Abstract
Background & aims
COVID-19 patients present a high hospitalization rate with a high mortality risk for those requiring intensive care. When these patients have other comorbid conditions and older age, the risk for severe disease and poor outcomes after ICU admission are increased. The present work aims to describe the preliminary results of the ongoing NUTRICOVID study about the nutritional and functional status and the quality of life of adult COVID-19 survivors after ICU discharge, emphasizing the in-hospital and discharge situation of this population.
Methods
A multicenter, ambispective, observational cohort study was conducted in 16 public hospitals of the Community of Madrid with COVID-19 survivors who were admitted to the ICU during the first outbreak. Preliminary results of this study include data retrospectively collected. Malnutrition and sarcopenia were screened at discharge using MUST and SARC-F; the use of healthcare resources was measured as the length of hospital stay and requirement of respiratory support and tracheostomy during hospitalization; other study variables were the need for medical nutrition therapy (MNT); and patients’ functional status (Barthel index) and health-related quality of life (EQ-5D-5L).
Results
A total of 176 patients were included in this preliminary analysis. Most patients were male and older than 60 years, who suffered an average (SD) weight loss of 16.6% (8.3%) during the hospital stay, with a median length of stay of 53 (27–89.5) days and a median ICU stay of 24.5 (11–43.5) days. At discharge, 83.5% and 86.9% of the patients were at risk of malnutrition and sarcopenia, respectively, but only 38% were prescribed MNT. In addition, more than 70% of patients had significant impairment of their mobility and to conduct their usual activities at hospital discharge.
Conclusions
This preliminary analysis evidences the high nutritional and functional impairment of COVID-19 survivors at hospital discharge and highlights the need for guidelines and systematic protocols, together with appropriate rehabilitation programs, to optimize the nutritional management of these patients after discharge.
Keywords: Coronavirus disease (COVID-19), Intensive care, Nutritional therapy, Malnutrition, Functional status, Health-related quality of life
1. Introduction
The coronavirus disease (COVID-19) pandemic spread rapidly around the world, impacting most healthcare systems [1], that had to adapt their resources to meet the need of tracking and testing people, ensuring adequate care of the population while fighting against this unprecedented situation [2]. At the same time, scientists and clinicians have been researching and learning about the disease's pathogenesis and the optimization of the management of affected patients [3,4].
Regarding the clinical management of the disease, a high hospitalization rate is observed in COVID-19 patients [5], with seriously ill ones being admitted into the intensive care units (ICU) and in most cases needing invasive mechanical ventilation, which ends up in higher mortality risk. Moreover, patients with comorbid conditions and older age are at risk for severe disease and poor outcomes after ICU admission [6], including impaired nutritional status and sarcopenia, independently of body mass index (BMI) [7,8]. In Spain, a prospective, multicenter, cohort study that enrolled 663 critically ill COVID-19 patients reported an ICU mortality rate of 31% [9].
Malnutrition during ICU stays has a prevalence ranging from 38% to 78% [10]. It is associated with loss of skeletal muscle mass and function, leading to poor quality of life, disability, and other morbidities that can affect individuals even long after their ICU discharge [10,11]. General clinical guidelines recommend medical nutrition therapy (MNT) for all ICU patients, mainly for more than 48 h [12], and the extension of the nutritional treatment after hospital discharge [11]. However, the prevalence of malnutrition in COVID-19 survivors [13] and the health-related effects that this malnutrition might bring in the long term are still unknown. Early during the pandemic, ESPEN published clinical guidance on the nutritional treatment of these patients focused to those in the ICU setting, elderly patients with comorbidities [11]. However, recommendations after hospital discharge are less known and a better knowledge about the nutritional and functional status of these individuals at hospital discharge and during the long-term recovery, would help clinicians and decision-makers optimize these patients' management in the community.
The present study aims to describe the nutritional and functional status and the quality of life of patients admitted in ICU due to confirmed COVID-19 during the first outbreak of the pandemic, and for one year after hospital discharge. In this work, we describe the preliminary results of this ongoing study, emphasizing the in-hospital and discharge situation of this population.
2. Materials and methods
2.1. Design and study population
This is a multicenter ambispective observational cohort study conducted in 16 public hospitals of the Community of Madrid, Spain. A list of the participating hospitals and the study collaborators involved can be found in Supplementary Table 1.
Eligible patients were adult patients with confirmed COVID-19, admitted in ICU between 1st March and 30th June 2020, who could be followed-up by the Nutrition and Endocrinology departments after hospital discharge. All participants voluntarily enrolled and signed an informed consent form.
The study was approved by the Ethics Committee of Hospital Clinico San Carlos and conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice.
2.2. Sample size and patient's selection
The sample size required to meet the study objective was calculated based on the number of patients admitted in ICUs from hospitals in the Community of Madrid from March (outbreak start) to May 2020 (3600 patients) [14], when the study protocol was developed. Assuming maximum variability criterium a minimum of 186 patients was required (95% unilateral confidence level and 7% detection precision). Considering a loss rate of 10% a sample size of 207 patients was estimated. Sample size was distributed to each participating hospital according to their capacity, calculated as number of hospital beds.
Then, a randomized selection of patients was performed to ensure a heterogeneous and representative sample of patients during all the period of the outbreak. For that, a random list of dates was provided to each participating hospital, including ICU admission dates from the first outbreak period. First patients admitted in the ICU due to confirmed COVID-19 for each date who met the previously mentioned inclusion criteria were invited.
2.3. Data collection
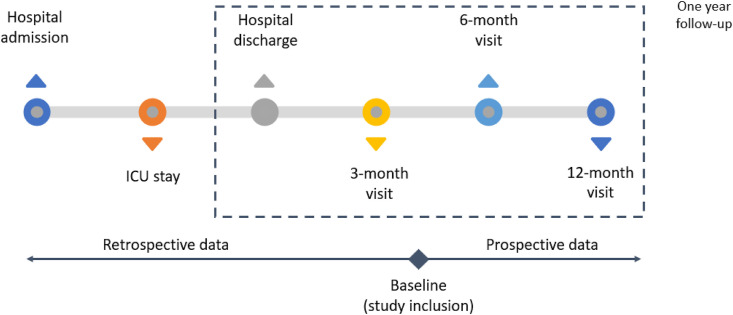
Patients were contacted by the Nutrition and Endocrinology departments after discharge and invited to participate in the study. At the study inclusion, researchers retrospectively collected the study variables related to previous care from electronic medical records (if available) or when interviewing the patients. After the inclusion, since our study is still ongoing, patients are being followed-up for 12 months after discharge and the study variables are being collected prospectively. The study diagram is presented in Fig. 1 . Follow-up visits are being performed through telemedicine or face-to-face visit depending on the feasibility of patients going to the hospital due to the pandemic.
Fig. 1.
Study diagram.
The study variables include sociodemographic characteristics (age and gender), comorbidities, patients' nutritional status (weight, height, and BMI, nutritional and sarcopenia screening), patients' use of resources in the hospital (length of stay, requirement of respiratory support, and tracheostomy) and MNT (parenteral [PN], enteral [EN] or oral nutritional supplement [ONS]) and time of MNT received (in-hospital and/or prescribed after discharge), patients’ functional status and health-related quality of life (HRQoL).
The nutritional screening was performed using the Malnutrition Universal Screening Tool (MUST). This tool uses three independent criteria to detect protein-energy malnutrition and the risk of malnutrition [15]: weight status using BMI, unintentional weight loss and acute disease effect producing or likely to produce no nutritional intake for more than 5 days. A score is given to each criterion, and the sum provides the overall risk of malnutrition [16].
The risk for sarcopenia was screened using SARC-F questionnaire, which assesses patient reported difficulty in doing daily activities such as walking, climbing stairs, or standing from a chair [17].
The patients' functional status was assessed using Barthel index [18], that is able to measure the performance of daily life activities and determine patients’ autonomy, classified into total, severe, moderate, or low dependency or independence.
Finally, HRQoL was measured using EuroQol-5 Dimension 5 levels (EQ-5D-5L) [19]. This tool comprises 5 dimensions regarding mobility, self-care, usual activities, pain or discomfort and anxiety or depression that can be graded into 5 levels from no problems, to slight, moderate, severe or extreme problems [20]. It also includes a visual analogue scale (EQ-VAS) that takes values between 100 “best imaginable health” and 0 “worst imaginable health”, on which patients provide a global assessment of their health.
2.4. Statistical analysis
A preliminary analysis of the available data from the study variables related to the hospital stay and discharge was performed. Qualitative variables are reported as relative and absolute frequencies, while quantitative variables are calculated with centrality and dispersion measures (mean, standard deviation [SD], median, quartiles [25th–75th percentiles, Q1-Q3], minimum and maximum). In addition, data on patient functional status at discharge based on Barthel index were analyzed according to the risk of sarcopenia and malnutrition that these patients presented at discharge. All statistical analyses were performed using the software STATA v.14 (Stata Corp, College Station, TX, USA).
3. Results
One hundred and seventy-six patients were included in this preliminary analysis of results. Therefore, 94.6% of the minimum sample size required was analyzed.
Sociodemographic and comorbidity data of the included patients are shown in Table 1 .
Table 1.
Sociodemographic and comorbidity data of the study population (n = 176).
| Age (mean [SD]) | 60.3 (10.5) |
| Gender (% [n]) | |
| Male | 71.6 (126) |
| Female | 28.4 (50) |
| Main comorbidities (% [n]) | |
| Obesity | 60.2 (106) |
| Dyslipidemia | 48.0 (84) |
| Hypertension | 46.3 (81) |
| Lung disease | 29.5 (52) |
| Diabetes mellitus | 18.8 (33) |
| Cardiovascular disease | 18.2 (32) |
| Liver disease | 10.2 (18) |
| Smoker | 9.7 (17) |
| Cancer | 9.1 (16) |
| Renal disease | 9.1 (16) |
3.1. Nutritional status
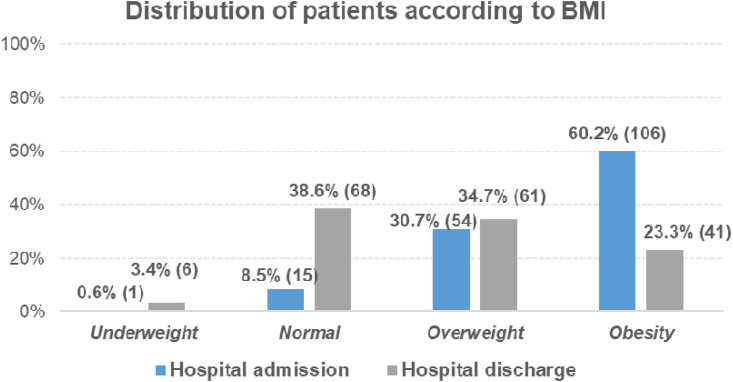
The mean (SD) weight at hospital admission was 89.6 (18.4) kg and the mean (SD) weight at hospital discharge was 74.4 (16.1) kg. This can be translated into an average (SD) weight loss of 16.6% (8.3%) during the hospital stay. Regarding the BMI, the median (Q1-Q3) BMI at hospital admission was 31.5 (27.2–35) kg/m2, whereas the median BMI at hospital discharge was 25.7 (22.6–29.5) kg/m2. At admission, 60.2% of patients were obese (BMI≥30 kg/m2), and 23.3% at hospital discharge (Fig. 2 ).
Fig. 2.
Distribution of patients according to their BMI at hospital admission and discharge.
According to MUST and SARC-F scores, 83.5% and 86.9% of the patients were considered at high risk of malnutrition, and at risk of sarcopenia, respectively, at hospital discharge (Table 2 ).
Table 2.
Distribution of patients according to their risk of sarcopenia and malnutrition at hospital discharge.
| Outcomes at discharge | Number of patients (%) | |
|---|---|---|
| Risk of sarcopenia (SARC-f) | With risk | 153 (86.9) |
| Without risk | 23 (13.1) | |
| Risk of malnutrition (MUST) | Low risk | 12 (6.8) |
| Medium risk | 17 (9.7) | |
| High risk | 147 (83.5) | |
Data on comorbidities according to nutritional and functional status are shown in Supplementary Table 2.
3.2. Use of resources
The median hospital length of stay was 53 (27–89.5) days, with a median ICU stay of 24.5 (11–43.5) days. It was observed that the length of hospital stay for patients at high risk of malnutrition at discharge (median 55 [IQR 27–93] days) was slightly higher than patients at low risk (median 45 [IQR 28–79] days). Moreover, those patients at high risk of malnutrition (MUST ≥2) at hospital discharge had required a longer UCI length of stay (median 26 [12–48] days, with a maximum of 142 days).
During the hospital stay, up to 88% of patients required invasive mechanical ventilation and up to 53.7% needed a tracheostomy (out of 175 patients). Regarding the use of respiratory support, those patients with a MUST ≥2 at discharge compared to those with a lower risk of malnutrition required more tracheostomy (57.5% vs. 40.4%), and invasive mechanical ventilation (89.0% vs. 5.5%).
Taking into account the risk of sarcopenia, data suggest that patients with risk at discharge had longer length of hospital and UCI stay (median 56 [IQR 28–96] days and 26 [IQR 12–44] days) than patients without risk (median 33 [IQR 20–57] days and 14 [IQR 10–26] days). Furthermore, those patients at risk of sarcopenia compared to those without risk seem to require more tracheostomy (56.2% vs 36.4%) and invasive mechanical ventilation (90.8% vs 69.6%).
During the hospitalization, 50.6% (out of 162) of the patients received PN, 85.2% EN, and 66.7% ONS. PN was used for a median time of 14 [[7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]] days, EN for 18.5 (9–34) days, and ONS for 12 [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]] days. At hospital discharge, ONS were prescribed in 36.8% (out of 163) patients (for at least 90 days in 76.3% of them) and 1.2% of the patients received home EN with a median duration of 62.5 (35–90) days.
3.3. Functional status and HRQoL
According to the Barthel index, only 20.5% of patients were independent or had a low level of dependency, the rest had moderate or total dependence (in 7.4% of them). It was observed that a high percentage of patients at risk of sarcopenia at discharge (86.3%) had moderate to total dependency, with a lower percentage in patients at no risk (34.8%). However, the percentage of patients at high and low risk of malnutrition who had moderate-total dependency were similar (82.3% and 83.3%, respectively).
HRQoL results are presented in Table 3 . Most of the patients, 71.2%, and 75.5% had important impairment (moderate to inability) of their mobility, and to conduct their usual activities, respectively. Finally, the median EQ-VAS score was 40 (25–50).
Table 3.
Results from the EQ-5D-5L in frequency, % (n).
| No problems | Slight problems | Moderate problems | Severe problems | Unable | |
|---|---|---|---|---|---|
| Mobility | 9.2 (15) | 19.6 (32) | 27.0 (44) | 26.4 (43) | 17.8 (29) |
| Self-care | 23.9 (39) | 25.2 (41) | 21.5 (35) | 11.7 (19) | 17.8 (29) |
|
Usual activities |
6.1 (10) |
18.4 (30) |
14.7 (24) |
19.6 (32) |
41.1 (67) |
|
No pain |
Slight pain |
Moderate pain |
Severe pain |
Extreme pain |
|
|
Pain/discomfort |
22.1 (36) |
16.6 (27) |
31.9 (52) |
24.5 (40) |
4.9 (8) |
|
Not anxious |
Slightly anxious |
Moderately anxious |
Severely anxious |
Extremely anxious |
|
| Anxiety/depression | 40.5 (66) | 20.2 (33) | 20.2 (33) | 11.0 (18) | 8.0 (13) |
4. Discussion
The preliminary results of this multicenter observational study describe the nutritional and functional status of COVID-19 survivors who were admitted to the UCI during the first outbreak of the pandemic in the Community of Madrid, during the hospital stay and at the time of hospital discharge. Due to the high mortality risk for ICU patients with COVID-19, the scientific community has focused on providing information concerning the characteristics of the affected patients and the associated risk factors with the short-term outcomes, and not so much in studying the long-term evolution of the surviving patients. This study will provide data of COVID-19 survivors evolution.
Most patients in our study were male and 60 years old on average, similar to other published studies [[21], [22], [23]].
A large proportion of patients in our cohort were obese (BMI ≥30 kg/m2) at the time of hospital admission. This fact, together with old age, have been associated with a worse clinical outcome [6]. However, scientific evidence is still limited about the exact pathophysiological mechanisms that relate obesity with COVID-19. Extrapolating the obesity behaviour in other infections such as the one caused by H1N1 influenza virus [24], it is shown that obese subjects present a more severe disease, related to immunity and inflammatory processes which make the individual more vulnerable to infections and less responsive to vaccinations, antiretroviral and antimicrobial treatments. A previous study carried out in patients with COVID-19 showed that patients with a BMI in the range 20–34.9 kg/m2 had a significantly increased risk of respiratory failure (odds ratio 2.32, 95%; confidence interval 1.31–4.09, p = 0.004) and required care in intensive care unit [24].
In addition, the sudden and significant weight loss that patients suffer during hospitalization turns them susceptible to developing disease-related malnutrition and sarcopenic obesity. Weight loss correlates with C-reactive protein levels, impaired kidney function, and longer duration of COVID-19 disease [25]. This means that prompt MNT of these patients should be implemented in order to decrease the consequences of malnutrition and muscle mass loss during hospitalization, and after discharge to prevent long-term disability due to acute COVID-19 disease.
The use of simple clinical tools for identifying nutritional risk and loss of muscle mass and function (MUST and SARC-F) that can be administered by telemedicine has been suggested in response to the COVID-19 pandemic crisis [26]. In our study, at least 8 out of 10 patients developed an impaired nutritional status. COVID-19 infection can cause sarcopenia because of the increased muscle wasting caused by the systematic inflammation, the reduced physical activity, and an inadequate nutrient intake caused by many reasons including anorexia, anosmia and social isolation. In this case, rehabilitation programs including physical activities together with protein supplementation can reverse sarcopenia during COVID-19 treatment [27].
Our results show that MNT has been necessary for most of the patients during hospitalization. However, MNT is prescribed in only 38% of patients after hospital discharge, mainly in the form of ONS. This low frequency of prescription after discharge draws attention. Despite improvements in the sensitivity of professionals and institutions in relation to MNT as basic therapy in all pathologies, the pandemic has come to highlight the need to value individualized nutritional treatment due to its effectiveness and efficiency [28]. The difficulties in face-to-face access to patients, the lack of protocols in the follow-up of these patients that incorporate structured MNT and its telematic control, and the magnitude of the first outbreak of the pandemic in our centers and the overburden of work could justify these results.
Barthel index has been used to predict the mortality risk from COVID-19 [29], however, the role of functional status in determining poor outcomes has not yet been fully established. In our study, results showed that most of patients (72.1%) reported a moderate to severe mobility dependency at discharge. Barthel and SARC-F scores at discharge suggested a tendency between the decline in functional status and a higher risk of sarcopenia.
In terms of quality of life, similar outcomes were reported, with most of the patients reporting difficulties in mobility (71.2%) and the performance of their daily life activities (75.5%). Appropriate rehabilitation programs have been recommended, as they can improve mobility issues and maximize the functional return of COVID-19 survivors [30].
To our knowledge this is the first published multicentric study on the topic of long-term nutritional, functional recovery and use of resources, of ICU admitted COVID-19 survivors. In this work we present the preliminary data of the patients included and at discharge. There are several limitations in this preliminary analysis. Firstly, the minimum sample size has not been fully accomplished. However, it is worth emphasizing that even though results and final conclusions cannot be reached yet, 94% of the total patient population is included in these preliminary results, and therefore we consider all the information valuable and worth of sharing. On the other hand, the sample size was not calculated to find correlations between variables or to compare subgroups, hence no statistically significant conclusions can be drawn from the results obtained. Secondly, even though the sample size is large, population belongs to only one region of Spain (Madrid). Nevertheless, to the best of our knowledge our preliminary results are in line with other studies. Lastly, many data were retrospectively collected through electronic medical records, that in some cases were not complete due to the overburden of work in the units during the pandemic crisis. However, some of these data were collected through the interviews with the patients.
This preliminary analysis of the NUTRICOVID study evidences the high nutritional and functional impairment of COVID-19 survivors at hospital discharge and highlights the need of a guidance/guideline and/or a systematic protocol, that help physicians in their clinical practice to adequately treat and follow-up not only COVID-19 patients, but also patients of future pandemics that may befall upon us.
Statement of authorship
C.C., A.J., participated in the study concept development, were involved in the design stage, and supervised the entire study. M.P. and C.E. were involved in designing the protocol study. All the members of the NUTRICOVID study group, conducted the research and were involved. C.C., A.J., M.P. and C.E. were involved in analyzing the data, the critical review, and final approval of the manuscript. A.S. and P.FJ. participated in the preparation, and drafting of the final article.
Funding sources
The study is sponsored by Sociedad de Endocrinología, Nutrición y Diabetes de la Comunidad de Madrid (SENDIMAD) and funded by non-restrictive financial support from Nutricia-Danone Specialized Nutrition.
Conflict of interest
Dr. C.C. reports personal fees from Takeda, personal fees from Fresenius Kabi, personal fees from Baxter, personal fees from Nutricia, personal fees from Persan Farma, outside the submitted work. The rest of the authors declare no conflict of interest related to this article. The authors declare that the funding provider was not involved in analyzing and dissemination of the study results, and that no conflict of interest exists with this organization.
Acknowledgements
The authors acknowledge the investigators from participating centers for their help and input during research. The authors also acknowledge the funding of this study by Nutricia-Danone Specialized Nutrition.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnu.2021.11.017.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organisation Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 at.
- 3.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Severe outcomes among patients with coronavirus disease 2019 (COVID-19) — United States, february 12–march 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laviano A., Koverech A., Zanetti M. Nutrition support in the time of SARS-CoV-2 (COVID-19) Nutrition. 2020;74:110834. doi: 10.1016/j.nut.2020.110834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puig-Domingo M., Marazuela M., Giustina A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68(1):2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrando C., Mellado-Artigas R., Gea A., Arruti E., Aldecoa C., Bordell A., et al. Patient characteristics, clinical course and factors associated to ICU mortality in critically ill patients infected with SARS-CoV-2 in Spain: a prospective, cohort, multicentre study. Rev Esp Anestesiol Reanim. 2020;67(8):425–437. doi: 10.1016/j.redar.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lew C.C.H., Yandell R., Fraser R.J.L., Chua A.P., Chong M.F.F., Miller M. Association between malnutrition and clinical outcomes in the intensive care unit: a systematic review [formula: see text] J Parenter Enter Nutr. 2017;41(5):744–758. doi: 10.1177/0148607115625638. [DOI] [PubMed] [Google Scholar]
- 11.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D., et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39(6):1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer P., Blaser A.R., Berger M.M., Alhazzani W., Calder P.C., Casaer M.P., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 13.Cawood A.L., Walters E.R., Smith T.R., Sipaul R.H., Stratton R.J. A review of nutrition support guidelines for individuals with or recovering from COVID-19 in the community. Nutrients. 2020;12(11) doi: 10.3390/nu12113230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ministerio de Sanidad Consumo y Bienestar Social . 2020. Actualización nº110 - enfermedad por el coronavirus (COVID-19) - 19.05.2020. [Google Scholar]
- 15.Stratton R.J., Hackston A., Longmore D., Dixon R., Price S., Stroud M., et al. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the 'malnutrition universal screening tool' ('MUST') for adults. Br J Nutr. 2004;92(5):799–808. doi: 10.1079/bjn20041258. [DOI] [PubMed] [Google Scholar]
- 16.BAPEN . 2011. Malnutrition universal screening tool. [Google Scholar]
- 17.Malmstrom T.K., Morley J.E. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14(8):531–532. doi: 10.1016/j.jamda.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Cid-Ruzafa J., Damián-Moreno J. [Disability evaluation: Barthel's index] Rev Esp Salud Publica. 1997;71(2):127–137. [PubMed] [Google Scholar]
- 19.EuroQol Group EuroQol 5 dimensions 5 levels. Qual Life Res. 2011;20(10):1727–1736. [Google Scholar]
- 20.EuroQoL Group About EQ-5D-5L. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/ at.
- 21.Estella A., Garcia Garmendia J.L., de la Fuente C., Machado Casas J.F., Yuste M.E., Amaya Villar R., et al. Medicina Intensiva; 2021. Predictive factors of six-week mortality in critically ill patients with SARS-CoV-2: a multicenter prospective study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Álvarez J., Lallena S., Bernal M. Nutrición y pandemia de la COVID-19. Medicine - Programa de Formación Médica Continuada Acreditado. 2020;13(23):1311–1321. doi: 10.1016/j.med.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anker M.S., Landmesser U., von Haehling S., Butler J., Coats A.J.S., Anker S.D. Weight loss, malnutrition, and cachexia in COVID-19: facts and numbers. J Cachexia Sarcopenia Muscle. 2021;12(1):9–13. doi: 10.1002/jcsm.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ž Krznarić, Bender D.V., Laviano A., Cuerda C., Landi F., Monteiro R., et al. A simple remote nutritional screening tool and practical guidance for nutritional care in primary practice during the COVID-19 pandemic. Clin Nutr. 2020;39(7):1983–1987. doi: 10.1016/j.clnu.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P.-y., Li Y., Wang Q. Sarcopenia: an underlying treatment target during the COVID-19 pandemic. Nutrition. 2021;84:111104. doi: 10.1016/j.nut.2020.111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuetz P., Fehr R., Baechli V., Geiser M., Deiss M., Gomes F., et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019;393(10188) doi: 10.1016/S0140-6736(18)32776-4. 2312-2321. [DOI] [PubMed] [Google Scholar]
- 29.Laosa O., Pedraza L., Álvarez-Bustos A., Carnicero J.A., Rodriguez-Artalejo F., Rodriguez-Mañas L. Rapid assessment at hospital admission of mortality risk from COVID-19: the role of functional status. J Am Med Dir Assoc. 2020;21(12) doi: 10.1016/j.jamda.2020.10.002. 1798-802.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halpin S.J., McIvor C., Whyatt G., Adams A., Harvey O., McLean L., et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93(2):1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.