Abstract
Introduction
The COVID-19 pandemic caused delays in breast cancer management forcing clinicians to potentially alter treatment recommendations. This study compared breast cancer stage at diagnosis and rates of neoadjuvant therapy among women presenting to our institution before and during COVID-19.
Methods
Retrospective chart review of patients with a new breast cancer diagnosis from March 2020–August 2020 (during-COVID-19) were compared with March 2019–August 2019 (pre-COVID-19). We compared stage at diagnosis, clinical/demographic features, and neoadjuvant therapy use between the time periods.
Results
A total of 573 patients included: 376 pre-COVID-19, 197 during-COVID-19. Method of cancer detection was by imaging in 66% versus 63% and by physical findings/symptoms in 34% versus 37% of patients comparing pre-COVID-19 to during-COVID-19, p = 0.47. Overall clinical prognostic stage did not differ significantly (p = 0.39) between the time periods, nor did cM1 disease (2% in each period); 23% pre-COVID-19 and 27% during-COVID-19 presented with cN+ disease (p = 0.38).
Neoadjuvant therapy use was significantly higher during-COVID-19 (39%) versus pre-COVID-19 (29%, p = 0.02) driven by increased neoadjuvant endocrine therapy (NET) use (7% to 16%, p = 0.002), whereas neoadjuvant chemotherapy use did not change (22% vs. 23%, p = 0.72). In HR+/HER2− disease, NET use increased from 10% pre-COVID-19 to 23% during-COVID-19 (p = 0.001) with a significant increase in stage I patients (7 to 22%, p < 0.001) and nonsignificant increases in stage II (18 to 23%, p = 0.63) and stage III (9 to 29%, p = 0.29).
Conclusions
Breast cancer stage at diagnosis did not differ significantly during-COVID-19 compared with pre-COVID-19. More patients during-COVID-19 were treated with NET, which was significantly increased in stage I HR+/HER2− disease.
The COVID-19 pandemic changed the way breast cancer was managed worldwide in an effort to conserve limited resources and triage patients according to medical necessity. This led to the temporary suspension of breast cancer screening programs and closures of operating rooms for elective procedures.1–3 Due to these restrictions, surgery was delayed for some patients with breast cancer, forcing clinicians to adjust their management strategies and look toward other therapeutic options.3–6 In Italy, for example, it was estimated 8125 breast cancer diagnoses would be missed by a 3-month suspension of breast-cancer screening and an additional 1300 patients would have delayed diagnosis or treatment due to COVID-19-related anxiety about seeking medical treatment during that period.1 In the United Kingdom, decrease in screening and prioritizing diagnostic interventions to the most critical is predicted to lead to an estimated 4.9–5.2% increase in breast cancer-related deaths (281–344 patients) over a 5-year period.2 Changes in management have been seen in the United States with the American College of Surgeons and other professional societies publishing consensus guidelines to assist with breast cancer patient triage and management during the pandemic.3–5 Patients with early-stage hormone receptor-positive (HR+) disease who traditionally undergo surgery first, for example, were being started on neoadjuvant endocrine therapy (NET) initially, while operating rooms were closed, to act as bridge therapy prior to surgery.6 Now that screening programs have restarted and operating rooms are again performing elective surgeries, traditional methods of breast cancer detection and management have returned, but the true extent of the pandemic on cancer diagnosis and outcomes is not yet known.
At Mayo Clinic Rochester, our breast clinic sees all patients with new breast cancer diagnoses both internally and externally referred. We hypothesized that patients were presenting with more advanced breast cancers since the COVID-19 pandemic began in March 2020 than those who presented prior to the pandemic, and that rates of neoadjuvant therapy use also increased. In the current study, we evaluated clinical and demographic features, breast cancer stage at diagnosis, and rates of neoadjuvant therapy among women presenting to our institution with newly diagnosed breast cancer before and during the COVID-19 pandemic. This information will help to highlight the importance of routine screening programs and continued access to multidisciplinary breast cancer care.
Methods
With Institutional Review Board approval, we performed a retrospective chart review of all patients presenting to our institution with a new breast cancer diagnosis from March 2020 to August 2020 (during-COVID-19) and compared them to patients presenting in the same time frame the prior year: March 2019 to August 2019 (pre-COVID-19). Patients presenting with recurrent ipsilateral breast cancer were excluded. We compared clinical and demographic features and breast cancer stage at diagnosis between the during-COVID-19 and pre-COVID-19 time periods and evaluated use of neoadjuvant therapy. Patients presenting with bilateral breast cancer were characterized according to the side presenting with the highest stage of disease.
Hormone receptor status was classified as positive with estrogen receptor (ER) and/or progesterone receptor (PR) > 1% nuclear staining. ER and PR negative were < 1% nuclear staining. HER2 positive (HER2+) was characterized by 3+ on IHC or positive by FISH. HER2 negative (HER2−) was 0 or 1+ on IHC or negative by FISH as per the College of American Pathologist guidelines.7 Clinical stage was determined based on tumor size nodal and metastatic burden using TNM staging as per AJCC 7th edition clinical anatomic staging8 and also according to AJCC 8th edition clinical prognostic staging with the 8th edition used for primary analysis.9 At our institution, axillary ultrasound is performed on all patients with invasive breast cancer. Any lymph node that has abnormal morphology or cortical thickness >3 mm undergoes fine needle aspiration (FNA). cN0 includes patients with normal lymph nodes on axillary ultrasound or who are FNA-negative. Variables collected from the chart review included method of cancer detection—either by imaging or physical examination—patient demographics, including race and ethnicity, distance traveled to our institution based on zip code, and insurance type. Surgical interventions also were collected, including breast operation (lumpectomy, unilateral mastectomy with or without reconstruction, and bilateral mastectomy with or without reconstruction) as well as axillary operation (no axillary staging, SLNB only, or ALND).
The pre-COVID-19 and during-COVID-19 time periods were compared by using Cochran-Armitage trend tests for ordinal variables, such as clinical prognostic stage, chi-square tests for nominal variables, and Wilcoxon rank-sum tests for continuous variables. A sensitivity analysis was conducted among the subgroup of local patients, defined as those residing within 100 miles of our facility, to confirm that our findings were not impacted by referral bias. Analysis was performed by using SAS (Version 9.4, SAS Institute Inc., Cary, NC). P values < 0.05 were considered statistically significant.
Results
A total of 573 patients were identified: 376 in the pre-COVID-19 period and 197 in the during-COVID-19 period. Sixteen patients (3%) had bilateral breast cancer (4% vs. 2%, pre- and during COVID, p = 0.34). Patient median age was 62 years pre-COVID-19 and 60 years during COVID-19 (p = 0.55). Method of cancer detection was by imaging in 66% of patients and by physical findings or symptoms in 34% pre-COVID-19, compared with 63% and 37%, respectively during COVID-19, p = 0.47. Demographic and clinical characteristics summarized for the two time periods are shown in Table 1.
Table 1.
Demographic and clinical characteristics compared between pre-COVID and during-COVID time periods
| Pre-COVID (N = 376) |
During-COVID (N = 197) |
Total (N = 573) |
p value | |
|---|---|---|---|---|
| Age (at visit) | 0.5491 | |||
| Mean (SD) | 60.5 (13.5) | 59.8 (13.6) | 60.3 (13.5) | |
| Median | 61.5 | 60.0 | 61.0 | |
| Q1, Q3 | 52.0, 69.5 | 50.0, 70.0 | 51.0, 70.0 | |
| Range | (18.0-99.0) | (26.0-87.0) | (18.0-99.0) | |
| Gender | 0.5552 | |||
| F | 373 (99.2%) | 197 (100.0%) | 570 (99.5%) | |
| M | 3 (0.8%) | 0 (0.0%) | 3 (0.5%) | |
| Race | 0.0302 | |||
| White | 351 (93.4%) | 183 (92.9%) | 534 (93.2%) | |
| Black or African American | 7 (1.9%) | 1 (0.5%) | 8 (1.4%) | |
| Asian | 6 (1.6%) | 7 (3.6%) | 13 (2.3%) | |
| American Indian/Alaskan Native | 0 (0.0%) | 3 (1.5%) | 3 (0.5%) | |
| Unknown or not reported | 12 (3.2%) | 3 (1.5%) | 15 (2.6%) | |
| Ethnicity | 0.8353 | |||
| Not Hispanic or Latino | 360 (95.7%) | 187 (94.9%) | 547 (95.5%) | |
| Hispanic or Latino | 10 (2.7%) | 7 (3.6%) | 17 (3.0%) | |
| Unknown or not reported | 6 (1.6%) | 3 (1.5%) | 9 (1.6%) | |
| Distance from facility (miles) | 0.0051 | |||
| N | 368 | 197 | 565 | |
| Mean (SD) | 215.3 (320.0) | 152.7 (207.5) | 193.5 (287.2) | |
| Median | 86.7 | 67.3 | 77.4 | |
| Q1, Q3 | 45.8, 261.7 | 20.9, 197.8 | 35.3, 248.6 | |
| Range | (1.9-2530.1) | (1.9-1225.4) | (1.9-2530.1) | |
| Distance within 100 miles | 0.0193 | |||
| Missing | 8 | 0 | 8 | |
| ≥100 miles | 178 (48.4%) | 75 (38.1%) | 253 (44.8%) | |
| <100 miles | 190 (51.6%) | 122 (61.9%) | 312 (55.2%) | |
| Did patient live in-state? | 0.0943 | |||
| Missing | 8 | 0 | 8 | |
| No | 167 (45.4%) | 75 (38.1%) | 242 (42.8%) | |
| Yes | 201 (54.6%) | 122 (61.9%) | 323 (57.2%) | |
| Did patient live in MN or surrounding 4 states? | 0.0763 | |||
| Missing | 8 | 0 | 8 | |
| No | 83 (22.6%) | 32 (16.2%) | 115 (20.4%) | |
| Yes | 285 (77.4%) | 165 (83.8%) | 450 (79.6%) | |
| Insurance type | 0.2713 | |||
| Missing | 3 | 1 | 4 | |
| Employee | 29 (7.8%) | 21 (10.7%) | 50 (8.8%) | |
| Govt | 123 (33.0%) | 54 (27.6%) | 177 (31.1%) | |
| Private | 221 (59.2%) | 121 (61.7%) | 342 (60.1%) | |
| Method of detection | 0.4723 | |||
| Missing | 11 | 4 | 15 | |
| Imaging detected | 240 (65.8%) | 121 (62.7%) | 361 (64.7%) | |
| Palpable abnormality/symptoms | 125 (34.2%) | 72 (37.3%) | 197 (35.3%) | |
| Bilateral cancer | 0.3393 | |||
| No | 363 (96.5%) | 193 (98.0%) | 556 (97.0%) | |
| Yes | 13 (3.5%) | 4 (2.0%) | 17 (3.0%) | |
| Clinical T category | 0.0874 | |||
| cTis | 68 (18.1%) | 29 (14.7%) | 97 (16.9%) | |
| cT1 | 182 (48.4%) | 92 (46.7%) | 274 (47.8%) | |
| cT2 | 88 (23.4%) | 48 (24.4%) | 136 (23.7%) | |
| cT3 | 28 (7.4%) | 19 (9.6%) | 47 (8.2%) | |
| cT4 | 10 (2.7%) | 9 (4.6%) | 19 (3.3%) | |
| Clinical N category | 0.3124 | |||
| cN0 | 305 (81.1%) | 152 (77.2%) | 457 (79.8%) | |
| cN1 | 53 (14.1%) | 34 (17.3%) | 87 (15.2%) | |
| cN2 | 7 (1.9%) | 3 (1.5%) | 10 (1.7%) | |
| cN3 | 11 (2.9%) | 8 (4.1%) | 19 (3.3%) | |
| Clinical node-positive | 0.2633 | |||
| No | 305 (81.1%) | 152 (77.2%) | 457 (79.8%) | |
| Yes | 71 (18.9%) | 45 (22.8%) | 116 (20.2%) | |
| Clinical M category | >0.992 | |||
| cM0 | 367 (97.6%) | 193 (98.0%) | 560 (97.7%) | |
| cM1 | 9 (2.4%) | 4 (2.0%) | 13 (2.3%) | |
| AJCC 7th edition clinical anatomic stage | 0.1234 | |||
| 0 | 68 (18.1%) | 28 (14.2%) | 96 (16.8%) | |
| I | 170 (45.2%) | 86 (43.7%) | 256 (44.7%) | |
| II | 99 (26.3%) | 55 (27.9%) | 154 (26.9%) | |
| III | 30 (8.0%) | 24 (12.2%) | 54 (9.4%) | |
| IV | 9 (2.4%) | 4 (2.0%) | 13 (2.3%) | |
| AJCC 8th edition clinical prognostic stage | 0.2334 | |||
| 0 | 68 (18.1%) | 28 (14.2%) | 96 (16.8%) | |
| I | 211 (56.1%) | 112 (56.9%) | 323 (56.4%) | |
| II | 65 (17.3%) | 35 (17.8%) | 100 (17.5%) | |
| III | 23 (6.1%) | 18 (9.1%) | 41 (7.2%) | |
| IV | 9 (2.4%) | 4 (2.0%) | 13 (2.3%) | |
| Grade | 0.9184 | |||
| Missing | 2 | 1 | 3 | |
| I | 110 (29.4%) | 54 (27.6%) | 164 (28.8%) | |
| II | 161 (43.0%) | 93 (47.4%) | 254 (44.6%) | |
| III | 103 (27.5%) | 49 (25.0%) | 152 (26.7%) | |
| ER status | 0.3823 | |||
| Missing | 1 | 0 | 1 | |
| Negative | 49 (13.1%) | 31 (15.7%) | 80 (14.0%) | |
| Positive | 326 (86.9%) | 166 (84.3%) | 492 (86.0%) | |
| PR status | 0.6503 | |||
| Missing | 4 | 0 | 4 | |
| Negative | 77 (20.7%) | 44 (22.3%) | 121 (21.3%) | |
| Positive | 295 (79.3%) | 153 (77.7%) | 448 (78.7%) | |
| HER2 status (among patients with invasive breast cancer) | 0.8963 | |||
| Missing | 2 | 3 | 5 | |
| Negative | 266 (86.9%) | 145 (87.3%) | 411 (87.1%) | |
| Positive | 40 (13.1%) | 21 (13.7%) | 61 (12.9%) | |
| Biologic subtype (among patients with invasive breast cancer) | 0.9993 | |||
| Missing | 3 | 3 | 6 | |
| HR+/HER2+ | 30 (9.8%) | 16 (9.6%) | 46 (9.8%) | |
| HR+/HER2- | 234 (76.7%) | 127 (76.5%) | 361 (76.6%) | |
| HR-/HER2+ | 9 (3.0%) | 5 (3.0%) | 14 (3.0%) | |
| HR-/HER2- | 32 (10.5%) | 18 (10.8%) | 50 (10.6%) |
1Wilcoxon
2Fisher Exact
3Chi-Square
4Armitage Trend Test
Tumor biology did not vary by period of presentation. Among patients with DCIS, 94% were HR+ pre-COVID-19 and 86% during COVID-19 (p = 0.19). Comparing invasive tumors pre-COVID-19 to during COVID-19, 77% versus 77% were HR+/HER2−, 10% versus 10% were HR+/HER2+, 3% versus 3% were HR-/HER2+, and 11% versus 11% were HR−/HER2− respectively (p > 0.99; Table 1).
Overall AJCC 8th edition clinical prognostic stage did not differ significantly (p = 0.39) between the two time periods with 74% stage 0–I, 17% stage II, 6% stage III, and 2% stage IV during the pre-COVID-19 period and a similar distribution during COVID-19: 71% stage 0–I, 18% stage II, 9% stage III, 2% stage IV, although a slightly higher percentage of patients presented with stage II–IV disease during-COVID-19 versus pre-COVID-19 (29% vs. 26%, p = 0.42). Similarly, AJCC 7th edition clinical anatomic stage did not differ between the two time periods (p = 0.12; Table 1). The percent of patients with DCIS only was 18% pre-COVID-19 and 14% during COVID-19 (p = 0.24). The percent with cM1 disease also was similar at 2% and 2%, respectively, p > 0.99. Among patients with invasive stage I-III disease, cT category was 59% cT1, 29% cT2, 9% cT3, and 3% cT4 pre-COVID-19. A similar distribution was observed during COVID-19 (54%, 28%, 11%, 5%, respectively, p = 0.22), whereas 23% pre-COVID-19 and 27% during COVID-19 presented with cN+ disease (p = 0.38).
Among the initial sample of 573 patients, no surgical treatment was planned for a total of 30 (20 [5.3%] pre-COVID-19 and 10 [5.1%] during COVID-19, p = 0.90) either due to cM1 disease (n = 10), stage I-III disease (n = 12) in very elderly patients or those with significant medical comorbidities, or in the setting of stage 0 (DCIS only) disease (n = 8) if the patient opted for nonsurgical treatment. Among the DCIS only patients who opted for no surgery, all eight had HR+ DCIS and were managed with endocrine therapy as the definitive treatment; the percent of DCIS patients opting for no surgery did not differ significantly between the two time periods (10.3% vs. 3.6%, p = 0.43). Among patients undergoing primary surgery, the time from diagnosis to initial treatment pre-COVID-19 versus during COVID-19 was similar (p = 0.93).
Type of Surgery
Treatment variables are summarized for 543 surgical patients (356 pre-COVID-19, 187 during COVID-19) in Table 2. At the time of this writing, two patients remain on neoadjuvant therapy but with surgical therapy planned upon completion. When looking at the type of breast surgery patients underwent, there was no significant difference between pre-COVID-19 compared with during COVID-19: 55% versus 48% underwent breast-conserving surgery (BCS); 10% versus 15% had unilateral mastectomy without reconstruction; 7% versus 7% had bilateral mastectomy without reconstruction; 10% versus 8% underwent unilateral mastectomy with reconstruction; and 18% versus 23% had bilateral mastectomy with reconstruction (p = 0.22). In terms of axillary staging, 64% had SLN surgery only, 10% had SLN surgery and completion ALND, 7% had ALND, and 19% did not have any axillary surgery in the pre-COVID-19 period compared with 65%, 9%, 8%, and 18%, respectively in the during COVID-19 period (p = 0.95). Additionally, no significant differences were found with respect to axillary surgery within the cN0 (p = 0.81) and cN+ patients (p = 0.37) analyzed separately.
Table 2.
Treatment characteristics among breast cancer patients undergoing surgery
| Pre-COVID (N = 356) |
During-COVID (N = 187) |
Total (N = 543) |
p value | |
|---|---|---|---|---|
| Axillary surgery | 0.9501 | |||
| Missing | 0 | 2 | 2 | |
| None | 66 (18.5%) | 33 (17.8%) | 99 (18.3%) | |
| SLN only | 229 (64.3%) | 121 (65.4%) | 350 (64.7%) | |
| SLN + ALND | 37 (10.4%) | 17 (9.2%) | 54 (10.0%) | |
| ALND only | 24 (6.7%) | 14 (7.6%) | 38 (7.0%) | |
| Breast operation | 0.2211 | |||
| Missing | 0 | 2 | 2 | |
| Breast-conserving surgery | 194 (54.5%) | 89 (48.1%) | 283 (52.3%) | |
| Unilateral mastectomy | 36 (10.1%) | 27 (14.6%) | 63 (11.6%) | |
| UM+reconstruction | 36 (10.1%) | 14 (7.6%) | 50 (9.2%) | |
| Bilateral mastectomy | 26 (7.3%) | 12 (6.5%) | 38 (7.0%) | |
| BM+reconstruction | 64 (18.0%) | 43 (23.2%) | 107 (19.8%) | |
| Surgery type | 0.1581 | |||
| Missing | 0 | 2 | 2 | |
| Breast-conserving surgery | 194 (54.5%) | 89 (48.1%) | 283 (52.3%) | |
| Mastectomy | 162 (45.5%) | 96 (51.9%) | 258 (47.7%) | |
| Reconstruction (among mastectomy patients) | 0.7081 | |||
| No reconstruction | 62 (38.3%) | 39 (40.6%) | 101 (39.1%) | |
| Reconstruction | 100 (61.7%) | 57 (59.4%) | 157 (60.9%) | |
| Neoadjuvant therapy | 0.0191 | |||
| No neoadjuvant therapy | 254 (71.3%) | 115 (61.5%) | 369 (68.0%) | |
| Neoadjuvant therapy | 102 (28.7%) | 72 (38.5%) | 174 (32.0%) | |
| Neoadjuvant chemotherapy | 0.7161 | |||
| No neoadjuvant chemotherapy | 279 (78.4%) | 144 (77.0%) | 423 (77.9%) | |
| Neoadjuvant chemotherapy | 77 (21.6%) | 43 (23.0%) | 120 (22.1%) | |
| Neoadjuvant endocrine therapy | 0.0021 | |||
| No neoadjuvant endocrine therapy | 331 (93.0%) | 158 (84.5%) | 489 (90.1%) | |
| Neoadjuvant endocrine therapy | 25 (7.0%) | 29 (15.5%) | 54 (9.9%) |
1Chi-square
Neoadjuvant Therapy
Use of neoadjuvant therapy was significantly higher during-COVID-19 (39%) compared with pre-COVID-19 (29%, p = 0.02) driven by an increased use of neoadjuvant endocrine therapy (NET) from 7% pre-COVID-19 to 16% during COVID-19 (p = 0.002). Neoadjuvant chemotherapy (NAC) use remained stable at 22% pre-COVID-19 and 23% during COVID-19 (p = 0.72).
Of patients treated with NET, 48% were clinical prognostic stage I pre-COVID-19 and 69% during-COVID-19 (p = 0.12). When looking at HR+/HER2− patients specifically, use of NET significantly increased from 10% pre-COVID-19 to 23% during COVID-19 (p = 0.001). Looking at NET use by stage in these patients (Fig. 1), there was a significant increase in NET use in clinical stage I patients going from 7% pre-COVID-19 to 22% during COVID-19 (p < 0.001). Similar trends were seen in clinical stage II (18% pre-COVID-19, 23% during COVID-19, p = 0.64) and III (9% pre-COVID-19, 29% during COVID-19, p = 0.29) patients as well, although not statistically significant.
Fig. 1.
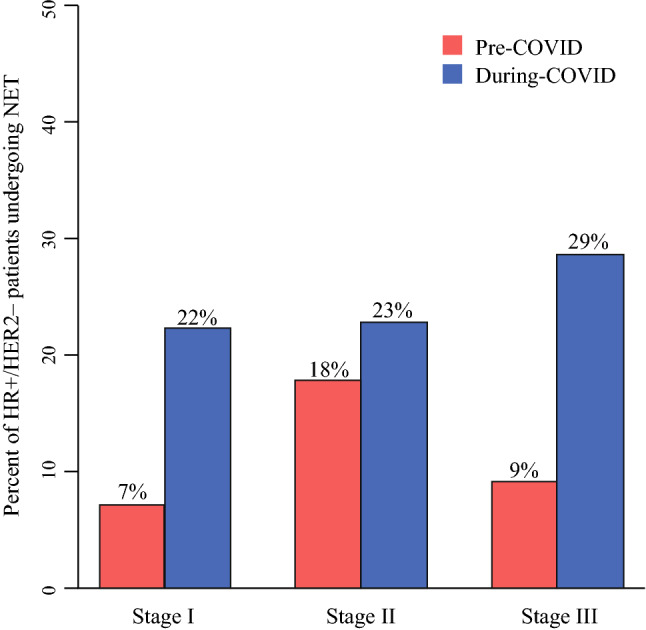
Percent of HR+/HER2- patients undergoing NET by AJCC 8th edition clinical prognotic stage
When looking at use of NAC by stage in triple-negative breast cancer (TNBC) patients, pre-COVID-19 compared with during-COVID-19, 33% versus 43% of stage I (p = 0.67), 70% versus 80% of stage II (p = 0.68), and 100% versus 100% of stage III (p = n/a) patients with TNBC underwent NAC. Similarly, no difference was seen in use of NAC by stage in HER2+ patients comparing pre-COVID-19 to during COVID-19 periods: 55% versus 56% of stage I (p = 0.96), 86% versus 100% of stage II (p = 0.25), and 100% versus 100% of stage III (p = n/a) patients with HER2+ breast cancer underwent NAC.
Impact of NET on Breast Surgery
Although more patients underwent NET during-COVID-19, this did not significantly change the distribution of breast surgery type. In the pre-COVID-19 period, 54.5% underwent BCS versus 45.5% mastectomy, whereas 48% during COVID-19 period had BCS and 52% had mastectomy overall (p = 0.16; Table 2). Among HR+/HER2− patients, however, the percent undergoing BCS was 57% pre-COVID compared with 51% during COVID (p = 0.32). When looking at surgery type in HR+/HER2− patients who received NET, 41% had BCS and 59% mastectomy pre-COVID-19 compared with 59% BCS and 41% mastectomy during COVID-19 (p = 0.20). Conversely, for those HR+/HER2− patients undergoing primary surgery, 66% had BCS and 34% mastectomy pre-COVID-19 compared with 54% BCS and 46% mastectomy during COVID-19 (p = 0.07). In looking at rates of BCS by clinical T stage in HR+/HER2− patients who received NET pre-COVID-19 compared with during COVID-19, respectively, they were 63% versus 87% in cT1 (p = 0.19), 27% versus 33% in cT2 (p = 0.77), and 33% versus 0% in cT3/cT4 (p > 0.99). The rates of BCS by clinical T stage in HR+/HER2− patients undergoing primary surgery pre-COVID-19 compared with during COVID-19, respectively, were 77% versus 68% (p = 0.21) in cT1, 30% versus 12% (p = 0.14) in cT2, and 25% versus 0% in cT3 (p = 0.31). This demonstrated non-significant increased use of BCS with NET in cT1 and cT2 patients during-COVID-19 (Fig. 2).
Fig. 2.
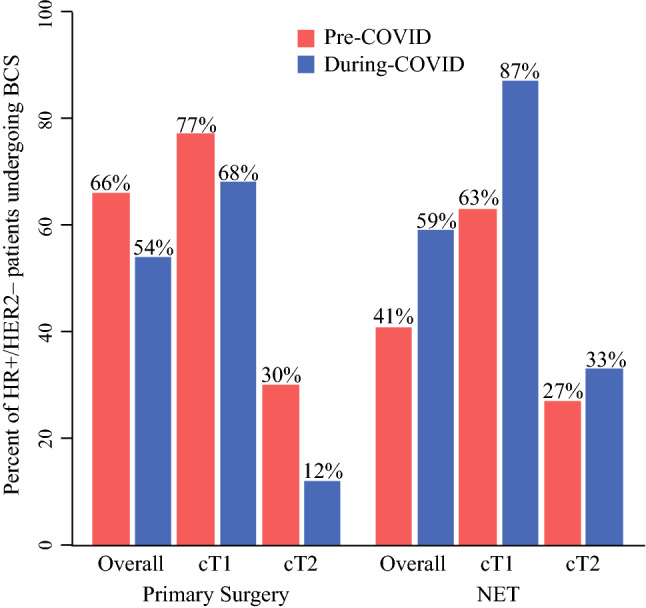
Percent of HR+/HER2- patients undergoing NET by BCS pre-COVID and during-COVID
Local Patient Subgroup Analysis
Looking just at local patients (those residing within 100 miles of our facility), results were consistent with the findings overall. Among this local patient subgroup, there was no difference between pre-COVID and during-COVID with respect to clinical prognostic stage (77.9% stage 0–I, 15.3% stage II, 6.8% stage III–IV pre-COVID compared with 77.0% stage 0–I, 15.6% stage II, 7.4% stage III–IV during COVID, p = 0.84), clinical nodal status (16.3% vs. 17.2% cN+, respectively, p = 0.84), or method of detection (69.7% vs. 63.9% imaging detected, respectively, p = 0.29). Type of breast surgery, axillary surgery, and use of reconstruction also did not differ significantly between the two time periods within this subgroup. However, as with the larger patient cohort, NET was used more frequently during-COVID (16.5%) compared with pre-COVID (4.5%), p = 0.001, among the local patient subgroup.
Discussion
The COVID-19 pandemic changed breast cancer management strategies. Reviewing our institutional data, we demonstrated a significant increase in use of neoadjuvant endocrine therapy during the COVID-19 pandemic in HR+/HER2− disease. Interestingly, we did not see a change in method of breast cancer detection or an increase in stage of breast cancer at diagnosis or a significant change in breast surgical procedures performed.
As a consequence of breast cancer screening programs being closed in the height of the pandemic, several studies predicted patients would present with more advanced disease resulting in a stage migration and possibly worse cancer outcomes.2,10,11 A recent study from a university referral center hospital in northern Italy looked at this issue. They performed a retrospective single-institution review of women diagnosed with breast cancer between May 2020 and July 2020 after they had a 2-month interruption in breast cancer screening and then fast-tracking those who had been delayed through their screening and compared them with patients diagnosed in the same period the year prior. They had 177 cancers in the 2020 group compared with 223 in the 2019 group. They did not see a significant difference in tumor biology or method of cancer detection; they did, however, see a significant increase in clinical stage III breast cancers and node-positive cancers at diagnosis as well as significantly fewer in situ cancers at diagnosis.12 Similar to their results, we did not see a difference in tumor biology or method of cancer detection. Although we predicted a similar stage migration among patients presenting during the COVID-19 pandemic, we did not see a significant difference in stage at diagnosis comparing pre-COVID-19 presentation to during-COVID-19 presentation, even though there was a nonsignificant shift with increased stage II-IV disease. It may be that longer follow-up is needed to see the predicted stage migration and the impact that lack of screening and delays in diagnosis have on stage at presentation. Further follow-up also is necessary to assess cancer outcomes in these patients.
As a known tertiary care referral center, Mayo Clinic Rochester sees patients from all over the world as well as locally. In our study, we collected data regarding distance traveled to assess the impact of the pandemic on our referral pattern and patient mix. During-COVID-19, patients did not travel as great a distance in miles as they did pre-COVID-19 with a nonsignificant trend of more patients during COVID-19 being from in-state or from the surrounding four states compared with pre-COVID-19. We also saw far fewer patients during COVID-19 than pre-COVID-19, which may be due to a myriad of reasons, including nationwide travel restrictions, potential patient fears of seeking medical attention during the pandemic, as well as patient triage protocols implemented at Mayo Clinic to conserve resources, which may have limited acceptance of certain types of outside referrals. Despite this potential difference in types of patient referrals and distances traveled for care, we did not see a difference in cancer stage at presentation. When looking at the more “local” patient population compared with those traveling greater distances, we also did not see a difference in stage at presentation.
Several societies recommended the use of neoadjuvant therapy whenever possible as a means of delaying surgical intervention during the pandemic.3–5 This guidance was implemented at our institution, and we saw an increase in use of neoadjuvant therapy overall, which was due to significantly increased utilization of NET. This was especially true of NET use in clinical stage I HR+/HER2− patients with similar though nonsignificant trends seen in clinical stage II and III HR+/HER2− patients. With more patients utilizing NET during COVID-19, a logical question is whether surgical management also changed. Although not significant, we did see a trend toward more BCS in cT1 and cT2 HR+/HER2− patients who received NET. No significant difference was seen regarding axillary surgery. Of note, we did not see a difference in breast reconstruction cases when comparing pre-COVID to during-COVID time periods. While the COVID-19 pandemic breast cancer consortium5 published recommendations to limit breast reconstruction amidst the pandemic, at our center we triaged patients based on their tumor biology and stage (similar to the published recommendations). Those patients meeting priority to proceed to the operating room were offered immediate reconstruction. The Mayo team decided that this was reasonable, because this was all within one procedure. Thus, a minimal increase in resources was utilized, and we had the operating room staff and plastic surgeons available for these cases. We successfully transitioned our tissue-expander reconstruction cases to outpatient without overnight stay, thus limiting hospital resources utilized.
Our study is limited by the inherent limitations on data collection of a retrospective chart review. We did not specifically analyze the time course of when patients presenting during COVID-19 underwent their screening mammograms. It may be that those presenting in the latter part of 2020, and therefore were due for screening amidst the height of the pandemic, present with higher stages at diagnosis than those who presented early in the pandemic and were potentially screened prior to closure of mammography centers. More extended analysis and longer follow-up is needed to assess this possibility and its impact on stage migration. Additionally, the short time course and limited follow-up period inhibits our ability to fully assess the impact of changes in management strategies with increased NET use and delays in surgical intervention during the COIVD-19 pandemic have had on cancer outcomes. Future study should focus on the long-term impact of more extensive and extended use of NET on patient management, surgical therapy, and outcome, especially in stage I patients who had not been traditionally managed this way prior to the COVID-19 pandemic.
Conclusions
Breast cancer stage at diagnosis, method of cancer detection, tumor biology, and breast and axillary operations performed did not differ significantly during COVID-19 compared with pre-COVID-19. During the COVID-19 pandemic, more patients were treated with neoadjuvant endocrine therapy. Neoadjuvant endocrine therapy use increased significantly in stage I HR+/HER2− disease.
Disclosures
Dr. Boughey receives research funding from Lilly for a clinical trial and is on a DSMB for Cairns Surgical.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vanni G, Pellicciaro M, Materazzo M, et al. Lockdown of breast cancer screening for COVID-19: possible scenario. In Vivo. 2020;34(5):3047–3053. doi: 10.21873/invivo.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maringe C, Spicer J, Morris M, et al. The impact of the COVID0-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19 Pandemic Breast Cancer Consortium 2020, COVID-19 Guidelines for Triage of Breast Cancer Patients, American College of Surgeons. Available at. https://www.facs.org/covid-19/clinical-guidance/elective-case/breast-cancer. Accessed 17 Sept 2020.
- 4.Society of Surgical Oncology. Resource for Management Options of Breast Cancer During COVID-10. https://www.surgonc.org/wp-content/uploads/2020/03/Breast-Resource-during-COVID-19-3.30.20.pdf. Accessed 19 March 2021.
- 5.Dietz JR, Moran MS, Isakoff SJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. The COVID-19 pandemic breast cancer consortium. Breast Cancer Res Treat. 2020;181(3):487–497. doi: 10.1007/s10549-020-05644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson CK, Lee MK, Baker JL, Attai DJ, DiNome ML. Taking a second look at neoadjuvant endocrine therapy for the treatment of early stage estrogen receptor positive breast cancer during the COVID-19 Outbreak. Ann Surg. 2020;272(2):e96–e97. doi: 10.1097/SLA.0000000000004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff AC, Hammond EH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med. 2018;142(11):1364–1382. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual, Seventh Edition. American Joint Committee on Cancer. 2010 [DOI] [PubMed]
- 9.Hortobagyi GN, Connolly JL, D’Orsi CJ, et al. AJCC Cancer Staging Manual. 8. Chicago, IL: The American College of Surgeons; 2018. [Google Scholar]
- 10.Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21(8):1035–1044. doi: 10.1016/S1470-2045(20)30392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patt D, Gordan L, Diaz M, et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform. 2020;4:1059–1071. doi: 10.1200/CCI.20.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toss A, Isca C, Venturelli M, et al. Two-month stop in mammographic screening significantly impacts on breast cancer stage at diagnosis and upfront treatment in COVID era. ESMO Open. 2021;6(2):100055. doi: 10.1016/j.esmoop.2021.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]


