The brain coordinates the body’s movements through the central nervous system (CNS). Hence, movement behaviors in infants reveal valuable information regarding their developing CNS (1). In infants, spontaneous movements often referred to as general movements (GMs) are an indicator of later neurological deficits (2). GMs are automatic, are complex, occur frequently, and can be observed accurately from early fetal life to 6 mo of age (3). Early observation and assessment of atypical GMs open up the possibility of therapeutic intervention in infants and rely on the neuroplasticity of the brain to avert potential negative outcomes (4, 5). Qualitative and quantitative monitoring of GMs currently requires clinical tests, medical history, video monitoring, and medical experts (6, 7). All these are time and resource intensive; therefore, they are not available to the wider population. In PNAS, Jeong et al. (8) demonstrate an artificial intelligence-enabled soft-electronic sensor network that monitors movements in infants for predicting later neurological deficits (Fig. 1A).
Fig. 1.
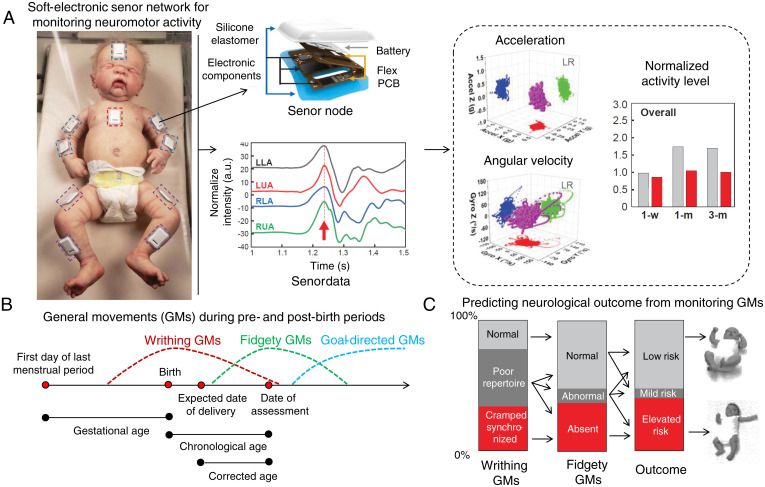
Soft-electronic sensor network for early detection of later neurological deficits in infants. (A) The sensors are placed on the forehead, chest, and limbs of the infants. These sensors are fabricated using flexible printed circuit boards (PCBs). Electronic components and batteries are assembled and encapsulated inside a waterproof silicone elastomer. Accelerometer and gyroscope data from the left upper arm (LUA), left lower arm (LLA), right upper arm (RUA), and right lower arm (RLA) are then interpreted to acceleration, angular velocity, and normalized activity levels. With machine-learning techniques, these three-axis accelerations and angular velocities are reconstructed to reveal typical/atypical GMs. Adapted from ref. 8. (B) GMs during pre- and postbirth periods. Three types of GMs: (1) writhing GMs, (2) fidgety GMs, and (3) goal-directed GMs are observed from early fetal life to 6 mo of age. Reprinted with permission from ref. 9. (C) Predicting neurological outcome from the occurrence of writhing and fidgety GMs. A longitudinal study on 130 infants showed that monitoring the writhing and fidgety GMs can be used to predict neurological outcome at 3 y of age. Adapted with permission from ref. 3.
Infants typically show three types of GMs during pre- and postbirth periods: 1) writhing GMs, 2) fidgety GMs, and 3) goal-directed GMs (Fig. 1B) (3, 9). Identifying these GMs, as well as quantifying their occurrence frequency, is essential for predicting patient outcomes. In postbirth scenarios, assessment of GMs in infants 10 to 20 wk of age showed 97% sensitivity and 89% specificity while predicting for later cerebral palsy (3, 10). Prechtl’s assessment of GMs, a widely used tool for assessing the infant nervous system, establishes two atypical GM patterns for reliably predicting later cerebral palsy: 1) a consistent pattern of cramped, synchronized GMs (these movements appear rigid and lack a smooth and fluent character) and 2) the absence of GMs of fidgety character, which are small movements of moderate speed with variable acceleration of the neck, trunk, arms, and legs in every direction (3). The cramped, synchronized GMs, when occurring often in infants, demonstrate a high possibility of later cerebral palsy (11). On the other hand, frequent occurrences of fidgety movements lower the possibility of later cerebral palsy (12). Einspieler and Prechtl (3) previously performed a longitudinal study on 130 infants by observing the occurrence of writhing and fidgety GMs and later associated them with the neurological outcome at 3 y of age (Fig. 1C). This assessment technique of GMs is not as resource intensive as other methods of neurological monitoring, such as MRI and brain ultrasound.
In general, monitoring and assessing GMs rely heavily on high-resolution imaging (13), motion tracking with wearable sensors (9), and recording electroencephalography and electromyography signals (12, 14). Most of the existing technologies are cumbersome to use due to their size, weight, and wired connections. In PNAS, Jeong et al. (8) present a soft-electronic sensor network composed of sensor nodes that are more than three times thinner, five times lighter, and two times smaller in overall volume compared with the most advanced commercialized sensor for motion capture (Xsens MTw Awinda; Xsens Technologies B.V.). These sensors, encapsulated in a thin silicone elastomer, feature a nordic nRF52832 Bluetooth transceiver, power management circuitry, 4-Gb flash memory, and six-axis inertial measurement unit. These sensors (32 × 21 × 3 mm in size and 2.6 g in weight) send synchronized timestamps, movement information from each sensor node, and static and dynamic orientations relative to the gravity vector that allow reconstruction of full-body motions.
In the networked mode, data from all the connected sensor nodes are collected in a time-synchronized fashion with a millisecond relative timing accuracy. This is then used to create three-dimensional motion models using a smartphone and a personal computer. In this time-synchronized manner, 10 sensor nodes stream three-axis digital accelerometer and gyroscope data to a host, which can be interpreted to reveal acceleration, angular velocity, and normalized activity levels (Fig. 1A). Furthermore, with machine-learning techniques, the three-axis accelerations and angular velocities from these sensor nodes are reconstructed to show typical/atypical GMs that remained hidden in the video analysis.
From a usability perspective, this sensor network is already deployable to hospitals and will not require expert supervision during data collection. Additionally, since captured data can be easily anonymized, medical data privacy concerns can be reduced compared with other photo- or image-based motion capture systems (15). Moreover, both motion and vital signs information can be recorded from the sensor network (16, 17). Extracting motion and vital signs requires appropriate filtering of the data. For example, motion data extraction requires a low-pass filter that cuts off frequencies higher than 20 Hz. From the motion data, Jeong et al. (8) generated a quantitative activity level to observe infants who were 1 wk, 1 mo, and 3 mo of age. Gross neuromotor deficiencies, such as trouble in lifting the head or holding the head up while in the prone position or in exhibiting stiffness in the limbs with little or no movements, were picked up using the soft sensor network, while these characteristics remained hidden in the visual and video analyses. Although further study will be required to determine the outcome of the infants in the study, the ability to monitor hidden clues in GMs is extremely encouraging.
The emergence of soft and flexible bioelectronics is bringing new sensing capabilities to clinicians (18). We have seen the use of these soft devices to understand complex diseases both from inside and from outside the body (19, 20). Adding to the existing library of soft bioelectronics, the work by Jeong et al. (8) introduces a low-cost, easy-to-use wireless sensor for quantitatively assessing movement patterns that enables the early detection of neurological deficits in infants. Despite the need of further longitudinal testing on a larger population set, this work demonstrates the feasibility and promise of using a soft sensor network in a vulnerable population (i.e., infants). In the future, given the nature of the measurement, we can envision in-home and remote assessments of GMs—enabling early detection and therapeutic intervention for treating neurological deficits in infants.
Acknowledgments
Y.K. and Z.B. acknowledge support from NSF Multimodal Sensor Systems for Precision Health Enabled by Data Harnessing, Artificial Intelligence, and Learning (SenSE) Program Grant 2037304.
Footnotes
The authors declare no competing interest.
See companion article, “Miniaturized wireless, skin-integrated sensor networks for quantifying full-body movement behaviors and vital signs in infants,” 10.1073/pnas.2104925118.
References
- 1.Kakei S., Hoffman D. S., Strick P. L., Muscle and movement representations in the primary motor cortex. Science 285, 2136–2139 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Prechtl H. F., Einspieler C., Is neurological assessment of the fetus possible? Eur. J. Obstet. Gynecol. Reprod. Biol. 75, 81–84 (1997). [DOI] [PubMed] [Google Scholar]
- 3.Einspieler C., Prechtl H. F., Prechtl’s assessment of general movements: A diagnostic tool for the functional assessment of the young nervous system. Ment. Retard. Dev. Disabil. Res. Rev. 11, 61–67 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Tilton A. H., Therapeutic interventions for tone abnormalities in cerebral palsy. NeuroRx 3, 217–224 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hur J. J., Review of research on therapeutic interventions for children with cerebral palsy. Acta Neurol. Scand. 91, 423–432 (1995). [DOI] [PubMed] [Google Scholar]
- 6.Einspieler C., Prechtl H. F., Ferrari F., Cioni G., Bos A. F., The qualitative assessment of general movements in preterm, term and young infants–review of the methodology. Early Hum. Dev. 50, 47–60 (1997). [DOI] [PubMed] [Google Scholar]
- 7.Einspieler C., et al., Early markers for cerebral palsy: Insights from the assessment of general movements. Future Neurol. 7, 709–717 (2012). [Google Scholar]
- 8.Jeong H., et al., Miniaturized wireless, skin-integrated sensor networks for quantifying full-body movement behaviors and vital signs in infants. Proc. Natl. Acad. Sci. U.S.A. 118, 10.1073/pnas.2104925118(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redd C. B., Barber L. A., Boyd R. N., Varnfield M., Karunanithi M. K., “Development of a wearable sensor network for quantification of infant general movements for the diagnosis of cerebral palsy” in 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (IEEE, 2019), pp. 7134–7139. [DOI] [PubMed]
- 10.Kwong A. K. L., Fitzgerald T. L., Doyle L. W., Cheong J. L. Y., Spittle A. J., Predictive validity of spontaneous early infant movement for later cerebral palsy: A systematic review. Dev. Med. Child Neurol. 60, 480–489 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Ferrari F., et al., Cramped synchronized general movements in preterm infants as an early marker for cerebral palsy. Arch. Pediatr. Adolesc. Med. 156, 460–467 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Hadders-Algra M., Van Eykern L. A., Klip-Van den Nieuwendijk A. W., Prechtl H. F., Developmental course of general movements in early infancy. II. EMG correlates. Early Hum. Dev. 28, 231–251 (1992). [DOI] [PubMed] [Google Scholar]
- 13.Hadders-Algra M., General movements: A window for early identification of children at high risk for developmental disorders. J. Pediatr. 145(2 suppl.), S12–S18 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Prechtl H. F., Ferrari F., Cioni G., Predictive value of general movements in asphyxiated fullterm infants. Early Hum. Dev. 35, 91–120 (1993). [DOI] [PubMed] [Google Scholar]
- 15.Enaizan O., et al., Electronic medical record systems: Decision support examination framework for individual, security and privacy concerns using multi-perspective analysis. Heal. Technol 10, 795–822 (2020). [Google Scholar]
- 16.Lee J., Ha I., Real-time motion capture for a human body using accelerometers. Robotica 19, 601–610 (2001). [Google Scholar]
- 17.Ni X., et al., Automated, multiparametric monitoring of respiratory biomarkers and vital signs in clinical and home settings for COVID-19 patients. Proc. Natl. Acad. Sci. U.S.A. 118, e2026610118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Someya T., Bao Z., Malliaras G. G., The rise of plastic bioelectronics. Nature 540, 379–385 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Boutry C. M., et al., Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 3, 47–57 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Gao Y., et al., A flexible multiplexed immunosensor for point-of-care in situ wound monitoring. Sci. Adv. 7, eabg9614 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]