Key Points
Question
Can the inhaled steroid ciclesonide reduce the time to alleviation of all COVID-19–related symptoms among nonhospitalized participants with symptomatic COVID-19 infection?
Findings
In this randomized clinical trial of 400 patients with symptomatic COVID-19, ciclesonide did not reduce the time to alleviation of all COVID-19–related symptoms. However, patients who were treated with ciclesonide had fewer subsequent emergency department visits or hospital admissions for reasons that were related to COVID-19.
Meaning
The results of this randomized clinical trial suggest that future studies of inhaled steroids are needed to explore their efficacy in patients with a high risk for disease progression and in reducing the incidence of long-term COVID-19 symptoms or postacute sequelae of SARS-CoV-2.
Abstract
Importance
Systemic corticosteroids are commonly used in treating severe COVID-19. However, the role of inhaled corticosteroids in the treatment of patients with mild to moderate disease is less clear.
Objective
To determine the efficacy of the inhaled steroid ciclesonide in reducing the time to alleviation of all COVID-19–related symptoms among nonhospitalized participants with symptomatic COVID-19 infection.
Design, Setting, and Participants
This phase 3, multicenter, double-blind, randomized clinical trial was conducted at 10 centers throughout the US and assessed the safety and efficacy of a ciclesonide metered-dose inhaler (MDI) for treating nonhospitalized participants with symptomatic COVID-19 infection who were screened from June 11, 2020, to November 3, 2020.
Interventions
Participants were randomly assigned to receive ciclesonide MDI, 160 μg per actuation, for a total of 2 actuations twice a day (total daily dose, 640 μg) or placebo for 30 days.
Main Outcomes and Measures
The primary end point was time to alleviation of all COVID-19–related symptoms (cough, dyspnea, chills, feeling feverish, repeated shaking with chills, muscle pain, headache, sore throat, and new loss of taste or smell) by day 30. Secondary end points included subsequent emergency department visits or hospital admissions for reasons attributable to COVID-19.
Results
A total of 413 participants were screened and 400 (96.9%) were enrolled and randomized (197 [49.3%] in the ciclesonide arm and 203 [50.7%] in the placebo arm; mean [SD] age, 43.3 [16.9] years; 221 [55.3%] female; 2 [0.5%] Asian, 47 [11.8%] Black or African American, 3 [0.8%] Native Hawaiian or other Pacific Islander, 345 [86.3%] White, and 1 multiracial individuals [0.3%]; 172 Hispanic or Latino individuals [43.0%]). The median time to alleviation of all COVID-19–related symptoms was 19.0 days (95% CI, 14.0-21.0) in the ciclesonide arm and 19.0 days (95% CI, 16.0-23.0) in the placebo arm. There was no difference in resolution of all symptoms by day 30 (odds ratio, 1.28; 95% CI, 0.84-1.97). Participants who were treated with ciclesonide had fewer subsequent emergency department visits or hospital admissions for reasons related to COVID-19 (odds ratio, 0.18; 95% CI, 0.04-0.85). No participants died during the study.
Conclusions and Relevance
The results of this randomized clinical trial demonstrated that ciclesonide did not achieve the primary efficacy end point of reduced time to alleviation of all COVID-19–related symptoms.
Trial Registration
ClinicalTrials.gov Identifier: NCT04377711
This randomized clinical trial examines the efficacy of the inhaled steroid ciclesonide in reducing the time to alleviation of all COVID-19–related symptoms among nonhospitalized participants with symptomatic COVID-19 infection.
Introduction
The SARS-CoV-2 COVID-19 pandemic has led to an ongoing global public health emergency. Symptoms of COVID-19 vary widely and include fever, cough, difficulty breathing, and loss of taste and smell. Reported illnesses have ranged from asymptomatic to severe illness and death among confirmed COVID-19 cases. To date, most therapeutic studies have focused on patients with severe disease that required hospitalization.
In the RECOVERY trial, dexamethasone resulted in lower 28-day mortality among patients with severe COVID-19.1 The role of inhaled corticosteroids for patients with mild to moderate coronavirus disease is less clear. Systemic corticosteroids have shown mixed results for these patient types.2 The potential antiinflammatory benefits of corticosteroids must be weighed against the potential risks of immunosuppression and other systemic steroid effects.3,4
Inhaled corticosteroids may also be beneficial in COVID-19 treatment, as they reduce the expression of key proteins that are involved in the entry of the virus into host cells.5 Inhaled corticosteroids have also been shown to cause downregulation of COVID-19 genes.6
Among the available inhaled corticosteroids, ciclesonide has emerged as a potential treatment option for COVID-19. In vitro, ciclesonide has been shown to have antiviral properties against COVID-19 and blocks COVID-19 viral replication.7,8 A case series described 3 elderly patients with hypoxia due to COVID-19 who recovered following treatment with ciclesonide.9 Clinical trials are needed to determine the effects of ciclesonide on COVID-19 in the clinical setting. This study examined the effects of ciclesonide vs placebo in nonhospitalized participants with symptomatic COVID-19 infection.
Methods
Study Design and Participants
This was a phase 3, multicenter, double-blinded, randomized placebo-controlled trial to assess the safety and efficacy of a ciclesonide metered-dose inhaler (MDI) for treating nonhospitalized patients with symptomatic COVID-19 infection (Supplement 1). Participants were eligible for inclusion if, at the time of enrollment, they (1) were at least age 12 years, (2) had a positive SARS-CoV-2 molecular or antigen diagnostic sample obtained during the previous 72 hours, (3) were not hospitalized or under consideration for hospitalization, (4) had an oxygen saturation level of at least 93% on room air, (5) were able to demonstrate successful use of an MDI, and (6) had at least 1 of the following symptoms of COVID-19: fever, cough, or dyspnea. An age of 12 years was selected as the age threshold for enrollment, which was consistent with the US Food and Drug Administration–approved prescribing information for ciclesonide for the maintenance treatment of asthma as prophylactic therapy. Participants were excluded if they (1) had a history of hypersensitivity to ciclesonide, (2) had taken an inhaled or intranasal corticosteroid within 14 days, (3) had taken oral corticosteroids within 90 days, (4) had participated in any other clinical trial or used any investigational agent within 30 days, (5) had a history of cystic fibrosis, (6) had a history of idiopathic pulmonary fibrosis, (7) were receiving treatment with hydroxychloroquine/chloroquine, or (8) were pregnant.
The study was conducted at 10 centers throughout the US. Public and private academic and nonacademic sites were represented among the centers. This study was approved by the Western Institutional Review Board. All participants, parents/legal guardians, or legally authorized representatives provided written informed consent.
Randomization and Masking
Approximately 400 eligible participants were planned to be randomized 1:1 to receive treatment with the ciclesonide MDI, 160 μg per actuation, for a total of 2 actuations (am and pm) twice a day (total daily dose, 640 μg) plus standard supportive care or placebo MDI twice a day plus standard supportive care for 30 days. The total daily dose of 640 μg was selected for this study, which was consistent with the highest recommend daily dose in the US Food and Drug Administration–approved prescribing information for ciclesonide for the maintenance treatment of asthma as prophylactic therapy.
The ciclesonide MDIs and placebo MDIs were identical in appearance. The randomization schedule was generated by the contract manufacturing organization and incorporated into the labeling of kits. The MDI kits were sent to the study sites in blocks of 6, with 3 active and 3 placebo kits randomized within each block. Individual site personnel dispensed individual kits in order and were masked to the assignment.
Procedures
Participant recruitment varied by site and included advertisements in traditional and social media, as well as referrals from testing sites and health care clinicians. In-person study visits were performed on day 0 (enrollment) and day 30. One site performed in-person visits at the participants’ homes, during which 1 member of the study team traveled to the participants’ homes while other members of the study team joined via audio/video telemedicine. At the remaining sites, participants traveled to the health care or research facilities of the sites or were already there, seeking medical care, at the time of enrollment. In all cases, COVID-19 precautions, such as proper personal protective equipment use, were followed based on local procedures. All other planned study contact was performed by telephone.
At the enrollment visit, study staff reviewed and documented participants’ comorbidities and concomitant medication use. All medications, including prescription and over the counter, were included. Each time study team members contacted a participant, they inquired about any changes in health status, comorbidities, or concomitant medications. A medication’s use was considered concomitant if its use was initiated or continued after the administration of an investigational product.
Race and ethnicity data were obtained based on participants’ self-reporting using predefined categories. This information was included in the study because certain racial and ethnic groups have been shown to be at higher risk for severe COVID-19 disease progression.10
Participants were instructed on how to self-administer the MDI at the initial visit by the study team. Metered-dose inhaler use was reviewed with participants during all follow-up calls. The MDI technique was not evaluated after the initial visits. Participants were dispensed a 30-day supply of investigational product, a pulse oximeter for at-home oxygen saturation level monitoring, and an electronic diary smartphone application (eDiary). Within 1 hour of self-administration of the investigational product, participants were to complete and record in their eDiary the presence of COVID-19–related symptoms. During each self-assessment, participants logged their individual COVID-19–related symptoms of cough, dyspnea, chills, and feeling feverish using a subjective 4-point scale. Absent symptoms were scored as 0. Mild, moderate, or severe symptoms were scored as 1, 2, or 3, respectively. During each self-assessment, participants also logged their individual COVID-19–related symptoms of repeated shaking with chills, muscle pain, headache, sore throat, and new loss of taste or smell as either absent or present. Participants received reminders to self-administer the study medication and log symptoms in the form of push notifications from their eDiary and scheduled phone calls from the study team. Qualified health care clinicians contacted participants on days 2, 4, 6, 8, 10, 12, 14, and 21 (or within 2 days before or after) and conducted a study visit on day 30 (or within 2 days before or after) for a health status check to collect adverse event and concomitant medication information and confirm and/or clarify information recorded in the eDiary. The health care clinicians also contacted participants on day 37 (or within 4 days before or after) and day 60 (or within 7 days before or after) to collect follow-up safety and outcome data.
Per the protocol, participants were instructed to seek emergency department evaluation if their oxygen saturation level was less than or equal to 92%. Participants (or their representatives) were asked to notify study personnel directly in the event that they visited an emergency department or were hospitalized during their participation in the study. Participants were instructed to continue using the study medication for 30 days, even if symptoms resolved earlier. Continuing the medication for 30 days standardized administration schemes for all patients and allowed for the evaluation of symptom-based and non–symptom-based outcome measures.
Supportive care was provided by the qualified health care clinicians (physicians and nurses) from the research team and was at the discretion of the individual site investigators. It was not protocolized, with the exception of the standardized recommendation to seek emergency care for hypoxia. In addition, participants were free to continue to receive care from nonstudy health care clinicians for COVID-19 and non–COVID-19–related conditions throughout the study. There was no unified standard for hospital admission. Once a participant sought emergency care, the decision to admit the patient to the hospital was made by the treating clinician and was outside the control of study team.
Outcomes
The primary outcome was time to alleviation of all COVID-19–related symptoms (cough, dyspnea, chills, feeling feverish, repeated shaking with chills, muscle pain, headache, sore throat, and new loss of taste or smell) by day 30. The time to alleviation of COVID-19–related symptoms was defined as reporting all COVID-19–related symptoms as absent for a continuous period of at least 24 hours (ie, at least 3 consecutive am/pm assessments), as self-reported in the participant’s eDiary.
The secondary outcomes were to assess whether ciclesonide MDI plus standard supportive care reduced the incidence of subsequent emergency department visits or hospital admissions for reasons related to COVID-19, reduced the incidence of hospital admissions or death, reduced all-cause mortality, reduced COVID-19–related mortality, increased the percentage of participants with alleviation of COVID-19–related symptoms, and increased the time to hospital admission or death compared with placebo plus standard supportive care. Alleviation of all COVID-19–related symptoms by days 7, 14, and 30 were also compared.
Statistical Analysis
The primary efficacy analysis was based on the intent-to-treat (ITT) population (all randomized participants). A sensitivity analysis was performed for the per-protocol (PP) population. Participant eDiary compliance of less than 65% was considered a major protocol deviation, as a proxy for medication noncompliance. To improve treatment-effect estimation and inference precision, preplanned baseline covariate adjustments were made for sex, age, race and ethnicity, and body mass index (calculated as weight in kilograms divided by height in meters squared), as these are known COVID-19 risk factors.10,11,12,13 A Cox proportional hazard model was used to include these additional covariates. The median time to event and 95% CIs were summarized by treatment arm, and Kaplan-Meier estimates of the survival curves were generated. A shift table depicting the change in severity of COVID-19–related symptoms of cough, dyspnea, chills, and feeling feverish from baseline was presented for each day and point.
The secondary efficacy analysis was based on the ITT population. A sensitivity analysis was performed for the PP population. The secondary efficacy end points were analyzed using a logistic regression model.
The ITT population was used to evaluate safety. All adverse events and serious adverse events were coded using Medical Dictionary for Regulatory Activities, version 23.0 (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use). The data monitoring committee conducted a review of masked safety data once 100 participants had been enrolled. P < .05 was the threshold for statistical significance.
Primary Outcome and Sample Size Changes
The original primary outcome registered was the percentage of participants with a subsequent emergency department visit or hospital admission for reasons related to COVID-19 by day 30. The enrollment target of 400 participants was based on sample size calculations for this original end point.
The primary end point was changed in subsequent versions of the protocol. The final primary outcome, which is the primary outcome reported in this article, was time to alleviation of all COVID-19–related symptoms by day 30. A primary outcome based on symptom resolution was chosen rather than one based on emergency department visits or hospital admission after preliminary data demonstrated substantially lower than expected rates of emergency department visits or hospitalizations among study participants. For the final primary outcome, a sample size of approximately 232 patients (116 per arm) was required to achieve 90% power at α = .05. This was based on the assumptions that there would be a median time to alleviation of symptoms of 7 days for the ciclesonide arm and 11 days for the placebo arm (hazard ratio [HR] of approximately 1.58) with a total study duration of 30 days. The larger enrollment target of 400 participants was maintained to account for an unknown dropout rate in this patient population, as well as other factors that may have affected the overall power of the study (eMethods in Supplement 2).
This trial was prospectively registered with ClinicalTrials.gov (NCT04377711). The changes made to the primary outcome were incorporated into the protocol, approved by the institutional review board, reflected in updates to the study registration, and had no effect on the data collection process. The final version of the protocol will be available on ClinicalTrials.gov.
Results
From June 11, 2020, to November 3, 2020, 413 participants were screened and 400 (96.9%) were enrolled and randomized (197 [49.3%] in the ciclesonide arm and 203 [50.7%] in the placebo arm) (eTable 1 in Supplement 2). Sixteen participants (4.0%) were younger than 18 years (9 in the ciclesonide arm and 7 in the placebo arm), with the youngest participant being age 13 years. All randomized participants received at least 1 dose of investigational product and were included in the ITT and safety populations. The PP population included 377 participants (94.3%; 184 [93.4%] participants in the ciclesonide arm and 193 [95.1%] participants in the placebo arm). One participant in the ciclesonide arm was excluded from the PP population because of a pregnancy test being performed before signing the informed consent, and the remaining 22 participants (12 in the ciclesonide arm, 10 in the placebo arm) were excluded from the PP population because of a diary compliance of less than 65%.
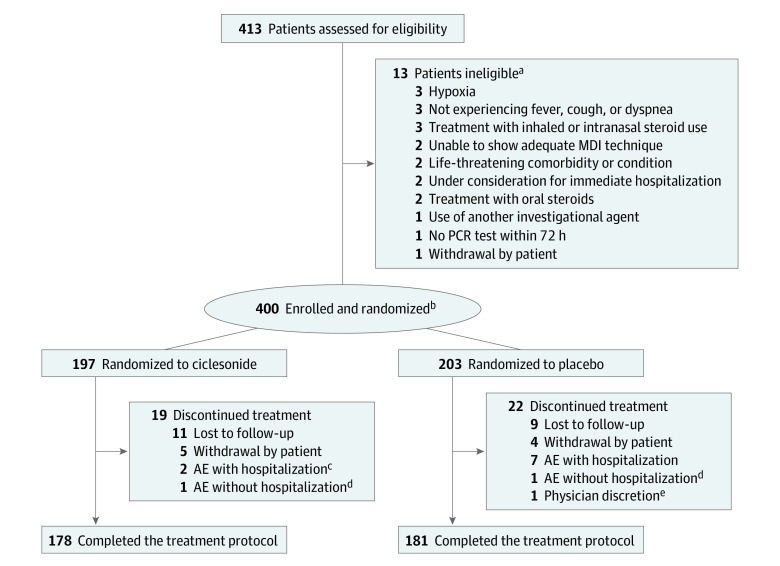
Overall, 359 participants (89.8%; 178 participants [90.4%] in the ciclesonide arm and 181 participants [89.2%] in the placebo arm) completed the study and 41 participants (10.3%; 19 [9.6%] and 22 [10.8%], respectively) discontinued participation. For participants in both arms, the most common reason for discontinuation was that participants were lost to follow-up (11 participants [5.6%] in the ciclesonide arm and 9 participants [4.4%] in the placebo arm) (Figure 1).
Figure 1. Trial Profile.
AE indicates adverse event; MDI, metered-dose inhaler; PCR, polymerase chain reaction.
aSome potential participants were excluded for multiple reasons.
bAll randomized participants were included in the intention to treat analysis.
cOne participant was hospitalized for a reason unrelated to COVID-19 (dog bite) but did not discontinue treatment.
dOne participant in each arm discontinued treatment because of a headache that was classified as an adverse event; neither was hospitalized.
eOne participant was withdrawn at the discretion of the physician in the absence of an adverse event.
In the ITT population, 221 participants (55.3%) were female, 47 (11.8%) were Black or African American, and 172 (43.0%) were Hispanic or Latino. The ciclesonide arm had higher rates of type 2 diabetes (22 participants [11.2%] in the ciclesonide arm and 8 participants [3.9%], P = .01) and asthma (18 participants [9.1%] in the ciclesonide arm and 8 participants [3.9%]; P = .04); otherwise, demographic characteristics, medical histories, and concomitant medications of interest were not different between the arms (Table 1). No participants were treated with remdesivir during the study.
Table 1. Participants’ Demographic Characteristics, Medical Histories, and Concomitant Medications.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Ciclesonide (n = 197) | Placebo (n = 203) | Overall (N = 400) | |
| Age, mean (SD) [range], y | 43.7 (17.53) [13-87] | 42.9 (16.28) [14-83] | 43.3 (16.89) [13-87] |
| Participants <18 y | 9 (4.6) | 7 (3.4) | 16 (4.0) |
| Sex | |||
| Male | 85 (43.1) | 94 (46.3) | 179 (44.8) |
| Female | 112 (56.9) | 109 (53.7) | 221 (55.3) |
| Race | |||
| Asian | 1 (0.5) | 1 (0.5) | 2 (0.5) |
| Black or African American | 20 (10.2) | 27 (13.3) | 47 (11.8) |
| Native Hawaiian or other Pacific Islander | 1 (0.5) | 2 (1.0) | 3 (0.8) |
| White | 174 (88.3) | 171 (84.2) | 345 (86.3) |
| Multiracial | 0 | 1 (0.5) | 1 (0.3) |
| Othera | 1 (0.5) | 1 (0.5) | 2 (0.5) |
| Ethnicity | |||
| Hispanic or Latino | 86 (43.7) | 86 (42.4) | 172 (43.0) |
| Not Hispanic or Latino | 111 (56.3) | 117 (57.6) | 228 (57.0) |
| Body mass index, mean (SD)b | 28.8 (6.06) | 30.0 (6.87) | 29.4 (6.50) |
| Medical historyc | |||
| Hypertension | 47 (23.9) | 42 (20.7) | 89 (22.3) |
| Drug hypersensitivityd | 21 (10.7) | 30 (14.8) | 51 (12.8) |
| Hyperlipidemia | 20 (10.2) | 16 (7.9) | 36 (9.0) |
| Type 2 diabetes | 22 (11.2) | 8 (3.9) | 30 (7.5) |
| Asthma | 18 (9.1) | 8 (3.9) | 26 (6.5) |
| Selected concomitant medicationse | |||
| Paracetamol | 105 (53.3) | 109 (53.7) | 214 (53.5) |
| Nonsteroidal antiinflammatory drugs | 39 (19.8) | 48 (23.6) | 87 (21.8) |
| Antibiotics | 12 (6.1) | 8 (3.9) | 20 (5.0) |
| Antivirals | 1 (0.5) | 3 (1.5) | 4 (1.0) |
| Monoclonal antibodies | 1 (0.5) | 0 | 1 (0.3) |
| Baseline symptom severityf | |||
| One or more severe symptoms | 5 (2.5) | 9 (4.4) | 14 (3.5) |
| One or more moderate or severe symptoms | 80 (40.6) | 74 (36.5) | 154 (38.5) |
Other was a single predefined category.
Calculated as weight in kilograms divided by height in meters squared.
Medical histories occurring at a difference of more than 2.0% between treatment arms.
A reported history of an allergy to any medication.
Selected concomitant medications of interest. A medication’s use was considered concomitant if it was initiated or continued after administration of investigational product. Concomitant medications were classified based on the Anatomical and Therapeutic Class of World Health Organization drug, preferred term, and treatment group.
Symptoms of cough, dyspnea, chills, and feeling feverish as subjectively rated by participants at time of enrollment.
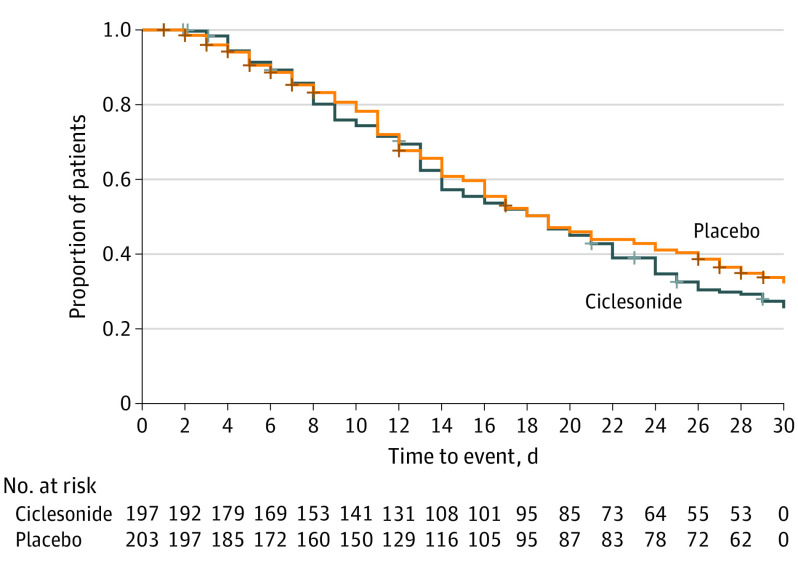
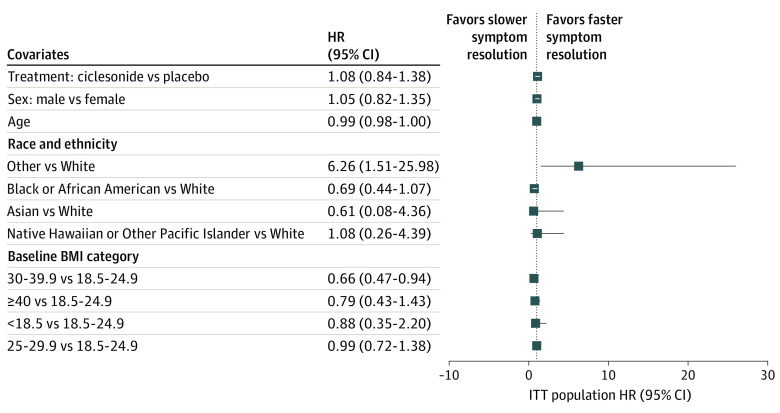
The primary efficacy outcome was to assess whether treatment with ciclesonide MDI plus standard supportive care resulted in improved time to alleviation of all COVID-19–related symptoms of cough, dyspnea, chills, feeling feverish, repeated shaking with chills, muscle pain, headache, sore throat, and new loss of taste or smell compared with placebo plus standard supportive care in nonhospitalized participants with symptomatic COVID-19 infection. In the ITT population, 139 of 197 participants (70.6%) in the ciclesonide arm and 129 of 203 participants (63.5%) in the placebo arm experienced alleviation of all symptoms. Kaplan-Meier estimates of the median time to alleviation of COVID-19–related symptoms were 19.0 days (95% CI, 14.0-21.0) in the ciclesonide arm and 19.0 days (95% CI, 16.0-23.0) in the placebo arm (Figure 2). The HR for the comparison of ciclesonide vs placebo based on a Cox proportional hazards regression model was 1.08 (95% CI, 0.84-1.38) (Figure 3). There was no difference in the time to alleviation of cough (HR, 1.09; 95% CI, 0.87-1.36), dyspnea (HR, 0.96; 95% CI; 0.77-1.19), loss of taste and smell (HR, 0.92; 95% CI, 0.75-1.13), or any of the other individual symptoms studied. There was also no difference in time to elevation of all symptoms exclusive of loss of taste and smell (HR, 1.10; 95% CI, 0.87-1.40).
Figure 2. Kaplan-Meier Curve of Time to Alleviation of COVID-19–Related Symptoms.
Figure 3. Cox Regression of Time to Alleviation of COVID-19–Related Symptoms.
BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); HR, hazard ratio; ITT, intent to treat.
Participants who received ciclesonide experienced fewer occurrences of emergency department visits or hospital admissions for reasons related to COVID-19 by day 30 compared with those who received placebo (1.0% vs 5.4%; odds ratio [OR], 0.18; 95% CI; 0.04-0.85; P = .03). No other secondary outcomes reached statistical significance. The most common symptoms reported on day 30 were cough (11.7% vs 12.3%; P = .88), muscle pain (9.6% vs 8.9%; P = .86), and dyspnea (10.2% vs 7.9%; P = .49).
The results of the analysis of secondary efficacy outcomes for the ITT population, including P values and ORs (95% CI) for the comparison of ciclesonide vs placebo based on a logistic regression model that was adjusted for baseline covariates of sex, age, and baseline body mass index, are shown in Table 2. Sensitivity analyses for the primary and secondary efficacy end points showed similar results as those from the corresponding primary analyses. Participants with at least 1 moderate or severe symptom of cough, dyspnea, chills, or feeling feverish had a similar time to alleviation of those symptoms than those who had only mild symptoms (eTable 2 in Supplement 2).
Table 2. Secondary Efficacy Outcomes.
| Secondary efficacy end point | No. (%) | Results, ciclesonide vs placebo, OR (95% CI) | P value | |
|---|---|---|---|---|
| Ciclesonide (n = 197) | Placebo (n = 203) | |||
| Participants with subsequent emergency department visit or hospital admission for reasons related to COVID-19 by day 30, % | 2 (1.0) | 11 (5.4) | 0.18 (0.04-0.85) | .03 |
| Participants with hospital admission or death by day 30, %a | 3 (1.5) | 7 (3.4) | 0.45 (0.11-1.84) | .26 |
| All-cause mortality by day 30 | 0 | 0 | NA | NA |
| COVID-19–related mortality by day 30 | 0 | 0 | NA | NA |
| Participants with alleviation of COVID-19–related symptoms by day 7, % | 28 (14.2) | 29 (14.3) | 0.92 (0.51-1.66) | .79 |
| Participants with alleviation of COVID-19–related symptoms by day 14, % | 81 (41.1) | 76 (37.4) | 1.19 (0.78-1.81) | .43 |
| Participants with alleviation of COVID-19–related symptoms by day 30, % | 139 (70.6) | 129 (63.5) | 1.28 (0.84-1.97) | .25 |
Abbreviations: NA, not applicable; OR, odds ratio.
Hazard ratio for time to hospital admission or death (ciclesonide vs placebo) (95% CI): 0.39 (0.09-1.65).
Adverse events were reported by 22 participants (11.2%) in the ciclesonide arm and 29 participants (14.3%) in the placebo arm (eTable 3 in Supplement 2). Most adverse events were mild to moderate in severity. Oral candidiasis was reported in 1 participant (0.5%) in each of the study arms. Dry mouth was reported in 3 participants (1.5%) in the ciclesonide arm and 1 participant (0.5%) in the placebo arm. Distinct from the participants’ eDiary reporting, headache was reported as an adverse event in 1 participant (0.5%) in the ciclesonide arm and 4 participants (0.5%) in the placebo arm. One participant (0.5%) in each arm reported a headache that was judged to be an adverse event in the opinion of the site principal investigators and was subsequently discontinued from the protocol. No participants died during the trial.
Discussion
No statistically significant difference was observed between participants who were treated with ciclesonide vs placebo for the primary efficacy end point.
These composite efficacy outcomes were based on resolution of all COVID-19 symptoms. It is not uncommon for patients with COVID-19 to continue to have 1 or more mild lingering symptoms as they convalesce. In particular, loss of smell is a frequently reported symptom of COVID-19 that can be present for 3 weeks or longer for many patients.14 The end point of complete symptom recovery across all COVID-19 symptoms may have masked a significant population who were able to safely return to their baseline activities and were no longer at high risk for transmission, but who did not yet have complete symptom resolution.
In this study, participants who were treated with ciclesonide were less likely to have a subsequent emergency department visit or hospital admission for reasons related to COVID-19 by day 30 (1.0% vs 5.4%; P = .03). The sensitivity analysis conducted on the PP population showed similar results. While secondary outcomes are considered exploratory, this was originally the primary outcome that was included in the initial study registration. This outcome may be more relevant to the patients and health care systems than complete resolution of symptoms. Inhaled steroids may represent a relatively low-cost intervention to prevent emergency department visits or hospital admissions due to COVID-19. In this study, the number needed to treat to prevent emergency department visits or hospital admissions due to COVID-19 was 23.
Most participants did not report any moderate or severe symptoms at the time of enrollment. Furthermore, there were few hospitalizations and no deaths in this study. It is possible that patients with more severe symptoms or who were at higher risk for disease progression may have been less likely to volunteer to participate in an outpatient study during the pandemic. A study that focused on patients with severe symptoms or who were at high risk for disease progression may have yielded different results. Further studies of the efficacy of inhaled steroids among populations of pediatric patients, geriatric patients, and patients with known risk factors are needed to explore the efficacy of inhaled steroids among patients at higher risk for severe disease progression, hospitalization, and death of COVID-19.
Corticosteroids are commonly used in treating various respiratory diseases.15 Specifically, ciclesonide has been shown to have antiinflammatory properties in lung and bronchial tissues with minimal systemic effects.15 Inhaled corticosteroids have been shown to downregulate angiotensin-converting enzyme 2 expression.6 Ciclesonide has been shown to inhibit the PAK1 enzyme in cells, a pathogenic pathway for COVID-19 that is associated with angiotensin-converting enzyme 2. By inhibiting PAK1, ciclesonide reduces immune suppression and decreases lung inflammation.16 In addition, ciclesonide is believed to target the nonstructural proteins of the COVID-19 virus. This targeting, in vitro, has been shown to hinder viral replication.7,17
Two recent open-label, randomized clinical trials of inhaled budesonide in the treatment of patients with COVID-19 demonstrated a decreased need for COVID-19–related urgent medical care18 and hospitalizations or death,19 which is consistent with this study’s findings. Unlike this study’s findings, the studies of budesonide also demonstrated a decreased time to symptom resolution.18,19
Among the various completed or ongoing trials (NCT04193878, NCT04330586, NCT04331054, NCT04331470, NCT04355637, NCT04356495, NCT04377711, NCT04381364, NCT04416399, and ISRCTN86534580) of inhaled corticosteroids for the treatment of COVID-19, few use a double-blinded design. A double-blinded design, like the one used in this study, is especially critical when relying on participant-dependent end points, such as self-reported symptoms and the decision to seek emergency department care.
This study followed participants for 60 days. As the pandemic continues, a growing population of patients with long-term COVID-19 symptoms or postacute sequelae of SARS-CoV-2 beyond 12 weeks has emerged.20 Longer-term studies are needed to better understand factors that may affect this growing subset of patients.
Limitations
COVID-19 has been noted to manifest with a wide variety of dynamically changing symptoms. Adverse events were evaluated by the site investigators without knowledge of the participants’ treatment assignment. Specific symptoms, such as headache, were adjudicated to be an adverse event if, in the opinion of the principal investigator, they were outside of what would be expected of the participant’s COVID-19 disease progression or if they were associated with a need for emergency care or hospitalization. This may have led to actual adverse event symptoms being incorrectly attributed to COVID-19 disease progression or symptoms of COVID-19 disease progression incorrectly adjudicated as adverse events.
In this study, symptom resolution was defined as 3 consecutive am/pm eDiary entries that reported no symptoms. Missed eDiary entries may have caused participants to appear to have a longer duration of symptoms then they actually had. Participants in the treatment arm were more likely to have asthma and type 2 diabetes, which are known risk factors for severe COVID-19 disease12; therefore, they may have been at higher risk for disease progression.
Participants younger than 18 years comprised only 4% of the sample; this small number prevented a meaningful analysis of the efficacy of the inhaled corticosteroids in pediatric patients with COVID-19. Use of inhaled or systemic steroids were part of the exclusion criteria. This may have excluded potential participants with comorbidities, such as moderate to severe asthma or chronic obstructive pulmonary disease, who may have benefited from the addition of an inhaled steroid.
With the exception of a standardized recommendation to seek emergency care for an oxygen saturation level of 92% or less, supportive care was not protocolized and was at the discretion of the site investigators. This and the fact that participants were free to obtain medical care from nonstudy health care clinicians may have led to variations in treatments among sites and individual participants.
Conclusions
Ciclesonide did not achieve the primary efficacy end point of reduction of time to alleviation of all COVID-19–related symptoms. Future studies of inhaled steroids are needed to explore their efficacy in patients with a high risk for disease progression and in reducing the incidence of long-term COVID-19 symptoms or postacute sequelae of SARS-CoV-2.
Trial protocol
eMethods. Changes to Primary Efficacy Endpoint and Sample Size Plan
eTable 1. Number of Participants Enrollment by Month and Location
eTable 2. Cox Regression of Time to Alleviation of COVID-19-Related Symptoms of Cough, Dyspnea, Chills, Feeling Feverish Based on Severity of Symptoms at time of Enrollment
eTable 3. Adverse Events
Data sharing statement
References
- 1.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693-704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shuto H, Komiya K, Yamasue M, et al. A systematic review of corticosteroid treatment for noncritically ill patients with COVID-19. Sci Rep. 2020;10(1):20935. doi: 10.1038/s41598-020-78054-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35(3):266-271. doi: 10.1007/s12250-020-00207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020;55(5):105955. doi: 10.1016/j.ijantimicag.2020.105955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters MC, Sajuthi S, Deford P, et al. COVID-19-related genes in sputum cells in asthma: relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020;202(1):83-90. doi: 10.1164/rccm.202003-0821OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milne S, Li X, Yang CX, et al. Inhaled corticosteroids downregulate SARS-CoV-2–related genes in COPD: results from a randomised controlled trial. Eur Respir J. 2021;58(1):2100130. doi: 10.1183/13993003.00130-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeon S, Ko M, Lee J, et al. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob Agents Chemother. 2020;64(7):e00819-e00820. doi: 10.1128/AAC.00819-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura H, Kurusu H, Sada M, et al. Molecular pharmacology of ciclesonide against SARS-CoV-2. J Allergy Clin Immunol. 2020;146(2):330-331. doi: 10.1016/j.jaci.2020.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwabuchi K, Yoshie K, Kurakami Y, Takahashi K, Kato Y, Morishima T. Therapeutic potential of ciclesonide inhalation for COVID-19 pneumonia: report of three cases. J Infect Chemother. 2020;26(6):625-632. doi: 10.1016/j.jiac.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross CP, Essien UR, Pasha S, Gross JR, Wang SY, Nunez-Smith M. Racial and ethnic disparities in population-level COVID-19 mortality. J Gen Intern Med. 2020;35(10):3097-3099. doi: 10.1007/s11606-020-06081-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jun T, Nirenberg S, Weinberger T, et al. Analysis of sex-specific risk factors and clinical outcomes in COVID-19. Commun Med. 2021;1:3. doi: 10.1038/s43856-021-00006-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mesas AE, Cavero-Redondo I, Álvarez-Bueno C, et al. Predictors of in-hospital COVID-19 mortality: a comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PLoS One. 2020;15(11):e0241742. doi: 10.1371/journal.pone.0241742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tartof SY, Qian L, Hong V, et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173(10):773-781. doi: 10.7326/M20-3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrocelli M, Cutrupi S, Salzano G, et al. Six-month smell and taste recovery rates in coronavirus disease 2019 patients: a prospective psychophysical study. J Laryngol Otol. 2021;135(5):436-441. doi: 10.1017/S002221512100116X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eedara BB, Alabsi W, Encinas-Basurto D, Polt R, Ledford JG, Mansour HM. Inhalation delivery for the treatment and prevention of COVID-19 infection. Pharmaceutics. 2021;13(7):1077. doi: 10.3390/pharmaceutics13071077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maruta H, He H. PAK1-blockers: potential therapeutics against COVID-19. Med Drug Discov. 2020;6:100039. doi: 10.1016/j.medidd.2020.100039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuyama S, Kawase M, Nao N, et al. The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells. J Virol. 2020;95(1):e01648-e20. doi: 10.1128/JVI.01648-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramakrishnan S, Nicolau DV Jr, Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;19(21):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu LM, Bafadhel M, Dorward J, et al. Inhaled budesonide for COVID-19 in people at higher risk of adverse outcomes in the community: interim analyses from the PRINCIPLE trial. medRxiv. Posted April 12, 2021. doi: 10.1101/2021.04.10.21254672 [DOI]
- 20.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods. Changes to Primary Efficacy Endpoint and Sample Size Plan
eTable 1. Number of Participants Enrollment by Month and Location
eTable 2. Cox Regression of Time to Alleviation of COVID-19-Related Symptoms of Cough, Dyspnea, Chills, Feeling Feverish Based on Severity of Symptoms at time of Enrollment
eTable 3. Adverse Events
Data sharing statement