Abstract
Background.
Candidemia is a common opportunistic infection causing substantial morbidity and mortality. Because of an increasing proportion of non-albicans Candida species and rising antifungal drug resistance, the Infectious Diseases Society of America (IDSA) changed treatment guidelines in 2016 to recommend echinocandins over fluconazole as first-line treatment for adults with candidemia. We describe candidemia treatment practices and adherence to the updated guidelines.
Methods.
During 2017–2018, the Emerging Infections Program conducted active population-based candidemia surveillance at 9 US sites using a standardized case definition. We assessed factors associated with initial antifungal treatment for the first candidemia case among adults using multivariable logistic regression models. To identify instances of potentially inappropriate treatment, we compared the first antifungal drug received with species and antifungal susceptibility testing (AFST) results from initial blood cultures.
Results.
Among 1835 patients who received antifungal treatment, 1258 (68.6%) received an echinocandin and 543 (29.6%) received fluconazole as initial treatment. Cirrhosis (adjusted odds ratio = 2.06; 95% confidence interval, 1.29–3.29) was the only underlying medical condition significantly associated with initial receipt of an echinocandin (versus fluconazole). More than one-half (n = 304, 56.0%) of patients initially treated with fluconazole grew a non-albicans species. Among 265 patients initially treated with fluconazole and with fluconazole AFST results, 28 (10.6%) had a fluconazole-resistant isolate.
Conclusions.
A substantial proportion of patients with candidemia were initially treated with fluconazole, resulting in potentially inappropriate treatment for those involving non-albicans or fluconazole-resistant species. Reasons for nonadherence to IDSA guidelines should be evaluated, and clinician education is needed.
Keywords: antifungal drug resistance, Candida, candidemia, echinocandins, fluconazole
Candidemia is among the most common opportunistic fungal infections worldwide [1] and results in prolonged, costly hospitalizations with high mortality rates [2–4]. In the United States, Candida albicans is the most common species causing candidemia, but the proportion of infections caused by non-albicans species has increased in recent decades [5, 6]. Non-albicans Candida species tend to be more resistant to antifungal drugs than C albicans, and the emergence of drug-resistance among both albicans and non-albicans Candida, including C auris, constitutes a major public health threat [7].
Early identification and treatment of candidemia with an appropriate antifungal drug improves morbidity and mortality [8, 9]. Before 2016, treatment guidelines from the Infectious Diseases Society of America (IDSA) recommended using an echinocandin (ie, anidulafungin, caspofungin, or micafungin) for candidemia treatment in neutropenic adult patients and either fluconazole or an echinocandin for treatment in non-neutropenic adult patients [10]. Because of the increasing frequency of infections caused by non-albicans Candida [5], rising levels of fluconazole resistance [11], and evidence that echinocandins are more effective than fluconazole [12], IDSA changed treatment guidelines in 2016 to strongly recommend echinocandins instead of fluconazole as the initial treatment of candidemia in all adults; the guidelines consider fluconazole to be an acceptable alternative to an echinocandin as initial therapy in noncritically ill patients and patients considered unlikely to have a fluconazole-resistant Candida species [13]. Even before the IDSA guidelines changed in 2016, echinocandin use for candidemia treatment was increasing, whereas fluconazole use was decreasing during 2012–2016 [6]. IDSA guidelines also strongly recommend performing fluconazole antifungal susceptibility testing (AFST) on all bloodstream Candida isolates because of the emergence of fluconazole resistance. The guidelines further recommend echinocandin AFST for C glabrata and C parapsilosis bloodstream isolates because of emerging resistance in these species [13].
Because it generally takes at least 2–4 days after blood culture collection to detect and identify Candida species, and because access to AFST may be limited or delayed, clinicians usually select initial antifungal treatment based on local species epidemiology, antifungal resistance patterns, and individual patient factors [13–16]. A common practice among clinicians is to initially treat candidemia with an echinocandin followed by deescalation to fluconazole after the patient shows clinical improvement [13]. AFST results, if available, may also be used to guide step-down treatment decisions and identify instances where treatment may be ineffective [17].
Emerging Infections Program (EIP) conducts population-based candidemia surveillance through a multisite collaboration among the US Centers for Disease Control and Prevention (CDC), state health departments, and academic partners [18]. The data collected are used to monitor the spread of antifungal resistance and other concerning epidemiologic trends. We analyzed 2017–2018 EIP surveillance data to characterize candidemia treatment practices for adults and assess adherence to the updated 2016 IDSA guidelines.
METHODS
During January 2017–December 2018, EIP conducted candidemia surveillance in specific counties in 9 US states: California, Colorado, Georgia, Maryland, Minnesota, New Mexico, New York, Oregon, and Tennessee. EIP candidemia surveillance methods have been previously described [6]. Surveillance personnel used standardized case report forms to collect demographic and clinical data from medical and laboratory records.
A case of candidemia was defined as a blood culture positive for a Candida species collected from a resident of the surveillance area. Any blood cultures positive for a Candida species within 30 days of the initial positive culture from the same patient were considered part of the same case, including if more than 1 Candida species was detected. A positive Candida culture after the 30-day period was considered a new case in the same patient.
All isolates from initial blood cultures positive for Candida spp. are speciated by inpatient and outpatient clinical, reference, or commercial laboratories serving the surveillance population as part of routine practices. Some laboratories also conducted AFST on certain isolates depending on laboratory testing and clinician ordering practices. We restricted our analysis to the index candidemia case (defined as the first case per patient within the analytic period) for adult patients (aged ≥18 years) during 2017–2018, using species identification and AFST results provided by the laboratories.
Case Characteristics and Initial Antifungal Treatment
We described the number, demographic characteristics, underlying medical conditions, fluconazole prophylaxis, candidemia risk factors (eg, recent abdominal surgery, injection drug use), healthcare encounters, and in-hospital survival of patients by initial antifungal drug administered. To assess possible treatment delays, we calculated the time between the date of first positive blood culture collection and initial receipt of antifungal drug treatment. We assessed differences in the characteristics of patients who received an echinocandin versus fluconazole as initial candidemia treatment using χ2 or Fisher exact test for proportions. To identify independent predictors of receiving an echinocandin versus fluconazole, we conducted a multivariable logistic regression analysis, beginning with all factors assessed in bivariate analyses and using backwards stepwise elimination of nonsignificant (α = 0.05) covariates; models were adjusted for age.
Initial Antifungal Drug Choice by Species and Antifungal Susceptibility
To assess for instances in which initial antifungal drug choices resulted in potentially inappropriate treatment, we compared the initial antifungal treatment given with Candida spp. and AFST results, when available. We also examined the species and AFST practices for patients whose drug class was switched during their treatment course. AFST results were interpreted based on species-specific Clinical and Laboratory Standards Institute breakpoints [19], and isolates were classified as resistant, not resistant, or no interpretation if a species with no Clinical and Laboratory Standards Institute–defined breakpoint was tested. If a culture grew more than 1 isolate, that specimen was considered drug resistant if any of the isolates were resistant.
We performed statistical analyses using SAS (version 9.4; SAS Institute, Cary, North Carolina). P values < .05 were considered statistically significant. This activity was reviewed by the CDC and was conducted consistent with applicable federal law and CDC policy (eg, 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.).
RESULTS
Initial Antifungal Treatment
During 2017–2018, 2415 candidemia cases occurred among 2271 adult patients residing within EIP surveillance areas. For the index cases of these 2271 patients, 1835 received an antifungal drug for candidemia treatment. Initial antifungal treatments included an echinocandin (n = 1258, 68.6%), fluconazole (n = 543, 29.6%), and other drugs (eg, amphotericin, non-fluconazole triazole) (n = 34, 1.9%); 436 patients received no antifungal treatment (Figure 1).
Figure 1.

Flow diagram showing initial treatment choices and drug class switching for adult patients with candidemia detected through the Emerging Infections Program surveillance system at 9 US surveillance sites during 2017–2018. SD, standard deviation. aPatients who received an antifungal drug other than an echinocandin or fluconazole as initial treatment were excluded from further analyses. Other antifungal drugs received as initial treatment included amphotericin (n = 18), azoles other than fluconazole (n = 14), and other antifungal drugs (n = 2). bReasons for not receiving an antifungal drug included death (n = 154) or discharge (n = 77) before the culture result was available to the clinician, the initiation of comfort care-only measures (n = 66), clinician interpretation that the culture was clinically insignificant (n = 20), refusal of treatment against medical advice (n = 14), and unknown reason (n = 105).
The median time between first positive blood culture collection and initial antifungal drug receipt was 2.0 days (mean 1.8, interquartile range [1.0, 3.0]). The remaining analyses described in this paper included the 1801 patients who received either fluconazole or echinocandin as initial candidemia treatment. The most common antifungal drug treatment sequences included: initiating treatment with an echinocandin without switching (n = 664, 36.9%), initiating treatment with an echinocandin and switching to fluconazole (n = 539, 29.9%, median days until switch = 3.0), initiating treatment with fluconazole without switching (n = 306, 17.0%), and initiating treatment with fluconazole and switching to an echinocandin (n = 226, 12.5%, median days until switch = 2.0) (Figure 1).
In bivariate analyses comparing initial antifungal treatment, patients who received an echinocandin were more likely to have cirrhosis (n = 110, 8.7%) than were patients who received fluconazole (n = 23, 4.2%) (P = .0008) (Table 1). Patients who received an echinocandin were less likely to have had a recent hospitalization (within 90 days before candidemia) than were patients who received fluconazole (n = 673, [53.5%] versus n = 323 [59.5%], P = .0190). There were no significant differences in the proportion of patients who received an echinocandin versus fluconazole by age group, sex, race/ethnicity, diabetes, transplant status, solid organ malignancy, neutropenia, history of fluconazole prophylaxis (within 14 days before candidemia), and time spent at a long-term acute care hospital or long-term care facility within 90 days before candidemia diagnosis. Among patients with available in-hospital mortality data (n = 1795, 99.7%), a higher percentage of patients who received an echinocandin (n = 355, 28.3%) versus fluconazole (n = 87, 16.1%) as initial treatment died during their candidemia-associated hospitalization (P < .0001).
Table 1.
Demographic Features, Clinical Characteristics, and Outcomes of Adult Patients With Candidemia (N = 1801) by Initial Antifungal Treatment Administered—9 surveillance sites, United States, 2017–2018
| Initial Antifungal Drug Administered | |||||
|---|---|---|---|---|---|
| Echinocandin (n = 1258) | % | Fluconazole (n = 543) | % | PValuea | |
| Characteristic | |||||
| Age group, y | .4730 | ||||
| 18–44 | 284 | 22.6 | 113 | 20.8 | |
| 45–64 | 476 | 37.8 | 199 | 36.6 | |
| ≥65 | 498 | 39.6 | 231 | 42.5 | |
| Sex b | .1108 | ||||
| Male | 700 | 55.6 | 280 | 51.6 | |
| Female | 558 | 44.4 | 263 | 48.4 | |
| Race/ethnicity | .0599 | ||||
| White, NH | 635 | 50.5 | 311 | 57.3 | |
| Black, NH | 341 | 27.1 | 133 | 24.5 | |
| Hispanic or Latino | 77 | 6.1 | 33 | 6.1 | |
| Asian, NH | 24 | 1.9 | 12 | 2.2 | |
| Otherc, NH | 15 | 1.2 | 3 | 0.6 | |
| Unknown race and ethnicity | 166 | 13.2 | 51 | 9.4 | |
| Surveillance site | <.0001 | ||||
| California | 104 | 8.3 | 36 | 6.6 | |
| Colorado | 135 | 10.7 | 46 | 8.5 | |
| Georgia | 335 | 26.6 | 166 | 30.6 | |
| Maryland | 233 | 18.5 | 54 | 9.9 | |
| Minnesota | 167 | 13.3 | 57 | 10.5 | |
| New Mexico | 24 | 1.9 | 17 | 3.1 | |
| New York | 56 | 4.5 | 24 | 4.4 | |
| Oregon | 63 | 5.0 | 40 | 7.4 | |
| Tennessee | 141 | 11.2 | 103 | 19.0 | |
| Underlying conditions and medications | |||||
| Diabetes | 435 | 34.6 | 210 | 38.7 | .0962 |
| Solid organ malignancy | 268 | 21.3 | 116 | 21.4 | .9776 |
| Hematologic malignancy | 74 | 5.9 | 28 | 5.2 | .5408 |
| Any liver disease | 243 | 19.3 | 66 | 12.2 | .0002 |
| Hepatitis C | 135 | 10.7 | 49 | 9.0 | .2722 |
| Cirrhosis | 110 | 8.7 | 23 | 4.2 | .0008 |
| Chronic renal disease | 336 | 26.7 | 144 | 26.5 | .9334 |
| Any transplant | 40 | 3.2 | 9 | 1.7 | .0684 |
| Solid organ transplant | 27 | 2.1 | 7 | 1.3 | .2200 |
| Stem cell transplant | 13 | 1.0 | 2 | 0.4 | .2563 |
| HIV | 32 | 2.5 | 16 | 2.9 | .6262 |
| Advanced HIV disease | 14 | 1.1 | 7 | 1.3 | .7492 |
| Injection drug use in the past 12 mo | 118 | 9.4 | 54 | 9.9 | .7082 |
| Abdominal surgery in the 90 d before candidemia diagnosis | 268 | 21.3 | 103 | 19.0 | .2608 |
| Any surgery in the 90 d before candidemia diagnosis | 229 | 18.2 | 92 | 16.9 | .5212 |
| Neutropeniad | 45 | 3.6 | 16 | 2.9 | .4973 |
| Fluconazole prophylaxise | 39 | 3.1 | 23 | 4.2 | .2251 |
| Healthcare encounters within 90 days before diagnosis | |||||
| Overnight stay in long-term acute care hospital | 20 | 1.6 | 12 | 2.2 | .3606 |
| Overnight stay in long-term care facility | 116 | 9.2 | 45 | 8.3 | .5239 |
| Previous hospitalization | 673 | 53.5 | 323 | 59.5 | .0190 |
| Outcome | |||||
| Died during candidemia-associated hospitalizationf | 355 | 28.3 | 87 | 16.1 | <.0001 |
Abbreviation: NH, not Hispanic or Latino.
P values were calculated using χ2 or Fisher’s exact test for proportions.
Three patients were transgender.
The other race/ethnicity category includes patients who were NH American Indian or Alaska Native (n = 7, 0.4%), NH Native Hawaiian or other Pacific Islander (n = 7, 0.4%), or NH multiracial (n = 4, 0.2%).
Neutropenia was documented on or within 2 days before index culture collection date.
Fluconazole prophylaxis was administered within 14 days before index culture collection date.
Mortality outcome data were missing for 6 patients.
In a multivariable logistic regression model adjusting for age, cirrhosis, recent hospitalization, and surveillance site were the only factors significantly associated with receipt of an echinocandin versus fluconazole (Table 2). Patients with cirrhosis had over twice the odds of receiving an echinocandin versus fluconazole as initial candidemia treatment (adjusted odds ratio: 2.06, 95% confidence interval, 1.29–3.29). Recent hospitalization was associated with a lower odds of receiving an echinocandin as first-line treatment (adjusted odds ratio: 0.80; 95% confidence interval, 0.65–0.98). The odds of receiving an echinocandin versus fluconazole as initial treatment varied significantly by surveillance site (P < .0001), with patients from certain sites having over twice the odds of receiving an echinocandin compared with others.
Table 2.
Multivariable Logistic Regression Model Assessing Factors Associated With Receipt of Echinocandin Versus Fluconazole for Initial Treatment of Adult Candidemia Patients (N = 1801)—9 Surveillance Sites, United States, 2017–2018
| Characteristic | Adjusted Odds Ratio | 95% Confidence Interval | PValue |
|---|---|---|---|
| Age group, y | .4062 | ||
| 18–44 (referent) | 1.00 | … | |
| 45–64 | .92 | .69–1.21 | |
| ≥65 | .83 | .63–1.10 | |
| Cirrhosis | 2.06 | 1.29–3.29 | .0026 |
| Hospitalized within 90 d before diagnosis | .80 | .65–.98 | .0332 |
| Surveillance site a | <.0001 | ||
| California | 2.08 | 1.31–3.30 | |
| Colorado | 2.06 | 1.35–3.15 | |
| Georgia | 1.46 | 1.07–2.01 | |
| Maryland | 3.09 | 2.09–4.57 | |
| Minnesota | 2.11 | 1.42–3.14 | |
| New Mexico | .96 | .48–1.89 | |
| New York | 1.64 | .95–2.83 | |
| Oregon | 1.15 | .72–1.84 | |
| Tennessee (referent)b | 1.00 | … |
The Emerging Infections Program conducted surveillance in select counties in each of these states.
Tennessee was chosen as the referent group because patients from this site had the lowest odds of receiving an echinocandin versus fluconazole in unadjusted odds ratios.
Antifungal Drug Choice by Species and Antifungal Susceptibility
Of 543 patients treated initially with fluconazole, 304 (56.0%) grew a non-albicans species, including 145 patients with C glabrata, 76 with C parapsilosis, and nine with C krusei (Table 3).
Table 3.
Antifungal Treatment Received and Antifungal Susceptibility Testing Result Availability by Candida Species of Adult Candidemia Patients (N = 1801)—9 Surveillance Sites, United States, 2017–2018
|
Candida Species From First Positive Blood Culturea |
Initial Antifungal Drug Administered |
Tested for Antifungal Susceptibility |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (N = 1801) | % | Echinocandin (n = 1258) | % | Fluconazole (n = 543) | % | Fluconazole (n = 968) | % | Echinocandin (n = 720) | % | |
| C albicans | 695 | 38.6 | 456 | 65.6 | 239 | 34.4 | 366 | 52.7 | 241 | 34.7 |
|
| ||||||||||
| C glabrata | 530 | 29.4 | 385 | 72.6 | 145 | 27.4 | 260 | 49.1 | 226 | 42.6 |
|
| ||||||||||
| C parapsilosis | 237 | 13.2 | 161 | 67.9 | 76 | 32.1 | 133 | 56.1 | 103 | 43.5 |
|
| ||||||||||
| C tropicalis | 129 | 7.2 | 96 | 74.4 | 33 | 25.6 | 67 | 51.9 | 45 | 34.9 |
|
| ||||||||||
| C krusei b | 32 | 1.8 | 23 | 71.9 | 9 | 28.1 | 32 | 100.0 | 16 | 50.0 |
|
| ||||||||||
| C lusitaniae | 31 | 1.7 | 19 | 61.3 | 12 | 38.7 | 18 | 58.1 | 15 | 48.4 |
|
| ||||||||||
| C dubliniensis | 23 | 1.3 | 18 | 78.3 | 5 | 21.7 | 16 | 69.6 | 14 | 60.9 |
|
| ||||||||||
| Other non-albicans species | 75 | 4.2 | 61 | 81.3 | 14 | 18.7 | 42 | 56.0 | 37 | 49.3 |
|
| ||||||||||
| >1 speciesc | 49 | 2.7 | 39 | 79.6 | 10 | 20.4 | 34 | 69.4 | 23 | 46.9 |
Abbreviation: C, Candicans.
Rows are mutually exclusive.
Fluconazole susceptibility results were considered known for all of these patients because C krusei is an inherently fluconazole-resistant species.
C albicans was one of the species present for 32 of the patients whose initial blood culture grew >1 species.
Among the 1801 patients treated with either an echinocandin or fluconazole, 968 (53.7%) received fluconazole AFST, and this proportion ranged substantially across surveillance sites from 11.5% to 95.1% (Figure 2). Fewer than half of patients (n = 720 [40.0%]) received echinocandin AFST, with site-specific percentages ranging from 5.3% to 95.1%. Echinocandin AFST was performed for 226 (42.6%) of 530 patients with C glabrata and for 103 (43.5%) of 237 patients with C parapsilosis. AFST for both fluconazole and an echinocandin was performed for 701 patients (38.9%, range among sites = 4.9%–95.1%).
Figure 2.
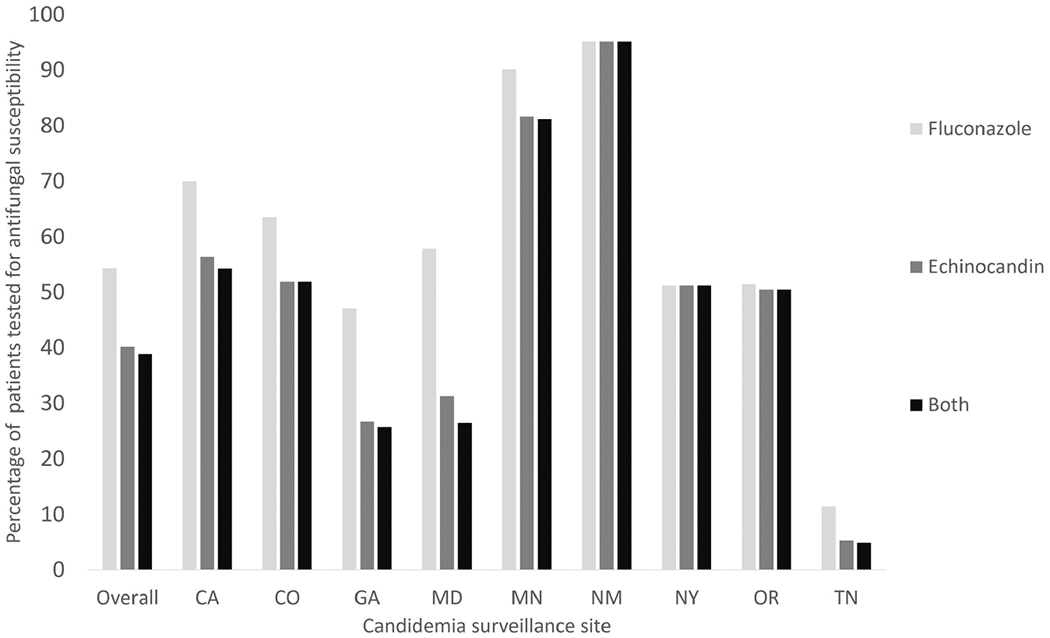
Histogram showing the percentage of candidemia patients (N = 1801) whose isolates received susceptibility testing for fluconazole, an echinocandin, and both fluconazole and an echinocandin at 9 US surveillance sites during 2017–2018. The vertical bars for overall and for each site are not mutually exclusive.
Among 265 patients initially treated with fluconazole and that received fluconazole AFST, 28 (10.6%) were ultimately found to have fluconazole-resistant isolates; of these 28 patients, 19 were switched to echinocandin, three were switched to another drug class, and six were not switched. Eight of 525 (1.5%) patients who were treated initially with an echinocandin and received echinocandin AFST grew an echinocandin-resistant Candida species. Among 539 patients who were switched from an echinocandin to fluconazole, 263 (48.8%) grew a non-albicans Candida species and 328 (60.9%) had fluconazole AFST performed. Of the 226 patients who were switched from fluconazole to an echinocandin, 157 (69.5%) grew a non-albicans Candida species and 110 (48.7%) had fluconazole AFST preformed.
DISCUSSION
Our analysis of candidemia treatment practices at 9 US surveillance sites found that most adult patients received an echinocandin rather than fluconazole as initial treatment for candidemia. However, a substantial proportion (29.6%) of patients initially received fluconazole, which resulted in potentially inappropriate treatment. More than one-half of patients started on fluconazole were later found to grow a non-albicans Candida species on initial blood cultures, and >10% of Candida isolates from patients who received fluconazole and whose specimens were tested for fluconazole susceptibility were resistant. Geographic area, but not diabetes, malignancy, and recent exposure to fluconazole (known risk factors for fluconazole resistance [13, 20]), was one of the strongest predictors of initial drug choice. This finding suggests that regional practices play a stronger role in initial candidemia therapy than patient-level factors and that clinician education regarding risk factors for drug-resistant candidemia may be needed.
Cirrhosis was the only medical condition significantly predictive of echinocandin over fluconazole as initial treatment. This might be due to several factors, including concerns for potential fluconazole-associated hepatotoxicity [21] or fungal infections as an emerging concern in patients with cirrhosis given the particularly poor outcomes of candidemia in this population [22, 23]. Patients with a history of recent hospitalization were also more likely to receive fluconazole than an echinocandin as initial candidemia treatment. Given these unexpected findings, further research is needed regarding how patient-level factors might influence clinician antifungal choices.
Geographic location was among the few independent predictors of whether a patient received an echinocandin as initial treatment, with patients at certain surveillance sites having over twice the odds of initially receiving an echinocandin compared with other sites. Differences in antifungal selection among sites are likely due to a combination of factors, including the local epidemiology of Candida spp. distributions, risk factors of the local population, antifungal susceptibility patterns, facility type, and clinician awareness and appropriate implementation of IDSA guidelines. Species and drug- resistance patterns are known to vary substantially by geography, facility, and even among units within the same facility [24, 25]. Therefore, variation in treatment patterns by surveillance site was not unexpected given the epidemiologic variety that likely results in a diversity of clinician practices across the United States; the impact of these factors on candidemia treatment decisions merits further research.
Approximately 25% of patients in our analysis first received antifungal treatment ≥3 days after the date of their first positive blood culture collection, which is consistent with previous research of candidemia treatment delays [26]. These possible treatment delays likely occurred because of the non-specific clinical presentation of candidemia, low sensitivity of blood cultures for candidemia detection, and the time needed to detect Candida in blood cultures [15, 27]. Furthermore, 1 in 4 patients received an antifungal drug on or during the 14 days before the specimen collection day of their first positive blood culture, suggesting that they may have received empiric therapy for suspected candidemia before Candida was detected. Although retrospective studies have suggested that empiric antifungal therapy might improve survival for select high-risk patients [28], a large, multicenter randomized control trial of empiric antifungal therapy in nonneutropenic critically ill patients did not find a significant difference in survival among patients treated empirically with micafungin versus placebo [29]. The appropriate role for empiric antifungal therapy for suspected candidemia remains controversial, and further investigation of this topic is needed [29]. Our findings underscore the continued need for the evaluation of culture-independent diagnostic tests to assist with earlier candidemia detection and treatment, as well as continued clinician education about candidemia in at-risk patients [15].
Our findings also suggest an opportunity for increased use of AFST because AFST results can be an important adjunct in clinical decision-making [30]. Although IDSA guidelines recommend fluconazole AFST for all bloodstream Candida isolates, nearly one-half of patients did not receive testing for fluconazole susceptibility. Compared with fluconazole AFST, echinocandin AFST was performed less frequently, which is expected because echinocandin AFST is not recommended for all bloodstream isolates [13]. However, contrary to IDSA guidelines, fewer than half of C glabrata or C parapsilosis isolates underwent echinocandin AFST, which is recommended because of concern for echinocandin resistance in these species. In our analysis, 39.1% of patients were switched from an echinocandin to fluconazole without receiving fluconazole AFST. Although AFST results are generally unavailable at the time treatment is initiated, the use of AFST, in conjunction with facility-specific antibiograms, may improve antifungal stewardship by facilitating the transition from echinocandins to fluconazole when appropriate [17, 31].
The proportion of patients with isolates that underwent AFST varied widely among surveillance sites. Although the capacity of laboratories in the United States to perform AFST has increased in recent years, the frequency of AFST performance and use in clinical decision-making might vary based on several factors, including cost, a lack of personnel trained in mycology, and reliance on off-site testing, which can substantially delay results (from ~3 days with on-site testing versus ~7 days for off-site testing) [14, 32]. Furthermore, a lack of reflexive AFST (ie, automatic testing versus requiring a clinician order) for Candida isolates, may further hinder timely receipt of appropriate treatment and the likelihood of clinicians to use AFST results for treatment decision-making. A recent analysis using National Healthcare Safety Network data found that reflexive AFST is lacking in approximately two-thirds of acute care hospitals [14]. As antifungal drug resistance continues to rise, efforts to increase local laboratory AFST capacity and integrate AFST into treatment practices are becoming critically important.
In our investigation, patients treated initially with an echinocandin were more likely to die during their candidemia-associated hospitalization than those treated with fluconazole. However, this finding is likely from confounding factors because critically ill patients are more likely to be treated with an echinocandin [33]. A randomized controlled trial, more robust for examining effects of treatment, found superior outcomes for patients who received an echinocandin as primary treatment for invasive candidiasis [12].
Our findings are subject to several limitations. Although EIP candidemia surveillance encompassed a source population of approximately 17.1 million, treatment practices observed in EIP surveillance sites may not reflect those throughout the country. EIP candidemia surveillance data are useful for analyzing broad national trends, but our analysis was limited by the lack of facility-level data needed to compare practices among facility types (eg, tertiary, academic, community hospitals), as well as a lack of data on setting of care (eg, ward, intensive care unit) and treating physician type (eg, infectious disease specialist) at the time of antifungal drug treatment initiation. Clinicians most likely choose candidemia treatments based on a combination of factors including local adoption of guidelines, drug cost and availability, ability to administer fluconazole orally, individual patient characteristics, facility type, and local epidemiology, which makes it difficult to generalize about treatment practices across the nation or to definitively state whether the treatment decisions were inappropriate. Further, EIP surveillance data lack information regarding why patients were switched from one drug class to another, how clinicians might use site-specific antibiograms or local candidemia species data, and information regarding the timing of when clinicians receive results of species testing and AFST. We noted that nearly one-half of patients started initially on fluconazole were then switched to an echinocandin, usually within several days; although the motivation driving this switch is unknown, we suspect that these changes could be due to treatment failure, receipt of AFST results, identification of additional species that warranted a new treatment plan, specialist consultation, or clinical worsening.
Nonetheless, our analysis of data from a large, population-based, and multisite candidemia surveillance system identified instances in which patients received potentially inappropriate candidemia treatment. Our findings highlight opportunities for closer adherence to IDSA guidelines on antifungal drug selection and AFST utilization, as well as the need for improved candidemia diagnostic tools. Further research regarding how clinicians choose antifungal drugs for candidemia and barriers to use of IDSA guidelines is also needed. Although treatment guidelines should never supplant clinician judgment when caring for individual patients, increased clinician education and stricter adherence to IDSA guidelines should be considered.
Acknowledgments.
Brenda Barnes, Karlyn Beer, Joanne Benton, Rebekah Blakney, Lindsay Bonner, Taylor Chambers, Christina Felsen, Anita Gellert , Caroline Graber, Sasha Harb , Sherry Hillis, Rosemary Hollick, Randy Kuykendall, Vijitha Lahanda Wadu, Zeyu Li, Kaytlynn Marceaux, Lewis Perry, and Andrew Revis.
Financial support.
This work was supported by the Centers for Disease Control and Prevention (CDC-RFA-CK17-1701).
Potential conflicts of interest.
C. A. C. reports support from Centers for Disease Control and Prevention (Emerging Infections Program) grant to the Colorado Department of Public Health and Environment) during the conduct of the study and outside the submitted work. E. P. reports grant funding to her institution from the Centers for Disease Control and Prevention, during the conduct of the study. H. L. J. reports that the Centers for Disease Control and Prevention provided funding to the Colorado Department of Public Health and Environment via the Emerging Infections Program to conduct this project. J. N. reports California Emerging Infections Program Cooperative Agreement from the Centers for Disease Control and Prevention during the conduct of the study. M. M. F. reports Centers for Disease Control and Prevention funding for the candida surveillance system (Cooperative Agreement) to her institution, during the conduct of the study. S. S. D. reports grant funding to her institution from the Centers for Disease Control and Prevention, during the conduct of the study. S. T. reports Emerging Infections Program funded through a Centers for Disease Control and Prevention cooperative agreement. W. S. reports support from the Centers for Disease Control and Prevention (Emerging Infections Program) during the conduct of the study. L. H. reports consulting fees from Sanofi Pasteur, GSK, ID Connect, Merck, and Pfizer outside submitted work and reports travel support from Pfizer, Sanofi Pasteur, GSK, and Merck outside the submitted work. W. S. reports consulting fees from VBI Vaccines outside the submitted work and serves as Medical Director for National Foundation for Infectious Diseases outside the submitted work. A. Z. reports support from the Centers for Disease Control and Prevention outside the submitted work. D. B. reports that the Colorado Department of Public Health receives grant funding from the Centers for Disease Control and Prevention to conduct the surveillance activities outlined in the manuscript. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Footnotes
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Vallabhaneni S, Mody RK, Walker T, Chiller T. The global burden of fungal diseases. Infect Dis Clin North Am 2016; 30:1–11. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007; 20:133–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedict K, Jackson BR, Chiller T, Beer KD. Estimation of direct healthcare costs of fungal diseases in the United States. Clin Infect Dis 2019; 68:1791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsay SV, Mu Y, Williams S, et al. Burden of candidemia in the United States, 2017. Clin Infect Dis 2020; 71:e449–e453. [DOI] [PubMed] [Google Scholar]
- 5.Lockhart SR, Iqbal N, Cleveland AA, et al. Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from 2008 to 2011. J Clin Microbiol 2012;50:3435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toda M, Williams SR, Berkow EL, et al. Population-based active surveillance for culture-confirmed candidemia—four sites, United States, 2012–2016. MMWR Surveill Summ 2019; 68:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC, 2019. [Google Scholar]
- 8.Andes DR, Safdar N, Baddley JW, et al. ; Mycoses Study Group. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis 2012; 54:1110–22. [DOI] [PubMed] [Google Scholar]
- 9.Ostrosky-Zeichner L, Kullberg BJ, Bow EJ, et al. Early treatment of candidemia in adults: a review. Med Mycol 2011; 49:113–20. [DOI] [PubMed] [Google Scholar]
- 10.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management candidiasis: 2009 update by the infectious diseases society of America. Clin Infect Dis 2009; 48:503–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berkow EL, Lockhart SR. Fluconazole resistance in Candida species: a current perspective. Infect Drug Resist 2017; 10:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reboli AC, Rotstein C, Pappas PG, et al. ; Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007; 356:2472–82. [DOI] [PubMed] [Google Scholar]
- 13.Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis 2016; 62:e1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallabhaneni S, Sapiano M, Weiner LM, Lockhart SR, Magill S. Antifungal susceptibility testing practices at acute care hospitals enrolled in the national healthcare safety network, United States, 2011–2015. Open Forum Infect Dis 2017; 4:ofx175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis 2013; 56:1284–92. [DOI] [PubMed] [Google Scholar]
- 16.Safavieh M, Coarsey C, Esiobu N, et al. Advances in Candida detection platforms for clinical and point-of-care applications. Crit Rev Biotechnol 2017; 37:441–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah DN, Yau R, Weston J, et al. Evaluation of antifungal therapy in patients with candidaemia based on susceptibility testing results: implications for antimicrobial stewardship programmes. J Antimicrob Chemother 2011; 66:2146–51. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. About EIP. Available at: https://www.cdc.gov/ncezid/dpei/eip/eip-about.html. Accessed 11 November.
- 19.Clinical and Laboratory Standards Institute. M60 performance standards for antifungal susceptibility testing of yeasts. 2nd ed. CLSI supplement M60. Wayne, PA: Clinical and Laboratory Standards Institute, 2020. [Google Scholar]
- 20.Déry M, Hasbun R. Fluconazole-resistant candida: mechanisms and risk factor identification. Curr Fungal Infect Rep 2011; 5:23–8. [Google Scholar]
- 21.Spernovasilis N, Kofteridis DP. Pre-existing liver disease and toxicity of antifungals. J Fungi 2018; 4:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeoh SF, Lee TJ, Chew KL, Lin S, Yeo D, Setia S. Echinocandins for management of invasive candidiasis in patients with liver disease and liver transplantation. Infect Drug Resist 2018; 11:805–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexopoulou A, Vasilieva L, Agiasotelli D, Dourakis SP. Fungal infections in patients with cirrhosis. J Hepatol 2015; 63:1043–5. [DOI] [PubMed] [Google Scholar]
- 24.Pfaller MA, Messer SA, Moet GJ, Jones RN, Castanheira M. Candida bloodstream infections: comparison of species distribution and resistance to echinocandin and azole antifungal agents in intensive care unit (ICU) and non-ICU settings in the SENTRY antimicrobial surveillance program (2008–2009). Int J Antimicrob Agents 2011; 38:65–9. [DOI] [PubMed] [Google Scholar]
- 25.Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. Candida bloodstream infections: comparison of species distributions and antifungal resistance patterns in community-onset and nosocomial isolates in the SENTRY antimicrobial surveillance program, 2008–2009. Antimicrob Agents Chemother 2011; 55:561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clancy CJ, Pappas PG, Vazquez J, et al. Detecting infections rapidly and easily for candidemia trial, part 2 (DIRECT2): a prospective, multicenter study of the T2Candida panel. Clin Infect Dis 2018; 66:1678–86. [DOI] [PubMed] [Google Scholar]
- 27.Guery BP, Arendrup MC, Auzinger G, et al. Management of invasive candidiasis and candidemia in adult non-neutropenic intensive care unit patients: part I. Epidemiology and diagnosis. Intensive Care Med 2009; 35:55–62. [DOI] [PubMed] [Google Scholar]
- 28.Parkins MD, Sabuda DM, Elsayed S, Laupland KB. Adequacy of empirical antifungal therapy and effect on outcome among patients with invasive Candida species infections. J Antimicrob Chemother 2007; 60:613–8. [DOI] [PubMed] [Google Scholar]
- 29.Timsit JF, Azoulay E, Schwebel C, et al. ; EMPIRICUS Trial Group. Empirical micafungin treatment and survival without invasive fungal infection in adults with ICU-acquired sepsis, Candida colonization, and multiple organ failure: the EMPIRICUS randomized clinical trial. JAMA 2016; 316:1555–64. [DOI] [PubMed] [Google Scholar]
- 30.Sanguinetti M, Posteraro B. Susceptibility testing of fungi to antifungal drugs. J Fungi 2018; 4:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander BD, Johnson MD, Pfeiffer CD, et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 2013; 56:1724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pai MP, Pendland SL. Antifungal susceptibility testing in teaching hospitals. Ann Pharmacother 2003; 37:192–6. [DOI] [PubMed] [Google Scholar]
- 33.Leroy O, Bailly S, Gangneux JP, et al. ; AmarCAND2 study group. Systemic antifungal therapy for proven or suspected invasive candidiasis: the AmarCAND 2 study. Ann Intensive Care 2016; 6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]


