Abstract
We analyzed the effects of a single 14 day course of teplizumab treatment on metabolic function and immune cells among participants in a randomized controlled trial of non-diabetic relatives at high-risk for Type 1 diabetes (T1D). In an extended follow up (923 day median) of a previous report of teplizumab treatment the median times to diagnosis were 59.6 and 27.1 months for teplizumab and placebo treated participants, respectively (HR=0.457, p=0.01). Fifty % of teplizumab-treated but only 22% of the placebo-treated remained diabetes free. Glucose tolerance, C-peptide area under the curve (AUC), and insulin secretory rates were calculated, and relationships to T cell subsets and function were analyzed. Teplizumab treatment improved beta cell function, reflected by average on-study C-peptide AUC (1.94 vs 1.72 pmol/ml; p=0.006). Drug treatment reversed a decline in insulin secretion prior to enrollment followed by stabilization of the declining C-peptide AUC seen with placebo treatment. Proinsulin:C-peptide ratios after drug treatment were similar between the treatment groups. The changes in C-peptide with teplizumab treatment were associated with increases in partially exhausted memory KLRG1+TIGIT+ CD8+ T cells (r=0.44; p=0.014) that showed reduced secretion of IFNγ and TNFα. A single course of teplizumab had lasting effects on delay of T1D diagnosis and improved beta cell function in high-risk individuals. Changes in CD8+ T cell subsets indicate that partially exhausted effector cells are associated with clinical response. This is the first trial to show improvement in metabolic responses and delay of diabetes with immune therapy.
Single Sentence Summary:
Teplizumab treatment of non-diabetic relatives at high-risk for T1D induces partially exhausted CD8+ T cells and improves beta cell function.
Introduction
Type 1 diabetes (T1D) is an autoimmune disease characterized by T-cell mediated destruction of insulin producing beta cells within the pancreatic islets of Langerhans. Longitudinal observational studies over more than 30 years have described the progression of the autoimmune disease from the first appearance of autoantibodies until beta cell function is critically impaired and the clinical diagnosis, often with ketoacidosis, occurs (1-5). T1D is associated with a need for lifelong exogenous insulin administration for survival, increased morbidity and mortality due to immediate (e.g. hypoglycemia) and long term complications (e.g. vascular, renal, and eye disease), and reduced life-span, life impairments, and considerable health-care-related costs (6-9). Thus, approaches to prevent progression to clinical T1D before irremediable beta cell destruction and insulin deficiency are of paramount importance.
Changes in beta cell function precede the clinical diagnosis of T1D and have been studied in natural history cohorts of individuals who are identified as at-risk for the disease based on the presence of islet autoantibodies (10-12). Some studies suggest an ongoing and intermittently progressive decline in beta cell function, that begins years before clinical diagnosis at a time when glucose tolerance is normal. During this period there are signs of ongoing autoimmunity: Based on the findings of natural history studies, individuals with two or more islet autoantibodies have been classified as stages of T1D, with further specification according to the level of metabolic dysfunction: stage 1 prior to glucose abnormalities, stage 2 with dysglycemia during an oral glucose tolerance test (OGTT), and stage 3 at clinical presentation with hyperglycemia (2, 13, 14). However, the relationships between changes in beta cell function and clinical disease remain poorly defined. It is known, for example, that glucose tolerance, defined through responses to an oral glucose tolerance test (OGTT), may fluctuate between abnormal and normal values within an individual who is at risk (15, 16). In addition, OGTT glucose tolerance classifications used to designate a clinical diagnosis, and beta cell function, measured by C-peptide responses to a metabolic challenge, may not be closely related and at diagnosis using an OGTT, many individuals have clinically meaningful C-peptide responses (15-18).
Based on successes from previous studies in patients with Stage 3 T1D (i.e. after clinical diagnosis) with teplizumab, an Fc receptor-nonbinding anti-CD3ε monoclonal antibody, that showed reduced decline in stimulated C-peptide responses compared to placebo or control participants (19-25), in the TrialNet TN10 anti-CD3 prevention trial, we conducted a randomized Phase II trial of teplizumab in individuals with Stage 2 disease, to test whether treatment would prevent or delay the clinical diagnosis of T1D (26). In this time-to-event study, we found a delay in the median time to diagnosis of 24 months with teplizumab vs placebo, and a reduction in the rate of diabetes diagnoses from 35.9% to 14.9% per year (26). This trial represented the first to show successful prevention or delay in the diagnosis of T1D with immune therapy (27-31).
Because the participants in the TrialNet TN10 anti-CD3 prevention trial were not diagnosed with clinical T1D at the time of study enrollment, the successful outcome of the trial has enabled us to evaluate, for the first time, the effects of the therapy on beta cell function and its relationship to immune modifications when the disease process was clinically silent.
To test the hypothesis that the immune therapy would improve beta cell function in the at-risk individuals from the TrialNet TN10 anti-CD3 prevention trial, we analyzed the results of metabolic studies performed before, during, and after conclusion of the original trial, as well as immune responses. Specifically, we collected data from OGTTs before randomization, as well as follow-up OGTT data from non-diabetic participants available through monitoring in the TrialNet TN01 Pathway to Prevention Study. Our data show a persistence of the duration of treatment effects on delay in clinical presentation of T1D. We show that a single course of treatment with teplizumab reversed a decline in C-peptide production prior to study entry and improved the beta cell response to oral glucose after treatment compared to placebo. Early insulin secretion was also improved with teplizumab, suggesting qualitative improvement in beta cell function, although we did not detect a difference in the ratio of proinsulin:C-peptide between treatment groups. After the initial 3-6 months following treatment, the C-peptide responses were stable compared to placebo until an abrupt decline in the response approximately 6 months prior to diagnosis in those who were diagnosed with clinical T1D. The improved C-peptide responses were associated with an increase in the frequency of TIGIT+KLRG1+ memory CD8 T cells, which exhibited reduced secretion of IFNγ and TNFα, two inflammatory cytokines linked to beta cell destruction (32). These studies indicate that even prior to clinical diagnosis, treatment with teplizumab can improve metabolic function associated with modulation of pathologic T cell signatures.
Results
Teplizumab treatment resulted in a sustained delay in T1D during extended follow-up studies:
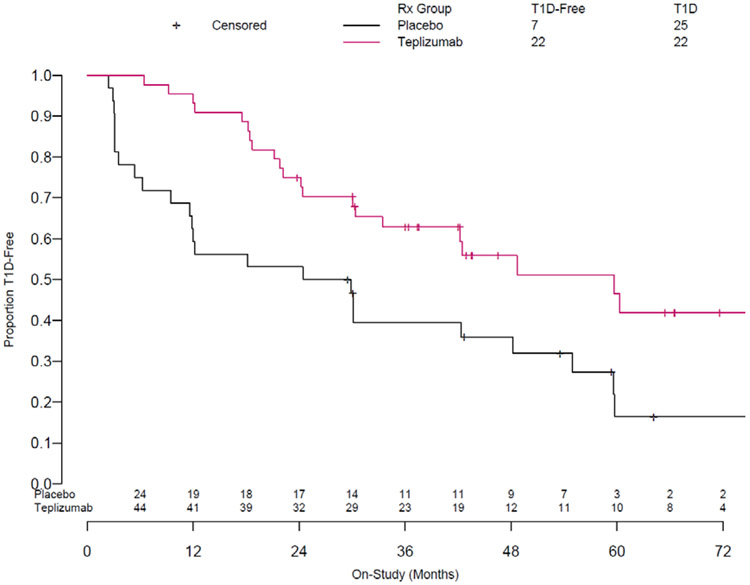
A total of 76 relatives at high-risk, but without a clinical diagnosis of T1D were enrolled into the TrialNet TN10 anti-CD3 prevention trial (26). The median age was 13 (range 8-49) and all participants had 2+ autoantibody tests within the 6 months prior to enrollment. We previously reported, after a median follow up of 742 days (range 74 to 2683) that 42 were diagnosed with T1D. We have since continued to follow the study participants for a median time of 923 days (range of 74-3,119) (Supplemental Figure 1). Over this extended period of follow-up, 25/32 (78%) of the placebo-treated and 22/44 (50%) of the teplizumab-treated participants were diagnosed with T1D (Figure 1) (Cox model adjusting for stratification and age: HR = 0.457 p = 0.01). The median times to diagnosis of T1D were 59.6 and 27.1 months in the teplizumab and placebo treatment groups respectively. Ten of thirteen subjects followed beyond 60 months were not diagnosed with T1D. Of these individuals, eight were in the teplizumab group and two were in the placebo group.
Figure 1. Teplizumab Treatment is Associated with a Sustained Effect on Type 1 Diabetes Progression Over 923 Days of Follow-up.
Updated Kaplan-Meier Curve based on 923 days of follow up (range 74-3,119 days). Hazard ratio for development of type 1 diabetes in teplizumab-treated participants vs. placebo was 0.457; p=0.01. The median time to diabetes was 27.1 and 59.6 months in the placebo and teplizumab treatment groups respectively. At the conclusion of this period, 7 (22%) and 22 (50%) respectively were not diagnosed with T1D.
Teplizumab treatment improved quantitative OGTT glucose AUC values over the course of the study:
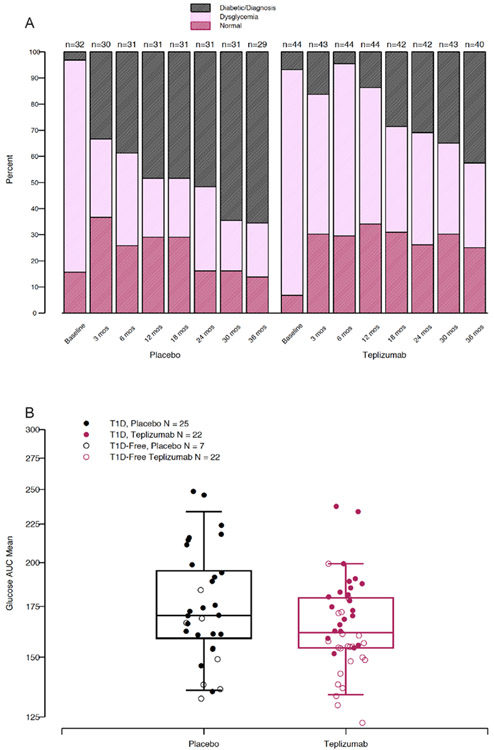
To determine how teplizumab treatment affected glucose tolerance, we classified the outcomes of the OGTTs as normal, dysglycemic, or diabetic at study entry and tallied the frequency of these outcomes at each study visit over the first 36 months of study and afterwards (Figure 2A, with individual results in Supplemental Figure 2). Study participants had been recruited on the basis of a dysglycemic OGTT test result. At randomization, and consistent with the known variability of OGTT results, a small number of subjects had normal (n=3) or diabetic (n=6) glucose tolerance at that visit. The clinical diagnosis of T1D, the primary endpoint of the study, required two consecutive diabetic OGTTs hence, participants could continue in the study with a single diabetic OGTT. At the 3 month visit after teplizumab or placebo administration, the frequency of dysglycemic OGTTs declined and the frequency of normal OGTTs increased in both groups (in teplizumab treated from 6.8% to 30.2%, McNemar test: p = 0.009) and in placebo from 15.6% to 36.7%, McNemar test: p = 0.02). Diabetic OGTTs also increased in both groups at this timepoint, particularly in the placebo group. Afterwards, the frequency of normal and dysglycemic OGTTs remained relatively constant in the teplizumab group: the frequency of diabetic OGTTs increased in both groups but at a slower rate in the teplizumab treated participants, who mostly maintained dysglycemic OGTT status.
Figure 2. Improved glycemia in teplizumab treated participants is associated with maintenance of dysglycemic status.
A. OGTT classifications for participants in each group over 36 months of follow-up. The data are shown to 36 months because of loss of placebo treated participants because of the clinical diagnosis of T1D (for individual participants see Supplemental Figure 2). B. Boxplot displaying median and interquartile ranges for on-study OGTT glucose AUC mean for participants from placebo and teplizumab treated groups. ANCOVA model incorporating baseline value, age, and treatment group showed that treatment group had a significant effect to decrease average on-study glucose AUC (ANCOVA teplizumab effect: 92.8%, p=0.03).
Changes in OGTT classifications could overlook more subtle effects of treatment on the OGTT glucose responses. We therefore calculated and compared an average on-study glucose AUC for each individual, which was corrected for the time in study. The average on-study glucose AUC was higher in those treated with placebo vs teplizumab (unadjusted mean (IQR)) 175 (159, 195) mg/dl vs 165 (154, 180) mg/dl, when adjusted for baseline glucose and age using ANCOVA, p=0.02). (Figure 2B, Supplemental Table 1). The individual glucose AUC at the time of study entry was a predictor of the average on-study glucose AUC (p=0.0008), but values at entry were similar between groups (unadjusted group geometric means of placebo and teplizumab: 155.5mg/dl for placebo and 162.2 mg/dl for teplizumab, p=0.25).
Average on-study Hemoglobin A1c (HbA1c) AUC was also calculated and analyzed. In contrast to glucose, the average on-study HbA1c AUC was not statistically different in those treated with placebo vs teplizumab (mean (IQR) 5.42 % (5.29, 5.57) vs 5.27 % (4.99, 5.55); when adjusted for age and baseline A1c using ANCOVA treatment, p=0.13). (Supplemental Figure 3, Supplemental Table 2). Because the frequency of diabetes was higher in the placebo group, the similarity in the HbA1c, a measure of chronic glucose exposure, the higher average on-study glucose AUC level in the placebo group most likely was due to acute rather than chronic changes in glucose levels.
Teplizumab treatment increased C-peptide responses:
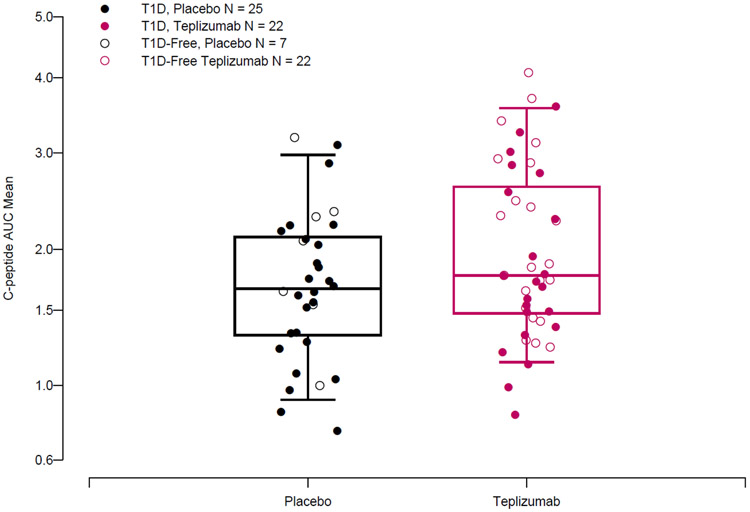
The average on-study C-peptide AUC was greater in the teplizumab treatment group vs placebo (unadjusted mean (IQR): 1.96 (1.48, 2.61) pmol/ml vs 1.68 (1.32, 2.11) pmol/ml and when adjusted for the age and baseline C-peptide AUC, the differences were highly significant (p=0.006) (Figure 3, Supplemental Table 3). To assess the relationship of this endpoint with diabetes development, we compared average on-study C-peptide AUC values between participants who did or did not develop T1D during the period of observation. For the entire study population, average on-study C-peptide AUC was greater in individuals that remained diabetes free compared to those that progressed to T1D (unadjusted mean (IQR) 2.07 (1.55, 2.47) pmol/ml (n=29) vs. 1.71 (1.33, 2.13) pmol/ml (n=47)(p=0.03). However, there was not a clear drug effect when the average C-peptide levels were compared in those who were diagnosed and remained diabetes free (T1D free (unadjusted mean IQR): placebo: 1.95(1.6, 2.33)(n=7) pmol/ml; teplizumab 2.1(1.55, 2.78) pmol/ml (n=22))(T1D: placebo: 1.61(1.28, 2.04) pmol/ml (n=25); teplizumab: 1.83(1.41, 2.49) pmol/ml (n=22), t-test, p=0.26) (Figure 3).
Figure 3. Teplizumab treatment was associated with increased average on-study C-peptide AUC.
Boxplot displaying median and interquartile ranges for average on-study OGTT C-peptide AUC mean for participants from placebo and teplizumab treated groups. An ANCOVA model including baseline C-peptide AUC and age showed that treatment was associated with higher average on-study C-peptide AUC (p=0.009).
Baseline C-peptide AUC (p<0.0001) was a significant determinant of the average on-study C-peptide AUC, but baseline values were similar between treatment groups (unadjusted group means (IQR) for placebo and teplizumab of 1.91(1.56, 2.36)(n=32) pmol/ml and 1.99(1.47, 2.18)(n=44) pmol/ml (p=0.661). Compared to a control group of and sex-matched autoantibody negative relatives, the mean C-peptide AUC values in the teplizumab and placebo groups were reduced at baseline (p=0.001 for comparison of participant baseline values to values of antibody negative relatives using Wilcoxon test, Supplemental Table 4).
There was also a direct relationship between the participant age and the average C-peptide AUC across both treatment arms and outcomes (Supplemental Figure 4) (from Pearson’s product-moment correlation, r= 0.44, p=0.0001) as has been noted previously in studies of individuals at risk and with new onset T1D (33). In contrast to relationships with time to clinical diagnosis (26) , HLA-DR4+, HLA-DR3−, or anti-ZnT8 antibody status did not show significant interactions with treatment and the average on-study C-peptide AUC. (Wald test: HLA-DR3 p=0.44, HLA-DR4 p=0.84, ZnT8 p=0.84; Supplemental Table 5).
Teplizumab treatment reverses declines in C-peptide AUC during the first 6 months of treatment:
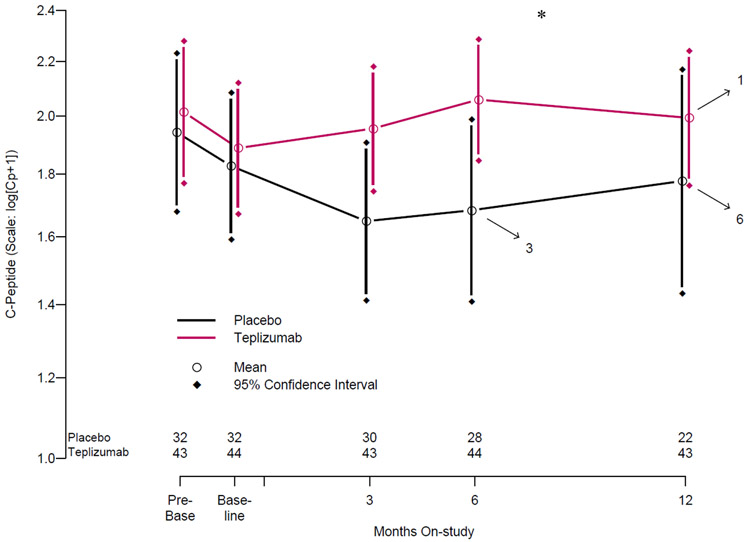
Because average on-study C-peptide AUC could obscure more pronounced between-group differences at individual study timepoints, we next analyzed the timing of the changes in C-peptide AUC relative to treatment and the insulin secretion patterns. As the participants had been recruited from the TN01 Natural History study, we were able to analyze the C-peptide response to OGTTs before enrollment, and compare these to values after enrollment in this study. Geometric-like group means over a median of 2.4 months prior to randomization, and over the 12 months after are shown in Figure 4. There was a decline in the C-peptide AUC prior to study enrollment in both groups (pre-baseline and baseline): placebo 1.94 (1.6, 2.37), and 1.83 pmol/ml (1.56, 2.36), teplizumab: 2.01(1.47, 2.59) and 1.89 pmol/ml (1.47, 2.17) with a pre-treatment slope of −0.0233 (−0.0557, 0.0175) for all participants (n=74). In the participants treated with placebo, the decline in C-peptide persisted at the same rate for the first 6 months after enrollment (mean C-peptide AUC of 1.68 pmol/ml (1.2, 2.15) at 6 months), with no significant differences in pre vs. post treatment slopes even after correction for age and the C-peptide at enrollment. In contrast, there was a significant increase in the C-peptide AUC in the teplizumab treated participants at 6 months after enrollment (6 month mean C-peptide AUC of 2.06 pmol/ml (1.55, 2.58), paired t-test p=0.02). The post-treatment slopes differed significantly between the placebo and teplizumab treated participants by ANCOVA after correcting for age and the pre-treatment slope (p=0.0015; Supplemental Table 6).
Figure 4. C-peptide over time in the two treatment arms over the first year.
The log-transformed mean C-peptide AUC is shown. Arrows indicate individual drop out from OGTT monitoring due to diabetes development at each timepoint. Median value for “pre-baseline” timepoint was 24. Months prior to randomization and median value for “baseline” timepoint was 0.85 months prior to randomization. *P<0.05 for comparisons of 6-month on-treatment C-peptide AUC values to baseline in the teplizumab group and 6-month C-peptide AUC values in the teplizumab group to 6-month C-peptide AUC values in the placebo group.
Insulin secretory dynamics are improved by teplizumab treatment:
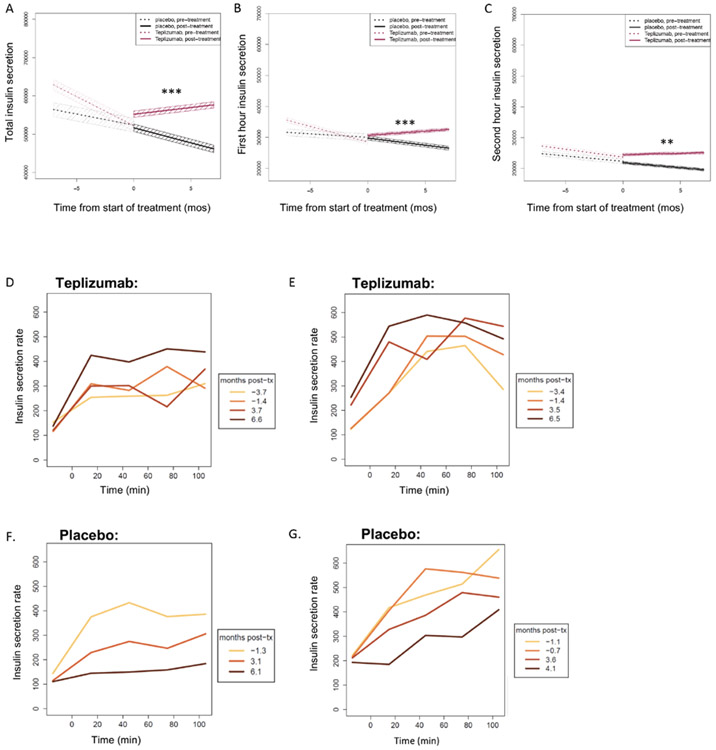
In addition to quantitative decreases in C-peptide AUC, studies by our group and others have identified qualitative abnormalities in beta cell secretory kinetics, with loss of early insulin secretion reflecting beta cell dysfunction prior to the onset of T1D (10, 33-36). To determine whether the quantitative improvement in C-peptide AUC was associated with qualitative changes in the kinetics of insulin secretion, we determined the insulin secretory rates (ISR) during the OGTTs using a two-compartment model, and evaluated the kinetics and total insulin secretion (Figure 5A-G, Table 1). We compared the OGTT insulin secretory responses and modeled the change in the secretory responses (slope) before and after drug treatment and between the two study arms. With this analysis of insulin secretion, we could distinguish the early and late secretory responses (i.e. first and second hour). The modeled slopes describing the change in the total, first, and second hour insulin secreted were similar in both groups prior to study enrollment (p=0.95). After treatment with teplizumab there was a significant increase in the total insulin secreted during the test in the teplizumab group that was significantly greater than in the placebo group (p=0.01, p=0.0004). The insulin secreted during the first hour continued to decline in the placebo group whereas it increased in the teplizumab group (p=0.007). The second hour of insulin secretion also improved in the teplizumab treatment group (p=0.03), but not in the placebo group (p=0.38) (Table 1). These results indicate that in the first 6 months after teplizumab treatment there is improvement in insulin secretion, particularly within the first hour of the OGTT, suggesting improved beta cell function, whereas there is continuing deterioration in insulin secretion in the placebo treated participants.
Figure 5. Insulin secretion after treatment with teplizumab or placebo.
Estimated slopes for the insulin secreted (pmol) during the total (A), first hour (B), and second hour (C) of the OGTT at the visits before enrollment and over the first 6 months after study drug treatment. Median values (and 95% confidence intervals in shaded colors) are shown. Significance for Wilcoxon signed-rank test for comparison of posttreatment slopes between treatment groups are shown in each panel. Please refer to Table 1 for full statistical analyses. (D and E) Representative insulin secretion rates during serial OGTTs for two teplizumab-treated participants who were not diagnosed with T1D (aged 11 and 12 years) and (F and G) two placebo-treated individuals (both aged 13 years) who were diagnosed with T1D. The colored lines indicate the time of the visits in relationship to study drug administration. tx, treatment. **P < 0.01 and ***P < 0.001.
Table 1:
Analysis of insulin secretion to oral glucose in the first 6 months after treatment
| Measure to be compared between arms | Medians | p-value | |
|---|---|---|---|
| Placebo | Teplizumab | ||
| First hour insulin interval secretion | |||
| Pre-treatment slope | −259.5 | −422.7 | 0.79 |
| Post-treatment slope | −476.2 | 371.0 | 0.0003 |
| Paired pre- vs. post-rx p-values*within arms | p = 0.86 | p = 0.007 | -- |
| Second hour insulin interval secretion | |||
| Pre-treatment slope | −728.2 | −383.6 | 0.78 |
| Post-treatment slope | −186.8 | 442.5 | 0.003 |
| Paired pre- vs. post-rx p-values*within arms | p = 0.38 | p = 0.03 | -- |
| Insulin interval secretion (2 hr) | |||
| Pre-treatment slope | −1245.0 | −1024.0 | 0.95 |
| Post-treatment slope | −1037.4 | 1085.8 | 0.0004 |
| Paired pre- vs. post-rx p-values*within arms | p = 0.80 | p = 0.01 | -- |
where diff = (post-rx slope) – (pre-rx slope)
p-values based on Wilcoxon signed rank (paired) test comparing the pre- vs. post-rx slopes by subject; evaluation of how much changes in these measures changed pre- vs. post-rx by subject and across those subjects in each treatment arm.
The relative level of proinsulin (i.e. proinsulin:C-peptide ratio) has been suggested as a measure of beta cell stress (37). Therefore, we compared the ratios of proinsulin:C-peptide at the times of the improved C-peptide and insulin secretion i.e. beginning 6 months after study drug treatment. Coincident fasting proinsulin and C-peptide measures were available for 22 placebo-treated participants and 41 teplizumab-treated participants. Unlike the increased secretion of insulin in the first hour of the OGTT or the total C-peptide AUC, we did not identify a significant difference in the ratios between the treatment arms at 6 months, after the C-peptide levels had improved in the teplizumab treated patients (placebo, median [range] 0.26 [0.05-2.14]; teplizumab 0.34 [0.1-2.44], p=ns), or when the average levels following treatment up to 36 months were compared (placebo average on-study PI:C ratio median [range]: 0.38 [0.05-10.7] vs. teplizumab: 0.42 [0.1-3.0]; p=0.57) and Supplemental Figure 3B.
Preservation of C-peptide is maintained until the last 6 months preceding clinical diagnosis:
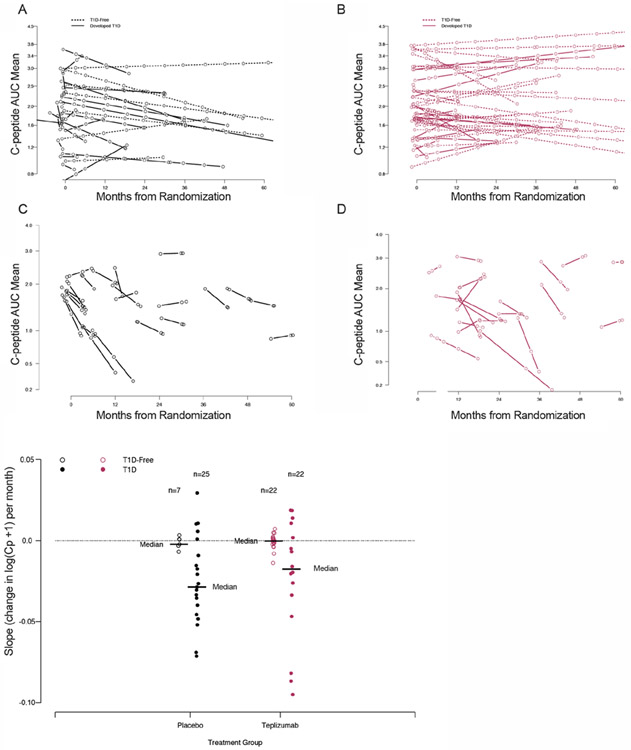
To determine the duration of these metabolic effects, we analyzed the C-peptide trajectories (least-square lines) over the entire study period or until the 6 months prior to when the participant was diagnosed with T1D. (Figure 6A, B). With this analysis, C-peptide AUC declined in the placebo group – the median slope was significantly less than 0 (median, IQR: −0.00382, −0.0107 to 0.000755, Wilcoxon 1-sample: p = 0.04). The loss of C-peptide in the placebo group was even more pronounced in the 6 months between the penultimate and final OGTT (mean slope (IQR) of −0.0242 (−0.0469, −0.0041); significantly non-zero (Wilcoxon 1-sample: p = 0.0001) (Figures 6C and E).
Figure 6. Teplizumab preserves C-peptide over the course of the study until the period surrounding diagnosis.
For all panels, data from teplizumab-treated participants are shown in blue, and placebo-treated participants are shown in maroon. A,B. Regression lines for C-peptide AUC values over the study period of OGTT monitoring from baseline study visit until diagnosis (teplizumab n=44, placebo n=32). C,D. Regression lines for C-peptide AUC values over 6 month period before diabetes diagnosis (placebo n=23, teplizumab n=22). E. Slopes of C-peptide AUC for 6-month period before diagnosis in those that developed T1D, and the last 6 months of study in individuals remaining T1D-free.
In contrast, the median slope for the teplizumab group until the end of the study period or until the 6 months prior to when the participant was diagnosed with T1D was not significantly different from 0 (mean (IQR): −0.000294 (−0.00372, 0.00304), Wilcoxon 1-sample: p = 0.63) (Figure 6B) and thus, less C-peptide AUC was lost over time compared to the placebo treated participants (Wilcoxon 2-sample: p = 0.04). In the participants treated with teplizumab who were diagnosed with T1D, there was also a decline in the C-peptide AUC in the peridiagnostic period, but the median was not significantly less than 0 (Wilcoxon 1-sample with a comparison to 0: p = 0.09)(Figure 6D) and was modestly greater than those in the placebo treatment arm who were diagnosed with T1D)(Wilcoxon 2-sample comparing placebo and teplizumab slopes prior to diagnosis: p = 0.06) (Figure 6E). A difference in insulin sensitivity between the two treatment arms was not a likely explanation for these findings since the C-peptide AUC/glucose AUCs were similar in the teplizumab and placebo groups at the time of T1D diagnosis (p = 0.23)(Supplemental Figure 5A, B).
C-peptide responses correlate with increases in partially exhausted CD8+ T cells:
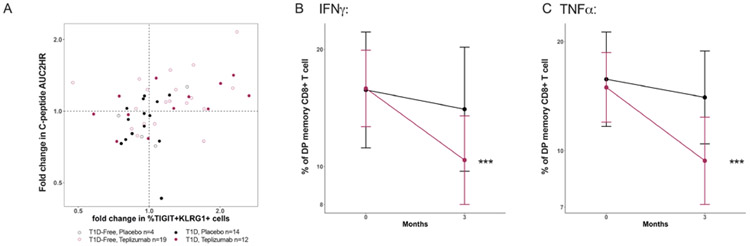
We postulated that the rapid improvement in metabolic responses was related to the effects of teplizumab on T cells. There was a transient decline in the number of circulating lymphocytes during teplizumab treatment but the number of cells was at the baseline level by day 42 (38). However, the improvement in C-peptide was not related to the changes in the number of circulating CD3+ (Pearson r=0.19, p=0.28), CD4+ (r=0.01, p=0.97), or CD8+ (r=0.24, p=0.17) cells (Supplemental Figure S6). We had previously described an increase in the frequency of memory CD8+ T cells with teplizumab treatment that we proposed were “partially exhausted” by expression of TIGIT and KLRG1+ (double positive cells), and a transcriptional activation/exhaustion signature that could be further reduced by ligation of TIGIT (21, 23, 25, 26, 39). Thus, we tested whether the frequency of these or other cells was associated with C-peptide AUC during or shortly after the drug treatment period and whether they were functionally exhausted. We observed a significant correlation of the change in frequency of CD8+KLRG1+TIGIT+ T cells at month 3, 6, and 18 but not CD4+ or other CD8 T cells with the fold change in C-peptide at month 6 (Table 2). The changes in T cell subsets most likely preceded the changes in C-peptide and therefore, we also analyzed the fold changes in the double positive CD8+ T cells at month 3 and the fold changes in C-peptide at month 6. There was a significant association between the frequency (p=0.014)(Figure 7A) and also the absolute number of these cells (p=0.02, Supplemental Figure S6D) and the change in C-peptide.
Table 2:
Pearson correlations between % change in C-peptide from baseline to month 6 and CD8+ T cell subpopulations
| @Month 3 | @Month 6 | @Month 18 | ||||
|---|---|---|---|---|---|---|
| CD8 T cell subsets | r | p | r | p | r | p |
| KLRG-1+TIGIT+ of CD8 Central Memory | 0.429 | 0.016 | 0.433 | 0.01 | 0.463 | 0.011 |
| KLRG-1+TIGIT+ of CD8 Effector Memory | 0.421 | 0.018 | 0.460 | 0.006 | 0.461 | 0.012 |
| KLRG1-TIGIT-naïve CD8 | 0.049 | 0.79 | 0.17 | 0.34 | −0.126 | 0.52 |
| CD4 T cell subsets | ||||||
| KLRG1+TIGIT+CD4 | 0.016 | 0.93 | 0.026 | 0.883 | 0.217 | 0.26 |
| PD1+TIGIT+ Memory CD4+Treg (FoxP3+, CD127lo) | −0.195 | 0.27 | −0.04 | 0.82 | 0.3 | 0.1 |
Figure 7. Functional changes in T cells are associated with improvements in metabolic function.
A. The changes in TIGIT+KLRG1+CD45RO+CD8+ T cells between baseline and 3 months and the change in the C-peptide AUC between the baseline and 6 months are shown. There was a significant correlation between the changes in this cell subset and C-peptide in the teplizumab (Pearson r=0.44, p=0.014, n=31) but not the placebo treated (r=0.28, p=0.25, n=18) participants. B, C. The frequency of double positive (DP) CD8+ memory cells that produce IFNγ or TNFα are shown for the placebo treated (●, n=16) and drug treated (●, n=24) participants at baseline and month 3. The frequency of the IFNγ and TNFα producing cells were reduced in the teplizumab treated participants (paired T-test, ***p<0.0001).
T cell exhaustion has been associated with reduced cytokine production following activation (40). We therefore measured intracellular cytokines after stimulation of PBMC with anti-CD3 and anti-CD28. Among the double positive CD8+ T cells the frequency of IFNγ-producing (p<0.0001, p=0.0004) and TNFα-producing cells (p<0.0001 for both) were decreased at 3 months (Figure 7B, C and Table 2) in the teplizumab but not placebo treated participants and the decline was associated with the improvement in C-peptide. In contrast, relative proportions of IFNγ and TNFα producing cells among the double positive memory CD8+ T cells remained stable in the placebo group at 3 months of follow-up.
Discussion
Studies of natural history cohorts have described changes in metabolic function during the progression to T1D in relatives at risk. Our successful intervention trial, with teplizumab, in the at-risk population has given us a unique opportunity to directly assess how changing immune cells can affect metabolic function and progression to the clinical diagnosis of T1D in relatives at high-risk. The trial design was a time-to-event analysis. The current work utilized an extended period of follow-up, as well as OGTT data obtained before enrollment in the TN10 anti-CD3 prevention trial to provide metabolic characterization of study participants. In this extended follow up we show that the effects of the single 14 day course of teplizumab treatment persists: The median time to diabetes in the teplizumab group was approximately 5 years compared to slightly over 2 years in the placebo treated participants, with 50% of the teplizumab treated participants vs 22% of placebo treated participants not diagnosed with T1D. Eighteen percent of the teplizumab-treated participants vs 6 percent of the placebo treated participants were followed for more than 5 years and were not diagnosed. Importantly, this is the first study to show successful modulation of the progression of beta cell failure prior to the diagnosis of T1D with immune intervention.
Teplizumab treatment improved beta cell function, even in those who were not diagnosed with T1D. The average OGTT glucose levels were lower and C-peptide responses higher with teplizumab treatment. There was improvement in total and early insulin secretion rates, which identifies a functional as well as a quantitative improvement in insulin release. The early secretion of insulin, a feature of normal beta cell function, was the most dramatically changed, indicating that the impaired “beta cell glucose sensitivity” that has been described in patients who progress to clinical diabetes was improved, and that effects on C-peptide AUC were not solely a reflection of improved beta cell mass (34). The metabolic changes were associated with an increased frequency of TIGIT+KLRG1+ memory CD8+ T cells and a reduced secretion of cytokines (TNFα and IFNγ) that have been associated with pathology in T1D, indicating that the T cells had functional exhaustion (21, 23, 25, 26, 39). In contrast, early increases in C-peptide were not correlated with individual changes in total number of CD3+ cells, or other T cell subsets. The lack of correlation between increases in C-peptide AUC and reductions in total CD3+ cells within the teplizumab-treated group, combined with the stabilization of C-peptide despite recovery of circulating CD3+cells, points away from a direct effect of the treatment-induced CD3+ nadir on beta cell function. Previous successful interventions that have shown preservation of C-peptide responses with immune or other interventions have all been done in patients with Stage 3 disease. This is the first analysis to show metabolic improvement even in the absence of hyperglycemia.
Because the clinical trial was designed as a time-to-event protocol, the variable time in the study for each participant created a challenge in analyzing the metabolic responses during the study OGTTs. Hence, we used the average on-study C-peptide, glucose, and HbA1c AUCs which included all of the available data for each participant. Moving forward, this approach could prove useful in metabolic analyses of other diabetes prevention studies designed as time-to-event trials.
Although the time in the trial was not a significant determinant of the average C-peptide AUC, there were time-dependent metabolic effects of the drug treatment. Beta cell function was declining in the participants as they were enrolling in the TN10 anti-CD3 prevention trial. Indeed in previous studies we found that the level of beta cell death was high among similarly high-risk individuals and other studies have documented beta cell dysfunction in the peridiagnosis period (35, 41, 42). This metabolic data together with the relatively short median time to diagnosis of T1D in the placebo group indicates that the screening methods utilized identified an active time of disease and individuals at very high risk for progression. Consistent with preclinical studies, the effects at this period of active disease support the concept that this intervention may be most effective when there is immune cell activation (43). The greatest increase in C-peptide occurred shortly after teplizumab treatment, followed by stabilization of beta cell function, whereas in the placebo group, beta cell function declined gradually over time. Consistent with prior reports, in those who developed clinical diabetes in both treatment arms, there was a precipitous decline in the stimulated C-peptide levels seen about 6 months prior to T1D onset (41, 42).
With teplizumab treatment, one measure of beta cell dysfunction, the impaired early secretion of insulin, improved, in addition to the total C-peptide AUC, but another, the ratio of proinsulin:C-peptide was not different between the groups at 6 months or over the 36 months after treatment. Moreover, the OGTTs did not uniformly normalize in those who were not diagnosed with T1D - the outcomes of the OGTTs fluctuated even within individuals that did and did not develop T1D. Most likely this variability reflects the tenuous level of residual insulin production, or even the ongoing presence of metabolic stressors. Consistent with this, we did not find a relationship between the average on-study glucose AUC and C-peptide AUC. These clinical outcomes are similar to the effects of anti-CD3 mAb in the NOD model of T1D, prior to the diagnosis of diabetes, in which insulin granularity was improved but beta cell mass did not recover to normal levels (44, 45). Thus, qualitative and quantitative measures of beta cell function may be impacted differently by the immune therapy. Further studies with metabolic clamps might refine our analysis of the metabolic function, but such studies were impractical in this clinical trial setting.
The factors that precipitated disease in the 6 months prior to clinical T1D diagnosis in both the treatment and placebo arms are not clear at this point. The similar relation between C-peptide and glucose in the two treatment arms among those who were diagnosed with T1D suggests that insulin insensitivity was not a precipitating factor for the diagnosis. Interestingly, even with progression to clinical diabetes, the decline in C-peptide tended to be less pronounced in the teplizumab group vs placebo, which suggests that the effects of drug treatment on C-peptide may persist even during and potentially after the clinical diagnosis. There may be waning of the effects of the anti-CD3 antibody on immune cells which we identified previously by tracking the CD8 memory double positive cells (23). Other observations in the field show that progression to clinical diabetes is associated with acquisition of effector T cell function, but it is possible that in this setting restored effector function may involve waning of the immune effects of teplizumab or even new or regenerated pathologic T cells repopulating the repertoire after the single course of drug. The median age at the time of treatment in the TN10 anti-CD3 prevention trial was 13.9 years, and in young children, the thymic output of T cells may be ongoing. In other studies of the long term outcomes of patients treated with teplizumab, there was an increased frequency of Programmed Cell Death Protein 1 (PD-1)+ memory CD8+ T cells in responders compared to non-responders and controls suggesting that changes in the phenotype and function of the CD8+ memory compartment may occur over time (46). Ongoing work tracking TCRs and single cell analyses will help to address these hypotheses and may suggest agents that could be used to extend the diabetes-free period possibly by blocking pathways needed for T-effector expansion (46).
There are limitations to our studies. Our methods do not allow us to distinguish the contribution of maintained beta cell mass vs. improved function of beta cells. Our analysis of the total C-peptide AUC suggests the former but the kinetics of insulin secretion also suggest the latter. Longer term follow-up of the half of patients who had not been diagnosed with T1D may address this question in a practical way. In addition, studies of beta cell killing are ongoing. The number of subjects was relatively small, and the study was powered to detect differences in diabetes incidence rather than changes in C-peptide AUC, insulin secretion, proinsulin:C-peptide ratio, or immune function. Our analysis of proinsulin:C-peptide could only be done in a subsets of participants with samples available after the 6 month time point. Additionally, the time-to-event design of the original study had some important implications for the analyses included here. We did not have OGTT analyses for most individuals after diagnosis of T1D which limited our ability to compare OGTT data between all members of placebo and teplizumab groups over the same time period, particularly for the placebo group, which exhibited more rapid progression to diabetes. The time-to-event design also limited our ability to compare the relationship between metabolic endpoints and T1D progression, as some individuals included in the study that did not progress to diabetes may ultimately develop T1D. Furthermore, given prior results showing teplizumab treatment preserved C-peptide in patients with recent-onset T1D (19-25), positive effects on C-peptide might also be expected to occur amongst individuals that developed diabetes during this study. Participants from both arms of the trial that developed diabetes were enrolled in the TrialNet Long Term Investigational Follow-up (LIFT) study, which performs longitudinal metabolic testing in participants who have been diagnosed with T1D (13).
In summary, we show extended delay in progression to T1D in at-risk subjects treated with teplizumab. Teplizumab treatment changed the biologic course of the disease by enhancing beta cell function reflected by the quantitative and qualitative improvements in insulin secretion. These changes were associated with modulation of the frequency and function of memory CD8+ T cells. The pronounced early efficacy of the drug followed by stabilization of beta cell function also suggests that repeated treatment, which previously has been safely implemented (19, 20, 47), or the addition of other complimentary agents, even drugs that act directly on beta cells, at key timepoints in the clinical course may be valuable to extend the delay or prevent the diagnosis of T1D. Finally, our findings have implications for other autoimmune diseases by showing how immune intervention can change the pathobiology even prior to disease diagnosis and lead to a clinically significant outcome.
Materials and Methods:
Trial Design
The design of this phase 2, randomized, placebo-controlled, double-blind trial (NCT01030861) has been previously been reported (26). Institutional-review-board approval was obtained at each participating site. The participants, their parents, or both provided written informed consent or assent before trial entry. The participants were identified through the TrialNet Pathway to Prevention study (TN01) (14, 48). In that study, OGTTs were performed at approximately 6 month intervals in islet autoantibody positive individuals (including anti-glutamic acid decarboxylase 65, micro insulin, anti-islet antigen 2, anti-zinc transporter 8, and/or islet-cell antibodies), and the glucose results from these tests were used to identify eligibility for the TrialNet TN10 anti-CD3 prevention trial and were used in this data analysis. Islet autoantibody testing, HLA genotyping, and OGTT testing were performed as previously described (4, 49). Briefly, eligibility criteria included age >=8 years at randomization, history of a relative with type 1 diabetes, positive titers for two or more islet autoantibodies, and dysglycemia on OGTT (fasting glucose 110-125 mg/dL (6.1-6.9 mmol/L), a 2-hour postprandial plasma glucose level of >= 140 mg/dL (7.8 mmol/L) and < 200 mg/dL (11.1 mmol/L), or an intervening postprandial glucose level at 30, 60, or 90 minutes of > 200 mg/dL. For participants who did not have a hemoglobin A1c available at the baseline visit, values obtained within the 3 months before treatment were utilized.
Participants were randomly assigned to teplizumab or saline and treated with a 14-day outpatient course administered as an IV infusion in a clinical research center. Teplizumab was dosed at 51 μg/m2 on day 0, 103 μg/m2 on day 1, 207 μg/m2 on day 2, 413 μg/m2 on day 3, followed by a dose of 826 μg/m2 on days 4 through 13. OGTTs were performed 3 months and 6 months after the infusions and every 6 months thereafter. Random screening glucose levels were evaluated at 3-month intervals, and an OGTT was performed if the random glucose level was > 200 mg/dL (11.1 mmol/). T1D was diagnosed using ADA criteria during an OGTT but only after the diabetic OGTT was sequentially confirmed. The date of diagnosis was identified as the time of the first of the 2 diagnostic tests (50). Six participants were clinically diagnosed with T1D outside of OGTT monitoring. The original trial end date was June 2019. Participants who had not been diagnosed with T1D were transferred into the TrialNet Pathway to Prevention Natural History study (TN01) for follow up OGTT monitoring. TN01 Natural History data from before randomization into the TN10 anti-CD3 prevention trial, as well as TN01 Natural History data from follow up after the TN10 study was completed, between July 2011 and March 2020, are included in this analysis. Participants that did develop T1D were offered enrollment in TrialNet’s Long Term Investigational Follow-up (LIFT) study for continued metabolic follow-up.
In addition, OGTT data from islet-autoantibody negative relatives obtained through TrialNet testing were also analyzed. Autoantibody negative relatives selected for analyses were matched to study participants by sex and age. For subjects < 19 years, age was matched within 6 months. Subjects >= 19 were matched within +/− 2 years with the exception of 5 subjects, which required +/− 6 years. Mean BMIs for the placebo group, placebo-matched controls, teplizumab group, and teplizumab-matched controls were similar and in the non-overweight range (22.0, 24.1, 22.0, 22.8).
Metabolic Analyses
OGTT C-peptide and glucose values were tested by Northwest Lipids Research Laboratories using the TOSOH C-peptide immunoassay and Roche glucose assay. Samples for measurement of fasting proinsulin levels were available after drug treatment (6, 12, 18, 24, 30, 36 months) from a subgroup of individuals according to allowable blood volumes. The proinsulin was measured using a TECO intact proinsulin ELISA. PI:C-peptide (PI:C) ratios were calculated by obtaining the equimolar ratio of intact proinsulin: fasting C-peptide x100 (51). OGTT results were assigned to the nearest study visit timepoint (within 3 months of the official timepoint assignment. OGTT results were classified as normal, dysglycemic, or diabetic based on above definitions used for study entry. The baseline OGTT was the study at the time or immediately prior to randomization.
Area under the curve (AUC) values for ISR, C-peptide, and glucose were calculated using the trapezoidal rule, where each of two consecutive timed measurements (e.g., 0 and 30 minute) form a trapezoid with a base of 30 minutes. The formula for the area of a trapezoid is [(Height1+Height2)/2] x Base. The areas of the four trapezoids are than added together to estimate the AUC. This sum is divided by 120 minutes to give an AUC mean (52). The on-study AUC means for C-peptide, glucose, and HbA1c were calculated by multiplying the AUC means for each OGTT visit and the visit intervals in days (as the trapezoidal base) to calculate a total study AUC, and then dividing by the days from the first to the last OGTT (confirmatory diabetic OGTT if developed T1D). Insulin secretory rates (ISRs) were calculated using the Chronobiological Series Analyzer (CSA) software, which uses a 2-compartment model for hormone clearance and standard kinetic parameters for C-peptide (35, 53-58). ISR calculations were performed using participant OGTT C-peptide and glucose values, as well as age, sex, height, and weight. The insulin secretion was divided into the amount (pmol) secreted over the 2 hr OGTT or in the first or second hour of the test.
Flow cytometry analysis:
Peripheral blood mononuclear cells (PBMC) were processed and stored at the NIDDK repository. Cryopreserved vials of PBMC were sent to ITN Core laboratory at Benaroya Research Institute for analysis by flow cytometry with antibody panels shown in Supplementary Tables 7 and 8. T-cell phenotyping was performed on thawed PBMC and the frequency of CD45RO+CD8+ T-cells that were TIGIT+KLRG1+CD57− was determined as described previously (59). Intracellular cytokine expression was measured after 6 hrs with stimulation of PBMC by plate-bound anti-CD3 (1 μg/ml ) and soluble anti-CD28 (10 μg/ml) in the presence of equimolar amounts of Golgi-stop. The frequency of TIGIT+KLRG1+ CD8+ memory (CD45RA−) T cells that produce IFNγ or TNFα were determined at baseline and month 3.
Instrument standardization was performed using 8 peak rainbow calibration beads (Spherotech, Lake Forest, IL) adjusting PMT voltages for consistent 7th peak mean fluorescent intensities. All samples from the same subject were run on the same day, and an internal control arm from the same subject was run each week. Sample acquisition was performed as previously described on an LSR-Fortessa (BD Biosciences) with FACS Diva software and analyzed with FlowJo software version 9.5 (Tree Star, Ashland, OR) (59). The quadrants were placed based on staining controls. Gated populations with <100 events were excluded from analysis.
Statistical Analysis
The original trial was designed as a time-to-event analysis and therefore participants who were diagnosed with T1D were not followed further in that study. The impact of teplizumab treatment on incidence of type 1 diabetes following enrollment was performed using a Cox proportional hazards model. For this analysis, metabolic parameters over the entire period of the trial included OGTT data in the visit immediately prior to and all OGTT data after study drug treatment (confirmatory diabetic OGTT for individuals diagnosed with diabetes, or last available OGTT for those remaining diabetes free). Slopes for changes in glucose and C-peptide prior to and after enrollment were calculated using linear regression analysis of available OGTT visit data for specified intervals. An impact of treatment on each endpoint was determined by fitting results to an ANCOVA model, with age, baseline value, and treatment group included as covariates. Wald tests were used to determine if covariates significantly impacted the model. Estimated slopes for changes in the insulin secretion rates were also calculated for each subject based on changes before treatment (time points up to 6 months prior to baseline) and for after initiation of treatment (time points up to 6 months after baseline) using linear regression models as well as mixed models for repeated measures. Insulin secretion rates were calculated across the overall 2-hour interval as well as specifically for the first hour and the second hour intervals of the OGTTs. Differences in these slopes before vs. after treatment were compared using Wilcoxon signed rank tests within and across treatment arms. Differences and percent changes in these slopes before vs. after treatment were also evaluated using a generalized linear model to assess the influence of treatment arm.
Flow cytometry data were log-transformed for statistical analysis. Pearson’s correlation coefficient was calculated to determine associations between fold changes in C-peptide AUC and frequency of TIGIT+KLRG1+ CD8+ memory T cells. Since our question was focused on (previously described) TIGIT+KLRG1+ CD8+ memory T cells and if their frequency associated with the changes in C-peptide, corrections for multiple comparisons were not performed for this analysis. The frequency of TIGIT+KLRG1+ CD8+ memory T cells producing IFNγ or TNFα were analyzed by paired t-test.
Analysis of proinsulin:C-peptide measures were done using log2-transformed measures. The average on-study proinsulin:C-peptide ratios were calculated using a trapezoidal rule calculation to get a study-wide AUC of proinsulin:C-peptide ratio over time for the post-baseline time points, divided by the time interval for those measures. If only one post-baseline time point was available (i.e. only at 6 months), the proinsulin:C-peptide ratio at that time point was used.
Supplementary Material
Acknowledgements:
The authors would like to thank Jorge Pardo and Lori Blanchfield from ITN for flow cytometry analysis, Jerry Nepom for reviewing data and providing feedback and the BRI HIP Core for performing the longitudinal flow cytometry acquisition and analysis. The mechanistic assay and data analysis were sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) under Award UM1AI109565. This report is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The sponsor of the trial was the Type 1 Diabetes TrialNet Study Group. The Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and The Eunice Kennedy Shriver National Institute of Child Health and Human Development, through the cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085453, U01 DK085461, U01 DK085465, U01 DK085466, U01 DK085476, U01 DK085499, U01 DK085504, U01 DK085509, U01 DK103180, U01 DK103153, U01 DK103266, U01 DK103282, U01 DK106984, U01 DK106994, U01 DK107013, U01 DK107014, UC4 DK106993, UC4 DK11700901, U01 DK 106693-02, and the JDRF. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the JDRF.
References
- 1.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, Winkler C, Ilonen J, Veijola R, Knip M, Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA : the journal of the American Medical Association 309, 2473–2479 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, Greenbaum CJ, Herold KC, Krischer JP, Lernmark A, Ratner RE, Rewers MJ, Schatz DA, Skyler JS, Sosenko JM, Ziegler AG, Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes care 38, 1964–1974 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krischer JP, Lynch KF, Lernmark A, Hagopian WA, Rewers MJ, She JX, Toppari J, Ziegler AG, Akolkar B, T. S. Group, Genetic and Environmental Interactions Modify the Risk of Diabetes-Related Autoimmunity by 6 Years of Age: The TEDDY Study. Diabetes care 40, 1194–1202 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahon JL, Sosenko JM, Rafkin-Mervis L, Krause-Steinrauf H, Lachin JM, Thompson C, Bingley PJ, Bonifacio E, Palmer JP, Eisenbarth GS, Wolfsdorf J, Skyler JS, C. TrialNet Natural History, G. Type 1 Diabetes TrialNet Study, The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatric diabetes 10, 97–104 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Siljander HT, Hermann R, Hekkala A, Lahde J, Tanner L, Keskinen P, Ilonen J, Simell O, Veijola R, Knip M, Insulin secretion and sensitivity in the prediction of type 1 diabetes in children with advanced beta-cell autoimmunity. Eur J Endocrinol 169, 479–485 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, Maahs DM, Tamborlane WV, Bergenstal R, Smith E, Olson BA, Garg SK, State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther 21, 66–72 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rawshani A, Sattar N, Franzen S, Rawshani A, Hattersley AT, Svensson AM, Eliasson B, Gudbjornsdottir S, Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 392, 477–486 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livingstone SJ, Levin D, Looker HC, Lindsay RS, Wild SH, Joss N, Leese G, Leslie P, McCrimmon RJ, Metcalfe W, McKnight JA, Morris AD, Pearson DW, Petrie JR, Philip S, Sattar NA, Traynor JP, Colhoun HM, g. Scottish Diabetes Research Network epidemiology, R. Scottish Renal, Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA : the journal of the American Medical Association 313, 37–44 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao B, Pietropaolo M, Atkinson M, Schatz D, Taylor D, Estimating the cost of type 1 diabetes in the U.S.: a propensity score matching method. PloS one 5, e11501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans-Molina C, Sims EK, DiMeglio LA, Ismail HM, Steck AK, Palmer JP, Krischer JP, Geyer S, Xu P, Sosenko JM, G. Type 1 Diabetes TrialNet Study, beta Cell dysfunction exists more than 5 years before type 1 diabetes diagnosis. JCI Insight 3, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sims EK, DiMeglio LA, Cause or effect? A review of clinical data demonstrating beta cell dysfunction prior to the clinical onset of type 1 diabetes. Mol Metab 27S, S129–S138 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koskinen MK, Helminen O, Matomäki J, Aspholm S, Mykkänen J, Mäkinen M, Simell V, Vähä-Mäkilä M, Simell T, Ilonen J, Reduced β-cell function in early preclinical type 1 diabetes. European Journal of Endocrinology 174, 251–259 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bingley PJ, Wherrett DK, Shultz A, Rafkin LE, Atkinson MA, Greenbaum CJ, Type 1 Diabetes TrialNet: A Multifaceted Approach to Bringing Disease-Modifying Therapy to Clinical Use in Type 1 Diabetes. Diabetes Care 41, 653–661 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenbaum CJ, Speake C, Krischer J, Buckner J, Gottlieb PA, Schatz DA, Herold KC, Atkinson MA, Strength in Numbers: Opportunities for Enhancing the Development of Effective Treatments for Type 1 Diabetes-The TrialNet Experience. Diabetes 67, 1216–1225 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathan BM, Boulware D, Geyer S, Atkinson MA, Colman P, Goland R, Russell W, Wentworth JM, Wilson DM, Evans-Molina C, Wherrett D, Skyler JS, Moran A, Sosenko JM, T. Type 1 Diabetes, G. Diabetes Prevention Trial-Type 1 Study, Dysglycemia and Index60 as Prediagnostic End Points for Type 1 Diabetes Prevention Trials. Diabetes care 40, 1494–1499 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sosenko JM, Palmer JP, Rafkin-Mervis L, Krischer JP, Cuthbertson D, Mahon J, Greenbaum CJ, Cowie CC, Skyler JS, G. Diabetes Prevention Trial-Type 1 Study, Incident dysglycemia and progression to type 1 diabetes among participants in the Diabetes Prevention Trial-Type 1. Diabetes care 32, 1603–1607 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer JP, C-peptide in the natural history of type 1 diabetes. Diabetes Metab Res Rev 25, 325–328 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenbaum CJ, Anderson AM, Dolan LM, Mayer-Davis EJ, Dabelea D, Imperatore G, Marcovina S, Pihoker C, S. S. Group, Preservation of beta-cell function in autoantibody-positive youth with diabetes. Diabetes care 32, 1839–1844 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagopian W, Ferry RJ Jr., Sherry N, Carlin D, Bonvini E, Johnson S, Stein KE, Koenig S, Daifotis AG, Herold KC, Ludvigsson J, I. Protege Trial, Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protege trial. Diabetes 62, 3901–3908 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, Boyle KD, Keyes-Elstein L, Aggarwal S, Phippard D, Sayre PH, McNamara J, Bluestone JA, A. T. E. S. T. Ab, Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 62, 3766–3774 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA, Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 346, 1692–1698. (2002). [DOI] [PubMed] [Google Scholar]
- 22.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L, Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 352, 2598–2608 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Long SA, Thorpe J, DeBerg HA, Gersuk V, Eddy J, Harris KM, Ehlers M, Herold KC, Nepom GT, Linsley PS, Partial exhaustion of CD8 T cells and clinical response to teplizumab in new-onset type 1 diabetes. Sci Immunol 1, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ Jr., Bode B, Aronoff S, Holland C, Carlin D, King KL, Wilder RL, Pillemer S, Bonvini E, Johnson S, Stein KE, Koenig S, Herold KC, Daifotis AG, I. Protege Trial, Teplizumab for treatment of type 1 diabetes (Protege study): 1-year results from a randomised, placebo-controlled trial. Lancet 378, 487–497 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tooley JE, Vudattu N, Choi J, Cotsapas C, Devine L, Raddassi K, Ehlers MR, McNamara JG, Harris KM, Kanaparthi S, Phippard D, Herold KC, Changes in T-cell subsets identify responders to FcR-nonbinding anti-CD3 mAb (teplizumab) in patients with type 1 diabetes. Eur J Immunol 46, 230–241 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, Gitelman SE, Gottlieb PA, Krischer JP, Linsley PS, Marks JB, Moore W, Moran A, Rodriguez H, Russell WE, Schatz D, Skyler JS, Tsalikian E, Wherrett DK, Ziegler AG, Greenbaum CJ, G. Type 1 Diabetes TrialNet Study, An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med 381, 603–613 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Näntö-Salonen K, Kupila A, Simell S, Siljander H, Salonsaari T, Hekkala A, Korhonen S, Erkkola R, Sipilä JI, Haavisto L, Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. The Lancet 372, 1746–1755 (2008). [DOI] [PubMed] [Google Scholar]
- 28.G. Diabetes Prevention Trial--Type 1 Diabetes Study, Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 346, 1685–1691 (2002). [DOI] [PubMed] [Google Scholar]
- 29.G. Writing Committee for the Type 1 Diabetes TrialNet Oral Insulin Study, Krischer JP, Schatz DA, Bundy B, Skyler JS, Greenbaum CJ, Effect of Oral Insulin on Prevention of Diabetes in Relatives of Patients With Type 1 Diabetes: A Randomized Clinical Trial. JAMA : the journal of the American Medical Association 318, 1891–1902 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elding Larsson H, Lundgren M, Jonsdottir B, Cuthbertson D, Krischer J, A.-I. T. S. G. Di, Safety and efficacy of autoantigen-specific therapy with 2 doses of alum-formulated glutamate decarboxylase in children with multiple islet autoantibodies and risk for type 1 diabetes: A randomized clinical trial. Pediatric diabetes 19, 410–419 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Gale EA, Bingley PJ, Emmett CL, Collier T, G. European Nicotinamide Diabetes Intervention Trial, European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 363, 925–931 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Eizirik DL, Colli ML, Ortis F, The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol 5, 219–226 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Tsai EB, Sherry NA, Palmer JP, Herold KC, The rise and fall of insulin secretion in type 1 diabetes mellitus. Diabetologia 49, 261–270 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Ferrannini E, Mari A, Nofrate V, Sosenko JM, Skyler JS, D. P. T. S. Group, Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset. Diabetes 59, 679–685 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herold KC, Usmani-Brown S, Ghazi T, Lebastchi J, Beam CA, Bellin MD, Ledizet M, Sosenko JM, Krischer JP, Palmer JP, G. Type 1 Diabetes TrialNet Study, beta cell death and dysfunction during type 1 diabetes development in at-risk individuals. J Clin Invest 125, 1163–1173 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherry NA, Tsai EB, Herold KC, Natural history of beta-cell function in type 1 diabetes. Diabetes 54 Suppl 2, S32–39 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Sims EK, Evans-Molina C, Tersey SA, Eizirik DL, Mirmira RG, Biomarkers of islet beta cell stress and death in type 1 diabetes. Diabetologia, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herold KC, Bucktrout SL, Wang X, Bode BW, Gitelman SE, Gottlieb PA, Hughes J, Joh T, McGill JB, Pettus JH, Potluri S, Schatz D, Shannon M, Udata C, Wong G, Levisetti M, Ganguly BJ, Garzone PD, R. N. W. Group, Immunomodulatory activity of humanized anti-IL-7R monoclonal antibody RN168 in subjects with type 1 diabetes. JCI Insight 4, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA, A Single Course of Anti-CD3 Monoclonal Antibody hOKT3{gamma}1(Ala-Ala) Results in Improvement in C-Peptide Responses and Clinical Parameters for at Least 2 Years after Onset of Type 1 Diabetes. Diabetes 54, 1763–1769 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLane LM, Abdel-Hakeem MS, Wherry EJ, CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu Rev Immunol 37, 457–495 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS, Patterns of metabolic progression to type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes care 29, 643–649 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Bogun MM, Bundy BN, Goland RS, Greenbaum CJ, C-Peptide Levels in Subjects Followed Longitudinally Before and After Type 1 Diabetes Diagnosis in TrialNet. Diabetes Care 43, 1–8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatenoud L, Primo J, Bach JF, CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol 158, 2947–2954 (1997). [PubMed] [Google Scholar]
- 44.Akirav EM, Baquero MT, Opare-Addo LW, Akirav M, Galvan E, Kushner JA, Rimm DL, Herold KC, Glucose and inflammation control islet vascular density and beta-cell function in NOD mice: control of islet vasculature and vascular endothelial growth factor by glucose. Diabetes 60, 876–883 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC, Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes 55, 3238–3245 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Perdigoto AL, Preston-Hurlburt P, Clark P, Long SA, Linsley PS, Harris KM, Gitelman SE, Greenbaum CJ, Gottlieb PA, Hagopian W, Woodwyk A, Dziura J, Herold KC, N. Immune Tolerance, Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis. Diabetologia 62, 655–664 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herold KC, Gitelman SE, Willi SM, Gottlieb PA, Waldron-Lynch F, Devine L, Sherr J, Rosenthal SM, Adi S, Jalaludin MY, Michels AW, Dziura J, Bluestone JA, Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia 56, 391–400 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Battaglia M, Anderson MS, Buckner JH, Geyer SM, Gottlieb PA, Kay TWH, Lernmark A, Muller S, Pugliese A, Roep BO, Greenbaum CJ, Peakman M, Understanding and preventing type 1 diabetes through the unique working model of TrialNet. Diabetologia 60, 2139–2147 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rana S, Yue S, Stadel D, Zoller M, Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol 44, 1574–1584 (2012). [DOI] [PubMed] [Google Scholar]
- 50.A. American Diabetes, 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 42, S13–S28 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Sims EK, Chaudhry Z, Watkins R, Syed F, Blum J, Ouyang F, Perkins SM, Mirmira RG, Sosenko J, DiMeglio LA, Evans-Molina C, Elevations in the Fasting Serum Proinsulin-to-C-Peptide Ratio Precede the Onset of Type 1 Diabetes. Diabetes care 39, 1519–1526 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX, The use of areas under curves in diabetes research. Diabetes Care 18, 245–250 (1995). [DOI] [PubMed] [Google Scholar]
- 53.Steele C, Hagopian WA, Gitelman S, Masharani U, Cavaghan M, Rother KI, Donaldson D, Harlan DM, Bluestone J, Herold KC, Insulin secretion in type 1 diabetes. Diabetes 53, 426–433 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Polonsky KS, Licinio-Paixao J, Given BD, Pugh W, Rue P, Galloway J, Karrison T, Frank B, Use of biosynthetic human C-peptide in the measurement of insulin secretion rates in normal volunteers and type I diabetic patients. J Clin Invest 77, 98–105 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Cauter E, Mestrez F, Sturis J, Polonsky KS, Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 41, 368–377 (1992). [DOI] [PubMed] [Google Scholar]
- 56.Ehrmann DA, Cavaghan MK, Imperial J, Sturis J, Rosenfield RL, Polonsky KS, Effects of metformin on insulin secretion, insulin action, and ovarian steroidogenesis in women with polycystic ovary syndrome. J Clin Endocrinol Metab 82, 524–530 (1997). [DOI] [PubMed] [Google Scholar]
- 57.Ghazi T, Rink L, Sherr JL, Herold KC, Acute metabolic effects of exenatide in patients with type 1 diabetes with and without residual insulin to oral and intravenous glucose challenges. Diabetes Care 37, 210–216 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polonsky KS, Given BD, Hirsch LJ, Tillil H, Shapiro ET, Beebe C, Frank BH, Galloway JA, Van Cauter E, Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. N Engl J Med 318, 1231–1239 (1988). [DOI] [PubMed] [Google Scholar]
- 59.Long SA, Thorpe J, Herold KC, Ehlers M, Sanda S, Lim N, Linsley PS, Nepom GT, Harris KM, Remodeling T cell compartments during anti-CD3 immunotherapy of type 1 diabetes. Cell Immunol 319, 3–9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.