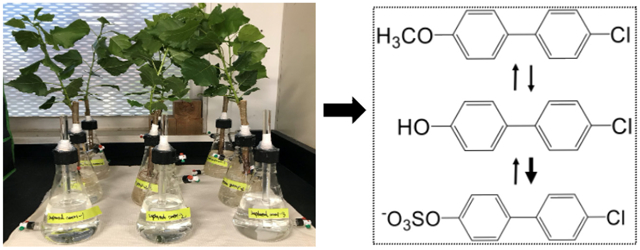
Abstract
Methoxylated polychlorinated biphenyls (MeO-PCBs) are overlooked metabolites of PCBs. In general, they are more toxic to plants than their parent congeners. However, information on the fate of MeO-PCBs and the relationship between methoxylated, hydroxylated and sulfated metabolites of PCBs in plants is scarce. In this work, poplar plants (Populus deltoides × nigra, DN34) were hydroponically and separately exposed to 4′-methoxy-4-monochlorobiphenyl (4′-MeO-PCB 3) and 4′-PCB 3 sulfate for 10 days to investigate the uptake, translocation and metabolism of MeO-PCBs and the relationship between methoxy-PCBs, hydroxyl-PCBs and PCB sulfates within plants. Results showed that 4′-MeO-PCB 3 and 4′-PCB 3 sulfate were taken up by the roots of poplar plants and translocated from roots to shoots and leaves. 4′-OH-PCB 3 and 4′-PCB 3 sulfate were identified as the hydroxylated metabolite and sulfate metabolite of 4′-MeO-PCB 3 in poplar, respectively. In the backward reaction, 4′-OH-PCB 3 and 4′-MeO-PCB 3 were found as metabolites of 4′-PCB 3 sulfate. For exposure groups, the yields of 4′-OH-PCB 3 produced from 4′-MeO-PCB 3 and 4′-PCB 3 sulfate were 1.29% and 0.13% respectively. The yield of 4′-PCB 3 sulfate which originated from 4′-MeO-PCB 3 in wood and root samples of exposure groups was only 0.02%. Only 0.04% of the initial mass of 4′-PCB 3 sulfate was transformed to 4′-MeO-PCB 3 in the exposure groups. The sulfation yield of 4′-OH-PCB 3 was higher than hydrolysis yield of 4′-PCB 3 sulfate, indicating that formation of PCB sulfates was predominant over the reverse reaction, the formation of hydroxyl-PCBs. These results provide new perspective on the transport, metabolism, and fate of MeO-PCBs, and also help to better understand sources of OH-PCBs and PCB sulfates in the environment. This study provides the first evidence of interconversion of sulfate metabolites from methoxy-PCBs and methoxy-PCBs from PCB sulfates.
Keywords: Interconversion, MeO-PCB 3, OH-PCB 3, PCB 3 sulfates, Poplar plants
Graphical Abstract
1. Introduction
Polychlorinated biphenyls (PCBs) are still ubiquitous in the environment and their troubling persistence has been reported repeatedly, even though their production was banned in 1970s (Desforges et al., 2018). PCBs were used worldwide in a broad range of industrial applications due to their exceptional physical and chemical properties (Grimm et al., 2015). Biotransformation of PCBs in various environmental media has been reported (Grimm et al., 2015; Grimm et al., 2017; Tehrani and Van Aken, 2014). However, metabolites of PCBs are possibly more toxic and equally persistent as the parent congeners (Grimm et al., 2015; Tehrani and Van Aken, 2014). Thus, PCB transformation products demand more attention based on their potential chronic environmental health risks.
As well-known metabolites of PCBs, hydroxylated polychlorinated biphenyls (OH-PCBs) and PCB sulfates are toxic to various organisms (Grimm et al., 2015; Subramanian et al., 2017). Hydroxylated-PCBs are prevalent across a range of environmental media, including air (Awad et al., 2016; Marek et al., 2017), water (Darling et al., 2004; Kuch et al., 2010; Ueno et al., 2007), sediments (Marek et al., 2013a; Sakiyama et al., 2007) and humans (Allmyr et al., 2006; Fängström et al., 2004; Fängström et al., 2005; Marek et al., 2013b; Nomiyama et al., 2009; Quinete et al., 2015; Zota et al., 2013). Toxicity of OH-PCBs is often greater than that of their parent congeners in humans. Besides acting as endocrine disruptors, they can also interrupt reproductive processes and brain function in mammals (Tehrani and Van Aken, 2014). OH-PCBs are substrates for sulfotransferase (SULT) enzymes and can further be transformed to PCB sulfates, even though there is evidence that OH-PCBs also have the ability to inhibit the activity of phenol SULTs (Li et al., 2010). Disruption to endocrine systems by PCB sulfates has been suggested. Lower chlorinated PCB sulfates display the highest binding affinities for major drug-binding sites such as human serum albumin (PCB sulfate ≥ OH-PCB > PCB) according to Rodriguez et al. (Rodriguez et al., 2016). A sulfate metabolite of PCB 11 was identified in human serum (Grimm et al., 2017). Although hydroxy- and sulfate-metabolites of PCBs have received increased environmental concern, information on their fate in the environment is limited. Their sources are underestimated (Sun et al., 2016a), and their fate processes are worthy of further investigation.
Recently, two methoxylated polychlorinated biphenyl (MeO-PCB) congeners, 3′-methoxy-2,3,5,6-tetrachlorobiphenyl (3′-MeO-PCB 65) and 4′-methoxy-2,2′,4,5,5′-pentachlorobiphenyl (4′-MeO-PCB 101), were detected in sewage sludge of China, which is the first report of MeO-PCBs in actual environmental samples. The concentration in sewage sludge was 0.58 ng/g dry weight for 3′-MeO-PCB 65 and 0.52 ng/g dry weight for 4′-MeO-PCB 101 (Sun et al., 2016b). Methoxylated metabolites of PCBs were reported both in vivo and in vitro (Kamei et al., 2006; Rezek et al., 2008; Safe et al., 1975). Higher toxicity of 4′-methoxy-2,3,4,5-tetrachlorobiphenyl (4′-MeO-PCB 61) compared to its parent congener, 2,3,4,5-tetrachlorobiphenyl (PCB 61) was reported in rice plants by Lin et al. (Lin et al., 2020). Thus, MeO-PCBs are often overlooked metabolites of PCBs, even though their lipophilicity and persistency are likely higher than that of OH-PCBs based on their chemical-physical properties (e.g. log Kow of 4′-MeO-PCB 3 and 4′-OH-PCB 3 are 4.48 and 3.92, respectively, according to EPI Suit) (Sun et al., 2016a). To understand the metabolism, bioaccumulation and elimination of MeO-PCBs in various organisms is of high priority.
Sun et al. investigated the interconversion between MeO-PCBs and OH-PCBs in plants (Sun et al., 2018; Sun et al., 2016a). However, only OH-PCB metabolites of highly chlorinated MeO-PCBs (≥ 4Cl) were studied in herbaceous plants. Interconversion between OH-PCBs and PCB sulfates in vitro was reported by Rodriguez et al. (Rodriguez et al., 2018). As primary producers at the first trophic level of the food chain, plants play an important role in the cycling of toxic organic contaminants (Collins et al., 2006; Li et al., 2017, 2019). However, there is scant information on interconversion between methoxy-, hydroxyl- and sulfate metabolites of PCBs in plants.
In this work, poplar plants (Populus deltoides × nigra, DN34) were hydroponically exposed to a ubiquitous, mobile, lightly-chlorinated methoxy-PCB, 4′-methoxy-4-monochlorobiphenyl (4′-MeO-PCB 3), and 4′-PCB 3 sulfate to study the fate and metabolism in a model plant system. Populus spp. are considered a model plant with a completely sequenced genome, global distribution, and wide usage in phytoremediation. Potential methoxylated, hydroxylated and sulfated metabolites of 4′-MeO-PCB 3 and 4′-PCB 3 sulfate were investigated to better understand the relationship among the three analogues and potential sources and fate of MeO-PCBs, OH-PCBs and PCB sulfates in the ecosystem. To our knowledge, it is the first report of a sulfate metabolite from MeO-PCBs and a methoxylated metabolite from PCB sulfates representing interconversion.
2. Materials and methods
2.1. Chemicals and regents
4′-Methoxy-4-monochlorobiphenyl (4′-MeO-PCB 3), 4′-hydroxy-4-monochlorobiphenyl (4′-OH-PCB 3), and 4′-PCB 3 sulfate were prepared and authenticated as described in Table S1. 3-F, 4′-PCB 3 sulfate was synthesized as described previously. The surrogate standards, 3′,4′-dichloro-4-[13C12]biphenylol (13C 4′-OH-PCB 12, 50 μg·mL−1 in toluene) and 2,4,5-trichloro-4′-methoxy[13C12]biphenyl (13C 4′-MeO-PCB 29, 50 μg·mL−1 in toluene), were purchased from Wellington Laboratories, Canada. dPCB 30 (2,4,6-trichlorobiphenyl-2′,3′,4′,5′,6′-d5, C/D/N Isotopes, Pointe-Claire, QC, Canada) was used as an internal standard. The purities of these standards were >98%.
Ion pair reagent dibutylamine acetate (0.5 M) and ammonium hydroxide solution were purchased from Sigma-Aldrich and Honeywell Fluka, respectively. Silica gel (70–230 mesh) and anhydrous sodium sulfate (Na2SO4) were activated at 450 °C overnight by the muffle furnace prior to use. Acid silica gel (30%, w/w) was a mixture of activated silica gel and concentrated H2SO4. Hexane (pesticide grade), dichloromethane (DCM, pesticide grade), acetone (pesticide grade), methanol (Optima™ LC/MS Grade) and methyltert butyl ether (MTBE, Optima™ LC/MS Grade), were purchased from Fisher Scientific, Hampton, NH. Deionized water (18.3 MΩ) was obtained from an ultrapure water system (Barnstead International, Dubuque, IA, USA). All other chemicals and reagents were of the highest purity commercially available.
2.2. Hydroponic exposure
Clonal poplar tree cuttings (Populus deltoides × nigra, DN34) were purchased from Hramor Nursery (Manistee, MI). Each cutting (8″) was fit snugly through the hole of a pre-drilled screw cap with a PTFE liner. The interface of the cutting and the cap was sealed with 100% silicone sealant (DAP Products Inc., Baltimore, MD). To prevent growth within the reactors, all buds below the cap were removed. Opaque plastic bins (25″ × 18″ × 7″) containing 20 L of half strength Hoagland nutrient solution were used to grow the cuttings. Growth conditions were 25 °C with 16 h light/8 h dark. After 25 days, healthy and uniform poplar plants were used for the exposure experiment.
Exposure groups, unplanted controls, and blank controls were arranged randomly in the exposure chamber. Reactors for hydroponic exposure were 500 mL glass conical flasks modified with a top injection port and bottom sampling port, sealed by a Mininert valve (Valco Instruments Co. Inc., Houston, TX). For exposure groups, 400 mL of half-strength Hoagland’s solution and 400 μL of exposure chemicals were mixed in each reactor. Initial exposure concentrations were 1 μg/mL for 4′-MeO-PCB 3 (dissolved in acetone) and 0.99 μg/mL for 4′-PCB 3 sulfate (dissolved in water: acetonitrile, 65:35, v/v). Then, a poplar tree cutting with leaves and roots was planted into the solution and sealed. Aluminum foil was used to wrap the whole reactor to provide darkness for the growth of roots and to avoid any possible photo-transformation of 4′-MeO-PCB 3 and 4′-PCB 3 sulfate unrelated to poplar plants. Above procedures were conducted in a laminar flow hood. Unplanted controls were spiked with 4′-MeO-PCB 3 or 4′-PCB 3 sulfate but without poplar plants. Glass rods instead of poplar plants were inserted in the unplanted control reactors to ascertain the potential for leaks (volatilization) of exposure group reactors. Blank controls were planted with poplar plants and spiked with 400 μL of acetone or water: acetonitrile (65:35, v/v) but without exposure chemicals to control and assess potential cross contamination. To sterilize the system, the reactors and glass rods were all autoclaved (120 °C for 20 min), and the Hoagland solution was filtered by a sterilized 0.2 μm bottle-top filter (Fisher Scientific, Hampton, NH). All the exposure groups and controls were set-up in triplicate. During the exposure, transpiration losses were replaced by adding autoclaved deionized water. Autoclaved deionized water was added into each reactor of the exposure groups and blank controls using a syringe (approximately 111 mL/day and 85 mL/day for 4′-MeO-PCB 3 and 4′-PCB 3 sulfate exposure, respectively). The hydroponic exposure lasted for 10 days.
2.3. Sampling and sample pretreatment
Poplar plants were sampled and sectioned into roots, bottom woods, bottom barks, top woods, top barks and shoots and leaves after 10 days exposure to investigate the translocation and distribution of parent chemicals and potential metabolites (Fig. 1). Roots of poplar plants were rinsed carefully with sterile, deionized water. The rinse water was combined with the solution to measure the parent chemicals and potential metabolites. All the plant samples were freeze-dried. Then, roots, shoots and leaves were ground by a ceramic mortar and pestle. Bark and wood samples were cut into very small pieces (<3 mm) before they were freeze-dried. All samples were stored at −10 °C before further analysis.
Fig. 1.
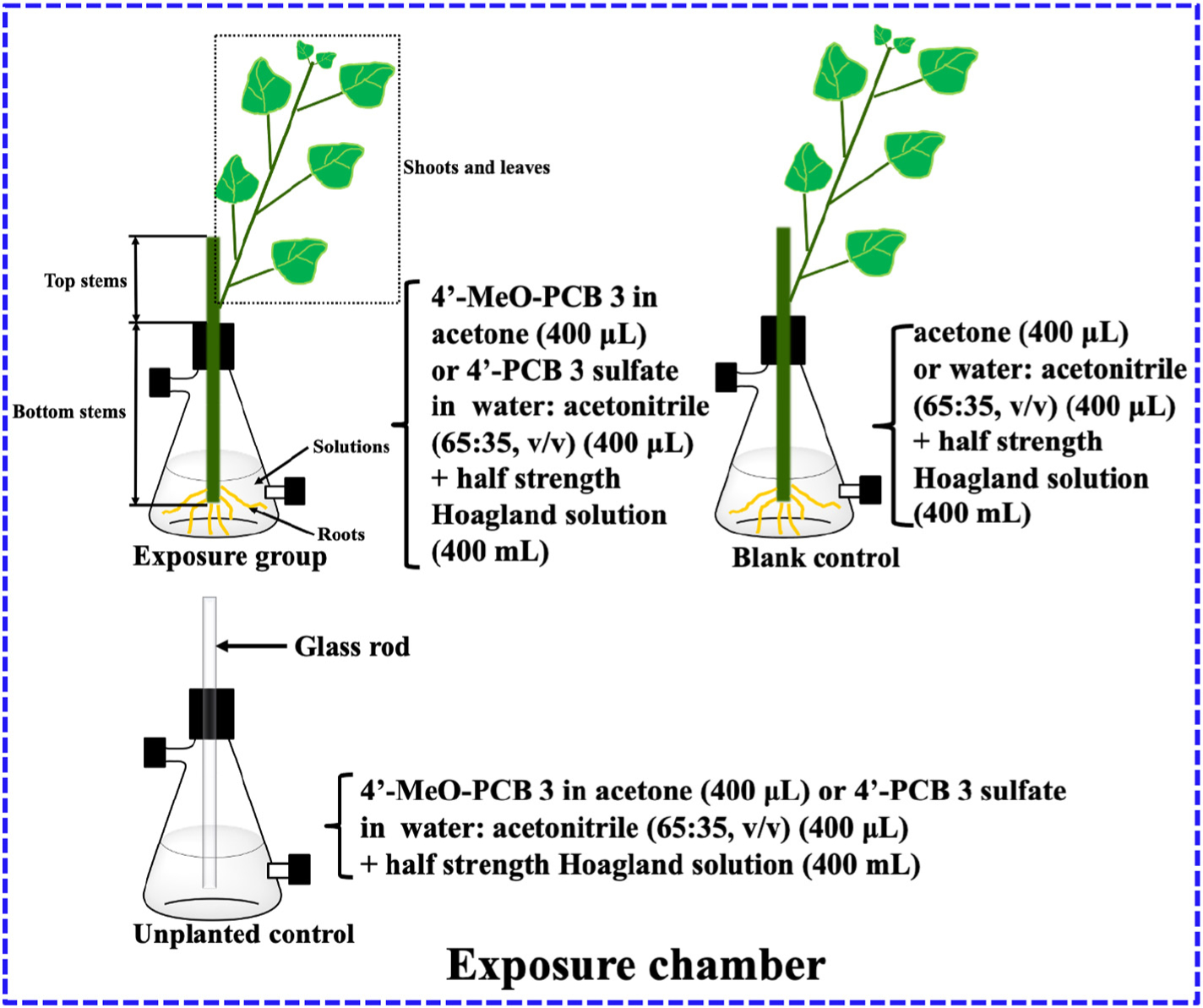
Experimental design for exposure and control groups. All the reactors were wrapped with aluminum foil during 10 days exposure to prevent potential photodegradation.
The extraction and cleanup method for MeO-PCBs, OH-PCBs and PCB sulfates were modified according to previous work (Sun et al., 2016a; Zhai et al., 2013). In brief, for MeO-PCBs and OH-PCBs, homogenized plant samples (0.05–0.63 g) were spiked with surrogate standards, and then ultrasonicated for 15 min with 6 mL of hexane/MTBE (1:1, v/v). After centrifugation (5 min at 3000 rpm), the organic extract was transferred to another clean tube. This extraction procedure was repeated three times. The combined extracts were dried under a gentle flow of nitrogen gas and redissolved in 30 mL of DCM. Lipid and other interferences were removed by adding 10 g of acid silica gel (30%, w/w), shaking vigorously and then filtering through an anhydrous sodium sulfate column (15 g). The anhydrous Na2SO4 column was then rinsed by 50 mL of DCM. The combined eluent was concentrated to dryness and re-dissolved in 1 mL of hexane. Half of the extract (500 μL in hexane) was transferred to a new GC vial and then spiked with an internal standard (25 ng of dPCB 30) for the analysis of MeO-PCBs on GC–MS/MS. The other 500 μL of extract in hexane was dried and re-dissolved in 200 μL of acetonitrile for the subsequent analysis of OH-PCBs on LC/MSD. The solution samples were liquid-liquid extracted with hexane/MTBE (1:1, v/v) after spiking with surrogate standards. The combined extracts were concentrated to dryness and re-dissolved in 30 mL of DCM. The subsequent purification process was similar to that of the plant samples.
For PCB sulfates, 0.15–0.96 g of plant samples were ultrasonically extracted with methanol (5 mL) overnight at 4 °C after spiking with 800 ng of surrogate standard (3-F, 4′-PCB 3 sulfate). After centrifugation (3000 rpm for 5 min), organic extract was transferred, concentrated to dryness, re-dissolved in water: acetonitrile (65:35, v/v), and then filtered by a 0.2 μm membrane filter (mdi syringe filter, SY13TF, PTFE) for LC/MSD analysis.
2.4. Instrumental analysis
Analysis of MeO-PCBs was performed on an Agilent 7890B gas chromatograph (GC) coupled with a 7000D triple quadrupole mass spectrometer (MS) equipped with electron ionization (EI) ion source, 7693 autosampler and a Supelco SPB-Octyl capillary column (5% phenyl methyl siloxane, 30 m × 0.25 mm × 0.25 μm) in multi-reaction monitoring (MRM) mode. The MRM precursor-product transitions are shown in Table S2. The carrier gas, helium, was at a constant flow of 0.75 mL/min. Nitrogen/argon was used as the collision gas. The temperature of the transfer line and electron ionization source was 280 °C and 250 °C, respectively. A solvent vent injection mode was used for GC analysis. The injection conditions were initially 45 °C (hold for 0.06 min), then ramped to inlet temperature 325 °C at a rate of 600 °C/min (4.4 psi). The oven temperature was initially 45 °C for 2 min, and increased to 75 °C at a rate of 100 °C/min and held for 5 min, 75 °C to 150 °C at a rate of 15 °C/min and held for 1 min, 150 °C to 220 °C at 2.5 °C and held for 1 min, 220 °C to 280 °C at 10 °C/min, and with a final hold for 10 min.
Analysis of OH-PCBs and PCB sulfates was performed on LC/MSD (Agilent 1260 infinity-6140) with a Agilent Zorbax Bonus RP column (2.1 × 150 mm, 5 μm). The electrospray in negative ionization mode of MS (LC-ESI (−)-MS) was utilized, and the injection volume was 20 μL. For OH-PCBs, the flow rate of mobile phase was 0.15 mL/min (room temperature). In mobile phase, the ratio of acetonitrile and water (pH 9.9) was 65:35. The ion mass detected in SIM was 203 for 4′-OH-PCB 3 and 249.1 for 13C 4′-OH-PCB 12. Fragmentor was at 115 V. Capillary voltage, gain, nebulizer pressure, drying gas flow and drying gas temperature were 3500 V, 7.00, 35 psi, 10 L/min and 250 °C, respectively. For PCB sulfates, a mobile phase consisting of acetonitrile and water (ion pair 5 mM) was used with a ratio of 35:65. The ion mass detected in SIM was 283 and 301 for 4′-PCB 3 sulfate and 3-F, 4′-PCB 3 sulfate, respectively. The fragmentor was 80 V for PCB 3 sulfate and 35 V for 3-F, 4′-PCB 3 sulfate. Drying gas flow and drying gas temperature were 13 L/min and 300 °C, respectively. Other analysis parameters of the LC/MSD were the same as that of OH-PCBs.
2.5. Quality assurance and quality control
All glassware was heated at 450 °C overnight, and rinsed with methanol, acetone and hexane sequentially to avoid potential contamination. Method blanks consisting of 0.4 g blank plant tissues (which were planted at a clean lab and without exposure chemicals) or 400 mL deionized water were extracted, analyzed, and quantified. Then, the method blanks were used to determine the method detection limits (MDLs), which was calculated as the upper limit of the 95% confidence interval (average mass of surrogate-corrected blanks plus two times the standard deviation) at five times measurements. MDLs for 4′-MeO-PCB 3, 4′-OH-PCB 3 and PCB 3 sulfate in plant samples were 0.03 ng/g, 0.77 ng/g and 1.54 ng/g, respectively. MDLs for the three chemicals in solutions were 0.08 pg/mL, 3.76 pg/mL and 0.16 ng/mL, respectively. Spiking recoveries of 4′-MeO-PCB 3, 4′-OH-PCB 3 and PCB 3 sulfate were in the ranges of 64.0%–94.3%, 38.5%–51.8% and 47.5%–66.7%, respectively. The recoveries of the surrogate standards, including 13C 4′-MeO-PCB 29, 13C 4′-OH-PCB 12 and 3-F, 4′-PCB3 sulfate, were 47.8%–106%, 39.5%–66.3%, and 31.1%–66.8%, respectively. All results were surrogate recovery-corrected.
Product yield was the percentage of the metabolized mass divided by the initial exposure mass of exposure chemicals. It was calculated based on the equimolar reaction between parent chemical and its demethylation and sulfated metabolites. The equation is shown as follow:
in which mB is the mass of metabolites, mA0 is the initial mass of exposure chemicals, MA and MB are the molecular weights of exposure chemicals and their metabolites, respectively.
3. Results and discussion
3.1. Uptake and translocation of exposure chemicals (4′-MeO-PCB 3 and 4′-PCB 3 sulfate) in poplar plants
The distributions of 4′-MeO-PCB 3 and 4′-PCB 3 sulfate in different plant tissues were analyzed to investigate the uptake and translocation of the exposure chemicals in poplar plants (shown as concentration and mass in Figs. 2, 3 and Fig. S1, S2, respectively). For exposed poplar plants, the uptaken concentration of 4′-MeO-PCB 3 was ranked as roots > bottom barks > bottom woods > top barks > shoots and leaves > top woods. For the exposure chemical, 4′-PCB 3 sulfate, the concentration in exposed plant tissue was also ranked as roots > bottom woods > top woods. It indicates that both exposure chemicals, 4′-MeO-PCB 3 and 4′-PCB 3 sulfate, were taken up by the roots and translocated from the roots to the shoots and leaves and woods in poplar plants. After a 10-day exposure, 51.6 ± 5.6% of 4′-MeO-PCB 3 mass was measured/recovered in the exposure system (Table 1). The largest portion of 4′-MeO-PCB 3 (32.3% of initial mass) was found in solution samples, followed by roots (10.2%). Only 0.07% of initial mass of 4′-MeO-PCB 3 was translocated and found in shoots and leaves. Only 0.24 ± 0.08% of 4′-PCB 3 sulfate mass was accumulated in woods and roots (Table 1). According to our previous work (Zhai et al., 2013), the extraction and detection of 4′-PCB 3 sulfate were greatly influenced by the matrixes in the bark and shoot and leaves samples. However, the matrices in wood and root samples showed little electrospray-induced ion suppression. Thus, 4′-PCB 3 sulfate in wood and root samples was successfully analyzed, but not in shoots, leaves and barks.
Fig. 2.
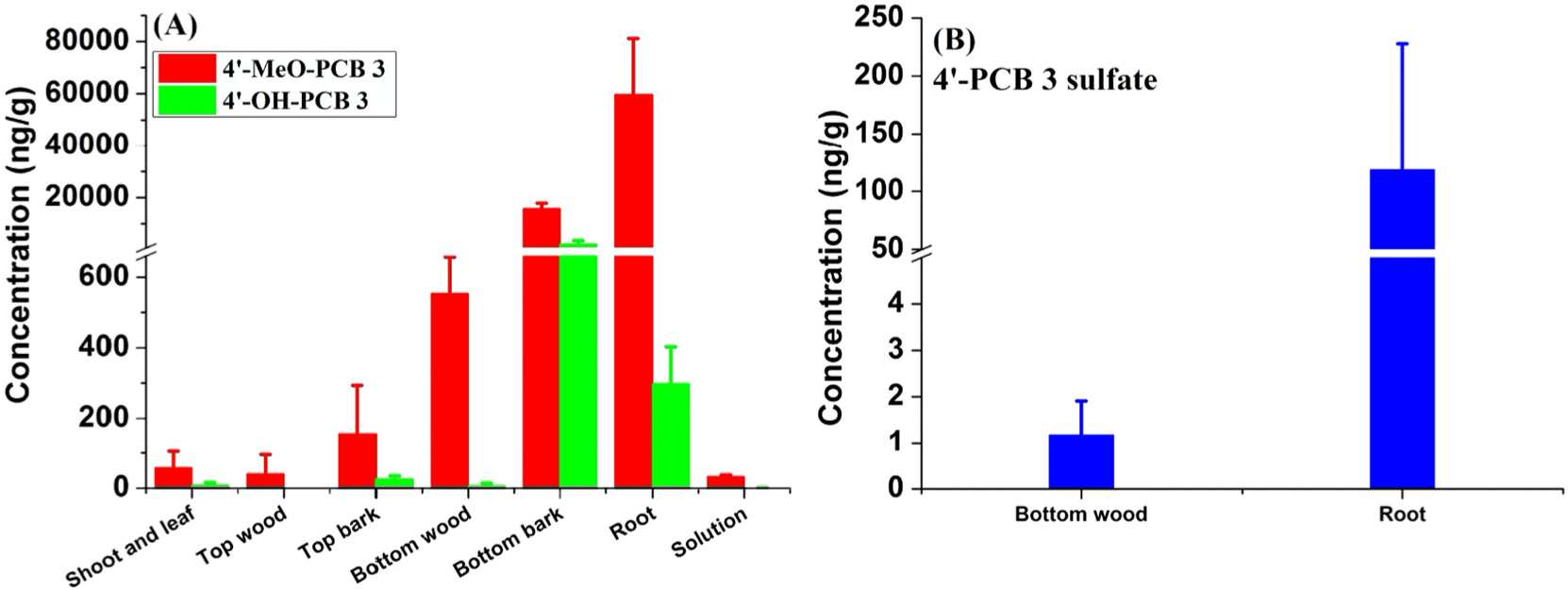
Concentrations of exposure chemical, 4′-MeO-PCB 3 (A), and its hydroxylated (A) and sulfated (B) metabolites in different components of exposure groups after 10 days exposure. No 4′-OH-PCB 3 and 4′-PCB 3 sulfate were detected in top woods of exposure groups.
Fig. 3.
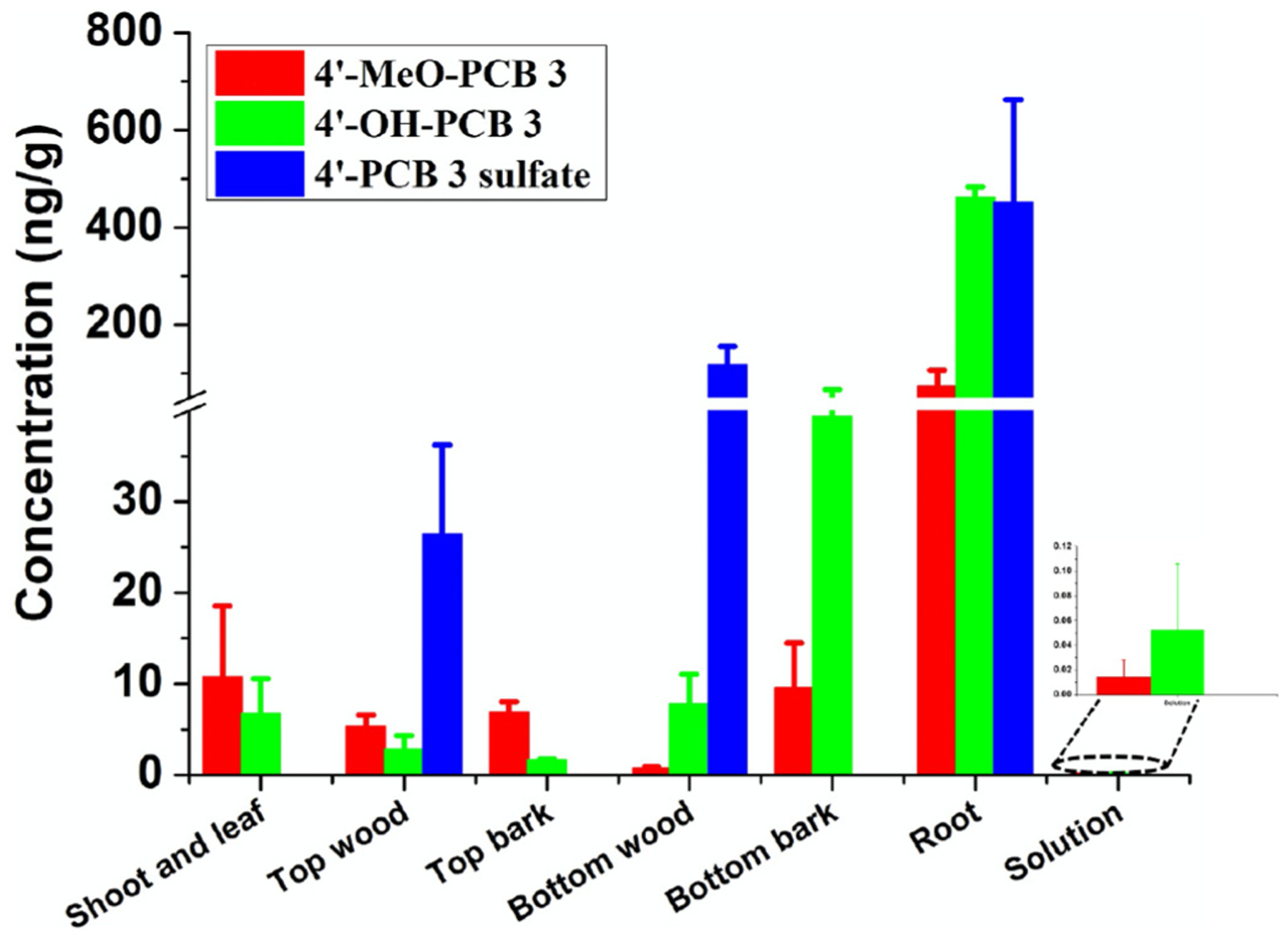
Concentrations of exposure chemical, 4′-PCB 3 sulfate, and its methoxylated and hydroxylated metabolites in different components of exposure groups after 10 days exposure. No 4′-PCB 3 sulfate were detected in solutions of exposure groups.
Table 1.
Mass balance and distributions of exposure chemicals (4′-MeO-PCB 3 and 4′-PCB 3 sulfate) (% of mass applied) in exposure groups and unplanted controls after 10 days hydroponic exposure.a
| Reactors | Samples | 4′-MeO-PCB 3 (%) | Total 4′-MeO-PCB 3 recovered (%)c | 4′-PCB 3 sulfate (%) | Total 4′-PCB 3 sulfate recovered (%) |
|---|---|---|---|---|---|
| Exposure groups | Shoots | 0.07 ± 0.06b | 51.6 ± 5.6 | _d | 0.24 ± 0.08 |
| Top woods | 0.02 ± 0.03 | 0.02 ± 0.01 | |||
| Top barks | 0.05 ± 0.05 | _ | |||
| Bottom woods | 0.57 ± 0.03 | 0.17 ± 0.06 | |||
| Bottom barks | 8.34 ± 1.48 | _ | |||
| Roots | 10.2 ± 2.0 | 0.04 ± 0.02 | |||
| Solutions | 32.3 ± 4.5 | n.d.e | |||
| Unplanted controls | Solutions | 72.1 ± 27.6 | 72.1 ± 27.6 | 47.9 ± 6.2 | 47.9 ± 6.2 |
The recovered 4′-MeO-PCB 3 and 4′-PCB 3 sulfate in different components expressed as the mass percentage of initial exposed chemical. The initial mass of 4′-MeO-PCB 3 and 4′-PCB 3 sulfate was 400 μg and 397 μg, respectively.
Mean value ± standard deviation, n = 3.
The summation of different components.
These samples were not analyzed in this work.
Non-detectable.
No exposure chemicals were found in blank controls, suggesting that there was no cross contamination between reactors or background contamination from the laboratory. The mean total recoveries of 4′-MeO-PCB 3 and 4′-PCB 3 sulfate in unplanted controls were 72.1 ± 27.6% and 47.9 ± 6.2%, respectively (Table 1). Approximately 27.9% and 52.1% of the initial dose of 4′-MeO-PCB 3 and 4′-PCB 3 sulfate were not recovered, respectively, and was likely lost due to volatilization and chemical transformation and metabolism. Lower recoveries of 4′-MeO-PCB 3 and 4′-PCB 3 sulfate in exposure groups compared to unplanted controls suggest that a large amount of 4′-MeO-PCB 3 and 4′-PCB 3 sulfate were metabolized by the poplar plants to unmeasured products and to phytovolatilization.
3.2. Interconversion of MeO-PCB 3, OH-PCB 3 and PCB 3 sulfate in poplar plants
The relationships between methoxylated, hydroxylated and sulfated metabolites of PCB 3 in whole poplar plants were investigated via hydroponic exposure in separate experiments by MeO-PCB 3 and PCB 3 sulfate. Transformations from 4′-MeO-PCB 3 to 4′-OH-PCB 3, and then to 4′-PCB 3 sulfate were found in exposed poplar plants (Fig. 2). Besides, transformations from 4′-PCB 3 sulfate to 4′-OH-PCB 3, and then to 4′-MeO-PCB 3 were also observed after 10 days exposure (Fig. 3). Importantly, interconversion between methoxylated and sulfated metabolites of PCB 3 was found for the first time.
The distribution of metabolites of exposure chemicals in different plant-compartments was also investigated. Results showed that the hydroxylated metabolite, 4′-OH-PCB 3, produced from 4′-MeO-PCB 3 and 4′-PCB 3 sulfate was identified throughout the exposed whole poplar plants (Figs. 2 and 3, Figs. S1, S2 and S3). The concentration distribution of 4′-OH-PCB 3 produced from 4′-MeO-PCB 3 in exposure groups was highest in bottom barks, followed by roots, top barks, shoots and leaves, bottom woods and solutions, which was similar to that of the original exposure chemical. The concentration of 4′-OH-PCB 3 produced from 4′-PCB 3 sulfate in exposure groups was ranked as roots > bottom barks > bottom woods > shoots and leaves > top woods > top barks > solutions. Results indicate that poplar plants can metabolize 4′-MeO-PCB 3 and 4′-PCB 3 sulfate into hydroxylated products via demethylation and sulfation, respectively, and that translocation of 4′-OH-PCB 3 occurred in the plants. However, no 4′-OH-PCB 3 (produced from 4′-MeO-PCB 3) was found in the top woods of exposed poplar plants. The relatively high concentration of 4′-OH-PCB 3 produced from 4′-MeO-PCB 3 in bottom bark was attributed to location of the bottom bark close to the hydroponic exposure solution, and due to direct contact of the gas phase with the bottom bark including any gaseous exposure chemical and its hydroxylated metabolite. The high concentration of 4′-PCB 3 sulfate and its metabolite, 4′-OH-PCB 3, in roots of exposure groups suggests that metabolism of 4′-PCB 3 sulfate occurred first in the roots and was then translocated.
The yields of 4′-OH-PCB 3 produced from 4′-MeO-PCB 3 and 4′-PCB 3 sulfate in exposure groups were 1.29 ± 0.95% and 0.13 ± 0.01%, respectively (Table 2). The demethylation yield of 4′-MeO-PCB 3 in poplar plants was lower than that of MeO-PCB 61 in rice plants which were hydroponically exposed to MeO-PCB 61 for 5 days (Sun et al., 2016a). Uptake and metabolism of PCBs and their metabolites in plants depends on the plant species and the reactivity of different congeners. According to the recovered exposure chemicals and the yield of 4′-OH-PCB 3 in exposure groups, there were still large amounts of unrecovered exposure chemicals, indicating that other metabolites were formed in exposure reactors besides the hydroxylated metabolite, 4′-OH-PCB 3. Losses due to phytovolatilization during transpiration are thought to be small based on the low concentrations of metabolites in the shoots and leaves. The enzyme glutathione S-transferase (GST) is known to bind with OH-PCBs (Bagnati et al., 2019; Subramanian et al., 2017). OH-PCBs can undergo transferase-mediated conjugation with glutathione, which is a potentially important detoxification pathway in plants (Bagnati et al., 2019; Tehrani and Van Aken, 2014).
Table 2.
Yields of metabolites in different compartments of exposure groups and unplanted controls after exposure to separate 4′-MeO-PCB 3 and 4′-PCB 3 sulfate for 10 days.
| Reactors | Samples | Exposure to 4′-MeO-PCB 3 | Exposure to 4′-PCB 3 sulfate | ||
|---|---|---|---|---|---|
| 4′-OH-PCB 3 (%) | 4′-PCB 3 sulfate (%) | 4′-MeO-PCB 3 (%) | 4′-OH-PCB 3 (%) | ||
| Exposure groups | Shoots and leaves | 0.01 ± 0.01a | _ | 0.01 ± 0.00 | 0.007 ± 0.003 |
| Top woods | n.d.b | n.d. | 0.006 ± 0.002 | 0.003 ± 0.001 | |
| Top barks | 0.01 ± 0.00 | _c | 0.004 ± 0.001 | 0.001 ± 0.000 | |
| Bottom woods | 0.01 ± 0.01 | 0.001 ± 0.001 | 0.001 ± 0.000 | 0.02 ± 0.01 | |
| Bottom barks | 1.17 ± 0.93 | _ | 0.008 ± 0.004 | 0.04 ± 0.02 | |
| Roots | 0.06 ± 0.03 | 0.02 ± 0.02 | 0.009 ± 0.004 | 0.06 ± 0.01 | |
| Solutions | 0.04 ± 0.04 | n.d. | 0.0005 ± 0.0005 | 0.007 ± 0.007 | |
| Unplanted controls | Solutions | 0.01 ± 0.00 | n.d. | n.d. | 0.002 ± 0.000 |
Mean value ± standard deviation, n = 3.
Non-detectable.
4′-PCB 3 sulfate in shoots and leaves and bark samples were not analyzed in this work due to the matrixes in these samples containing pigments which can greatly influence the extraction and detection of 4′-PCB 3 sulfate (Zhai et al., 2013).
Sulfated metabolites of MeO-PCBs have not been reported previously in vivo or in vitro. However, the sulfate metabolite, 4′-PCB 3 sulfate, was identified in exposed poplar plants after being hydroponically exposed to 4′-MeO-PCB 3 for 10 days in this work (Fig. 2 and Fig. S4). Only 4′-PCB 3 sulfate in wood and root samples was successfully analyzed due to matrix interferences. The concentration of 4′-PCB 3 sulfate produced from 4′-MeO-PCB 3 was 1.17 ± 0.74 ng/g in bottom woods and 119 ± 109 ng/g in roots (Fig. 2B). No 4′-PCB 3 sulfate was detected in top woods of the exposure groups, which was consistent with our previous work (Zhai et al., 2013). The total yield of 4′-PCB 3 sulfate in bottom woods and roots was about 0.02% (Table 2), which was much lower than that of 4′-OH-PCB 3 (also produced from 4′-MeO-PCB 3) in wood and root samples. The concentration ratio of 4′-PCB 3 sulfate to 4′-OH-PCB 3 in bottom woods and roots was 1: 4.90 and 1: 2.50, respectively. Results indicate that 4′-PCB 3 sulfate was mainly produced in roots and then upwardly translocated from roots to bottom woods. The function of OH-PCBs as substrates for sulfotransferase (SULT) enzymes was reported (Grimm et al., 2015; Parker et al., 2018). OH-PCBs were able to be further metabolized to PCB sulfates by SULT in vitro and in vivo (Dhakal et al., 2012; Dhakal et al., 2014; Zhai et al., 2013). In our previous work, sulfate metabolites of PCBs were detected, however, the ratio of sulfate metabolites to hydroxyl-PCBs was affected by the further formation of methoxylated metabolites (Zhai et al., 2013). The concentration ratio of 4′-PCB 3 sulfate to 4′-OH-PCB 3 in roots was higher when poplar plants were exposed to methoxy-PCB 3 than to PCB 3 (1: 16.3) (Zhai et al., 2013).
Interconversion between OH-PCB 61 and MeO-PCB 61 in rice was reported. But in this work the production of methoxylated metabolites of 4′-PCB 3 sulfate was also investigated. 4′-MeO-PCB 3 produced from 4′-PCB 3 sulfate was found in exposure groups (Fig. 2 and Fig. S2). The concentration of 4′-MeO-PCB 3 produced from 4′-PCB 3 sulfate in exposure groups was 10.8 ± 7.8 ng/g in shoots and leaves, 5.36 ± 1.22 ng/g in top woods, 6.91 ± 1.13 ng/g in top barks, 0.77 ± 0.21 ng/g in bottom woods, 9.54 ± 4.93 ng/g in bottom barks, 73.5 ± 33.7 ng/g in roots and 0.01 ± 0.01 ng/mL in solutions. The concentration of 4′-MeO-PCB 3 (originating from 4′-PCB 3 sulfate) in roots (p < 0.05), bottom barks (p = 0.14) and bottom woods (p = 0.21) was lower than that of 4′-OH-PCB 3 (produced from 4′-PCB 3 sulfate). However, the concentration of 4′-MeO-PCB 3 (originating from 4′-PCB 3 sulfate) in shoots and leaves (p > 0.05), top woods (p = 0.09) and top barks (p < 0.01) was higher than that of 4′-OH-PCB 3 (produced from 4′-PCB 3 sulfate). The yield of 4′-MeO-PCB 3 from 4′-PCB 3 sulfate was 0.04 ± 0.01%.
No metabolites were found in blank controls after 10 days exposure, indicating there was not background contamination. For unplanted controls, no sulfated metabolite of 4′-MeO-PCB 3, 4′-PCB 3 sulfate, and no methoxylated metabolite of 4′-PCB 3 sulfate, 4′-MeO-PCB 3, were detected. Small amounts of 4′-OH-PCB 3 were found in unplanted controls (Table 2). However, the yield of 4′-OH-PCB 3 produced from 4′-MeO-PCB 3 (1.29 ± 0.95%) and 4′-PCB 3 sulfate (0.13 ± 0.01%) was much higher than that in unplanted controls (0.01 ± 0.00%, p = 0.08; 0.002 ± 0.000%, p < 0.01), respectively. Thus, metabolism of exposure chemicals, demethylation, sulfation, methylation and hydrolysis, were mediated by poplar plants and further confirmed.
According to the mass balance (Tables 1 and 2), the total recovered parent chemicals (including the yields of hydroxyl-PCB, methoxy-PCB and PCB sulfate) in exposed plants were lower than that in unplanted controls, which is not surprising for two reasons. The PCB sulfate accumulated in barks and shoots and leaves was not quantitatively analyzed in this work due to matrix interferences, which was the main reason for the low recovery of exposure chemical, 4′-PCB 3 sulfate, in exposure groups. Second, it is likely that other metabolites were formed by plants in the experiments with exposed plants that have not been measured, like glutathione complexes. Considering that the sulfate metabolite was transformed from hydroxyl-PCB, the total yield of sulfate metabolite in the exposure system was lower than that of hydroxylated metabolite. Thus, the main reason for the lower recovery of exposure chemical in exposed plants compared to the unplanted controls was likely due to plant metabolic reactions and unmeasured metabolites.
3.3. Metabolism pathways
The proposed metabolism pathways of methoxy-PCB, hydroxyl-PCB and PCB sulfate in poplar plants are shown in Fig. 4, including the demethylation, sulfation, methylation and hydrolysis. The metabolism pathways of exposure chemicals in this work suggest that 4′-MeO-PCB 3 and 4′-PCB 3 sulfate were first transformed to 4′-OH-PCB 3 intermediate. Then, 4′-OH-PCB 3 was further transformed to 4′-PCB 3 sulfate and 4′-MeO-PCB 3, respectively.
Fig. 4.
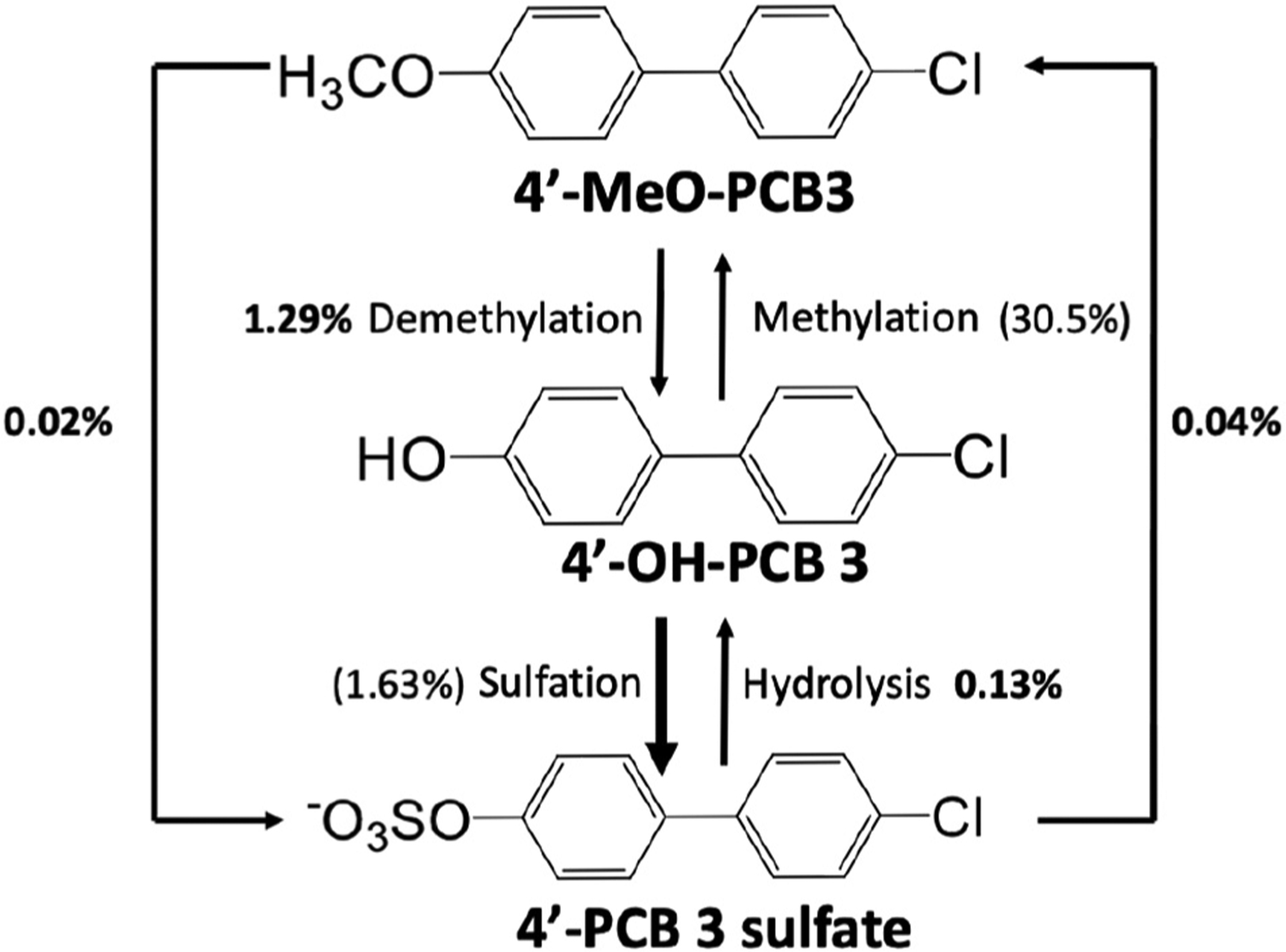
Metabolic pathways of exposure chemicals in whole poplar plants. 4′-PCB 3 sulfate was only analyzed in wood and root samples due to influence of matrices. The yield percentages in bold face without any parentheses were measured from results of the experiments while the others (with parentheses) are calculated (inferred from the mass balance and reaction pathways).
The yields between 4′-MeO-PCB 3, 4′-OH-PCB 3 and 4′-PCB 3 sulfate are shown in Fig. 4. The methylation yield was 30.5% if we assume that all the OH-PCB 3 becomes final product, 4′-MeO-PCB 3, which was much higher than the demethylation yield (1.29%) when 4′MeO-PCB 3 was transformed to 4′-OH-PCB 3. In contrast, Sun et al. reported that methylation yield of 4′-OH-CB-61 was lower than demethylation yield of 4′-MeO-CB-61 (Sun et al., 2016a). The sulfation yield of 4′-OH-PCB 3 (1.63%) was higher than hydrolysis yield of 4′-PCB 3 sulfate (0.128%) even though only the 4′-PCB 3 sulfate in wood and root samples was analyzed. It indicates that formation of sulfated metabolites was greater than the reverse reaction to hydroxy-PCBs.
4. Environmental implication
This work provides the first evidence for interconversion between methoxylated, hydroxylated and sulfated metabolites of PCB 3 in whole plants. Though the exposure concentration was higher than typical environmental concentrations, the demethylation, sulfation, methylation and hydrolysis of methoxy-PCBs and PCB sulfates by plants were confirmed. Data regarding environmental occurrence of toxic MeO-PCBs are scarce at present. As we know, the bioavailability of organic chemicals in hydroponic exposure systems is higher than that in a real soil-plant system, but this simple system facilitated documentation of a difficult-to-detect and previously unrecognized biotransformation. In this work, we used poplar plants as model plants for exposure. The species differences between poplar and other plants may affect the transformation results. However, out results still confirmed the interconversion between metabolites of PCBs in plants. Although the interconversion between methoxy-PCBs and PCB sulfates was at very low level, the results of this research provide insight into possible metabolism pathways of MeO-PCBs and even new sources of OH-PCBs and PCB sulfates, which are proving to be long-lived “emerging” chemicals from legacy PCBs. This paper does not conclusively prove the environmental hazard associated with transformation and interconversion of methoxy-, hydroxy-, and sulfated PCBs in plants. However, it does demonstrate the potential for those transformations to result in more toxic products than the parent compounds.
5. Conclusion
Relationship between methoxylated, hydroxylated and sulfate metabolites of PCB 3 was explored. Poplar plants can uptake, accumulate, translocate and metabolize 4′-MeO-PCB 3, 4′-OH-PCB 3 and 4′-PCB 3 sulfate. It was found that sulfation ratio was higher than hydrolysis ratio, indicating that formation of PCB sulfates (produced from OH-PCBs) was easier than formation of OH-PCBs (produced from PCB sulfates). For the first time, interconversion between methoxylated, hydroxylated and sulfate metabolites of PCB 3 in plants was demonstrated.
Supplementary Material
HIGHLIGHTS.
Sulfation of methoxy-PCBs in plants was demonstrated for the first time.
First evidence of methylation of PCB sulfates in plants
Interconversion demonstrated between methoxy-, hydroxyl- and PCB sulfates in poplar
Acknowledgements
This work was supported by the Iowa Superfund Basic Research Program (SBRP), Grant Number P42ES013661. We thank Dr. Ram C. Dhakal for authenticating the standards of 4′-MeO-PCB 3, 4′-OH-PCB 3, 4′-PCB 3 sulfate and 3-F, 4′-PCB 3 sulfate. This is a contribution from the W.M.Keck Phytotechnologies Laboratory at the University of Iowa.
Footnotes
CRediT authorship contribution statement
Yanlin Li: Conceptualization, Methodology, Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. Christian M. Bako: Methodology, Writing – review & editing. Panithi Saktrakulkla: Methodology, Writing – review & editing. Hans-Joachim Lehmler: Project administration, Resources, Writing – review & editing, Funding acquisition. Keri C. Hornbuckle: Funding acquisition, Project administration, Resources, Writing – review & editing. Jerald L. Schnoor: Conceptualization, Methodology, Writing – review & editing, Supervision, Project administration, Funding acquisition, Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.147341.
References
- Allmyr M, Adolfsson-Erici M, McLachlan MS, Sandborgh-Englund G, 2006. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. Sci. Total Environ 372, 87–93. [DOI] [PubMed] [Google Scholar]
- Awad AM, Martinez A, Marek RF, Hornbuckle KC, 2016. Occurrence and distribution of two Hydroxylated polychlorinated biphenyl congeners in Chicago air. Environ. Sci. Technol. Let 3, 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnati R, Terzaghi E, Passoni A, Davoli E, Fattore E, Maspero A, Palmisano G, Zanardini E, Borin S, Di Guardo A, 2019. Identification of sulfonated and hydroxy-sulfonated Polychlorinated Biphenyl (PCB) metabolites in soil: new classes of intermediate products of PCB degradation? Environ. Sci. Technol 53, 10601–10611. [DOI] [PubMed] [Google Scholar]
- Collins C, Fryer M, Grosso A, 2006. Plant uptake of non-ionic organic chemicals. Environ. Sci. Technol 40, 45–52. [DOI] [PubMed] [Google Scholar]
- Darling C, Alaee M, Campbell L, Pacepavicius G, Ueno D, Muir D, 2004. Hydroxylated PCBs in abiotic environmental matrices: precipitation and surface waters. Organohalogen Compd. 66, 1470–1475. [Google Scholar]
- Desforges JP, Hall A, McConnell B, Rosing-Asvid A, Barber JL, Brownlow A, De Guise S, Eulaers I, Jepson PD, Letcher RJ, Levin M, Ross PS, Samarra F, Vikingson G, Sonne C, Dietz R, 2018. Predicting global killer whale population collapse from PCB pollution. Science 361, 1373–1376. [DOI] [PubMed] [Google Scholar]
- Dhakal K, He X, Lehmler HJ, Teesch LM, Duffel MW, Robertson LW, 2012. Identification of sulfated metabolites of 4-chlorobiphenyl (PCB3) in the serum and urine of male rats. Chem. Res. Toxicol 25, 2796–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal K, Uwimana E, Adamcakova-Dodd A, Thorne PS, Lehmler H-J, Robertson LW, 2014. Disposition of phenolic and sulfated metabolites after inhalation exposure to 4-Chlorobiphenyl (PCB3) in female rats. Chem. Res. Toxicol 27, 1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fängström B, Strid A, Athanassiadis I, Grandjean P, Weihe P, Bergman Å, 2004. A retrospective time trend study of PBDEs and PCBs in human milk from the Faroe Islands. Organohalogen Comp. 66, 2829–2833. [Google Scholar]
- Fängström B, Strid A, Grandjean P, Weihe P, Bergman Å, 2005. A retrospective study of PBDEs and PCBs in human milk from the Faroe Islands. Environ. Health 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm FA, Hu D, Kania-Korwel I, Lehmler HJ, Ludewig G, Hornbuckle KC, Duffel MW, Bergman A, Robertson LW, 2015. Metabolism and metabolites of polychlorinated biphenyls. Crit. Rev. Toxicol 45, 245–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm FA, Lehmler HJ, Koh WX, DeWall J, Teesch LM, Hornbuckle KC, Thorne PS, Robertson LW, Duffel MW, 2017. Identification of a sulfate metabolite of PCB 11 in human serum. Environ. Int 98, 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei I, Kogura R, Kondo R, 2006. Metabolism of 4,4′-dichlorobiphenyl by white-rot fungi Phanerochaete chrysosporium and Phanerochaete sp. MZ142. Appl. Microbiol. Biotechnol 72, 566–575. [DOI] [PubMed] [Google Scholar]
- Kuch B, Kern F, Metzger JW, von der Trenck KT, 2010. Effect-related monitoring: estrogen-like substances in groundwater. Environ. Sci. Pollut. R 17, 250–260. [DOI] [PubMed] [Google Scholar]
- Li XS, Parkin S, Duffel MW, Robertson LW, Lehmler H-J, 2010. An efficient approach to sulfate metabolites of polychlorinated biphenyls. Environ. Int 36, 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YL, Hou XW, Yu M, Zhou QF, Liu JY, Schnoor JL, Jiang GB, 2017. Dechlorination and chlorine rearrangement of 1,2,5,5,6,9,10-heptachlorodecane mediated by the whole pumpkin seedlings. Environ. Pollut 224, 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YL, Hou XW, Chen WF, Liu JY, Zhou QF, Schnoor JL, Jiang GB, 2019. Carbon chain decomposition of short chain chlorinated paraffins mediated by pumpkin and soybean seedlings. Environ. Sci. Technol 53 (12), 6765–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FJ, Sun JT, Liu N, Zhu LZ, 2020. Phytotoxicity and metabolic responses induced by tetrachlorobiphenyl and its hydroxylated and methoxylated derivatives in rice (Oryza sative L.). Environ. Int 139, 105–695. [DOI] [PubMed] [Google Scholar]
- Marek RF, Martinez A, Hornbuckle KC, 2013a. Discovery of Hydroxylated Polychlorinated Biphenyls (OH-PCBs) in sediment from a Lake Michigan waterway and original commercial aroclors. Environ. Sci. Technol 47, 8204–8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek RF, Thorne PS, Wang K, DeWall J, Hornbuckle KC, 2013b. PCBs and OH-PCBs in serum from children and mothers in urban and rural US communities. Environ. Sci. Technol 47, 3353–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek RF, Thome PS, Herkert NJ, Awad AM, Hornbuckle KC, 2017. Airborne PCBs and OH-PCBs inside and outside urban and rural US schools. Environ. Sci. Technol 51, 7853–7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomiyama K, Yonehara T, Yonemura S, Yamamoto M, Koriyama C, Akiba S, Shinohara R, Koga M, 2009. Determination and characterization of hydroxylated polychlorinated biphenyls (OH-PCBs) in serum and adipose tissue of Japanese women diagnosed with breast cancer. Environ. Sci. Technol 44, 2890–2896. [DOI] [PubMed] [Google Scholar]
- Parker VS, Squirewell EJ, Lehmler HJ, Robertson LW, Duffel MW, 2018. Hydroxylated and sulfated metabolites of commonly occurring airborne polychlorinated biphenyls inhibit human steroid sulfotransferases SULT1E1 and SULT2A1. Environ. Toxicol. Phar 58, 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinete N, Kraus T, Belov VN, Aretz C, Esser A, Schettgen T, 2015. Fast determination of hydroxylated polychlorinated biphenyls in human plasma by online solid phase extraction coupled to liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 888, 94–102. [DOI] [PubMed] [Google Scholar]
- Rezek J, Macek T, Mackova M, Triska J, Ruzickova K, 2008. Hydroxy-PCBs, methoxy-PCBs and hydroxy-methoxy-PCBs: metabolites of polychlorinated biphenyls formed in vitro by tobacco cells. Environ. Sci. Technol 42, 5746–5751. [DOI] [PubMed] [Google Scholar]
- Rodriguez EA, Li XS, Lehmler HJ, Robertson LW, Duffel MW, 2016. Sulfation of lower chlorinated polychlorinated biphenyls increases their affinity for the major drug-binding sites of human serum albumin. Environ. Sci. Technol 50, 5320–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez EA, Vanle BC, Doorn JA, Lehmler H-J, Robertson LW, Duffel MW, 2018. Hydroxylated and sulfated metabolites of commonly observed airborne polychlorinated biphenyls display selective uptake and toxicity in N27, SH-SY5Y, and HepG2 cells. Environ. Toxicol. Phar 62, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S, Hutzinger O, Jones D, 1975. Mechanism of chlorobiphenyl metabolism. J. Agric. Food Chem 23, 851–853. [DOI] [PubMed] [Google Scholar]
- Sakiyama T, Yamamoto A, Kakutani N, Fukuyama J, Okumura T, 2007. Hydroxylated polychlorinated biphenyls (OH-PCBs) in the aquatic environment : levels and congener profiles in sediments from Osaka, Japan. Organohalogen Comp. 69, 1380–1383. [Google Scholar]
- Subramanian S, Schnoor JL, Van Aken B, 2017. Effects of Polychlorinated Biphenyls (PCBs) and their hydroxylated metabolites (OH-PCBs) on Arabidopsis thaliana. Environ. Sci. Technol 51, 7263–7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JT, Pan LL, Su ZZ, Zhan Y, Zhu LZ, 2016a. Interconversion between methoxylated and hydroxylated polychlorinated biphenyls in rice plants: an important but overlooked metabolic pathway. Environ. Sci. Technol 50, 3668–3675. [DOI] [PubMed] [Google Scholar]
- Sun JT, Zhu LZ, Pan LL, Wei Z, Song Y, Zhang YD, Qu LP, Zhan Y, 2016b. Detection of methoxylated and hydroxylated polychlorinated biphenyls in sewage sludge in China with evidence for their microbial transformation. Sci. Rep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JT, Pan LL, Chen J, Li KL, Zhu LZ, 2018. Uptake, translocation, and metabolism of hydroxylated and methoxylated polychlorinated biphenyls in maize, wheat, and rice. Environ. Sci. Pollut. Res. Int 25, 12–17. [DOI] [PubMed] [Google Scholar]
- Tehrani R, Van Aken B, 2014. Hydroxylated polychlorinated biphenyls in the environment: sources, fate, and toxicities. Environ. Sci. Pollut. Res. Int 21, 6334–6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno D, Darling C, Alaee M, Campbell L, Pacepavicius G, Teixeira C, Muir D, 2007. Detection of hydroxylated polychlorinated biphenyls (OH-PCBs) in the abiotic environment: surface water and precipitation from Ontario, Canada. Environ. Sci. Technol 41, 1841–1848. [DOI] [PubMed] [Google Scholar]
- Zhai GS, Lehmler HJ, Schnoor JL, 2013. Sulfate metabolites of 4-monochlorobiphenyl in whole poplar plants. Environ. Sci. Technol 47, 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Linderholm L, Park J-S, Petreas M, Guo T, Privalsky ML, Zoeller RT, Woodruff TJ, 2013. Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California. Environ. Sci. Technol 47, 11776–11784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


