Abstract
Guillain–Barré syndrome is often caused by Campylobacter jejuni infection that has induced antibodies to the lipo-oligosaccharide (LOS) that cross-react with gangliosides at peripheral nerves causing polyneuropathy. To examine fine specificities of anti-ganglioside antibodies and develop a more robust platform for diagnosis and disease monitoring, we developed a chemoenzymatic approach that provided an unprecedented panel of oligosaccharides composed of the inner-core of the LOS of C. jejuni extended by various ganglioside mimics. The compounds and corresponding ganglio-oligosaccharides were printed as a microarray to examine binding specificities of lectins, anti-ganglioside antibodies, and serum antibodies of GBS patients. Although lectins and anti-ganglioside antibodies did not differentiate the ganglio-oligosaccharides and mimics, the patient serum samples bound much more strongly to the ganglioside mimics. The data indicate that antibodies have been elicited to a foreign epitope that includes a heptosyl residue unique of bacterial LOS and that these antibodies subsequently cross-react with lower affinity to gangliosides. The microarray detected anti-GM1a antibodies with high sensitivity and will be attractive for diagnosis, disease monitoring, and immunological research.
Graphical Abstract
INTRODUCTION
Guillain–Barré syndrome (GBS) is a potentially life-threatening disease of the peripheral nervous system characterized by rapid and progressive limb weakness.1 The clinical course and severity of GBS are variable, and approximately a quarter of the patients develop respiratory failure or severe autonomic dysfunction.2,3 GBS often occurs after a respiratory or gastrointestinal tract infection, and previous studies have shown some of these infections induce antibodies that cross-react with gangliosides at peripheral nerves thereby causing polyneuropathy.4 The type of infection and the specificity of the resulting anti-ganglioside antibodies are important determinants of the subtype and clinical course of GBS.5 The most common pathogen causing the antecedent infection of GBS is Campylobacter jejuni (C. jejuni), which is associated with acute motor axonal neuropathy subtype of GBS and a severe clinical course.6 Other infections that have been associated with GBS include Mycoplasma pneumoniae, hepatitis E virus, cytomegalovirus, Epstein–Barr virus, and Zika virus.7–9
C. jejuni is a Gram-negative, non-spore-forming bacterium that is a common cause of gastroenteritis and is transmitted to humans through ingestion of insufficiently cooked poultry, contaminated milk, and water.10 It produces a lipo-oligosaccharide (LOS) that often terminates in a structure resembling the saccharide moieties of gangliosides. In particular, LOSs of C. jejuni that are associated with GBS often produce structures mimicking the oligosaccharide moieties of GM1a and GD1a.11–13 In addition, strains have been isolated that express GD3, GM2, GM3, and GT1a mimics.14 Clinical and serologic data support a model in which the LOS of specific C. jejuni strains elicit antibodies that recognize both bacterial molecules and gangliosides, and recognition of the latter biomolecules, which are abundantly expressed in the nervous system where they are involved in neurotransmission, causes neurological disfunction.11,15 Anti-GM1a antibodies are the most frequently observed antibodies in GBS and associated with a severe and pure motor clinical phenotype.16,17
Although there is strong scientific support for the involvement of anti-ganglioside antibodies in the pathogenesis of GBS,2 molecular mechanisms by which immunotolerance is broken leading to autoimmune-like responses are not well understood. Furthermore, the role of anti-ganglioside antibodies in diagnosis is fraught with difficulties, and in particular the frequency and specificity of anti-ganglioside antibodies are low leading to false negative results. The positive predictive value of anti-ganglioside antibodies, especially those of the IgM class, is also compromised because these can occur in other diseases. Detection of anti-ganglioside antibodies is mainly performed by ELISA using gangliosides,18 usually obtained by isolation from natural sources. These compounds are often not homogeneous, and only a limited number of structures are readily available, which is impeding comprehensive analysis of structure–binding relationships and mechanisms by which they promote nerve damage.
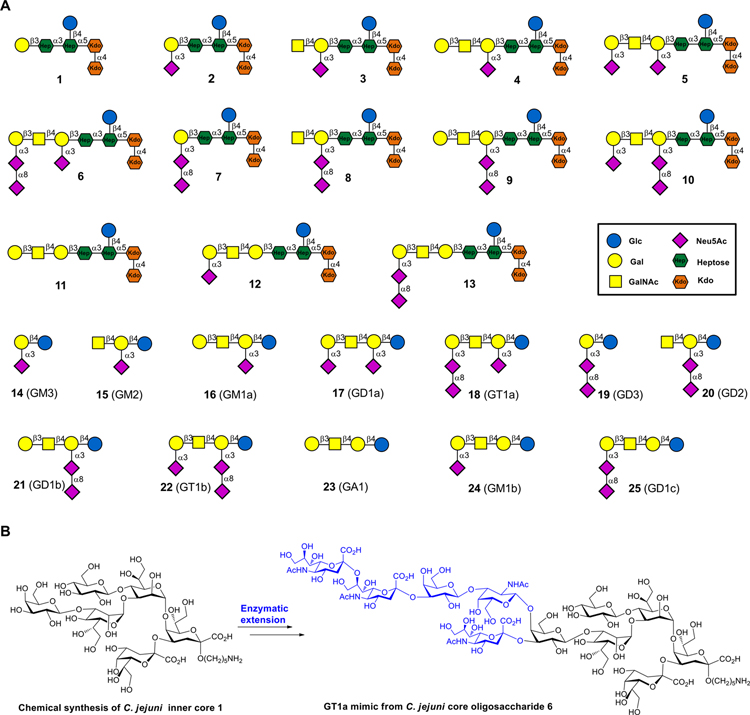
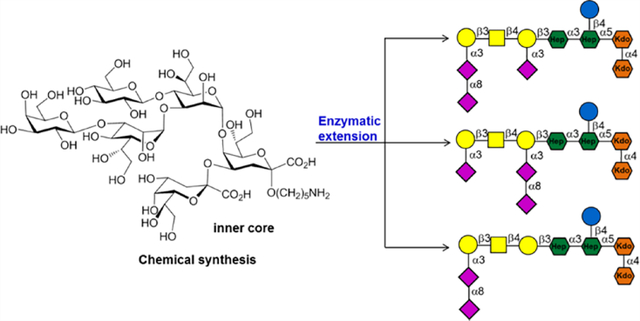
During natural infections, the immune system is primed by LOS of C. jejuni strains,19 and thus we hypothesized that anti-ganglioside antibodies elicited during C. jejuni infection are directed to epitopes that straddle the inner core region of LOS and the ganglioside structural analogs. To test this mode of immune recognition, we synthesized a large panel of oligosaccharides composed of the inner core oligosaccharide of the LOS of C. jejuni extended by various ganglioside mimics. Compound 1 resembles the inner core oligosaccharide of C. jejuni, and compounds 2–13 are derived from this structure but extended by the oligosaccharide mimics of GM1 (2), GM2 (3), GM1a (4), GD1a (5), GT1a (6), GD3 (7), GD2 (8), GD1b (9), GT1b (10), GA1 (11), GM1b (12), and GD1c (13). We also prepared the corresponding ganglioside oligosaccharides 14–25 (Figure 1). Compounds 2–13 were obtained by a chemoenzymatic strategy in which inner core oligosaccharide 1 was prepared chemically and then extended using appropriate microbially derived glycosyltransferases to install ganglio-mimetics. The ganglioside oligosaccharides 14–25 were prepared starting from spacer modified lactose which was enzymatically extended to provide the target compounds. The oligosaccharides were printed as a glycan microarray, which was used to investigate the binding reactivities of a range of lectins, anti-ganglioside antibodies, and serum antibodies of normal and GBS patients. Although the lectins and anti-ganglioside antibodies recognized a ganglio-oligosaccharide and corresponding mimic equally well, the patient serum samples bound much more strongly to the mimics. This observation indicates that GBS patients have not generated primary autoreactive antibodies but cross-reactive antibodies, and most likely epitopes targeted by serum antibodies include a heptosyl residue unique of bacterial LOS. Such an epitope will be perceived as foreign for mammalian immune cells, thereby providing a rationale for breaking immune-tolerance. It was also observed that the microarray platform detects with greater sensitivity anti-GM1a antibodies compared to traditional ELISA using bovine extracted gangliosides, and this will be attractive for diagnosis, disease monitoring, and immunological research.
Figure 1.
Synthetic ganglioside mimics from C. jejuni core oligosaccharides and normal ganglio-oligosaccharide library by a chemoenzymatic approach: (A) synthetic ganglioside mimic and ganglioside oligosaccharide library; (B) chemically synthesized inner core hexasaccharide 1 and enzymatic extension of 1 to afford compounds such as GT1a ganglioside mimic 6.
RESULTS AND DISCUSSION
Chemical Synthesis of Inner Core Oligosaccharide (1).
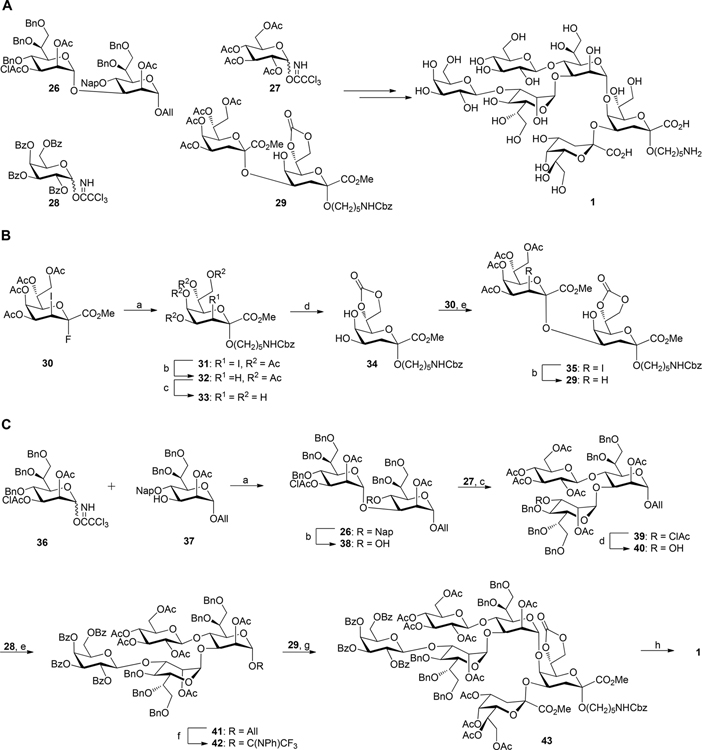
The chemical synthesis of the inner core hexasaccharide 1 is challenging due to the low acceptor reactivity of the C5-hydroxyl of Kdo-α-(2→4)-Kdo acceptor 2920–22 and the need to install glycosidic bonds to form a highly crowded 3,4-branched heptoside.23,24 We envisaged that fully protected hexasaccharide 1 could be synthesized by a convergent and stereocontrolled [4 + 2] approach (Scheme 1) relying on the use of flexible disaccharide intermediate 26, which is modified by the orthogonal protecting groups 2-naphthylmethyl (Nap) ether, chloroacetyl (ClAc) ester, and allyl (All) ether. The ability to remove the Nap ether and ClAc ester protecting groups in different orders made it possible to establish the optimal glycosylation sequence, using glycosyl 27 and 28, to prepare tetrasaccharide 41 having a crowded 3,4-branched heptoside. Removal of the anomeric allyl ether of 41 and conversion of the resulting lactol into a donor followed by glycosylation with 29 would install the challenging α(1→5)-linked Hep-Kdo glycosidic linkage affording the protected hexasaccharide 43. Global deprotection of the latter compound would provide the required inner core hexasaccharide 1.
Scheme 1. (A) Chemical Synthesis of Inner Core Hexasaccharide 1 and (B) Synthesis of Kdo2 Glycosyl Acceptor 29a.
aReagents and conditions: (a) HO(CH2)5NHCbz, BF3·Et2O, CH2Cl2, 3 Å molecular sieves, 0 °C to rt, 2.5 h, 75%; (b) lauroyl peroxide, ClCH2CH2Cl/cyclohexane 1:7, reflux at 90 °C, 2 h, 83% for 32, 87% for 29; (c) NaOCH3/CH3OH, rt, 4 h, 92%; (d) diphosgene, sym-collidine, THF, −25 °C, 30 min, 76%; (e) BF3·Et2O, CH2Cl2, 3 Å molecular sieves, 0 to 15 °C, 2 h, 80%. (C) Assembly of hexasaccharide 1, reagents and conditions: (a) TMSOTf, CH2Cl2, 4 Å molecular sieves, −20 to −10 °C, 30 min, 89%; (b) DDQ, CH2Cl2/PBS buffer (100 mM, pH 7.4) 10:1, rt, 1.5 h, 78%; (c) TMSOTf, CH2Cl2, 4 Å molecular sieves, 0 to 5 °C, 70 min, 80%; (d) thiourea, sym-collidine, CH2Cl2/CH3OH 2:3, 70 °C, 24 h, 85%; (e) TMSOTf, CH2Cl2, 4 Å molecular sieves, −10 to 0 °C, 60 min, 90%; (f) (i) PdCl2, CH2Cl2/MeOH 1:5, rt, 2.5 h, 86%; (ii) CF3C(NPh)Cl, Cs2CO3, CH2Cl2, rt, 4 h, 85%; (g) TMSOTf, CH2Cl2, 5 Å molecular sieves, rt, 2 h, 40%; (h) (i) 1 M NaOH(aq)/CH3OH/dioxane 1:1:3, rt, 16 h, (ii) H2, Pd(OH)2/C, t-BuOH/H2O/HOAc 3:2:0.02, rt, 18 h, 42% over 2 steps.
First, α(2→4)-linked Kdo dimer 29 was prepared which has a free hydroxyl at C5 for further glycosylation at this position (Scheme 1B). Glycosylation using a Kdo donor with an iodide at C3 as a stereodirecting group is attractive because it provides only α-anomeric products.25 Thus, coupling of donor 30 with benzyloxycarbonyl protected 5-aminopentanol in the presence of BF3·Et2O (2 equiv) as the promoter gave ketoside 31 in a yield of 75% as only the 2,3-trans-isomer. Only a small amount of the glycal byproduct (8%) was isolated due to a competing elimination reaction. The 3-iodo-substituent of 31 was readily removed by hydrogen atom transfer from cyclohexane induced by lauroyl peroxide in 1,2-dichloroethane to afford spacer modified α-linked Kdo 32.26 1H NMR analysis of 32 showed a low-field shift of H-4 (δ = 5.26 ppm), which was typical for an α-anomeric Kdo linkage in 4-O-acylated Kdo derivatives.27,28 Treatment of 32 with a catalytic amount of sodium methoxide in methanol resulted in removal of the acetyl esters to give tetraol 33. The heteronuclear coupling constant of C-1 with the axial proton at C-3 position (3JC1–H3ax < 1 Hz) further confirmed the α-anomeric configuration of Kdo.27 The 7,8-diol of 33 was selectively protected as a cyclic carbonate by treating with diphosgene (1 equiv) in the presence of sym-collidine at −25 °C to give 34 having a free diol at C-4,5. Careful control of the reaction conditions including the equivalence of diphosgene and temperature was important to avoid further reaction of the 4,5-O-diol. The equatorial C4 hydroxyl of 34 has higher glycosyl acceptor reactivity compared to the axial hydroxyl at C5, and thus it was expected that 34 is an appropriate acceptor for glycosylation at C4 without a need for further protecting group manipulations. Indeed, a BF3·Et2O promoted glycosylation of 30 with 34 resulted in the selective formation of α(1,4)-linked ketoside 35 in high yield. Dehalogenation of the latter compound was achieved using standard conditions providing the desired dimeric Kdo acceptor 29.
Next, attention was focused on the preparation of 3,4-branched heptosyl donor 42 (Scheme 1C). Thus, a TMSOTf promoted glycosylation of 36 with 37 gave disaccharide 26 as only α-anomer (JC1–H1 = 176 Hz) due to neighboring group participation by the acetyl ester at C-2 of the donor. To give 38, the Nap ether of 26 was oxidatively removed using 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ)29 in a mixture of CH2Cl2 and phosphate buffer (pH 7.4) without affecting any other protecting groups. A glycosylation of the latter acceptor with glycosyl donor 27, using TMSOTf (1.3 equiv) as the activator, afforded the 3,4-branched trisaccharide 39. A relatively large quantity of TMSOTf was required to avoid the formation of an orthoester byproduct (Figure S1). Furthermore, the use of per-benzyolated glucosyl trichloroacetimidate as donor did not provide the corresponding trisaccharide probably due to steric hindrance.23 Next, the chloroacetyl (ClAc) ester30,31 of 39 was selectively cleaved by treatment with thiourea in the presence of sym-collidine to give trisaccharide 40, which was glycosylated with glycosyl donor 28 using a catalytic amount of TMSOTf as promotor to afford tetrasaccharide 41 in 90% yield. Trifluoro-N-phenylacetimidate 42 was prepared by a two-step procedure involving selective removal of the ally ether of 41 using PdCl2 to give a lactol, which was reacted with N-phenyltrifluoroacetimidoyl chloride in the presence of Cs2CO3.32,33
Having glycosyl acceptor 29 and donor 42 in hand, attention was focused on the preparation of inner core hexasaccharide 1. Preactivated of glycosyl acceptor 29 using a catalytic amount of TMSOTf followed by slow addition of glycosyl donor 42 at room temperature resulted in the formation of hexasaccharide 43. Inverse glycosylation34,35 at room temperature was essential, and standard glycosylation conditions gave only trace amounts of 43, and in this case mainly decomposition of the glycosyl donor and acceptor was observed. The low reactivity of the sterically hindered C-5 hydroxyl of 29 probably causes a mismatch in reactivity with glycosyl donor 42 resulting in a challeging glycosylation. Finally, global deprotection of 43 to give hexasaccharide 1 was accomplished by saponification of the esters and carbonate using sodium hydroxide, followed by catalytic hydrogenolysis over Pd-(OH)2/C to remove the benzyl ethers. The NMR data of 1 confirmed the anomeric configurations of all glycosidic linkages.
Previously, we36 and others23,37,38 have employed a 7,8-O-isopropylidene protected Kdo acceptor for glycosylations to increase the reactivity of the axial C5-hydroxyl. However, the use of such an acceptor proved incompatible with BF3·Et2O mediated glycosylation and resulted in the formation of a complex mixture of products probably due to isopropylidene cleavage (Figure S2). Our studies demonstrate that a 7,8-carbonate is an attractive protecting group for Kdo containing oligosaccharides being extended at the anomeric center and C-4 and C-5. We also explored an alternative glycosylation sequence for the preparation of 1 by constructing the challenging α-Hep-(1→5)-Kdo glycosidic linkage to give α-Hep-(1→5)-Kdo-α-(2→4)-Kdo trisaccharide that was converted into a glycosyl acceptor for coupling with a β-Gal-(1→3)-Hep donor to form a properly protected pentasaccharide for further glucosylation (Figure S3). Although the pentasaccharide was detected by MALDI-MS, it was difficult to purify and the yield was low (<10%). Probably steric hindrance at the C-3″ hydroxyl of α-Hep-(1→5)-Kdo-α-(2→4)-Kdo trisaccharide led to failure of the glycosylation.
Enzymatic Synthesis of Ganglioside Oligosaccharides and Ganglioside Mimics.
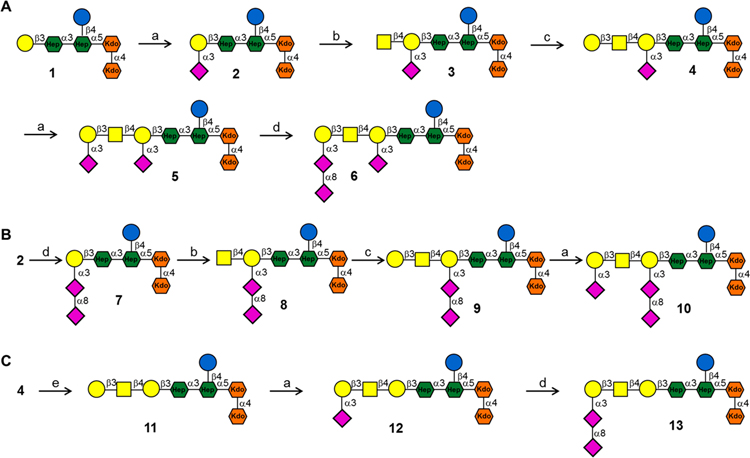
Having inner core hexasaccharide 1 in hand, attention was focused on enzymatic extension to give ganglioside mimics 2–13 (Scheme 2). Glycosyltransferases from microbes such as C. jejuni and Pasteurella multocida have been identified that can assemble ganglio-series oligosaccharides. These include the α2,3-sialyltransferase PmST139 from Pasteurella multocida that can form GM3; the β1,4-N-acetylgalactosaminyltransferase (CgtA) from C. jejuni which can synthesize GM2; β1,3-galactosyltransferase (CgtB)40 from C. jejuni that can prepare GM1; and the bifunctional sialyltransferase CstII41 from C. jejuni that has both α2,3-sialyltransferase (GM3 oligosaccharide synthase) and α2,8-sialyltransferase (GD3 oligosaccharide synthase) activity. Starting from lactose, these enzymes have been used to prepare several oligosaccharide moieties of gangliosides including GM3, GM2, GM1a, GD3, GD2, and GD1b.42 The enzyme toolbox has, however, not made it possible to prepare a number of other ganglio-oligosaccharides including GA1, GM1b, and GD1c. Here, we introduce a synthetic strategy that addressed the latter deficiency and made it possible to prepare an unprecedented collection of ganglio-oligosaccharides (14–25). It was expected that the galactosyl moiety of inner-core oligosaccharide 1 would provide a proper starting structure for extension by the glycosyltransferases24,42–44 and was amenable to our strategy to give access to ganglioside mimics 2–12.
Scheme 2. Enzymatic Extension of 1 to Install Various Outer Core Ganglioside Epitopesa.
aReagents and conditions: (a) PmST1, CMP-Neu5Ac, 100 mM Tris-HCl (pH 8.0), 37 °C, 81% for 2, 75% for 5, 74% for 10, 76% for 12; (b) CgtA, UDP-GalNAc, 10 mM MgCl2, 50 mM Tris-HCl (pH 7.5), 37 °C, 92% for 3, 88% for 8; (c) CgtB, UDP-Gal, 10 mM MgCl2, 50 mM Tris-HCl (pH 7.5), 37 °C, 83% for 4, 80% for 9; (d) CstII, CMP-Neu5Ac, 100 mM Tris-HCl (pH 8.0), 37 °C, 76% for 6, 77% for 7, 74% for 13; (e) α2–3,6,8,9 neuraminidase A from Arthrobacter ureafaciens, 5 mM CaCl2, 50 mM sodium acetate (pH 5.5), 37 °C, 94%.
First, inner-core hexasaccharide 1 was sialylated with PmST1 and cytidine-5′-monophospho-N-acetylneuraminic acid (CMP-Neu5Ac) to give GM3 mimic 2. The progress of the reaction was carefully monitored by ESI-MS because PmST1 has residual hydrolytic activity and, after consumption of CMP-NeuAc, can hydrolyze the product.45 Oligosaccharide 2 was smoothly converted into GM2 mimic 3 using CgtA and uridine 5′-diphospho-N-acetylgalactosamine (UDP-GalNAc). Next, the synthesis of GM1a mimic 4 was accomplished by treatment of 3 with CgtB in the presence of uridine 5′-diphosphogalactose (UDP-Gal). To accomplish a high yield, it was critical to employ only 1 equiv of UDP-Gal because an excess of this reagent resulted in further galactosylation of 4. α2,3-Sialylation of 4 with PmST1 and CMP-Neu5Ac resulted in the formation of GD1a mimic 5, which was further sialylated using α(2,8)-sialyltransferase CstII in the presence of CMP-Neu5Ac and calf intestine alkaline phosphatase (CIAP) to provide GT1a mimic 6 (Scheme 2A). 1H NMR analysis of 6 showed a downfield shift of the H-8 (from 3.73 to 4.02 ppm) compared to the same proton of the terminal α(2,3)-Neu5Ac-II residue of 5. In addition, a NOESY spectrum revealed a correlation between the H-3ax (δ = 1.580) of terminal α(2,8)-Neu5Ac-III and H-8 (δ = 4.016) of α(2,3)-Neu5Ac-II43 (Figure S16), further confirming that the α(2,8)-Neu5Ac moiety was attached to the terminal α(2,3)-Neu5Ac. Thus, the results demonstrate that CstII only extends the terminal α2,3-linked sialoside.
In parallel, 2 was converted into GD3 mimic 7 using CstII and CMP-Neu5Ac (Scheme 2B). The amount of CMP-Neu5Ac was carefully controlled to avoid further α(2,8)-sialylation.44 Furthermore, it was observed that the rate of α(2,8)-sialylation of 2 is faster than for 6. Next, 7 was subjected to CgtA in the presence of UDP-GalNAc to form GD2 mimic 8. Treatment of the latter compound with CgtB and 1 equiv UDP-Gal resulted in the formation of GD1b mimic 9, which was further sialylated by PmST1 to provide GT1b mimic 10.
The synthesis of GA1, GM1b, and GD1c mimics 11, 12, and 13 is challenging because previous studies have shown that CgtA46 requires an α(2,3)-linked sialic acid at the terminal galactoside to install a β(1,4)-GalNAc moiety, and thus compound 1 cannot be extended by this enzyme and the enzyme toolbox does not provide entry into 11–13. To address this difficulty, we opted to treat 4 with a sialidase to give 11 which can then be further elaborated with the glycosyltransferases to provide 12 and 13. Commercially available α2–3,6,8 neuraminidases from Clostridium perfringens and Vibrio cholerae were not able to cleave the internal and branched Neu5Ac moiety. Gratifyingly, α2–3,6,8,9 neuraminidase A from Arthrobacter ureafaciens could remove the Neu5Ac residue of 4 to give GA1 mimic 11. A relatively high concentration of this neuraminidase and a prolonged incubation time were required to cleave the internal Neu5Ac residue. Sialylation of 11 using PmST1 and CMP-Neu5Ac afforded GM1b mimic 12, which could be further sialylated using CstII to provide GD1c mimic 13 (Scheme 2C).
The corresponding ganglioside oligosaccharides 14–25 (Figure S11) were prepared by a similar strategy starting from lactose modified by an anomeric aminopropyl linker. The preparation of GA1, GM1b, and GD1c did not require expensive α2–3,6,8,9 neuraminidase, and in this case, treatment of 16 with 2 M acetic acid resulted in clean removal of the internal Neu5Ac to give GA1 oligosaccharide 23 (Figure S11),47 which was further extended using appropriate glycosyltransferases to provide GM1b and GD1c oligosaccharides 24 and 25, respectively. It is important to note that treatment of 4 with acetic acid results in cleavage of the Kdo glycosidic linkages.
The enzymatic conversions were monitored by ESI-MS, and products were purified by Bio-Gel P-2 or P-4 size exclusion chromatography and HPLC using a HILIC column (XBridge Amide 5 μm, 10 mm × 250 mm, Waters), and the structural identity of each compound was confirmed by NMR spectroscopy (1D and 2D) and HR-MS.
Glycan Recognition of Lectins, mAbs, and Serum Antibodies Using a Glycan Micoarray Platform.
The synthetic oligosaccharides 1–25 were employed to examine specificities of serum antibodies of patients in the acute stage of GBS following a C. jejuni infection, and the results were compared to healthy control serum samples. For this purpose, a glycan microarray was constructed by piezoelectric non-contact printing of the aminopentyl containing glycans onto N-hydroxysuccinimide (NHS) ester activated glass in replicates of six. To validate the array, the slides were examined for binding by the plant lectins RCA I, SBA, MAL II, and WGA. The subarrays were also probed for binding by a number of commercially available anti-ganglioside antibodies.
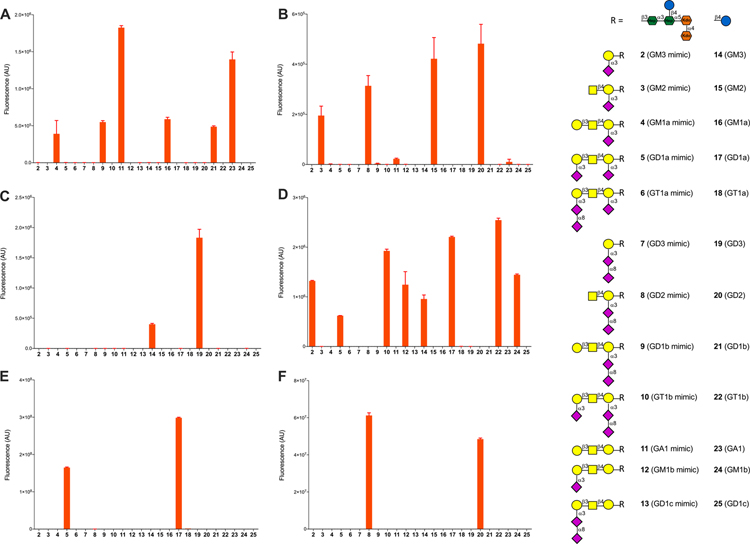
RCA I binds galactose, and as expected, all compounds bearing such a terminal residue (16/4 GM1a/GM1a mimic; 21/9 GD1b/GD1b mimic; 23/11 GA1/GA1 mimic) were recognized (Figure 2A). SBA preferentially binds GalNAc but also recognizes Gal residues although with much lower affinity.48 Binding to SBA was observed for compounds having a terminal GalNAc including compounds 15 (GM2), 20 (GD2), and their corresponding mimics 3 and 8 (Figure 2B). Weak binding was detected for compounds 23/11 (GA1/GA1 mimic) having a terminal Gal residue, and no binding was observed for 16/4 (GM1a/GM1a mimic) and 21/9 (GD1b/GD1b mimic) due to the inhibitory effect of Neu5Ac.49 MAL-II binds Neu5Ac(α2–3)Gal(β1–4)GlcNAc/Glc moieties,50 and as anticipated, compounds 14 and 19, having this epitope, were bound by this lectin (Figure 2C).49 The corresponding ganglioside mimics 2 and 7, respectively, were not recognized most likely because the glucose moiety of the binding epitope is replaced by heptose. Finally, we examined WGA, which preferentially binds GlcNAc moieties and also interacts with glycoproteins via terminal sialic acid residues. As expected, only the ganglioside oligosaccharides having a terminal α(2,3)Neu5Ac moiety (14, 17, 22, 24) and the corresponding mimics (2, 5, 10, 12) showed binding (Figure 2D).
Figure 2.
Microarray results of the synthetic ganglioside library at 100 μM with (A) RCA I (20 μg/mL), (B) SBA (20 μg/mL), (C) MAL II (20 μg/mL), (D) WGA (10 μg/mL), (E) anti-GD1a mAb, clone GD1a-1 (2 μg/mL), and (F) anti-GD2 mAb, clone 14.G2a (2 μg/mL). Bars represent the mean ± SD.
Next, binding profiles of several anti-ganglioside antibodies were determined. As expected, an anti-GD1a mAb (Figure 2E) only showed binding to 17 (GD1a) and 5 (GD1a mimic), and an anti-GD2 mAb (Figure 2F) only bound to 20 (GD2) and 8 (GD2 mimic). A polyclonal GM1 antibody (Figure S12A) exhibited some promiscuity and recognized compounds 16/4 (GM1a/GM1a mimic), 21/9 (GD1b/GD1b mimic), and 23/11 (GA1/GA1 mimic), indicating the terminal Gal(β1,3)-GalNAc moiety of these compounds is recognized.51 The subarrays were also treated with neuraminidase A (N-A), which cleaves terminal α2,3-, α2,6-, and α2,8-linked sialic acid residues, and then reprobed by this antibody, showing the expected binding patterns (Figure S12B). Importantly, the antibodies recognized the corresponding ganglio-oligosaccharides and ganglio-mimics with similar intensities indicating that the underlying inner-core oligosaccharide of the mimics does not influence recognition.
Having established the glyco-microarray is appropriate for examining specificities of anti-ganglioside antibodies, attention was focused on determining IgG and IgM antibodies binding profiles of 12 serum samples from patients that suffered from Guillain–Barré syndrome and had an antecedent C. jejuni infection (Table S2) and showed positive serological responses in a traditional ELISA for anti-ganglioside antibodies (Table S3).
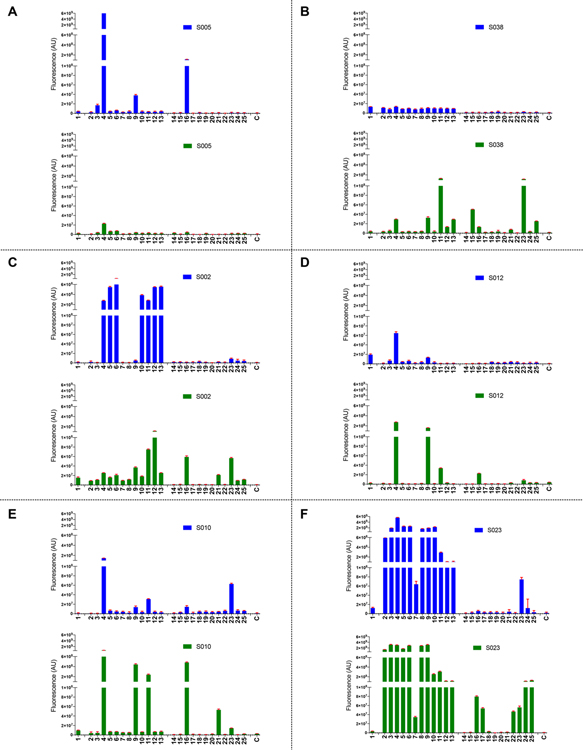
Representative array results are presented in Figure 3, and the data for all patient serum samples are shown in Figure S13A. In each sample, IgM and/or IgG antibodies were observed that bound one or more of the ganglioside oligosaccharides (14–25). Anti-GM1a (16), anti-GM1b (24), and anti-GD1a (17) antibodies have been associated with the motor form of GBS after C. jejuni infection.11,12,52 The array data showed that the majority of the patients had elicited IgM and/or IgG antibodies targeting GM1a. Anti-GM1b and GD1a antibody responses were observed in only a few serum samples. Interestingly, many patients had elicited antibodies against GA1 (23); however, the clinical significance of this observation needs validation. As expected, on slide treatment of 14 and 19 with neuraminidase to create lactose abolished binding confirming lactose is not recognized by the serum antibodies.
Figure 3.
Microarray results of the synthetic ganglioside library (1–25) at 100 μM with serum samples (1:500) (A) S005, (B) S038, (C) S002, (D) S012, (E) S010, and (F) S023. For each sample, the graph at the top shows in blue IgG responses and the graph at the bottom in green IgM responses. Bars represent the mean ± SD. C indicates blank control. Assays are performed in the same session, and results are depicted at the same scale.
It was observed that there is a significant correlation between the recognition of specifc ganglio-oligosaccharides and the corresponding mimic indicating cross-reactivity (see Figure S14 for 4 vs 16). However, for almost all of the examined samples, the binding of the ganglioside mimics (2–13) was much stronger than that of the corresponding ganglioside oligosaccharides (14–25). Furthermore, in most cases, no or a low response was observed for the inner-core oligosaccharide (1). These observations indicate that the GBS patients had elicited antibodies mainly to the outer core region of LOS of C. jejuni. The finding that the ganglioside oligosaccharides are not as well recognized as the mimics indicates that gangliosides are suboptimal ligands for the serum antibodies. In this respect, the ganglio-oligosaccharides have a lactosyl (Galβ(1,4)Glc) moiety at the reducing end, whereas the mimics have a Galβ(1,3)Hep epitope at this position. Thus, it is likely that the serum antibodies recognize an epitope that encompasses the heptosyl residue of the inner-core and the ganglio-oligosaccharide of the outer core. A heptoside poorly mimics the glucosyl moiety of gangliosides, thereby providing a rationale for the differences in responsiveness of ganglio-oligosaccharides and mimics. Thus, it appears that antibodies elicited during C. jejuni infection leading to GBS are directed to epitopes that straddle the inner- and outer-core region of LOS that can cross-react with autologous gangliosides. This finding is in accordance with the clinical observation that GBS has a monophasic course in which patients tend to recover after clearing of the infection.
Although commonalities were observed in the antibody responses, also differences were noted, which probably is due to the clinical heterogeneity of GBS. First, the magnitude of the antibody responses differed considerably between the various serum samples. For one patient, mainly IgG antibodies were observed (S005), whereas for another one predominantly IgM antibodies were detected (S038). However, most patients had elicited IgM and IgG antibodies, but in these cases also differences in binding patterns were observed. For several patients (S010, S014, S023, S035, S039) IgM and IgG antibodies were detected that bound to the ganglio-oligosaccharides as well as to the mimics. Other samples (S002, S007, S012) showed strong IgG antibody responses to the ganglioside mimics (2–14) with little- or no binding to the corresponding ganglio-oligosaccharides. In these cases, IgM antibodies were present that bound the ganglioside-oligosaccharides. Probably, class switching and antibody affinity maturation resulted in IgG antibodies with high affinity for the mimics but a low affinity for the gangliosides. It can, however, not be excluded that antibodies binding potently to gangliosides have been scavenged by nerves leaving antibodies to the mimics behind.53
We also examined five serum samples (S017, S019, S024, S033, S037) of patients that had suffered GBS but for whom no anti-ganglioside antibodies had been detected by traditional ELISA (Table S3). In three of these samples (S017, S024, S033), the microarray detected anti-GM1a IgM antibody indicating the new platform is more sensitive to detect such antibodies (Figures S13B and S15). In addition, we analyzed antibody responses of 10 control serum samples, and as expected, no or very low responses were measured (Figure S13C).
CONCLUSIONS
It is widely accepted that antibodies elicited to the outer core oligosaccharide of the LOS of C. jejuni, which structurally resembles the oligosaccharide moiety of gangliosides, cause neural injury and clinical symptoms of GBS.1–3 Despite their importance in the pathogenesis of GBS, the fine specificities of anti-ganglioside serum antibodies have been difficult to examine, and for example it is not known if elements of the inner core are part of antigenic epitopes. It is the expectation that knowledge of the fine specificities of anti-ganglioside antibodies elicited by GBS patients will provide a more comprehensive understanding of the microbial factors important for the pathogenesis of GBS and give opportunties to develop more robust platforms for diagnosis and monitoring of disease activity. Such investigations require a comprehensive set of well-defined and pure lipo-oligosaccharides of C. jejuni in which the conserved inner core is extended by various ganglioside mimetics. It also necessitates a complementary range of ganglio-oligosaccharides to systematically compare antigenic responses with the ganglio-mimics. To obtain a comprehensive range of ganglioside mimics, we drew inspiration from enzymatic approaches to prepare ganglio-oligosaccharides by extension of lactose by various microbial glycosyl transferases41,42,44,54 and envisaged that an inner core terminating in galactose would be also an appropriate precursor for these enzymes. We devised an efficient synthetic approach for C. jejuni inner core hexasaccharide based on a convergent and stereo–controlled [4 + 2] oligosaccharide assembly. It employed orthogonally protected building blocks that offered flexibility in the assembly of the hexasaccharide. Enzymatic extension of the hexasaccharide by a panel of glycosyltransferases afforded core oligosaccharides having various outer core ganglioside mimics. In parallel, we developed a chemoenzymatic approach for the gangliosides including the previously inaccessible GA1, GM1b, and GD1c by chemical or enzymatic removal of the sialoside of GM1a followed by enzymatic extension. The synthetic efforts provide an unprecedented collection of ganglio-oligosaccharides and their mimics including GM1, GM2, GM1a, GD1a, GT1a, GD3, GD2, GD1b, GT1b, GA1, GM1b, and GD1c.
The oligosaccharides were printed as a microarray to examine fine specificities of serum antibodies of GBS patients that had suffered an antecedent C. jejuni infection. The results showed that the majority of GBS patients had elicited IgM and/or IgG antibodies targeting GM1a (16). Interestingly, in almost all cases the GM1a mimic (4) was bound much more strongly indicating that in addition to the outer core, which mimics GM1a, part of the inner core is also recognized. The inner core (1) by itself gave low or no responsiveness highlighting that the outer core contains the critical antigenic component. Collectively, these observations indicate that antibodies elicited during the preceding C. jejuni infection leading to GBS are primarily raised to an epitope on the pathogen (LOS) that cross-react to the autologous gangliosides. Thus, although the outer core resembles a self-structure, it is attached to heptosyl residue thereby creating a foreign antigen for mammalian immune cells. It was also observed that the microarray platform can more sensitively detect anti-GM1a antibodies compared to a traditional ELISA format using bovine extracted gangliosides, which was especially the case for IgM antibodies targeting GM1a. This may be due to the covalent binding of the antigens allowing the use of detergents during washing steps reducing nonspecific binding. Furthermore, a good correlation was found between the responsiveness of GM1a and its mimic (Figure S14), and thus the ganglioside mimics, which were recognized much more potently, may be attractive for the detection of serum antibodies associated with GBS. A glycan microarray has many advantages for the development of the next generation platform to examine the presence of anti-ganglioside antibodies to facilitate GBS diagnoses and disease monitoring. It requires minute quantities of precious oligosaccharides that can be printed in a format that allows in one operation the examination of a large number of compounds and samples. The assay is robust because compounds are chemically immobilized, and it is more sensitive and has a greater dynamic range compared to traditional ELISA. Antibodies that recognize ganglioside complexes have been observed in GBS patients.55 A microarray platform makes it possible to print many different combinations of ganglio-oligosaccharides allowing the detection of such antibodies. To establish it as a platform for GBS diagnoses and monitoring of disease activity, future efforts will focus on the preparation of additional ganglioside mimics to determine the minimal epitope for binding by serum antibodies. In particular, attention will be focused on compounds having truncated inner core oligosaccharides to determine to what extent it is recognized by the serum antibodies. In addition, a large number of serum samples will be screened to establish threshold values of clinically relevant antibody responses.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. J. Glushka for assisting with NMR experiments, A. P. Tio-Gillen and W. van Rijs for technical assistance with ELISAs, and Dr. L. Liu for assisting with microarray printing and calculations. This research was supported by the National Institute of General Medical Sciences from the U.S. National Institutes of Health (Grant U01GM120408 to G.-J.B.) and the Dutch Prinses Beatrix Spierfonds (Grant W.OR09-04 to B.C.J), cofinanced by Stichting Spieren voor Spieren.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c08583.
Synthetic protocols, microarray procedures, compound characterization, and NMR and LC–MS spectra (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/jacs.0c08583
The authors declare no competing financial interest.
Contributor Information
Tiehai Li, Complex Carbohydrate Research Center, University of Georgia, Athens, Georgia 30602-4712, United States.
Margreet A. Wolfert, Complex Carbohydrate Research Center, University of Georgia, Athens, Georgia 30602-4712, United States; Department of Chemical Biology and Drug Discovery, Utrecht Institute for Pharmaceutical Sciences, and Bijvoet Center for Biomolecular Research, Utrecht University, 3584 Utrecht, The Netherlands.
Na Wei, Complex Carbohydrate Research Center, University of Georgia, Athens, Georgia 30602-4712, United States.
Ruth Huizinga, Department of Immunology, Erasmus MC, University Medical Center Rotterdam, 3015 GD Rotterdam, The Netherlands.
Bart C. Jacobs, Department of Immunology and Department of Neurology, Erasmus MC, University Medical Center Rotterdam, 3015 GD Rotterdam, The Netherlands.
Geert-Jan Boons, Complex Carbohydrate Research Center and Department of Chemistry, University of Georgia, Athens, Georgia 30602-4712, United States; Department of Chemical Biology and Drug Discovery, Utrecht Institute for Pharmaceutical Sciences, and Bijvoet Center for Biomolecular Research, Utrecht University, 3584 Utrecht, The Netherlands.
REFERENCES
- (1).Goodfellow JA; Willison HJ Guillain-Barré syndrome: a century of progress. Nat. Rev. Neurol. 2016, 12, 723–731. [DOI] [PubMed] [Google Scholar]
- (2).Willison HJ; Jacobs BC; van Doorn PA Guillain-Barré syndrome. Lancet 2016, 388, 717–727. [DOI] [PubMed] [Google Scholar]
- (3).Kondziella D Autonomic dysfunction in Guillain-Barré syndrome puts patients at risk. Neurocrit. Care 2020, 32, 86–87. [DOI] [PubMed] [Google Scholar]
- (4).Kaida K; Ariga T; Yu RK Antiganglioside antibodies and their pathophysiological effects on Guillain-Barré syndrome and related disorders - a review. Glycobiology 2009, 19, 676–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).van den Berg B; Walgaard C; Drenthen J; Fokke C; Jacobs BC; van Doorn PA Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat. Rev. Neurol. 2014, 10, 469–482. [DOI] [PubMed] [Google Scholar]
- (6).Rees JH; Soudain SE; Gregson NA; Hughes RAC Campylobacter jejuni Infection and Guillain-Barré syndrome. N. Engl. J. Med. 1995, 333, 1374–1379. [DOI] [PubMed] [Google Scholar]
- (7).Jacobs BC; Rothbarth PH; van der Meche FG; Herbrink P; Schmitz PI; de Klerk MA; van Doorn PA The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology 1998, 51, 1110–1115. [DOI] [PubMed] [Google Scholar]
- (8).van den Berg B; van der Eijk AA; Pas SD; Hunter JG; Madden RG; Tio-Gillen AP; Dalton HR; Jacobs BC Guillain-Barré syndrome associated with preceding hepatitis E virus infection. Neurology 2014, 82, 491–497. [DOI] [PubMed] [Google Scholar]
- (9).Cao-Lormeau VM; Blake A; Mons S; Lastere S; Roche C; Vanhomwegen J; Dub T; Baudouin L; Teissier A; Larre P; Vial AL; Decam C; Choumet V; Halstead SK; Willison HJ; Musset L; Manuguerra JC; Despres P; Fournier E; Mallet HP; Musso D; Fontanet A; Neil J; Ghawche F Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016, 387, 1531–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Young KT; Davis LM; DiRita VJ Campylobacter jejuni: molecular biology and pathogenesis. Nat. Rev. Microbiol. 2007, 5, 665–679. [DOI] [PubMed] [Google Scholar]
- (11).Yuki N; Susuki K; Koga M; Nishimoto Y; Odaka M; Hirata K; Taguchi K; Miyatake T; Furukawa K; Kobata T; Yamada M Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barré syndrome. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 11404–11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Goodfellow JA; Bowes T; Sheikh K; Odaka M; Halstead SK; Humphreys PD; Wagner ER; Yuki N; Furukawa K; Furukawa K; Plomp JJ; Willison HJ Overexpression of GD1a ganglioside sensitizes motor nerve terminals to anti-GD1a antibody-mediated injury in a model of acute motor axonal neuropathy. J. Neurosci. 2005, 25, 1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Godschalk PC; Kuijf ML; Li J; St. Michael F; Ang CW; Jacobs BC; Karwaski MF; Brochu D; Moterassed A; Endtz HP; van Belkum A; Gilbert M Structural characterization of Campylobacter jejuni lipooligosaccharide outer cores associated with Guillain-Barré and Miller Fisher syndromes. Infect. Immun. 2007, 75, 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Gilbert M; Karwaski MF; Bernatchez S; Young NM; Taboada E; Michniewicz J; Cunningham AM; Wakarchuk WW The genetic bases for the variation in the lipo-oligosaccharide of the mucosal pathogen, Campylobacter jejuni. Biosynthesis of sialylated ganglioside mimics in the core oligosaccharide. J. Biol. Chem. 2002, 277, 327–337. [DOI] [PubMed] [Google Scholar]
- (15).Nachamkin I; Allos BM; Ho T Campylobacter species and Guillain-Barré syndrome. Clin. Microbiol. Rev. 1998, 11, 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Yuki N; Yoshino H; Sato S; Miyatake T Acute axonal polyneuropathy associated with anti-GM1 antibodies following Campylobacter enteritis. Neurology 1990, 40, 1900–1902. [DOI] [PubMed] [Google Scholar]
- (17).Jacobs BC; van Doorn PA; Schmitz PI; Tio-Gillen AP; Herbrink P; Visser LH; Hooijkaas H; van der Meche FG Campylobacter jejuni infections and anti-GM1 antibodies in Guillain-Barré syndrome. Ann. Neurol. 1996, 40, 181–187. [DOI] [PubMed] [Google Scholar]
- (18).Ravindranath MH; Ravindranath RMH; Morton DL; Graves MC Factors affecting the fine specificity and sensitivity of serum antiganglioside antibodies in ELISA. J. Immunol. Methods 1994, 169, 257–272. [DOI] [PubMed] [Google Scholar]
- (19).van Belkum A; van den Braak N; Godschalk P; Ang W; Jacobs B; Gilbert M; Wakarchuk W; Verbrugh H; Endtz H A Campylobacter jejuni gene associated with immune-mediated neuropathy. Nat. Med. 2001, 7, 752–753. [DOI] [PubMed] [Google Scholar]
- (20).Pokorny B; Kosma P First and stereoselective synthesis of an alpha-(2→5)-linked disaccharide of 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo). Org. Lett. 2015, 17, 110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Kong L; Vijayakrishnan B; Kowarik M; Park J; Zakharova AN; Neiwert L; Faridmoayer A; Davis BG An antibacterial vaccination strategy based on a glycoconjugate containing the core lipopolysaccharide tetrasaccharide Hep2Kdo2. Nat. Chem. 2016, 8, 242–249. [DOI] [PubMed] [Google Scholar]
- (22).Trattnig N; Farcet JB; Gritsch P; Christler A; Pantophlet R; Kosma P Synthesis of a pentasaccharide fragment related to the inner core region of rhizobial and agrobacterial lipopolysaccharides. J. Org. Chem. 2017, 82, 12346–12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Bernlind C; Oscarson S Synthesis of a branched heptose- and KDO-containing common tetrasaccharide core structure of Haemophilus influenzae lipopolysaccharides via 1,6-anhydro-L-glycero-beta-D-manno-heptopyranose intermediate. J. Org. Chem. 1998, 63, 7780–7788. [Google Scholar]
- (24).Yoshida F; Yoshinaka H; Tanaka H; Hanashima S; Yamaguchi Y; Ishihara M; Saburomaru M; Kato Y; Saito R; Ando H; Kiso M; Imamura A; Ishida H Synthesis of the core oligosaccharides of lipooligosaccharides from Campylobacter jejuni: A putative cause of Guillain-Barré syndrome. Chem. - Eur. J. 2019, 25, 796–805. [DOI] [PubMed] [Google Scholar]
- (25).Pokorny B; Kosma P Synthesis of chlamydia lipopolysaccharide haptens through the use of alpha-specific 3-iodo-Kdo fluoride glycosyl donors. Chem. - Eur. J. 2015, 21, 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Pokorny B; Kosma P Synthesis of 5-O-oligoglucosyl extended alpha-(2→4)-Kdo disaccharides corresponding to inner core fragments of Moraxellaceae lipopolysaccharides. Carbohydr. Res. 2016, 422, 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Unger FM; Stix D; Schulz G The anomeric configurations of the two ammonium (methyl 3-deoxy-d-manno-2-octulopyranosid)-onate salts (methyl α- and β-ketopyranosides of KDO). Carbohydr. Res. 1980, 80, 191–195. [Google Scholar]
- (28).Kosma P; Strobl M; Allmaier G; Schmid E; Brade H Synthesis of pentasaccharide core structures corresponding to the genus-specific lipopolysaccharide epitope of Chlamydia. Carbohydr. Res. 1994, 254, 105–132. [DOI] [PubMed] [Google Scholar]
- (29).Xia J; Abbas SA; Locke RD; Piskorz CF; Alderfer JL; Matta KL Use of 1,2-dichloro 4,5-dicyanoquinone (DDQ) for cleavage of the 2-naphthylmethyl (NAP) group. Tetrahedron Lett. 2000, 41, 169–173. [Google Scholar]
- (30).Masaki M; Kitahara T; Kurita H; Ohta M A new method for the removal of chloroacetyl groups. J. Am. Chem. Soc. 1968, 90, 4508–4509. [Google Scholar]
- (31).Kováč P; Yeh HJC; Glaudemans CPJ Synthesis of methyl O-(3-deoxy-3-fluoro-β-d-galactopyranosyl)-(1→6)-β-d-galactopyranoside and methyl O-(3-deoxy-3-fluoro-β-d-galactopyranosyl)-(1→6)-O-β-d-galactopyranosyl-(1→6)-β-d-galactopyranoside. Carbohydr. Res. 1985, 140, 277–288. [DOI] [PubMed] [Google Scholar]
- (32).Yu B; Tao HC Glycosyl trifluoroacetimidates. Part 1: Preparation and application as new glycosyl donors. Tetrahedron Lett. 2001, 42, 2405–2407. [Google Scholar]
- (33).Zhu XM; Schmidt RR New principles for glycoside-bond formation. Angew. Chem., Int. Ed. 2009, 48, 1900–1934. [DOI] [PubMed] [Google Scholar]
- (34).Toepfer A; Schmidt RR An efficient synthesis of the Lewis X (Lex) antigen family. Tetrahedron Lett. 1992, 33, 5161–5164. [Google Scholar]
- (35).Liu M; Yu B; Hui Y First total synthesis of 25(R)-ruscogenin-1-yl β-D-xylopyranosyl-(1→3)-[β-D-glucopyranosyl-(1→2)]-β-D-fucopyranoside, an ophiopogonis saponin from the tuber of Liriope muscari (Decne.). Tetrahedron Lett. 1998, 39, 415–418. [Google Scholar]
- (36).Boltje TJ; Zhong W; Park J; Wolfert MA; Chen W; Boons GJ Chemical synthesis and immunological evaluation of the inner core oligosaccharide of Francisella tularensis. J. Am. Chem. Soc. 2012, 134, 14255–14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Yang Y; Martin CE; Seeberger PH Total synthesis of the core tetrasaccharide of Neisseria meningitidis lipopolysaccharide, a potential vaccine candidate for meningococcal diseases. Chem. Sci. 2012, 3, 896–899. [Google Scholar]
- (38).Huang W; Zhou Y; Pan X; Zhou X; Lei J; Liu D; Chu Y; Yang J Stereodirecting effect of C5-carboxylate substituents on the glycosylation stereochemistry of 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo) thioglycoside donors: Stereoselective synthesis of α- and β-Kdo glycosides. J. Am. Chem. Soc. 2018, 140, 3574–3582. [DOI] [PubMed] [Google Scholar]
- (39).Yu H; Chokhawala H; Karpel R; Yu H; Wu B; Zhang J; Zhang Y; Jia Q; Chen X A multifunctional Pasteurella multocida sialyltransferase: a powerful tool for the synthesis of sialoside libraries. J. Am. Chem. Soc. 2005, 127, 17618–17619. [DOI] [PubMed] [Google Scholar]
- (40).Bernatchez S; Gilbert M; Blanchard M-C; Karwaski M-F; Li J; DeFrees S; Wakarchuk WW Variants of the β1,3-galactosyltransferase CgtB from the bacterium Campylobacter jejuni have distinct acceptor specificities. Glycobiology 2007, 17, 1333–1343. [DOI] [PubMed] [Google Scholar]
- (41).Yu H; Cheng J; Ding L; Khedri Z; Chen Y; Chin S; Lau K; Tiwari VK; Chen X Chemoenzymatic synthesis of GD3 oligosaccharides and other disialyl glycans containing natural and non-natural sialic acids. J. Am. Chem. Soc. 2009, 131, 18467–18477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Yu H; Li Y; Zeng J; Thon V; Nguyen DM; Ly T; Kuang HY; Ngo A; Chen X Sequential one-pot multienzyme chemoenzymatic synthesis of glycosphingolipid glycans. J. Org. Chem. 2016, 81, 10809–10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Gilbert M; Brisson JR; Karwaski MF; Michniewicz J; Cunningham AM; Wu Y; Young NM; Wakarchuk WW Biosynthesis of ganglioside mimics in Campylobacter jejuni OH4384. Identification of the glycosyltransferase genes, enzymatic synthesis of model compounds, and characterization of nanomole amounts by 600-MHz 1H and 13C NMR analysis. J. Biol. Chem. 2000, 275, 3896–3906. [DOI] [PubMed] [Google Scholar]
- (44).Blixt O; Vasiliu D; Allin K; Jacobsen N; Warnock D; Razi N; Paulson JC; Bernatchez S; Gilbert M; Wakarchuk W Chemoenzymatic synthesis of 2-azidoethyl-ganglio-oligosaccharides GD3, GT3, GM2, GD2, GT2, GM1, and GD1a. Carbohydr. Res. 2005, 340, 1963–1972. [DOI] [PubMed] [Google Scholar]
- (45).Sugiarto G; Lau K; Qu J; Li Y; Lim S; Mu S; Ames JB; Fisher AJ; Chen X A sialyltransferase mutant with decreased donor hydrolysis and reduced sialidase activities for directly sialylating Lewisx. ACS Chem. Biol. 2012, 7, 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Wen LQ; Zheng Y; Jiang K; Zhang MZ; Kondengaden SM; Li SS; Huang K; Li J; Song J; Wang PG Two-step chemoenzymatic detection of N-acetylneuraminic acid-alpha(2–3)-galactose glycans. J. Am. Chem. Soc. 2016, 138, 11473–11476. [DOI] [PubMed] [Google Scholar]
- (47).Varki A; Diaz S The release and purification of sialic acids from glycoconjugates: methods to minimize the loss and migration of O-acetyl groups. Anal. Biochem. 1984, 137, 236–247. [DOI] [PubMed] [Google Scholar]
- (48).Rao VSR; Lam K; Qasba PK Three dimensional structure of the soybean agglutinin Gal/GalNAc complexes by homology modeling. J. Biomol. Struct. Dyn. 1998, 15, 853–860. [DOI] [PubMed] [Google Scholar]
- (49).’t Hart IME; Li T; Wolfert MA; Wang S; Moremen KW; Boons GJ Chemoenzymatic synthesis of the oligosaccharide moiety of the tumor-associated antigen disialosyl globopentaosylceramide. Org. Biomol. Chem. 2019, 17, 7304–7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Goldstein IJ; Winter HC; Poretz RD In New Comprehensive Biochemistry; Elsevier, 1997; Vol. 29, Part B, pp 403–474. [Google Scholar]
- (51).Steck A; Yuki N; Graus F Antibody testing in peripheral nerve disorders. Handb. Clin. Neurol. 2013, 115, 189–212. [DOI] [PubMed] [Google Scholar]
- (52).Kusunoki S; Iwamori M; Chiba A; Hitoshi S; Arita M; Kanazawa I GM1b is a new member of antigen for serum antibody in Guillain-Barré syndrome. Neurology 1996, 47, 237–242. [DOI] [PubMed] [Google Scholar]
- (53).Cunningham ME; McGonigal R; Meehan GR; Barrie JA; Yao D; Halstead SK; Willison HJ Anti-ganglioside antibodies are removed from circulation in mice by neuronal endocytosis. Brain 2016, 139, 1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Meng X; Yao W; Cheng J; Zhang X; Jin L; Yu H; Chen X; Wang F; Cao H Regioselective chemoenzymatic synthesis of ganglioside disialyl tetrasaccharide epitopes. J. Am. Chem. Soc. 2014, 136, 5205–5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Rinaldi S; Brennan KM; Kalna G; Walgaard C; van Doorn P; Jacobs BC; Yu RK; Mansson JE; Goodyear CS; Willison HJ Antibodies to heteromeric glycolipid complexes in Guillain-Barré syndrome. PLoS One 2013, 8, No. e82337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.