Abstract
Background:
Aerobic exercise and environmental enrichment have been shown to enhance brain function. Virtual reality (VR) is a promising method for combining these activities in a meaningful and ecologically valid way.
Objective:
The purpose of this Phase 2 pilot study was to calculate relative change and effect sizes to assess the impact of simultaneous exercise and cognitive training in VR on brain health and cognition in older adults.
Methods:
Twelve cognitively normal older adults (64.7±8.8 years old, 8 female) participated in a 12-week intervention, 3 sessions/week for 25–50 minutes/session at 50–80% HRmax. Participants cycled on a custom-built stationary exercise bike while wearing a VR head-mounted display and navigating novel virtual environments to train spatial memory. Brain and cognitive changes were assessed using MRI imaging and a cognitive battery.
Results:
Medium effect size (ES) improvements in cerebral flow and brain structure were observed. Pulsatility, a measure of peripheral vascular resistance, decreased 10.5% (ES(d) = 0.47). Total grey matter volume increased 0.73% (ES(r) = 0.38), while thickness of the superior parietal lobule, a region associated with spatial orientation, increased 0.44% (ES(r) = 0.30). Visual memory discrimination related to pattern separation showed a large improvement of 68% (ES(ηp2) = 0.43). Cognitive flexibility (Trail Making Test B) (ES(r) = 0.42) and response inhibition (ES(W) = 0.54) showed medium improvements of 14% and 34%, respectively.
Conclusions:
Twelve weeks of simultaneous exercise and cognitive training in VR elicits positive changes in brain volume, vascular resistance, memory, and executive function with moderate-to-large effect sizes in our pilot study.
Keywords: Virtual reality, simultaneous exercise and cognitive training, older adults, brain health, cognition
INTRODUCTION
Cognitive decline is a significant public health concern in older adults. Alzheimer’s disease (AD), the leading cause of cognitive decline among older adults, affects more than 33 million people worldwide with numbers expected to triple over the next 30 years [1]. Due to the gradual progression of the disease, a significant burden is placed on the healthcare system, with costs approaching $300 billion a year [2]. AD is characterized by progressive memory loss, with deficits in episodic memory observed in the preclinical stage of the disease up to 6 years prior to diagnosis [3]. This includes spatial memory, a subtype of episodic memory responsible for spatial location, spatial pattern, and object location recall [4, 5]. Deficits in spatial memory have been shown to result in difficulty navigating familiar spaces, ultimately compromising safety, autonomy, and quality of life [6, 7]. To date, there are no effective disease-modifying treatments for AD, as existing FDA-approved drugs have only been shown to ameliorate symptoms and not target the underlying etiology [8, 9]. As a result, there has been a paradigm shift towards primary prevention and exploring new ways to maintain brain health.
Recent studies have shown that 50% of AD cases are attributable to seven modifiable lifestyle factors. Among these lifestyle factors, exercise and cognitive enrichment have been associated with the greatest reduction in the risk of developing AD in the US and worldwide, respectively. This has been supported by several studies which have shown that individuals with a lifestyle rich in mental and physical stimulation experience less age-related cognitive decline. In one such study, the time between the onset of cognitive decline and clinical diagnosis was found to be 7 years longer in highly educated older adults compared to those with low education [10]. In another study, exercise training was associated with an increase in hippocampal volume and improvement in spatial memory [11]. Emerging evidence has also shown that there may be a synergistic effect between exercise and cognitive stimulation, such that the benefits of engaging simultaneously in both activities are greater than either one alone. This is supported in several studies, including one in which older adults with dementia engaged in a 12-week intervention consisting of cycling and cognitive enrichment comprised of tasks assessing response inhibition, task switching, and processing speed [12]. The study found moderate improvements in psychomotor speed compared to exercise-only controls.
Virtual reality (VR) has emerged as a promising technology for the prevention of dementia and is ideally suited for simultaneous aerobic exercise and cognitive enrichment activities. VR has already been integrated into the field of medicine with applications in motor rehabilitation, cognitive rehabilitation, pain management, and the treatment of psychological disorders [13]. The utility of VR in medicine is driven by its ability to provide a safe, controlled, and adaptable environment for patients, allowing for studies that may otherwise not be possible in the real world. VR has also been shown to be an ecologically valid tool for assessing spatial navigation deficits in healthy older adults and individuals with Alzheimer’s disease [14]. This has been supported by several studies, including one that showed a high correlation between real-world and computerized spatial navigation in a hospital environment (r = 0.73) [14]. Furthermore, immersive VR head-mounted displays create a sense of presence and engagement which is important for performance on spatial navigation tasks [15].
There is limited research on the impact of simultaneous aerobic exercise and targeted spatial memory engagement in virtual reality on cognition and brain health. Therefore, in this study, we conducted a Phase 2, 12-week intervention in older adults that engages these simultaneous activities and assessed changes in neuroimaging and neuropsychological measures of brain health, including memory, executive function, and brain morphometry. Cerebrospinal fluid (CSF) and cerebral blood (CBF) flow were also measured due to their association with cerebrovascular health, a risk factor for developing AD [16]. Studies have also theorized that CSF and CBF flow support the removal of amyloid from the brain, suggesting a potential role as an early biomarker of AD progression [17]. We hypothesized our intervention would elicit improvements in memory and executive function, increases in hippocampal and total grey matter volumes, and exercise-related changes in CSF and CBF flow.
METHODS
Study population
Sixteen healthy older adults (65.0±8.3 years old; 52–80 years; 8 females) provided written consent and participated in this pilot study. Study procedures were approved by the Institutional Review Board at the University of Southern California and performed in accordance with the Declaration of Helsinki. Participants were recruited from a convenience sample of community-dwelling older adults using the LEARNit clinical trial (NCT02000622) database, local community flyers, and online advertisements. Participants were eligible for the study if they were 50–85 years of age and were physically capable of cycling and engaging in moderate-to-vigorous physical activity. Exclusion criteria included dementia, depression, stroke, neurological disorders, history of traumatic brain injury, or contraindications to MRI.
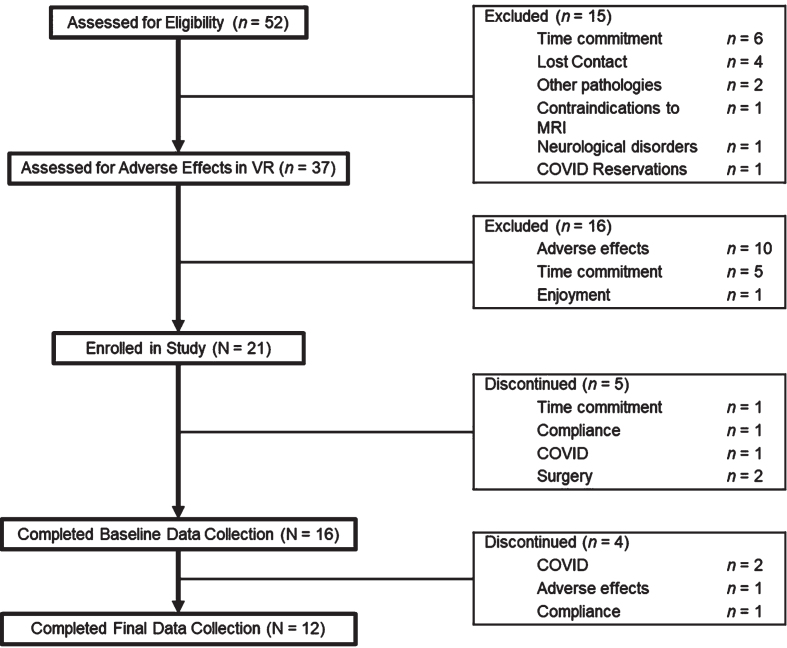
A flow diagram summarizing participant eligibility, participation, and attrition throughout the study is shown in Fig. 1. Briefly, 52 older adults were contacted via phone to assess study eligibility in accordance with the inclusion and exclusion criteria described above. Thirty-seven eligible older adults came in for a 1-hour on-campus visit to assess adverse effects associated with cycling and navigating a virtual park environment while wearing a VR head-mounted display. Adverse effects were assessed using the Simulator Sickness Questionnaire (SSQ), with a total score of greater than 15 used as guidance for discontinuing further participation in the study [18, 19]. Twenty-one participants enrolled in the study, with 16 completing baseline data collection. Twelve participants completed the entire study, including VR training and all data collection timepoints.
Fig. 1.
Flow diagram summarizing participant eligibility, participation, and attrition.
Study design
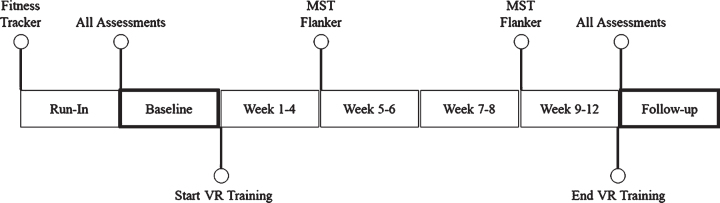
Figure 2 summarizes the timeline of events for the study. First, all enrolled participants were assigned and required to wear a fitness tracker (GENEActiv) for a 14-day run-in period to assess baseline levels of physical fitness. After the run-in period, participants participated in a full-day baseline visit consisting of neuroimaging and cognitive performance assessments. Following the baseline visit, participants started a 12-week intervention consisting of 36 VR training visits. Participants were advised maintain their pre-intervention lifestyle during the intervention. If participants had planned vacations or absences, the intervention was extended until all VR training visits were completed. Cognitive assessments, Flanker and MST, were administered at baseline, 4 weeks, 8 weeks, and 12 weeks. At the end of the intervention, participants participated in another full-day visit consisting of the same neuroimaging and cognitive assessments performed at baseline. The order of assessments was kept constant between the baseline and follow-up visits when possible.
Fig. 2.
Timeline of assessments and VR training during study.
Intervention
Participants engaged in simultaneous exercise and spatial navigation training in VR 3 times per week, alternating between a training day and rest day to allow for exercise recovery. Each visit was comprised of VR training and a set of pre-post questionnaires. VR training consisted of cycling on a custom-built stationary exercise bike at moderate-to-vigorous intensity levels for 25–50 minutes while navigating a cognitively challenging virtual environment. The Short Symptom Checklist (SSC) questionnaire was administered to check for adverse effects that are commonly associated with immersive VR, including nausea, eye strain, dizziness with eyes closed, and stomach awareness [20]. The Borg Rate of Perceived Exertion Scale (RPE) was utilized to obtain an additional surrogate measurement of exertion [21]. Blood pressure was measured at the beginning of each week to track changes associated with the intervention.
Aerobic exercise
Aerobic exercise was prescribed using a linear periodized schedule (Table 1), with cycling intensity levels increasing from 50% HR max at the onset of the intervention to 80% HR max at 12 weeks. Maximal HR was estimated according to a regression equation established by Tanaka et al. [22] Periodization consisted of two 6-week phases, moderate and vigorous, with intensity levels increasing by 5% every two weeks within each phase. In the first 6 weeks, intensity levels increased from 50% to 60% HR max with a corresponding increase in volume from 120 to 150 minutes per week. In the last 6 weeks, intensity levels increased from 70% to 80% HR max with volumes decreasing from 120 to 75 minutes per week. Cycling intensity and associated volumes were calculated according to the Surgeon General recommendations for physical activity in older adults and the American College of Sports Medicine [23]. Heart rate was tracked using a Polar H7 chest strap monitor and cycling resistance levels were adjusted at the beginning of each visit to ensure participants were within their target heart range.
Table 1.
Summary of aerobic exercise and cognitive training progression
| Week | Aerobic cycling | Cognitive training | |
| Intensity (% HR Max) | Duration (minutes) | Difficulty (Avg. # turns) | |
| 1 | 50% | 40 | 4 |
| 2 | 50% | 45 | 8 |
| 3 | 55% | 50 | 10 |
| 4 | 55% | 50 | 11 |
| 5 | 60% | 50 | 10* |
| 6 | 60% | 50 | 7 |
| 7 | 70% | 40 | 12 |
| 8 | 70% | 35 | 13 |
| 9 | 75% | 30 | 22 |
| 10 | 75% | 25 | 22 |
| 11 | 80% | 25 | 19* |
| 12 | 80% | 25 | 16* |
*Indicates that the specified training week contains trials requiring participants to navigate in the reverse direction (end point to starting point).
Cognitive training
Cognitive training was conducted in an immersive virtual reality environment viewed through an HTC Vive Pro head mounted display (HMD). The VR environment was designed to enhance motivation and enjoyment in older adults engaging in aerobic exercise and cognitively challenging tasks. This involved creating immersive storylines and gameplay and integrating it into a cognitive training paradigm (Fig. 3). Briefly, participants were assigned the role of a park ranger and required to complete missions consisting of monitoring and caring for the animals and performing routine park maintenance. This included rescuing animals that have escaped the park, feeding and treating injured animals, fighting wildfires, and repairing broken equipment. In-game promotions and rewards were awarded to the participant as they completed each mission to promote adherence and gameplay enjoyment [24].
Fig. 3.
Cognitively challenging gameplay and immersive storylines in VR. (top left) Rescue an animal that has escaped the sanctuary; (top right) Strategic landmarks placed at each intersection and guiding arrow for learning route; (bottom left) Participant cycling on stationary exercise to navigate VR environment; (bottom right) Top-down view of road network. Green dots denote participant’s path, red dot indicates starting point, and purple dot indicates destination.
For the cognitive training paradigm, participants were tasked with navigating along a network of roads in the environment, via pedaling on the stationary exercise bike, and completing a set of cognitive tasks targeting spatial memory and attention. Cognitive tasks were administered in five unique virtual environments, including an urban park, aviary, savannah, desert, and jungle, to facilitate learning under different conditions. Each environment contained a unique set of landmarks strategically placed at every road intersection to serve as visual cues during navigation.
Spatial memory was targeted using a cued route-learning paradigm consisting of three types of trials: cued learning, immediate recall, and delayed recall. In the cued learning trial, arrows were placed at each road intersection to guide the participant while they learned a new route. In the immediate recall trial, participants were tasked with navigating that same route, but without the assistance of arrows. In the delayed recall trial, participants were required to navigate a route they learned from a previous training visit without the assistance of arrows. At each training visit, participants completed five trials consisting of a delayed recall, two cued learning, and two immediate recall trials. The difficulty of the routes, defined by the number of decision points (intersections), progressively increased each week throughout the study (Table 1).
The cognitive demand of the intervention was enhanced by including an attention task, creating a dual-task paradigm. Participants collected specific items located along the left and right side of the road using their corresponding handlebar brakes. Briefly, each environment had a specific mission, including rescuing, feeding, and healing animals, as well as fighting fires and repairing power plants. Each route contained items associated with that environment’s mission as well as distractor items. While navigating a route, participants were tasked with collecting items specific to that environment and ignoring distractors.
Neuroimaging
All participants received an MRI scan at baseline and at 12-week follow-up after completing the intervention. MRI scans were acquired on a 3T Siemens Prisma using a 32-channel head coil (Siemens Medical Solutions, Erlangen, Germany). A localizer and scout scan were acquired at the beginning of the session to locate and align the participant’s head for subsequent scan sequences.
Cerebral flow
Cerebrospinal fluid flow (CSF) and cerebral blood flow (CBF) measurements were acquired using a 2D cine-PC (PC-MRI) pulse sequence with retrospective cardiac gating. CSF flow was measured at the cerebral aqueduct (CA) and the subarachnoid space (SS) at the level between the second and third cervical vertebrae (C2-C3). CBF flow was measured at the C2-C3 level at the internal carotid arteries (ICA) and vertebral arteries (VA). PC-MRI data was acquired with the following parameters: frames, 32; TR/TE, 27-28/ 8-9 ms; flip angle, 25°; field of view (FOV), 140×140×5 mm; matrix, 336×336; velocity encoding, 10 (CA), 5 (SS), 80 (ICA/VA) cm/s.
Perfusion
Whole-brain CBF perfusion measurements were acquired using a Pseudo-Continuous Arterial Spin Labeling (PCASL) sequence. PCASL images were acquired with background suppression and the following parameters: TR/TE/post-label decay, 4300/38.3/2000 ms; resolution, 2.5 mm isotropic; label duration, 700 ms. PCASL data was processed using an automated in-house processing pipeline involving motion correction of the label and control images, generation of perfusion-weighted images, and principal component analysis-based denoising [25]. An established kinetic model for the ASL signal was utilized to generate CBF time series images which were then averaged to create a mean CBF image [26].
Structural volume
Structural volume measurements were acquired using three sequences: (1) T1-weighted (T1w) magnetization-prepared rapid acquisition gradient echo (MP-RAGE); (2) T2-weighted SPACE; and (3) T2-weighted (T2w) turbo spin echo (TSE). Three-dimensional images of the entire brain were obtained from the T1w MP-RAGE and T1w SPACE sequences, both acquired in the sagittal plane. T1w MP-RAGE images were acquired with the following parameters: TR/TE, 2400/2.22 ms; FOV, 256×256×208 mm; resolution, 0.8 mm3 isotropic. T2w SPACE images were acquired with the following parameters: TR/TE, 3200/563 ms; FOV, 256×256×208 mm; resolution, 0.8 mm3 isotropic. A high-resolution image of the hippocampal subfields was obtained using a T2w TSE acquired at an angle perpendicular to the long axis of the hippocampus with the following parameters: TR/TE, 8020/50 ms; FOV, 175×175×28 mm; resolution, 0.4×0.7×2.0 mm.
Cerebral flow analysis
PC-MRI data was processed using BioFlow v3.1.2, a free medical imaging analysis software [27]. Each anatomical region of interest (ROI), including the CA, SS, and left and right ICA and VA, was manually traced and segmented to generate a waveform consisting of 32 flow rate (mm3/s) measurements over a cardiac cycle. The ICA and VA waveforms were summed to create a single arterial flow waveform. Background correction was applied to the CA and SS / arterial waveforms by subtracting out signal from stationary tissue located at the midbrain and C2 posterior spinous process, respectively. Stroke volume (SV) and flush peak (FshP) were calculated from the CA and SS CSF waveforms. Stroke volume represents the total volume of CSF displaced in the caudal and cranial directions, while flush peak represents the peak flow rate in the caudal direction [28, 29]. Pulsatility index (PI) and resistivity index (RI), two surrogate measures of peripheral vascular resistance, were calculated from the arterial waveform [30, 31]. PI represents the difference in peak systolic and diastolic flow divided by mean arterial flow, while RI represents the difference in peak systolic and diastolic flow divided by peak systolic flow [32].
Structure volume analysis
T1w MP-RAGE data was processed using the longitudinal pipeline in FreeSurfer v6, a software package for segmenting and labeling neuroanatomical structures [33, 34]. Grey matter volumes and cortical thickness were obtained using the recon-all script, which performed motion correction, intensity normalization, Talairach coordinate transformation, skull-stripping, registration, and white and grey matter segmentation [35, 36]. Segmented regions of interest (ROI) included the superior parietal lobule, hippocampus, and frontal gyrus due to their association with spatial orientation, working memory, and attention and executive function, respectively [37–39]. Total grey matter volume was also measured to provide a global assessment of brain structure.
T2w TSE data was processed using ASHS, a free open-source software for automated segmentation of the medial temporal lobe [40]. T2w TSE, T1w MP-RAGE, and an atlas package available online through the NITRC image repository, were provided as inputs to the ASHS pipeline [41, 42]. The atlas package consisted of manually segmented hippocampal images of older adult participants obtained from a research study on aging conducted at the Penn Memory Center at the University of Pennsylvania [40]. ASHS performed registration, multi-atlas segmentation using joint label fusion, and correction of the consensus segmentation using corrective machine learning classifiers [40]. CA1, dentate gyrus (DG), and entorhinal cortex (ERC) volumes were obtained from the output of the segmentation pipeline.
Cognitive battery
Six neuropsychological tests were administered to assess global cognition, executive function, and memory. Global cognition was assessed using the Montreal-Cognitive Assessment (MOCA) [43, 44]. Three core components of executive function was assessed using the D-KEFS Trail Making Test (TMT) [45], Eriksen Flanker Task [46]; and Digit Symbol Substitution Test [47]. TMT condition A and B scores were used to assess to cognitive flexibility. Flanker interference scores, calculated as the difference in mean reaction time between the congruent trials and incongruent trials, were used to assess inhibitory control [48]. DSST scores, calculated as the total number of correct pairings within 90 seconds, were used to assess processing speed and attention [49]. Memory was assessed using the Rey Auditory Verbal Learning Test (RAVLT) [50] and Mnemonic Similarity Task (MST) [52]. For the RAVLT, an immediate recall score was calculated as the total number of recalled words from list A across the 5 learning trials [50]. A delayed recall score was also calculated as the number of words recalled from List A after a 30-minute delay following the presentation of List B [50]. For the MST, a lure discrimination index (LDI) score was calculated as the probability of similar responses given to lures (Similar | Lure) minus the probability of similar responses given to foils (Similar | Foil) [52]. All tests were administered at baseline and 12 weeks, with Flanker and MST administered at 2 additional timepoints of 4 weeks and 8 weeks.
Primary outcomes
Proof-of-concept primary outcome measures were selected for brain function and cognition. This included executive function, memory, and cerebral blood flow. Cerebral blood flow measurements included arterial pulsatility index (PI) and resistivity index (RI), surrogate measures of peripheral vascular resistance which have been shown to be associated with cognitive dysfunction in individuals with Alzheimer’s disease [58]. Executive function and memory measurements included the Flanker interference control and MST lure discrimination index, measures of inhibitory control and pattern separation, respectively. These measures were selected as they were hypothesized to be the most sensitive to the changes elicited by the prescribed intervention over a 12-week period.
Secondary outcomes
The following measures were secondary outcomes. This included CA and SS CSF flow measurements, stroke volume and flush peak, and whole brain mean CBF perfusion. Structural measurements included total grey matter volume, hippocampal volume, superior parietal lobule thickness, and middle frontal gyrus thickness. Cognitive measurements included the MOCA, TMT-B, and Digit Symbol scores.
Statistical analysis
Statistical analyses were performed using SPSS (IBM v27, 2020) [59]. Given the limited sampled size and exploratory nature of this pilot study, effect sizes were utilized to examine the impact of the intervention on all neuroimaging and cognitive outcomes. A paired t-test was performed to calculate effect sizes for all outcome measures collected at 2 timepoints, baseline and 12 weeks. Normality assumptions were assessed through visual inspection of the distribution of pre-post difference scores on a histogram and QQ plot. Effect sizes were reported using Cohen’s D (d), with values of.2,.5, and.8 interpreted as small, medium, and large, respectively [60]. Outcome measures that did not satisfy the assumptions for a paired t-test were assessed using the Wilcoxon matched-pairs signed-ranks test, with effect size (r) values of.1,.3, and.5 interpreted as small, medium, and large, respectively [60]. One participant was excluded from the perfusion, CSF, and CBF flow analysis as they wore a facemask during MRI image acquisition which has been shown to impact respiration and cerebral blood flow [61–63].
A one-way repeated measures ANOVA was performed to calculate effect sizes on all outcome measures collected at 4 timepoints, including the MST and Flanker. Normality assumptions were assessed through visual inspection of the distribution of the residuals on a histogram and QQ plot. Homoscedasticity was assessed using Mauchly’s test for sphericity. Effect sizes were reported using partial eta squared (ηp2), with values of.01,.06, and.14 interpreted as small, medium, and large, respectively [60]. Outcome measures that failed the assumptions for the ANOVA were assessed using the Friedman’s test with Kendall’s W effect size values of.2,.5, and.8 interpreted as small, medium, and large, respectively [60]. One participant was excluded from the MST analysis due to missing data at the baseline timepoint.
Percent mean and standard deviation change scores, defined as the difference in scores pre- to post-intervention divided by the pre-intervention score, were reported for all outcome measures that were analyzed using parametric tests. Percent median and IQR change scores were reported for all measures analyzed using non-parametric tests.
RESULTS
Cognitive battery
Global cognition
No differences in global cognition, as measured by the MOCA, were found pre- (26.0±2.7) to post-intervention (25.8±3.7).
Table 2.
Participant demographics and physical characteristics
| Characteristics | N = 2 |
| Age (year) | 65±8.8 |
| Gender, n | 8 F |
| Race, n | |
| Asian | 1 |
| African American | – |
| White | 9 |
| Hispanic | 2 |
| Education (year) | 17±2 |
| Weight (Ibs) | 166±25 |
| Body fat (%) | 31±9 |
| Blood pressure | |
| Systolic | 132±14 |
| Diastolic | 80±9 |
| Physical activity (mins/day)a | |
| Light | 316±81 |
| Moderate | 48±21 |
| Vigorous | 9±8 |
Mean, standard deviation, minimum, and maximum values are shown. Physical activity measurements were recorded using a GENEActiv accelerometer. Light, moderate, and vigorous physical activity levels were defined according to 30, 100, and 200 mg threshold levels, respectively. The reported values represent the average number of minutes in which accelerometer measurements were greater than the light/moderate/vigorous activity thresholds for at least 80% of a 1-minute bout [64]. aOne participant excluded due to missing accelerometer data.
Table 3.
Descriptive statistics and effect sizes for all cognitive outcomes
| Measure | Baseline | 4 Weeks | 8 Weeks | 12 Weeks | Effect size |
| MOCAa | 26.0±2.7 | – | – | 25.8±3.7 | 0.06 |
| DKEFS TMT | |||||
| Part B | 80.0±42.0 | 68.5±42.0 | 0.42* | ||
| Part Aa | 35.1±15.8 | 33.0±11.1 | 0.23 | ||
| Part B - A | 49.5±30.0 | 39.0±38.0 | 0.38* | ||
| Digit Symbol Substitutiona | 68.4±14.4 | 71.3±13.6 | 0.42 | ||
| Flanker | |||||
| Incongruentb | 718.8±89.7 | 674.3±94.4 | 685.0±109.2 | 641.8±81.7 | 0.73** |
| Congruent | 579.1±77.7 | 556.5±139.5 | 584.7±174.6 | 551.8±113.7 | 0.04 |
| Interference Control | 127.0±47.8 | 107.4±42.6 | 92.2±36.6 | 83.2±36.8 | 0.54* |
| RAVT | |||||
| Immediate Recall | 49.0±13.0 | 50.0±14.0 | 0.26 | ||
| Delayed Recalla | 9.6±3.8 | 9.7±3.8 | 0.03 | ||
| MSTc | |||||
| Lure Discrimination Indexb | 0.19±0.24 | 0.27±0.16 | 0.34±0.15 | 0.32±0.18 | 0.43** |
Descriptive statistics are reported as median±IQR and effect sizes reported using r or Kendall W for data collected at 2 timepoints and 3 + timepoints, respectively, unless otherwise specified. aData normally distributed so statistics reported as mean±SD and effect sizes reported using Cohen’s d. bData normally distributed so statistics reported as mean±SD and effect sizes reported as using ηp2. cOne participant excluded due to missing data at baseline timepoint. *Medium effect sizes associated with pre- to post-intervention changes. **Large effect sizes associated with pre- to post-intervention changes.
Executive function
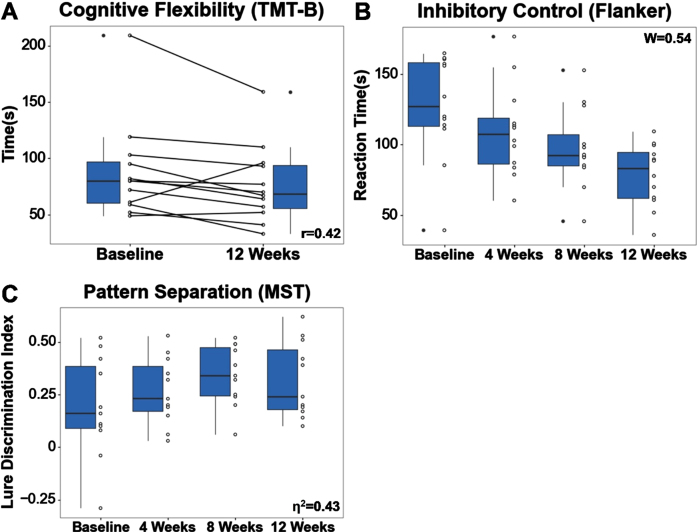
Two core aspects of executive function improved during the intervention: cognitive flexibility and inhibitory control (Fig. 4). Cognitive flexibility as measured by TMT-B showed improvement of medium effect size, with completion times decreasing 14.4% pre- to post-intervention (median = –14.4%, IQR = 0.0%, r = 0.42). This improvement was slightly diminished but remained moderate even after controlling for processing speed by subtracting out TMT A completion time (TMT B-A) (median = –21.2%, IQR = 26.6%, r = 0.38). Inhibitory control as measured by Flanker task reaction times also showed medium effect size improvement. Pairwise comparisons showed a decrease in reaction time across all timepoints, including baseline to 4 weeks (median =–15.4%, IQR = –10.9%), 4 weeks to 8 weeks (median = –14.2%, IQR = –14.1%), and 8 weeks to 12 weeks (median = –9.8%, IQR = 0.5%). Overall, reaction times decreased 34.4%pre- to post-intervention (median = –34.4%, IQR = –23.0%, W = 0.54). No differences in processing speed and attention, as measured by the Digit Symbol Substitution Test, were found pre- (68.4±14.4) to post-intervention (71.3±13.6).
Fig. 4.
(A) Cognitive flexibility, as measured by TMT-B, improved pre- to post-intervention. (B) Inhibitory control, as measured by Flanker, improved across each of the 4 timepoints. (C) Visual discrimination related to pattern separation, as measured by MST, improved pre- to post-intervention. Effect sizes are shown in the bottom or top right corner of each boxplot.
Memory
Visual memory discrimination related to pattern separation, as measured by the MST lure discrimination index (LDI), showed large effect size improvement with scores increasing 68.4% pre- to post-intervention (+68.4% ± –25.0%, η2 = 0.43) (Fig. 4). Pairwise comparisons showed an increase in scores across the first three timepoints, including baseline to 4 weeks (+42.1% ± –33.3%) and 4 weeks to 8 weeks (+25.9% ± –6.3%). No differences in verbal memory, as measured by RAVLT, were found pre- to post-intervention on either the immediate (pre: median = 49.0, IQR = 13.0; post: median = 50.0, IQR = 14.0) or 30-minute delayed (pre: 9.6±3.8; post: 9.7±3.8) word-list recalls.
Neuroimaging
Structural volumes
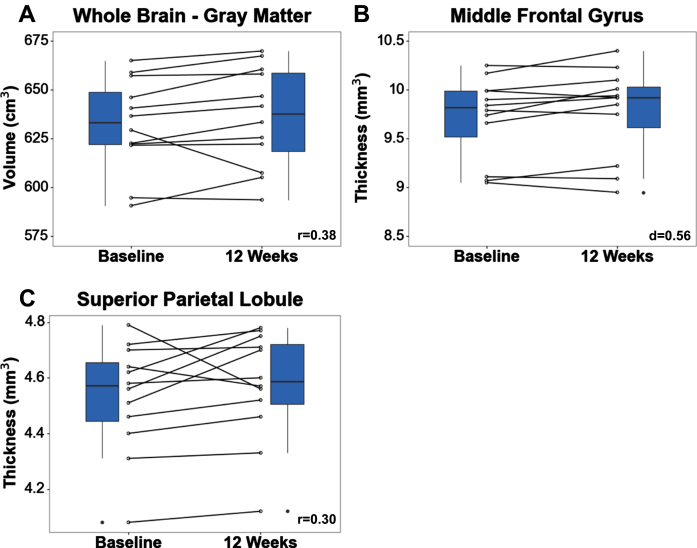
Analysis of whole brain volumes showed changes of medium effect size, with total grey matter volume increasing 0.73% pre- to post-intervention (median =+0.73%, IQR = –102.1%, r = 0.38) (Fig. 5). No changes were found at the hippocampus (pre: 7.74±0.57; post: 7.75±0.51 cm3) pre to post-intervention. Analysis of the hippocampal substructures found no changes pre- to post-intervention at the CA1 (pre: 2.69±0.27 cm3; post: 2.69±0.29 cm3), dentate gyrus (pre: 1.65±0.14 cm3; post: 1.63±0.14 cm3), or entorhinal cortex (pre: 0.92±0.10 cm3; post: 0.92±0.10 cm3). Within the cerebral cortex, thickness of the superior parietal lobule and middle frontal gyrus showed changes of medium effect size, increasing 0.4% (median = +0.4%, IQR = 0.0%, r = 0.30) and 0.72% (0.72% ± 9.5%, d = 0.56), respectively, pre- to-post intervention (Fig. 5). No changes in thickness were found at the superior frontal gyrus (pre: 5.30±0.23; post: 5.32±0.26, r = 0.26) or inferior frontal gyrus (pre: 15.07±0.89; post: 15.17±1.14, r = 0.15)
Fig. 5.
Whole brain volume (A), superior parietal lobule thickness (B), and middle frontal gyrus thickness (C) increased pre- to post-intervention. Effect sizes are shown in the bottom right corner of each boxplot.
Table 4.
Descriptive statistics and effect sizes for all neuroimaging outcomes
| Measure | Baseline | 12 Weeks | Effect Size |
| Structural volume (cm3) | |||
| Total grey mattera | 633.0±32.7 | 637.6±25.6 | 0.38* |
| Hippocampus | 7.74±0.57 | 7.75±0.51 | 0.05 |
| CA1 | 2.69±0.27 | 2.69±0.29 | 0.07 |
| Dentate gyrus | 1.65±0.14 | 1.63±0.14 | 0.26 |
| Entorhinal cortex | 0.92±0.10 | 0.92±0.10 | 0.02 |
| Cortical thickness (mm3) | |||
| Superior parietal lobulea | 4.57±0.27 | 4.59±0.27 | 0.30* |
| Superior frontal gyrus | 5.30±0.23 | 5.32±0.26 | 0.26 |
| Middle frontal gyrus | 9.71±0.42 | 9.78±0.46 | 0.56* |
| Inferior frontal gyrusa | 15.07±0.89 | 15.17±1.14 | 0.15 |
| CB flow - ICA and VAb | |||
| Pulsatility index | 1.24±0.41 | 1.11±0.38 | 0.47 |
| Resistivity index | 0.67±0.11 | 0.65±0.10 | 0.30 |
| CSF flow - Subarachnoid spaceb | |||
| Stroke volume (mm3)a | 436.2±177.0 | 527.3±297.3 | 0.28 |
| Flush peak (mm3/s) | 1972±572 | 2194±776 | 0.51* |
| CSF flow - Aqueductb | |||
| Stroke volume (mm3)a | 47.8±48.5 | 57.6±30.5 | 0.19 |
| Flush peak (mm3/s) | 209.1±74.7 | 220.8±58.2 | 0.20 |
| Cerebral perfusionb,c | 36.6±9.3 | 38.2±7.4 | 0.13 |
Descriptive statistics are reported as mean±SD and effect sizes reported using Cohen’s d unless otherwise specified. aData not normally distributed so statistics reported as median±IQR and effect sizes reported using test statistic from non-parametric Wilcoxon signed rank test. bOne participant was excluded due to wearing a facemask while in MRI scanner. cPCASL sequence not acquired in one participant. *Medium effect sizes associated with pre- to post-intervention changes.
Cerebral flow
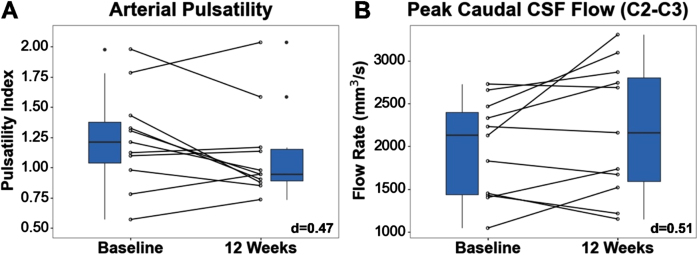
Changes in PC-MRI measurements of both cerebral blood flow and cerebrospinal fluid flow were found pre to post-intervention (Fig. 6). CBF flow at the internal carotid and vertebral arteries showed changes approaching medium effect size, with arterial pulsatility decreasing 10.5% (–10.5% ± 0.5%, d = 0.47), indicating a reduction in the peripheral resistance of the cerebral arteries. CSF flow at the C2-C3 subarachnoid space showed changes of medium effect size, with flush peak (craniocaudal) flow rates increasing 11.3% pre- to post-intervention (11.3% ± 35.7%, d = 0.51). However, no differences were found in stroke volume (pre: 436.2±177.0 mm3; post: 527.3±297.3 mm3). For CSF flow at the cerebral aqueduct, no differences were found for both stroke volume (pre: median = 47.8, IQR = 48.5 mm3; post: 57.6, IQR = 30.5 mm3) and flush peak (pre: 209.1±74.7 mm3/s; post: 220.8±58.2 mm3/s). Finally, no differences were found in PCASL whole brain cerebral blood perfusion (pre: 36.6±9.3 ml/100 g/min; post: 38.2±7.4 ml/100 g/min).
Fig. 6.
(A) Lower arterial pulsatility suggests reduced peripheral vascular resistance. (B) Higher cranio-caudal peak CSF flow through C2-C3 SS suggests improved circulation. Effect sizes are shown in the bottom right corner of each boxplot.
Aerobic exercise and cognitive training
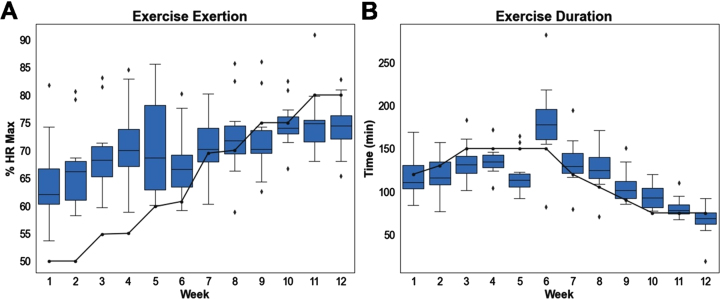
Participants were within or above their target heart-rate zone while cycling in VR 61% of the time. Participants were below their target heart-rate zone 39% of the time. On a per-week basis, participants cycled well above their target HR for the first 8 weeks and slightly below it the last 4 weeks (Fig. 7). Participants spent an average of 118 minutes per week cycling in VR. On a per-week basis, average time spent cycling closely tracked weekly target times, with participants cycling slightly longer the first 5 weeks and slightly shorter the last 7 weeks (Fig. 7). Overall, participants cycled for approximately the prescribed amount of time, but at higher than recommended exertion levels in the moderate phase and lower than recommended exertion levels in the vigorous phase according to the exercise periodization protocol shown in Table 1.
Fig. 7.
(A) Exertion levels, defined as percentage of HR max, per week. Solid line indicates target exertion based on aerobic exercise schedule. (B) Time spent cycling per week. Solid line indicates target volume based on aerobic exercise schedule.
Table 5.
Summary of aerobic exercise and cognitive training performance in virtual reality
| Performance | Mean±SD |
| Avg. HR (bpm) | 112.6±8.9 |
| Time in or above target HR (%) | 61.4±6.6 |
| Less than target (%) | 38.6±6.6 |
| RPE | |
| Baseline | 7.5±1.2 |
| Final | 12.1±1.7 |
| Peak | 13.6±2.3 |
| Distance traveled (miles)a | 269±12 |
| Avg. speed (mph)a | 11.3±1.5 |
| Time in VR | |
| Total (hours) | 23.5±3.8 |
| Week (mins) | 118±19 |
| Visit (mins) | 39±6 |
| Correct decisions (%) | |
| Forward | 92.9±8.2 |
| Reverse | 82.1±16.0 |
| Immediate recall | 93.5±8.0 |
| Delayed recall | 90.1±11.9 |
aSpeed and distance traveled were estimated based on the wheelbase and gear ratio of the stationary exercise bike.
Current study VR intervention outcomes vs. published control group outcomes
The design of this proof-of-concept pilot study did not include a formal control group. As an alternative method to account for possible practice effects or natural changes in brain health that occur over time, control groups from previously published randomized controlled exercise and cognitive training interventions were reviewed (Table 6) [65–68]. These reference studies had similar participant demographics to our VR intervention, as they were conducted in cognitively normal older adults with mean ages ranging from 62 to 72 years old. Moreover, all but one reference study was 12 weeks in duration. The control group was non-active in three of the five reference studies and consisted of light stretching activities in the other two studies. Table 6 shows a comparison between our VR intervention and the reference studies control groups for the outcome measures that had medium-to-large effect sizes. For each outcome measure, the relative change from baseline to 12 weeks was larger for our VR intervention compared to the control group.
Table 6.
VR intervention outcomes vs control group outcomes
| Measure | Control groups from other studies | VR intervention | |||
| Study characteristics | Baseline | 12 Weeks | Baseline | 12 Weeks | |
| Structural volume (cm3) | |||||
| Total grey matter | McCauley et al.c | 548.1±46.2 | 548.2±49.3 | 633.0±32.7 | 637.6±25.6 |
| Cortical thickness (mm3) | n = 20 | ||||
| Superior parietal lobule | Age: 69±5.8 | 4.16±0.24 | 4.17±0.27 | 4.57±0.27 | 4.59±0.27 |
| Middle frontal gyrus | Sex: 70% F | 9.22±0.32 | 9.16±0.36 | 9.71±0.42 | 9.78±0.46 |
| CSF flush peak (mm3/s) | Length: 12 weeks | 1972±572 | 2194±776 | ||
| CBF flow pulsatility indexa | Activity: None | 0.99±0.23 | 1.01±0.25 | 1.24±0.41 | 1.11±0.38 |
| Y-Balanceb | 396.4±38.7 | 407.7±37.3 | 363.9±88.0 | 386.1±66.5 | |
| MST - Lure discrimination index | Kovacevic et al.252 | ||||
| n = 23 | |||||
| Age: 72±6.6 | 0.12±0.12 | 0.17±0.17 | 0.19±0.24 | 0.32±0.18 | |
| Sex: 65% F | |||||
| Length: 12 weeks | |||||
| Activity: Stretching | |||||
| TMT - Part B | Juliano et al.253 | ||||
| n = 20 | |||||
| Age: 67±11.7 | 78.1±29.0 | 79.6±35.2 | 80.0±42.0 | 68.5±42.0 | |
| Sex: 60% F | |||||
| Length: 12 weeks | |||||
| Activity: None | |||||
| TMT - Part B - A | Nishiguchi et al.254 | ||||
| n = 24 | |||||
| Age: 74±5.6 | 37.9±20.7 | 41.5±30.7 | 49.5±30.0 | 39.0±38.0 | |
| Sex: 46% F | |||||
| Length: 12 weeks | |||||
| Activity: None | |||||
| Flanker - Interference controlb | Gothe et al.255 | ||||
| n = 57 | |||||
| Age: 62±5.6 | 186.7 | 179.1 | 127.0±47.8 | 83.2±36.8 | |
| Sex: 75% F | |||||
| Length: 8 weeks | |||||
| Activity: Stretching | |||||
aPulsatility calculations for the control group and intervention were based on two different MRI acquisition sequences. The control group measurements were acquired using an in-house vascular compliance sequence, while the intervention measurements were acquired using a PC-MRI sequence. bInterference control values for the control group were calculated as the difference in mean incongruent (Incon) and congruent (Con) reaction times at baseline and 8- weeks based on the following reported values in the study: baseline (Incon: 790.6±137.3, Con: 603.9±81.3). 8-weeks (Incon: 770.4±182.2, Con: 591.3±86.4). cControl group values are shown for a 12-week control period between baseline and the start of a periodized aerobic exercise intervention conducted in older adults by Dr. Tim McCauley at the CERC at USC. Participants were advised to continue their normal daily activities during this control period.
DISCUSSION
In this phase 2 pilot study, we conducted a 12-week intervention assessing the impact of simultaneous exercise and cognitive training in VR on brain health and cognition in a cohort of healthy, cognitively normal older adults. This novel multi-modal approach required subjects to cycle on a stationary exercise bike while engaging in cognitively challenging spatial memory tasks in a virtual environment. Our findings showed improvements across several MRI imaging and neuropsychological measures of brain health. Specifically, we found positive changes, of medium-to-large effect size, in vascular resistance and brain structure, including total grey matter volume, superior parietal lobule thickness, and middle frontal thickness. Cognitive changes were found as well, with improvements seen in memory discrimination, cognitive flexibility, and response inhibition.
Cognitive performance
Among the neuropsychological measures, improvements in key aspects of memory and executive function were identified. Improvements in pattern separation, a hallmark feature of episodic memory and a sensitive measure of hippocampal function, were found pre- to post-intervention [69]. This is a key finding as pattern separation has been shown to decline across the lifespan, with older adults performing poorly compared to younger adults on recognition memory tasks requiring visual pattern separation due to age-related alterations in hippocampal integrity [70, 71]. Our findings support previous studies which have shown that pattern separation can be improved in older adults through exercise and environmental enrichment. In one such environmental enrichment study, older adults showed significant improvement in MST lure discrimination performance after a 4-week intervention involving spatial exploration and learning in the real world [72]. In another exercise-only study, a 30% improvement in MST lure discrimination performance was found in older adults after a 12-week intervention involving high-intensity interval training [73].
Three core subcomponents of executive function: attention, cognitive flexibility, and inhibitory control were assessed. Improvements in both cognitive flexibility and inhibitory control, but not attention, were observed. These improvements in executive function are consistent with previous studies, including a meta-analysis assessing the impact of aerobic exercise on executive function in older adults [74]. In the meta-analysis, aerobic exercise interventions were associated with modest, but significant improvements on a battery of assessments measuring executive function [74]. This included cognitive flexibility, in which 5 studies, 6 to 17 weeks in duration, showed significant improvement in TMT-B completion times [74].
Cerebral flow
Among the neuroimaging measures, positive changes in cerebral blood flow (CBF) pulsatility at the arteries in the cervical C2-C3 region were found. Pulsatility is an important measure of cardiovascular health and is commonly used as an indicator of peripheral vascular resistance [75]. Aging is associated with a gradual stiffening of the arteries [76]. Arterial stiffening causes excessive pulsatile flow which induces microvascular damage and leads to increased peripheral vascular resistance [76]. Long-term dysregulation of cerebral blood flow can also cause hypertension, a risk factor for developing Alzheimer’s disease [1]. As a result, pulsatility may be a potential early biomarker for AD. This is supported in several studies, including one which showed that individuals with MCI and AD had higher arterial pulsatility relative to healthy older adults [77].
The improvements in CBF flow found in our study are likely driven by exercise which has been shown to lower arterial stiffness and may play a key role in reversing cerebrovascular dysfunction. Moreover, exercise is a modifiable risk factor for AD, suggesting that interventions that incorporate aerobic exercise may be useful for preventing cognitive decline. This has been supported in several studies, including a cross-sectional study on middle-aged adults, in which higher aerobic fitness was associated with lower arterial stiffness and better cognitive performance [78]. In another 12-week study in older adults, it was found that the aerobic exercise group had significantly lower pulsatility compared to the control group at the basilar, vertebral, posterior, anterior, and middle cerebral arteries [79]. This supports the findings from our study, which show lower pulsatility at the vertebral and carotid arteries.
Cerebrospinal fluid flow (CSF) at the cerebral aqueduct and C2-C3 region was measured as an exploratory analysis. CSF flow dysfunction in these two regions is commonly associated with normal pressure hydrocephalus [80], syringomyelia [81], and Chiari malformations [82]. However, recent studies have also shown that CSF flow alterations occur with normal aging. In one such study, it was shown that stroke volumes and flush peak flow rates at the C2-C3 region, and stroke volumes at the cerebral aqueduct, were lower in older adults compared to younger adults [28]. While studies assessing CSF flow in AD are limited, there is evidence to suggest it plays an important role in the clearance of amyloid plaques from the brain. This clearance pathway is characterized by arterial pulsatility which drives CSF flow through a network of perivascular channels, facilitating elimination of waste from the interstitial space [83]. Taken together, this suggests that low CSF flow may be associated with impaired clearance.
Studies assessing the effects of exercise on CSF flow are limited. However, as exercise has been shown to improve CBF flow, it is expected that changes in CSF flow would occur as well due to the dynamic link between CBF and CSF flow in the brain established by the Monroe-Kellie doctrine [84]. While we did not find any differences in CSF flow at the aqueduct, we did find CSF flow changes at the C2-C3 region. Specifically, we found improvements in peak cranio-caudal CSF flow rates. Taken together, this suggests that exercise induces alterations in CSF and CBF flow that may enhance waste clearance from the brain.
Structural volumes
Structural changes in the brain, including increases in total grey matter volume, superior parietal lobule thickness, and middle frontal thickness, were observed. This increase is a key finding as normal aging is associated with brain volume loss, with estimates ranging from 0.32% to 0.55% per year in healthy adults 70 years of age or older [85]. Moreover, regions of brain volume loss are differentially impacted by age, with larger losses occurring in the frontal, parietal, and temporal lobe [86]. Improvements in brain volume have been shown in several exercise studies, including one which found significant increases in grey matter volume in older adults who engaged in 6-months of moderate aerobic exercise [86]. In another study, it was shown that 82% of grey matter volume in the brain was associated with physical activity, including aerobic exercise, weight lifting, running, martial arts, and sports [87]. Taken together, this supports the findings in our study and suggests that brain volume in older adults may be preserved and enhanced after only 3 months of aerobic exercise at moderate-to-vigorous intensity levels. In addition to exercise, cognitive enrichment may also play an important role in enhancing brain volume. This is supported by our findings at the superior parietal lobule, an important region for forming egocentric representations of space in spatial memory [88]. Improvements observed specifically at this region suggests that our cognitive training paradigm targeting spatial memory engagement may have subserved the impact of aerobic exercise on brain volume.
Interestingly, changes in hippocampal volumes were not observed. This included the hippocampal subfields CA1, dentate gyrus, and entorhinal cortex. Preserving hippocampal volumes is of critical importance as the dentate gyrus is the primary site of neurogenesis while the CA1 and the entorhinal cortex are important regions for spatial memory. Moreover, the hippocampus is highly sensitive to the effects of aging, with volume loss estimated to be as high as 1.1% per year in older adults [89]. Previous studies assessing the impact of aerobic exercise on hippocampal volumes have yielded results with significant heterogeneity. In one such study, hippocampal volumes increased 2% after 1 year of aerobic exercise consisting of walking at moderate intensities [11]. However, in another study in which older adults exercised at moderate-to-vigorous intensities over 16 weeks, it was shown that hippocampal volumes were preserved but not enhanced [90]. This was supported in a meta-analysis, which showed that exercise prevented decreases in hippocampal volume over time [91]. Overall, the impact of exercise on hippocampal volumes remains unclear. It is also possible that our 3-month intervention was not long enough to elicit significant changes to hippocampal volumes.
Participant adherence to the intervention
Another objective of this pilot study was to assess how well older adults could adhere to an aerobic exercise protocol while engaging in cognitive training in VR. Our periodized aerobic exercise routine was designed for participants to cycle at moderate (50%–60% HR max) intensity levels the first 6 weeks and vigorous (70%–80% HR max) intensity levels the last 6 weeks. We found that participants cycled well above their moderate target exertion levels the first 6 weeks. This may be due to age, as older adults have a lower estimated HR max which yields a target HR that is close to their resting HR at low prescribed intensity levels. Participants cycled approximately within their target exertion levels in weeks 7 through 10, but slightly below in weeks 11 and 12. This may be due to the design of the cognitive training paradigm, where participants did not have enough time to get up to the required heart rate before the end of the trial. Overall, however, participants were successfully able to reach target exertion levels, as they cycled within or above their target HR zone 61% of the time. Moreover, participants were also able to cycle for the prescribed duration, as the average cycling time for participants tracked closely with the target times each week. Collectively, this suggests that it is possible to incorporate an aerobic exercise routine that adheres to the Surgeon General guidelines for exercise in older adults while simultaneously engaging in a cognitive training paradigm in virtual reality.
Cognitive training
The cognitive training paradigm was designed to progressively increase in difficulty throughout the intervention. Overall, all participants performed well on the navigation tasks, making correct decisions at 93% ± 8% of the intersections (min: 52%, max 100%) in the forward direction and 82% ± 16% (min: 33%, max 100%) in the reverse direction. The high performance on these tasks indicates that the older adult participants were successfully able to navigate and learn new routes in virtual reality. This suggests that the visual cues in the virtual environments, including the landmarks at each intersection, were sufficient for spatial learning. Due to the high performance on the navigation tasks, participants may benefit from a higher level of route difficulty. This can be achieved by adding more intersections or reducing the number of cued learning trials.
Participant retention and attrition
A total of fifty-two older adults were contacted and screened for eligibility to participate in this study. Thirty-seven eligible older adults completed an additional VR screen, with 27% (n = 10) excluded from further participation due to self-reported symptoms of simulator sickness associated with cycling in a virtual environment. This attrition rate is expected based on previously published studies assessing adverse effects associated with locomotion in virtual reality, including one which found that 25% of younger and older adults reported significant sickness symptoms when cycling while wearing an immersive VR head-mounted display [19]. Among the 27 participants that passed the VR screen, sixteen completed baseline data collection with twelve completing the entire 12-week intervention and all data collection timepoints, yielding a 25% attrition rate. While the attrition rate appears relatively high, it was confounded by the onset of COVID. Indeed, two participants discontinued due to safety concerns regarding COVID and not the intervention itself. After excluding for this unprecedented pandemic, the attrition rate dropped to 13%, which is well within previously established guidelines for clinical trials [92]. Moreover, in a systematic review on exercise studies in sedentary adults, it was found that the average rate of attrition for prescribed weightlifting and aerobic exercise periodization routines was 9.5% [93]. In this study, only 6% of the study sample (n = 1) discontinued due to non-compliance with the prescribed intervention, which is lower than the attrition rates seen in other exercise studies.
Adverse effects in virtual reality
The primary concern with coupling locomotion and virtual reality while wearing an immersive HMD is adverse effects due to sensory incongruence induced by a mismatch between perceived motion in VR and actual motion in the real world [94–96]. In this study, only one participant discontinued due to significant adverse effects, suggesting the VR screen was successful in preemptively excluding individuals with high sensitivity to simulator sickness. A few participants did experience mild adverse effects, including one participant that experienced slight nausea and stomach awareness sporadically throughout the intervention. Two participants also experienced slight nausea within the first week, but quickly acclimated to VR and reported no adverse effects on any of the subsequent visits afterwards. Overall, symptoms were temporary and self-resolved in all cases. A preliminary acclimation period that gradually increases the time spent in VR may help prevent these initial transient symptoms.
Limitations
There were several notable limitations to this pilot study. The primary limitation was a small sample size of 12 participants, which is insufficient for assessing statistical significance. Instead, effect sizes and percent changes were reported for each measure. Another limitation of the present study was the lack of a control group. While control groups are not required for proof-of-concept pilot studies, [57] its exclusion makes it difficult to assess natural change that occurs in the brain over time or practice effects that occur due to repeated neuropsychological testing. As a surrogate, we utilized control groups from previously published randomized controlled studies with similar participant demographics. The control groups showed no brain structure or cerebral blood flow changes over 12 weeks. Moreover, no practice effects were observed for the neuropsychological tests, including Flanker, DKEFS TMT-B, and MST. This is supported by a few studies, including one in which MST was empirically validated to show no practice effects with repeated short-term testing [52]. Overall, however, test-retest reliability for several of our cognitive outcomes have been reported to vary considerably, generally ranging from fair-to-good, depending on the specific task and condition [51, 53–56]. As a result, the brain and cognition improvements reported in our study may be partially attributed to natural brain changes or practice effects which cannot be fully excluded without an exercise-only and cognitive-only control group.
An additional limitation of this study was the heterogeneity of the participant demographics. Our study included all adults between 50 and 85 years of age, spanning almost the entire range of older adulthood. This may have confounded our findings as age differentially impacts brain structure and cognitive function. Similarly, our sample was imbalanced by gender, with twice as many women participating in the study as men. Furthermore, the participants in our study were physically active and highly educated. Self-reported measures of physical activity showed that most participants engaged in weightlifting or aerobic exercise, including walking and cycling, prior to the start of the intervention. As a result, the positive changes in brain health associated with aerobic exercise and cognitive training in this active, well-educated cohort may underestimate the true changes observed in a more typical, sedentary older adult population. Finally, while the benefits of aerobic exercise and cognitive training have been shown to appear within 12 weeks, longer intervention durations may be needed to elicit changes in key regions such as the hippocampus.
CONCLUSION
In this pilot study we report that a 12-week intervention consisting of simultaneous aerobic exercise and cognitive training in virtual reality elicits positive changes in executive function, memory, brain structure, and cerebral blood flow. We also demonstrate that this intervention can be conducted in older adults, ages 50–85, with high compliance and low attrition. Future steps include conducting this study in a larger, randomized controlled clinical trial with careful consideration of control arms, such as aerobic exercise-only and cognitive training-only to understand the relative benefits of engaging in combined activities over each one individually.
Ethics approval and consent to participate
All subjects provided written consent to participate in this study, which was approved by the Institutional Review Board at the University of Southern California and performed in accordance with the Declaration of Helsinki.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
FUNDING
None.
ACKNOWLEDGMENTS
None.
REFERENCES
- [1]. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. The Lancet Neurology. 2011;10(9):819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Alzheimer’s Association, Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2019;15(3):321–87. [Google Scholar]
- [3]. Bäckman L, Small BJ, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain. 2001;124(1):96–102. [DOI] [PubMed] [Google Scholar]
- [4]. Kessels RP, Feijen J, Postma A. Implicit and explicit memory for spatial information in Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2005;20(2-3):184–91. [DOI] [PubMed] [Google Scholar]
- [5]. Vlček K, Laczó J. Neural correlates of spatial navigation changes in mild cognitive impairment and Alzheimer’s disease. Frontiers in Behavioral Neuroscience. 2014;8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA. Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Medicine. 2010;12(1):27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Allison SL, Fagan AM, Morris JC, Head D. Spatial navigation in preclinical Alzheimer’s disease. Journal of Alzheimer’s Disease. 2016;52(1):77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, Booker L, Oremus M. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Annals of Internal Medicine. 2008;148(5):379–97. [DOI] [PubMed] [Google Scholar]
- [9]. Herrmann N, Chau SA, Kircanski I, Lanctot KL. Current and emerging drug treatment options for Alzheimer’s disease. Drugs. 2011;71(15):2031–65. [DOI] [PubMed] [Google Scholar]
- [10]. Amieva H, Mokri H, Le Goff M, Meillon C, Jacqmin-Gadda H, Foubert-Samier A, Orgogozo JM, Stern Y, Dartigues JF. Compensatory mechanisms in higher-educated subjects with Alzheimer’s disease: a study of 20 years of cognitive decline. Brain. 2014;137(4):1167–75. [DOI] [PubMed] [Google Scholar]
- [11]. Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences. 2011;108(7):3017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Karssemeijer EG, Aaronson JA, Bossers WJ, Donders R, Rikkert MG, Kessels RP. The quest for synergy between physical exercise and cognitive stimulation via exergaming in people with dementia: a randomized controlled trial. Alzheimer’s Research & Therapy. 2019;11(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Schultheis MT, Rizzo AA. The application of virtual reality technology in rehabilitation. Rehabilitation Psychology. 2001;46(3):296. [Google Scholar]
- [14]. Cushman LA, Stein K, Duffy CJ. Detecting navigational deficits in cognitive aging and Alzheimer disease using virtual reality. Neurology. 2008;71(12):888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Maguire EA, Burgess N, O’Keefe J. Human spatial navigation: cognitive maps, sexual dimorphism, and neural substrates. Current Opinion in Neurobiology. 1999;9(2):171–7. [DOI] [PubMed] [Google Scholar]
- [16]. Lange-Asschenfeldt C, Kojda G. Alzheimer’s disease, cerebrovascular dysfunction and the benefits of exercise: from vessels to neurons. Experimental Gerontology. 2008;43(6):499–504. [DOI] [PubMed] [Google Scholar]
- [17]. Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B. Clearance systems in the brain—implications for Alzheimer disease. Nature Reviews Neurology. 2015;11(8):457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Kennedy RS, Lane NE, Berbaum KS, Lilienthal MG. Simulator sickness questionnaire: An enhanced method for quantifying simulator sickness. The International Journal of Aviation Psychology. 1993;3(3):203–20. [Google Scholar]
- [19]. Sakhare AR, Yang V, Stradford J, Tsang I, Ravichandran R, Pa J. Cycling and spatial navigation in an enriched, immersive 3D virtual park environment: A feasibility study in younger and older adults. Frontiers in Aging Neuroscience. 2019;11:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Cobb SV, Nichols S, Ramsey A, Wilson JR. Virtual reality-induced symptoms and effects (VRISE). Presence: Teleoperators & Virtual Environments. 1999;8(2):169–86. [Google Scholar]
- [21]. Borg GA, Psychophysical bases of perceived exertion. Medicine & Science in Sports & Exercise. 1982. [PubMed]
- [22]. Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. Journal of the American College of Cardiology. 2001;37(1):153–6. [DOI] [PubMed] [Google Scholar]
- [23]. U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. Washington, DC: U.S. Department of Health and Human Services; 2018.
- [24]. Glen K, Eston R, Loetscher T, Parfitt G. Exergaming: Feels good despite working harder. Plos one. 2017;12(10):e0186526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Albrecht D, Isenberg AL, Stradford J, Monreal T, Sagare A, Pachicano M, Sweeney M, Toga A, Zlokovic B, Chui H, Joe E. Associations between vascular function and tau PET are associated with global cognition and amyloid. Journal of Neuroscience. 2020;40(44):8573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, Van Osch MJ. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine. 2015;73(1):102–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Balédent O, Idy-peretti I. Cerebrospinal fluid dynamics and relation with blood flow: a magnetic resonance study with semiautomated cerebrospinal fluid segmentation. Investigative Radiology. 2001;36(7):368–77. [DOI] [PubMed] [Google Scholar]
- [28]. Stoquart-ElSankari S, Balédent O, Gondry-Jouet C, Makki M, Godefroy O, Meyer ME. Aging effects on cerebral blood and cerebrospinal fluid flows. Journal of Cerebral Blood Flow & Metabolism. 2007;27(9):1563–72. [DOI] [PubMed] [Google Scholar]
- [29]. Nitz WR, Bradley WG Jr, Watanabe AS, Lee RR, Burgoyne B, O’sullivan RM, Herbst MD. Flow dynamics of cerebrospinal fluid: assessment with phase-contrast velocity MR imaging performed with retrospective cardiac gating. Radiology. 1992;183(2):395–405. [DOI] [PubMed] [Google Scholar]
- [30]. de Riva N, Budohoski KP, Smielewski P, Kasprowicz M, Zweifel C, Steiner LA, Reinhard M, Fábregas N, Pickard JD, Czosnyka M. Transcranial Doppler pulsatility index: what it is and what it isn’t. Neurocritical Care. 2012;17(1):58–66. [DOI] [PubMed] [Google Scholar]
- [31]. Bude RO, Rubin JM. Relationship between the resistive index and vascular compliance and resistance. Radiology. 1999;211(2):411–7. [DOI] [PubMed] [Google Scholar]
- [32]. Harloff A, Albrecht F, Spreer J, Stalder AF, Bock J, Frydrychowicz A, Schöllhorn J, Hetzel A, Schumacher M, Hennig J, Markl M. 3D blood flow characteristics in the carotid artery bifurcation assessed by flow-sensitive 4D MRI at 3T. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2009;61(1):65–74. [DOI] [PubMed] [Google Scholar]
- [33]. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–94. [DOI] [PubMed] [Google Scholar]
- [34]. Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–55. [DOI] [PubMed] [Google Scholar]
- [35]. Anderson-Hanley C, Barcelos NM, Zimmerman EA, Gillen RW, Dunnam M, Cohen BD, Yerokhin V, Miller KE, Hayes DJ, Arciero PJ, Maloney M. The aerobic and cognitive exercise study (ACES) for community-dwelling older adults with or at-risk for mild cognitive impairment (MCI): neuropsychological, neurobiological and neuroimaging outcomes of a randomized clinical trial. Frontiers in Aging Neuroscience. 2018;10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Sepehrband F, Barisano G, Sheikh-Bahaei N, Choupan J, Cabeen RP, Crawford MS, Mack WJ, Chui HC, Ringman JM, Toga AW, Alzheimer’s Disease Neuroimaging Initiative, Alteration of perivascular spaces in early cognitive decline: Neuroimaging/Optimal neuroimaging measures for early detection. Alzheimer’s & Dementia. 2020;16, e045605. [Google Scholar]
- [37]. Karnath HO. Spatial orientation and the representation of space with parietal lobe lesions. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1997;352(1360):1411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behavioral and Neural Biology. 1993;60(1):9–26. [DOI] [PubMed] [Google Scholar]
- [39]. Japee S, Holiday K, Satyshur MD, Mukai I, Ungerleider LG. A role of right middle frontal gyrus in reorienting of attention: a case study. Frontiers in Systems Neuroscience. 2015;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Yushkevich PA, Pluta JB, Wang H, Xie L, Ding SL, Gertje EC, Mancuso L, Kliot D, Das SR, Wolk DA. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Human Brain Mapping. . 2015;36(1):258–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Kennedy DN, Haselgrove C, Riehl J, Preuss N, Buccigrossi R. The NITRC image repository. NeuroImage. 2016;124:1069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. “ASHS: Automatic Segmentation of Hippocampal Subfields.” N I T R C, http://www.nitrc.org/frs/?group_id=370
- [43]. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695–9. [DOI] [PubMed] [Google Scholar]
- [44]. Nazem S, Siderowf AD, Duda JE, Ten Have T, Colcher A, Horn SS, Moberg PJ, Wilkinson JR, Hurtig HI, Stern MB, Weintraub D. Montreal cognitive assessment performance in patients with Parkinson’s disease with “normal” global cognition according to mini-mental state examination score. Journal of the American Geriatrics Society. 2009;57(2):304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8(3):271–6. [Google Scholar]
- [46]. Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16(1):143–9. [Google Scholar]
- [47]. Wechsler D. WAIS-R manual: Wechsler adult intelligence scale-revised. Psychological Corporation; 1981.
- [48]. Sanders LM, Hortobágyi T, Balasingham M, Van der Zee EA, van Heuvelen MJ. Psychometric properties of a flanker task in a sample of patients with dementia: A pilot study. Dementia and Geriatric Cognitive Disorders Extra. 2018;8(3):382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Jaeger M. Digit symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. Journal of Clinical Psychopharmacology. 2018;38(5):513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Ricci M, Graef S, Blundo C, Miller LA. Using the Rey Auditory Verbal Learning Test (RAVLT) to differentiate Alzheimer’s dementia and behavioural variant fronto-temporal dementia. The Clinical Neuropsychologist. 2012;26(6):926–41. [DOI] [PubMed] [Google Scholar]
- [51]. de Sousa Magalhães, Sabrina, Leandro Fernandes Malloy-Diniz and Amer Cavalheiro Hamdan, “Validity convergent and reliability test-retest of the rey auditory verbal learning test. ” Clinical Neuropsychiatry 9.3 (2012).
- [52]. Stark SM, Kirwan CB, Stark CE. Mnemonic similarity task: A tool for assessing hippocampal integrity. Trends in Cognitive Sciences. 2019;23(11):938–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Stark SM, Stevenson R, Wu C, Rutledge S, Stark CE. Stability of age-related deficits in the mnemonic similarity task across task variations. Behavioral Neuroscience. 2015;129(3):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Bruijnen CJ, Dijkstra BA, Walvoort SJ, Budy MJ, Beurmanjer H, De Jong CA, Kessels RP. Psychometric properties of the Montreal Cognitive Assessment (MoCA) in healthy participants aged 18–70. International Journal of Psychiatry in Clinical Practice. 2020;24(3):293–300. [DOI] [PubMed] [Google Scholar]
- [55]. Sanders LM, Hortobágyi T, Balasingham M, Van der Zee EA, van Heuvelen MJ. Psychometric properties of a flanker task in a sample of patients with dementia: A pilot study. Dementia and Geriatric Cognitive Disorders Extra. 2018;8(3):382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Swanson J. The Delis-Kaplan executive function system: a review. Canadian Journal of School Psychology. 2005;20(1-2):117–28. [Google Scholar]
- [57]. Birckhead B, Khalil C, Liu X, Conovitz S, Rizzo A, Danovitch I, Bullock K, Spiegel B. Recommendations for methodology of virtual reality clinical trials in health care by an international working group: iterative study. JMIR Mental Health. 2019;6(1):e11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Lim JS, Lee JY, Kwon HM, Lee YS. The correlation between cerebral arterial pulsatility and cognitive dysfunction in Alzheimer’s disease patients. Journal of the Neurological Sciences. 2017;373:285–8. [DOI] [PubMed] [Google Scholar]
- [59]. IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp.
- [60]. Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. Journal of Experimental Psychology: General. 2012;141(1):2. [DOI] [PubMed] [Google Scholar]
- [61]. Dreha-Kulaczewski S, Joseph AA, Merboldt KD, Ludwig HC, Gärtner J, Frahm J. Inspiration is the major regulator of human CSF flow. Journal of Neuroscience. 2015;35(6):2485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Chen L, Beckett A, Verma A, Feinberg DA. Dynamics of respiratory and cardiac CSF motion revealed with real-time simultaneous multi-slice EPI velocity phase contrast imaging. Neuroimage. 2015;122:281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Law CS, Lan PS, Glover GH. Effect of wearing a face mask on fMRI BOLD contrast. NeuroImage. 2021;229:117752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Menai M, Van Hees VT, Elbaz A, Kivimaki M, Singh-Manoux A, Sabia S. Accelerometer assessed moderate-to-vigorous physical activity and successful ageing: results from the Whitehall II study. Scientific Reports. 2017;7(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65]. Kovacevic A, Fenesi B, Paolucci E, Heisz JJ. The effects of aerobic exercise intensity on memory in older adults. Applied Physiology, Nutrition, and Metabolism. 2020;45(6):591–600. [DOI] [PubMed] [Google Scholar]
- [66]. Iuliano E, di Cagno A, Aquino G, Fiorilli G, Mignogna P, Calcagno G, Di Costanzo A. Effects of different types of physical activity on the cognitive functions and attention in older people: A randomized controlled study. Experimental Gerontology. 2015;70:105–10. [DOI] [PubMed] [Google Scholar]
- [67]. Nishiguchi S, Yamada M, Tanigawa T, Sekiyama K, Kawagoe T, Suzuki M, Yoshikawa S, Abe N, Otsuka Y, Nakai R, Aoyama T. A 12-week physical and cognitive exercise program can improve cognitive function and neural efficiency in community-dwelling older adults: a randomized controlled trial. Journal of the American Geriatrics Society. 2015;63(7):1355–63. [DOI] [PubMed] [Google Scholar]
- [68]. Gothe NP, Kramer AF, McAuley E. Hatha yoga practice improves attention and processing speed in older adults: results from an 8-week randomized control trial. The Journal of Alternative and Complementary Medicine. 2017;23(1):35–40. [DOI] [PubMed] [Google Scholar]
- [69]. Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends in Neurosciences. 2011;34(10):515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Stark SM, Stark CE. Age-related deficits in the mnemonic similarity task for objects and scenes. Behavioural Brain Research. 2017;333:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learning & Memory. 2009;16(5):338–42. [DOI] [PubMed] [Google Scholar]
- [72]. Kolarik BS, Stark SM, Stark CE, Enriching hippocampal memory function in older adults through real-world exploration. Frontiers in Aging Neuroscience. 2020;12. [DOI] [PMC free article] [PubMed]
- [73]. Kovacevic A, Fenesi B, Paolucci E, Heisz JJ. The effects of aerobic exercise intensity on memory in older adults. Applied Physiology, Nutrition, and Metabolism. 2020;45(6):591–600. [DOI] [PubMed] [Google Scholar]
- [74]. Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosomatic Medicine. 2010;72(3):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Wielicka M, Neubauer-Geryk J, Kozera G, Bieniaszewski L. Clinical application of pulsatility index. Medical Research Journal. 2020;5(3):201–10. [Google Scholar]
- [76]. Steppan J, Barodka V, Berkowitz DE, Nyhan D, Vascular stiffness and increased pulse pressure in the aging cardio vascular system. Cardiology Research and Practice. 2011; 2011. [DOI] [PMC free article] [PubMed]
- [77]. El Sankari S, Gondry-Jouet C, Fichten A, Godefroy O, Serot JM, Deramond H, Meyer ME, Balédent O. Cerebrospinal fluid and blood flow in mild cognitive impairment and Alzheimer’s disease: a differential diagnosis from idiopathic normal pressure hydrocephalus. Fluids and Barriers of the CNS. 2011;8(1):1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78]. Tarumi T, Gonzales MM, Fallow B, Nualnim N, Pyron M, Tanaka H, Haley AP. Central artery stiffness, neuropsychological function, and cerebral perfusion in sedentary and endurance-trained middle-aged adults. Journal of Hypertension. 2013;31(12):2400–9. [DOI] [PubMed] [Google Scholar]
- [79]. Zheng G, Chen B, Fang Q, Lin Q, Tao J, Chen L. Baduanjin exercise intervention for community adults at risk of ischamic stroke: a randomized controlled trial. Scientific Reports. 2019;9(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80]. Battal B, Kocaoglu M, Bulakbasi N, Husmen G, Tuba Sanal H, Tayfun C. Cerebrospinal fluid flow imaging by using phase-contrast MR technique. The British Journal of Radiology. 2011;84(1004):758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81]. Vandertop WP. Syringomyelia. Neuropediatrics. 2014;45(01):003–9. [DOI] [PubMed] [Google Scholar]
- [82]. Ellenbogen RG, Armonda RA, Shaw DW, Winn HR. Toward a rational treatment of Chiari I malformation and syringomyelia. Neurosurgical Focus. 2000;8(3):1–0. [DOI] [PubMed] [Google Scholar]
- [83]. Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B. Clearance systems in the brain—implications for Alzheimer disease. Nature Reviews Neurology. 2015;11(8):457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84]. ElSankari S, Balédent O, Van Pesch V, Sindic C, De Broqueville Q, Duprez T. Concomitant analysis of arterial, venous, and CSF flows using phase-contrast MRI: a quantitative comparison between MS patients and healthy controls. Journal of Cerebral Blood Flow & Metabolism. 2013;33(9):1314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85]. Schippling S, Ostwaldt AC, Suppa P, Spies L, Manogaran P, Gocke C, Huppertz HJ, Opfer R. Global and regional annual brain volume loss rates in physiological aging. Journal of Neurology. 2017;264(3):520–8. [DOI] [PubMed] [Google Scholar]
- [86]. Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006;61(11):1166–70. [DOI] [PubMed] [Google Scholar]
- [87]. Batouli SA, Saba V. At least eighty percent of brain grey matter is modifiable by physical activity: A review study. Behavioural Brain Research. 2017;332:204–17. [DOI] [PubMed] [Google Scholar]
- [88]. Weniger G, Ruhleder M, Wolf S, Lange C, Irle E. Egocentric memory impaired and allocentric memory intact as assessed by virtual reality in subjects with unilateral parietal cortex lesions. Neuropsychologia. 2009;47(1):59–69. [DOI] [PubMed] [Google Scholar]
- [89]. Fraser MA, Shaw ME, Cherbuin N. A systematic review and meta-analysis of longitudinal hippocampal atrophy in healthy human ageing. Neuroimage. 2015;112:364–74. [DOI] [PubMed] [Google Scholar]
- [90]. Frodl T, Strehl K, Carballedo A, Tozzi L, Doyle M, Amico F, Gormley J, Lavelle G, O’Keane V, Aerobic exercise increases hippocampal subfield volumes in younger adults and prevents volume decline in the elderly. Brain Imaging and Behavior. 2019:1-1. [DOI] [PubMed]
- [91]. Firth J, Stubbs B, Vancampfort D, Schuch F, Lagopoulos J, Rosenbaum S, Ward PB. Effect of aerobic exercise on hippocampal volume in humans: a systematic review and meta-analysis. Neuroimage. 2018;166:230–8. [DOI] [PubMed] [Google Scholar]
- [92]. Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. The Lancet. 2002;359(9308):781–5. [DOI] [PubMed] [Google Scholar]
- [93]. Strohacker K, Fazzino D, Breslin WL, Xu X. The use of periodization in exercise prescriptions for inactive adults: A systematic review. Preventive Medicine Reports. 2015;2:385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94]. Chance SS, Gaunet F, Beall AC, Loomis JM. Locomotion mode affects the updating of objects encountered during travel: The contribution of vestibular and proprioceptive inputs to path integration. Presence. 1998;7(2):168–78. [Google Scholar]
- [95]. Sharples S, Cobb S, Moody A, Wilson JR. Virtual reality induced symptoms and effects (VRISE): Comparison of head mounted display (HMD), desktop and projection display systems. Displays 2008;29(2):58–69. [Google Scholar]
- [96]. Boletsis C. The new era of virtual reality locomotion: A systematic literature review of techniques and a proposed typology. Multimodal Technologies and Interaction. 2017;1(4):24. [Google Scholar]