Highlights
-
•
Arbuscular mycorrhizal fungi (AMF) and plant symbiosis.
-
•
Role AMF in root development and plant growth promotion.
-
•
AMF influence and plant response under strigolactone (SL) and SL-GR24 application.
-
•
Effects and functions of SL in root development and interaction with AMF.
Keywords: AMF, Symbiosis, SLs, GR24, Plant growth promotion
Abstract
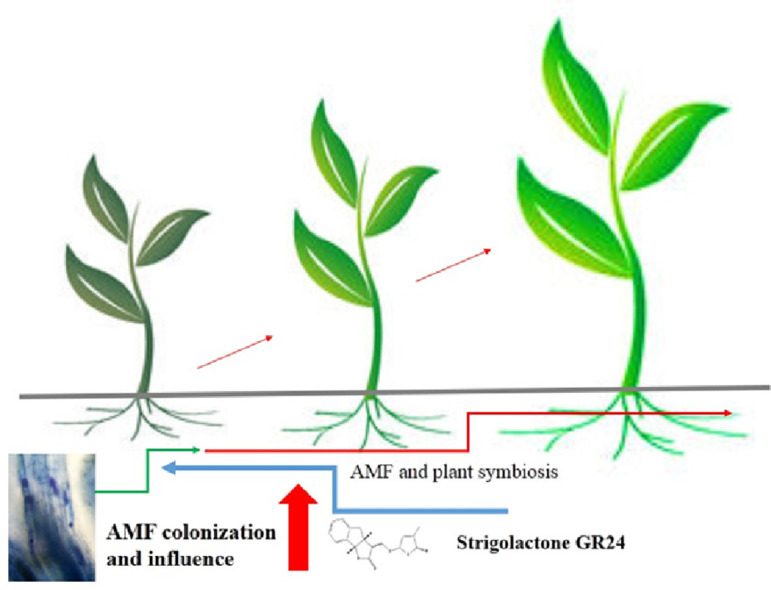
Arbuscular mycorrhizal fungi (AMF) and plant symbiosis is the old, fascinating and beneficial relation that exist on earth for the plants. In this review, we have elaborated that the strigolactones (SLs) are released from the roots and function with root parasite, seeds and symbiotic AMF as contact chemicals. They are transported through the xylem in the plants and can regulate plant architecture, seed germination, nodule formation, increase the primary root length, influence the root hairs and physiological reactions to non-living agents by regulating their metabolism. SLs first evolved in ancient plant lineages as regulators of the basic production processes and then took a new role to maintain the growing biological complexities of terrestrial plant. SLs belongs to a diversified category of butenolide‐bearing plant hormones related to various processes of agricultural concern. SLs also arouses the development of spores, the divergence and enlargement of hyphae of AMF, metabolism of mitochondria, reprogramming of transcription process, and generation of chitin oligosaccharides which further stimulate the early response of symbiosis in the host plant, results from better communication in plant and ability of coexistence with these fungi. The required nutrients are transferred from the roots to the shoots, which affect the physiological, biochemical, and morphological characteristics of the plant. On the other hand, the plant provides organic carbon in the form of sugars and lipids to the fungi, which they use as a source of energy and for carried out different anabolic pathways. SLs also lead to alteration in the dynamic and structure of actin in the root region as well as changes the auxin's transporter localization in the plasma membrane. Thus, this study reveals the functions that SLs play in the growth of roots, as well as their effect and interaction with AMF that promote plant growth.
Graphical abstract
Introduction
In the existing consequence of increasing price of synthetic fertilizers and the widening gap between the supply and demand of the nutrients, the utilization of plant growth-promoting AMF offers a remarkable opportunity to use low-cost and eco-friendly nutrient supplements for the improvement of crops yield and growth (Mitra et al., 2019; Chaudhary et al., 2019). In arbuscular mycorrhizal (AM) symbiosis, the mycelial fungal network spreads under the plant roots and facilitates the absorption of nutrients that are usually inaccessible to the plants (Begum et al., 2019). The mycelium of fungi inhabits the roots of a wide number of shrubberies, even though they belong to a variety of species. AM symbiosis has long been thought to be a promiscuous relationship of more than >100,000 plant species with some 100 AMF moral morphotypes (Chen et al., 2018; Melo et al., 2019). However, the typical species definition is problematical in the sense of AMF because of its relatively distinctive morphological characteristics (mostly associated with spores) and basic asexual mode of propagation (Chen et al., 2018). AMF have never been undergone sexual fusion or experience sexual stages, but in the lack of classical recombination and meiosis, they may undergo a hyphal fusion and exchange of genetic materials to replenish their genomes and to produce the new diversity of genes (Chagon, 2014). This feature may theoretically be used as an additional criterion for defining the taxonomic groups, apart from spore morphotypes, depending upon its genetic relationship (Chagon, 2014). AMF are field fungi that are synchronized with plant roots. Various host benefits are due to AMF, including improved soil absorption of immobile nutrients and mostly phosphorous (P). An awareness of the impacts of agronomic activities on these fungal species will help to ensure that symbiosis is used and contribute to the achievements of sustainability (Mitra et al., 2020). The symbiosis of AMF is related with more than 80% of plants in the land between mandating bio-trophic fungi. The host plant releases important metabolites during the pre-symbiotic process in order to cause fungal production and root colonization (Fig. 1). These fungi form such symbiotic relationships and inhabit inside and around the plant root organization in order to develop internal edifices that simplify the transmission of photo-synthetically derived carbon in the form of carbohydrates and starches to the AMF. This nutrient delivery indorses host plant growth. For instance, AMF uptake many diverse nutrients, but the nitrogen (N) to P ratio in an organization is found to be a resilient driver of the growth profit that AMF has provide to the crop plants (Johnson et al., 2015). AMF supports host plants to propagate energetically under unfavorable circumstances by facilitating a series of multifaceted communicating actions between fungus and plant leading to an improved rate of photosynthesis and other traits related to gaseous exchange, respectively (Birhane et al., 2012). The multifarious cellular connection between the AMF and host roots necessitates a series of signals that directs the proper formation and maintenance of AMF in the plant roots (Kaldorf, M. and Ludwig‐Müller, 2000). In ectomycorrhizal symbiotic process, numerous examples are well known where indole-3-acetic acid (IAA) is formed by the fungal species which results in the growth of lateral roots (LA) and consequently directs the promotion of the symbiotic relationship (Karabaghli-Degron et al., 1998). It was also reported that maize root colonized with Rhizophagus irregularis (formerly Glomus intraradices) when inoculated with AMF shows an increased amount of total indole-3-butyric acid (IBA) in the young roots (Ludwig-Müller et al., 1997). AMF, being usual root symbionts, deliver some vigorous inorganic nutrients to different species of plants, thereby enlightening yield and growth under unfavorable and favorable conditions. AMF equally helps host plants in order to up-regulate the mechanism related to tolerance and thereby down-regulate the significant metabolic pathways (Begum et al., 2019). In the host's roots, AMF forms hyphae, arbuscules and vesicles and also develops hyphae and spores in the rhizospheric region. The development of hyphal network by the AMF with the roots of plant ominously boosts the access of roots to the greater surface of the soil, which triggers the enhancement in the growth of plant (Bowles et al., 2016). It is also reported that AMF can effectively overcome several abiotic prompts such as extreme temperatures, cold stress, alkali stress, nutrient stress, drought, salinity and therefore increases the per hectare yield of huge number of vegetables and crop plants (Begum et al., 2019). Therefore, for the promotion of bio healthy agriculture, AMF convention is considered to be the enormous one for current global agricultural systems and their constant sustainability.
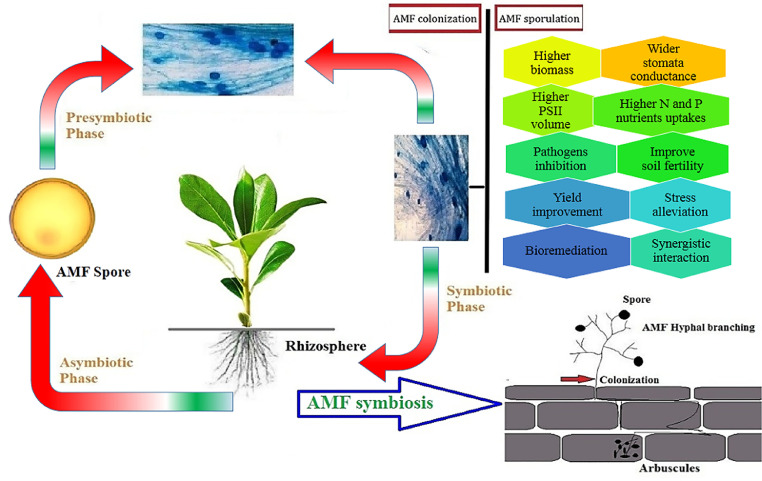
Fig. 1.
AMF symbiotic relationship, metabolic process and phases of symbiosis in the host system.
AMF-plant-soil interactions: symbiosis, nutrient acquisition and plant growth promotion
The AM symbiosis is an old and best symbiosis on the Earth. Example, in the Aglaophyton, hyphaes and arbuscules, isolated from the Rhynie chert, have been identified and shown the presence of AM symbiotic process in the early Devonian. Besides, the data of molecular clock based on 18-year nucleotide divergence rDNA indicated that between 350 and 460 million years ago, Glomales evolved and symbiosis was a key factor for efficient plant colonization. The symbiosis of AMF in about 10 percent of plants, such as entire angiosperm groups, was lost during development. Distinguishable stages of AMF colonization include a series of complicated morphogenetic alterations in fungi (Fig. 2):
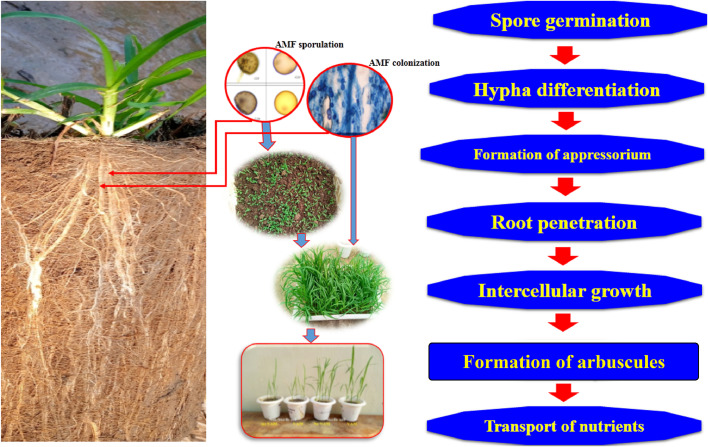
Fig. 2.
AMF symbiosis with plant, growth promotion and nutrients supply.
AMF are mandatory biotrophic that rely on the abilities of a host plant for the completion of their life cycle (Fig. 2). Additionally, in the unavailability of the host plant, the growth of fungal species stops after about 25–30 days of culture (Harrier, 2001). Their compulsory status and coenocytic nature of their spores are the biological aspects of the fungi which impede their study and the symbiosis with the roots of plants. Later one is especially important because the normal state of most plants is a mycorrhizal root (Harrier, 2001).
A symbiotic relationship with rhizospheric microorganisms not only improves the status of nutrients in the crops but also can upgrade the quality of different crops. Furthermore, the soil inoculated with AMF, forms a high constant mass and extra-radical hyphal mycelium than the non-AMF containing soil. Glomalin-related soil protein is reported to maintain the content of water into the soil under the exposure of various abiotic stresses, which further regulates the frequency of water between the plants and the soil, automatically raises the development of plant varieties (Begum et al., 2019). Such as, the strawberry colonized with AMF exhibited a high level of phytocompounds which ultimately improved their antioxidant property reported by Castellanos-Morales et al. (2010). Although the benefits of AMF symbiotic relationship have been well identified the information regarding its circadian chronobiology still needs further investigation (Nanjundappa et al., 2019).
SLs: a novel recognize plant hormone and biosynthesis pathway
SLs, have newly been documented as plant hormone which are comprised in orchestrating root and shoot architecture. These SLs are derivative of carotenoid which is one of the major class of plant secondary metabolites, formed by assorted plant species (Kumar et al., 2015) and has gained a lot of attention since their identification as a novel group of plant hormone. The initial study on SL was reported in 1996 from the root exudates of cotton which revealed its potential role as a stimulant for the germination of Striga and Orobanche, the parasitic plant seeds. However, later on SLs have been recognized as stimulants of AMF directed colonization of root and hyphal branching. SLs are majorly produced in root and various other parts of the plant such as internode and epicotyl tissue; however, the known SLs till date are more commonly found in the roots (Yoneyama et al., 2013). Being released in the rhizospheric region, SLs played a potential role in the microbes which proliferate around the plant roots in the soil. Major focus has been rewarded so far to the rhizobia, AMF, and symbiotic microbe (Kapulnik and Koltai, 2014; Lanfranco et al., 2017; Rozpądek et al., 2018; Waters et al., 2017), while very few studies have been reported that how pathogenic and saprotrophic fungi respond to SLs (Akiyama et al., 2010; Carvalhais et al., 2019; Choi et al., 2020; Lanfranco et al., 2017).
AMF and SLs interaction: physiological roles and signaling in root development
Various evidence accumulated from the different investigations revealed that SLs involve in the growth of plant roots. It also directs the elongation of adventitious and primary/seminal roots and represses the formation of LR, respectively (Kapulnik et al., 2011a). Shen et al. (2020) reported that this role of SLs could be reversed by the addition of GR24 (active and synthetic SL) in SL-deficient plants and wild-type, but not in the case of SL-insensitive mutant. Interestingly, in the case of rice, SLs positively regulate the adventitious roots (ARs) regulation as reported by Arite et al. (2012); Sun et al. (2014). Conversely, Wang et al. (2020) find out that the SL mutant of rice produced very few numbers of ARs at the stage of seedling and a low number per tiller at the stage of maturation. The number of AR in the case of synthetic mutant of SL (d10), not in the signaling mutant (d3) of SL, was complemented by GR24 addition. P and N are the main nutrients needed for the growth of plant. SLs play an important role in the adaptive responses to P and N inadequacy due to increased level of SL in the roots of plant (Marzec et al., 2013; Sun et al., 2016; Xie et al., 2013). For example, SLs promote the symbiotic relation with AMF by inducing the branching of hyphae and they also adjust the architecture of shoot by restricting the outgrowth of tiller bud in order to adapt the condition of N or P deficiency (Czarnecki et al., 2013; Sun et al., 2020). Recently, it was determined that SLs controls the response or perception of plants to phosphorous-limited conditions (Mayzlish-Gati et al., 2012). Interestingly, various studies determined that SLs played a potential role in the root growth of rice plant in case of P and N deficiencies (Mayzlish-Gati et al., 2012).
SLs are the phytohormones that may become increasingly popular in plant science (Zwanenberg and Blanco-Ania, 2018) and play a vital role in plant and its root growth (Friml, 2003). They are terpenoid lactones derived from carotenoids (Waldie et al., 2010), often made up of four rings. Three rings form a tricyclic lactone through an enol ether bridge which is linked to the fourth butenolide ring (Akiyama and Hayashi, 2006). Over the last sixty years, germination stimulants such as Striga, Phelipanche and Orobanche have been identified for parasites [Table 1; (Cook et al., 1966)]. Various new bio-properties of SLs have been found, such as the AMF branching factor, architecture of plant (inhibition of shoot branching and bud outgrowth), and reaction to abiotic factors, etc. (Akiyama et al., 2005; Zwanenberg and Blanco-Ania, 2018). Interestingly, SLs have been identified as the plant hormones that regulate the architecture of above-ground plants by masking different effects and eventually restricting the outgrowth of bud (Gomez-Roldan et al., 2008; Umehara et al., 2008. The finding that SLs cause parasites to germinate and recruit the provision of AMF nutrients indicated that parasites can be managed by controlling the colonization of AMF (Akiyama et al., 2005). Germination of Striga hermonthica was significantly decreased in inoculated maize and sorghum plant varieties with AMF (Lendzemo et al., 2007). Some investigations reported that in response to AMF inoculation, there is a reduction in the Striga's germination that could be partly due to down-regulation of the SLs. Indeed, this is likely to constitute a mechanism known as auto regulation, for the host plant to avoid excessive colonization that could be metabolically costly (Staehelin et al., 2011). AMF hyphal branching-stimulatory activity is critically impaired due to the structural variations in SLs (Akiyama et al., 2010). According to a report, in AMF (Gigaspora margarita), 5-deoxystrigol, which has not any hydroxyl group, was 30-times more active than the sorgomol, which has a hydroxyl group in the first tricyclic lactone ring. 5-deoxystrigol was exclusively secreted from maize (Zea mays L.), while the cultivar of resistant type exuded sorgomol in the primarily (Yoneyama et al., 2015). The cultivar which are Striga-susceptible are also find to secrete 5-deoxystrigol primarily in Sorghum bicolor (L.) Moench (Yoneyama et al., 2010), whereas the resistant cultivar produced orobanchol primarily, which has a hydroxy group on the second tricyclic lactone ring (Mohemed et al., 2016). These authors first recorded the existence of orobanchol in the variety of sorghum and it's near association with field resistance to Striga. A comprehensive genetic evaluation of a Striga-resistant sorghum cultivar resulted in the recognition of a mutation of the sulfotransferase gene that decreases the S. hermonthica germination by imposing orobanchol P production over 5-deoxystrigolol (Gobena et al., 2017). Thus, seeds of S. hermonthica can distinguish host varieties or species based on the composition of SL. Striga's low sensitivity to orobanchol indicated that selection for producers of high obanchol could be served as an effective strategy for obtaining genotypes with higher acquisition of phosphorous and AMF responsiveness but lower activity in inducing germination of Striga. The impact on hyphal branching of the AMF (e.g. G. margarita) of various natural SLs may be highly variable. For example, obanchol was found to be more effective than 5-deoxystrigol in inducing AMF hyphal branching (Akiyama et al., 2010).
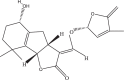
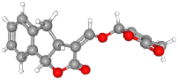
Table 1.
SLs and SL analog GR24 information from PubChem database.
| Compound | PubChem CID | Molecular Formula | Molecular Weight (g/mol) | SMILES | 2D Structure | 3D Structure |
|---|---|---|---|---|---|---|
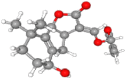
| Strigol | 5,281,396 | C19H22O6 | 346.4 | CC1=CC(OC1=O)OC
C2C3CC4=C(C3OC2=O) C(CCC4O)(C)C |
 |
 |
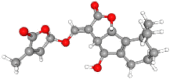
| Orobanchol | 10,665,247 | C19H22O6 | 346.4 | CC1=CC(OC1=O)OC
C2C3C(C4=C(C3OC2=O) C(CCC4)(C)C)O |
 |
 |
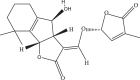
| Strigolactone GR24 | 123,343,697 | C17H14O5 | 298.29 | CC1=CC(OC1=O)OC
C2C3CC4=CC CC C4C3OC2=O |
 |
 |
Signaling crosstalk between SLs and their derivatives for the enhancements of root growth in plant
Numerous studies described the prominence of newly recognized SLs and their derivatives in hormonal crosstalk while the development of root in many plant varieties (Kapulnik et al., 2011a; Ruyter-Spira et al., 2011; Mayzlish-Gati et al., 2012; Sun et al., 2014; De Cuyper et al., 2014). SLs production increases significantly when P and N levels decrease in order to regulate adaptive plant responses to these nutrients deficiencies (Umehara et al., 2008; Yoneyama et al., 2007). They have been reported to play an essential role in plant communication in the rhizospheric region. Increasing evidence shows the importance of auxins in regulating the formation of LR in plants (Goh et al., 2012; Xuan et al., 2016). SL signaling acts upstream of auxins in regulating LR positioning, initiation, and elongation (Kapulnik et al., 2011b; Mayzlish-Gati et al., 2012; Ruyter-Spira et al., 2011; Sun et al., 2014). However, the positive or adverse action of SLs depends on the condition of experiments and different plant species, respectively. The primary root length was found to be decreased in mutants of SL in Arabidopsis than in case of wild type due to a decline of cell number in the meristem. In the case of LR formation, a comparison between the wild type (WT) and mutants in Arabidopsis plants revealed an enhanced density of LR in SL-synthesis mutant (more axillary growth4) and an SL-signaling mutant (more axillary growth2), while the addition of GR24 decreased the density of LR via the signaling gene MAX2 (Kapulnik et al., 2011a; Kohlen et al., 2011; Ruyter-Spira et al., 2011). Nevertheless, another investigation done by Sun et al. (2014) has shown the non-significant difference in the density of LR between d and WT mutants in rice at the stage of seedling and a low number of ARs per tiller at the stage of maturation. It has been hypothesized that SLs control the transport of auxin in roots which latter is primarily mediated by PIN-FORMED (PIN) genes. The addition of GR24 on Arabidopsis roots declined PIN proteins levels in the tips of primary root (PIN7, PIN3, and PIN1) which are the main auxin efflux carriers in plants (Friml, 2003; Wiśniewska et al., 2006). Conversely, in the availability of exogenous auxin, GR24 does not affect the PIN levels (Ruyter-Spira et al., 2011). The formation of LR in Arabidopsis and rice were repressed via the GR24 application in SL and WT-synthetic mutant (more axillary growth4/d10), but not in an SL-signaling mutant (more axillary growth2/d3) via the reduction of auxin levels and distribution in roots as a result of down-regulation of expression of PIN family genes in roots (Ruyter-Spira et al., 2011; Sun et al., 2014). In other terms, SLs may inhibit the formation of LR by decreasing the PIN proteins levels in plant roots which reduces auxin levels. These results were confirmed with the examination of [3H] IAA transport and GUS::DR5 activity after the addition of GR24 (Sun et al., 2014, 2018).
Beneficial interactions between growth-promoting rhizobacteria, plant roots and AMF can enhance the health of plant by improving its immune systems and thereby enhancing nutrient production (Pérez-de-Luque, 2017). AMF of the Glomeromycotina ancient monophyllic group are the significant contributor in the cycling of nutrients and (Lanfranco et al., 2017) minerals in the plants and also increases the tolerance capability of different plant species to biotic and abiotic stress (Spatafora et al., 2016). AMF is a linking biotrophy of coenocytic and asexual spores, while researchers have recently suggested the occurrence of secret sexual events also (Corradi and Brachmann, 2017). Since AMF interacts with more than 80 percent of soil plants, including crop plants and Bryophytes (Bonfante and Genre, 2008) and can also host endobacteria in its cytoplasm (Bonfante and Desirò, 2017), AM symbiosis is an outstanding example for discussing inter- and intra- domain signal molecular exchanges. Plants must differentiate between helper bacteria and pathogens around themselves, while the AMF must identify the photosynthetic host to ensure a decreased carbon flow. Some studies have shown that host plants also provide lipids to fungal species, not just the sugars only as believed for many years (Bravo et al., 2017; Jiang et al., 2017; Keymer et al., 2017; Luginbuehl et al., 2017). In exchange, mineral nutrients are passed by AMF to the host plants. These interactions are mainly found in cortical root cells, which are called arbuscules with much ramified fungal hyphae (Gutjahr and Parniske, 2013). Bonfante and Genre (2015) suggested the key roles of the molecules included in inter kingdom-symbiotic signals, including cutin monomers, SLs and molecules similar to chitin, originally not associated with symbiosis. The symbiotic function of these molecules therefore depends on the co-developed ability of the AMF partners to interpret them as signals. SLs have possible effects on microbes that are released into the region of rhizosphere in the soil around the root (Bonfante and Genre, 2008). SL treatment has improved fungal metabolism, resulting in increased ATP production and division of mitochondria (Lanfranco et al., 2017). Pleiotropic effects of SLs affect root development as reported by Marzec and Melzer (2018). SLs also participate in the response of plants to nutrient deficiency by directing association with symbiotic organizations and by promoting root lengthening in addition to their role in the growth of roots and plant architecture in general. Based on recent observations, the restriction of the accidental formation of roots by ethylene is independent of SLs (Marzec and Melzer, 2018). Furthermore, root exposure to SLs has been demonstrated to contribute in the build-up of secondary metabolites, including flavonols and antioxidants, respectively.
Genes of SLs biosynthesis, regulation and their role
A strictly organized interplay between different plant hormones must result bacterial infection and nodule organ formation. Once the receptors of nodulation (Nod) are viewed as the Nod factors, spatiotemporal designs of gene expression can bring rhizobial infection and partition of cortical cells. Nearly all characteristic plant hormones are tangled in the expansion of an efficient nodule in a multifaceted network of connections (Ferguson and Mathesius, 2014). As a consequence, cytokinin's are the important phytohormone that control nodule organ development; sensation mutants in the cytokine receptor eradicate nodulation, whereas spontaneous nodules ensue in cytokine receptor mutants, which are attainment function (Murray et al., 2007). Soto et al. (2010), Foo and Davies (2011), and Ur Rehman et al. (2018) findings indicate that SLs play a vital function in the nodulation process as well. In Medicago sativa (alfa), Pisum sativum (pisum) and soybean, and number of legume species, the use of synthetic SL rac-GR24 had a positive effect on the amount of nodule formation. Also, fewer nodules are developed when SL biosynthesis genes are silenced or get mutated reported by Foo and Davies (2011); Foo et al. (2013); Liu et al. (2013); Haq et al. (2017). These information specify that nodulation by SLs has an optimistic function. For example, in A. thaliana additional axillary branches 2 (Max2) mutants, which practice portion of the SL receptor situation, the number of the nodule in the SL-insensitive ramosus4 (rms4) mutant has been increased (Foo et al., 2013). Moreover, the rac-GR24 treatment distresses the nodule-dependent amount of M. truncatula, with a very low rac-GR24 stimulating effect and an undesirable influence at higher concentrations (De Cuyper et al., 2014). The nodulation process is modulated by SLs. The nodulation action of SL can depend, however on their concentration and the particular sensitivity of the vegetable species to SL. In addition, it should be noted that along with KARRIKIN-INSENSITIVE 2 (KAI2), MAX2/RMS4 contribute likewise in the insight of smoke from karrikine and the uncharacteristic endogenous ligand (Waters et al., 2017). Therefore, the other carotenoid-based molecules apparent by a MAX2/RMS4 and KAI2 complex can show a part at the nodulation stage which cannot be excluded. Rac-GR24 also consists of two diastereoisomers, dual seem in Arabidopsis to remain recognized by KAI2 (Flematti et al., 2016; Scaffidi et al., 2014). To create strict relations amongst plant mutants, metabolites and function, a vigilant study of the role of the SL and carrikin signaling should therefore be carried out in future research (Table 2).
Table 2.
Gene of SLs in plant and their regulation.
| AMF | SLs | Gene(s) | Role/effect in plant growth | Reference |
|---|---|---|---|---|
| – | + | HIGH TILLERING AND DWARF17/DWARF1 (D17/HTD1) | Raises tiller number and improves the yield of grains in rice | (Wang et al., 2020) |
| – | + | WRKY45 | rice (Oryza sativa) / an established determinant of plant immunity | (Zhou et al., 2020) |
| – | + | CAROTENOID CLEAVAGE DIOXYGENASE 7 (NaCCD7: important enzyme in the biosynthesis of SL), max2-like (NaSMXL6/7) DWARF 14 (NaD14: the receptor for SL), and NaMAX2 | Native tobacco, (Nicotiana attenuate) / significant alterations in the accumulated amount of defensive compounds such as nicotine and phenolamides. | (Li et al., 2020) |
| – | + | Oryza sativa 9-CIS-EPOXYCAROTENOID DIOXYGENASE 1 (OsNCED1) | Rice | (Liu et al., 2020) |
| – | + | One LBO gene, two MAX1, and one D27 were involved in the biosynthesis of SL, and two D53, one D3, and one D14 genes were related to the signaling of SL. SL receptor gene FveD14, SL biosynthetic genes FveMAX1B, FveLBO, FveMAXIB, FveMAXIA, FveCCD8, FveCCD7, FveD27 and signaling genes FveD53B, FveD53A, and FveD3 | Increased in the receptacle after pollination and decreased during the development of receptacle in case of woodland strawberry (Fragaria vesca) | (Wu et al., 2019) |
| – | + | PROTEIN KINASE 3 (PKS3) and 1-AMINOCYCLOPROPANE-1-CARBOXYLATE SYNTHASE 4 (ACC4) | Arabidopsis | (Kumar et al., 2019) |
| – | + | MAX3 and MAX4 | SLs directs leaf senescence by increasing the action of ethylene in A. thaliana | (Ueda and Kusaba, 2015) |
| + | – | LjSultr1;2 | Lotus japonicus /improve the status of sulfur under sulfur deficiency | (Giovannetti et al., 2014) |
| – | + | DWARF14 (D14) | inhibits rice tillering | (Arite et al., 2012) |
| – | + | MORE AXILLARY GROWTH 1 (MAX1) | Arabidopsis | (Zhang et al., 2014) |
During nodulation, tissue-specific expression patterns of SL-related genes could indicate the SL role for nodule formation (Liu et al., 2010; van Zeijl et al., 2015; McAdam et al., 2017; Haq et al., 2017). During nodulation, a pattern of expression dominated by the Nod factor signaling route was up-regulated with various SL biosynthesis genes. Tissue-specific tests in the early infection zone of indeterminate, and mature M. truncatular nodules showed that the promoters were involved in the nodule meristem and primordium. This phrase design would match with a role in the regulation of cell division in interplay with cytokinin and auxin, since the operation occurred at sites in cell division. In different SL-mutants of various leguminous species, no changes in the structure of nodule were observed and this function could not be significant. This pattern of expression may also be symptomatic of a function in autoregulation of nodulation (AON), which regulates the amount of fixed N, used by vegetative plants for controlling the nodules number per plant. Studies with AON mutants have however failed to support this hypothesis (Foo et al., 2013; De Cuyper et al., 2014).
In previous stages of the symbiotic root nodule establishment, SLs may already be behaving. The usage of rac-GR24 had a nugatory impact on the growth of infection threads and the expression of an inflammatory marker gene in M. truncatula. In ethylene perception, mutants that cause major infections and this negative effect are abolished (Breakspear et al., 2014; De Cuyper et al., 2014). Instead, a pea mutant of the SL biosynthesis had less infection threads than the wild form (MsAdam et al., 2017). Therefore, implying a role for SLs in controlling the creation of infection threads. However, the contrast findings from the rac-GR24 treatment suggest that the complex of the karrikin receptor requires nodulation, or that a near regulation of the SL concentration is sufficient for the desired rhizobic outcome of the legume interaction. Different legumes can also exhibit different SL susceptibility. Furthermore, it may not always be the same sites of hormone action and biosynthesis. SLs in the dividing cells could therefore be formed but they function at the stage of rhizobial infection. Analysis of the SL perception genes with comprehensive cell-specific expression may give you a better understanding of the validity of the hypothesis. Detailed research is generally required in order to understand the function of SLs in the case of nodulation.
SLs and its application
AMF activation in the rhizosphere, its formation and hyphal branching play the most important role in the production of SLs in microbe-plant interactions. Basically, SLs are released to direct the beneficial symbiont but can also adversely affect the plant, since SLs enable the germination of parasite weeds in the rhizosphere (Rochange et al., 2019). Reproduction activities are intended to reduce the exudation of SL from plants to prevent parasite infestations of plants. The degree to which this affects, AM symbiosis will be necessary to understand. SL genes shift to synthetically alter SL forms (Waters et al., 2017), other approaches may also be intended. Since AMF replies to a variety of SL molecules (Akiyama et al., 2010), it might have potential to design plants that radiate types of SL that rouses AMF, but don't cause adjustable parasitic weeds to germinate.
The way in which SLs control the nodulation is mysterious and the effect of D14 or KAI2 mediated signaling on infection thread formation and nodulation by rac-GR24 is still not understood very well. This can be discovered as leguminous mutants become available in each receptor gene. The genetically or pharmacologically modified SL roots concentrations have reported some conflicting findings in M. truncatula and pea, respectively. There could be a different sensitivity of both species to SLs. Alternatively, the difference may be due to the growth conditions. The biosynthesis of SL (Yoneyama et al., 2012; Nagata et al., 2015) and the pathways of other hormones are affected by light intensity or nutrient concentration, for example the modifications in symmetry and/or communication of numerous hormones will accordingly cause the SL effects disparities. This hypothesis can be answered by structural side-by-side assessments of nodulation effects with diverse growth circumstances (Rochange et al., 2019).
Conclusion
This review shows that SLs were expected to play special roles in order to withstand the accumulative biological complexity of land plants. They are involved in many processes related to plant physiology and development, making them a major regulator in plants that helps the plant to adapt certain stressed conditions. The production and secretion of SLs from plant roots can regulate rhizobium bacteria's coexistence with legumes and the coexistence of plants with AMF can improve plant development and growth, and further control the metabolism of plant under normal and stressed conditions. Furthermore, the impairment of either its signaling or synthesis may compromise with pathogen-specific resistance in plant. SLs are very much essential for the recognition of plants by symbiotic fungi, mainly AMF. SLs can create a strong and extensive root system in plants in order to raise the mineral and water absorption in conditions of deficiency of these substances while AMF needs to recognize a photosynthetic partner which guarantees a flow of reduced carbon. The part of SLs in plants, consequently equivocal as to the perturbation of SL biosynthesis and presented signaling to alter the metabolism of other hormones, which also contributes in the establishment of the AM symbiotic relation. Also, SLs seem to regulate two processes such as phosphate metabolism and morphogenesis of root in the host plants, which are recognized to be, to some degree, under the influence of the AM symbiosis. This makes the SLs an important target for developing synthetic chemicals for a more sustainable and modern agriculture system. Our much-improved understanding of SLs biosynthesis, perception, and downstream signaling is vital for the development of efficient possibilities. More fundamental research on the SLs roles in and outside the host plant is required which will further lead to the alteration of this into an effective tool that we will need to make a sustainable agricultural system. Thus, understanding the complicated relationship between these networks and understanding its details requires further research in the future.
Intellectual property
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Research ethics
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Author contributions
DM, KVR, PC, JR, MSS, HB was involved in the writing, image presentation & table preparation; PKDM, PP, DM was involved in manuscript refinement & important intellectual content discussion; DM, PP, PKDM initiated the idea of the literature review study.
Funding
None
Declaration of Competing Interest
We wish to confirm that there are no known conflicts of interest associated with this publication.
Acknowledgments
The authors are thankful to Raiganj University, Raiganj, India; University of Tehran, Karaj, Iran, Gujarat Arts and Science College, Ellisbridge, India; Banasthali University, India; Cadi Ayyad University, Marrakesh, Morocco and ICAR – National Rice Research Institute, Cuttack, India. D. Mitra is grateful to Government of West Bengal, India for Swami Vivekananda Merit Cum Means Ph.D. Scholarship. D. Mitra is thankful to Prof. Sergio de los Santos Villalobos and Dr. Fannie I. Parra Cota, Laboratorio de Biotecnología del Recurso Microbiano Instituto Tecnológico de Sonora, Sonora, México and Guest Editors of the Special Issue “Current trends in plant growth-promoting microorganisms research for sustainable food security” in Current Research in Microbial Sciences for the invitation. The authors are grateful to reviewers for their valuable suggestions to increase the scientific quality of the manuscript.
Contributor Information
Pradeep K. Das Mohapatra, Email: pkdmvu@gmail.com.
Periyasamy Panneerselvam, Email: panneerccri@rediffmail.com.
References
- Akiyama K., Hayashi H. Strigolactones: chemical signals for fungal symbionts and parasitic weeds in plant roots. Ann. Bot. 2006;97:925–931. doi: 10.1093/aob/mcl063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K., Matsuzaki K.-i., Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- Akiyama K., Ogasawara S., Ito S., Hayashi H. Structural requirements of strigolactones for hyphal branching in AM fungi. Plant. Cell. Physiol. 2010;51:1104–1117. doi: 10.1093/pcp/pcq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arite T., Kameoka H., Kyozuka J. Strigolactone positively controls crown root elongation in rice. J. Plant. Growth. Regul. 2012;31:165–172. doi: 10.1007/s00344-011-9228-6. [DOI] [Google Scholar]
- Begum N., Qin C., Ahanger M.A., Raza S., Khan M.I., Ashraf M., Zhang L. Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front. Plant. Sci. 2019;10:1068. doi: 10.3389/fpls.2019.01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birhane E., Sterck F.J., Fetene M., Bongers F., Kuyper T.W. Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia. 2012;169:895–904. doi: 10.1007/s00442-012-2258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfante P., Desirò A. Who lives in a fungus? The diversity, origins and functions of fungal endobacteria living in Mucoromycota. ISME. J. 2017;11:1727–1735. doi: 10.1038/ismej.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfante P., Genre A. Plants and arbuscular mycorrhizal fungi: an evolutionary-developmental perspective. Trends. Plant. Sci. 2008;13:492–498. doi: 10.1016/j.tplants.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Bonfante P., Genre A. Arbuscular mycorrhizal dialogues: do you speak ‘plantish’ or ‘fungish’? Trends. Plant. Sci. 2015;20:150–154. doi: 10.1016/j.tplants.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Bowles T.M., Barrios-Masias F.H., Carlisle E.A., Cavagnaro T.R., Jackson L.E. Effects of arbuscular mycorrhizae on tomato yield, nutrient uptake, water relations, and soil carbon dynamics under deficit irrigation in field conditions. Sci. Total. Environ. 2016;566:1223–1234. doi: 10.1016/j.scitotenv.2016.05.178. [DOI] [PubMed] [Google Scholar]
- Bravo A., Brands M., Wewer V., Dörmann P., Harrison M.J. Arbuscular mycorrhiza- specific enzymes FatM and RAM2 fine-tune lipid biosynthesis to promote development of arbuscular mycorrhiza. New. Phytol. 2017;214:1631–1645. doi: 10.1111/nph.14533. [DOI] [PubMed] [Google Scholar]
- Breakspear A., Liu C., Roy S., Stacey N., Rogers C., Trick M., Morieri G., Mysore K.S., Wen J., Oldroyd G.E.D., Downie J.A., Murray J.D. The root hair “infectome” of medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for auxin signaling in rhizobial infection. Plant. Cell. 2014;26:4680–4701. doi: 10.1105/tpc.114.133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalhais L.C., Rincon-Florez V.A., Brewer P.B., Beveridge C.A., Dennis P.G., Schenk P.M. The ability of plants to produce strigolactones affects rhizosphere community composition of fungi but not bacteria. Rhizosphere. 2019;9:18–26. doi: 10.1016/j.rhisph.2018.10.002. [DOI] [Google Scholar]
- Castellanos-Morales V., Villegas J., Wendelin S., Vierheiling H., Eder R., Cardenas-Navarro R. Root colonization by the arbuscular mycorrhizal fungus Glomus intraradices alters the quality of strawberry fruit (Fragaria ananassa Duch.) at different nitrogen levels. J. Sci. Food Agric. 2010;90:1774–1782. doi: 10.1002/jsfa.3998. [DOI] [PubMed] [Google Scholar]
- Chaudhary P., Jain D., Anand K., Mitra D. Microbial Resources for Sustainable Agriculture. LAP LAMBERT Academic Publishing; 2019. Biofertilizers: a sustainable approach for plant and soil health; pp. 106–119. [Google Scholar]
- Chen M., Arato M., Borghi L., Nouri E., Reinhardt D. Beneficial services of arbuscular mycorrhizal fungi–from ecology to application. Front. Plant. Sci. 2018;9:1270. doi: 10.3389/fpls.2018.01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Lee T., Cho J., Servante E.K., Pucker B., Summers W., Bowden S., Rahimi M., An K., An G. The negative regulator SMAX1 controls mycorrhizal symbiosis and strigolactone biosynthesis in rice. Nat. Commun. 2020;11:1–13. doi: 10.1038/s41467-020-16021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C.E., Whichard L.P., Turner B., Wall M.E., Egley G.H. Germination of Witchweed (Striga lutea Lour.): isolation and Properties of a Potent Stimulant. Science. 1966;154:1189–1190. doi: 10.1126/science.154.3753.1189. [DOI] [PubMed] [Google Scholar]
- Corradi N., Brachmann A. Fungal mating in the most widespread plant symbionts? Trends. Plant. Sci. 2017;22:175–183. doi: 10.1016/j.tplants.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Czarnecki O., Yang J., Weston D.J., Tuskan G.A., Chen J.-.G. A dual role of strigolactones in phosphate acquisition and utilization in plants. Int. J. Mol. Sci. 2013;14:7681–7701. doi: 10.3390/ijms14047681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cuyper C., Fromentin J., Yocgo R.E., De Keyser A., Guillotin B., Kunert K., Boyer F.-.D., Goormachtig S. From lateral root density to nodule number, the strigolactone analogue GR24 shapes the root architecture of Medicago truncatula. J. Exp. Bot. 2014;66:137–146. doi: 10.1093/jxb/eru404. [DOI] [PubMed] [Google Scholar]
- Ferguson B.J., Mathesius U. Phytohormone regulation of legume-rhizobia interactions. J. Chem. Ecol. 2014;40:770–790. doi: 10.1007/s10886-014-0472-7. [DOI] [PubMed] [Google Scholar]
- Flematti G.R., Scaffidi A., Waters M.T., Smith S.M. Stereospecificity in strigolactone biosynthesis and perception. Planta. 2016;243:1361–1373. doi: 10.1007/s00425-016-2523-5. [DOI] [PubMed] [Google Scholar]
- Foo E., Davies N.W. Strigolactones promote nodulation in pea. Planta. 2011;234:1073. doi: 10.1007/s00425-011-1516-7. [DOI] [PubMed] [Google Scholar]
- Foo E., Yoneyama K., Hugill C.J., Quittenden L.J., Reid J.B. Strigolactones and the Regulation of Pea Symbioses in Response to Nitrate and Phosphate Deficiency. Mol. Plant. 2013;6:76. doi: 10.1093/mp/sss115. [DOI] [PubMed] [Google Scholar]
- Friml J. Auxin transport — Shaping the plant. Curr. Opin. Plant. Biol. 2003;6:7–12. doi: 10.1016/S1369526602000031. [DOI] [PubMed] [Google Scholar]
- Giovannetti M., Tolosano M., Volpe V., Kopriva S., Bonfante P. Identification and functional characterization of a sulfate transporter induced by both sulfur starvation and mycorrhiza formation in Lotus japonicus. New. Phytol. 2014;204:609–619. doi: 10.1111/nph.12949. [DOI] [PubMed] [Google Scholar]
- Gobena D., Shimels M., Rich P.J., Ruyter-Spira C., Bouwmeester H., Kanuganti S., Mengiste T., Ejeta G. Mutation in sorghum LOW GERMINATION STIMULANT 1 alters strigolactones and causes Striga resistance. Proc. Natl. Acad. Sci. U.S.A. 2017;114:4471–4476. doi: 10.1073/pnas.1618965114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh T., Kasahara H., Mimura T., Kamiya Y., Fukaki H. Multiple AUX/IAa ARF modules regulate lateral root formation: the role of Arabidopsis SHY2/IAA3-mediated auxin signalling. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2012;367:1461–1468. doi: 10.1098/rstb.2011.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roldan V., Fermas S., Brewer P.B., Puech-Pagès V., Dun E.A., Pillot J.-.P., Letisse F., Matusova R., Danoun S., Portais J.-.C. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- Gutjahr C., Parniske M. Cell and developmental biology of arbuscular mycorrhiza symbiosis. Annu. Rev. Cell. Dev. Biol. 2013;29:593–617. doi: 10.1146/annurev-cellbio-101512-122413. [DOI] [PubMed] [Google Scholar]
- Haq B.U., Ahmad M.Z., ur Rehman N., Wang J., Li P., Li D., Zhao J. Functional characterization of soybean strigolactone biosynthesis and signaling genes in Arabidopsis MAX mutants and GmMAX3 in soybean nodulation. BMC Plant Biol. 2017;17:259. doi: 10.1186/s12870-017-1182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrier L. The arbuscular mycorrhizal symbiosis: a molecular review of the fungaldimension. J. Exp. Bot. 2001;52:469–478. doi: 10.1093/jxb/52.suppl_1.469. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Wang W., Xie Q., Liu N., Liu L., Wang D., Zhang X., Yang C., Chen X., Tang D. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science. 2017;356:1172–1175. doi: 10.1126/science.aam9970. [DOI] [PubMed] [Google Scholar]
- Johnson N.C., Wilson G., Wilson J., Miller M., Bowker M. Mycorrhizal phenotypes and the law of the minimum. New. Phytol. 2015;205:1473–1484. doi: 10.1111/nph.13172. [DOI] [PubMed] [Google Scholar]
- Kaldorf M., Ludwig-Müller J. AM fungi might affect the root morphology of maize by increasing indole-3-butyric acid biosynthesis. Physiol. Plant. 2000;109:58–67. doi: 10.1034/j.1399-3054.2000.100109.x. [DOI] [Google Scholar]
- Kapulnik Y., Delaux P.-.M., Resnick N., Mayzlish-Gati E., Wininger S., Bhattacharya C., Séjalon-Delmas N., Combier J.-.P., Bécard G., Belausov E. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta. 2011;233:209–216. doi: 10.1007/s00425-010-1310-y. [DOI] [PubMed] [Google Scholar]
- Kapulnik Y., Koltai H. Strigolactone involvement in root development, response to abiotic stress, and interactions with the biotic soil environment. Plant. Physiol. 2014;166:560–569. doi: 10.1104/pp.114.244939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapulnik Y., Resnick N., Mayzlish-Gati E., Kaplan Y., Wininger S., Hershenhorn J., Koltai H. Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. J. Exp. Bot. 2011;62:2915–2924. doi: 10.1093/jxb/erq464. [DOI] [PubMed] [Google Scholar]
- Karabaghli-Degron C., Sotta B., Bonnet M., Gay G., Le Tacon F. The auxin transport inhibitor 2, 3, 5-triiodobenzoic acid (TIBA) inhibits the stimulation of in vitro lateral root formation and the colonization of the tap-root cortex of Norway spruce (Picea abies) seedlings by the ectomycorrhizal fungus Laccaria bicolor. New. Phytol. 1998;140:723–733. doi: 10.1046/j.1469-8137.1998.00307.x. [DOI] [PubMed] [Google Scholar]
- Keymer A., Pimprikar P., Wewer V., Huber C., Brands M., Bucerius S.L., Delaux P.-.M., Klingl V., von Röpenack-Lahaye E., Wang T.L. Lipid transfer from plants to arbuscular mycorrhiza fungi. Elife. 2017;6:e29107. doi: 10.7554/eLife.29107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlen W., Charnikhova T., Liu Q., Bours R., Domagalska M.A., Beguerie S., Verstappen F., Leyser O., Bouwmeester H., Ruyter-Spira C. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant. Physiol. 2011;155:974–987. doi: 10.1104/pp.110.164640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Kim I., Kim Y.-.K., Heo J.B., Suh M.C., Kim H.U. Strigolactone signaling genes showing differential expression patterns in Arabidopsis max. Mutants. Plants. 2019;8:352. doi: 10.3390/plants8090352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Pandya-Kumar N., Kapulnik Y., Koltai H. Strigolactone signaling in root development and phosphate starvation. Plant. Signal. Behav. 2015;10 doi: 10.1080/15592324.2015.1045174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranco L., Fiorilli V., Venice F., Bonfante P. Strigolactones cross the kingdoms: plants, fungi, and bacteria in the arbuscular mycorrhizal symbiosis. J. Exp. Bot. 2017;69:2175–2188. doi: 10.1093/jxb/erx432. [DOI] [PubMed] [Google Scholar]
- Lendzemo V.W., Kuyper T.W., Matusova R., Bouwmeester H.J., Ast A.V. Colonization by Arbuscular Mycorrhizal Fungi of Sorghum Leads to Reduced Germination and Subsequent Attachment and Emergence of Striga hermonthica. Plant. Signal. Behav. 2007;2:58–62. doi: 10.4161/psb.2.1.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Joo Y., Cao D., Li R., Lee G., Halitschke R., Baldwin G., Baldwin I.T., Wang M. Strigolactone signaling regulates specialized metabolism in tobacco stems and interactions with stem-feeding herbivores. PLoS. Biol. 2020;18 doi: 10.1371/journal.pbio.3000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Novero M., Charnikhova T., Ferrandino A., Schubert A., Ruyter-Spira C., Bonfante P., Lovisolo C., Bouwmeester H.J., Cardinale F. Carotenoid cleavage dioxygenase 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model legume Lotus japonicus. J. Exp. Bot. 2013;64:1967–1981. doi: 10.1016/j.molp.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.-.Q., Allan D.L., Vance C.P. Systemic signaling and local sensing of phosphate in common bean: cross-talk between photosynthate and MicroRNA399. Mol. Plant. 2010;3:428–437. doi: 10.1093/mp/ssq008. [DOI] [PubMed] [Google Scholar]
- Liu X., Hu Q., Yan J., Sun K., Liang Y., Jia M., Meng X., Fang S., Wang Y., Jing Y., Liu G., Wu D., Chu C., Smith S.M., Chu J., Wang Y., Li J., Wang B. ζ-Carotene isomerase suppresses tillering in rice through the coordinated biosynthesis of strigolactone and abscisic acid. Mol. Plant. 2020;13:1784–1801. doi: 10.1016/j.molp.2020.10.001. [DOI] [PubMed] [Google Scholar]
- Ludwig-Müller J., Kaldorf M., Sutter E.G., Epstein E. Indole-3-butyric acid (IBA) is enhanced in young maize (Zea mays L.) roots colonized with the arbuscular mycorrhizal fungus Glomus intraradices. Plant. Sci. 1997;125:153–162. doi: 10.1016/S0168-9452(97)00064-2. [DOI] [Google Scholar]
- Luginbuehl L.H., Menard G.N., Kurup S., Van Erp H., Radhakrishnan G.V., Breakspear A., Oldroyd G.E., Eastmond P.J. Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science. 2017;356:1175–1178. doi: 10.1126/science.aan0081. [DOI] [PubMed] [Google Scholar]
- Marzec M., Melzer M. Regulation of root development and architecture by strigolactones under optimal and nutrient deficiency conditions. Int. J. Mol. Sci. 2018;19:1887. doi: 10.3390/ijms19071887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M., Muszynska A., Gruszka D. The role of strigolactones in nutrient-stress responses in plants. Int. J. Mol. Sci. 2013;14:9286–9304. doi: 10.3390/ijms14059286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayzlish-Gati E., De-Cuyper C., Goormachtig S., Beeckman T., Vuylsteke M., Brewer P.B., Beveridge C.A., Yermiyahu U., Kaplan Y., Enzer Y., Wininger S., Resnick N., Cohen M., Kapulnik Y., Koltai H. Strigolactones are involved in root response to low phosphate conditions in Arabidopsis. Plant. Physiol. 2012;160:1329–1341. doi: 10.1104/pp.112.202358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam E.L., Hugill C., Fort S., Samain E., Cottaz S., Davies N.W., Reid J.B., Foo E. Determining the site of action of strigolactones during nodulation. Plant. Physiol. 2017;175:529–542. doi: 10.1104/pp.17.00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo C.D., Walker C., Krüger C., Borges P.A.V., Luna S., Mendonça D., Fonseca H.M.A.C., Machado A.C. Environmental factors driving arbuscular mycorrhizal fungal communities associated with endemic woody plant Picconiaazorica on native forest of Azores. Ann. Microbiol. 2019;69:1309–1327. doi: 10.1007/s13213-019-01535-x. [DOI] [Google Scholar]
- Mitra D., Anđelković S., Panneerselvam P., Senapati A., Vasić T., Ganeshamurthy A., Chauhan M., Uniyal N., Mahakur B., Radha T. Phosphate-solubilizing microbes an biocontrol agent for plant nutrition and protection: current perspective. Commun. Soil. Sci. Plant. Anal. 2020;51:645–657. doi: 10.1080/00103624.2020.1729379. [DOI] [Google Scholar]
- Mitra D., Uniyal N., Panneerselvam P., Senapati A., Ganeshamurthy A. Role of mycorrhiza and its associated bacteria on plant growth promotion and nutrient management in sustainable agriculture. Int. J. Life. Sci. Applied. Sci. 2019;1:1–10. http://www.ijlsas.com/?mno=302644881 [Google Scholar]
- Mohemed N., Charnikhova T., Bakker E.J., van Ast A., Babiker A.G., Bouwmeester H.J. Evaluation of field resistance to Striga hermonthica (Del.) Benth. in Sorghum bicolor (L.) Moench. The relationship with strigolactones. Pest. Manag. Sci. 2016;72:2082–2090. doi: 10.1002/ps.4426. [DOI] [PubMed] [Google Scholar]
- Murray J.D., Karas B.J., Sato S., Tabata S., Amyot L., Szczyglowski K. A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science. 2007;315:101–104. doi: 10.1126/science.1132514. [DOI] [PubMed] [Google Scholar]
- Nagata M., Yamamoto N., Shigeyama T., Terasawa Y., Anai T., Sakai T., Inada S., Arima S., Hashiguchi M., Akashi R., Nakayama H., Ueno D., Hirsch A.M., Suzuki A. Red/far red light controls arbuscular mycorrhizal colonization via jasmonic acid and strigolactone signaling. Plant. Cell. Physiol. 2015;56:2100–2109. doi: 10.1093/pcp/pcv135. [DOI] [PubMed] [Google Scholar]
- Nanjundappa A., Bagyarai D.J., Saxena A.K., Kumar M., Chakdar H. Interaction between arbuscular mycorrhizal fungi and Bacillus spp. in soil enhancing growth of crop plants. Fungal Biol. Biotechnol. 2019;6 doi: 10.1186/s40694-019-0086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-de-Luque A. Interaction of nanomaterials with plants: what do we need for real applications in agriculture? Front. Environ. Sci. 2017;5:12. doi: 10.1038/s41598-017-16697-4. [DOI] [Google Scholar]
- Rochange S., Goormachtig S., Lopez-Raez J.A., Gutjahr C. Strigolactones-Biology and Applications. Springer; 2019. The role of strigolactones in plant–microbe interactions; pp. 121–142. [DOI] [Google Scholar]
- Rozpądek P., Domka A.M., Nosek M., Ważny R., Jędrzejczyk R.J., Wiciarz M., Turnau K. The role of strigolactone in the cross-talk between Arabidopsis thaliana and the endophytic fungus Mucor sp. Front. Microbiol. 2018;9:441. doi: 10.3389/fmicb.2018.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyter-Spira C., Kohlen W., Charnikhova T., van Zeijl A., van Bezouwen L., de Ruijter N., Cardoso C., Lopez-Raez J.A., Matusova R., Bours R., Verstappen F., Bouwmeester H. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant. Physiol. 2011;155:721–734. doi: 10.1104/pp.110.166645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi A., Waters M.T., Sun Y.K., Skelton B.W., Dixon K.W., Ghisalberti E.L., Flematti G.R., Smith S.M. Strigolactone hormones and their stereoisomers signal through two related receptor proteins to induce different physiological responses in Arabidopsis. Plant. Physiol. 2014;165:1221–1232. doi: 10.1104/pp.110.166645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Xue Z., Sun L., Zhao C., Sun S., Liu J., Zhao Y., Liu J. Effect of GR24 concentrations on biogas upgrade and nutrient removal by microalgae-based technology. Bioresour. Technol. 2020;312 doi: 10.1016/j.biortech.2020.123563. [DOI] [PubMed] [Google Scholar]
- Soto M.J., Fernández-Aparicio M., Castellanos-Morales V., García-Garrido J.M., Ocampo J.A., Delgado M.J., Vierheilig H. First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa) Soil. Biol. Biochem. 2010;42:383–385. doi: 10.1016/j.soilbio.2009.11.007. [DOI] [Google Scholar]
- Spatafora J.W., Chang Y., Benny G.L., Lazarus K., Smith M.E., Berbee M.L., Bonito G., Corradi N., Grigoriev I., Gryganskyi A. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia. 2016;108:1028–1046. doi: 10.3852/16-042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin C., Xie Z.-.P., Illana A., Vierheilig H. Long-distance transport of signals during symbiosis. Plant. Signal. Behav. 2011;6:372–377. doi: 10.4161/psb.6.3.13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Tao J., Bi Y., Hou M., Lou J., Chen X., Zhang X., Luo L., Xie X., Yoneyama K., Zhao Q., Xu G., Zhang Y. OsPIN1b is involved in rice seminal root elongation by regulating root apical meristem activity in response to low nitrogen and phosphate. Sci. Rep. 2018;8:13014. doi: 10.1038/s41598-018-29784-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Tao J., Gu P., Xu G., Zhang Y. The role of strigolactones in root development. Plant. Signal. Behav. 2016;11 doi: 10.1080/15592324.2015.1110662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Tao J., Liu S., Huang S., Chen S., Xie X., Yoneyama K., Zhang Y., Xu G. Strigolactones are involved in phosphate- and nitrate-deficiency-induced root development and auxin transport in rice. J. Exp. Bot. 2014;65:6735–6746. doi: 10.1093/jxb/eru02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Chen F., Yuan L., Mi G. The physiological mechanism underlying root elongation in response to nitrogen deficiency in crop plants. Planta. 2020;251:84. doi: 10.1007/s00425-020-03376-4. [DOI] [PubMed] [Google Scholar]
- Ueda H., Kusaba M. Strigolactone regulates leaf senescence in concert with ethylene in Arabidopsis. Plant. Physiol. 2015;169:138–147. doi: 10.1104/pp.15.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara M., Hanada A., Yoshida S., Akiyama K., Arite T., Takeda-Kamiya N., Magome H., Kamiya Y., Shirasu K., Yoneyama K., Kyozuka J., Yamaguchi S. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- Ur Rehman N., Ali M., Ahmad M.Z., Liang G., Zhao J. Strigolactones promote rhizobia interaction and increase nodulation in soybean (Glycine max) Microb. Pathog. 2018;114:420–430. doi: 10.1016/j.micpath.2017.11.049. [DOI] [PubMed] [Google Scholar]
- van Zeijl A., Liu W., Xiao T.T., Kohlen W., Yang W.-.C., Bisseling T., Geurts R. The strigolactone biosynthesis gene DWARF27 is co-opted in rhizobium symbiosis. BMC. Plant. Biol. 2015;15:260. doi: 10.1186/s12870-015-0651-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldie T., Hayward A., Beveridge C.A. Axillary bud outgrowth in herbaceous shoots: how do strigolactones fit into the picture? Plant. Mol. Biol. 2010;73:27–36. doi: 10.1007/s11103-010-9599-2. [DOI] [PubMed] [Google Scholar]
- Wang Y., Shang L., Yu H., Zeng L., Hu J., Ni S., Rao Y., Li S., Chu J., Meng X., Wang L., Hu P., Yan J., Kang S., Qu M., Lin H., Wang T., Wang Q., Hu X., Chen H., Wang B., Gao Z., Guo L., Zeng D., Zhu X., Xiong G., Li J., Qian Q. A strigolactone biosynthesis gene contributed to the green revolution in rice. Mol. Plant. 2020;13:923–932. doi: 10.1016/j.molp.2020.03.009. [DOI] [PubMed] [Google Scholar]
- Waters M.T., Gutjahr C., Bennett T., Nelson D.C. Strigolactone signaling and evolution. Annu. Rev. Plant. Biol. 2017;68 doi: 10.1146/annurev-arplant-042916-040925. [DOI] [PubMed] [Google Scholar]
- Wiśniewska J., Xu J., Seifertová D., Brewer P.B., Růžička K., Blilou I., Rouquié D., Benková E., Scheres B., Friml J. Polar PIN localization directs auxin flow in plants. Science. 2006;312 doi: 10.1126/science.1121356. 883-883. [DOI] [PubMed] [Google Scholar]
- Wu H., Li H., Chen H., Qi Q., Ding Q., Xue J., Ding J., Jiang X., Hou X., Li Y. Identification and expression analysis of strigolactone biosynthetic and signaling genes reveal strigolactones are involved in fruit development of the woodland strawberry (Fragaria vesca) BMC Plant. Biol. 2019;19:73. doi: 10.1186/s12870-019-1673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Yoneyama K., Kisugi T., Uchida K., Ito S., Akiyama K., Hayashi H., Yokota T., Nomura T., Yoneyama K. Confirming stereochemical structures of strigolactones produced by rice and tobacco. Mol. Plant. 2013;6:153–163. doi: 10.1093/mp/sss139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan W., Band L.R., Kumpf R.P., Van Damme D., Parizot B., De Rop G., Opdenacker D., Möller B.K., Skorzinski N., Njo M.F., De Rybel B., Audenaert D., Nowack M.K., Vanneste S., Beeckman T. Cyclic programmed cell death stimulates hormone signaling and root development in Arabidopsis. Science. 2016;351:384–387. doi: 10.1126/science.aad2776. [DOI] [PubMed] [Google Scholar]
- Yoneyama K., Awad A.A., Xie X., Yoneyama K., Takeuchi Y. Strigolactones as germination stimulants for root parasitic plants. Plant. Cell. Physiol. 2010;51:1095–1103. doi: 10.1093/pcp/pcq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama, K., Kisugi, T., Xie, X., Yoneyama, K., 2013. Chemistry of strigolactones: why and how do plants produce so many strigolactones?, molecular microbial ecology of the rhizosphere. pp. 373–379. doi: 10.1002/9781118297674.ch34.
- Yoneyama K., Xie X., Kim H.I., Kisugi T., Nomura T., Sekimoto H., Yokota T., Yoneyama K. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta. 2012;235:1197–1207. doi: 10.1007/s00425-011-1568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama K., Yoneyama K., Takeuchi Y., Sekimoto H. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta. 2007;225:1031–1038. doi: 10.1007/s00425-006-0410-1. [DOI] [PubMed] [Google Scholar]
- Yoneyama K., Arakawa R., Ishimoto K., Kim H.I., Kisugi T., Xie X., Nomura T., Kanampiu F., Yokota T., Ezawa T., Yoneyama K. Difference in striga-susceptibility is reflected in strigolactone secretion profile, but not in compatibility and host preference in arbuscular mycorrhizal symbiosis in two maize cultivars. Plant. Physiol. 2015;206(3):983–989. doi: 10.1111/nph.13375. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Yan C., Liu J., Lu H., Duan H., Du J., Wang W. Silicon alleviation of cadmium toxicity in mangrove (Avicennia marina) in relation to cadmium compartmentation. J. Plant. Growth. Regul. 2014;33:233–242. doi: 10.1038/nchembio.1660. [DOI] [Google Scholar]
- Zhou X., Liu L., Li Y., Li K., Liu X., Zhou J., Yang C., Liu X., Fang C., Luo J. Integrative metabolomic and transcriptomic analyses reveal metabolic changes and its molecular basis in rice mutants of the strigolactone pathway. Metabolites. 2020;10:425. doi: 10.3390/metabo10110425. [DOI] [PMC free article] [PubMed] [Google Scholar]