Abstract
In dentistry, problems of craniofacial, osteochondral, periodontal tissue, nerve, pulp or endodontics injuries, and osteoarthritis need regenerative therapy. The use of stem cells in dental tissue engineering pays a lot of increased attention, but there are challenges for its clinical applications. Therefore, cell-free-based tissue engineering using exosomes isolated from stem cells is regarded an alternative approach in regenerative dentistry. However, practical use of exosome is restricted by limited secretion capability of cells. For future regenerative treatment with exosomes, efficient strategies for large-scale clinical applications are being studied, including the use of ceramics-based scaffold to enhance exosome production and secretion which can resolve limited exosome secretory from the cells when compared with the existing methods available. Indeed, more research needs to be done on these strategies going forward.
Keywords: : cell-free-based therapy, ceramics-based scaffold, exosome, MSC, regenerative dentistry, tissue engineering
Graphical abstract
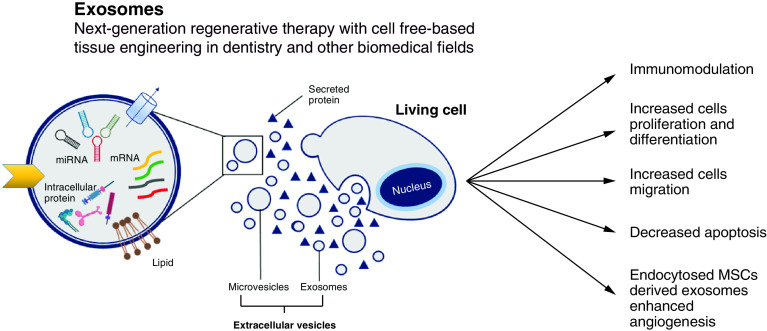
Lay abstract
Application of stem cells in dental tissue engineering such as in osteochondral, periodontal, pulp, salivary gland or nerve regeneration as well as in osteoarthritis, mucosal, skin and oral wound healing, is still problematic, especially for its large clinical scales. Therefore, cell-free-based tissue engineering using exosomes isolated from stem cells is regarded next-generation treatment in dentistry. In this study, basic understanding on the exosomes, status, the potential for regenerative therapy including challenges and strategies for the clinical applications are reviewed. Future approaches to increase production and secretion from the cells are also proposed to resolve limitation in exosomes availability.
There is a limitation of the regenerative potential in human tissue. Therefore, regenerative medicine exists to stimulate and induce healing and regeneration of human tissue or organ. In regenerative medicine, tissue engineering is considered an extremely important area involving the use of materials, cells and to a large extent, signaling molecules. Tissue engineering approach has the goal to understand tissue function and enable tissue or organ on the body to be made de novo. To achieve very important long-range objective of tissue engineering, research in many areas with collective interdisciplinary views are required.
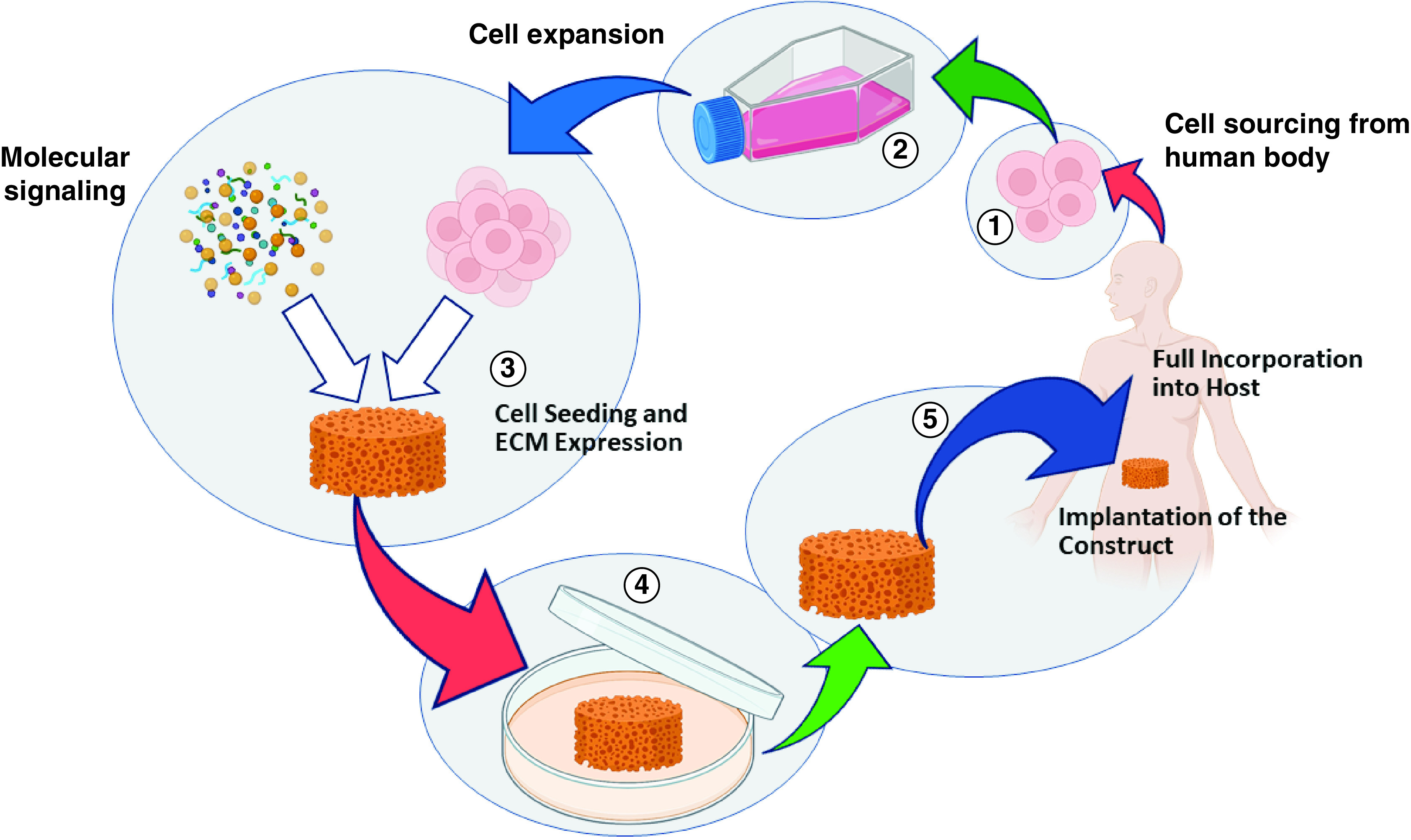
Based on tissue engineering paradigm, materials are necessary to design then provide proper scaffold to support the constructive remodeling of injured, damaged or missing tissues or organ. Scaffold can be engineered to have specific structural, mechanical, physical and chemical properties that closely approximate those of extracellular matrix of the tissue replaced. The scaffold should facilitate the attachment, migration, proliferation, differentiation and three-dimensional spatial organization of the cell population required for structural and functional replacement of the targeted tissue or organ [1–3]. Any scaffold materials will be subjected to in vivo remodeling which covers the process of host response to the scaffold materials, the degradation and replacement of scaffold by new host tissue, and the organization and differentiation of the new host tissue in relationship to surrounding structures to fully incorporate into the host toward tissue regeneration (Figure 1). The in vivo remodeling is influenced by several factors such as blood supply, pH, concentration of oxygen and carbon dioxide, mechanical stressors and host–scaffold interface [4,5].
Figure 1. . Tissue engineering paradigm with the scaffold as the central construct to provide 3D spatial for cells to attach, growth as well as organize structural and functional replacement of the tissue or organ.
The use of cells in tissue engineering attracted scientists to pay a lot of attention to stem cells, especially mesenchymal stem cells (MSCs), because their defining properties make them an ideal candidate to cure diseases. In fact, embryonic and fetal stem cells have the greatest potential to differentiate into different cell types, but their application is limited due to ethical issues and the danger of unlimited and uncontrolled cells division. The use of stem or progenitor cells have been expanded widely for treating many types of diseases in the framework of tissue engineering. It was known from previous studies that MSCs can stimulate regeneration of several tissues or organs after injury both preclinically and in clinical trials [6–15]. Mesenchymal stem cell (MSC) is a cell that has not been differentiated and able to regenerate itself through cell division, with the ability to differentiate into other cells [16]. These cells are widely used in the field of tissue engineering to regenerate bone tissue because they can differentiate into osteogenic cells [17,18].
Despite the observed beneficial effects, there is no consistent evidence that the cells employed generate organ-specific cell population, able to replace the cell loss after injury [6]. A major MSC limit for its clinical applications is the inherent heterogenicity and variation associated with cell expansion [19,20]. Changes that may increase the risk of MSC therapeutic application could also be induced during in vitro cell processing and expansion. The risk of unwanted differentiation in vivo is also a problem due to its clinical applications. Therefore, nowadays cell-free-based therapy is considered an alternative treatment in tissue engineering, which also includes extensive research on the identification of the molecules involved in paracrine action of stem cells to open new therapeutics options.
Due to the theory of paracrine stimulation, upon the application, transplanted cells will affect residing cells by secretion of bioactive molecules into the extracellular space [20]. A lot of studies have been conducted to purify growth factors (GF) and apply the GF to stimulate regeneration, including the use of platelet-rich plasma and platelet-rich fibrin [21,22]. However, the role of purified GFs in stimulating regeneration is not as effective as expected due to their short half-life in the extracellular space [23]. Therefore, there is a need to develop a strategy to overcome the disadvantages of a cell-based approach in tissue engineering and regenerative medicine. The MSCs extracellular vesicles (EVs) which contain biologically active molecules, such as GFs, cytokines and functional RNAs known as exosomes have become a particular interest for cell-free regenerative therapy due to their epigenetic capacity and cargos.
To some extent, in tissue-engineering-based regenerative therapy, there is a ‘construct’ to promote the repair and/or regeneration of tissues. As explained previously, the ‘construct’ which is provided as a scaffold and cells/biomolecules delivery system, plays an important role as a conductive strategy to interfere regenerative process by enabling the desired host cells to populate the regeneration site [3,24]. This is because scaffold is intended to support cell migration, growth, differentiation and guide tissue development and organization into a mature and healthy state [24]. Meanwhile, it is also recognized that ceramics containing construct functions as instructive extracellular microenvironment for morphogenesis [3].
In view of the current advancement and challenges, in this study, a comprehensive, hence, concise review on the role of MSCs derived exosomes in regenerative therapy applied in dentistry is elaborated. To understand the role of exosomes in regenerative therapy, overview on the origin, functions and potentials of exosomes are described. The important aspect on the osteoconductive strategy by scaffold containing ceramics is also discussed in this study.
What is MSC-derived exosomes & why?
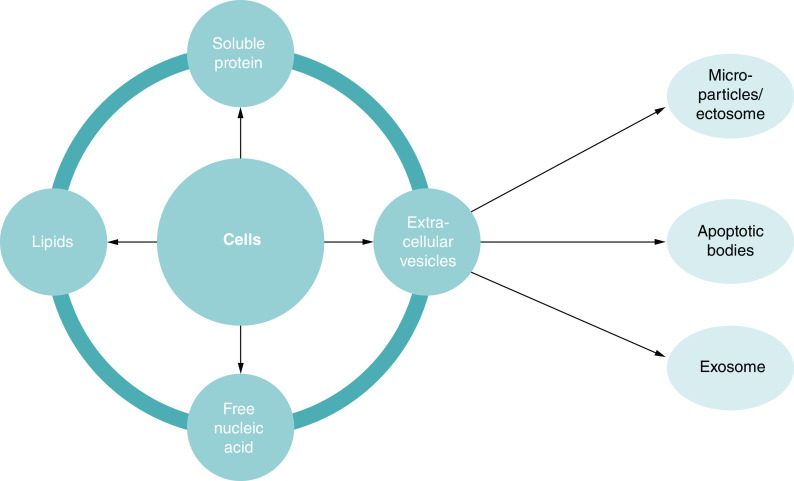
The cells (including MSC) produce a set of factors or molecules secreted to the extracellular space. The secreted factors include, among others, soluble proteins, free nucleic acids, lipids and EVs. The latter, EVs, can be subdivided into apoptotic bodies, microparticles or microvesicles and exosomes [25], which are differentiated based upon their biogenesis, release pathways, size, content and function. Table 1 shows type, size and origin of EVs [26]. Figure 2 describes diagrammatically the organization of cell secretome. Meanwhile, Figure 3 summarizes the position of exosome as a part of EVs and clarify the organization of EVs in more detailed explanation based on some of the literature and guidelines from the International Society for Extracellular Vesicles in the Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018) on nomenclature, isolation and characterization [26,27]. The secretome of individual cells and tissues is specific, and changes in response to fluctuations in physiological states or pathological conditions. The exosome is part of the EVs originating from the endosome with a size of 30–100 nm [28]. Exosomes are known to be an important substance to facilitate cellular communication by delivering functional cargos, for example, protein, mRNA and microRNA (miRNA), as described by Wang and co-workers [29]. Exosomes from stem cells are composed of a lipid bilayer, with the ability to efficiently protect, transport, and deliver a wide variety of molecules contained in it [30]. Exosomes also play an irreplaceable role in normal physiological processes such as nerve function and neurodegenerative diseases.
Table 1. . Types, size and origin of extracellular vesicles according to the MISEV 2018.
| Types | Size (nm) | Origin |
|---|---|---|
| Exosome | 50–100 | Endosome |
| Micro vesicles | 100–1000 | Plasma membrane |
| Apoptotic bodies | 1000–5000 | Plasma membrane |
Data taken from [26].
Figure 2. . Cells secreted bodies into the extracellular space are called secretome, which contains soluble proteins, free nucleic acid, lipids and extracellular vesicles.
The EVs can be divided into apoptotic bodies, microparticles and exosomes. Microparticles are also indicated with other names: nanoparticles, microvesicles, shedding vesicles or shedding bodies, exovesicles, secretory vesicles and oncosomes. Exosomes contains growth factors, cytokines and functional RNA.
EV: Extracellular vesicle.
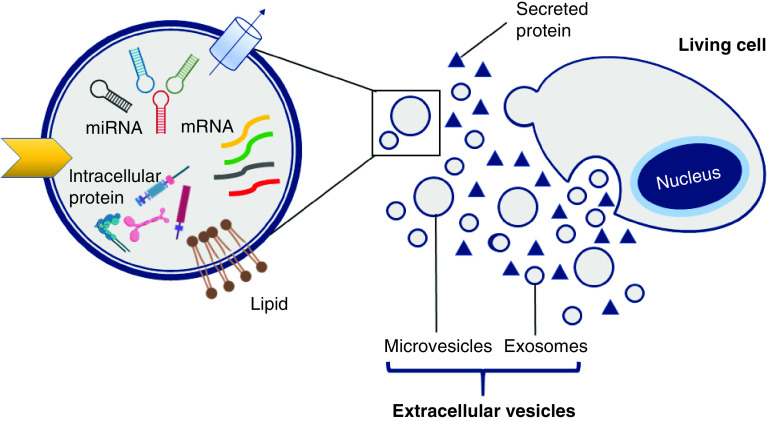
Figure 3. . A living cell releases proteins and extracellular vesicles (microvesicles and exosomes) into extracellular spatial.
The exosomes isolated from MSCs have a more effective therapeutic ability due to their small size and role in cell communication. According to Chopra and co-workers [31], the effectiveness of exosomes derived from MSCs may be due to its ability to migrate to the areas which need repair so that they are ideal for therapeutic use. At this point, it will be a potential method in regenerative treatment if MSCs derived exosomes are used because MSC can be obtained from Wharton's Jelly which is a waste. Zhang et al. [32] proved that exosomes originating from MSCs can help repair cartilage. Exosomes can also help periodontal regeneration by increasing the migration and proliferation of the periodontal ligament cells [33]. However, the use of exosomes as a therapeutic agent currently still has obstacles, including the small number of exosomes produced [31,32,34].
Compared with stem cells or cell-based applications, the use of exosomes provides key advantages over cell-based therapy. The use of exosomes resolves several problems associated with the transplantation of living and proliferative cells population, which cannot be fully controllable in vivo. Besides, the immune compatibility, tumorigenicity, emboli formation and the transmission of infections can also be prevented. Moreover, the preparation prior to its applications can be evaluated for its safety, dosage and potency in a method analogous to the preparation of conventional pharmaceutical agents and the use of toxic cryo-preservative agent can also be avoided [35,36]. Eiro and co-workers [35] also predicted that mass production is possible through tailor-made cell lines under controlled laboratory conditions, allow it to provide off-the-shelf exosome therapies immediately.
Biogenesis, functions & potential of exosomes
As previously described, the secretome of MSC consists of soluble factors and EVs. The EV contains biologically active molecules, such as growth factors, cytokines and functional RNAs. Exosomes are released by MSC. At the time of this release, biological factors contained in the exosomes can exert an effect on the cells of the cellular environment or reach distant organs via the bloodstream, including the central nervous system, which processes depend on the permeability of the blood–brain barrier.
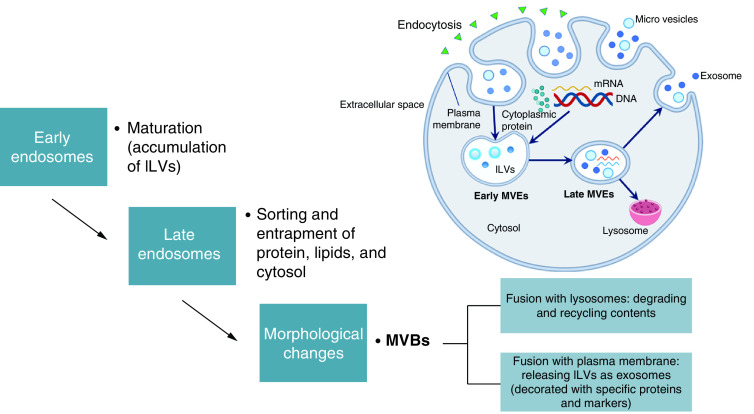
Exosomes are originated from endosomal and the size ranges from 30 to 100 nm [6,28], or 50 to 100 nm according to the Minimal Information for Studies of Extracellular Vesicles (MISEV) [26]. Its biogenesis is known to be regulated by specific cellular pathways [37]. Constitutive exocytosis, which occurs in almost all cell types and involved in the secretion of newly synthesized protein, triggers exosomes release. Exocytosis is initiated by invagination of endocytic vesicles that fused with early endosome, continued by endosomal formation [38]. In this way, exosomes are the result of the secretion process of the endosomal components of the cells.
Unlike the microvesicles or ectosomes and apoptotic bodies which are directly shed from the plasma membrane, exosomes are released upon the fusion of late endosomes and multivesicular bodies with the plasma membrane [39]. A highly dynamic endocytic pathway started the first step of exosomes release. The release is started by the accumulation of intraluminal vesicles (ILVs) as early endosomes mature into late endosomes. The ILVs sort and entrap proteins, lipids and cytosol within the late endosomes. The endosomal sorting complex required for transport (ESCRT) is found to control these initial steps of exosomes secretion [40]. The entrapment leads to morphological changes that result in multivesicular bodies (MVBs), as described in the previous studies [41–43]. In most cases, MVBs fuse with lysosome for the degrading and recycling their contents. Certain MVBs are patterned with specific proteins and markers to ensure the fusion with the plasma membrane, allow the release of their contents to extracellular space, and become known as exosomes. This mechanism is dependent, controlled by ESCRT and involves 30 different proteins which help sequester specific biomolecules in the MVBs and guide their releases through the plasma membrane as exosomes [39,43–45]. The diagrammatic cascade of exosomes biogenesis is shown in Figure 4.
Figure 4. . Biogenesis of extracellular vesicles.
Microvesicles and apoptotic bodies are originated from plasma membrane, while exosomes are derived from the endosomal compartments. Through an ESCRT-dependent pathway, proteins, lipids, nucleic acids and other cargo are sequestered within the ILVs. The MVBs which fuse with plasma membrane will release ILVs into extracellular space as exosomes.
ESCRT: Endosomal sorting complex required for transport; ILV: Intraluminal vesicle; MVB: Multivesicular body.
Exosomes associate with the progression of diseases, among them are neurodegenerative disease, cardiovascular diseases and cancer. The association is related to the involvement of exosomes in many physiological processes such as antigen presentation, RNA transfer or tissue repair [31–35,46]. Evidence from the previous studies shows that exosomes have specialized functions and key roles in coagulation, intercellular signaling and waste products management. Their functions include immune regulation, vascular regeneration promoting, mediation of cell proliferation, differentiation, migration, and apoptosis, preserving the body physiological condition and partaking in disease processes [20].
MSC-derived exosomes (MSC-exosomes) exhibit high potential for cell-free-based therapy in regenerative medicine. Since MSCs derived exosomes have the characteristics of the resource cells, thus it can promote cell self-repair, regenerate tissue, restore homeostasis of the tissue and accelerate wound healing [47]. A lot of research shows that MSCs have a strong ability to produce exosomes [45–48] and MSCs derived exosomes are believed as the main effective paracrine component which plays almost equivalent biological effects to those of whole MSCs.
In comparison with the whole MSCs, exosomes fuse directly with the targeted cells, thus exhibit more intense biological effects. Exosomes derived from MSCs can be easily stored and transported at -70°C for a long time. Their main components are effectively protected by the exosome's plasma membrane which make them not easily destroyed. The concentration, dosage, route, and time of use are also easily controlled. Moreover, there is no risk of immune rejection and tumorigenesis caused by cell transplantation therapy [47].
Potential application of exosomes in regenerative dentistry
An engine search by MEDLINE (PubMed) database with relevant key words such as exosome, regenerative therapy, regeneration, tissue engineering and/or dentistry was used to search relevant articles to this review published up to 31 January 2021. When the terms ‘exosome’ AND ‘dentistry’ AND ‘regeneration’ were used, 60 articles were found. After the screening of the titles, among 60 there were 27 titles directly related or relevant to the study. Among 27, one article provided an experiment related to antibacterial activity of exosome, but after further reading, it was found that the authors only used exosome-liked vesicles from three bee products, in other words, honey, royal jelly and bee pollen [49]. A review article on the exosome derived from saliva [50] was also excluded from the study. Although the article [50] provided description on regeneration of wound healing by salivary exosome, but it was considered not directly relevant to this review. There were also other four review articles concerning exosome or cell-free-based tissue engineering, regenerative therapy and nanotechnology [51–54] which are not specifically related to regenerative dentistry. Therefore, we finally excluded them from the study, remaining 21 articles for further analysis. Table 2 summarizes search results from the 21 articles [55–75].
Table 2. . Summary of the search results from the MEDLINE (PubMed) database for the articles concerning the application of exosome in regenerative dentistry.
| Functions in dentistry | Summary | Type of the study | Study | Ref. |
|---|---|---|---|---|
| To regenerate periodontal ligament (PDL) | MSC exosome-loaded collagen-sponge-enhanced periodontal regeneration in an immunocompetent rat periodontal defect model | In vivo | Chew et al. | [55] |
| To induce bone, cartilage, dentin, mucosa and pulp tissue formation | Functions of MSC exosome in relation to oral and craniofacial tissue engineering | Review | Cooper et al. | [56] |
| To repair critical size osteochondral defects | Exosome enhanced matrix synthesis and a regenerative immune phenotype in osteochondral defect | In vivo | Zhang et al. | [57] |
| Exosome derived human embryonic MSCs promoted osteochondral regeneration | In vivo | Zhang et al. | [58] | |
| To enhance angiogenesis in oral wounds | Possible implication of exosome for therapeutic induction of angiogenesis in the oral wounds | Review | Zimta et al. | [59] |
| To function as small molecule drug to enhance chondrogenesis | Exosome improved efficient delivery of kartogenin to synovial fluid derived MSCs for chondrogenic differentiation | In vitro and in vivo | Xu et al. | [60] |
| To treat OA (osteoarthritis) in TMJ (temporo mandibular joint) | Potential of exosome in regenerating cartilage and osseous compartments in TMJ, thus restoring injured, dysfunction, and pain tissues | Review | Lee et al. | [61] |
| Exosome attenuated inflammation and restored matrix homeostasis | In vitro | Zhang et al. | [62] | |
| To control dental-pulp derived pain and inflammation | Potential of exosome to modulate thermo-sensitive receptor potential cation channel in pain and inflammation management in everyday dental practices | Review | Schuh et al. | [63] |
| To promote oral mucosal wound healing | Exosome isolated from clinical grade production of oral mucosal epithelial cells stimulated epithelial regeneration and showed pro-regenerative effects on skin wound healing | In vitro and in vivo | Sjöqvist et al. | [64] |
| To enhance endodontics and pulp regeneration | Potential of exosome as an approach to enhance regenerative endodontics | Review | Tatullo et al. | [65] |
| Potential of exosome to trigger pulp regeneration (including pulp angiogenesis), regulate proliferation, migration and differentiation and provide neuroprotection | Review | Yu et al. | [66] | |
| Exosome was isolated from DPCs culture supernatant and examined on its roles to HUVEC proliferation, pro-angiogenic factors expression and tubular formation. It was found that exosome-derived DPCs have vital roles in angiogenesis and tubular morphogenesis | In vitro | Xian et al. | [67] | |
| To enhance cutaneous wound healing | Exosome from neonatal serum used to pre-treat MSCs improved MSCs biological functions in enhancing angiogenesis | In vitro | Qiu et al. | [68] |
| To enhance nerve regeneration, increase number and diameter of nerve fibers and promote myelin formation | Exosome was isolated from gingival MSCs, combined with biodegradable chitin conduits and applied to rat sciatic nerve defect. Number and diameter of nerve fibers increased significantly. There was also significant increased proliferation of Schwann cells and dorsal root ganglions by the treatment | In vivo | Rao et al. | [69] |
| To inhibit cancer growth | Potential of exosome to inhibit cancer growth since it may transduce apoptosis-inducing factors | Review | Stefanska et al. | [70] |
| To regulate bone remodeling and function as therapeutics agent in orthodontics | Potential of exosome to enhance communication networks integrating bone cells osteoblast, osteoclast, osteocyte) and linking bone to other tissues. These potentials are significant to augment bone remodeling associated with orthodontic force application or required for the repair of craniofacial bone | Review | Holliday et al. | [71] |
| To induce dentinogenesis in regenerative endodontics procedure | Stem cells from apical papilla derived exosomes were applied into the root fragment containing bone marrow MSCs and transplanted subcutaneously into immunodeficient mice. It was observed that dental-pulp like tissues were present and newly formed dentine was deposited onto the existing root canal. It was also observed that dentine sialophosphoprotein and mineralized nodule were significantly increased | In vitro and in vivo | Zhuang et al. | [72] |
| To regenerate and repair tissue through cell-free-based tissue engineering with sustained release capability | Potential of exosome as mediators for tissue regeneration. The review describes exosome involvement in a multitude of physiological processes, such as development, cell differentiation and angiogenesis | Review | Alqurashi et al. | [73] |
| To accelerate craniofacial regeneration when combined with three-dimensional block co-polymer | Exosome derived from human dental pulp stem cells loaded into biodegradable triblock copolymer microspheres of poly(lactic-co-glycolic-acid) or PLGA and poly(ethylene glycol) or PEG facilitated bone marrow stromal cells. It was also observed that direct insertion of the construct into calvaria defect of the mouse accelerated bone healing in vivo | In vitro and in vivo | Swanson et al. | [74] |
| To regenerate salivary gland | Potential of exosome to ameliorate salivary gland injury by combination of three-dimensional bioprinting or bio assembly spheroid or organoid cell transplantation | Review | Chansaenroj et al. | [75] |
DPC: Dental pulp cell; MSC: Mesenchymal stem cell.
In his review, Cooper and co-workers [56] mentioned that exosomes carry with them informative cargo from the MSCs to targeted cells. The informative cargo is needed to regulate fundamental cellular processes for lineage-specific differentiation, migration and apoptosis. Regarding bone regeneration, key protein factors carried by exosomes will mediate a series of signaling pathway [76]. For example, an important transcription factor RUNX2 in exosome will promote differentiation of pluripotent stem cells into osteoblast and at the same time inhibit osteoblast maturation [77] or repress osteogenic transcription factors such as OPN, BSP, OSX, dan OCN [78]. Table 3 shows key bone regeneration factors mediated by protein carried by exosomes [76–106].
Table 3. . Key bone regeneration factors mediated by protein or cytokine carried by exosomes.
| Bone regeneration factor | Function | Study | Ref. |
|---|---|---|---|
| RUNX2 | Key transcription factor for the differentiation of stem cells into osteoblast and inhibition of osteoblast maturation by suppression of OPN, BSP, OSX and OCN expression | Wang et al.; Deng et al. | [77,78] |
| PI3K-AKT | Key transcription factor with phosphatidylinositol 3-kinase (PI3K) and Akt/protein kinase B proteins involved. This signal transduction pathway promotes metabolism, proliferation, cell survival, growth and angiogenesis in response to extracellular signals | Xu et al.; Zhao et al. | [79,80] |
| Wnt | Key transcription factor for signaling pathway related to bone remodeling and repair. Wnt signaling system is also known to be a key factor to maintain bone mass | Komiya and Habbas; Issack et al.; Grigorie et al.; De Santis et al. | [81–84] |
| RANKL-RANK | Key signaling pathway responsible for homeostasis of bone metabolism determined by dynamic balance between osteoblast and osteoclast | Theoleyre et al.; Wada et al.; Leibbrandt et al.; Huynh et al. | [85–88] |
| BMP2 | It is multi-functional growth factors which belong to superfamily TGF-β. The BMPs play critical roles in cartilage development, and specifically has been utilized for the therapeutics of bone defects, bone fractures, osteoporosis, spinal fusion and root canal surgery | Chen et al. | [89] |
| BMP9 | BMP9 is known to have highest osteogenic potentials compared with other BMPs family. However, it is also revealed that BMP9 exerts broad range biological functions such as adipogenesis, angiogenesis, neurogenesis, oncogenesis and/or tumorigenesis and metabolism | Mostafa et al.; Bharadwaz and Jayasuriya | [90,91] |
| SPP1 (OPN) | SPP1 is also known as BSP1 or OPN. Among its diverse biological functions, OPN is known to regulate biomineralization because its calcium binding sites. As a member of SIBLING (Small Integrin-Binding Ligand, N-linked Glycoprotein) family, it can interact directly with extracellular matrix including fibronectin | Chen et al.; Mukherjee et al.; Fisher et al.; White et al.; Lund et al.; Singh et al.; Si et al. | [92–98] |
| OCN | Produced by osteoblast, OCN is the most abundant non-collagenous protein in bone. It regulates bone mineralization and coordinates mineral ions homeostasis. Bone quality is regulated by OCN because it aligns biological apatite parallel to the collagen fibrils | Wei and Karsenty; Komori | [99,100] |
| COL1 | Type I collagen is the most abundant collagen and a key structural composition of bone tissue that is also expressed in almost all connective tissue as predominant component of interstitial tissue membrane | Henriksen and Karsdal | [101] |
| TGFβ1 | TGFβ1 is abundant in bone, responsible for bone formation and resorption. It stimulates matrix protein synthesis and at the same time inhibits both osteoclast formation and activity | Bonewald and Mundy; Mundy | [102,103] |
| VEGF | This growth factor, VEGF belongs to PDGF super family. It regulates angiogenesis and vascular permeability | Risau; Shibuya | [104,105] |
| PDGF | When being activated, PDGF stimulates cell growth, changes cell shape by reorganization of actin filament and affects chemotaxis which directs cells motility. Its role is important during embryonic development and wound healing | Heldin and Westermark | [106] |
The table is summarized from a chapter written by Yang et al. [76].
BMP: Bone morphogenetic protein; BSP: Bone sialoprotein; COL1: Type I collagen; OCN: Osteocalcin; OPN: Osteopontin; OSX: Osterix; SPP1: Secreted phosphoprotein 1
It is also notified that generally exosome enhanced regeneration by increasing cellular mobilization and proliferation. In case of PDL, Chew and co-workers used PDL cell cultures and found that exosome increased PDL migration and proliferation through CD73-mediated adenosine receptor activation of pro-survival AKT and ERK signaling [55]. Meanwhile, the mechanism of exosomes involvement in promoting bone regeneration process can be observed from the process in which proteins carried by exosomes upregulate bone regeneration factors, as depicted in Figure 5. For example, exosome may induce high expression of BMP2 which in turn promotes osteogenic differentiation and osteogenesis by cascade activation of OSX factor [107]. Similarly, when OPN or Type I collagen is upregulated by exosome, bone mineralization cascade will be activated followed by other bone regeneration processes. High expression of other bone regeneration factors such as BMP9, TGF-b1, VEGF and PDGF induced by exosomes will activate osteogenic differentiation and angiogenesis pathway to enhance bone regeneration [76]. In view of the potential of exosome in PDL and bone regeneration, the implication in regenerative dentistry can be clearly elaborated, such as for craniofacial regeneration, implant dentistry, oral-maxillofacial regeneration in general, PDL regeneration, and in orthodontics for application related to bone remodeling control to avoid relapse post orthodontics treatment.
Figure 5. . Mesenchymal stem cells derived exosomes with the potential to enhance regeneration in clinical dentistry.
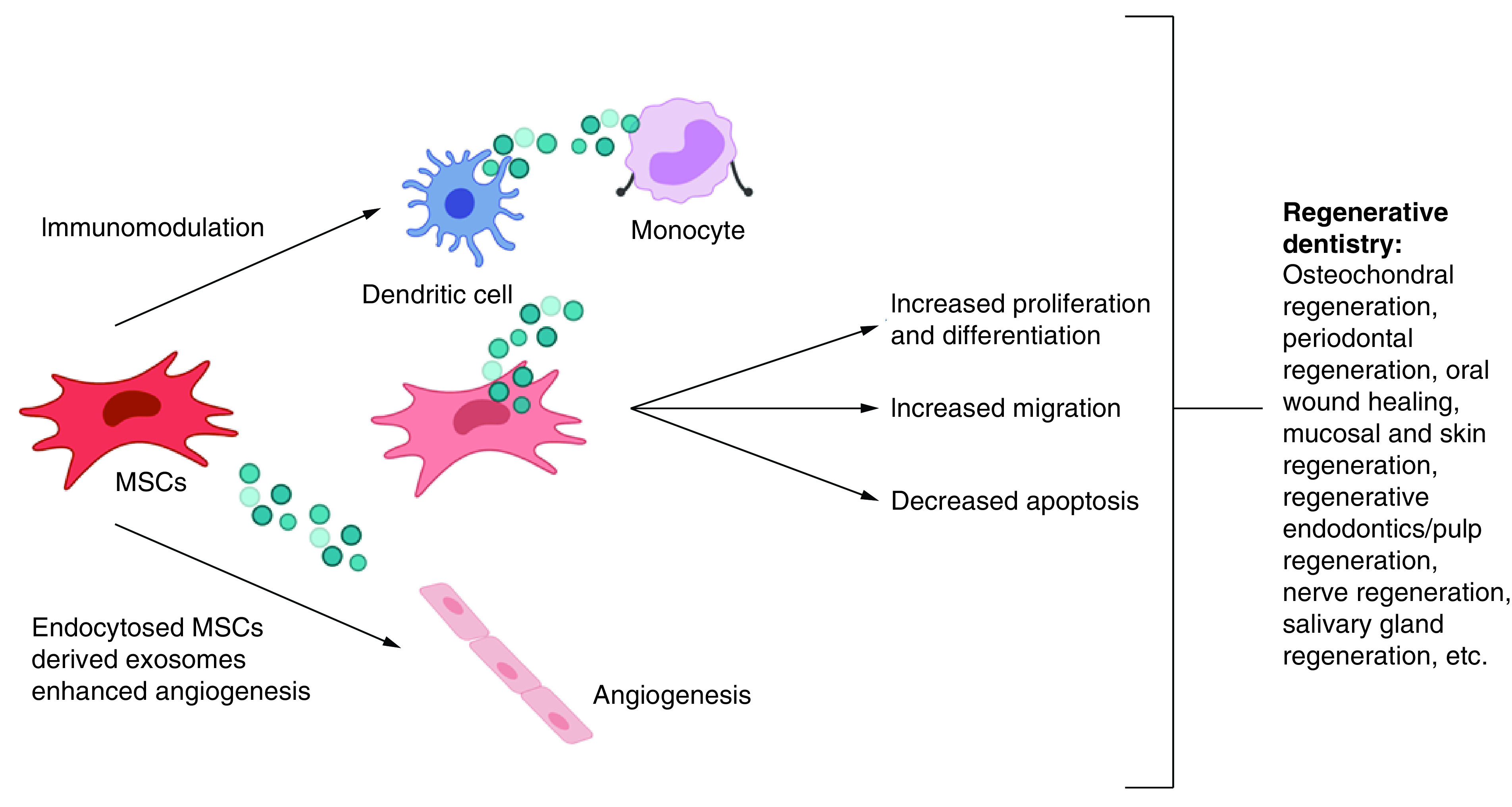
Related to the role of exosome in cartilage regeneration, firstly it is known that cartilage is a connective tissue with limited capacity for intrinsic regeneration upon injury or lesions. Cartilage injury can also be further aggravated by several joint diseases such as osteoarthritis (OA) and rheumatoid arthritis (RA). In clinics, OA is characterized by joint pain, tenderness, crepitus, stiffness, limitation of movement due to occasional effusion with various degrees of local inflammation [108,109]. It is the most frequent chronic joint disease, with progressive breakdown of articular cartilage [110]. Meanwhile, RA is characterized by dysregulated inflammatory processes in the synovium of the joint. The inflammatory process eventually leads to the destruction of cartilaginous and bony elements of the joint, resulting pain and disability [111]. It is an autoimmune disorder in which dysregulated inflammation and T-cells induce pain and joint degradation [112].
Zhang et al. [58] demonstrated that weekly intra-articular injections of human embryonic MSC exosome has successfully induced an orderly cartilage regeneration and subchondral bone in a rat model, marked by the development of hyaline cartilage and underlying subchondral bone. The osteochondral defect repair is characterized by increased cellular proliferation and infiltration, enhanced matrix synthesis, and a regenerative immune phenotype [57,58]. The results of the study by Zhang and co-workers [58] corroborated with a previous study used exosome-derived synovial fibroblasts of RA patient which found that exosome from RA synovial fibroblast of patient (RASF) has a membrane bound form of TNF-a, which leads to apoptotic resistance of T-cells in RA because the lack of apoptotic machinery for T-cells progresses RA [113]. When apoptotic resistance of T-cells increases, there would be delayed onset of RA. In regenerative dentistry, these findings are relevant for the treatment of TMJ disorders because TMJ injury, dysfunction, and pain are closely related to the manifestation of OA and RA in dentistry [61,62].
Generally, during regeneration process, vascularization is essential because new blood vessel formation improves diffusion of oxygen and nutrients in the regeneration area. It has been demonstrated that exosome can stimulate proliferation, migration, and tube formation of endothelial cells (ECs) [114,115]. As it is widely known, angiogenesis is a process of blood vessel formation and stability which is controlled and dictated by VEGF [59,104,105]. Beside having action on vascular endothelial cells, VEGF also enhances bone development by stimulating vascular endothelial cells. Therefore, in some studies, it was known that osteogenesis is closely related to vascularization through cell-to-cell communication between vascular endothelial cells and osteoblasts. In other words, sufficient vascularization is considerably promoting osteogenesis [116–119]. Moreover, it is also revealed from the previous study that exosome-derived MSCs contained abundant levels of VEGF, which enhanced angiogenesis and contributed to periodontal tissue [73,120], oral epithelial [64], and dental pulp or endodontics [65] regenerations.
As depicted schematically in Figure 6, angiogenesis is composed of several stages [59]. Regarding angiogenesis which leads to neovascularization in regeneration area, exosomes are known as mediators for intercellular communication and can be used to maximize the local pro-angiogenic potential at the wound site. Abundant number of pro- and anti-angiogenic microRNAs were found [59] and this modulates the behavior of endothelial cells and could be included in the design of exosome delivery of pro-angiogenic factors. Exosome with its miRNA cargoes direct the process during neovascularization, for example miRNA-21 activates AKT and ERK pathway, thus leading to the VEGF-increased production. Besides, when generating exosomes, MSCs are maintained in hypoxic conditions. This hypoxic condition is predicted to stimulate enhancement of pro-angiogenic capacity of the exosomes [121]. The underlying mechanism is possibly due to overloading of exosomes with miR-135b which targets the factor inhibiting HIF-1 (FIH-1) gene, an inhibitor of hypoxia-inducible factor 1 (HIF-1), known as pro-angiogenic factor [122].
Figure 6. . Angiogenesis is composed of several stages, as depicted schematically.
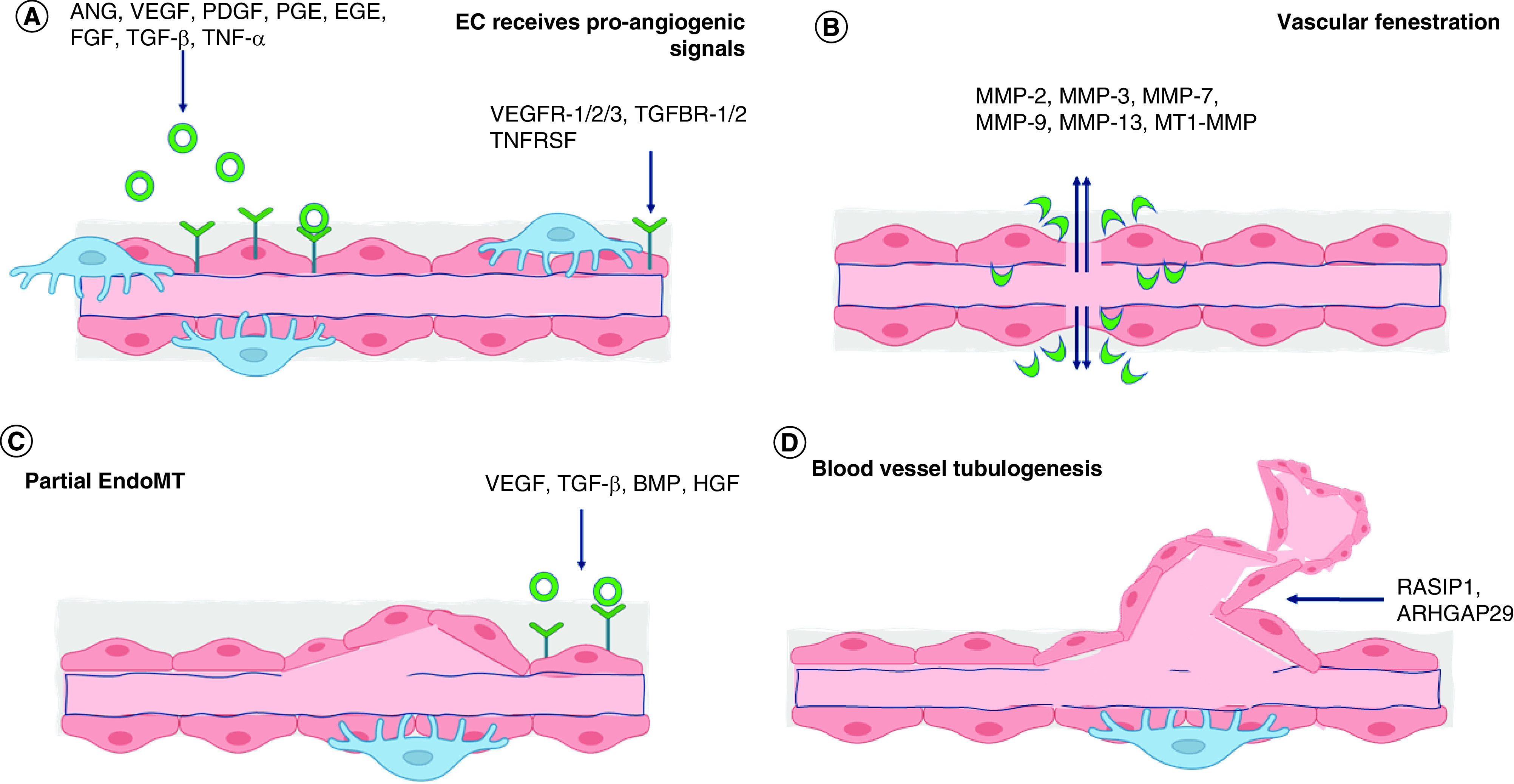
The diagram is modified from and referred to the work Zimta et al. [59]. At first stage, pro-angiogenic factors, which bind to their corresponding receptors, are released by surrounding cells into local environment. (A) The ECs found at the outer surface of a blood vessel receive pro-angiogenic signals from ANG, VEGF, PDGF, PGF, EGF, FGF, TGF-β, and TTNF-α. On the surface of EC, there are several receptors corresponding to pro-angiogenic factors, for instance VEGFR1/2/3, TGFBR1/2, and TNFRSF. After signal transduction in the EC, cells start to proliferate and produce metalloproteinases (MMP). (B) Concurrently, blood vessel pores increase in size and fenestration happens. This will enable MMPs to escape from the blood vessels and degrade basement membrane. (C) The ECs start to migrate. This process is called partial endothelial to mesenchymal transition (partial EndoMT), proliferate at the fenestration area, and result a new blood vessel budding. (D) As the new tube forms, multiple signals from the environment such as RASIP1 and ARHGAP29 will enhance the development of 3D structure and organization of the newly formed network. By the final stage of the process, pericytes found at the exterior of the blood vessel responsible for blood vessel contraction begin to populate the newly formed network.
EC: Endothelial cell.
Beside cartilage, bone, oral mucosal, and PDL regenerations, nerve regeneration pays a lot of attention in dentistry due to prevalent cases of nerve injuries caused by oral-maxillofacial traumas and/or surgeries. From several studies, it was observed that exosome could stimulate Schwann cell proliferation and increased expression of cyclin Ki67 as an indication of exosome potentials in enhancing neurite length of dorsal root ganglion (DRG) neurons [69,123]. The capability of exosome in enhancing nerve regeneration is due to the presence of neuronal growth factors such as brain-derived neurotrophic factor (BDNF), insulin growth factor-1 (IGF-1), nerve growth factor (NGF), fibroblast growth factor-1 (FGF-1), and glial cell-derived neurotrophic factor (GDNF) [124,125]. Based on the presence of key neural growth factors, exosome could potentially provide new approaches to nerve regeneration in medicine and dentistry but further research to uncover underlying neurogenesis mechanism and roles of specific signaling molecules in relation to neural regeneration pathways are needed.
Therapeutic potential of exosome in healing & regeneration process
From the previous description, it can be concluded that exosomes have potential in the process of regeneration, thus exosomes can be used as therapeutics agent in medicine and dentistry. These potentials cannot be separated from the three overlapping stages in regeneration process: inflammation, proliferation, and remodeling phases. Inflammation is a human body self-defense mechanism against harmful stimuli. It is a regulated acute response beneficial for wound healing [126]. If the inflammation phase were chronic and dysregulated, wound healing would be delayed and it would promote fibrosis, excessive scar formation, and inhibited proliferation or re-epithelization [127].
During the inflammation process, macrophages are a major component of the mononuclear phagocyte system and play critical roles in initiation, maintenance, and resolution of inflammation. Macrophage functions as antigen presenting cells and produce cytokine and growth factors for immunomodulation [128]. Macrophages are activated and deactivated during the inflammatory process. Activation of inflammation induces M1 phenotype to release signals, including cytokines such as interferon gamma (IFN-g), tumor necrosing factor (TNF), bacterial lipopolysaccharide (LPS), ECM proteins, and other chemical mediators. In concert with microbial products, such as LPS, and cytokines, such as TNF, IFN-g will activate M1 [129] indicated by high interleukin-12 (IL-12) and IL-23, as well as high toxic intermediates such as nitric oxide (NO) and reactive oxygen intermediates (ROI) productions [130]. Inflammation deactivation happens by removal of mediators to permit host to repair damage tissues in which M2 phenotype releases anti-inflammatory cytokines (interleukin 10 or IL-10 and TGF-b) and cytokines antagonist [128–130].
Since macrophages produce a wide range biologically active molecules to autoregulate in the inflammatory process, therapeutic interventions using exosome which targets macrophage may open new avenues for inflammatory disease control. Exosome can regulate activation, differentiation, and proliferation of B-lymphocytes and suppress T-lymphocyte proliferation at the same time. It can also convert activated T-lymphocytes into T-regulatory phenotype to exert immunosuppressive effects [126,131,132], as depicted in Figure 7, summarized from a review on the role of EVs in autoimmunity [133]. The regulation of inflammatory factors plays important roles in regeneration process. With the capacity to deliver a cargo of protein, lipids, nucleic acids, or other cellular components to neighboring or distant cells, exosomes may polarize the inflammatory response through down-regulation of pro-inflammatory enzymes like inducible NO, cyclooxygenase (COX)-2 and of cytokines such as TNF-a, IL-1b, monocyte chemoattractant protein (MCP)-1. Table 4 summarizes possible mechanism of exosomes in immune regulation and inflammatory responses, as the key points for therapeutics and regenerative treatment [124,129–132].
Figure 7. . Exosomes and microvesicles (MSC's derived EV) exert immunomodulatory effect on innate and adaptive immune reactions mediated by many immune cells (T and B lymphocytes, natural killer cells, dendritic cells, and macrophages).
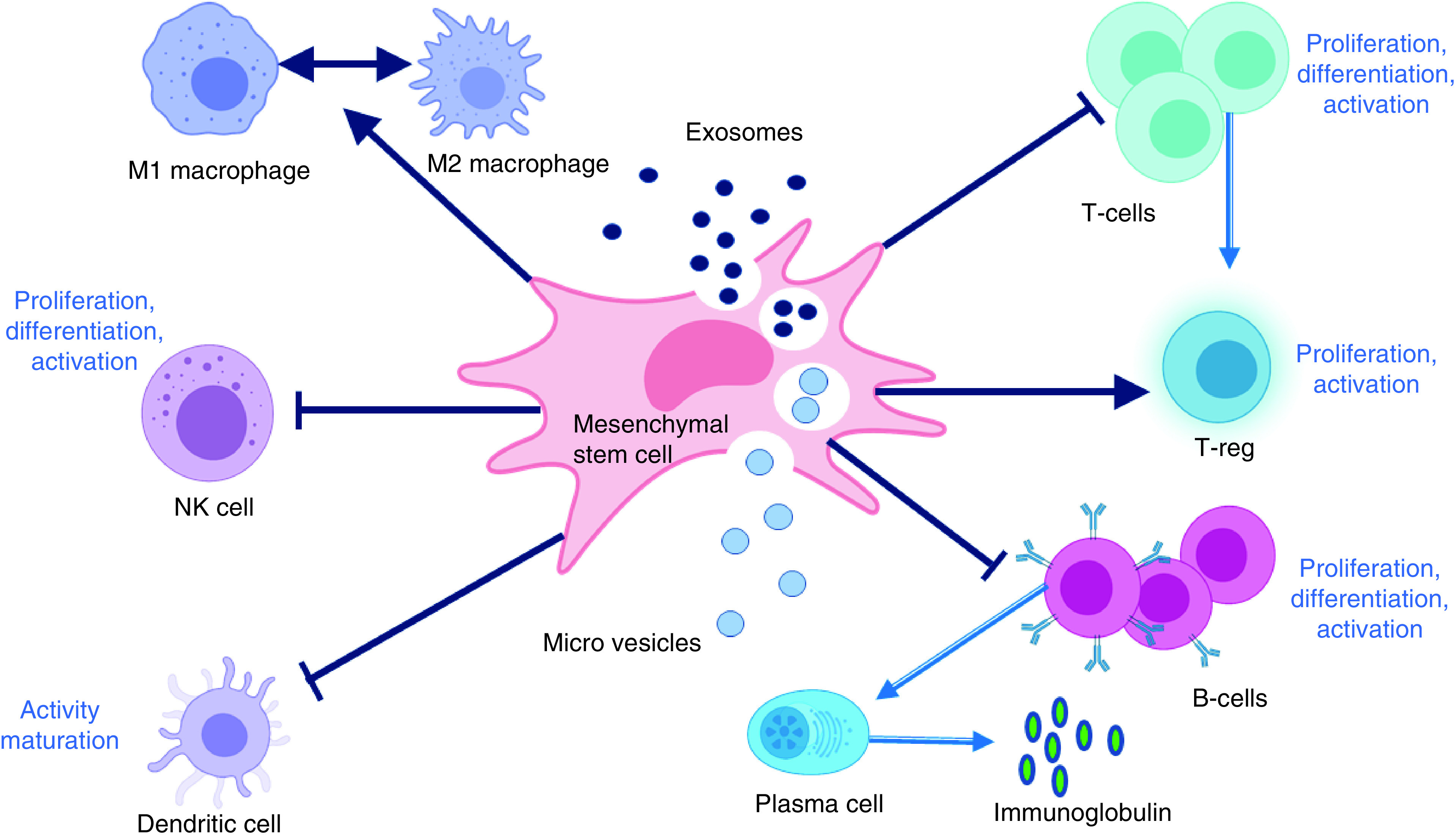
Exosomes and microvesicles can inhibit the proliferation, differentiation, and activation of T, B, and natural killer cells and the pathogen-presenting function of dendritic cells and macrophages. Macrophage polarization can also be regulated under different microenvironment in accordance with EVs application.
Figure was derived and further modified from Wang and co-workers [133].
Table 4. . Possible mechanism of exosomes in immune regulation and inflammatory responses.
| Target | Effect | Mechanism |
|---|---|---|
| CD4+ and CD8+ T-cells | Inhibiting differentiation toward effector or memory T-cell phenotypes | Mediated by anti-CD3/CD2/CD28 stimulation |
| T-cells in general | Inhibiting activation | Reduction of interferon-gamma (IFN-γ) secretion |
| Macrophage | Increasing mRNA levels of M2-related arginase-1 and interleukin-10 (IL-10) | |
| Inducing macrophage polarization toward anti-inflammatory M2 phenotypes | Transactivation of arginase-1 by active signal transducer and activator of transcription 3 (STAT-3) | |
| Inhibiting macrophage inflammatory responses | Stimulated by lipopolysaccharide (LPS) and IFN-γ | |
| Inflammatory response (in vivo in mouse model) | Regulating inflammatory responses | Reduction of inflammatory cytokines sech as IL-4, IL-23, IL-31, and tumor necrosis factor-alpha (TNF-α) |
| Inflammatory response (in vivo in mice after sepsis syndrome) | Improving survival and suppressing inflammatory responses | Increase inflammatory mediators such as MMP-9, macrophage migration inhibitory factor, TNF-α, nuclear factor kappa-B (NF kappa-B), and IL-1β |
During proliferation phase, angiogenesis plays crucial roles in wound healing and repair. For this, MSCs derived exosomes are enriched with various angiogenesis related proteins and other miRNAs that could activate signaling pathways in endothelial cells, including up regulating angiogenesis related molecules found in vascular endothelial cells such as VEGF, VEGF receptor (VEGFR), FGF-1, E-selectin, angiopoetin-1 and endothelial nitric oxide synthase (eNOS), chemokine ligand 16 and IL-8 [126]. Furthermore, ECM reconstruction is the key point for tissue reconstruction during remodeling phase. It was observed that exosomes promoted synthesis of type I collagen, type III collagen and elastin during the early stage and inhibited collagen synthesis in the late stage of remodeling to inhibit scar tissue formation [126]. In this context MSCs derived exosomes will be potential candidate as a therapeutic agent to regulate inflammatory, proliferation and remodeling phase in tissue regeneration.
Moreover, because of their ideal native structure and characteristics, exosome is also indicated as a promising nanocarriers for clinical use due to its ideal small size to penetrate deep tissues, slightly negative zeta potential for long circulation, deformable cytoskeleton and similarity to cell membranes [133,134]. Exosomes can also exhibit an increased capacity to escape degradation or clearance by immune system [133].
Challenges & strategies
Based on the previous review, the MSCs derived exosomes and their potential as cell-free-based therapy in tissue engineering applied in dentistry, the challenges for the application include extensive research on the identification of the molecules involve in paracrine action of stem cells to open new therapeutics options using exosomes, the production, processing and manufacturing aspects, as well as strategies to develop the cost-effective system for clinical applications.
The initial challenges to be considered are exosome isolation, purification and characterization. In general, exosomes can be isolated from the conditioned media of cultured cells and almost any biological fluids [45]. Comprehensively, Li and co-workers wrote an update on various exosome isolation techniques and described the advantages and disadvantages of isolation techniques based on ultracentrifugation, size, immunoaffinity captured, precipitation and microfluidics [135]. To prepare clinical grade exosome, good manufacturing practices (GMPs) and quality control become an utmost important factor. In view of this, cell source and state including microenvironmental conditions must be kept uniform to provide consistent exosome quality and yield [45].
The development and translational framework of human EV-based therapeutics in general, or specifically exosomes, is regulated under biological medicinal products category [136]. A biological medicine contains one or more active substances made by or derived from biological cells, and to some extent equivalent to biologic drugs, biologicals or biopharmaceuticals [137–140]. Although regulatory framework for manufacturing and clinical trials exists in Europe, Australia and USA, but research related to establishment of special guidelines targeting EV-based therapeutics relevant to isolation, purification, characterization and their valorization is considered important area to be investigated. In the context of exosome-based therapeutics valorization and clinical translation, mechanism of action (MoA) is essential and iterative because the dissection between exosome as an active substance and excipients are important to control the quality and safety of the exosome-based therapy. Further challenges related to the changes and differentiation of residing stem cells surrounding treatment area also need special attention since exosome may influence cell behavior [141,142] including the risk of factors transduction and other cell to cell communication [143]. For clinical application of exosomes in regenerative therapy, currently there has no standardized procedure yet. The isolation and storage are critical, even more the manufacturing requires adequate and appropriate technology with quality system for the safety of both donor and recipients.
In case of manufacturing for instance, although extensive research has been conducted, but still, the practical use of exosome is restricted by limited exosome secretion capability of cells [144]. Moreover, a large dose of exosome is required in actual clinical administration [136–140,144–146]. On the other hands, several studies show that increased intracellular calcium can lead to the formation and/or production of EVs [135]. In view of this, scaffold which contains calcium may resolve the problems by creating microenvironment with a capability to adjust cell exosome production. As shown in the previous study by Wu and co-authors [144], bioactive glass (BG) ceramic ion products significantly promoted MSCs to secrete exosome without changing the morphology, size distribution and internalization of the MSCs, and simultaneously improved their biological functions.
Typical BG is composed of SiO2, Na2O, CaO and P2O5 [147,148] which has been widely used for wound repair and regeneration [149–151]. As reported by Wu et al. [144], BG ion products significantly enhanced pathways that generate intracellular exosome vesicles and release mature exosome. Wu and co-authors [144] proposed the underlying mechanism that BG ion products enhanced MSCs to generate exosomes by upregulating the expression of nSMase2 and promoted MSCs to release exosomes by upregulating the expression of Rab27a. Simultaneously, the pathways will promote vascularization of the recipient cells by regulating the levels of miR-342-5p and miR-1290 in cargoes.
From the reports [34], it is known that ceramics-based scaffold can affect the behavior of single type of cells and cell–cell interactions to enhance exosome generation. Regarding this aspect, previous research have been extensively conducted to investigate synergetic effects of ceramics-based scaffold and exosome in regenerative treatment [3,17], however, they elaborated more on the therapeutics effects, not on the effect and mechanism on exosome generation by calcium containing construct such as ceramics-based scaffold.
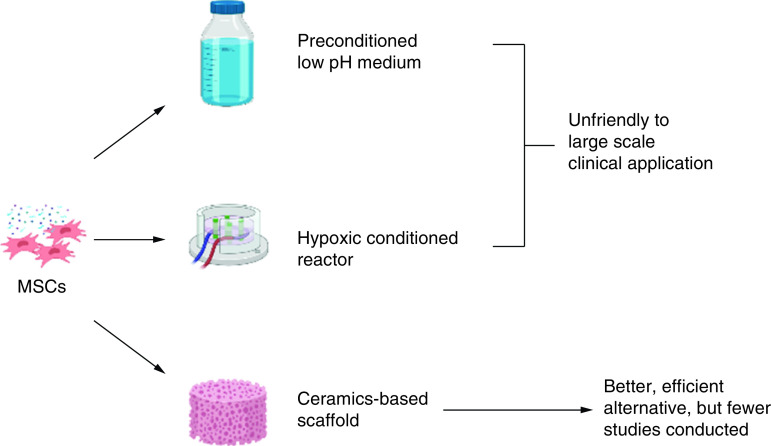
In view of this, several ceramics-based scaffold can be explored and developed as an effective strategy to either enhance exosome production or yield and improve biological activity of the secreted exosomes. The use of ceramics-based scaffold can be a better alternative strategy to overcome limited exosome secretory from the cells when compared with modulation of cell culture conditions under hypoxia and low pH microenvironment, because it will be difficult to maintain healthy cells in such unfriendly conditions for large-scale clinical applications, as concluded diagrammatically in Figure 8. Therefore, a lot of ceramics-based scaffolds are awaiting to be investigated further as an alternative strategy to enhance exosome production from MSCs or other cells, such as hydroxyapatite, carbonate apatite, calcium carbonate, some calcium orthophosphate including biphasic calcium phosphate, tricalcium phosphate, bioactive glass [3,17] or other mineral-doped scaffold [152]. Specifically, ceramics-based scaffold can function as biomaterial for exosome retention and delivery vehicles for exosome to reach targeted cells. Meanwhile, in general challenges in cell-free-based tissue engineering therapy and therapeutics using exosomes in dentistry and medicine are summarized in Figure 9.
Figure 8. . Although limited studies have been conducted, but ceramics-based scaffold has high potential to enhance exosome generation, production and secretion from MSCs due to the release of ion products from the construct such as calcium ions since the increased intracellular calcium can lead to the formation and/or production of extracellular vesicles.
The use of ceramics-based scaffold is also beneficial for exosome retention and controlled release delivery system.
Figure 9. . A lot of further in vitro and in vivo investigations are awaiting regarding isolation, characterization, production and storage of mesenchymal stem cells derived exosome for real clinical application.
Mechanism of action, biosafety and biocompatibility on the use of exosomes for cell-free-based tissue engineering and new therapeutics approach are wide open area to be studied before translating exosomes as an investigational new drugs into clinical application.
Conclusion
Clinical application on the use of MSC is limited due to its inherent heterogenicity, variation associated with cell expansion and risk of unwanted differentiation. Therefore, nowadays cell-free-based therapy with MSCs derived exosomes is considered an alternative treatment in dental tissue engineering. This is because exosomes carry with them informative cargos from the MSCs to targeted cells, which is needed to regulate fundamental cellular processes for lineage-specific proliferation, differentiation, migration, apoptosis and modulation of a series of signaling pathway. In the current state, exosome is found to be potential candidate as a therapeutic agent to regulate inflammatory, proliferation and remodeling phase in tissue regeneration. As a conclusion, exosomes could potentially provide new approaches to dental tissue engineering, but further research to uncover underlying mechanism and roles of specific signaling molecules in relation to dental tissue regeneration pathways are needed.
In addition to the first conclusion, currently there has no standardized procedure for the isolation, storage, and manufacturing technology with quality system for the safety of both donor and recipients in large-scale valorization. In case of manufacturing for instance, although extensive research has been conducted, but still, the practical use of exosome is restricted by limited exosome secretion capability of cells. The limitation on the application of exosome in clinics can be resolved by ceramics-based scaffold which can affect the behavior of single type of cells and cell–cell interactions to enhance exosome generation. Therefore, a lot of further investigations are awaiting to develop ceramics-based scaffold that functions in large-scale clinical application for cell-free-based therapy with exosomes to provide better alternative strategy to overcome limited exosome secretory from the cells, because ceramics-based scaffold produces important ions to enhance production and secretion of exosomes from the cells.
Future perspective
Exosomes are potential as an alternative future treatment in dental tissue engineering and in regenerative medicine. Exosomes carry with them informative cargos from the MSCs to regulate fundamental cellular processes for lineage-specific proliferation, differentiation, migration, apoptosis and modulation of a series of signaling pathway in the targeted cells. Because of that, exosomes are very potential for next generation therapeutic agent to regulate inflammatory, proliferation and remodeling phase in tissue regeneration. However, to date, there are still limited studies conducted to uncover underlying mechanism and roles of specific signaling molecules in relation to dental tissue regeneration pathways. There are also limited research involving exosomes for modulating extracellular signaling and intracellular reprogramming, which have been a significant approaches in tissue engineering.
On the other hands, advances in materials synthesis, protein engineering, molecular self-assembly, bio, micro and nanofabrication technology, nanotechnology as well as micro and nanopatterning technology have contributed to the high possibility of developing future therapy. When it is combined with exosomes technology, this could be resolution in regenerative therapy. Furthermore, progressive advancement in materials sciences may resolve problems and overcome limited capacity of the cells regarding production and secretion of EVs, including exosomes, because hybrid materials containing certain ions can also be designed and directed to enhance generation and secretion of exosomes for large-scale clinical applications.
To achieve future goals, better relevant and predictive in vitro or ex vivo models are needed to predict efficacy and safety of the cell-free-based therapy with exosomes. In this context, the development of microfluidic organ system known as ‘organ-on-chip’ may be crucial to capture phenomenon happens in body tissues and model diseases to provide accurate personalized medicine, involving a complex of exosomes and other biomolecules with hybrid biomaterials. A microfluidic system will allow researchers to study living tissues and organ in a more complex way and will contribute a lot to uncover underlying concept of exosomes as next generation therapeutic agent, including their novel delivery system. Interdisciplinary research to find out proper hybrid biomaterials to deliver, target, increase production, and increase secretion of exosomes involving bio-nanofabrication are significant to be conducted. Besides, standardized procedure for the isolation, storage, and manufacturing technology with quality system for the safety of both donor and recipients in large-scale valorization must be investigated. Their translational steps into dental clinics and, to large extent, into biomedical applications, are also important to be studied in the near future.
Executive summary.
In tissue engineering, the use of exosomes released have become a particular interest for cell-free regenerative therapy due to their epigenetic capacity and cargos.
To valorize the use of exosomes in large-scale clinical setting, better relevant and predictive in vitro or ex vivo models are needed to predict efficacy and safety of the cell-free-based therapy with exosomes, as well as to capture phenomenon happens in body tissues and model diseases.
Interdisciplinary research to find out proper hybrid biomaterials to deliver, target, increase production and increase secretion of exosomes involving bio-nanofabrication are significant to be conducted to uncover underlying concept of exosomes as next generation therapeutic agent.
Their translational steps into dental clinics and biomedical applications are also important to be studied in the future.
Acknowledgments
Authors used BioRender to create some figures in this study.
Footnotes
Author contributions
ID Ana reviewed the literature, analyzed the literature, drafted the manuscript and prepared figures. A Barlian, AC Hidajah, CH Wijaya, HB Notobroto and TDK Wungu reviewed and edited the manuscript. All authors read and approved the manuscript.
Financial & competing interests disclosure
This study is supported by Indonesia Collaborative Research (RKI) Funding Scheme under World Class University Acceleration Program from the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia, with a contract number 810/UN1/Ditlit/Dit-Lit/PT/2020. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Creative Commons Attribution 4.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Caplan AI. Design parameters for functional tissue engineering. In: Functional Tissue Engineering. Guilak I, Butler DL, Goldstein SA, Mooney DJ (Eds). Springer-Verlag, Amsterdam, The Netherlands, 129–130 (2003). [Google Scholar]
- 2.Badylak S, Gilbert T, Myers-Irvin J. The extracellular matrix as a biologic scaffold for tissue engineering. In: Tissue Engineering. Van Blitterswijk CA, Thomsen P, Lindahl Aet al.. et al. (Eds). Academic Press, CA, USA, 121–143 (2008). [Google Scholar]
- 3.Ana ID, Satria GAP, Dewi AH, Ardhani R. Bioceramics for clinical application in regenerative dentistry. In: Novel Biomaterials for Regenerative Medicine. Chun HJ, Park K, Kim CH, Khang G (Eds). Springer, Singapore, 309–316 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Badylak S. Xenogenic extracellular matrix as a scaffold for tissue reconstruction. Transpl. Immunol. 12(3–4), 367–377 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Farah-Carson MC, Wagner RC, Kiick KL. Extracellular matrix: structure, function, and applications to tissue engineering. In Tissue Engineering. Fisher JP, Mikos AG, Bronzino JD (Eds). CRC Press, FL, USA, 1–17 (2007). [Google Scholar]
- 6.Lopatina T, Deregibus MC, Cantaluppi V, Camussi G. Stem cell-derived microvesicles: a cell free therapy approach to the regenerative medicine. Curr. Biotechnol. 1, 11–22 (2012). [Google Scholar]
- 7.Togel F, Westenfelder C. The role of multipotent marrow stromal cells (MSCs) in tissue regeneration. Organogenesis 7(2), 96–100 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubina K, Kalinina N, Efmenko A. Adipose stromal cells stimulate angiogenesis via promoting progenitor cell differentiation, secretion of angiogenic factors, and enhancing vessel maturation. Tissue. Eng. Part A 15(8), 2039–2050 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Hare JM, Traverse JH, Henry TD. A randomized, double blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 54(24), 2277–2286 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyth S, Schroeder J, Liebergall M. Stem cells in bone diseases: current clinical practice. Br. Med. Bull. 99, 199–210 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Mingliang R, Bo Z, Zhengguo W. Stem cells for cardiac repair: status, mechanisms, and new strategies. Stem Cells. Int. 3, 109–128 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casteilla L, Planat-Benard V, Laharrague P, Cousin B. Adipose derived stromal cells: their identity and uses in clinical trials, an update. World. J. Stem. Cells 3(4), 25–33 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kunter U, Rong S, Boor P. Mesenchymal stem cells prevent progressive experimental renal failure but mal differentiate into glomerular adipocytes. J. Am. Soc. Nephrol. 18(6), 1754–1764 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Michalopoulos GK. Liver regeneration. J. Cell. Physiol. 213(2), 286–300 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokoo T, Matsumoto K, Yokote S. Potential use of stem cells for kidney regeneration. Int. J. Nephrol. 5, 917–931 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zakrzewski W, Dobrzyński M, Szymonowicz M, Zbigniew R. Stem cells: past, present, and future. Stem Cell. Res. Ther. 10(68), 1165–1186 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahanani ES, Ana ID, Bachtiar I. Human mesenchymal stem cells behavior on synthetic coral scaffold. Key. Eng. Mater. 696, 205–211 (2019). [Google Scholar]
- 18.Wang J, Ye F, Cheng L et al. Osteogenic differentiation of mesenchymal stem cells promoted by overexpression of connective tissue growth factor. J. Zhejiang Univ. Sci. B 10(5), 355–367 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breitbach M, Bostani T, Roell W. 2007. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 110(4), 1362–1369 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Gomzikova MO, Rizvanov AA. Current trends in regenerative medicine: from cell to cell-free therapy. Bio. Nano. Sci. 7, 240–245 (2017). [Google Scholar]; •• Clarifies the importance, advantages and future perspective of cell and cell-free based therapy.
- 21.Inchingolo F, Tatullo M, Marrelli M et al. Regenerative surgery performed with platelet-rich plasma used in sinus lift elevation before dental implant surgery: a useful aid in healing and regeneration of bone tissue. Eur. Rev. Med. Pharmacol. Sci. 16(9), 1222–1226 (2012). [PubMed] [Google Scholar]
- 22.Ge R, Lv Y, Li P, Xu L, Feng X, Qi H. Upregulated microRNA-126 induces apoptosis of dental pulp stem cell via mediating PTEN-regulated Akt activation. J. Clin. Lab. Anal. 35(2), e23624 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 12, 260–270 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutmacher D, Woodfield T, Dalton P, Lewis J. Scaffold design and fabrication. In: Tissue Engineering. Van Blitterswijk CA, Thomsen P, Lindahl Aet al.. et al. (Eds). Academic Press, CA, USA, 404–454 (2008). [Google Scholar]
- 25.Beer L, Mildner M, Ankersmith HJ. Cell secretome based drug substances in regenerative medicine: when regulatory affairs meet basic science. Ann. Transl. Med. 5, 170–172 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Théry C, Witwer KW, Aikawa E. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7(1), 1535750 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witwer KW, Van Balkom BWM, Bruno S et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 8(1), 1609206 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun B, Peng J, Wang S et al. Applications of stem cell-derived exosomes in tissue engineering and neurological diseases. Rev. Neurosci. 29(5), 531–546 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Wang B, Xing D, Zhu Y, Dong S, Zhao B. The State of exosomes research: a global visualized analysis. Biomed. Res. Int. 2019, 1–11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maguire G, Friedman P, Mc Carthy D, Friedman R, Maniotis A. Stem cell released molecules and exosomes in tissue engineering. Procedia. Eng. 59, 270–278 (2013). [Google Scholar]
- 31.Chopra N, Dutt Arya B, Jain N et al. Biophysical characterization, and drug delivery potential of exosomes from human wharton's jelly-derived mesenchymal stem cells. ACS Omega 4(8), 13143–13152 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis, and modulating immune reactivity. Biomaterials 156, 16–27 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Chew JRJ, Chuah SJ, Teo KYW et al. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 15(89), 252–264 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J. Extracell. Vesicles 7(1), 1522236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eiró N, Sendon-Lago J, Seoane S et al. Potential therapeutic effect of the secretome from human uterine cervical stem cells against both cancer and stromal cells compared with adipose tissue stem cells. Oncotarget 5, 10692–10708 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 18, 1852–1875 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9(8), 581–593 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Melliana A, Dewi NM, Wijaya A. Mesenchymal stem cell secretome: cell-free therapeutic strategy in regenerative medicine. Indones. Biomed. J. 11(2), 113–124 (2019). [Google Scholar]
- 39.Van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 19, 213–228 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat. Rev. Mol. Cell. Biol. 11(10), 688–699 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Simons M, Raposo G. Exosomes – vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21(4), 575–581 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Thery C, Zitgovel L, Amigorena S. 2002. Exosomes: composition, biogenesis, and functions. Nat. Rev. Immunol. 2, 569–579 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Hessvik NP, Liorente A. Current knowledge on biogenesis and release. Cell. Mol. Life Sci. 75, 193–208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell. Dev. Biol. 30, 255–289 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Ramasubramanian L, Kumar P, Wang A. Engineering extracellular vesicles as nanotherapeutics for regenerative medicine. Biomolecules 10, 48–70 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin. Cell. Dev. Biol. 40, 82–88 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, The BJ. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv. Drug. Deliv. Rev. 65(3), 336–341 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Lu K, Li HY, Yang K, Wu JL, Cai XW, Zhou Y. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: invitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell. Res. Ther. 8(1), 108–118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuh CMAP, Aguayo S, Zavala G, Khoury M. Exosome-like vesicles in Apis mellifera bee pollen, honey and royal jelly contribute to their antibacterial and pro-regenerative activity. J. Exp. Biol. 222, 1–7 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Brand HS, Veerman EC. Saliva and wound healing. Chin. J. Dent. Res. 16(1), 7–12 (2013). [PubMed] [Google Scholar]
- 51.Haque N, Widera D, Govindasamy V, Soesilawati P, Abu Kasim NH. Extracellular vesicles from stem and progenitor cells for cell-free regenerative therapy. Curr. Mol. Med. (epub ahead of print) (2021). [DOI] [PubMed] [Google Scholar]
- 52.Kayambashi P, Iyer J, Pillai S, Upadhyay A, Zhang Y, Tran SD. Hydrogel encapsulation of mesenchymal stem cells and their derived exosomes for tissue engineering. Int. J. Mol. Sci. 22(2), 684 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niedermair T, Lukas C, Li S et al. Influence of extracellular vesicles isolated from osteoblasts of patients with Cox-Arthrosis and/or osteoporosis on metabolism and osteogenic differentiation of BMSCs. Front. Bioeng. Biotechnol. 8, 615520 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin GZ. Current nanoparticle-based technology for osteoarthritis therapy. Nanomaterials (Basel) 10(12), 2368 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chew JRJ, Chuah SJ, Teo KYW et al. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 89, 252–264 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Cooper LF, Ravindran S, Huang CC, Kang M. A role of exosome in craniofacial tissue engineering and regeneration. Front. Physiol. 10, 1569 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh SW. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 156, 16–27 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Zhang S, Chu WC, Lai RC, Lim SK, Hui JHP, Toh SW. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 24(12), 2135–2140 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Zimta AA, Baru O, Badea M, Buduru SD, Berindan-Neagoe I. The role of angiogenesis and pro-angiogenetic exosome in regenerative dentistry. Int. J. Mol. Sci. 20(2), 406–427 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu X, Lian Y, Li X et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials 269, 120539 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Lee YH, Park HK, Auh QS et al. Emerging potential of exosomes in regenerative medicine for temporomandibular joint osteoarthritis. Int. J. Mol. Sci. 21(4), 1541 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang S, Teo K, Chuah SJ, Lai RC, Lim SK, Toh WS. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 200, 35–47 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Schuh CMAP, Benso B, Aguayo S. Potential novel strategies for the treatment of dental pulp-derived pain: pharmacological approaches and beyond. Front. Pharmacol. 10, 1068 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • An interesting study which describes novel strategy for the treatment of dental pulp-derived pain with exosomes.
- 64.Sjöqvist S, Ishikawa T, Shimura D et al. Exosomes derived from clinical-grade oral mucosal epithelial cell sheets promote wound healing. J. Extracell. Vesicles 8(1), 1565264 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tatullo M, Marelli B, Palmieri F et al. Exosomes from human periapical cyst-MSCs: theranostic application in parkinson's disease. Int. J. Environ. Res. Public. Health 17(1), 3001 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu S, Chen H, Gao B. Potential of exosomes in regenerative endodontics. Arch. Oral Biol. 120, 104946 (2020). [DOI] [PubMed] [Google Scholar]
- 67.Xian X, Gong Q, Li C, Guo B, Jiang JH. Exosomes with highly angiogenic potential for possible use in pulpregeneration. J. Endod. 44(5), 751–758 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Qiu X, Liu J, Zheng C et al. Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis. Cell Prolif. 53(8), e12830 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rao F, Zhang D, Fang T et al. Exosomes from human gingiva-derived mesenchymal stem cells combined with biodegradable chitin conduits promote rat sciatic nerve regeneration. Stem Cells Int. 2019, 2546367 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stefańska K, Mehr K, Wieczorkiewicz M et al. Stemness potency of human gingival cells-application in anticancer therapy and clinical trials. Cells 9(8), 1916 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holliday LS, McHugh KP, Zuo J, Aguirre JI, Neubert JK, Rody WJ Jr. Exosomes: novel regulators of bone remodeling and potential therapeutic agents for orthodontics. Orthod. Craniofac. Res. 20(Suppl. 1), 95–99 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhuang X, Ji L, Jiang H et al. Exosomes derived from stem cells from the apical papilla promote dentine-pulp complex regeneration by inducing specific dentinogenesis. Stem Cells Int. 2020, 5816723 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alqurash H, Ortega A, Lambert DW. The emerging potential of extracellular vesicles in cell-free tissue engineering and regenerative medicine. Tissue Eng. B 2020, 0222 (2020). [DOI] [PubMed] [Google Scholar]
- 74.Swanson WB, Zhang Z, Xiu K et al. Scaffolds with controlled release of pro-mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomater. 118, 215–232 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chansaenroj A, Yodmuang S, Ferreira J. Trends in salivary gland tissue engineering: from stem cells to secretome and organoid bioprinting. Tissue Eng. Part. B 2020, 0149 (2020). [DOI] [PubMed] [Google Scholar]
- 76.Yang J, Zhu W, Lu J, Xie K, Fang S, Kan L. Potential therapeutic applications of exosomes in bone regenerative medicine. In book: Osteogenesis and Bone Regeneration. IntechOpen, 1–21 (2018). [Google Scholar]
- 77.Wang KX, Xu LL, Rui YF et al. The effect of secretion factors from umbilical cord derived mesenchymal stem cells on osteogenic differentiation of mesenchymal stem cells. PLoS ONE 10, e120593 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deng Y, Zhou H, Gu P, Fan X. Repair of canine medial orbital bone defects with miR-31 modified bone marrow mesenchymal stem cells. Invest. Ophthalmol. Vis. Sci. 55, 6016–6023 (2014). [DOI] [PubMed] [Google Scholar]
- 79.Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh. Migr. 9(4), 317–324 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao B, Xie Z, Wang Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 7(1), 136 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis 4(2), 68–75 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Issack PS, Helfet DL, Lane JM. Role of Wnt signaling in bone remodeling and repair. HSS J. 4(1), 66–70 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grigorie D, Lerner UH. The crucial role of the Wnt system in bone remodeling. Acta Endocrinol. (Buchar). 14(1), 90–101 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Santis M, Di Matteo B, Chisari E et al. The role of Wnt pathway in the pathogenesis of OA and its potential therapeutic implications in the field of regenerative medicine. Biomed. Res. Int. 2018, 7402947 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 15(6), 457–475 (2004). [DOI] [PubMed] [Google Scholar]
- 86.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 12(1), 17–25 (2006). [DOI] [PubMed] [Google Scholar]
- 87.Leibbrandt A, Penninger JM. RANK/RANKL: regulators of immune responses and bone physiology. Ann. NY Acad. Sci. 1143, 123–150 (2008). [DOI] [PubMed] [Google Scholar]
- 88.Huynh N, VonMoss L, Smith D et al. Characterization of regulatory extracellular vesicles from osteoclasts. J. Dent. Res. 95, 673–679 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors 22(4), 233–241 (2004). [DOI] [PubMed] [Google Scholar]
- 90.Mostafa S, Pakvasa M, Coalson E et al. The wonders of BMP9: from mesenchymal stem cell differentiation, angiogenesis, neurogenesis, tumorigenesis, and metabolism to regenerative medicine. Genes Dis. 6(3), 201–223 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bharadwaz A, Jayasuriya AC. Osteogenic differentiation cues of the bone morphogenetic protein-9 (BMP-9) and its recent advances in bone tissue regeneration. Mater. Sci. Eng. C 120, 111748 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen Y, Bal BS, Gorski JP. Calcium and collagen binding properties of osteopontin, bone sialoprotein, and bone acidic glycoprotein-75 from bone. J. Biol. Chem. 267(34), 24871–24878 (1992). [PubMed] [Google Scholar]
- 93.Mukherjee BB, Nemir M, Beninati S et al. Interaction of osteopontin with fibronectin and other extracellular matrix molecules. Ann. NY Acad. Sci. 760, 201–212 (1995). [DOI] [PubMed] [Google Scholar]
- 94.Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem. Biophys. Res. Commun. 280(2), 460–465 (2001). [DOI] [PubMed] [Google Scholar]
- 95.White FJ, Burghardt RC, Hu J, Joyce MM, Spencer TE, Johnson GA. Secreted phosphoprotein 1 (Osteopontin) is expressed by stromal macrophages in cyclic and pregnant endometrium of mice but is induced by estrogen in luminal epithelium during conceptus attachment for implantation. Reproduction 132(6), 919–929 (2006). [DOI] [PubMed] [Google Scholar]
- 96.Lund SA, Giachelli CM, Scatena M. The role of osteopontin in inflammatory processes. J. Cell. Commun. Signal. 3(3–4), 311–322 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singh A, Gill G, Kaur H, Ahmed M, Jakhu H. Role of osteopontin in bone remodeling and orthodontic tooth movement: a review. Prog. Orthod. 19(1), 18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Si J, Wang C, Zhang D, Wang B, Zhou Y. Osteopontin in bone metabolism and bone diseases. Med. Sci. Monit. 26, e919159 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wei J, Karsenty G. An overview of the metabolic functions of osteocalcin. Rev. Endocr. Metab. Disord. 16(2), 93–98 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Komori T. Functions of osteocalcin in bone, pancreas, testis, and muscle. Int. J. Mol. Sci. 21(20), 7513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heriksen K, Karsdal MA. Type I collagen. In book: Biochemistry of Collagens, Laminins and Elastin. Academic Press, 1–11 (2016). [Google Scholar]
- 102.Bonewald LF, Mundy GR. Role of transforming growth factor-beta in bone remodeling. Clin. Orthop. Relat. Res. 250, 261–276 (1990). [PubMed] [Google Scholar]
- 103.Mundy GR. The effects of TGF-beta on bone. Ciba. Found. Symp. 157, 137–143 (1991). [PubMed] [Google Scholar]
- 104.Risau W. Mechanisms of angiogenesis. Nature 386(6626), 671–674 (1997). [DOI] [PubMed] [Google Scholar]
- 105.Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2(12), 1097–1105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 79(4), 1283–1316 (1999). [DOI] [PubMed] [Google Scholar]
- 107.Martins M, Ribeiro D, Martins A, Reis RL, Neves NM. 2016. Extracellular vesicles derived from osteogenically induced human bone marrow mesenchymal stem cells can modulate lineage commitment. Stem Cell Rep. 6, 284–291 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pereira D, Ramos E, Branco J. Osteoarthritis. Acta Med. Port. 28(1), 99–106 (2015). [DOI] [PubMed] [Google Scholar]
- 109.Xia B, Chen D, Zhang J, Hu S, Jin H, Tong P. Osteoarthritis pathogenesis: a review of molecular mechanisms. Calcif. Tissue Int. 95(6), 495–505 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bertrand J, Cromme C, Umlauf D, Frank S, Pap T. Molecular mechanisms of cartilage remodeling in osteoarthritis. Int. J. Biochem. Cell Biol. 42(10), 1594–1601 (2010). [DOI] [PubMed] [Google Scholar]
- 111.Gibofsky A. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: a synopsis. Am. J. Manag. Care 20(Suppl. 7), S128–S135 (2014). [PubMed] [Google Scholar]
- 112.Burke J, Hunter M, Kolhe R, Isales C, Hamrick M, Fulzele S. Stem cell-derived exosomes: a potential alternative therapeutic agent in orthopedics. Stem Cells Int. 2016, 5802529 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang HG, Liu C, Su K et al. A membrane form of TNF-alpha presented by exosomes delays T-cell activation-induced cell death. J. Immunol. 176(12), 7385–7393 (2006). [DOI] [PubMed] [Google Scholar]
- 114.Takeuchi R, Katagiri W, Endo S, Kobayashi T. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS ONE 14(11), e0225472 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang W, Wray LS, Rnjak-Kovacina J et al. Vascularization of hollow channel-modified porous silk scaffolds with endothelial cells for tissue regeneration. Biomaterials 56, 68–77 (2015). [DOI] [PubMed] [Google Scholar]
- 116.Dicarlo M, Bianchi N, Ferretti C, Orciani M, Di Primio R, Mattioli-Belmonte M. Evidence supporting a paracrine effect of IGF-1/VEGF on human mesenchymal stromal cell commitment. Cells Tissues Organs 201(5), 333–341 (2016). [DOI] [PubMed] [Google Scholar]
- 117.Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol. 26(8), 434–441 (2008). [DOI] [PubMed] [Google Scholar]
- 118.Saran U, Gemini PS, Chatterjee S. Role of angiogenesis in bone repair. Arch. Biochem. Biophys. 561, 109–117 (2014). [DOI] [PubMed] [Google Scholar]
- 119.Rouwkema J, Khademhosseini A. Vascularization, and angiogenesis in tissue engineering: beyond creating static networks. Trends Biotechnol. 34(9), 733–745 (2016). [DOI] [PubMed] [Google Scholar]
- 120.Kawai T, Katagiri W, Osugi M, Sugimura Y, Hibi H, Ueda M. Secretomes from bone marrow-derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy 17(4), 369–381 (2015). [DOI] [PubMed] [Google Scholar]
- 121.Fan GC. Hypoxic exosomes promote angiogenesis. Blood 123(25), 3669–3670 (2014). [DOI] [PubMed] [Google Scholar]
- 122.Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. (Berl.) 92(4), 387–397 (2014). [DOI] [PubMed] [Google Scholar]
- 123.Bucan V, Vaslaitis D, Peck CT, Strauß S, Vogt PM, Radtke C. Effect of exosomes from rat adipose-derived mesenchymal stem cells on neurite outgrowth and sciatic nerve regeneration after crush injury. Mol. Neurobiol. 56(3), 1812–1824 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hong P, Yang H, Wu Y, Li K, Tang Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Res. Ther. 10, 242 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu Z, Kalyan BS, Chen L. Therapeutic potential role of exosomes for ischemic stroke. BSA 5(2), 128–143 (2020). [Google Scholar]
- 126.Hu P, Yang Q, Wang Q et al. Mesenchymal stromal cells-exosomes: a promising cell-free therapeutic tool for wound healing and cutaneous regeneration. Burn Trauma 7, 38 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell. Mol. Life Sci. 73, 3861–3885 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Adams DO, Hamilton TA. The cell biology of macrophage activation. Annu. Rev. Immunol. 2, 283–318 (1984). [DOI] [PubMed] [Google Scholar]
- 129.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 4(3), 281–286 (2005). [DOI] [PubMed] [Google Scholar]
- 130.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25(12), 677–686 (2004). [DOI] [PubMed] [Google Scholar]
- 131.Sugimoto MA, Sousa LP, Pinho V, Peretti M, Teixeira MM. Resolution of inflammation: what controls its onset? Front. Immunol. 7, 160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev. Mol. Med. 13, e23 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang JH, Liu XL, Sun JM, Yang JH, Xu DH, Yan SS. Role of mesenchymal stem cell derived extracellular vesicles in autoimmunity: a systematic review. World J. Stem Cells 12(8), 879–896 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv. Drug. Deliv. Rev. 106, 148–156 (2016). [DOI] [PubMed] [Google Scholar]
- 135.Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 38, 754–763 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The potential of exosomes for next-generation therapeutic agents are well described in this study.
- 136.Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics 7(3), 789–804 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lener T, Gimona M, Aigner L et al. Applying extracellular vesicles-based therapeutics in clinical trials - an ISEV position paper. J. Extracell. Vesicles. 4, 30087 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ilic N, Savic S, Siegel E, Atkinson K, Tasic L. Examination of the regulatory frameworks applicable to biologic drugs (including stem cells and their progeny) in Europe, the U.S., and Australia: part I – a method of manual documentary analysis. Stem Cells Transl. Med. 1, 898–908 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ilic N, Savic S, Siegel E, Atkinson K, Tasic L. Examination of the regulatory frameworks applicable to biologic drugs (including stem cells and their progeny) in Europe, the U.S., and Australia: part II – a method of software documentary analysis. Stem Cells Transl. Med. 1, 909–920 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reiner AT, Witwer KW, Van Balkom BWM et al. Concise review: developing best-practice models for the therapeutic use of extracellular vesicles. Stem Cells Transl. Med. 6(8), 1730–1739 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Marrelli M, Codispoti B, Shelton RM et al. Dental pulp stem cell mechanoresponsiveness: effects of mechanical stimuli on dental pulp stem cell behavior. Front. Physiol. 26(9), 1685 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ballini A, Boccaccio A, Saini R, Van Pham P, Tatullo M. dental-derived stem cells and their secretome and interactions with bioscaffolds/biomaterials in regenerative medicine: from the in vitro research to translational applications. Stem Cells Int. 2017, 6975251 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Inchingolo F, Tatullo M, Abenavoli FM et al. Non-Hodgkin lymphoma affecting the tongue: unusual intra-oral location. Head Neck Oncol. 3, 1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu Z, He D, Li H. Bioglass enhances the production of exosomes and improves their capability of promoting vascularization. Bioact. Mater. 6(3), 823–835 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Since the production and secretion of the exosomes from cells are still a challenge, this study provides solution to resolve the problem based on the role of calcium-containing scaffold.
- 145.Watson DC, Yung BC, Bergamaschi C et al. Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. J. Extracell. Vesicles 7(1), 1442088 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Patel DB, Luthers MJ, Fisher JP, Steven MJ. Enhanced extracellular vesicle production and ethanol-mediated vascularization bioactivity via a 3D-printed scaffold-perfusion bioreactor system. Acta Biomater. 95, 236–244 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Technology to enhance extracellular vesicle production is described in this study, and this can be a good reference for the application of tissue engineering with exosome and biomaterials.
- 147.Hench LL. The story of bioglass. J. Mater. Sci. Mater. Med. 17, 967–978 (2006). [DOI] [PubMed] [Google Scholar]
- 148.Ana ID, Anggraeni R. Development of bioactive resin modified glass ionomer cement for dental biomedical applications. Heliyon 7(1), e05944 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dong X, Chang J, Li H. 2017. Bioglass promotes wound healing through modulating the paracrine effects between macrophages and repairing cells. J. Mater. Chem. B 5, 5240–5250 (2017). [DOI] [PubMed] [Google Scholar]; •• This study is an interesting study to elucidate the mechanism of Bioglass in enhancing wound healing by switching of macrophages and paracrine effects between the macrophages and repairing cells in wound healing, thus is beneficial in understanding the roles of calcium in wound healing and regeneration in combination with cells or their secretomes.
- 150.Xu Y, Peng J, Li H, Chang J. Combined chemical and structural signals of biomaterials synergistically activate cell-cell communications for improving tissue regeneration. Acta Biomater. 55, 249–261 (2017). [DOI] [PubMed] [Google Scholar]; •• This is a good study to be referred related to signals important for cell–cell communications, including the role of exosome in combination with biomaterials.
- 151.Li H, Xue N, Kong K, Chang J. Silicate bioceramics enhanced vascularization and osteogenesis through stimulating interactions between endothelia cells and bone. Biomaterials 35, 3803–3818 (2014). [DOI] [PubMed] [Google Scholar]; •• This study clearly described the roles of biomaterials in cell–cell interaction, thus important to further studies employing biomaterials and exosomes.
- 152.Tatullo M, Spagnuolo G, Codispoti B et al. PLA-based mineral-doped scaffolds seeded with human periapical cyst-derived MSCs: a promising tool for regenerative healing in dentistry. Materials (Basel). 12(4), 597 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]