Abstract
In complement-driven thrombotic microangiopathies, failure to regulate complement activation leads to end-organ damage. The modified Ham (mHam) test measures complement-mediated killing of a nucleated cell in vitro but lacks a confirmatory assay and reliable positive controls. We demonstrate that C5b-9 accumulation on the surface of TF1 PIGAnull cells correlates with cell killing in the mHam. We also show that Sialidase treatment of cells or addition of Shiga toxin 1 to human serum serve as a more reliable positive control for the mHam than cobra venom factor or lipopolysaccharide. Simultaneously performing the mHam and measuring C5b-9 accumulation either in GVB++ or GVB0 MgEGTA buffer with the addition of complement pathway specific inhibitors (anti-C5 antibody or a factor D inhibitor, ACH-145951) can be used to localize defects in complement regulation. As more targeted complement inhibitors become available, these assays may aid in the selection of personalized treatments for patients with complement-mediated diseases.
Keywords: Complement, Modified HAM assay, Sialidase, Shiga toxin 1, Atypical hemolytic uremic syndrome, Complement inhibitors
1. Introduction
Failure to regulate complement activation on cell surfaces primarily drives the pathogenesis of complement-mediated thrombotic microangiopathies (TMA) [1,2]. Atypical hemolytic uremic syndrome (aHUS) is characterized by microangiopathic hemolytic anemia, thrombocytopenia, renal impairment, and preserved ADAMTS13 function [3,4]. Endothelial damage in aHUS is complement-mediated and up to 50% of patients harbor germline defects in complement regulatory genes or autoantibodies against complement regulatory proteins that modulate the alternative pathway of complement [5,6]. Penetrance is variable and development of clinically significant disease appears to require a supervening process. aHUS is commonly associated with a trigger, such as infection, surgery, pregnancy, cancer or autoimmunity. HELLP syndrome (hemolysis, elevated liver function tests, and low platelets) is a rare TMA that occurs in up to 0.5% of pregnancies, typically in the third trimester when complement levels are highest [7]. Similar to aHUS, up to 50% of cases harbor rare germline variants in genes that regulate the alternative pathway of complement [8,9]. Typical HUS caused by Shiga toxin-producing Escherichia coli (STEC-HUS) and post-transplant associated TMA are also thought to be complement-driven TMAs but are less well characterized [10–13].
The diagnosis of these disorders, especially aHUS, is challenging and often a diagnosis of exclusion. C5 inhibition with eculizumab is effective in treating aHUS, but uncertainty in the diagnosis coupled with the high cost of the drug often lead to the delays in treatment [14–16]. Previously, we developed the modified Ham (mHam) assay to measure complement-mediated killing of nucleated cells in vitro [17]. The assay is performed by mixing human serum with TF1 PIGAnull cells, an engineered cell line that is devoid of the downstream cell-surface complement regulators, CD55 and CD59. Non-viability (cell killing) is measured in a colorimetric assay and ≥ 20% cell killing after 2 h of incubation distinguishes aHUS from thrombotic thrombocytopenic purpura (TTP) with >95% sensitivity and specificity [17]. The mHam is also positive (>20% cell killing) in most cases of HELLP, post-transplant TMAs, catastrophic antiphospholipid antibody syndrome (CAPS), and the acute phase of STEC-HUS [8,10,11,18].
To date, there are several challenges that limit more widespread clinical use of the mHam. One such limitation is the lack of a reliable positive control. Cobra venom factor (CVF) and lipopolysaccharide (LPS) activate complement activities on cells but yield variable results in the mHam assay. Previously, serum from aHUS patients served as a positive control, however, fresh serum from patients is not always available. Secondly, a confirmatory assay to corroborate results from the mHam is needed. Additionally, there are three pathways that can lead to terminal complement activation: classical, lectin and alternative. The mHam assay detects cell death caused by all three complement pathways. In ordered to predict responses to different complement inhibitors, assays that can isolate the specific complement pathway, which is dysregulated, are required.
In the present study, we demonstrate that Sialidase (Sia) and Shiga toxin 1 (Stx1) are reliable positive controls for the mHam assay. We further find that C5b-9 deposition measured by flow cytometry on TF1 PIGAnull cells correlates with cell killing in the mHam and that a critical threshold of complement deposition on the cell surface is required to achieve cell killing in the mHam. Lastly, we describe a flow cytometry-based assay that is pathway specific and aids in the distinction of complement-driven TMAs in patient samples.
2. Materials and methods
2.1. Cells and reagents
TF1 PIGAnull cells were derived from TF1 cells as previously described [19] and cultured in RPMI 1640 supplemented with 1 ng/ml Granulocyte Macrophage Colony-Stimulating Factor (GM-CSF), 2 mM L-glutamine, 1% Penn/Strep (P/S), and 10% fetal bovine serum (FBS). Cells were maintained at a density of 500,000 cells/ml daily [17]. Gelatin veronal buffer with Ca++ and Mg++ (GVB++, cat. B102) was used to facilitate activation of all complement pathways. An alternative complement pathway-specific buffer GVB° with 10 mM MgEGTA (GVB°MgEGTA, AP Buffer) was prepared by mixing gelatin veronal buffer without Ca++ and Mg++ (GVB°, cat. B103) with 100 mM MgEGTA (MgEGTA, cat. B106) in a 9:1 ratio. Normal human serum (NHS, cat. NHS) was heated at 56°C for 30 min to inactivate complement activity (NHS (H)). All reagents were purchased from Complement Technology Inc. (Tyler, TX). The cell proliferation reagent WST-1 (cat. 11644807001, Roche, Switzerland) was diluted in RPMI 1640 without phenol red (cat. 32404014, Gibco) at a ratio of 1:9 before use.
2.2. Complement activators (proteins) and inhibitors
2.2.1. Complement mediated activators (proteins)
α2–3,6,8 Neuraminidase, also called Sialidase (Sia), was purchased from New England Bio Labs (Cat. P0720L, Ipswich MA). Shiga toxin 1 (Stx1) was purchased from Sigma-Aldrich (Cat. SML0562, St. Louis, MO). Cobra venom factor (CVF) was purchased from Complement Technology Inc. (Cat. CVF, Tyler, TX) and Quidel (Cat. A600, Athens, OH). Lipopolysaccharide (LPS) was purchased from Cell Signaling Technology (Cat. 14001, Boston, MA) and Sigma-Aldrich (Cat. L5293, St. Louis, MO).
2.2.2. Complement activity inhibitors
Factor D inhibitor (ACH-145951) is a small molecule synthesized and provided by Achillion Pharmaceuticals (New Haven, CT). Factor D inhibitor is specific for the alternative pathway of complement. Anti-C5 monoclonal antibody (Cat. Clone M5G1.1), which binds to the terminal complement component 5 (C5) and prevents membrane attack complex (MAC) formation, was a gift from Alexion Pharmaceuticals (New Haven, CT). C5-deplete (C5-Dpl) serum was purchased from complement technology, Inc. (Cat. A320, Tyler, TX).
2.3. The modified Ham (mHam) assay to evaluate complement activity by complement activation proteins
Complement activators (Stx1, CVF, and LPS) were added to NHS and pre-incubated at 37 °C for 15 min. Stx1, CVF, and LPS were prepared at concentrations of 0.625, 1.25, 2.5, 5, and 10 μg/ml. Next, TF1 PIGAnull cells were plated in 96-well round-bottom plates at a density of 6700 cells per well in 80 μl GVB++. 20 μl of NHS with and without the complement activators was added to each well.
For Sia samples, the cells were plated at a density of 6700 cells per well in 80 μl GVB++ and Sia was added at doses of 6.25, 12.5, 25, 50, and 100 units/ml. Samples were incubated at 37°C for 15 min, and then 20 μl NHS was added per well. Sia was added to cells rather than serum since it acts on the sialic acid linkages that bind factor (FH) to the cell surface and thus acts on the cell surface rather than in the soluble phase.
The reaction plate was incubated at 37°C for 45 min with shaking. After incubation, the plate was centrifuged at 1900 rpm (844 × g) for 3 min, the supernatant was removed, and cell pellets were washed once in 1× PBS. 100 μl WST-1 solution (WST-1 diluted in RPMI 1640, at a ratio of 1:9) per well was added to cells and incubated at 37 °C for 2 h.
Absorbance was measured in a plate reader (ELX808, BioTeK, Winooski, VT) at 450 nm with a reference wavelength at 630 nm. The measured sample absorbance was normalized by subtracting the absorbance of WST-1 solution alone (blank). The percentage of live cells was calculated as the ratio of sample absorbance (A450–630nM) over the absorbance of heat-inactivated normal human serum [NHS (H)] multiplied by 100. (Formula: % live cells = [(sample A450–630nM – blank A450–630nM) / (NHS (H) A450–630nM – blank A450–630nM) × 100]). NHS (H) represents 100% viable cells, as heat inactivates complement activity in the serum. The percentage of non-viable cells (cell killing) was calculated by subtracting the percentage of live cells from 100. Based on prior experiments, we established ≥20% non-viable cells as a positive test [17]. Normal human serum (NHS) was used as a negative control (≤10% cell killing). Samples were performed in triplicate and each assay was performed three times.
2.4. Detection of complement activity by complement activation proteins in flow cytometry analysis
C3c, C4d, and C5b-9 deposition on TF1 PIGAnull cells was analyzed by flow cytometry. Cells were seeded in a 96-well V-bottom plate at a concentration of 1.0 to 1.2 × 105 cells per well. Addition of different buffers at a volume of 80 μl was used to elicit specific complement pathway activation. GVB++ buffer (pH 7.4) was used to allow activation of all complement pathways, whereas GVB° MgEGTA (AP) buffer (pH 6.4) allows for alternative pathway activation only. This was followed by addition of 20 μl (20%) NHS or acidified NHS (aNHS) pre-incubated with Stx1 (final concentration from 2.5 μg/ml to 10 μg/ml). This was then incubated at 37 °C for 15 min with shaking.
For Sia treatment, the cells were seeded in 80 μl of either GVB++ or AP buffer and incubated with Sia (final dose from 6.3 to 100 units/ml) for 15 min. After incubation for 15 min, 20 μl (20%) NHS or 20 μl aNHS was added to the cells and incubated at 37 °C for 15 min with shaking. NHS with 5 mM of ethylenediaminetetraacetic acid (EDTA) was used as the negative control.
After incubating the cells for 15 min, FACS buffer supplemented with 1% BSA and 15 mM EDTA in PBS was added to stop the reaction. Cell pellets were separated by centrifugation at 1900 rpm (844 × g) for 3 min at room temperature (RT), washed once with PBS, and stained with C5b-9 monoclonal primary antibody (Cat. sc-58935, Santa Cruz Biotechnology, Inc., 1:50 dilution). The plate was incubated for 30 min on ice. After incubation, the cells were washed with PBS. Alexa 647 conjugated secondary antibody (Cat. Ab172325, Abcam, 1:500 dilution) and Alexa 488-conjugated anti-C3c antibody (Cat. 4212, Abcam, 1:100 dilution) were added to the cells and incubated for another 30 min on ice. The cells were also labeled with anti-C4d biotinylated monoclonal antibody (Cat. A704, Quidel, dilution at 1:50) and PE-Streptavidin (Cat. 554061, BD Pharmingen, 1:500 dilution). After staining, cells were washed and transferred to FACS tubes. FACS was performed using a BD FACScalibur. Unstained cells and isotype control were used to set the gate and compensation. Ten thousand events were recorded and analyzed using FlowJo software version 10.5.3 (FlowJo Inc).
2.5. Inhibition of complement activity using factor D inhibitor and anti-C5 antibody
Inhibition of complement activation by a small molecule factor D inhibitor (ACH-145951, Achillion, Inc) and anti-C5 antibody (anti-C5Ab) was assessed on TF1 PIGAnull cells. NHS was incubated with either ACH-145951 diluted in dimethyl sulfoxide (DMSO) (final concentrations ranging from 0.3 μM to 1.0 μM) or anti-C5Ab diluted in GVB++ (final concentrations ranging from 25 μg/ml to 100 μg/ml) on ice for 5–15 min. NHS with and without inhibitors was subsequently incubated with 10 μg/ml of Stx1, CVF, or LPS at 37 °C for 15 min. For the mHam assay, the NHS mixture was added to the cells (6700 cells/well) in 80 μl GVB++. For FACS, the NHS was added to the cells (1.0 to 1.2 × 105 cells/well) in either GVB++ buffer for all pathway activation or AP buffer for alternative pathway activation. For the Sia treatment, cells were pre-incubated with Sia (50 U/ml) for 15 min before exposure to NHS with and without complement inhibitors. NHS acted as the negative control and NHS with either Sia or Stx1 served as positive controls for all experiments. For the mHam assay, the NHS negative control is established as less than 10% non-viable cells and positive controls are greater than 20% non-viable cells.
2.6. C3b loading assay
C3b loading was performed by adding C3, factor B, and factor D to TF1 PIGAnull cells for up to 4 cycles. The reaction buffer was prepared by mixing 50 mM NiCl2 and GVB° buffer (cat. B103) in a ratio of 1:50. Ten million TF1 PIGAnull cells were collected and washed twice in PBS before loading. For cycle one, the cells were resuspended in 77 μl reaction buffer, and 17.5 μg C3 (Cat. A113), 4 μg factor B (Cat. A135), and 0.1 μg factor D (Cat. A136) were added. The loading tube was kept at RT for 20 min.
The cells loaded with C3b were washed with GVB° three times and two million cells were collected in a new tube for cycle one. The remaining cells were reloaded with 2 μg factor B and 0.1 μg factor D for 3 min at RT and then treated with 0.25 M EDTA and 17.5 μg C3 for 15 min at 37 °C. After each loading cycle, two million cells were collected, and the remaining cells were again loaded with factors B and D and treated with EDTA and C3 as above. After four consecutive loading cycles, all cells collected were washed in GVB° buffer and the mHam and FACS assays were performed.
2.7. Detection of complement activity in patient serum
We used samples from three patients with aHUS and one with APS patients, who were diagnosed according to standardized criteria as previously published [17,18]. Whole blood and serum from patients were collected in EDTA and serum separator (SST) tubes. This study was approved by the Institutional Review Board and conducted in accordance with the Declaration of Helsinki. All patients in the study provided written, informed consent.
The mHam assay was performed by incubating 20 μl patient serum with TF1 PIGAnull cells in 80 μl GVB++ at 37 °C for 45 min, as described in section 2.3. Heat-inactivated patient serum was used to calculate percent viability for the patient sample. C3c, C4d, and C5b-9 deposition on TF1 PIGAnull cells was determined by incubating 20 μl patient serum with cells (120,000 cells/well) in 80 μl GVB++ or AP buffer at 37 °C for 15 min, as described in section 2.4.
2.8. Statistics
All data were analyzed by One-way ANOVA and multiple comparisons using Prism (Graphad Software, San Diego, CA).
3. Results
3.1. C3b accumulation on TF1 PIGAnull cells leads to C5b-9 deposition and cell killing with a threshold effect
We hypothesized that C5b-9 accumulation on the cell surface would correlate with cell killing detected in the mHam assay. To test this, TF1 PIGAnull cells were loaded with C3b and C5b-9 deposition was quantified by flow cytometry. Cell viability in the mHam was measured after adding normal human serum.
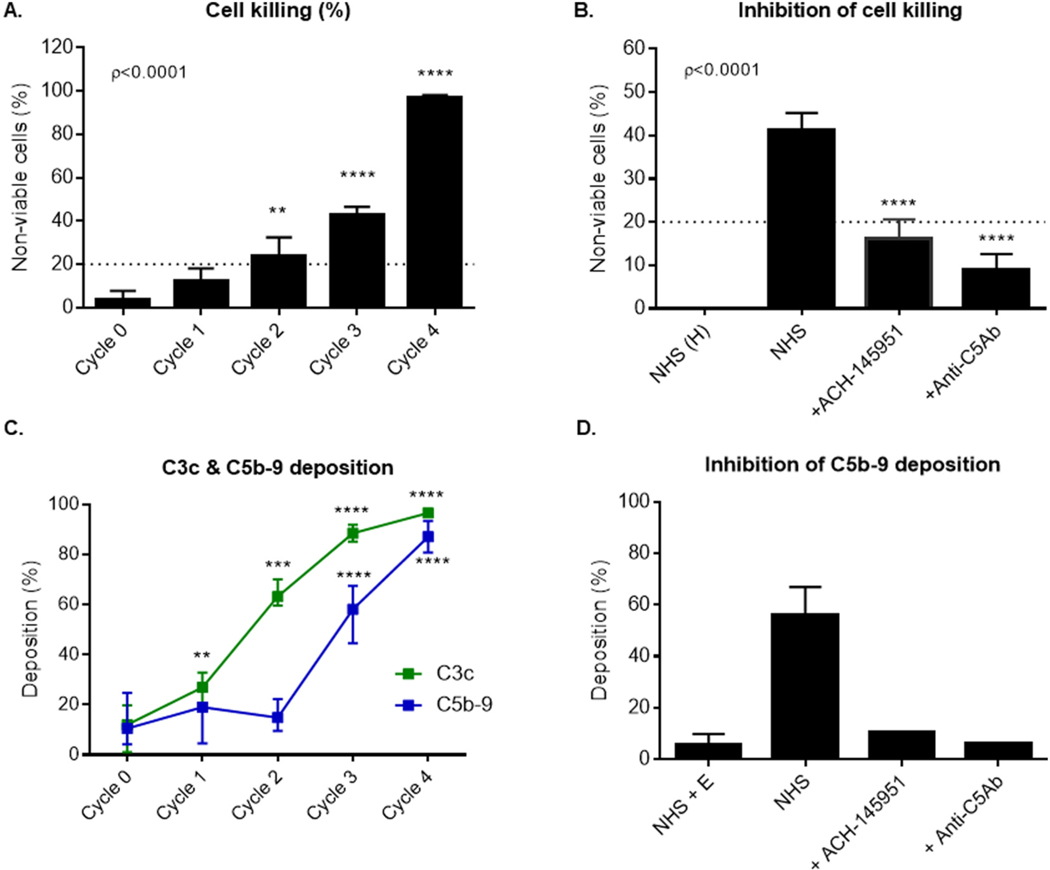
Cells loaded with one or two cycles of C3b demonstrated minimal C5b-9 deposition (Fig. 1C and Supplementary Appendix Fig. 1) and minimal cell killing (<20%) in the mHam assay (Fig. 1A). After three cycles of C3b loading, a marked increase in C5b-9 deposition (Fig. 1C and Supplementary Appendix Fig. 1) was observed, which correlated with elevated complement-mediated cell killing in the mHam assay (Fig. 1A). Both complement inhibitors, anti-C5Ab and factor D inhibitor (ACH-145951) blocked cell killing (Fig. 1B). Addition of anti-C5Ab or ACH-145951 after three cycles of C3b loading reduced C5b-9 deposition to the level of the negative control (NHS + EDTA) (Fig. 1D). Together, these data show that complement-mediated cell killing in the mHam correlates with accumulation of C5b-9 deposition on the cell surface detected by flow cytometry and that a certain threshold of C3 deposition on the cell surface is needed to activate cell killing in the mHam.
Fig. 1.
C3b accumulation on the cell surface leads to increased cell killing and C5b-9 deposition, which is blocked by complement inhibitors. (A) TF1 PIGAnull cells loaded with one to four cycles of C3b were incubated in 20% human serum and cell killing was measured using the mHam. (B) Cells loaded with 3 cycles of C3b were incubated with NHS alone, NHS and ACH-145951 (0.3 μM), or NHS and anti-C5 antibody (50 μg/ml). Cell killing was measured using the mHam. (C) Cells loaded with one to four cycles of C3b were incubated in 20% human serum. C3c and C5b-9 deposition was measure by flow cytometry. (D) Cells loaded with 3 cycles of C3b were incubated with NHS, NHS and ACH-145951 (0.3 μM), or NHS and anti-C5Ab (50 μg/ml). Data shown as mean ± SEM of triplicate wells and representative of three independent experiments. Normal Human Serum (NHS), Heat Inactivated Normal Human Serum (NHS(H)), factor D inhibitor (ACH-145951), anti-C5 antibody (anti-C5Ab), Normal Human Serum and EDTA (NHS + E), ** p < 0.01, ****p < 0.0001.
3.2. Sialidase and Shiga toxin 1 activate complement-mediated cell death in the mHam assay
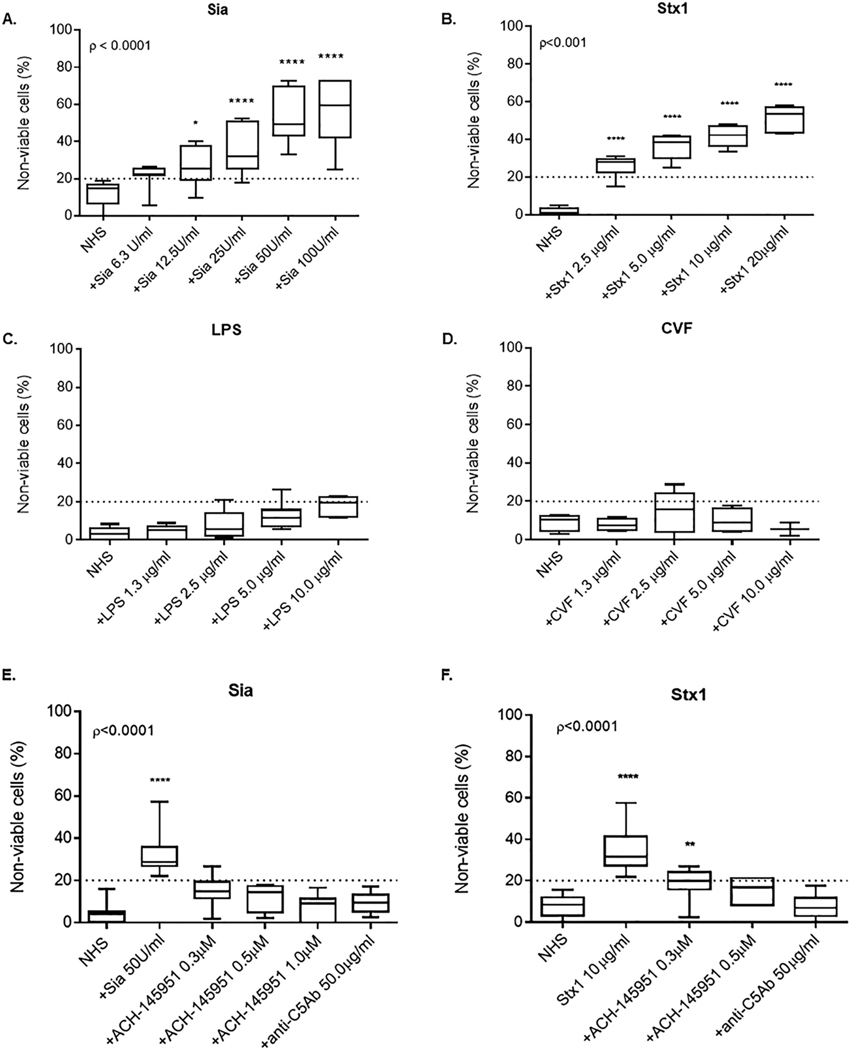
Historically, we have used sera from a patient with an established diagnosis of aHUS as the positive control for the mHam, but fresh sera is not readily available. To establish a commercially-available positive control, we measured cell killing in the mHam as an indicator of terminal complement activation using the following: (1) Sialidase (Sia), which cleaves sialic acid residues on the cell surface thereby diminishing the inhibitory effect of factor H on the alternative pathway; (2) Shiga toxin 1 (Stx1), which activates complement through all three complement pathways [20,21]; (3) cobra venom factor (CVF), which binds to factor B and upregulates the formation of C3b and C5b [22]; and (4) lipopolysaccharide (LPS), which is known to induce C3 convertase [23]. Addition of Sia and Stx1 led to a marked increase in cell killing in the mHam (Fig. 2A and B). Sia added to cells at a dose of 50 units/ml increased complement-mediated cell killing by more than 3-fold compared to NHS alone (Fig. 2A). Stx1 at a concentration of 10 μg/ml increased cell death by more than 4-fold compared to NHS alone (Fig. 2B). Increasing doses of CVF and LPS produced minimal cell death (< 20% cell killing) (Fig. 2C and D).
Fig. 2.
Sialidase and Shiga toxin type 1 induce complement-mediated cell killing in a dose-dependent manner that is blocked by addition of ACH-145951 and anti-C5 antibody.
Cell killing measured by the mHam with the addition of (A) Sialidase, (B) Shiga toxin 1, (C) Lipopolysaccharide and (D) Cobra venom factor at escalating doses. (E) Sia-induced cell killing in the mHam in the presence of ACH-145951 and anti-C5Ab. (F) Stx1-induced cell killing in the presence of ACH-145951 and anti-C5Ab. Data shown as mean ± SEM of triplicate wells and are representative of three independent experiments. Normal Human Serum (NHS), Sialidase (Sia), Shiga toxin 1 (Stx1), Lipopolysaccharide (LPS), Cobra venom factor (CVF), factor D inhibitor (ACH-145951), anti-C5 antibody (anti-C5Ab) * p < 0.05, ** p < 0.01, ****p < 0.0001.
When NHS was incubated with increasing concentrations of anti-C5Ab or ACH-145951, the complement-mediated killing induced by Sia was inhibited in a dose-dependent manner (Fig. 2E). ACH-145951 at a concentration of 0.3 μM reduced cell killing of Sia-treated cells to the level of NHS alone (Fig. 2E). This observation is consistent with the mechanism of Sia-induced complement activation through upregulation of the alternative pathway.
In contrast, Stx1 induced cell killing was more effectively blocked by anti-C5Ab than ACH-145951 (Fig. 2F). Complement inhibition with ACH-145951 on Stx1-treated cells was concentration dependent (Fig. 2F). However, 0.3 μM ACH-145951 only partially blocked Stx1-induced cell killing, whereas anti-C5Ab blocked this completely, suggesting additional complement activation by Stx1 occurs outside of the alternative pathway.
3.3. Sia promotes C5b-9 deposition predominantly via the alternative pathway
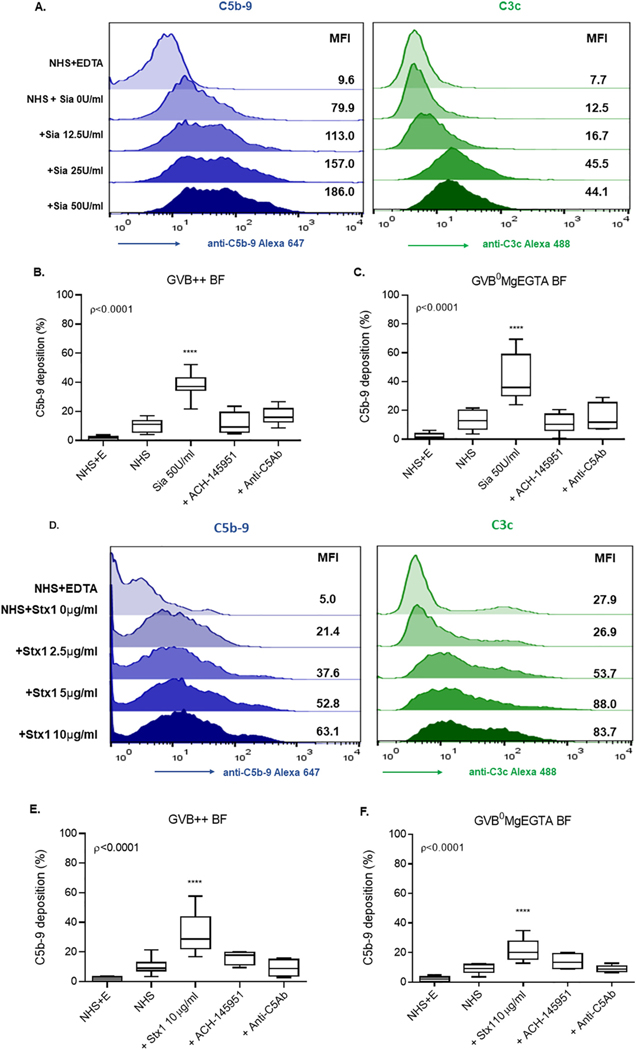
We next evaluated C5b-9 and C3c deposition on TF1 PIGAnull cells after Sia treatment in two different buffers: all complement pathway buffer (GVB++, GVB with Ca++ and Mg++) and alternative pathway (AP) specific buffer (GVB° plus MgEGTA). When treating cells in GVB++ buffer, Sia triggered high C5b-9 deposition on cell surfaces in a dose-dependent manner (Fig. 3A) that correlated with cell killing in the mHam (Fig. 2A). Treating cells with 50 units/ml of Sia induced more than 2-fold higher C5b-9 deposition (Fig. 3A and Supplementary Appendix Fig. 2) and 4-fold higher C3c deposition than untreated cells. Anti-C5Ab (50 μg/ml) and ACH-145951 (0.3 μM) inhibited C5b-9 deposition on Sia-treated cells to the level of untreated cells (Fig. 3B).
Fig. 3.
Sia and Stx1 induce C5b-9 and C3c deposition on the cell surface. (A) Histogram overlays showing C5b-9 (blue) and C3c (green) deposition following Sia treatment of cells in GVB++ buffer. C5b-9 deposition on Sia treated cells in the presence of ACH-145951 (1.0 μM) and anti-C5Ab (50 μg/ml) in (B) GVB++ or (C) GVB°MgEGTA. (D) Histogram overlays showing C5b-9 (blue) and C3c (green) deposition following addition of Stx1 in GVB++. C5b-9 deposition in the presence of ACH-145951 (1.0 μM) and anti-C5Ab (50 μg/ml) in (F)GVB++ and (E) GVB° MgEGTA. Data shown as mean ± SEM of triplicate wells and are representative of three independent experiments. Normal Human Serum (NHS), Sialidase (Sia), Normal Human Serum and EDTA (NHS+E), Factor D Inhibitor (ACH-145951), Anti-C5 Antibody (anti-C5Ab), Shiga toxin 1 (Stx1), ****p < 0.0001.
Treating cells with Sia in AP specific buffer resulted in C5b-9 deposition comparable to that observed in the GVB++ buffer (Fig. 3C and Supplementary Appendix Fig. 2). ACH-145951 (0.3 μM) completely inhibited C5b-9 deposition and reduced it to the level of the NHS control (Fig. 3C). However, complete inhibition with anti-C5Ab was achieved only at high concentrations (100 μg/ml). Additionally, Sia did not significantly increase C4d deposition on cell surface (Supplementary Appendix Fig. 3). C4d is activated by the classical and lectin pathways. Taken together, these results indicate that complement activation by Sia occurs mainly via the alternative pathway and is more effectively blocked by ACH-145951 than anti-C5Ab.
3.4. Stx1 induces C5b-9 deposition via both the alternative and classical pathways
Dose response experiments showed increased C5b-9 and C3c deposition on TF1 PIGAnull cells treated with increasing concentrations of Stx1 (Fig. 3D), which correlated with cell killing with the mHam (Fig. 2B). In GVB++, NHS with 10 μg/ml Stx1 doubled C5b-9 and C3c deposition on cells versus NHS alone. Addition of 50 μg/ml anti-C5 antibody reduced C5b-9 deposition induced by Stx1 to a level similar to NHS alone. ACH-145951 led to a partial reduction in C5b-9 deposition. Even at a high concentration (1.0 μM) of the ACH-145951, C5b-9 deposition remained higher than NHS alone (Fig. 3E).
Incubating NHS with Stx1 in the presence of GVB°MgEGTA (AP) buffer reduced Stx1 induced C5b − 9 deposition by 50% as compared to GVB++(Fig. 3F and Supplementary Appendix Fig. 2). Addition of anti-C5 antibody (50 μg/ml) reduced Stx1-induced C5b-9 deposition to the level of NHS alone. ACH-145951 also inhibited C5b-9 deposition (Fig. 3F). In addition, NHS with 10 μg/ml of Stx1 induced a 4-fold increase in C4d deposition on cell surfaces versus NHS alone in GVB++ buffer (Supplementary Appendix Fig. 3). These data demonstrate that the alternative pathway is only partially involved in complement activation by Stx1, and Stx1-induced complement activity is more effectively inhibited by an anti-C5 antibody than a factor D inhibitor (ACH-145951) (Fig. 3E and F).
3.5. aHUS and APS serum lead to increased C5b-9 deposition on TF1 PIGAnull cells that correlates with a positive mHam
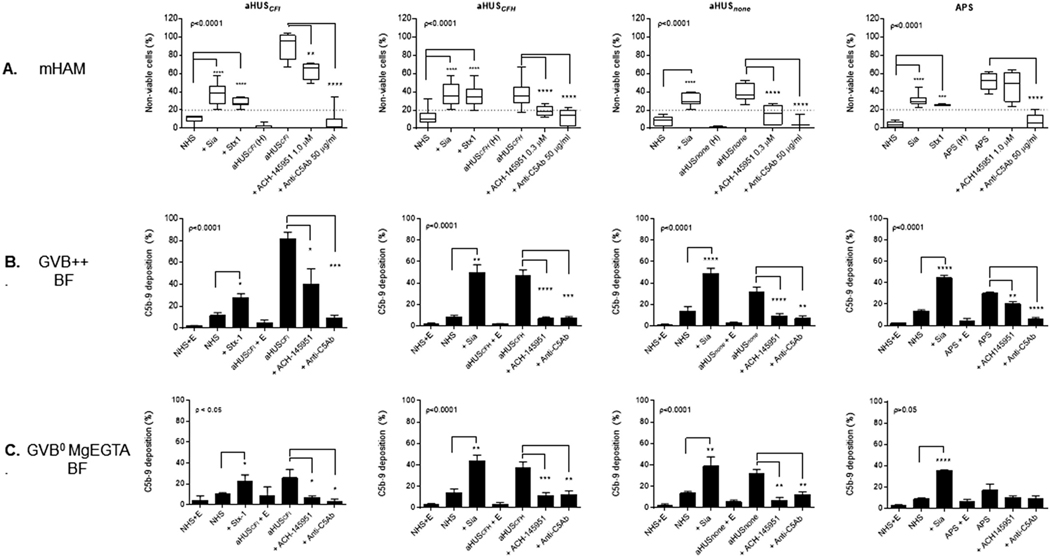
We previously demonstrated that aHUS serum consistently led to positive results (≥ 20% cell killing) in the mHam [17]. We hypothesized that serum from aHUS patients would lead to increased C5b-9 deposition on TF1 PIGAnull cells, which would correlate with high cell killing in the mHam. Moreover, detecting C5b-9 deposition using different buffers that allow for selective activation of different complement pathways may be used to determine whether complement-mediated cell killing in the mHam is alternative pathway specific.
To test this hypothesis, we incubated TF1 PIGAnull cells with 20% normal human serum (NHS only as a negative control and NHS with Sia or Stx1 as a positive control) or 20% serum from aHUS patients (aHUSCFI, aHUSCFH, aHUSnone). The mHam and flow cytometry analysis were performed simultaneously. All 3 aHUS patients (two with known germline mutations in complement) (Table 1) had positive mHam results that were blocked by adding anti-C5Ab (Fig. 4A). ACH-145951 reduced complement-mediated cell killing to near the level of the NHS baseline in the patient with no known complement gene mutations (aHUSnone) and the patient with a complement factor H mutation (aHUSCFH). Addition of ACH-145951 did not inhibit cell killing in the patient with a complement factor I mutation (aHUSCFI), which interferes with the regulation of all complement pathways (Fig. 4A).
Table 1.
Clinical characteristics of atypical hemolytic uremic syndrome and antiphospholipid antibody syndrome patients
| Patient | Diagnosis | Complement Mutations | Age (yr) | Sex | Eculizumab Treatment | Creatinine (mg/dL) |
LDH (U/L) |
PLT (K/cu mm) |
mHam (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At Diagnosis | Post Ecu | At Diagnosis | Post Ecu | At Diagnosis | Post Ecu | |||||||
| aHUSCFH | aHUS | CFH1 Gln950His heterozygous, del (CFHR1-CHFR3) homozygous | 53 | F | yes | 1.6 | 0.9 | 2505 | 180 | 9 | 327 | 43.2 ± 0.125 |
| aHUSnone | aHUS | None detected | 27 | F | yes | 6.3 | 1.2 | 2820 | 195 | 62 | 251 | 36.1 ± 0.099 |
| aHUSCFI | aHUS | CFI Ile416Leu heterozygous, Thr300Ala homozygous | 53 | F | yes | 4.8 | On iHD |
3459 | 231 | 5 | N/A | 90.3 ± 13.18 |
| APS | APS | Not performed | 43 | M | no | 11.2 | N/A | 168 | N/A | 61 | N/A | 51.7 ± 9.36 |
LDH Lactate Dehydrogenase, PLT Platelet count, Ecu Eculizumab. iHD intermittent hemodialysis.
Fig. 4.
Complement-mediated cell killing in the mHam correlates with increased C5b-9 deposition in aHUS and APS patients. (A) Cell killing was measured by the mHam assay in sera from four patients, three patients with aHUS and mutations in complement factor I (CFI), complement factor H (CFH) and no identified germline mutations (none), respectively, and one patient with APS in the presence and absence of ACH-145951 or Anti-C5Ab. C5b-9 deposition on PIGAnull TF1 cells after incubation with the four patients’ sera was analyzed by flow cytometry in (B) GVB++ and (C) GVB0MgEGTA in presence and absence of ACH-145951 or Anti-C5Ab. Data shown as mean ± SEM of triplicate wells and are representative of three independent experiments. Normal Human Serum (NHS), Sialidase (Sia), Shiga toxin 1 (Stx1), heat inactived serum from atypical Hemolytic Uremic Syndrome (aHUS (H)), Atypical Hemolytic Uremic Syndrome (aHUS) serum, factor D inhibitor (ACH-145951), anti-C5 antibody (anti-C5Ab), Antiphospholipid Antibody Syndrome (APS) serum, APS serum and EDTA (APS + E), Normal Human Serum and EDTA (NHS + E).* p < 0.05, **p < 0.001, *** p < 0.001, **** p < 0.0001.
Consistent with our hypothesis, C5b-9 deposition was markedly increased on cells treated with aHUS sera, with C5b-9 levels similar to or higher than that observed in the positive controls (Fig. 4B). Anti-C5Ab and ACH-145951 effectively blocked C5b-9 deposition in aHUSCFH and aHUSnone. C5b-9 deposition with the serum from the aHUSCFI patient was completely blocked after the addition of 50 μg/ml anti-C5 antibody but only 50% reduced with addition of 1.0 μM ACH-145951 (Fig. 4B). The inhibitory effect of anti-C5 antibody and ACH-145951 on C5b-9 deposition reflected the pattern of cell killing in the mHam.
Treating cells with serum from aHUSnone and aHUSCFH in the alternative pathway-specific (AP) buffer induced levels of C5b-9 deposition similar to those observed in the all complement pathway buffer (Fig. 4C), whereas C5b-9 deposition triggered by aHUSCFI serum was reduced by greater than 50% (Fig. 4C). In all three cases, blocking factor D with ACH-145951 resulted in complete inhibition of C5b-9 deposition on cell surface in AP buffer (Fig. 4C).
Finally, we studied a patient with antiphospholipid antibody syndrome (APS) (Fig. 4). Similar to our recent publication [18], the positive mHam assay with the patient’s serum was inhibited by anti-C5Ab inhibition but not ACH-145951 (factor D inhibitor) suggesting complement-mediated cell killing in APS is not alternative pathway driven (Fig. 4A). This was confirmed by showing that the majority of the C5b-9 deposition on the cells occurred in GVB++ rather than GVB°MgEGTA (AP) (Fig. 4B and C). Collectively, the patient data confirms that C5b-9 accumulation in GVB++ correlates with killing in the mHam and using pathway specific buffers and inhibitors provides insight into the genes/pathways driving disease.
4. Discussion
Several new drugs that block the complement cascade at different levels have been developed to treat complementopathies [2]. Functional assays that distinguish the specific pathway driving complement dysregulation in nucleated human cells are needed both as an adjunct to clinical diagnoses and to assess the potential effects of these drugs. The mHam represents such a functional assay but several challenges have limited its broader applicability to this point. Here, we demonstrate that Sialidase and Shiga toxin 1 are reproducible positive controls for the mHam assay. Further, C5b-9 deposition on TF1 PIGAnull cells correlates with complement-dependent killing in the mHam and can serve as a validation for this functional assay. Lastly, complement inhibitors and pathway specific buffers can provide insights into the specific pathway (s) of complement activation driving a patient’s disease.
Simultaneously performing the mHam and measuring C5b-9 accumulation on TF1 PIGAnull cells in the presence and absence of pathway specific inhibitors (inhibitors of C5 and FD) provides insight into which complement pathways are being activated. For example, Sialidase activates the alternative pathway by disabling factor H binding [25,26]; Shiga toxin appears to activate the classical and/or lectin pathways since cell killing was attenuated in the alternative (AP) pathway buffer and was not completely inhibited by the factor D specific inhibitor ACH-145951. We successfully used the mHam and flow cytometry to elucidate the complement pathway being triggered using clinical unknowns (Fig. 4). All three aHUS patients studied had a positive mHam, which was blocked by C5 inhibition. Factor D inhibition was effective in blocking cell killing in the patient with a CFH mutation and the patient without detected mutations in complement genes but only partly inhibited cell killing in the patient with a CFI mutation. CFI is important for regulation of both the AP and classical/lectin C5 convertases. Similarly, the APS patient serum displayed strong killing in the mHam that was inhibited by a C5 inhibitor but not a factor D inhibitor, suggesting greater activation of the classical/lectin pathways. In addition to localizing which complement pathway(s) is driving disease, these assays provide insight into which complement inhibitor would provide the best therapeutic efficacy.
The mHam has several advantages as a tool to diagnosis complement-driven diseases. Other functional complement assays often use non-human (rabbit or sheep) erythrocytes. In red cell assays, such as the HAM test, a single membrane attack complex (MAC) can lyse an erythrocyte [24], whereas scores of MACs are needed to kill nucleated cells used in the mHam. Nonfunctional assays alone, such as monitoring C3c or C5b-9 deposition, can be misleading since there is a threshold effect where C3 fragments must accumulate to a critical level before correlative cell killing is observed (Fig. 1A and C). For the mHam to become positive, cells need to have a high density of C5b-9 on their surface (Fig. 1 and Supplementary Appendix Fig. 1). This specificity has allowed us to prospectively employ the mHam to uncover a role of complement in disorders, such as HELLP and CAPS. This role is further supported by the over-representation of rare germline variants in genes that regulate complement in these diseases [9,18]. This threshold effect may also in part explain why patients with genetic defects in complement regulation only experience clinical manifestations with some triggers and not others.
We have addressed several of the challenges in translating the mHam into clinical practice, such as the lack of a reliable positive control and confirmatory assay. Notwithstanding these advances, the need to continuously maintain the TF1 PIGAnull in log-phase for reproducibility represents a continued challenge.
In summary, we have developed a companion assay to the mHam, which provides insight into the specific complement pathway that is dysregulated in complement-driven disorders, such as aHUS, HELLP, and APS/CAPS. As a role for complement is identified in a growing number of diseases and multiple complement inhibitors become available in clinical practice, novel assays that can identify patients likely to respond to complement-directed therapies and predict responses to specific complement inhibitors are needed.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) [R01HL133113 2016] and NIH [K08 HL138142].
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2020.108616.
References
- [1].Merrill SA, Brodsky RA, Complement-driven anemia: more than just paroxysmal nocturnal hemoglobinuria, Hematology Am Soc Hematol Educ Program 2018 (1) (2018) 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gavriilaki E, Brodsky RA, Complementopathies and precision medicine, J. Clin. Invest 130 (5) (2020) 2152–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nester CM, Barbour T, de Cordoba SR, Dragon-Durey MA, Fremeaux-Bacchi V, Goodship TH, Kavanagh D, Noris M, Pickering M, Sanchez-Corral P, Skerka C, Zipfel P, Smith RJ, Atypical aHUS: state of the art, Mol. Immunol 67 (1) (2015) 31–42. [DOI] [PubMed] [Google Scholar]
- [4].Baines AC, Brodsky RA, Complementopathies, Blood Rev. 31 (4) (2017) 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nester CM, Barbour T, de Cordoba SR, Dragon-Durey MA, Fremeaux-Bacchi V, Goodship THJ, Kavanagh D, Noris M, Pickering M, Sanchez-Corral P, Skerka C, Zipfel P, Smith RJH, Atypical aHUS: state of the art, Mol. Immunol 67 (1) (2015) 31–42. [DOI] [PubMed] [Google Scholar]
- [6].Noris M, Remuzzi G, Glomerular diseases dependent on complement activation, including atypical hemolytic uremic syndrome, Membranoproliferative glomerulonephritis, and C3 Glomerulopathy: Core curriculum 2015, Am. J. Kidney Dis 66 (2) (2015) 359–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vaught AJ, Braunstein E, Chaturvedi S, Blakemore K, Brodsky RA, A review of the alternative pathway of complement and its relation to HELLP syndrome: is it time to consider HELLP syndrome a disease of the alternative pathway, J. Matern. Fetal Neonatal Med (2020) 1–9. [DOI] [PubMed]
- [8].Vaught AJ, Gavriilaki E, Hueppchen N, Blakemore K, Yuan X, Seifert SM, York S, Brodsky RA, Direct evidence of complement activation in HELLP syndrome: a link to atypical hemolytic uremic syndrome, Exp. Hematol 44 (5) (2016) 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vaught AJ, Braunstein EM, Jasem J, Yuan X, Makhlin I, Eloundou S, Baines AC, Merrill SA, Chaturvedi S, Blakemore K, Sperati CJ, Brodsky RA, Germline mutations in the alternative pathway of complement predispose to HELLP syndrome, JCI Insight 3 (6) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brady TM, Pruette C, Loeffler LF, Weidemann D, Strouse JJ, Gavriilaki E, Brodsky RA, Typical Hus: evidence of acute phase complement activation from a daycare outbreak, J Clin Exp Nephrol 1 (2) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rotz SJ, Luebbering N, Dixon BP, Gavriilaki E, Brodsky RA, Dandoy CE, Jodele S, Davies SM, In vitro evidence of complement activation in transplantation-associated thrombotic microangiopathy, Blood Adv 1 (20) (2017) 1632–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gavriilaki E, Sakellari I, Anagnostopoulos A, Brodsky RA, Transplant-associated thrombotic microangiopathy: opening Pandora’s box, Bone Marrow Transplant. 52 (10) (2017) 1355–1360. [DOI] [PubMed] [Google Scholar]
- [13].Noris M, Mescia F, Remuzzi G, STEC-HUS, atypical HUS and TTP are all diseases of complement activation, Nat Rev Nephrol 8 (11) (2012) 622–633. [DOI] [PubMed] [Google Scholar]
- [14].Merrill SA, Brittingham ZD, Yuan X, Moliterno AR, Sperati CJ, Brodsky RA, Eculizumab cessation in atypical hemolytic uremic syndrome, Blood 130 (3) (2017) 368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C C. Bingham, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nürnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C, Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome, New Engl. J. Med 368 (23) (2013) 2169–2181. [DOI] [PubMed] [Google Scholar]
- [16].Noris M, Galbusera M, Gastoldi S, Macor P, Banterla F, Bresin E, Tripodo C, Bettoni S, Donadelli R, Valoti E, Tedesco F, Amore A, Coppo R, Ruggenenti P, Gotti E, Remuzzi G, Dynamics of complement activation in aHUS and how to monitor eculizumab therapy, Blood 124 (11) (2014) 1715–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gavriilaki E, Yuan X, Ye Z, Ambinder AJ, Shanbhag SP, Streiff MB, Kickler TS, Moliterno AR, Sperati CJ, Brodsky RA, Modified ham test for atypical hemolytic uremic syndrome, Blood 125 (23) (2015) 3637–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chaturvedi S, Braunstein EM, Yuan X, Yu J, Alexander A, Chen H, Gavriilaki E, Alluri R, Streiff MB, Petri M, Crowther MA, McCrae KR, Brodsky RA, Complement activity and complement regulatory gene mutations are associated with thrombosis in APS and CAPS, Blood 135 (4) (2020) 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Savage WJ, Barber JP, Mukhina GL, Hu R, Chen G, Matsui W, Thoburn C, Hess AD, Cheng L, Jones RJ, Brodsky RA, Glycosylphosphatidylinositol-anchored protein deficiency confers resistance to apoptosis in PNH, Exp. Hematol 37 (1) (2009) 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ozaki M, Kang Y, Tan YS, Pavlov VI, Liu B, Boyle DC, Kushak RI, Skjoedt MO, Grabowski EF, Taira Y, Stahl GL, Human mannose-binding lectin inhibitor prevents Shiga toxin-induced renal injury, Kidney Int. 90 (4) (2016) 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Orth D, Khan AB, Naim A, Grif K, Brockmeyer J, Karch H, Joannidis M, Clark SJ, Day AJ, Fidanzi S, Stoiber H, Dierich MP, Zimmerhackl LB, Wurzner R, Shiga toxin activates complement and binds factor H: evidence for an active role of complement in hemolytic uremic syndrome, J. Immunol 182 (10) (2009) 6394–6400. [DOI] [PubMed] [Google Scholar]
- [22].Vogel CW, Smith CA, Muller-Eberhard HJ, Cobra venom factor: structural homology with the third component of human complement, J. Immunol 133 (6) (1984) 3235–3241. [PubMed] [Google Scholar]
- [23].Nygren H, Dahlen G, Moller G, Bacterial lipopolysaccharides bind selectively to lymphocytes from lipopolysaccharide high-responder mouse strains, Scand. J. Immunol 10 (6) (1979) 555–561. [DOI] [PubMed] [Google Scholar]
- [24].Meri S, Morgan BP, Davies A, Daniels RH, Olavesen MG, Waldmann H, Lachmann PJ, Human protectin (CD59), an 18,000–20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers, IMMUNOLOGY 71 (1) (1990) 1–9. [PMC free article] [PubMed] [Google Scholar]
- [25].Hyvarinen S, Meri S, Jokiranta TS, Disturbed sialic acid recognition on endothelial cells and platelets in complement attack causes atypical hemolytic uremic syndrome, Blood 127 (22) (2016) 2701–2710. [DOI] [PubMed] [Google Scholar]
- [26].Harder MJ, Anliker M, Ho-Nchsmann B, Simmet T, Huber-Lang M, Schrezenmeier H, Ricklin D, Lambris JD, Barlow PN, Schmidt CQ, Comparative analysis of novel complement-targeted inhibitors, MiniFH, and the natural regulators factor H and factor H-like protein 1 reveal functional determinants of complement regulation, J. Immunol 196 (2) (2016) 866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.