Highlights
-
•
Bacteriocinogenic Enterococcus faecium strains were evaluated for their beneficial and safety properties.
-
•
Safety of the strains were evaluated based on phenotypic and bio-molecular approaches.
-
•
The beneficial properties of the strains were demonstrated.
-
•
High survivability under simulated GIT conditions and inhibition of Listeria spp. were demonstrated.
-
•
The strains were found to carry genes coding for GABA production.
Keywords: Enterococcus faecium, bacteriocins, probiotics, safety, virulence
Abstract
Enterococcus spp., known for their wide ecological distribution, have been associated with various fermented food products of plant and animal origin. The strains used in this study, bacteriocinogenic Enterococcus faecium previously isolated from artisanal soybean paste, have shown strong activity against Listeria spp. and vancomycin-resistant enterococci. Although their antimicrobial activity is considered beneficial, the potential application of enterococci is still under debate due to concerns about their safety for human and other animal consumption. Therefore, this study not only focuses on the screening of potential virulence factors, but also the auxiliary beneficial properties of the strains Ent. faecium ST651ea, ST7119ea, and ST7319ea. Phenotypic screening for gelatinase, hemolysin, and biogenic amine production showed that the strains were all safe. Furthermore, the antibiogram profiling showed that all the strains were susceptible to the panel of antibiotics used in the assessment except for erythromycin. Yet, Ent. faecium ST7319ea was found to carry some of the virulence genes used in the molecular screening for safety including hyl, esp, and IS16. The probiotic potential and other beneficial properties of the strains were also studied, demonstrating high aggregation and co-aggregation levels compared to previously characterized strains, in addition to high survivability under simulated gastrointestinal conditions, and production of numerous desirable enzymes as evaluated by APIZym, indicating diverse possible biotechnological applications of these strains. Additionally, the strains were found to carry genes coding for γ-aminobutyric acid (GABA) production, an auxiliary characteristic for their probiotic potential. Although these tests showed relatively favorable characteristics, it should be considered that these assays were carried out in vitro and should therefore also be assessed under in vivo conditions.
Graphical abstract
1. Introduction
Lactic acid bacteria (LAB) are associated with a wide variety of ecological niches, including different fermented food products based on the plant phyllosphere and animal, dairy origin, and also with the human intestinal tract (Franz et al., 2010; dos Santos et al., 2020; Vizoso Pinto et al., 2006). Although LAB require a nutrient-rich environment, some members of this group are clearly adapted to a broader range of environmental conditions relative to the other members. These include the autochthonous distributions of some species belonging to the genera Pediococcus, Lactobacillus, and Enterococcus. Even though the applications of enterococci are still under debate, a proposed two-clade classification of Enterococcus faecium appears to be feasible to separate pathogenic strains from well-evaluated beneficial strains (Lebreton et al., 2018; Holzapfel et al., 2018). This paved the way to the exploitation and earnest scientific interest towards a better understanding of both the pathogenicity and probiotic properties of this species. The probiotic potential of some representatives of the genus Enterococcus has been explored particularly regarding its antibacterial properties for application as biocontrol contaminants in dairy food products (De Vuyst et al., 2003; Franz et al., 2003; Omar et al., 2004) while several strains are applied as starter cultures (Foulquié Moreno et al., 2006), and some are established as probiotics (Holzapfel et al., 2018). On the other hand, reports on some clinically associated enterococci suggest that some strains may pose a serious health risk, especially those specifically harboring virulence factors and carrying antibiotic resistance genes, including resistance to vancomycin. Moreover, some strains of Ent. faecalis and Ent. faecium isolated from fresh produce were shown to harbor antibiotic resistance against ciprofloxacin, tetracycline, and nitrofurantoin (Johnston and Jaykus, 2004). A survey of the prevalence of Enterococcus spp. in Brazilian food products conducted by Gomes et al. (2008) showed that the distribution of Ent. faecium and Ent. faecalis in cheeses, milk, meat, and vegetable food products, alongside with the assessment of associated potential virulence factors, reported that dominantly, although not all, Ent. faecalis harbor higher potential virulence genes compared to Ent. faecium isolates.
The applications of Enterococcus spp. as probiotics have long been debated due to safety concerns. Despite this, some of the Enterococcus spp. play major roles in the food production industry, include contribution to cheese ripening, and also fermentation of raw meat and vegetable-based foods such as kimchi and sauerkraut, and even fermented milk like kefir (Centeno et al., 1996;Giraffa, 2002). Also, some of the known probiotic applications of Enterococcus spp. include both human and animal consumption (Franz et al., 2011; Holzapfel et al., 2018).
Enterococci probiotics designed for human consumption were considered advantageous due to their ability to produce multiple beneficial metabolites that also contributes to the stability of microorganisms in the gastrointestinal tract, considering that they are natural gut commensals. Ent. faecium SF68, one of the safe enterococcal strains that has long been commercialized and applied as a probiotic, has specifically been used as an alternative to antibiotics for the treatment for diarrhea caused by food-borne pathogens including Shigella spp., Escherichia coli, Enterobacter spp., Campylobacter spp., and some serovars of Salmonella enterica (Bellomo et al., 1980; Fugaban et al., 2021b). Additionally, the administration of probiotic preparations, composed of enterococci has been done to patients suffering from antibiotic-associated diarrhea (AAD) and irritable bowel syndrome patients (Wunderlich et al., 1989; Fan et al., 2006). The bile hydrolase enzyme produced by Enterococcus spp. allows them to deconjugate bile salts. This feature has been exploited for application in lowering blood cholesterol levels, as exemplified in commercially available probiotic fermented milk called gaio, fermented with a human-derived probiotic strain of Ent. faecium. The product was prepared with two other Streptococcus thermophilus strains and reported to have cholesterol level lowering properties (Agerholm-Larsen et al., 2000). Additionally, applications of Enterococcus spp. as probiotics were not only limited to humans but they are also well employed in the swine industry (Taras et al., 2006; Pollmann et al., 2005), the poultry industry (Vahjen and Manner, 2003; Mountzouris et al., 2007), in cattle farming (Ghorbani et al., 2002; Emmanuel et al., 2007; Nocek et al., 2002) and even for domestic pets (Vahjen and Manner, 2003). Although, various evidence for the application of Enterococcus strains is promising and encouraging for continuous innovation for the applications of beneficial strains belonging to this group, the debate on their safety as probiotics still continues.
This study aimed to evaluate the safety and beneficial potential of previously characterized bacteriocinogenic Ent. faecium strains ST651ea, ST7119ea, and ST7319ea (Fugaban et al., 2021a), found to be active against food-borne pathogens, including Listeria monocytogenes, along with emerging-pathogen vancomycin-resistant Enterococcus (VRE) strains of clinical origin.
2. Materials and methods
2.1. Bacterial cultures
Ent. faecium strains ST651ea, ST7119ea, and ST7319ea, were previously isolated from locally outsourced doenjang (Korean traditional fermented soybean paste) and identified (Fugaban et al., 2021a). These strains were investigated together with other organisms in this study and were stored in presence of glycerol (10% v/v) at -80 °C. The strains were revived under aerobic conditions in de Man, Rogosa and Sharpe (MRS) broth (Difco, Franklin Lakes, NJ, USA), BHI (Difco) and grown at 37 °C for 24–48 h until robust growth was observed.
2.2. Phenotypic evaluation of the safety of Enterococcus faecium strains
2.2.1. Gelatinase enzyme production
The production of the gelatinase enzyme was determined from 18 h old cultures grown in MRS broth at 37 °C. Tubes containing 10 mL BHI broth supplemented with 4% gelatin were inoculated with 100 µL (estimated 105 cells/mL) of each strain, and incubated for 24 h at 37 °C, followed by cooling at 4 °C for 30 min. Positive results, indicating production of gelatinase enzyme were associated with retention of the liquid phase after refrigeration. A previously characterized Bacillus amyloliquefaciens ST109 (Fugaban et al., 2021b) was used as positive control, while Lactobacillus plantarum ATCC14917 and an untreated medium were used as negative controls (dos Santos et al., 2020; Omar et al., 2004). All tests were performed in three independent experiments.
2.2.2. Hemolysin production
Hemolytic activity for the studied strains was evaluated on Columbia Blood Agar (Oxoid LTD, Basingstoke, UK) with 5% defibrinized horse blood according to dos Santos et al. (2020). Strains (18h-old cultures grown in MRS at 37 °C) were spot-plated (10 µL) on the agar surface. Positive hemolytic activity is indicated by clear yellow zones around the bacterial growth. Reference strains used were Streptococcus pneumoniae ATCC49619, B. cereus ATCC27348, and Lb. plantarum ATCC42917 as producers of α-, β-, γ- hemolysins, respectively. All experiments were performed in triplicate.
2.2.3. Biogenic amine production
The detection of amino acid decarboxylase enzyme production was performed as suggested by Bover-Cid and Holzapfel (1999). Previously grown strains were subjected to an induction assay (subsequent sub-culture of strains for 5 days) using MRS broth supplemented with respective amino acid precursors comprising tyrosine (Samchun Chemicals, Daejeon, Republic of Korea), histidine monohydrochloride (Daejung Chemicals, Gyeonggi, Republic of Korea), lysine monohydrochloride (Sigma-Aldrich, St. Louis, MO, USA), and ornithine monohydrochloride (Sigma Aldrich) at concentrations 1% (w/v). The final batch was streaked on MRS agar (1.5%, w/v) supplemented with the corresponding amino acid precursor. Inoculated plates were incubated for at least three days at 37 °C. Biogenic amine production is indicated by purplish coloration around the colonies. Reference strains used for the biogenic amine production were E. coli ATCC25922 (positive control) and Lb. plantarum ATCC14917 (negative control). All test organisms were tested in at least two independent experiments.
2.2.4. Antimicrobial susceptibility profiling
The antimicrobial susceptibility testing (AST) was performed according to the standards recommended by the Clinical and Laboratory Standards Institute (CLSI) (2012) on Performance Standards for Antimicrobial Susceptibility Testing for Enterococcus spp. The assay was performed by microbroth dilution using the antibiotics ampicillin, chloramphenicol, ciprofloxacin, clindamycin, erythromycin, gentamycin, kanamycin, streptomycin, tetracycline (all from Sigma-Aldrich), and vancomycin (CheilJedang Pharma Co., Republic of Korea) on cation-adjusted Mueller-Hinton broth supplemented with MRS (5.0 g/L). The assay was performed in a 96-well microplate (SPL Life sciences, Pocheon-si, Gyeonggi-do, Republic of Korea) and comprised 10 antibiotic dilutions in two-fold and controls (growth and sterility controls). The inocula were adjusted to 0.5 McFarland units (approximately 107 CFU/mL) and distributed accordingly to obtain a final concentration of 105 CFU/mL. The plates were incubated at 35 ± 1 °C for 18 h. The lowest concentration with complete bacterial inhibition was recorded as the MIC and analyzed according to the standards set for Enterococcus spp. (Rychen et al., 2017; Wiegand et al., 2008).
2.3. Biomolecular safety tests
2.3.1. Screening for the presence of potential virulence genes
Bacterial cells grown overnight in MRS at 37 °C were used for the DNA isolation using ZR Fungal/Bacterial DNA Kit (Zymo Research, Irvine, CA, USA) according to the manufacturer's recommendations. The concentration and purity of the DNA were determined using SPECTROstar Nano nanodrop (BMG LABTECH, Rotenberg, Germany).
Bacterial DNA from the studied strains was screened by PCR assay for the presence of potential virulence genes including efaA (endocarditis specific proteins), cyt (cytolysin), IS16 (Enterococcus pathogenicity island), esp (enterococcal surface protein), asa1 (aggregation substance protein), and hyl (hyaluronidase) as suggested by EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) (Rychen et al., 2017). Oligonucleotide sequences of primers are shown in Table 1. PCR reactions were performed in a Veriti 96 well Thermal Cycler (Applied Biosystems, Thermo Scientific, Waltham, MA, USA), and amplicons were separated on 1.5% (w/v) agarose gel (1x TAE buffer, 100 V, 1 h) and stained with SYBR©Safe DNA gel stain (0.02 μL/mL) (Thermo Scientific) (GH-200 Genera Biosystems, Victoria, Australia; Elite 300 Plus Power Supply, Wealtec Bioscience Co., Ltd., Taiwan) and visualized using an Omega Lum™ G gel documenter (Aplegen, Inc., CA, USA).
Table 1.
Primers used in the molecular-based screening of safety and beneficial properties.
| Genes | Primer Oligonucleotide Sequence (5’ – 3’) | References |
|---|---|---|
| Vancomycin resistance-associated genes | ||
| vanA | F: GTA GGC TGC GAT ATT CAA AGC | |
| R: CGA TTC AAT TGC GTA GTC CAA | ||
| vanB | F: GTA GGC TGC GAT ATT CAA AGC | |
| R: GCC GAC AAT CAA ATC ATC CTC | ||
| vanC | F: ATC CAA GCT ATT GAC CCG CT | Fugaban et al. (2021) |
| R: TGT GGC AGG ATC GTT TTC AT | ||
| vanD | F: TGT GGG ATG CGA TAT TCA A | |
| R: TGC AGC CAA GTA TCC GGT AA | ||
| vanE | F: TGT GGT ATC GGA GCT GCA G | |
| R: GTC GAT TCT CGC TAA TCC | ||
| vanG | F: GAA GAT GGT ACT TTG CAG GGC A | |
| R: AGC CGC TTC TTG TAT CCG TTT T | ||
| Adhesion genes | ||
| EF1249 | F: GCG GTC GAC AAA CGA GGG ATT TAT TAT G | Todorov et al. (2010), Castilho et al. (2019), dos Santos et al. (2020) |
| R: CTG GCG GCC GCG TTT AAT ACA ATT AGG AAG CAG A | ||
| EF2380 | F: GCG GTC GAC GAC ATC TAT GAA AAC AAT | |
| R: TCC GCG CCG CCT TAA ACT TTC TCC TT | ||
| EF2662 | F: GGC GTC GAC CAC TTA AAC TGA TAG AGA GGA AT | |
| R: CGC GCC GCA ATT AAT TAT TAA CTA GTT TCC | ||
| EF-Tu | F: TTC TGG TCG TAT CGA TCG TG | |
| R: CCA CGT AAT AAC GCA CCA AC | ||
| mapA | F: TGG ATT CTG CTT GAG GTA AG | |
| R: GAC TAG TAA TAA CGC GAC CG | ||
| mub | F: GTA GTT ACT CAG TGA CGA TCA ATG | |
| R: TAA TTG TAA AGG TAT AAT CGG AGG | ||
| prgB | F: GCC GTC GAC TCG AGG AGA ATG ATA CAT GAA T | |
| R: CCT GCG GCC GCG TCC TTC TTT TCG TCT TCA A | ||
| Potential Virulence Genes | ||
| hyl | F: ACA GAA GAG CTG CAG GAA ATG | Vankerckhoven et al. (2004) |
| R: GAC TGA CGT CCA AGT TTC CAA | ||
| esp | F: AGA TTT CAT CTT TGA TTC TTG G | Vankerckhoven et al. (2004) |
| R: AAT TGA TTC TTT AGC ATC TGG | ||
| efaA | F: GCCAATTGGGACAGACCCTC | Martín-Platero et al. (2009) |
| R: CGC CTT CTG TTC CTT CTT TGG C | ||
| asa1 | F: GCA CGC TAT TAC GAA CTA TGA | Vankerckhoven et al. (2004) |
| R: TAA GAA AGA ACA TCA CCA CGA | ||
| cylA | F: ACT CGG GGA TTG ATA GGC | Vankerckhoven et al. (2004) |
| R: GCT GCT AAA GCT GCG CTT | ||
| IS16 | F: CAT GTT CCA CGA ACC AGA G | Werner et al. (2011) |
| R: TCA AAA AGT GGG CTT GGC | ||
| GABA-operon | ||
| gad | F:CCT CGA GAA GCC GAT CGC TTA GTT CG | Bajić et al. (2020) |
| R: TCA TAT TGA CCG GTA TAA GTG ATG CCC | ||
| Folate (Vitamin B9) | ||
| pabC | F: CGG ACA AGC ATA ATG AAT ACT CGG AAT | Meucci et al. (2018) |
| R: GGA TTG ATA ACC GCT TCT ATT GCC GA | ||
| pabB | F: CCT CAA TTC ATA CAA CCC TCT CAC A | |
| R: CAG ACA AAT CTT CAC TCA CGC CAT AA | ||
| folK-Q | F: CAC TAG TGT CTA TTG ACT CAA ATA TTT T | |
| R: CGT TTT TAT GGC TAT CAC GGG GCT | ||
| folP-E | F: GAG ATA GTC TTA ACG ACA TCA CGA TT | |
| R: GCA GTC TAT CAA TTA TTG GAA GCT TT |
2.3.2. Screening for the presence of vancomycin-resistance associated genes
Detection of vancomycin-resistance-related genes was performed by PCR assay using the genes vanA, vanB, vanC, vanD, vanE, and vanG (Fugaban et al., 2021b). The oligonucleotide primer sequences and PCR conditions are mentioned in Table 1. Separation, visualization, and analysis of the PCR products were performed as described before.
2.4. Assessment of the probiotic potential of bacteriocinogenic Enterococcus faecium strains
2.4.1. Enzyme production profiling
Enzyme production of the evaluated strains was profiled using APIZym (bioMérieux, Marcy l'Etoile, France), carried out according to the manufacturer's instructions. Bacterial cells were obtained from an 18 h-old culture grown in MRS agar at 37 °C and were inoculated on the provided strips, followed by incubation at 37 °C for 4 h. Enzyme production was monitored through color changes and interpretation of results was carried out based on the guidelines provided by the manufacturer.
2.4.2. D/L-lactic acid production
Measurement of the D- and L-lactic acid produced by the strains Ent. faecium ST651ea, ST7119ea, and ST7319ea was performed using Megazyme D-/L-lactic acid assay kit (Megazyme, Bray, Wicklow, Ireland). The cell-free supernatant of each strain was obtained by centrifugation (12000 x g, 5 min) from 18 h-old culture grown in MRS at 37 °C for 24 h. The assay was performed according to the manufacturer's instructions. Analysis of results was calculated using the corresponding spreadsheet provided by the manufacturer available on their customer assistance page (http://www.megazyme.com). The produced lactic acid by each strain was interpreted in proportion showing the amounts of both D- and L-lactic acid.
2.4.3. Screening for adhesion genes
Bacterial cells in the stationary phase were harvested by centrifugation (8000 x g, 10 min, 4 °C) and the genomic DNA of the evaluated strains was extracted using QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's suggestion with few modifications. DNA concentration and purity were measured by SPECTROstar Nano nanodrop (BMG LABTECH, Otterberg, Germany). Adhesion genes, mapA (mucus adhesion associated gene), mub (mucus adhesion associated gene), EF-Tu (adhesion-like factor), prgB (aggregation substance gene), EF2662 (choline-binding protein gene), EF1249 (fibronectin-binding genes), and EF2380 (membrane-associated zinc metalloprotease gene) were used in the PCR-based adhesion gene detection assay (Todorov et al., 2010; Castilho et al., 2019; dos Santos et al., 2020). Sequences of the oligonucleotide primers are shown in Table 1. PCR products were separated through gel electrophoresis on agarose gel (1, 2%, w/v), and stained and visualized as described before.
2.4.4. Bacterial cell-surface hydrophobicity
Bacterial adherence was evaluated according to the assay proposed by Rosenberg (1984) using hydrocarbons (n-hexadecane, Sigma-Aldrich) with modification as follows. Cells of the evaluated strains were collected (4000 × g, 20 min, 4 °C) and washed twice using 100 mM potassium phosphate buffer (pH 6.5) and re-suspending using the same buffer to achieve an optical density reading (OD560nm ≈ 1.0, refered as A0). This was followed by the addition of n-hexadecane to the cell suspensions in the ratio 1:5 before emulsification (vortex mixing) for 2 min. Set-ups were incubated for 1 h at 37 °C. After separation of the phases, n-hexadecane on the surface of the cell suspension was gradually removed before measuring the optical density for A1 (after 1 h incubation) of the hydrophilic phase at OD560nm, and % hydrophobicity was quantified using the equation:
The cell surface hydrophobicity quantification assay was performed in at least two independent experiments wherein each set-up was measured in triplicate.
2.4.5. Aggregation properties
Auto-aggregation: The ability of the evaluated strains to auto-aggregate was evaluated according to dos Santos et al. (2020). Each strain was grown in MRS broth for 18 h at 37 °C. The cells were harvested through centrifugation (4000 × g, 20 min, 4 °C) and washed twice and re-suspended in sterile phosphate buffer saline (Lonza, Basel, Switzerland). The suspensions were then readjusted using the same buffer to obtain a final OD660nm with reading ≈ 0.3 using a UV-vis spectrophotometer (Optizen, Daejeon, Republic of Korea). Set-ups were incubated for one hour at 37 °C and centrifuged for 20 min at 300 × g before measuring the optical density (OD660nm) of the top phase. Calculations for the % auto-aggregation were carried out using the formula:
where ODo and OD1 refer to optical density readings before and after incubation, respectively. All experiments were performed in triplicates in at least two independent assays.
Co-aggregation: The evaluation of the strains to co-aggregate with other microorganisms was evaluated using partner strains classified as either pathogenic or beneficial strains. The partner microorganisms L. monocytogenes ATCC15313, Listeria innocua ATCC33090, Ent. faecium VRE19, were cultured in BHI broth and Lactobacillus rhamnosus LGG, Lactobacillus sakei HEM802, Ent. faecium HEM1108, and Lactobacillus fermentum HEM792 in MRS broth. All cultures were grown for 24 h at 37 °C before cell harvesting as previously described. Partner organisms adjusted to OD660nm ≈ 0.3 were individually mixed with strains Ent. faecium ST651ea, Ent. faecium ST7119ea, and Ent. faecium 7319ea (OD660nm ≈ 0.3) in a 1:1 ratio. The OD0 (at OD660nm) was recorded for 60 s after mixing and OD1 (at OD660nm) was measured after one-hour incubation at 37 °C. Calculations for % co-aggregation were carried out as follows:
where OD0 and OD1 are turbidity readings right after mixing of partner strains and after one-hour incubation, respectively (dos Santos et al., 2020). All experiments were performed in at least two independent repetitions comprising assays carried out in triplicates.
2.5. Molecular identification of potential beneficial metabolites
2.5.1. γ-Aminobutyric acid (GABA)
Identification of some of the functional properties of LAB includes detection of putative beneficial metabolites such as GABA. This has been performed by molecular-based detection of the GABA production associated genes. Oligonucleotide sequences of the primers as presented in Table 1, were used to carry out this assay (Bajić et al., 2020). PCR products were determined and analyzed by gel electrophoresis on agarose gel (2%, w/v) and stained and visualized as described before.
2.5.2. Vitamin B12 (folate)
PCR-based detection assay for the genes responsible for bacterial biosynthesis of folate was carried out according to the suggestions of Meucci et al. (2018). Sequences of the primers are shown in Table 1. Gel electrophoresis (agarose gel 1, 2%) visualization and analysis have been carried out as previously described.
2.6. Gastrointestinal tract simulation assay
2.6.1. Gastrointestinal survival assay Enterococcus faecium strains ST651ea, ST7119ea, and ST7319ea
The survivability of the evaluated strains under mimicked gastrointestinal conditions has been evaluated as suggested by dos Santos et al. (2020). For the GIT simulation assay cultures in the mid-stationary phase were inoculated in 100 mL of MRS broth and incubated at 37 °C for 18 h.
The initial viable bacterial counts (t0) were quantified (CFU/mL) by using one mL of the previously prepared bacterial culture and serial dilution in a sterile saline solution and plated on MRS supplemented with 1.5% (w/v) agar before incubation at 37 °C for 72 h under anaerobic conditions (anaerobic jars, Oxoid, Basingstoke, Hampshire, United Kingdom).
Simulation of the gastric fluid conditions was performed by preparing electrolyte solutions composed of sodium chloride (NaCl, 6.20 g/L), potassium chloride (KCl, 2.20 g/L), calcium chloride (CaCl2, 0.22 g/L), and sodium bicarbonate (NaHCO3, 1.20 g/L) adjusted to a final pH of 2.5 and supplemented with 0.3% of pepsin (Sigma-Aldrich). Six mL of previously prepared bacterial culture were obtained and added to 10 mL of simulated gastric juice solution and incubated for 1 h at 37 °C with continuous agitation (150 rpm) under anaerobic conditions. Samples were withdrawn, serially diluted, and quantified as previously described to obtain CFU/mL for t1.
Duodenal pass simulation was performed by taking 2 mL of the sample from the previous set-up and adding to 8 mL of artificial duodenum solution prepared by formulating an electrolyte solution comprising NaHCO3 (6.4 g/L), KCl (1.28 g/L), NaCl (1.28 g/L) adjusted to a final pH of 7.2 and supplemented with 0.5% bile salt (Oxgall, Difco) and 0.1% pancreatin (Sigma-Aldrich). The bacterial suspension was incubated in an anaerobic system for 3 h at 37 °C under continuous agitation (150 rpm) before quantification (CFU/mL, t2) as previously described. Calculations for the survival rate were determined as:
where N0 and N1 are viable cell counts of strains Ent. faecium ST651ea, Ent. faecium ST7119ea, and Ent. faecium ST7319ea before and after the simulation passes. All experiments were performed in at least three independent experiments comprising three replicates for each set-up.
2.6.2. GIT survival of test organisms in competition with the studied Enterococcus faecium strains
The GIT competition assay was performed using the same component assay as previously described. Test organisms L. monocytogenes ATCC15313, L. innocua ATCC33090, were grown in 100 mL BHI broth at 37 °C for 18 h. Antagonistic strains, Ent. faecium strains ST651ea, ST7119ea, and ST7319ea, on the other hand, were grown in MRS broth. Before performing the assays, bacterial cells from all the above strains were all adjusted to obtain OD600 = 0.5 to standardize the cell concentrations used in the experiments. Each test organism was measured against all bacteriocinogenic strains being evaluated for their safety. Serial dilutions of adjusted bacterial suspensions were plated using respective growth media for determining the initial viable cell population (CFU/mL). The obtained counts are designated as t0.
Following the same assay for the gastric pass and duodenum pass simulations, all antagonistic set-ups with the test organisms L. monocytogenes ATCC15313 and L. innocua ATCC33090 were plated in Listeria selective medium (RAPID’L.mono Medium, BioRad, Hercules, CA, USA). All plates were incubated for 72 h at 37 °C before viable cell counting. Calculations for survival rate were carried out as previously described. All experiments were performed in at least three independent experiments comprising three replicates for each set-up.
2.7. Statistical and data analysis
All quantification was carried out in at least two independent experiments with at least three replicates. The statistical analysis and data visualization were carried out in GraphPad Prism 9.
3. Results and discussions
3.1. Phenotypic evaluation of the safety of previously characterized bacteriocinogenic Enterococcus faecium strains
3.1.1. Production of gelatinase, hemolysin, and biogenic amines
Beneficial LAB have been empirically employed for centuries in the food chain until their existence and functions, including their advantages and disadvantages in the food systems, have gradually been scientifically elucidated. Although several LAB strains are generally recognized as safe (GRAS), some species in this group are considered to be at the crossroads between safety and potential hazards, particularly members of the genus Enterococcus (Franz et al., 1999, 2003). The debate on the application of Enterococcus spp. in the food chain is on-going for a long time. Even though these bacteria are naturally residing in the gut of humans and animals, relatively higher health risks are associated with this group in comparison with the other members of the LAB. Various putative virulence factors and the production of enzymes and potentially harmful metabolites (e.g., gelatinase, hemolysins, and biogenic amines) should also be considered. Gelatinase, a protease that hydrolyses bioactive peptides, can potentially degrade collagen, hemoglobin, casein, and other bioactive peptides that play a significant role as a defense line for humans and animals during infection (Coque et al., 1995). Additionally, severe cases of endocarditis in murine models and dermal necrosis in rabbit skin were associated with the ability of enterococci to produce hemolysins (Coque et al., 1995; Franz et al., 1999). In this study, the evaluation of Ent. faecium strains ST651ea, ST7119ea, and ST7319ea, all of which were isolated from locally produced Korean fermented soybean paste, were evaluated for hemolytic and gelatinase activity with no strain showing positive results as indicated in Table 2, including other virulence factors that may aid the enterococci to evade, invade, and embed in membrane surfaces to successfully cause pathological changes and infections (Coque et al., 1995; Jett et al., 1994). Thus, it is an imperative to evaluate Enterococcus spp. strains before any intended application particularly as probiotic strain or starter culture for food and feed industries.
Table 2.
Phenotypic assessment of gelatinase and haemolysin productions of strains E. faecium strains ST651ea, ST7119ea, and ST7319ea in vitro.
| Strains Evaluated | Results |
|---|---|
| Gelatinase Activity | |
| E. faecium ST651ea | - |
| E. faecium ST7119ea | - |
| E. faecium ST7319ea | - |
| B. amyloliquefaciens ST109 (positive control) | + |
| Lb. plantarum ATCC14917 (negative control) | - |
| Haemolytic Activity | |
| Ent. faecium ST651ea | - |
| Ent. faecium ST7119ea | - |
| Ent. faecium ST7319ea | - |
| S. pneumoniae ATCC49619 (α-haemolysis control) | + |
| B. cereus ATCC27348 (β-haemolysis control) | + |
| Lb. plantarum ATCC14917 (γ-haemolysis control) | + |
Production of biogenic amines was assessed for all the evaluated strains, showing that no strain produced nitrogenous by-products when supplemented with histidine, lysine, ornithine, or tyrosine (as shown in Table 3). Although biogenic amine (BA) production is not directly associated with the virulence of Enterococcus spp., their accumulation from exogenous (food) sources may have toxic effects (BA intoxication i.e., scombroid poisoning and tyramine intoxication), when consumed in amounts beyond the safety threshold. According to Smit et al. (2005) and Fernández et al. (2007), some LAB strains are major producers of biogenic amine in fermented food systems. Formerly considered to be strain-specific, Ladero et al. (2012) suggested that this characteristic may be generalized for some species; this has been supported by continuous advancements in the techniques and approaches employed. Among the LAB, Enterococcus spp. are known to represent some of the strongest producers of BAs. This group has been particularly associated with the decarboxylation of arginine to putrescine while most strains typically produce tyrosine decarboxylase (of Ent. faecalis, Ent. faecium, and Ent. mundii) with the formation of tyramine (Bargossi et al., 2015; Fernández et al., 2007; Kučerová et al., 2010; Ladero et al., 2012). Although the BA production of enterococci plays a significant role in the continuous debate on their safety and their involvement in the food chain, the presence and use of Enterococcus spp. in fermented food have long been recognized, and their beneficial properties were considered to outweigh this negative aspect. Their specific role is acknowledged in the development of crucial metabolites contributing to desirable sensory characteristics of particular foods such as in cheese ripening and fermentation of meat products and sausages (Smit et al., 2005).
Table 3.
Phenotypic demonstration of biogenic amine decarboxylation of E. faecium strains evaluated.
| Strain evaluated | Biogenic amine amino acid precursor used | |||
|---|---|---|---|---|
| Tyrosine | Histidine | Lysine | Ornithine | |
| E. faecium ST651ea | - | - | - | - |
| E. faecium ST7119ea | - | - | - | - |
| E. faecium ST7319ea | - | - | - | - |
| E. coli ATCC25922 | + | + | + | + |
| Lb. plantarum ATCC14917 | - | - | - | - |
3.1.2. Antimicrobial susceptibility profiling
Antibiotic susceptibility profiles of Ent. faecium strains ST651ea, ST7119ea, and ST7319ea (Table 2) were determined against ampicillin, vancomycin, gentamicin, kanamycin, streptomycin, erythromycin, clindamycin, tetracycline, chloramphenicol, and ciprofloxacin through micro-broth dilution and were compared based on the breakpoints from the European Food Safety Authority (EFSA)(2011) as stated in the guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. Results showed (Table 3) that the resistance of all strains evaluated were below the stated breakpoints except erythromycin. It has been noted that most members of enterococci are known to inherently possess resistance to antibiotics especially to ciprofloxacin, penicillin, and erythromycin with occurrence rates of 56.3%, 45.8%, and 27.1%, accordingly (Franz et al., 2003). Thus, it is imperative to identify the antibiogram profile of any candidate strain intended for human and animal use; this is especially important for distinctive antibiotics that may potentially control enterocccal proliferation in aberrant niches. Ent. faecium associated infections were previously described to be only associated with <10% of hospital-associated infections worldwide, but the alarming increase in the associated vancomycin-resistant enterococci infections has now reached 30% of nosocomial-acquired infections (Centers of Disease Control and Prevention, 2019). The World Health Organization (WHO) has indeed declared that the search for alternative treatments for VRE-associated infections is now classified as high priority along with AMR pathogens such as methicillin-resistant Staphylococcus aureus (MRSA), clarithromycin-resistant Helicobacter pylori (CRHP), and fluoroquinolone-resistant Salmonella (WHO, 2018).
3.2. Biomolecular characterization of safety
3.2.1. Screening for the presence of potential virulence genes
The development of rapid techniques to screen the safety of strains has come a long way. The use of various molecular techniques (genomics) has allowed presumptive assessment of the safety of various microorganisms circulating in the food chain. In this study, the presence of various potential virulence factors involved in the attachment, evasion, and translocation was determined by PCR-based assay including genes associated with endocarditis specific proteins (efaA), cytolysin (cyt), Enterococcus pathogenicity island (IS16), enterococcal surface protein (esp), aggregation substance protein (asa1), and hyaluronidase (hyl). The detection technique showed that only Ent. faecium ST7319ea harbors the genes hyl, esp, and IS16 (Table 4). The screening for these genes was carried out according to the guidelines suggested by the EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) (Rychen et al., 2017) for the safety assessment of Ent. faecium in animal nutrition, whereas it was stated that screening for specific virulence genes IS16, esp, and hyl should be conducted when all strains are found to be susceptible to the key antibiotics suggested in the guidelines.
Table 4.
Antimicrobial susceptibility profiles of the Enterococcus faecium strains.
| Antibiotics | Experimental Minimum Inhibitory Concentration (µg/mL) | EFSA Cutoff | ||
|---|---|---|---|---|
| Ent. faecium ST651ea | Ent. faecium ST7119ea | Ent. faecium ST7319ea | ||
| Ampicillin | 1 | 1 | 1 | 2 |
| Vancomycin | 1 | 2 | 1 | 4 |
| Gentamicin | 32 | 8 | 16 | 32 |
| Kanamycin | 64 | 128 | 256 | 1024 |
| Streptomycin | 64 | 64 | 64 | 128 |
| Erythromycin | 64 | 64 | 32 | 4 |
| Clindamycin | 0.5 | 0.5 | 0.5 | 4 |
| Tetracycline | 0.5 | 0.5 | 0.5 | 4 |
| Chloramphenicol | 16 | 16 | 16 | 16 |
| Ciprofloxacin | ≤0.25 | ≤0.25 | ≤0.25 | n.r. |
The gene IS16 (insertion sequence involved in ampicillin resistance), characterized by Leavis et al. (2007) as a novel identifier for the emerging multi-drug resistant strains of Ent. faecium, has been a key discriminator for the diversification of Ent. faecium into the two-clade subdivision suggested by Palmer et al. (2012), and which was established primarily based on the whole genome sequences of the strains evaluated. In this classification, a subpopulation termed as Clade A typically encompasses clinically isolated ampicillin-resistant Ent. faecium, while the other subpopulation classified as Clade B predominantly includes Ent. faecium of both human and animal origin that are susceptible to ampicillin (EFSA, 2011; Leavis et al., 2007; Palmer et al., 2012; Novais et al., 2016).
Another virulence factor associated with one of the largest pathogenicity islands ( in Ent. faecium strains associated with infections (Leavis et al., 2004), the esp gene, has been screened for in the evaluated strains; this feature was found to be linked in the ability of members of enterococci to form a biofilm. According to Tendolkar et al. (2004), the enterococcal surface protein enhances biofilm formation by aiding in the initial attachment of planktonic cells on surfaces. In the same study, comparative biofilm formation characterization of both esp- and non-esp-carrying strains were carried out and showed association of biofilm formation and the presence of esp genes could be demonstrated.
According to Rice et al. (2003), the detection of open reading frames of hyaluronidase coding genes (hyl) are predominantly characterized in Ent. faecium strains isolated from nosocomial settings. Hyaluronidases of Gram-positive bacterial species were found to facilitates initial infection or colonization of pathogenic microorganisms such as Cl. perfringens, Staph. uberis, Staph. aureus, Str. pyogenes, and Str. pneumoniae (Hynes and Walton, 2000), in which it aids in the breakdown of hyaluronate commonly found in animal skin cells and tissues. The detection of this key potential virulence factor provides an additional level of protection in screening strains that can potentially be introduced into the food chain. Although detection of these genes only signifies possible expression, further characterization of the said virulence genes should be implemented.
3.2.2. Screening for the presence of vancomycin-resistance associated genes
Vancomycin, one of the last resort drugs usually administered against various systemic infections, is a bactericidal antibiotic that acts by interfering in the cell wall synthesis of target microorganisms. The ability of these microorganisms to adapt to the presence of vancomycin has been attributed to modifications in various clusters of genes which are central to the level(s) of resistance of these strains (Faron et al., 2016; Manson et al., 2003). In this study, the PCR-based assay for the detection of vancomycin-resistance (VR) gene clusters in Ent. faecium strains ST651ea, ST7119ea, and ST7319ea was carried out for van A, B, C, D, E, and G. The screening showed that all vancomycin resistance-associated gene clusters were all undetected in the strains evaluated as indicated in Table 4. The structural genes used were known to be individually or in clustered, responsible for glycopeptide ligase-aided resistance to vancomycin and other related (glycopeptide) antibiotics. Distinct antibiotic resistance characteristics are associated with each cluster; the vanA operon mediated resistance has been described predominantly for Ent. faecium and Ent. faecalis with modifications in the terminal peptides of N-acetylmuramic acid (NAM) subunits of the cell wall causing a significant decrease in the affinity for vancomycin; such strains were also found to be phenotypically resistant to teicoplanin– another glycopeptide antibiotic (Faron et al., 2016). The vanB resistance, on the other hand, causes resistance in variable concentrations of vancomycin (reaching up to ≥250 µg/mL), although this type of resistance is less prevalent than the latter (Coombs et al., 2014; Faron et al., 2016; Walsh et al., 1996). The vanC-operon mediated resistance is typically known for intrinsic resistance to vancomycin at low levels (2 to 32 µg/mL) relative to the other VR genotypes and is not mobile (chromosomally associated) as compared to the previous two gene clusters (plasmid-associated) (Courvalin, 2006; Reynolds and Courvalin, 2005). Thus, the vanC-type of VR resistance is also chromosomally located and should typically be characterized as susceptible but this pathway is rendered malfunctional due to IS19 associated insertion in a specific gene in the operon thereby resulting in resistance (Courvalin, 2006; Depardieu et al., 2004). The vanE-type, on the other hand, has the same genotypic organization and location as vanC but differs in the modifications of the target and is typically resistant at levels between 8 and 32 µg/mL of vancomycin (Abadía Patiño et al., 2002; Courvalin, 2006). As for the latter three gene clusters, vanG has low resistance to vancomycin (16 µg/mL), but is susceptible to teicoplanin (Courvalin, 2006; McKessar et al., 2000; Du et al. 2019). Albeit that the studied strains have been found susceptible to all the screened antibiotics, especially vancomycin, it is still essential to identify the presence of these gene clusters in these strains to assess possible risks for their application as probiotics for both animal and human consumption.
3.3. Assessment of the probiotic/beneficial potential of bacteriocinogenic Enterococcus faecium strains
3.3.1. Enzyme production profiling
Enzyme production has been one of the most exploited applications of various beneficial strains in industry. In addition, physiological and functional advantages and properties of the strains are also aided by their capacity to produce enzymes. In this study, the strains were profiled for their ability to produce different enzymes using the APIZym (BioMerieux) assay. Collectively, these Ent. faecium strains were shown to produce the following enzymes: esterase (C4), esterase lipase (C8), acid phosphatase, arylamidases (leucine, valine, cysteine), α-chymotrypsin, naphthol-AS-BI-phosphohydrolase, β-galactosidase,) and N-acetyl-B-glycosaminidase (Table 6). These enzymes play various key roles in different industrial applications. According to Ramakrishnan et al. (2012), the production of esterase plays a significant role in the development of flavor, consistency and texture of fermented food products. Additionally, characterization of an intracellular esterase from Ent. faecium ACA-CDC 237, isolated from Greek feta cheese, was found to play a synergistic role with lipolytic enzymes for maturation during cheese making (Tsakalidou et al., 1994).
Table 5.
Molecular-based assay for the detection of adhesion and various virulence genes in strains Ent. faecium ST651ea, ST711ea, and ST7319ea.
| Genes Evaluated | Strains Evaluated | ||
|---|---|---|---|
| Ent. faecium ST651ea | Ent. faecium ST7119ea | Ent. faecium ST7319ea | |
| Vancomycin-resistance genes | |||
| vanA | - | - | - |
| vanB | - | - | - |
| vanC | - | - | - |
| vanD | - | - | - |
| vanE | - | - | - |
| vanG | - | - | - |
| Adhesion genes | |||
| EF1249 | - | - | - |
| EF2380 | - | - | - |
| EF2662 | - | - | - |
| EF-Tu | - | - | - |
| mapA | - | - | - |
| mub | - | - | - |
| prgB | - | - | - |
| Other potential virulence factors | |||
| hyl | - | - | + |
| esp | - | - | + |
| efaA | - | - | - |
| asa1 | - | - | - |
| cyt | - | - | - |
| IS16 | - | - | + |
Table 6.
APIZym profiles of the studied Enterococcus faecium strains.
| Enzyme assayed for | Ent. faecium ST651ea | Ent. faecium ST7119ea | Ent. faecium ST7319ea |
|---|---|---|---|
| Control | 0* | 0 | 0 |
| Alkaline phosphatase | 0 | 0 | 0 |
| Esterase (C 4) | 0 | 3 | 2 |
| Esterase Lipase (C 8) | 1 | 3 | 2 |
| Lipase (C 14) | 2 | 2 | 1 |
| Leucine arylamidase | 5 | 5 | 4 |
| Valine arylamidase | 5 | 4 | 3 |
| Cysteine arylamidase | 2 | 4 | 3 |
| Trypsin | 0 | 0 | 0 |
| α-chymotrypsin | 2 | 0 | 0 |
| Acid phosphatase | 3 | 4 | 3 |
| Naphthol-AS-BI-phosphohydrolase | 5 | 5 | 5 |
| α-galactosidase | 1 | 0 | 0 |
| β-galactosidase | 1 | 5 | 5 |
| ß-glucuronidase | 0 | 0 | 0 |
| α-glucosidase | 0 | 0 | 0 |
| β-glucosidase | 0 | 1 | 0 |
| N-acetyl-ß-glucosaminidase | 2 | 2 | 2 |
| α-mannosidase | 0 | 0 | 0 |
| α-fucosidase | 0 | 0 | 0 |
0 = no acitivity; 1, 2 = weak activity; 3–5 = strong enzymatic activity.
It has been noted that enterococci are dominantly present as compared to the other LAB in artisanal cheeses (51% to 49%), wherein it was observed that the dominant species were Ent. faecium. All the strains produced acid phosphatases, an enzyme that is optimally active at pH 5.0, and usually associated with the hydrolysis of most mono-phosphorylated substrates and phytate, commonly associated with the raw materials used in the fermentation of various products of plant and animal origin (Palacios et al., 2005). Additionally, from a physiological vantage point, the presence of acid phosphatases aids in the adjustment of these microorganisms to stress by sequestering toxic compounds that usually accumulate in an acidic environment during successful fermentation processes. Aside from this, it has been observed that the strains can produce high amounts of various peptidases (leucine, valine, and cysteine arylamidase) that can be advantageous in cheese ripening industries coupled with their low production of proteases as exhibited by the low production of α-chymotrypsin, a ubiquitously available proteolytic enzyme. In their study on the assessment of technological and safety assessment of various beneficial enterococci isolates, Jaouani et al. (2015) suggested this as a desirable trait for good adjunct/starter cultures; this will serve to support cheese ripening and enhance cheese flavor development, also by reduction of bitterness texture improvement the of the product. Furthermore, the presence of these enzymes can also be attributed to the high-protein soybean products from which the strains have been isolated.
Based on positive naphthol-AS-BI-phosphate reactions, all the evaluated strains were shown to be producers of hydrolases. This feature was described by Colombo et al. (2018) to be inherent to most LAB; this enzyme supports the antioxidant and anti-inflammatory effects of beneficial strains employed as probiotics in various food products. It was also notably observed that Ent. faecium strains ST7119ea and ST7319ea were strong producers of β-galactosidase, the enzyme responsible for the cleavage of lactose to its respective carbohydrate subunits (Saqib et al., 2017). This enzyme has been exploited in the production of commercially available lactose-free fermented milk products.
No evidence of any other enzymes in the evaluation APIZym panel (as shown in Table 6) could be detected for the Ent. faecium strains ST651ea, ST7119ea, and ST7319ea.
3.3.2. D/L-lactic acid production
Named after the primary metabolite of carbohydrate metabolism, the LAB produce either the L(+)-or D(-) isomer of lactic acid, or a combination of both isomers. Although L(+)-lactic acid has been known to be majorly produced during fermentation, traces of D(-)-lactic acid may still be produced. Some species such as Lactobacillus delbrueckii subsp. bulgaricus and all Leuconostoc species, typically produce D(-)-lactic acid. High intake of this isomer, especially by infants, has been associated with acidosis; this may play a role in the etiology of some chronic diseases (Vitetta et al., 2017). Thus, the evaluation of the ratio between these two isomers is one of the criteria used for the evaluation of a good probiotic candidate. In this study, the amounts D(-)- and L(+)-lactic acids produced by the strains were: 0.769 g/L and 14.609 g/L for Ent. faecium ST651ea, 0.641 g/L and 10.231 g/L for Ent. faecium ST7119ea, 1.025 g/L and 16.677 g/L for Ent. faecium ST7319ea. These data are in agreement with the values and observations reported by Bhagwat and Annapure (2019), which showed mean total lactic acid produced by various Enterococcus spp. strains isolated from humans ranges between 5 and 12 g/L, while Yoshimune et al., (2017), on the other hand, demonstrated that Enterococcus spp. majorly produces L(+)-lactic acid in a fermentation system with trace amounts of D(-)-lactic.
3.3.3. Bacterial cell-surface hydrophobicity
Good probiotic candidates are not only screened for their ability to survive in the gut but also for their ability to attach on mucosal surfaces. This physiological aspect was used as a preliminary indicator for their ability to colonize and persist in the gut (Ouwehand et al., 1999; Rosenberg, 1984, 2006). In this study, the cell surface adherence was measured using the BATH assay, whereby hydrophobicity is measured as indication of potential adherence ability. Low levels with values ranging between 14 and 16% have been measured for these strains. Similar observations were also reported by dos Santos et al. (2015) using the same assay for bacteriocinogenic Ent. faecium strains EM485 and EM925 isolated from Brazilian cheeses. Although these results may suggest a relatively low adherence potential, this property should be further evaluated in an ex vivo model for further characterization of the strains.
3.3.4. Aggregation properties
Auto-aggregation, also referred to as bacterial flocculation or auto-agglutination, is defined as the formation of multicellular clumps of the same type of bacterial cell. This phenomenon has been linked to various bacterial cell functionalities including their ability to adapt to stressful environments and evade host immune responses during infections. Additionally, auto-aggregation has been linked to the initial step of biofilm formation (Angmo et al., 2016; Collado et al., 2008; Trunk et al., 2018). While these characteristics are associated with pathogenic microorganisms, aggregation has also been considered as an advantage to beneficial strains, including probiotics, considering that it predicts possible adhesion properties in vitro to the mucosal surfaces, thereby aiding in successful niche colonization (Collado et al., 2008; García-Cayuela et al., 2014). On the other hand, co-aggregation is defined as the ability of genetically distinct bacterial strains to flocculate or clump together. This phenomenon has been associated with the formation of various multispecies biofilms (Rickard et al., 2003), thus making it an imperative property to be evaluated particularly for strains intended for the food chain.
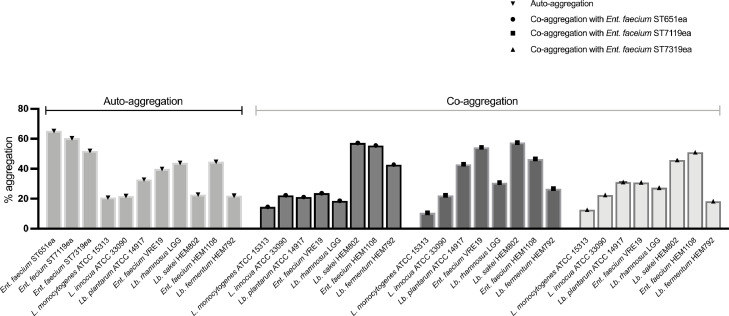
In this study, auto-aggregation and co-aggregation of the three strains of Ent. faecium, compared with previously characterized beneficial strains and pathogenic reference strains, were measured in vitro (Fig. 1). High levels of mean auto-aggregation (>50%) were exhibited by Ent. faecium ST651ea, ST7119ea, and ST7319ea; these were relatively higher compared to the other microorganisms included in the panel including previously characterized probiotic strains and pathogenic strains (Fig. 1). Furthermore, co-aggregation value wear observed to be higher for the evaluated strains partnered with the strains Lb. sakei HEM802, Ent. faecium HEM1108, and Lb. fermentum HEM792, all of which have previously been characterized as beneficial strains.
Fig. 1.
Rates of aggregation and co-aggregation of Ent. faecium ST651ea, Ent. faecium ST7119ea, and Ent. faecium ST7319ea with their corresponding partner microorganisms.
Although the generalization and correlation of aggregation and cell surface attachment must still be validated further, we can summarize that the evaluated strains, Ent. faecium ST651ea, ST7119ea, and ST7319ea, have strong aggregation capabilities and that correlation with auto-aggregation can only be assumed on strain level and variations can be observed within taxonomic clusters (dos Santos et al., 2015; Zommiti et al., 2018). Based on the co-aggregation rate of the strains being studied, low rates (10–15%) for all the strains paired with L. innocua and L. monocytogenes strains were noted, while high aggregation was observed between Ent. faecium ST651ea, ST7119ea, and ST7319ea and Ent. faecium VRE19 with rates ranging between 24 and 54% for all strains. However, this high rate of aggregation with a pathogenic microorganism is considered detrimental especially if the pathogen is innately resistant to the bacteriocins produced by the strains being studied (Del Re et al., 2000). The cell-to-cell contact between the producer and target cell (susceptible to the bacteriocins of the producer cell) would increase exposure of the susceptible pathogenic strain to the antimicrobial peptides, thus, from this angle, it may be considered as an advantageous characteristic for the strains evaluated.
3.4. Molecular identification of potentially beneficial metabolites
Gamma-aminobutyric acid (GABA), a ubiquitous and naturally occurring inhibitory neurotransmitter in humans and other animals, aids in the physiological regulation of various body systems including the cardiovascular and the central nervous system. It is a four-carbon molecule that acts as the primary inhibitory neurotransmitter in the brain. GABA production has long been associated with LAB, some of which were used as starter cultures and are commonly found in fermented food products. Some studies have also banked on this property of LAB as an application for various immunological and physiological mediatory functions (Boonstra et al., 2015; Cui et al., 2020). The efficacy of GABA has been attributed to its ability to pass through the blood-brain barrier, and serving as the intermediate molecule between various beneficial strains that have been found to play a significant role in the gut-brain axis (Bajić et al., 2020). According to Cui et al. (2020), several well-characterized GABA producing strains have been isolated from traditional fermented foods such as kimchi, yogurt, fermented soybeans, cheeses, among others; wherein representative(s) from the genus Lactobacillus, Enterococcus, Leuconostoc, Pediococcus, and Weissella was/were GABA producer(s). In this study, the ability of the Ent. faecium strains ST651ea, ST7119ea, and ST7319ea, all isolated from artisanal produced fermented soybean paste, were screened for the presence of GABA-production associated genes. The PCR-based screening assay showed that all the strains were positive for the gene assayed, thereby showing the strains to be potential GABA producers. Their ability to express the gene and the physiological ability to produce this compound must be evaluated further.
Another functional property associated with LAB includes folate production. This has been investigated in strains of Str. thermophilus (Iyer et al., 2010; Meucci et al., 2018; Tarrah et al., 2018), Lb. plantarum, Bif. adolescentis, and Bif. pseudocatelinatum (Rossi et al., 2011). Although results of our screening assay indicated that the strains do not have the complete operon responsible for producing vitamin B12, screening for this beneficial property may reveal an additional benefit. Indeed, some Ent. faecium strains have been reported as novel sources of this metabolite (Li et al., 2017). This has also been highlighted by Meucci et al. (2018), also mentioning that the ability of LAB to synthesize this metabolite is highly strain dependent as demonstrated for various strains of Str. thermophilus, Lb. delbrueckii subsp. bulgaricus, and Lactococcus spp.
3.5. Gastrointestinal survival assay Enterococcus faecium strains ST651ea, ST7119ea, and ST7319ea
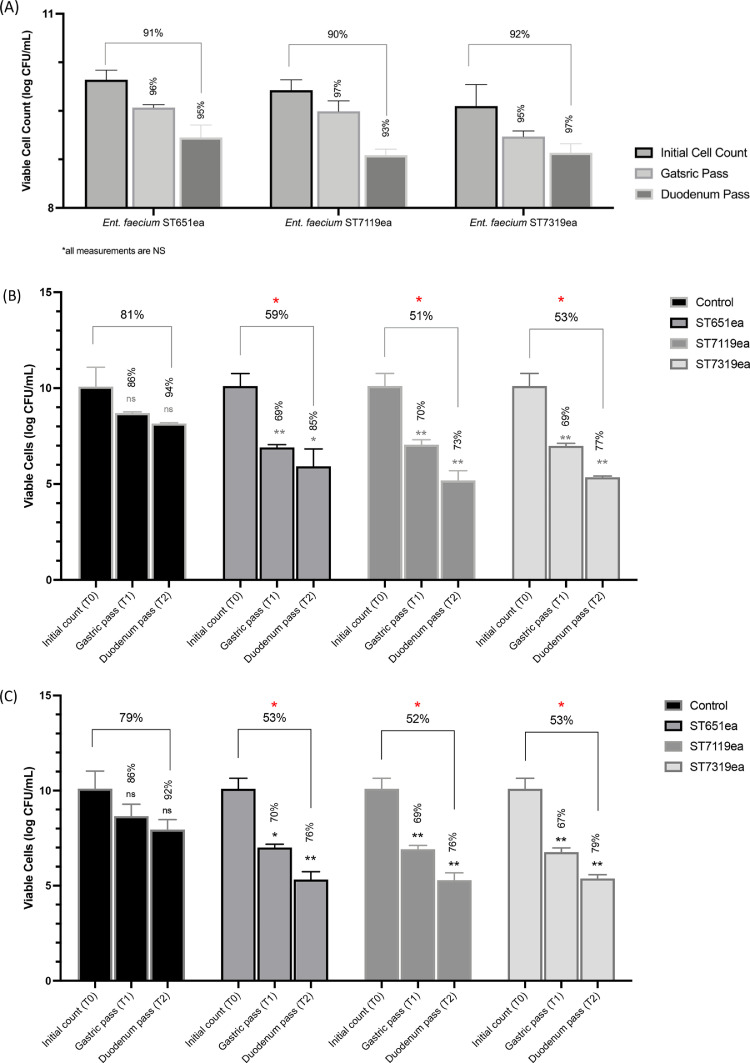
Enterococcus species are considered as typical members of the lower digestive tract. Their ability to survive in a wide range of environmental pH-values relative to other LAB allows autochthonous enterococci to dominate various niches. One of the criteria that needs to be met to consider a strain as a good probiotic candidate is a high survival under the conditions of the gastrointestinal tract in order to ensure sufficiently high viable dose of the probiotic to reach the target organ as intended for its application. Results showed no significant decrease in population CFU numbers between T0, even after the duodenum simulation passage (T2), as shown in Fig. 2A. This demonstrates high survivability and viability of the evaluated strains.
Fig. 2.
Survival rates of (A) Ent. faecium strains ST651ea, ST7119ea, ST7319ea; and test organisms (B) L. monocytogenes ATCC15313 and (C) L. innocua ATCC33090 in competition with Ent. faecium strains in the gastrointestinal simulation model. Survival rates in comparison with the previous timepoint are indicated on the top of the correspond boxplots. Asterisks above box plots demonstrates statistical significance as determined by Welch's test by comparing the mean log values of each viable bacterial count from the corresponding passage with that of the control. Significance as demonstrated by each asterisk is as follows: *p ≤ 0.05, **p ≤ 0.001.
Statistical significance for overall passage were quantified by two-way ANOVA and Turkey's test post-hoc analysis of *p ≤ 0.05.
Similar observations were reported by Amaral et al. (2017) indicating the high tolerance of enterococci to gastrointestinal tract conditions, even in the presence of key active enzymes as demonstrated in vitro. It was also stated by Nazzaro et al. (2012) that a high number of cells should be left viable (∼log 6 to 7) upon reaching the GIT to facilitate the intended functionality of a probiotic in the host. Although this consideration is of primary importance, the type of vector should also be considered – may it be food products or any other industrial applications – and that may significantly affect their viability. Furthermore, in vitro assessment of survivability in the gastrointestinal conditions only provides a prediction of how these strains function in the host system, thus, in vivo tests should be conducted to confirm the viability of these strains under practical conditons.
3.6. GIT survival of the test organisms in competition with Enterococcus faecium strains
Aside from high survivability in the GIT, a good probiotic candidate should also be successful in competing and establishing a niche under practical conditions especially in the presence of undesirable members of the gut microbiota. In this study, the same conditions were applied to evaluate the ability of the strains to compete with presentative strains of two known food-contaminant microorganisms, L. monocytogenes and L. innocua. The survival rates for L. innocua ATCC33090 in competition with Ent. faecium ST651ea, ST7119ea, and 7319ea were all significant after the gastric simulation passage relative to the controls demonstrated with the rates of 70%, 69%, and 67%, respectively against the control (Fig. 2B). Whereas L. monocytogenes ATCC15313 had survival rates after the gastric simulation of 60%, 70%, and 69% when co-incubated in this condition with Ent. faecium ST651ea, ST7119ea, and ST7319ea, accordingly (Fig. 2C). Further reduction was observed after duodenum simulation passage for both test organisms with an overall survival rate of around 52 to 53% in all competition set-ups for L. innocua ATCC33090, while rates for L. monocytogenes ATCC15313 in competition set-ups had the highest decrease observed against Ent. faecium ST7119ea and lowest against Ent. faecium ST651ea (Fig. 2). These variabilities in the observations can be attributed to the differences among strains (Amaral et al., 2017). Additionally, limitations of the employed methods should also be taken into consideration especially that these observations were made only in vitro. Thus, this information, although promising, can only provide possible behavior of the strains when applied to the target hosts and should be evaluated further in vivo to assess the ability of the strains to produce their bacteriocins and further assess the extent of efficiency of inhibition by the bacteriocinogenic strains.
4. Conclusions
Previously identified and characterized bacteriocinogenic Ent. faecium strains ST651ea, ST7119ea, and ST7319ea showed to be safe according to phenotypic evaluation of various potential virulence factors including production of gelatinase, hemolysin, and biogenic amine production. Furthermore, all the strains were sensitive to key therapeutic antibiotics and did not have genes associated with vancomycin resistance. Yet, strain Ent. faecium ST7319ea was found to harbor some of the virulence genes screened including hyl, IS16, and esp, thus, as suggested in the EFSA guidelines on the use of Enterococcus spp. as feed additives, this strain may not be considered safe. On the other hand, both safe strains may serve as good adjunct, starter cultures, or probiotic candidates. This is supported by promising enzyme production profiles, high GIT survival and aggregation, and ability to outcompete harmful/undesirable microorganisms such as L. monocytogenes and L. innocua, in vitro.
CRediT authorship contribution statement
Joanna Ivy Irorita Fugaban: Methodology, Formal analysis, Investigation, Data curation, Writing – original draft. Wilhelm Heinrich Holzapfel: Writing – review & editing, Funding acquisition. Svetoslav Dimitrov Todorov: Formal analysis, Conceptualization, Funding acquisition, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abadía Patiño L., Courvalin P., Perichon B. VanE gene cluster of vancomycin-resistant Enterococcus faecalis BM4405. J. Bacteriol. 2002;184(23):6457–6464. doi: 10.1128/jb.184.23.6457-6464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agerholm-Larsen L., Bell M.L., Grunwald G.K., Astrup A. The effect of a probiotic milk product on plasma cholesterol: a meta-analysis of short-term intervention studies. Eur. J. Clin. Nutr. 2000;54(11):856–860. doi: 10.1038/sj.ejcn.1601104. [DOI] [PubMed] [Google Scholar]
- Angmo K., Kumari A., Savitri T.C.B. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT Food Sci. Technol. 2016;66:428–435. doi: 10.1016/j.lwt.2015.10.057. [DOI] [Google Scholar]
- Amaral D.M.F., Silva L.F., Casarotti S.N., Nascimento L.C.S., Penna A.L.B. Enterococcus faecium and Enterococcus durans isolated from cheese: Survival in the presence of medications under simulated gastrointestinal conditions and adhesion properties. J. Dairy Sci. 2017;100(2):933–949. doi: 10.3168/jds.2016-11513. [DOI] [PubMed] [Google Scholar]
- Bajić S.S., Đokić J., Dinić M., Tomić S., Popović N., Brdarić E., Golić N., Tolinački M. GABA potentiate the immunoregulatory effects of Lactobacillus brevis BGZLS10-17 via ATG5-dependent autophagy in vitro. Sci. Rep. 2020;10(1):1347. doi: 10.1038/s41598-020-58177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargossi E., Gardini F., Gatto V., Montanari C., Torriani S., Tabanelli G. The capability of tyramine production and correlation between phenotypic and genetic characteristics of Enterococcus faecium and Enterococcus faecalis strains. Front. Microbiol. 2015;6 doi: 10.3389/fmicb.2015.01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo G., Mangiagli A., Nicastro L., Frigerio G. A controlled double-blind study of SF68 strain as a new biological preparation for the treatment of diarrhoea in pediatrics. Curr. Ther. Res. 1980;28:927–936. http://doi.org/10.101/0168-1605(95)00029-j. [Google Scholar]
- Bhagwat A., Annapure U.S. In vitro assessment of metabolic profile of Enterococcus strains of human origin. J. Genet. Eng. Biotechnol. 2019;17:11. doi: 10.1186/s43141-019-0009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra E., de Kleijn R., Colzato L.S., Alkemade A., Forstmann B.U., Nieuwenhuis S. Neurotransmitters as food supplements: the effects of GABA on brain and behavior. Front. Psychol. 2015;6 doi: 10.3389/fpsyg.2015.01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bover-Cid S., Holzapfel W.H. Improved screening procedure for biogenic amine production by lactic acid bacteria. Int. J. Food Microbiol. 1999;53(1):33–41. doi: 10.1016/s0168-1605(99)00152-x. 9. h 1999 Dec 1. [DOI] [PubMed] [Google Scholar]
- Castilho N.P.A., Colombo M., Oliveira L.L., Todorov S.D., Nero L.A. Lactobacillus curvatus UFV-NPAC1 and other lactic acid bacteria isolated from calabresa, a fermented meat product, present high bacteriocinogenic activity against Listeria monocytogenes. BMC Microbiol. 2019;19(1):63. doi: 10.1186/s12866-019-1436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno J.A., Menéndez S., Rodríguez-Otero J.L. Main microbial flora present as natural starters in Cebreiro raw cow's-milk cheese (Northwest Spain) Int. J. Food Microbiol. 1996;33(2):307–313. doi: 10.1016/0168-1605(96)01165-8. [DOI] [PubMed] [Google Scholar]
- Centers of Disease and Control (CDC). (2019). Antibiotic resistance threats and reports. Extracted from https://www.cdc.gov/DrugResistance/Biggest-Threats.html, accessed: October, 2020.
- Clinical and Laboratory Standards Institute (CLSI) Clinical and Laboratory Standards Institute; Wayne, PA: 2012. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—9th Edition. CLSI document M07-A9. [Google Scholar]
- Collado M.C., Meriluoto J., Salminen S. Adhesion and aggregation properties of probiotic and pathogen strains. Eur. Food Res. Technol. 2008;226(5):1065–1073. doi: 10.1007/s00217-007-0632-x. [DOI] [Google Scholar]
- Colombo M., Castilho N.P.A., Todorov S.D., Nero L.A. Beneficial properties of lactic acid bacteria naturally present in dairy production. BMC Microbiol. 2018;18(1):219. doi: 10.1186/s12866-018-1356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs G.W., Pearson J.C., Daley D.A., Le T., Robinson O.J., Gottlieb T., Howden B.P., Johnson P.D.R., Bennett C.M., Stinear T.P., Turnidge J.D. Molecular epidemiology of enterococcal bacteremia in Australia. J. Clin. Microbiol. 2014;52(3):897–905. doi: 10.1128/JCM.03286-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coque T.M., Patterson J.E., Steckelberg J.M., Murray B.E. Incidence of hemolysin, gelatinase, and aggregation substance among enterococci isolated from patients with endocarditis and other infections and from feces of hospitalized and community-based persons. J. Infect. Dis. 1995;171(5):1223–1229. doi: 10.1093/infdis/171.5.1223. [DOI] [PubMed] [Google Scholar]
- Courvalin P. Vancomycin resistance in gram-positive cocci. Clin. Infect. Dis. 2006;42:S25–S34.. doi: 10.1086/491711. Supplement_1. [DOI] [PubMed] [Google Scholar]
- Cui Y., Miao K., Niyaphorn S., Qu X. Production of gamma-aminobutyric acid from lactic acid bacteria: a systematic review. Int. J. Mol. Sci. 2020;21(3):995. doi: 10.3390/ijms21030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Re B., Sgorbati B., Miglioli M., Palenzona D. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett. Appl. Microbiol. 2000;31(6):438–442. doi: 10.1046/j.1365-2672.2000.00845.x. [DOI] [PubMed] [Google Scholar]
- Depardieu F., Perichon B., Courvalin P. Detection of the van alphabet and identification of enterococci and staphylococci at the species level by multiplex PCR. J. Clin. Microbiol. 2004;42(12):5857–5860. doi: 10.1128/JCM.42.12.5857-5860.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vuyst L., Foulquié Moreno M.R., Revets H. Screening for enterocins and detection of hemolysin and vancomycin resistance in enterococci of different origins. Int. J. Food Microbiol. 2003;84(3):299–318. doi: 10.1016/s0168-1605(02)00425-7. [DOI] [PubMed] [Google Scholar]
- dos Santos K.M.O., Vieira A.D.S., Salles H.O., Oliveira J., da S., Rocha C.R.C., Borges M., de F., Bruno L.M., Franco B.D.G.M., Todorov S.D. Safety, beneficial and technological properties of Enterococcus faecium isolated from Brazilian cheeses. Braz. J. Microbiol. 2015;46(1):237–249. doi: 10.1590/S1517-838246120131245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos K.M.O., de Matos C.R., Salles H.O., Franco B.D.G.M., Arellano K., Holzapfel W.H., Todorov S.D. Exploring beneficial/virulence properties of two dairy-related strains of streptococcus infantarius subsp. infantarius. Probiotics Antimicrob. Proteins. 2020;12(4):1524–1541. doi: 10.1007/s12602-020-09637-8. [DOI] [PubMed] [Google Scholar]
- Du F., Lv X., Duan D., Wang L., Huang J. Characterization of a linezolid- and vancomycin-resistant streptococcus suis isolate that harbors optrA and vanG operons. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02026. http://doi.org/10.3389/fmicb.2019.02026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychen G., Aquilina G., Azimonti G., Bampidis V., Bastos M., de L., Bories G., Chesson A., Cocconcelli P.S., Flachowsky G., Gropp J., Kolar B., Kouba M., López-Alonso M., López Puente S., Mantovani A., Mayo B., Ramos F., Saarela M., de Villa R., Wallace R., Wester P., Anguita M., Galobart J., Innocenti M., Martino L., EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) Guidance on the assessment of the safety of feed additives for the target species. EFSA J. 2017;15(10) doi: 10.2903/j.efsa.2017.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanuel D.G.V., Jafari A., Beauchemin K.A., Leedle J.A.Z., Ametaj B.N. Feeding live cultures of Enterococcus faecium and Saccharomyces cerevisiae induces an inflammatory response in feedlot steers. J. Anim. Sci. 2007;85(1):233–239. doi: 10.2527/jas.2006-216. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority (EFSA) EFSA approaches to risk assessment in the area of antimicrobial resistance. EFSA Suppor. Publ. 2011;8(10) doi: 10.2903/j.efsa.2011.196. [DOI] [Google Scholar]
- European Food Safety Authority (EFSA) Guidance on the safety assessment of Enterococcus faecium in animal nutrition. EFSA J. 2011;10(5):2682. doi: 10.2903/j.efsa.2012.2682. [DOI] [Google Scholar]
- Fan Y., Chen S., Yu Y., Si J., Liu B. A probiotic treatment containing Lactobacillus, Bifidobacterium and Enterococcus improves IBS symptoms in an open label trial. J. Zhejiang Univ. Science. B. 2006;7(12):987–991. doi: 10.1631/jzus.2006.B0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faron M.L., Ledeboer N.A., Buchan B.W. Resistance mechanisms, epidemiology, and approaches to screening for vancomycin-resistant Enterococcus in the health care setting. J. Clin. Microbiol. 2016;54(10):2436–2447. doi: 10.1128/JCM.00211-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández M., Linares D.M., Rodríguez A., Alvarez M.A. Factors affecting tyramine production in Enterococcus durans IPLA 655. Appl. Microbiol. Biotechnol. 2007;73(6):1400–1406. doi: 10.1007/s00253-006-0596-y. [DOI] [PubMed] [Google Scholar]
- Foulquié Moreno M.R., Sarantinopoulos P., Tsakalidou E., De Vuyst L. The role and application of enterococci in food and health. Int. J. Food Microbiol. 2006;106(1):1–24. doi: 10.1016/j.ijfoodmicro.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Franz C., Stiles M.E., Schleifer K.H., Holzapfel W.H. Enterococci in foods—a conundrum for food safety. Int. J. Food Microbiol. 2003;88(2–3):105–122. doi: 10.1016/S0168-1605(03)00174-0. [DOI] [PubMed] [Google Scholar]
- Franz C.M.A.P., Holzapfel W.H., Stiles M.E. Enterococci at the crossroads of food safety? Int. J. Food Microbiol. 1999;47(1–2):1–24. doi: 10.1016/S0168-1605(99)00007-0. [DOI] [PubMed] [Google Scholar]
- Franz C.M.A.P., Cho G.S., Holzapfel W.H., Gálvez A. In: Biotechnology of Lactic Acid Bacteria. Mozzi F., Raya R., Vignolo., editors. John Wiley & Sons, Ltd; 2010. Safety of lactic acid bacteria; pp. 341–359. [DOI] [Google Scholar]
- Franz C.M.A.P., Huch M., Abriouel H., Holzapfel W., Gálvez A. Enterococci as probiotics and their implications in food safety. Int. J. Food Microbiol. 2011;151(2):125–140. doi: 10.1016/j.ijfoodmicro.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Fugaban J.I.I., Vazquez Bucheli J.E., Holzapfel W.H., Todorov S.D. Characterization of partially purified bacteriocins produced by Enterococcus faecium strains isolated from soybean paste active against Listeria spp. and vancomycin-resistant enterococci. Microorganisms. 2021;9(5):1085. doi: 10.3390/microorganisms9051085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugaban J.I.I., Vazquez Bucheli J.E., Holzapfel W.H., Todorov S.D. Bacteriocinogenic Bacillus spp. isolated from Korean fermented cabbage (Kimchi)—beneficial or hazardous? Fermentation. 2021;7(2):56. doi: 10.3390/fermentation7020056. [DOI] [Google Scholar]
- García-Cayuela T., Korany A.M., Bustos I., Gómez de Cadiñanos P., Requena L., Peláez T., C., Martínez-Cuesta M.C. Adhesion abilities of dairy Lactobacillus plantarum strains showing an aggregation phenotype. Food Res. Int. 2014;57:44–50. doi: 10.1016/j.foodres.2014.01.010. [DOI] [Google Scholar]
- Giraffa G. Enterococci from foods. FEMS Microbiol. Rev. 2002;26(2):163–171. doi: 10.1111/j.1574-6976.2002.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Ghorbani G.R., Morgavi D.P., Beauchemin K.A., Leedle J.A.Z. Effects of bacterial direct-fed microbials on ruminal fermentation, blood variables, and the microbial populations of feedlot cattle. J. Anim. Sci. 2002;80(7):1977–1985. doi: 10.2527/2002.8071977x. [DOI] [PubMed] [Google Scholar]
- Gomes B.C., Esteves C.T., Palazzo I.C.V., Darini A.L.C., Felis G.E., Sechi L.A., Franco B.D.G.M., De Martinis E.C.P. Prevalence and characterization of Enterococcus spp. isolated from Brazilian foods. Food Microbiol. 2008;25(5):668–675. doi: 10.1016/j.fm.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Holzapfel W., Arini A., Aeschbacher M., Coppolecchia R., Pot B. Enterococcus faecium SF68 as a model for efficacy and safety evaluation of pharmaceutical probiotics. Benef. Microbes. 2018;9(3):375–388. doi: 10.3920/BM2017.0148. [DOI] [PubMed] [Google Scholar]
- Hynes W.L., Walton S.L. Hyaluronidases of gram-positive bacteria. FEMS Microbiol. Lett. 2000;183(2):201–207. doi: 10.1111/j.1574-6968.2000.tb08958.x. [DOI] [PubMed] [Google Scholar]
- Iyer R., Tomar S.K., Kapila S., Mani J., Singh R. Probiotic properties of folate producing Streptococcus thermophilus strains. Food Res. Int. 2010;43(1):103–110. doi: 10.1016/j.foodres.2009.09.011. [DOI] [Google Scholar]
- Jaouani I., Abbassi M.S., Ribeiro S.C., Khemiri M., Mansouri R., Messadi L., Silva C.C.G. Safety and technological properties of bacteriocinogenic enterococci isolates from Tunisia. J. Appl. Microbiol. 2015;119(4):1089–1100. doi: 10.1111/jam.12916. [DOI] [PubMed] [Google Scholar]
- Jett B.D., Huycke M.M., Gilmore M.S. Virulence of enterococci. Clin. Microbiol. Rev. 1994;7(4):462–478. doi: 10.1128/cmr.7.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L.M., Jaykus L.A. Antimicrobial resistance of Enterococcus species isolated from produce. Appl. Environ. Microbiol. 2004;70(5):3133–3137. doi: 10.1128/AEM.70.5.3133-3137.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kučerová K., Svobodová H., Tůma Š., Ondráčková I., Plocková M. Production of biogenic amines by enterococci. Czech J. Food Sci. 2010;27(Special Issue 2):50–55. doi: 10.17221/673-CJFS. [DOI] [Google Scholar]
- Ladero V., Fernández M., Calles-Enríquez M., Sánchez-Llana E., Cañedo E., Martín M.C., Alvarez M.A. Is the production of the biogenic amines tyramine and putrescine a species-level trait in enterococci? Food Microbiol. 2012;30(1):132–138. doi: 10.1016/j.fm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Leavis H., Top J., Shankar N., Borgen K., Bonten M., van Embden J., Willems R.J.L. A novel putative enterococcal pathogenicity island linked to the esp virulence gene of Enterococcus faecium and associated with epidemicity. J. Bacteriol. 2004;186(3):672–682. doi: 10.1128/JB.186.3.672-682.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavis H.L., Willems R.J.L., Wamel W.J.B., Schuren F.H., Caspers M.P.M., Bonten M.J.M. Insertion sequence–driven diversification creates a globally dispersed emerging multiresistant subspecies of E. faecium. PLoS Pathog. 2007;3(1):e7. doi: 10.1371/journal.ppat.0030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton F., Valentino M.D., Schaufler K., Earl A.M., Cattoir V., Gilmore M.S. Transferable vancomycin resistance in clade B commensal-type Enterococcus faecium. J. Antimicrob. Chemother. 2018;73(6):1479–1486. doi: 10.1093/jac/dky039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Gu Q., Wang Y., Yu Y., Yang L., Chen J.V. Novel vitamin B12-producing Enterococcus spp. and preliminary in vitro evaluation of probiotic potentials. Appl. Microbiol. Biotechnol. 2017;101(15):6155–6164. doi: 10.1007/s00253-017-8373-7. [DOI] [PubMed] [Google Scholar]
- Manson J.M., Keis S., Smith J.M.B., Cook G.M. A clonal lineage of VanA-type enterococcus faecalis predominates in vancomycin-resistant Enterococci isolated in New Zealand. Antimicrob. Agents Chemother. 2003;47(1):204–210. doi: 10.1128/aac.47.1.204-210.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Platero A.M., Valdivia E., Maqueda M., Martínez-Bueno M. Characterization and safety evaluation of enterococci isolated from Spanish goats’ milk cheeses. Int. J. Food Microbiol. 2009;132(1):24–32. doi: 10.1016/j.ijfoodmicro.2009.03.010. [DOI] [PubMed] [Google Scholar]
- McKessar S.J., Berry A.M., Bell J.M., Turnidge J.D., Paton J.C. Genetic characterization of vanG, a novel vancomycin resistance locus of Enterococcus faecalis. Antimicrob. Agents Chemother. 2000;44(11):3224–3228. doi: 10.1128/AAC.44.11.3224-3228.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci A., Rossetti L., Zago M., Monti L., Giraffa G., Carminati D., Tidona F. Folates biosynthesis by Streptococcus thermophilus during growth in milk. Food Microbiol. 2018;69:116–122. doi: 10.1016/j.fm.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Mountzouris K.C., Tsirtsikos P., Kalamara E., Nitsch S., Schatzmayr G., Fegeros K. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 2007;86(2):309–317. doi: 10.1093/ps/86.2.309. [DOI] [PubMed] [Google Scholar]
- Nazzaro F., Fratianni F., Nicolaus B., Poli A., Orlando P. The prebiotic source influences the growth, biochemical features, and survival under simulated gastrointestinal conditions of the probiotic Lactobacillus acidophilus. Anaerobe. 2012;18(3):280–285. doi: 10.1016/j.anaerobe.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Nocek J.E., Kautz W.P., Leedle J.A.Z., Allman J.G. Ruminal supplementation of direct-fed microbials on diurnal pH variation and in situ digestion in dairy cattle. J. Dairy Sci. 2002;85(2):429–433. doi: 10.3168/jds.S0022-0302(02)74091-5. [DOI] [PubMed] [Google Scholar]
- Novais C., Tedim A.P., Lanza V.F., Freitas A.R., Silveira E., Escada R., Roberts A.P., Al-Haroni M., Baquero F., Peixe L., Coque T.M. Co-diversification of Enterococcus faecium core genomes and PBP5: evidences of pbp5 horizontal transfer. Front. Microbiol. 2016;7:1581. doi: 10.3389/fmicb.2016.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar N.B., Castro A., Lucas R., Abriouel H., Yousif N.M.K., Franz C.M.A.P., Holzapfel W.H., Rubén P.P., Martínez-Canãmero M., Gálvez A. Functional and safety aspects of Enterococci isolated from different Spanish foods. Syst. Appl. Microbiol. 2004;27(1):118–130. doi: 10.1078/0723-2020-00248. [DOI] [PubMed] [Google Scholar]
- Ouwehand A.C., Kirjavainen P.V., Grönlund M.M., Isolauri E., Salminen S.J. Adhesion of probiotic micro-organisms to intestinal mucus. Int. Dairy J. 1999;9(9):623–630. doi: 10.1016/S0958-6946(99)00132-6. [DOI] [Google Scholar]
- Palacios M.C., Haros M., Rosell C.M., Sanz Y. Characterization of an acid phosphatase from Lactobacillus pentosus: regulation and biochemical properties. J. Appl. Microbiol. 2005;98(1):229–237. doi: 10.1111/j.1365-2672.2004.02447.x. [DOI] [PubMed] [Google Scholar]
- Palmer K.L., Godfrey P., Griggs A., Kos V.N., Zucker J., Desjardins C., Cerqueira G., Gevers D., Walker S., Wortman J., Feldgarden M., Haas B., Birren B., Gilmore M.S. Comparative genomics of Enterococci: variation in Enterococcus faecalis, clade structure in E. faecium, and defining characteristics of E. gallinarum and E. casseliflavus. MBio. 2012;3(1) doi: 10.1128/mBio.00318-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollmann M., Nordhoff M., Pospischil A., Tedin K., Wieler L.H. Effects of a probiotic strain of Enterococcus faecium on the rate of natural chlamydia infection in swine. Infect. Immun. 2005;73(7):4346–4353. doi: 10.1128/IAI.73.7.4346-4353.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan V., Balakrishnan B., Rai A.K., Narayan B., Halami P.M. Concomitant production of lipase, protease and enterocin by Enterococcus faecium NCIM5363 and Enterococcus durans NCIM5427 isolated from fish processing waste. Int. Aquat. Res. 2012;4(1):14. doi: 10.1186/2008-6970-4-14. [DOI] [Google Scholar]
- Reynolds P.E., Courvalin P. Vancomycin resistance in Enterococci due to synthesis of precursors terminating in d-alanyl-d-serine. Antimicrob. Agents Chemother. 2005;49(1):21–25. doi: 10.1128/AAC.49.1.21-25.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice L.B., Carias L., Rudin S., Vael C., Goossens H., Konstabel C., Klare I., Nallapareddy S.R., Huang W., Murray B.E. A potential virulence gene, hylEfm, predominates in Enterococcus faecium of clinical origin. J. Infect. Dis. 2003;187(3):508–512. doi: 10.1086/367711. [DOI] [PubMed] [Google Scholar]
- Rickard A.H., Gilbert P., High N.J., Kolenbrander P.E., Handley P.S. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 2003;11(2):94–100. doi: 10.1016/s0966-842x(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Bacterial adherence to hydrocarbons: a useful technique for studying cell surface hydrophobicity. FEMS Microbiol. Lett. 1984;22(3):289–295. doi: 10.1111/j.1574-6968.1984.tb00743.x. [DOI] [Google Scholar]
- Rosenberg M. Microbial adhesion to hydrocarbons: twenty-five years of doing MATH. FEMS Microbiol. Lett. 2006;262(2):129–134. doi: 10.1111/j.1574-6968.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- Rossi M., Amaretti A., Raimondi S. Folate production by probiotic bacteria. Nutrients. 2011;3(1):118–134. doi: 10.3390/nu3010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saqib S., Akram A., Halim S.A., Tassaduq R. Sources of β-galactosidase and its applications in food industry. 3 Biotech. 2017;7(1) doi: 10.1007/s13205-017-0645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit G., Smit B.A., Engels W.J.M. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005;29(3):591–610. doi: 10.1016/j.femsre.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Taras D., Vahjen W., Macha M., Simon O. Performance, diarrhea incidence, and occurrence of Escherichia coli virulence genes during long-term administration of a probiotic Enterococcus faecium strain to sows and piglets. J. Anim. Sci. 2006;84(3):608–617. doi: 10.2527/2006.843608x. [DOI] [PubMed] [Google Scholar]
- Tarrah A., Castilhos J., Rossi R.C., Duarte V., da S., Ziegler D.R., Corich V., Giacomini A. In vitro probiotic potential and anti-cancer activity of newly isolated folate-producing streptococcus thermophilus strains. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tendolkar P.M., Baghdayan A.S., Gilmore M.S., Shankar N. Enterococcal surface protein, esp, enhances biofilm formation by Enterococcus faecalis. Infect. Immun. 2004;72(10):6032–6039. doi: 10.1128/IAI.72.10.6032-6039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov S.D., Wachsman M., Tomé E., Dousset X., Destro M.T., Dicks L.M.T., Franco B.D.G.M., Vaz-Velho M., Drider D. Characterisation of an antiviral pediocin-like bacteriocin produced by Enterococcus faecium. Food Microbiol. 2010;27(7):869–879. doi: 10.1016/j.fm.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Trunk T., Khalil H.S., Leo J.C. Bacterial auto aggregation. AIMS Microbiol. 2018;4(1):140–164. doi: 10.3934/microbiol.2018.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakalidou E., Dalezios I., Kalantzopoulos G. Isolation and partial characterization of an intracellular esterase from Enterococcus faecium ACA-DC 237. J. Biotechnol. 1994;37(3):201–208. doi: 10.1016/0168-1656(94)90127-9. [DOI] [Google Scholar]
- Vahjen W., Männer K. The effect of a probiotic Enterococcus faecium product in diets of healthy dogs on bacteriological counts of Salmonella spp., Campylobacter spp. and Clostridium spp. in faeces. Arch. Tierernahr. 2003;57(3):229–233. doi: 10.1080/0003942031000136657. [DOI] [PubMed] [Google Scholar]
- Vankerckhoven V., Van Autgaerden T., Vael C., Lammens C., Chapelle S., Rossi R., Jabes D., Goossens H. Development of a multiplex PCR for the detection of asa1, gelE, cylA, esp, and hyl genes in Enterococci and survey for virulence determinants among European hospital isolates of Enterococcus faecium. J. Clin. Microbiol. 2004;42(10):4473–4479. doi: 10.1128/JCM.42.10.4473-4479.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizoso Pinto M.G., Franz C.M.A.P., Schillinger U., Holzapfel W.H. Lactobacillus spp. with in vitro probiotic properties from human faeces and traditional fermented products. Int. J. Food Microbiol. 2006;109(3):205–214. doi: 10.1016/j.ijfoodmicro.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Vitetta L., Coulson S., Thomsen M., Nguyen T., Hall S. Probiotics, D–Lactic acidosis, oxidative stress and strain specificity. Gut Microbes. 2017;8(4):311–322. doi: 10.1080/19490976.2017.1279379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C.T., Fisher S.L., Park I.S., Prahalad M., Wu Z. Bacterial resistance to vancomycin: five genes and one missing hydrogen bond tell the story. Chem. Biol. 1996;3(1):21–28. doi: 10.1016/S1074-5521(96)90079-4. [DOI] [PubMed] [Google Scholar]
- Werner G., Fleige C., Geringer U., van Schaik W., Klare I., Witte W. IS element IS16 as a molecular screening tool to identify hospital-associated strains of Enterococcus faecium. BMC Infect. Dis. 2011;11:80. doi: 10.1186/1471-2334-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand I., Hilpert K., Hancock R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008;3(2):163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2018. Antimicrobial Resistance and Primary Health Care.https://apps.who.int/iris/handle/10665/326454 License: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Wunderlich P.F., Braun L., Fumagalli I., D'Apuzzo V., Heim F., Karly M., Lodi R., Politta G., Vonbank F., Zeltner L. Double-blind report on the efficacy of lactic acid-producing Enterococcus SF68 in the prevention of antibiotic-associated diarrhoea and in the treatment of acute diarrhoea. J. Int. Med. Res. 1989;17(4):333–338. doi: 10.1177/030006058901700405. [DOI] [PubMed] [Google Scholar]
- Yoshimune K., Yamamoto M., AoyagIi T., Yumoto I. High and rapid L-lactic acid production by Alkaliphilic Enterococcus sp. by adding wheat bran hydrolysate. Ferment. Technol. 2017;6:138. doi: 10.4172/2167-7972.1000138. [DOI] [Google Scholar]
- Zommiti M., Cambronel M., Maillot O., Barreau M., Sebei K., Feuilloley M., Ferchichi M., Connil N. Evaluation of probiotic properties and safety of Enterococcus faecium isolated from artisanal tunisian meat “dried ossban. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.01685. [DOI] [PMC free article] [PubMed] [Google Scholar]