Highlights
-
•
Endophytic bacterial diversity of root, stem, and leaf tissues of Physalis ixocarpa was deciphered.
-
•
Unique and shared species were found for each plant compartment analyzed.
-
•
Extensive screening of various isolates exhibited antagonism against fungal pathogens.
-
•
Diverse endophytes stimulated the growth of Physalis ixocarpa seedlings.
-
•
Neobacillus drentensis CH23 stood out as an excellent plant growth-promoting bacterium.
Keywords: Plant growth-promoting endophytes, Biocontrol, Plant microbiome, Agricultural crops
Abstract
The endophytic bacterial diversity of root, stem, and leaf tissues of Mexican husk tomato plants (Physalis ixocarpa) was compared and deciphered, and screened for their plant growth-promoting activity and antagonism against fungal phytopathogens. Total 315 isolates (108 roots, 102 stems, and 105 leaves) were obtained and characterized by 16S ribosomal gene sequencing. The most abundant genera were Bacillus, Microbacterium, Pseudomonas, and Stenotrophomonas. Unique species were found for each tissue analyzed, along with B. thuringiensis, B. toyonensis, Neobacillus drentensis, Paenibacillus castaneae, P. fluorescens, P. poae, and S. maltophilia present throughout the plant. Biodiversity indices did not show significant differences, but root tissues showed the highest abundance of bacterial endophytes. Several isolates showed excellent promotion activities in Physalis ixocarpa seedlings, increasing the length and weight of the root, total biomass, and chlorophyll content. Various isolates also exhibited antagonism against fungal pathogens. Among screened isolates, Neobacillus drentensis CH23 was found in all plant compartments, exhibiting growth-promoting activity and fungal antagonism. Strain CH23 and other endophytes showed the production of indoleacetic acid, siderophores, proteases, and solubilization of phosphates. These results demonstrate that the husk tomato plant endobiome has a high potential as a bioinoculating agent for agriculturally important crops.
1. Introduction
Agricultural crops can be severely affected by various abiotic and biotic factors. Abiotic factors include nutrient-poor soils, salinity, drought, and extreme pH, etc., while biotic factors include microbial pathogens, which cause serious economic losses annually (Chakraborty and Newton, 2011; Kandel et al., 2017). To overcome such factors, chemical fertilizers and pesticides are used as a first solution, which are highly toxic for animals and humans as well as contaminate agro-ecosystems. Therefore, bioinoculants containing microorganisms, such as plant-associated fungi and bacteria, have emerged as an alternative (Avis et al., 2008). An interesting group of microorganisms that has shown various beneficial activities in plants is endophytic bacteria (Liu et al., 2017). Endophytic bacteria are part of the plant endobiome, and have the ability to colonize and survive the internal tissues of plants without causing any apparent damage (Santoyo et al., 2016). Some of them can also stimulate the plant growth as well as defenses against potential pathogens by facilitating the acquisition of nutrients through the production of siderophores or solubilization of phosphates, produce antimicrobial compounds such as 2, 4-diacetylphloroglucinol, phenazines, or volatile organic compounds (such as hydrogen cyanide or dimethyl disulfide), produce phytohormones (e. g., auxins, such as indoleacetic acid), or reduce stress levels through the hydrolysis of ethylene precursors (ACC deaminase) (Glick, 2012). The species of various genera of endophytic bacteria such as Aureobacterium, Azoarcus, Bacillus, Burkholderia, Chitinophaga, Flavobacterium, Paenibacillus, Phyllobacterium, Pseudomonas, Stenotrophomonas, Micrococcus, Pantoea, and Microbacterium (Kandel et al., 2017; Hardoim et al., 2012; Mahmood et al., 2016), have shown to be excellent agricultural growth-stimulating agents in wheat (Triticum spp.), soybean (Glycine max), sugarcane (Saccharum officinarum), maize (Zea mays L.), peanut (Arachis hypogaea L.), onion (Allium spp.), etc. (Orozco-Mosqueda and Santoyo, 2020).
The husk tomato, tomatillo or Mexican husk tomato (Physalis ixocarpa), in addition to being endemic of Mexico, is a plant whose fruit, in pre-Columbian era, was an important diet for the Aztecs and Mayans (Marquez-Santacruz et al., 2010). In a previous study, the endophytic bacterial diversity in the roots of husk tomato plants was determined through the library construction of 16S ribosomal genes, in which the dominant genera were Stenotrophomonas (21.9%), Microbacterium (17.1%), Burkholderia (14.3%), Bacillus (14.3%), and Pseudomonas (10.5%). Interestingly, some of these ribosomal genes were detected in the rhizosphere of the same host plant, such as Stenotrophomonas, Burkholderia, Bacillus, and Pseudomonas, indicating that the bacterial endobiome is a subset of rhizospheric populations with potentially beneficial activities (Marquez-Santacruz et al., 2010). Although a large part of these 16S rDNA genes belong to bacterial endophytes with potential plant growth-promoting activities, this hypothesis has not been proven.
Therefore, the aim of this study was to estimate the culturable bacterial endophytic tissue-specific diversity in the roots, stems, and leaves of husk tomato plants and characterize their plant growth-stimulating activity and antagonism against fungal plant pathogens (Botrytis cinerea, Fusarium oxysporum, F. solani, and Rhizoctonia solani). Additionally, screening mechanisms such as IAA, siderophore, and protease production as well as phosphorous solubilization, were also evaluated.
2. Material and methods
2.1. Plants sample and isolation of endophytic strains from different plant tissues
Twenty-four healthy plants in a vegetative stage of Physalis ixocarpa Brot. ex Horm. were collected from an organic agricultural land in Zamora, Michoacán México (20°01´42.8´´N 102°15´31.6´´W) during the fall of 2018. Endophytic bacteria were isolated using a previously described protocol (Contreras et al., 2016). Briefly, plant tissues were separated into leaves, stems, and roots. Soil particles were carefully removed from roots. One gram of each tissue from each plant was collected (hence, total 24 g of each tissue, i.e., roots, stems, and leaves) and immersed in 70% ethanol for 30 s, then in a 2.5% solution of commercial bleach for 5 min, followed by five times washing with sterile distilled water. To confirm the sterilization process, an aliquot from the last rinse of sterile distilled water was cultured on nutrient agar (NA) plates and incubated at 30 °C for 72 h. The plant tissues were macerated using sterile mortars and cultured on plates containing nutrient agar medium (Merck) and incubated at 30 °C for 48 h. Thereafter, CFU were determined per gram of fresh weight from every tissue (roots, stems, and leaves). The latter assay was performed in triplicate. Statistical/ecological analyses consisted of measuring the alpha diversity with the Shannon and Simpson ecological indexes. The sampling representativeness was measured with a rarefaction curve, in which the variables used were the number of observed rRNA sequences by the number of obtained ribosomal gene reads (rRNA 16S), using PAST software 3.15 (Paleontological Statistics).
2.2. Characterization of bacterial strains
A single colony of each endophytic strain was cultured in 3 ml of nutritive liquid medium (BD Bioxon) at 30 °C overnight with agitation. Genomic DNA was extracted using the The Wizard® Genomic DNA Purification Kit (Promega), following the manufacturer's instructions. The 16S rDNA genes of each the bacterial isolates were PCR amplified using the universal primers Fd1 5´-CAGAGTTGATCCTGGCTCAG-3´ and rD1 5´-AAGGAGGTGATCCAGCC-3´ (Weisburg et al., 1991). The PCR conditions were as follows: an initial denaturation for 3 min at 95 °C, 30 cycles of denaturation for 1 min at 95 °C, alignment for 1 min at 53 °C, extension for 2 min at 72 °C, and a final extension at 72 °C for 5 min. The PCR products were purified and sequenced. Sequences with a minimum of 1200 nucleotides were obtained. All the sequences obtained were analyzed for homology searches using the Basic Local Alignment Search Tool (BLAST) against the nucleotide sequences present in the GenBank (NCBI) database. Non-redundant 16S rDNA sequences are available under accession numbers MW386502–MW386621. The accession number for Neobacillus drentensis strain CH23 accession number is MW397039. The 16S rDNA -based phylogenetic tree was constructed using MEGA 7 (Kumar et al., 2016b) via the maximum likelihood method, with a bootstrap support of 1000 replications.
2.3. Evaluation of plant growth promotion
Plant growth promoting activities were evaluated by inoculating single strains on their host seedlings. First, husk tomato seeds were sterilized with 95% ethanol for 5 min and 20% sodium hypochlorite for 5 min, followed by 5 washes with sterile distilled water. The seeds were germinated and grown in 0.2 × MS medium (Murashine Skoog). Five days after germination, selection of five seedlings with homogeneous growth were transplanted into plates containing MS medium supplemented with 10% NA medium and at the extreme of the plate a 10 µL inoculum of each endophyte bacterium, previously grown on nutrient liquid medium (OD 600 nm = 0.05). The plates were incubated with a photoperiod of 16/8 h day/night in a growth chamber (Percival Scientific). After 7 d of incubation, the total fresh weight, length and weight of the aerial part and roots, and length and number of lateral roots were measured. Chlorophyll content was quantified using an MC-100 Apogee chlorophyll meter. To provide a reference for plant growth-stimulating activity (positive control), two well-studied bacterial strains (Pseudomonas fluorescens strains UM256 and UM270) were included in the analysis for their plant growth-promoting capacities (Hernández-León et al., 2015; Rojas-Solis et al., 2016).
2.4. Evaluation of antifungal activity
The evaluation of fungal antagonism was performed using petri dish bioassays as described previously (Hernández-León et al., 2015). Briefly, the bacterial isolates were simultaneously deposited with pathogenic fungi (Botrytis cinerea, Fusarium oxysporum, F. solani, and Rhizoctonia solani) on PDA media. Bacterial endophytes were streaked onto plates in a cross shape, and a mycelial plug of 4 mm was deposited in the center of each quadrant. The plates were incubated in the dark at 30 °C, and the mycelial growth diameter was measured on days 6–8, depending on the fungal species. Antifungal experiments were independently performed for a minimum of three times, and the percentage of mycelium inhibition (compared to controls) was determined.
2.5. Identification of siderophores, IAA, proteases, and phosphate solubilization activities
The production of siderophores was determined by growing bacteria on chromium azurol-S (CAS) and detecting an orange-yellow halo (Santoyo et al., 2019). To detect proteolytic activity, skimmed milk (SM) medium was employed (Kumar et al., 2016a). Phosphate-solubilizing activity was detected by growing the bacterial endophytes on petri dishes containing pikovskaya (PVK) agar and observing a halo (Yaish et al., 2015). The production of indoleacetic acid and other related products was determined by the capacity of these strains to utilize 500 µg ml−1 of L-tryptophan in minimal medium (MM) to produce IAA and similar molecules. The production of IAA was colorimetrically measured for all the isolated strains as described by Yaish et al. (2015), using Salkowski's reagent as the colorimetric reagent. The bacterial endophyte strains were also inoculated in MM medium lacking tryptophan, which was used as a negative control in the experiment. The amount of IAA produced was estimated from a calibration curve of pure indolacetic acid (Sigma) (Hernández-León et al., 2015).
3. Results
3.1. Diversity and abundance of bacterial endophytes
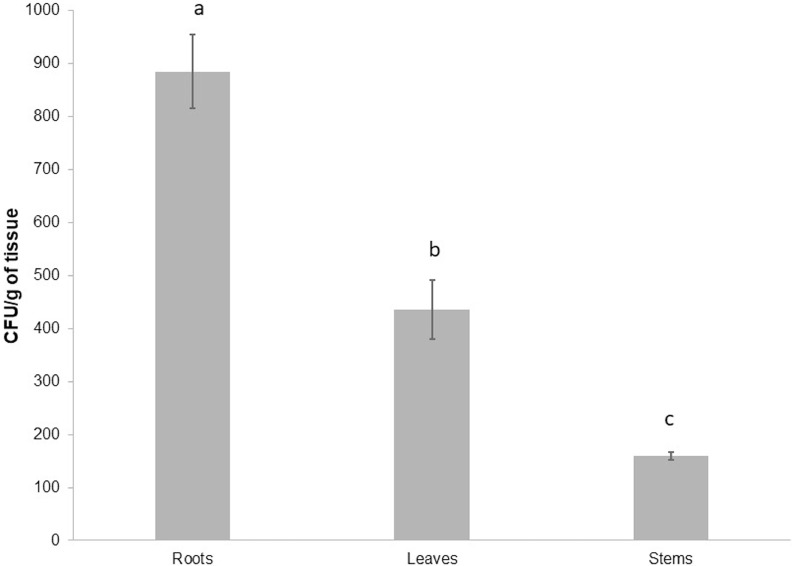
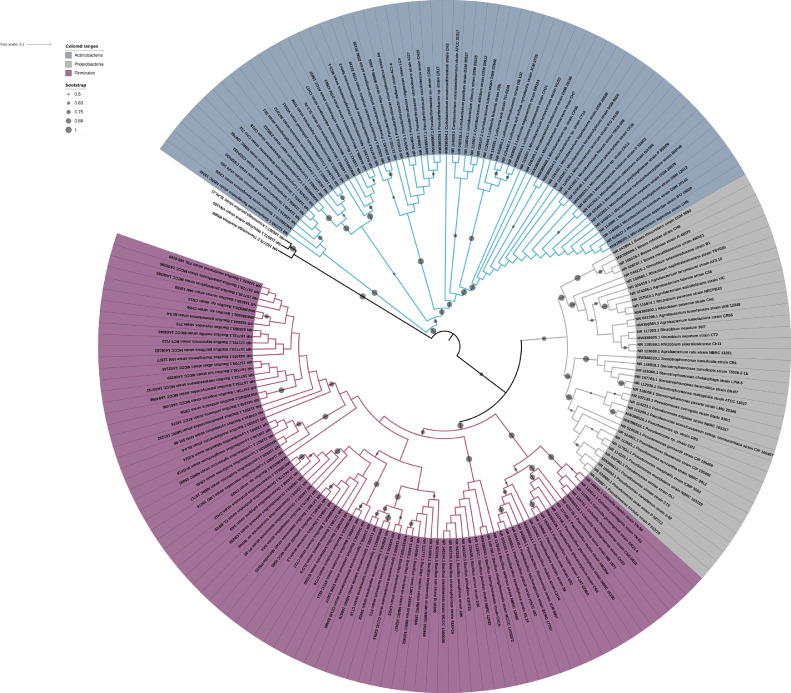
In this study, the abundance and diversity of cultivable endophytic bacteria (Physalis ixocarpa), isolated from the leaf, stem, and root tissues of husk tomato plants, which were collected from an agricultural field in the State of Michoacán, Mexico, were determined. The results showed that the highest abundance of endophytic bacteria was found in the root (8.84 × 10−2), followed by the leaves (4.35 × 10−2) and stems (1.59 × 10−2) (Fig. 1). Total 315 isolates (108 from roots, 102 from stems, and 105 from leaves) from husk tomato plants were isolated and characterized. The cultivable diversity found in tomato husk plants comprised of total 19 genera, the most abundant being Bacillus, Microbacterium, Pseudomonas, and Stenotrophomonas. Table 1 shows a summary of the number of bacterial isolates found by tissue type. The diversity indices (Shannon and Simpson) did not show significant differences in tissue-specific diversity, and neither was dominance at the observed phylum level. Fig. 2 also show the phylogenetic tree describing the most representative species and strains of the three main groups (Actinobacteria, Proteobacteria and Firmicutes) found as endophytes in the plant compartments. Preliminary taxonomic affiliation and the construction of the phylogeny is based on 16S rRNA sequences.
Fig. 1.
The cultivable abundance of bacterial endophytes found in tomato husk plant endospheres, including roots, stems, and leaves. Endophytic bacterial abundance, measured as Colony Forming Units per gram of fresh tissue (CFU/gram of tissue), were higher in roots, followed by leaves and stems of husk tomato plants. The three plant compartments exhibited significant differences in the abundance of bacterial endophytes.
Table 1.
Number of bacterial endophyte isolates in husk tomato plant tissues and their respective diversity indexes.
| Species detected in husk tomato | Phylum/Subphylum | Roots | Stems | Leaves |
|---|---|---|---|---|
| Arthrobacter chlorophenolicus | Actinobacteria | 1 | 0 | 0 |
| Arthrobacter niigatensis | Actinobacteria | 2 | 0 | 0 |
| Arthrobacter phenanthrenivorans | Actinobacteria | 2 | 3 | 0 |
| Corynebacterium doosanense | Actinobacteria | 1 | 0 | 0 |
| Curtobacterium oceanosedimentum | Actinobacteria | 1 | 0 | 1 |
| Leifsonia shinshuensis | Actinobacteria | 0 | 7 | 1 |
| Microbacterium foliorum | Actinobacteria | 0 | 4 | 5 |
| Microbacterium oxydans | Actinobacteria | 13 | 0 | 0 |
| Microbacterium paraoxydans | Actinobacteria | 1 | 0 | 0 |
| Microbacterium phyllosphaerae | Actinobacteria | 0 | 12 | 14 |
| Microbacterium testaceum | Actinobacteria | 0 | 0 | 4 |
| Micromonospora citrea | Actinobacteria | 0 | 0 | 3 |
| Micromonospora echinospora | Actinobacteria | 0 | 0 | 5 |
| Micromonospora inositola | Actinobacteria | 0 | 0 | 3 |
| Actinobacteria % | 19.4% | 25.5% | 34.3% | |
| Agrobacterium tumefaciens | α-proteobacteria | 2 | 0 | 0 |
| Bosea robiniae | α-proteobacteria | 0 | 0 | 6 |
| Rhizobium neporum | α-proteobacteria | 0 | 3 | 0 |
| Rhizobium pusense | α-proteobacteria | 0 | 0 | 2 |
| Pseudomonas libanensis | γ-proeobacteria | 1 | 0 | 0 |
| Pseudomonas poae | γ-proeobacteria | 2 | 4 | 4 |
| Stenotrophomonas maltophilia | γ-proeobacteria | 8 | 3 | 2 |
| Streptomyces flavogriseus | γ-proeobacteria | 0 | 0 | 2 |
| Pseudomonas fluorescens | γ-proeobacteria | 7 | 24 | 28 |
| Pseudomonas gessardii | γ-proeobacteria | 2 | 5 | 0 |
| Proteobacteria | Proteobacteria % | 20.4% | 38.2% | 41.9% |
| Bacillus anthracis | Firmicutes | 6 | 1 | 0 |
| Bacillus cereus | Firmicutes | 2 | 0 | 0 |
| Bacillus circulans | Firmicutes | 0 | 3 | 0 |
| Bacillus desertis | Firmicutes | 0 | 2 | 1 |
| Neobacillus drentensis | Firmicutes | 2 | 1 | 1 |
| Bacillus safensis | Firmicutes | 2 | 0 | 1 |
| Bacillus thuringiensis | Firmicutes | 29 | 20 | 1 |
| Bacillus toyonensis | Firmicutes | 6 | 2 | 1 |
| Bacillus trypoxylicola | Firmicutes | 1 | 0 | 0 |
| Bacillus vireti | Firmicutes | 0 | 0 | 1 |
| Brevibacterium frigoritolerans | Firmicutes | 0 | 0 | 2 |
| Cohnella ginsengisoli | Firmicutes | 0 | 0 | 2 |
| Lysinibacillus fusiformis | Firmicutes | 3 | 0 | 0 |
| Oceanobacillus polygoni | Firmicutes | 0 | 0 | 1 |
| Paenibacillus castaneae | Firmicutes | 2 | 3 | 6 |
| Paenibacillus lautus | Firmicutes | 4 | 0 | 0 |
| Paenibacillus pabuli | Firmicutes | 0 | 0 | 2 |
| Paenibacillus turicensis | Firmicutes | 0 | 0 | 1 |
| Sporosarcina aquimarina | Firmicutes | 0 | 0 | 1 |
| Sporosarcina contaminans | Firmicutes | 1 | 0 | 0 |
| Sporosarcina koreensis | Firmicutes | 0 | 0 | 1 |
| Firmicutes % | 53.7% | 31.4% | 21.0% | |
| Not determined | 7 | 5 | 3 | |
| Not determined | Not determined % | 6.5% | 4.9% | 2.9% |
| Total | 108 | 102 | 105 | |
| Shannon´s index | 2.521 | 2.286 | 2.725 | |
| Simpson´s Index | 0.8648 | 0.8596 | 0.8868 |
Fig. 2.
Phylogenetic tree of the three main groups (Actinobacteria, Proteobacteria and Firmicutes) found as endophytes in the plant compartments of husk tomato plants.
3.2. Tissue-specific diversity
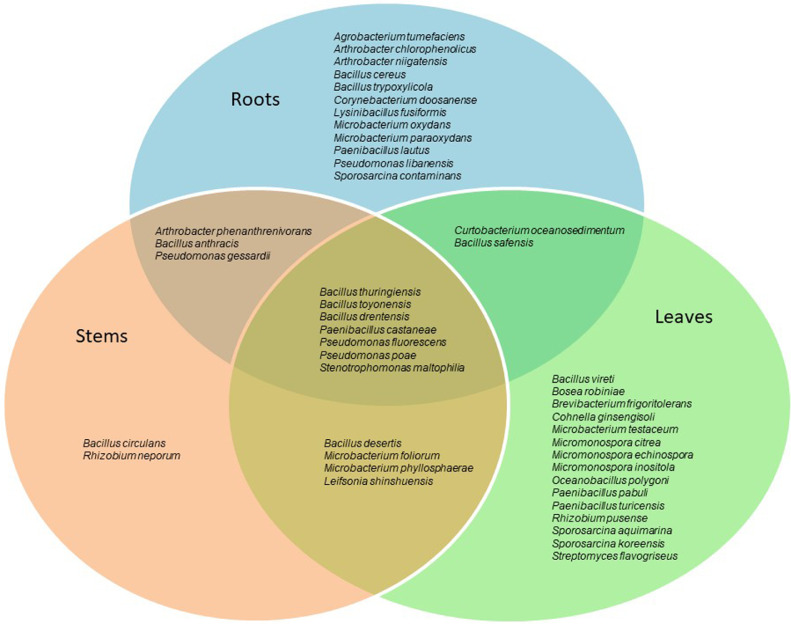
Fig. 3 is a Venn diagram showing the species found in each of the three tissues analyzed (root, stem, and leaf) as well as those shared between the tissues. Some species, such as Agrobacterium tumefaciens, Corynebacterium doosanense, Lysinibacillus fusiformis, and Sporosarcina contaminans were detected only in roots, while others such as Bacillus circulans and Rhizobium neporum were only isolated from stems. Species such as Bosea robiniae, Cohnella ginsengisoli, Micromonospora spp., Oceanobacillus polygoni, and Sporosarcina spp. were leaf-specific. It should be noted that some species, such as B. thuringiensis, B. toyonensis, N. drentensis, Paenibacillus castaneae, P. fluorescens, P. poae, and S. maltophilia were present throughout the plant, showing a high plant tissue colonization capability.
Fig. 3.
Venn diagram showing the common and unique bacterial endophytic diversity in the inner plant compartments, such as roots, stems, and leaves.
3.3. Plant growth stimulation of husk tomato seedlings
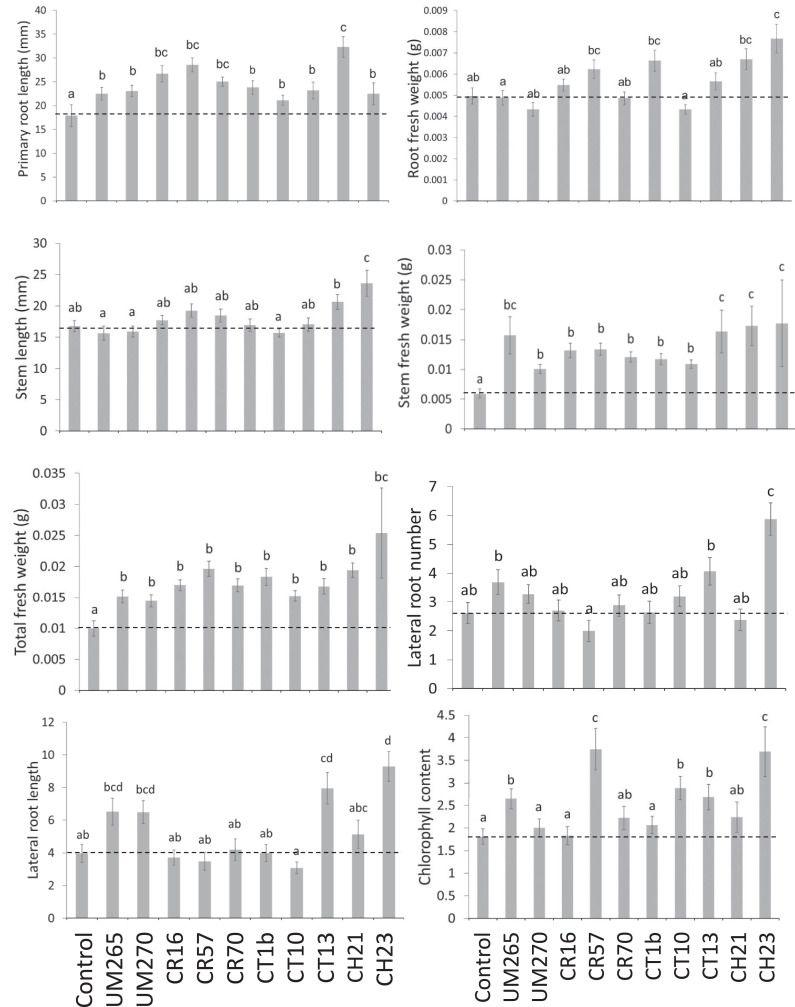
Endophytic bacteria can perform beneficial functions by interacting with their host plants. Therefore, the potential of bacterial endophytes to stimulate plant growth in Physalis ixocarpa seedlings was evaluated in vitro. For reference, the growth-promoting effect of each of the bacterial endophytes was compared with two bacterial strains (Pseudomonas fluorescens strains UM256 and UM270) well studied for their plant growth-promoting capacities (including Physalis ixocarpa) (Hernández-León et al., 2015; Rojas-Solis et al., 2016). Fig. 4 shows eight endophytic isolates having similar or greater growth promotion activities of husk tomato plants to that of the Pseudomonas strains. The root isolates CR16 (Microbacterium oxydans), CR57 (Sp. Undetermined), and CR70 (Stenotrophomonas maltophilia) as well as stem isolates CT1b (Bacillus toyonensis), CT10 (Microbacterium foliorum), and CT13 (Leifsonia shinshuensis) and leaf isolates CH21 (Sp. undetermined) and CH23 (Neobacillus drentensis), increased the length of the primary root, the number and length of lateral roots, the fresh weight of the root and stem, and total weight of the plant as well as the chlorophyll content. The strain CH23 stands out from this analysis, showing significant differences from the control seedlings and those inoculated with the Pseudomonas strains in several of the parameters analyzed.
Fig. 4.
Eight endophyte isolates with similar or greater growth-stimulating activities of husk tomato seedlings than those of plant growth-promoting Pseudomonas are shown, highlighting the endophyte Neobacillus drentensis CH23.
3.4. Evaluation of antagonism against fungal pathogens
One of the indirect mechanisms of plant growth promotion is antagonism towards potential plant pathogens, as shown by beneficial endophytic bacteria. In this analysis, 49 of the endophytic isolates showed some type of inhibitory action against one, two, or more of the pathogens evaluated (Table 2). Antifungal activities were classified as mild inhibition, ranging from 1% to 25% inhibition of mycelial growth (+), medium, 26–50% (++), and strong, 51–75% (+++). In this test, the strain N. drentensis CH23 stood out, showing antagonism against all four pathogens evaluated, as a medium for Fusarium oxysporum, F. solani, and Rhizoctonia solani. Finally, strain CH23 exhibited strong mycelial growth inhibition against Botrytis cinerea.
Table 2.
Antifungal activity of the bacterial endophyte isolates of husk tomato plants.
| Bacterial endophyte species | Strain | B. cinerea | F. oxysporum | F. solani | R. solani |
|---|---|---|---|---|---|
| Agrobacterium tumefaciens | CR22 | + | |||
| Arthrobacter niigatensis | CR60 | ++ | + | ||
| Bacillus anthracis | CT4b | ++ | + | ||
| Bacillus cereus | CR10 | + | + | ||
| Neobacillus drentensis | CH23 | +++ | ++ | ++ | ++ |
| Bacillus thuringiensis | CR11CR83CR33CR47CR31CR79CR25CR34CR7CR12 | +++++++++++ | +++ | + | ++ |
| Bacillus toyonensis | CT1CT1bCR15CR87CR30CR82CT1bCT1 | +++++++++ | ++++++ | ++++ | |
| Bacillus trypoxylicola | CR37 | + | |||
| Curtobacterium oceanosedimentum | CH3 | ++ | ++ | + | |
| Leifsonia shinshuensis | CT11CH11 | +++ | ++ | ||
| Microbacterium foliorum | CT10 | + | + | ||
| Microbacterium oxydans | CR88CR26CR13CR92CR90 | + | +++++ | + | |
| Microbacterium phyllosphaerae | CT3CT3bCH5 | ++ | ++ | + | |
| Pseudomonas gessardii | CR3 | + | |||
| Rhizobium pusense | CH1 | + | |||
| Sporosarcina aquimarina | CT19 | + | |||
| Sporosarcina contaminans | CR42 | + | |||
| Stenotrophomonas maltophilia | CR48CR27CR69CR70 | +++ | +++++ | + | + |
| Not determined | CR45bCR45CR57CT5CR53b | +++++ | ++ | + |
The antifungal activity was determined in in vitro bioassays as previously done (Hernández-León et al., 2015). The measurements indicate an inhibition of the growth of the diameter of the mycelium of the phytopathogens of between 1% and 25% (+), 26% and 50% (++) and 51% and 75% (+++). The results were repeated at least three times.
3.5. Screening of plant growth-promoting mechanisms
Plant growth-promoting bacterial endophytes are well known for producing diverse stimulating and biocontrol determinants. Here, bacterial endophytes were screened to evaluate some of their possible mechanisms that are important when interacting with their plant hosts. Table 3 summarizes the bacterial endophytes that showed the best indoleacetic acid (and similar compounds) production activities, siderophores, proteases, and phosphate solubilization. Notably, a higher percentage of endophytes that showed IAA production (≥ 3 µg/mL) were isolated from the root (34 of 108), compared to those isolated from the stem and leaf. Other endophytic strains that stand out are M. oxydans CR16, B. toyonensis CT1, M. foliorum CT10, and N. drentensis CH23, which are good producers of IAA, siderophores, and proteases, in addition to showing good solubilization of phosphates. These mechanisms may be involved in direct and indirect activities to promote plant growth.
Table 3.
Screening of plant growth-promoting mechanisms in endophytic strains of roots, stems, and leaves of Physalis ixocarpa. Different capacity to siderophores and proteases is denoted by a corresponding number of + signs. IAA and similar compounds as µg ml−1, where standard deviations were less than 20% of the given means and therefore were omitted to preserve the table's clarity. N.D. denoted no activity or product detected in the assays.
| Endophytic species | Strain | IAA and similar compounds (μg/mL) | Siderophore production | Phosphate solubilization | Proteases |
|---|---|---|---|---|---|
| Roots | |||||
| B. thuringiensis | CR72 | 11.3377 | ++ | ++ | +++ |
| B. thuringiensis | CR91 | 8.5609 | ++ | + | ++ |
| Undetermined | CR57 | 8.1669 | ++ | N.D. | ++ |
| B. thuringiensis | CR61 | 7.3789 | ++ | + | ++ |
| B. thuringiensis | CR32 | 7.0975 | N.D. | + | +++ |
| M. oxydans | CR88 | 6.8911 | ++ | + | +++ |
| B. thuringiensis | CR63 | 6.4221 | N.D. | + | ++ |
| Undetermined | CR45b | 6.1782 | ++ | + | +++ |
| B. anthracis | CR56 | 5.8404 | N.D. | + | +++ |
| B. thuringiensis | CR34 | 5.5403 | N.D. | N.D. | +++ |
| B. toyonensis | CR30 | 5.1275 | ++ | ++ | +++ |
| B. thuringiensis | CR83 | 4.9774 | N.D. | + | ++ |
| S. contaminans | CR42 | 4.9023 | ++ | + | +++ |
| P. lautus | CR6 | 4.8085 | N.D. | + | N.D. |
| M. oxydans | CR16 | 4.6584 | + | + | +++ |
| B. anthracis | CR51 | 4.5834 | N.D. | + | ++ |
| Undetermined | CR78 | 4.1894 | N.D. | ++ | N.D. |
| B. thuringiensis | CR99 | 4.1519 | ++ | + | ++ |
| B. thuringiensis | CR33 | 4.0956 | +++ | + | +++ |
| B. thuringiensis | CR79 | 4.0956 | N.D. | + | +++ |
| A. tumefaciens | CR22 | 4.0393 | +++ | N.D. | ++ |
| M. oxydans | CR80 | 3.9267 | N.D. | N.D. | N.D. |
| Undetermined | CR21 | 3.8704 | ++ | N.D. | ++ |
| P. lautus | CR72 | 3.6828 | N.D. | N.D. | N.D. |
| L. fusifirmis | CR23 | 3.5702 | N.D. | + | N.D. |
| B. thuringiensis | CR81 | 3.5702 | ++ | + | ++ |
| M. paraoxydans | CR96 | 3.5702 | N.D. | + | N.D. |
| Undetermined | CR45 | 3.5327 | ++ | ++ | ++ |
| A. niigatensis | CR53 | 3.5327 | + | +++ | N.D. |
| B. thuringiensis | CR38 | 3.4952 | ++ | + | +++ |
| B. safensis | CR100 | 3.4577 | N.D. | + | + |
| S. maltophilia | CR49 | 3.2513 | + | + | ++ |
| B. thuringiensis | CR28 | 3.1762 | ++ | ++ | ++ |
| B. thuringiensis | CR50b | 3.1200 | ++ | ++ | +++ |
| Stems | |||||
| M. foliorum | CT10 | 5.2213 | ++ | + | ++ |
| Undetermined | CT5 | 5.2025 | ++ | + | ++ |
| Undetermined | CT22 | 4.9211 | N.D. | + | N.D. |
| B. toyonensis | CT1 | 3.9455 | ++ | +++ | ++ |
| B. anthracis | CT4b | 3.7391 | ++ | +++ | ++ |
| B. thuringiensis | CT14 | 3.1387 | ++ | + | ++ |
| Leaves | |||||
| C. oceanosedimentum | CH3 | 4.2457 | ++ | + | ++ |
| B. vireti | CH21 | 5.2401 | +++ | + | N.D. |
| N. drentensis | CH23 | 6.9474 | +++ | ++ | ++ |
4. Discussion
Some studies have suggested that all 300,000 plant species on the planet contain microbial endophytes in their internal tissues, with bacteria being the most abundant and diverse (Smith et al., 2008). Additionally, bacterial endophytes perform beneficial interactions and functions, such as promoting the growth of their hosts and protecting them from the attack of pathogens (Santoyo et al., 2016) . Recent robust studies employing a metagenomics approach (independent of the culture) have found that Firmicutes, Proteobacteria, Actinobacteria, Verrucomicrobia, Bacteroidetes and Acidobacteria, among the most abundant in the endobiome associated with various plant species (as well as residents of the rhizosphere) (Fitzpatrick et al., 2018), including those of agricultural interest (Mendes et al., 2011; Compant et al., 2010).
In another recent study but employing a culturable bacterial approach, the phylogenetic diversity of bacterial endophytes of mountain-cultivated ginseng plants (MCG, Panax ginseng Meyer) was determined and grouped into four phyla: Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria. Proteobacteria predominated in this plant species, representing ~63% of the total isolates. Among all the reported genera, Pseudomonas (Gamma-Proteobacteria) was the most abundant genus, comprising 48% of the total characterized isolates (Chowdhury et al., 2017). It should be noted that the isolation of endophytes may be restricted by the culture media used. In this way, differences between works with metagenomic and culturable approaches are expected since bacteria like Verrucomicrobia, Bacteroidetes and Acidobacteria are difficult to grow on a general culture media (Janssen et al., 2002; O'Sullivan et al., 2004). In the present work, our interest to explore husk tomato as source of PGPB lead us to employ a culturable bacterial approach. A wide diversity of bacterial endophytes was found, all of the bacteria belonging to the three major cultivable phyla, such as Firmicutes, Protobacteria and Actinobacteria, with not a clear dominance of one specific phylum. Among them the vast majority belonging to genera or species that are widely known as plant growth-promoting bacteria (PGPB). It should be noted that the diversity found in this study coincides with a previous study, where the presence of genera such as Bacillus, Stenotrophomonas, Microbacterium, and Pseudomonas have already been reported as endophytes (Marquez-Santacruz et al., 2010). This suggests that the husk tomato plant endobiome could be strongly associated with these bacterial endophytes, since the sampled plants were collected at different times and from different places. A massive 16S gene sequencing study in husk tomato plants from various regions and different varieties could reinforce this hypothesis.
Several Bacillus species, such as B. thuringiensis, N. drentensis, and B. toyonensis were found as endophytes, in addition to Pseudomonas, Stenotrophomonas, and Microbacterium isolates, which have been reported as endophytes in other plants. For example, Kumar et al. (2016a) isolated and characterized fourteen bacterial endophytes from the rhizome of Curcuma longa L., by their morphological, biochemical characteristics, and sequence analysis of the 16S rRNA gene. Strains of Bacillus cereus, Bacillus thuringiensis, Bacillus sp., Bacillus pumilis, and Pseudomonas putida, among others, were identified in these isolates. These isolates showed good capacities to produce IAA and siderophores, in addition to solubilizing phosphates. Additionally, some Bacillus endophytes exhibited antimicrobial activity against Alternaria alternata, Aureobasidium pullulans, Byssochlamys fulva, and Fusarium solani.
Recently, Chowdhury et al. (2017) analyzed the phylogenetic diversity of bacterial endophytes of mountain-cultivated ginseng plants (MCG, Panax ginseng Meyer) was determined and grouped into four phyla: Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria. Proteobacteria predominated in this study, representing ~63% of the total isolates. Among all the reported genera, Pseudomonas (Gamma-proteobacteria) was the most abundant genus, comprising 48% of the total characterized isolates. It should be noted that the isolation of endophytes may be restricted by the culture media used, which would be convenient for expanding the use of other bacterial culture media that offer the possibility of isolating lesser-known genera. It should also be noted that metagenomic studies (independent of the culture) also corroborate this premise, where Firmicutes, Proteobacteria, and Actinobacteria, among other phyla, are the most abundant in the endobiome associated with various plant species (as well as residents of the rhizosphere), including those of agricultural interest (Compant et al., 2010).
The diversity of microbial endophytes in plants as well as residents of the rhizosphere (which can potentially colonize the internal tissues of the roots), can be subjected to various environmental factors, such as the salinity of soils, the availability of water, and the temperature or altitude of the region (Liu et al., 2017), as well as some biotic factors, such as plant species, development stage, nutritional and health status, and type of tissue analyzed (Hardoim et al., 2012; Afzal et al., 2019; Brown et al., 2020; Maggini et al., 2019). In the present work, species were found that were unique to a particular tissue analyzed, i.e., root, stem, or leaf. While some species, such as B. thuringiensis, B. toyonensis, N. drentensis, Paenibacillus castaneae, P. fluorescens, P. poae, and S. maltophilia were isolated from all the three tissues, suggesting an active colonization of these endophytes in each part of the host. Similar results were recently published by Brown and colleagues (Brown et al., 2020). They detected different abundances of bacterial endophytes as well as a decreased bacterial diversity between rhizosphere and endosphere compartments in Medicago truncatula plants, such as roots, nodules, and leaves.
The tissue-specific dynamics of the endophytic bacterial communities of the Arctic pioneer plant Oxyria digyna were analyzed through massive sequencing of the 16S ribosomal gene. The authors evaluated the endophytic bacterial communities in the leaves and roots of the plants after one growing season and one year in the field, and compared them with those of the wild plants growing in the same site. It was interesting to observe that the OTUs found revealed that the endophytic communities in the roots were more diverse than in the leaves, and the diversity in the plants was greater in the field, and even more in the wild plants. Likewise, the type of tissue had a strong impact on the structure of the endophytic bacterial community. For example, Firmicutes were abundant in the foliar communities, while Proteobacteria and Bacteroidetes were more abundant in the roots. Finally, several of the endophytic OTUs of Oxyria digyna represented diazotrophic bacterial taxa and phosphate solubilizers, suggesting an important role in nutrient acquisition, particularly in nutrient-poor arctic soils (Given et al., 2020).
By interacting with the plant, bacterial endophytes can fulfill important functions, such as stimulating growth and development, increasing production as well as producing antibiotic compounds, in addition to igniting plant defense mechanisms against pathogens and increasing tolerance levels to multiple types of environmental stress (Afzal et al., 2019; Given et al., 2020; Ullah et al., 2019). It has been shown that endophytic root microbiomes harbor a wealth of as yet unknown functional traits that in fact, can protect the plant inside out, especially against fungal attacks (Carrión et al., 2019). Here, the mechanisms of plant growth stimulation and antagonism against fungal pathogens were evaluated, where multiple isolates reported excellent antifungal activities and IAA production, proteases, siderophores, and phosphate solubilization. Studies have shown that these mechanisms are strongly associated directly with promoting plant growth and indirectly with antimicrobial action growth (Fadiji and Babalola, 2020; Singh et al., 2019; Wu et al., 2019). Strategies to introduce beneficial endophytic bacteria in the plant endobiome, avoiding the rhizospheric competence, have been tested, i.e. inoculation of flowers with endophytic bacteria in parental plants leads to the inclusion of these endophytes in the microbiomes of progeny seeds (Mitter et al., 2017), and inoculation endophytic bacteria introduced during in vitro micropropagated strawberry plants remain as endophytic colonizers in the long term seedlings, stimulating enhanced strawberry production under greenhouse experiments (Hernández-Soberano et al., 2020).
Neobacillus drentensis CH23 (previously known as Bacillus drentensis) found to be an excellent growth promoter of husk tomato plants and an antagonist against the four fungal pathogens evaluated (Mahmood et al., 2016). The CH23 strain was among the main isolates, which produced IAA, proteases, siderophores, and solubilized phosphates. Preliminary results show that the strain CH23 also has good ACC deaminase activity, an additional mechanism that could be involved in the beneficial interaction with Physalis ixocarpa. Neobacillus drentensis has been previously reported as a growth promoter in mung bean, rice, and medicinal and aromatic plants (Mahmood et al., 2016; Pereira et al., 2016; Yadav et al., 2011). Finally, these results suggest that the Mexican husk tomato endobiome is composed of endophytic bacteria with beneficial activities, which have enormous potential as bioinoculating agents in field studies.
CRediT authorship contribution statement
Claudia E. Hernández-Pacheco: Investigation. Ma del Carmen Orozco-Mosqueda: Investigation. Aurora Flores: Investigation. Eduardo Valencia-Cantero: Resources, Supervision, Writing – review & editing. Gustavo Santoyo: Conceptualization, Supervision, Resources, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
G.S. thanks CONACYT-México (Proposal: A1-S-15956) and CIC-UMSNH (2020–2021) for financial support. We also thank Miguel Contreras-Pérez and Daniel Rojas-Solis for their technical help.
References
- Afzal I., Khan Shinwari Z., Sikandar S., Shahzad Shaheen. Plant beneficial endophytic bacteria: mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019;221:36–49. doi: 10.1016/j.micres.2019.02.001. February. [DOI] [PubMed] [Google Scholar]
- Avis T.J., Gravel V., Antoun H., Tweddell R.J. Multifaceted beneficial effects of rhizosphere microorganisms on plant health and productivity. Soil Biol. Biochem. 2008;40(7):1733–1740. 10.1016/j.soilbio.2008.02.013. [Google Scholar]
- Brown S.P., Grillo M.A., Podowski J.C., Heath K.D. Soil origin and plant genotype structure distinct microbiome compartments in the model legume medicago truncatula. Microbiome. 2020;8(1):1–17. doi: 10.1186/s40168-020-00915-9. 10.1186/s40168-020-00915-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión V.J., Perez-Jaramillo J., Cordovez V., Tracanna V., de Hollander M., Ruiz-Buck D., Mendes L.W., et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science. 2019;366(6465):606–612. doi: 10.1126/science.aaw9285. 10.1126/science.aaw9285. [DOI] [PubMed] [Google Scholar]
- Chakraborty S., Newton A.C. Climate change, plant diseases and food security: an overview. Plant Pathol. 2011;60(1):2–14. 10.1111/j.1365-3059.2010.02411.x. [Google Scholar]
- Chowdhury E., Khan J.J., Soon O.R., Young H.P., Seung Kyu L., Hanhong B. Composition, diversity and bioactivity of culturable bacterial endophytes in mountain-cultivated ginseng in Korea. Sci. Rep. 2017;7(1):1–10. doi: 10.1038/s41598-017-10280-7. 10.1038/s41598-017-10280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compant S., Clément C., Sessitsch A. Plant Growth-Promoting Bacteria in the Rhizo- and Endosphere of Plants: Their Role, Colonization, Mechanisms Involved and Prospects for Utilization. Soil Biology and Biochemistry. 2010;42(5):669–678. 10.1016/j.soilbio.2009.11.024. [Google Scholar]
- Contreras M., Loeza P.D., Villegas J., Farias R., Santoyo G. A glimpse of the endophytic bacterial diversity in roots of blackberry plants (Rubus fruticosus) Genet. Mol. Res. 2016;15(3) doi: 10.4238/gmr.15038542. [DOI] [PubMed] [Google Scholar]
- Fadiji A.E., Babalola O.O. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 2020;8:1–20. doi: 10.3389/fbioe.2020.00467. May10.3389/fbioe.2020.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick C.R., Copeland J., Wang P.W., Guttman D.S., Kotanen P.M., Johnson M.T.J. Assembly and ecological function of the root microbiome across angiosperm plant species. Proc. Natl. Acad. Sci. U.S.A. 2018;115(6):E1157–E1165. doi: 10.1073/pnas.1717617115. 10.1073/pnas.1717617115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given C., Häikiö E., Kumar M., Nissinen R. Tissue-specific dynamics in the endophytic bacterial communities in arctic pioneer plant oxyria digyna. Front. Plant Sci. 2020;11:1–13. doi: 10.3389/fpls.2020.00561. May10.3389/fpls.2020.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick, B.R. 2012. “Plant Growth-Promoting Bacteria : Mechanisms and Applications” 2012. [DOI] [PMC free article] [PubMed]
- Hardoim P.R., Hardoim C.C.P., van Overbeek L.S., van Elsas J.D. Dynamics of seed-borne rice endophytes on early plant growth stages. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-León R., Rojas-Solís D., Contreras-Pérez M., Orozco-Mosqueda M.D.C., Macías-Rodríguez L.I., Reyes-de la Cruz H., Valencia-Cantero E., Santoyo G. Characterization of the antifungal and plant growth-promoting effects of diffusible and volatile organic compounds produced by pseudomonas fluorescens strains. Biol. Control. 2015;81 10.1016/j.biocontrol.2014.11.011. [Google Scholar]
- Hernández-Soberano, C., L.F. Ruiz-Herrera and E. Valencia-Cantero 2020. "Endophytic bacteria Arthrobacter agilis UMCV2 and Bacillus methylotrophicus M4-96 stimulate achene germination, in vitro growth, and greenhouse yield of strawberry (Fragaria × ananassa)". Scientia Horticulturae 261 (2020): 109005. doi: 10.1016/j.scienta.2019.109005. [DOI]
- Janssen P.H., Yates P.S., Grinton B.E., Taylor P.M., Sait M. Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions acidobacteria, actinobacteria, proteobacteria, and verrucomicrobia. Appl. Environ. Microbiol. 2002;68(5):2391–2396. doi: 10.1128/AEM.68.5.2391-2396.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel S.L., Firrincieli A., Joubert P.M., Okubara P.A., Leston N.D., McGeorge K.M., Mugnozza G.S., Harfouche A., Kim S.H., Doty S.L. An in vitro study of bio-control and plant growth promotion potential of Salicaceae endophytes. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.00386. MAR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Singh R., Yadav A., Giri D.D., Singh P.K., Pandey K.D. Isolation and characterization of bacterial endophytes of Curcuma Longa L. 3 Biotech. 2016;6(1):1–8. doi: 10.1007/s13205-016-0393-y. 10.1007/s13205-016-0393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Carvalhais L.C., Crawford M., Singh E., Dennis P.G., Pieterse C.M.J., Schenk P.M. Inner plant values: diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 2017;8:1–17. doi: 10.3389/fmicb.2017.02552. DEC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggini V., Mengoni A., Gallo E.R., Biffi S., Fani R., Firenzuoli F., Bogani P. Tissue specificity and differential effects on in vitro plant growth of single bacterial endophytes isolated from the roots, leaves and rhizospheric soil of echinacea purpurea. BMC Plant Biol. 2019;19(1):1–9. doi: 10.1186/s12870-019-1890-z. 10.1186/s12870-019-1890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood S., Daur I., Al-Solaimani S.G., Ahmad S., Madkour M.H., Yasir M., Hirt H., Ali S., Ali Z. Plant growth promoting rhizobacteria and silicon synergistically enhance salinity tolerance of mung bean. Front. Plant Sci. 2016;7 doi: 10.3389/fpls.2016.00876. JUNE2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Santacruz H.A., Hernandez-Leon R., Orozco-Mosqueda M.C., Velazquez-Sepulveda I., Santoyo G. Diversity of bacterial endophytes in roots of Mexican husk tomato plants (Physalis Ixocarpa) and their detection in the rhizosphere. Genet. Mol. Res.: GMR. 2010;9(4) doi: 10.4238/vol9-4gmr921. [DOI] [PubMed] [Google Scholar]
- Mendes R., Kruijt M., de Bruijn I., Dekkers E., van der Voort M., Schneider J.H.M., Piceno Y.M., et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science. 2011;332(6033):1097–1100. doi: 10.1126/science.1203980. 10.1126/science.1203980. [DOI] [PubMed] [Google Scholar]
- Mitter B., Pfaffenbichler N., Flavell R., Compant S., Antonielli L., Petric A., Berninger T., et al. A new approach to modify plant microbiomes and traits by introducing beneficial bacteria at flowering into progeny seeds. Front. Microbiol. 2017;8:1–10. doi: 10.3389/fmicb.2017.00011. JAN10.3389/fmicb.2017.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan L.A., Fuller K.E., Thomas E.M., Turley C.M., Fry J.C., Weightman A.J. Distribution and culturability of the uncultivated ‘AGG58 cluster’ of the bacteroidetes phylum in aquatic environments. FEMS Microbiol. Ecol. 2004;47(3):359–370. doi: 10.1016/S0168-6496(03)00300-3. 10.1016/S0168-6496(03)00300-3. [DOI] [PubMed] [Google Scholar]
- del Carmen Orozco-Mosqueda M., Gustavo S. Plant-microbial endophytes interactions: scrutinizing their beneficial mechanisms from genomic explorations. Curr. Plant Biol. 2020 no. xxxx: 100189. 10.1016/j.cpb.2020.100189. [Google Scholar]
- Pereira S.I.A., Monteiro C., Vega A.L., Castro P.M.L. Endophytic culturable bacteria colonizing Lavandula dentata L. plants: isolation, characterization and evaluation of their plant growth-promoting activities. Ecol. Eng. 2016;87:91–97. 10.1016/j.ecoleng.2015.11.033. [Google Scholar]
- Rojas-Solis D., Hernandez-Pacheco C.E., Santoyo G. Evaluation of Bacillus and Pseudomonas to colonize the rhizosphere and their effect on growth promotion in tomato (Physalis Ixocarpa Brot. Ex Horm.) Rev. Chapingo Serie Hortic. 2016;22(1):45–57. 10.5154/r.rchsh.2015.06.009. [Google Scholar]
- Santoyo G., Moreno-Hagelsieb G., del Carmen Orozco-Mosqueda M., Glick B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016 doi: 10.1016/j.micres.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Santoyo, G., J.M. Sánchez-Yáñez, and S. de los Santos-Villalobos. 2019. “Methods for Detecting Biocontrol and Plant Growth-Promoting Traits in Rhizobacteria.” In . 10.1007/978-981-13-5767-1_8.
- Singh M., Kumar A., Pandey K.D. Biochemical and molecular identification of Solanum Lycopersicum L. temperature tolerant bacterial endophytes. Biocatal. Agric. Biotechnol. 2019;22 10.1016/j.bcab.2019.101409. [Google Scholar]
- Smith S.A., Tank D.C., Boulanger L.A., Bascom-Slack C.A., Eisenman K., Kingery D., Babbs B., et al. Bioactive endophytes warrant intensified exploration and conservation. PLoS One. 2008;3(8):1–4. doi: 10.1371/journal.pone.0003052. 10.1371/journal.pone.0003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah A., Nisar M., Ali H., Hazrat A., Hayat K., Keerio A.A., Ihsan M., et al. Drought tolerance improvement in plants: an endophytic bacterial approach. Appl. Microbiol. Biotechnol. 2019;103(18):7385–7397. doi: 10.1007/s00253-019-10045-4. [DOI] [PubMed] [Google Scholar]
- Weisburg W.G., Barns S.M., Pelletier D.A., Lane D.J. 16S Ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991;173(2):697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhou J., Li C., Ma Y. Antifungal and plant growth promotion activity of volatile organic compounds produced by Bacillus amyloliquefaciens. MicrobiologyOpen. 2019;8(8):1–14. doi: 10.1002/mbo3.813. 10.1002/mbo3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S., Kaushik R., Saxena A.K., Arora D.K. Diversity and phylogeny of plant growth-promoting bacilli from moderately acidic soil. J. Basic Microbiol. 2011;51(1):98–106. doi: 10.1002/jobm.201000098. [DOI] [PubMed] [Google Scholar]
- Yaish M.W., Irin A., Glick B.R. Isolation and characterization of endophytic plant growth-promoting bacteria from date palm tree (Phoenix Dactylifera L.) and their potential role in salinity tolerance. Antonie van Leeuwenhoek, Int. J. Gen. Mol. Microbiol. 2015;107(6):1519–1532. doi: 10.1007/s10482-015-0445-z. 10.1007/s10482-015-0445-z. [DOI] [PubMed] [Google Scholar]