Abstract
The emergence and clinical application of immunotherapy is considered a promising breakthrough in cancer treatment. According to the literature, immune checkpoint blockade (ICB) has achieved positive clinical responses in different cancer types, although its clinical efficacy remains limited in some patients. The main obstacle to inducing effective antitumor immune responses with ICB is the development of an immunosuppressive tumor microenvironment. Myeloid-derived suppressor cells (MDSCs), as major immune cells that mediate tumor immunosuppression, are intimately involved in regulating the resistance of cancer patients to ICB therapy and to clinical cancer staging and prognosis. Therefore, a combined treatment strategy using MDSC inhibitors and ICB has been proposed and continually improved. This article discusses the immunosuppressive mechanism, clinical significance, and visualization methods of MDSCs. More importantly, it describes current research progress on compounds targeting MDSCs to enhance the antitumor efficacy of ICB.
Keywords: Immunotherapy, immunosuppression, MDSCs, ICB, compounds
Introduction
As cancer progresses, a complex tumor microenvironment (TME) is gradually formed through the interaction of immune cells infiltrating the tumor tissue with cancer cells1. To cope with the pathological changes in the body, the immune system activates effectors that exert antitumor effects2. However, myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and other immunosuppressive cells are induced by tumors and antagonize the effector function of cytotoxic T lymphocytes (CTLs), thereby preventing their infiltration into tumor tissues3,4. Moreover, the inhibitory immune checkpoint molecules programmed death 1 (PD1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA4), among other molecules expressed on T cells, bind their ligands and subsequently participate in mediating T cell inhibition5. These inhibitory reactions impair T cell activation and are considered the main mechanisms promoting tumor progression and immune escape6. Because the immune system has plasticity and can be reprogramed to exert antitumor effects, several immunotherapies emerged and quickly received widespread attention7.
Immunotherapies, including monoclonal antibody (mAb) therapy and immune cell immunotherapy, have shown potentially beneficial results in clinical applications8,9. Phase III clinical trials with immune checkpoint blockade (ICB) therapies, including anti-PD1/PD-L1 or anti-CTLA4 mAbs, have shown positive antitumor activity and prolonged overall survival in the treatment of various solid and hematological malignancies10. However, some cancer patients show little or no response to ICB therapy11,12. The efficacy of immunotherapy depends on enhancing effective CTL responses to tumor-associated antigens, and patients with low immunogenic tumors may lack sufficient preexisting tumor-infiltrating lymphocytes (TILs), thus resulting in a limited response to ICB therapy13,14. Therefore, an urgent need remains to improve the responses of patients with various types of cancer to ICB therapy and to enhance its antitumor efficacy.
Relieving immunosuppression, a typical feature of the TME15, is a potent strategy to enhance the efficacy of ICB therapy. Among the immunosuppressive cells infiltrating into tumors, MDSCs are significantly associated with intratumoral immunosuppression through multiple mechanisms, and their inhibitory activity is closely involved in disease progression and poor prognosis in cancer patients16,17. Vigorously amplified and activated MDSCs in cancer patients lead to T cell suppression and impair the antitumor immune response18. In addition, cancer tissues with high MDSC infiltration have been shown to be associated with patient resistance to various immunotherapies15. The aim of ICB in cancer patients is to reverse the immunosuppressive signals in the TME19, and the levels of MDSCs in cancer patients may be a prerequisite for initiating ICB therapy20. Therefore, the development of combined immunotherapies that target MDSC-mediated immunosuppressive pathways to improve the antitumor efficacy of ICB therapy has very broad prospects21.
Given the gradually increasing clinical application of combined treatments, comprehensive and updated literature reviews on the current combination of MDSC inhibition and ICB are lacking. In this review, we describe the immunosuppressive properties, clinical value, and visualization methods of MDSCs. Importantly, we focus on strategies for targeting MDSCs and compounds combined with ICB therapy.
The role of MDSCs in the TME
Classification of MDSCs
A heterogeneous population of myeloid-derived cells is defined as MDSCs22. MDSCs consist of 2 groups of cells termed polymorphonuclear MDSCs (PMN-MDSCs) and monocytic MDSCs (M-MDSCs)23. In humans, PMN-MDSCs are characterized by CD11b+CD33+HLA-DR−/CD14−CD15+, and M-MDSCs are characterized by CD11b+CD33+HLA-DR−/CD14+CD15−24. In mice, the Gr-1-specific antibody binds Ly6G and Ly6C22. PMN-MDSCs have a CD11b+Ly6G+Ly6Clow phenotype, and M-MDSCs have a CD11b+Ly6G−Ly6Chi phenotype22.
In tumor tissues, M-MDSCs dominate the MDSC classification, whereas in peripheral lymphoid organs, the proportion of PMN-MDSCs is much greater than that of M-MDSCs25. M-MDSCs mainly upregulate the expression of arginase 1 (ARG1), inducible nitric oxide synthase (iNOS) and transforming growth factor β (TGFβ), thus causing nonspecific T cell inactivation26. PMN-MDSCs produce excessive reactive oxygen species (ROS) and reactive nitrogen species (RNS), which cause effector T cells to lose their response to antigen-specific stimulation but retain their ability to respond to nonspecific stimulation27. Therefore, the inhibitory characteristics of the MDSCs present in tumors and peripheral lymphoid organs differ. In addition, owing to the biochemical and functional heterogeneity of MDSCs, differences exist in the MDSC phenotypes present in different cancer types, and these phenotypes change with the cancer environmental conditions28. In most types of cancer, PMN-MDSCs account for more than 80% of all MDSCs23.
Immunosuppressive function of MDSCs
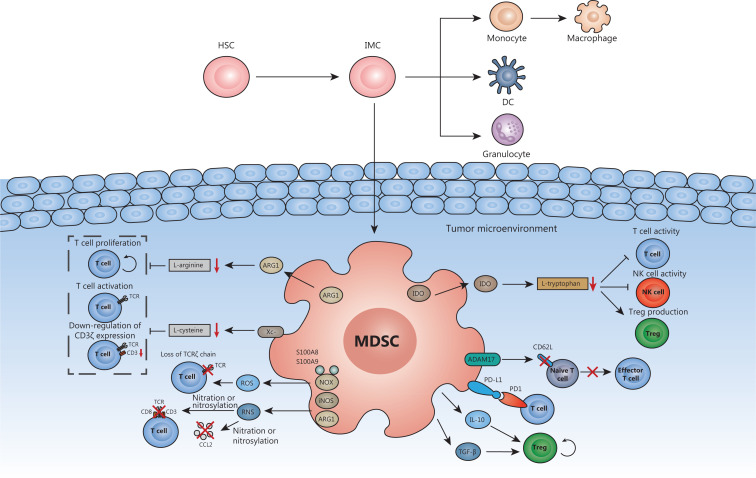
Under physiological conditions, only a small number of MDSCs exist in the circulation and participate in regulating tissue repair and immune responses15. In the tumor-driven microenvironment, the population of MDSCs is greatly expanded by the induction of tumor-derived growth factors and proinflammatory cytokines derived from the tumor stroma29. Activated MDSCs then induce anergy in effector T cells, thus impairing the innate and adaptive immune responses through multiple mechanisms (Figure 1).
Figure 1.
Mechanisms of MDSC-mediated immunosuppression. In healthy individuals, hematopoietic stem cells (HSCs) differentiate into immature myeloid cells (IMCs); IMCs can differentiate into granulocytes or monocytes, or further differentiate into mature macrophages or dendritic cells (DCs). In cancer patients, the maturation process of IMCs is disrupted, thus resulting in a dramatic expansion of the MDSC population. The activated MDSCs in the TME (i) express ARG1 and Xc−, thus depriving T cells of L-arginine and L-cysteine, which are essential for proliferation and activation; (ii) consume L-tryptophan by expressing IDO, thus inhibiting the activity of T cells and NK cells and increasing Treg production; (iii) release ROS and RNS, which mediate loss of the TCR ζ-chain and the nitration or nitrosylation of TCR signaling complex components and CCL2; (iv) express ADAM17, which cleaves CD62L, thereby preventing naive T cells from migrating to tumors or lymph nodes and subsequently forming effector T cells; (v) express PD-L1, which binds PD1 on T cells; and (vi) secrete IL-10 and TGF-β, which stimulate Treg activation and expansion.
Enrichment in MDSCs results in excessive consumption of L-arginine and L-cysteine—amino acids necessary for T cell proliferation and activation30,31. After stimulation with cytokines including interferon γ (IFN-γ), interleukin 10 (IL-10), and tumor necrosis factor β (TNF-β), MDSCs overexpress ARG1 and consequently consume L-arginine32. MDSCs express cystine–glutamate transporters (Xc−) and compete with antigen-presenting cells for the uptake of extracellular L-cysteine, thereby preventing T cells from importing L-cysteine31. In addition, the elimination of these necessary amino acids decreases T cell-CD3ζ, interferes with T cell Janus kinase/signal transduction and transcription activator (JAK/STAT) signaling proteins and inhibits MHC class II molecules, thereby suppressing T cells32,33.
L-Tryptophan is also an important amino acid for T cell function34. MDSCs express indoleamine-2,3-dioxygenase (IDO), which consumes L-tryptophan, and the resultant metabolite kynurenine diminishes the activity of T cells and natural killer (NK) cells and increases Treg production35,36. IDO helps tumors evade immune surveillance by depleting tryptophan in the TME and induces immunosuppressive responses by inhibiting the functions of CTLs37. These disordered T cells secrete IFN-γ, thus further increasing the expression of IDO and perpetuating the immunosuppressive cycle38.
Oxidative stress mediated by ROS and RNS is a crucial mechanism in MDSC-mediated immunosuppression33. The NADPH oxidase (NOX) complex, composed of S100A8, S100A9, and gp91phox, regulates ROS generation by MDSCs. High ROS levels and their interaction with nitric oxide (NO) contribute to the formation of strong biological RNS such as peroxynitrite (ONOO−) through the regulation of ARG1, NOX, and iNOS227,33. Tumor-associated myeloid cells release ROS such as hydrogen peroxide (H2O2), which mediates the loss of the TCR ζ-chain and consequently inhibits T cell activation33. RNS cause the nitration or nitrosylation of CC-chemokine ligand 2 (CCL2), thus inhibiting the infiltration of TILs into the tumor core39. More importantly, the generation of ROS and RNS by MDSCs eliminates the ability of CD8+ T cells to bind peptide-MHC complexes and weakens the antigen-specific responses of peripheral CD8+ T cells by modifying TCR and CD8 molecules27.
In addition to the main mechanisms described above, MDSCs inhibit T cells through other means. MDSCs decrease the L-selectin levels on effector T cells through the plasma membrane expression of a disintegrin and metalloproteinase 17 (ADAM17), thus preventing T cells from homing to tumors or lymph nodes40. MDSCs accumulating at tumor sites are exposed to a hypoxic and inflammatory microenvironment25. Hypoxia-inducible factor-1α (HIF-1α) has been reported to participate in the regulation of high levels of PD-L1 expressed by MDSCs; PD-L1 binds PD1 and subsequently induces T cell failure41. Furthermore, MDSCs stimulate the activation and expansion of Tregs by secreting IL-10 and TGF-β42. Excessive Treg infiltration in the TME promotes tumor progression and is associated with poor prognosis in cancer patients43.
Clinical importance and visualization of MDSCs
The prognostic value of MDSCs
Compared with those in healthy individuals, circulating MDSCs in cancer patients in all stages are significantly elevated, and MDSC levels are strongly associated with the clinical cancer stage and metastatic tumor burden44.
MDSCs promote tumor angiogenesis45,46 and can induce epithelial-mesenchymal transition and cancer cell stemness or cancer stem cell expansion, thereby promoting metastasis47. Before tumor cells reach premetastatic sites, MDSCs significantly decrease IFN-γ levels; increase proinflammatory cytokine and matrix metalloproteinase 9 (MMP9) production; and promote vascular remodeling, thereby forming an inflammatory and immunosuppressive environment48. MDSCs have also been reported to interfere with the senescence-related secretory phenotypes of tumors through secreting interleukin-1 receptor antagonists (IL-1Rα), thereby antagonizing tumor cell senescence49. On the basis of this evidence, high levels of MDSCs in cancer patients predict poor prognosis.
The influence of MDSCs on treatment effects
In cancer patients, expanded MDSC populations and immunosuppressive states arise by the time at which precancerous lesions are present and gradually become aggravated with tumor progression50. However, few effector T cells are found in preinvasive lesions, owing to MDSC infiltration into tumors in a mutually exclusive manner51. Therefore, blocking MDSCs early in the course of immunotherapy is important.
Patients diagnosed with non-small cell lung cancer who cannot be treated surgically have lower overall survival if they have high M-MDSC levels in the peripheral blood before receiving chemotherapy52. In addition, among patients with pancreatic adenocarcinoma undergoing chemotherapy, those with progressive disease have clearly higher MDSC levels in the peripheral blood than those with stable disease53.
Studies have shown that low baseline percentages of peripheral MDSCs before ICB therapy or their decrease during treatment indicates positive outcomes54. Thus, effective detection of the dynamic distribution of MDSCs in vivo would provide favorable information for evaluating cancer patients’ responses to various therapies, as well as their prognoses.
Methods for visualizing MDSCs
Traditional MDSC detection is mainly dependent on measurements in vitro or invasive methods50. Because the imaging of S100A8/A9 released by MDSCs reflects the abundance of MDSCs in premetastatic sites and the establishment of an immunosuppressive environment, antibody-based single-photon emission computed tomography has been applied to detect S100A8/A9 in vivo and has made substantial progress55. A premodified CD11b-specific mAb has been used to radiolabel PMN- and M-MDSCs, and positron emission tomography (PET) imaging has subsequently been used to noninvasively and quantitatively monitor the migration of MDSCs in multiple cancer types56. Moreover, some RNA aptamers that specifically recognize tumor-infiltrating MDSCs have been identified. These aptamers can be used not only to detect MDSCs but also to conjugate them to chemotherapeutic drugs, and improve antitumor efficacy and targeted delivery of drugs to the TME57.
Near-infrared II (NIR-II) fluorescence imaging overcomes the barriers of penetration/contrast in the field of visible imaging58; on the basis of this technology, NIR-IIa and NIR-IIb PbS/CdS quantum dot-based nanoprobes conjugated with 2 MDSC-specific antibodies have been created to target MDSCs in vivo59. This nanoprobe can clearly reveal the real-time dynamic distribution of MDSCs in cancer patients in a non-traumatic manner through the colocalization of two-color fluorescence; therefore, it has important clinical value for the evaluation of MDSC-targeted immunotherapy.
Strategies and compounds for targeting MDSCs
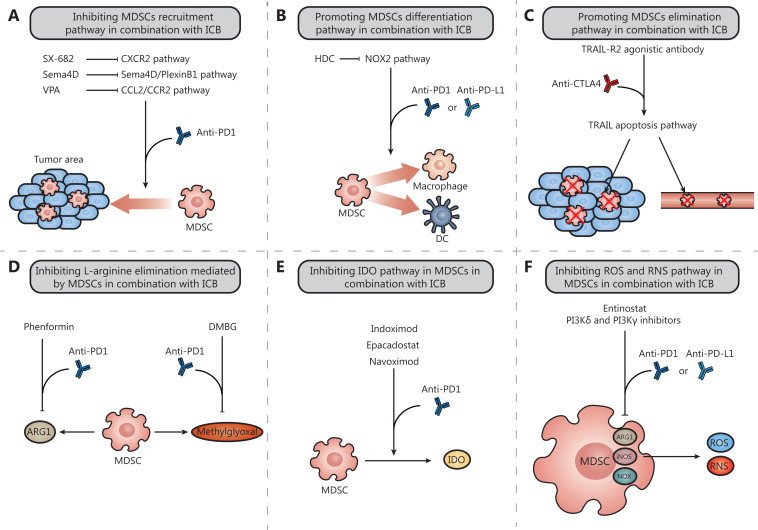
MDSCs promote tumor progression and metastasis and contribute to tumor immune escape through a variety of mechanisms. The accumulation of MDSCs with substantial immunosuppressive activity in tumor tissues is associated with the resistance of cancer patients to multiple immunotherapies and with poor prognosis47. Strategies for targeting MDSCs to improve the antitumor effects of immunotherapies have sparked widespread interest and have made positive progress. As shown in Figure 2, these strategies comprise those that (1) prevent the recruitment of MDSCs; (2) promote the differentiation of MDSCs into mature cells; (3) deplete MDSCs in the circulation and the tumor; (4) inhibit the elimination of L-arginine mediated by MDSCs; (5) inhibit the activation of IDO in MDSCs; and (6) decrease the levels of ROS and RNS in MDSCs. We have selected several compounds reported in recent years that affect MDSCs through these pathways. The results are divided into 6 categories according to the strategies used to target MDSCs (Table 1).
Figure 2.
Combinations of MDSC-targeted compounds and ICB. A. SX-682, the Sema4D mAb and VPA inhibit the MDSC recruitment pathway; B. HDC promotes the MDSC differentiation pathway; C. The TRAILR2 agonistic antibody promotes the MDSC elimination pathway; D. Phenformin and DMBG inhibit the L-arginine elimination pathway mediated by MDSCs; E. Indoximod, epacadostat, and navoximod inhibit the IDO pathway in MDSCs; F. Entinostat and inhibitors of PI3Kδ and γ inhibit the ROS and RNS pathways in MDSCs.
Table 1.
Compounds targeting MDSCs
| Strategy | Compound | Mechanisms of action | Clinical state | Reference |
|---|---|---|---|---|
| Preventing the recruitment of MDSCs | AZ13381758 (CXCR2 SM) | Inhibition of CXCR2 | Trial in mice bearing pancreatic tumors | 64 |
| TG100-115 | Inhibition of PI3-kinase isoform p110γ | Trial in mice bearing tumors | 65 | |
| Zoledronate | Inhibition of MMP-9 activity | FDA approved for multiple cancers | 82 | |
| 1α,25-Dihydroxyvitamin D3 | Inhibition of IL6/p-STAT3 | Trial in mice bearing esophageal squamous cell carcinoma | 83 | |
| Bevacizumab | Blocking of VEGF signaling | FDA approved for multiple cancers | 84 | |
| SX-682 | Inhibition of CXCR2 signaling | Phase I trial in melanoma | 85 | |
| Sema4D mAb | Inhibition of MAPK-dependent chemokines such as CXCL1, 2, and 5 | Trial in mice bearing oral cancer | 86 | |
| VPA | Inhibition of HDAC activity and downregulation of CCR2 | FDA approved for multiple cancers | 87 | |
| Promoting the differentiation of MDSCs into mature cells | Icariin and ICT | Downregulation of S100A8/9, STAT3, and AKT | Phase I trial in solid tumors; Phase III trial in hepatocellular carcinoma | 69 |
| ATRA | Activation of ERK1/2 and upregulation of the ROS scavenger GSH | Phase II and Phase III trials in multiple cancers; Phase IV trial in acute promyelocytic leukemia | 70 | |
| ATX | Activation of the Nrf2 signaling pathway to induce the synthesis of GSH | Early Phase I trial in healthy volunteers; trial in mice bearing colon tumors | 71 | |
| Curcumin | Inhibition of activation of NF-κB and STAT3 signaling | Phase II and Phase III trials in multiple cancers | 88 | |
| MPSSS | Stimulation of the Myd88-dependent NF-κB signaling pathway | Trial in mice bearing tumor | 89 | |
| Docetaxel | Decrease in pSTAT3 in MDSCs | FDA approved for multiple cancers | 90 | |
| WGP | Activation of dectin-1 receptor | Phase II trial in non-small cell lung cancer | 91 | |
| HDC | Inhibition of NOX2 | Phase IV trial in acute myeloid leukemia | 92 | |
| Depleting MDSCs in the circulation and tumor infiltration | Anti-Ly6G mAb; anti-Ly6C mAb | Depletion of MDSCs | Trials in mice bearing multiple types of tumors | 61 |
| BI 836858 | Depletion of MDSCs by ADCC | Phase I trial in acute myeloid leukemia | 74 | |
| Doxorubicin | Triggering of the apoptosis program of MDSCs | FDA approved for multiple cancers | 75 | |
| Gemcitabine | Induction of MDSC apoptosis and necrosis | FDA approved for multiple cancers | 76 | |
| 5-FU | Activation of caspase-3 and caspase-7 to induce MDSC apoptosis | FDA approved for multiple cancers | 77 | |
| Celecoxib | Inhibition of COX-2 and decrease in ROS | FDA approved for multiple cancers | 93 | |
| RGX-104 | Promotion of MDSC apoptosis | Phase I trial in malignant neoplasms | 94 | |
| Anti-IL4Rα aptamer | Inhibition of IL-4Rα–STAT6 signaling | Trial in mice bearing mammary carcinoma | 95 | |
| Peptibody | Depletion of MDSCs by complement-dependent cytotoxicity or ADCC and induction of MDSC apoptosis | Trials in mice bearing multiple types of tumors | 96 | |
| DS-8273a | Induction of MDSC apoptosis by agonist of TRAIL-R2 | Phase I trial in patients with advanced cancers | 97 | |
| Inhibiting the elimination of L-arginine mediated by MDSCs | Phenformin | Decrease in the levels of ARG1 and S100A8/A9 | Phase I trial in melanoma | 98 |
| LPS | Interference with the HMGB1-TLR4 axis | Phase I and Phase II trials in lymphoma | 99 | |
| DMBG | Neutralization of the glycosylation function of methylglyoxal | Trial in melanoma-bearing mice | 100 | |
| Inhibiting the activation of IDO in MDSCs | Indoximod | Inhibition of IDO | Phase III trials in multiple cancers | 101 |
| Navoximod | Inhibition of IDO | Phase I trial in patients with recurrent/advanced solid tumors | 101 | |
| Epacadostat | Inhibition of IDO | Phase III trial in multiple cancers | 101 | |
| BMS-986205 | Inhibition of IDO | Phase I and Phase II trials in melanoma; Phase I trial in various other types of advanced tumors | 101 | |
| 1-MT | Inhibition of IDO | Phase I and Phase II trials in multiple cancers | 34 | |
| Decreasing the levels of ROS and RNS in MDSCs | Sildenafil | Downregulation of ARG1 and NOS2 expression | FDA approved for multiple cancers | 78 |
| CDDO-Me | Inhibition of STAT3 activity, upregulation of antioxidant genes, and decrease in ROS | Phase I and Phase II trials in multiple cancers | 81 | |
| AT38 | Downregulation of ARG1 and NOS2, and decrease in RNS and N-CCL2 | Trials in mice bearing multiple types of tumors | 39 | |
| NAC | Inhibition of ROS production | Phase I trial in multiple cancers; Phase II trial in patients at risk of melanoma | 102 | |
| WA | Decrease in TNFα, IL6, IL10, and ROS | Trials in mice bearing multiple types of tumors | 103 | |
| Entinostat | Decrease in ARG1, iNOS, and COX2 | Phase I and Phase II trials in multiple cancers; Phase III trial in breast carcinoma | 104 | |
| IPI-145 | Inhibition of PI3Kδ and PI3Kγ | Phase I and Phase II trials in head and neck squamous cell carcinoma; Phase III trial in lymphoma | 105 |
VPA, valproic acid; CCR2, CC-chemokine receptor 2; ATRA, all-trans retinoic acid; ATX, astaxanthin; WGP, whole β-glucan particles; HDC, histamine dihydrochloride; ADCC, antibody-dependent cellular cytotoxicity; 5-FU, 5-fluorouracil; LPS, lipopolysaccharides; DMBG, dimethylbiguanide; 1-MT, 1-methyl-L-tryptophan; NAC, N-acetylcysteine; WA, Withaferin A.
Preventing the recruitment of MDSCs
The factors that control the recruitment of MDSCs to tumors are essentially the same as those that regulate the migration of monocytes and neutrophils26. The absence of peripheral proliferation by MDSC subgroups suggests that targeting the chemokine system to inhibit the migration of MDSCs to tumors is a potential therapeutic method for eliminating MDSCs from tumor tissues60.
MDSCs are recruited to tumor sites through GM-CSF; monocyte chemoattractant protein 1 (MCP1); CXCL1, 2, 5, and 12; IL-8; CSF1; prokineticin 2 (Prok2); CCL2; S100A8/9; and other factors derived from the TME47,61,62. In addition, the hypoxic environment at the primary tumor site induces the production of growth factors and cytokines that recruit MDSCs and decrease the cytotoxic effector functions of NK cell populations, thereby creating a premetastatic niche63.
In pancreatic ductal adenocarcinoma treated with CXCR2 signaling inhibitors, the migration of MDSCs and the formation of a niche at the metastatic site are significantly suppressed64. The phosphatidylinositol 3-kinase (PI3K) isoform p110γ is required for the integrin α4β1-mediated adhesion of myeloid cells. After activation, p110γ-dependent α4β1 recruits myeloid cells to tumor sites. In mice with breast carcinoma treated with the p110γ inhibitor TG100-115, MDSC recruitment is significantly decreased65.
Promoting the differentiation of MDSCs into mature cells
In the tumor-driven microenvironment, proinflammatory cytokines such as prostaglandin-E2 (PG-E2), macrophage colony-stimulating factor (M-CSF), granulocyte colony-stimulating factor (G-CSF), GM-CSF, stem cell factor (SCF), and vascular endothelial growth factor (VEGF) are involved in the induction of chronic inflammation in cancer26. The signaling pathways in MDSCs triggered by cytokines converge on members of the JAK protein family and STAT3, thereby participating in the regulation of cell proliferation and differentiation22. STAT3 suppresses dendritic cell (DC) differentiation and increases MDSC accumulation and activation by upregulating the expression of S100A8 and S100A9, the transcription factor CCAAT-enhancer-binding protein β (C/EBPβ), interferon regulatory factor 8 (IRF8), and other important proteins33,66. STAT3 also upregulates NOX2 components and increases ROS levels, thereby enhancing MDSC inhibition67. In addition, the interaction of T cell immunoglobulin 3 (TIM3) on T cells and galectin 9 (Gal9) on MDSC precursors promotes MDSC proliferation and inhibits T cell responses68. Therefore, targeting proinflammatory cytokines to promote the maturation and differentiation of MDSCs is a promising strategy to decrease their population and abolish their immunosuppressive function.
When using the compound ICT, a derivative of icariin, to process MDSCs in vitro, the expression of S100A8 and S100A9 and the activation of STAT3 and protein kinase B (AKT) in MDSCs are significantly inhibited, thus leading to a lower percentage of MDSCs and their differentiation into DCs and macrophages. Moreover, the conversion of this cell type is accompanied by the downregulation of IL-10, IL-6, and TNF-α production69. All-trans-retinoic acid (ATRA) is a retinoid receptor agonist that inhibits retinoic acid signaling. By mediating the accumulation of glutathione (GSH) and neutralizing ROS, ATRA promotes the differentiation of MDSCs into mature myeloid cells70. The carotenoid astaxanthin (ATX) has considerable antioxidant activity. In ATX-treated tumor- bearing mice, the Nrf2 signaling pathway in MDSCs is activated and induces the synthesis of GSH, which in turn promotes further differentiation of MDSCs into macrophages or DCs71. These drugs not only weaken the immunosuppressive effects of MDSCs but also enhance the body’s innate immunity.
Depleting MDSCs in the circulation and in tumors
IL-4, IL-13, and IFN-γ, ligands for Toll-like receptors (TLRs) and TGF-β produced by activated T cells and tumor stromal cells, initiate multiple signaling pathways, such as STAT1, STAT6, and nuclear factor κB (NF-κB), thereby regulating MDSC activity72. The formation of a positive feedback loop between PG-E2 and cyclooxygenase 2 (COX2) contributes to stabilizing the phenotype of MDSCs and regulating their inhibitory function73.
In addition to inhibiting the activation of MDSCs, inducing their apoptosis is an effective strategy to deplete MDSCs in the circulation and in tumors. Preclinical studies have used anti-Ly6C or anti-Ly6G antibodies to treat multiple tumor-bearing mouse models or to systematically eliminate MDSCs in mice, and enhanced antitumor effects have been observed61. In addition, the fully humanized, Fc-modified monoclonal antibody BI 836858 for CD33 has been found to consume MDSCs through antibody-dependent cell-mediated cytotoxicity74.
According to the evidence that the differentiation of MDSCs relies on the signal transduction of cellular tyrosine kinases, sunitinib treatment in cancer patients significantly decreases the effects of c-Kit and vascular endothelial growth factor receptor on MDSCs, thereby diminishing MDSC levels15. Furthermore, low-dose chemotherapy effectively eliminates MDSC populations in tumor-bearing mice26. Multiple chemotherapeutics, such as doxorubicin75, gemcitabine76, and 5-fluorouracil (5-FU)77, have been reported to induce MDSC apoptosis, thus enhancing antitumor immune activity.
Inhibiting the immunosuppressive function of MDSCs
Eliminating MDSC-mediated immunosuppression and reversing the suppressed states of T cells are the main objectives of immunotherapy. In this process, effective inhibition of the key molecules in MDSC suppressive pathways is crucial. These strategies can be further divided into inhibiting the elimination of L-arginine mediated by MDSCs, inhibiting the activation of IDO, and decreasing the levels of ROS and RNS in MDSCs.
The phosphorylation of STAT3 is required to induce MDSCs to upregulate the expression of IDO. Therefore, IDO-induced MDSC immunosuppressive activity can be blocked by the STAT3 antagonist JSI-124 or the IDO inhibitor 1-methyl-L-tryptophan34.
Sildenafil, a a phosphodiesterase 5 (PDE5) inhibitor, downregulates the expression of IL-4Rα on MDSCs, and decreases ARG1 and iNOS levels, thereby inhibiting MDSC inhibitory activity78. PDE5 inhibitors also increase tumor-infiltrating CD8+ T cells and decrease Treg proliferation, thereby enhancing immune-mediated antitumor activity79.
The anti-inflammatory triterpenoid CDDO-Me stimulates the nuclear factor-erythroid 2-related factor 2 (Nrf2) pathway and consequently upregulates multiple antioxidant genes80. The application of CDDO-Me to target MDSCs effectively decreases ROS production, thus abolishing the immunosuppressive effect of MDSCs81.
Compounds targeting MDSCs applied in combination with ICB
As discussed above, activated MDSCs significantly inhibit T cell infiltration into lesion sites and antitumor activity through specific and nonspecific mechanisms. MDSCs are strongly associated with tumor progression and metastasis. Therefore, expanded MDSCs in the TME are considered crucial in ICB therapy resistance among cancer paitents106. With the rise of combination immunotherapies, reports combining MDSC-targeting strategies with ICB to treat cancer are increasingly being updated87,107. Here, we elaborate on several representative reports on the combination of compounds targeting MDSCs through multiple mechanisms and ICB (Figure 2).
Inhibiting the MDSC recruitment pathway in combination with ICB
SX-682
SX-682, a small-molecule allosteric inhibitor of CXCR2, selectively inhibits CXCR2+ PMN-MDSC transport into tumors and consequently decreases the MDSC population in tumors85. CXCR2 is a G protein-coupled receptor for the human CXC chemokines CXCL1, 2, 3, 5, 6, 7, and 864. CXCL1/2 attracts CD11b+Gr-1+ myeloid cells to tumors, thus enhancing the survival of cancer cells through the generation of chemokines, including S100A8/9108. In various tumor models, the CXCR2 signaling pathway has been observed to recruit PMN-MDSCs to the TME, and drive tumor invasion and metastasis109,110.
CXCR2 blockade promotes T cell infiltration into tumors and improves sensitivity to immunotherapy. The combination of a CXCR2 inhibitor (CXCR2 SM) with an anti-PD1 antibody to treat mice bearing pancreatic ductal adenocarcinoma clearly inhibits metastasis and enhances antitumor efficacy64. In addition, PD-L1 is highly expressed in murine rhabdomyosarcoma, but treatment with PD1 blockade alone has limited persistent effects. When combined with anti-CXCR2, anti-PD1 elicits enhanced antitumor effects111.
Accumulation of CD8+ TILs and increased PD-L1 expression on tumor cells has been observed in tumor-bearing mice treated with SX-68285. Combining SX-682 with an anti-PD1 antibody to treat mice significantly inhibits tumor growth and increases mouse survival rates85,112. Importantly, combined treatment with SX-682 and the anti-PD1 antibody pembrolizumab has been tested in phase I clinical trials for metastatic melanoma26.
Semaphorin 4D mAb
The interactions of Semaphorin 4D (Sema4D) with its receptor Plexin-B1 regulate angiogenesis and tumor invasive growth113. Sema4D, derived from cancer cells, induces the formation of peripheral blood mononuclear cells into MDSCs in vitro. Additionally, the function of Sema4D in promoting tumor progression has been confirmed in various malignant tumors in human and animal models, and associated with a poor prognosis114.
A decrease in PMN-MDSC recruitment has been observed after the use of Sema4D mAb to treat murine oral cancer 1 (MOC1); this phenomenon is associated with decreased expression of MAPK-dependent chemokines such as CXCL1, 2, and 5 in tumor cells86. The decrease in PlexinB1 downstream ERK and STAT3-dependent arginase production also weakens PMN-MDSC-induced T cell inhibition. Moreover, IFN-γ production increases in the TME. These changes increase the infiltration of CD8+ TILs into tumors and enhance the activation of T lymphocytes in draining lymph nodes.
The combination of the Sema4D mAb and anti-PD1 in MOC1 or Lewis lung carcinoma mouse models suppresses tumor growth and significantly improves survival in both models. The decrease in PMN-MDSC recruitment and inhibition of immunosuppressive functions caused by the Sema4D mAb enhances the specific responses of T cells to tumor antigens, thus potentially explaining the increased immune response to PD1 blockade86. Similar combined effects have been observed in models of colon carcinoma115.
Valproic acid
When GM-CSF-stimulated murine bone marrow cells are exposed to histone deacetylases (HDACs), M-MDSCs substantially expand, and the proliferation of allogeneic T cells is inhibited116. The antiepileptic drug valproic acid (VPA) has been shown to be a strong class I HDAC inhibitor that may inhibit HDAC activity by binding the catalytic center117. VPA effectively alleviates tumor burden by decreasing the number of M-MDSCs infiltrating into tumors, and the antitumor immune response induced by anti-PD1 has been found to be improved by combination treatment with VPA87.
In anti-PD1-sensitive EL4 and anti-PD1-resistant B16-F10 tumor-bearing mouse models, the combined application of VPA and anti-PD1 clearly increases CD8+ T cell infiltration into tumors and suppresses tumor progression87. The overexpression of the chemokine CCL2 and its receptor CCR2 has been observed in multiple cancer types118,119. The CCL2/CCR2 pathway is strongly associated with MDSC migration into tumors, and a lack of these cytokines can impair the tumor-promoting effects of MDSCs120. In both mouse models, the application of VPA inhibits the activity of HDACs and decreases CCR2 expression on M-MDSCs, thereby inhibiting the recruitment of M-MDSCs to tumors. The decrease in histone acetylation in MDSCs caused by VPA may explain the observed downregulation of CCR2 expression. VPA also promotes CD8+ T cell and NK cell expansion and reactivation in the TME. In addition, the decrease in ARG1 and prostaglandin E synthase levels in the PMN-MDSCs of VPA-treated mice indicates the ability to avoid the immunosuppression caused by PMN-MDSCs87,121.
Promoting the MDSC differentiation pathway in combination with ICB
Histamine dihydrochloride
Histamine dihydrochloride (HDC) can be decomposed into histamine in solution92. Controlled by the STAT3 transcription factor, the upregulation of NOX2 activity increases the ROS levels in MDSCs and the suppression of T cell function. In the absence of NOX2 activity, MDSCs cannot effectively inhibit T cell responses and they will rapidly differentiate into mature DCs and macrophages67. Histamine, an inhibitor of myeloid NOX2, promotes the maturation of myeloid cells that produce ROS. By decreasing the production and extracellular release of ROS, histamine also helps to retain the function of NK cells and promote the NK cell-mediated removal of malignant cells122. In a tumor-bearing mouse model, MDSC accumulation and tumor progression are promoted in histamine-deficient mice compared with wild-type mice123.
In 3 murine cancer models, EL4 lymphoma, MC38 colorectal carcinoma, and 4T1 mammary carcinoma, which exhibit MDSC accumulation, HDC treatment delays tumor growth92. In the EL4 and 4T1 models, HDC decreases MDSC accumulation and the level of NOX2-derived ROS. The negative correlation between the percentage of MDSCs in the tumor and tumor-infiltrating CD8+ T cells is clear in both models, thereby suggesting that HDC relieves MDSC-induced immunosuppression92. However, HDC has not been found to affect tumor progression in Nox2-KO mice or mice lacking Gr-1, thus indicating that its antitumor efficacy requires NOX2+Gr-1+ cells96. More importantly, the combination of HDC and anti-PD1/anti-PD-L1 to treat mice with MC38 or EL4, respectively, has been found to be superior to any monotherapy in inhibiting tumor development. This finding might be associated with the increase in the proportion of CD8+ T cells showing an effector phenotype caused by anti-PD1/anti-PD-L1 treatment92.
Promoting the MDSC elimination pathway in combination with ICB
TRAIL-R2 agonistic antibody
TNF-related apoptosis-inducing ligand (TRAIL) is an effective stimulator of apoptosis, and the TRAIL pathway is a promising target for promoting MDSC elimination. TRAIL ligates 2 receptor types: the death receptors TRAILR1 and TRAILR2 (also known as DR5) and the decoy receptors TRAILR3 and TRAILR4124. On the basis of the function of TRAIL receptors (TRAILRs) to selectively inhibit MDSCs, the efficacy of an agonistic DR5 antibody has been verified125.
In cancer patients with high MDSC levels, DS-8273a, an agonistic antibody to TRAILR2, rapidly eliminates MDSCs without affecting neutrophils, monocytes, or other myeloid and lymphoid cells97. In tumor-free mice, the endoplasmic reticulum stress response causes changes in the expression of TRAILRs in MDSCs, thus resulting in a shorter lifespan for MDSCs than their counterparts (PMNs and monocytes). In tumor-bearing mice treated with the agonistic DR5 antibody (MD5-1 mAb), MDSCs are selectively inhibited. Combining the MD5-1 mAb and anti-CTLA4 to treat mice increases the sensitivity of tumors to anti-CTLA4 and significantly delays tumor progression125.
Inhibiting the L-arginine elimination pathway mediated by MDSCs in combination with ICB
Phenformin
Previous reports have suggested that biguanides, such as phenformin, exhibit antitumor activity both in vivo and in vitro126,127. After treatment with phenformin, the numbers of PMN-MDSCs but not M-MDSCs significantly decrease in the spleens of mice with melanoma. The effects of phenformin on PMN-MDSCs are dependent on AMP-activated protein kinase, a major mediator of its antitumor activity98. Phenformin diminishes the PMN-MDSC population by inhibiting proliferation and promoting apoptosis128,129. These results suggest that phenformin has a selective inhibitory effect on PMN-MDSC-driven immunosuppression.
The combination of phenformin and an anti-PD1 antibody to treat mouse models of BRAF/PTEN melanoma effectively inhibits tumor growth98. In these models, phenformin significantly decreases the levels of proteins such as ARG1, S100A8, and S100A9, which are critical to the immunosuppressive activity of MDSCs. In addition, this combination clearly decreases the ratio of PMN-MDSCs in the tumor and spleen and synergistically promotes CD8+ T cell infiltration, thus further indicating its positive prospects98.
Dimethylbiguanide
The metabolism of MDSCs in tumors and inflammatory tissues is greatly diminished, and this response may be associated with the accumulation of methylglyoxal in MDSCs100. Methylglyoxal can be administered to CD8+ T cells by cell-to-cell transfer; it then causes the consumption of L-arginine inside T cells and the deactivation of L-arginine-containing proteins through glycosylation, thereby inhibiting their effector functions. Dimethylbiguanide (DMBG)-containing guanidine groups neutralize the glycosylation function of methylglyoxal and release the suppressed states of CD8+ T cells conferred by MDSCs100.
After isolation from hepatocellular carcinoma patients, methylglyoxal has been detected in M-MDSCs but not in PMN-MDSCs, thus indicating one difference between human and mouse models100. Strong and lasting tumor regression has been observed in mice with melanoma specifically expressing ovalbumin after the combined application of DMBG and an anti-PD1 antibody. Tumor cells grown after treatment with this combined therapy lose ovalbumin expression100. These results clearly indicate that DMBG relieves MDSC inhibition of CD8+ T cells against tumor-specific antigens, and its combination with an anti-PD1 antibody synergistically increases tumor-specific immune responses.
Inhibiting the IDO pathway in MDSCs in combination with ICB
IDO pathway inhibitors
IDO is activated by MDSCs in many human cancers, and its overexpression tends to be associated with poor prognosis130. Because IDO is regarded as an important target for cancer treatment, IDO pathway inhibitors have been applied in various types of cancer models and clinical trials37,101. In addition, IDO expression is associated with some immune checkpoints, such as PD-L1 and CTLA4, thus supporting a combined targeting strategy131.
In non-Hodgkin lymphoma mouse models, the application of the IDO inhibitor indoximod decreases the number of Tregs in tumor-draining lymph nodes and effectively inhibits tumor growth132. In patients with melanoma, the combination of a PD1 antibody and indoximod has achieved positive disease control rates101. In addition, other IDO inhibitors, such as epacadostat and navoximod, when combined with PD1 blockade to treat head and neck squamous cell carcinoma, melanoma, and other solid tumors, have shown enhanced antitumor activity compared with treatment with PD1 blockade alone133.
Inhibiting the ROS and RNS pathways in MDSCs in combination with ICB
Entinostat
Entinostat, a class I-specific HDAC inhibitor, impairs the dynamic interactions between host immune surveillance and the TME134. Entinostat enhances tumor cell immunogenicity in animals bearing tumors or in cancer patients by activating tumor antigen expression, antigen presentation, and costimulatory molecules135,136. Furthermore, entinostat inhibits the immunosuppressive functions of MDSCs infiltrating into tumors by significantly decreasing the levels of ARG1, iNOS, and COX2104.
In one report, 2 mouse models bearing Lewis lung carcinoma or renal cell (RENCA) carcinoma have been used to assess the combined efficacy of entinostat and PD1 blockade. Enhanced antitumor effects have been observed in both models. In the entinostat and anti-PD1 combination group, compared with the control, entinostat alone or anti-PD1 alone group, MDSC function was suppressed, the FoxP3 protein level in CD4+FoxP3+ cells was strongly decreased, and CD8+ T cell infiltration into the TME was increased. With clear changes in cytokine/chemokine release in vivo, the microenvironment changed from immunosuppressive to tumor suppressive, thus indicating that entinostat promotes the antitumor response to anti-PD1104.
PI3Kδ and γ inhibitors
PI3Ks are part of a family of signal transducing enzymes that mediate critical cellular functions in immunity and cancer137. p110δ and p110γ, class I PI3K isoforms, activate MDSCs, and both are associated with MDSC-mediated immunosuppression in solid tumors65,138,139.
In mice with oral cancer, MDSCs significantly accumulate in the periphery and TME, thus resulting in the inhibition of T lymphocyte function105. The expression of the PI3Kδ and γ isoforms is higher in PMN-MDSCs than MOC cells. The inhibition of PI3Kδ and γ with IPI-145 in vitro partially reverses the immunosuppressive phenotype of peripheral and tumor-infiltrating PMN-MDSCs by altering the expression of ARG1 and NOS2. The combination of IPI-145 and anti-PD-L1 to treat tumor-bearing mice enhances the sensitivity of the mice to anti-PD-L1 and significantly improves the antigen-specific T-lymphocyte response105.
Other drugs
In addition to the drugs listed above, other MDSC-targeting compounds have been reported to have potential for combination with ICB. For instance, Prim-O-glucosylcimifugin impedes the proliferation and activity of PMN-MDSCs, mainly by suppressing the metabolism of arginine and proline and the citric acid cycle140. Cabozantinib and BEZ235 inhibit PI3K-AKT-mTOR signaling in tumors and decrease CCL5, CCL12, CD40, and hepatocyte growth factor levels, thus inhibiting the recruitment and activity of MDSCs112. The combination of these compounds with ICB relieves the suppressed state of effector T cells and increases their infiltration into tumors, thereby improving the responses of cancer patients to ICB and enhancing antitumor efficacy112,140.
Conclusions
The mechanisms of MDSC-mediated immunosuppression in the TME have been described. Additionally, the combined application of MDSC-targeting compounds and ICB to enhance antitumor effects has been shown to have broad prospects, and substantial progress has been made in this field. However, some challenges remain to be overcome in future clinical applications.
The distributions of MDSCs and the dominant MDSC phenotypes in various cancer types are known to differ28. In addition, differences exist in the baseline percentages of MDSCs among individuals52,54. These differences can lead to failure of MDSC-targeted therapies in patients. Therefore, precise detection of the phenotype and the dynamic distribution of MDSCs in cancer patients is beneficial and necessary for personalized cancer therapy. Timely and accurate MDSC visualization methods will provide important reference values for evaluating the effectiveness and durability of immunotherapy, and more effective and less invasive tools also must be developed.
Molecular imaging is a tracking method that reflects specific molecular events in disease progression, thus providing an important foundation for personalized cancer therapy. The use of specific molecules expressed on MDSCs as targets for real-time imaging can provide guidance for the combination of molecular imaging with molecular therapy. The targeting specificity of mAbs combined with the excellent resolution and sensitivity of PET has allowed immunoPET imaging to become an emerging molecular imaging technology with far-reaching value in the development of cancer diagnosis and personalized medicine141,142. Several immunoPET probes targeting anti-PD1 or anti-PD-L1 have been developed to detect the dynamic distribution of these antibodies in the body through precise imaging143–145. In future research, the exploration of immunoPET probes targeting MDSCs may be a meaningful strategy to select patients who are sensitive to MDSC-targeted therapy for further treatment, and to direct patients with little or no response to therapy toward multidisciplinary treatment. In addition, molecular imaging technologies based on ultrasound microbubbles146 or MRI147 are being applied in cancer treatment strategies, and these technologies are expected to be further developed for MDSC-targeted therapies.
Another challenge is the limited clinical trials targeting MDSCs, particularly those testing the combination of targeted MDSC therapy and ICB therapy. Most combination strategies have been evaluated only in the preclinical stage or in small numbers of cancer patients. Furthermore, other effects of related drugs targeting MDSCs on the TME are not clear. Some MDSC-targeting compounds such as HDC have limitations in triggering the influx of CD8+ T cells into tumors, thus indicating that related combination therapies must be properly adjusted92. In the next phase, more clinical trials testing combination therapies are expected to be performed, and the patient cohorts must be expanded to further evaluate the effects of these therapies on patient prognosis and accompanying adverse effects.
Theranostics is a new type of biomedical technology that effectively combines the diagnosis and treatment of diseases, and the emergence of nanoscale agents provides a new opportunity for its further development148. Because of their unique biological properties149, nanomaterials can be considered for integration into MDSC-targeting compounds with molecular markers for imaging to construct specific nanotheranostics. However, how to improve the specific uptake of MDSC-targeting nanotheranostics by tumor tissues and promote their penetration into the tumor core remains a challenge requiring further investigation.
MDSCs intimately participate in mediating immunosuppression in the TME and cancer patients’ resistance to ICB therapy. Therefore, exploration of the clinical diagnosis and MDSC-targeted therapies and visualization methods has very far-reaching value. More importantly, with the in-depth study of MDSC expansion, recruitment and immunosuppressive mechanisms, research dedicated to combining MDSC-targeting strategies with ICB therapies has gradually emerged and made positive progress. Given that many MDSC-targeted compounds have been approved by the FDA or are in different stages of clinical trials, more effective compounds and ICB combination strategies must be further explored to evaluate their antitumor efficacy.
Footnotes
Conflict of interest statement No potential conflicts of interest are disclosed.
Author contributions
Conceived and designed the analysis: Hongchao Tang, Hao Li, Zhijun Sun.
Collected the data and analysis: Hongchao Tang, Hao Li.
Figure preparation: Hao Li, Hongchao Tang.
Wrote and revised the paper: Hongchao Tang, Hao Li, Zhijun Sun.
Grant support
This work was supported by the National Nature Science Foundation of China (Grant Nos. 82072996, 81874131, and 81672668) and Innovative Research Team of High-level Local Universities in Shanghai (Grant No. SSMU-ZLCX20180500). Zhijun Sun was supported by Fundamental Research Funds for the Central Universities of China (Grant No. 2042017kf0171) (Outstanding Young Researchers) and Hubei Province Nature Science Funds for Distinguished Young Scholar (Grant No. 2017CFA062).
References
- 1.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 2.Song H, Park H, Park G, Kim YS, Lee HK, Jin DH, et al. Corticotropin-releasing factor induces immune escape of cervical cancer cells by downregulation of NKG2D. Oncol Rep. 2014;32:425–30. doi: 10.3892/or.2014.3191. [DOI] [PubMed] [Google Scholar]
- 3.Zhang E, Gu J, Xu H. Prospects for chimeric antigen receptor-modified T cell therapy for solid tumors. Mol Cancer. 2018;17:7. doi: 10.1186/s12943-018-0759-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Retseck J, VanderWeele R, Lin HM, Lin Y, Butterfield LH, Tarhini AA. Phenotypic and functional testing of circulating regulatory T cells in advanced melanoma patients treated with neoadjuvant ipilimumab. J Immunother Cancer. 2016;4:38. doi: 10.1186/s40425-016-0141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haile ST, Dalal SP, Clements V, Tamada K, Ostrand-Rosenberg S. Soluble CD80 restores T cell activation and overcomes tumor cell programmed death ligand 1-mediated immune suppression. J Immunol. 2013;191:2829–36. doi: 10.4049/jimmunol.1202777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151–67. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 8.Kiyozumi Y, Baba Y, Okadome K, Yagi T, Ogata Y, Eto K, et al. Indoleamine 2, 3-dioxygenase 1 promoter hypomethylation is associated with poor prognosis in patients with esophageal cancer. Cancer Sci. 2019;110:1863–71. doi: 10.1111/cas.14028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang S, Fu H, Xu Q, Zhou Y. miR-20a regulates sensitivity of colorectal cancer cells to NK cells by targeting MICA. Biosci Rep. 2019;39 doi: 10.1042/BSR20180695. BSR20180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elia AR, Caputo S, Bellone M. Immune checkpoint-mediated interactions between cancer and immune cells in prostate adenocarcinoma and melanoma. Front Immunol. 2018;9:1786. doi: 10.3389/fimmu.2018.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ventola CL. Cancer immunotherapy, part 3: challenges and future trends. P T. 2017;42:514–21. [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Liu Z, Zhang A, Han C, Shen A, Jiang L, et al. NQO1 targeting prodrug triggers innate sensing to overcome checkpoint blockade resistance. Nat Commun. 2019;10:3251. doi: 10.1038/s41467-019-11238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escors D. Tumour immunogenicity, antigen presentation and immunological barriers in cancer immunotherapy. New J Sci. 2014;2014:734515. doi: 10.1155/2014/734515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipson EJ, Forde PM, Hammers HJ, Emens LA, Taube JM, Topalian SL. Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol. 2015;42:587–600. doi: 10.1053/j.seminoncol.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Draghiciu O, Lubbers J, Nijman HW, Daemen T. Myeloid derived suppressor cells-an overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology. 2015;4:e954829. doi: 10.4161/21624011.2014.954829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–12. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 18.Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91:167–81. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang L, Li Y, Du Y, Zhang Y, Wang X, Ding Y, et al. Mild photothermal therapy potentiates anti-PD-L1 treatment for immunologically cold tumors via an all-in-one and all-in-control strategy. Nat Commun. 2019;10:4871. doi: 10.1038/s41467-019-12771-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toor SM, Elkord E. Therapeutic prospects of targeting myeloid-derived suppressor cells and immune checkpoints in cancer. Immunol Cell Biol. 2018;96:888–97. doi: 10.1111/imcb.12054. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell TC, Hamid O, Smith DC, Bauer TM, Wasser JS, Olszanski AJ, et al. Epacadostat plus pembrolizumab in patients with advanced solid tumors: phase I results from a multicenter, open-label phase I/II trial (ECHO-202/KEYNOTE-037) J Clin Oncol. 2018;36:3223–30. doi: 10.1200/JCO.2018.78.9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umansky V, Blattner C, Gebhardt C, Utikal J. The role of myeloid-derived suppressor cells (MDSC) in cancer progression. Vaccines (Basel) 2016;4:36. doi: 10.3390/vaccines4040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37:208–20. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Law AMK, Valdes-Mora F, Gallego-Ortega D. Myeloid-derived suppressor cells as a therapeutic target for cancer. Cells. 2020;9:561. doi: 10.3390/cells9030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–35. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci. 2014;1319:47–65. doi: 10.1111/nyas.12469. [DOI] [PubMed] [Google Scholar]
- 29.Condamine T, Mastio J, Gabrilovich DI. Transcriptional regulation of myeloid-derived suppressor cells. J Leukoc Biol. 2015;98:913–22. doi: 10.1189/jlb.4RI0515-204R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24:302–6. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldron TJ, Quatromoni JG, Karakasheva TA, Singhal S, Rustgi AK. Myeloid derived suppressor cells: targets for therapy. Oncoimmunology. 2013;2:e24117. doi: 10.4161/onci.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu J, Du W, Yan F, Wang Y, Li H, Cao S, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through ido expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190:3783–97. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 35.Della Chiesa M, Carlomagno S, Frumento G, Balsamo M, Cantoni C, Conte R, et al. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKP46- and NKG2D-activating receptors and regulates NK-cell function. Blood. 2006;108:4118–25. doi: 10.1182/blood-2006-03-006700. [DOI] [PubMed] [Google Scholar]
- 36.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–8. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tierney JF, Vogle A, Finnerty B, Zarnegar R, Ghai R, Gattuso P, et al. Indoleamine 2,3-dioxygenase-1 expression in adrenocortical carcinoma. J Surg Res. 2020;256:90–5. doi: 10.1016/j.jss.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Seeber A, Klinglmair G, Fritz J, Steinkohl F, Zimmer KC, Aigner F, et al. High IDO-1 expression in tumor endothelial cells is associated with response to immunotherapy in metastatic renal cell carcinoma. Cancer Sci. 2018;109:1583–91. doi: 10.1111/cas.13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–62. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183:937–44. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. Pd-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–90. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010;70:99–108. doi: 10.1158/0008-5472.CAN-09-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. 2016;28:401–9. doi: 10.1093/intimm/dxw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tartour E, Pere H, Maillere B, Terme M, Merillon N, Taieb J, et al. Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 2011;30:83–95. doi: 10.1007/s10555-011-9281-4. [DOI] [PubMed] [Google Scholar]
- 46.Qu X, Zhuang G, Yu L, Meng G, Ferrara N. Induction of Bv8 expression by granulocyte colony-stimulating factor in CD11b+Gr1+ cells: key role of Stat3 signaling. J Biol Chem. 2012;287:19574–84. doi: 10.1074/jbc.M111.326801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Condamine T, Ramachandran I, Youn JI, Gabrilovich DI. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med. 2015;66:97–110. doi: 10.1146/annurev-med-051013-052304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–49. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Mitri D, Toso A, Chen JJ, Sarti M, Pinton S, Jost TR, et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature. 2014;515:134–7. doi: 10.1038/nature13638. [DOI] [PubMed] [Google Scholar]
- 50.Zhao F, Obermann S, von Wasielewski R, Haile L, Manns MP, Korangy F, et al. Increase in frequency of myeloid-derived suppressor cells in mice with spontaneous pancreatic carcinoma. Immunology. 2009;128:141–9. doi: 10.1111/j.1365-2567.2009.03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–27. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 52.Vetsika EK, Koinis F, Gioulbasani M, Aggouraki D, Koutoulaki A, Skalidaki E, et al. A circulating subpopulation of monocytic myeloid-derived suppressor cells as an independent prognostic/predictive factor in untreated non-small lung cancer patients. J Immunol Res. 2014;2014:659294. doi: 10.1155/2014/659294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Markowitz J, Brooks TR, Duggan MC, Paul BK, Pan X, Wei L, et al. Patients with pancreatic adenocarcinoma exhibit elevated levels of myeloid-derived suppressor cells upon progression of disease. Cancer Immunol Immunother. 2015;64:149–59. doi: 10.1007/s00262-014-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hernandez C, Arasanz H, Chocarro L, Bocanegra A, Zuazo M, Fernandez-Hinojal G, et al. Systemic blood immune cell populations as biomarkers for the outcome of immune checkpoint inhibitor therapies. Int J Mol Sci. 2020;21:2411. doi: 10.3390/ijms21072411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eisenblaetter M, Flores-Borja F, Lee JJ, Wefers C, Smith H, Hueting R, et al. Visualization of tumor-immune interaction – target-specific imaging of S100A8/A9 reveals pre-metastatic niche establishment. Theranostics. 2017;7:2392–401. doi: 10.7150/thno.17138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffmann SHL, Reck DI, Maurer A, Fehrenbacher B, Sceneay JE, Poxleitner M, et al. Visualization and quantification of in vivo homing kinetics of myeloid-derived suppressor cells in primary and metastatic cancer. Theranostics. 2019;9:5869–85. doi: 10.7150/thno.33275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De La Fuente A, Zilio S, Caroli J, Van Simaeys D, Mazza EMC, Ince TA, et al. Aptamers against mouse and human tumor-infiltrating myeloid cells as reagents for targeted chemotherapy. Sci Transl Med. 2020;12:eaav9760. doi: 10.1126/scitranslmed.aav9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu S, Tian R, Antaris AL, Chen X, Dai H. Near-infrared-II molecular dyes for cancer imaging and surgery. Adv Mater. 2019;31:e1900321. doi: 10.1002/adma.201900321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu GT, Luo MY, Li H, Chen S, Huang B, Sun ZJ, et al. Molecular targeting nanoprobes with non-overlap emission in the second near-infrared window for in vivo two-color colocalization of immune cells. ACS Nano. 2019;13:12830–9. doi: 10.1021/acsnano.9b05038. [DOI] [PubMed] [Google Scholar]
- 60.Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008;111:5457–66. doi: 10.1182/blood-2008-01-136895. [DOI] [PubMed] [Google Scholar]
- 61.Davis RJ, Van Waes C, Allen CT. Overcoming barriers to effective immunotherapy: MDSCs, TAMs, and tregs as mediators of the immunosuppressive microenvironment in head and neck cancer. Oral Oncol. 2016;58:59–70. doi: 10.1016/j.oraloncology.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–31. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 63.Sceneay J, Chow MT, Chen A, Halse HM, Wong CS, Andrews DM, et al. Primary tumor hypoxia recruits CD11B+/Ly6Cmed/Ly6g+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72:3906–11. doi: 10.1158/0008-5472.CAN-11-3873. [DOI] [PubMed] [Google Scholar]
- 64.Steele CW, Karim SA, Leach JDG, Bailey P, Upstill-Goddard R, Rishi L, et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell. 2016;29:832–45. doi: 10.1016/j.ccell.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmid MC, Avraamides CJ, Dippold HC, Franco I, Foubert P, Ellies LG, et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kgamma, a single convergent point promoting tumor inflammation and progression. Cancer Cell. 2011;19:715–27. doi: 10.1016/j.ccr.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waight JD, Netherby C, Hensen ML, Miller A, Hu Q, Liu S, et al. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest. 2013;123:4464–78. doi: 10.1172/JCI68189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sakuishi K, Jayaraman P, Behar SM, Anderson AC, Kuchroo VK. Emerging tim-3 functions in antimicrobial and tumor immunity. Trends Immunol. 2011;32:345–9. doi: 10.1016/j.it.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou J, Wu J, Chen X, Fortenbery N, Eksioglu E, Kodumudi KN, et al. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int Immunopharmacol. 2011;11:890–8. doi: 10.1016/j.intimp.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 2007;67:11021–8. doi: 10.1158/0008-5472.CAN-07-2593. [DOI] [PubMed] [Google Scholar]
- 71.Jeong SM, Kim YJ. Astaxanthin treatment induces maturation and functional change of myeloid-derived suppressor cells in tumor-bearing mice. Antioxidants (Basel) 2020;9:350. doi: 10.3390/antiox9040350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Law AMK, Lim E, Ormandy CJ, Gallego-Ortega D. The innate and adaptive infiltrating immune systems as targets for breast cancer immunotherapy. Endocr Relat Cancer. 2017;24:X1. doi: 10.1530/ERC-16-0404e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Obermajer N, Wong JL, Edwards RP, Odunsi K, Moysich K, Kalinski P. PGE(2)-driven induction and maintenance of cancer-associated myeloid-derived suppressor cells. Immunol Invest. 2012;41:635–57. doi: 10.3109/08820139.2012.695417. [DOI] [PubMed] [Google Scholar]
- 74.Eksioglu EA, Chen X, Heider KH, Rueter B, McGraw KL, Basiorka AA, et al. Novel therapeutic approach to improve hematopoiesis in low risk MDS by targeting MDSCs with the Fc-engineered CD33 antibody BI 836858. Leukemia. 2017;31:2172–80. doi: 10.1038/leu.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, et al. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014;74:104–18. doi: 10.1158/0008-5472.CAN-13-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 77.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 78.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weed DT, Vella JL, Reis IM, De la Fuente AC, Gomez C, Sargi Z, et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:39–48. doi: 10.1158/1078-0432.CCR-14-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thimmulappa RK, Fuchs RJ, Malhotra D, Scollick C, Traore K, Bream JH, et al. Preclinical evaluation of targeting the NRF2 pathway by triterpenoids (CDDO-im and CDDO-Me) for protection from LPS-induced inflammatory response and reactive oxygen species in human peripheral blood mononuclear cells and neutrophils. Antioxid Redox Signal. 2007;9:1963–70. doi: 10.1089/ars.2007.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16:1812–23. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007;67:11438–46. doi: 10.1158/0008-5472.CAN-07-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen PT, Hsieh CC, Wu CT, Yen TC, Lin PY, Chen WC, et al. 1alpha,25-dihydroxyvitamin D3 inhibits esophageal squamous cell carcinoma progression by reducing IL6 signaling. Mol Cancer Ther. 2015;14:1365–75. doi: 10.1158/1535-7163.MCT-14-0952. [DOI] [PubMed] [Google Scholar]
- 84.Kusmartsev S, Eruslanov E, Kubler H, Tseng T, Sakai Y, Su Z, et al. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J Immunol. 2008;181:346–53. doi: 10.4049/jimmunol.181.1.346. [DOI] [PubMed] [Google Scholar]
- 85.Sun L, Clavijo PE, Robbins Y, Patel P, Friedman J, Greene S, et al. Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight. 2019;4:e126853. doi: 10.1172/jci.insight.126853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clavijo PE, Friedman J, Robbins Y, Moore EC, Smith E, Zauderer M, et al. Semaphorin4D inhibition improves response to immune-checkpoint blockade via attenuation of MDSC recruitment and function. Cancer Immunol Res. 2019;7:282–91. doi: 10.1158/2326-6066.CIR-18-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xie Z, Ikegami T, Ago Y, Okada N, Tachibana M. Valproic acid attenuates CCR2-dependent tumor infiltration of monocytic myeloid-derived suppressor cells, limiting tumor progression. Oncoimmunology. 2020;9:1734268. doi: 10.1080/2162402X.2020.1734268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu D, You M, Xu Y, Li F, Zhang D, Li X, et al. Inhibition of curcumin on myeloid-derived suppressor cells is requisite for controlling lung cancer. Int Immunopharmacol. 2016;39:265–72. doi: 10.1016/j.intimp.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 89.Wu H, Tao N, Liu X, Li X, Tang J, Ma C, et al. Polysaccharide from lentinus edodes inhibits the immunosuppressive function of myeloid-derived suppressor cells. PLoS One. 2012;7:e51751. doi: 10.1371/journal.pone.0051751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583–94. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Albeituni SH, Ding C, Liu M, Hu X, Luo F, Kloecker G, et al. Yeast-derived particulate beta-glucan treatment subverts the suppression of myeloid-derived suppressor cells (MDSC) by inducing polymorphonuclear MDSC apoptosis and monocytic MDSC differentiation to APC in cancer. J Immunol. 2016;196:2167–80. doi: 10.4049/jimmunol.1501853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grauers Wiktorin H, Nilsson MS, Kiffin R, Sander FE, Lenox B, Rydstrom A, et al. Histamine targets myeloid-derived suppressor cells and improves the anti-tumor efficacy of PD-1/PD-L1 checkpoint blockade. Cancer Immunol Immunother. 2019;68:163–74. doi: 10.1007/s00262-018-2253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Aerts JG, et al. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer. 2010;10:464. doi: 10.1186/1471-2407-10-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liang H, Shen X. Lxr activation radiosensitizes non-small cell lung cancer by restricting myeloid-derived suppressor cells. Biochem Biophys Res Commun. 2020;528:330–5. doi: 10.1016/j.bbrc.2020.04.137. [DOI] [PubMed] [Google Scholar]
- 95.Roth F, De La Fuente AC, Vella JL, Zoso A, Inverardi L, Serafini P. Aptamer-mediated blockade of IL4Ralpha triggers apoptosis of MDSCs and limits tumor progression. Cancer Res. 2012;72:1373–83. doi: 10.1158/0008-5472.CAN-11-2772. [DOI] [PubMed] [Google Scholar]
- 96.Qin H, Lerman B, Sakamaki I, Wei G, Cha SC, Rao SS, et al. Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat Med. 2014;20:676–81. doi: 10.1038/nm.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dominguez GA, Condamine T, Mony S, Hashimoto A, Wang F, Liu Q, et al. Selective targeting of myeloid-derived suppressor cells in cancer patients using DS-8273a, an agonistic TRAIL-R2 antibody. Clin Cancer Res. 2017;23:2942–50. doi: 10.1158/1078-0432.CCR-16-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim SH, Li M, Trousil S, Zhang Y, Pasca di Magliano M, Swanson KD, et al. Phenformin inhibits myeloid-derived suppressor cells and enhances the anti-tumor activity of PD-1 blockade in melanoma. J Invest Dermatol. 2017;137:1740–8. doi: 10.1016/j.jid.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 99.Tachibana M. [The immunosuppressive function of myeloid-derived suppressor cells is regulated by the HMGB1-TLR4 axis] Yakugaku Zasshi. 2018;138:143–8. doi: 10.1248/yakushi.17-00158. [DOI] [PubMed] [Google Scholar]
- 100.Baumann T, Dunkel A, Schmid C, Schmitt S, Hiltensperger M, Lohr K, et al. Regulatory myeloid cells paralyze T cells through cell-cell transfer of the metabolite methylglyoxal. Nat Immunol. 2020;21:555–66. doi: 10.1038/s41590-020-0666-9. [DOI] [PubMed] [Google Scholar]
- 101.Prendergast GC, Malachowski WP, DuHadaway JB, Muller AJ. Discovery of IDO1 inhibitors: from bench to bedside. Cancer Res. 2017;77:6795–811. doi: 10.1158/0008-5472.CAN-17-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12:230–8. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sinha P, Ostrand-Rosenberg S. Myeloid-derived suppressor cell function is reduced by Withaferin A, a potent and abundant component of Withania somnifera root extract. Cancer Immunol Immunother. 2013;62:1663–73. doi: 10.1007/s00262-013-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Orillion A, Hashimoto A, Damayanti N, Shen L, Adelaiye-Ogala R, Arisa S, et al. Entinostat neutralizes myeloid-derived suppressor cells and enhances the antitumor effect of PD-1 inhibition in murine models of lung and renal cell carcinoma. Clin Cancer Res. 2017;23:5187–201. doi: 10.1158/1078-0432.CCR-17-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davis RJ, Moore EC, Clavijo PE, Friedman J, Cash H, Chen Z, et al. Anti-PD-L1 efficacy can be enhanced by inhibition of myeloid-derived suppressor cells with a selective inhibitor of PI3Kdelta/gamma. Cancer Res. 2017;77:2607–19. doi: 10.1158/0008-5472.CAN-16-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Park YJ, Kuen DS, Chung Y. Future prospects of immune checkpoint blockade in cancer: from response prediction to overcoming resistance. Exp Mol Med. 2018;50:109. doi: 10.1038/s12276-018-0130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature. 2016;539:443–7. doi: 10.1038/nature20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–78. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S, et al. Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Discov. 2016;6:80–95. doi: 10.1158/2159-8290.CD-15-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3007974. 237ra67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lu X, Horner JW, Paul E, Shang X, Troncoso P, Deng P, et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature. 2017;543:728–32. doi: 10.1038/nature21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ch’ng ES, Kumanogoh A. Roles of Sema4d and Plexin-B1 in tumor progression. Mol Cancer. 2010;9:251. doi: 10.1186/1476-4598-9-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Younis RH, Han KL, Webb TJ. Human head and neck squamous cell carcinoma-associated semaphorin 4D induces expansion of myeloid-derived suppressor cells. J Immunol. 2016;196:1419–29. doi: 10.4049/jimmunol.1501293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Evans EE, Jonason AS, Jr, Bussler H, Torno S, Veeraraghavan J, Reilly C, et al. Antibody blockade of semaphorin 4D promotes immune infiltration into tumor and enhances response to other immunomodulatory therapies. Cancer Immunol Res. 2015;3:689–701. doi: 10.1158/2326-6066.CIR-14-0171. [DOI] [PubMed] [Google Scholar]
- 116.Rosborough BR, Castellaneta A, Natarajan S, Thomson AW, Turnquist HR. Histone deacetylase inhibition facilitates GM-CSF-mediated expansion of myeloid-derived suppressor cells in vitro and in vivo. J Leukoc Biol. 2012;91:701–9. doi: 10.1189/jlb.0311119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–78. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Strom SR, Burdick MD, et al. Macrophage infiltration in human non-small-cell lung cancer: the role of CC chemokines. Cancer Immunol Immunother. 2000;49:63–70. doi: 10.1007/s002620050603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Conti I, Rollins BJ. CCL2 (monocyte chemoattractant protein-1) and cancer. Semin Cancer Biol. 2004;14:149–54. doi: 10.1016/j.semcancer.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 120.Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252:86–92. doi: 10.1016/j.canlet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 121.Xie Z, Ago Y, Okada N, Tachibana M. Valproic acid attenuates immunosuppressive function of myeloid-derived suppressor cells. J Pharmacol Sci. 2018;137:359–65. doi: 10.1016/j.jphs.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 122.Martner A, Wiktorin HG, Lenox B, Ewald Sander F, Aydin E, Aurelius J, et al. Histamine promotes the development of monocyte-derived dendritic cells and reduces tumor growth by targeting the myeloid nadph oxidase. J Immunol. 2015;194:5014–21. doi: 10.4049/jimmunol.1402991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang XD, Ai W, Asfaha S, Bhagat G, Friedman RA, Jin G, et al. Histamine deficiency promotes inflammation-associated carcinogenesis through reduced myeloid maturation and accumulation of CD11b+LY6G+ immature myeloid cells. Nat Med. 2011;17:87–95. doi: 10.1038/nm.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Johnstone RW, Frew AJ, Smyth MJ. The trail apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–98. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 125.Condamine T, Kumar V, Ramachandran IR, Youn JI, Celis E, Finnberg N, et al. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J Clin Invest. 2014;124:2626–39. doi: 10.1172/JCI74056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pollak M. Potential applications for biguanides in oncology. J Clin Invest. 2013;123:3693–700. doi: 10.1172/JCI67232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yuan P, Ito K, Perez-Lorenzo R, Del Guzzo C, Lee JH, Shen CH, et al. Phenformin enhances the therapeutic benefit of BRAF(V600E) inhibition in melanoma. Proc Natl Acad Sci U S A. 2013;110:18226–31. doi: 10.1073/pnas.1317577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ju D, Wang X, Xie Y. Dyclonine and alverine citrate enhance the cytotoxic effects of proteasome inhibitor MG132 on breast cancer cells. Int J Mol Med. 2009;23:205–9. [PMC free article] [PubMed] [Google Scholar]
- 129.Yadlapalli S, Yamashita YM. DNA asymmetry in stem cells – immortal or mortal? J Cell Sci. 2013;126:4069–76. doi: 10.1242/jcs.096024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Godin-Ethier J, Hanafi LA, Piccirillo CA, Lapointe R. Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res. 2011;17:6985–91. doi: 10.1158/1078-0432.CCR-11-1331. [DOI] [PubMed] [Google Scholar]
- 131.Chevolet I, Speeckaert R, Schreuer M, Neyns B, Krysko O, Bachert C, et al. Characterization of the in vivo immune network of IDO, tryptophan metabolism, PD-L1, and CTLA-4 in circulating immune cells in melanoma. Oncoimmunology. 2015;4:e982382. doi: 10.4161/2162402X.2014.982382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nakamura N, Hara T, Shimizu M, Mabuchi R, Nagano J, Ohno T, et al. Effects of indoleamine 2,3-dioxygenase inhibitor in non-Hodgkin lymphoma model mice. Int J Hematol. 2015;102:327–34. doi: 10.1007/s12185-015-1835-8. [DOI] [PubMed] [Google Scholar]
- 133.Epacadostat shows value in two SCCHN trials. Cancer Discov. 2017;7:OF2. doi: 10.1158/2159-8290.CD-NB2017-100. [DOI] [PubMed] [Google Scholar]
- 134.Shen L, Ciesielski M, Ramakrishnan S, Miles KM, Ellis L, Sotomayor P, et al. Class I histone deacetylase inhibitor entinostat suppresses regulatory T cells and enhances immunotherapies in renal and prostate cancer models. PLoS One. 2012;7:e30815. doi: 10.1371/journal.pone.0030815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kroesen M, Gielen P, Brok IC, Armandari I, Hoogerbrugge PM, Adema GJ. HDAC inhibitors and immunotherapy; a double edged sword? Oncotarget. 2014;5:6558–72. doi: 10.18632/oncotarget.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shen L, Orillion A, Pili R. Histone deacetylase inhibitors as immunomodulators in cancer therapeutics. Epigenomics. 2016;8:415–28. doi: 10.2217/epi.15.118. [DOI] [PubMed] [Google Scholar]
- 137.Zhang X, Shen L, Liu Q, Hou L, Huang L. Inhibiting PI3 kinase-gamma in both myeloid and plasma cells remodels the suppressive tumor microenvironment in desmoplastic tumors. J Control Release. 2019;309:173–80. doi: 10.1016/j.jconrel.2019.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shi X, Li X, Wang H, Yu Z, Zhu Y, Gao Y. Specific inhibition of PI3Kdelta/gamma enhances the efficacy of anti-PD1 against osteosarcoma cancer. J Bone Oncol. 2019;16:100206. doi: 10.1016/j.jbo.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ali K, Soond DR, Pineiro R, Hagemann T, Pearce W, Lim EL, et al. Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510:407–11. doi: 10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gao W, Zhang X, Yang W, Dou D, Zhang H, Tang Y, et al. Prim-o-glucosylcimifugin enhances the antitumour effect of PD-1 inhibition by targeting myeloid-derived suppressor cells. J Immunother Cancer. 2019;7:231. doi: 10.1186/s40425-019-0676-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Knowles SM, Wu AM. Advances in immuno-positron emission tomography: antibodies for molecular imaging in oncology. J Clin Oncol. 2012;30:3884–92. doi: 10.1200/JCO.2012.42.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wei W, Rosenkrans ZT, Liu J, Huang G, Luo QY, Cai W. Immunopet: concept, design, and applications. Chem Rev. 2020;120:3787–851. doi: 10.1021/acs.chemrev.9b00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Natarajan A, Mayer AT, Reeves RE, Nagamine CM, Gambhir SS. Development of novel immunoPET tracers to image human PD-1 checkpoint expression on tumor-infiltrating lymphocytes in a humanized mouse model. Mol Imaging Biol. 2017;19:903–14. doi: 10.1007/s11307-017-1060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Natarajan A, Mayer AT, Xu L, Reeves RE, Gano J, Gambhir SS. Novel radiotracer for immunopet imaging of PD-1 checkpoint expression on tumor infiltrating lymphocytes. Bioconjug Chem. 2015;26:2062–9. doi: 10.1021/acs.bioconjchem.5b00318. [DOI] [PubMed] [Google Scholar]
- 145.Kikuchi M, Clump DA, Srivastava RM, Sun L, Zeng D, Diaz-Perez JA, et al. Preclinical immunoPET/CT imaging using Zr-89-labeled anti-PD-L1 monoclonal antibody for assessing radiation-induced Pd-L1 upregulation in head and neck cancer and melanoma. Oncoimmunology. 2017;6:e1329071. doi: 10.1080/2162402X.2017.1329071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen H, Hwang JH. Ultrasound-targeted microbubble destruction for chemotherapeutic drug delivery to solid tumors. J Ther Ultrasound. 2013;1:10. doi: 10.1186/2050-5736-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhou H, Qian W, Uckun FM, Zhou Z, Wang L, Wang A, et al. IGF-1 receptor targeted nanoparticles for image-guided therapy of stroma-rich and drug resistant human cancer. Proc SPIE Int Soc Opt Eng. 2016;9836:98361O. doi: 10.1117/12.2224914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen H, Zhang W, Zhu G, Xie J, Chen X. Rethinking cancer nanotheranostics. Nat Rev Mater. 2017;2:17024. doi: 10.1038/natrevmats.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lim EK, Kim T, Paik S, Haam S, Huh YM, Lee K. Nanomaterials for theranostics: recent advances and future challenges. Chem Rev. 2015;115:327–94. doi: 10.1021/cr300213b. [DOI] [PubMed] [Google Scholar]