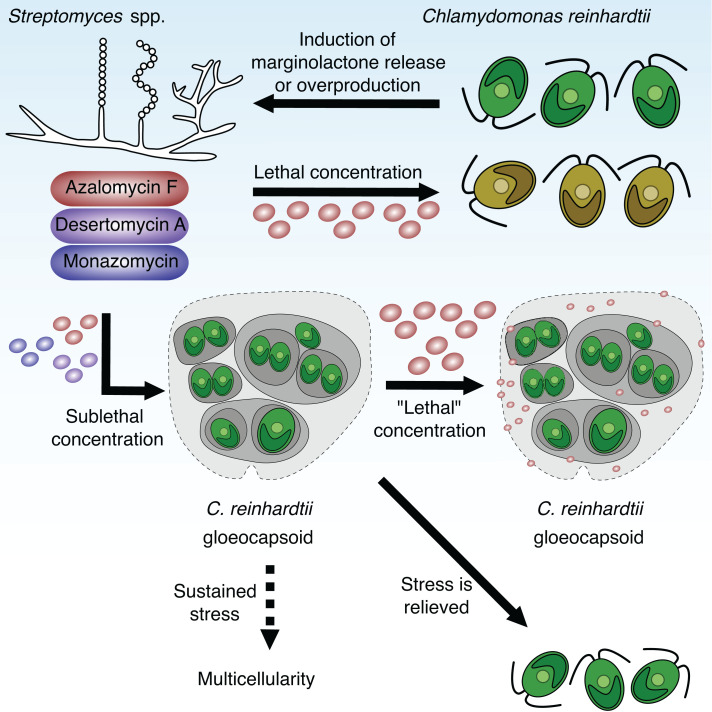
Microorganisms face everchanging conditions in their respective habitats. Because of acids or bases released by microorganisms or farming, the environmental pH values can vary considerably between 3 and 10 (27, 41). By the uptake of acetate, C. reinhardtii increased the pH around its colonies. Under these conditions, the natural product azalomycin F produced by S. iranensis displayed reduced algicidal activity (SI Appendix, Fig. 2). This explains why a higher azalomycin F concentration was needed to induce gloeocapsoids in C. reinhardtii at pH 8 than at pH 7. It is remarkable that gloeocapsoids protect C. reinhardtii both against alkaline pH and azalomycin F since streptomycetes can increase their surrounding pH by the secretion of ammonia (27). Thus, an increase in pH in combination with algicidal marginolactones are indicators for the presence of competing streptomycetes. As a response, C. reinhardtii forms gloeocapsoids that represent a protective response to the presence of these harmful bacteria (Fig. 5).
Fig. 5.
Schematic overview of the interaction of Streptomyces spp. with C. reinhardtii. C. reinhardtii induces the release of the marginolactone azalomycin F as well as the overproduction and release of desertomycin A and monazomycin in cocultures with the bacteria Streptomyces iranensis, Streptomyces macronensis, and Streptomyces mashuensis. Lethal concentrations of these marginolactones lead to bleaching and death of C. reinhardtii. Sublethal concentrations trigger the formation of multicellular gloeocapsoids in which the individual cells are surrounded by multiple cell walls, membranes, and a spacious polysaccharide matrix. These aggregates protect the individual cells against otherwise lethal azalomycin F concentrations as well as alkaline pH, indicating the presence of competing streptomycetes. We hypothesize that the additional membranes and the acidic polysaccharide matrix sequester the positively charged azalomycin F, and hence, the algicide cannot reach the individual algal cells. After stress is relieved, the gloeocapsoids disassemble and motile single cells emerge. We hypothesize that sustained sublethal stress leads to the evolution of a multicellular organism exhibiting differentiated cell types and division of labor.
We have shown that C. reinhardtii required up to 7 d to develop gloeocapsoids, and therefore concentrations of 10 and 15 μg ⋅ mL−1 of azalomycin F killed non-pretreated algal cells within 24 h (Fig. 5) (24). C. reinhardtii only formed resistant gloeocapsoids at sublethal azalomycin F concentrations. This reflects an ecological scenario in which C. reinhardtii is not instantly confronted with high concentrations of an algicide but rather with a concentration gradient. It is worth noting that this might be a reason for the unusual release mechanism of azalomycin F. S. iranensis steadily produces the compound but only secretes it in the presence of C. reinhardtii (24). Production, storage, and massive release of the algicide upon contact with C. reinhardtii might be a strategy of the bacteria to circumvent the algal resistance mechanism by instantly creating a high concentration of azalomycin F.
Multicellularity Is a Widespread Protective Mechanism against Environmental Stressors.
We provide evidence that aggregation facilitates the protection of single cells against environmental stressors. This is in accordance with observations made for, e.g., Pseudomonas aeruginosa, that produces biofilms in contact with antibiotics (42) or when experiencing DNA replication stress (43). A number of stressors induce the formation of palmelloids and actively formed aggregates in C. reinhardtii (14). Most likely, they play a role in protecting C. reinhardtii against these stressors. Since the main difference of NaCl- and sodium citrate–induced palmelloids and azalomycin F–induced gloeocapsoids is their protection against azalomycin F (Fig. 4 and SI Appendix, Figs. 11–14), we assigned gloeocapsoids to the family of palmelloids as specialized structures for defense against azalomycin F.
The matrix of gloeocapsoids is composed of acidic polysaccharides, which may have the capacity to buffer alkaline pH and might help to maintain an internal pH near 7. Similarly, sulfate-reducing bacteria buffer the surrounding pH by secretion of exopolysaccharides (44). The acidic polysaccharide matrix could also explain the resistance of gloeocapsoids against azalomycin F (Fig. 4 C and D and SI Appendix, Fig. 10B). We hypothesize that the guanidyl moiety of azalomycin F is positively charged at physiological pH and thus may be sequestered by the negatively charged acidic polysaccharides of the extracellular matrix. Furthermore, membranes are known targets of azalomycin F (24). Since there are several membrane layers present in gloeocapsoids (SI Appendix, Fig. 4A), this might promote sequestration of azalomycin F over a larger surface area (Fig. 2B). Possibly, the additional surrounding membranes sequester marginolactones and thus protect the cytoplasmic membranes of cells lying underneath (Fig. 5). A similar protective mechanism appears to be realized in the tripartite system examined recently. The mycelium of A. nidulans is proposed to provide an increased surface area composed of polar lipids that function to sequester free azalomycin F, thereby protecting the cohabitating C. reinhardtii (24).
The formation of multicellular gloeocapsoids represents a protective strategy that does not require any other microbial partner organism. From an evolutionary point of view, such a strategy is in accordance with the theory that multicellularity initially evolved for survival in a hostile environment (45). Multicellularity is the fundamental step in the evolution of all higher organisms such as animals (46, 47) and enables maintenance of internal homeostasis even in the presence of osmotic stress, extreme pH, toxins, or desiccation (45, 48). This is also evident in the multicellular volvocine algae of the order Chlamydomonadales, Pleodorina and Volvox (49). These algae produce extracellular matrices, which enable the differentiation of soma and germ cells and also protect offspring developing inside the parent spheroid (49). Here, we report that C. reinhardtii produces a similar type of polysaccharide matrix. We highlight that the ability to form polysaccharide matrices is already present in the most basal member of the volvocine algae and might have been fundamental for the evolution of true multicellularity. This is also reflected in the low number of species-specific genes in Volvox carteri compared to C. reinhardtii (50). However, an essential difference found between Volvox and C. reinhardtii gloeocapsoids is the missing cytoplasmic connection between the individual cells, which enables Volvox cells to synchronize their behavior (51). Additionally, the polysaccharide matrix found in gloeocapsoids is linked to specific bacterial triggers and thus is formed for protection against harmful bacteria and could have later resulted in an evolution of multicellularity as suggested by similar experiments from Khona et al. (6). We hypothesize that multicellular green algae might have evolved by the sustained presence of bacterial marginolactones granting protection against these widespread algicides.
Algicidal Marginolactones Are Widespread in Nature.
The marginolactones azalomycin F, desertomycin A, and monazomycin exhibit similar structures and target the cell membrane (37, 38, 52, 53). Sublethal doses of these natural products induce the formation of gloeocapsoids in C. reinhardtii. By contrast, amphotericin B and daptomycin, which also target the membrane by binding to ergosterol (39) or interfering with membrane microdomains (54), respectively, did not induce formation of gloeocapsoids (SI Appendix, Figs. 7–9). Thus, it is not merely membrane stress that leads to gloeocapsoids but rather the specific activity of marginolactones. For desertomycin A, it was shown that its activity depends on the primary amine on the side chain (55). This is supported by the observation that inactive members of the desertomycin family, the oasomycins, lack a primary amine in their side chain. The substitution of this side chain with side chains containing a primary or secondary amine created oasomycin derivatives with antibacterial activity (55). Our data further support the notion that the activity of marginolactones relies on their positively charged side chain.
Since marginolactones have been isolated from a number of actinobacteria, it can be expected that these compounds are widespread in soil habitats (56, 57). Bioinformatic analyses show that the azalomycin F biosynthetic gene cluster is present in a number of streptomycetes isolated from diverse soil samples across the world (58–61). We previously showed that azalomycin F was specifically released by S. iranensis in the presence of C. reinhardtii and possesses algicidal activity against several algal species (24). Similarly, we demonstrated here that S. macronensis and S. mashuensis overproduced their respective marginolactones in the presence of C. reinhardtii (Fig. 3). It is tempting to speculate that the streptomycetes create a spatially confined lethal concentration of marginolactones in soil to kill neighboring algae. It is worth noting that desertomycin G was isolated from a marine streptomycete present on the surface of a macroalga (62).
Our data suggest that marginolactones may have evolved to target algae. The widespread distribution of marginolactone-producing bacteria makes it likely that the compounds affect C. reinhardtii in its natural habitat. The alga in turn developed a particular strategy for defense against these compounds that includes the aggregation of cells, production of a polysaccharide matrix, and maintenance of multiple cell membranes. This structure, which we named gloeocapsoid, allows C. reinhardtii to cope with marginolactones and alkaline pH stress.
Materials and Methods
Microbial Strains and Plasmids.
Microbial strains used in this study are listed in SI Appendix, Table 1.
Production and Purification of Azalomycin F from S. iranensis.
Azalomycin F was produced and purified as described in Krespach et al. (24).
Preparation of Solid TAP Medium with pH Indicator and for Evaluation of Killing of C. reinhardtii by S. iranensis.
TAP agar was prepared as published by Gorman and Levine (63). As a pH indicator, 0.03 g ⋅ L−1 bromothymol blue (Sigma-Aldrich) was added prior to the autoclaving of the medium. For TAP agar without acetate, the pH of the agar was adjusted to 6 by the addition of HCl. C. reinhardtii was spot inoculated and incubated at 26 °C and 30 μE ⋅ m−2 ⋅ s−1.
For the evaluation of the killing of the algae by S. iranensis, C. reinhardtii was centrifuged with 2,000 × g for 2 min and inoculated into agar that was allowed to cool to ∼35 °C. The final optical density at a wavelength of 750 nm (OD750) was adjusted to 2. S. iranensis was washed with TAP medium (centrifugation 12,000 × g for 1 min), and 15 μL was spot inoculated onto agar containing C. reinhardtii. The coculture was incubated at 26 °C and 30 μE ⋅ m−2 ⋅ s−1 and after 7 d, pictures were taken of the agar plates.
Staining Methods.
The staining of C. reinhardtii with concanavalin A, Alcian blue, SYTOX blue, CellMask, and Nile red are explained in SI Appendix, Staining methods.
Induction of Formation of Gloeocapsoids for Resistance Assays.
For the standardized induction of the formation of gloeocapsoids, 200 μL C. reinhardtii cell suspension (OD750 = 1) was precultured in TAP medium pH 7 for 7 d at 26 °C and 120 rpm at 30 μE ⋅ m−2 ⋅ s−1 with 3 μg ⋅ mL−1 azalomycin F in 96-well plates. Control cells were not treated with the compound. After 7 d, cells from the same treatment were pooled and centrifuged at 2,000 × g for 2 min. The pellet was resuspended in fresh TAP medium to create a suspension with a final autofluorescence of 3,000. For the evaluation of resistance against pH, the pH of the fresh TAP medium was set to a final pH of 9 to 11 using NaOH. The test was carried out by the inoculation of 200 μL cell suspension into each well of a 96-well plate.
For testing resistance against azalomycin F, the cell pellet was resuspended in TAP medium of pH 7 to achieve a final autofluorescence of 3,000 (9 × 105 – 1 × 106 cells mL−1), and 200 μL culture was inoculated into each well of a 96-well plate. Either 10 or 15 μg ⋅ mL−1 azalomycin F were added to each well when indicated. Incubation was carried out at 26 °C, 120 rpm, and 30 μE ⋅ m−2 ⋅ s−1. Each 96-well plate was incubated at 26 °C, 120 rpm, and 30 μE ⋅ m−2 ⋅ s−1. Autofluorescence, a proxy for the viability of C. reinhardtii (28), was measured after excitation at 480 nm, and emission was recorded at 684 nm on a Tecan Infinite M200 pro microplate reader (Tecan Trading AG).
Electron Microscopy.
Sample preparation and electron microscopy are described in SI Appendix, Electron microscopy.
Supplementary Material
Acknowledgments
We thank Maria Mittag and Daniel Scheer for sharing their valuable expertise in the biology of C. reinhardtii. Volker Schroeckh is thanked for his continuous support and helpful critical discussions. Christina Täumer is acknowledged for excellent technical support. We are grateful to Hendrik Huthoff for the critical reading of the manuscript. This work was funded by the Deutsche Forschungsgemeinschaft (German Research Foundation)—Project-ID 239748522—Sonderforschungsbereich (SFB) 1127, A02, B01, B02, and the Cluster of Excellence Balance of the Microverse—Project-ID 390713860, Gepris 2051.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2100892118/-/DCSupplemental.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2100892118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
Change History
July 22, 2022: The editor’s affiliation has been updated to correct an error. Jens Nielsen’s affiliation is: BioInnovation Institute, DK2200 Copenhagen, Denmark.
References
- 1.Netzker T., et al. , Microbial interactions trigger the production of antibiotics. Curr. Opin. Microbiol. 45, 117–123 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Sasso S., Stibor H., Mittag M., Grossman A. R., From molecular manipulation of domesticated Chlamydomonas reinhardtii to survival in nature. eLife 7, e39233 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimmel S. M., Darley W. M., Productivity and density of soil algae in an agricultural system. Ecology 66, 1439–1447 (1985). [Google Scholar]
- 4.Merchant S. S., et al. , The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318, 245–250 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vrieze J., The littlest farmhands. Science 349, 680–683 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Khona D. M., et al. , Characterization of salt stress-induced palmelloids in the green alga, Chlamydomonas reinhardtii. Algal Res. 16, 434–448 (2016). [Google Scholar]
- 7.Iwasa K., Murakami S., Palmelloid formation of Chlamydomonas I. Palmelloid induction by organic acids. Physiol. Plant. 21, 1224–1233 (1968). [Google Scholar]
- 8.Iwasa K., Murakami S., Palmelloid formation of Chlamydomonas II. Mechanism of palmelloid formation by organic acids. Physiol. Plant. 22, 43–50 (1969). [Google Scholar]
- 9.Nakamura K., Bray D. F., Wagenaar E. B., Ultrastructure of Chlamydomonas eugametos palmelloids induced by chloroplatinic acid treatment. J. Bacteriol. 121, 338–343 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen Y., Knutsen G., Lien T., Characteristics of phosphorus limitation in Chlamydomonas reinhardtii (Chlorophycaceae) and its palmelloids. J. Phycol. 19, 313–319 (1983). [Google Scholar]
- 11.Visviki I., Santikul D., The pH tolerance of Chlamydomonas applanata (Volvocales, Chlorophyta). Arch. Environ. Contam. Toxicol. 38, 147–151 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Cheloni G., Slaveykova V. I., Morphological plasticity in Chlamydomonas reinhardtii and acclimation to micropollutant stress. Aquat. Toxicol. 231, 105711 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Bajguz A., Dinan L., Effects of ecdysteroids on Chlorella vulgaris. Physiol. Plant. 121, 349–357 (2004). [Google Scholar]
- 14.de Carpentier F., Lemaire S. D., Danon A., When unity is strength: The strategies used by Chlamydomonas to survive environmental stresses. Cells 8, 1307 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sathe S., Durand P. M., Cellular aggregation in Chlamydomonas (Chlorophyceae) is chimaeric and depends on traits like cell size and motility. Eur. J. Phycol. 51, 129–138 (2016). [Google Scholar]
- 16.Fan J., Zheng L., Bai Y., Saroussi S., Grossman A. R., Flocculation of Chlamydomonas reinhardtii with different phenotypic traits by metal cations and high pH. Front. Plant Sci. 8, 1997 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lurling M., Beekman W., Palmelloids formation in Chlamydomonas reinhardtii: Defence against rotifer predators? Ann. Limnol. 42, 65–72 (2006). [Google Scholar]
- 18.Herron M. D., et al. , De novo origins of multicellularity in response to predation. Sci. Rep. 9, 2328 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brakhage A. A., Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 11, 21–32 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Aiyar P., et al. , Antagonistic bacteria disrupt calcium homeostasis and immobilize algal cells. Nat. Commun. 8, 1756 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeckh V., et al. , Intimate bacterial-fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc. Natl. Acad. Sci. U.S.A. 106, 14558–14563 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stroe M. C., et al. , Targeted induction of a silent fungal gene cluster encoding the bacteria-specific germination inhibitor fumigermin. eLife 9, e52541 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer J., et al. , Chromatin mapping identifies BasR, a key regulator of bacteria-triggered production of fungal secondary metabolites. eLife 7, e40969 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krespach M. K. C., et al. , Lichen-like association of Chlamydomonas reinhardtii and Aspergillus nidulans protects algal cells from bacteria. ISME J. 14, 2794–2805 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox G., Benson D., Dwarte D. M., Ultrastructure of a cave-wall cyanophyte-Gloeocapsa NS4. Arch. Microbiol. 130, 165–174 (1981). [Google Scholar]
- 26.Shiraiwa Y., Goyal A., Tolbert N. E., Alkalization of the medium by unicellular green algae during uptake dissolved inorganic carbon. Plant Cell Physiol. 34, 649–657 (1993). [Google Scholar]
- 27.Avalos M., Garbeva P., Raaijmakers J. M., van Wezel G. P., Production of ammonia as a low-cost and long-distance antibiotic strategy by Streptomyces species. ISME J. 14, 569–583 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pouneva I., Evaluation of algal culture viability and physiological state by fluorescent microscopic methods. Bulg. J. Plant Physiol. 13, 67–76 (1997). [Google Scholar]
- 29.Van Boekel W., Phaeocystis colony mucus components and the importance of calcium ions for colony stability. Mar. Ecol. Prog. Ser. 87, 301–305 (1992). [Google Scholar]
- 30.Pollio A., et al. , Chlamydomonas pitschmannii Ettl, a little known species from thermoacidic environments. Protist 156, 287–302 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Harris E. H., “Cell division” in The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use, Harris E. H., Stern D. B., Witman G. B., Eds. (Elsevier Science, 2009), vol. 1, pp. 65–87. [Google Scholar]
- 32.Harris E. H., “Basal bodies, flagellar roots, and cellular filaments” in The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use, Harris E. H., Stern D. B., Witman G. B., Eds. (Elsevier Science, 2009), vol. 1, pp. 44–48. [Google Scholar]
- 33.Raposo G., Stahl P. D., Extracellular vesicles: A new communication paradigm? Nat. Rev. Mol. Cell Biol. 20, 509–510 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Cai Q., He B., Weiberg A., Buck A. H., Jin H., Small RNAs and extracellular vesicles: New mechanisms of cross-species communication and innovative tools for disease control. PLoS Pathog. 15, e1008090 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zerlin M., Thiericke R., Common principles in macrolactone (marginolactone) biosynthesis. Studies on the desertomycin family. J. Org. Chem. 59, 6986–6993 (1994). [Google Scholar]
- 36.Benallaoua S., Coulon J., Bonaly R., Membrane phospholipid composition in Saccharomyces uvarum cells grown in the presence of subinhibitory doses of amphotericin B and desertomycin. Res. Microbiol. 143, 695–702 (1992). [DOI] [PubMed] [Google Scholar]
- 37.Andersen O. S., Muller R. U., Monazomycin-induced single channels. I. Characterization of the elementary conductance events. J. Gen. Physiol. 80, 403–426 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becucci L., Guidelli R., Kinetics of channel formation in bilayer lipid membranes (BLMs) and tethered BLMs: Monazomycin and melittin. Langmuir 23, 5601–5608 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Anderson T. M., et al. , Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 10, 400–406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Müller A., et al. , Daptomycin inhibits cell envelope synthesis by interfering with fluid membrane microdomains. Proc. Natl. Acad. Sci. U.S.A. 113, E7077–E7086 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slessarev E. W., et al. , Water balance creates a threshold in soil pH at the global scale. Nature 540, 567–569 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Linares J. F., Gustafsson I., Baquero F., Martinez J. L., Antibiotics as intermicrobial signaling agents instead of weapons. Proc. Natl. Acad. Sci. U.S.A. 103, 19484–19489 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gotoh H., et al. , Pseudomonas aeruginosa, under DNA replication inhibition, tends to form biofilms via Arr. Res. Microbiol. 159, 294–302 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Braissant O., et al. , Exopolymeric substances of sulfate‐reducing bacteria: Interactions with calcium at alkaline pH and implication for formation of carbonate minerals. Geobiology 5, 401–411 (2007). [Google Scholar]
- 45.Bonner J. T., The origins of multicellularity. Integr. Biol. Issues News Rev. 1, 27–36 (1998). [Google Scholar]
- 46.Brunet T., King N., The origin of animal multicellularity and cell differentiation. Dev. Cell 43, 124–140 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woznica A., et al. , Bacterial lipids activate, synergize, and inhibit a developmental switch in choanoflagellates. Proc. Natl. Acad. Sci. U.S.A. 113, 7894–7899 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyons N. A., Kolter R., On the evolution of bacterial multicellularity. Curr. Opin. Microbiol. 24, 21–28 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hallmann A., Evolution of reproductive development in the volvocine algae. Sex. Plant Reprod. 24, 97–112 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prochnik S. E., et al. , Genomic analysis of organismal complexity in the multicellular green alga Volvox carteri. Science 329, 223–226 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoops H. J., Nishii I., Kirk D. L., “Cytoplasmic bridges in Volvox and its relatives” in Cell-Cell Channels, Baluska F., Volkmann D., Barlow P. W., Eds. (Springer, 2006), pp. 65–84. [Google Scholar]
- 52.Bax A., Aszalos A., Dinya Z., Sudo K., Structure elucidation of the antibiotic desertomycin through the use of new two-dimensional NMR techniques. J. Am. Chem. Soc. 108, 8056–8063 (1986). [Google Scholar]
- 53.Moore B. S., Hertweck C., Biosynthesis and attachment of novel bacterial polyketide synthase starter units. Nat. Prod. Rep. 19, 70–99 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Taylor S. D., Palmer M., The action mechanism of daptomycin. Bioorg. Med. Chem. 24, 6253–6268 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Kretzschmar G., Krause M., Radics L., Chemistry and biological activity of oasomycin macrolactones. Tetrahedron 53, 971–986 (1997). [Google Scholar]
- 56.Song X., Yuan G., Li P., Cao S., Guanidine-containing polyhydroxyl macrolides: Chemistry, biology, and structure-activity relationship. Molecules 24, 3913 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berlinck R. G. S., Bertonha A. F., Takaki M., Rodriguez J. P. G., The chemistry and biology of guanidine natural products. Nat. Prod. Rep. 34, 1264–1301 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Blin K., et al. , antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 47, W81–W87 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamedi J., et al. , Streptomyces iranensis sp. nov., isolated from soil. Int. J. Syst. Evol. Microbiol. 60, 1504–1509 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Horn F., et al. , Draft genome sequence of Streptomyces iranensis. Genome Announc. 2, e00616-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim K. H., et al. , Natalamycin A, an ansamycin from a termite-associated Streptomyces sp. Chem. Sci. (Camb.) 5, 4333–4338 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Braña A. F., et al. , Desertomycin G, a new antibiotic with activity against Mycobacterium tuberculosis and human breast tumor cell lines produced by Streptomyces althioticus MSM3, isolated from the Cantabrian Sea Intertidal macroalgae Ulva sp. Mar. Drugs 17, 114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gorman D. S., Levine R. P., Cytochrome f and plastocyanin: Their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc. Natl. Acad. Sci. U.S.A. 54, 1665–1669 (1965). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.