Abstract
Background
Camrelizumab (a PD-1 inhibitor) has been used as a potential therapy in unresectable advanced esophageal squamous cell carcinoma (ESCC) along with adjuvant treatment in locally advanced ESCC, exhibiting an acceptable efficacy and safety profile. This pilot study was designed to further investigate the clinical value and tolerance of neoadjuvant camrelizumab plus chemotherapy in locally advanced ESCC.
Methods
A total of 16 patients with locally advanced ESCC were recruited. Patients received 2 cycles of neoadjuvant therapy including 2 doses of camrelizumab concurrent with 2 cycles of paclitaxel plus carboplatin followed by surgery 4 weeks afterward. Then, the treatment response after neoadjuvant therapy, R0 resection rate, tumor regression grade (TRG), and pathological complete remission (pCR) rate were measured. Besides, adverse events were documented. At last, progression-free survival (PFS) and overall survival (OS) were assessed.
Results
Generally, objective remission rate (ORR) was 81.3% whereas disease control rate (DCR) was 100% after neoadjuvant therapy. Concerning TRG grade, 31.3, 37.5, 18.8, and 12.5% patients reached TRG0, TRG1, TRG2, and TRG3, respectively. Then, pCR rate and R0 resection rate were 31.3 and 93.8%, respectively. Besides, mean PFS and OS were 18.3 months (95%CI: (16.2–20.5) months) and 19.2 months (95%CI: (17.7–20.7) months), respectively, with a 1-year PFS of 83% and OS of 90.9%. Adverse events included white blood cell decrease (37.5%), neutrophil decrease (31.3%), reactive cutaneous capillary endothelial proliferation (37.5%), and nausea or vomiting (25.0%), which were relatively mild and manageable.
Conclusion
Neoadjuvant camrelizumab plus chemotherapy exhibits good efficacy and acceptable tolerance in patients with locally advanced ESCC.
Keywords: Camrelizumab, Chemotherapy, Neoadjuvant therapy, Esophageal squamous cell carcinoma, Efficacy and safety
Introduction
Esophageal squamous cell carcinoma (ESCC), with a proportion of 87% in all esophageal cancer, exhibits a more extensive intratumor heterogeneity and a higher mortality [1–3]. Surgery is the most common treatment modality for the ESCC patients; however, as to patients with locally advanced ESCC, they always lose surgical opportunities because of the large tumor size. Fortunately, the emergence of neoadjuvant therapy not only decreases the tumor size but also brings more surgical opportunities to the patients with locally advanced ESCC [4, 5].
Chemotherapy is the first prior neoadjuvant therapy recommended by several guidelines [6–8] for these patients. Recently, several researches indicate that some certain targeted drugs (such as apatinib and nivolumab) in combination with chemotherapy probably promote the survival profile in locally advanced ESCC [9–11].
Programmed cell death protein 1 (PD-1), expressed by activated lymphocytes, blocks immune responses and facilitates immune escape by binding to its ligands including programmed cell death ligand 1 (PD-L1), which further contributes to tumorigenesis and disease progression in various malignancies [12, 13]. PD-1 inhibitor, as a novel developed immune therapy, blocks the PD-1/PD-L1 linkage and has been widely applied in numerous carcinomas [12, 14]. In addition, in locally advanced ESCC, nivolumab and pembrolizumab combination with chemotherapy was recently used for neoadjuvant therapy, which exhibited an acceptable therapeutic response, progression-free survival (PFS), and overall survival (OS) [9]. Camrelizumab, a domestic product developed in China, is a novel IgG4-kappa anti-PD-1 inhibitor that has been used for treatment of a variety of malignancies, such as refractory classical Hodgkin’s lymphoma and gastric or gastroesophageal junction adenocarcinoma [15, 16]. Moreover, camrelizumab has been previously witnessed for adjuvant or second-line therapy of locally advanced ESCC [17, 18]. However, the clinical value of neoadjuvant camrelizumab plus chemotherapy in the treatment for locally advanced ESCC has not been reported before.
Thus, this study aimed to explore the therapeutic response, survival, and safety profiles of neoadjuvant camrelizumab plus chemotherapy in treating locally advanced ESCC.
Methods
Patients
The current study was a prospective study. A total of 16 patients with locally advanced ESCC who were recruited between July 1, 2019, and December 31, 2020, and received camrelizumab combined with chemotherapy as neoadjuvant therapy before surgery in our hospital. The inclusion criteria were as follows: (i) histologically confirmed as ESCC; (ii) potentially curable and locally advanced ESCC, which was defined as cT1N1-3M0 or cT2-4aN0-3M0 (Union for International Cancer Control, UICC Version 8.0) [19]; (iii) aged >18 years old; (iv) Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 1; (v) willing to receive the neoadjuvant therapy of camrelizumab combined with paclitaxel and carboplatin; and (vi) able to be regularly follow-up. The exclusion criteria were as follows: (i) poorly controlled underlying diseases or insufficient hepatic, hematological, and kidney functions that led to be unsuitable for neoadjuvant therapy; (ii) hypersensitivity to the study drugs; (iii) difficult to perform gastric tube reconstruction after esophagectomy; (iv) known concurrent malignancies; (v) severe infections; (vi) history of use of camrelizumab, paclitaxel or carboplatin; and (vii) pregnancy. Ethical permission for this study was obtained from the Institutional Review Board with the approval number of KS1951 on 11th May 2019, and the written informed consents were acquired from all patients. Our study was registered on Chinese Clinical Trial Registry (https://www.chictr.org.cn/index.aspx) with the approval number of “ChiCTR2100051903”.
Neoadjuvant therapy and surgery
All patients received 2 cycles of neoadjuvant therapy, including 2 doses of camrelizumab concurrent with 2 cycles of paclitaxel plus carboplatin. In detail, the camrelizumab was administered intravenously at a dose of 200 mg each time, every 3 weeks (a treatment cycle). Simultaneously, the paclitaxel was administered by intravenous drip at a dose of 100 mg/m2 of body-surface area on days 1 and 8, and the carboplatin was administered by intravenous drip and targeted at an area under the curve of 5 mg/ml per minute on day 1. The surgery was performed approximately 4 weeks after completion of 2 cycles of neoadjuvant therapy, and the type of surgery included minimally invasive esophagectomy, right transthoracic open esophagectomy, or hybrid approaches (using video-assisted thoracoscopy and laparotomy) with a total 2-field lymphadenectomy. Complete thoracoabdominal two-field lymph node dissection (standard thoracoabdominal two-field plus mediastinum, especially bilateral recurrent laryngeal nerve chain lymph nodes) was performed for the patients without suspicious enlarged lymph nodes in the neck and the patients with middle and lower thoracic esophageal cancer. Cervical-thoracoabdominal three-field lymph node dissection (upper and lower neck and supraclavicular complete two-field lymph nodes mentioned above) was performed for the patients with suspiciously swollen lymph nodes in the neck and patients with upper thoracic esophageal cancer. As for adjuvant therapy, patients received paclitaxel plus carboplatin regimen for about 2–4 cycles.
Treatment response evaluation and safety assessment
The primary endpoint was pathological complete remission (pCR), and the secondary endpoints included treatment response, tumor regression grade (TRG), R0 resection rate, adverse events, progression-free survival (PFS), and overall survival (OS). After the completion of 2 cycles of neoadjuvant therapy, a preoperative computed tomography (CT) examination was performed to assess the treatment response according to the Response Evaluation Criteria In Solid Tumors (RECIST Version 1.1) [20]. Meanwhile, objective remission rate (ORR) and disease control rate (DCR) were calculated as follows: ORR = complete remission (CR) rate plus partial remission (PR) rate and DCR = CR rate plus PR rate plus stable disease (SD) rate. After surgery, pathological examination of the resection specimens was conducted to evaluate the resection margin status and tumor regression grade (TRG). R0 resection was defined as no cancer cells at resection margins. The TRG was used for assessment of the degree of histomorphological degeneration, which was classified as follows: grade 0, no residual cancer cells (defined as pCR); grade 1, single cell or small groups of cancer cells; grade 2, residual cancer cells outgrown by fibrosis; and grade 3, minimum or no treatment effect and extensive residual cancer cells [21, 22]. In addition, adverse events occurred during the neoadjuvant therapy were documented in detail to access safety profiles, which consisted mainly of hematologic adverse events and non-hematologic adverse events.
Survival assessment
For assessment of progression-free survival (PFS) and overall survival (OS), the postoperative surveillance and follow-up for patients were implemented every 3 months in the first year then every 6 months in the following years.
Statistical analysis
Data processing and graph plotting were carried out with the use of SPSS 26.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 6.02 (GraphPad Software Inc., San Diego, CA, USA). Descriptive analysis was completed with the use of the statistics including mean with standard deviation (SD), median with interquartile range (IQR), and frequency with percentage. Survival curves were plotted using Kaplan-Meier method.
Results
Baseline characteristics
In total, 16 patients with locally advanced ESCC were enrolled in present study (Table 1). The age was 60.9±7.8 years. Regarding gender, the number of male and female patients was 14 (87.5%) and 2 (12.5%), respectively. Moreover, 13 (81.3%) patients were scored as ECOG PS score 0, while 3 (18.8%) patients were scored as ECOG PS score 1. Besides, the distribution of tumor location was listed as follows: 3 (18.8%) patients with proximal third, 8 (50.0%) patients with middle third, and 5 (31.3%) patients with distal third. Furthermore, there were 0 (0.0%), 4 (25.0%), 10 (62.5%), and 2 (12.5%) patients were diagnosed as TNM stage I, stage II, stage III, and stage IV, respectively. The detailed characteristics were shown in Table 1.
Table 1.
Characteristics of locally advanced ESCC patients
| Characteristics | ESCC patients (N = 16) |
|---|---|
| Age (years) | |
| Median (IQR) | 60.5 (56.0-67.3) |
| Mean±SD | 60.9±7.8 |
| Male, No. (%) | 14 (87.5) |
| ECOG PS, no. (%) | |
| 0 | 13 (81.3) |
| 1 | 3 (18.8) |
| Tumor location, no. (%) | |
| Proximal third | 3 (18.8) |
| Middle third | 8 (50.0) |
| Distal third | 5 (31.3) |
| Histological grade, no. (%) | |
| Well | 1 (6.3) |
| Moderate | 8 (50.0) |
| Poor | 7 (43.7) |
| Clinical T stage, no. (%) | |
| cT1 | 0 (0.0) |
| cT2 | 2 (12.5) |
| cT3 | 13 (81.3) |
| cT4 | 1 (6.3) |
| Clinical N stage, no. (%) | |
| N0 | 2 (12.5) |
| N1 | 10 (62.5) |
| N2 | 3 (18.8) |
| N3 | 1 (6.3) |
| Clinical TNM stage, no. (%) | |
| Stage I | 0 (0.0) |
| Stage II | 4 (25.0) |
| Stage III | 10 (62.5) |
| Stage IV | 2 (12.5) |
ESCC esophageal squamous cell carcinoma, IQR interquartile range, SD standard deviation, ECOG PS Eastern Cooperative Oncology Group performance status
Treatment response
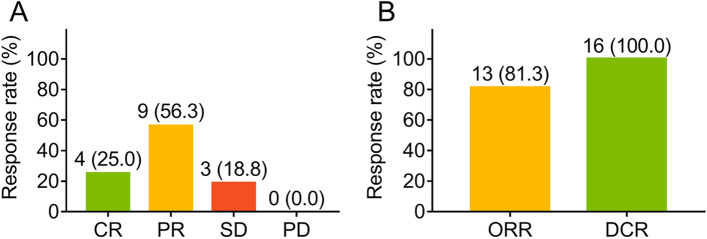
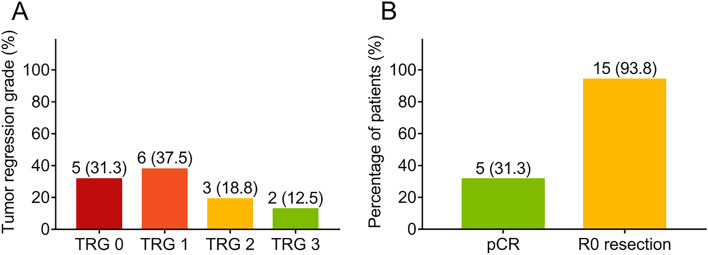
After neoadjuvant therapy, 4 (25.0%) patients achieved CR, 9 (56.3%) patients reached PR and 3 (18.8%) patients remained SD, whereas no one got progressive disease (PD) (Fig. 1a). To sum up, ORR was 81.3%; meanwhile, DCR was 100% (Fig. 1b). Regarding tumor regression grade, 5 (31.3%), 6 (37.5%), 3 (18.8%), and 2 (12.5%) patients reached TRG 0, TRG 1, TRG 2, and TRG 3, respectively (Fig. 2a). Furthermore, 5 (31.3%) patients achieved pCR (Fig. 2b). Besides, 15 (93.8%) patients realized R0 resection.
Fig. 1.
Treatment response rate in patients with locally advanced ESCC. Treatment response rate concerning CR, PR, SD, and PD (a). Treatment response rate concerning ORR and DCR (b) adopting neoadjuvant camrelizumab plus chemotherapy in locally advanced ESCC. CR, complete remission; PR, partly remission; SD, stable disease; PD, progressive disease; ORR, objective remission rate; DCR, disease control rate; ESCC, esophageal squamous cell carcinoma
Fig. 2.
Pathological response rate in patients with locally advanced ESCC. TRG rate (a), pCR rate and R0 resection rate (b) of neoadjuvant camrelizumab plus chemotherapy in locally advanced ESCC. TRG, tumor regression rate; pCR, pathological complete response; ESCC, esophageal squamous cell carcinoma
Survival profiles
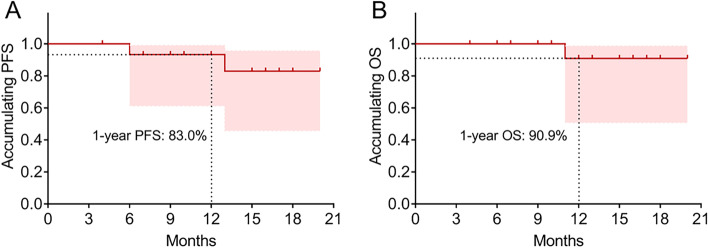
The mean PFS was 18.3 months (95%CI: (16.2–20.5) months) with a 1-year PFS rate of 83% (Fig. 3a); meanwhile, the mean OS was 19.2 months (95%CI: (17.7–20.7) months) with a 1-year OS rate of 90.9% (Fig. 3b). In addition, the detailed information of each patient in terms to baseline characteristics, responses, and survival data was listed in Table 2.
Fig. 3.
PFS and OS in patients with locally advanced ESCC. The PFS (a) and OS (b) adopting neoadjuvant camrelizumab plus chemotherapy in locally advanced ESCC. PFS, progression-free survival; OS, overall survival; ESCC, esophageal squamous cell carcinoma
Table 2.
Key features and treatment outcomes of each advanced ESCC patient
| No. | Age (years) | Gender | ECOG PS | Location | Clinical T stage | Clinical N stage | Clinical TNM stage | Treatment response | TRG | pCR | R0 resection | Progression | PFS (months) | Death | OS (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 | M | 0 | Proximal third | T3 | N0 | Stage II | CR | 1 | No | Yes | No | 16 | No | 16 |
| 2 | 64 | M | 0 | Proximal third | T2 | N1 | Stage II | CR | 0 | Yes | Yes | No | 13 | No | 13 |
| 3 | 61 | M | 0 | Middle third | T3 | N1 | Stage III | PR | 0 | Yes | Yes | No | 20 | No | 20 |
| 4 | 65 | M | 0 | Proximal third | T3 | N2 | Stage III | PR | 2 | No | Yes | No | 18 | No | 18 |
| 5 | 52 | M | 0 | Distal third | T2 | N1 | Stage II | SD | 1 | No | Yes | No | 16 | No | 16 |
| 6 | 68 | M | 0 | Middle third | T3 | N1 | Stage III | PR | 0 | Yes | Yes | No | 7 | No | 7 |
| 7 | 70 | M | 0 | Distal third | T3 | N0 | Stage II | PR | 0 | Yes | Yes | No | 10 | No | 10 |
| 8 | 58 | M | 0 | Middle third | T3 | N1 | Stage III | PR | 1 | No | Yes | No | 17 | No | 17 |
| 9 | 53 | M | 1 | Distal third | T3 | N2 | Stage III | PR | 1 | No | Yes | Yes | 13 | No | 16 |
| 10 | 72 | F | 1 | Middle third | T3 | N3 | Stage IV | SD | 3 | No | No | Yes | 6 | Yes | 11 |
| 11 | 56 | M | 1 | Middle third | T3 | N1 | Stage III | CR | 1 | No | Yes | No | 9 | No | 9 |
| 12 | 46 | M | 0 | Distal third | T3 | N1 | Stage III | PR | 2 | No | Yes | No | 6 | No | 6 |
| 13 | 62 | M | 0 | Distal third | T3 | N2 | Stage III | PR | 1 | No | Yes | No | 12 | No | 12 |
| 14 | 60 | F | 0 | Middle third | T3 | N1 | Stage III | CR | 0 | Yes | Yes | No | 15 | No | 15 |
| 15 | 56 | M | 0 | Middle third | T4 | N1 | Stage IV | PR | 3 | No | Yes | No | 4 | No | 4 |
| 16 | 75 | M | 0 | Middle third | T3 | N1 | Stage III | SD | 2 | No | Yes | No | 16 | No | 16 |
ESCC esophageal squamous cell carcinoma, ECOG PS Eastern Cooperative Oncology Group performance status, TRG tumor regression grade, pCR pathologic complete remission, PFS progression-free survival, OS overall survival, M male, F female, CR complete remission, PR partial remission, SD stable disease, PD progressive disease
Adverse events
Regarding hematological adverse events, 6 (37.5%), 5 (31.3%), 2 (12.5%), and 2 (12.5%) patients experienced white blood cell (WBC) reduction, neutrophil decreased, thrombocytopenia, and anemia (Table 3). Regarding non-hematologic adverse events, 6 (37.5%), 4 (25.0%), 3 (18.8%), 3 (18.8%), 2 (12.5%), 2 (12.5%), 1 (6.3%), and 1 (6.3%) patients suffered from reactive cutaneous capillary endothelial proliferation (RCCEP), nausea or vomiting, decreased appetite, baldness, diarrhea, constipation, fatigue, and rash.
Table 3.
Adverse events during neoadjuvant therapy
| Adverse events | ESCC patients (N = 16) |
|---|---|
| Hematologic, no. (%) | |
| WBC decreased | 6 (37.5) |
| Neutrophil decreased | 5 (31.3) |
| Anemia | 2 (12.5) |
| Thrombocytopenia | 2 (12.5) |
| Non-hematologic, no. (%) | |
| RCCEP | 6 (37.5) |
| Nausea or vomiting | 4 (25.0) |
| Decreased appetite | 3 (18.8) |
| Baldness | 3 (18.8) |
| Diarrhea | 2 (12.5) |
| Constipation | 2 (12.5) |
| Fatigue | 1 (6.3) |
| Rash | 1 (6.3) |
ESCC esophageal squamous cell carcinoma, WBC white blood cell, RCCEP reactive cutaneous capillary endothelial proliferation
Discussion
In present study, it was revealed that (1) after the neoadjuvant therapy, ORR was 81.3% and DCR was 100%. (2) In surgery, the pCR rate and R0 resection rate were 31.3 and 93.8%, respectively. (3) The 1-year PFS and OS were 83 and 90.9%, respectively. (4) All adverse events were slight and tolerable.
Currently, the chemotherapy and radiotherapy are recommended neoadjuvant therapy for locally advanced ESCC patients [6–8]. What’s more, some recent studies point out that targeted drugs (such as apatinib, pembrolizumab, and nivolumab) combined with chemotherapy could possibly exhibit an even better efficacy for the locally advanced ESCC patients. However, there is little evidence around neoadjuvant camrelizumab combined with chemotherapy in treating locally advanced ESCC patients. Subsequently, the current study turn attention to camrelizumab, a PD-1 immune checkpoint inhibitor, which blocks the binding site of PD-1 expressed on activated T lymphocytes, B cells, and natural killer cells to PD-L1 overexpressed on certain cancer cells. So far, several studies report that camrelizumab combined with chemotherapy has been used in adjuvant and second-line therapy of locally advanced ESCC and exhibits a promising antitumor efficacy [17, 18]. However, the clinical value of neoadjuvant camrelizumab combined with chemotherapy in locally advanced ESCC is still unclear. In present study, patients receiving neoadjuvant camrelizumab combined with chemotherapy reached an ORR of 81.3% and DCR of 100% in locally advanced ESCC patients. Possible explanations might be that (1) camrelizumab blocked the binding of PD-1 to PD-L1; promoted the activation of monocytes, T cells, B cells, dendritic cells, and tumor-infiltrating lymphocytes to exert tumor suppressive function; and furthermore contributed to better therapeutic response [23]. (2) Besides, chemotherapy might help to activate tumor-specific T cells by promoting tumor antigen presentation and by destroying immunosuppressive factors and further promotes the antitumor efficacy of camrelizumab [24]. Hence, synergic effect of cytotoxic chemotherapy plus camrelizumab probably results in a favorable therapeutic response.
Regarding pathological response, previous studies exhibited that an R0 resection rate was 96.3% and a pCR rate was 33.3% in patients with locally advanced ESCC receiving neoadjuvant nivolumab and pembrolizumab plus chemotherapy [9]. Likewise, in another study, pCR rate was 34.21%; meanwhile, R0 resection rate was 92.11% in locally advanced ESCC patients receiving neoadjuvant camrelizumab and pembrolizumab plus chemotherapy [25]. In this present study, 5 (31.3%), 6 (37.5%), 3 (18.8%), and 2 (12.5%) patients reached TRG 0, TRG 1, TRG 2, and TRG 3, respectively; 31.3% patients had pCR; meanwhile, 93.8% patients were witnessed with R0 resection. Possible explanations were as follows: (1) Camrelizumab may promote the cytotoxic effect of chemotherapy in tumor cells through elevating the chemosensitivity in patients with locally advanced ESCC; therefore, camrelizumab plus chemotherapy might present a better tumor suppressive effect than neoadjuvant chemotherapy alone, contribute to improve R0 resection rate, pCR rate, and tumor regression effect [26]. (2) Camrelizumab suppresses tumor angiogenesis and stimulates antineoplastic activities, which could be facilitated by chemotherapy [27]. Hence, the neoadjuvant camrelizumab plus chemotherapy might elevate the antitumor efficiency and further achieve a favorable R0 resection rate, pCR rate, and tumor regression effect in patients with locally advanced ESCC.
So far, few studies investigate the survival profile of neoadjuvant PD-1 inhibitors plus chemotherapy in patients with locally advanced ESCC. In the present study, it was revealed that with neoadjuvant camrelizumab plus chemotherapy treatment, the mean PFS and OS were 18.3 months and 19.2 months with a 1-year PFS of 83% and 1-year OS of 90.9%, respectively. These findings might be explained by the following hypotheses: (1) Camrelizumab may promote the cytotoxic effect of chemotherapy on tumor cells through increasing the chemosensitivity. Furthermore, camrelizumab combined with chemotherapy might present a better tumor suppressing efficacy particularly in less immunogenic, chemo-sensitive tumors [26]. (2) Neoadjuvant camrelizumab combined with chemotherapy achieves a good treatment response and satisfactory pCR rate, as discussed above, which may contribute to improve prognosis in present research.
Concerning camrelizumab for the treatment of gastric and esophagus cancer, the major adverse events were discovered in the skin, gastrointestinal tract, endocrine glands, liver, and lung; meanwhile, most of them were mild and manageable [28]. As to the current study, the major adverse events include white blood cell (WBC) reduction, neutrophil decreased, RCCEP, nausea, or vomiting, most of which were mild and controllable. It is indicated that neoadjuvant camrelizumab combined with chemotherapy was well-tolerant as the neoadjuvant therapy of locally advanced ESCC.
Limitations were as followed: (1) The relatively small sample size of eligible patients might potentially reduce the reliability of the results. (2) As this was a single-arm study, it lacks a control group; hence, further randomized, controlled study would be desirable. (3) Follow-up period was relatively short, and further study with a longer follow-up period was necessary to determine the long-term efficacy of neoadjuvant camrelizumab combined with chemotherapy in patients with locally advanced ESCC. (4) The economic evaluation of camrelizumab combined with chemotherapy was neglected in the current study, which needs further exploration. (5) In the present study, patients’ selection bias might exist to affect the survival profile.
Conclusions
Collectively, neoadjuvant camrelizumab plus chemotherapy exhibits good efficacy and acceptable tolerance in treating patients with locally advanced ESCC, while further validation of the efficacy in larger cohort is needed.
Acknowledgements
Not applicable.
Abbreviations
- ESCC
Esophageal squamous cell carcinoma
- TRG
Tumor regression grade
- pCR
Pathological complete remission
- PFS
Progression-free survival
- OS
Overall survival
- ORR
Objective remission rate
- DCR
Disease control rate
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed cell death ligand 1
- CT
Computed tomography
- CR
Complete remission
- PR
Partial remission
- SD
Stable disease
- IQR
Interquartile range
Authors’ contributions
XZ is responsible for the conception and design. PY gave administrative support. PY, XY, and YW provided the study materials or patients. TS, SF, and XM did the collection and assembly of data. PY and XZ carried out the data analysis and interpretation. All authors contributed in the manuscript writing. The authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available because the data are confidential patient data but are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Ethical permission for this study was obtained from the Institutional Review Board, and the written informed consents were acquired from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peng Yang and Xiao Zhou contributed equally to this work.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Cheng YF, Chen HS, Wu SC, Chen HC, Hung WH, Lin CH, et al. Esophageal squamous cell carcinoma and prognosis in Taiwan. Cancer Med. 2018;7(9):4193–4201. doi: 10.1002/cam4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Z, Wang Y, Li F, Gao J, Han B, Wang R, et al. Comparison of clinicopathological features and prognosis between adenocarcinoma of esophagogastric junction and adenocarcinoma of gastric antrum. Zhonghua Wei Chang Wai Ke Za Zhi. 2019;22(2):149–155. [PubMed] [Google Scholar]
- 4.Pasquali S, Yim G, Vohra RS, Mocellin S, Nyanhongo D, Marriott P, et al. Survival after neoadjuvant and adjuvant treatments compared to surgery alone for resectable esophageal carcinoma: a network meta-analysis. Ann Surg. 2017;265(3):481–491. doi: 10.1097/SLA.0000000000001905. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36(27):2796–2803. doi: 10.1200/JCO.2018.79.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao XF, He XT, Ji L, Xiao J, Lv J. Effects of neoadjuvant radiochemotherapy on pathological staging and prognosis for locally advanced esophageal squamous cell carcinoma. Dis Esophagus. 2009;22(6):477–481. doi: 10.1111/j.1442-2050.2008.00910.x. [DOI] [PubMed] [Google Scholar]
- 7.de Gouw D, Klarenbeek BR, Driessen M, Bouwense SAW, van Workum F, Futterer JJ, et al. Detecting pathological complete response in esophageal cancer after neoadjuvant therapy based on imaging techniques: a diagnostic systematic review and meta-analysis. J Thorac Oncol. 2019;14(7):1156–1171. doi: 10.1016/j.jtho.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(7):855–883. doi: 10.6004/jnccn.2019.0033. [DOI] [PubMed] [Google Scholar]
- 9.Shen D, Chen Q, Wu J, Li J, Tao K, Jiang Y. The safety and efficacy of neoadjuvant PD-1 inhibitor with chemotherapy for locally advanced esophageal squamous cell carcinoma. J Gastrointest Oncol. 2021;12(1):1–10. doi: 10.21037/jgo-20-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukuoka E, Yamashita K, Tanaka T, Sawada R, Sugita Y, Arimoto A, et al. Neoadjuvant chemotherapy increases PD-L1 expression and CD8(+) tumor-infiltrating lymphocytes in esophageal squamous cell carcinoma. Anticancer Res. 2019;39(8):4539–4548. doi: 10.21873/anticanres.13631. [DOI] [PubMed] [Google Scholar]
- 11.Hu L, Kong Z, Meng Q, Wang J, Zhou M, Yu J, et al. The safety and efficacy of apatinib treatment in addition to concurrent chemoradiotherapy in patients with nonoperative locally advanced esophageal squamous cell carcinoma. Med Sci Monit. 2020;26:e927221. doi: 10.12659/MSM.927221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto S, Kato K, Daiko H, Kojima T, Hara H, Abe T, et al. Feasibility study of nivolumab as neoadjuvant chemotherapy for locally esophageal carcinoma: FRONTiER (JCOG1804E) Future Oncol. 2020;16(19):1351–1357. doi: 10.2217/fon-2020-0189. [DOI] [PubMed] [Google Scholar]
- 13.Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10(3):727–742. [PMC free article] [PubMed] [Google Scholar]
- 14.Geisler AN, Phillips GS, Barrios DM, Wu J, Leung DYM, Moy AP, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83(5):1255–1268. doi: 10.1016/j.jaad.2020.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Y, Wang Z, Yan C, Yan M, Hou Z, Zheng R, et al. Protocol for a randomized controlled trial of perioperative S-1 plus oxaliplatin combined with apatinib and camrelizumab in patients with resectable, locally advanced gastric or gastroesophageal junction adenocarcinoma. Ann Transl Med. 2020;8(24):1684. doi: 10.21037/atm-20-7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Wang C, Li X, Dong L, Yang Q, Chen M, et al. Improved clinical outcome in a randomized phase II study of anti-PD-1 camrelizumab plus decitabine in relapsed/refractory Hodgkin lymphoma. J Immunother Cancer. 2021;9(4):e002347. doi: 10.1136/jitc-2021-002347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang B, Qi L, Wang X, Xu J, Liu Y, Mu L, et al. Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first-line treatment of advanced esophageal squamous cell carcinoma. Cancer Commun. 2020;40(12):711–720. doi: 10.1002/cac2.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Yan C, Gao X, Li X, Cao F, Zhao G, et al. Safety and feasibility of radiotherapy plus camrelizumab for locally advanced esophageal squamous cell carcinoma. Oncologist. 2021;26(7):e1110–e1e24. doi: 10.1002/onco.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang W. Interpretation of 2017 National Comprehensive Cancer Network (NCCN) guidelines for the diagnosis and treatment of esophageal squamous cell carcinoma through the new TNM staging of esophageal carcinoma (eighth edition) by the Union for International Cancer Control (UICC) and the American Cancer Commission (AJCC) Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20(10):1122–1126. [PubMed] [Google Scholar]
- 20.Schwartz LH, Litiere S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-Update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::AID-CNCR2820731105>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 22.Chirieac LR, Swisher SG, Ajani JA, Komaki RR, Correa AM, Morris JS, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103(7):1347–1355. doi: 10.1002/cncr.20916. [DOI] [PubMed] [Google Scholar]
- 23.Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 Checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G, Emens LA. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother. 2013;62(2):203–216. doi: 10.1007/s00262-012-1388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Z, Zheng Q, Chen H, Xiang J, Hu H, Li H, et al. Efficacy and safety of neoadjuvant chemotherapy and immunotherapy in locally resectable advanced esophageal squamous cell carcinoma. J Thorac Dis. 2021;13(6):3518–3528. doi: 10.21037/jtd-21-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chowdhury PS, Chamoto K, Honjo T. Combination therapy strategies for improving PD-1 blockade efficacy: a new era in cancer immunotherapy. J Intern Med. 2018;283(2):110–120. doi: 10.1111/joim.12708. [DOI] [PubMed] [Google Scholar]
- 27.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21(1):15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D, Zhang S, Ding P, Zhao Y, Zhang X, Zhao Q. Immune-related adverse events mimicking Behcet’s disease in a gastric cancer patient following camrelizumab treatment. Iran J Immunol. 2020;17(2):167–171. doi: 10.22034/iji.2020.85507.1717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available because the data are confidential patient data but are available from the corresponding author upon reasonable request.