Highlights
-
•
S. pneumoniae invades brain endothelium through a novel dynamin independent endocytosis pathway.
-
•
Invasion through dynamin independent pathway is aided by SPN adhesin and host receptor interaction.
-
•
Entry through dynamin independent route promotes enhanced intracellular persistence.
Keywords: Streptococcus pneumoniae, Blood-brain barrier, Clathrin mediated endocytosis, Caveolae, Dynamin independent endocytosis
Abstract
Adoption of an endocytosis route promoting safe intracellular trafficking is a pre-requisite for development of invasive diseases by Streptococcus pneumoniae (SPN). We aim to explore the contribution of various endocytic routes in internalization and survival of SPN in blood brain barrier (BBB), a key event in development of pneumococcal meningitis. Pneumococcal entry and survival in brain endothelial cells were evaluated following treatment with combinations of inhibitors to block multiple endocytosis pathways leaving a single entry port open. Entry of SPN into brain endothelium through a novel dynamin independent pathway dictates a separate downstream trafficking itinerary. This allows SPN to evade lysosomal degradation, potentially promoting safe transit across BBB, leading to development of meningitis.
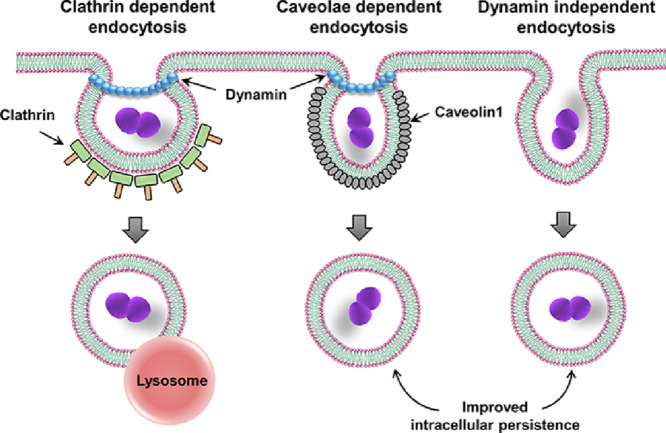
Graphical abstract
1. Introduction
Endocytosis is a fundamental process involved in cellular functions such as nutrient and fluid uptake, receptor internalization and signaling, promoting growth and homeostasis at the cellular level (Kirkham and Parton, 2005). The different endocytic routes recognized are broadly classified as clathrin-dependent and clathrin-independent pathways. While the clathrin-dependent endocytosis is the most widely studied pathway, recently there has been a surge in the discovery, morphological and molecular characterization of various clathrin-independent endocytic pathways, for example; caveolae mediated endocytosis, macropinocytosis, clathrin and dynamin independent internalized cargo - GPI enriched early endosomal compartments (CLIC/GEEC) mediated pathway, flotillin pathway and Arf6 dependent pathway (Kirkham and Parton, 2005). Clathrin-mediated endocytosis is initiated by the recruitment of clathrin coat components to the plasma membrane, followed by assembly of clathrin triskelions assisted by adaptor proteins (APs) into a curved polygonal web that ultimately drives the deformation of the anchored membrane to form a clathrin-coated pit (Takei et al., 2005). Caveolae are distinct membrane invaginations seen in plasma membrane structured by caveolin- family proteins. The membrane lipid in this case is highly hydrophobic commonly termed as Lipid rafts (Kiss and Botos, 2009). Both of the pathways are completed by the fission reaction, in which dynamin GTPase acts as a molecular scissor (Hinshaw and Schmid, 1995). Many pathogens can hijack these endocytic routes for invasion into various host cells either to support their intracellular life or for dissemination across host cell barriers (Veiga and Cossart, 2005; Ellington et al., 1999; Rohde et al., 2003; Wooldridge et al., 1996; Lemire et al., 2012; Schneider et al., 2007; Watarai et al., 2002; Loh et al., 2017).
The Gram positive bacterium, Streptococcus pneumoniae (SPN, pneumococcus), is a common asymptomatic nasopharyngeal colonizer in humans. However, its transition into an invasive pathogen causes debilitating diseases like pneumonia, sepsis and meningitis which contribute significantly to the global disease burden (Henriques-Normark and Tuomanen, 2013). Pneumococcal invasion into the brain requires its safe trafficking across the blood-brain barrier (BBB) leading to meningitis (Ring et al., 1998; Iovino et al., 2013; Orihuela et al., 2009). This involves a series of complex interactions between SPN and the host triggering bacterial internalization, evasion of lysosomal fusion and its exit at the basolateral side of the brain endothelium. In this study, using widely used endocytosis pathway inhibitors, we investigated the contribution of different endocytic routes in pneumococcal uptake and survival inside the BBB. Though majority of the SPN following invasion in brain endothelium gets targeted towards lysosomal killing (Radin et al., 2005), few SPN can cross the BBB as a live pathogen to cause CNS infection. We therefore attempted to compare the fate of pneumococci entering via different endocytic pathways, with the supposition that, a particular endocytic route might provide survival benefit to SPN by preventing or delaying lysosomal fusion and thus promoting its safe transit across BBB.
2. Materials and methods
2.1. Reagents and antibodies
The following chemicals and antibodies are used in this study: chlorpromazine hydrochloride (CPZ; C8138), Dansylcadaverine (MDC; D4008), Methyl-β cyclodextrin (Mβ-CD; C4555), Dynasore hydrate (D7693), 5-(N-Ethyl-N-isopropyl) amiloride (EIPA; A3085), Cholera toxin B subunit (CTxB; C1655) and FITC-Dextran (FD70) from Sigma Aldrich; DyngoⓇ 4a (ab120689) from Abcam; Anti-Transferrin antibody from Pierce (PA3913). CPZ and Mβ-CD was dissolved in water, Dynasore and Dyngo-4a in DMSO and methanol was used to solubilize EIPA.
2.2. Bacterial strains and cell culture
SPN strain R6 (serotype 2, unencapsulated) was grown in Todd-Hewitt broth (THB) supplemented with 1.5% yeast extract at 37⁰C in 5% CO2. Human brain microvascular endothelial cells (hBMEC) were cultured in RPMI 1640 medium (Gibco) supplemented with 10% Fetal Bovine Serum (Gibco), 10% Nu-Serum (Corning), and 1% Minimum Essential Medium Non-Essential Amino acids (Gibco) at 37 °C in 5% CO2 (Stins et al., 2001).
2.3. Chemical inhibitions and bacterial infections
Infection assays were performed as described earlier (Uchiyama et al., 2009). Briefly, fully confluent hBMEC monolayers were pretreated (1 h) with different chemical inhibitors at indicated concentrations or vehicle (agents used for solubilizing the inhibitors) and infected with exponentially grown SPN (OD600 nm ∼ 0.4) for 1 h at a multiplicity of infection (MOI) of 10. No significant change in bacterial CFU count was observed during this 1 h infection period. For adherence, following infection hBMECs were thoroughly washed with PBS, trypsinized (0.025% trypsin EDTA), lysed (0.025% Triton X-100) and lysates were spread plated for enumeration of the bacterial count. The levels of adherence were calculated as follows: (recovered CFU/CFU in inoculum) × 100%. For invasion, after infection with SPN as described in the above adhesion assays, hBMEC monolayers were incubated in medium containing penicillin (10 µg/ml) and gentamicin (400 µg/ml) for 2 h to kill extracellular SPN. Subsequently, cells were trypsinized (0.025% trypsin EDTA), lysed (0.025% Triton X-100) and lysates were plated for enumeration of the intracellular bacterial count. Bacterial invasion efficiency was calculated as (recovered CFU/inoculum CFU) × 100%. Intracellular survival of SPN was assessed similarly by plating infected cell lysates at indicated time intervals following antibiotic treatment (10 µg/ml penicillin and 400 µg/ml gentamicin throughout the assay) and was represented as percent survival at indicated time points relative to 0 h.
Inhibitor concentrations were optimized by assessing uptake of pathway specific cargos following inhibitor treatment. The following specific cargo/s were used: Transferrin (clathrin-mediated endocytosis, 100 ng/ml, 2 min), FITC-Cholera toxin B (caveolae-mediated endocytosis, 500 ng/ml, 15 min) and FITC-Dextran 70 KDa (dynamin independent endocytosis, 1 mg/ml, 15 min). Subsequently, cells were washed and processed for fluorescence microscopy.
2.4. MTT assay
Cell viability of hBMEC following treatment with different endocytic pathway inhibitors was assessed using MTT assay kit (HiMedia, India) according to manufacturer's protocol. Vehicles (H2O, DMSO or methanol at concentrations used to dissolve the inhibitors) and Triton X-100 (0.025%) were used as negative and positive control, respectively.
2.5. Fluorescence imaging and analysis
For immunofluorescence, hBMECs were grown on 1% collagen coated glass cover slips. Following treatments or infection, cells were washed with RPMI, fixed with 4% paraformaldehyde, permeabilized using 0.1% Triton X-100 and blocked in 3% BSA. After treatment with appropriate primary and secondary antibody, coverslips were mounted with VectaShieldⓇ (Vector Laboratories) and visualized with a Laser Scanning Confocal microscope (Zeiss Axio-Observer Z1) with 63X oil objectives. The images were acquired after optical sectioning and then processed using Imaris software (Version 5.0.). Total cellular fluorescence intensity was calculated using ImageJ.
2.6. Trypsinization of SPN and protein analysis
Mid exponentially grown SPN cultures (OD600 nm ∼ 0.4) were treated with 0.85 µg/ml chloramphenicol for 1 h at 37 °C to block protein synthesis. Pneumococcal cells were then trypsinized (1 µg/ml) for 1 h to shave surface proteins. Mid logarithmic phase grown bacteria was chosen for surface shaving to match the protein expression during infections. To evaluate shaving efficiency, supernatant of trypsin treated SPN was precipitated with 10% TCA and loaded on to a 12% SDS-PAGE gel. Cell lysates of non-treated bacteria served as a control. The trypsinized pneumococci were used for invasion assay.
2.7. Statistical analysis
All statistical analysis was done using GraphPad Prism software (version 5). Statistical tests undertaken for individual experiments are mentioned in the respective figure legends. Statistical significance was accepted at p < 0.05.
3. Results
3.1. SPN invades BBB via clathrin- and caveloae-mediated endocytic pathways
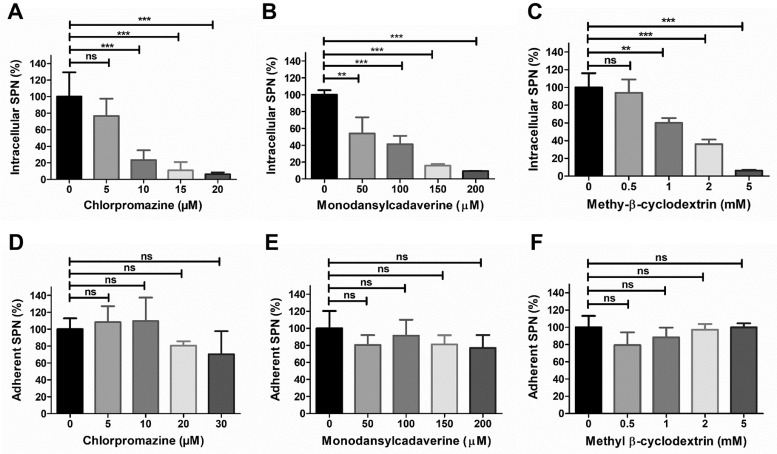
To evaluate the role of different endocytic pathways in pneumococcal BBB invasion, we inhibited various endocytic routes by treating cells with different inhibitors followed by invasion assays with SPN. Our results showcased a steady decline in SPN internalization due to treatment with increasing concentrations of the clathrin pathway inhibitors, chlorpromazine (CPZ) and monodansylcadaverine (Fig. 1A, B) as well as with methyl-β-cyclodextrin (Mβ-CD), a disrupter of cholesterol-enriched lipid rafts and thus inhibitor of caveolae-mediated endocytic pathway (Fig. 1C). However, no significant changes in pneumococcal adherence efficiencies to hBMECs were observed even at highest inhibitor concentrations (Fig. 1D-F). Also, MTT assay results revealed that the inhibitors were non-cytotoxic at the concentrations employed (Suppl. Fig. 1), suggesting that pneumococcus invades BBB via both clathrin- and caveolae-mediated endocytic pathways.
Fig. 1.
S. pneumoniae invades brain endothelium using clathrin and caveolae dependent endocytic pathways. Intracellular (A-C) or adherent (D-F) SPN to hBMEC were determined following treatment with different concentrations of clathrin pathway inhibitors Chlorpromazine (A, D) and Monodansylcadaverine (B, E) or caveolae pathway inhibitor Methyl β-cyclodextrin (C, F) for 1 h. Confluent hBMEC monolayers were infected with exponentially grown SPN at MOI 10 and adherence and invasion assays were performed as described in methods. The levels of invasion or adherence were normalized to vehicle treated cells. Data are represented as mean ± SD of triplicate experiments and representative graphs are shown. Statistical tests were performed using One-way ANOVA followed by Tukey's multiple comparison test. ns, non-significant; **p < 0.01; ***p < 0.001.
3.2. BBB invasion by SPN also involves entry through dynamin independent pathway
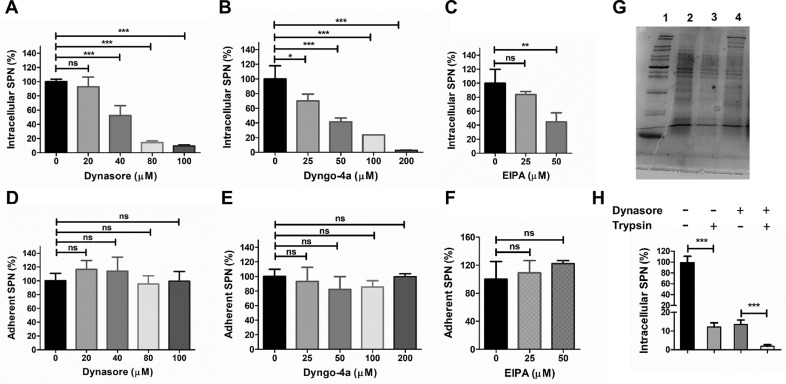
To assess the combined contribution of clathrin and caveolae mediated endocytosis in pneumococcal internalization into the brain endothelium, we inhibited activity of dynamin, a GTPase involved in scission of both clathrin and caveolae coated pits from the plasma membrane by pretreating hBMECs with Dynasore and Dyngo-4a. Our findings reconfirm the role of dynamin-dependent pathways in pneumococcal invasion of brain endothelium as steady reduction in intracellular bacterial counts was observed with increasing inhibitor concentrations without affecting the adherence (Fig. 2A, B, D, E). Intriguingly, the results also suggest possible utilization of a dynamin-independent pathway by SPN for entry into the BBB, as pneumococcal uptake was not completely blocked even at highest non-cytotoxic concentrations of the dynamin inhibitors (residual survival; 9.28% for Dynasore at 100 µM and 2.6% for Dyngo-4a at 200 µM) (Fig. 2A, B).
Fig. 2.
Pneumococcus adopts dynamin independent pathway for entry into the brain endothelium. Invasion (A-C) and adherence (D-F) of SPN to hBMECs as measured by gentamicin protection assays following treatment with various concentrations of dynamin inhibitors Dynasore (A, D) and Dyngo-4a (B, E) or dynamin independent pathway inhibitor EIPA (C, F). Exponentially grown SPN were allowed to infect confluent hBMEC monolayers at MOI 10 and adherence and invasion assays were performed as described in methods. The levels of invasion or adherence were normalized to vehicle treated cells. (G) Verification of pneumococcal cell surface protein shaving following trypsinization by SDS-PAGE. Lane 1: Mol. wt. marker; Lane 2: SPN lysate; Lane 3: lysate of trypsin treated SPN; Lane 4: supernatant of trypsin treated SPN. (H) Role of cell surface proteins in pneumococcal invasion of brain endothelium through dynamin independent pathway. Chloramphenicol (0.85 µg/ml, for blocking protein synthesis) treated SPN was trypsinized (1 µg/ml) and allowed to invade hBMECs through dynamin independent pathway following inhibition of dynamin dependent endocytosis with Dynasore (100 μM). The levels of invasion were normalized to vehicle treated cells. In all cases, results are represented as mean ± SD of triplicate experiments and representative graphs are shown. Statistical tests were performed using One-way ANOVA followed by Tukey's multiple comparison test. ns, non-significant; *p < 0.05; ***p < 0.001.
We further validated SPN internalization via dynamin-independent pathway by treating hBMECs with EIPA (5-(N-Ethyl-N-isopropyl) amiloride), an inhibitor of the NHE-1Na+/H+ exchanger that blocks endocytic pathways like the CLIC-GEEC and macropinocytosis in host cells. Enumeration of intracellular SPN following penicillin-gentamicin protection assay revealed significant reduction of SPN invasion (55.5%) following 50 µM EIPA treatment that was non-cytotoxic to hBMECs (Fig. 2C) although the adherence remains unchanged (Fig. 2F). Collectively, these findings suggest that apart from the traditional clathrin and caveolae pathways, pneumococcus can employ a novel dynamin-independent pathway for entry of into the BBB.
3.3. Bacterial uptake via dynamin-independent pathway requires pneumococcal cell surface ligands
Receptor-mediated endocytosis is well established for both clathrin and caveolae mediated pathways. However, dynamin-independent pathway like micropinocytosis is assumed to be a passive non-selective fluid sampling mechanism. Hence, in order to test an alternate possibility of induction of dynamin independent pathway due to interaction between specific host cell surface receptors with pneumococcal invasins, we performed invasion assays with surface shaved pneumococci in presence of dynasore, as it is most widely used dynamin inhibitor. Presence of differential protein bands in supernatant of trypsin treated SPN that are absent in the bacterial lysate (trypsin treated or untreated) indicates efficient shaving of pneumococcal surface proteins as well as intactness of pneumococcal cells following trypsinization (Fig. 2G and Suppl. Fig. 2). Moreover, significant reduction in bacterial invasion was observed following trypsinization of SPN implying involvement of pneumococcal surface proteins in receptor-mediated bacterial endocytosis in hBMECs as suggested by previous reports (Iovino et al., 2016). Interestingly, we observed similar reduction in pneumococcal uptake in dynasore treated hBMECs upon trypsinization (Fig. 2H), suggesting that pneumococcal entry into BBB via dynamin independent pathway is also governed by specific interaction between pneumococcal surface invasins and host cell receptors.
3.4. SPN displays longer intracellular persistence in brain endothelium following entry via dynamin independent pathway
After establishing SPN uptake via multiple endocytic pathways (clathrin, caveolae and dynamin-independent pathway), we were interested in exploring pneumococcal fate upon internalization via the specific endocytic route. In order to investigate this, we designed an intracellular survival assay in presence of inhibitor combinations that blocked multiple pathways concurrently allowing SPN internalization via a single endocytic pathway.
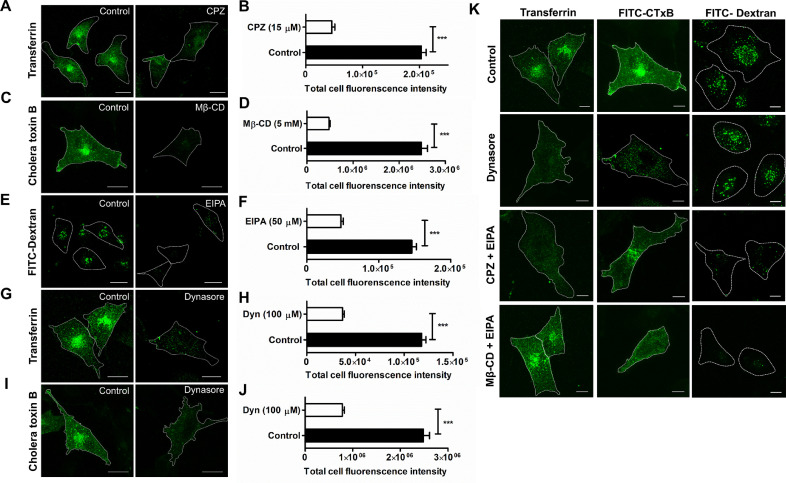
Firstly, we verified the endocytosis hindering action of the different inhibitors individually at their highest non-cytotoxic concentrations by checking for uptake of endocytic cargos known to get internalized via specific endocytic routes employing fluorescence microscopy. Internalization of transferrin, which is known to navigate via clathrin-mediated endocytosis displayed extensive perinuclear clustering. This was almost completely abolished following treatment with 15 µM CPZ (Fig. 3A). Quantification of large number of such fluorescence images encompassing approximately 200 cells, revealed significant reduction (4.4 fold) in total cellular fluorescence intensity of transferrin signal (203,100 ± 8264 A.U. vs. 45,780 ± 5344 A.U.) following CPZ treatment (Fig. 3B). Similarly, FITC-conjugated cholera toxin B (CTxB) and fluid-phase marker FITC Dextran (70 kDa) were used as indicators of caveolae and dynamin-independent endocytosis, respectively. Immunofluorescence analysis suggested that Mβ-CD at 5 mM and 50 µM of EIPA substantially impeded CTxB (∼ 5.2 fold decrease; 2,483,000 ± 132,000 A.U. vs. 477,800 ± 27,160 A.U.) and FITC-Dextran (3.1 fold reduction; 146,100 ± 6190 A.U. vs. 47,460 ± 3304 A.U.) internalization, respectively, as compared to non-treated cells (Fig. 3C-F). Inhibitory activity of Dynasore (100 µM) was similarly analyzed for uptake of both transferrin and CTxB (Fig. 3G, I). Fluorescence intensity measurements displayed approximately 3.2-fold reduction in both transferrin (117,900 ± 4455 A.U. vs. 36,590 ± 1861 A.U.) and CTxB uptake (2,483,000 ± 132,000 A.U. vs. 780,900 ± 42,810 A.U.) (Fig. 3H, J). Thus, the concentration of dynasore employed is able to effectively block both the dynamin dependent endocytic pathways, viz; clathrin and caveolae in hBMECs. We presume that the residual fluorescence observed in inhibitor treated cells was primarily due to the contribution of extracellular surface bound cargos that couldn't be excluded while calculating the total cellular fluorescence.
Fig. 3.
Efficiency and specificity of chemical inhibitors blocking different endocytic pathways. Confocal micrographs showing inhibition of internalization of transferrin, FITC-CTxB and FITC-dextran following treatment with (A) CPZ (15 μM), (C) Mβ-CD (5 mM) and (E) EIPA (50 μM), respectively. Dynasore (100 μM) treatment blocked entry of both (G) transferrin and (I) CtxB. hBMECs were allowed to internalize transferrin (100 ng/ml, for 2 min), FITC-CTxB (500 ng/ml, 15 min) or FITC-Dextran (1 mg/ml, 15 min) following treatment with inhibitors. Fluorescence images were captured following fixation and incubation of cells with necessary primary and secondary Ab and representative images are shown. Scale bar, 5 μm. Total fluorescence intensity of internalized transferrin (B, H), FITC-CTxB (D, J) and FITC-dextran (F) in presence or absence of non-cytotoxic concentrations of CPZ (B), Mβ-CD (D), EIPA (F) and Dynasore (H, J) were calculated from fluorescence images (n ≥ 200 cells per coverslip in triplicate) of cells following fixation at indicated time points and incubation with necessary primary and secondary Ab. Data are presented as mean ± SEM of triplicate experiments. Statistical tests were performed using unpaired Student's t-test, ***p < 0.001. (K) Confocal micrographs depicting specificity of the endocytic inhibitors. hBMECs were treated with combinations of inhibitors to block multiple endocytosis pathways leaving a single port of entry open. The following inhibitor combinations were used: Dynasore (Blocked pathways: clathrin and caveolae; operating pathway: dynamin independent pathways); CPZ + EIPA (Blocked pathways: clathrin and dymanin independent pathways; operating pathway: caveolae) and MβCD + EIPA (Blocked pathways: caveolae and dynamin independent pathways; operating pathway: clathrin). Cells were treated with designated endocytic inhibitors at the earlier determined concentration for the desired time period as described above. Fluorescence images (n ≥ 50) were captured following fixation and incubation of cells with necessary primary and secondary Ab and representative images are shown. Scale bar, 5 μm.
We further tested whether the inhibitors when used in combination could block multiple pathways, but simultaneously allowed the open endocytosis pathway to function normally. Fluorescence microscopy displayed normal uptake of the specific cargos via the open pathways in hBMECs, viz; transferrin uptake on Mβ-CD + EIPA treatment (open pathway: Clathrin), CTxB uptake on CPZ + EIPA treatment (open pathway: Caveolae) and Dextran (70 kDa) uptake on Dynasore treatment (open pathway: Dynamin-independent). As expected, internalization of the specific cargos via the blocked pathways was completely inhibited (Fig. 3K).
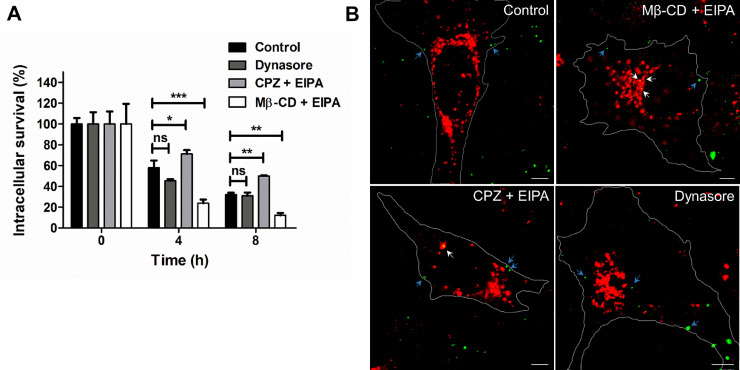
Following confirmation of inhibitor concentrations when used in combination for inhibition of multiple endocytosis pathways keeping only one selective pathway functional, intracellular survival assays were performed to determine the survival outcome of pneumococcus entering via specific endocytic pathway. Interestingly, higher survival counts were observed for SPN entering via caveloae (CPZ + EIPA) and the unexplored dynamin-independent (dynasore) pathway (Fig. 4A), in comparison to SPN invading via clathrin pathway (Mβ-CD + EIPA). Pneumococci entering via clathrin pathway displayed drastic reduction in intracellular persistence with time suggestive of their efficient lysosomal killing, the general fate of any cargo entering via clathrin-mediated endocytosis. Indeed, SPN entered through clathrin pathway exhibits effective association with lysosomal marker cathepsin B, which is negligible for pneumococcal entry through either caveolae or dynamic independent pathway (Fig. 4B), suggesting SPN adopting clathrin endocytosis pathway are preferentially destined for lysosomal killing. Thus, our findings indicate that entry via non-clathrin endocytic routes, seems to provide a survival advantage to pneumococci by virtue of not allowing association with lysosomes during its trafficking across the BBB.
Fig. 4.
Entry through clathrin independent pathways promotes improved intracellular persistence of SPN. (A) Intracellular survival efficiency of WT SPN in hBMECs following pre-treatment with combination of endocytosis inhibitors (Dynasore, MβCD + EIPA and CPZ + EIPA). Exponentially grown SPN were allowed to infect confluent hBMEC monolayers at MOI 10 and after killing extracellular SPN with penicillin (10 µg/ml) and gentamycin (400 µg/ml), survival ability of internalized SPN at indicated time points were calculated by enumerating bacterial numbers following plating cellular lysates on BHI plates. Survival efficiency were calculated as percent survival at indicated time points relative to 0 h. Data are presented as mean ± SD of triplicate experiments. Statistical analysis was performed using two-way ANOVA (Bonferroni test). ns, non-significant; *p < 0.05; **p < 0.01, ***p < 0.001. (B) Confocal micrographs showing association of SPN (green) with lysosomal marker cathepsin B (red) at 6 h post infection. hBMECs were infected with SPN and stained with anti-Cathepsin B Ab. Multiple images (n ≥ 50) were captured using laser scanning confocal microscope and representative images are shown. White arrow designates SPN present inside lysosomes, while blue arrow depicts non-lysosome associated SPN. Scale bar, 5 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Pneumococcus’ potential to cause meningitis is dependent on its ability to invade and safely transcytose across the BBB. In the present work, we deciphered exploitation of multiple endocytic routes by SPN for its internalization inside the brain endothelium. Our investigation re-established employment of clathrin and caveolae-mediated endocytosis by SPN (Radin et al., 2005; Gradstedt et al., 2013) but crucially led to the discovery of previously unexplored dynamin-independent pathway as a novel route of entry utilized by SPN for efficient trafficking through BBB.
Although many pathogens exploit the clathrin-dependent pathway for entry into host cells (McMahon and Boucrot, 2011), few intracellular bacteria have evolved mechanisms to alter the de novo fate of lysosomal degradation by either taking a safe refuge inside self-modified endosomes or rupturing endosomes and escaping into the cytosol (Shaughnessy et al., 2006; Tranchemontagne et al., 2015). Availing intracellular residence not only protects pathogens from extracellular immune defense mechanisms of the host but also provides a nutrient rich niche that facilitates their survival, proliferation and further dissemination. Accruing evidences have alternately demonstrated bacterial internalization via clathrin-independent endocytic routes prevent lysosomal fusion and degradation, thus imparting survival advantage onto the invading pathogens. For example, the FimH adhesin of E. coli interacts with macrophage cell surface receptor CD48 promoting bacterial uptake via caveolae pathway. This triggers longer intracellular persistence inside non-acidic compartments as compared to the opsonized E. coli taken up by conventional phagocytosis which quickly fuse with lysosomes leading to its degradation (Baorto et al., 1997). Similarly, A. felis and B. abortus entering host cells via lipid raft-mediated macropinocytic pathway demonstrate enhanced survival and replication inside the host cells (Schneider et al., 2007; Watarai et al., 2002). These evidences strongly support the notion that the internalization route influences the fate of bacteria inside host cells.
It is well established that majority of SPN entering the brain endothelium get degraded inside lysosomes except for a few that traffic across the barrier (Gradstedt et al., 2013; Surve et al., 2018). In this study we observed enhanced intracellular survival of SPN entering via the caveolae- and dynamin-independent endocytic pathways as compared to the SPN entering via clathrin-mediated endocytosis. This points towards the possibility that few pneumococci entering the brain endothelium via the unconventional clathrin-independent endocytic pathways may contribute to the sub-population which evades lysosomal degradation and successfully transcytoses across the BBB. Unlike, the well-characterised clathrin-dependent pathway which is known to terminate into the lysosomes, the non-clathrin-mediated endocytic pathways are relatively less studied with regards to their intracellular trafficking route and their ultimate destination. Hence, further studies need to be performed to molecularly characterize the fate of SPN entering via these clathrin-independent endocytic routes to gain a better mechanistic understanding of their ability for longer persistence and contribution to BBB trafficking.
We have performed the experiments with a non-encapsulated strain of SPN, which is classically considered as avirulent. However, recent emergence of such non-encapsulated strains in clinical settings has generated major interest in their persistence and evolution (Keller et al., 2016). Although these non-encapsulated strains are primarily involved in conjunctivitis, lately they have been found to be associated with pneumonia, sepsis and other pneumococcal invasive diseases (Dixit et al., 2016; Bradshaw et al., 2018). This may be due to the fact that in these strains surface proteins are not masked by a capsule, promoting more favorable interactions with the host cell receptors. This triggers greater adherence, a prerequisite for invasive disease. Pneumococcus expresses a myriad of cell surface molecules that promote its attachment and invasion into host cells. For example, PCho on the pneumococcal surface binds to PAFr present on the brain endothelial cell surfaces for uptake of SPN in a β-arrestin-1 mediated clathrin-dependent pathway (Radin et al., 2005). Similarly, pneumococcal PspC interaction with pIgR was shown to mediate SPN entry via both the clathrin and caveolae-mediated endocytic pathway in epithelial cells (Asmat et al., 2014). Dynamin-independent pathways are characterised for uptake of GPI-anchored proteins localised in cholesterol sensitive lipid-raft microdomains and require small GTPase belonging to either the Rho family members like the CDC42, Rac1 or RhoA or the Arf family protein, ARF6 (Kirkham and Parton, 2005). Recently, pneumococcal cell wall was reported to be internalised via an actin-dependent macropinocytosis-like fluid uptake pathway following interaction of the cell wall teichoic acid with TLR2 ligand on host cells (Loh et al., 2017).This entry pathway was sensitive to EIPA treatment and induced downstream signaling by activating Rac1, CDC42 and PI3K which contributed to the host inflammatory responses to pneumococcal cell wall. Our experiment involving pneumococcal surface shaving by trypsinization followed by invasion assays in presence or absence of Dynasore suggests the engagement of pneumococcal surface proteins with host cell receptors triggering bacterial entry via the unexplored dynamin-independent pathways. Collectively, our findings imply employment of a smart invasion strategy, such as the unconventional clathrin-independent endocytic pathways, adopted by SPN for longer intracellular persistence which may lead to efficient trafficking through the brain endothelium, facilitating pneumococcal entry into the central nervous system.
CRediT authorship contribution statement
Manalee V. Surve: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization. Shruti Apte: Methodology, Validation, Formal analysis, Investigation, Writing - review & editing, Visualization. Smita Bhutda: Methodology. Kshama G. Kamath: Validation. Kwang S. Kim: Resources. Anirban Banerjee: Conceptualization, Formal analysis, Resources, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We acknowledge confocal microscopy and BSL-2 facility of IIT Bombay. MVS, SB and SA received doctoral fellowship from UGC and IIT Bombay, respectively. Financial support for this work was provided by research funding from IIT Bombay (13IRSGHC002) to AB. The authors declare no conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.crmicr.2020.08.001.
Appendix. Supplementary materials
References
- Asmat T.M., Agarwal V., Saleh M., Hammerschmidt S. Endocytosis of streptococcus pneumoniae via the polymeric immunoglobulin receptor of epithelial cells relies on clathrin and caveolin dependent mechanisms. Int. J. Med. Microbiol. 2014;304(8):1233–1246. doi: 10.1016/j.ijmm.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Baorto D.M., Gao Z., Malaviya R., et al. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature. 1997;389(6651):636–639. doi: 10.1038/39376. [DOI] [PubMed] [Google Scholar]
- Bradshaw J.L., Pipkins H.R., Keller L.E., Pendarvis J.K., McDaniel L.S. Mucosal infections and invasive potential of nonencapsulated streptococcus pneumoniae are enhanced by oligopeptide binding proteins AliC and AliD. MBio. 2018;9(1):e02017–e02097. doi: 10.1128/mBio.02097-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit C., Keller L.E., Bradshaw J.L., Robinson D.A., Swiatlo E., McDaniel L.S. Nonencapsulated streptococcus pneumoniae as a cause of chronic adenoiditis. IDCases. 2016;4:56–58. doi: 10.1016/j.idcr.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington J.K., Reilly S.S., Ramp W.K., Smeltzer M.S., Kellam J.F., Hudson M.C. Mechanisms of Staphylococcus aureus invasion of cultured osteoblasts. Microb. Pathog. 1999;26(6):317–323. doi: 10.1006/mpat.1999.0272. [DOI] [PubMed] [Google Scholar]
- Gradstedt H., Iovino F., Bijlsma J.J. Streptococcus pneumoniae invades endothelial host cells via multiple pathways and is killed in a lysosome dependent manner. PLoS ONE. 2013;8(6):e65626. doi: 10.1371/journal.pone.0065626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques-Normark B., Tuomanen E.I. The pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring Harb. Perspect. Med. 2013;3(7) doi: 10.1101/cshperspect.a010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw J.E., Schmid S.L. Dynamin self-assembles into rings suggesting a mechanism for coated vesicle budding. Nature. 1995;374(6518):190–192. doi: 10.1038/374190a0. [DOI] [PubMed] [Google Scholar]
- Iovino F., Orihuela C.J., Moorlag H.E., Molema G., Bijlsma J.J. Interactions between blood-borne Streptococcus pneumoniae and the blood-brain barrier preceding meningitis. PLoS ONE. 2013;8(7):e68408. doi: 10.1371/journal.pone.0068408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovino F., Seinen J., Henriques-Normark B., van Dijl J.M. How does streptococcus pneumoniae invade the brain? Trends Microbiol. 2016;24(4):307–315. doi: 10.1016/j.tim.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Keller L.E., Robinson D.A., McDaniel L.S. Nonencapsulated streptococcus pneumoniae: emergence and pathogenesis. MBio. 2016;7(2):e01792. doi: 10.1128/mBio.01792-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham M., Parton R.G. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim. Biophys. Acta. 2005;1745(3):273–286. doi: 10.1016/j.bbamcr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Kiss A.L., Botos E. Endocytosis via caveolae: alternative pathway with distinct cellular compartments to avoid lysosomal degradation? J. Cell Mol. Med. 2009;13(7):1228–1237. doi: 10.1111/j.1582-4934.2009.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire P., Houde M., Segura M. Encapsulated group B Streptococcus modulates dendritic cell functions via lipid rafts and clathrin-mediated endocytosis. Cell Microbiol. 2012;14(11):1707–1719. doi: 10.1111/j.1462-5822.2012.01830.x. [DOI] [PubMed] [Google Scholar]
- Loh L.N., McCarthy E.M.C., Narang P., Khan N.A., Ward T.H. Escherichia coli K1 utilizes host macropinocytic pathways for invasion of brain microvascular endothelial cells. Traffic. 2017;18(11):733–746. doi: 10.1111/tra.12508. [DOI] [PubMed] [Google Scholar]
- Loh L.N., Gao G., Tuomanen E.I. Dissecting bacterial cell wall entry and signaling in eukaryotic cells: an actin-dependent pathway parallels platelet-activating factor receptor-mediated endocytosis. MBio. 2017;8(1):e02016–e02030. doi: 10.1128/mBio.02030-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon H.T., Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011;12(8):517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- Orihuela C.J., Mahdavi J., Thornton J., et al. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J. Clin. Invest. 2009;119(6):1638–1646. doi: 10.1172/JCI36759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J.N., Orihuela C.J., Murti G., Guglielmo C., Murray P.J., Tuomanen E.I. beta-Arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect. Immun. 2005;73(12):7827–7835. doi: 10.1128/IAI.73.12.7827-7835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring A., Weiser J.N., Tuomanen E.I. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J. Clin. Invest. 1998;102(2):347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde M., Müller E., Chhatwal G.S., Talay S.R. Host cell caveolae act as an entry-port for group A streptococci. Cell Microbiol. 2003;5(5):323–342. doi: 10.1046/j.1462-5822.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- Schneider B., Schueller C., Utermoehlen O., Haas A. Lipid microdomain dependent macropinocytosis determines compartmentation of Afipia felis. Traffic. 2007;8(3):226–240. doi: 10.1111/j.1600-0854.2006.00525.x. [DOI] [PubMed] [Google Scholar]
- Shaughnessy L.M., Hoppe A.D., Christensen K.A., Swanson J.A. Membrane perforations inhibit lysosome fusion by altering pH and calcium in Listeria monocytogenes vacuoles. Cell Microbiol. 2006;8(5):781–792. doi: 10.1111/j.1462-5822.2005.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stins M.F., Badger J., Sik Kim K. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb. Pathog. 2001;30(1):19–28. doi: 10.1006/mpat.2000.0406. [DOI] [PubMed] [Google Scholar]
- Surve M.V., Bhutda S., Datey A., et al. Heterogeneity in pneumolysin expression governs the fate of Streptococcus pneumoniae during blood-brain barrier trafficking. PLoS Pathog. 2018;14(7) doi: 10.1371/journal.ppat.1007168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K., Yoshida Y., Yamada H. Regulatory mechanisms of dynamin-dependent endocytosis. J. Biochem. 2005;137(3):243–247. doi: 10.1093/jb/mvi052. [DOI] [PubMed] [Google Scholar]
- Tranchemontagne Z.R., Camire R.B., O'Donnell V.J., Baugh J., Burkholder K.M. Staphylococcus aureus Strain USA300 Perturbs Acquisition of Lysosomal Enzymes and Requires Phagosomal Acidification for Survival inside Macrophages. Infect. Immun. 2015;84(1):241–253. doi: 10.1128/IAI.00704-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama S., Carlin A.F., Khosravi A., et al. The surface-anchored NanA protein promotes pneumococcal brain endothelial cell invasion. J. Exp. Med. 2009;206(9):1845–1852. doi: 10.1084/jem.20090386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga E., Cossart P. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat. Cell Biol. 2005;7(9):894–900. doi: 10.1038/ncb1292. [DOI] [PubMed] [Google Scholar]
- Watarai M., Makino S., Fujii Y., Okamoto K., Shirahata T. Modulation of Brucella-induced macropinocytosis by lipid rafts mediates intracellular replication. Cell Microbiol. 2002;4(6):341–355. doi: 10.1046/j.1462-5822.2002.00195.x. [DOI] [PubMed] [Google Scholar]
- Wooldridge K.G., Williams P.H., Ketley J.M. Host signal transduction and endocytosis of Campylobacter jejuni. Microb. Pathog. 1996;21(4):299–305. doi: 10.1006/mpat.1996.0063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.