Highlights
-
•
The first ever detection in the meconium of the methanogen M. smithii.
-
•
These data position M. smithii among the early inhabitants of the human gut.
-
•
These data suggest the contribution of methanogens to the perinatal development of intestinal microbiota and physiology.
Keywords: Methanogens, Methanobrevibacter smithii, Meconium, Preterm, Neonate, Gut microbiota
Abstract
To understand the dynamics of methanogens in the human intestinal microbiota, we investigated the presence of methanogens in meconium using a polyphasic approach including microscopy and PCR-sequencing in 33 meconium samples collected from 33 pre-term neonates, in accordance with current ethics regulation. In the presence of negative controls, 90.9% samples were real-time PCR-positive for methanogens and 69.7 % were PCR-sequencing positive, identified as Methanobrevibacter (M.) smithii. Further, auto-fluorescent analysis detected methanogens in the two meconium samples analyzed, with a morphology suggesting M. smithii. Multispacer Sequence Typing found M. smithii genotypes ST1 and ST2, previously described as intestinal microbiota inhabitants. C-section delivery and non-use of peripartum antibiotics significantly correlated with PCR-detection of methanogens in meconium. These data position M. smithii among the early inhabitants of the human gut, detectable immediately after birth and suggest the contribution of methanogens to the perinatal development of intestinal microbiota and physiology.
Introduction
Meconium, the first stool of the newborn, lines the intestinal tract of the fetus during pregnancy (Gosalbes et al., 2013). Its composition varies during fetal development and comprises 72 to 80% water, intestinal secretions, cellular desquamations, bile pigments, inflammatory proteins, and blood (Gosalbes et al., 2013). Meconium, found in the fetal digestive system at the end of the first trimester of pregnancy, results from the ingestion of amniotic fluid during pregnancy (Gosalbes et al., 2013; Hu et al., 2013; Moles et al., 2013). Physiologically, the emission of the first meconium occurs 24 to 48 hours after birth, being most often expelled by the newborn at the time of birth reflex and usually abundantly (Gosalbes et al., 2013; Hu et al., 2013; Moles et al., 2013). In some studies, bacteria have been isolated and/or detected by Polymerase Chain Reaction (PCR) in amniotic fluid (Bearfield et al., 2002; Hitti et al., 1997; Oh et al., 2010), umbilical cord blood (Jiménez et al., 2005), meconium (Ardissone et al., 2014; Dominguez-Bello et al., 2010; Gosalbes et al., 2013; Hu et al., 2013; Jiménez et al., 2008; Madan et al., 2012; Moles et al., 2013; Mshvildadze et al., 2010), placenta (Aagaard et al., 2014; Satokari et al., 2009; Stout et al., 2013), and fetal membranes (Rautava et al., 2012; Steel et al., 2005) without any clinical or histological evidence of infection or inflammation in the mother or the newborn. However, intestinal methanogens have recently gained attention as players of immune-mediated diseases (Sereme et al., 2019).
Methanogens are strict aero-intolerant archaea that produce methane in the presence of H2, CO2 and other substrates (Guindo, 2020; Nkamga et al., 2017; Sereme et al., 2019; Sogodogo et al., 2019). They are divided into three groups according to the substrates used in the production of methane: hydrogenotroph methanogens use CO2 and formate as substrates; methylotroph methanogens use methyl compounds as substrates; and acetogenotroph methanogens use acetate as substrate (Guindo, 2020; Nkamga et al., 2017; Sereme et al., 2019; Sogodogo et al., 2019). Methanogens are part of the human microbiota, in particular the intestinal representing 10 % of the anerobic microorganisms in the human digestive tract and oral microbiota (Guindo, 2020; Nkamga et al., 2017; Sereme et al., 2019; Sogodogo et al., 2019). They have even been found in colostrum and breast milk (Togo et al., 2019), in blood during infectious endocarditis (Drancourt et al., 2020), in vagina only in case of vaginosis (Grine et al., 2019a) and in urinary tract during urinary tract infections (Grine et al., 2019b). They are present in humans from birth (Grine et al., 2017).
Detection of methanogens requiring specific laboratory methods is not routinely developed in clinical microbiology. Accordingly, the detection of methanogens in the meconium has not been reported. Here, as part of a clinical research protocol on the development of the intestinal microbiota and immune status in a cohort of premature infants, we have used such specific methods we are mastering, to explore the presence of methanogens in the meconium of premature newborns.
Results
Clinical data
Thirty-three meconium samples collected from 33 pre-term neonates were investigated in this study. The mean birth weight was 1.190 g (range 440–2.130 g), mean gestational age of 30 weeks (range 25–32 weeks) and mean height of 39 cm (range 32–45 cm) (Table 1). Of these 33 subjects, 5/33 (15.15%) were moderate preterm premature, 19/33 (57.57%) were very preterm, 9/33 (27.27%) were extremely preterm, 28/33 (84.84%) were from C-section delivery, 5/33 (15.15%) were from vaginal delivery, and 7/33 (21.21%) were from mothers with a history of peripartum antibiotic therapy (Table 2).
Table 1.
Clinical data for 33 premature newborns here investigated for the presence of meconial methanogens.
| Code | Peripartum maternal antibiotic therapy | Mode of delivery | Gestational age | Weight | Size |
|---|---|---|---|---|---|
| 1 | Yes | Cesarean section | 26 | 925 | 35 |
| 2 | No | Cesarean section | 32 | 1260 | 39 |
| 3 | No | Vaginal delivery | 30 | 1480 | 41 |
| 4 | No | Vaginal delivery | 30 | 1460 | 38 |
| 5 | No | Cesarean section | 29 | 1565 | 42 |
| 6 | No | Cesarean section | 29 | 880 | 35 |
| 7 | Yes | Vaginal delivery | 30 | 1670 | 43 |
| 8 | No | Cesarean section | 31 | 1120 | 38 |
| 9 | No | Cesarean section | 24 | 530 | 31 |
| 10 | No | Cesarean section | 28 | 890 | 33 |
| 11 | No | Cesarean section | 26 | 925 | 35 |
| 12 | No | Cesarean section | 26 | 565 | 31 |
| 13 | Yes | Cesarean section | 27 | 925 | 34 |
| 14 | Yes | Cesarean section | 27 | 680 | 31 |
| 15 | Yes | Cesarean section | 30 | 1335 | 39 |
| 16 | Yes | Cesarean section | 30 | 1355 | 47 |
| 17 | No | Cesarean section | 30 | 1480 | 39 |
| 18 | No | Cesarean section | 31 | 1570 | 43 |
| 19 | No | Cesarean section | 32 | 1155 | 39 |
| 20 | No | Cesarean section | 29 | 1050 | 39 |
| 21 | No | Cesarean section | 29 | 1190 | 38 |
| 22 | No | Cesarean section | 32 | 1930 | 44 |
| 23 | No | Cesarean section | 32 | 1575 | 44 |
| 24 | No | Cesarean section | 25 | 870 | 34 |
| 25 | No | Cesarean section | 30 | 1750 | 43 |
| 26 | No | Cesarean section | 30 | 1360 | 39 |
| 27 | No | Cesarean section | 27 | 600 | 29 |
| 28 | No | Vaginal delivery | 25 | 750 | 32 |
| 29 | No | Cesarean section | 31 | 980 | 36 |
| 30 | No | Cesarean section | 31 | 1410 | 39 |
| 31 | No | Cesarean section | 30 | 770 | 33 |
| 32 | Yes | Vaginal delivery | 32 | 1568 | 41 |
| 33 | No | Cesarean section | 30 | 1680 | 39 |
Table 2.
Distribution of newborns according to gestational age, mode of delivery and maternal antibiotic intake.
| Moderate preterm | Very preterm | Extremely preterm | Cesarean section | Vaginal delivery | Peripartum maternal antibiotic therapy |
|---|---|---|---|---|---|
| 5/33(15.15%) | 19/33(57.57%) | 9/33(27.27%) | 28/33(84.84%) | 5/33(15.15%) | 7/33(21.21%) |
Methanobrevibacter smithii is very frequently detected in meconium by PCR
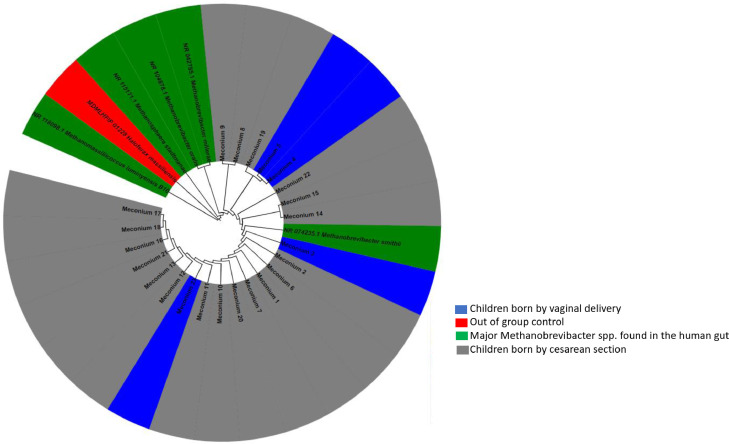
DNA extraction yielded 22.04 ± 4.96 ng/µL and incorporating 16S rRNA archaeal gene PCR primers recently designed in our laboratory into real-time PCR, we detected the presence of methanogen DNA in 30 (90.9%) of meconium samples here investigated and none of the negative controls. PCR-sequencing yielded M. smithii in 23 cases (69.69%) and sequences exhibited a 99.5% similarity with the reference 16S rRNA gene sequence of M. smithii ATCC 35061 (accession NCBI: NR_074235). The phylogenetic tree showed that all these 23 sequences clustered with M. smithii previously detected in the human digestive tract (Fig. 1). MST genotyping revealed the presence of genotype ST1 in 22/23 (95.65%) meconium sample, and the genotype ST2 in one meconium sample (4.34%) (Table 3).
Fig. 1.
Molecular phylogenetic analysis, based on 16S rRNA partial gene, showed the position of Methanobrevibacter smithii sequences detected in meconium samples. The evolutionary history was inferred by using the Maximum Likelihood method based on the Kimura 2-parameter model. The tree with the highest log likelihood (-1716.60) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 29 nucleotide sequences. All positions containing gaps and missing data were eliminated. There was a total of 473 positions in the final dataset. Evolutionary analyses were conducted in MEGA 7.
Table 3.
Multispacer sequence typing of 23 Methanobrevibacter smithii detected in 23 meconium preterm babies.
| Samples | Peripartum maternal antibiotic therapy | Mode of delivery | Gestational age | Genotype | ||||
|---|---|---|---|---|---|---|---|---|
| Spacer1 | Spacer2 | Spacer 3 | Spacer 4 | Spacer type* | ||||
| 1 | Yes | Cesarean | 26 | X | X | X | X | 1 |
| 2 | No | Cesarean | 32 | X | X | X | X | 1 |
| 3 | No | Vaginal delivery | 30 | X | X | X | X | 1 |
| 4 | No | Vaginal delivery | 30 | X | X | X | X | 1 |
| 5 | Yes | Vaginal delivery | 30 | X | X | X | X | 1 |
| 6 | No | Cesarean | 28 | X | X | X | 2 | |
| 7 | Yes | Cesarean | 27 | X | X | X | X | 1 |
| 8 | Yes | Cesarean | 30 | X | X | X | X | 1 |
| 9 | No | Cesarean | 30 | X | X | X | X | 1 |
| 10 | No | Cesarean | 31 | X | X | X | X | 1 |
| 11 | No | Cesarean | 32 | X | X | X | X | 1 |
| 12 | No | Cesarean | 29 | X | X | X | X | 1 |
| 13 | No | Cesarean | 29 | X | X | X | X | 1 |
| 14 | No | Cesarean | 32 | X | X | X | X | 1 |
| 15 | No | Cesarean | 32 | X | X | X | X | 1 |
| 16 | No | Cesarean | 25 | X | X | X | X | 1 |
| 17 | No | Cesarean | 30 | X | X | X | X | 1 |
| 18 | No | Cesarean | 30 | X | X | X | X | 1 |
| 19 | No | Cesarean | 27 | X | X | X | X | 1 |
| 20 | No | Vaginal delivery | 25 | X | X | X | X | 1 |
| 21 | No | Cesarean | 31 | X | X | X | X | 1 |
| 22 | No | Cesarean | 31 | X | X | X | X | 1 |
| 23 | Yes | Vaginal delivery | 32 | X | X | X | X | 1 |
*Spacer type was determined according to references (Grine et al., 2017; Guindo et al., 2021; Nkamga et al., 2015).
Microscopic observation is compatible with the presence of M. smithii in meconium
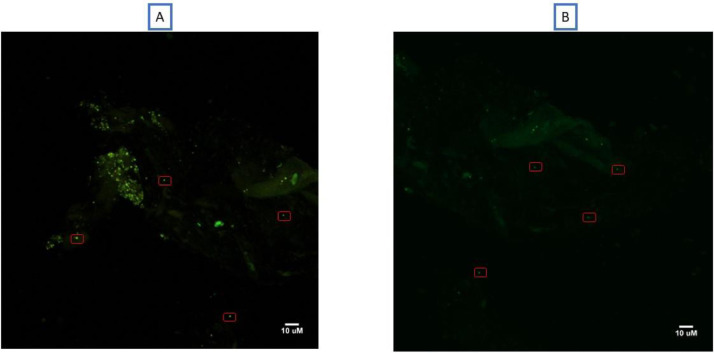
By using confocal microscopy, we were able to observe fluorescent microorganisms exhibiting a green fluorescent and diplococcus morphology characteristic of M. smithii supporting the presence of methanogens in two meconium specimens (Fig. 2)
Fig. 2.
Fluorescent microorganisms exhibiting a green fluorescent and diplococcus morphology characteristic of M. smithii from two meconium samples (A and B) using a confocal microscope at 63X magnification.
The presence of methanogens in meconium is correlated with c-section delivery
Further exploitation of the five available clinical variables (peripartum maternal antibiotic therapy, mode of delivery, gestational age, sex and weight) indicated no significant correlation between gestational age (pvalue = 0.318), weight at birth (pvalue = 0.229), sex (pvalue = 0.476) and the detection of methanogens in meconium specimens. However, we observed a significant correlation between the detection of methanogens in meconium specimens and c-section delivery (pvalue = 0.004) and with non-use of antibiotic peripartum (pvalue = 0.038) (Table 4).
Table 4.
Results of Principal Component Analysis (PCA) (Results of PCA. Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘’ 1).
| Estimate | Standard error | zvalue | Statistical significance | |
|---|---|---|---|---|
| Intercept | -4.0665 | 1.1784 | -3.451 | 0.0005*** |
| Peripartum maternal antibiotic therapy (No) | 0.8014 | 1.3026 | 0.615 | 0.0381* |
| Peripartum maternal antibiotic therapy (Yes) | -0.3257 | 1.104 | -0.295 | 0.7679 |
| Cesarean section delivery | 2.4192 | 1.233 | 1.962 | 0.0049** |
| Vaginal delivery | -0.3673 | 1.3532 | 0.271 | 0.7769 |
| Gestational age | 1.4219 | 1.4268 | 0.997 | 0.3189 |
| Weight | -0.3642 | 1.3234 | -0.292 | 0.2292 |
| Sex | 0.671 | 1.822 | 0.152 | 0.4766 |
Discussion
We are reporting the first ever detection in the meconium of the methanogen M. smithii, firmly identified by a polyphasic approach including microscopy and PCR-sequencing. All data here reported were ascertained by the negativity of negative controls and the fact that concordant results were obtained by different techniques.
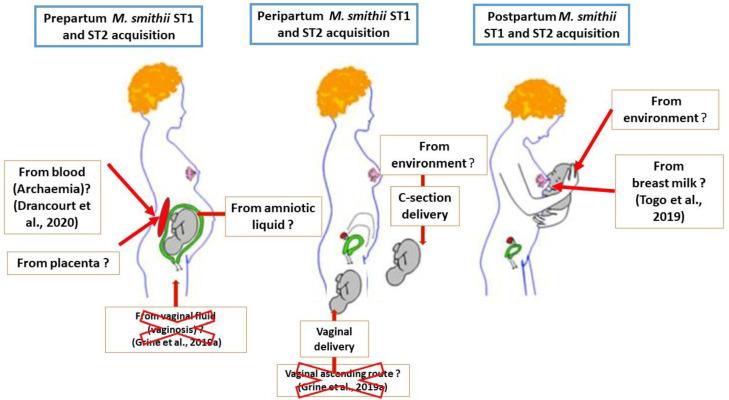
The data here reported are in line with previous detection of M. smithii in 50/50 (100%) of gastric juice samples collected from one-day-old newborns (Grine et al., 2017), pushing back the colonization of the newborn digestive tract most probably during the in-utero period of life; questioning the sources of such a colonization. In this perspective, herein genotyping meconial M. smithii using the sequence-based MST method yielded ST1 previously found in one-day newborns’ gastric juice and in adult gut microbiota (Grine et al., 2017; Nkamga et al., 2015); and ST2 also part of the adult gut microbiota (Nkamga et al., 2015). Viable M. smithii and Methanobrevibacter oralis (M. oralis) colonize the colostrum and the mother milk (Togo et al., 2019) but M. oralis was not detected in meconium in the present study, in line with its lack of detection in one-day newborns’ gastric juice (Grine et al., 2017). This observation and the fact that, here, meconium samples have been collected prior to any feeding, plea against the hypothesis that mother milk was the source of M. smithii in preterm newborns. Likewise, M. smithii has been detected in the vaginal fluid only in the case of vaginosis, making the vaginal fluid an unlikely source of M. smithii in this study (Grine et al., 2019a). Accordingly, meconial M. smithii was here significantly associated with cesarean section, further ruling-out the vaginal fluid as a source of M. smithii (Figure 3). It has been suggested that, during pregnancy, gut microorganisms translocating through the intestinal epithelium move to the placenta via the bloodstream (Rodríguez et al., 2015). M. smithii could also be found in the blood, after we recently reported a series of M. smithii archaemia in febrile adult patients, including three cases of infectious endocarditis (Drancourt et al., 2020). Indeed, dendritic cells present in the intestinal barrier, do recover bacteria as well as the intestinal methanogens Methanosphaera stadtmanae and M. smithii, eventually transported to lymphoid organs (Rodríguez et al., 2015). There, methanogens activate the adaptive immune response, as illustrated in rabbit and mouse models of immunization (Macario et al., 1984, 1983) (Figure 3).
Fig. 3.
Peripartum hypothetical routes of transmission of M. smithii in preterm infants. In this study, M. smithii were genotyped using multi-spacer typing, indicating spacer type 1(ST1) and spacer type 2 (ST2). Hypothetical routes of transmission of M. smithii prior, during and after delivery of preterm infants were derived from data gathered from this study and up-to-date literature (references are shown in brackets). Unlikely routes of transmission are indicated by red crosses.
In conclusion, this study demonstrating the presence of M. smithii as an in-utero member of the gut microbiota. This finding, together with previous literature showing the absence of M. smithii in infant life-threatening kwashiorkor (Million et al., 2016), suggests that M. smithii is an early and crucial player of gut microbiota and immunity in infants.
Methods
Patients and sampling
Premature newborns were included in the “Influence of Intestinal Microbiota Implantation in Preterm Infants on Microbiota and Immune Orientation at 3 Years” (NCT02738411, principal investigator AF) cohort after written informed parental consent was obtained for each preterm. This research project was approved by the Ethics Committee on Clinical Research of Nîmes and Montpellier University Hospitals. To be eligible for enrolment, preterm neonates must have been born at a gestational age ≤ 32 weeks. First spontaneously evacuated meconium was collected by the medical staff at the Nîmes, and Montpellier University Hospitals noticed peripartum maternal antibiotic therapy, mode of delivery, gestational age, and weight of premature newborns (Table 1). Collections were done between May and September 2018. Before sampling, the pediatrician washed his or her hands with alcoholic solution and then put gloves on before manipulating meconium samples and each meconium sample was transferred from a diaper to one sterile Falcon tube (Sigma-Aldrich, Saint Quentin Fallavier, France) using a sterile tongue depressor by the medical staff and stored at -80°C until analysis.
Molecular analysis
DNA extraction was performed as described previously (Dridi et al., 2009). Briefly, 0.2 g of each meconium sample has been mixed with 500 μL of G2 buffer (QIAGEN, Hilden, Germany), then, shaked with 0.3 g of acid-washed beads ≤ 106 μm (Sigma-Aldrich, Saint-Quentin Fallavier, France) in a FastPrep BIO 101 device (MP Biomedicals, Illkirch, France) for 45 s. 20 µL of proteinase K (QIAGEN) was added to a volume of 180-µL mixture, then incubated 56 °C overnight. Total DNA was finally extracted with the EZ1 Advanced XL extraction kit (QIAGEN) and 50 μL eluted volume. In each DNA extraction run, we used sterile PBS as a negative control.
Once the DNA Extracted, a real-time PCR targeting a 156 pb 16S rRNA regions (691 and 843) was performed using Metha_16S_2_MBF: 5′-CGAACCGGATTAGATACCCG -3′ and Metha_16S_2_MBR: 5′-CCCGCCAATTCCTTTAAGTT-3′ primers and the FAM_Metha_16S_2_MBP 6FAM- CCTGGGAAGTACGGTCGCAAG probe targeting the 16S DNA gene of methanogens (Eurogentec, Angers, France) designed according the following steps, the 16S rRNA gene of Methanobrevibacter smithii ATCC 35061 (GenBank accession number CP000678), Methanosphaera stadtmanae DSM 3091 (GenBank accession number NC 007681), Methanobrevibacter oralis M2 CSUR P5920 (GenBank accession number GCA_900289035.1), Methanobrevibacter arboriphilus ANOR1 (GenBank accession number GCA_000513315.1), Methanomassiliicoccus luminiyensis B10 (GenBank accession number GCA_000308215.1) was targeted using MEGA7 software (https://www.megasoftware.net/). Using the online Primer 3 program(http://biotools.umassmed.edu/bioapps/primer3_www.cgi), we found that all these published genomes exhibit only one copy of the 16S rRNA gene. The specificity of the PCR primers and probes have been verified by testing experimentally the DNA extracted from 30 bacterial species representative of common gut inhabitants and in silico using the BLAST program at NCBI (http://www.ncbi.nlm.nih.gov/BLAST).
The real-time PCR amplification reaction was performed as previously described (Guindo et al., 2021) following this program: 50 °C for 2 min, followed by 39 cycles of 95°C for 45 s, 95 °C for 5 s and finally 60 °C for 30 s. The amplifications were carried out in CFX96 thermocycler (BioRad, Marnes-la-Coquette, France). We considered as positive all meconium samples which PCR exhibited a CT<40. Gene amplification and PCR sequencing were performed as previously described (Grine et al., 2018, 2017; Guindo et al., 2020; Nkamga et al., 2015).
Multispacer sequence typing
The multispacer sequence typing (MST) technique was performed on meconium as previously described (Grine et al., 2017; Guindo et al., 2021; Nkamga et al., 2015). Briefly, all positive PCR products were sequenced in both directions using the same primers as used for PCRs in a 2720 Thermal Cycler (Applied Biosystems, Foster City, USA) with an initial 1-minute denaturation step at 96°C, followed by 25 cycles denaturation for 10-second each at 96°C, a 20-second annealing step at 50°C and a 4-minute extension step at 60°C. Sequencing products were purified using the MultiScreen 96-well plates Millipore (Merck, Molsheim, France) containing 5 % of Sephadex G-50 (Sigma-Aldrich) and sequences were analyzed on an ABI PRISM 31309 Genetic Analyzer (Applied Biosystem) and edited using the ChromasPro software (version 1.42; Technelysium Pty Ltd). MST genotypes were defined as a unique combination of the four spacer sequences (Grine et al., 2017; Guindo et al., 2021; Nkamga et al., 2015).
Phylogenetic analyses
Sequences were edited using ChromasPro software (ChromasPro 1.7, Technelysium Pty Ltd., Tewantin, Australia). Molecular phylogenetic and evolutionary analyses were conducted in MEGA7 as previously described (Kumar et al., 2016). We used sequences of the major methanogens present in the human digestive tract (Methanobrevibacter smithii, Methanosphaera stadtmanae, Methanobrevibacter oralis, Methanobrevibacter millerae and Methanomassiliicoccus luminiyensis) in the construction of the phylogenetic trees. The non-methanogen archaea species Haloferax massiliensis was used as an out-of-group control.
Direct microscopic examination
Based on factor 420 carried by methanogens (Dridi, 2012), the presence of methanogens in meconium has been investigated by confocal microscopy on the only two fresh meconium samples as follows. Briefly, meconium suspension was prepared with distilled water. A drop of the prepared meconium suspension deposited on a microscopy slide was observed at 63X magnification using a confocal microscope (LSM800 Airyscan Zeiss, Oberkochen, Germany).
Statistical analyses
All statistical processes were done using the open-source statistical language R (R Development Core Team, 2010). The threshold of 0.05 was the maximal p-value for each statistical conclusion. The model hypothesis was that the presence of methanogens could be associated with the peripartum maternal antibiotic therapy, mode of delivery, gestational age, sex and weight. We tested this hypothesis using a Principal Component Analysis (PCA) (Groth et al., 2013) with the functions of the FactoMineR (https://cran.rproject.org/web/packages/FactoMineR/index.html) and factoextra (https:/cran.r-project.org/web/packages/factoextra/index.html).
Authors contributions
YS and COG conducted the experiments, analyzed the data and wrote the paper; AF and TA contributed to the collection of samples; JV, PC and MD designed the project, participated in the writing of the paper and provided great support carrying out the experiments; GG designed the project, helped conduct the experiments and participated in the writing of the paper.
Funding
YS and COG benefit from PhD grants from the Fondation Méditerranée Infection, Marseille, France. This work was supported by the French Government under the «Investissements d'avenir» (Investments for the Future) program managed by the Agence Nationale de la Recherche (ANR, fr: National Agency for Research), (reference: Méditerranée Infection 10-IAHU-03).
Declaration of Competing Interest
The authors declare no competing interests in relation to this study. Outside this study, JV reports speaker and consultancy fees in the past 5 years from Meda Pharma (Mylan), Novartis, Sanofi, Thermo Fisher Scientific, outside the submitted work.
We confirm that the manuscript has been read and approved by all named authors.
We confirm that the order of authors listed in the manuscript has been approved by all named authors.
Acknowledgments
YS and COG benefit from Ph.D. grants from the Fondation Méditerranée Infection, Marseille, France. This work was supported by the French Government under the «Investissements d'avenir» (Investments for the Future) program managed by the Agence Nationale de la Recherche (ANR, fr: National Agency for Research), (reference: Méditerranée Infection 10-IAHU-03).
References
- Aagaard K., Ma J., Antony K.M., Ganu R., Petrosino J., Versalovic J. The Placenta harbors a unique microbiome. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3008599. 237ra65–237ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardissone A.N., de la Cruz D.M., Davis-Richardson A.G., Rechcigl K.T., Li N., Drew J.C., Murgas-Torrazza R., Sharma R., Hudak M.L., Triplett E.W., Neu J. Meconium microbiome analysis identifies bacteria correlated with premature birth. PLoS One. 2014;9:e90784. doi: 10.1371/journal.pone.0090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearfield C., Davenport E.S., Sivapathasundaram V., Allaker R.P. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. Br. J. Obstet. Gynaecol. 7. 2002;109(5):527–533. doi: 10.1111/j.1471-0528.2002.01349.x. [DOI] [PubMed] [Google Scholar]
- Dominguez-Bello M.G., Costello E.K., Contreras M., Magris M., Hidalgo G., Fierer N., Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drancourt M., Djemai K., Gouriet F., Grine G., Loukil A., Bedotto M., Levasseur A., Lepidi H., Bou-Khalil J., Khelaifia S., Raoult D. Methanobrevibacter smithii archaemia in febrile patients with bacteremia, including those with endocarditis. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa998. ciaa998. [DOI] [PubMed] [Google Scholar]
- Dridi B. Laboratory tools for detection of archaea in humans. Clin. Microbiol. Infect. 2012;18:825–833. doi: 10.1111/j.1469-0691.2012.03952.x. [DOI] [PubMed] [Google Scholar]
- Dridi B., Henry M., El Khéchine A., Raoult D., Drancourt M. High prevalence of methanobrevibacter smithii and methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol. PLoS One. 2009;4:e7063. doi: 10.1371/journal.pone.0007063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosalbes M.J., Llop S., Vallès Y., Moya A., Ballester F., Francino M.P. Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clin. Exp. Allergy. 2013;43:198–211. doi: 10.1111/cea.12063. [DOI] [PubMed] [Google Scholar]
- Grine G., Boualam M.A., Drancourt M. Methanobrevibacter smithii, a methanogen consistently colonising the newborn stomach. Eur. J. Clin. Microbiol. Infect. Dis. 2017;36:2449–2455. doi: 10.1007/s10096-017-3084-7. [DOI] [PubMed] [Google Scholar]
- Grine G., Drouet H., Fenollar F., Bretelle F., Raoult D., Drancourt M. Detection of Methanobrevibacter smithii in vaginal samples collected from women diagnosed with bacterial vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38:1643–1649. doi: 10.1007/s10096-019-03592-1. [DOI] [PubMed] [Google Scholar]
- Grine G., Lotte R., Chirio D., Chevalier A., Raoult D., Drancourt M., Ruimy R. Co-culture of Methanobrevibacter smithii with enterobacteria during urinary infection. EBioMedicine. 2019;43:333–337. doi: 10.1016/j.ebiom.2019.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grine G., Terrer E., Boualam M.A., Aboudharam G., Chaudet H., Ruimy R., Drancourt M. Tobacco-smoking-related prevalence of methanogens in the oral fluid microbiota. Sci. Rep. 2018;8:9197. doi: 10.1038/s41598-018-27372-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth D., Hartmann S., Klie S., Selbig J. In: Computational Toxicology, Methods in Molecular Biology. Reisfeld B., Mayeno A.N., editors. Humana Press; Totowa, NJ: 2013. Principal Components Analysis; pp. 527–547. [DOI] [PubMed] [Google Scholar]
- Guindo C.O. Digestive tract methanodrome: Physiological roles of human microbiota-associated methanogens. Microb. Pathog. 2020;149:104425. doi: 10.1016/j.micpath.2020.104425. [DOI] [PubMed] [Google Scholar]
- Guindo C.O., Davoust B., Drancourt M., Grine G. Diversity of Methanogens in Animals’ Gut 10. Microorganisms. 2021;9(1):13. doi: 10.3390/microorganisms9010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindo C.O., Terrer E., Chabrière E., Aboudharam G., Drancourt M., Grine G. Culture of salivary methanogens assisted by chemically produced hydrogen. Anaerobe. 2020;61 doi: 10.1016/j.anaerobe.2019.102128. [DOI] [PubMed] [Google Scholar]
- Hitti J., Riley D.E., Krohn M.A., Hillier S.L., Agnew K.J., Krieger J.N., Eschenbach D.A. Broad-spectrum bacterial rDNA Polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin. Infect. Dis. 1997;24:1228–1232. doi: 10.1086/513669. [DOI] [PubMed] [Google Scholar]
- Hu J., Nomura Y., Bashir A., Fernandez-Hernandez H., Itzkowitz S., Pei Z., Stone J., Loudon H., Peter I. Diversified microbiota of meconium is affected by maternal diabetes status. PLoS One. 2013;8:e78257. doi: 10.1371/journal.pone.0078257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez E., Fernández L., Marín M.L., Martín R., Odriozola J.M., Nueno-Palop C., Narbad A., Olivares M., Xaus J., Rodríguez J.M. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr. Microbiol. 2005;51:270–274. doi: 10.1007/s00284-005-0020-3. [DOI] [PubMed] [Google Scholar]
- Jiménez E., Marín M.L., Martín R., Odriozola J.M., Olivares M., Xaus J., Fernández L., Rodríguez J.M. Is meconium from healthy newborns actually sterile? Res. Microbiol. 2008;159:187–193. doi: 10.1016/j.resmic.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macario E.C., de König H., Macario A.J., Kandler O. Six antigenic determinants in the surface layer of the archaebacterium Methanococcus vannielii revealed by monoclonal antibodies. J. Immunol. 1984;132:883–887. [PubMed] [Google Scholar]
- Madan J.C., Salari R.C., Saxena D., Davidson L., O'Toole G.A., Moore J.H., Sogin M.L., Foster J.A., Edwards W.H., Palumbo P., Hibberd P.L. Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch. Dis. Child. - Fetal Neonatal Ed. 2012;97:F456–F462. doi: 10.1136/fetalneonatal-2011-301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles L., Gómez M., Heilig H., Bustos G., Fuentes S., de Vos W., Fernández L., Rodríguez J.M., Jiménez E. Bacterial diversity in meconium of preterm neonates and evolution of their fecal microbiota during the first month of life. PLoS One. 2013;8:e66986. doi: 10.1371/journal.pone.0066986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mshvildadze M., Neu J., Shuster J., Theriaque D., Li N., Mai V. Intestinal microbial ecology in premature infants assessed with non–culture-based techniques. J. Pediatr. 2010;156:20–25. doi: 10.1016/j.jpeds.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkamga V.D., Henrissat B., Drancourt M. Archaea: Essential inhabitants of the human digestive microbiota. Hum. Microbiome J. 2017;3:1–8. doi: 10.1016/j.humic.2016.11.005. [DOI] [Google Scholar]
- Nkamga V.D., Huynh H.T.T., Aboudharam G., Ruimy R., Drancourt M. Diversity of human-associated methanobrevibacter smithii isolates revealed by multispacer sequence typing. Curr. Microbiol. 2015;70:810–815. doi: 10.1007/s00284-015-0787-9. [DOI] [PubMed] [Google Scholar]
- Oh K.J., Lee S.E., Jung H., Kim G., Romero R., Yoon B.H. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J. Perinat. Med. 2010;38 doi: 10.1515/jpm.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing; Vienna: 2010. a language and environment for statistical computing: reference index. [Google Scholar]
- Rautava S., Collado M.C., Salminen S., Isolauri E. Probiotics modulate host-microbe interaction in the placenta and fetal gut: a randomized, double-blind, placebo-controlled trial. Neonatology. 2012;102:178–184. doi: 10.1159/000339182. [DOI] [PubMed] [Google Scholar]
- Rodríguez J.M., Murphy K., Stanton C., Ross R.P., Kober O.I., Juge N., Avershina E., Rudi K., Narbad A., Jenmalm M.C., Marchesi J.R., Collado M.C. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015;26:26050. doi: 10.3402/mehd.v26.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satokari R., Grönroos T., Laitinen K., Salminen S., Isolauri E. Bifidobacterium and Lactobacillus DNA in the human placenta. Lett. Appl. Microbiol. 2009;48:8–12. doi: 10.1111/j.1472-765X.2008.02475.x. [DOI] [PubMed] [Google Scholar]
- Sereme Y., Mezouar S., Grine G., Mege J.L., Drancourt M., Corbeau P., Vitte J. Methanogenic Archaea: emerging partners in the field of allergic diseases. Clin. Rev. Allergy Immunol. 2019;57:456–466. doi: 10.1007/s12016-019-08766-5. [DOI] [PubMed] [Google Scholar]
- Sogodogo E., Drancourt M., Grine G. Methanogens as emerging pathogens in anaerobic abscesses. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38:811–818. doi: 10.1007/s10096-019-03510-5. [DOI] [PubMed] [Google Scholar]
- Steel J.H., Malatos S., Kennea N., Edwards A.D., Miles L., Duggan P., Reynolds P.R., Feldman R.G., Sullivan M.H.F. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr. Res. 2005;57:404–411. doi: 10.1203/01.PDR.0000153869.96337.90. [DOI] [PubMed] [Google Scholar]
- Stout M.J., Conlon B., Landeau M., Lee I., Bower C., Zhao Q., Roehl K.A., Nelson D.M., Macones G.A., Mysorekar I.U. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am. J. Obstet. Gynecol. 2013;208:226.e1–226.e7. doi: 10.1016/j.ajog.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo A.H., Grine G., Khelaifia S., des Robert C., Brevaut V., Caputo A., Baptiste E., Bonnet M., Levasseur A., Drancourt M., Million M., Raoult D. Culture of Methanogenic Archaea from Human Colostrum and Milk. Sci. Rep. 2019;9:18653. doi: 10.1038/s41598-019-54759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]