To the Editor: In a small group of recipients of messenger RNA (mRNA) vaccines against coronavirus disease 2019 (Covid-19), the occurrence of erythematous and indurated skin reactions has been reported at an average of 8 days after the first or second injection.1 The identification of such hypersensitivity responses has been a focus of study for many years by researchers investigating various systems. Such reactions to mRNA vaccines strongly resemble the primary first-dose flare and secondary skin-test responses that we reported 45 years ago in volunteers who were injected with foreign proteins2 (Figure 1A). At that time, we noted that the studied reactions were similar to those described as Jones–Mote responses (JMR) to rabbit serum proteins by Dr. T. Duckett Jones and a medical student, John Mote, in 1934 in the Journal.3
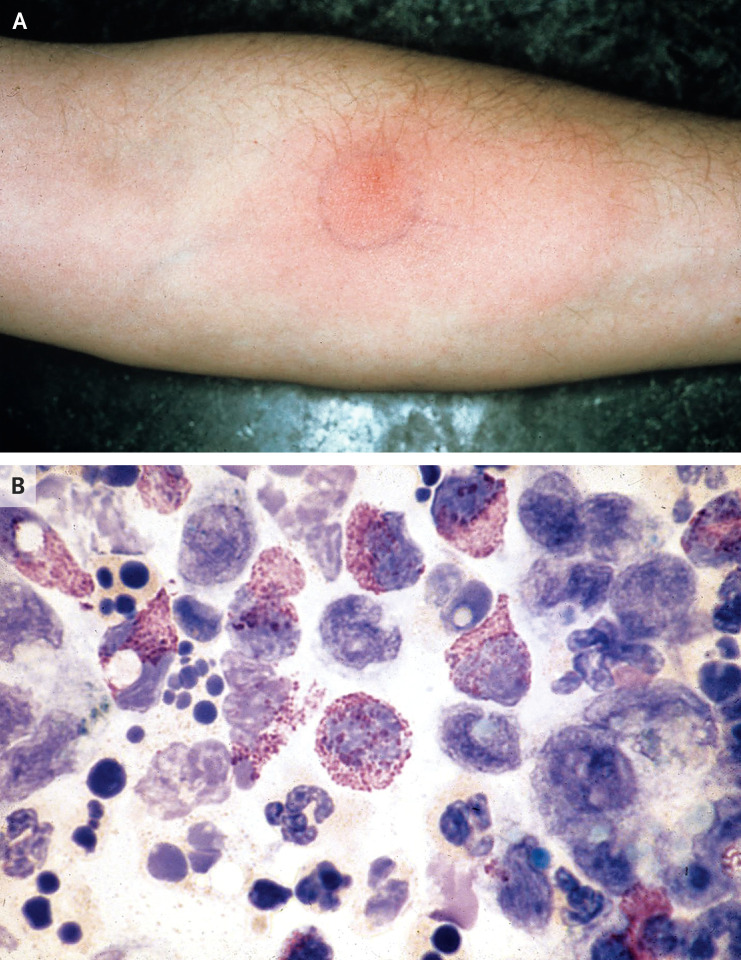
Figure 1. Cutaneous Basophil Hypersensitivity Response after Skin-Test Antigen Injection.
Shown are the results of a hypersensitivity reaction in a volunteer who received an intradermal skin-test injection of 20 μg of keyhole limpet hemocyanin (KLH) protein 1 week after priming with an intradermal immunization with 222 μg of KLH protein in a 1976 study.3 Panel A shows the delayed cutaneous erythematous and indurated JMR reaction on day 7 after the second test injection. Panel B shows the results of a Rebuck skin-window examination (in which the top layer of skin is scraped off to facilitate the identification of responding inflammatory cells) after 36 hours. In the numerous basophils that are shown, the cytoplasm is packed with metachromatic purple staining granules that obscure the nucleus. Polymorphonuclear neutrophils and mononuclear cells are also visible.
In our 1976 case report, the antigen that was used was the copper-carrying (and thus blue) protein called keyhole limpet hemocyanin (KLH), which is found in a Pacific Ocean marine gastropod. We identified the cutaneous JMR to KLH as a strong example of cutaneous basophil hypersensitivity (CBH).2 This delayed reaction was caused by a T-cell response that is rich in basophils to remnants of an intradermal skin-test injection of a strong foreign antigen, as described by Ann and Hal Dvorak in 19704 (Figure 1B). Our study volunteers who had these JMR and CBH reactions were 20 White Yale medical students. At the time, I spoke with John Mote, then retired at 82 years of age, who visited us and confirmed that the reactions that we observed could indeed be classified as JMR.
It seems that the rare cutaneous hypersensitivity reactions among recipients of mRNA vaccines are responses to the translated spike protein of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). JMR and CBH reactions to the KLH protein in our skin-immunized volunteers were highly antigen-specific in vivo T-cell–dependent responses to residual antigen that was left at the local site from the first injection, to which the host had a primary immune T-cell JMR. Then similar reactions were elicited to secondary skin-test exposures. Such reactions also include antigen-specific T-cell proliferation with distinct kinetics, reactions that now are ripe for more modern molecular analysis.
Recognition that responses to the mRNA Covid-19 vaccines resemble JMR and CBH reactions may lead to skin testing in patients and to other related studies to better understand SARS-CoV-2 infections. Perhaps so-called “long Covid” has a similar pathogenesis and could respond to treatments appropriate to JMR and CBH reactions. An example may be the improvement that was seen in patients with long Covid who were treated with combined antihistamines, since the source of histamine may be the basophils.5
Disclosure Forms
Footnotes
Disclosure forms provided by the author are available with the full text of this letter at NEJM.org.
References
- 1.Blumenthal KG, Freeman EE, Saff RR, et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med 2021;384:1273-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askenase PW, Atwood JE. Basophils in tuberculin and “Jones-Mote” delayed reactions of humans. J Clin Invest 1976;58:1145-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duckett Jones T, Mote JR. The phases of foreign protein sensitization in human beings. N Engl J Med 1934;210:120-123. [Google Scholar]
- 4.Richerson HB, Dvorak HF, Leskowitz S. Cutaneous basophil hypersensitivity. I. A new look at the Jones-Mote reaction, general characteristics. J Exp Med 1970;132:546-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glynne P, Tahmasebi N, Gant V, Gupta R. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med 2021. October 05 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.